Abstract
Heavy drinking can induce early-onset dementia and increase the likelihood of the progression and severity of Alzheimer’s Disease and related dementias (ADRD). Recently, we showed that alcohol-drinking by mature adult C57BL/6J mice induces more signs of cognitive impairment in females versus males without worsening age-related cognitive decline in aged mice. Here, we immunoblotted for glutamate receptors and protein markers of ADRD-related neuropathology within the hippocampus and prefrontal cortex (PFC) of these mice after three weeks of alcohol withdrawal to determine protein correlates of alcohol-induced cognitive decline. Irrespective of alcohol history, age-related changes in protein expression included a male-specific decline in hippocampal glutamate receptors and an increase in the expression of a beta-site amyloid precursor protein cleaving enzyme (BACE) isoform in the PFC as well as a sex-independent increase in hippocampal amyloid precursor protein. Alcohol-drinking was associated with altered expression of glutamate receptors in the hippocampus in a sex-dependent manner, while all glutamate receptor proteins exhibited significant alcohol-related increases in the PFC of both sexes. Expression of BACE isoforms and phosphorylated tau varied in the PFC and hippocampus based on age, sex, and drinking history. The results of this study indicate that withdrawal from a history of alcohol-drinking during later life induces sex- and age-selective effects on glutamate receptor expression and protein markers of ADRD-related neuropathology within the hippocampus and PFC of potential relevance to the etiology, treatment and prevention of alcohol-induced dementia and Alzheimer’s Disease.
Keywords: Binge-drinking, Alcohol, Aging, AMPA receptor, NMDA receptor, Group 1 metabotropic glutamate receptor, Phosphorylated tau, Alzheimer’s disease and related dementias
1. Introduction
Heavy alcohol drinking (> 14 drinks/week) significantly increases the likelihood of developing dementia [1–4] and reduces the age of dementia-onset in both humans [5] and laboratory rodents [e.g., 6–8]. In fact, alcohol use disorders (AUDs) account for approximately 60% of early-onset dementia cases and thus are the strongest modifiable risk factor for this condition [9]. Moreover, cognitive decline is associated even with light-to-moderate alcohol consumption (< 8 drinks/week) [e.g., 10]. Women are nearly twice as likely to develop Alzheimer’s Disease (AD) and related dementias (ADRDs) than men [11,12] and are also more vulnerable to other alcohol-induced pathologies, including cancer, heart and liver disease [c.f., 13]. Thus, it is particularly concerning that the prevalence of AUD in women has increased by 84% over the past 10 years versus a 35% increase in men [14]. Adding to this concern, a published survey of individual drinking patterns during the early part of the coronavirus (COVID-19) pandemic indicated a 41% increase in heavy drinking reported by mature adult women (those between the ages of 30–59), over their pre-pandemic baseline [15]. Finally, more than half of the binge-drinks consumed within the United States are consumed by those aged 35+, with individuals aged 65+ reporting more frequent binge-drinking than younger age groups [16]. Thus, is it imperative that we investigate how biological sex interacts with a history of heavy drinking during mature adulthood to impact the brain and induce cognitive dysfunction.
Animal models of heavy alcohol-drinking or binge-drinking provide a powerful tool with which to study simultaneously the pathobiology of excessive drinking and its behavioral correlates, without the influence of variables that confound the interpretation of cause-effect relations in humans with AUD [17,18]. In this regard, a number of studies have employed transgenic mouse models of Alzheimer’s Disease (AD) to demonstrate that alcohol can accelerate both cognitive impairment and AD-related neuropathology [e.g., 5,7,19–21]. Aligning with these data, studies of wild-type, inbred, C57BL/6 (B6) mice also indicate that alcohol can induce microglial activation [22] and upregulate the expression of ADRD-related genes in brain [e.g., 7,23,24]. In terms of behavior, a history of binge-drinking by adolescent B6 mice is reported to induce signs of cognitive impairment when assessed in later adulthood [25] and we recently showed that binge-drinking by B6 mice during mature adulthood (i.e., 6 months of age; [26]) accelerates cognitive decline to a level comparable to aged (18 month-old) animals [8]. Although we did not detect a sex difference in age-related cognitive decline in our recent study, in two replicate studies of B6 mice, mature adult female alcohol-drinking mice exhibited more signs of cognitive impairment than their male counterparts [8], arguing that mature adult females are more vulnerable than males to an alcohol-induced acceleration of cognitive decline.
Here, we begin to probe the potential neurobiological bases for the Age by Alcohol by Sex interaction observed in our recent behavioral study of B6 mice [8]. Based on the evidence that alcohol can induce ADRD-related gene expression in B6 mice [7,23,24], we evaluated changes in the expression of certain ADRD-related proteins within the hippocampus and prefrontal cortex (PFC) of alcohol-drinking animals, two regions that are critical for a number of cognitive functions and known to be affected in ADRDs. Two major pathological hallmarks of AD are the deposition of amyloid plaques mainly composed of the β-amyloid peptide (Aβ), and neurofibrillary tangles comprised of hyperphosphorylated microtubule-associated protein tau [c.f., 27,28]. The deposition of Aβ occurs with normal aging as diffuse plaques [29,30] and the activity of β-site amyloid precursor protein-cleaving enzyme (BACE) has been implicated in both age- [30] and ADRD-related amyloid plaque formation [31,32]. Thus, we determined whether or not a history of alcohol-drinking increased the expression of BACE isoforms and amyloid precursor protein (APP). Tau phosphorylation at Thr217 is considered a highly specific biomarker of AD in both preclinical and advanced stages of AD [33,34], while phosphorylation at Ser396 is considered to be one of the earliest events in ADRD neuropathology [35]; thus, we also immunoblotted for both of these forms of phosphorylated tau. Based on the decades of evidence implicating glutamate anomalies in the pathogenesis of ADRD [for recent reviews, 36–41] and our results demonstrating that a month-long history of binge-drinking is sufficient to induce a hyper-glutamate state throughout the mesocorticolimbic system of younger adult (2–3 month-old) B6 mice [e.g., 42–47], we additionally probed for changes in the expression of Group 1 metabotropic glutamate receptors (mGlu1, mGlu5) and subunits of ionotropic glutamate receptors(β-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors) within these regions. We hypothesized that both age-related and alcohol-induced cognitive impairment would be associated with anomalies in hippocampal and PFC protein expression, with mature adult female binge-drinking mice exhibiting a protein profile comparable to that of aged mice.
2. Methods
2.1. Subjects, alcohol-drinking and behavioral testing procedures
The present study examined hippocampal and PFC tissue obtained from the mice described in Study 3 of our recent behavioral report [8] and the housing, binge-drinking and behavioral testing procedures are detailed therein. In brief, both male and female 5 month-old and 17 month-old mice were purchased from Jackson Laboratories (Sacramento, CA) and housed in same-sex and same experimental condition groups of 3–4 under standard ventilated caging and reverse cycle housing (lights off: 1100 h) conditions for 1 month prior to commencing binge-alcohol or water-drinking procedures. Binge-drinking consisted of 30 consecutive days of concurrent access to unadulterated 10, 20 and 40% (v/v) ethanol in tap water for 2 h/day, starting at 3 h after lights out, and was conducted in individual drinking cages located on a free-standing rack in the holding room. As discussed in detail in Jimenez Chavez et al. [8], the large scale of this study precluded simultaneous single-housing of the water control mice. As such, water control mice were handled daily by being placed, with their cage mates, into a novel drinking cage on same free-standing rack as the binge-drinking mice and mice were presented with a single sipper tube containing water, as conducted previously by our group [48–50].
The procedural time-line of behavioral testing of the mice prior to tissue collection is provided in Fig. 1A. As detailed previously [8], on the day following the 30-day drinking period, mice were first subjected to a 1-day behavioral test battery for negative affect with males and females tested on separate days. Then, all mice underwent a 5-day Morris water maze procedure, followed by a 14-day radial arm maze procedure. Tissue collection occurred two days following completion of radial arm maze testing, approximately 3 weeks following cessation of alcohol/water-drinking.
Fig. 1. Summary of procedural details and behavioral results.
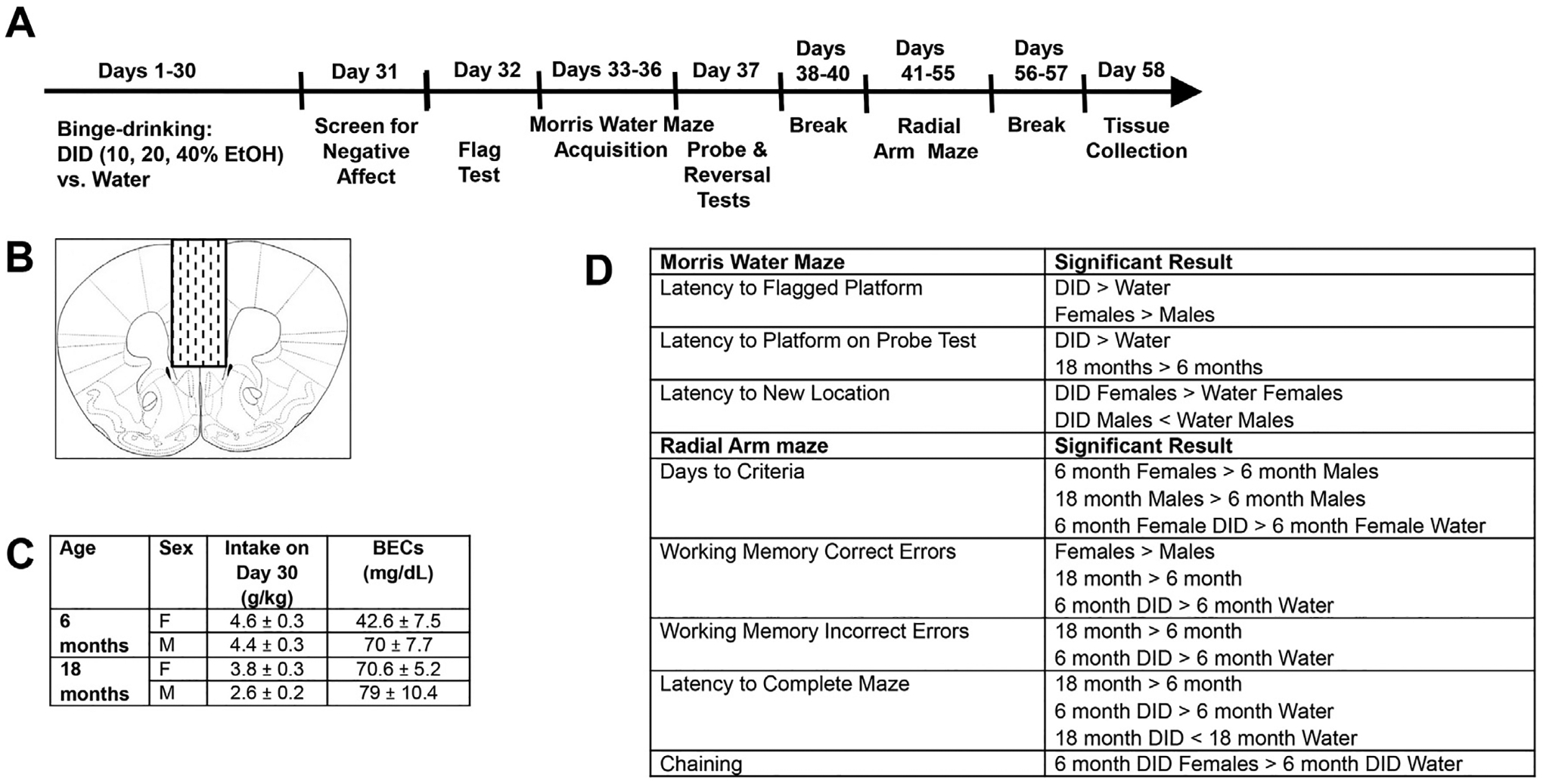
(A) Procedural time-line of the behavioral study published in Jimenez Chavez et al. (2022), in which male and female 6 month- and 18-month-old mice underwent 1 month of alcohol-drinking under Drinking-in-the-Dark (DID) procedures or 1 month of water-drinking (Water) and then were tested for anxiety, followed by cognitive function in the Morris water and radial arm mazes. (B) Cartoon depicting the gross dissection of the prefrontal cortex conducted 2–3 days following completion of behavioral testing. (C) Table summarizing the alcohol intakes and resultant BECs attained on the last day of the 30-day drinking period. (D) Table summarizing the significant effects and interactions from the Morris water maze (top) and radial arm maze (bottom). See Ref 8 for details.
Food and water were available ad libitum except during the 2-h drinking session or behavioral testing. Throughout, cages were lined with sawdust bedding, nesting materials and a plastic enrichment device in accordance with vivarium protocols. All experimental procedures were in compliance with The Guide for the Care and Use of Laboratory Animals (2014) and approved by the Institutional Animal Care and Use Committee of the University of California, Santa Barbara.
2.2. Tissue dissection and immunoblotting
To determine the protein correlates of cognitive dysfunction, mice were decapitated approximately 48 h following the last radial arm maze session. Brains were extracted and cooled on ice, then the forebrain was sectioned in 1 mm-thick coronal slices at the level of the PFC and striatum. Using blunt forceps, the PFC was dissected out as illustrated in Fig. 1B and then the remainder of the brain was sectioned along the sagittal plane and the hippocampus removed from each hemisphere.
Immunoblotting was performed on whole cell homogenates and the procedures for preparing the tissue homogenates, the immunofluorescent detection of protein expression and protein quantification were similar to those employed in recent studies of glutamate receptor expression [51,52] and ADRD-related pathology [e.g., 28–30,35,36]. The following rabbit primary antibodies were used: mGlu5 (metabotropic glutamate receptor 5; 1:1000 dilution; Millipore; AB5675), GluN1 (NMDA receptor subunit 1; 1:250 dilution; Cell Signaling Technology; 5704S), GluA1 (AMPA receptor subunit 1; 1:200 dilution; Millipore; AB1504), APP (1:1000 dilution; Millipore-Sigma; 07–667), tau (1:1000 dilution; Sigma-Aldrich, SAB4501822), p(Ser396)-tau (1:500 dilution; Abcam; ab109390), and p(Thr217)-tau (1:500 dilution; Invitrogen, 44–744). The following mouse primary antibodies were also employed: mGlu1 (metabotropic glutamate receptor 1; 1:1000 dilution; BD Biosciences; 610965), GluN2b (NMDA subunit 2b; 1:500 dilution; Invitrogen; MA1–2014), GluA2 (AMPA receptor subunit 2; 1:500 dilution; Synaptic Systems; 182 111), and BACE (1:250 dilution; Millipore Sigma; MAB5308). Note that as reported in our earlier study [52], our selected mGlu1 antibody failed to reliably detect the dimer form of the receptor (see Suppl. Figure 1A,A’). As such, only the monomer form of this receptor is reported herein. Note that we attempted to immunoblot for the monomer and multimer forms of Aβ1–42 in both hippocampal and PFC tissue using a commercially available rabbit antibody (dilutions ranging from 1:200 to 1:1000 dilution; Millipore Sigma-Aldrich; A9810), we were unsuccessful at detecting the predicted monomer band at 4 kD, nor any reliable multimer bands below approximately 20 kD (see Suppl. Figure 1B). Thus, we cannot report on any alcohol-induced accumulation of Aβ1–42, in relation to BACE or APP expression. Glyceraldehyde3-phosphate dehydrogenase (GAPDH) or calnexin expression was employed to control for protein loading and transfer using a rabbit or mouse primary anti-calnexin antibody (for rabbit primary, 1:1000 dilution; Enzo Life Sciences; ADI-SPA-860; for mouse primary, 1:500 dilution; Enzo Life Sciences; ADI-SPA-860-D) or a mouse anti-GAPDH primary (1:1000 dilution; ThermoFisher Scientific; MA5–15738-D680). However, technical issues occurred when initially immunoblotting the PFC tissue from the female mice for glutamate receptor-related proteins and we were unsuccessful at detecting either GAPDH or calnexin, despite robust expression for glutamate receptor proteins. Thus, for our assays of glutamate receptor proteins only, we opted to employ a mouse anti-CaMKIIα antibody (calcium/calmodulin-dependent kinase IIα; 1:1000 dilution; Millipore; 05–532) as a loading control for this tissue as we failed to detect any changes in CaMKIIα expression in either the hippocampus or PFC tissue from males (see Supplemental Fig. 1C), or in recent studies of cocaine-experienced rats [52]. Following primary antibody incubation, the membranes were washed with phosphate-buffered saline with tween (PBST), incubated in either a goat anti-rabbit IRDye 800CW secondary antibody (1:10,000 dilution; Li-Cor; 925–3221) or a goat anti-mouse IRDye 680RD secondary antibody (1:10,000 dilution; Li-Cor; 925–68070), and imaged on an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). Raw values for each band were measured, and first normalized to their corresponding calnexin/GAPDH/CaMKIIα signal and then to the average value of the 6-month water control (i.e., 6 month-Water).
Due to the large number of experimental groups in this study, immunoblotting was conducted on the tissue from males and females separately as two independent experiments. While this approach precluded any direct determination of sex differences in protein expression, it enabled a direct examination of the effects of alcohol and age, as well as their interaction, within each sex of relevance to the interpretation of our sex- and age-selective behavioral results [see Ref [8]].
2.3. Statistical analyses
The immunoblotting data were expressed as a percent of the 6 month-Water controls on each gel and then analyzed using two-tailed univariate Alcohol X Age analyses of variance (ANOVAs), separately for each sex. For all analyses, significant interactions were followed up with t-tests, when appropriate, with significance defined as p < 0.05. Pearson’s correlational analysis was conducted to determine the relationship between total alcohol intake and blood ethanol concentration.
3. Results
3.1. Summary of prior behavioral results
The behavioral results from this study are detailed in Experiment 3 of Jimenez Chavez et al. [8] and are briefly summarized here. On average, over the 30-day course of drinking, female mice consumed more alcohol than males, and the 6 month-old mice consumed more alcohol than the 18 month-old animals. On the 30th and last day of drinking, blood ethanol concentration (BEC) determinations indicated both an age- and sex-difference that reflected lower BECs in females versus males and in the 6 month-old versus 18 month-old mice (Fig. 1C). As summarized in Fig. 1C, the BECs of the 18 month-old mice and the 6 month-old males were within error of the 80 mg/dl criterion for binge-drinking, while that of the 6 month-old females was inexplicably half that exhibited by the other groups, despite comparable intake to their 6 month-old male counterparts. As such, there was no significant correlation between the total alcohol intake of the mice on Day 30 and the BECs attained at the end of the 2-h drinking period (r=−0.133, p = 0.368, n = 48).
The key significant findings from the cognitive testing of these mice are summarized in Fig. 1D. As highlighted, both age and drinking history negatively impacted a number of measures under both Morris water and radial arm maze procedures, with a few overall sex differences also observed. We also detected several interactions between sex, binge-drinking history and/or age of drinking onset that prompted this follow-up biochemical study. For one, alcohol-drinking (Drinking-in-the-dark or DID) females of both ages took longer than their water controls to locate a repositioned platform during reversal learning in the Morris water maze; males showed the opposite pattern of effect. Second, 6 month-old DID mice of both sexes committed more working memory correct and incorrect errors (respectively, the number of re-entries into an arm that formerly contained a platform and the number of re-entries into an arm that never contained a platform). Consequently, 6 month-old DID mice took longer to complete the radial arm maze than the 6 month-old water-consuming (Water) controls; no alcohol-induced working memory impairments were detected in the 18 month-old mice. As aged mice successfully navigated the radial arm maze, the lack of an alcohol effect in the 18 month-old mice cannot be readily attributed to a floor effect on behavior. Together, these radial arm maze results suggest that mature adult mice are more prone than aged mice to alcohol-induced working memory dysfunction.
Finally, as evidence that mature adult females may be more sensitive than males to alcohol-induced cognitive impairment, the 6 month-old female mice required significantly more days to reach the criteria for radial arm maze acquisition and engaged in significantly more chaining behavior (a non-memory strategy in which mice simply visit adjacent arms to navigate the radial arm maze) than their female water controls, with no alcohol-water difference detected in older mice or in 6 month-old males. Notably, these latter results indicating more signs of alcohol-induced cognitive decline in mature adult females versus males aligns with findings from the two other studies presented in our prior report [8], prompting the current examination of sex by age interactions in the effects of binge-drinking on protein expression.
3.2. Sex differences in the effects of age and alcohol-drinking on glutamate receptor expression within hippocampus
Immunoblotting for AMPA receptor subunits in the hippocampus of male mice indicated significant Age effects for both GluA1 (Fig. 2A) [F(1,46)=9.098, p = 0.004; other p’s>0.254] and GluA2 (Fig. 2B) [F(1,46)=6.838, p = 0.012; other p’s>0.168], irrespective of the prior alcohol-drinking history of the animals (Alcohol effects and interactions, p’s>0.168). Similarly, we also detected an age-related reduction in the hippocampal expression of the obligatory GluN1 NMDA receptor subunit (Fig. 2C) [F(1,47)=4.428, p = 0.041; other p’s>0.500]. Although both the NMDA receptor subunit GluN2b (Fig. 2D) and the monomer form of mGlu1 (Fig. 2E) also appeared to be lower in 18 versus 6 month-old mice, these differences were not statistically significant (Age effects: for GluN2b, p = 0.088; for mGlu1, p = 0.141) nor were the apparent interactions in protein expression (Age X Alcohol: for GluN2b, p = 0.095; for mGlu1 monomer, p = 0.104). However, both hippocampal GluN2b (Fig. 2D) and mGlu1 (Fig. 2E) exhibited an alcohol-induced increase in expression, as did the dimer form of mGlu5 (Fig. 2F) [Alcohol effects: for GluN2b, F(1,46)=4.464, p = 0.040; for mGlu1, F(1,47)=6.755, p = 0.013; for mGlu5 dimer, F(1,46)=6.679, p = 0.013; other p’s>0.243]. No group differences were detected for the hippocampal expression of the mGlu5 monomer (Fig. 2G; all p’s>0.230).
Fig. 2. Expression of glutamate receptor-related proteins within the hippocampus.
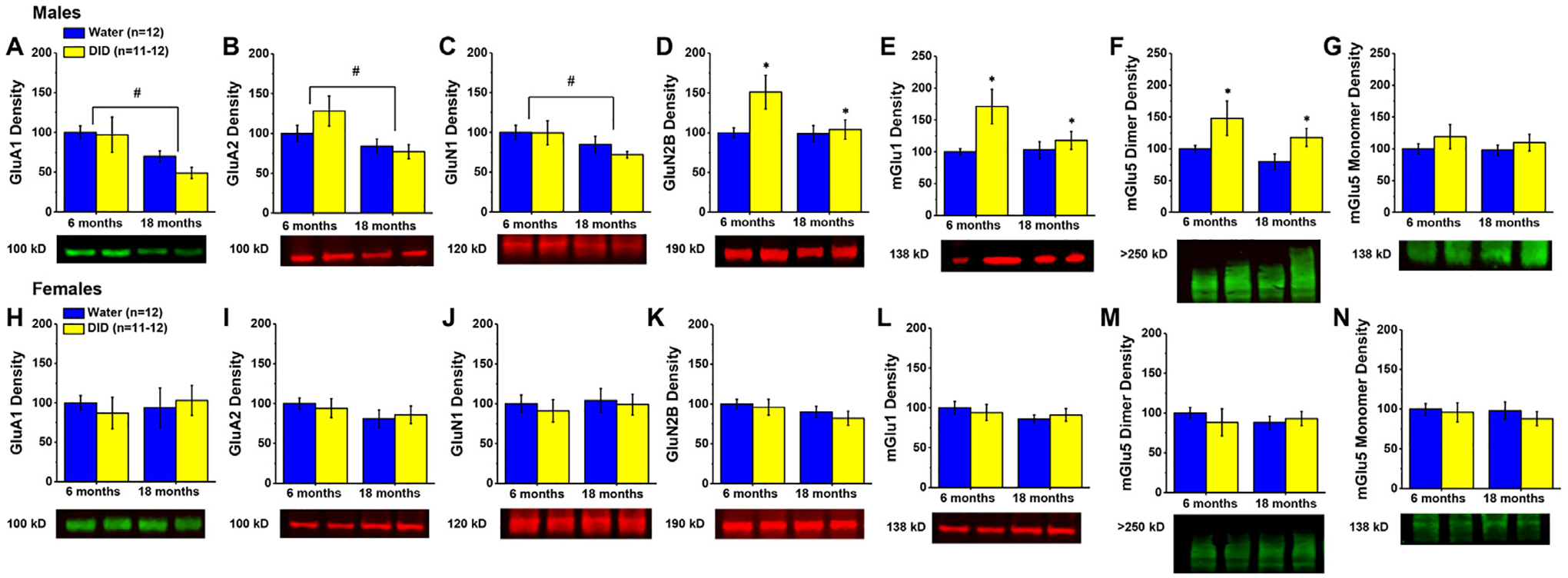
Immunoblotting conducted on the hippocampus of males indicated lower expression of (A) GluA1, (B) GluA2 and (C) GluN1 in 18 month- versus 6 month-old mice. Irrespective of age, male mice with a history of alcohol-drinking (DID) exhibited higher levels of (D) GluN2b; (E) mGlu1 and (F) the mGlu5 dimer versus water-drinking controls (Water). (G) No age or alcohol effects were detected for the mGlu5 monomer in male mice. (H–N) Immunoblotting conducted in female hippocampus did not detect any age- or alcohol-related changes in protein expression. Data are normalized to the average protein densities of the 6 month-old water controls for either sex and represented as means ± SEMs (n = 11–12). * p<0.05, main effect of alcohol-drinking; #p<0.05, main effect of age.
In striking contrast to the males, we detected no group differences in the hippocampal expression of glutamate receptor-related proteins in our female mice (Fig. 2H–N; Age X Alcohol ANOVAs: for GluA1, all p’s>0.550, for GluA2, all p’s>0.207; for GluN2b, all p’s>0.164; for mGlu1, all p’s>0.358; for mGlu5 monomer, all p’s>0.480; for mGlu5 dimer, all p’s>0.446). Thus, the hippocampus of male mice appears to be more alcohol- and age-sensitive than that of females.
3.3. Sex by age interactions in the effects of alcohol-drinking on ADRD-related proteins within hippocampus
Given the many studies linking alcohol-drinking to accelerated cognitive decline and the development of dementia, we next examined the expression of proteins associated with ADRD. Specifically, we examined the expression of amyloid precursor protein (APP), beta-site APP-cleaving enzyme (BACE), total tau, and phosphorylated tau at serine 396 (Ser396) and threonine 217 (Thr217). Many BACE antibodies (including the one employed in this study) detect multiple bands, likely reflecting the presence of multiple isoforms of this enzyme. Bands at 56 kD and 70 kD were selected for analysis.
3.3.1. Amyloid-related proteins
18 month-old males exhibited higher levels of APP within the hippocampus, with no evidence of any alcohol effect on protein expression (Fig. 3A) [Age effect: F(1,46)=8.480, p = 0.006; other p’s>0.142]. Although no group differences were apparent for hippocampal BACE 70 kD levels (Fig. 3B; Alcohol X Age ANOVA, all p’s>0.168), alcohol-drinking history increased BACE 56 kD expression selectively in 6 month-old males (Fig. 3C) [Alcohol X Age interaction: F(1,47)=6.198, p = 0.017; for 6 month-olds, t(22)=2.834, p = 0.010; for 18 month-olds,p = 0.556].
Fig. 3. Expression of neuropathology-related proteins within the hippocampus.
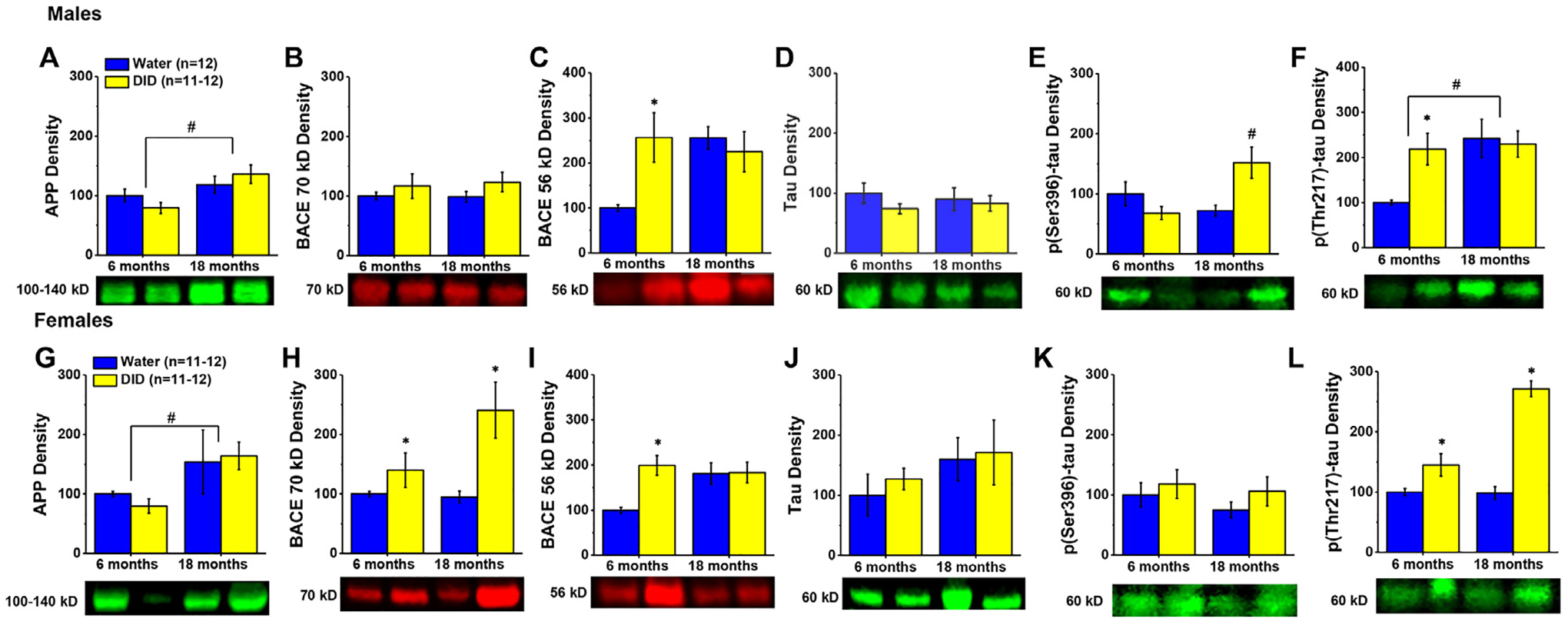
Immunoblotting conducted on the hippocampus of males (A-F) revealed both age- and/or alcohol-related increases in the expression of protein markers of Aβ and tau pathology, the majority of which were distinct from those observed in the hippocampus of females (G-L). Data are normalized to the average protein densities of the 6 month-old water controls for either sex and represented as means ± SEMs (n = 11–12). *p<0.05, main or specific effect of alcohol-drinking; #p<0.05, main or specific effect of age.
Females also exhibited an age-related increase in hippocampal APP, irrespective of alcohol history (Fig. 3G) [Age effect: F(1,43)=5.013, p = 0.031; other p’s>0.624]. However, alcohol-drinking significantly elevated BACE 70 kD levels in females of both ages (Fig. 3H) [Alcohol effect: F(1,46)=10.821, p = 0.002; other p’s>0.073], while an alcohol-induced increase in hippocampal BACE 56 kD expression was observed only in the 6 month-old females (Fig. 3I) [Alcohol X Age: F(1,46)=5.932, p = 0.019; for 6 month-olds, t(22)=4.369, p<0.0001; for 18 month-olds, p = 0.938], in a manner akin to males.
3.3.2. Phosphorylated tau
We detected no group differences in the hippocampal expression of total tau in male mice (Fig. 3D; Alcohol X Age ANOVA, all p’s>0.181). In contrast, we detected a significant Alcohol X Age interaction for hippocampal levels of p(Ser396)-tau (Fig. 3E) [F(1,44)=6.020, p = 0.018]. However, deconstruction of this interaction along the Age factor failed to detect significant water-alcohol differences for either age group (t-tests: for 6 months, p = 0.172; for 18 months, p = 0.061). Thus, the interaction was deconstructed along the Alcohol factor, which revealed a significant age-dependent increase in hippocampal p(Ser396)-tau expression in DID mice [t(20)=3.152, p = 0.005], which was not apparent in Water controls (t-test, p = 0.205). In contrast to p(Ser396)-tau, 18 month-old males exhibited higher hippocampal p(Thr217)-tau expression overall compared to their 6 month-old counterparts (Fig. 3F) [Age effect: F(1,47)=6.002, p = 0.018] and alcohol-drinking history elevated hippocampal p(Thr217)-tau expression selectively in 6 month-old males [Alcohol X Age interaction: F(1,47)=4.406, p = 0.042; for 6 month-olds, t(22)=3.360, p = 0.003; for 18 month-olds, p = 0.881].
Similar to males, no group differences were detected for the expression of total tau (Fig. 3J; Alcohol X Age ANOVA, all p’s>0.129 or p(Ser396)-tau (Fig. 3K; Alcohol X Age ANOVA, all p’s>0.261) in the hippocampus of females. In contrast, alcohol drinking-history increased hippocampal expression of p(Thr217)-Tau in females of both ages (Fig. 3L) [Alcohol effect: F(1,46)=10.263, p = 0.003]. While the magnitude of the alcohol-induced increase appeared to be larger in 18 versus 6 month-old females, we failed to detect any significant Age effect or interaction for this tau pathology marker (p’s>0.068).
These data provide evidence that sex differences exist with respect to alcohol-induced neuropathology within the hippocampus and indicate that hippocampal BACE 56 kD is a biochemical correlate of the age-selective effect of alcohol-drinking on cognitive function that is sex-independent.
3.4. No age- or sex-related differences in the robust effects of alcohol-drinking on glutamate receptor expression in the PFC
Irrespective of the age of drinking onset, males with a history of alcohol-drinking exhibited a robust increase in the PFC expression of GluA1 (Fig. 4A) [Alcohol effect: F(1,47)=5.268, p = 0.027; other p’s>0.250], GluA2 (Fig. 4B) [Alcohol effect: F(1,47)=17.689, p<0.0001; other p’s>0.510], GluN1 (Fig. 4C) [F(1,47)=7.572, p = 0.009; other p’s>0.900], GluN2b (Fig. 4D) [Alcohol effect: F(1,47)=17.000, p<0.0001; other p’s>0.750], mGlu1 (Fig. 4E) [Alcohol effect: F(1,47)=42.657, p<0.0001; other p’s>0.260], and mGlu5 dimer (Fig. 4F) [Alcohol effect: F(1,47)=29.866, p<0.0001; other p’s>0.730]. Although the magnitude of the alcohol-induced increase in mGlu5 monomer expression in male PFC was not as large as that for the dimer form of this receptor, mGlu5 monomer levels were also significantly higher in alcohol-drinking males versus their water controls (Fig. 4G) [Alcohol effect: F(1,47)=4.870, p = 0.033; other p’s>0.330].
Fig. 4. Expression of glutamate receptor-related proteins within the PFC.
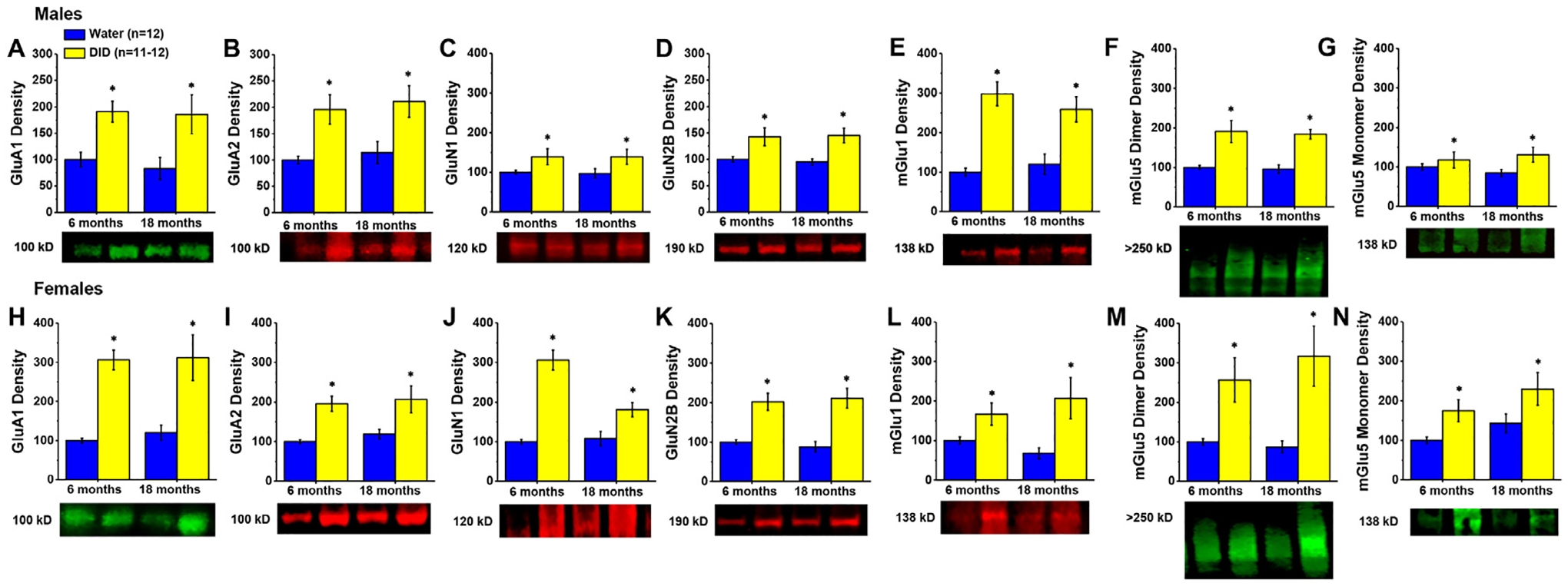
Immunoblotting conducted on the PFC of both male (A-G) and female (H–N) mice with a history of alcohol-drinking (DID) indicated higher expression of every glutamate receptor-related protein examined, irrespective of age. Data are normalized to the average protein densities of the 6 month-old water controls for either sex and represented as means ± SEMs (n = 11–12). *p<0.05, main effect of alcohol-drinking.
Similarly, females exhibited a large alcohol-induced increase in the expression of PFC levels of all the glutamate-related proteins examined. As illustrated in Fig. 4H–K, GluA1 [F(1,46)=35.577, p<0.0001; other p’s>0.690], GluA2 [F(1,46)=19.522, p<0.0001; other p’s>0.480], GluN1 [F(1,46)=22.413, p<0.0001; other p’s>0.275], and GluN2b [F(1,46)=38.156, p<0.0001; other p’s>0.550] expression was significantly higher in alcohol-drinking females, relative to their water controls, irrespective of age. Likewise, the levels of mGlu1 (Fig. 4L) [F(1,46)=11.005, p = 0.002; other p’s>0.250], as well as both the mGlu5 dimer (Fig. 4M) [F(1,46)=16.150, p<0.0001; other p’s>0.440] and mGlu5 monomer (Fig. 4N) [F(1,46)=8.182, p = 0.007; Age effect: p = 0.087; interaction, p = 0.823] were higher in the PFC of alcohol-drinking females, again, irrespective of the age of drinking onset. These data indicate that a history of binge-drinking during later life results in a robust increase in the expression of glutamate receptors within the PFC of both male and female mice.
3.5. Sex differences in the effects of age and alcohol-drinking on ADRD-related proteins within PFC
3.5.1. Amyloid-related proteins
As observed in the hippocampus, we detected no group differences in the expression of either APP (Fig. 5A; Alcohol X Age ANOVA, all p’s>0.158) or BACE 70 kD (Fig. 5B; Alcohol X Age ANOVA, all p’s>0.632) in the PFC of male mice. In contrast, a significant Age effect [F(1,47)=7.356, p = 0.010] and Alcohol X Age interaction [F(1,47)=4.377, p = 0.042] were noted for PFC BACE 56 kD expression (Fig. 5C). Post-hoc comparisons of water-alcohol differences in BACE 56 kD expression failed to detect significant alcohol effects for either the 6 or 18 month-old males (t-tests, respectively: p = 0.136 and p = 0.143). Deconstruction of this interaction along the Alcohol factor revealed an age-related increase in BACE 56 kD expression in water controls [t(23)=3.621, p = 0.001], which was not apparent in alcohol-drinking males (t-test, p = 0.716).
Fig. 5. Expression of neuropathology-related proteins within the PFC.
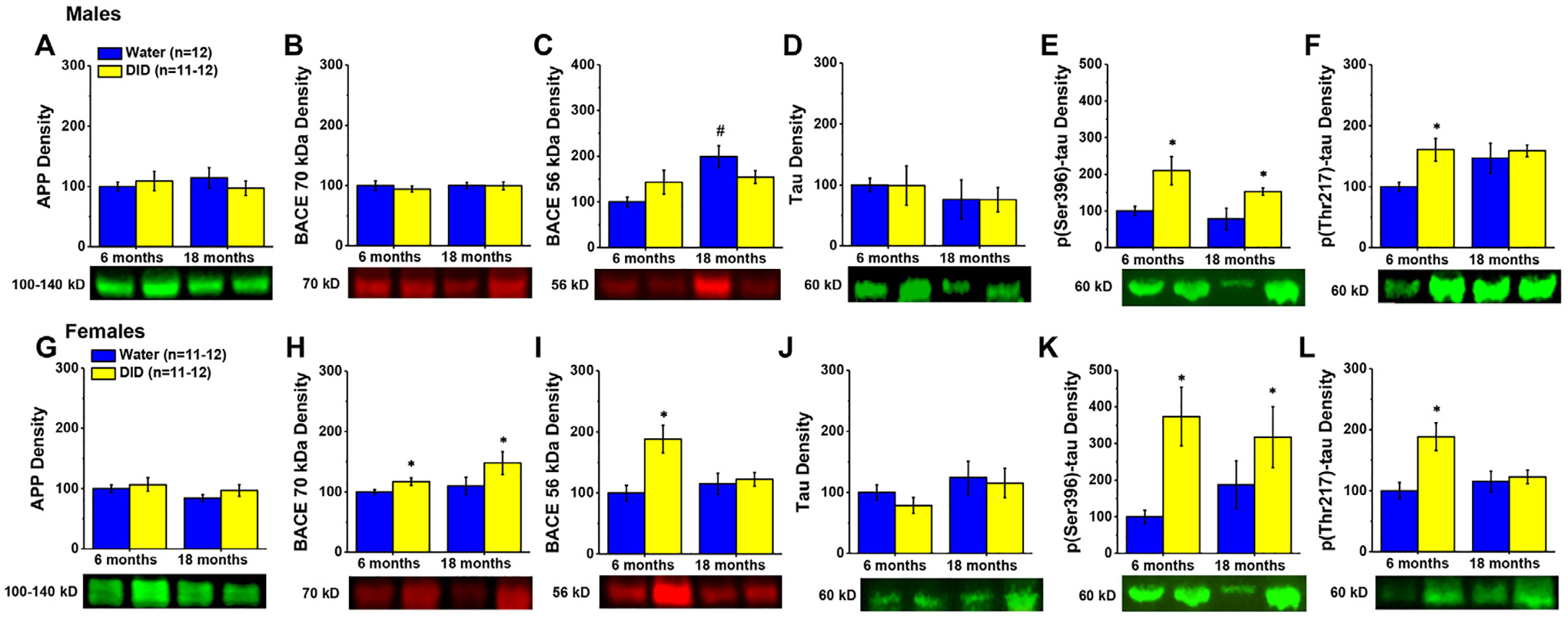
Immunoblotting conducted on the PFC of males (A-F) indicated little effect of aging, but some alcohol-induced increases in the expression of protein markers of Aβ and tau pathology in the PFC. While female PFC (G-L) tended to be more sensitive to alcohol-induced increases in BACE isoform expression (H,I) than males, female PFC exhibited a similar tau protein profile as males (J-L). Data are normalized to the average protein densities of the 6 month-old water controls for either sex and represented as means ± SEMs (n = 11–12). * p<0.05, main or specific effect of alcohol-drinking; #p<0.05, main or specific effect of age.
Akin to males, we detected no group differences in APP levels within PFC of females (Fig. 5G; Alcohol X Age: all p’s>0.158). However, alcohol-drinking history increased the PFC expression of BACE 70 kD in females of both ages (Fig. 5H) [Alcohol effect: F(1,41)=4.750, p = 0.036; other p’s>0.107], while the expression of BACE 56 kD was increased selectively in 6 month-old female alcohol-drinking mice (Fig. 5I) [Alcohol X Age: F(1,46)=6.017, p = 0.018; for 6 month-olds, t(22)=4.456, p<0.0001; for 18 month-olds, p = 0.774].
3.5.2. Phosphorylated tau
Males exhibited no group differences in PFC levels of total tau (Fig. 5D; Alcohol X Age ANOVA, all p’s>0.392). However, both 6 and 18 month-old mice with a history of alcohol-drinking exhibited elevated expression of p(Ser396)-tau (Fig. 5E) [Alcohol effect: F(1,43)=9.438, p = 0.004; other p’s>0.196], while an Alcohol X Age interaction was detected for PFC levels of p(Thr217)-tau (Fig. 5F) [F(1,47)=4.377, p = 0.042], reflecting an alcohol-induced increase in p(Thr217)-tau expression in the 6 month-old DID mice [t(22)=3.417, p = 0.002] that was not apparent in the 18 month-old animals (t-test, p = 0.916).
The pattern of total and phosphorylated tau expression in female PFC was similar to that exhibited by males, with no group differences detected for total tau (Fig. 5J; Alcohol X Age ANOVA, all p’s>0.243), and an alcohol-induced increase in p(Ser396)-tau levels observed in both mature adult and aged mice (Fig. 5K) [Alcohol effect: F(1,42)=8720, p = 0.005; other p’s>0.442). Alcohol also increased PFC expression of p(Thr217)-tau selectively in the PFC of 6 month-old females (Fig. 5L) [Alcohol X Age: F(1,46)=6.017, p = 0.018; for 6 month-olds, t(22)=3.396, p = 0.003; for 18 month-olds, p = 0.714).
These data provide evidence that sex differences exist with respect to alcohol-induced neuropathology also within the PFC, with females exhibiting more indices of neuropathology than males. Moreover, these data indicate that PFC p(Thr396)-tau is a biochemical correlate of the age-selective effect of alcohol-drinking on cognitive function that is sex-independent, while PFC BACE 56 kD expression is a biochemical correlate of the cognitive dysfunction apparent only in alcohol-drinking mature adult females.
4. Discussion
Globally, the population of individuals aged 65+ is growing at a faster rate than all other age groups and with this will bring a marked rise in the prevalence of age-related disease, including ADRDs [53]. An overactive glutamate system is posited to be a putative early biomarker and therapeutic target for ADRD [54–56]. Despite the closing of the gender gap in binge-drinking across all age groups [57], the fact that more than 50% of binge-drinks consumed those aged 35+ years, and evidence that those aged 65+ years binge-drink more frequently than other age groups [16], clear gaps exist in our knowledge of how sex interacts with older age to impact cognitive function, let alone its underlying neuropathology [see 58,59]. Heavy drinking associated with an AUD is one of the strongest modifiable risk factors for early cognitive decline in humans [9]. Consistent with this, we demonstrated recently in a series of mouse studies that a month-long history of binge-drinking during mature adulthood induces cognitive impairments during withdrawal that are comparable to that exhibited by alcohol-naïve aged mice. Moreover, aligning with evidence that women are nearly twice as likely to develop ADRDs than men [11,12], mature adult female mice exhibited more signs of alcohol-induced cognitive impairment during withdrawal than their male counterparts in two replicate experiments, while we detected relatively few alcohol-related effects in either male or female aged mice (see Fig. 1D) [8]. A month-long history of binge-drinking upregulates glutamate receptor expression within mesocorticolimbic structures during early withdrawal in young adult mice (i.e., 2–3 months old) [43–45] and alcohol-induced hyper-activation of glutamate receptors, notably GluN2b-containing NMDA receptors and mGlu5, have been implicated in pathological Aβ accumulation and tau hyper-phosphorylation underpinning cognitive decline [1,37,60–64]. Thus, we hypothesized at the outset of this study that the cognitive effects of binge-drinking observed in our prior report [8] might reflect age- and/or sex-dependent perturbations in glutamate receptor expression and indices of ADRD-related neuropathology within the hippocampus and the PFC.
4.1. Age- and alcohol-related changes in hippocampal glutamate receptor expression are male-selective
Contrary to our hypothesis that females would exhibit more signs of glutamate receptor anomalies than males, we observed a male-selective age-related reduction of hippocampal ionotropic glutamate receptors (iGluR) and an alcohol-induced upregulation of these proteins, with no age- or alcohol-related effects observed in female mice (Fig. 2). Further, trends towards an age-dependent decline in hippocampal mGlu1 and mGlu5 dimer expression were also male-selective (Fig. 2E,F). Thus, as reported for young adult rats [65], alcohol-drinking produces a male-selective increase in hippocampal NMDA receptor subunit expression in older mice. Moreover, these findings are consistent with prior reports demonstrating that a history of binge-drinking can produce sex-specific changes in glutamate receptor mRNA expression within the nucleus accumbens of young adult C57BL/6J mice [e.g., 66,67]. While several studies have examined sex differences in age-related cognitive decline in laboratory rodents [e.g., 68–71], to the best of our knowledge, little work has directly examined the interactions between normal aging and biological sex with respect to glutamate receptor expression in the brain. Nevertheless, the male-selectivity of our aging effect aligns with evidence from humans indicating that an age-related decrease in brain glutamate concentrations can be larger in men than in women [72] as well as studies of C57BL/6J mice indicating sex differences in age-related changes in neurometabolic activity within hippocampus [73]. Further, they are consistent with other studies, conducted exclusively in male rodents, demonstrating an age-dependent reduction in hippocampal NMDA [e.g., 74–78] and AMPA [e.g., [79–82]; but see Ref. [82] receptor subunit expression or binding.
Why females appear to be resilient to both alcohol- and age-related changes in hippocampal glutamate receptor expression is unclear at the present time, but is not likely related to circulating ovarian hormones as ovariectomy does not alter GluA1 expression in the female hippocampus [83] nor does it alter alcohol-induced changes in GluN1 expression in this region [84]. Conversely, an age-related decline in androgens [85,86] might contribute to the lower iGluR expression in aged versus young adult males as orchidectomy is reported to lower hippocampal GluA1 expression in male mice [83]. Even if this were the case for males, it is clear from the present immunoblotting results that the interactions between sex and the age of drinking onset for alcohol withdrawal-induced cognitive dysfunction (Fig. 1D) are not overtly related to changes in the total protein expression of any of the glutamate receptor-related proteins examined herein.
4.2. Alcohol-induced increases in PFC protein expression are both sex- and age-independent
Also contrary to our hypothesis, and very different from our observations in the hippocampus (Fig. 2), we detected no age-related change in PFC protein expression in the present study (Fig. 4). Further, although an inspection of the immunoblotting results for the hippocampus argues a smaller alcohol effect size for GluN2b and mGlu1 in aged versus mature adult male mice (Fig. 2), alcohol up-regulated PFC glutamate receptor expression to a similar extent in 6 month-and 18 month-old mice (Fig. 4). The lack of an aging effect or alcohol-age interaction was rather surprising given the decades of evidence demonstrating an age-related decline in PFC glutamate receptor expression in humans [87], as well as in a wide variety of laboratory animals [e.g., 74,81–83]. One possible explanation for our negative results might reflect our study of whole cell homogenates, which cannot delineate the subcellular localization of receptor expression. Indeed, Zhao et al. [74] demonstrated more robust NMDA receptor subunit changes and correlations between subunit expression within PFC and cognitive performance in assays of the synaptic membrane fraction versus whole cell homogenate. Related to this, we did not assay for changes in the expression of glutamate receptor scaffolding proteins (e.g., Homer, postsynaptic density protein of 95 kDa or PSD-95, glutamate receptor-interacting protein or GRIP) in the present study. The levels of these scaffolding proteins change with aging [e.g., 88–90], as does their capacity to bind specific receptors and receptor subunits, which can regulate their synaptic location and function to impact cognitive function [88,90]. Indeed, altered glutamate receptor scaffolding has been implicated in aberrant signaling through NMDA and mGlu5 receptors that can lead to Aβ accumulation, hyperphosphorylated tau and cognitive impairment [60,37,61–64].
Also, in contrast to the hippocampus, we detected a robust, sex-independent and seemingly ubiquitous effect of binge-drinking on PFC glutamate receptor expression (Fig. 4). These findings extend the results of a recent RNA-Seq study conducted in younger adult mice demonstrating that the PFC is particularly sensitive to binge-drinking-induced changes in its molecular profile [23]. Moreover, our findings extend the results of earlier studies employing the chronic, intermittent, ethanol (CIE) model in young adult rats indicating an increase in GluN2b expression within PFC [91,92]. However, in contrast to these rat studies in which PFC GluN2b levels returned to the level of water-drinking controls following 21 days of abstinence, the alcohol-induced increases in both hippocampal and PFC glutamate receptor expression observed in the present study were apparent 21+ days into abstinence. Whether or not this persistence reflects genuine long-lasting neuroadaptations in response to the month-long binge-drinking procedure (versus responses to the behavioral testing or to the alcohol withdrawal itself), is unique to older animals (i.e., 6+ months-old), and is species- or alcohol delivery procedure-specific are all important questions that should be addressed in future work. It is notable also that the robust alcohol-induced increase in PFC glutamate receptor expression observed herein contrasts with the study by Crowley et al. [93] in which 8 days of binge-drinking under the more traditional single-bottle (20% alcohol) DID procedure resulted in a female-specific reduction in the synaptic membrane expression of GluN1 and GluNA2/3 within the prelimbic subregion of the PFC. As we sampled from the entire PFC in this study (see Fig. 1B) and assayed protein expression in whole cell homogenates following a month-long, 3-bottle-choice, DID procedure, it is difficult at present to decipher which procedural variables might account for the discrepancy in findings. Nevertheless, our results for the PFC demonstrating that the alcohol-induced increase in PFC glutamate receptor expression is both age- and sex-independent cannot directly account for the observed interactions between these variables with respect to alcohol-induced cognitive decline (Fig. 1D) [8].
This study is not the first to report a dissociation between sex differences in alcohol-induced cognitive impairment and glutamate receptor protein expression within hippocampus and PFC [65,94]. However, rendering the interpretation of the apparent dissociations between our behavioral and immunoblotting data more difficult, the vast majority of studies examining sex differences in the glutamate-related neurobiology of alcohol-induced cognitive impairment in wild-type (i.e., genetically unmanipulated) rodents have been conducted in models of Fetal Alcohol Syndrome (i.e., prenatal, neonatal, or early postnatal alcohol exposure) or adolescent alcohol-drinking [c.f., 58]. Unfortunately, the results of such studies are likely not directly comparable to the mature adult and aged brain due to developmental changes in iGluR and mGluR expression, iGluR subunit composition, and receptor scaffolding that affect: (1) their pharmacological sensitivity to both endogenous ligands (incl. glutamate, serine and polyamines), as well as exogenous ligands like alcohol; (2) their subcellular localization, not only within the synaptic membrane but between synaptic and extrasynaptic sites within the synaptic membrane; (3) their activation kinetics and cation-selective gating in the case of iGluRs; and (4) their capacity to activate specific intracellular signaling pathways.
4.3. Sex- and age-selective changes in the capacity of alcohol to increase protein markers of ADRD-related neuropathology
Consistent with evidence that a history of alcohol-drinking induces ADRD-related gene expression in B6 mice [7,23,24], we detected alcohol-induced increases in markers of both Aβ and tau pathology within hippocampus and PFC, some of which were sex- and/or age-specific (see summary in Table 1). Notable sex differences included female-specific alcohol-induced increases in the 70 kD BACE isoform within hippocampus (Fig. 3H) and increases in both the 70 and 56 kD BACE isoforms within PFC (Fig. 5H,I), the latter of which occurred only in the 6 month-old females (Fig. 5I). While both male and female 6 month-old mice exhibited an alcohol-induced increase in the hippocampal expression of the prototypical biomarker of AD, p(Thr217)-tau [33,34] (Fig. 3F,L), alcohol history elevated the hippocampal expression of p(Thr217)-tau only in aged females (Fig. 3L) and p(Ser396)-tau expression only in aged males (Fig. 3E). Two other notable age-specific effects of alcohol drinking history are increases in hippocampal BACE 56 kD (Fig. 3C,I) and in PFC p(Thr217)-tau expression (Fig. 5F,L), both of which were apparent only in 6 month-old animals. Thus, in contrast to glutamate receptor expression, interactions were detected between age of drinking-onset and/or sex with respect to alcohol-induced changes in our protein markers of AD-related neuropathology within hippocampus and PFC that align with, and may contribute, to the sex- and/or age-selective effects of alcohol-drinking during later life on cognitive function (Fig. 1D) [8].
Table 1.
Comparison of the main effects of, and interactions between, age (6 versus 18 months), alcohol drinking history (DID versus Water) and sex on the expression of glutamate receptor- and neuropathology-related protein expression within hippocampus (left) and PFC (right).
| Protein | Result Summary | |
|---|---|---|
| Hippocampus | PFC | |
| GluA1 | 18 month < 6 month (males only) | DID > Water |
| GluA2 | 18 month < 6 month (males only) | DID > Water |
| GluN1 | 18 month < 6 month (males only) | DID > Water |
| GluN2B | DID > Water (males only) | DID > Water |
| mGlu1 | DID > Water (males only) | DID > Water |
| mGlu5 dimer | DID > Water (males only) | DID > Water |
| mGlu5 monomer | DID = Water 18 month = 6 month | DID > Water |
| APP | 18 month > 6 month | DID = Water 18 month = 6 month |
| BACE 70 kD | DID > Water (females only) | DID > Water (females only) |
| BACE 56 kD | DID > Water (6 month only) | DID > Water (6 month females only) 18 month > 6 month (males only) |
| Tau | DID = Water 18 month = 6 month | DID = Water 18 month = 6 month |
| p(Ser396)-tau | DID > Water (18 month males only) | DID > Water |
| p(Thr217)-tau | DID > Water (6 month males, both 6 and 18 month females) 18 month > 6 month (males only) | DID > Water (6 month only) |
Our results for BACE are particularly interesting given that its mRNA and pro-protein/protein levels are reported to be stable across normal aging [29,31] and relatively unaffected in AD or mouse models of AD [90,95]. Herein, we observed age-dependent increases in BACE 56 kD expression in both male and female hippocampus (Fig. 3C,I), as well as male PFC (Fig. 5C), the former of which was not detected by our statistical analyses owing to the large alcohol-induced increase in isozyme expression in 6 month-old alcohol-drinking mice (Fig. 3C,I). In contrast to its expression [29,31,90,95], BACE enzymatic activity increases with both age and AD progression [29–33]. As we conducted immunoblotting exclusively in this study, it remains to be determined whether group differences in the alcohol-induced increase in BACE expression translate into sex- and/or age-related difference in enzymatic activity, Aβ over-production and neurotoxicity. While total APP expression did not vary as a function of age or alcohol-drinking history in either brain region in the present study (Fig. 3A,G; Fig. 5A,G) and we could not detect Aβ1–42 in our tissue (Suppl. Figure 1C), BACE over-expression [96] and knock-out [97,98] increases and reduces, respectively, the amount of Aβ in mouse brain, raising the possibility that the alcohol-age interactions in cognitive function reported in our previous study (Fig. 1D) [8] might relate, at least in part, to increased BACE 56 kD enzymatic activity. p(Thr217)-tau is considered a highly specific biomarker of AD in both preclinical and advanced stages of AD [33,34], while p(Ser396)-tau is considered one of the earliest markers of AD-related neuropathology [35]. Given this, we predicted at the outset of this study that if alcohol-drinking induces cognitive deficits via increases in tau phosphorylation, then any alcohol-induced increase in tau phosphorylation should be more robust in mature adult versus aged mice and/or in females versus males. Our results for p(Thr217)-tau in both the hippocampus and PFC are largely consistent with our hypothesis (Table 1), while those for p(Ser396)-tau did not map onto group differences in cognitive function. Tau phosphorylation at Thr217 occurs via activation of several proline-directed kinases, including glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (Cdk5), protein kinase A (PKA) and calcium/calmodulin-dependent kinase II (CaMKII) [99]. Alcohol increases the expression and/or activational state of these kinases in brain and this activation has been implicated in both alcohol-induced neurotoxicty and alcohol reward [e.g., 100–106]. Thus, important next steps in future research will be to determine which, if any, of these kinases drives the alcohol-induced hyper-phosphorylation of Thr217, whether they differ as a function of the age of drinking-onset, whether the activation of specific glutamate receptors may be involved [c.f., 37,38,107] and the functional consequences of increased p(Thr217)-tau levels for alcohol-induced cognitive dysfunction during later life.
4.4. Caveats and conclusions
The persistent overactivation of hippocampal and PFC glutamate receptors, including their upregulation by chronic alcohol exposure, can lead to pathological Aβ accumulation, tau hyper-phosphorylation and neurotoxicity [1,37,38,60–74,107]. To the best of our knowledge, no study has examined the effects of alcohol-drinking during later life on protein expression of markers of ADRD-related neuropathology in wild-type rodents, let alone in relation to glutamate receptor expression. Contrary to our prediction, a comparison of our immunoblotting results indicates no overt relationship between the hippocampal and PFC expression of glutamate receptor-related proteins and the neuropathology-related markers examined herein (Table 1). It should be noted, however, that we only immunoblotted for a limited number of Aβ-and tauopathyrelated markers and did not examine for neurotoxicity directly. Importantly, we did not conduct functional assays of receptor or enzymatic activity, which would better inform as to the potential mechanisms through which a history of alcohol-drinking during mature adulthood, but not old age, negatively impacts cognitive function. Lastly, we did not test the functional relevance of AMPA, NMDA or Group1 mGlu receptor activation for alcohol-induced changes in BACE or phosphorylated tau expression in relation to cognitive function. Nevertheless, the present results indicate that the interactions between sex, older age and alcohol-drinking on cognitive function can be dissociated from the total protein expression of AMPA, NMDA and Group1 mGlu receptor expression within the hippocampus and PFC, but may reflect increases in BACE and p(Thr217)-tau of relevance to the neurobiology of alcohol-induced cognitive decline.
Supplementary Material
Acknowledgements
The authors would like to thank Ms. Frida Finnestrand (Laguna Blanca High School, Santa Barbara) for her assistance with the immunoblotting studies during her internship in the laboratory.
Funding
This work was supported by the National Institutes of Health (grant number AA024044). BME was supported by the UCSB California Alliance for Minority Participation (CAMP) program, LEM was supported by a UCSB Undergraduate Research and Creative Activities Award and SLS was supported by a Young Investigator Award from the Brain and Behavior Research Foundation (28691).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2023.100099.
Data availability
Data will be made available on request.
References
- [1].Huang D, Yu M, Yang S, Lou D, Zhou W, Zheng L, et al. , Ethanol alters APP processing and aggravates alzheimer-associated phenotypes, Mol. Neurobiol 55 (2018) 5006–5018. [DOI] [PubMed] [Google Scholar]
- [2].Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, Kivimaki M, Singh-Manoux A, Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study, BMJ 362 (2018) k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weyerer S, Schaufele M, Wiese B, Maier W, Tebarth F, van den Bussche H, Pentzek M, et al. , German AgeCoDe study g. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older, Age Ageing 40 (2011) 456–463. [DOI] [PubMed] [Google Scholar]
- [4].Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu JT, Alcohol consumption and dementia risk: a dose response meta-analysis of prospective studies, Eur. J. Epidemiol 32 (2017) 31–42. [DOI] [PubMed] [Google Scholar]
- [5].Ledesma JC, Rodriguez-Arias M, Gavito AL, Sanchez-Perez AM, Vina J, Medina Vera D, Rodriguez de Fonseca F, Minarro J, Adolescent binge-ethanol accelerates cognitive impairment and beta-amyloid production and dysregulates endocannabinoid signaling in the hippocampus of APP/PSE mice, Addict. Biol 26 (2021) e12883. [DOI] [PubMed] [Google Scholar]
- [6].Crews FT, Vetreno RP, (2014) Neuroimmune basis of alcoholic brain damage, Int. Rev. Neurobiol 118 (2020) 315–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoffman JL, Faccidomo S, Kim M, Taylor SM, Agoglia AE, May AM, et al. , Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg-AD mouse model of Alzheimer’s disease, Int. Rev. Neurobiol 148 (2019) 169–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jimenez Chavez CL, Van Doren E, Matalon J, Ogele N, Kharwa A, Madory L, Kazerani I, Herbert J, Torres-Gonzalez J, Rivera E, K Szumlinski K, Alcohol-drinking under limited-access procedures during mature adulthood accelerates the onset of cognitive impairment in mice, Front. Behav. Neurosci 16 (2022) 732375, doi: 10.3389/fnbeh.2022.732375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J, Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study, Lancet Public Health 3 (2018) e124–e132. [DOI] [PubMed] [Google Scholar]
- [10].Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, et al. , Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study, BMJ 357 (2017) j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, et al. , Sex differences in Alzheimer disease - the gateway to precision medicine, Nat. Rev. Neurol 14 (2018) 457–469. [DOI] [PubMed] [Google Scholar]
- [12].Hebert LE, Weuve J, Scherr PA, Evans DA, Alzheimer disease in the United States (2010–2050) estimated using the 2010 census, Neurology 80 (2013) 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Agabio R, Pisanu C, Gessa GL, Franconi F, Sex differences in alcohol use disorder, Curr. Med. Chem 24 (2017) 2661–2670. [DOI] [PubMed] [Google Scholar]
- [14].Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. , Sex differences in stress-related alcohol use, Neurobiol. Stress 10 (2019) 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pollard MS, Tucker JS, Green HD Jr., Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US, JAMA Netw. Open 3 (2020) e2022942, doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD, Annual total binge drinks consumed by U.S. adults, 2015, Am. J. Prev. Med 54 (2018) 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Brien CP, Gardner EL, Critical assessment of how to study addiction and its treatment: human and non-human animal models, Pharmacol. Ther 108 (2005) 18–58, doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- [18].Volkow ND, Li TK, Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences, Pharmacol. Ther 108 (2005) 3–17, doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- [19].Hu Y, Ding WT, Zhu XN, Wang XL, A mini review: tau transgenic mouse models and olfactory dysfunction in Alzheimer’s disease, Zhongguo Ying Yong Sheng Li Xue Za Zhi 31 (2015) 481–490. [PubMed] [Google Scholar]
- [20].Jankowsky JL, Zheng H, Practical considerations for choosing a mouse model of Alzheimer’s disease, Mol. Neurodegener 12 (2017) 89, doi: 10.1186/s13024-017-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ, Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models, Front. Genet 5 (2014) 88, doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grifasi IR, Evans WA, Rexha AD, Sako LW, Marshall SA, A comparison of hippocampal microglial responses in aged and young rodents following dependent and non-dependent binge drinking, Int. Rev. Neurobiol 148 (2019) 305–343, doi: 10.1016/bs.irn.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu M, Guo S, Huang D, Hu D, Wu Y, Zhou W, Song W, Chronic alcohol exposure alters gene expression and neurodegeneration pathways in the brain of adult mice, J. Alzheimers Dis 86 (2022) 315–331, doi: 10.3233/JAD-215508. [DOI] [PubMed] [Google Scholar]
- [24].Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW, Moderate alcohol drinking and the amygdala proteome: identification and validation of calcium/calmodulin dependent kinase II and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol, Biol. Psychiatry 79 (2016) 430–4342, doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Van Hees L, Didone V, Charlet-Briart M, Van Ingelgom T, Alexandre A, Quertemont E, Nguyen L, Laguesse S, Voluntary alcohol binge-drinking in adolescent C57Bl6 mice induces delayed appearance of behavioural defects in both males and females, Addict. Biol 27 (2022) e13102, doi: 10.1111/adb.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flurkey KCJ, Harrison DE, The mouse in aging research, in: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A (Eds.), The Mouse in Biomedical Research, 2nd ed, Elsevier, Burlington, MA, 2007, pp. 637–672. [Google Scholar]
- [27].Glenner GG, Wong CW, Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein, Biochem. Biophys. Res. Commun 120 (1984) 885–890. [DOI] [PubMed] [Google Scholar]
- [28].Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI, Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology, Proc. Natl. Acad. Sci. U.S.A 83 (1986) 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T, Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques, Am. J. Pathol 148 (1996) 259–265. [PMC free article] [PubMed] [Google Scholar]
- [30].Wisniewski HM, Ghetti B, Terry RD, Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys, J. Neuropathol. Exp. Neurol 32 (1973) 566–584. [DOI] [PubMed] [Google Scholar]
- [31].Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC, Beta-secretase activity increases with aging in human, monkey, and mouse brain, Am. J. Pathol 164 (2004) 719–725, doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Holsinger D, McLean CA, Beyreuther K, Masters CL, Evin G, Increased expression of amyloid precursor β-secretase in Alzheimer’s disease, Ann. Neurol 51 (2002) 783–786. [DOI] [PubMed] [Google Scholar]
- [33].Fukumoto H, Cheung BS, Hyman BT, Irizarry MC, Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease, Arch. Neurol 59 (2002) 1381–1389. [DOI] [PubMed] [Google Scholar]
- [34].Johnson GV, Stoothoff WH, Tau phosphorylation in neuronal cell function and dysfunction, J. Cell Sci 117 (2004) 5721–5729, doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- [35].Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC, Proctor NK, Chai X, Shcherbinin S, Sims JR, Triana-Baltzer G, Theunis C, Slemmon R, Mercken M, Kolb H, Dage JL, Hansson O, Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease, Nat. Commun 11 (2020) 1683, doi: 10.1038/s41467-020-15436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mondragón-Rodríguez S, Perry G, Luna-Muñoz J, Acevedo-Aquino MC, Williams S, Phosphorylation of tau protein at sites Ser(396–404) is one of the earliest events in Alzheimer’s disease and down syndrome, Neuropathol. Appl. Neurobiol 40 (2014) 121–135, doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- [37].Abd-Elrahman KS, Ferguson SSG, Noncanonical metabotropic glutamate receptor 5 signaling in alzheimer’s disease, Annu. Rev. Pharmacol. Toxicol 62 (2022) 235–254, doi: 10.1146/annurev-pharmtox-021821-091747. [DOI] [PubMed] [Google Scholar]
- [38].Babaei P, NMDA and AMPA receptors dysregulation in Alzheimer’s disease, Eur. J. Pharmacol 908 (2021) 174310, doi: 10.1016/j.ejphar.2021.174310. [DOI] [PubMed] [Google Scholar]
- [39].Czapski GA, Strosznajder JB, Glutamate and GABA in microglia-neuron cross-talk in alzheimer’s disease, Int. J. Mol. Sci 22 (2021) 11677, doi: 10.3390/ijms222111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fairless R, Bading H, Diem R, Pathophysiological ionotropic glutamate signalling in neuroinflammatory disease as a therapeutic target, Front. Neurosci 15 (2021) 741280, doi: 10.3389/fnins.2021.741280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guo C, Ma YY, Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders, Front. Neural Circuits 15 (2021) 711564, doi: 10.3389/fncir.2021.711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Srivastava A, Das B, Yao AY, Yan R, Metabotropic glutamate receptors in alzheimer’s disease synaptic dysfunction: therapeutic opportunities and hope for the future, J. Alzheimers Dis 78 (2020) 1345–1361, doi: 10.3233/JAD-201146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Campbell RR, Domingo RD, Williams AR, Wroten MG, McGregor HA, Waltermire RS, Greentree DI, Goulding SP, Thompson AB, Lee KM, Quadir SG, Jimenez Chavez CL, Coelho MA, Gould AT, von Jonquieres G, Klugmann M, Worley PF, Kippin TE, Szumlinski KK, Increased alcohol-drinking induced by manipulations of mGlu5 phosphorylation within the bed nucleus of the stria terminalis, J. Neurosci 39 (2019) 2745–2761, doi: 10.1523/JNEU-SCI.1909-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK, Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures, Alcohol Clin. Exp. Res 36 (2012) 1623–1633, doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK, Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala, Neuropsychopharmacology 39 (2014) 435–444, doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK, Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism, J. Neurosci 29 (2009) 8655–8668, doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS, Accumbens neurochemical adaptations produced by binge-like alcohol consumption, Psychopharmacology (Berl) 190 (2007) 415–431, doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- [48].Lee KM, Coelho MA, Class MA, Szumlinski KK, mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice, Drug Alcohol Depend 184 (2018) 1–11, doi: 10.1016/j.drugalcdep.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jimenez Chavez CL, Coelho MA, Brewin LW, Swauncy I, Tran T, Albanese T, Laguna A, Gabriela I, K Szumlinski K, Incubation of negative affect during protracted alcohol withdrawal is age-, but not sex-selective, Brain Sci 10 (2020) 405, doi: 10.3390/brainsci10060405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Szumlinski KK, Coelho MA, Lee KM, Tran T, Sern KR, Bernal A, Kippin TE, DID it or DIDn’t it? Exploration of a failure to replicate binge-like alcohol-drinking in C57BL/6J mice, Pharmacol. Biochem. Behav 178 (2019) 3–18, doi: 10.1016/j.pbb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chiu AS, Kang MC, Huerta Sanchez LL, Fabella AM, Holder KN, Barger BD, Elias KN, Shin CB, Jimenez Chavez CL, Kippin TE, Szumlinski KK, Preclinical evidence to support repurposing everolimus for craving reduction during protracted drug withdrawal, Neuropsychopharmacology 46 (2021) 2090–2100, doi: 10.1038/s41386-021-01064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huerta Sanchez LL, Sankaran M, Li TL, Doan H, Chiu A, Shulman E, Shab G, Kippin TE, K Szumlinski K, Profiling prefrontal cortex protein expression in rats exhibiting an incubation of cocaine craving following short-access self-administration procedures, Front. Psychiatry 13 (2023) 1031585, doi: 10.3389/fpsyt.2022.1031585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].United NationsWorld Population Ageing 2019: Highlights, Department of Economic and Social Affairs, Population Division, 2019. ST/ESA/SER.A/430. [Google Scholar]
- [54].Hascup KN, Findley CA, Sime LN, Hascup ER, Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice, Sci. Rep 10 (2020) 14503, doi: 10.1038/s41598-020-71587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Findley CA, Bartke A, Hascup KN, Hascup ER, Amyloid beta-related alterations to glutamate signaling dynamics during alzheimer’s disease progression, ASN Neuro 11 (2019) 2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ghatak S, Dolatabadi N, Trudler D, Zhang X, Wu Y, Mohata M, Ambasudhan R, Talantova M, Lipton SA, Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls, Elife 8 (2019) e50333, doi: 10.7554/eLife.50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Keyes KM, Li G, Hasin DS, Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis, Alcohol Clin. Exp. Res 35 (2011) 2101–2112, doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cortez I, Rodgers SP, Kosten TA, Leasure JL, Sex and age effects on neurobehavioral toxicity induced by binge alcohol, Brain Plast 6 (2020) 5–25, doi: 10.3233/BPL-190094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA, Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity, Pflugers Arch 465 (2013) 643–654, doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang R, Reddy PH, Role of glutamate and NMDA receptors in Alzheimer’s disease, J. Alzheimers Dis 57 (2017) 1041–1048, doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Abd-Elrahman KS, Albaker A, de Souza JM, Ribeiro FM, Schlossmacher MG, Tiberi M, Hamilton A, Ferguson SSG, Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer’s disease model mice in a sex-selective manner, Sci. Signal 13 (2020) eabd2494, doi: 10.1126/scisignal.abd2494. [DOI] [PubMed] [Google Scholar]
- [62].Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, et al. , Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5, Neuron 66 (2010) 739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE, RS)-2-chloro-5- hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus, Neuropharmacology 36 (1997) 265–267. [DOI] [PubMed] [Google Scholar]
- [64].Cox MF, Hascup ER, Bartke A, & Hascup KN Friend or Foe? Defining the role of glutamate in aging and Alzheimer’s disease. Front. Aging 3 (2022) 929474. doi: 10.3389/fragi.2022.929474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Devaud LL, Morrow AL, Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus, Eur. J. Pharmacol 369 (1999) 331–334, doi: 10.1016/s0014-2999(99)00103-x. [DOI] [PubMed] [Google Scholar]
- [66].Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA, Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice, Neuropharmacology 105 (2016) 164–174, doi: 10.1016/j.neuropharm.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Finn DA, Hashimoto JG, Cozzoli DK, Helms ML, Nipper MA, Kaufman MN, Wiren KM, Guizzetti M, Binge ethanol drinking produces sexually divergent and distinct changes in nucleus accumbens signaling cascades and pathways in adult C57BL/6J mice, Front. Genet 9 (2018) 325, doi: 10.3389/fgene.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J, Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity, Neuroscience 137 (2006) 413–423, doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- [69].Hernandez AR, Truckenbrod LM, Campos KT, Williams SA, Burke SN, Sex differences in age-related impairments vary across cognitive and physical assessments in rats, Behav. Neurosci 134 (2020) 69–81, doi: 10.1037/bne0000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Markowska AL, Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle, J. Neurosci 19 (1999) 8122–8133, doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhvania MG, Japaridze N, Tizabi Y, Lomidze N, Pochkhidze N, Lordkipanidze T, Age-related cognitive decline in rats is sex and context dependent, Neurosci. Lett 765 (2021) 136262, doi: 10.1016/j.neulet.2021.136262. [DOI] [PubMed] [Google Scholar]
- [72].Sailasuta N, Ernst T, Chang L, Regional variations and the effects of age and gender on glutamate concentrations in the human brain, Magn. Reson. Imaging 26 (2008) 667–675, doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Duarte JM, Do KQ, Gruetter R, Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy, Neurobiol. Aging 35 (2014) 1660–1668, doi: 10.1016/j.neurobiolaging.2014.01.135. [DOI] [PubMed] [Google Scholar]
- [74].Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR, The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory, Neuroscience 162 (2009) 933–945, doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Das SR, Magnusson KR, Changes in expression of splice cassettes of NMDA receptor GluN1 subunits within the frontal lobe and memory in mice during aging, Behav. Brain Res 222 (2011) 122–133, doi: 10.1016/j.bbr.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Magnusson KR, Aging of glutamate receptors: correlations between receptor binding and spatial memory performance in C57Bl mice, Mech. Ageing Dev 104 (1998) 227–248. [DOI] [PubMed] [Google Scholar]
- [77].Pelleymounter MA, Beatty G, Gallagher M, Hippocampal 3H-CPP binding and spatial learning deficits in aged rats, Psychobiology 18 (1990) 298–304. [Google Scholar]
- [78].Peterson C, Cotman CW, Strain-dependent decrease in glutamate binding to the N-methyl-D-aspartic acid receptor during aging, Neurosci. Lett 104 (1989) 309–313, doi: 10.1016/0304-3940(89)90594-6. [DOI] [PubMed] [Google Scholar]
- [79].Nicoletti VG, Condorelli DF, Dell’Albani P, Ragusa N, Giuffrida Stella AM, AMPA-selective glutamate receptor subunits in the rat hippocampus during aging, J. Neurosci. Res 40 (1995) 220–224, doi: 10.1002/jnr.490400210. [DOI] [PubMed] [Google Scholar]
- [80].Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK, Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis, Exp. Neurol 206 (2007) 70–79, doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yu DF, Wu PF, Fu H, Cheng J, Yang YJ, Chen T, Long LH, Chen JG, Wang F, Aging-related alterations in the expression and distribution of GluR2 and PICK1 in the rat hippocampus, Neurosci. Lett 497 (2011) 42–45, doi: 10.1016/j.neulet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [82].Ve H, Cabana VC, Gouspillou G, Lussier MP, Quantitative immunoblotting analyses reveal that the abundance of actin, tubulin, synaptophysin and EEA1 proteins is altered in the brains of aged mice, Neuroscience 442 (2020) 100–113, doi: 10.1016/j.neuroscience.2020.06.044. [DOI] [PubMed] [Google Scholar]
- [83].Bian C, Zhu K, Yang L, Lin S, Li S, Su B, Zhang J, Gonadectomy differentially regulates steroid receptor coactivator-1 and synaptic proteins in the hippocampus of adult female and male C57BL/6 mice, Synapse 66 (2012) 849–857, doi: 10.1002/syn.21574. [DOI] [PubMed] [Google Scholar]
- [84].Devaud LL, Morrow AL, Nguyen UT, Ovariectomy has minimal effects on neuroadaptations associated with ethanol dependence in female rats, Neurochem. Int 37 (2000) 433–442, doi: 10.1016/s0197-0186(00)00052-8. [DOI] [PubMed] [Google Scholar]
- [85].Aribas E, van Lennep JER, De Rijke YB, Laven JSE, Ikram MA, Peeters RP, Kavousi M, Sex steroids and sex steroid-binding globulin levels among middle-ged and elderly men and women from general population, Eur. J. Clin. Invest (2022) e13866 epub ahead of print, doi: 10.1111/eci.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Longitudinal effects of aging on serum total and free testosterone levels in healthy men, J. Clin. Endocrinol. Metab 86 (2001) 724–731. [DOI] [PubMed] [Google Scholar]
- [87].Piggott MA, Perry EK, Perry RH, Court JA, [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging, Brain Res 588 (1992) 277–286. [DOI] [PubMed] [Google Scholar]
- [88].Ménard C, Quirion R, Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways, PLoS One 7 (2012) e28666, doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM, Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome, J. Neurochem 113 (2010) 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Irizarry MC, Locascio JJ, Hyman BT, β-Site APP cleaving enzyme mRNA expression in APP transgenic mice: anatomic overlap with transgene expression and static levels with aging, Am. J. Pathol 158 (2001) 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mann K, Ackerman K, Croissant B, Mundle G, Nakovics H, Diehl A, Neuroimaging of gender differences in alcohol dependence: are women more vulnerable, Alcohol Clin. Exp. Res 29 (2005) 896–901. [DOI] [PubMed] [Google Scholar]
- [92].Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF, Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects, Alcohol Clin. Exp. Res 32 (2008) 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Crowley NA, Magee SN, Feng M, Jefferson SJ, Morris CJ, Dao NC, Brockway DF, & Luscher B Ketamine normalizes binge drinking-induced defects in glutamatergic synaptic transmission and ethanol drinking behavior in female but not male mice [DOI] [PMC free article] [PubMed]
- [94].Devaud LL, Alele PE, differential effects of chronic ethanol administration and withdrawal on βaminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain, Alcohol Clin. Exp. Res 28 (2004) 957–965. [DOI] [PubMed] [Google Scholar]
- [95].Yasojima K, McGeer EG, McGeer PL, Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain, Brain Res 919 (2001) 115–121. [DOI] [PubMed] [Google Scholar]
- [96].Bodendorf U, Simone D, Fischer F, Stefani M, Sturchler-Pierrat C, K.−.H. Wiederhold, M. Staufenbiel, P. Paganetti, Expression of human β-secretase in mouse brain increases the steady-state level of β-amyloid, J. Neurochem 80 (2002) 799–806. [DOI] [PubMed] [Google Scholar]
- [97].Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R, Mice deficient in BACE1, the Alzheimer’s betasecretase, have normal phenotype and abolished beta-amyloid generation, Nat. Neurosci 4 (2001) 231–232. [DOI] [PubMed] [Google Scholar]
- [98].Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L, BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics, Hum. Mol. Genet 10 (2001) 1317–1324. [DOI] [PubMed] [Google Scholar]
- [99].Wang JZ, Grundke-Iqbal I, Iqbal K, Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration, Eur. J. Neurosci 25 (2007) 59–68, doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Luo J, GSK3beta in ethanol neurotoxicity, Mol. Neurobiol 40 (2009) 108–21, doi: 10.1007/s12035-009-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ji Z, Yuan L, Lu X, Ding H, Luo J, Ke ZJ, Binge alcohol exposure causes neurobehavioral deficits and GSK3β activation in the hippocampus of adolescent rats, Sci. Rep 8 (2018) 3088, doi: 10.1038/s41598-018-21341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA, Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking, Alcohol. Clin. Exp. Res 30 (2006) 1322–1335. [DOI] [PubMed] [Google Scholar]
- [103].Behl T, Yadav HN, Sharma PL, Alcoholic neuropathy: involvement of multifaceted signalling mechanisms, Curr. Mol. Pharmacol 14 (2021) 2–10, doi: 10.2174/1874467213666200512114943. [DOI] [PubMed] [Google Scholar]
- [104].Morisot N, Ron D, Alcohol-dependent molecular adaptations of the NMDA receptor system, Genes Brain Behav 16 (2017) 139–148, doi: 10.1111/gbb.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lim JR, Lee HJ, Jung YH, et al. , Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation, Cell Commun. Signal 18 (2020) 123, doi: 10.1186/s12964-020-00572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Easton AC, Lucchesi W, Lourdusamy A, Lenz B, Solati J, Golub Y, Lewczuk P, Fernandes C, Desrivieres S, Dawirs RR, Moll GH, Kornhuber J, Frank J, Hoffmann P, Soyka M, Kiefer F, et al. , αCaMKII autophosphorylation controls the establishment of alcohol drinking behavior, Neuropsychopharmacology 38 (2013) 1636–1647 Erratum in: Neuropsychopharmacology 38 (2013) 2735, doi: 10.1038/npp.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Revett TJ, Baker GB, Jhamandas J, Kar S, Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology, J. Psychiatry Neurosci 38 (2013) 6–23, doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


