Abstract
Critical knowledge gaps regarding infection with Mycobacterium ulcerans, the cause of Buruli ulcer (BU), have impeded development of new therapeutic approaches and vaccines for prevention of this neglected tropical disease. Here, we review the current understanding of host–pathogen interactions and correlates of immune protection to explore the case for establishing a controlled human infection model of M. ulcerans infection. We also summarise the overarching safety considerations and present a rationale for selecting a suitable challenge strain.
1. Background
1.1. Disease burden
The neglected tropical disease, Buruli ulcer (BU), is a bacterial infection of skin and subcutaneous tissue. The causative bacteria Mycobacterium ulcerans was first described in contemporary literature by Australian researchers (MacCallum and colleagues) in 1948 [1]. Shortly thereafter, clinicians in African countries reported similar lesions, notably in the Buruli county (now Nakasongola District) of Uganda [2]. Cases of BU have since been identified globally, predominantly in West and Central Africa [3]. BU is the third most commonly reported mycobacterial infection after tuberculosis and leprosy, with an estimated 5,000 to 6,000 cases per year from 2004 to 2010 [4] although reducing in subsequent years [3]. In Africa, focal prevalence during outbreaks may reach as high as 15% [5].
In Victoria, Australia, where the climate is temperate, cases have continued to increase, with calls for an urgent scientific response [6]. Since first recognition of the “Bairnsdale ulcer” in the 1930’s [7], cases have been reported focally in regional, predominantly coastal areas, with an unpredictable pattern of geographical distribution. In early 2021, the epidemiology shifted unexpectedly, with an increase in incidence in the inner north-western suburbs of the Victorian state capital city Melbourne. Although investigations are ongoing, suggested explanations for this new urban focus have included increased rainfall due to multiple consecutive La Niña weather events, and the evolving complex relationship with native possums, which are known to be natural hosts [8] manifesting clinical disease and being geographically linked to human cases [9]. Like many neglected tropical diseases [10], the incidence of M. ulcerans is prone to shift in response to climate change and the increasingly complex relationship between humans and their environment.
1.2. Clinical disease
In Victoria, Australia, exposure is followed by a long incubation period, averaging 4 to 5 months [11,12]. M. ulcerans typically produces a localised skin and subcutaneous infection due to its growth requirement for cooler temperatures. Lesions often begin as a nodule, plaque or more rarely, oedema [13]. Ulcers typically develop slowly, and are characteristically painless, with an undermined edge, surrounding swelling and central necrosis. Left untreated, ulcers may either heal spontaneously, as reported in Australia [14], or progress in size and severity, leading to permanent contracture, deformity, and disability [15]. Contiguous infection of underlying bone has also been reported, principally in Africa [16]. Antibiotic treatment generally results in very high cure rates without the need for surgery, although treatment duration is long, and side-effects are common [17]. Although the disease is uncommonly lethal, vulnerable populations with inadequate access to healthcare are at risk of long-term disability due to advanced disease and scarring, particularly if antibiotic treatment (or surgery) is delayed.
1.3. Pathogenesis
The pathogenesis of BU is driven by production of the toxin mycolactone (ML), which has potent immunosuppressive properties [18]. Mycolactone acts by binding to the Sec61 translocon complex, preventing transport of newly synthesised proteins across the membrane of the endoplasmic reticulum [19]. By this mechanism, mycolactone irreversibly inhibits the production of numerous cytokines [20], including interleukin-2 (IL-2) [21,22] and interferon gamma (IFN-γ) [23]. The importance of IFN-γ is apparent in IFN-γ knockout mice, which have reduced capacity to kill intracellular bacilli [24] and faster disease progression [25]. Prolonged Sec61 inhibition also stimulates cytotoxic stress responses, with apoptosis [26] and dose-dependent necrosis [27], liberating bacilli [26,28] and inhibiting leukocyte infiltration [29] within the ulcer’s core. In the tissue surrounding this necrotic core, where mycolactone is less concentrated, a band of infiltrating leukocytes, comprising predominantly macrophages, contain intracellular bacilli [29,30]. This enables the centrifugal propagation of infection [31]. In mice, ML-negative isolates are avirulent, with a mononuclear infiltrate, intracellular bacilli, and granulomatous inflammation [29].
1.4. Human immune responses to M. ulcerans: Current knowledge
1.4.1. Local cell-mediated immunity in humans
An understanding of the local and systemic cell-mediated immunity (CMI) to BU in humans, particularly during healing (following surgery, antibiotic therapy, or spontaneous healing) may uncover correlates of protection. Studies have consistently demonstrated that patients with BU have suppressed IFN-γ production during clinical disease, with relative suppression of Th1 responses, creating an environment where M. ulcerans evades mechanisms that usually control intracellular infection. As local immunity is likely to be more relevant than systemic immunity in this subcutaneous disease, intralesional immune profiles have been explored in human studies. Prévot and colleagues analysed the lesions of 14 patients in French Guiana, demonstrating high IL-10 but low IFN-γ mRNA levels in ulcers compared to high intralesional IFN-γ but low IL-10 mRNA levels in nodular lesions [32]. Kiszewski and colleagues analysed skin and subcutaneous tissue from 11 patients in Benin with BU, concluding that early ulcerative lesions had a predominantly immunosuppressive cytokine profile accompanied by high bacillary counts, whereas older lesions showed a mixed cytokine pattern, dominated by IFN-γ, together with low bacillary loads and granuloma formation [33]. Granuloma formation therefore likely represents a late event of the ulcerative stage, probably preceding healing. Schipper and colleagues studied 23 patients with active BU in Ghana, 7 with pre-ulcerative lesions and 16 with ulcerative disease, as well as 22 patients with healed BU [34]. They showed that patients with ulcerative and healed BUs produce significantly higher levels of IFN-γ compared to healthy controls, after ex vivo whole-blood stimulation with tuberculin purified protein derivative (PPD), and that patients with a granulomatous response produce higher IFN-γ levels than those without. As demonstrated by immunostaining of lesions at various stages of disease, further evidence supporting the protective effect of granulomatous inflammation in BU is the modest IFN-γ expression in the organising stage and marked IFN-γ expression during the granulomatous (healing) stage [34]. Schipper and colleagues were also the first to show that local IFN-γ production in granulomatous tissue was reflected by a systemic type 1 immune response to M. ulcerans, with a granulomatous response accompanied by high IFN-γ levels after whole-blood stimulation with tuberculin PPD [34].
1.4.2. Systemic cell-mediated immunity in humans
There is inconsistency in the literature regarding the degree of systemic immunosuppression in BU. Gooding and colleagues analysed cellular immune responses in 14 patients from Victoria, Australia, 4 patients with active BU and 10 with healed lesions after surgical excision. They reported significantly reduced production of IFN-γ in response to stimulation with M. ulcerans whole cells compared to healthy controls [23]. Gooding and colleagues also studied 23 patients and 25 household contacts in far north Queensland (FNQ), finding that affected patients produced significantly greater Th2-type cytokines (IL-4, -5, -6, and -10) than unaffected household contacts [35]. Notably, only 1 patient had active disease, with the remainder having distant illness (mean of 9.4 years since illness) [35]. The only prospective analysis of the human immune response was a household contact participant of the larger trial in FNQ, who demonstrated a type 2 cytokine response 3 months after surgical excision of BU, failing to produce IFN-γ and IL-12, but with preserved IL-4 and IL-10 production upon peripheral blood mononuclear cell (PBMC) stimulation [36]. Prévot and colleagues also observed decreased IFN-γ production in stimulated PBMCs in patients with ulcerative lesions, compared to patients with nodular forms [32].
In a study performed in Ghana, Westenbrink and colleagues compared 23 patients with early-stage BU to 16 patients with late-stage BU. Using in vitro whole-blood stimulation with phytohemagglutinin (PHA) and tuberculin PPD, they reported that patients with early-stage BU produced significantly lower levels of IFN-γ compared to late-stage BU and control patients; systemic IL-10 and IL-4 levels did not differ between patients and controls in this study [37]. Schipper and colleagues reasoned that reduced systemic IFN-γ responses previously reported [23,32,35,36] may be because patients may have not had enough time to develop a type 1 immune response. This is unlikely to explain the observed differences for the studies performed by Gooding and colleagues, which predominantly included patients late in the course of their disease, while the duration of illness in the patients analysed by Prévot and colleagues was indeed shorter [32]. An alternative explanation is that patients in Australia and French Guiana may have sought treatment earlier or had improved access to healthcare than African patients. Studies suggest that systemic suppression of M. ulcerans-specific IFN-γ resolves 5 to 10 months after surgical excision of BU [38] and 1 to 2 months following curative antibiotic treatment [13].
In a subsequent study evaluating cytokine and chemokine responses to BU in Ghana, Phillips and colleagues not only found that PHA stimulation-induced production of IFN-γ in whole blood was reduced in patients compared to healthy controls, but also that Th2 and Th17 responses were down-regulated in patients with BU. This systemic immunosuppression was reported to resolve following treatment. They also demonstrated that patients with nodular lesions had suppressed circulating chemokines, and suppression of these chemokines persisted during the ulcerative stage of disease [22]. Phillips and colleagues also reported that serum IL-4 was significantly up-regulated in patients with BU during antibiotic treatment compared to healthy control participants, possibly explaining paradoxical reactions in patients during antibiotic therapy, due to increased B- and T-cell proliferation and Th-2 cell differentiation [22]. The inhibition of professional phagocyte function has also been reported in dendritic cells (DCs). Coutanceau and colleagues found that ML suppresses the capacity of DCs to prime cellular immune responses, which may help explain the inhibition of IFN-γ production by T-cells and the fact that once BU lesions are excised, there is an intact Th1 response to the organism [39].
1.4.3. Humoral immunity
Like in most mycobacterial infections, humoral immunity seems to play a lesser role in controlling M. ulcerans infection. A number of studies have shown that patients with BU demonstrate an antibody response to infection [23,40]. Exposed controls can also have similar antibody responses to infected patients [35]. Antibodies targeting an immunodominant 18kDa heat-shock protein (Hsp18) without close orthologues in M. bovis or M. tuberculosis were frequently found in the sera of patients with BU (75%) and in healthy household contacts (38%) but rarely in controls from non-endemic regions [41]. Pidot and colleagues used comparative genomics to identify 45 potential M. ulcerans-specific proteins, of which they were able to express and purify 33 in Escherichia coli [42]. When sera from 30 patients with BU, 24 healthy controls from the same endemic region and 30 healthy controls from a non-endemic region in Benin were screened for M. ulcerans-specific antibodies to these proteins, 7 proteins (including PKS domains ER, AT propionate, and KR-A) showed a significant difference between patient and non-endemic controls. They also found that IgG responses of endemic controls were not significantly different to those of patients for any of the 7 proteins [42]. In the largest serosurvey to date, Hsp18 IgG titres in supposedly non-endemic areas showed comparable seropositivity rates to endemic areas (33% versus 31%, respectively) [43]. This suggests that unrecognised host factors may influence the development of clinical disease.
The production of ML-specific antibodies in laboratory animals has been challenging because of the molecule’s immunosuppressive nature and its small, lipid structure. Foulon and colleagues demonstrated the presence of neutralising antibodies in a mouse strain that displays spontaneous healing properties following infection with M. ulcerans. These neutralising antibodies were present in local tissue, but not in sera. Although ML is recognised by antibodies recovered from the lesions of 60% of patients from Benin with PCR-confirmed BU [44], the role of these antibodies in clinical disease remains unclear.
2. Role of a controlled human infection model for M. ulcerans
Due to the sporadic and highly focal nature of the epidemiology of BU, clinical trials testing vaccine efficacy among participants living in endemic areas are likely to be underpowered and expensive. A systematic review of BU vaccines [45] has illustrated the progress made towards vaccine development, with 28 studies describing vaccination approaches using various targets. The reviewers highlighted that, although several early stage candidate vaccines have emerged, other than M. bovis bacille Calmette–Guérin (BCG), none have progressed to efficacy testing [45]. More recent systems immunology approaches have resulted in the identification of additional vaccination candidates with attractive properties [46]. Thus, a number of promising candidates merit further investigation, but there is a lack of a path to efficacy testing.
Testing vaccines on healthy human volunteers, deliberately infected with M. ulcerans in a controlled fashion with a typical clinical phenotype, offers the potential for establishing safety and protective efficacy of a vaccine candidate much faster than would be possible using a randomised controlled trial in the field. A BU controlled human infection model (CHIM) could take a prominent position early in the development pipeline of vaccines or experimental therapies. CHIMs have been established to study a range of viral, bacterial, and parasitic pathogens. They are particularly valuable for complex and uncommon pathogens, allowing researchers to comprehensively interrogate the immunobiology of disease, while providing a biological signal of vaccine efficacy and identifying immunological correlates of protection, efficiently enabling subsequent stratification of vaccine development [47].
Vaccine efficacy can be studied in a CHIM of 10 to 100 participants, providing confidence to proceed to Phase 2 and 3 clinical trials where sample sizes of thousands of participants are typically required, with some confidence that the experimental intervention would be effective [48]. With a high (universal) infection rate in control participants, the number of participants required is small and readily defined [49]. For example, in a simple two-arm (placebo versus vaccine) trial with 90% power to detect a dichotomous outcome (lesion versus no lesion) at a p value of 0.05 and with vaccine efficacy of 70%, a CHIM with 100% attack rate would require 14 participants (7 participants per arm). If the attack rate was reduced to 80%, then a vaccine with 70% efficacy would require 18 participants per arm. In anticipation of dropouts, the sample size could be increased by 10% to 20% to compensate, without significantly affecting the economic feasibility of the study.
Beyond prophylactic interventions such as vaccines, other experimental therapeutic interventions may be tested using this model (Table 1). These interventions may be tested in parallel with vaccinations, as breakthrough infections may be diagnosed using experimental tests and treated using an experimental therapeutic product, with standard antibiotic therapy as a backup in case of treatment failure. Although a detailed review is beyond the scope of this paper, Roestenberg and colleagues have summarised the experimental infection of human volunteers and described the key ethical considerations, including novel models of neglected diseases [48].
Table 1. Questions that a BU CHIM may answer.
| 1 | What is the natural history of natural infection? |
| 2 | What are the systemic and local immunological responses to low dose infection? |
| 3 | What are the immunological correlates of protection? |
| 4 | What proportion of people develop antibodies against M. ulcerans-specific antigens? |
| 5 | What kind of antibodies do people develop against M. ulcerans? |
| 6 | What is the immunological basis for paradoxical reactions? |
| 7 | Can paradoxical reactions be predicted based on systemic immunological parameters? |
| 8 | What are the immunological correlates of spontaneous healing? |
| 9 | Can M. bovis BCG protect people from low-dose M. ulcerans infection? [45] |
| 10 | Can candidate vaccines delay or prevent clinical disease? |
| 11 | Can clinical disease be prevented by using early, short courses of antibiotics prior to the onset of clinical disease? (i.e., chemoprophylaxis) |
| 12 | Can abbreviated antibiotic courses (e.g., 4 to 6 weeks of rifampicin and clarithromycin) be used to treat early disease? |
| 13 | Can ultra-short treatment with telacebac [52] successfully treat BU in humans? |
| 14 | Can M. ulcerans-targeted bacteriophages be used in the management of BU? |
| 15 | Can non-antibiotic options be useful in the treatment of early (pre-ulcerative or small) lesions? For example, local heat therapy (thermotherapy) [53] |
BU, Buruli ulcer; CHIM, controlled human infection model.
There is no precedent for an M. ulcerans CHIM, with M. bovis BCG being the only mycobacteriosis to be studied in a CHIM setting [50]. Minassian and colleagues at the Jenner Institute (Oxford, United Kingdom) developed the model to test intradermal BCG vaccination as a surrogate for M. tuberculosis infection (because there is presently no safe tuberculosis CHIM available) [50]. They hypothesised that an effective vaccine against M. tuberculosis should reduce replication of M. bovis BCG, in their study population. In this model, intradermal BCG load was quantified using skin biopsy specimens by both quantitative polymerase chain reaction (qPCR) and culture using colony-forming units (CFUs); immunological correlates of protection were investigated by comparing pre-challenge immune profiles against M. bovis BCG load after challenge [50].
Because M. ulcerans infection is not restricted to humans, animal models may provide preclinical proof-of-concept, but the genetic and immunological differences make it difficult to extrapolate vaccine efficacy to humans [51]. In summary, although there are valuable clues in retrospective human studies, attempts to find immunological correlates of immune protection by retrospective analyses are hampered by long follow-up timeframes and confounders associated with observational methodology. Prospectively collected data that links clinical disease and immunological responses from baseline (pre-exposure) to infection and disease resolution, with or without exposure to a candidate vaccine or other intervention, has the potential to address numerous questions in BU research.
3. Approach to BU CHIM development
3.1. Safety considerations
Human models have been safely established for pathogens that can cause severe clinical syndromes, including Streptococcus pneumoniae, Streptococcus pyogenes, Vibrio cholerae, Plasmodium falciparum, dengue virus, and SARS-CoV-2 [47]. Compared to these pathogens, natural infections with M. ulcerans are slower, less severe, and relatively non-invasive, with low mortality, usually restricted to skin and subcutaneous tissue. Nevertheless, the safety of participants is paramount and residual risks to volunteers need to be minimised using a range of approaches, including (1) a cautious approach to strain selection and meticulous manufacturing processes in accordance with Good Manufacturing Practice (GMP) standards and requirements for regulatory approval; (2) selection of healthy adults, according to eligibility criteria approved by a human research ethics committee; (3) close clinical monitoring and support throughout the study; (4) prompt administration of curative antibiotic therapy; and (5) end-to-end oversight by a safety committee that includes investigators independent of the study team.
Initial studies to develop new CHIMs are generally restricted to healthy adult volunteers, following a thorough informed consent process [54]. For a BU CHIM, exclusion criteria would include a history of keloid formation and risk factors for delayed wound healing (e.g., smoking). BU severity and complications are particularly problematic in children [55] and the elderly [56], so young adult participants are preferred. They should also be screened for blood-borne viruses, primary and secondary immunodeficiencies, as well as comorbidities such as diabetes, which is a known risk factor for severe oedematous lesions and may impair wound healing [57]. Drug–drug interactions are a particular concern for both rifampicin and clarithromycin, so any concurrent medications should be carefully checked for interactions. A history of BCG vaccination and previous exposure to tuberculosis is also relevant due to potential baseline cross-protection.
A clinically relevant BU model would have a longer incubation period than for most CHIM studies, although the Schistosoma mansoni model is a good example of an established model with a long incubation period [58]. As in the schistosomiasis model, participants must be able to adhere to an outpatient follow-up schedule that could incorporate telehealth and in-person appointments as appropriate to facilitate prompt clinical evaluation, initiation of timely antibiotic treatment (or the intervention being tested), and wound care. Frequent follow-up will be needed until the lesion has fully healed, and until 12 months from treatment initiation, after which relapse or paradoxical reactions are uncommon [59,60]. In the absence of a lesion, follow-up beyond the longest reported incubation period is proposed (e.g., 12 months after challenge). Follow-up would be more frequent after any pre-ulcerative lesion is noted, with more extensive local and systemic sampling procedures. Transmission is thought to be from a puncture wound or mosquito bite, and human-to-human transmission is not thought to occur [61] but is nevertheless readily prevented by covering wounds.
In broad terms, a human model of infection can only be established for non-severe disease syndromes that are self-limited or reliably treated, with very rare acute or chronic complications [54]. Oral antibiotic therapy is now the standard of care for BU, with surgery reserved for very large ulcers (to achieve skin coverage) or unusual cases that respond incompletely to antibiotics [62]. Surgery may also be useful for those who cannot tolerate or decline antibiotic therapy or to shorten antibiotic treatment [62]. The WHO recommended antibiotic regimen is oral rifampicin (10 mg/kg once daily) and clarithromycin (7.5 mg/kg twice daily) for 8 weeks, following the results of a randomised trial that demonstrated all-oral therapy was non-inferior to injectable aminoglycoside antibiotic therapy and cured 96% of participants with early, limited BU [63]. Notably, the majority of participants with an unsuccessful outcome in this trial were lost to follow up or did not adhere to protocol-directed wound care. Of 146 participants, 9 (6%) prescribed oral combination antibiotic therapy experienced an adverse event, none serious. One participant prescribed rifampicin/clarithromycin experienced ototoxicity and 2 experienced non-severe QTc prolongation [63]. Retrospective observational Australian data suggests that antibiotic complications are not uncommon, particularly in those of advanced age [56] and renal impairment [17]. In retrospective Australian studies, treatment failure is rare (approximately 1%), and identified risk factors include patient weight >90 kg, male sex, and immunosuppression, highlighting the importance of careful participant selection [64].
Spontaneous healing without treatment has been reported in a small number of immunocompetent patients [14], although it is unclear how and why some patients mount sterilising immune responses and others do not. After antibiotic completion, early limited lesions typically heal after a median of 16 weeks (interquartile range 8 to 25 weeks) [63]. Approximately 7% of BU patients in Victoria’s Bellarine Peninsula develop an acute oedematous form of BU and may benefit from preemptive treatment with corticosteroids to minimise tissue destruction [65]. Paradoxical reactions may give the impression of clinical deterioration despite appropriate therapy. These reactions are observed after a median of 39 days [59] in approximately 20% of patients undergoing antibiotic treatment [56,59], occasionally requiring corticosteroids to blunt the exaggerated immunological response [66] and prolonged antibiotic treatment in selected cases [59]. These reactions are associated with high bacterial loads [67], emphasising the importance of careful definition of the challenge inoculum.
We envisage that in a BU human challenge trial, participants would begin antibiotic treatment shortly after a lesion develops. Although intervening this early in the disease process should preclude the need for surgical intervention, it is possible that the cosmetic result after complete excision of a small and early limited lesion might be preferred by some participants, may allow for a shortened duration of antibiotic therapy, and have the benefit of supplying tissue samples of significant research value. Participants would require more regular outpatient monitoring once treatment was started, for wound care and to observe any adverse effects from antibiotic therapy. Prompt initiation of treatment, prior to the development of significant soft tissue necrosis, would reduce the likelihood of secondary infection, while wound care also minimises environmental exposure [68].
3.2. Guiding criteria for M. ulcerans CHIM strain selection
With the overarching goals of promoting participant safety and clinical relevance of a BU CHIM, we propose the following considerations for selecting M. ulcerans challenge strains.
Suggested characteristics of an ideal M. ulcerans challenge strain
The ideal Mycobacterium ulcerans challenge strain
Is not associated with severe clinical disease, either in its individual provenance or in geographically associated cases.
Causes a typical infection phenotype.
Is amenable to a biologically plausible route of entry.
Is susceptible to clinically relevant antibiotics.
Can be cultured in a non-toxic, animal-free medium without genetic or chemical modification.
Can be accurately enumerated to ensure consistent challenge dosing.
Retains viability after cryopreservation.
Produces the key virulence factor after in vitro culture (i.e., mycolactone).
Maintains a conserved repertoire of genes encoding candidate vaccine antigens.
Remains genetically stable during manufacture and challenge.
1. Is not epidemiologically associated with severe clinical disease (either in its individual provenance or in geographically associated cases)
A suitable M. ulcerans challenge strain should not be associated with a severe clinical phenotype. For example, the Australian isolate JKD8049 has been previously studied in murine models of M. ulcerans infection [69,70]. JKD8049 was obtained from an adult male in 2004, during an outbreak in Point Lonsdale, Victoria. The presentation was of a characteristic painless ulcer on the posterior calf, which was first noticed incidentally by an allied health practitioner.
Although Australian isolates of M. ulcerans belong to the classical lineage that also causes BU in Africa, infections acquired in Australia demonstrate notably different clinical characteristics in observational studies and serve as a useful comparison [71]. In Point Lonsdale, Victoria, Australia, an outbreak began abruptly in 2002, with BU affecting 79 people (48 residents and 31 visitors) [72]. A clinical description of 180 cases across the entire Bellarine Peninsula (an area including Point Lonsdale) during this period noted that 95% of patients had a single lesion and the majority of patients had a nodule or ulcer [73]. Only 5% of patients presented with oedema, the majority of whom were over 60 years of age, similar to observed disease severity across Victoria [74]. One patient had septic bursitis, and another had BU osteomyelitis [73]; this contrasts with osteomyelitis rates of approximately 6% in Benin [16]. In Benin, osteomyelitis has been observed to occur some distance from active or apparently healed BU lesions and even in some patients without any history of BU skin lesion [16]. This phenomenon has rarely been reported in Australia [75]. Given that M. ulcerans grows optimally at 30° to 33°C, the subacute haematogenous spread suggested by these cases is surprising, although isolates from Africa are more thermotolerant than those from temperate regions [76].
2. Reproduces a typical infection phenotype
A successful CHIM should be clinically relevant, safely and reliably reproducing a clinical infection endpoint that faithfully resembles what is seen after natural exposure [49]. In Australia, the most common site of infection in humans is the lower limb, between the knee and ankle [77]. In an Australian setting, using an Australian isolate such as JKD8049, the posterior calf appeals as a site for inoculation, equidistant from the knee and ankle joints, and separated from the underlying bone by the large gastrocnemius muscle, reducing the (already low) risk of septic arthritis and osteomyelitis by contiguous spread, and contractures due to scarring. A lesion at this site is also easily amenable to surgical excision, if required. Observational studies also suggest that it is not a site with an increased likelihood of oedematous lesions such as those involving the hand, elbow, or ankle [57]. M. bovis BCG vaccination provides a useful reference point for the expected local superficial scar in a BU CHIM.
Unlike other pathogens tested in CHIMs, M. ulcerans is not restricted to humans, and has been identified in numerous zoonotic hosts. The selected challenge strain may therefore be characterised phenotypically in a murine model, in order to demonstrate predictable patterns of virulence and immunological responses to infection. At least in the case of JKD8049, remarkably low doses are known to establish infection in mice. For this isolate, the infectious dose to infect 50% of mice (ID50) is known to be approximately 2.6 CFU [69], although 104 to 106 CFU have been used in most vaccine/challenge murine studies, using a variety of challenge strains [45]. Such unrealistically high doses are likely to be far greater than what is required to establish natural infection and may overwhelm immune responses and underestimate the true efficacy of candidate vaccines. The CHIM should therefore use a realistically low dose of M. ulcerans. The initial study to establish a human challenge model must also incorporate a dose-finding design, as the infectious dose in humans is unknown.
In order to understand the typical disease phenotype, it is anticipated that the study end point will be reached at the onset of ulceration, or alternatively, when any pre-ulcerative lesion has been present for a significant period of time. If a cellulitic or oedematous presentation is encountered, the study end point will be reached, and treatment will need prompt initiation.
3. Is amenable to a biologically plausible route of entry
Studies have previously demonstrated that M. ulcerans isolate JKD8049 can cause infection after inoculation procedures mimicking the hypothesised mosquito bite route of entry, and also after subcutaneous injection, using realistically low doses in mice [69]. Studies have also shown that abrasions are not amenable to initiating clinical infection, at least in a guinea pig model [78]. The ID50 curve reported by Wallace and colleagues [69] demonstrates that, according to this model, the number of organisms required to establish infection in ≥90% of mice is approximately 20 CFU. Needlestick puncture has shown that a low-dose mouse tail infection was achieved in 21 of 24 (88%) of mice (average approximately 30 CFU) [69], while approximately 14 to 20 CFU resulted in infection in a mouse tail model, with at least 80% of mice infected [79]. Therefore, an initial target range of approximately 20 to 30 CFU may be considered, although minor deviations above this range are unlikely to have a clinically significant impact.
Given the success of previous experiments at successfully initiating infection using a low-dose mechanical model of infection, skin-puncturing microtrauma is proposed as the most biologically plausible method of inoculation for a BU CHIM. Specifically, a 25-gauge (or smaller) hypodermic needle, used to inject up to 0.1 mL of culture material subcutaneously, approximately 2 to 3 mm under the skin, is proposed. This depth approximates the length of a mosquito proboscis [80], which is postulated to be a possible route of infection in Australia [69,72,81].
4. Is susceptible to clinically relevant antibiotics
Although clarithromycin is the preferred companion drug to rifampicin, fluoroquinolones (such as ciprofloxacin or moxifloxacin) may be used in combination with rifampicin when clarithromycin is unavailable, contraindicated, or poorly tolerated [82]. Australian guidelines [82] recommend the use of clarithromycin alongside a fluoroquinolone when rifampicin cannot be used, based on effectiveness in mouse models [83]. Antibiotic resistance in Australian isolates has not been reported, unlike African isolates, where rifampicin resistance is described [84]. An ideal challenge strain would therefore be susceptible to rifampicin, clarithromycin, and fluoroquinolones in vitro, and a known allergy to these antibiotics would be an exclusion criterion. Although there were previously no defined clinical breakpoints for in vitro susceptibility testing, the Clinical and Laboratory Standards Institute (CSLI) guidelines offer suggested susceptibility ranges to interpret minimum inhibitory concentrations (MICs) for slow-growing non-tuberculous mycobacteria such as M. ulcerans [85]. Authorities suggest that laboratories should establish their own in-house validation for fastidious non-tuberculous mycobacterial species [85]. If externally validated, such methodology may standardise M. ulcerans MIC testing for future work.
5. Can be cultured in a nontoxic and safe minimal media, with minimal chemical modification to enrich growth
One of the major limitations in M. ulcerans research is the organism’s slow growth, due to a reported doubling time of approximately 48 h. This results in the requirement for long incubation periods, generally up to 12 weeks, within a relatively narrow temperature window. The long incubation period also increases the opportunity for contamination, reinforcing the importance of performing all experimental work within strictly sterile conditions. Although there are numerous M. ulcerans isolates available for consideration, geographically diverse isolates are remarkably conserved, with minimal genetic diversity [86]. Nevertheless, the growth characteristics of various isolates should be investigated; a faster time to culture positivity would be an attractive option, minimising the opportunity for contamination. However, an isolate with rapid growth characteristics is not necessarily a requirement for a candidate CHIM strain; rather, an isolate which reliably reproduces a typical clinical phenotype should be prioritised. The standard for M. ulcerans liquid culture, Middlebrook 7H9, requires the addition of albumin and catalase to enrich growth; both are generally derived from bovine sources. This introduces the small but not insignificant risk of bovine spongiform encephalopathy (BSE), particularly if the origin of the product is from an endemic region.
Sauton’s medium is an alternative liquid culture medium, which is free of animal products, and has an established history of use for the culture of M. bovis BCG [87]. It contains nontoxic ingredients and is pH neutral [88]. Previous studies have demonstrated that M. bovis BCG retains virulence properties when cultured in this medium compared to research media [87], while M. ulcerans reportedly also retains the ability to produce mycolactone in this medium [89]. Although often routinely added to reduce clumping, surfactants (e.g., polysorbate/Tween) are ideally avoided, aiming to minimise chemical modification, particularly considering the presence of hydrophobic lipid-rich structures, including mycolactone, in M. ulcerans. Other alternatives to explore may include replacing animal containing supplements with synthetic proteins or proteins of non-animal origin. Such additional nutritional supplementation is anticipated to enhance M. ulcerans growth; vegetable protein alternatives, which are free of animal protein and nongenetically modified, also have a history of use in a CHIM [49].
6. Can be enumerated with accuracy to ensure precise challenge dosing
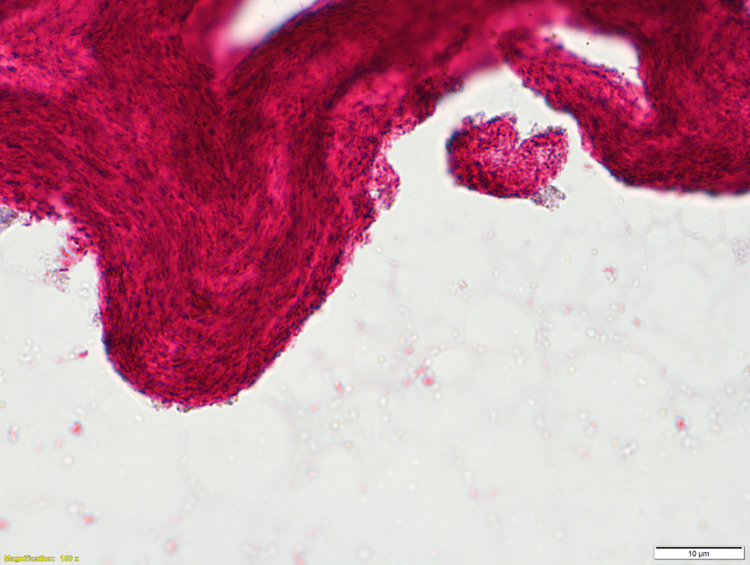
An issue that is familiar to researchers of slow-growing mycobacteria, and particularly M. ulcerans, is the propensity to form clumps and biofilm, potentially containing hundreds or thousands of bacilli (Fig 1). M. ulcerans has a known dose-dependent relationship with clinical phenotype; mice receiving larger doses of M. ulcerans demonstrate earlier onset of ulceration and more rapid tissue loss [90]. Therefore, a single clump may significantly overdose challenge participants, considering the low doses proposed. In the absence of detergent, mechanical de-clumping methodologies will need to be explored.
Fig 1. Ziehl–Neilson stain of M. ulcerans JKD8049 (×100 magnification with oil immersion) demonstrating clumping and cording characteristics, with innumerable acid-fast bacilli per clump when cultured in Sauton’s media without detergent.
7. Remains viable after frozen storage
Challenge doses of M. ulcerans created by any manufacturing process will require storage, generally by cryopreservation in a nontoxic substance (such as glycerol). Quality control processes of this sample will need to quantify its viability, including pre- and post-cryopreservation, and its stability after thawing using viable CFU count and/or quantitative PCR analysis.
8. Produces the key virulence factor after in vitro culture (mycolactone)
A clinically relevant BU model of infection would require that the selected isolate produces the main virulence toxin, mycolactone. Mycolactone production should be further characterised because minor sequence modifications in the ML genes of the pMUM plasmid (that encode the enzymes that synthesise the toxin) have produced a variety of ML congeners with variable virulence (A/B, C, D, E, and F). ML A/B (interconverting stereoisomers) produced by African strains are the most cytotoxic, while the potency is thought to be more attenuated in ML C (produced by Australian strains) and the other structural variants [91,92]. In murine fibroblast L929 cells treated with a series of synthetic mycolactones, ML F has been shown to be about 2 times less active and ML C about 15 times less active than ML A/B, respectively [93]. In addition to mycolactone C, Australian M. ulcerans strains also produce a fraction of ML A/B; Scherr and colleagues posit that the ML A/B portion may be more important for the pathogenesis caused by Australian strains than ML C [93]. In summary, the ability to produce mycolactone in vitro should be confirmed with liquid chromatography-mass spectroscopy.
9. Maintains a conserved repertoire of genes encoding candidate vaccine antigens
In order to test candidate vaccines against cell-surface antigens, whole-genome sequencing should confirm the presence of these genes in the challenge strain; these have been reviewed elsewhere [45]. Due to niche adaptation, global M. ulcerans isolates are remarkably conserved, so it is anticipated that most isolates should display a conserved repertoire of relevant antigens [94].
10. Remains genetically stable during manufacture and challenge
Serial whole-genome sequencing of the isolate, at multiple time points along the manufacturing cycle and following challenge, should (1) evaluate for the presence of spontaneous nucleotide polymorphisms; and (2) confirm the presence of an intact pMUM virulence plasmid.
Conclusions
Our limited understanding of human BU immunology comes from small retrospective analyses. Prospective studies are needed to comprehensively interrogate correlates of protection. Although there are several challenges to establishing a safe and clinically relevant human infection model of BU, a human model offers the potential to revolutionise understanding of host–pathogen interactions and represents an appealing platform for evaluating vaccines and therapeutic interventions for this neglected disease. We have introduced the framework to guide the characterisation of candidate challenge strains and provided study design recommendations to carry towards first-in-human studies. Future work must examine the ethical framework for such a model and explore protocol considerations.
Funding Statement
SM is supported by a Postgraduate Scholarship from the National Health and Medical Research Council (NHMRC) of Australia (GNT1191368), JO is supported by an NHMRC EL1 Investigator grant (GNT2009548); JM is supported by an NHMRC L3 Investigator grant (GNT2016396). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60(1):93–122. [PubMed] [Google Scholar]
- 2.Clancey J, Dodge R, Lunn HF. Study of a mycobacterium causing skin ulceration in Uganda. Ann De La Société Belge De Médecine Tropicale. 1962;42:585–590. [PubMed] [Google Scholar]
- 3.O’Brien DP, Jeanne I, Blasdell K, Avumegah M, Athan E. The changing epidemiology worldwide of Mycobacterium ulcerans. Epidemiol Infect. 2019;147:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molyneux DH, Savioli L, Engels D. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet. 2017;389(10066):312–325. doi: 10.1016/S0140-6736(16)30171-4 [DOI] [PubMed] [Google Scholar]
- 5.Marston BJ, Diallo MO, Horsburgh CR, Diomande I, Saki MZ, Kanga JM, et al. Emergence of Buruli ulcer disease in the Daloa region of Cote D’Ivoire. Am J Trop Med Hyg. 1995;52(3):219–224. doi: 10.4269/ajtmh.1995.52.219 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien DP, Athan E, Blasdell K, Barro PD. Tackling the worsening epidemic of Buruli ulcer in Australia in an information void: time for an urgent scientific response. Med J Australia. 2018;208(7):287–289. doi: 10.5694/mja17.00879 [DOI] [PubMed] [Google Scholar]
- 7.Alsop DG. The Bairnsdale ulcer. Aust NZ J Surg. 1972;41(4):317–319. [PubMed] [Google Scholar]
- 8.Fyfe JAM, Lavender CJ, Handasyde KA, Legione AR, O’Brien CR, Stinear TP, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Neglect Trop Dis. 2010;4(8):e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson C, Lavender CJ, Handasyde KA, O’Brien CR, Hewitt N, Johnson PDR, et al. Potential wildlife sentinels for monitoring the endemic spread of human Buruli ulcer in south-east Australia. PLoS Neglect Trop Dis. 2014;8(1):e2668. doi: 10.1371/journal.pntd.0002668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth M. Climate change and the neglected tropical diseases. Adv Parasitol. 2018;100:39–126. doi: 10.1016/bs.apar.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trubiano JA, Lavender CJ, Fyfe JAM, Bittmann S, Johnson PDR. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Neglect Trop Dis. 2013;7(10):e2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftus MJ, Trubiano JA, Tay EL, Lavender CJ, Globan M, Fyfe JAM, et al. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection) in Victoria, Australia–Remains similar despite changing geographic distribution of disease. PLoS Neglect Trop Dis. 2018;12(3):e0006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarfo FS, Phillips RO, Ampadu E, Sarpong F, Adentwe E, Wansbrough-Jones M. Dynamics of the cytokine response to Mycobacterium ulcerans during antibiotic treatment for M. ulcerans disease (Buruli ulcer) in humans. Clin Vaccine Immunol. 2009;16(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien DP, Murrie A, Meggyesy P, Priestley J, Rajcoomar A, Athan E. Spontaneous healing of Mycobacterium ulcerans disease in Australian patients. Plos Neglect Trop Dis. 2019;13(2):e0007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agbenorku P, Edusei A, Agbenorku M, Diby T, Nyador E, Nyamuame G, et al. Buruli ulcer induced disability in Ghana: A study at Apromase in the Ashanti region. Plastic Surg Int. 2012;2012:752749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pommelet V, Vincent QB, Ardant MF, Adeye A, Tanase A, Tondeur L, et al. Findings in patients from Benin with osteomyelitis and polymerase chain reaction–confirmed Mycobacterium ulcerans infection. Clin Infect Dis. 2014;59(9):1256–1264. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien DP, Friedman D, Hughes A, Walton A, Athan E. Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Intern Med J. 2017;47(9):1011–1019. [DOI] [PubMed] [Google Scholar]
- 18.Baron L, Paatero AO, Morel JD, Impens F, Guenin-Macé L, Saint-Auret S, et al. Mycolactone subverts immunity by selectively blocking the Sec61 translocon. J Exp Med. 2016;213(13):2885–2896. doi: 10.1084/jem.20160662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demangel C, High S. Sec61 blockade by mycolactone: A central mechanism in Buruli ulcer disease. Biol Cell. 2018;110(11):237–248. doi: 10.1111/boc.201800030 [DOI] [PubMed] [Google Scholar]
- 20.Hall BS, Hill K, McKenna M, Ogbechi J, High S, Willis AE, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10(4):e1004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pahlevan AA, Wright DJ, Andrews C, George KM, Small PL, Foxwell BM. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-κB function. J Immunol. 1999;7(163):3928–3935. [PubMed] [Google Scholar]
- 22.Phillips R, Sarfo FS, Guenin-Macé L, Decalf J, Wansbrough-Jones M, Albert ML, et al. Immunosuppressive signature of cutaneous Mycobacterium ulcerans infection in the peripheral blood of patients with Buruli ulcer disease. J Infect Dis. 2009;200(11):1675–1684. [DOI] [PubMed] [Google Scholar]
- 23.Gooding TM, Johnson PDR, Campbell DE, Hayman JA, Hartland EL, Kemp AS, et al. Immune response to infection with Mycobacterium ulcerans. Infect Immun. 2001;69(3):1704–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieri R, Bolz M, Ruf MT, Pluschke G. Interferon-γ Is a crucial activator of early host immune defense against Mycobacterium ulcerans infection in mice. Plos Neglect Trop Dis. 2016;10(2):e0004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrado E, Fraga AG, Logarinho E, Martins TG, Carmona JA, Gama JB, et al. IFN-γ–dependent activation of macrophages during experimental infections by Mycobacterium ulcerans is impaired by the toxin mycolactone. J Immunol. 2010;184(2):947–955. [DOI] [PubMed] [Google Scholar]
- 26.Bieri R, Scherr N, Ruf MT, Dangy JP, Gersbach P, Gehringer M, et al. The macrolide toxin mycolactone promotes Bim-dependent apoptosis in Buruli ulcer through inhibition of mTOR. ACS Chem Biol. 2017;12(5):1297–1307. doi: 10.1021/acschembio.7b00053 [DOI] [PubMed] [Google Scholar]
- 27.Adusumilli S, Mve-Obiang A, Sparer T, Meyers W, Hayman J, Small PLC. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol. 2005;7(9):1295–1304. [DOI] [PubMed] [Google Scholar]
- 28.Ogbechi J, Hall BS, Sbarrato T, Taunton J, Willis AE, Wek RC, et al. Inhibition of Sec61-dependent translocation by mycolactone uncouples the integrated stress response from ER stress, driving cytotoxicity via translational activation of ATF4. Cell Death Dis. 2018;9(3):397. doi: 10.1038/s41419-018-0427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira MS, Fraga AG, Torrado E, Castro AG, Pereira JP, Filho AL, et al. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect Immun. 2005;73(10):6299–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruf MT, Steffen C, Bolz M, Schmid P, Pluschke G. Infiltrating leukocytes surround early Buruli ulcer lesions, but are unable to reach the mycolactone producing mycobacteria. Virulence. 2017;8(8):1918–1926. doi: 10.1080/21505594.2017.1370530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrado E, Fraga AG, Castro AG, Stragier P, Meyers WM, Portaels F, et al. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans. Infect Immun. 2007;75(2):977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prévot G, Bourreau E, Pascalis H, Pradinaud R, Tanghe A, Huygen K, et al. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect Immun. 2004;72(2):958–965. doi: 10.1128/IAI.72.2.958-965.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiszewski AE, Becerril E, Aguilar LD, Kader ITA, Myers W, Portaels F, et al. The local immune response in ulcerative lesions of Buruli disease. Clin Exp Immunol. 2006;143(3):445–451. doi: 10.1111/j.1365-2249.2006.03020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schipper HS, Rutgers B, Huitema MG, Etuaful SN, Westenbrink BD, Limburg PC, et al. Systemic and local interferon-gamma production following Mycobacterium ulcerans infection. Clin Exp Immunol. 2007;150(3):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gooding TM, Johnson PDR, Smith M, Kemp AS, Robins-Browne RM. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect Immun. 2002;70(10):5562–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gooding TM, Kemp AS, Robins-Browne RM, Smith M, Johnson PDR. Acquired T-helper 1 lymphocyte anergy following infection with Mycobacterium ulcerans. Clin Infect Dis. 2003;36:1076–1077. [DOI] [PubMed] [Google Scholar]
- 37.Westenbrink BD, Stienstra Y, Huitema MG, Thompson WA, Klutse EO, Ampadu EO, et al. Cytokine responses to stimulation of whole blood from patients with Buruli ulcer disease in Ghana. Clin Diagn Lab Immunol. 2005;12(1):125–129. doi: 10.1128/CDLI.12.1.125-129.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeboah-Manu D, Peduzzi E, Mensah-Quainoo E, Asante-Poku A, Ofori-Adjei D, Pluschke G, et al. Systemic suppression of interferon-γ responses in Buruli ulcer patients resolves after surgical excision of the lesions caused by the extracellular pathogen Mycobacterium ulcerans. J Leukoc Biol. 2006;79(6):1150–1156. [DOI] [PubMed] [Google Scholar]
- 39.Coutanceau E, Decalf J, Martino A, Babon A, Winter N, Cole ST, et al. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J Exp Med. 2007;204(6):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobos KM, Spotts EA, Marston BJ, Horsburgh CR, King CH. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg Infect Dis. 2000;6(2):158–164. doi: 10.3201/eid0602.000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diaz D, Döbeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, Soder N, et al. Use of the immunodominant 18-kilodalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin Vaccine Immunol. 2006;13(12):1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pidot SJ, Porter JL, Marsollier L, Chauty A, Migot-Nabias F, Badaut C, et al. Serological evaluation of Mycobacterium ulcerans antigens identified by comparative genomics. PLoS Neglect Trop Dis. 2010;4(11):e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeboah-Manu D, Röltgen K, Opare W, Asan-Ampah K, Quenin-Fosu K, Asante-Poku A, et al. Sero-epidemiology as a tool to screen populations for exposure to Mycobacterium ulcerans. PLoS Neglect Trop Dis. 2012;6(1):e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foulon M, Pouchin A, Manry J, Khater F, Robbe-Saule M, Durand A, et al. Skin-specific antibodies neutralizing mycolactone toxin during the spontaneous healing of Mycobacterium ulcerans infection. Sci Adv. 2020;6(9):eaax7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muhi S, Stinear TP. Systematic review of M. bovis BCG and other candidate vaccines for Buruli ulcer prophylaxis. Vaccine. 2021;39(50):7238–7252. doi: 10.1016/j.vaccine.2021.05.092 [DOI] [PubMed] [Google Scholar]
- 46.Ishwarlall TZ, Adeleke VT, Maharaj L, Okpeku M, Adeniyi AA, Adeleke MA. Identification of potential candidate vaccines against Mycobacterium ulcerans based on the major facilitator superfamily transporter protein. Front Immunol. 2022;13:1023558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choy RKM, Bourgeois AL, Ockenhouse CF, Walker RI, Sheets RL, Flores J. Controlled human infection models to accelerate vaccine development. Clin Microbiol Rev. 2022;35(3):e00008–e00021. doi: 10.1128/cmr.00008-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roestenberg M, Kamerling IMC, de Visser SJ. Controlled human infections as a tool to reduce uncertainty in clinical vaccine development. Front Med. 2018;5:297. doi: 10.3389/fmed.2018.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osowicki J, Azzopardi KI, McIntyre L, Rivera-Hernandez T, Ong C, Baker C, et al. A controlled human infection model of group A streptococcus pharyngitis: Which strain and why? Msphere. 2019;4(1):e00647–e00618. doi: 10.1128/mSphere.00647-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minassian AM, Satti I, Poulton ID, Meyer J, Hill AVS, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guérin. J Infect Dis. 2012;205(7):1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noll KE, Ferris MT, Heise MT. The Collaborative Cross: A systems genetics resource for studying host-pathogen interactions. Cell Host Microbe. 2019;25(4):484–498. doi: 10.1016/j.chom.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida DV, Converse PJ, Omansen TF, Tyagi S, Tasneen R, Kim J, et al. Telacebec for ultrashort treatment of Buruli ulcer in a mouse model. Antimicrob Agents Chemother. 2020;64(6). doi: 10.1128/AAC.00259-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel M, Bayi PF, Ruf MT, Bratschi MW, Bolz M, Boock AU, et al. Local heat application for the treatment of Buruli ulcer: Results of a phase II open label single center non comparative clinical trial. Clin Infect Dis. 2016;62(3):342–350. doi: 10.1093/cid/civ883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bambery B, Selgelid M, Weijer C, Savulescu J, Pollard AJ. Ethical criteria for human challenge studies in infectious diseases. Public Health Ethics. 2015;9(1):92–103. doi: 10.1093/phe/phv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker G, Friedman DN, O’Brien MP, Cooper C, McDonald A, Callan P, et al. Paediatric Buruli ulcer in Australia. J Paediatr Child Health. 2020;56(4):636–641. doi: 10.1111/jpc.14704 [DOI] [PubMed] [Google Scholar]
- 56.O’Brien DP, Friedman ND, Cowan R, Pollard J, McDonald A, Callan P, et al. Mycobacterium ulcerans in the elderly: more severe disease and suboptimal outcomes. PLoS Neglect Trop Dis. 2015;9(12):e0004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien DP, Friedman ND, McDonald A, Callan P, Hughes A, Athan E. Clinical features and risk factors of oedematous Mycobacterium ulcerans lesions in an Australian population: beware cellulitis in an endemic area. PLoS Neglect Trop Dis. 2014;8(1):e2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langenberg MCC, Hoogerwerf MA, Koopman JPR, Janse JJ, Oosterhoud JK van, Feijt C, et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med. 2020;26(3):326–32. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien DP, Robson M, Friedman ND, Walton A, McDonald A, Callan P, et al. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infect Dis. 2013;13(1):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wynne JW, Stinear TP, Athan E, Michalski WP, O’Brien DP. Low incidence of recurrent Buruli ulcers in treated Australian patients living in an endemic region. PLoS Neglect Trop Dis. 2018;12(8):e0006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Brien DP, Wynne JW, Buultjens AH, Michalski WP, Stinear TP, Friedman ND, et al. Exposure risk for infection and lack of human-to-human transmission of Mycobacterium ulcerans disease. Australia Emerg Infect Dis. 2017;23(5):837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien DP, Callan P, Friedman ND, Athan E, Hughes A, McDonald A. Mycobacterium ulcerans disease management in Australian patients: the re-emergence of surgery as an important treatment modality. ANZ J Surg. 2019;89(6):653–658. [DOI] [PubMed] [Google Scholar]
- 63.Phillips RO, Robert J, Abass KM, Thompson W, Sarfo FS, Wilson T, et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet. 2020;395(10232):1259–1267. doi: 10.1016/S0140-6736(20)30047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Brien DP, Friedman ND, Walton A, Hughes A, Athan E. Risk factors associated with antibiotic treatment failure of Buruli ulcer. Antimicrob Agents Chemother. 2020;64(9). doi: 10.1128/AAC.00722-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien DP, Huffam S. Pre-emptive steroids for a severe oedematous Buruli ulcer lesion: a case report. J Med Case Reports. 2015;9(1):98. doi: 10.1186/s13256-015-0584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman ND, McDonald AH, Robson ME, O’Brien DP. Corticosteroid use for paradoxical reactions during antibiotic treatment for Mycobacterium ulcerans. PLoS Neglect Trop Dis. 2012;6(9):e1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frimpong M, Agbavor B, Duah MS, Loglo A, Sarpong FN, Boakye-Appiah J, et al. Paradoxical reactions in Buruli ulcer after initiation of antibiotic therapy: Relationship to bacterial load. PLoS Neglect Trop Dis. 2019;13(8):e0007689. doi: 10.1371/journal.pntd.0007689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pluschke G, Röltgen K, editors. Buruli ulcer, Mycobacterium ulcerans disease. 2019. [PubMed] [Google Scholar]
- 69.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Neglect Trop Dis. 2017;11(4):e0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Omansen TF, Porter JL, Johnson PDR, van der Werf TS, Stienstra Y, Stinear TP. In-vitro activity of avermectins against Mycobacterium ulcerans. PLoS Neglect Trop Dis. 2015;9(3):e0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Käser M, Rondini S, Naegeli M, Stinear T, Portaels F, Certa U, et al. Evolution of two distinct phylogenetic lineages of the emerging human pathogen Mycobacterium ulcerans. BMC Evol Biol. 2007;7(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson PDR, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13(11):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyd SC, Athan E, Friedman ND, Hughes A, Walton A, Callan P, et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Australia. 2012;196(5):341–344. [DOI] [PubMed] [Google Scholar]
- 74.Tai AYC, Athan E, Friedman ND, Hughes A, Walton A, O’Brien DP. Increased severity and spread of Mycobacterium ulcerans, southeastern Australia. Emerg Infect Dis. 2018;24(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loftus MJ, Kettleton-Butler N, Wade D, Whitby RM, Johnson PD. A severe case of Mycobacterium ulcerans (Buruli ulcer) osteomyelitis requiring a below-knee amputation. Med J Australia. 2018;208(7):290–291. [DOI] [PubMed] [Google Scholar]
- 76.Eddyani M, Portaels F. Survival of Mycobacterium ulcerans at 37°C. Clin Microbiol Infect. 2007;13(10):1033–1035. [DOI] [PubMed] [Google Scholar]
- 77.Yerramilli A, Tay EL, Stewardson AJ, Kelley PG, Bishop E, Jenkin GA, et al. The location of Australian Buruli ulcer lesions—Implications for unravelling disease transmission. PLoS Neglect Trop Dis. 2017;11(8):e0005800. doi: 10.1371/journal.pntd.0005800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PLC. Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: Implications for transmission. PLoS Neglect Trop Dis. 2014;8(4):e2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mangas KM, Buultjens AH, Porter JL, Baines SL, Marion E, Marsollier L, et al. Vaccine-specific immune responses against Mycobacterium ulcerans infection in a low-dose murine challenge model. Infect Immun. 2019;88(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dixon AR, Vondra I. Biting innovations of mosquito-based biomaterials and medical devices. Dent Mater. 2022;15(13):4587. doi: 10.3390/ma15134587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, et al. Ecology and transmission of Buruli ulcer disease: A systematic review. PLoS Neglect Trop Dis. 2010;4(12):e911. doi: 10.1371/journal.pntd.0000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien DP, Jenkin G, Buntine J, Steffen CM, McDonald A, Horne S, et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Australia. 2014;200(5):267–270. [DOI] [PubMed] [Google Scholar]
- 83.Ji B, Chauffour A, Robert J, Lefrançois S, Jarlier V. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2007;51(10):3737–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Owusu E, Newman MJ, Kotey NK, Akumwena A, Bannerman E. Susceptibility profiles of Mycobacterium ulcerans isolates to streptomycin and rifampicin in two districts of the eastern region of Ghana. Int J Microbiol. 2016;2016:8304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clinical and Laboratory Standards Institute. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actinomycetes; approved standard. Second edition. 2023. [PubMed] [Google Scholar]
- 86.Röltgen K, Stinear TP, Pluschke G. The genome, evolution and diversity of Mycobacterium ulcerans. Infect Genet Evol. 2012;12(3):522–529. [DOI] [PubMed] [Google Scholar]
- 87.Venkataswamy MM, Goldberg MF, Baena A, Chan J, Jacobs WR, Porcelli SA. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine. 2012;30(6):1038–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parish T, Stoker NG, editors. Mycobacteria Protocols. 1998. [Google Scholar]
- 89.Mve-Obiang A, Remacle J, Palomino JC, Houbion A, Portaels F. Growth and cytotoxic activity by Mycobacterium ulcerans in protein-free media. FEMS Microbiol Lett. 1999;181(1):153–157. [DOI] [PubMed] [Google Scholar]
- 90.Bénard A, Sala C, Pluschke G. Mycobacterium ulcerans mouse model refinement for pre-clinical profiling of vaccine candidates. PLoS ONE. 2016;11(11):e0167059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong H, Stinear T, Porter J, Demangel C, Leadlay PF. A novel mycolactone toxin obtained by biosynthetic engineering. Chembiochem. 2007;8(17):2043–2047. doi: 10.1002/cbic.200700411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mve-Obiang A, Lee RE, Portaels F, Small PLC. Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: Implications for virulence. Infect Immun. 2003;71(2):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scherr N, Gersbach P, Dangy JP, Bomio C, Li J, Altmann KH, et al. Structure-activity relationship studies on the macrolide exotoxin mycolactone of Mycobacterium ulcerans. PLoS Neglect Trop Dis. 2013;7(3):e2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]