Abstract
Background:
There is uncertainty surrounding use of direct oral anticoagulants (DOACs) in patients with kidney dysfunction.
Methods:
Using the COMBINE AF database, we performed an individual patient-level network meta-analysis to evaluate the safety and efficacy of DOACs vs warfarin across the continuous spectrum of creatinine clearance (CrCl), leveraging individual patient data from the ROCKET AF, ARISTOTLE, RE-LY and ENGAGE AF-TIMI 48 trials. To accomplish this, a multivariable stratified Cox proportional hazard model including a treatment-by-CrCl interaction with random effects was fitted to estimate hazard ratios (HRs) for pairs of treatment strategies with respect to stroke/systemic embolism (S/SE), major bleeding, intracranial hemorrhage (ICH) and death for patients randomized to standard dose DOAC, lower dose DOAC or warfarin.
Results:
Across 71,683 patients, median age was 70.6 (IQR 9.4). Of these, 37.3% (26,715) were female, median CrCl was 75.5 (IQR 30.5) and median follow-up was 23.1 months. The incidence of S/SE, major bleeding, ICH and death all significantly increased with worsening kidney function. Across all CrCl values down to at least 25ml/min, the hazard of major bleeding did not change for patients randomized to standard dose DOAC vs warfarin with changing CrCl (decrease in HR by 0.7% per 10mL/min decrease in CrCl, interaction p=0.61). Compared with warfarin, standard dose DOAC resulted in significantly lower hazard of ICH at CrCl values <122 mL/min, with a trend for increased safety with DOAC as CrCl decreased (decrease in HR by 6.2% per 10mL/min decrease in CrCl, p interaction p=0.08). Compared with warfarin, standard dose DOAC had significantly lower hazard for S/SE when CrCl was <97 mL/min, with a significant treatment-by-CrCl effect (decrease in HR by 4.8% per 10mL/min decrease in CrCl, interaction p=0.01). Hazard of death was significantly lower with standard dose DOAC for patients with a CrCl <77mL/min, with a trend towards increasing benefit with lower CrCl values (HR decrease 2.1%, interaction p=0.08). Use of lower dose DOAC rather than standard dose DOAC was not associated with a significant difference in incidence of bleeding or ICH in patients with reduced kidney function, but was associated with a higher incidence of both death and S/SE.
Conclusion:
The use of standard dose DOAC is safer and more effective than warfarin down to an CrCl of at least 25 ml/min. The use of lower dose DOAC does not result in significantly lower incidence of bleeding, or ICH compared to standard dose DOAC, but is associated with higher incidence of both death and S/SE. These findings support the use of standard dose DOAC over warfarin in patients with reduced kidney function.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is a major risk factor for stroke and systemic embolism (S/SE) as well as an independent predictor of mortality. These risks are amplified by the presence of kidney dysfunction, which increases the risk not only of AF,1 but also the subsequent risks of thromboembolic events, bleeding, and death patients with AF.2,3 This makes treatment decisions surrounding stroke prevention in patients with kidney dysfunction and comorbid atrial fibrillation of critical importance. Direct oral anticoagulants (DOACs) are first-line therapy for the prevention of stroke in AF, based on randomized data from multiple trials demonstrating similar or lower incidence of stroke with DOACs and a similar or lower risk of major bleeding in comparison to warfarin.4–6 Currently, dabigatran is recommended for stroke prevention in AF down to a CrCl of 30ml/min, with dose reduction for patients with CrCl of 15-30 ml/min, edoxaban and rivaroxaban are recommended down to a CrCl of 50 ml/min (and to 15ml/min with dose adjustment), and apixaban is recommended down to a CrCl of 25ml/min, with dose adjustment for patients meeting at least 2 clinical criteria surrounding weight, age and creatinine.7–10 However, DOACs are all partially renally clearance, ranging from 27% (apixaban) to 80% (dabigatran), leading to possible safety concerns for this population. As a result, DOACs are still less frequently used and often underdosed in patients with kidney insufficiency.11,12
Though patients were eligible for inclusion in these trials down to a creatinine clearance of 25-30 mL/min, relatively few patients in any given DOAC trial had severely reduced kidney function,. 13–19 Sub-analyses using these smaller cohorts have supported the use of DOAC over warfarin in patients with reduced kidney function, but these analyses were limited by low numbers of patients with kidney dysfunction. 17,20–22 Prior meta-analyses assessing the safety and efficacy of DOACs in patients with renal dysfunction have been limited to categorical analyses of CrCl based on previously published summary data from each individual trial.23 Like all study-level meta-analyses, these studies were also impacted by inconsistent follow-up time, absence of individual time to event results, and were unable to robustly evaluate for heterogeneity between trials. Individual patient data meta-analyses, which are only possible with access to granular patient data such as what is offered through COMBINE AF, address these limitations. Given the granular nature of this data, patient network meta-analyses are also not limited to the first event that occurred for a given patient (e.g., stroke or bleeding event, but not both), and can reflect the time to first event for each adjudicated patient outcome, in addition to incorporating inconsistent follow-up time better and increasing insight and power. Perhaps most importantly, the use a network individual patient analysis allows for both individual patient-level time to event data as well as for analyses of continuous variables.”
The COMBINE AF database incorporates individual patient data from the 4 pivotal trials of DOACs vs warfarin in AF, including 71,683 patients overall, and 24,369 patients with a CrCl <60. This represents the largest and most complete dataset of DOACs vs warfarin, which we leveraged using an individual network meta-analysis in order to achieve the most robustly powered and reliable estimate of DOAC vs warfarin use in patients with kidney dysfunction.24 We therefore specifically evaluated safety and efficacy outcomes of DOACs and warfarin across the continuous spectrum of kidney function (down to a CrCl of 25 ml/min) among patients in the COMBINE AF database, with a particular focus on patients with reduced kidney function, where hesitation surrounding DOAC use may still exist.
Methods
Analysis Design
The design and rationale of COMBINE AF has been described previously.24 Briefly, COMBINE AF incorporated individual patient data from 77,282 de-identified patients from 5 major randomized clinical trials of comparing DOACs to warfarin or aspirin in patients with AF. We included all patients from RE-LY, ROCKET AF, ARISTOTLE, ENGAGE AF-TIMI 48 who were randomized to warfarin or DOAC, yielding a cohort size of 71,683 for our analysis. (Patients from AVERROES, which assessed apixaban vs aspirin, were not included in these analyses.)
Patients were analyzed according to their study drug randomization: standard dose DOAC, lower dose DOAC, or warfarin. These analyses were not impacted by dose adjustment due to individual clinical characteristics such as age or weight. Standard dose DOAC was defined as standard dose used in ROCKET or ARISTOTLE (with protocol-specified dose-adjustment based on pre-specified trial criteria of age, weight or kidney function) and as the DOAC randomization arm with the higher dosing regimen in RE-LY (dabigatran 150mg twice daily) or ENGAGE AF-TIMI 48 (edoxaban 60mg once daily or 30mg once daily for patients with pre-specified trial criteria for dose adjustment). Lower-dose DOAC was defined as the DOAC randomization arm with the lower dosing regimen in RE-LY (dabigatran 110mg twice daily) or ENGAGE AF-TIMI 48 (edoxaban 30mg once daily or 15mg once daily for patients with pre-specified trial criteria for dose adjustment).
Outcomes
Outcome definitions in COMBINE AF have been described previously.24 Briefly, all outcomes were adjudicated in each of the constituent trials, which used a time-to-first-event design. Efficacy outcomes for our analysis included stroke/systemic embolism and all-cause mortality. Safety outcomes for this analysis included major bleeding, as defined by the International Society on Thrombosis and Haemostasis and intracranial hemorrhage (ICH).
Study Population
For efficacy outcomes, the intention-to-treat population was used. To account for different follow-up durations across trials and set a comparable follow-up duration in this network meta-analysis, subjects were censored when less than 10% of subjects were at risk in each study.24 For safety and composite outcomes, the safety population was used, as defined by each of the individual trials, but typically including participants who received at least one dose of a study drug and were followed for events occurring between date the participant began treatment with study drug and up to 2 days after participant discontinued study drug.
Statistical Analyses
The Cockcroft-Gault equation was used to calculate CrCl. Though our primary analyses were conducted across the continuous spectrum of CrCl, we additionally prespecified CrCl groups at baseline as follows: <30, 30-44, 45-59, 60-89, and ≥90 mL/min. These pre-specified groups were used to assess baseline characteristics, and for assessment of raw event rates.
To understand if CrCl is associated with event incidence and the treatment effects, we first assessed raw event incidence per 100 patient-years by CrCl category. To assess the impact of CrCl on event rates continuously, a quasi-poisson regression model including continuous CrCl and logarithm of event time (follow-up time if censored) as offset was fitted to estimate event rates with respect to each outcome. The quasi-poisson model was used due to the over-dispersion of the outcomes. We considered linear and non-linear association between CrCl and outcomes. For nonlinear associations, we considered a cubic spline with 3 knots, and a linear piecewise model with 1 or 3 knots. The model using assuming linear CrCl-by-outcome relationship has lowest QAIC for all outcomes. We plotted the event rates per 100 patient-year by decreasing CrCl and present here the change of event rate in percentage per 10 mL/min CrCl decrease with statistical significance assessed by the p-value.
We then performed a patient-level network meta-analysis to evaluate treatment effects across CrCl values. A multivariable stratified Cox proportional hazard model including a treatment-by-CrCl interaction was fitted to estimate hazard ratios (HRs) for pairs of treatment strategies with respect to each outcome. The model allows random effects on treatment effect coefficients to account for heterogeneity across trials. We did not add random effects on the CrCl or treatment-by-CrCl interaction because doing so did not improve model fit based on the Akaike information criterion. We considered continuous and categorical CrCl in two separate models, and Cox models assuming linear and non-linear association between CrCl and outcomes were fitted when CrCl was used continuously. For nonlinear associations, we considered a cubic spline model with 3 knots, and a linear piecewise model with 1 or 3 knots. In Cox regression models, non-linear associations were not observed and results from linear models were selected. Additionally, proportional hazard assumptions were assessed using the Schoenfeld residuals test 25 and graphical assessment of Kaplan-Meier curves in each trial.26 To assess if treatment effects differ with varying kidney function, we present HR change rate per 10 mL/min CrCl decrease with statistical significance assessed by the treatment-by-CrCl interaction. Between-study heterogeneity of the treatment effect was assumed to differ by treatment comparison and quantified by the standard deviation of random effects. All analyses were conducted using the coxme (version 2.2) and survival (version 3.3) packages on R, version 4.2.0 (The R Foundation).27
Results
Baseline Characteristics
Baseline characteristics of 71,683 patients by CrCl category and overall are presented in Table 1. Lower CrCl groups tended to include patients with older age, female sex, lower body weight, and prior diagnoses of heart failure, coronary artery disease, and bleeding. Patients in lower CrCl groups also tended to have higher CHA2DS2-VASc scores, to be more likely to use antiplatelet agents, and to have permanent or persistent AF vs paroxysmal AF. Median follow-up time was 23.1 months across all trials. For a brief list of patient demographics and median follow-up in individual trials, see Supplemental Table 1.
Table 1. Baseline Demographics by CrCl category.
CrCl calculated at baseline, using the Cockcroft-Gault formula. Frequencies are shown as n (percentage), and continuous variables as mean (SD), with the exception of CHA2DS2-VASc, which is shown as median (IQR). AF: atrial fibrillation; BMI: body mass index; CrCl: creatinine clearance; GIB: gastrointestinal bleed; HTN: hypertension; VKA: vitamin K antagonist
| CrCl Category | <30 | 30-44 | 45-59 | 60-89 | ≥90 | Overall |
|---|---|---|---|---|---|---|
| n | 510 | 8409 | 15477 | 28891 | 18277 | 71683 |
| Female | 335 (65.7%) | 4721 (56.1%) | 7118 (46.0%) | 10068 (34.8%) | 4425 (24.2%) | 26715 (37.3%) |
| Age | 80.2 (6.8) | 78.5 (6.6) | 75.3 (6.8) | 70.6 (7.7) | 62.6 (8.7) | 70.6 (9.4) |
| CrCl | 26.6 (3.0) | 38.7 (4.1) | 52.7 (4.3) | 73.5 (8.5) | 116.1 (26.7) | 75.5 (30.5) |
| BMI | 23.6 (4.4) | 25.2 (4.4) | 26.7 (4.4) | 28.9 (4.7) | 33.6 (6.4) | 29.2 (5.9) |
| Weight | 59.9 (13.3) | 66.3 (13.3) | 72.9 (13.6) | 82.5 (14.7) | 101.5 (20.3) | 83.2 (20.0) |
| Smoking | 190 (37.3%) | 2984 (35.5%) | 6104 (39.4%) | 12659 (43.8%) | 9283 (50.8%) | 31265 (43.6%) |
| Diabetes | 129 (25.3%) | 2118 (25.2%) | 4212 (27.2%) | 8607 (29.8%) | 6996 (38.3%) | 22087 (30.8%) |
| Stroke | 129 (25.3%) | 2517 (29.9%) | 4737 (30.6%) | 8376 (29.0%) | 4361 (23.9%) | 20147 (28.1%) |
| Prior VKA Use | 310 (60.8%) | 5433 (64.6%) | 10328 (66.7%) | 19698 (68.2%) | 13056 (71.4%) | 48892 (68.2%) |
| Antiplatelet Use | 196 (38.4%) | 3168 (37.7%) | 5711 (36.9%) | 10142 (35.1%) | 6213 (34.0%) | 25464 (35.5%) |
| CHA2DS2-VASc | 4.9 (1.4) | 4.8 (1.4) | 4.5 (1.4) | 3.9 (1.5) | 3.2 (1.4) | 4.0 (1.5) |
| AF type | ||||||
| Paroxysmal | 89 (17.5%) | 1880 (22.4%) | 3574 (23.1%) | 6870 (23.8%) | 4174 (22.8%) | 16609 (23.2%) |
| Persistent/Permanent | 421 (82.5%) | 6526 (77.6%) | 11903 (76.9%) | 22015 (76.2%) | 14098 (77.1%) | 55059 (76.8%) |
| Coronary Disease | 201 (39.4%) | 2753 (32.7%) | 5037 (32.5%) | 9137 (31.6%) | 5513 (30.2%) | 22674 (31.6%) |
| HTN | 444 (87.1%) | 7285 (86.6%) | 13465 (87.0%) | 25191 (87.2%) | 16380 (89.6%) | 62863 (87.7%) |
| Heart Failure | 271 (53.1%) | 4107 (48.8%) | 6884 (44.5%) | 12750 (44.1%) | 9231 (50.5%) | 33276 (46.4%) |
| Prior GIB | 23 (4.5%) | 320 (3.8%) | 506 (3.3%) | 750 (2.6%) | 429 (2.3%) | 2030 (2.8%) |
| Prior Non-GIB | 55 (10.8%) | 500 (5.9%) | 861 (5.6%) | 1517 (5.3%) | 1049 (5.7%) | 3989 (5.6%) |
Overall Event Incidence by Creatinine Clearance
When we evaluated the event incidence for safety and efficacy outcomes in the pooled dataset of all patients, we found that the incidence of major bleeding, ICH, S/SE and death all significantly increased with decreasing kidney function (Figure 1, Supplemental Table 2).
Figure 1: Raw Event by Category.
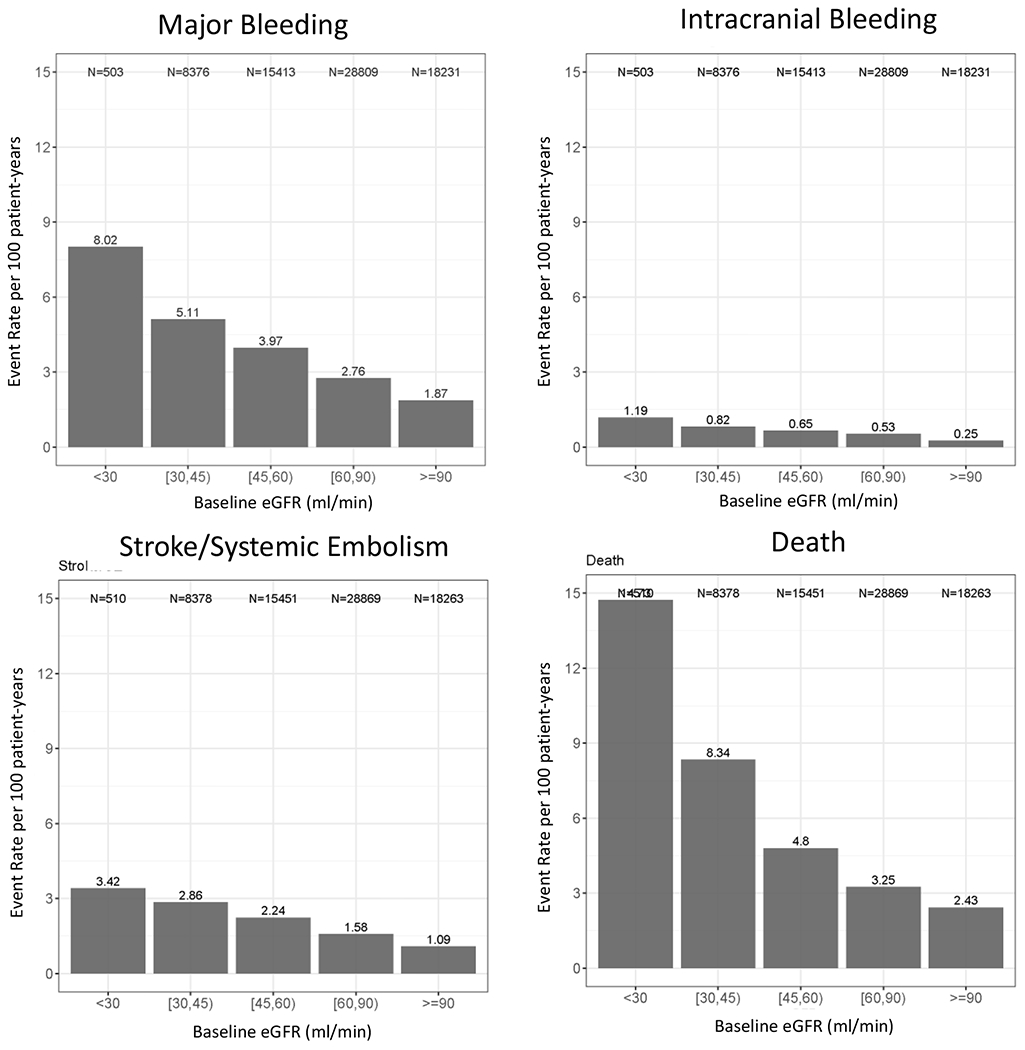
shown per 100 person-years. Panel A: Major Bleeding Panel B: Intracranial Hemorrhage Panel C: Stroke Panel D: Mortality. ICH: intracranial hemorrhage
Hazard of Major Bleeding Events by Continuous Creatinine Clearance
Analyzing the hazard of bleeding across the spectrum of continuous kidney function, we found that patients randomized to standard dose DOAC vs warfarin had a numerically lower hazard of bleeding at all CrCl values, though this was not statistically significant. There was no was no significant treatment-by-CrCl interaction for on the hazard of major bleeding for standard dose DOAC vs warfarin (Figure 2, Supplemental Table 3).
Figure 2: Hazard Ratios for Standard Dose DOAC vs Warfarin Across CrCl.
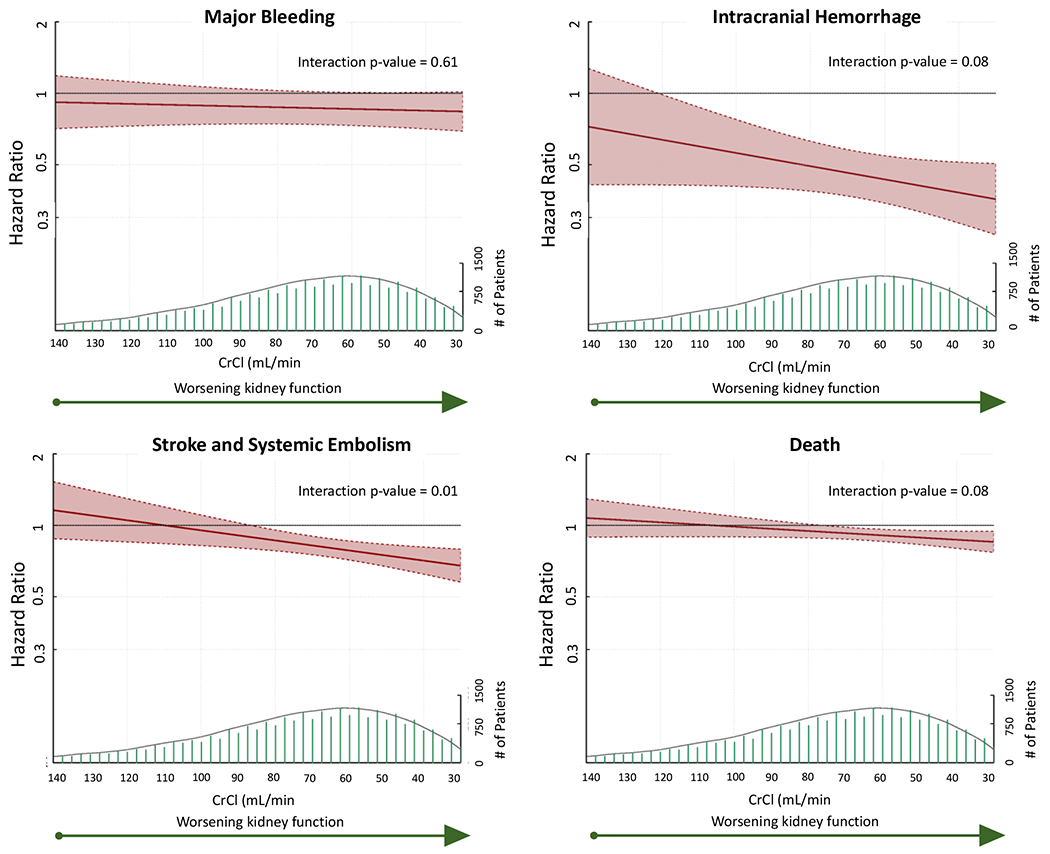
Hazard Ratio and 95% CI shown in red (left y axis), with population at each CrCl value shown in green directly below (right y axis). Cox models assume linear associations between CrCl and each outcome. HR above 1 favors warfarin, below 1 favors standard dose DOAC. Interaction p value represents significance of the treatment-by-CrCl effect. CrCl: creatinine clearance
Hazard of Intracranial Hemorrhage by Creatinine Clearance
Patients randomized to standard dose DOAC had a significantly lower risk of ICH than patients randomized to warfarin at any CrCl <122 mL/min, with a trend towards a positive treatment-by-CrCl interaction, such that patients tended to derive a greater relative benefit from standard dose DOAC vs warfarin as kidney function worsened (HR decrease 6.2% for every 10mL/min decrease in CrCl, 95% CI −0.7 to 12.6%, p=0.08, Figure 2, Supplemental Table 3).
Hazard of Stroke and Systemic Embolism by Creatinine Clearance
Patients randomized to standard dose DOAC had a significantly lower hazard of S/SE than those randomized to warfarin when CrCl was <87 mL/min. There was a significant treatment-by-CrCl interaction noted on the hazard of S/SE with standard dose DOAC vs warfarin, such that patients derived a greater benefit from standard dose DOAC with decreasing kidney function (HR decrease for every 10mL/min decrease in CrCl 4.8%, 95% CI 1.3-8.1%, p=0.01) (Figure 2, Supplemental Table 3).
Mortality Hazards by Creatinine Clearance
The hazard of death was significantly lower for patients randomized to standard dose DOAC vs warfarin for patients with a CrCl <77 mL/min, with a trend towards increasing benefit from standard dose DOAC as kidney function decreased (HR decrease 2.1% for every 10mL/min decrease in CrCl, 95% CI −0.3-4.4%, p=0.08, Figure 2, Supplemental Table 3).
Hazard of Composite Endpoints by Creatinine Clearance
Patients randomized to standard dose DOAC vs warfarin had a significantly lower hazard of a composite of bleeding or death when CrCl was between 42 and 109 mL/min, and a significantly lower hazard of a composite of bleeding, death or stroke/systemic embolism when CrCl was between 30mL/min and 96 mL/min. However, there was no significant interaction-by-CrCl on the hazard of either composite for patients randomized to standard dose DOAC vs warfarin (Supplemental Table 3).
Hazard of Events by Continuous Creatinine Clearance in Patients Randomized to Lower Dose DOAC vs Warfarin or Standard Dose DOAC
Patients randomized to lower dose DOAC vs warfarin had a had a significantly lower hazard of bleeding compared to warfarin for across all CrCl values >35 mL/min, and a lower risk of death for CrCl values between 30 and 76 mL/min. Similarly, a lower risk of ICH was seen with lower dose DOAC vs warfarin for all CrCl values, though without any significant interaction-by CrCl effect. There was no CrCl value for which lower dose DOAC had a significantly different hazard of S/SE. (Supplemental Table 3, Supplemental Figure).
Patients randomized to lower dose DOAC vs standard dose DOAC had a lower hazard of bleeding with CrCl values between 77 and 140 mL/min and a lower hazard of ICH with CrCl between 47mL/min and 106mL/min. However, these patients also had a significantly higher hazard of death (for CrCl values between 30mL/min and 42 mL/min) and of S/SE (with CrCl values of 30 to 98 mL/min). There was no significant treatment-by-CrCl effect observed on the hazard of bleeding, ICH, or stroke/systemic embolism for patients randomized to lower dose DOAC vs either warfarin or standard dose DOAC. However, patients randomized to lower dose DOAC had a significantly higher hazard of S/SE than patients randomized to standard dose DOAC at CrCl values less than 98 mL/min, and a significantly higher hazard of death than patients randomized to standard dose DOAC at CrCl values less than 42mL/min. Patients taking lower dose DOAC also faced a significant increase in hazard of death with worsening kidney function as compared to either warfarin (3.5% increase in hazard of death with low dose DOAC vs warfarin for every 10mL/min decrease in CrCl, p=0.03) or standard dose DOAC (5.8% increase in hazard of death with low dose DOAC vs standard dose DOAC for every 10 mL/min decrease in Cr Cl, p=−.001) (Supplemental Table 3, Supplemental Figure).
Hazard Ratio of Events by Categorical Creatinine Clearance
Similar results were found when hazards of major bleeding, ICH, and S/SE and death were assessed through Cox modeling across CrCl categories (Supplemental Table 4). For patients randomized to standard dose DOAC vs warfarin, the hazards of major bleeding, ICH, S/SE and death were numerically lower for each CrCl category <90 mL/min. This was statistically significant for CrCl values between 30 and 89 mL/min for ICH and S/SE, and between 30 and 59 for death. In Cox regression models, little or no between-study heterogeneity was observed for all outcomes with standard deviation of random effects close to zero (Supplemental Table 5).
Discussion
In this network meta-analysis of 71,683 patients across the major AF anticoagulation trials, we find that the benefits of DOAC over warfarin are retained in patients with reduced kidney function. In a Cox model analysis, patients with reduced CrCl randomized to standard dose DOAC vs warfarin had lower hazards of ICH, S/SE and death, with no difference in incidence of bleeding, down to a CrCl of at least 25ml/min. Furthermore, patients with low CrCl randomized to standard dose DOAC vs lower dose DOAC had a significantly lower hazard of S/SE and death with no significantly increased hazard of bleeding or ICH. There was no CrCl value for which standard dose DOAC use resulted in higher risk of bleeding, ICH, S/SE, or death than warfarin in this analysis.
In the case of S/SE, there was a significant treatment-by-CrCl interaction, such that there was a greater relative reduction in risk with standard dose DOAC vs warfarin with worsening kidney function, with non-significant trends towards greater benefit with standard dose DOAC over warfarin seen for reduction in risk of ICH and death with worsening kidney function. Much more important than any CrCl cut-off, these findings suggest that beyond being as safe and effective as warfarin in patients with diminished kidney function, the benefits of DOACs over warfarin are actually amplified as kidney function worsens, with increasing efficacy as well as a trend towards greater safety.
Despite concerns regarding safety with use of drugs that are in part renally eliminated, these results are reassuring and show that the safety of DOACs are preserved and efficacy even greater in patients with impaired kidney function, down to an eGFR of at least 25mL/min. We did not appreciate significant heterogeneity between trials, despite the varying renal clearance of different DOACs. These results suggest that DOACs are safer and more effective than warfarin at lower CrCl and that the benefits of DOAC over warfarin may in fact be amplified in patients with poor kidney function. Our findings are consistent with prior sub-analyses from individual trials, which have preserved safety and efficacy of dabigatran,17 apixaban,20 edoxaban21 and rivaroxaban.22 These findings are of particular importance given the observed increased risk of S/SE, bleeding, ICH and death with decreased kidney function, which we note in our results, and which have also been reported previously, including estimates that the risk of S/SE increases by 7% with every 10mL decrease in CrCl. 2,3,17,20–22 As patients with worse kidney function are at higher risk for complications related to both AF and anticoagulation, the safety and efficacy benefits seen with DOAC vs warfarin are even more important.
These results also suggest that it is inappropriate, and even dangerous, to reduce DOAC dose with kidney dysfunction unless the patient meets pre-specified criteria for dose reduction, as doing so may result a higher incidence of stroke and death without providing any safety benefit in terms of bleeding or ICH. Patients in RE-LY and ENGAGE AF-TIMI 48 were randomized to either standard dose DOAC, lower dose DOAC, or warfarin. This is different than the dose adjustment criteria used in ARISTOTLE, ROCKET and ENGAGE AF-TIMI 48, which was not random and instead based on patient criteria, including age, body weight, and creatinine. Our analysis stratified patients based on their randomized DOAC dose (standard vs low), not based on trial-specific dose adjustments made for kidney clearance or other non-randomized patient factors. Our findings show that at low levels of kidney function (below ~45mL/min), patients randomized to lower dose DOAC had significantly higher hazards of both death and stroke/systemic embolism, with no significant difference in risk of bleeding or ICH as compared to those randomized to standard dose DOAC. Furthermore, we find that there was a significant interaction of kidney function on hazard of death for patients randomized to lower dose DOAC vs both warfarin and standard dose DOAC, such that lower dose DOAC actually became more dangerous (i.e., was associated with a significantly higher hazard of death) with decreasing kidney function.
These findings are consistent with a prior smaller secondary analysis of patients with 0 vs 1 dose reduction criteria in ARISTOTLE, all of whom received either warfarin or to standard dose apixaban, without any dose reduction (because they had 1 but not 2 criteria for dose reduction). Importantly, the authors found no difference in HR for patients with 0 vs 1 dose reduction criteria for any outcome, nor did they find a significant difference based on type of dose reduction criteria (weight, age, or kidney function).28 Taken together with our findings, these results strongly suggest that there is no role for this reduction in patients not meeting criteria for dose reduction, and that standard dose DOAC maintains a comparable safety profile to lower dose DOAC while simultaneously preventing more strokes and more deaths. This is of critical importance, since patients with kidney dysfunction who do not meet criteria for dose reduction in their DOAC are frequently underdosed in an attempt to reduce risk of bleeding or other complications from anticoagulation.
Limitations and Strengths
There are limitations to our work. Our analyses were conducted using baseline CrCl, and we did not account for changes in CrCl over time. Because of natural variation in CrCl there were patients included in our analysis with baseline CrCl as low as 11 mL/min, though patients were only eligible for inclusion in the individual AF DOAC trials down to a CrCl of 25 (in the case of ROCKET AF and RE-LY) to 30 mL/min (ENGAGE AF TIMI-48, and ARISTOTLE). Therefore, there were relatively few events at the lowest values (<25 mL/min) of CrCl. However, this analysis still represents the single largest examination of anticoagulation across kidney function in patients with AF randomized to DOAC vs warfarin to date. Furthermore, our analysis shows linear trends towards greater, not diminishing, benefit with decreasing kidney function. CrCl is by nature an estimated measure of kidney function. There is also variation between DOACs in degree of kidney clearance. Despite this, there was little heterogeneity seen between trials for hazard ratios or interaction-by-CrCl treatment effects.
Strengths of these analyses include that they were performed on the largest and highest quality set of randomized data available for AF anticoagulation, and were performed using individual patient data in a patient-level meta analysis. Furthermore, these analyses were conducted using kidney function as a continuous variable, rather than being limited to categorical analyses.
Conclusion
We find that standard dosing strategies with DOACs are safer and more effective than warfarin in patients with kidney dysfunction down to a CrCl of at least 25 ml/min, with additional evidence that patients derive a greater relative benefit from standard dose DOAC over both warfarin and lower dose DOAC with decreasing kidney function. Furthermore, we find that in patients with the worst kidney function (down to a CrCl of 25 mL/min), use of lower dose rather than standard dose DOAC was associated with a higher risk of S/SE and death without any significant reduction in incidence of bleeding or ICH. Taken together, these results support the use of DOAC over warfarin down to a CrCl of at least 25 mL/min, and emphasize the important of prescribing guideline-supported doses of DOAC in the prevention of S/SE.
Supplementary Material
CLINICAL PERSPECTIVE.
What is New?
As kidney function worsens (down to a creatinine clearance of at least 25 ml/min), patients derive a larger relative benefit from direct oral anticoagulants (DOACs) vs warfarin in terms of hazard of stroke and systemic embolism, with similar trends seen for rates of death and intracranial hemorrhage.
Patients with kidney dysfunction randomized to lower dose DOAC did not have a significantly lower incidence of bleeding, or intracranial hemorrhage compared to standard dose DOAC, but did have a higher incidence of death and stroke and systemic embolism
What Are the Clinical Implications?
The use of standard dose DOAC is safer and more effective than warfarin down to a CrCl of at least 25 ml/min.
Patients should only receive dose reduction in their DOAC if they meet the clinical criteria for dose reduction.
Disclosures:
JH and AC completed work for this while on T32 grant T32HL069749. KH and HH have no relevant disclosures. SHH has received consulting fees Abbott, Bayer Healthcare, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiome, Gilead, Janssen, Johnson & Johnson, Pfizer, Portola, Sanofi Aventis, Servier, and Zoll, and lecture fees from Abbott, Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Pfizer, Sanofi Aventis). KAAF has received grant support from Bayer and AstraZeneca, and has served as a consultant for Bayer and Sanofi/Regeneron. ZH receives research support from The Swedish Society for Medical Research (S17-0133), Hjärt-Lungfonden (The Swedish Heart-Lung Foundation, 20200722), and Uppsala University Hospital, Sweden; and reports lecture and consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Roche Diagnostics. RDL has received research grants or contracts from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, Sanofi-Aventis, funding for educational activities or lectures from Pfizer and Daiichi Sankyo, and funding for consulting or other services from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk. LW reports research support from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Roche Diagnostics. RPG has received research support from Amgen, Anthos Therapeutics, Daiichi Sankyo, and Ionis, honoraria from Amgen, Centrix, Daiichi Sankyo, Dr. Reddy’s Laboratories, Medical Education Resources (MER), Medscape, Menarini, Merck, Pfizer, SAJA Pharmaceuticals, Servier, Shanghai Medical Telescope and Voxmedia, and has served as a consultant for Amarin, Amgen, Bayer, Boston Scientific, Caladrius, CryoLife, CSL Behring, CVS Caremark, Daiichi Sankyo, Esperion, Gilead, Hengrui, Inari, Janssen, Novartis, Paratek, Pfizer, PhaseBio Pharmaceuticals and Samsung.
Abbreviations
- AF
atrial fibrillation
- CrCl
creatinine clearance
- ICH
intracranial hemorrhage
- DOAC
direct oral anticoagulant
- S/SE
stroke/systemic embolism
Citations
- 1.Roberts PR, Green D. Arrhythmias in chronic kidney disease. Heart. 2011;97:766–773. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D. Anticoagulation in Concomitant Chronic Kidney Disease and Atrial Fibrillation: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2019;74:2204–2215. [DOI] [PubMed] [Google Scholar]
- 3.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrology Dialysis Transplantation. 2015;30:1162–1169. [DOI] [PubMed] [Google Scholar]
- 4.January Craig T, Samuel Wann L., Calkins Hugh, Chen Lin Y., Cigarroa Joaquin E., Cleveland Joseph C., Ellinor Patrick T., Ezekowitz Michael D., Field Michael E., Furie Karen L., Heidenreich Paul A., Murray Katherine T., Shea Julie B., Tracy Cynthia M., Yancy Clyde W. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. European Heart Journal. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6.Carnicelli AP, Hong H, Connolly SJ, Eikelboom J, Giugliano RP, Morrow DA, Patel MR, Wallentin L, Alexander JH, Cecilia Bahit M, Benz AP, Bohula EA, Chao T-F, Dyal L, Ezekowitz M, A A Fox K, Gencer B, Halperin JL, Hijazi Z, Hohnloser SH, Hua K, Hylek E, Toda Kato E, Kuder J, Lopes RD, Mahaffey KW, Oldgren J, Piccini JP, Ruff CT, Steffel J, Wojdyla D, Granger CB, COMBINE AF (A Collaboration Between Multiple Institutions to Better Investigate Non-Vitamin K Antagonist Oral Anticoagulant Use in Atrial Fibrillation) Investigators. Direct Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials With Interaction Testing by Age and Sex. Circulation. 2022;145:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingelheim B Pradaxa® (dabigatran etexilate) for U.S. Healthcare Professionals [Internet]. Pradaxa. [cited 2022 Dec 1]; Available from: https://pro.boehringer-ingelheim.com/us/products/pradaxa/ [Google Scholar]
- 8.Savaysa (edoxaban) dosing, indications, interactions, adverse effects, and more [Internet]. [cited 2022 Dec 1]; Available from: https://reference.medscape.com/drug/savaysa-edoxaban-999979
- 9.XARELTO® (rivaroxaban) | Healthcare Professional Website [Internet]. XARELTO® (rivaroxaban). 2021. [cited 2022 Dec 1]; Available from: https://www.xareltohcp.com/dosing-all-indications
- 10.Eliquis (apixaban) dosing, indications, interactions, adverse effects, and more [Internet]. [cited 2022 Dec 1]; Available from: https://reference.medscape.com/drug/eliquis-apixaban-999805
- 11.Barra ME, Fanikos J, Connors JM, Sylvester KW, Piazza G, Goldhaber SZ. Evaluation of Dose-Reduced Direct Oral Anticoagulant Therapy. Am J Med. 2016;129:1198–1204. [DOI] [PubMed] [Google Scholar]
- 12.Campitelli MA, Bronskill SE, Huang A, Maclagan LC, Atzema CL, Hogan DB, Lapane KL, Harris DA, Maxwell CJ. Trends in Anticoagulant Use at Nursing Home Admission and Variation by Frailty and Chronic Kidney Disease Among Older Adults with Atrial Fibrillation. Drugs Aging. 2021;38:611–623. [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 17.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961–970. [DOI] [PubMed] [Google Scholar]
- 18.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 19.Turpie AGG, Purdham D, Ciaccia A. Nonvitamin K antagonist oral anticoagulant use in patients with renal impairment. Ther Adv Cardiovasc Dis. 2017;11:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. [DOI] [PubMed] [Google Scholar]
- 21.Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of Renal Function on Outcomes With Edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation. 2016;134:24–36. [DOI] [PubMed] [Google Scholar]
- 22.Fox KAA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, Paolini JF, Hankey GJ, Mahaffey KW, Patel MR, Singer DE, Califf RM. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen PB, Lane DA, Rasmussen LH, Lip GYH, Larsen TB. Renal function and non-vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: a systemic review and meta-regression analysis. Clin Res Cardiol. 2015;104:418–429. [DOI] [PubMed] [Google Scholar]
- 24.Carnicelli AP, Hong H, Giugliano RP, Connolly SJ, Eikelboom J, Patel MR, Wallentin L, Morrow DA, Wojdyla D, Hua K, Hohnloser SH, Oldgren J, Ruff CT, Piccini JP, Lopes RD, Alexander JH, Granger CB. Individual Patient Data from the Pivotal Randomized Controlled Trials of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation (COMBINE AF): Design and Rationale: From the COMBINE AF (A Collaboration between Multiple institutions to Better Investigate Non-vitamin K antagonist oral anticoagulant use in Atrial Fibrillation) Investigators. American Heart Journal. 2021;233:48–58. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld D Chi-Squared Goodness-of-Fit Tests for the Proportional Hazards Regression Model. Biometrika. 1980;67:145–153. [Google Scholar]
- 26.Persson I Khamis HJ. A Comparision of Graphical Methods for Assessing the Proportional Hazards Assumptions in the Cox Model. Journal of Statistics and Applicaitons. 2007;2:1–32. [Google Scholar]
- 27.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 28.Alexander JH, Andersson U, Lopes RD, Hijazi Z, Hohnloser SH, Ezekowitz JA, Halvorsen S, Hanna M, Commerford P, Ruzyllo W, Huber K, Al-Khatib SM, Granger CB, Wallentin L, Apixaban for Reduction of Stroke and Other Thromboembolic Complications in Atrial Fibrillation (ARISTOTLE) Investigators. Apixaban 5 mg Twice Daily and Clinical Outcomes in Patients With Atrial Fibrillation and Advanced Age, Low Body Weight, or High Creatinine: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016;1:673–681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


