Abstract
Models of sexual conflict over mating, including conflict over indirect benefits of mate choice, have generally presumed that female resistance to male coercion must involve direct confrontation, which can lead to sexually antagonistic coevolutionary arms-races. We built a quantitative model examining the largely ignored possibility that females may evolve new, additional mate preferences for new male traits that undermine male capacity to coerce. Thus, females may “remodel” the coercive capacity of the male phenotype in order to enhance their own sexual autonomy—a novel alternative mechanism by which females may avoid arms-races. We demonstrate that evolutionary “remodeling” is possible, in spite of costs to males, because females that prefer males with protective, autonomy-enhancing traits (traits correlated with lower coercion effectiveness) are likelier to gain indirect benefits of having attractive mates. Our analysis reveals new possibilities for the evolution of systems of sexual conflict over indirect benefits, showing that autonomy-enhancing male traits can act as a “public good,” benefiting all females regardless of mating preferences, leading to oscillatory dynamics; and that preferences for more protective male traits will often be favored relative to preferences for less protective traits, potentially leading to an evolutionary “snowball” of expanding sexual autonomy.
Keywords: sexual conflict, sexual coercion, sexual selection, mate choice, multimodal mate preference, population-genetic model
Introduction
Sexual conflict over mating, wherein the evolutionary interests of males and females are misaligned in mating or fertilization, is typically characterized by the evolution of female resistance to male sexual coercion, often leading to striking sexually antagonistic coevolutionary arms-races (Arnqvist & Rowe, 2002, 2005; Gavrilets & Hayashi, 2006; Gavrilets et al., 2001; Lessells, 2006; Morrow & Arnqvist, 2003; Parker, 2006; Rowe & Arnqvist, 2002; Rowe et al., 2003). Recent work has further established that female resistance can evolve in response to sexual conflict over indirect benefits (fitness benefits of mate choice accruing to females via their offspring in terms of their attractiveness and/or viability; Brennan & Prum, 2012; Snow et al., 2019). Thus, female resistance can reinforce existing female mating preferences, rather than leading simply to the avoidance of physical harm (Brennan & Prum, 2012; Snow et al., 2019). Waterfowl provide a well-known example of resistance to retain the indirect genetic benefits of mating with their preferred social partners: in response to the evolution of elaborate male penis morphologies that facilitate the forced fertilization of females, females of some species of waterfowl have coevolved convoluted genital tracts that physically hinder unwanted fertilization (Brennan & Prum, 2012; Brennan et al., 2007, 2010; Snow et al., 2019).
Most previous models characterizing evolutionary outcomes of sexual conflict over mating have presumed that evolved female responses to male coercion require direct female interaction with the coercive male morphology/behavior; females can either resist more intensely or become less sensitive to the coercive attacks (e.g., Arnqvist & Rowe, 2005; Gavrilets & Hayashi, 2006; Rowe et al., 2005, but see Rosenthal & Servedio, 1999). Either response can produce selection for further exaggeration of coercive traits in males (Arnqvist & Rowe, 2005; Gavrilets & Hayashi, 2006; Gavrilets et al., 2001; Härdling & Smith, 2005; Lessells, 2006; Parker, 2006; Snow et al., 2019). As an alternative to direct confrontation and the classic arms-race scenario, in this article we explore the largely ignored possibility that females may evolve new, additional mating preferences that undermine male capacity to coerce and lead to reduced coercion, and increased freedom of mate choice.
In pursuit of mathematical simplicity, most previous models of sexual conflict over mating do not distinguish between male coercion and display, or female choice or resistance (e.g., Arnqvist & Rowe, 2005; Brooks & Griffith, 2010; Cordero & Eberhard, 2003; Eberhard, 2005; Holland & Rice, 1998; Kokko, 2005; reviewed in Brennan & Prum, 2012). However, examination of sexual conflict over indirect benefits reveals that these potentially concurrent processes can be meaningfully distinguished when sexual coercion is defined as any (male) action biasing mating in a way that subverts an existing (female) mating preference for a display (Brennan & Prum, 2012; Snow et al., 2019). Previous work has shown that coercion resistance traits in females will be selectively favored as long as females can attain some indirect benefits of mating with their preferred males based on display, and coercion causes them to mate with preferred males less often than otherwise (Snow et al., 2019). These insights open the door to inquiry into more complex systems that include features of simultaneous preference for male display and male coercion, as well as reimagining the diverse ways that females resist sexual coercion.
Prum (2015, 2017) has suggested a novel alternative mechanism by which females may mitigate sexual conflict over indirect benefits without direct confrontation by evolving new mate preferences that actively “remodel” male coercive capacities. Rather than evolving resistance traits that directly interfere with male coercion and potentially inciting an arms race, females may evolve an additional mate preference for a new male trait that has the benefit of enhancing female freedom of mate choice (referred to as “sexual autonomy”; Brennan & Prum, 2012; Prum, 2017; Snow et al., 2019). We will call a novel female mating preference for a male display trait with influence on coercion efficacy a “remodeling preference,” and the corresponding display trait an “autonomy-enhancing trait.”
Imagine a population in which fertilization determination mechanisms are in equilibrium between female mate choice based on male display and male sexual coercion. We hypothesize that a new female mating preference for a novel male trait incidentally correlated with lower effectiveness of sexual coercion can evolve because it disrupts this equilibrium in favor of female mate choice, reducing the efficacy of sexual coercion, and expanding female sexual autonomy.
This proposal is distinct from females altering their preferences for a display (e.g., Kokko, 2005). Male coercion specifically functions to subvert females’ ability to realize an existing mating preference, and therefore females must evolve independent resistance (Snow et al., 2019), or in this case, additional preferences unaffected by the existing male coercion mechanism, in order to reduce the effectiveness of coercion and expand sexual autonomy. Similarly, remodeling preferences are distinct from classic sexual selection, wherein females can be said to actively shape male phenotypes, because the classic preference is not tied to male coercive capacity and therefore the efficacy of another preference.
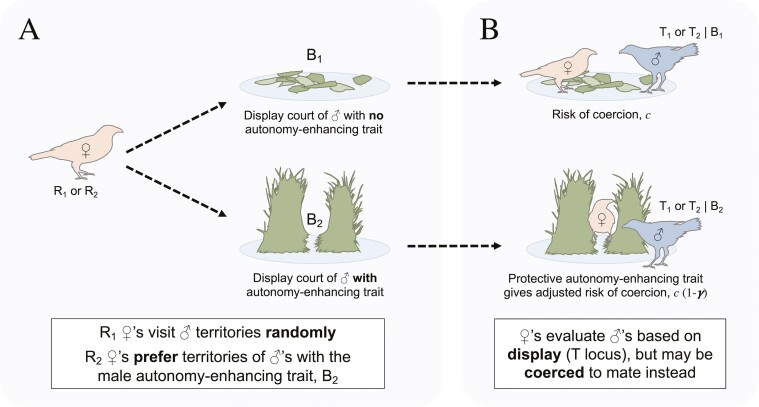
Male “autonomy-enhancing traits” that are subject to these novel preferences may be particular morphologies such as reduced weapons, behavioral cues such as displays that can be seen from a safe distance or display postures or orientations that reduce the likelihood of coercion, or aspects of extended phenotype that interfere with a males’ own ability to sexually coerce. For example, male bowerbirds (Ptilonorhynchidae) build courtship display bowers with a distinctive architectural design—such as an “avenue bower” that allows a female to perch between two walls of sticks—that protects females from the males’ own potential forced copulation attempts (Figure 1A; Borgia, 1995; Borgia & Presgraves, 1998; Patricelli et al., 2002; Prum, 2015, 2017). Females that prefer to visit males with autonomy-enhancing traits will have an advantage because they are less likely to be coerced, and thus more likely than other females to gain the indirect benefits of mating with attractive males. By this mechanism, females may be able to evolutionarily remodel male coercive capacity in spite of coercion success and potential costs to males, and mitigate sexual conflict over indirect benefits without evolving direct resistance that would possibly incite a sexually antagonistic co-evolutionary arms race (Brooks & Griffith, 2010; Kokko, 2005).
Figure 1.
Schematic of the process of the distribution of matings captured by the model, using the example of bowerbirds (Ptilonorhynchidae). (A) First, females choose to visit male territories according to their remodeling preference (coded at the R locus) for the corresponding autonomy-enhancing trait in males (coded at the B locus; represented by the protective architecture of the courtship display “avenue bower” built by the male). (B) Once on a territory, a female can accept or reject a male based his display attractiveness (coded at the T locus), or she may be coerced into mating without the opportunity to reject (with probability c). Females that associate with B2 males are physically protected from the male’s own coercive attempts by the bower structure [giving an adjusted risk of coercion c(1−γ)].
The plausibility of the remodeling mechanism is difficult to intuit because the hypothesis requires complex interactions among the effects of several traits—an attractive male display, female preference for that display, male coercion, a female preference for an additional “remodeled” autonomy-enhancing trait, and the autonomy-enhancing trait in males—which implies multiple higher-order effects propagated through genetic correlations arising from indirect selection. Specifically, we want to know if a male autonomy-enhancing trait can evolve by female choice because it enhances female sexual autonomy—that is, it increases the proportion of matings free from male coercion. We ask whether indirect selection on female preference promoting the evolution of autonomy-enhancing traits via genetic correlations with male attractiveness could be enough to overcome the costs incurred by males in terms of potential lower viability and lost coercive mating opportunities. We built a quantitative proof-of-concept theoretical model to examine this new possibility for the evolutionary trajectory of systems in sexual conflict over indirect benefits.
The model
We built a three-locus population genetic model. For simplicity, we assumed a polygynous, haploid system with two alleles per locus based on classic sexual selection models (Barton & Turelli, 1991; Kirkpatrick, 1982), expanded to incorporate three loci. All loci are autosomal, but with sex-limited expression. Males express two trait loci: T for a display trait, and B for an autonomy-enhancing trait. Females express “remodeling” preference for the male autonomy-enhancing trait at locus R. Males with the T2 allele produce an attractive display ornament while T1 males do not; B2 males produce the autonomy-enhancing trait while B1 males do not. Females with the R2 allele have a biased behavioral preference for visiting males with the autonomy-enhancing trait (B2 males), whereas R1 females visit males randomly with respect to males’ alleles at the B locus (See Table 1 for a summary of terms).
Table 1.
Summary of terms used in the three-locus population genetic model.
| T2/T1 | Allele for male attractive display trait/allele for unattractive display trait; lowercase denotes the respective allele frequencies |
| B2/B1 | Allele for the male autonomy-enhancing trait/allele for the lack of the autonomy-enhancing trait |
| R2/R1 | Allele for female preference for visiting males with the autonomy-enhancing trait (B2 males)/Allele for visiting males randomly with respect to males’ alleles at the B locus |
| s t | Viability cost to T2 males of producing an attractive display trait |
| s b | Viability cost to B2 males of producing the autonomy-enhancing trait |
| s r | Viability cost to R2 females of preferring males with the autonomy-enhancing trait (B2 males) |
| u | Mutation bias parameter: percent of the frequency of the T2 allele that mutates to the T1 allele each generation after mating and recombination |
| a t +1 | Factor by which all females prefer to mate with T2 males |
| a b +1 | Factor by which R2 females prefer to visit males with the autonomy-enhancing trait (B2 males) |
| c | Likelihood that a female gets coerced into mating without the opportunity to accept or reject a male based on his display upon visiting a male that lacks the autonomy-enhancing trait |
| γ | Effectiveness of the protection from coercion conferred by the autonomy-enhancing trait in B2 males |
For tractability, female preference for display and male coercion are treated as follows: (a) all females in the population have a preference for mating with attractive T2 males by a factor of (at + 1) relative to T1 males and (b) when visited by a female, all males have the capacity to attempt coercing females into mating with them without affording females the opportunity to choose based on their display. Our aim is to examine the plausibility of the evolution of female preferences for autonomy-enhancing traits in males via selection to mitigate sexual conflict over indirect benefits; having female preference for display and male coercion fixed allows us to set up a scenario of sexual conflict over indirect benefits (Snow et al., 2019; see Analyses) and investigate the dynamics of interest while using the simplest model framework (i.e., tracking the fewest loci) possible.
Further, we assume biased mutation at the male display (T) locus, such that a percentage of the frequency of the attractive T2 allele, u, mutates to T1 every generation after mating and recombination (Pomiankowski et al., 1991). This assumption is commonly used in sexual selection models as a straightforward mechanism to maintain diversity at key loci (which would otherwise be unrealistically depleted by selection in our simple model; Houle, 1992; Rowe & Houle, 1996), allowing for the maintenance of the indirect benefits of mate choice associated with, for example, Fisherian ornaments (Pomiankowski & Iwasa, 1993; Pomiankowski et al., 1991) or viability mediated by an ornament, in the case of good-genes models (Iwasa & Pomiankowski, 1994, 1999; Iwasa et al., 1991). Others have assumed a constant frequency to approximate a quantitative trait of constant variance in order to achieve a similar effect of allowing sexual selection to be ongoing in a simple model framework (Bank et al., 2012; Dhole et al., 2018; Snow et al., 2019; Tazzyman et al., 2014). Although the assumption of biased (as opposed to bidirectional) mutation is not strictly necessary for the maintenance of variation in the simple biallelic framework employed here (Veller et al., 2020), we use mutation bias to promote conceptual continuity with other quantitative sexual selection frameworks. For the simulation results we present in the main text, we employ a large mutation rate (0.1) that corresponds more closely to an assumption of constant variance while still allowing for exact recursions. We also explored a version with a more traditional small mutation rate, one order of magnitude smaller than selection on the display trait, which yielded qualitatively similar results (compare Supplementary Figures S1 and S2). The key consideration is that any selection on female preference is on the same order of magnitude as the mutation rate (or one order smaller, depending on the structure of the model) in order for the possibility of mutation–selection balance to maintain costly preference (Iwasa & Pomiankowski, 1994; Iwasa et al., 1991; Pomiankowski et al., 1991). In the present case, because indirect benefits of mating with attractive males are mediated by the autonomy-enhancing trait, (similar to how viability “good genes” are mediated by a display ornament) sr must be roughly one order smaller than mutation.
Ecological and behavioral assumptions
We assume that the attractive display (T2) and autonomy-enhancing trait (B2) each come with a viability cost such that a T2 male’s survival until mating season is reduced by a factor of (1−st), and a B2 male’s survival is reduced by a factor of (1−sb), relative to T1 or B1 males, respectively. We also assume a small cost of the female remodeling preference such that R2 females have a (1−sr) chance of surviving to mate relative to R1 females.
After viability selection, mating occurs. All surviving females are ultimately fertilized by one male each, but males may mate multiply and vary in relative success. Male mating success is determined by a combination of the attractiveness of their autonomy-enhancing trait, the attractiveness of their display, and their ability to coerce females. Females seek out mates by visiting males at their territories (Figure 1A; this model could also be interpreted as females choosing to associate with particular males). Females without a preference for the male autonomy-enhancing trait (R1 females) visit territories randomly. Females with a remodeling preference (R2 females) bias their territory visits toward males with the autonomy-enhancing trait (B2 males) by a factor of (ab + 1) relative to males that lack it.
Once on a male’s territory, the female can choose to accept or reject the male based on his display (his T allele). Females will choose to accept a T1 male with a probability of 1/(at + 2), and a T2 male with a probability of (at + 1)/(at + 2). Thus, we assume the baseline probability of accepting or rejecting a given male for a female without preference for display (at = 0) is ½. Alternatively, the visiting female may be coerced into mating without being afforded the opportunity to possibly reject him (Figure 1B). Females that visit the territories of males lacking the autonomy-enhancing trait (B1 males) have a probability c of getting coerced to mate. The autonomy-enhancing trait, B2, lowers a male’s ability to coerce, and thus offers protection and increased autonomy to females. Females that visit B2 males have an adjusted probability of being coercively mated of c*(1 − γ), where γ is the effectiveness of the protection conferred by the male trait. (See Table 2 for a matrix of the relative frequencies of mating between the various genotypes.) After matings are distributed, there is standard free recombination of alleles among gametes and finally biased mutation at the T locus (from T2 to T1 alleles at rate u) yielding genotype frequencies for the next generation (see Supplementary Appendix S1 for development of model equations and Supplementary File S1 for full model code).
Table 2.
Frequencies of matings between each of the eight genotypes. Although the model follows all genotypes independently, to aid in interpretation, each row gathers together all male genotype frequencies having the same alleles at the T and B loci; since these traits are sex-limited, these groups of genotypes function identically in mating. Each term is the sum of the frequencies of males with genotype TiBj (TiBjR1 and TiBjR2). Similarly, each column gathers all female genotype frequencies having the allele R1 or R2, yielding and . The prime notation is a reminder that the genotype frequencies have already been adjusted by viability selection. The “z” terms are normalizing factors, where , and z1 and z2 are the sums of the numerators in their columns divided by and respectively.
| Males | Females | |
|---|---|---|
| … R1 | … R2 | |
| T1B1 … | ||
| T2B1 … | ||
| T1B2 … | ||
| T2B2 … | ||
We hypothesize that R2 females, which prefer the autonomy-enhancing male trait, B2, will be selectively favored because B2 will become genetically correlated with the indirect benefits of mating with attractive T2 males. Therefore, if B2 persists in the population, we expect to see correlations arise between R2 and B2, between T2 and B2, and between R2 and T2. Further, we will explore the effect of at, ab, sr, c, and γ on whether the B2 allele is able to persist.
Analyses
We analyzed behavior of the model and equilibria using numerical simulation of the recursion equations, as the complexity of the model precludes standard eigenvalue analysis (Supplementary File S1). For our numerical simulations, we began with a population at an internal equilibrium for the frequency of the T2 allele (calculated with the R2 and B2 alleles fixed at zero; See Supplementary Appendix S2 and Supplementary File S2):
This means that there is a stable balance between the combined effect of sexual selection on the male display trait and the effect of male sexual coercion (the latter of which weakens the effect of sexual selection), mutation, and viability selection on the display trait. Although both display and coercion bias females’ mating, it is important to note that these processes are distinct and function in a noninterchangeable way: the term sexual coercion is meaningful because this behavior functions in a system where females have an existing mating preference for a display. Since coercion specifically circumvents females’ preference for display, it does not simply add to the effect of display (as an additional ornament might; for example, Pomiankowski & Iwasa, 1993), and since it dampens—but does not negate—the benefits females attain though mate choice, it has the property of producing sexual conflict over indirect benefits (Snow et al., 2019). The “broad-sense” conception of sexual coercion (Brennan & Prum, 2012) invoked here includes obvious physical restraint and harassment, but also other diverse mechanisms such as male–male competition that might interfere with female mate choice (Wong & Candolin, 2005). Note that employing coercion and display thusly means that this paper does not explore parameter conditions for which coercion (c) is so strong relative to female preference (at) that sexual selection is insufficient to maintain the male display trait in mutation-selection balance given the costs (st). We can get a sense of the overall effect that coercion has on the system by deriving an expression for the adjusted realized magnitude of the parameter for female preference strength, at, under the conditions for the nonzero initial equilibrium frequency for the T2 allele (see Supplementary Appendix S2 and Supplementary File S2). Essentially, we can ask what adjusted value of at would produce the equivalent equilibrium value of t2 if coercion were absent:
We can see that adjusted at under coercion will always be smaller than the actual at. This of course does not indicate that coercion causes females to have weaker preferences, but rather shows that coercion causes females’ preferences to be realized less effectively. As c approaches 1, approaches zero, demonstrating that when coercion is perfect, females have no opportunity to exercise their preferences. The expression for holds as along as the conditions for the stable initial nonzero T2 equilibrium are met (Supplementary Appendix S2).
Previous work has shown that sexual coercion concurrent with females retaining some indirect benefits of mating with attractive males represents a potentially unstable condition sufficient to produce selection for direct female resistance (Snow et al., 2019). Here we used this balance between sexual selection and coercion to examine the plausibility of the evolution of remodeling preferences as an alternative to direct confrontation with the male coercive strategy. Accordingly, into this equilibrium condition, we then introduced the R2 and B2 alleles at very low frequencies.
To establish whether, and under what conditions, selection for increased indirect benefits of autonomous mate choice could drive the evolution of female remodeling preferences and the corresponding male traits, we used the numerical simulations to explore the effect of the various parameters on whether these traits became established in the population. To gain further insight, we graphically inspected the evolutionary trajectories of the traits and genetic correlations in sample numerical simulations representative of outcomes where either the autonomy-enhancing male trait persisted or did not. Moreover, we undertook a weak selection approximation of the model equations to examine the leading-order mechanisms and forces involved in more detail. Finally, we looked at whether more protective male autonomy-enhancing traits would be favored relative to less protective ones by examining a version of the model that controls for the effects of arbitrary, Fisherian sexual selection on the autonomy-enhancing trait.
We performed all analyses and numerical simulations using Wolfram Mathematica (Wolfram, 2016). We ran numerical simulations until all genotype frequencies and genetic correlations were constant to seven decimal places, and then, to avoid incorrect conclusions arising from slow convergence on an unstable point, we applied a small random perturbation and allowed the simulation to continue until again constant to seven decimal places. For a full summary of parameter ranges investigated, see Supplementary Figures S1 and S2.
Results
Remodeling preferences and traits can evolve
The remodeling preference and trait—R2 and B2 alleles—fail to become established in the baseline case where the autonomy-enhancing trait does not protect females from coercion (γ = 0; Dashed lines in Figure 2). Because we assumed a small viability cost to females with remodeling preference, arbitrary Fisherian sexual selection is insufficient to allow B2 and R2 to persist at equilibrium in the absence of additional sources of selection, regardless of initial allele frequencies (Kirkpatrick & Barton, 1997; Pomiankowski et al., 1991).
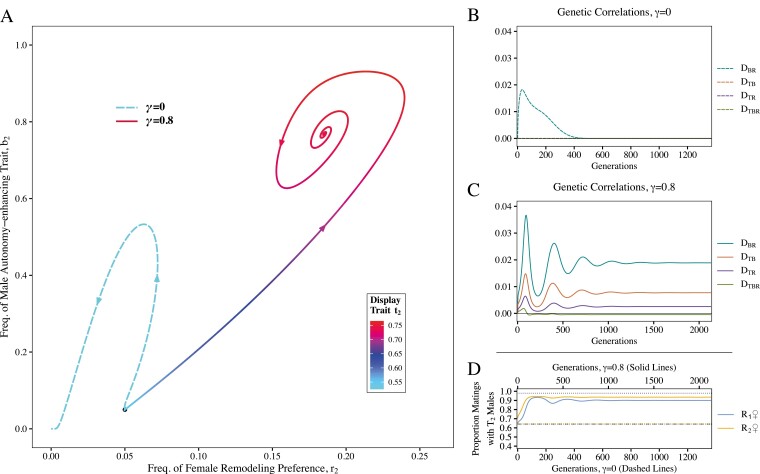
Figure 2.
Remodeling preferences and traits evolve to enhance female sexual autonomy, as shown in the case where the male autonomy-enhancing trait confers protection to females against male coercion (solid lines; γ = 0.8), in contrast to the case where the autonomy-enhancing trait is not protective (dashed lines; γ = 0). (A) Allele frequencies through time for γ = 0 and γ = 0.8. The black dot is the initial frequency (b2 = r2 = 0.05); arrows show the trajectory of the allele frequencies through time. The lines’ colors correspond to the frequency of the male attractive display trait (t2; initial freq. = 0.525). (B) Two- and three-way genetic correlations (linkage disequilibria) among the three loci though time for the nonprotective case. (C) Genetic correlations among the three loci though time for the protective case. Dashed lines for DTB, DTR, and DTBR are essentially zero throughout and therefore overlap. (D) Proportion of matings in the population with attractive T2 males through time. The lower black dotted line is the initial proportion and the upper black dotted line is the proportion of matings with T2 males that females would attain if there were no coercion (i.e., full sexual autonomy). The orange lines are the proportion of T2 males attained by R2 females preferring the autonomy-enhancing trait; the blue lines are the proportion for R1 females that mate randomly with respect to the autonomy-enhancing trait. Dashed lines for the γ = 0 case overlap with each other and the bottom black dotted line—the autonomy-enhancing trait is not protective and thus there is no increase in autonomy or advantage to having the R2 allele in this scenario. Other parameters are the same in both cases: at = 12, ab = 12, c = 0.5, st = 0.1, sb = 0.1, u = 0.1, sr = 0.01. See Supplementary Figure S3 for allele-frequency-by-time representations of the data shown in (A).
In contrast, when protection conferred by the autonomy-enhancing trait is at a requisite level (e.g., γ = 0.8; solid lines in Figure 2), the male trait increases in frequency and persists in the population at equilibrium, due to its evolved correlation with females’ sexual autonomy (Figure 2A, solid line). Unlike in the γ = 0 case, females are protected from coercion and can mate with males having an attractive display (T2) at a frequency closer to what they would attain without coercion (Figure 2D). We can see that as the autonomy-enhancing trait proliferates, the frequency of the T2 allele concurrently increases as sexual selection on display is strengthened (shift in color of solid line from blue to red; Figure 2A). How closely the final frequency of the T2 allele approaches the expectation under full autonomy depends on the combined influence of the protection of the autonomy-enhancing trait and the final frequency of the B2 allele in reducing the overall sexual selection-dampening effect of coercion (Equation 1; Supplementary Appendix S2).
The evolution of the B2 allele involves genetic correlations among the loci (Figure 2B and C). Whether the male trait effectively enhances female autonomy or not, it initially evolves due to sexual selection via females carrying the R2 allele, producing correlation between the autonomy-enhancing trait and female preference for it (DBR; Figure 2B and C). In the case that the male autonomy-enhancing trait confers protection (γ = 0.8; solid lines), the autonomy-enhancing trait becomes correlated with the T2 allele because females who visit the territories of B2 males are more likely to mate with attractive males (DTB; Figure 2C). Additionally, this produces positive correlation between the display trait and the female remodeling preference (DTR; Figure 2C), which produces additional positive indirect selection on the R2 allele, which boosts sexual selection on B2, and so on. This feedback loop allows the female preference for the autonomy-enhancing trait to overcome its viability cost, sr, and allows B2 to increase in frequency and persist at equilibrium (b2 > 0), as opposed to being lost (b2 = 0).
In cases where the male autonomy-enhancing trait persists, as the population approaches equilibrium, we often observe oscillations when there is a viability cost (sr > 0) to remodeling preference (Figure 2A). This result reveals an interesting aspect of the process of remodeling: male autonomy-enhancing traits will benefit females who don’t explicitly prefer them. As B2 proliferates, fewer matings are coerced since females that visit the territories of B2 males are protected (Figure 2D). Females with a preference for the autonomy-enhancing trait are at an advantage because they are able to more reliably mate with attractive T2 males (Figure 2D, solid orange line). However, once the autonomy-enhancing trait is common enough, R1 females without remodeling preference are likely to mate at a B2 male’s territory simply by chance (Figure 2D, solid blue line). Thus, R1 females can reap the benefits of enhanced autonomy of mate choice without paying the costs of seeking it out, so R1 females become favored until B2 decreases again (yielding a spiraling pattern; Figure 2A). In all our numerical simulations where they occur (Supplementary Figure S1) these oscillations invariably converge to a stable equilibrium as in Figure 2A.
Weak selection approximation
We examined the leading terms of a weak selection approximation of the model equations to gain further insight into the mechanisms underlying the evolution of male autonomy-enhancing traits and female preferences for them (Full calculation available in Supplementary File S4). For the approximation, we assumed weak selection (small st, sb, and sr), weak mutation (small u), weak female preferences (small at and ab), and weak male coercion (c), while allowing the key parameter of effectiveness of the male autonomy-enhancing trait in diminishing the likelihood of coercion (γ) to remain at any strength. We approximated expressions for Δr2, Δb2, and Δt2 to the first order with respect to the parameters assumed to be weak and under the assumption of quasi-linkage equilibrium for the genetic correlations, , among the T2, B2, and R2 alleles:
where and .
The terms θ and β are, to the first order, the coefficients of direct selection at the T and B loci, respectively. The θ term encapsulates the opposing forces of sexual selection on the display trait (at) and viability selection (st). The β term shows that the evolution of the male autonomy-enhancing trait hinges on the existence and strength of female preference for it (abr2) and that this is countered by viability selection (sb), the magnitude of coercion c, and interestingly, the effectiveness of autonomy-enhancing trait protection, γ. If γ is nonzero, having the autonomy-enhancing trait (B2 allele) hinders a male’s ability to coerce mates while B1 males remain free to coerce, and the more effective coercion is (the larger c is), the more B2 males stand to lose. Thus, the male autonomy-enhancing trait will only be favored with strong and prevalent enough female preference for it, so it is important to examine the factors contributing to the evolution of the R2 allele.
Looking at the expression for Δr2_approx, we can see that R2 can have positive evolution only via indirect selection mediated by its correlations with B2 and T2 ( and , respectively). We determined the values of all genetic correlations at QLE to the third order under the assumptions of weak selection, preferences, and coercion as follows:
The correlation between female remodeling preference and the male display trait, , is mediated by (and increasing in) at, ab, c, γ, as well as the variation at the B locus, demonstrating how the protectiveness of the autonomy-enhancing trait is key in producing an association between female remodeling preference and the attractive male display trait, which in turn can bolster the evolution of R2 in females. In our model, this is the force that allows female remodeling preference to persist at equilibrium if it is costly (sr > 0; Figures 2, 3, and 5).
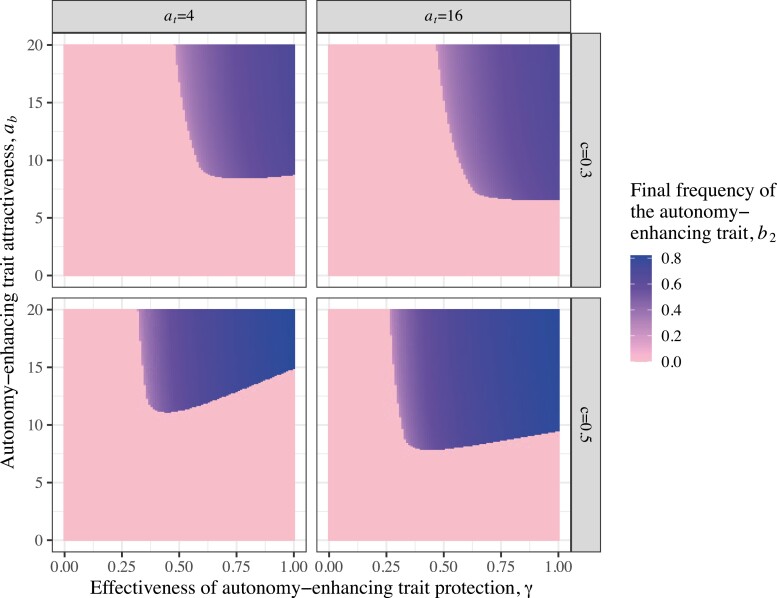
Figure 3.
Results of numerical simulations of the model for the persistence of the male autonomy-enhancing trait (B2) through parameter space. Color represents the final frequency of the B2 allele at equilibrium. Parameter sets shown here are: at = {4,16}, c = {0.3,0.5}, st = 0.1, sb = 0.1, u = 0.1, sr = 0.01, ab = 0–20 at increments of 0.1, and γ = 0–1 at increments of 0.01.
Figure 5.
The effect of viability cost (sr) to female remodeling preference on the persistence of the male autonomy-enhancing trait B2 at equilibrium through parameter space for ab and γ. In this example, at = 12, c = 0.4, st = 0.1, sb = 0.1, and u = 0.1.
The expression for is more complex: if γ = 0, arises solely due to Fisherian sexual selection, and is increasing in ab. Indeed, Fisherian sexual selection comprises the leading (first-order) term for in which γ and c do not appear. For nonzero values of γ, graphical inspection suggests various ways that protectiveness of the autonomy-enhancing trait can (weakly) influence the association between R2 and B2. For example, for a given level of coercion c, lower values of ab result in a monotonically decreasing relationship between and γ, whereas intermediate values yield a hump-shaped relationship, and finally larger values cause the relationship between and γ to be monotonically increasing (Supplementary Figure S4A). At low ab, increasing γ causes B2 males to lose mating opportunities relative to B1 males (as seen in the β term), but it additionally causes them to lose relatively more R2 females to their coercive competitors than they would otherwise, which disrupts the would-be correlation between R2 and B2. At higher ab, increasing γ instead augments because remodeling preference is strong enough that R2 females can take advantage of the indirect benefits of mating with B2 males and also of reliably mating with attractive T2 males, as indicated by the presence of at and t2 in the third-order term of the expression. By the same logic, increasing c for a given ab affects the system such that it causes larger γ to possibly disrupt rather than augment (Supplementary Figure S4B). In all, female preference for the male autonomy-enhancing trait (and therefore the autonomy-enhancing trait itself) will persist if preference is sufficiently strong and γ is optimally large (the magnitude of and large enough), such that these forces overcome the negative effect of γ on the coefficient of direct selection on B2 and any direct negative viability selection (sr) on R2.
We can also see, as suggested above, that the evolution of female preference for the male autonomy-enhancing trait is indirectly affected by the correlation between the male display trait and autonomy-enhancing trait () insofar as this term bolsters the initial evolution of B2 via indirect selection, to which c and γ both contribute positively.
Note that the expressions for the linkages, apart from , only appear at second or third order because they arise from compounded interactions among coercion, female preference, and protectiveness. In reality, we expect many of these parameters to possibly be large. Thus, we must be careful in our interpretations of the first-order allele frequency recursion approximations. They should be taken as a simplified idea of the main dynamics, though they are missing higher-order features that come into play when selection is strong.
Effect of key parameters on the evolution of the autonomy-enhancing trait
Naturally, stronger female preferences for attractive male display (at) and for the autonomy-enhancing trait itself (ab) generally promote the evolution of the autonomy-enhancing trait (evolutionary equilibria where b2 > 0; Figure 3). The effectiveness of the autonomy-enhancing trait’s protection from coercion (γ) allows the evolutionary persistence of B2 as long as it is large enough, but also, for intermediate values of ab, not too large (Figures 3 and 4). For a given level of ab, the γ parameter must be large enough that sufficient genetic correlation builds up between the female remodeling preference and male attractive display trait (DTR; Figures 2C and 4), allowing the co-evolutionary feedback loop to overcome the costs, including viability costs to R2 females and B2 males, and loss of male sexual success through the reduced efficiency of sexual coercion. It is possible, however, for γ to be so large that although R2 females reliably attain the benefits of mating with attractive T2 males (DTR is strong enough to overcome the costs of remodeling preference), males with the autonomy-enhancing trait do not receive enough mating benefits via the attraction of R2 females to outweigh the loss of coercive mating opportunities and the cost, sb, so B2 fails to increase and persist (Figure 4E). Because stronger female remodeling preferences give B2 males more of an advantage relative to B1 males, this effect is overcome in instances with higher values of ab (Figure 3). In cases where the autonomy-enhancing trait persists at equilibrium, higher γ also leads to stronger oscillations as described above because R1 females are even more likely to have the autonomy to successfully choose an attractive T2 male when they encounter a protective B2 male by chance (Figure 4D).
Figure 4.
For certain parameter sets, (such as shown here: ab = 15, at = 8, c = 0.6) the effectiveness conferred by the male autonomy-enhancing trait, (γ; having values at increments of 0.25 across panels A–E) can potentially be either too weak (A and B) or too strong (E) to permit the evolution of stable, non-zero frequencies of the female remodeling preference and the male autonomy-enhancing trait. The black dots are the initial frequency (b2 = r2 = 0.05). The lines’ colors show the frequency of the male attractive display trait (t2; initial freq. = 0.299). Other parameters are: st = 0.1, sb = 0.1, u = 0.1, sr = 0.01. See Supplementary Figure S5 for additional allele-frequency- and genetic-correlation-by-time data.
The viability cost of female remodeling preference (sr) also has an important effect on the evolution of the autonomy-enhancing trait. When sr = 0, the minimum threshold for γ disappears because there is no longer a cost to overcome, and indeed even an ineffective autonomy-enhancing trait (γ = 0) and remodeling preference can evolve to persist at equilibrium simply due to arbitrary Fisherian sexual selection alone (Figure 5; Kirkpatrick, 1982; Pomiankowski et al., 1991; Prum, 2010). When sr = 0, and γ > 0 (but not too large), B2 can evolve to fixation because there is no more “freeloading” effect: R2 females are able to outcompete R1 females even when B2 is at high frequency. Overall, higher sr means higher threshold values of γ and ab are required to build up enough indirect selection to support the stable evolution of female remodeling preference and therefore the persistence of the autonomy-enhancing trait (Figure 5).
Finally, somewhat counter-intuitively, a higher likelihood of successful coercion (larger c) shifts the minimum γ threshold lower for the same strength of remodeling preference (ab), and moreover allows for higher frequency of the male autonomy-enhancing trait at equilibrium where it evolves to persist (Figure 3). This is because higher likelihoods of successful coercion mean bigger rewards to females in terms of sexual autonomy for preferring the protective male trait—as we saw in the weak selection analysis, DTR is increasing in c. At the same time, higher c results in higher ab necessary to allow for the autonomy-enhancing trait to evolve because B2 males have more to lose in terms of coercive mating opportunities for a given level of protectiveness (γ). However, as indicated by the β expression in the weak selection approximation, c and γ both contribute (to leading order) to direct selection against B2 in the same way. Interestingly, this manifests such that lower c allows for higher possible magnitudes of γ for a given strength of female remodeling preference (Figure 3).
Selection for more protective autonomy-enhancing traits
Because of the additional indirect selection arising from increases in sexual autonomy, invading preferences for more effective autonomy-enhancing traits with higher γ will be subject to stronger positive selection than preferences of similar strength for less-protective autonomy-enhancing traits as long as the overall strength of female preference is great enough. We can demonstrate this by examining a version of the model in which R2 females prefer B2 males by a factor of (ab + 1), but instead of randomly visiting territories, R1 females have an equally strong preference (also parameterized as ab + 1) for non-autonomy-enhancing B1 males (see Supplementary Appendix S3 and Supplementary File S3). We set sr and sb to zero to compare invasion scenarios wherein the only difference is the value for γ. We start the population at r2 = 0.5 and b2 = 0.5 so that the effect of sexual selection will be equally strong on both the B1 and B2 alleles. We can then explore the influence of adding an autonomy-enhancing effect (γ) to B2 by asking whether the remodeling preference R2 increases as a result. Graphical and simulation analysis of the model recursion equations reveals that for a given value of ab, there is a threshold value of γ past which the remodeling preference will be favored, and the R2 allele will increase more with larger γ (Figure 6). For larger values of ab, the protectiveness threshold becomes lower until R2 is favored for all γ > 0 (Figures 6 and 7). Essentially, R2 will be favored as long as females have enough specificity of their preferences to allow the fitness advantage of mating more reliably with T2 males to overcome effect of the loss of coercive mating opportunities to B2 males. Therefore, given strong enough female preference, preferences for autonomy-enhancing traits with stronger γ will invade more readily than arbitrary, Fisherian preferences or preferences for comparatively less protective autonomy-enhancing traits. In addition, similar to the main model, lower likelihood of successful coercion (c) promotes the invasion of preferences for comparatively more protective autonomy-enhancing traits by shifting the threshold preference strength lower, especially for lower values of γ (Figure 7). Given these results and observations from the main model that, for a given level of ab, (a) higher coercion allows for more weakly protective traits to evolve and (b) lower coercion permits the evolution of more strongly protective traits (Figure 3), it is plausible that once conditions allow for autonomy-enhancing traits to persist in the population, incremental advances in coercion protection efficiency (resulting in lower effective coercion) may evolve into a “sexual autonomy snowball” that gradually dismantles sexual coercion.
Figure 6.
Examples of the change in frequency of the R2 allele across values for the attractiveness of the trait at the B locus (ab) and the effectiveness of the protection (γ) conferred by the male autonomy-enhancing trait, B2, for a version of the model where R2 females have a preference for protective autonomy-enhancing traits (B2 males), while R1 females have a preference of equal strength for nonprotective traits, and all else being equal (r2 = b2 = 0.5; sr = sb = 0; other parameters are: at = 5; c = 0.6, st = 0.1, u = 0.1). ΔR2 shown represents the change in the allele frequency in generation two (the first generation in which the R2 allele can evolve by indirect selection following the initial buildup of genetic correlations with the T and B loci).
Figure 7.
Analysis of the “invasion” of a female preference for protective autonomy-enhancing traits (the R2 allele) over a preference of equal strength for nonprotective traits, all else being equal (r2 = b2 = 0.5; sr = sb = 0), across values for the attractiveness of the trait at the B locus (ab), and the effectiveness of the protection (γ) conferred by the male autonomy-enhancing trait. Blue domains represent parameter sets where the R2 allele is initially favored (the R2 allele increases in generation two following the initial buildup of genetic correlations with the T and B loci). Red domains are where the R2 allele is not favored by selection. Parameter sets shown in this figure are: at = {4,16}, c = {0.3,0.5}, ab = 0 to 20 at increments of 0.1, γ = 0 to 1 at increments of 0.01, u = 0.1, and st = 0.1.
Discussion
We have demonstrated that a male “autonomy-enhancing” trait can plausibly evolve by female mate choice because it enhances female sexual autonomy, establishing a new evolutionary possibility for systems involving sexual conflict over the indirect benefits of mate choice. Rather than possibly engaging in an arms race by evolving direct resistance to male coercion, females may actively remodel male coercive capacity to expand their own sexual autonomy. This highlights the importance of allowing female reproductive traits greater complexity and biological realism than has been considered in some past theory on sexual conflict. As hypothesized, females that prefer to mate with males having the autonomy-enhancing trait can be at an advantage because they are protected from male coercion and thus more likely to mate with an attractive male (Figure 2D). The evolution of genetic correlation between the autonomy-enhancing trait, attractive display trait, and female “remodeling” preference for the autonomy-enhancing trait (Figure 2C) produces a co-evolutionary feedback loop that causes indirect selection on female remodeling preference. As long as the loss of coercive mating opportunities as a result of producing the protective autonomy-enhancing trait is not too large, this process enables the male autonomy-enhancing trait and the corresponding female preference to persist in the population because the force of sexual selection can overcome the costs to females and males in terms of viability (Figure 4). Our numerical simulations demonstrate that when faced with male coercion, females evolve preferences for male autonomy-enhancing traits that persist in the population through a significant portion of parameter space (Figure 3), increasing females’ ability to freely choose mates at equilibrium (Figures 2A and D and 4). We additionally showed that greater protectiveness conferred by the autonomy-enhancing trait (larger γ) often produces greater indirect selection on female remodeling preference, and thus autonomy-enhancing traits conferring more protection can often invade more readily than less protective traits, or nonprotective, arbitrarily attractive ones (Figures 6 and 7). Coupled with the observation that, for a given level of female remodeling preference, decreasing effective coercion allows for more strongly protective male traits persisting at equilibrium (Figure 3 and weak selection approximation), this suggests that the initial evolution of remodeling preferences and autonomy-enhancing traits can potentially lead to a sexual autonomy “snowball” effect of increasing protection. This dynamic may be offset, or more complex, under different cost structures, such as the cost of male autonomy-enhancing traits scaling with their protectiveness.
In the case where female preference for the autonomy-enhancing trait is costly to females (sr > 0), we revealed oscillatory dynamics unanticipated by previous verbal formulations (Prum, 2015, 2017). The protection from coercion conferred by male autonomy-enhancing traits acts as a sexually dimorphic “public good,” allowing females in the population to potentially attain the benefits of expanded sexual autonomy without paying the costs of seeking it out. Intriguingly, this suggests that when investigating the remodeling process in nature, researchers could expect to see potentially large variation in female preference for autonomy-enhancing traits. In our model, the possibility of freeloading produced by viability costs to female remodeling preference also prevents the male autonomy-enhancing trait from going to fixation since whenever it approaches high frequency, the advantage to females for preferring the autonomy-enhancing trait diminishes in favor of females who do not pay any costs (e.g., Figure 2A). Further, although higher protectiveness allows for higher equilibrium frequency of the male autonomy-enhancing trait, it also causes stronger oscillations (Figure 4). Thus, observed variation in male strategies is also potentially consistent with an ongoing remodeling process in nature. However, we assumed a fixed cost to female remodeling preference; a different assumption such as costs that depend on the frequency of the preferred male trait (e.g., Kokko et al., 2015) would likely dampen these dynamics. Of course, whether there are differential viability costs to females preferring protective autonomy-enhancing traits as opposed to other attractive aspects of male phenotype is an empirical question.
It is likely that the remodeling process is more complex and possibly less constrained than suggested by the necessarily simple model presented here. For tractability, we assumed that all males would attempt coercion if given the opportunity. In a more complex model in which coercion were allowed to evolve, in cases when the selective environment shifts toward favoring the evolution of the autonomy-enhancing trait, we expect that the coercion strategy would become disfavored (Snow et al., 2019). This dynamic could promote the evolution and persistence of the protective autonomy-enhancing trait beyond the parameter space we observed under our relatively more restrictive assumptions (Figures 2–4). Interestingly, given the counter-intuitive result that higher likelihood of successful coercion can frequently produce greater selection for remodeling (since there are higher rewards for females that prefer the autonomy-enhancing trait; Figure 3), it is also possible that even more complex or cyclical dynamics would arise as coercion evolved to be rarer.
However, we also assumed a fixed female preference for the attractive male display trait allele. A more complicated model with an evolving locus for female preference for display (in addition to female remodeling preference) could possibly involve initially weaker indirect benefits of sexual autonomy via remodeling if the starting condition featured the female preference allele at an intermediate frequency. This is because sexual selection on the display trait would be weaker, so it would be less beneficial for females to visit males with autonomy-enhancing traits.
Evolving preference for display and an additional locus allowing for evolving coercion (heritable variation in coercive ability among males) would introduce the possibility of indirect benefits of coercion in terms of the coercive ability of sons (Holland & Rice, 1998; Kokko, 2005), even in the absence male autonomy-enhancing traits. This could hinder or promote the evolution of remodeling preferences under certain conditions, but it is challenging to predict, particularly if coercive males must pay a viability cost relative to noncoercive males. Regardless, the model presented here captures the dynamics of remodeling within the domain in which it is possible, i.e., in situations where coercion is not so strong that it overwhelms any benefits of display.
Finally, we assumed differential viability costs to males producing the autonomy-enhancing trait, but one can imagine alternative scenarios. For example, if male coercive behavior is aided by a costly morphological weapon, “remodeled” males with smaller or less functional weapons will be less able to coerce but may not pay differential costs or may even have higher viability. This “deweaponization” scenario (Prum, 2017) would likely promote the evolution of the autonomy-enhancing trait beyond what we have presented. Additionally, as male coercion becomes constrained by remodeling and females gain sexual autonomy, this may open the door to advances in other areas of sexual conflict along “axes” not treated by our model, such as parental investment (Prum, 2017).
Although our results suggest that the remodeling process is a plausible alternative to direct resistance, and that it may be self-reinforcing once initiated, our model also demonstrates and clarifies several reasons why the evolutionary dismantling of sexual coercion via remodeling is not inevitable or ubiquitous. First, there must be phenotypic variation available in males that incidentally provides protection to females, and under costly remodeling preferences the threshold level of protectiveness (γ) can be quite high. Furthermore, females must be sufficiently choosy for remodeled males to attain a sufficient mating advantage to overcome the costs of having a protective phenotype in terms of lost coercive opportunities. The initiation of a remodeling process and its specific form will also be highly dependent on the ecological, developmental, physiological, and behavioral details of a given system. Remodeling can occur when males can subvert female preference and coerce females without giving them the opportunity to evaluate a display, while females can exercise agency by evolving new preferences that act in a manner independent of coercion’s influence. Thus, remodeling is likely to be more constrained in situations where females are able to evaluate males’ display to some extent from afar. Conversely, in the case of remodeling preferences that rely on evaluation of autonomy-enhancing traits from a safe distance, females must have a perceptual system that can operate on such a scale. For example, in waterfowl, females could hypothetically evolve a remodeling alternative to direct genital resistance by developing a way to detect males with reduced/de-weaponized genitals before choosing to associate with them. However, this would hinge on factors such as the distribution of food resources allowing for females to realistically avoid getting close to coercive males.
Expansion of sexual autonomy via remodeling as an alternative to direct resistance not only has been implicated previously in bowerbirds (Borgia, 1995; Prum, 2015, 2017), but also in the evolution of reduced body size and reduced canine tooth dimorphism in early hominids (Prum, 2017). Sexual coercion and infanticide are the social norm in many modern Old World monkeys and apes (Muller & Wrangham, 2009). Rather than possibly engaging in arms-race dynamics by evolving direct defenses or simply acquiescing to all mating attempts, early female hominins could have used their available behavioral agency to associate preferentially with less violent mates of similar size and with smaller canines. One way they could have accomplished this is through the formation of coalitions with other females or male “friends” (Hare et al., 2012; Palombit, 2009), or through choosing which groups to join during social group fission or individual dispersal (Palombit, 2014). Although our model demonstrates that it can be difficult to disentangle the effects of remodeling from typical sexual selection, comparative data are consistent with selection for traits associated with remodeled hominin coercive capacity, including the reduced body size dimorphism, canine size, and aggression, with increased prosociality that we see in modern humans and recent ancestors (Gordon, 2006; Hare, 2017; Hare & Tomasello, 2005; Hare et al., 2012; Lieberman, 2011; Prum, 2017; Wrangham et al., 2006). Our model results also suggest that perhaps a small percentage of females succeeding in selecting for remodeled male traits in this way could have been enough to provide benefits to all females and promote further advances in sexual autonomy.
Remodeling of sexual coercion may be an unappreciated factor shaping the evolution of many courtship displays, particularly in polygynous systems where a female must approach a male on his territory in order to experience a courtship display performance directed specifically at her, or where the evaluation of the display or ornament is similarly spatial- or time-scale dependent. For example, review of the lek display behaviors of male manakins (Pipridae; Bostwick, 2000; Prum, 1990) shows that multiple displays in numerous genera include elements with the male oriented backwards, or even approaching the female in backwards orientation. Display repertoires of the Pipra, Ceratopipra, and Machaeropterus clade include a homologous backward slide element where the male assumes a bowed, lowered-head posture, and approaches the female backwards on the display perch with tiny, rapid steps. Species in the Masius and Corapipo clade perform various displays on mossy fallen logs or buttress roots; these assorted bowing, bill-pointing, and wings-shiver displays and postures are inevitably oriented backwards toward the female. Species in the genus Chiroxiphia perform a cooperative “cartwheel” display with two or more males successively fluttering in a circle above a perch, and sliding along the perch after landing. The display is performed with the male flights oriented away from the perched female. In all of these cases, the displaying males’ orientation and directions of movement suggest the absence of coercive opportunity. Similarly, in birds of paradise, male Paradisaea rudolphi perform elaborate courtship displays upside-down, and Seleucidis melanoleuca and Ptiloris victoriae perch on broken trunks, to be approached by the female from below (Frith & Beehler, 1998). Both orientations obviate the possibility of forced copulation. Thus, it is likely that remodeling male coercive potential through female choice has had a broad impact on the specific form of courtship displays in polygynous species.
This is the first quantitative exploration of the remodeling mechanism; now that we have begun to shed light on this novel mechanism by which females may expand their sexual autonomy as a result of sexual conflict over indirect benefits, we hope that our model results can help identify previously unappreciated examples in nature. In particular, beyond polygynous species, our results reveal how commonly observed multi-stage and/or multimodal mate preferences (e.g., Candolin, 2003; Uy & Safran, 2013) may function as part of a remodeling process, with implications for female sexual autonomy and the evolutionary trajectory of male ornaments and behaviors.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1122492, the National Institutes of Health Predoctoral Training Program in Genetics under grant no. 5T32GM007499-38, the Yale University EEB Chair’s Fund, and the Agence Nationale de la Recherche under the Investments for the Future (Investissements d’Avenir) program (ANR-17-EUR-0010) to S.S.S., and the W.R. Coe Fund. We are grateful to M.R. Servedio and S.H. Alonzo for advice on earlier versions of this manuscript. Thanks also to L.U. Taylor and P. Bayer for discussion and to B. Evans and The Yale Center for Research Computing for support and access to high-performance computing resources.
Contributor Information
Samuel S Snow, Institute for Advanced Study in Toulouse, University of Toulouse 1 Capitole, Toulouse, Occitanie, France; Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA.
Richard O Prum, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA.
Data availability
Computer code is provided in the electronic supporting information. Data archival location: Dryad Digital Repository (datadryad.org) (https://doi.org/10.5061/dryad.t1g1jwt6s).
Authors contributions
S.S.S. and R.O.P conceived of the study. S.S.S. provided the models and analyses, and initial drafts of the manuscript. S.S.S. and R.O.P. developed the ideas and the manuscript.
Conflict of interest: The authors declare no conflict of interest.
References
- Arnqvist, G., & Rowe, L. (2002). Antagonistic coevolution between the sexes in a group of insects. Nature, 415(6873), 787–789. 10.1038/415787a [DOI] [PubMed] [Google Scholar]
- Arnqvist, G., & Rowe, L. (2005). Sexual conflict. Princeton University Press. [Google Scholar]
- Bank, C., Hermisson, J., & Kirkpatrick, M. (2012). Can reinforcement complete speciation? Evolution, 66(1), 229–239. 10.1111/j.1558-5646.2011.01423.x [DOI] [PubMed] [Google Scholar]
- Barton, N. H., & Turelli, M. (1991). Natural and sexual selection on many loci. Genetics, 127(1), 229–255. 10.1093/genetics/127.1.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgia, G. (1995). Why do bowerbirds build bowers? American Scientist, 83, 542–547. [Google Scholar]
- Borgia, G., & Presgraves, D. C. (1998). Coevolution of elaborated male display traits in the spotted bowerbird: An experimental test of the threat reduction hypothesis. Animal Behaviour, 56(5), 1121–1128. 10.1006/anbe.1998.0908 [DOI] [PubMed] [Google Scholar]
- Bostwick, K. S. (2000). Display behaviors, mechanical sounds, and evolutionary relationships of the club-winged manakin (Machaeropterus deliciosus). The Auk, 117(2), 465–478. 10.1093/auk/117.2.465 [DOI] [Google Scholar]
- Brennan, P. L. R., Clark, C. J., & Prum, R. O. (2010). Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proceedings of the Royal Society B: Biological Sciences, 277, 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, P. L. R., & Prum, R. O. (2012). The limits of sexual conflict in the narrow sense: New insights from waterfowl biology. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1600), 2324–2338. 10.1098/rstb.2011.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, P. L. R., Prum, R. O., McCracken, K. G., Sorenson, M. D., Wilson, R. E., & Birkhead, T. R. (2007). Coevolution of male and female genital morphology in waterfowl. PLoS One, 2(5), e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, R. C., & Griffith, S. C. (2010). Mate choice. In Westneat D. F., & Fox C. W. (Eds.), Evolutionary behavioral ecology (pp. 416–433). Oxford University Press. [Google Scholar]
- Candolin, U. (2003). The use of multiple cues in mate choice. Biological Reviews of the Cambridge Philosophical Society, 78(4), 575–595. 10.1017/s1464793103006158 [DOI] [PubMed] [Google Scholar]
- Cordero, C., & Eberhard, W. G. (2003). Female choice of sexually antagonistic male adaptations: A critical review of some current research. Journal of Evolutionary Biology, 16(1), 1–6. 10.1046/j.1420-9101.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Dhole, S., Stern, C. A., & Servedio, M. R. (2018). Direct detection of male quality can facilitate the evolution of female choosiness and indicators of good genes: Evolution across a continuum of indicator mechanisms. Evolution, 72(4), 770–784. 10.1111/evo.13466 [DOI] [PubMed] [Google Scholar]
- Eberhard, W. G. (2005). Evolutionary conflicts of interest: Are female sexual decisions different?. The American Naturalist, 165(Suppl 5), S19–S25. 10.1086/429348 [DOI] [PubMed] [Google Scholar]
- Frith, C. B., & Beehler, B. M. (1998). The birds of paradise: Paradisaeidae. Oxford University Press. [Google Scholar]
- Gavrilets, S., Arnqvist, G., & Friberg, U. (2001). The evolution of female mate choice by sexual conflict. Proceedings of the Royal Society B: Biological Sciences, 268, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets, S., & Hayashi, T. I. (2006). The dynamics of two- and three-way sexual conflicts over mating. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1466), 345–354. 10.1098/rstb.2005.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, A. D. (2006). Scaling of size and dimorphism in primates I: Microevolution. International Journal of Primatology, 27, 27–61. 10.1007/s10764-005-9003-2 [DOI] [Google Scholar]
- Härdling, R., & Smith, H. G. (2005). Antagonistic coevolution under sexual conflict. Evolutionary Ecology, 19(2), 137–150. 10.1007/s10682-004-7917-3 [DOI] [Google Scholar]
- Hare, B. (2017). Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annual Review of Psychology, 68, 155–186. 10.1146/annurev-psych-010416-044201 [DOI] [PubMed] [Google Scholar]
- Hare, B., & Tomasello, M. (2005). Human-like social skills in dogs? Trends in Cognitive Sciences, 9(9), 439–444. 10.1016/j.tics.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Hare, B., Wobber, V., & Wrangham, R. (2012). The self-domestication hypothesis: Evolution of bonobo psychology is due to selection against aggression. Animal Behaviour, 83(3), 573–585. 10.1016/j.anbehav.2011.12.007 [DOI] [Google Scholar]
- Holland, B., & Rice, W. R. (1998). Perspective: Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution, 52(1), 1–7. 10.1111/j.1558-5646.1998.tb05132.x [DOI] [PubMed] [Google Scholar]
- Houle, D. (1992). Comparing evolvability and variability of quantitative traits. Genetics, 130, 195–204. 10.1093/genetics/130.1.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa, Y., & Pomiankowski, A. (1994). The evolution of mate preferences for multiple sexual ornaments. Evolution, 48(3), 853–867. 10.1111/j.1558-5646.1994.tb01367.x [DOI] [PubMed] [Google Scholar]
- Iwasa, Y., & Pomiankowski, A. (1999). Good parent and good genes models of handicap evolution. Journal of Theoretical Biology, 200(1), 97–109. 10.1006/jtbi.1999.0979 [DOI] [PubMed] [Google Scholar]
- Iwasa, Y., Pomiankowski, A., & Nee, S. (1991). The evolution of costly mate preferences II. The ‘Handicap’ principle. Evolution, 45(6), 1431–1442. 10.1111/j.1558-5646.1991.tb02646.x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, M. (1982). Sexual selection and the evolution of female choice. Evolution, 36(1), 1–12. 10.1111/j.1558-5646.1982.tb05003.x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, M., & Barton, N. H. (1997). The strength of indirect selection on female mating preferences. Proceedings of the National Academy of Sciences of the United States of America, 94(4), 1282–1286. 10.1073/pnas.94.4.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko, H. (2005). Treat ‘em mean, keep ‘em (sometimes) keen: Evolution of female preferences for dominant and coercive males. Evolutionary Ecology, 19(2), 123–135. 10.1007/s10682-004-7919-1 [DOI] [Google Scholar]
- Kokko, H., Booksmythe, I., & Jennions, M. D. (2015). Mate-sampling costs and sexy sons. Journal of Evolutionary Biology, 28(1), 259–266. 10.1111/jeb.12532 [DOI] [PubMed] [Google Scholar]
- Lessells, C. M. (2006). The evolutionary outcome of sexual conflict. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1466), 301–317. 10.1098/rstb.2005.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, D. (2011). The evolution of the human head. Harvard University Press. [Google Scholar]
- Morrow, E. H., & Arnqvist, G. (2003). Costly traumatic insemination and a female counter-adaptation in bed bugs. Proceedings of the Royal Society B: Biological Sciences, 270, 2377–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, M. N., & Wrangham, R. W. (2009). Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females. Harvard University Press. [Google Scholar]
- Palombit, R. A. (2009). “Friendship” with males: A female counterstrategy to infanticide in chacma baboons of the Okavango Delta. In Muller M. N. & Wrangham R. W. (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 377–409). Harvard University Press. [Google Scholar]
- Palombit, R. A. (2014). Infanticide as sexual conflict: Coevolution of male strategies and female counterstrategies. In Rice W. R. & Gavrilets S. (Eds.), The genetics and biology of sexual conflict (pp. 199–228). Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, G. A. (2006). Sexual conflict over mating and fertilization: An overview. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 235–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli, G. L., Uy, J. A. C., Walsh, G., & Borgia, G. (2002). Male displays adjusted to female’s response. Nature, 415(6869), 279–280. 10.1038/415279a [DOI] [PubMed] [Google Scholar]
- Pomiankowski, A., & Iwasa, Y. (1993). Evolution of multiple sexual preferences by Fisher’s runaway process of sexual selection. Proceedings of the Royal Society of London B, 253, 173–181. [Google Scholar]
- Pomiankowski, A., Iwasa, Y., & Nee, S. (1991). The evolution of costly mate preferences. I. Fisher and biased mutation. Evolution, 45(6), 1422–1430. 10.1111/j.1558-5646.1991.tb02645.x [DOI] [PubMed] [Google Scholar]
- Prum, R. O. (1990). Phylogenetic analysis of the evolution of display behavior in the neotropical manakins (Aves: Pipridae). Ethology, 84(3), 202–231. [Google Scholar]
- Prum, R. O. (2010). The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: Implications for meaning, honesty, and design in intersexual signals. Evolution, 64(11), 3085–3100. 10.1111/j.1558-5646.2010.01054.x [DOI] [PubMed] [Google Scholar]
- Prum, R. O. (2015). The role of sexual autonomy in evolution by mate choice. In Parker G. A., Pizzari T., & Hoquet T. (Eds.), Current perspectives on sexual selection, history, philosophy and theory of the life sciences (pp. 237–262). Springer. [Google Scholar]
- Prum, R. O. (2017). The evolution of beauty: How Darwin’s forgotten theory of mate choice shapes the animal world—and us. Doubleday. [Google Scholar]
- Rosenthal, G. G., & Servedio, M. R. (1999). Chase-away sexual selection: Resistance to “Resistance.” Evolution, 53(1), 296–299. 10.1111/j.1558-5646.1999.tb05356.x [DOI] [PubMed] [Google Scholar]
- Rowe, L., & Arnqvist, G. (2002). Sexually antagonistic coevolution in a mating system: Combining experimental and comparative approaches to address evolutionary processes. Evolution, 56(4), 754–767. 10.1111/j.0014-3820.2002.tb01386.x [DOI] [PubMed] [Google Scholar]
- Rowe, L., Cameron, E., & Day, T. (2003). Detecting sexually antagonistic coevolution with population crosses. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1528), 2009–2016. 10.1098/rspb.2003.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, L., Cameron, E., & Day, T. (2005). Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. American Naturalist, 165(S5), S5–S18. 10.1086/429395 [DOI] [PubMed] [Google Scholar]
- Rowe, L., & Houle, D. (1996). The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society of London. Series B: Biological Sciences, 263, 1415–1421. [Google Scholar]
- Snow, S. S., Alonzo, S. H., Servedio, M. R., & Prum, R. O. (2019). Female resistance to sexual coercion can evolve to preserve the indirect benefits of mate choice. Journal of Evolutionary Biology, 32(6), 545–558. 10.1111/jeb.13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzyman, S. J., Iwasa, Y., & Pomiankowski, A. (2014). The handicap process favors exaggerated, rather than reduced, sexual ornaments. Evolution, 68(9), 2534–2549. 10.1111/evo.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy, J. A. C., & Safran, R. J. (2013). Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behavioral Ecology and Sociobiology, 67(9), 1499–1511. 10.1007/s00265-013-1492-y [DOI] [Google Scholar]
- Veller, C., Muralidhar, P., & Haig, D. (2020). On the logic of Fisherian sexual selection. Evolution, 74(7), 1234–1245. 10.1111/evo.13944 [DOI] [PubMed] [Google Scholar]
- Wolfram, S. (2016). Mathematica. Wolfram Research. [Google Scholar]
- Wong, B. B. M., & Candolin, U. (2005). How is female mate choice affected by male competition? Biological Reviews of the Cambridge Philosophical Society, 80, 559–571. [DOI] [PubMed] [Google Scholar]
- Wrangham, R. W., Wilson, M. L., & Muller, M. N. (2006). Comparative rates of violence in chimpanzees and humans. Primates, 47(1), 14–26. 10.1007/s10329-005-0140-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Computer code is provided in the electronic supporting information. Data archival location: Dryad Digital Repository (datadryad.org) (https://doi.org/10.5061/dryad.t1g1jwt6s).