PURPOSE
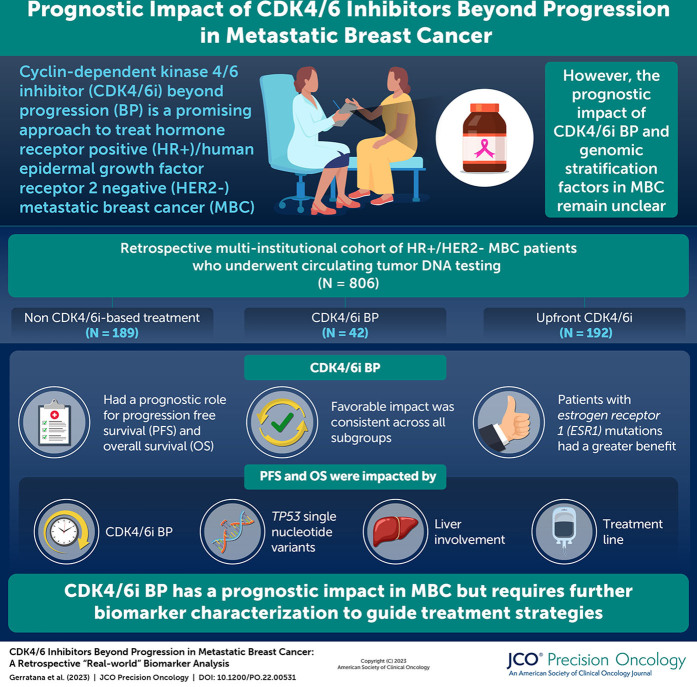
As the continuation beyond progression (BP) of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) is becoming increasingly attractive for the treatment of patients with hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative metastatic breast cancer (MBC), the definition of resistance factors is crucial. The aim of the study was to investigate the impact of CDK 4/6i BP and to explore potential genomic stratification factors.
MATERIALS AND METHODS
We retrospectively analyzed a multi-institutional cohort of patients with HR-positive HER2-negative MBC characterized for circulating tumor DNA through next-generation sequencing before treatment start. Differences across subgroups were analyzed by chi-square test, and survival was tested by univariable and multivariable Cox regression. Further correction was applied by propensity score matching.
RESULTS
Among the 214 patients previously exposed to CDK4/6i, 172 were treated with non–CDK4/6i-based treatment (non-CDK) and 42 with CDK4/6i BP. Multivariable analysis showed a significant impact of CDK4/6i BP, TP53 single-nucleotide variants, liver involvement, and treatment line on both progression-free survival (PFS) and overall survival (OS). Propensity score matching confirmed the prognostic role of CDK4/6i BP both for PFS and OS. The favorable impact of CDK4/6i BP was consistent across all subgroups, and a differential benefit was suggested for ESR1-mutated patients. ESR1 and RB1 mutations were more represented in the CDK4/6i BP subgroup with respect to CDK4/6i upfront.
CONCLUSION
The study highlighted a significant prognostic impact of the CDK4/6i BP strategy with a potential added benefit in patients with ESR1 mutations suggesting the need for an extensive biomarker characterization.
INTRODUCTION
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in combination with endocrine therapy (ET) have significantly affected both progression-free survival (PFS) and overall survival (OS) in patients with hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC).1,2 Although this association is now considered first-line standard-of-care therapy, most patients will experience a disease progression in a time frame between 12 and 36 months.1,2 In this scenario, predicting the onset of primary and acquired resistances is crucial for the early detection of disease progression and the definition of subsequent treatment lines.3-5
CONTEXT
Key Objective
The study analyzed the prognostic impact of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) beyond progression (BP) and explored the role of circulating tumor DNA features for patients' stratification and biomarker discovery.
Knowledge Generated
CDK4/6i BP has an important role in terms of progression-free survival and overall survival, particularly in patients with ESR1 mutations. Although both ESR1 and RB1 mutations were more represented in the CDK4/6i BP subgroup with respect to CDK4/6i upfront, the former has an unfavorable prognostic impact in the upfront subgroup because of the aromatase inhibitor backbone, while the latter is a negative prognostic factor for CDK4/6i BP.
Relevance
Although being a promising approach, not all patients will benefit from CDK4/6i BP. Biomarker characterization will be, therefore, essential to guide future treatment algorithms to maximize sequence strategies.
In this context, the clinical deployment of high-throughput sequencing technologies, such as circulating tumor DNA (ctDNA), is enabling a deep and longitudinal characterization of tumor biology and evolution.6,7 Several ET resistance mechanisms have been highlighted, such as TP53, ERBB2, PIK3CA, and ESR1 alterations.8,9 However, the definition of acquired resistance to CDK4/6i appears to be more complex, with preliminary data showing different resistance candidates across inhibitors and, potentially, different cross-resistance mechanisms.10
Although guidelines for patient management in the first-line setting are well established, ongoing efforts to define the optimal therapeutic approach after CDK4/6i progression have been focused on a variety of hormonal and targeted agents.2 As a matter of fact, although being supported by positive clinical trials, both ET combinations with phosphatidylInositol 3-kinase (PI3K) or mammalian target of rapamycin inhibitors (mTORi) show small or nonexisting subgroups of patients pretreated with CDK4/6i and have contraindications in the presence of specific comorbidities.2 In addition, promising alternative targets such as AKT inhibitors have more significant toxicities compared with CDK4/6i.11 An emerging alternative is being represented by the use of CDK4/6i beyond progression (BP) after switching the ET backbone, with the rationale of overcoming ET resistance while taking full advantage of residual CDK4/6i activity.
The primary objective of this study was to describe the impact of continued CDK 4/6i among patients previously treated with CDK4/6i stratified by ctDNA results in a multi-institutional cohort, while also highlighting potential biomarkers candidate to select and guide this emerging approach and future clinical trial design for patients with breast cancer.
MATERIALS AND METHODS
Study Population and Design
This study retrospectively analyzed a multi-institutional cohort of 806 patients with HR-positive HER2-negative MBC with ctDNA next-generation sequencing (NGS) sampling before starting a new treatment (Fig 1). Samples were collected from patients who underwent standard-of-care ctDNA testing at Northwestern University (Chicago, IL), Massachusetts General Hospital (Boston, MA), and Washington University in St Louis (St Louis, MO) between 2015 and 2020. Data were combined and shared under a data use agreement and approved by the institutional review boards (IRBs) of the three sites (Washington University School of Medicine St Louis, MO; IRB#202101147; Northwestern University, Chicago, IL; IRB#STU00214133; and Massachusetts General Hospital, Boston, MA; IRB#2013P000848). The requirement for informed consent was waived by the IRB for this deidentified analysis. The study was performed in concordance with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki.
FIG 1.
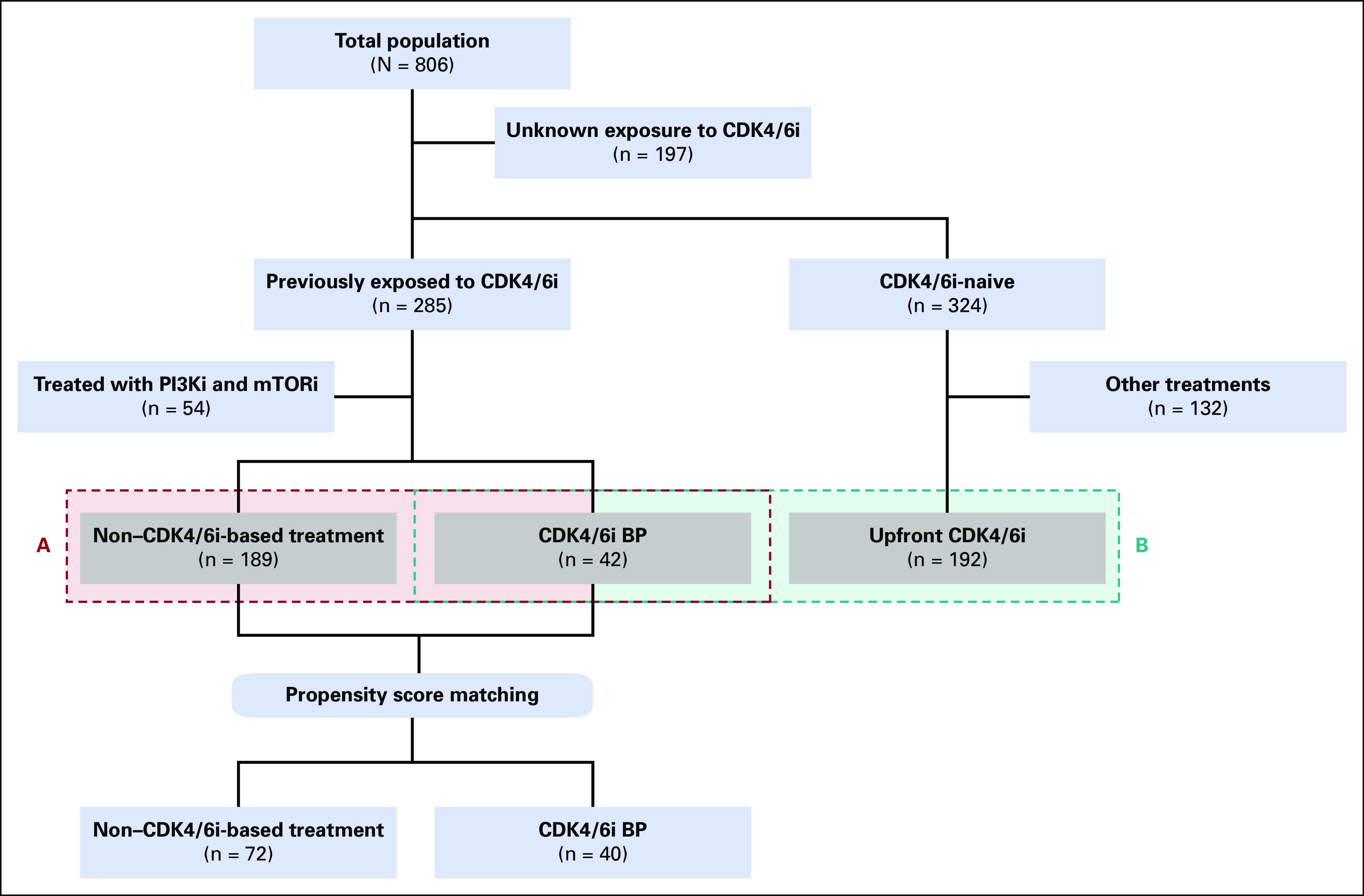
Study overview. The study examined a retrospective multi-institutional cohort of 806 patients with HR-positive HER2-negative MBC who had ctDNA NGS testing before starting a new treatment. The cohort was then restricted to 214 patients who had previously been exposed to CDK4/6i and were treated with CDK4/6i, single-agent endocrine therapy, or chemotherapy, depending on the treating physician's preference (A in red). The prognostic impact of ctDNA features in CDK4/6i-naive and pretreated patients was then compared in an additional subgroup of 192 patients treated with upfront CDK4/6i (B in blue). BP, beyond progression; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; ctDNA, circulating tumor DNA; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MBC, metastatic breast cancer; mTORi, mammalian target of rapamycin inhibitors; NGS, next-generation sequencing; PI3Ki, phosphatidyllnositol 3-kinase inhibitor.
The study cohort was then narrowed to the 214 patients previously exposed to CDK4/6i and treated with CDK4/6i, single-agent ET or chemotherapy, according to the treating physician's choice (Fig 1A). Type of CDK4/6i was not per-protocol controlled. An additional subgroup of 192 patients treated with upfront CDK4/6i was then analyzed (Fig 1B) to compare the prognostic impact of ctDNA features in CDK4/6i-naïve and pretreated patients. Baseline imaging (eg, computed tomography, positron emission tomography) was performed before ctDNA collection and start of therapy according to the treating physician's choice.
ctDNA Sample Collection and Analysis
Two 10-mL samples of blood were collected for each patient through stabilizing tubes (Streck, NE) and analyzed using the commercial Guardant360 NGS platform (Guardant Health, CA), a 72-gene panel on the basis of single-molecule digital sequencing, able to detect somatic single-nucleotide variants (SNVs), insertions/deletions (indels), gene fusions/rearrangements, and copy-number variations (CNVs).12-14 Mutations were annotated through the OncoKB database according to their effect (loss of function, gain of function) and pathogenicity.15 Only pathogenic mutations on the basis of OncoKB were included in the analyses.
Statistical Analysis
Clinical and pathologic variables were reported using descriptive analyses. Categorical variables were reported as frequency distributions, whereas continuous variables were described through median and IQRs.
Differences in distributions across subgroups of interest were analyzed through chi-square or Fisher's exact test according to sample size.
PFS was defined as the time from the baseline ctDNA blood draw to progression or death from any cause, whichever came first, while OS was defined as the time from the baseline ctDNA blood draw to death from any cause. Patients without an end point event at the last follow-up visit were censored. Differences in survival were tested using log-rank test and univariable and multivariable Cox regression with 95% CI and represented by Kaplan-Meier estimator plot. Correction for ctDNA features, main clinical characteristics, and line of treatment was applied to the multivariable model after univariable testing. Only SNVs and CNVs with at least a 10% prevalence were included in the prognostic models (ie, MYC CNVs, CCND1 CNVs, FGFR1 CNVs, TP53 SNVs, ESR1 SNVs, and PIK3CA SNVs).
Further correction was applied by propensity score matching through the MatchIt package with a 0.2 caliper and a 1:2 matching algorithm for factors that were found to be imbalanced across treatment types (ie, progesterone receptor status, previous chemotherapy, and visceral involvement) or potentially affecting treatment and prognosis (ie, center and treatment line).
Statistical analysis was conducted using StataCorp 2019 Stata Statistical Software: Release 16.1 (College Station, TX), R (version 4.1.0; The R foundation for Statistical Computing, Vienna, Austria), and JMP (version 16; SAS Institute, Cary, NC).
RESULTS
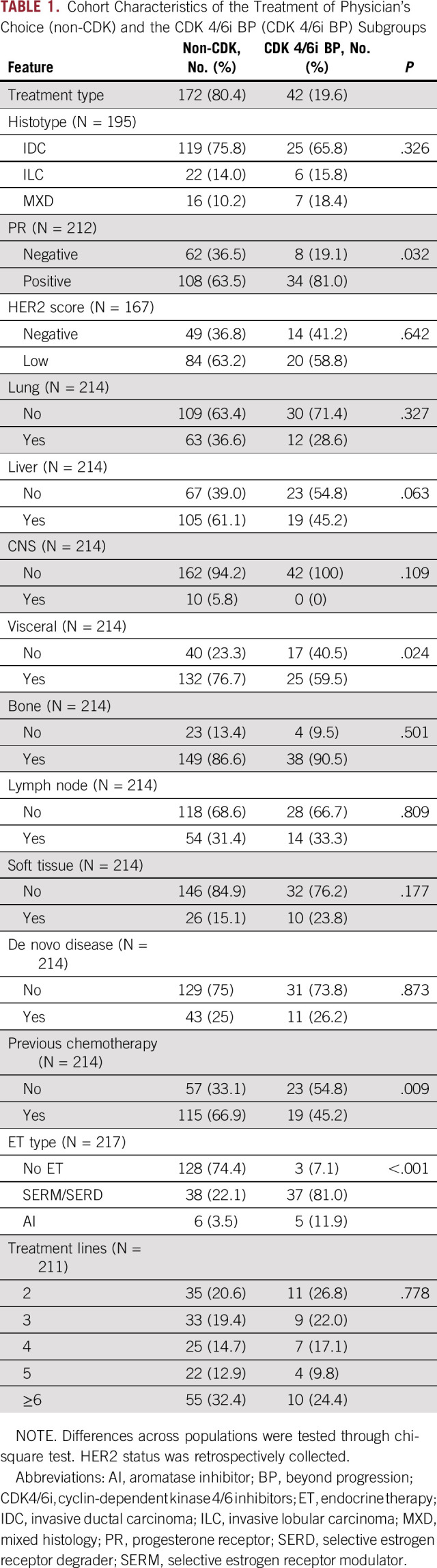
Among the 214 patients previously exposed to CDK4/6i (Fig 1A), 172 (80.4%) were treated with non–CDK4/6i-based treatment (non-CDK) and 42 patients (19.6%) were treated with CDK4/6i BP (Fig 1A). Upfront and BP CDK4/6i were prescribed according to physician's choice. The main histology was ductal carcinoma (75.8% and 65.8%, respectively, in non-CDK and CDK4/6i; Table 1), patients with HER2 low MBC were, respectively, 63.2% and 58.8% (Table 1), and metastatic sites were mainly bone (86.6% v 90.8%) and liver (61.1% in non-CDK v 45.5% in CDK4/6i). Patients with de novo disease were 25% and 26.2% for non-CDK and CDK4/6i, respectively. A previous chemotherapy was received by 66.9% and 45.2% of patients, respectively (Table 1). Non-CDK consisted in ET for 44 patients while, among patients treated with CDK4/6i, three received it as single agent (Table 1).
TABLE 1.
Cohort Characteristics of the Treatment of Physician's Choice (non-CDK) and the CDK 4/6i BP (CDK 4/6i BP) Subgroups
CDK4/6i BP Significantly Affected Both PFS and OS Independently From Clinical and ctDNA-Based Prognostic Factors
The prognostic impact of CDK4/6i BP was tested through univariable analysis highlighting a favorable role both in terms of PFS (hazard ratio [HR], 0.56; 95% CI, 0.38 to 0.83; P = .003; log-rank test; P = .0028) and OS (HR, 0.43; 95% CI, 0.27 to 0.70; P = .001; log-rank test; P = .0003; Figs 2A and 2B). Median PFS was, respectively, 3.7 and 10.23 months for non-CDK and CDK4/6i BP, while PFS at 6 and 12 months were, respectively, 32% and 19% for non-CDK, and 70% and 39% for CDK4/6i BP.
FIG 2.
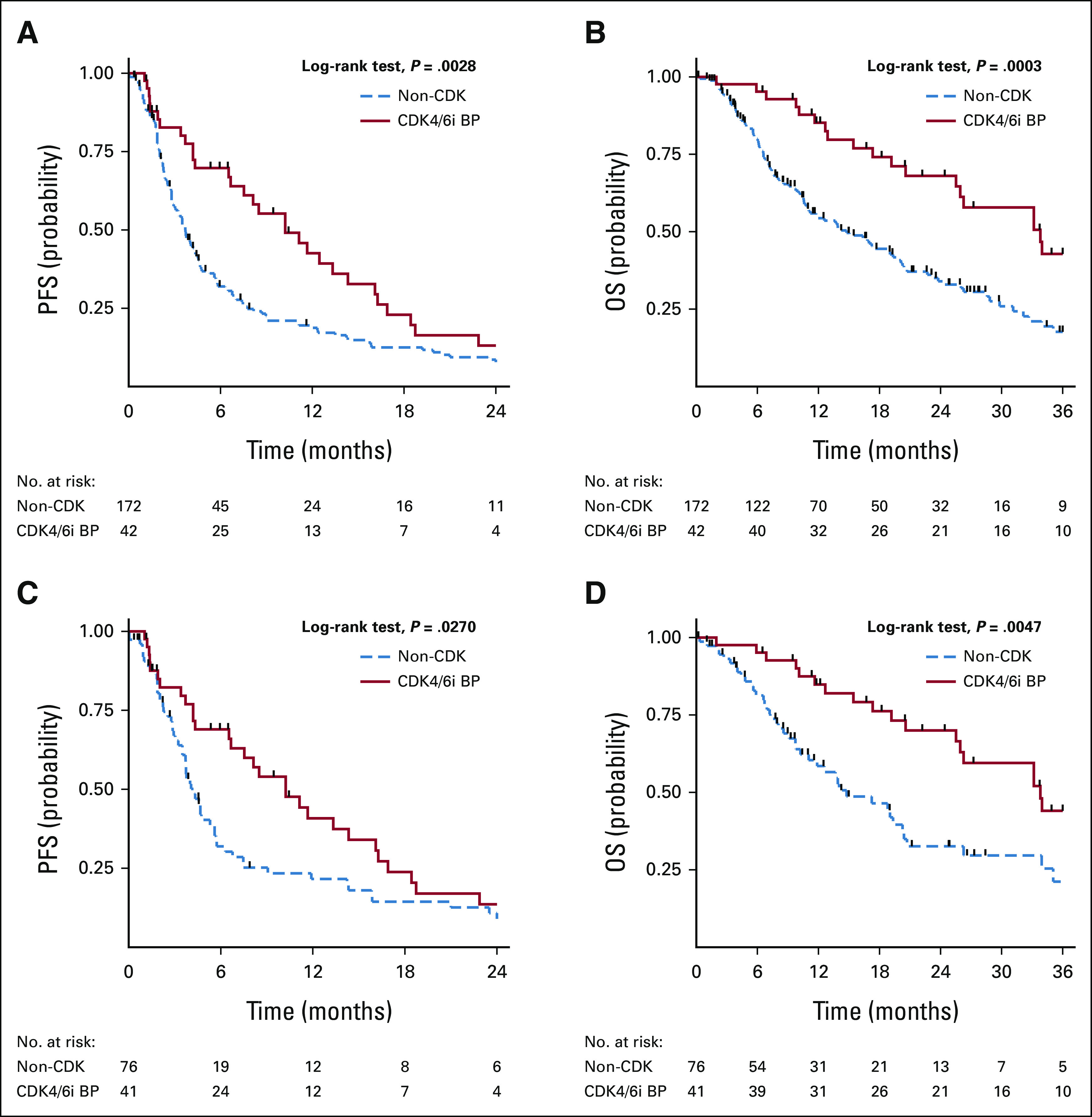
Kaplan-Meier curves on the impact of treatment strategy on PFS and OS in the overall study population (A and B) and after propensity score matching (C and D). A favorable prognostic impact of CDK4/6i BP was highlighted both in terms of (A) PFS (P = .0028) and (B) OS (P = .0003) Median PFS was, respectively, 3.7 and 10.23 months for non-CDK and CDK4/6i BP. Propensity score matching was applied for progesterone receptor status, previous chemotherapy, visceral involvement, treating center, and treatment line for bias correction. The prognostic role of CDK4/6i BP was confirmed for both (C) PFS and (D) OS (P = .0270 and P = .0047, respectively). BP, beyond progression; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; OS, overall survival; PFS, progression-free survival.
Propensity score matching was then applied for progesterone receptor status, previous chemotherapy, visceral involvement, treating center, and treatment line for bias correction, confirming the prognostic role of CDK4/6i BP both for PFS and OS (respectively, HR, 0.60; 95% CI, 0.38 to 0.93; P = .020; log-rank test; P = .0270 and HR, 0.42; 95% CI, 0.24 to 0.72; P = .002; log-rank test; P = .0047; Figs 2C and 2D).
The prognostic interplay of treatment strategy, clinical prognostic factors, and ctDNA-detectable gene alterations was then investigated. After multivariable analysis in terms of PFS, CDK4/6i BP retained its significance (HR, 0.54; 95% CI, 0.35 to 0.83; P = .005) together with ESR1 SNVs (HR, 1.48; 95% CI, 1.05 to 2.09; P = .024), TP53 SNVs (HR, 2.15; 95% CI, 1.51 to 3.08; P < .001), liver metastatic involvement (HR, 2.15; 95% CI, 1.51 to 3.08; P < .001), and treatment line (Table 2). CCND1 CNVs and PIK3CA SNVs had a prognostic impact only in univariable analysis (Appendix Table A1).
TABLE 2.
Multivariable Cox Regression Analysis in Terms of PFS
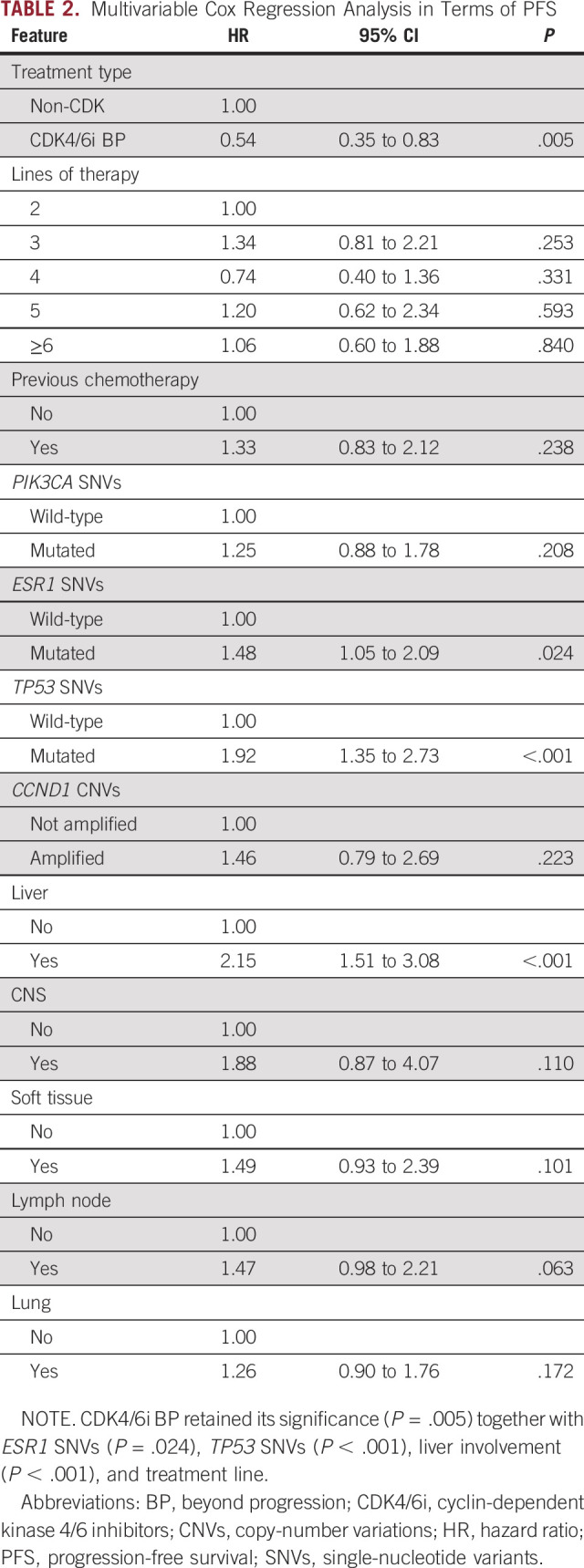
The multivariable OS model confirmed the impact for CDK4/6i BP (HR, 0.39; 95% CI, 0.23 to 0.67; P = .001). A prognostic role was also confirmed for TP53 SNVs (HR, 1.59; 95% CI, 1.07 to 2.36; P = .022), CCND1 CNVs (HR, 1.89; 95% CI, 1.04 to 3.42; P = .037) together with liver (HR, 2.24; 95% CI, 1.46 to 3.44; P < .001), CNS (HR, 5.42; 95% CI, 2.27 to 12.93; P < .001), soft tissue involvement (HR, 2.08; 95% CI, 1.18 to 3.65; P = .011), and treatment line (Appendix Table A3). Univariable models are reported in Appendix Table A2.
The Impact on PFS of CDK4/6i BP Is Higher in the ESR1-Mutated Subgroup
The impact of the treatment strategy was investigated across the main detected gene alterations and subgroups of clinical interest (Fig 3). The favorable impact of CDK4/6i BP was consistent across all subgroups, apart from the ESR1-mutated one, where a differential benefit is suggested (Fig 3).
FIG 3.
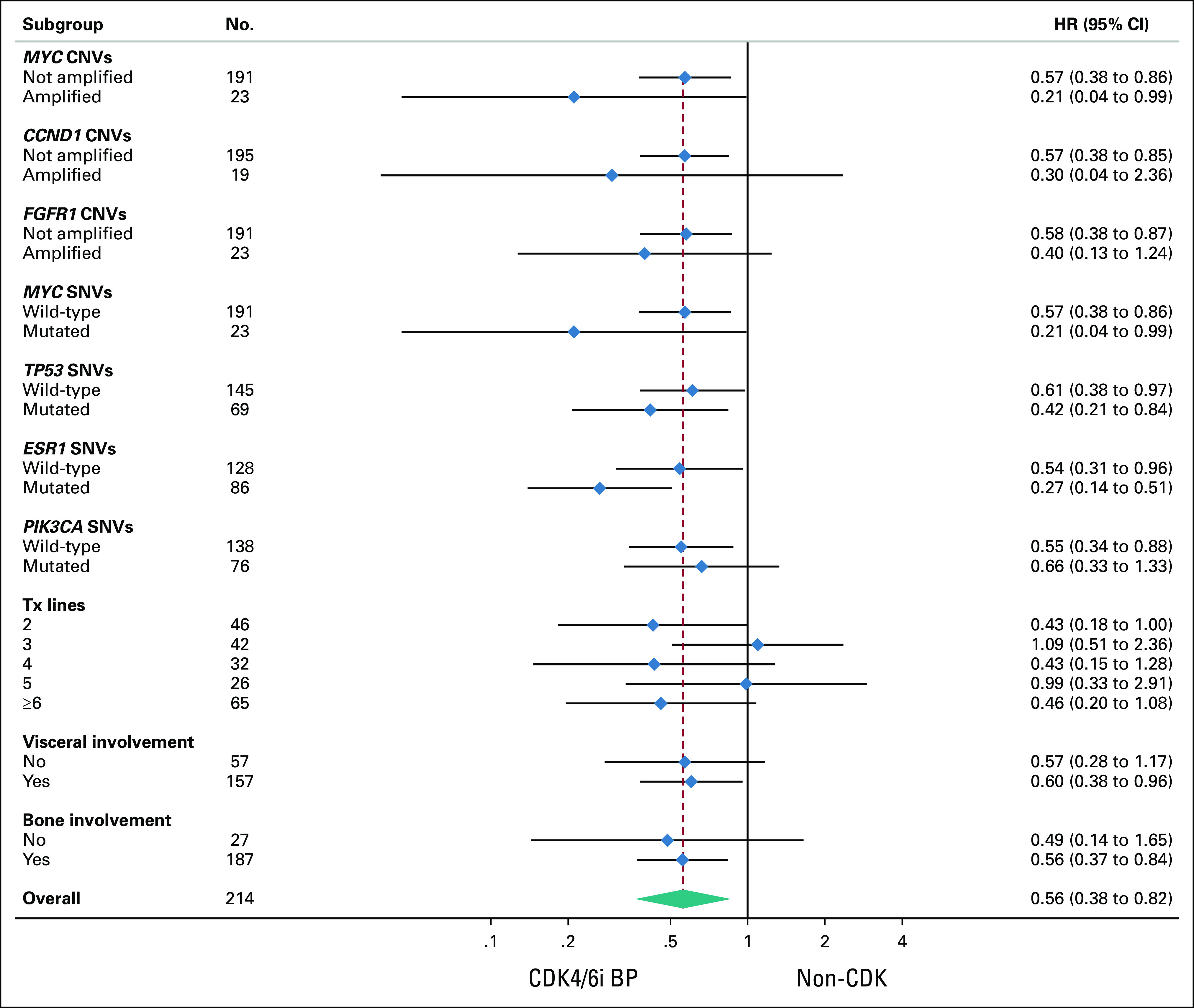
Subgroup analysis in terms of progression-free survival for CDK4/6i BP. The favorable impact of CDK4/6i BP was consistent across all subgroups, apart from ESR1-mutated, where a differential benefit is suggested, HR, 0.27; 95% CI, 0.14 to 0.51. BP, beyond progression; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CNVs, copy-number variations; HR, hazard ratio; SNVs, single nucleotide variants.
Upfront and BP CDK4/6i Are Characterized by a Different Resistance Background
The CDK4/6i BP subgroup was then compared with patients treated with CDK4/6i upfront (Fig 1B). The ET backbone was significantly more likely a selective estrogen receptor degrader (SERD)/selective estrogen receptor modulator (SERM) in the BP groups with respect to first-line CDK4/6i-based therapy (87%; 34 observed v 21.7 expected; P < .001), while aromatase inhibitors (AIs) were more represented in the upfront group (51%; 96 observed v 83.7 expected; P = .001; Fig 1B). The top five altered genes detected at baseline were ESR1 (N: 48; 19.67%), TP53 (N: 24; 9.84%), PIK3CA (N: 20; 8.20%), NF1 (N: 12; 4.92%), and ARID1A (N: 11; 4.51%) for CDK4/6i BP (Fig 4A), and PIK3CA (69; 14.26%), TP53 (58; 11.98%), ESR1 (35; 7.23%), FGFR1 (23; 4.75%), and GATA3 (22; 4.55%), for the upfront subgroup (Fig 4B).
FIG 4.
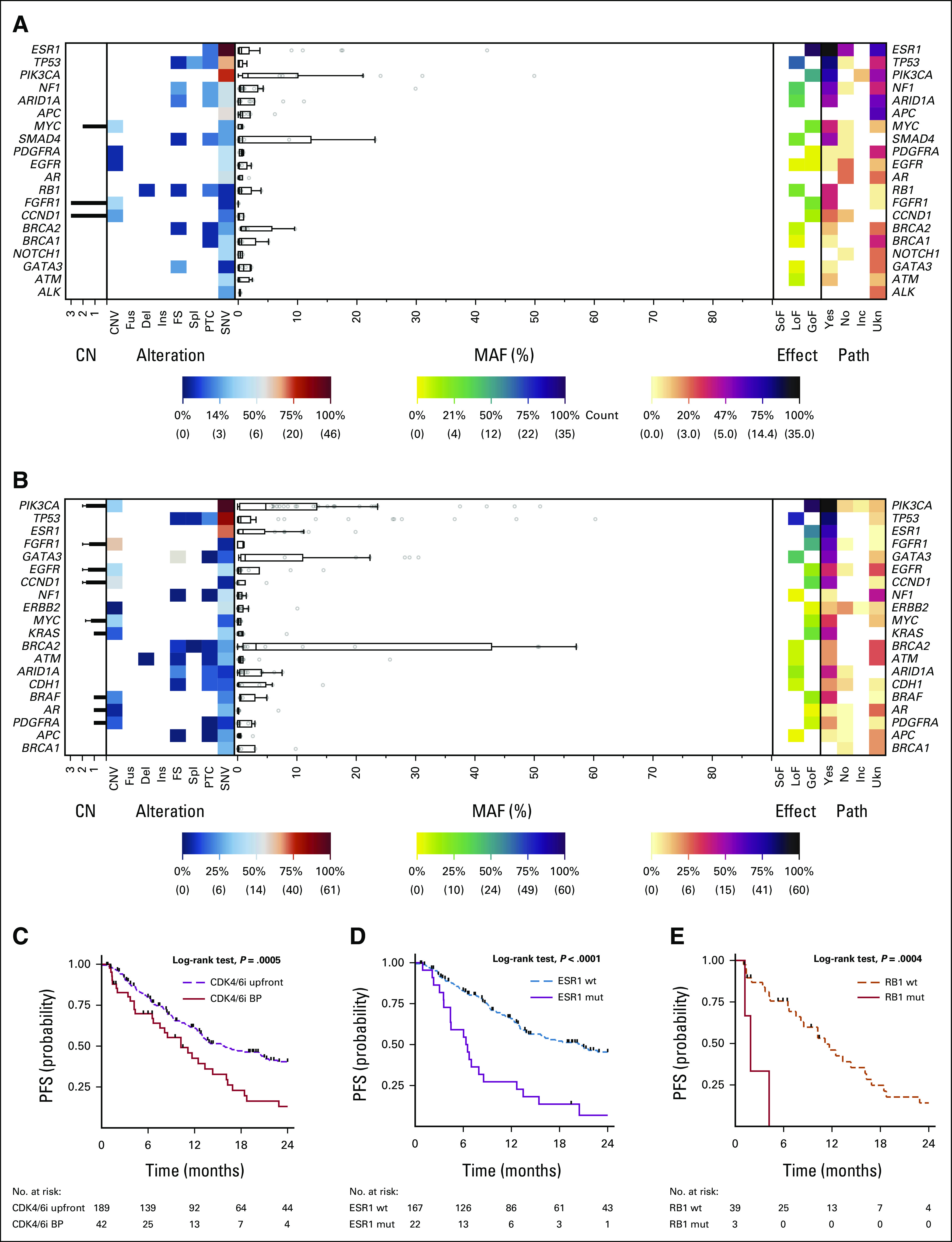
Landscape plot of all detectable aberrations in ctDNA samples in the CDK4/6i (A) BP and (B) upfront subgroups. PFS impact of (C) upfront and BP CDK4/6i and for (D) ESR1 and (E) RB1 SNVs in the upfront and BP subgroups. Incidence of the single aberrations (CNV, Fus, Del, Ins, FS, Spl, PTC, and SNV) is represented on the left. The MAF of each mutation is shown in the middle. Effect (GoF, LoF, and SoF) and pathogenicity (Yes, No, Ukn, and Inc) of all the detected aberrations are shown on the right. (C) PFS was significantly worse in for CDK4/6i BP (P = .0005), (D) ESR1 significantly affected in the upfront group (P <.001), and (E) RB1 had an unfavorable role in CDK4/6i BP (P = .007). BP, beyond progression; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CNV, copy-number variations; ctDNA, circulating tumor DNA; Del, deletions; FS, frameshift; Fus, fusions; GoF, gain of function; Inc, inconclusive; Ins, insertions; LoF, loss of function; MAF, mutant allele frequency; mut, mutated OS, overall survival; PFS, progression-free survival; PTC, premature termination codons; SNVs, single-nucleotide variants; SoF, switch of function; Spl, splicing variants; Ukn, unknown; wt, wild-type.
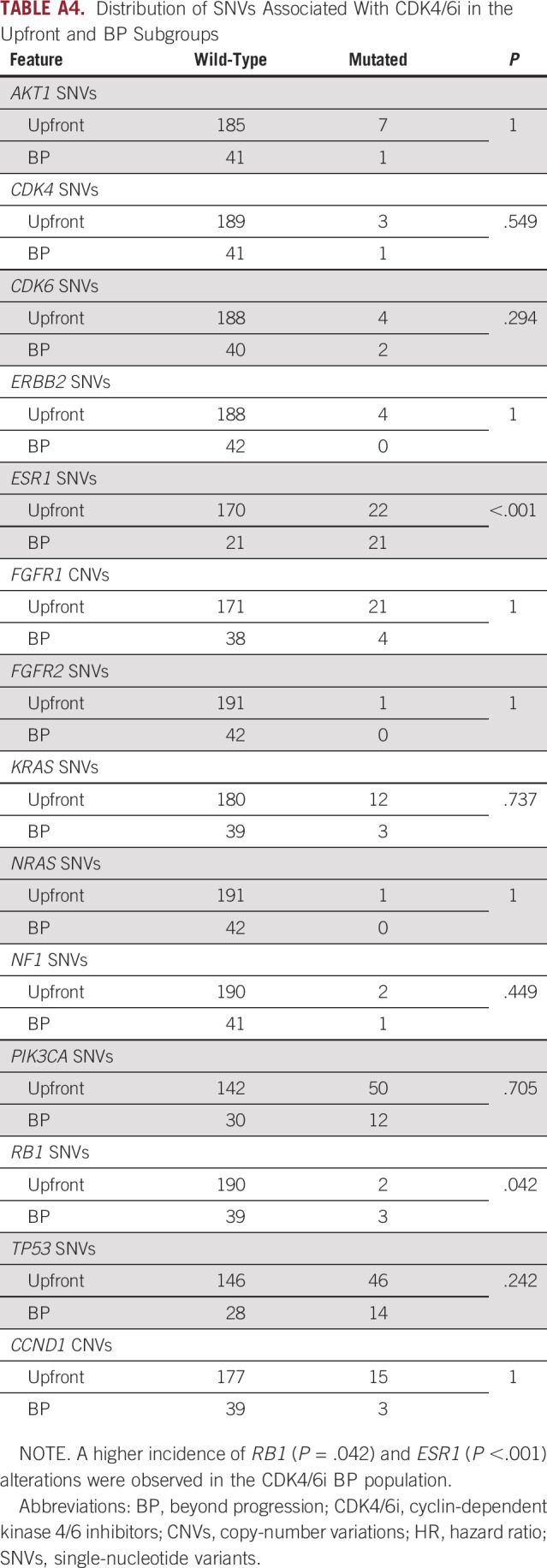
The distribution of gene alterations known to be associated with ET resistance (ie, AKT1, CDK4, CDK6, ERBB2, ESR1, FGFR1, FGFR2, KRAS, NRAS, NF1, PIK3CA, RB1, and TP53) was then tested, showing a higher incidence of RB1 SNVs (7.1% v 1.0%; P = .042) and ESR1 SNVs (50.0% v 11.5%; P < .001) in the CDK4/6i BP subgroup3 (Appendix Table A4).
PFS was significantly worse in for CDK4/6i BP (HR, 1.99; 95% CI, 1.34 to 2.97; P = .001; log-rank test; P = .0005); the median PFS for CDK4/6i upfront was 15.9 months (v 10.23 months for CDK4/6i BP), and PFS rates at 6 and 12 months were, respectively, 79% and 61% (Fig 4C).
Although both alterations had an independent impact on PFS in the overall CDK4/6i population (respectively, HR, 3.19; 95% CI, 1.27 to 7.99; P = .014, and HR, 2.84; 95% CI, 1.95 to 4.14; P < .001), ESR1 was significant only in the upfront group (HR, 3.32; 95% CI, 2.02 to 5.43; P < .001; Fig 4D) and RB1 exclusively in CDK4/6i BP (HR, 6.59; 95% CI, 1.68 to 25.88; P = .007; Fig 4E).
DISCUSSION
On the basis of a retrospective multi-institutional cohort to test the impact of CDK4/6i BP and by leveraging uniform ctDNA characterization across sites, the study identified possible candidate biomarkers to inform future clinical algorithms and trials for patients with HR-positive MBC. A significant prognostic impact for CDK4/6i BP was highlighted and confirmed after propensity score matching to further mitigate potential known confounding factors. The extraordinary availability of novel treatment options for MBC has revolutionized this setting, leaving the great unmet need of optimizing treatment sequencing strategies. This shortfall will become even more compelling as new data in the adjuvant setting are generated, emphasizing the importance of studies like the current one in addressing critical knowledge gaps.16
In this study, the subgroup analysis showed an interaction highlighting a potential added benefit in presence of ESR1 mutations, suggesting that the CDK4/6i BP strategy could be promising in patients with a predominant ET resistance that could be circumvented by switching the ET backbone, without hindering the action of CDK4/6i. This concept is supported by the PADA-1 phase III study, which investigated the impact of switching from letrozole to fulvestrant with the identification of ESR1 mutations in plasma before clinical progression.17 This strategy may be, therefore, considered an alternative implementation of CDK4/6i BP driven by the early detection of molecular progression instead of standard imaging. Patients underwent centralized ctDNA screening every 2 months and were randomly assigned between continuing letrozole or switching to fulvestrant when an ESR1 mutation was detected in the absence of clinical progression.17
The median PFS of patients who switched before disease progression was more than twice that of those who remained on letrozole (HR, 0.61; P = .005). The preplanned subgroup analysis found no differential benefit across patient characteristics.17
Continuation of palbociclib BP was associated with a median PFS of 10.5 in our cohort, which is similar to what has been observed in another small cohort of patients with HR-positive HER2-negative MBC treated in a nonclinical trial setting (median PFS of approximately 11 months).18 In terms of clinical trials, the MAINTAIN phase II study prospectively investigated the role of ribociclib in patients who experienced a progression to CDK4/6i.19 Similar to our cohort, ET was heterogeneous; patients treated with prior fulvestrant received exemestane and vice versa.19 Palbociclib was the main CDK4/6i (84%), followed by ribociclib (11%) and abemaciclib (2%).19 Consistent with our results, a statistically significant PFS improvement for the CDK4/6i BP strategy was observed in the overall population and in the fulvestrant subgroup (respectively, HR, 0.56; 95% CI, 0.37 to 0.83; P = .004, and HR, 0.59; 95% CI, 0.38 to 0.91; P = .02).19
Differently from our data, MAINTAIN's subgroup analysis suggested a lack of benefit in the ESR1-mutated population.19 Although prospectively enrolled, this subgroup was particularly small and characterized by the co-occurrence of known resistance factors to CDK4/6i and 50% of patients with ESR1 mutations also had an amplification of CCND1 and/or FGFR1. It has been previously observed that these alterations may significantly affect CDK4/6i and, therefore, the CDK4/6i BP strategy. Consistently, it has been observed that patients with an early progression to abemaciclib BP were characterized by RB1 and FGFR1 alterations.3,20-22
This imbalance, not observed in our study, may have impaired the action of CDK4/6i BP in MAINTAIN, underestimating its benefit and underlining how coalterations may have a nuanced impact in this setting. These results further underlined the importance of preplanned biomarker analyses since CCND1, FGFR1, and, more generally, receptor tyrosine kinase (RTK) alterations are important mechanisms of resistance to CDK4/6i, suggesting that an alternative strategy should be considered in these patients.5 However, resistance mechanisms mainly targeting the endocrine backbone can be overcome by switching to a different ET.17
Similarly to our study, Brett et al investigated if a multigene resistance panel (ie, CDKi-R), instead of individual genetic markers, might predict CDK4/6i resistance in patients with ESR1-mutated MBC treated with abemaciclib after progression to a palbociclib-based ET.22,23 Median PFS was 7.0 months for the CDKi-R negative subgroup, compared with 3.5 months for CDKi-R positive (HR, 2.8; P = .03).23 Consistently, CDKi-R mutations, but not ESR1 mutations, were associated with abemaciclib resistance in T47D and patient-derived circulating tumor cell lines.23 These results further support the concept of a granular definition of ET resistance that differentiates between CDK4/6i-targeted mechanisms from those specifically restricted to the ET backbone.
In this regard, the phase II study PACE investigated the activity of continuing CDK4/6i BP, with a switch in ET to fulvestrant.24 Given the strong preclinical rationale, it also tested the role of immunotherapy by combining the PD-L1 inhibitor avelumab with ET.25,26 Although adding palbociclib to fulvestrant after progression did not improve PFS (HR, 1.11; 95% CI, 0.79 to 1.55; P = .62), baseline ctDNA analyses suggest that the impact of targeted agents varies depending on mutational status (HR, 0.68 and HR, 1.70, respectively, in the ESR1-mutated and wild-type subgroups).26 Furthermore, combining avelumab with fulvestrant and palbociclib resulted in a longer PFS, an intriguing finding that merits further investigation.26
Additional prospective studies, such as GIM24 and postMONARCH, are actively investigating the CDK4/6i BP scenario and, together with PACE, will be crucial in better understanding the right implementation of the CDK4/6i BP concept because of their control of the ET companion (ie, fulvestrant), treatment line (ie, second line), and extensive biomarker characterization through tissue and ctDNA sequencing (PACE, GIM24, and postMONARCH) and circulating tumor cell enumeration (PACE).24,27,28
The comparison with the CDK4/6i upfront subgroup further underlines the connection between type of resistance and ET backbone. Despite being significantly less common, ESR1 mutations had a significant prognostic impact in the upfront subgroup, where AIs were the most common type of ET. However, CDK4/6i BP, where fulvestrant was predominant, was significantly more affected by RB1 mutations, which are rare but strong resistance factors to CDK4/6i.3,20 The differential role of ESR1 across ET backbones was analyzed in the SoFEA and PALOMA3 trials.29 Patients with ESR1 mutations had an improved PFS in the fulvestrant subgroup compared with exemestane, whereas patients with wild-type ESR1 had similar PFS after receiving either treatment arms.29 Consistently, palbociclib in association with fulvestrant improved PFS both in the ESR1 mutant and wild-type subgroups of the PALOMA3 trial.29 ESR1 mutations, moreover, were associated with acquired resistance to prior AI and were polyclonal.29
The study's main limitations are inherited by the retrospective design, as both the endocrine backbone and treatment line were considerably heterogeneous. Unknown resistance mechanisms derived by previous lines and heterogeneity in the ET companion may have introduced potential biases despite the application of propensity score matching.
In conclusion, this study analyzed a retrospective multi-institutional cohort highlighting a significant prognostic impact for CDK4/6i BP with a potential added benefit in patients with ESR1 mutations. These data further support the concept of CDK4/6i continuation after progression but suggest the need for an extensive biomarker characterization to correctly select patients who may benefit from this strategy and effectively guide treatment sequences.
APPENDIX
TABLE A1.
Univariable Cox Regression Analysis in Terms of Progression-Free Survival
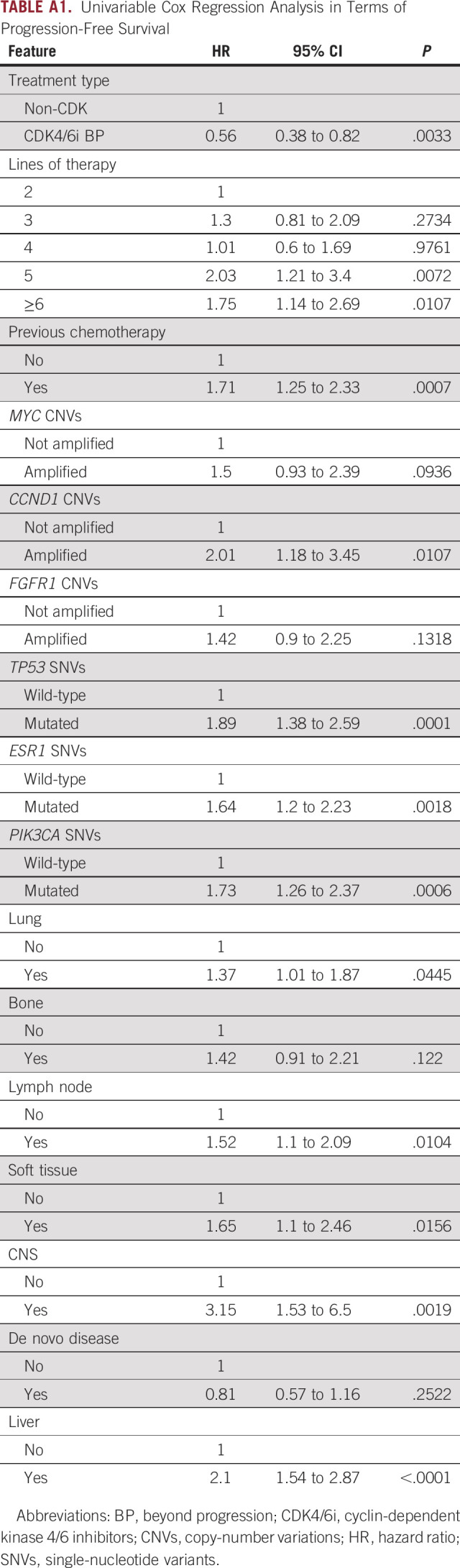
TABLE A2.
Univariable Cox Regression Analysis in Terms of Overall Survival
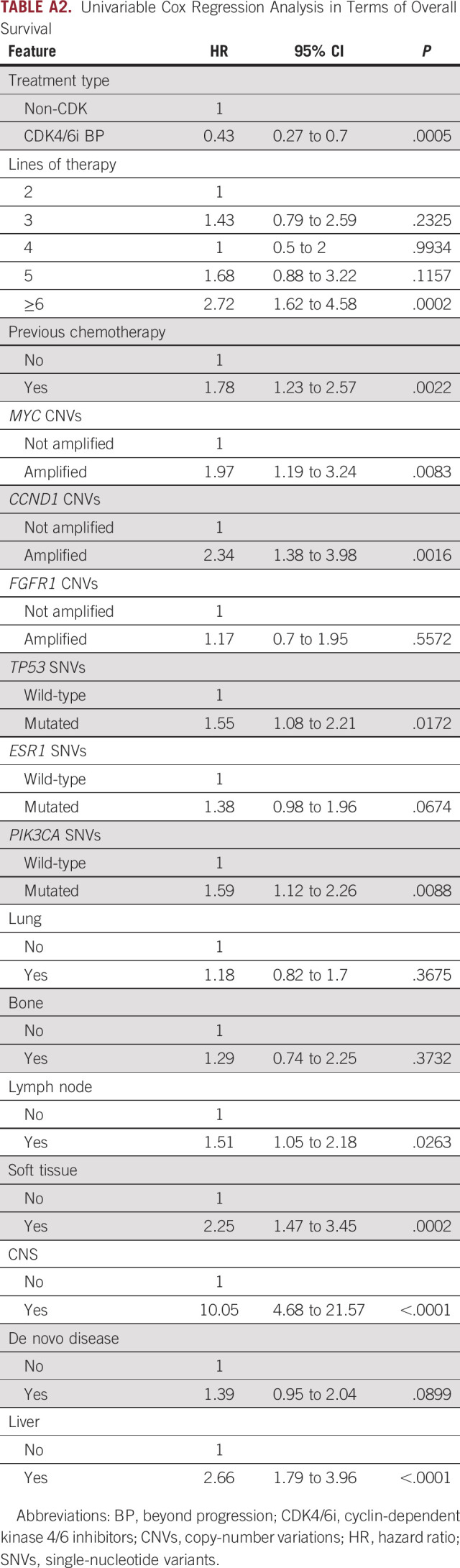
TABLE A3.
Multivariable Cox Regression Analysis in Terms of Overall Survival
TABLE A4.
Distribution of SNVs Associated With CDK4/6i in the Upfront and BP Subgroups
Lorenzo Gerratana
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, GlaxoSmithKline, Incyte
Research Funding: AstraZeneca (Inst)
Andrew A. Davis
Consulting or Advisory Role: Pfizer, bioTheranostics
Marko Velimirovic
Stock and Other Ownership Interests: AbbVie, Amgen
Carolina Reduzzi
Research Funding: Menarini Silicon Biosystems
Katherine Clifton
Employment: Zing Health
Stock and Other Ownership Interests: WellCare, Centene
Consulting or Advisory Role: Pfizer, bioTheranostics
Leslie Bucheit
Employment: Guardant Health, Myriad Genetics
Stock and Other Ownership Interests: Guardant Health
Ami N. Shah
Consulting or Advisory Role: AstraZeneca/MedImmune, Gilead Sciences
Paolo D'Amico
Employment: Merck
Stock and Other Ownership Interests: Merck
Research Funding: Roche Italy (Inst)
Arielle Medford
Honoraria: Natera
Consulting or Advisory Role: Natera, Illumina
Seth A. Wander
Consulting or Advisory Role: Foundation Medicine, Puma Biotechnology, Veracyte, Lilly, Hologic, Pfizer, Biovica
Speakers' Bureau: Lilly, Guardant Health
Research Funding: Genentech, Lilly, Pfizer, Regor Therapeutics, Nuvation Bio
William J. Gradishar
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Pfizer, Puma Biotechnology, Seattle Genetics, Merck, BeyondSpring Pharmaceuticals
Amir Behdad
Honoraria: Roche China, Lilly, Leica Biosystems, Caris Life Sciences
Consulting or Advisory Role: Caris Life Sciences, Leica Biosystems
Speakers' Bureau: Lilly, Roche China
Travel, Accommodations, Expenses: Bayer, Foundation Medicine, Pfizer
Cynthia X. Ma
Honoraria: PlusOne Health GmbH, Guardant Health
Consulting or Advisory Role: Novartis, Seattle Genetics, Agendia, AstraZeneca, Athenex, Bayer HealthCare Pharmaceuticals, Biovica Inc, Olaris, Sanofi-Genzyme, Gilead Sciences, Pfizer, Lilly, Tempus
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate: Authorship—served as a coauthor on the subject of endocrine therapy for metastatic breast cancer and receives compensation as an author, less than a few hundred dollars a year. It does not affect my patient care or research
Travel, Accommodations, Expenses: San Antonio Breast Cancer Symposium, Total Health Conferencing
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Pierre Fabre, Daiichi Sankyo, Eisai, Lilly, Pfizer, Exact Sciences
Consulting or Advisory Role: Roche, Amgen, Novartis, Pfizer, Eisai, Seattle Genetics, Pierre Fabre, AstraZeneca/Daiichi Sankyo, Viatris, Lilly, Gilead Sciences, Daiichi Sankyo Europe GmbH
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene, GlaxoSmithKline, Amgen, Astrazeneca, MSD, Novartis, Lilly, Pfizer
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Spectrum Pharmaceuticals, bioTheranostics, Merck, Radius Health, Immunomedics (Inst), Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Immunomedics, Sanofi, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Foundation Medicine, Philips Healthcare
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Massimo Cristofanilli
Honoraria: Pfizer
Consulting or Advisory Role: Lilly, Menarini, Olaris, AstraZeneca/Daiichi Sankyo, Syantra, Sermonix Pharmaceuticals, Celcuiy
Research Funding: Lilly, Angle, Merck, AstraZeneca, Menarini Silicon Biosystems
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Lynn Sage Cancer Research Foundation, OncoSET Precision Medicine Program, Italian Ministry of Health—Ricerca Corrente; REDCap support was funded by the National Institutes of Health UL1TR001422.
AUTHOR CONTRIBUTIONS
Conception and design: Lorenzo Gerratana, Fabio Puglisi, Aditya Bardia, Massimo Cristofanilli
Provision of study materials or patients: Andrew A. Davis, Firas Wehbe, William J. Gradishar, Cynthia X. Ma, Aditya Bardia, Massimo Cristofanilli
Collection and assembly of data: Andrew A. Davis, Marko Velimirovic, Whitney L. Hensing, Ami N. Shah, Charles S. Dai, Paolo D'Amico, Arielle Medford, Amir Behdad, Cynthia X. Ma, Aditya Bardia, Massimo Cristofanilli
Data analysis and interpretation: Lorenzo Gerratana, Andrew A. Davis, Carolina Reduzzi, Katherine Clifton, Leslie Bucheit, Tania Pivetta, Firas Wehbe, Seth A. Wander, William J. Gradishar, Amir Behdad, Fabio Puglisi, Aditya Bardia, Massimo Cristofanilli
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lorenzo Gerratana
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, GlaxoSmithKline, Incyte
Research Funding: AstraZeneca (Inst)
Andrew A. Davis
Consulting or Advisory Role: Pfizer, bioTheranostics
Marko Velimirovic
Stock and Other Ownership Interests: AbbVie, Amgen
Carolina Reduzzi
Research Funding: Menarini Silicon Biosystems
Katherine Clifton
Employment: Zing Health
Stock and Other Ownership Interests: WellCare, Centene
Consulting or Advisory Role: Pfizer, bioTheranostics
Leslie Bucheit
Employment: Guardant Health, Myriad Genetics
Stock and Other Ownership Interests: Guardant Health
Ami N. Shah
Consulting or Advisory Role: AstraZeneca/MedImmune, Gilead Sciences
Paolo D'Amico
Employment: Merck
Stock and Other Ownership Interests: Merck
Research Funding: Roche Italy (Inst)
Arielle Medford
Honoraria: Natera
Consulting or Advisory Role: Natera, Illumina
Seth A. Wander
Consulting or Advisory Role: Foundation Medicine, Puma Biotechnology, Veracyte, Lilly, Hologic, Pfizer, Biovica
Speakers' Bureau: Lilly, Guardant Health
Research Funding: Genentech, Lilly, Pfizer, Regor Therapeutics, Nuvation Bio
William J. Gradishar
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Pfizer, Puma Biotechnology, Seattle Genetics, Merck, BeyondSpring Pharmaceuticals
Amir Behdad
Honoraria: Roche China, Lilly, Leica Biosystems, Caris Life Sciences
Consulting or Advisory Role: Caris Life Sciences, Leica Biosystems
Speakers' Bureau: Lilly, Roche China
Travel, Accommodations, Expenses: Bayer, Foundation Medicine, Pfizer
Cynthia X. Ma
Honoraria: PlusOne Health GmbH, Guardant Health
Consulting or Advisory Role: Novartis, Seattle Genetics, Agendia, AstraZeneca, Athenex, Bayer HealthCare Pharmaceuticals, Biovica Inc, Olaris, Sanofi-Genzyme, Gilead Sciences, Pfizer, Lilly, Tempus
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate: Authorship—served as a coauthor on the subject of endocrine therapy for metastatic breast cancer and receives compensation as an author, less than a few hundred dollars a year. It does not affect my patient care or research
Travel, Accommodations, Expenses: San Antonio Breast Cancer Symposium, Total Health Conferencing
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Pierre Fabre, Daiichi Sankyo, Eisai, Lilly, Pfizer, Exact Sciences
Consulting or Advisory Role: Roche, Amgen, Novartis, Pfizer, Eisai, Seattle Genetics, Pierre Fabre, AstraZeneca/Daiichi Sankyo, Viatris, Lilly, Gilead Sciences, Daiichi Sankyo Europe GmbH
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene, GlaxoSmithKline, Amgen, Astrazeneca, MSD, Novartis, Lilly, Pfizer
Aditya Bardia
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Spectrum Pharmaceuticals, bioTheranostics, Merck, Radius Health, Immunomedics (Inst), Novartis (Inst), Genentech/Roche (Inst), Pfizer (Inst), Radius Health (Inst), Innocrin Pharma (Inst), Immunomedics, Sanofi, Puma Biotechnology, Daiichi Sankyo/Astra Zeneca, Foundation Medicine, Philips Healthcare
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Merck (Inst), Sanofi (Inst), Radius Health (Inst), Immunomedics (Inst), AstraZeneca/Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/523675
Massimo Cristofanilli
Honoraria: Pfizer
Consulting or Advisory Role: Lilly, Menarini, Olaris, AstraZeneca/Daiichi Sankyo, Syantra, Sermonix Pharmaceuticals, Celcuiy
Research Funding: Lilly, Angle, Merck, AstraZeneca, Menarini Silicon Biosystems
No other potential conflicts of interest were reported.
REFERENCES
- 1. Fontanella C, Giorgi CA, Russo S, et al. Optimizing CDK4/6 inhibitors in advanced HR+/HER2- breast cancer: A personalized approach. Crit Rev Oncol Hematol. 2022;180:103848. doi: 10.1016/j.critrevonc.2022.103848. [DOI] [PubMed] [Google Scholar]
- 2. Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 3. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9:896. doi: 10.1038/s41467-018-03215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bardia A, Su F, Solovieff N, et al. Genomic profiling of premenopausal HR+ and HER2– metastatic breast cancer by circulating tumor DNA and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. JCO Precision Oncol. 2021 doi: 10.1200/PO.20.00445. 10.1200/PO.20.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerratana L, Davis AA, Zhang Q, et al. Longitudinal dynamics of circulating tumor cells and circulating tumor DNA for treatment monitoring in metastatic breast cancer. JCO Precision Oncol. 2021 doi: 10.1200/PO.20.00345. 10.1200/PO.20.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hrebien S, Citi V, Garcia-Murillas I, et al. Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol. 2019;30:945–952. doi: 10.1093/annonc/mdz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerratana L, Basile D, Franzoni A, et al. Plasma-based longitudinal evaluation of ESR1 epigenetic status in hormone receptor-positive HER2-negative metastatic breast cancer. Front Oncol. 2020;10:550185. doi: 10.3389/fonc.2020.550185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mastoraki S, Strati A, Tzanikou E, et al. ESR1 methylation: A liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res. 2018;24:1500–1510. doi: 10.1158/1078-0432.CCR-17-1181. [DOI] [PubMed] [Google Scholar]
- 10. Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24:17. doi: 10.1186/s13058-022-01510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One. 2015;10:e0140712. doi: 10.1371/journal.pone.0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forbes SA, Beare D, Boutselakis H, et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zill OA, Banks KC, Fairclough SR, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res. 2018;24:3528–3538. doi: 10.1158/1078-0432.CCR-17-3837. [DOI] [PubMed] [Google Scholar]
- 15. Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. 2017 doi: 10.1200/PO.17.00011. 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bidard F-C, Hardy-Bessard A-C, Dalenc F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367–1377. doi: 10.1016/S1470-2045(22)00555-1. [DOI] [PubMed] [Google Scholar]
- 18. Samuel Eziokwu A, Varella L, Lynn Kruse M, et al. Real-world outcomes of cyclin-dependent kinase inhibitors continued beyond first disease progression in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2021;21:205–209. doi: 10.1016/j.clbc.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 19. Kalinsky K, Accordino MK, Chiuzan C, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J Clin Oncol. 2022;40 suppl 17; abstr LBA1004. [Google Scholar]
- 20. Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor–positive metastatic breast cancer. Cancer Discov. 2020;10:1174–1193. doi: 10.1158/2159-8290.CD-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10:1373. doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wander SA, Han HS, Zangardi ML, et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: A multicenter experience. J Natl Compr Cancer Netw. 2021 doi: 10.6004/jnccn.2020.7662. 10.6004/jnccn.2020.7662 [DOI] [PubMed] [Google Scholar]
- 23.Brett JO, Dubash TD, Johnson GN, et al. A gene panel associated with abemaciclib utility in ESR1-mutated breast cancer after prior CDK4/6-inhibitor progressionJCO Pres Oncol. 10.1200/PO.22.005322023
- 24.Mayer E.https://clinicaltrials.gov/ct2/show/NCT03147287 Palbociclib after CDK and endocrine therapy (PACE): A randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer 2022.
- 25. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer EL, Ren Y, Wagle N, et al. Abstract GS3-06: GS3-06 Palbociclib after CDK4/6i and endocrine therapy (PACE): A randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer Cancer Res 83(5_Supplement):GS3-06-GS3-06, 2023 [Google Scholar]
- 27.https://clinicaltrials.gov/ct2/show/NCT04318223 Consorzio Oncotech: Palbociclib plus fulvestrant in women with hormone receptor positive and human epidermal growth factor receptor type 2 negative locally advanced or metastatic breast cancer previously treated with a CDK4/6 inhibitor in combination with hormonal therapy: A multicenter, phase II trial, 2020.
- 28.https://clinicaltrials.gov/ct2/show/NCT05169567 Eli Lilly and Company: postMONARCH: A randomized, double blind, placebo-controlled, phase 3 study to compare the efficacy of abemaciclib plus fulvestrant to placebo plus fulvestrant in participants with HR+, HER2-, advanced or metastatic breast cancer following progression on a CDK4 & 6 inhibitor and endocrine therapy, 2022.
- 29. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961–2968. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]