PURPOSE
To investigate the efficacy of PD-1/PD-L1 inhibitors plus chemotherapy versus anti–PD-1/PD-L1 monotherapy in advanced microsatellite instability (MSI)/mismatch repair-deficient (dMMR) gastrointestinal cancers.
METHODS
We retrospectively recruited patients with MSI/dMMR gastrointestinal cancer who received anti–PD-1/PD-L1 with or without chemotherapy and compared objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) of PD-1/PD-L1 inhibitor plus chemotherapy (chemo-anti–PD-1/PD-L1 group) and PD-1/PD-L1 inhibitor alone (anti–PD-1/PD-L1 group). Propensity score–based overlap weighting analysis was conducted to adjust the baseline covariable imbalance. Sensitivity analysis was performed to confirm the stability of the results by propensity score matching and multivariable Cox and logistic regression models.
RESULTS
A total of 256 patients were eligible, with 68 and 188 receiving chemo-anti–PD-1/PD-L1 and anti–PD-1/PD-L1, respectively. The chemo-anti–PD-1/PD-L1 group showed significant improvements versus the anti–PD-1/PD-L1 group in ORR (61.8% v 38.8%; P = .001), DCR (92.6% v 74.5%; P = .002), PFS (median PFS [mPFS], not reached [NR] v 27.9 months; P = .004), and OS (median OS [mOS], NR v NR; P = .014). After overlap weighting, the improvements tended to be more significant with chemo-anti–PD-1/PD-L1 versus anti–PD-1/PD-L1 in ORR (62.5% v. 38.3%; P < .001), DCR (93.8% v 74.2%; P < .001), PFS (mPFS, NR v 26.0 months; P = .004), and OS (mOS, NR v NR; P = .010). These results were solidified through sensitivity analysis.
CONCLUSION
Chemo-anti–PD-1/PD-L1 is superior to anti–PD-1/PD-L1 in MSI/dMMR gastrointestinal cancers with improved efficacy.
BACKGROUND
Among the top 10 most common cancers globally, gastrointestinal cancers account for four, with colorectal cancer (CRC) ranking third and gastric cancer (GC), liver cancer, and esophagus cancer ranking fifth through seventh. In terms of mortality, gastrointestinal cancers make up half of the top 10 worldwide, with CRC, liver cancer, and GC ranking second through fourth.1 Moreover, gastrointestinal cancers are also a heavy burden to China and contribute to three and four of the top five cancers in incidence and mortality, respectively.2,3 Microsatellite instability (MSI) or mismatch repair-deficient (dMMR) is a distinct subtype featured by defects in the mismatch repair mechanisms and accumulation of mutant antigens, presenting in approximately 5% of metastatic CRC (mCRC) and GC (mGC).4,5
CONTEXT
Key Objective
PD-1/PD-L1 inhibitors are the standard therapy for microsatellite instability (MSI)/mismatch repair-deficient (dMMR) gastrointestinal cancers. However, about 30%-40% of patients progressed on anti–PD-1/PD-L1 monotherapy. Here, we performed a retrospective multicenter study in China to explore whether anti–PD-1/PD-L1 plus chemotherapy (chemo-anti–PD-1/PD-L1) was superior to anti–PD-1/PD-L1 monotherapy in MSI/dMMR gastrointestinal cancers. To reduce the interference of confounding factors, propensity score–based overlap weighting analysis, and sensitivity analysis were adopted.
Knowledge Generated
Chemo-anti–PD-1/PD-L1 achieved significantly better efficacy than anti–PD-1/PD-L1 monotherapy in MSI/dMMR gastrointestinal cancers in both unweighted and weighted analyses. These results were solidified through sensitivity analysis.
Relevance
Our results support the application of chemo-anti–PD-1/PD-L1 in patients with MSI/dMMR gastrointestinal cancer to improve efficacy and prognosis, especially for patients with potential resistance factors to anti–PD-1/PD-L1 monotherapy. However, considering the retrospective design, the conclusions need to be further confirmed in prospective studies.
MSI has been assessed as a pan-cancer biomarker for immune checkpoint inhibitor (ICI) therapy.6 With growing evidence demonstrating remarkable efficacy, ICI has been specified as the standard treatment for MSI gastrointestinal cancers and even the first-line treatment for MSI mCRC in the latest guideline.7,8 However, not all patients with MSI/dMMR gastrointestinal cancer benefit from ICI therapy. In the phase III KEYNOTE177 trial, the objective response was observed in only 44% of patients with MSI/dMMR mCRC treated with pembrolizumab versus an objective response rate (ORR) of 33% in patients receiving conventional chemotherapy with or without targeted treatment.7 Considering that approximately 30%-46% of patients have primary resistance to immune monotherapy,7,9 growing studies are assessing how to improve the efficacy through combined therapy. It has been reported that chemotherapy could enhance the benefits of ICI therapy by activating effector cells, inhibiting immunosuppressive cells, or/and increasing the production of neoantigens.10 It was reported in a retrospective study that patients with MSI/dMMR gastrointestinal cancer who had progressed on PD-1/PD-L1 inhibitors could still benefit from anti–PD-1/PD-L1 plus chemotherapy.11 Recently, the combination strategy of anti–PD-1/PD-L1 and chemotherapy has been proven to be more effective than chemotherapy and as the standard of care in many cancers, including lung cancer, GC, and esophagus cancer.12-15 Notably, in non–small-cell lung cancer (NSCLC), the PFS and OS curves of anti–PD-1/PD-L1 versus chemotherapy were always crossed in prior studies.16-18 However, the crossover disappeared when comparing the efficacy of anti–PD-1/PD-L1 plus chemotherapy with chemotherapy or anti–PD-1/PD-L1 monotherapy,19,20 indicating the potential superior efficacy of chemotherapy plus immunotherapy versus anti–PD-1/PD-L1 monotherapy.
There have been a few studies with small sample sizes that assessed the efficacy of chemotherapy plus immunotherapy in advanced MSI/dMMR gastrointestinal cancers. For example, in the subgroup analysis of the phase III KEYNOTE-062 study, nivolumab combined with chemotherapy achieved a slightly higher ORR (64.7% v 57.1%) but a lower 12-month overall survival (OS) rate (71.0% v 79.0%) compared with nivolumab monotherapy as first-line therapy for patients with MSI GC.8 A retrospective study also showed that chemotherapy plus immunotherapy achieved a higher ORR than immunotherapy alone in patients with advanced MSI GC (61.5% v 25%), although the difference was not significant.21 As for CRC, the ORR and disease control rate (DCR) of PD-1/PD-L1 inhibitors monotherapy were 31%-44% and 51%-69%, respectively.7,9,22 In a phase II trial, 10 patients with MSI/dMMR mCRC received FOLFOXIRI plus bevacizumab and nivolumab, and the ORR and DCR were 70% and 100%, respectively.23 Moreover, in a phase I study, four patients with MSI/dMMR mCRC were treated with FOLFOX plus pembrolizumab, and three patients achieved a partial response (PR), of whom one showed a durable response.24 These results indicated a potential efficacy advantage of chemotherapy plus PD-1/PD-L1 inhibitors versus anti–PD-1/PD-L1 monotherapy in MSI/dMMR gastrointestinal cancers, but the sample size was too small to obtain a definite conclusion.
In an online survey conducted among 1,257 clinicians in China, 42% and 58% supported chemotherapy plus PD-1/PD-L1 inhibitors and PD-1/PD-L1 inhibitors monotherapy for MSI/dMMR gastrointestinal cancers, respectively.25 The combination of chemotherapy with PD-1/PD-L1 inhibitors was performed in clinical practice by the clinicians who supported it, although this strategy lacked high-level evidence of improved efficacy over PD-1/PD-L1 inhibitors alone. Therefore, here we collected data of 256 patients from 30 hospitals to compare the efficacy of anti–PD-1/PD-L1 monotherapy and anti–PD-1/PD-L1 plus chemotherapy.
METHODS
Patients
This retrospective study collected data from 30 hospitals in China from February 2016 to April 2022. The relevant clinicopathologic and genetic characteristics were extracted from the medical records for each patient when available. All patients included satisfied the following criteria: (1) age 18 years and older; (2) histologically confirmed gastrointestinal cancer; (3) diagnosed as stage IV according to the 8th American Joint Committee on Cancer staging system, or staged II-III but were unresectable or unsuitable for surgery; (4) confirmed as dMMR through immunohistochemistry or MSI through polymerase chain reaction (PCR) or next-generation sequencing (NGS). In case of any inconsistency between the results of PCR/NGS and immunohistochemistry, the prior shall prevail; (5) received anti–PD-1/PD-L1 monotherapy or anti–PD-1/PD-L1 plus chemotherapy for at least one dose. For patients receiving anti–PD-1/PD-L1 plus chemotherapy, targeted therapy was allowed; (6) could be evaluated for response according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.126; and (7) normal function of heart, liver, and kidney. Patients with the following conditions were excluded: (1) a previous history of malignant tumors within 5 years; (2) received anti–PD-1/PD-L1 plus anti–cytotoxic T lymphocyte antigen-4 (CTLA-4); (3) received anti–PD-1/PD-L1 plus targeted therapy; and (4) additional local therapy, including radiotherapy, radiofrequency ablation, and transcatheter arterial chemoembolization. The flowchart of patient selection is presented in Figure 1.
FIG 1.
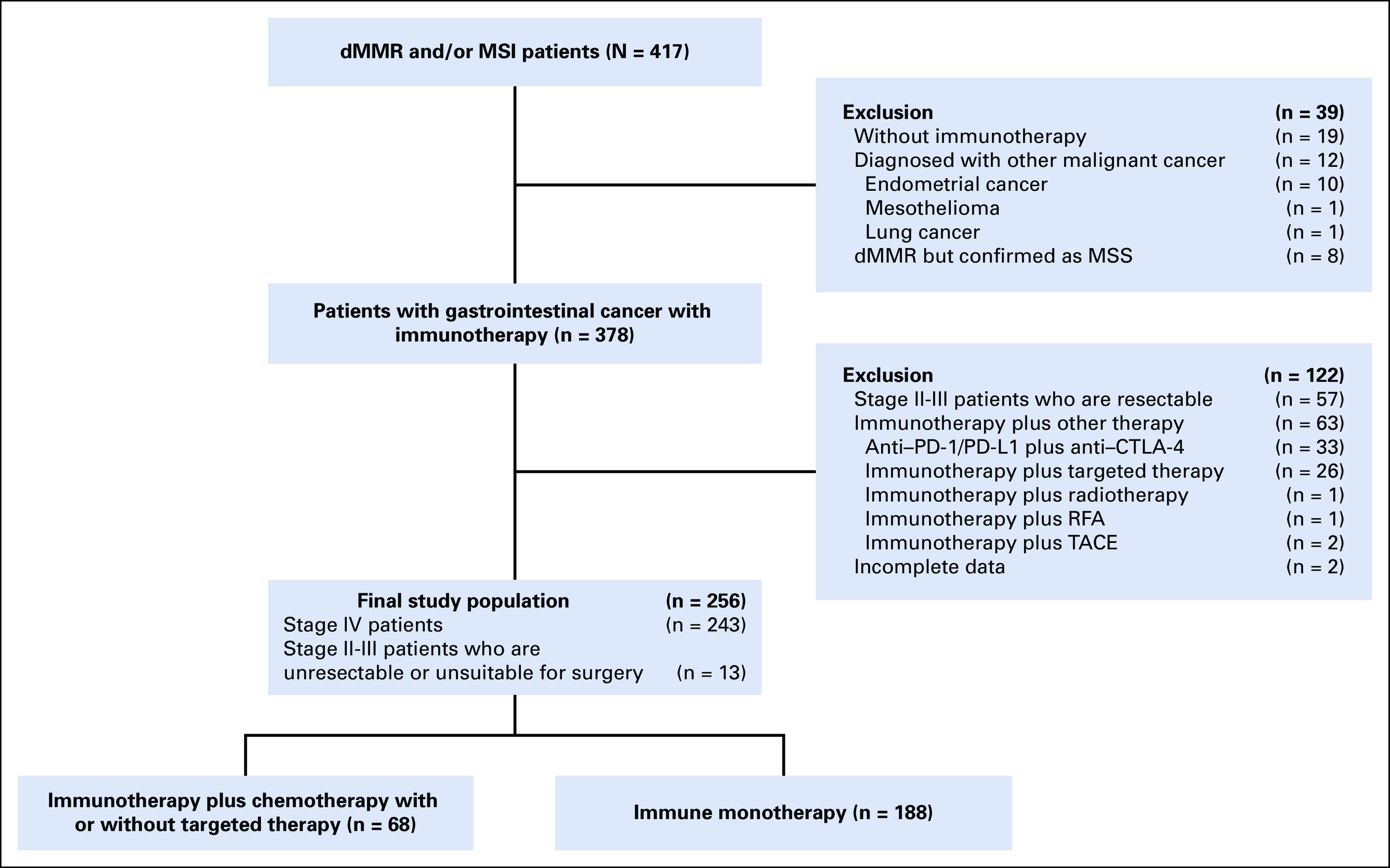
Flowchart of patient selection. chemo-anti–PD-1/PD-L1, combination of chemotherapy plus anti–PD-1/PD-L1 therapy; CTLA-4, cytotoxic T-lymphocyte–associated antigen-4; dMMR, mismatch repair-deficient; MSI, microsatellite instability; MSS, microsatellite stability; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Outcome Evaluation
All patients received CT or MR scans to evaluate efficacy regularly. The best response to therapy was assessed as complete response (CR), PR, stable disease (SD), or progressive disease (PD) on the basis of RECIST 1.1 by clinicians from different centers. Clinical outcomes included ORR, DCR, PFS, and OS. ORR was defined as the proportion of patients whose best response was CR or PR. DCR was defined as the proportion of patients with the best response of CR, PR, or SD. PFS was defined as the time from the beginning of therapy to disease progression or death from any cause, whichever happened first. OS was defined as the time from the beginning of therapy to death from any cause.
Statistical Analysis
Baseline clinicopathologic characteristics were presented as percentages. Categorical variables were assessed using Pearson's chi-square test or Fisher's exact test, as appropriate. PFS and OS were analyzed through the Kaplan-Meier method with the log-rank test. The logistic regression model and the Cox proportional hazard regression model were applied to calculate the odds ratio (OR) and hazard ratio (HR) along with corresponding 95% CI, respectively. To reduce the interference of confounding factors in this retrospective study, overlap weighting analysis was used, which is a propensity score method aimed at imitating the effects of randomized controlled trials (RCTs).27,28 Standardized mean differences (SMD) before and after propensity score analysis were compared with test equilibrium. Weighted Cox regression and logistic regression analyses were conducted in each subgroup to assess the efficacy of these two treatment regimens. Sensitivity analysis was performed using propensity score matching, multivariate Cox regression, and multivariate logistic regression to evaluate the robustness of the results. In Cox and logistic regression analysis, the significant factors (P ≤ .1) that might influence survival and response in the univariate analysis were further submitted into multivariate analysis. Bilateral P < .05 was defined as statistically significant in all analyses. All analyses were conducted using R software (version 4.0.3).
All participants provided written informed consent.
Human samples and clinical data were collected and used in accordance with the principles of the Declaration of Helsinki and approved by the institutional review board of Beijing Cancer Hospital (approval ID: 2021YJZ34).
RESULTS
Characteristics of Patients
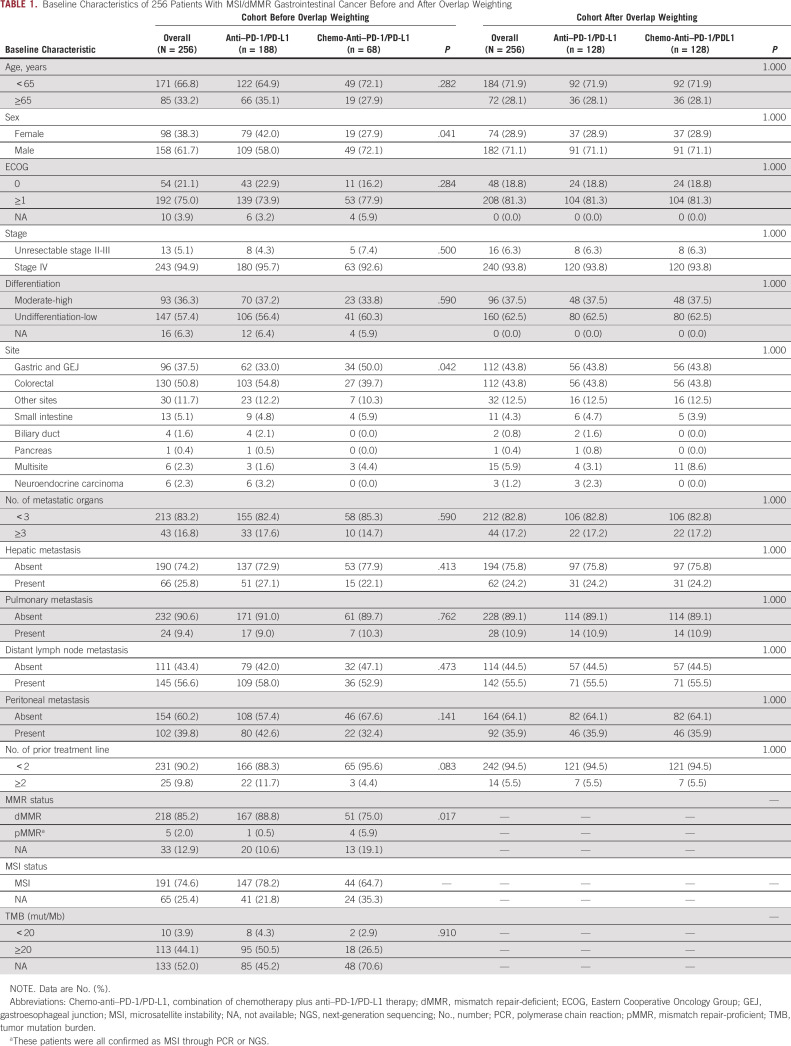
A total of 417 patients who were diagnosed with dMMR and/or MSI cancers were reviewed. Finally, 256 patients who met the inclusion criteria were included. The median follow-up time was 15.8 months. Table 1 presents the patients' baseline characteristics. In this cohort, 188 patients received PD-1/PD-L1 inhibitors alone (anti–PD-1/PD-L1 group), and 68 patients received the combination regimen of chemotherapy and PD-1/PD-L1 inhibitors (chemo-anti–PD-1/PD-L1 group). In the chemo-anti–PD-1/PD-L1 group, 18 patients received concurrent targeted therapy, including anti–vascular endothelial growth factor, anti–epidermal growth factor receptor, or anti–human epidermal growth factor receptor-2. The two treatment groups were comparable in all baseline characteristics except sex and the tumor site. The anti–PD-1/PD-L1 group had a higher proportion of female patients and patients with CRC than the chemo-anti–PD-1/PD-L1 group.
TABLE 1.
Baseline Characteristics of 256 Patients With MSI/dMMR Gastrointestinal Cancer Before and After Overlap Weighting
Altogether, 218 (85.2%) and 191 (74.6%) patients were confirmed as dMMR by staining four mismatch repair (MMR) proteins and MSI by NGS or PCR, respectively (Table 1 and Appendix Fig A1A). It should be mentioned that five patients with immunohistochemistry diagnoses of mismatch repair-proficient (pMMR) were later determined to be MSI by PCR or NGS and were incorporated into the analysis. As shown in Appendix Fig A1B, the loss of coexpression of MLH1/PMS2 was the most prevalent pattern in both dMMR CRC and GEJ/GC, and the loss of coexpression of MSH2/MSH6 was also common in dMMR CRC. Among the 123 patients with available results of tumor mutation burden (TMB), 113 (91.9%) patients had TMB values of 20 mut/Mb or more. Besides, gene sequencing results were available in 88 patients, among whom 16 lacked detailed information about PMS2 gene. Altogether, nine patients were found to have Lynch syndrome with germline mutations in MMR genes, and 43 patients were found to have somatic MMR gene mutations (Appendix Figs A1C and A1D).
Efficacy in the Whole Population
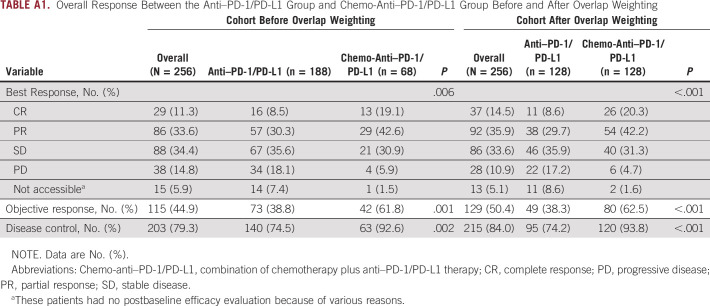
In the whole population, ORR and DCR were 44.9% and 79.3%, respectively. Compared with the anti–PD-1/PD-L1 group, the chemo-anti–PD-1/PD-L1 group achieved significantly better ORR (61.8% v 38.8%; P = .001) and DCR (92.6% v 74.5%; P = .002; Appendix Table A1). PFS was significantly improved in the chemo-anti–PD-1/PD-L1 group compared with the anti–PD-1/PD-L1 group (median PFS [mPFS], not reached [NR] v 27.9 months; HR, 0.44; 95% CI, 0.25 to 0.78; log-rank P = .004; Fig 2A). Median OS (mOS) was not reached in neither of the two groups. Besides, the OS curves of the two groups were observed to be crossed (HR, 0.36; 95% CI, 0.15 to 0.85; log-rank P = .014; Fig 2B). In the chemo-anti–PD-1/PD-L1 group and anti–PD-1/PD-L1 group, the 2-year PFS rates were 68.6% (95% CI, 51.0 to 86.2) and 52.5% (95% CI, 44.1 to 60.9), respectively, and the 2-year OS rates were 85.6% (95% CI, 72.9 to 98.3) and 69.3% (95% CI, 61.3 to 77.3), respectively.
FIG 2.
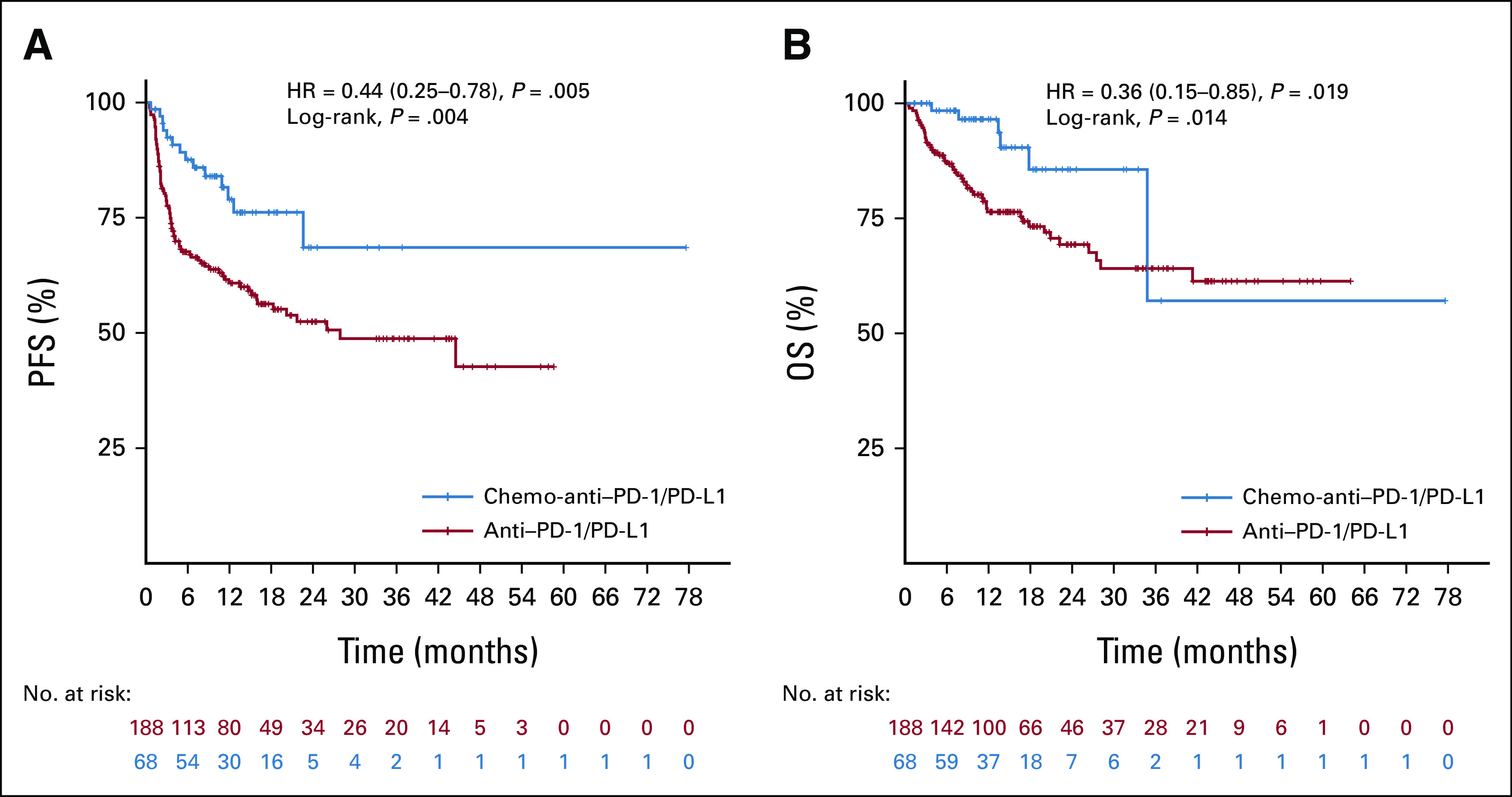
Kaplan-Meier curves of (A) PFS and (B) OS for patients with MSI/dMMR gastrointestinal cancer who received anti–PD-1/PD-L1 therapy and chemo-anti–PD-1/PD-L1 therapy. Tick marks mean censored data. The chemo-anti–PD-1/PD-L1 group showed significantly longer PFS (P = .004) than the anti–PD-1/PD-L1 group with log-rank analysis. Although the log-rank P was .014 for the OS curves, there is a crossing in the two curves. chemo-anti–PD-1/PD-L1, combination of chemotherapy plus anti–PD-1/PD-L1 therapy; dMMR, mismatch repair-deficient; HR, hazard ratio; MSI, microsatellite instability; OS, overall survival; PFS, progression-free survival.
Until the data cutoff, the therapy status of the 68 patients in the chemo-anti–PD-1/PD-L1 group was presented in Appendix Figure A2. Generally, all patients received combined therapy for four to eight cycles and then maintenance therapy. Among the 46 patients who experienced maintenance therapy, 34 received anti–PD-1/PD-L1 monotherapy, seven received anti–PD-1/PD-L1 plus targeted therapy, four received anti–PD-1/PD-L1 plus oral chemotherapy agents, and one received anti–PD-1/PD-L1 plus oral chemotherapy agent and targeted therapy. Altogether, 32 patients had terminated therapy at data cutoff. Except for patients who discontinued therapy for disease progression, adverse events, and willingness, 12 patients achieved the status of no evidence of disease (NED) or pathologic CR (pCR) and discontinued therapy after clinicians' advice, and the duration of therapy was up to 6 months for four patients, 1 year for four patients, and 2 years for four patients, respectively.
Overlap Weighting Analysis
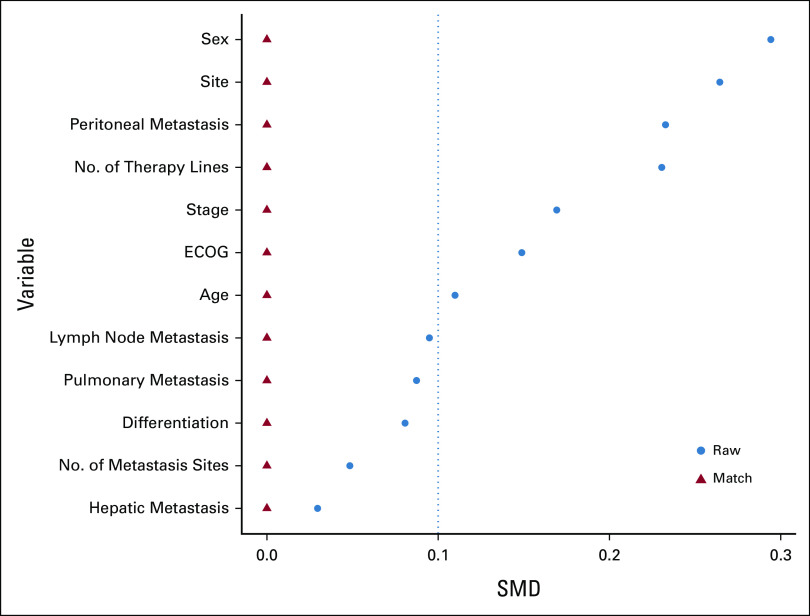
To reduce the influence of covariables on results, we performed an overlap weighting analysis to mimic the RCT process. After propensity score weighting, the anti–PD-1/PD-L1 group and the chemo-anti–PD-1/PD-L1 group were well balanced, with SMD <0.1 for all characteristics (Table 1 and Appendix Fig A3). Compared with the anti–PD-1/PD-L1 weighted group, the chemo-anti–PD-1/PD-L1 weighted group presented significantly better ORR (62.5% v 38.3%; P < .001), DCR (93.8% v 74.2%; P < .001), PFS (mPFS, NR v 26.0 months; HR, 0.40; 95% CI, 0.21 to 0.73; log-rank P = .004), and OS (mOS, NR v NR; HR, 0.29; 95% CI, 0.11 to 0.76; log-rank P = .010; Appendix Table A1 and Figs 3A and 3B).
FIG 3.
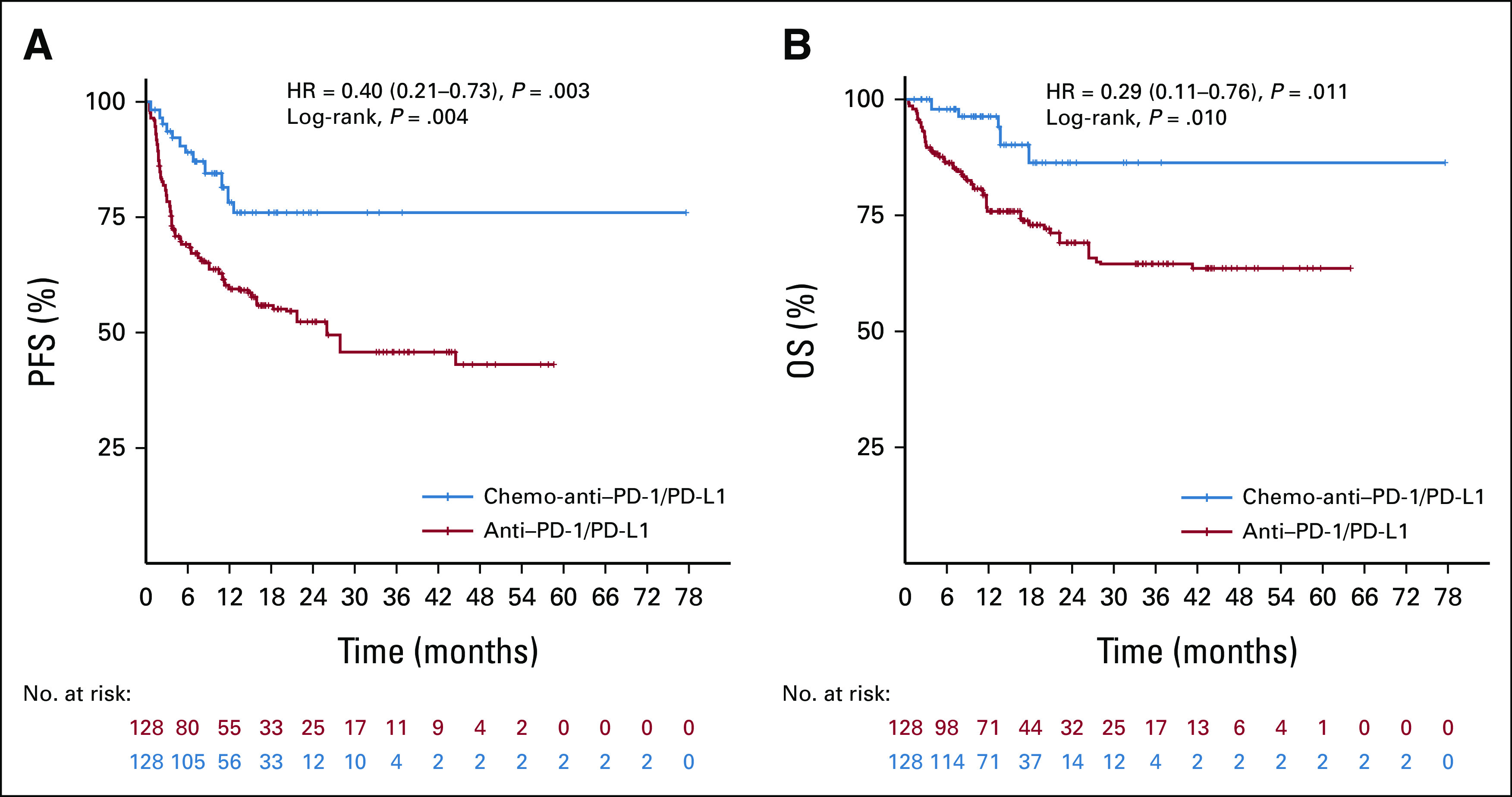
Kaplan-Meier curves of (A) PFS and (B) OS for patients with MSI/dMMR gastrointestinal cancer in the anti–PD-1/PD-L1 weighted group and chemo-anti–PD-1/PD-L1 weighted group. Tick marks indicate censored data. After overlap weighting analysis, PFS (P = .004) and OS (P = .010) were significantly longer in patients receiving chemo-anti–PD-1/PD-L1 than patients receiving anti–PD-1/PD-L1. chemo-anti–PD-1/PD-L1, combination of chemotherapy plus anti–PD-1/PD-L1 therapy; dMMR, mismatch repair-deficient; HR, hazard ratio; MSI, microsatellite instability; OS, overall survival; PFS, progression-free survival.
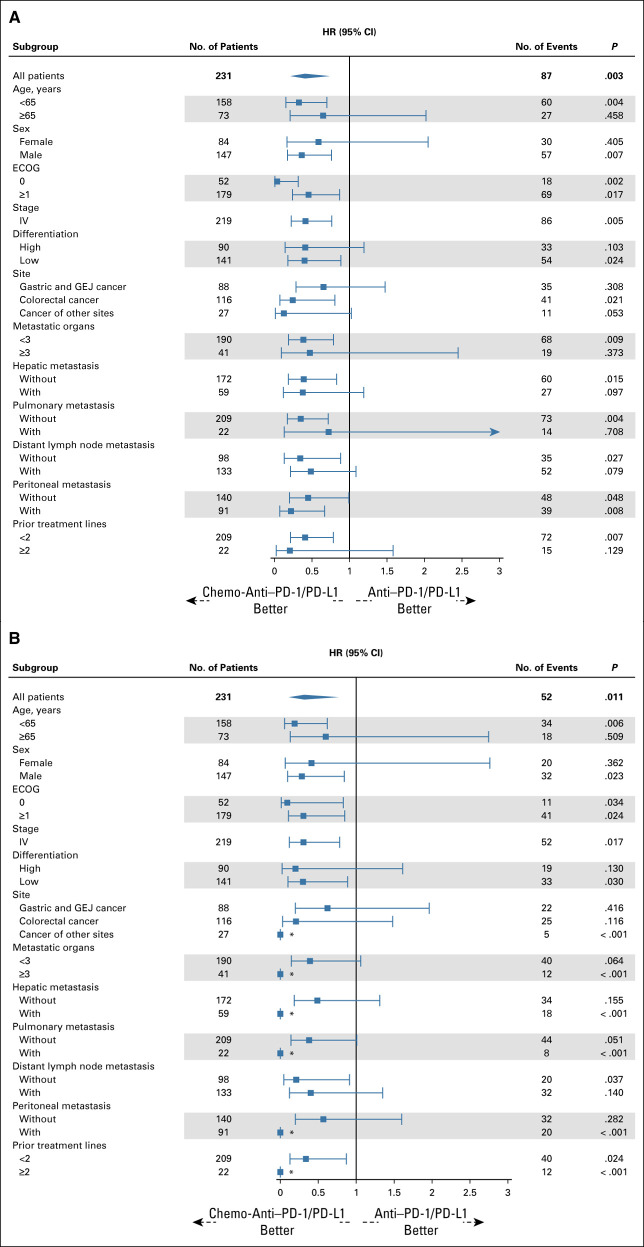
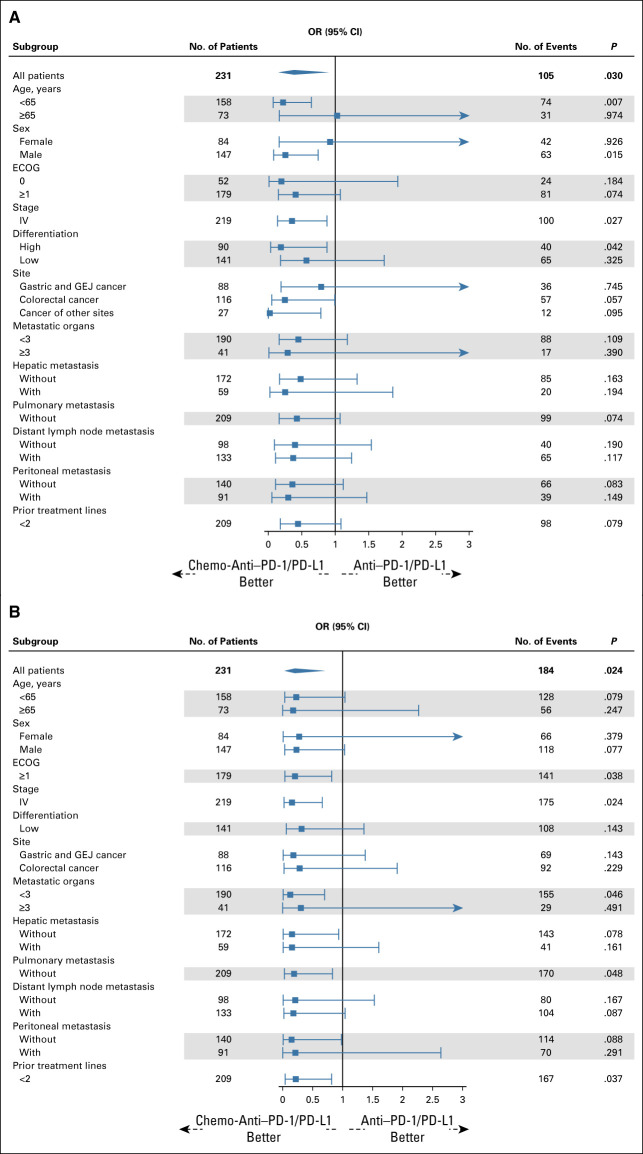
Then, we conducted subgroup analyses using weighted Cox regression and logistic regression according to baseline characteristics. The prolongation of PFS and OS with chemo-anti–PD-1/PD-L1 was generally consistent across all subgroups, including age, sex, ECOG, stage, differentiation, site, metastatic organs, and prior therapy lines (Figs 4A and 4B). Similarly, the improvement of ORR and DCR with chemo-anti–PD-1/PD-L1 was generally consistent across all subgroups, except that female and older (65 years and older) patients seemed to experience similar ORR from these two treatment regimens (Figs 5A and 5B).
FIG 4.
(A) PFS and (B) OS in subgroups of patients receiving chemo-anti–PD-1/PD-L1 compared with anti–PD-1/PD-L1. Weighted subgroup analysis was performed to evaluate the survival of chemo-anti–PD-1/PD-L1 therapy and anti–PD-1/PD-L1 therapy in each subgroup (age, sex, ECOG, stage, differentiation, site, metastatic organs, and prior therapy lines). The propensity score was calculated, and overlap weighting was conducted in each subgroup. Overlap weighting-based Cox regression was performed to calculate HRs, representing the effects of chemo-anti–PD-1/PD-L1 therapy compared with anti–PD-1/PD-L1 therapy. The prolongation of PFS and OS with chemo-anti–PD-1/PD-L1 was generally consistent across all subgroups. *These extreme values were due to that no deaths occurred in the chemo-anti–PD-1/PD-L1 group. chemo-anti–PD-1/PD-L1, combination of chemotherapy plus anti–PD-1/PD-L1 therapy; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; HR, hazard ratio; No., number; OS, overall survival; PFS, progression-free survival.
FIG 5.
(A) ORR and (B) DCR in subgroups of patients receiving chemo-anti–PD-1/PD-L1 compared with anti–PD-1/PD-L1. Weighted subgroup analysis was performed to evaluate the efficacy of chemo-anti–PD-1/PD-L1 therapy and anti–PD-1/PD-L1 therapy in each subgroup (age, sex, ECOG, stage, differentiation, site, metastatic organs, and prior therapy lines). The propensity score was calculated, and overlap weighting was conducted in each subgroup. Overlap weighting-based logistic regression was performed to calculate ORs, representing the effects of chemo-anti–PD-1/PD-L1 therapy compared with anti–PD-1/PD-L1 therapy. The improvement of ORR and DCR with chemo-anti–PD-1/PD-L1 was generally consistent across all subgroups, except that female and older patients seemed to experience similar ORR from these two treatment regimens. chemo-anti–PD-1/PD-L1, combination of chemotherapy plus anti–PD-1/PD-L1 therapy; DCR, disease control rate; dMMR, mismatch repair-deficient; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; MSI, microsatellite instability; No., number; OR, odds ratio; ORR, objective response rate.
Sensitivity Analysis
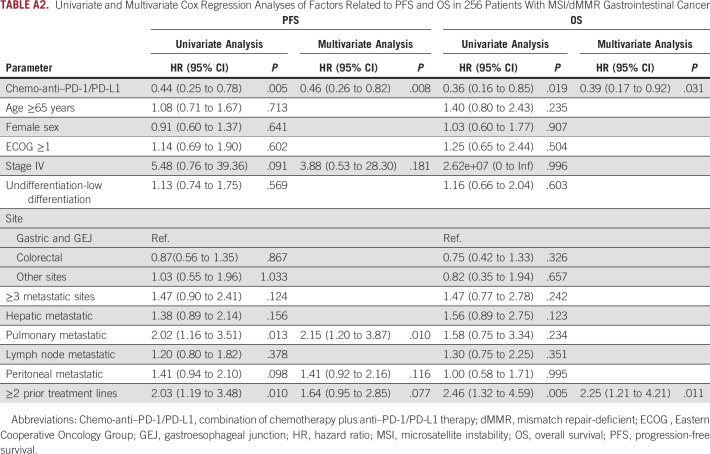
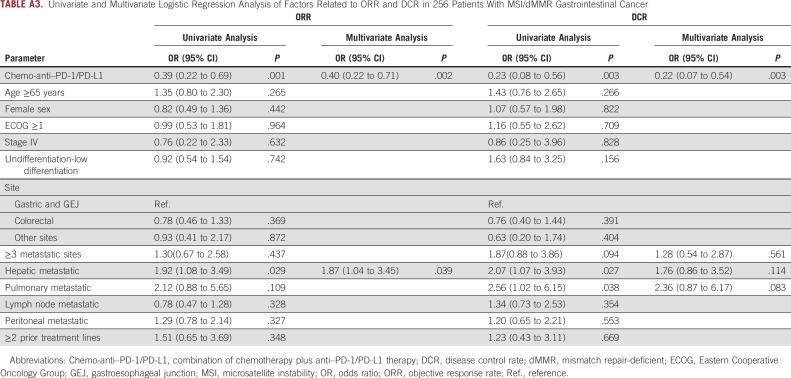
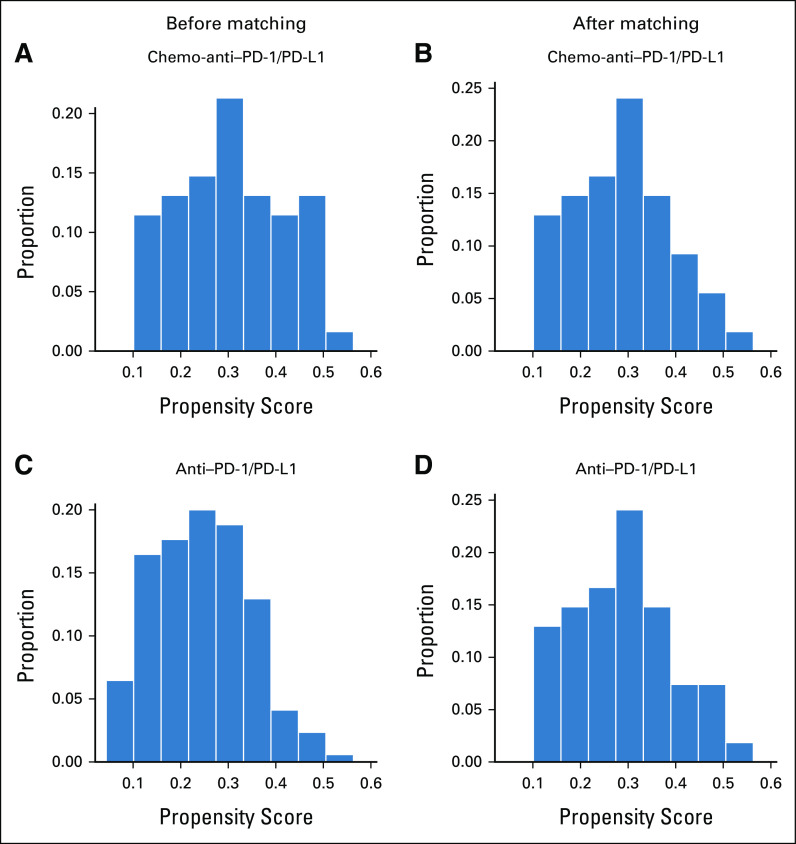
Sensitivity analysis was conducted using propensity score matching and multivariate Cox regression and logistic regression analysis. Patients were matched at 1:1 in the two groups, and a total of 108 patients were matched successfully with improved distribution of propensity scores (Appendix Figs A4A–A4D). Consistent with the above results, the chemo-anti–PD-1/PD-L1 matched group showed significant improvement versus the anti–PD-1/PD-L1 matched group in ORR (64.8% v 38.9%; P = .007), DCR (94.4% v 70.4%; P = .002), PFS (mPFS, NR v NR; HR, 0.40; 95% CI, 0.19 to 0.85; log-rank P = .013), and OS (mOS, NR v NR; HR, 0.26; 95% CI, 0.09 to 0.81, log-rank P = .012; Appendix Figs A5A and A5B). In addition, univariate and multivariate Cox analyses were conducted to investigate the effects of all baseline characteristics on PFS and OS. As demonstrated in Appendix Table A2, chemo-anti–PD-1/PD-L1 therapy was superior to anti–PD-1/PD-L1 monotherapy in improving PFS (HR, 0.46; 95% CI, 0.26 to 0.82; P = .008) and OS (HR, 0.39; 95% CI, 0.17 to 0.92; P = .031). We also investigated the effects of all baseline characteristics on ORR and DCR by univariate and multivariate logistic analysis. Similarly, chemo-anti–PD-1/PD-L1 therapy was significantly superior to anti–PD-1/PD-L1 monotherapy in improving ORR (P = .002) and DCR (P = .003; Appendix Table A3).
Additionally, Cox analyses also showed that the PFS of patients with pulmonary metastasis was significantly worse than patients without pulmonary metastasis (P = .010), although OS was not affected. Besides, in patients who received ≥2 lines of prior treatment, the PFS was impaired (P = .077), and OS was significantly worse (P = .011). The logistic analysis also presented that patients with hepatic metastasis had significantly worse ORR than those without hepatic metastasis (P = .039), and patients with pulmonary metastasis tended to have worse DCR than those without (P = .083).
DISCUSSION
To our knowledge, this is the first study to systematically compare the efficacy of anti–PD-1/PD-L1 plus chemotherapy versus anti–PD-1/PD-L1 monotherapy in MSI/dMMR gastrointestinal cancers. In this multicenter study covering most regions of China with 256 patients, the ORR for the anti–PD-1/PD-L1 group was 38.8%, similar to the results of prior prospective studies.7,9,29 Both unweighted and weighted analyses showed that the addition of chemotherapy to anti–PD-1/PD-L1 significantly improved ORR, DCR, PFS, and OS. These results were confirmed by different statistical analysis methods, further supporting the superiority of PD-1/PD-L1 blockade plus chemotherapy.
A retrospective study demonstrated that anti–PD-1/PD-L1 plus chemotherapy achieved significantly longer PFS (mPFS, 15.5 months v 4.6 months; HR, 0.43; 95% CI, 0.32 to 0.58; P < .001) and OS (mOS, NR v 24.8 m; HR, 0.30; 95% CI, 0.17 to 0.51; P < .001) than anti–PD-1/PD-L1 in advanced NSCLC,20 indicating that anti–PD-1/PD-L1 plus chemotherapy was more effective than anti–PD-1/PD-L1 monotherapy. In KEYNOTE177, the PFS curves of anti–PD-1/PD-L1 and chemotherapy were observed to be crossed in MSI CRC, and the rate of PD was higher with anti–PD-1/PD-L1 versus chemotherapy (29.4% v 12.3%).7 It was encouraging to note that our study discovered that the PFS curves of chemo-anti–PD-1/PD-L1 and anti–PD-1/PD-L1 were separated completely, and the rate of PD was lower with chemo-anti–PD-1/PD-L1 versus anti–PD-1/PD-L1 (5.9% v 18.1%), indicating this combination strategy to be feasible and promising. Nevertheless, the OS curves of chemo-anti–PD-1/PD-L1 and anti–PD-1/PD-L1 were observed to be crossed in our study, which might be because the follow-up time was not long enough to generate enough events in the group of chemo-anti–PD-1/PD-L1. It was worth noting that after weighted analysis, the two curves were separate. We look forward to the results of the RCT (ClinicalTrials.gov identifier: NCT02997228), which compares the efficacy of mFOLFOX6/bevacizumab with or without atezolizumab as first-line therapy in MSI/dMMR mCRC.
In weighted subgroup analysis, almost all subgroup patients receiving chemo-anti–PD-1/PD-L1 therapy demonstrated an advantage in improving PFS, OS, ORR, and DCR compared with those with anti–PD-1/PD-L1 therapy, except for female and older (65 years and older) patients who seemed to experience similar ORR from these two regimens. Generally, older patients were thought to have worse foundation conditions and more comorbidities, resulting in poor tolerance and efficacy to chemotherapy. Moreover, since their immune function was weakened, the efficacy of stimulating immunity through the addition of chemotherapy was also discounted,30 which might partly explain the insufficient advantage of chemo-anti–PD-1/PD-L1 compared with anti–PD-1/PD-L1 therapy in older patients. As for female patients, it was reported that because of the protective effect of estrogen, the body of female patients was better at producing inflammation and immune response.31 Therefore, the extra addition of chemotherapy might be less effective in female patients than male patients, indicating that for special patients, sometimes less is more. Moreover, we observed that patients of different tumor sites all showed an advantage in efficacy when receiving chemo-anti–PD-1/PD-L1, although the advantage seemed to be greater in patients with mCRC. As mentioned above, the superiority of the first-line treatment with PD-L1 antibody plus chemotherapy is being investigated in an RCT (ClinicalTrials.gov identifier: NCT02997228). In our subgroup analysis of second-line or later treatment, the benefit of chemo-anti–PD-1/PD-L1 versus anti–PD-1/PD-L1 was still observed, indicating chemo-anti–PD-1/PD-L1 therapy could also be considered in patients receiving two or more lines of systemic therapy, but more evidence was needed. In addition, past work by our group and others found that the activation of the PI3K-AKT-mTOR pathway affected the tumor immune microenvironment and was identified as potential predictors of primary resistance to ICIs in the patients with MSI/dMMR gastrointestinal cancer.32–35 Therefore, for patients who carried mutations in the PI3K-AKT-mTOR pathway, such as PIK3CA and AKT1, chemo-anti–PD-1/PD-L1 therapy was worth trying.
As mentioned in the background, the combination strategy of anti–PD-1/PD-L1 and chemotherapy has been adopted in many cancer types. In relative clinical trials, patients were usually given four to six cycles of anti–PD-1/PD-L1 plus chemotherapy and then maintenance anti–PD-1/PD-L1 agents for up to 2 years, with or without maintenance chemotherapy.12,13,15,19 In our study, most patients received anti–PD-1/PD-L1 monotherapy as maintenance therapy, whereas a small portion received anti–PD-1/PD-L1 plus oral chemotherapy or targeted therapy. Further studies are needed to explore the pros and cons of additional chemotherapy or targeted therapy in maintenance therapy. At data cutoff, approximately half of the patients had received maintenance therapy for more than one year. It was worth noting that eight patients who achieved the status of NED or pCR received therapy for only 1 year or less and achieved durable response. Actually, the optimal duration of maintenance therapy for MSI/dMMR mCRC is still unknown. In the KEYNOTE142 study, the patients with previously treated MSI/dMMR mCRC who received nivolumab plus ipilimumab for four cycles followed by nivolumab for up to 2 years had a 3-year PFS rate of 60% and a 3-year OS rate of 71%.36 Although the GERCOR NIPICOL study presented a 3-year PFS rate of 70% and a 3-year OS rate of 73% when nivolumab maintenance therapy is given for up to 1 year,37 which indicated that for patients with MSI/dMMR mCRC, maintenance treatment for up to 1 year might be enough.
Our study has some limitations. The most salient limitation is its retrospective design, and the results need to be further verified in a prospective study. All patients in the current study were from China; therefore, caution must be taken when extrapolating these findings to other populations. Additionally, the short follow-up time restricts the observation and collection of sufficient end points. Moreover, the retrospective nature of our study limits the complete collection of adverse events, and the safety of PD-1/PD-L1 inhibitors plus chemotherapy should be further explored.
In conclusion, this multicenter retrospective cohort study demonstrated that anti–PD-1/PD-L1 plus chemotherapy significantly improved the prognosis of patients with MSI/dMMR gastrointestinal cancer compared with anti–PD-1/PD-L1 monotherapy, but the conclusions need to be further confirmed in prospective trials.
ACKNOWLEDGMENT
The authors thank Xiaobin Wu from the School of Basic Medical Sciences, Peking University, for his guidance in statistical analysis and application of R software. The authors thank Feilong Zhao from 3D Medicines Inc and Sai Ge from Peking University Cancer Hospital & Institute for their guidance in data analysis.
APPENDIX
TABLE A1.
Overall Response Between the Anti–PD-1/PD-L1 Group and Chemo-Anti–PD-1/PD-L1 Group Before and After Overlap Weighting
TABLE A2.
Univariate and Multivariate Cox Regression Analyses of Factors Related to PFS and OS in 256 Patients With MSI/dMMR Gastrointestinal Cancer
TABLE A3.
Univariate and Multivariate Logistic Regression Analysis of Factors Related to ORR and DCR in 256 Patients With MSI/dMMR Gastrointestinal Cancer
FIG A1.
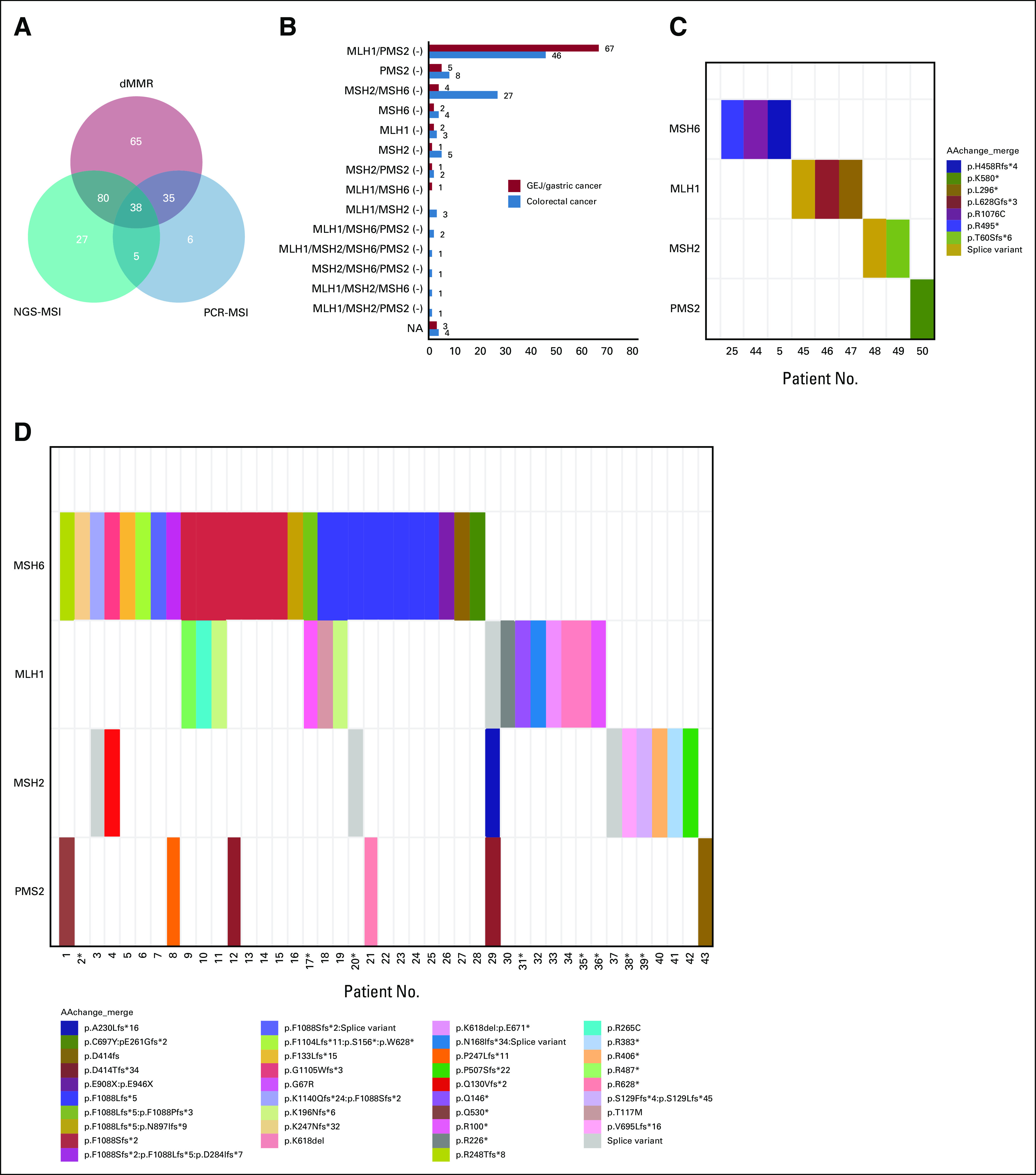
The results of MMR protein expression by immunohistochemistry and MMR gene mutations by NGS. (A) A total of 218 patients were confirmed as dMMR by immunohistochemistry, and 191 patients were confirmed as MSI by PCR or NGS. Specifically, 86 GEJ/GC patients and 108 CRC patients were confirmed as dMMR by immunohistochemistry. (B) The co-expression of MLH1/PMS2 loss was the most common pattern in both GEJ/GC and CRC. Besides, among 88 patients with the results of NGS, (C) nine patients were found to carry germline MMR gene mutations, and (D) 43 patients were found to carry somatic MMR gene mutations (D). *These patients lacked detailed information on PMS2 gene. GEJ, gastroesophageal junction; MSI, microsatellite instability; NA, not available; NGS, next-generation sequencing; PCR, polymerase chain reaction.
FIG A2.
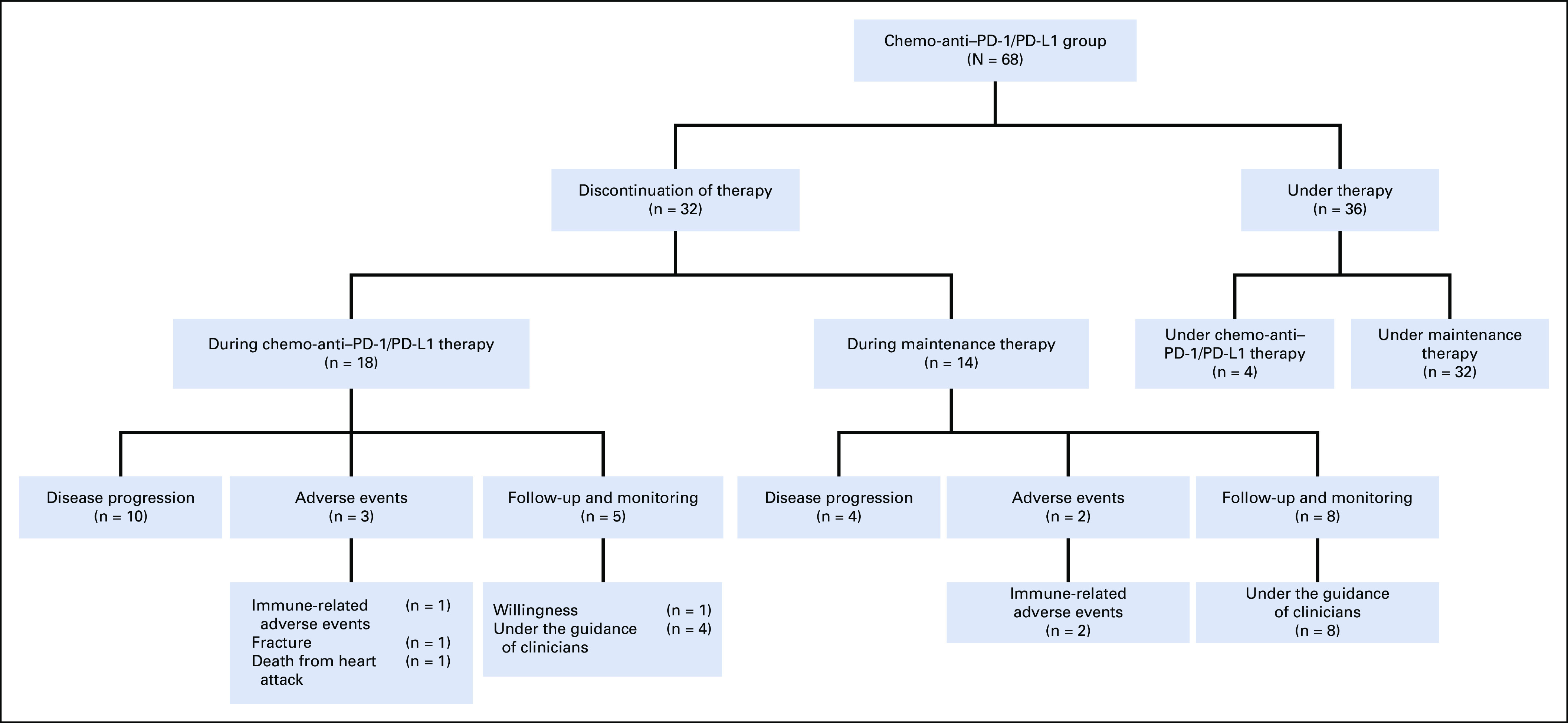
The therapy status of patients in the chemo-anti-PD1/PD-L1 group at data cutoff. At data cutoff, 36 patients in the chemo-anti-PD1/PD-L1 group were still under therapy. Among 32 patients who had discontinued therapy, 12 patients achieved pCR or NED and terminated therapy under the guidance of clinicians, while the others discontinued therapy for disease progression, adverse events, or willingness.
FIG A3.
Love plot of SMD between the anti-PD1/PD-L1 group and chemo-anti-PD1/PD-L1 group before and after overlap weighting analysis. SMD between the two treatment regimens was compared before and after overlap weighting analysis, and SMD less than 0.1 was considered balanced. A love plot was adopted to visualize the results. After overlap weighting, baseline characteristics were well balanced in the two treatment groups. ECOG, Eastern Cooperative Oncology Group; SMD, standardized mean differences.
FIG A4.
Distribution of the propensity score between the chemo-anti-PD1/PD-L1 group and anti-PD1/PD-L1 group before and after matching. The histograms show the propensity score between (A-B) the chemo-anti-PD1/PD-L1 group and (C-D) anti-PD1/PD-L1 group before and after matching analysis. After propensity score matching, the distribution of the propensity score was improved, indicating that the two groups were well balanced.
FIG A5.
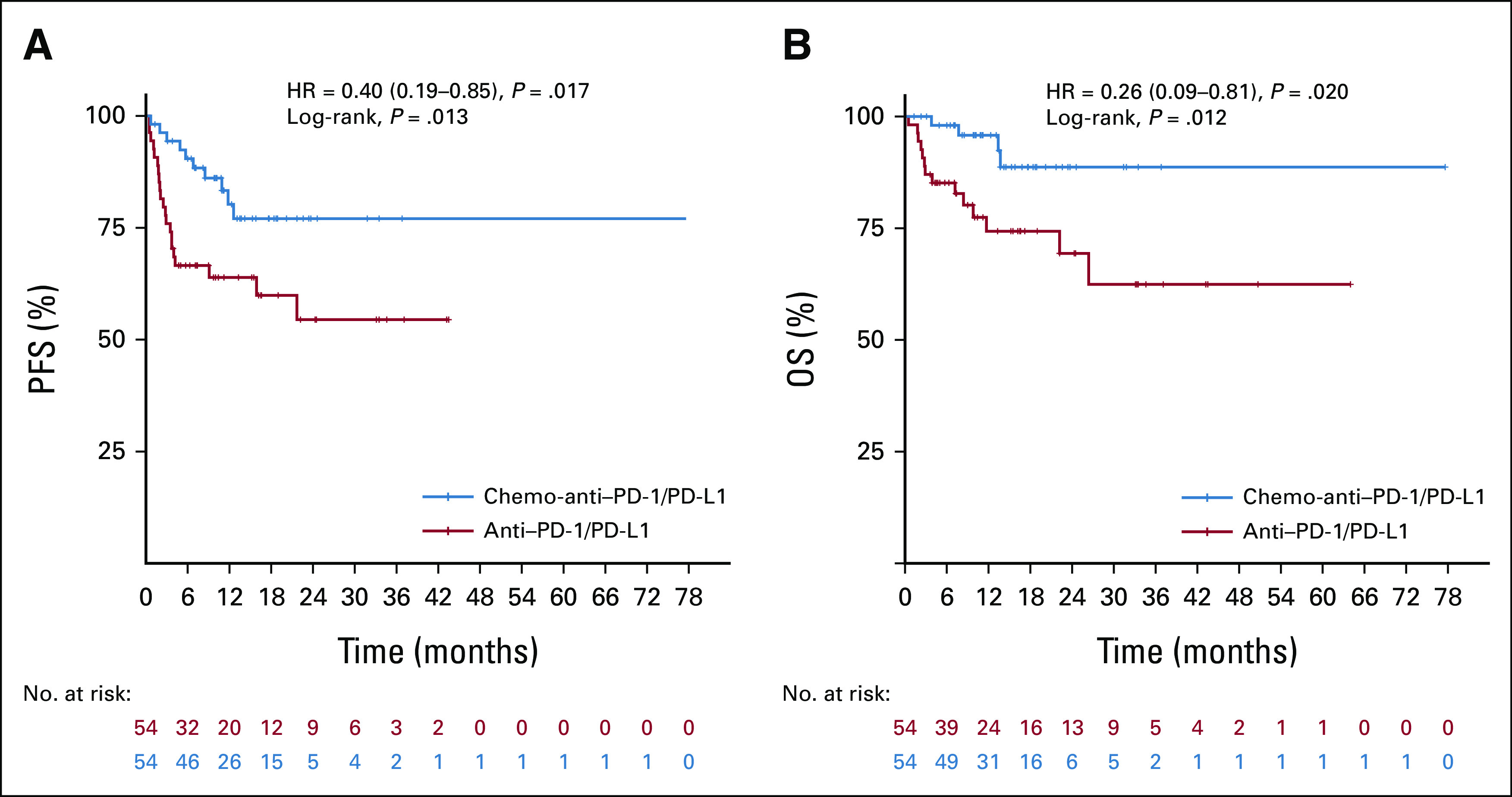
Kaplan–Meier curves of PFS and OS for anti-PD1/PD-L1 group and chemo-anti-PD1/PD-L1 group in propensity score matching analysis. Shown are Kaplan–Meier curves of PFS (A) and OS (B) for MSI/dMMR gastrointestinal cancer patients in the anti-PD1/PD-L1 matched group and chemo-anti-PD1/PD-L1 matched group, respectively. Tick marks mean censored data. We performed a 1:1 nearest neighbor propensity score matching within the caliper width of 0.1. After propensity score matching analysis, PFS (P = .013) and OS (P = .012) were significantly longer in patients receiving chemo-anti-PD1/PD-L1 than in patients receiving anti-PD1/PD-L1. HR, hazard ratio; PFS, progression-free survival.
Lin Shen
Consulting or Advisory Role: MSD, Bristol-Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology (Inst), Beijing Xiantong Biomedical Technology (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Major Research Program of National Natural Science Foundation of China (No. 91959130), the Beijing Municipal Natural Science Foundation (No. 7224323), the National Natural Science Foundation of China (No. 81902514), and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZYLX202116).
M.C., Z.W., Z.L., T.D., X.W., and Z.C. contributed equally to this work.
DATA SHARING STATEMENT
Data are available upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Mifen Chen, Zhenghang Wang, Zimin Liu, Ting Deng, Xiaodong Wang, Zhiwei Chang, Lin Shen, Jian Li
Financial support: Zhenghang Wang, Sai Ge, Jian Li
Administrative support: Lin Shen, Jian Li
Provision of study materials or patients: Mifen Chen, Zhenghang Wang, Zimin Liu, Ting Deng, Xiaodong Wang, Zhi Ji, Zhiwei Chang, Lin Shen, Jian Li
Collection and assembly of data: Mifen Chen, Zhenghang Wang, Zimin Liu, Ting Deng, Xiaodong Wang, Zhiwei Chang, Qi Zhang, Ning Liu, Zhi Ji, Xiaotian Zhang, Xicheng Wang, Zhi Peng, Yi Li, Yujuan Cao, Xuan Jin, Hongxia Lu, Huajun Qu, Yong Tang, Chunlei Xu, Weijia Fang, Hangyu Zhang, Dong Yan, Li Wang, Jiayi Li, Jingdong Zhang, Qiwei Wang, Liying Xue, Fei Yin, Guangjie Han, Zhiqiang Cheng, Qing Liu, Yongdong Jin, Yinjie Zhang, Lanxing Li, Baoshan Cao, Yanhong Yao, Zhiyu Chen, Jianling Zou, Jieer Ying, Qing Wei, Tiantian Tian, Weifeng Zhao, Longmei Li, Tong Zhang, Fanghua Song, Ya-er Ba, Na Li, Hui Gao, Yinghua Ji, Liying Bao, Lin Shen, Jian Li
Data analysis and interpretation: Mifen Chen, Zhenghang Wang, Zimin Liu, Ting Deng, Xiaodong Wang, Zhiwei Chang, Wenlei Yang, Xiaochen Zhao, Jinping Cai, Zheping Yuan, Lin Shen, Jian Li
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lin Shen
Consulting or Advisory Role: MSD, Bristol-Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology (Inst), Beijing Xiantong Biomedical Technology (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Sun D, Cao M, Li H, et al. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129–139. doi: 10.21147/j.issn.1000-9604.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao W, Chen H-D, Yu Y-W, et al. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 5. Cortes-Ciriano I, Lee S, Park W-Y, et al. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. André T, Shiu K-K, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 8. Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7:895–902. doi: 10.1001/jamaoncol.2021.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 11. Chen M, Wang Z, Liu Z, et al. The optimal therapy after progression on immune checkpoint inhibitors in MSI metastatic gastrointestinal cancer patients: A multicenter retrospective cohort study. Cancers. 2022;20:5158. doi: 10.3390/cancers14205158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 13. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 14. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 16. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 17. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 18. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 19. Zhou C, Wang Z, Sun Y, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23:220–233. doi: 10.1016/S1470-2045(21)00650-1. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Niu X, An N, et al. Comparative efficacy and safety of immunotherapy alone and in combination with chemotherapy for advanced non-small cell lung cancer. Front Oncol. 2021;11:611012. doi: 10.3389/fonc.2021.611012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan W-L, Ma Y, Cui Y-H, et al. The impact of mismatch repair status on prognosis of patients with gastric cancer: A multicenter analysis. Front Oncol. 2021;11:712760. doi: 10.3389/fonc.2021.712760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damato A, Bergamo F, Antonuzzo L, et al. Phase II study of nivolumab in combination with FOLFOXIRI/bevacizumab as first-line treatment in patients with advanced colorectal cancer RAS/BRAF mutated (mut): NIVACOR trial (GOIRC-03-2018) J Clin Oncol. 2022;40 doi: 10.1186/s12885-020-07268-4. supp 16; abstr 3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herting CJ, Farren MR, Tong Y, et al. A multi-center, single-arm, phase Ib study of pembrolizumab (MK-3475) in combination with chemotherapy for patients with advanced colorectal cancer: HCRN GI14-186. Cancer Immunol Immunother. 2021;70:3337–3348. doi: 10.1007/s00262-021-02986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://mp.weixin.qq.com/s/8UdTC7vLdsSZmcl5PYFVpA China Medical Tribune: The Gastrointestinal Cancer Immunity Pioneer Forum, 2022.
- 26. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 28. Thomas LE, Li F, Pencina MJ. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323:2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Deng Y, Zhang W, et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol Oncol. 2021;14:95. doi: 10.1186/s13045-021-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Granier C, Gey A, Roncelin S, et al. Immunotherapy in older patients with cancer. Biomed J. 2021;44:260–271. doi: 10.1016/j.bj.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haupt S, Caramia F, Klein SL, et al. Sex disparities matter in cancer development and therapy. Nat Rev Cancer. 2021;21:393–407. doi: 10.1038/s41568-021-00348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Z, Zhang Q, Qi C, et al. Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer. J Immunother Cancer. 2022;10:e004703. doi: 10.1136/jitc-2022-004703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chida K, Kawazoe A, Kawazu M, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res. 2021;27:3714–3724. doi: 10.1158/1078-0432.CCR-21-0401. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Wang X, Xu Y, et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022;20:133. doi: 10.1186/s12916-022-02327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collins NB, Al Abosy R, Miller BC, et al. PI3K activation allows immune evasion by promoting an inhibitory myeloid tumor microenvironment. J Immunother Cancer. 2022;10:e003402. doi: 10.1136/jitc-2021-003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33:1052–1060. doi: 10.1016/j.annonc.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 37. Cohen R, Bennouna J, Meurisse A, et al. RECIST and iRECIST criteria for the evaluation of nivolumab plus ipilimumab in patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The GERCOR NIPICOL phase II study. J Immunother Cancer. 2020;8:e001499. doi: 10.1136/jitc-2020-001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.