Abstract
Background
Coffee is the most commonly consumed beverage among children and adolescences. Caffeine was demonstrated to be associated with bone metabolism. However, the relationship between caffeine intake and BMD in children and adolescents remains unclear. This study aimed to identified relationship between caffeine consumption and bone mineral density (BMD) in children and adolescents.
Methods
Based on National Health and Nutrition Examination Survey (NHANES), we conducted an epidemiological cross-section study to measure the relationship between caffeine consumption and BMD in children and adolescents by multivariate linear regression models. Then, five methods of Mendelian randomization (MR) analyses were performed to estimate their causal relationship between coffee and caffeine intake and BMD in children and adolescents. MR-Egger and inverse-variance weighted (IVW) were used to evaluate the heterogeneity effect of instrumental variables (IVs).
Results
In epidemiological studies, individuals with the highest quartile of caffeine intake do not have a significant change in femur neck BMD (β = 0.0016, 95% CI: -0.0096, 0.0129, P = 0.7747), total femur BMD (β = 0.0019, P = 0.7552), and total spine BMD (β = 0.0081, P = 0.1945) compared with the lowest quartile. In MR analysis, the IVW-random effect indicates no causal relationship between coffee consumption and TB- BMD (β = 0.0034, P = 0.0910). Other methods of MR analyses and sensitivity analysis reveals consistent findings. Similarly, the fixed-effects IVW method shows no causal association between caffeine intake and TB-BMD in children and adolescents (β = 0.0202, P = 0.7828).
Conclusions
Our study does not support a causal relationship between caffeine consumption and BMD in children and adolescents. However, more studies are needed to verify our findings, such as its underlying molecular mechanisms and the long-term impact of early caffeine exposure at a younger age.
Background
Osteoporosis (OP) is a bone disorder featured by decreased bone mass density (BMD) and impaired microarchitecture, causing bone pain and a higher risk of fragility fracture for the old [1]. The global prevalence of osteoporosis among adults aged 50–59, 60–69, and 70–79 was 11.4%, 24.8, and 37.6%, respectively [2]. In 2013, twenty-two million females and 5.5 million males in European Union (EU) countries suffered from osteoporosis, leading to 3.5 million fragility fractures annually [3]. The peak bone mass (PBM) in adolescence is a crucial factor in the process of OP and brittle fractures in the elderly [4]. A previous study found that a 10% PBM increase was linked to a 50% decrease in fragility fracture risk in older age [5]. Boreham et al. reported that a 6.4% reduction in PBM during childhood and adolescence could double the risk of fragility fractures in adulthood [6]. Studies have indicated that PBM formation could be influenced by genetics, diet and nutrition, physical activity, some diseases, and other factors [7–9].
Coffee is a trendy beverage worldwide, and approximately 73% of children drink caffeinated products daily [10]. Caffeinated soft drinks are a major source of caffeine for children and adolescents, containing between 50–500 mg of caffeine per bottle (equivalent to 5 cups of coffee) [11]. In recent decades, much speculation has been about the potential link between caffeine intake and osteoporosis. Many studies have been carried out to explore their relationship from the angle of clinical studies and molecular mechanism investigation [12–16]. Liu et al. [17] claimed in their study that caffeine could reduce BMD by enhancing osteoclastogenesis in rats. In addition, Rapuri et al. [18] proved that caffeine could impair osteoblast activation by reducing the vitamin D receptor expression on human osteoblasts and alkaline phosphatase activity. However, clinical research has always drawn inconsistent conclusions with different populations, study designs, or doses of caffeine intake [13, 19, 20]. A recent study of the National Health and Nutrition Examination Survey (NHANES) by Wang et al. [19] indicated caffeine could increase lumbar spine BMD in women aged 30–39 but could have a negative effect on men aged 40–49. In another study of young adult women aged 19–26, caffeine consumption was proven not to be a predictor of lower BMD [13]. Nevertheless, no studies investigate the relationship between caffeine consumption and BMD in children and adolescents [21]. Therefore, it is essential to uncover the relationship between caffeine consumption and BMD in children and adolescents as they obtain most of the PBM at the end of adolescence.
In order to fully evaluate associations between caffeine consumption and bone mineral density in children and adolescents, we conducted a cross-sectional study based on NHANES to determine the relationship between caffeine consumption and BMD in children and adolescents. Then, we implemented a two-sample Mendelian randomization (MR) analysis to evaluate their causal relationship at the genetic level. Two-sample MR is a novel method that uses a genetic variation of) to assess the causal relationship of exposure with outcome [22]. MR analysis could avoid confounding factors and infer causality since the alleles of exposure genetic variants are randomly assigned [23].
Methods
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the NHANES Institutional Review Board. The use of data was approved by the ethics review board of the National Center for Health Statistics. The study procedures were structured in line with the Declaration of Helsinki. Written consent was obtained from all subjects and their legal guardian(s).
Observational epidemiological analysis
NHANES project is an American national nutrition survey using a stratified, multi-stage random sampling design. We chose two cycles of NHANES (2007–2008, 2009–2010) since the data on BMD in children and adolescence existed in the two cycles. In the beginning, 4,264 subjects aged 8–19 were found in NHANES 2007–2008 and 2009–2010. After excluding 949 subjects without caffeine intake and 321 subjects without BMD (femur and lumbar spine BMD), 2,994 eligible subjects were included in our analysis. The flow chart of the research was exhibited in S1 Appendix.
Variables included in the observational cross-sectional study
Based on two 24-hour dietary recall interviews, the individual’s caffeine intake was calculated by counting all caffeinated beverages and foods, including coffee, tea, soda and chocolate. The analysis used the average caffeine intake from two 24-hour recalls. We selected femur BMD (femur neck and total femur BMD) and lumbar spine BMD as the outcome variable in our analysis. Professionals collected and standardized BMD measured by Dual-energy X-ray absorptiometry (DXA) examinations. Covariates in the multiple regression analysis were chosen based on previous studies [8, 24, 25]. All data collection and processing were detailed at https://www.cdc.gov/nchs/nhanes/.
Sources of two-sample MR
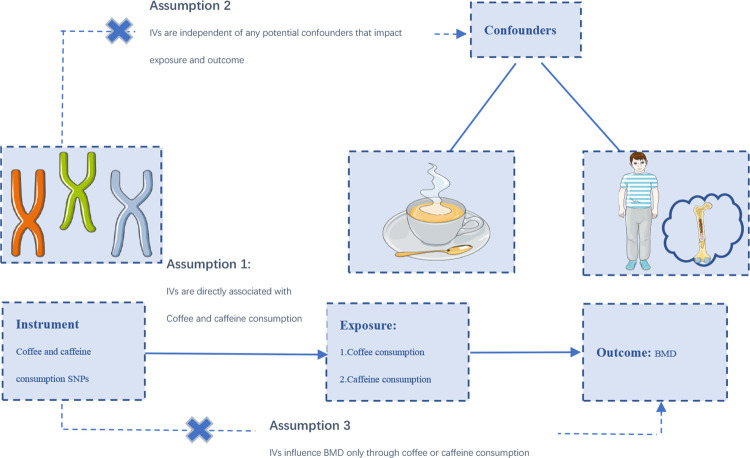
MR analysis was conducted based on three main assumptions (Fig 1) [26]. Fifteen SNPs strongly associated with coffee consumption were extracted from a meta-analysis of Genome-wide association studies (GWASs) on habitual coffee consumption [27], which adjusted for age, sex, BMI, total energy, and top 20 nutrients. In this genome-wide association study, over 370,000 adults of European ancestry were used to determine SNPs related to coffee consumption. Coffee consumption was available from assessment center visit and 24-h recalls. The study begins with a discovery analysis UK Biobank followed by replication in three independent US cohorts. Three SNPs (rs12699844, rs4719497, rs117692895) were excluded due to linkage disequilibrium (LD (r2> 0.01 and clumping distance <10,000kb). Finally, 12 independent SNPs were used as instrumental variables (IVs) for coffee consumption. Two SNPs closely related to caffeine consumption (P< 5E−8) were selected as IVs from a meta-analysis of GWAS (9,876 European descendants) [28]. Caffeine consumption was measured with caffeine-related metabolites in plasma and urine. The two SNPs explained about 1.31% of the variance in caffeine consumption.
Fig 1. The study design of two‐sample MR analysis.
Summary-level data for total body BMD (TB-BMD) of children and adolescents were obtained from a GWAS meta-analysis, which comprised 30 epidemiological studies of 11,807 individuals aged 0–15 years [29]. The GWAS meta-analysis was adjusted for age, weight, height, genomic principal components in a linear regression model.
Statistical analysis
In observational epidemiological analysis, participants were grouped based on caffeine intake quartiles (quartile 1: 0–1.5 mg/d; quartile 2: 1.5–13.5 mg/d; quartile 3: 13.5–43.5 mg/d; quartile 4: >43.5 mg/d). We performed weighted chi-square tests for analyzing categorical variables and linear regression models for continuous variables. Multivariate linear regression models were performed to evaluate the relationship of caffeine intake (quartile) with femur and spine BMD. We built three models: model 1 (unadjusted model), model 2 (adjusting race/ethnicity, gender, age), and model 3 (adjusting race/ethnicity, gender, age, BMI, PIR, serum phosphorus, and serum calcium). A P-value less than 0.05 is considered statistical significance. MR is a method that uses genetic variation to estimate the causality between exposure and outcome. We employed five methods to examine the causal association between coffee consumption and TB-BMD in children and adolescents, including inverse-variance weighted (IVW), MR-Egger, weighted mode, weighted median, and simple model, among which IVW was the primary method [30]. Fixed-effects IVW (<3 SNPs) was performed to assess the genetic predictions between caffeine consumption and TB-BMD. The MR-Egger method and MR pleiotropy residual sum and outlier (MR-PRESSO) were used to test its horizontal pleiotropic. Outlier SNPs were detected by MR-PRESSO packages and then deleted. Cochran’s Q statistic was applied to examine the heterogeneity of individual SNPs in IVW and MR-Egger tests. Sensitivity analysis was performed by removing single SNP one by one. All data were analyzed by EmpowerStats software and R software.
Results
Epidemiological observation and analysis
Overall, 2,994 subjects were included in our analysis, with a mean age of 13.48 ± 3.32. Of these subjects, 6.27% are other Hispanic, 13.33% are Mexican American,13.46% are non-Hispanic black, 59.81% are non-Hispanic white, and 7.12% are other races (including multiracial population). Weighted baseline characteristics of all subjects were as described by quartiles of caffeine intake (Table 1). Then, we describe the baseline characteristics study population with caffeine consumption and no caffeine consumption. (S2 Appendix). Table 2 displays the results of the weighted multivariate regression analysis. In the unadjusted model, caffeine intake was positively correlated with total spine BMD, total femur BMD and Femur neck BMD (P for trend< 0.001). Nevertheless, no correlations were in the adjusted models (models 2 and 3). Individuals with the highest quartile of caffeine intake do not have a significant change in femur neck BMD (β = 0.0016, 95% CI: -0.0096, 0.0129, P = 0.77), total femur BMD (β = -0.0019, 95% CI: -0.0141, 0.0102, P = 0.7552), and total spine BMD (β = 0.0065, 95% CI: -0.0046, 0.0176, P = 0.2536) compared with the lowest quartile in the fully adjusted model (Table 2).
Table 1. Characteristics of the study population based on caffeine intake quartiles.
| Caffeine intake quartiles(mg/d) | ||||||
|---|---|---|---|---|---|---|
| Total | Q1 (0–1.5) | Q2 (1.5–13.5) | Q3 (13.5–43.5) | Q4 (>43.5) | P value | |
| Number of subjects (n) | 2994 | 742 | 747 | 756 | 749 | |
| Age (years) | 13.48 ± 3.32 | 12.91 ± 3.26 | 12.19 ± 3.11 | 13.12 ± 3.18 | 15.10 ± 2.96 | <0.001 |
| Gender (%) | 0.176 | |||||
| Men | 52.39 | 52.49 | 53.33 | 48.95 | 54.25 | |
| Women | 47.61 | 47.51 | 46.67 | 51.05 | 45.75 | |
| Race/ethnicity (%) | <0.001 | |||||
| Mexican American | 13.33 | 15.2 | 15.05 | 16.05 | 8.69 | |
| Other Hispanic | 6.27 | 6.19 | 6.44 | 8.08 | 4.83 | |
| Non-Hispanic White | 59.81 | 48.53 | 55.33 | 56.52 | 73.47 | |
| Non-Hispanic Black | 13.46 | 22.19 | 13.81 | 13.71 | 6.95 | |
| Other Race (Including Multi-Racial) | 7.12 | 7.89 | 9.37 | 5.65 | 6.06 | |
| BMI | 22.12 ± 5.47 | 21.93 ± 5.58 | 20.76 ± 5.01 | 22.12 ± 5.51 | 23.28 ± 5.45 | <0.001 |
| PIR | 2.63 ± 1.63 | 2.65 ± 1.66 | 2.67 ± 1.64 | 2.55 ± 1.61 | 2.65 ± 1.61 | 0.51 |
| Serum total calcium (mmol/L) | 2.40 ± 0.06 | 2.40 ± 0.06 | 2.41 ± 0.05 | 2.40 ± 0.06 | 2.40 ± 0.07 | 0.013 |
| Serum phosphorus (mmol/L) | 1.41 ± 0.17 | 1.41 ± 0.16 | 1.43 ± 0.16 | 1.41 ± 0.17 | 1.39 ± 0.19 | <0.001 |
| Lumbar spine BMD (g/cm2) | 0.85 ± 0.20 | 0.83 ± 0.20 | 0.79 ± 0.20 | 0.83 ± 0.20 | 0.93 ± 0.18 | <0.001 |
| Total femur BMD (g/cm2) | 0.90 ± 0.19 | 0.89 ± 0.19 | 0.85 ± 0.18 | 0.89 ± 0.18 | 0.96 ± 0.18 | <0.001 |
| Femur neck BMD (g/cm2) | 0.84 ± 0.17 | 0.82 ± 0.17 | 0.79 ± 0.16 | 0.83 ± 0.17 | 0.89 ± 0.16 | <0.001 |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model. (%) for categorical variables
The P value was calculated by the weighted chi-square test. Abbreviation: BMD, bone mineral density. BMI, Body mass index. PIR, poverty income ratio
Table 2. The association between caffeine intake and BMD in children and adolescence.
| Total spine BMD (g/cm2) | Total femur BMD (g/cm2) | Femur neck BMD (g/cm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | |
| Caffeine intake categories (mg/d) | |||||||||
| Q1 (0–1.5) | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Q2 (1.5–13.5) | -0.0440 (-0.0646, -0.0234) <0.001 | -0.0041 (-0.0166, 0.0084) 0.5212 | 0.0015 (-0.0098, 0.0129) 0.7890 | 0.0405 (-0.0600, | -0.0058 (-0.0195, 0.0078) 0.4027 | 0.0003 (-0.0121, 0.0127) 0.9622 | -0.0343 (-0.0520, -0.0166) 0.0001 | -0.0039 (-0.0170, 0.0092) 0.5572 | 0.0028 (-0.0086, 0.0143) 0.6289 |
| -0.0210) <0.0001 | |||||||||
| Q3 (13.5–43.5) | 0.0054 (-0.0151, 0.0258) 0.6058 | -0.0017 (-0.0141, 0.0106) 0.7829 | -0.0025 (-0.0137, 0.0088) 0.6657 | 0.0010 (-0.0183, 0.0204) 0.9165 | 0.0000 (-0.0136, 0.0136) 0.9989 | -0.0009 (-0.0132, 0.0114) 0.8879 | 0.0048 (-0.0128, 0.0223) 0.5938 | 0.0045 (-0.0084, 0.0175) 0.4926 | 0.0035 (-0.0078, 0.0149) 0.5430 |
| Q4 (>43.5) | 0.1029 (0.0836, 0.1222) <0.0001 | 0.0081 (-0.0041, 0.0203) 0.1945 | 0.0065 (-0.0046, 0.0176) 0.2536 | 0.0760 (0.0578, 0.0943) <0.0001 | -0.0000 (-0.0135, 0.0134) 0.9942 | -0.0019 (-0.0141, 0.0102) 0.7552 | 0.0665 (0.0500, 0.0830) <0.0001 | 0.0038 (-0.0090, 0.0166) 0.5616 | 0.0016 (-0.0096, 0.0129) 0.7747 |
| P for trend | <0.001 | 0.158 | 0.72 | <0.001 | 0.786 | 0.359 | <0.001 | 0.344 | 0.777 |
Model 1 unadjusted. Model 2 adjusted for age, gender, and race/ethnicity. Model3 adjusted for race/ethnicity, gender, age, BMI, PIR, serum phosphorus, and serum calcium. Abbreviation: BMD, bone mineral density. BMI, body mass index. PIR, poverty income ratio.
Coffee consumption and TB-BMD
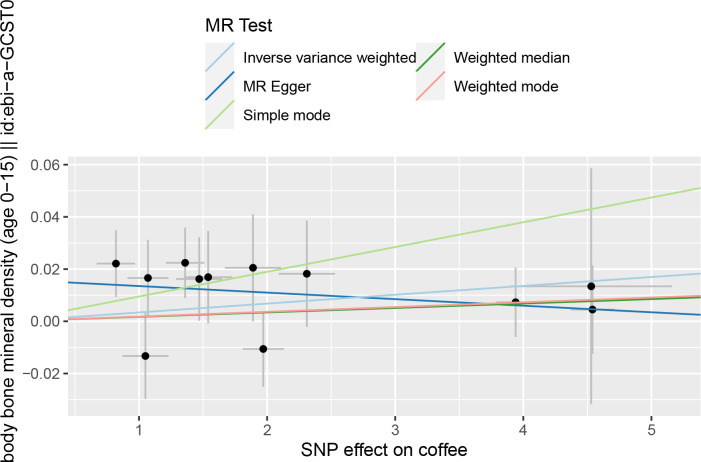
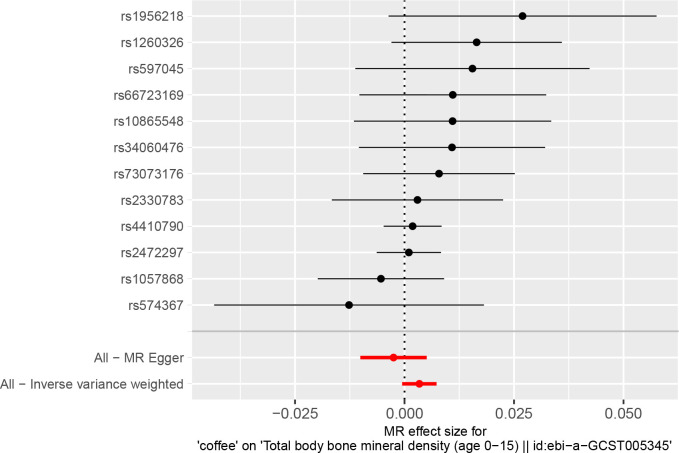
Details on SNPs for caffeine and coffee consumption were summarized in Table 3. The result of the MR analysis was shown in Fig 2 and Table 4. The IVW results found no causal relationship between coffee consumption and the TB-BMD of children and adolescents (β = 0.0034, 95% CI: -0.0005, 0.0073, P = 0.0910). Similarly, no causal association was observed in the MR-Egger test, weighted mode, weighted median, and simple mode, either. The Cochran’s Q statistic of MR-Egger (Cochran’s Q = 6.362, P = 0.784) and IVW methods (Cochran’s Q = 9.546, P = 0.572) indicated no significant heterogeneity between IVs. There were no horizontal pleiotropic by MR-Egger regression (P = 0.105) and MR-PRESSO global test (P = 0.621). The forest plots of coffee consumption and TB-BMD were shown in Fig 3. The results were also consistent after the sensitivity analysis (S3 Appendix).
Table 3. Characteristics of SNPs associated with coffee and caffeine consumption.
| SNP-exposure (coffee and caffeine consumption) | SNP-outcome (TB-BMD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Nearby Gene | Chr | EA | OA | EAF | Beta | SE | P | Beta | SE | P |
| Coffee consumption | |||||||||||
| rs1057868 | POR | 7 | T | C | 0.29 | 1.97 | 0.16 | 5.26E-33 | -0.0106 | 0.0145 | 0.4669 |
| rs10865548 | TMEM18 | 2 | G | A | 0.83 | 1.54 | 0.19 | 4.46E-15 | 0.0169 | 0.0177 | 0.3401 |
| rs1260326 | GCKR | 2 | C | T | 0.61 | 1.36 | 0.15 | 2.62E-19 | 0.0224 | 0.0135 | 0.0982 |
| rs1956218 | AKAP6 | 14 | G | A | 0.56 | 0.82 | 0.15 | 3.62E-08 | 0.0221 | 0.0128 | 0.0843 |
| rs2330783 | SPECC1L- | 22 | G | T | 0.99 | 4.53 | 0.63 | 1.57E-12 | 0.0134 | 0.0452 | 0.7669 |
| ADORA2A | |||||||||||
| rs2472297 | CYP1A1/2 | 15 | T | C | 0.27 | 4.54 | 0.17 | 5.19E-155 | 0.0045 | 0.017 | 0.79 |
| rs34060476 | LOC101927630 | 7 | G | A | 0.13 | 1.89 | 0.22 | 5.06E-18 | 0.0205 | 0.0205 | 0.3157 |
| rs4410790 | AHR | 7 | C | T | 0.63 | 3.94 | 0.15 | 5.59E-141 | 0.0073 | 0.0133 | 0.5828 |
| rs574367 | SEC16B | 1 | T | G | 0.21 | 1.05 | 0.18 | 8.06E-09 | -0.0133 | 0.0165 | 0.4215 |
| rs597045 | OR8U8 | 11 | A | T | 0.69 | 1.07 | 0.16 | 6.62E-11 | 0.0166 | 0.0146 | 0.256 |
| rs66723169 | MC4R | 18 | A | C | 0.23 | 1.47 | 0.18 | 9.88E-17 | 0.0162 | 0.016 | 0.3108 |
| rs73073176 | MLXIPL | 7 | C | T | 0.87 | 2.31 | 0.22 | 5.56E-25 | 0.0182 | 0.0204 | 0.3721 |
| Caffeine consumption | |||||||||||
| rs2470893 | CYP1A1 | 15 | T | C | 0.31 | 0.12 | 0.016 | 5.15E-14 | -0.005 | 0.0157 | 0.7485 |
| rs4410790 | AHR | 7 | C | T | 0.62 | 0.15 | 0.017 | 2.36E-19 | 0.0073 | 0.0133 | 0.5828 |
P value < 5×10−8 for reporting genome-wide significance. Abbreviations: EA, effect allele. OA, other allele, EAF effect allele frequency. MR, Mendelian randomization. SE, standard error; SNP, single nucleotide polymorphism. TB-BMD, total body bone mineral density.
Fig 2. The scatter plot for MR analyses of causal associations between coffee consumption SNP and TB-BMD.
Abbreviations: TB-BMD: total body bone mineral density. IVW: inverse-variance weighted.
Table 4. Causal effect of coffee and caffeine consumption and TB-BMD.
| Exposure | Outcome | Method | β | Lo CI | Up CI | P |
|---|---|---|---|---|---|---|
| Coffee consumption | TB-BMD of Children and adolescence | IVW | 0.0034 | -0.0005 | 0.0073 | 0.0910 |
| MR Egger | -0.0025 | -0.0101 | 0.0051 | 0.5310 | ||
| Weighted median | 0.0017 | -0.0032 | 0.0066 | 0.4956 | ||
| Simple mode | 0.0095 | 0.0009 | 0.0180 | 0.0523 | ||
| Weighted mode | 0.0018 | -0.0030 | 0.0068 | 0.4921 | ||
| MR Egger: Cochran’s Q = 6.362, P = 0.784 | ||||||
| IVW: Cochran’s Q = 9.546, P = 0.572 | ||||||
| MR-Egger intercept = 0.016, P = 0.105 | ||||||
| MR-PRESSO global test = 0.621 | TB-BMD of Children and adolescence | IVW | 0.0202 | -0.1236 | 0.1641 | 0.7828 |
| Caffeine consumption | ||||||
| IVW: Cochran’s Q = 6.362, P = 0.568 |
No outlier was observed in the MR-PRESSO analysis in MR analysis in coffee consumption and TH-BMD. Abbreviations: CI, confidence interval; MR, Mendelian randomization
IVW, inverse-variance weighted; TB-BMD, total body bone mineral density.
Fig 3. Forest plot to visualize causal effect of each single SNP of coffee consumption and TB-BMD.
Caffeine consumption and TB-BMD
Similarly, the fixed-effects IVW analysis suggested that there was no causal effect of caffeine intake on TB-BMD of children and adolescents (β = 0.0202, 95% CI: -0.1236, 0.1641, P = 0.7828) (Table 4). IVW methods showed no significant heterogeneity existing (Cochran’s Q = 6.362, P = 0.568). MR-PRESSO and MR-Egger intercept tests could not be performed because of the absence of sufficient IVs.
Discussion
This work employed a large observational epidemiological analysis and a two-sample MR analysis to explore the association between caffeine intake and BMD in children and adolescents. Our study found no causal association between caffeine intake and BMD, providing novel evidence for caffeine’s potential roles in the bone health of children and adolescents.
Caffeine is very common in the diet of people worldwide. Soda, tea, specialty coffee drinks, and food products like candy bars, potato chips, and gum [31] contain caffeine. Over the past few decades, there has been a keen interest in exploring the impact of these popular foods on human health [32–34]. Extensive studies demonstrated that caffeine was related to a decreased risk of all-cause mortality, Parkinson’s disease, liver diseases, cardiovascular mortality, type 2 diabetes, and so on [35, 36]. However, the association between caffeine intake and BMD or osteoporosis remains controversial from molecular studies and epidemiological studies of adult or elderly populations only [37–39]. In a prospective study from Taiwan between 2006 and 2014, 2,682 Taiwanese aged 30 years and older were included in the analysis [37]. Each completed questionnaire included weekly coffee consumption frequency, which was identified by asking subjects about the frequency of coffee drinking per week. BMD was evaluated by quantitative ultrasound (QUS) at the calcaneus [37]. As a result, caffeine consumption was positively linked with T-scores for both sexes. In another cross-sectional study of postmenopausal women in Korea, individuals with the highest caffeine consumption quartile were at a lower risk of osteoporosis than individuals in the lowest quartile (OR = 0.64; 95%CI, 0.43–0.95). This finding was consistent in osteoporosis of the femoral neck and lumbar spine (OR = 0.55 and 0.65, respectively). In addition, coffee intake was positively related to the lumbar spine and femoral neck BMD. By contrast, a Swedish longitudinal study of 61,433 females showed that high coffee consumption (≥4 cups per day) was linked to a 4% reduction in BMD compared with low consumption (<1 cup daily). However, it did not increase the fracture risk (OR = 0.99; 95%CI, 0.98–1.00) [38]. In an early study, Barrett-Connor et al. [40] found that a lifetime intake of two cups of caffeinated coffee per day is associated with decreased BMD in older women. However, for daily milk drinkers, coffee-related osteoporosis was offset. In another study of young adult women aged 19–26, caffeine consumption was not related to BMD after adjusting age, height, BMI, calcium and protein intake, and alcohol use in linear regression models. In conclusion, the evidence of caffeine consumption and bone health was always inconsistent, However, clinical studies evaluating the effects of caffeine intake on BMD in children and adolescents were few. Our study using MR analysis which could avoid confounding factors and infer causality, indicates no causal association between caffeine intake and BMD, providing novel evidence for caffeine’s possible roles in the bone health of children and adolescents.
In vitro and animal studies, caffeine has been proven to exert biological effects that may affect bone metabolism and BMD. Evidence has shown that a major mechanism of caffeine in organs is its direct and indirect effects on bone metabolism through competitive inhibition of four adenosine receptors, including A1, A2A, A2B and A3 adenosine, which are expressed in undifferentiated osteoblast precursors and differentiated osteoblasts [41, 42]. Bone metabolism appears to be significantly regulated by A2A and A2B receptors. Mediero et al. [43] found that reduced extracellular adenosine levels can lead to decreased bone mass in ovariectomized mice, and A2B and A2A agonists can reverse this effect. In vitro and animal models, antagonism of A2A receptors influenced by caffeine promotes osteoclast formation and function, while antagonism of A2B receptors inhibits osteoblast formation [41]. In addition, caffeine blockade of A1 receptors can lead to increased osteoblast activity, in contrast to the pro-osteoporotic effect of caffeine on A2 receptors [44]. Thus, the effect of caffeine on bone metabolism depends on its ability to block A1 and A2 receptors and the importance of the affected signaling pathways. Furthermore, caffeine could disrupt calcium metabolism and alter the vitamin D receptor (VDR) to regulate bone metabolism, reducing bone mass in mice [18, 39]. In conclusion, at the molecular level, the conflicting effects of caffeine on A1 versus A2a and A2b receptors and which one predominates remain to be elucidated. Therefore, the multiple effects of caffeine on bone metabolism remain to be investigated by further molecular mechanisms and clinical research.
Our study has some advantages. First, the caffeine consumption in the cross-sectional study was based on average caffeine intake from two 24-hour recalls, which makes our results more reliable. Second, in addition to epidemiological observation, MR analysis was conducted to examine their causal association. The results of the five methods of MR analysis were consistent, which added to the evidence of our research. Third, the MR analysis’s overall heterogeneity was low, verified by MR-Egger and IVW methods. No horizontal pleiotropic was found by MR-Egger regression and MR-PRESSO global test. In addition, the results were also consistent after the sensitivity analysis. However, some potential limitations could not be avoided. First, there may be personal questionnaire survey bias when recalling caffeine consumption because of the nature of questionnaire survey. In addition, other confounding factors such as hormonal status, medication use, and genetic factors were not included in linear regression models because of the data limitation. Second, we only took children older than eight into account since the limitation of the NHANES database. More research should be carried out to investigate the relationship between caffeine intake and BMD in children under 8 years old. Third, we found only one GWAS of BMD in children and children and adolescents. Furthermore, some SNPs related to coffee consumption were excluded due to linkage disequilibrium. However, the exclusion of certain SNPs due to linkage disequilibrium may have affected the power of the MR analysis. Further MR analysis based on updated GWASs is required to verify the results of our study. Fourth, the current MR study used data from GWAS meta-analysis with a predominantly European population. Whether the findings of this study can be generalized to other populations remains to be looked at, and more studies involving other races and populations are needed in the future. Fifth, due to the nature of epidemiology, the study may contain residual errors, such as measurement error. So, the result of this study should be interpreted with caution.
Conclusions
Based on the cross-sectional study and Mendelian randomization study, our study does not support a causal association between caffeine consumption and BMD in children and adolescents. However, more studies with more robust evidence are needed to verify our findings, such as its underlying molecular mechanisms and the long-term impact of early caffeine exposure at a younger age.
Supporting information
(PDF)
(DOCX)
(PDF)
Acknowledgments
We acknowledge the data from the National Health and Nutrition Examination Survey (NHANES).
Availability of data and materials
The datasets generated and/or analysed during the study are available in the [NHANES] repository, [https://www.cdc.gov/nchs/nhanes/].
List of abbreviations
- BMD
Bone mineral density
- OP
Osteoporosis
- NHANES
National Health and Nutrition Examination Survey
- MR
Mendelian randomization
- TB-BMD
Total body BMD
- IVs
Instrumental variables
- SNPs
Heterogeneous single-nucleotide polymorphisms
- MR-PRESSO
MR pleiotropy residual sum and outlier
- IVW
Inverse-variance weighted
- EU
European Union
- PBM
Peak bone mass
- GWASs
Genome-wide association studies
- QUS
Quantitative ultrasound
- VDR
Vitamin D receptor
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Shaanxi Provincial Department of Science and Technology, Innovative Talents Promotion Plan - Youth Science and Technology Star Project (2021KJXX-57) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Styrkarsdottir U, Thorleifsson G, Gudjonsson SA et al. Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Nat Commun 2016,7:10129. doi: 10.1038/ncomms10129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao PL, Cui AY, Hsu CJ et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int 2022. [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013,8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matkovic V, Jelic T, Wardlaw GM et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest 1994,93:799–808. doi: 10.1172/JCI117034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenberg S, Bruyère O, Bergmann P et al. How to manage osteoporosis before the age of 50. Maturitas 2020,138:14–25. doi: 10.1016/j.maturitas.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Boreham CA, McKay HA. Physical activity in childhood and bone health. Br J Sports Med 2011,45:877–9. doi: 10.1136/bjsports-2011-090188 [DOI] [PubMed] [Google Scholar]
- 7.Karlsson MK, Rosengren BE. Exercise and Peak Bone Mass. Curr Osteoporos Rep 2020,18:285–290. doi: 10.1007/s11914-020-00588-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui A, Xiao P, Hu B et al. Blood Lead Level Is Negatively Associated With Bone Mineral Density in U.S. Children and Adolescents Aged 8–19 Years. Front Endocrinol (Lausanne) 2022,13:928752. doi: 10.3389/fendo.2022.928752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fewtrell MS, Williams JE, Singhal A, Murgatroyd PR, Fuller N, Lucas A. Early diet and peak bone mass: 20 year follow-up of a randomized trial of early diet in infants born preterm. Bone 2009,45:142–9. doi: 10.1016/j.bone.2009.03.657 [DOI] [PubMed] [Google Scholar]
- 10.Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among U.S. children and adolescents. Pediatrics 2014,133:386–93. doi: 10.1542/peds.2013-2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soós R, Gyebrovszki Á, Tóth Á, Jeges S, Wilhelm M. Effects of Caffeine and Caffeinated Beverages in Children, Adolescents and Young Adults: Short Review. Int J Environ Res Public Health 2021,18. doi: 10.3390/ijerph182312389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grainge MJ, Coupland CA, Cliffe SJ, Chilvers CE, Hosking DJ. Cigarette smoking, alcohol and caffeine consumption, and bone mineral density in postmenopausal women. The Nottingham EPIC Study Group. Osteoporos Int 1998,8:355–63. [DOI] [PubMed] [Google Scholar]
- 13.Conlisk AJ, Galuska DA. Is caffeine associated with bone mineral density in young adult women? Prev Med 2000,31:562–8. doi: 10.1006/pmed.2000.0742 [DOI] [PubMed] [Google Scholar]
- 14.Trimpou P, Landin-Wilhelmsen K, Odén A, Rosengren A, Wilhelmsen L. Male risk factors for hip fracture-a 30-year follow-up study in 7,495 men. Osteoporos Int 2010,21:409–16. doi: 10.1007/s00198-009-0961-7 [DOI] [PubMed] [Google Scholar]
- 15.Kamagata-Kiyoura Y, Ohta M, Cheuk G, Yazdani M, Saltzman MJ, Nakamoto T. Combined effects of caffeine and prostaglandin E2 on the proliferation of osteoblast-like cells (UMR106-01). J Periodontol 1999,70:283–8. doi: 10.1902/jop.1999.70.3.283 [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP. Effects of caffeine on bone and the calcium economy. Food Chem Toxicol 2002,40:1263–70. doi: 10.1016/s0278-6915(02)00094-7 [DOI] [PubMed] [Google Scholar]
- 17.Liu SH, Chen C, Yang RS, Yen YP, Yang YT, Tsai C. Caffeine enhances osteoclast differentiation from bone marrow hematopoietic cells and reduces bone mineral density in growing rats. J Orthop Res 2011,29:954–60. doi: 10.1002/jor.21326 [DOI] [PubMed] [Google Scholar]
- 18.Rapuri PB, Gallagher JC, Nawaz Z. Caffeine decreases vitamin D receptor protein expression and 1,25(OH)2D3 stimulated alkaline phosphatase activity in human osteoblast cells. J Steroid Biochem Mol Biol 2007,103:368–71. doi: 10.1016/j.jsbmb.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Fang ZB, Liu DL, Chu SF, Li HL, Zhao HX. Association between caffeine intake and lumbar spine bone mineral density in adults aged 20–49: A cross-sectional study. Front Endocrinol (Lausanne) 2022,13:1008275. doi: 10.3389/fendo.2022.1008275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de França NA, Camargo MB, Lazaretti-Castro M, Peters BS, Martini LA. Dietary patterns and bone mineral density in Brazilian postmenopausal women with osteoporosis: a cross-sectional study. Eur J Clin Nutr 2016,70:85–90. doi: 10.1038/ejcn.2015.27 [DOI] [PubMed] [Google Scholar]
- 21.Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: a longitudinal analysis. Bone 2006,38:576–83. doi: 10.1016/j.bone.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 22.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol 2016,27:3253–3265. doi: 10.1681/ASN.2016010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Q, Burgess S, Turman C et al. Body mass index and breast cancer survival: a Mendelian randomization analysis. Int J Epidemiol 2017,46:1814–1822. doi: 10.1093/ije/dyx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JJ. Calcium, phosphorus and human bone development. J Nutr 1996,126:1153s–8s. doi: 10.1093/jn/126.suppl_4.1153S [DOI] [PubMed] [Google Scholar]
- 25.Li T, Xie Y, Wang L et al. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients 2022,14. doi: 10.3390/nu14071523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 2018,362:k601. doi: 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong VW, Kuang A, Danning RD et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet 2019,28:2449–2457. doi: 10.1093/hmg/ddz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornelis MC, Kacprowski T, Menni C et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet 2016,25:5472–5482. doi: 10.1093/hmg/ddw334 [DOI] [PubMed] [Google Scholar]
- 29.Medina-Gomez C, Kemp JP, Trajanoska K et al. Life-Course Genome-wide Association Study Meta-analysis of Total Body BMD and Assessment of Age-Specific Effects. Am J Hum Genet 2018,102:88–102. doi: 10.1016/j.ajhg.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013,37:658–65. doi: 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heckman MA, Sherry K, De Mejia EG. Energy Drinks: An Assessment of Their Market Size, Consumer Demographics, Ingredient Profile, Functionality, and Regulations in the United States. Compr Rev Food Sci Food Saf 2010,9:303–317. doi: 10.1111/j.1541-4337.2010.00111.x [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Cao Q, Chen P et al. An updated dose-response meta-analysis of coffee consumption and liver cancer risk. Sci Rep 2016,6:37488. doi: 10.1038/srep37488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis 2010,20 Suppl 1:S221–38. doi: 10.3233/JAD-2010-091525 [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: a meta-analysis of prospective studies. Eur J Nutr 2014,53:25–38. doi: 10.1007/s00394-013-0603-x [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Woolf B, Gill D. Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients 2022,14. doi: 10.3390/nu14091697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li BH, Yan SY, Li XH et al. Coffee and caffeine consumption and risk of renal cell carcinoma: A Mendelian randomization study. Front Nutr 2022,9:898279. doi: 10.3389/fnut.2022.898279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang HC, Hsieh CF, Lin YC et al. Does coffee drinking have beneficial effects on bone health of Taiwanese adults? A longitudinal study. BMC Public Health 2018,18:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallström H, Byberg L, Glynn A, Lemming EW, Wolk A, Michaëlsson K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol 2013,178:898–909. doi: 10.1093/aje/kwt062 [DOI] [PubMed] [Google Scholar]
- 39.Massey LK, Whiting SJ. Caffeine, urinary calcium, calcium metabolism and bone. J Nutr 1993,123:1611–4. doi: 10.1093/jn/123.9.1611 [DOI] [PubMed] [Google Scholar]
- 40.Barrett-Connor E, Chang JC, Edelstein SL. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. Jama 1994,271:280–3. doi: 10.1001/jama.1994.03510280042030 [DOI] [PubMed] [Google Scholar]
- 41.Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends Endocrinol Metab 2013,24:290–300. doi: 10.1016/j.tem.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mediero A, Wilder T, Perez-Aso M, Cronstein BN. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. Faseb j 2015,29:1577–90. doi: 10.1096/fj.14-265066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mediero A, Perez-Aso M, Cronstein BN. Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br J Pharmacol 2013,169:1372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih YV, Liu M, Kwon SK et al. Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss. Sci Adv 2019,5:eaax1387. doi: 10.1126/sciadv.aax1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.
The datasets generated and/or analysed during the study are available in the [NHANES] repository, [https://www.cdc.gov/nchs/nhanes/].