Abstract
Background
Cognitive impairments affect functional ability in people with dementia. Cognitive rehabilitation (CR) is a personalised, solution‐focused approach that aims to enable people with mild‐to‐moderate dementia to manage everyday activities and maintain as much independence as possible.
Objectives
To evaluate the effects of CR on everyday functioning and other outcomes for people with mild‐to‐moderate dementia, and on outcomes for care partners.
To identify and explore factors that may be associated with the efficacy of CR.
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group Specialised Register, which contains records from MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS, and other clinical trial databases, and grey literature sources. The most recent search was completed on 19 October 2022.
Selection criteria
We included randomised controlled trials (RCTs) comparing CR with control conditions and reporting relevant outcomes for the person with dementia and/or the care partner.
Data collection and analysis
We extracted relevant data from published manuscripts and contacted trial authors if necessary. Within each of the comparisons, we pooled data for each outcome of interest and conducted inverse‐variance, random‐effects meta‐analyses. We evaluated the certainty of the evidence using GRADEpro GDT.
Main results
We identified six eligible RCTs published in English between 2010 and 2022, which together included 1702 participants. The mean age of participants ranged from 76 to 80 and the proportion of male participants was between 29.4% and 79.3%. Most participants, in the studies where the type of dementia was reported, had a diagnosis of Alzheimer’s disease (AD; n = 1002, 58.9% of the whole sample, 81.2% of the participants for whom the specific diagnosis was reported).
Risk of bias in the individual studies was relatively low. The exception was a high risk of bias in relation to blinding of participants and practitioners, which is not usually feasible with psychosocial interventions.
Our primary outcome of everyday functioning was operationalised in the included studies as goal attainment in relation to activities targeted in the intervention. For our main comparison of CR with usual care, we pooled data for goal attainment evaluated from three perspectives (self‐rating of performance, informant rating of performance, and self‐rating of satisfaction with performance) at end of treatment and at medium‐term follow‐up (3 to 12 months). We could also pool data at these time points for 20 and 19 secondary outcomes respectively. The review findings were strongly driven by one large, high‐quality RCT.
We found high‐certainty evidence of large positive effects of CR on all three primary outcome perspectives at the end of treatment: participant self‐ratings of goal attainment (standardised mean difference (SMD) 1.46, 95% confidence interval (CI) 1.26 to 1.66; I2 = 0%; 3 RCTs, 501 participants), informant ratings of goal attainment (SMD 1.61, 95% CI 1.01 to 2.21; I2 = 41%; 3 RCTs, 476 participants), and self‐ratings of satisfaction with goal attainment (SMD 1.31, 95% CI 1.09 to 1.54; I2 = 5%; 3 RCTs, 501 participants), relative to an inactive control condition. At medium‐term follow‐up, we found high‐certainty evidence showing a large positive effect of CR on all three primary outcome perspectives: participant self‐ratings of goal attainment (SMD 1.46, 95% CI 1.25 to 1.68; I2 = 0%; 2 RCTs, 432 participants), informant ratings of goal attainment (SMD 1.25, 95% CI 0.78 to 1.72; I2 = 29%; 3 RCTs, 446 participants), and self‐ratings of satisfaction with goal attainment (SMD 1.19, 95% CI 0.73 to 1.66; I2 = 28%; 2 RCTs, 432 participants), relative to an inactive control condition.
For participants at the end of treatment we found high‐certainty evidence showing a small positive effect of CR on self‐efficacy (2 RCTs, 456 participants) and immediate recall (2 RCTs, 459 participants).
For participants at medium‐term follow‐up we found moderate‐certainty evidence showing a small positive effect of CR on auditory selective attention (2 RCTs, 386 participants), and a small negative effect on general functional ability (3 RCTs, 673 participants), and we found low‐certainty evidence showing a small positive effect on sustained attention (2 RCTs, 413 participants), and a small negative effect on memory (2 RCTs, 51 participants) and anxiety (3 RCTs, 455 participants).
We found moderate‐ and low‐certainty evidence indicating that at the end of treatment CR had negligible effects on participant anxiety, quality of life, sustained attention, memory, delayed recall, and general functional ability, and at medium‐term follow‐up on participant self‐efficacy, depression, quality of life, immediate recall, and verbal fluency.
For care partners at the end of treatment we found low‐certainty evidence showing a small positive effect on environmental aspects of quality of life (3 RCTs, 465 care partners), and small negative effects of CR on level of depression (2 RCTs, 32 care partners) and on psychological wellbeing (2 RCTs, 388 care partners).
For care partners at medium‐term follow‐up we found high‐certainty evidence showing a small positive effect of CR on social aspects of quality of life (3 RCTs, 436 care partners) and moderate‐certainty evidence showing a small positive effect on psychological aspects of quality of life (3 RCTs, 437 care partners).
We found moderate‐ and low‐certainty evidence at the end of treatment that CR had negligible effects on care partners’ physical health, psychological and social aspects of quality of life, and stress, and at medium‐term follow‐up for the physical health aspect of care partners’ quality of life and psychological wellbeing.
Authors' conclusions
CR is helpful in enabling people with mild or moderate dementia to improve their ability to manage the everyday activities targeted in the intervention. Confidence in these findings could be strengthened if more high‐quality studies contributed to the observed effects. The available evidence suggests that CR can form a valuable part of a clinical toolkit to assist people with dementia in overcoming some of the everyday barriers imposed by cognitive and functional difficulties. Future research, including process evaluation studies, could help identify avenues to maximise CR effects and achieve wider impacts on functional ability and wellbeing.
Keywords: Humans, Male, Activities of Daily Living, Alzheimer Disease, Anxiety, Cognitive Training, Dementia
Plain language summary
What are the benefits and risks of cognitive rehabilitation for people with mild‐to‐moderate dementia?
Key messages
Cognitive rehabilitation helps people living with dementia to manage everyday activities that are important to them.
Future studies could explore how to use cognitive rehabilitation to also improve overall functioning and wellbeing.
What is dementia?
Dementia is a group of symptoms caused by changes in the brain that get worse over time. People with some types of dementia have difficulties with memory, planning, concentrating, and communicating. These and other thinking difficulties are collectively described by the umbrella term, 'cognitive impairment'. Cognitive impairment makes it harder to do daily activities and stay independent for as long as possible.
What is cognitive rehabilitation?
Cognitive rehabilitation is a personalised intervention. People have one‐to‐one sessions with a practitioner, usually in their own home. People identify everyday activities and tasks that they would like to manage better or do more independently. The practitioner suggests strategies and works with them to help achieve these improvements in the activities that are important to them. Family members are often involved as well.
What did we want to find out?
We explored whether cognitive rehabilitation was better than usual treatment for: doing a chosen task or activity that matters to the person; managing daily activities; feeling confident about being able to manage things; feeling depressed or anxious; having a sense of wellbeing.
We also explored whether cognitive rehabilitation was better for ensuring the wellbeing of the care partner ‐ usually a husband, wife, or other close family member.
What did we do? We searched for studies that rigorously tested the effects of cognitive rehabilitation for people with mild‐to‐moderate dementia. In these studies, some people had their usual treatment and others had their usual treatment plus cognitive rehabilitation. This made it possible to see whether cognitive rehabilitation was more helpful than usual treatment alone. We compared and summarised the results of the studies. We rated our confidence in the evidence the studies provided, based on the methods used and the numbers of people involved.
What did we find?
We found six studies. They involved 1702 people with mild‐to‐moderate dementia, who had between 8 and 14 sessions with a cognitive rehabilitation practitioner. Alzheimer’s disease was the most common dementia diagnosis (59% of all participants, 82% of participants with the specific diagnosis reported).
The main findings are that, compared to people who just had their usual treatment, people who had cognitive rehabilitation got better at doing their chosen tasks or activities.
This improvement was seen by the people with dementia and by their care partners.
The improvement was seen straight after cognitive rehabilitation and was still noticeable 3 to 12 months later.
Other results
Straight after cognitive rehabilitation, compared to people who just had their usual treatment, people with dementia may feel more confident about how they are managing.
There might not be any differences in the wellbeing of people with dementia and their care partners.
We are not sure if there are any differences for people with dementia in managing other tasks or activities or in feeling depressed.
Three to 12 months after cognitive rehabilitation, compared to usual treatment, care partners may have better psychological wellbeing.
There may not be any differences in how well people with dementia manage other tasks or activities, in how confident or depressed they feel, or in their wellbeing.
What are the limitations of the evidence?
Our review included six studies, but the findings are based mostly on information from one large study. We do not know if the effects of cognitive rehabilitation last more than a year. Results for several effects of cognitive rehabilitation were not clear.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to October 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Cognitive rehabilitation compared to inactive control condition for people with mild‐to‐moderate dementia (at the end of therapy).
| Cognitive rehabilitation compared to inactive control condition for people with mild‐to‐moderate dementia (at the end of therapy) | ||||||
| Patient or population: people with mild‐to‐moderate dementia (at the end of therapy) Setting: community dwelling Intervention: cognitive rehabilitation Comparison: inactive control condition | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with inactive control condition | Risk with cognitive rehabilitation | |||||
| Functional ability in targeted activities: personal goals ‐ performance (participant self‐report) Assessed with: BGSI, COPM Follow‐up: range 2 to 3 months | — | SMD 1.46 higher (1.26 higher to 1.66 higher) | — | 501 (3 RCTs) | ⊕⊕⊕⊕ High | Cognitive rehabilitation improves functional ability in targeted activities (performance in relation to personal goals, as self‐reported by participant) |
| Functional ability in targeted activities: personal goals ‐performance (informant report of participant) Assessed with: BGSI, DMT Follow‐up: range 1 to 3 months | — | SMD 1.61 higher (1.01 higher to 2.21 higher) | — | 476 (3 RCTs) | ⊕⊕⊕⊕ High | Cognitive rehabilitation improves functional ability in targeted activities (performance in relation to personal goals, as reported by informant) |
| General functional ability (informant report of participant) Assessed with: DAD, BADL Follow‐up: range 1 to 3 months | — | SMD 0.05 SD higher (0.1 lower to 0.2 higher) | — | 673 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | Cognitive rehabilitation may result in little or no difference in general functional ability |
| Self‐efficacy (participant self‐report) Assessed with: GSES Scale from: 10 to 40 Follow‐up: range 2 to 3 months | The mean self‐efficacy (participant self‐report) was 0 | MD 0.71 higher (0.12 higher to 1.3 higher) | — | 456 (2 RCTs) | ⊕⊕⊕⊕ High | Cognitive rehabilitation slightly improves self‐efficacy of participants |
| Mood: depression (participant self‐report) Assessed with: HADS Scale from: 0 to 21 Follow‐up: range 2 to 3 months | The mean mood: depression (participant self‐report) was 0 | MD 1.45 higher (0.39 lower to 3.29 higher) | — | 502 (3 RCTs) | ⊕⊝⊝⊝ Very lowd,e | We are uncertain whether cognitive rehabilitation makes a change to depressive symptoms in participants |
| Quality of life (participant self‐report) Assessed with: QoL‐AD, DQoL, DEMQOL, WHO QoL (composite) Follow‐up: range 1 to 3 months | — | SMD 0.06 SD lower (0.19 lower to 0.08 higher) | — | 853 (5 RCTs) | ⊕⊕⊕⊝ Moderatec | Cognitive rehabilitation probably results in little or no difference in overall quality of life of participants |
| Quality of life: psychological (care partner self‐report) Assessed with: WHOQOL‐BREF Scale from: 4 to 20 Follow‐up: range 2 to 3 months | The mean quality of life: psychological (care partner self‐report) was 0 | MD 0.22 higher (0.28 lower to 0.71 higher) | — | 464 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | Cognitive rehabilitation probably results in little or no difference in the psychological aspect of quality of life of care partners |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BGSI: Bangor Goal‐Setting Interview; CI: confidence interval; COPM: Canadian Occupational Performance Measure; DAD: Disability Assessment for Dementia; DEMQOL: DEMentia Quality Of Life; DMT: Direct Measure of Training; DQoL: Dementia Quality of Life; ED5D3L: Euroqol Questionnaire ‐ short; FAQ: Functional Activities Questionnaire; GSES: Generalized Self‐Efficacy Scale, HADS: Hospital Anxiety and Depression Scale; MD: mean difference; QoL‐AD: Quality of Life in Alzheimer's Disease; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference; WHOQOL‐BREF: World Health Organization’s Quality of Life Instrument (short version) | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_435474071382494872. | ||||||
aDowngraded by 1 point as there are serious concerns related to risk of bias: in addition to no blinding of participants we noted potential selective reporting or incomplete outcome data in the included studies. bDowngraded by 1 point as there are serious concerns related to imprecision: the confidence interval crosses two interpretation categories (including both the benefit and harm categories). cDowngraded by 1 point as there are serious concerns related to imprecision: the confidence interval crosses two interpretation categories (including both the benefit and harm categories). dDowngraded by 2 points as there are very serious concerns regarding relatively large and statistically significant heterogeneity in effect size. eDowngraded by 2 points as there are very serious concerns related to imprecision: the confidence interval crosses three interpretation categories (including both the benefit and harm categories).
Summary of findings 2. Summary of findings table ‐ Cognitive rehabilitation compared to inactive control condition for people with mild‐to‐moderate dementia (at medium‐term follow‐up).
| Cognitive rehabilitation compared to inactive control condition for people with mild‐to‐moderate dementia (at medium‐term follow‐up) | ||||||
| Patient or population: people with mild‐to‐moderate dementia (at medium‐term follow‐up) Setting: community dwelling Intervention: cognitive rehabilitation Comparison: inactive control condition | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with inactive control condition | Risk with cognitive rehabilitation | |||||
| Functional ability in targeted activities: personal goals ‐ performance (participant self‐report) Assessed with: BGSI, COPM, BADL Follow‐up: range 6 to 9 months | — | SMD 1.46 higher (1.25 higher to 1.68 higher) | — | 432 (2 RCTs) | ⊕⊕⊕⊕ High | Cognitive rehabilitation improves functional ability in targeted activities (performance in relation to personal goals, as self‐reported by participant) |
| Functional ability in targeted activities: personal goals ‐ performance (informant report of participant) Assessed with: BGSI, DMT Follow‐up: range 3 to 9 months | — | SMD 1.25 higher (0.78 higher to 1.72 higher) | — | 446 (3 RCTs) | ⊕⊕⊕⊕ High | Cognitive rehabilitation improves functional ability in targeted activities (performance in relation to personal goals, as reported by informant) |
| General functional ability (informant report of participant) Assessed with: DAD, FAQ Follow‐up: range 3 to 6 months | — | SMD 0.23 SD lower (0.43 lower to 0.03 lower) | — | 380 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | Cognitive rehabilitation may result in a slight decline in participants' general functional ability |
| Self‐efficacy (participant self‐report) Assessed with: GSES Scale from: 10 to 40 Follow‐up: range 6 to 9 months | The mean self‐efficacy (participant self‐report) was 0 | MD 0.58 higher (0.05 lower to 1.21 higher) | — | 417 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | Cognitive rehabilitation probably makes little or no difference to self‐efficacy of participants |
| Mood: depression (participant self‐report) Assessed with: HADS Scale from: 0 to 21 Follow‐up: range 6 to 9 months | The mean mood: depression (participant self‐report) was 0 | MD 0.14 lower (0.49 lower to 0.2 higher) | — | 456 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | Cognitive rehabilitation probably makes little or no difference to participants' depressive symptoms |
| Quality of life (participant self‐report) Assessed with: QoL‐AD, DQoL, DEMQOL, WHO QoL (composite) Follow‐up: range 3 to 9 months | — | SMD 0.05 SD lower (0.32 lower to 0.22 higher) | — | 783 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | Cognitive rehabilitation probably makes little or no difference to participants' quality of life |
| Quality of life: psychological (care partner self‐report) Assessed with: WHOQOL‐BREF Scale from: 4 to 20 Follow‐up: range 6 to 9 months | The mean quality of life: psychological (care partner self‐report) was 0 | MD 0.4 higher (0.24 lower to 1.05 higher) | — | 437 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | Cognitive rehabilitation probably slightly improves the psychological aspect of quality of life of care partners |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BGSI: Bangor Goal‐Setting Interview; CI: confidence interval; COPM: Canadian Occupational Performance Measure; DAD: Disability Assessment for Dementia; DEMQOL: DEMentia Quality Of Life; DMT: Direct Measure of Training; DQoL: Dementia Quality of Life; ED5D3L: Euroqol Questionnaire ‐ short; FAQ: Functional Activities Questionnaire; GSES: Generalized Self‐Efficacy Scale, HADS: Hospital Anxiety and Depression Scale; MD: mean difference; QoL‐AD: Quality of Life in Alzheimer's Disease; SD: standard deviation; SMD: standardised mean difference; WHOQOL‐BREF: World Health Organization’s Quality of Life Instrument (short version) | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_435474166653226654. | ||||||
aDowngraded by 1 point as there are serious concerns related to imprecision: the analysis is based on fewer than 400 participants. bDowngraded by 1 point as there are serious concerns related to imprecision: the confidence interval crosses two interpretation categories (including both the benefit and harm categories).
Background
Description of the condition
Dementia is a general term for a number of progressive neurodegenerative conditions arising predominantly in later life. The prevalence of dementia increases with age (ADI 2015), and Alzheimer’s Disease International estimates that there are over 55 million people living with dementia worldwide (ADI 2023). Changes in lifestyle, and consequently in health status and life expectancy, translate into differences in incidence and prevalence rates between countries and generations. Monitoring the prevalence of dementia is challenging. Data collected in different countries and across various studies cannot be easily compared due to variations in the diagnostic process and evolving diagnostic criteria (Wu 2017). The general trend is for people to live longer, so regardless of these limitations, the number of people with dementia is expected to increase to 74.7 million by 2030, and to 131.5 million by 2050. The risk of dementia is higher for those with poorer cardiovascular health, and with worse access to education and healthcare (Prince 2015; Wu 2017). Alzheimer’s disease is the most common form of dementia, which accounts for approximately 62% of cases, followed by vascular dementia (17%), and mixed Alzheimer’s disease and vascular dementia (10%) (Prince 2014). Rarer forms of dementia include the Parkinsonian dementias, Parkinson’s disease dementia (PDD, 2%), and dementia with Lewy bodies (DLB, 4%), and the behavioural and semantic variants of frontotemporal dementia (FTD, 2%).
Each type of dementia in the mild‐to‐moderate stages has its own profile of cognitive changes, which can be demonstrated on neuropsychological testing, although as dementia progresses further, the differences become less distinguishable. A useful summary is provided by Weintraub 2012. Alzheimer’s disease is characterised by impairments in episodic memory; other cognitive domains, such as executive function, are also affected. In vascular dementia, episodic memory may be less impaired, while executive functioning, attention, and perception are more affected. Parkinsonian dementias are characterised by impairment in attention, executive function, and visual perception (Kudlicka 2011). Among the frontotemporal dementias, semantic dementia is characterised by loss of conceptual knowledge and vocabulary; the behavioural variant is characterised by executive dysfunction (Chare 2014; Hodges 1992).
Cognitive impairments affect functional ability (Martyr 2012a; Royall 2007). Impaired ability to function in daily life is a core feature of dementia, progressing from mild difficulty with instrumental activities of daily living in the early stages to dependence on others for basic activities of daily living in the later, severe stages (Boyle 2002; Njegovan 2001). Even in the early stages of dementia, impaired functional ability impacts on independence, and may result in loss of confidence and withdrawal from activities, leading to what has been termed ‘excess’ or unnecessary additional disability (Reifler 1990). Impairments in functional ability, and associated excess disability, contribute significantly to caregiver burden (Martyr 2014; Razani 2007). Supporting functional ability by enabling people with dementia to function at their best level given their underlying impairments is potentially an important target for intervention (Poulos 2017).
Description of the intervention
Cognitive rehabilitation (CR) is a personalised approach, based on a problem‐solving framework, which enables people with dementia to engage in, or manage, everyday activities, function optimally, and maintain as much of their independence as possible. Rehabilitation denotes a positive approach to enabling people to make the most of their functional ability; in some settings, especially community settings, reablement is a more commonly used descriptor (Poulos 2017). The terms CR and the equivalent, neuropsychological rehabilitation, were first introduced to differentiate this approach from rehabilitation for physical disabilities. Cognitive, or neuropsychological, indicates that the intervention addresses the impact of cognitive impairments on everyday life, and on engagement in everyday activities. None of these terms imply that the underlying impairment can be removed, or that there are attempts to restore or improve cognitive function; instead, they emphasise a solution‐focused approach to managing the everyday challenges that result from the impairment (McLellan 1991).
Originally developed for people living with cognitive impairment as a result of brain injury (Wilson 2002), the CR approach was adapted for people with dementia, and is consistent with the values of person‐centred dementia care (Clare 2017). Its goal is to support independence and social participation, in line with many European and worldwide organisations that promote strategies to maximise functional ability in the older population and in people with dementia (EIPAHA 2012; Myshra 2016; WHO 2018). The term also recognises the right of people with dementia to receive support that enables them to reach their best possible level of functioning. This may be important for the sustainability of healthcare systems, as improved functioning in everyday activities may potentially reduce the need for paid support and unnecessary hospitalisation (Clare 2017), and prevent premature admission to residential care (Amieva 2016). CR practitioners may be drawn from various professional backgrounds, such as neuropsychology, clinical psychology, occupational therapy, or nursing. Often, a qualified practitioner will supervise less qualified staff, such as assistant psychologists or occupational therapy technicians. Other groups of staff, such as home support workers, may be trained to implement this approach under supervision.
The aim of CR is to improve functioning in areas that the recipient identifies as personally relevant and important (Clare 2008). These targeted areas are typically outlined in the form of personal goals that the individual wishes to attain. CR for people with dementia is usually conducted in the person’s home setting, or the environment in which the targeted activities generally occur. Transferring new learning to different situations is a challenge in behavioural interventions, and this can be avoided by working directly in the context in which the new skills will be used. Consequently, CR is usually offered as an individual intervention, rather than in group formats.
If cognitive impairments have progressed to the point where the person does not readily understand or engage in the rehabilitation process, the practitioner may use the CR approach to help care partners (e.g. family members, care workers, care home staff, or home support staff) develop more effective strategies to support and enable the person with dementia. However, this review will consider interventions for people with mild‐to‐moderate dementia who are still able to engage in the process of identifying their rehabilitation goals.
During the goal‐setting process, the CR practitioner works with individuals to identify the areas of daily life that they wish to manage better. The practitioner assesses three areas:
The person. The practitioner needs to understand the person’s current level of functioning, where difficulties arise and why, and whether the person could potentially function better if secondary issues such as loss of confidence or lack of necessary support were to be addressed.
The context. The practitioner needs to understand the environment in which the person is operating, and factors that could either facilitate or hinder progress towards the achievement of personal goals. This includes the nature of the relationship with family members or friends, and the level of support that might be forthcoming. Family members may have their own priority areas to be addressed, and negotiation may be required to arrive at a set of goals that meets the needs and wishes of both parties.
The activity. The practitioner needs to understand the nature and demands of each activity or task that the person wishes to manage better, the steps involved in completing it, and what strategies, if any, have already been attempted. If the person is currently doing the activity, the practitioner needs to identify where any problems or difficulties arise, and what needs to change to enable the activity to be undertaken more successfully.
Based on this assessment, the practitioner clarifies the goals, ensures they are realistic, and draws on a set of evidence‐based or practice‐tested methods and techniques to prepare an individual rehabilitation plan. This may include the following methods.
Engender procedural learning through developing habits and routines; for example, designating and using a specific place to leave important personal items, learning to make calls and send messages on a smartphone, or using a dosette box to manage medication.
Reactivate previous knowledge; for example, remembering and using the names of one’s grandchildren.
Compensate for known difficulties and challenges, modifying tasks or the environment, or introducing assistive technology; for example, developing strategies to avoid being distracted and lose concentration when preparing meals.
Build individual strategies to support functioning in specific situations or re‐engaging in a previously enjoyed activity; for example, joining the conversation at the family dinner table.
Address specific dementia‐related difficulties; for example, reactivating knowledge of vocabulary and concepts for people with semantic dementia.
Evidence‐based techniques used in CR interventions include both enhanced learning methods and introduction of compensatory strategies. Enhanced learning methods include modelling, prompting with gradual fading of prompts, and expanding rehearsal of information (Clare 2008). While errorless learning approaches are sometimes recommended, evidence suggests that reducing or removing errors during learning does not confer benefits for people with dementia (Dunn 2007; Voigt‐Radloff 2017), although making fewer errors may make learning more congenial by reducing the experience of failure. Some activities can be broken down into steps and practised one step at a time until the whole sequence of steps has been mastered. Compensatory strategies and memory aids may be introduced with the support of the CR practitioner where appropriate.
The CR practitioner works with the person, and where appropriate with his or her family or other supporters, to implement the rehabilitation plan. The practitioner encourages supporters to learn the techniques so that they can facilitate between‐session practice. As people differ in how they respond to particular strategies and techniques, the practitioner may need to try more than one strategy to identify the approach that works best for a given individual. Therefore, the practitioner might adapt the rehabilitation plan, based on ongoing evaluation of its progress and assessment of the extent to which goals are achieved. Additional elements may be incorporated into the intervention where needed; for example, an individual may need to develop anxiety management skills before advancing to selected goals. The level of support may vary in length and number of sessions, and the extent to which the broader personal and social context is addressed; for example, it may include help to manage depression and anxiety or advice for family members.
In research trials, the CR approach may be adapted to allow more defined methods of evaluation. For example, a researcher who is not the treating practitioner may be involved in eliciting goals and rate progress; this means that practitioners may be working with goals on which they had no prior input. Goals may also be selected from a pre‐defined list, rather than developing them de novo with the individual. Progress may be evaluated through self‐ or informant ratings in relation to goals, observation of performance, or objective tests, rather than practitioner evaluation of outcomes (Clare 2019; Voigt‐Radloff 2017).
How the intervention might work
CR is a behaviour change intervention, based on an understanding of the cognitive changes seen in mild‐to‐moderate dementia, which builds on relatively preserved cognitive abilities to address and overcome the impact of cognitive impairment. It has long been understood that people with mild‐to‐moderate dementia have considerable retained cognitive and behavioural capacities, and are capable of behaviour change and some new learning, given appropriate support (Backman 1992; Fernández‐Ballesteros 2003; Little 1986). For example, in Alzheimer’s disease, vascular dementia, and mixed dementia memory problems are common. Neuropsychological models distinguish different types and processes of memory, and experimental studies show that these different types of memory are differentially affected; episodic memory (memory for events and personal experiences) is impaired, but procedural memory (learned habits and routines) is relatively spared in people with mild‐to‐moderate stages of these types of dementia (Squire 1995). Therefore, by providing strategies that draw on relatively preserved processes, it is possible to compensate for the results of more severe impairment in other areas (Bahar‐Fuchs 2013).
Psychologically, the experience of successfully achieving goals and improving everyday function could increase feelings of self‐efficacy and help to counter negative consequences of dementia, such as loss of confidence, thus reducing excess disability (Marshall 2005).
Family members or other supporters may benefit in several ways. They may feel less burdened as the person with dementia functions better in targeted areas of daily life. They are supported to learn some of the rehabilitative strategies themselves and can apply them when new difficulties arise after the therapy sessions end. Involvement in the therapy process can improve understanding of dementia and the person’s behaviour, which in turn enables care partners to have more patience with the person with dementia, and improves the relationship overall (Clare 2019).
Why it is important to do this review
Impairments in functional ability form part of the diagnostic criteria for dementia and are a defining characteristic of the condition (APA 2013; WHO 2018). Among people with dementia, better functional ability is associated with higher self‐ and informant‐ratings of quality of life (Bosboom 2012; Clare 2022b; Dourado 2016; Martyr 2018; Woods 2014). In mild‐to‐moderate dementia, there is a significant decline in ability to carry out instrumental activities of daily living. Diminished functional ability limits independence, adds to caregiver burden, and can result in a loss of confidence and withdrawal from activities (McLaughlin 2010). Despite this, limited attention has been paid to strategies that support functional ability. CR, if effective, could form a valuable component of support for people with dementia and their families.
In previous Cochrane Reviews, CR was included with cognitive training, and the most recent update found only one randomised controlled trial of CR (Bahar‐Fuchs 2013). Because the volume of evidence relating to CR has increased since that time, these two very different interventions (see Table 1 in Bahar‐Fuchs 2019) are now the subject of separate reviews.
Objectives
To evaluate the effects of CR on everyday functioning and other outcomes for people with mild‐to‐moderate dementia, and on outcomes for care partners.
To identify and explore factors that may be associated with the efficacy of CR.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared cognitive rehabilitation (CR) with an inactive control condition (treatment as usual or a waiting‐list), a non‐specific active control intervention, and/or an alternative treatment. Trials with a cross‐over design were eligible if there were sufficient data available for the first period only (Elbourne 2002). We excluded other study designs to limit the risk of bias in estimates of treatment effects (Reeves 2011). We did not impose any language or date restrictions in the search strategy. For possibly relevant studies published in a language other than English, we attempted to obtain translations. Where a translation was not available prior to submission of the completed review, we filed the studies under 'awaiting classification'.
Studies had to include, at a minimum, baseline and post‐treatment evaluations. Further follow‐up, where available, could be of any duration.
Types of participants
Participant characteristics: adults of any age and background. They might, or might not, have an unpaid caregiver (spouse or partner, family member, or friend) who supported their participation and provided relevant information.
Diagnosis: dementia, of any type, made according to established clinical and research criteria, as indicated in the following examples.
The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM‐V, APA 2013), or earlier versions (APA 1995)
The International Classification of Diseases, 11th revision (ICD‐11, WHO 2018), or earlier versions (ICD‐10)
The National Institute of Neurological and Communicative Disorders ‐ Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA, McKhann 1984)
The National Institute of Health ‐ Alzheimer’s Association (NIA‐AA, McKhann 2011)
The Association Internationale pour la Recherché et l’Enseignement en Neurosciences (NINDS‐AIREN, Román 1993)
Vascular Impairment of Cognition Classification Consensus Study (McKeith 1996; McKeith 2006; McKeith 2017)
The International Behavioural Variant FTD Criteria Consortium (FTDC, Skrobot 2018)
Stage of dementia: mild‐to‐moderate level of severity, on average, as indicated by group mean scores, score ranges, or individual scores, on measures used to indicate dementia severity. We used an internationally recognised dementia staging system, the Clinical Dementia Rating (CDR), as a reference, along with equivalent scores on other screening tests (Hughes 1982). Mild‐to‐moderate level of severity was indicated by scores of 0.5 to 2 on the CDR; 11 or above on the Mini‐Mental State Examination (MMSE; Folstein 1975); a Montreal Cognitive Assessment (MoCA) raw score of 5 or above (Nasreddine 2005; Roalf 2013); or an Addenbrooke's Cognitive Examination (ACE‐III and ACE‐R) score of 27 or above (Matías‐Guiu 2018; Perneczky 2006). We did not set an upper limit for screening test scores, as the study participants had to have a diagnosis of dementia. We included studies where fewer than 20% of participants fell outside the mild‐to‐moderate level of severity, provided this information was clearly indicated.
Pharmacological treatment: participants in both the intervention and control groups could be receiving concurrent pharmacological treatment for dementia as a randomly distributed covariate. Where available, we noted information about participants’ use of such medication, including information about whether participants were receiving a stable dose.
Types of interventions
We included interventions that met our definition of CR. We acknowledged that terminology in the field of non‐pharmacological interventions for people with dementia is inconsistent, and researchers might use alternative terms such as reablement or remediation. In some cases, the term cognitive rehabilitation could be incorrectly applied to describe different approaches, such as cognitive training or cognitive stimulation. CR protocols vary considerably across clinical practice and research trials. For example, CR could form part of a comprehensive programme that includes formal therapy for mood disorders and counselling for family members, or the term could refer to a set of techniques that address memory or attention difficulties (Kudlicka 2018). For consistency, we defined CR as a therapy encompassing the following elements.
Focuses on functioning in everyday activities.
Addresses specific targeted activities chosen or identified as important by each individual participant, with the selected activities usually expressed in terms of personal goals that the participant wishes to achieve.
Applies an individual, personalised therapy plan, aimed at improving performance in, or management of, these activities, based on an assessment of the person’s current functioning and intrinsic capacity and on an evaluation of the demands of the targeted activities.
Uses recognised rehabilitative strategies and methods to enable the person to compensate for, manage, or overcome functional limitations regarding the targeted activities.
For the purposes of selecting studies for this review, we operationalised this definition in the following way.
The intervention aims to improve functioning in everyday activities (i.e. not on abstract exercises, puzzles, or tests).
-
The intervention is personalised, as indicated by at least one of the following features.
The therapy objective is chosen by the person with dementia, or a family supporter, or both, although it may be selected from a pre‐defined list.
The therapy plan is based on an assessment of the person’s current functioning and capacity.
The therapy strategies reflect the person’s ability and therapy objectives (i.e. the intervention does not use the same method for every person, every goal, or both).
-
The intervention uses recognised cognitive rehabilitation techniques, including at least one of the following techniques.
Graded activity
Modelling
Action‐based learning
Expanding rehearsal (also known as spaced retrieval)
Prompting and fading
Altering features of the person’s environment and surroundings
Mnemonics, elaboration, and vanishing cues for learning or relearning information
Introducing compensatory strategies such as memory aids
We expected that the practitioner would deliver the intervention in the person’s home setting, or in the everyday environment in which the targeted activities were undertaken, and provide it on a one‐to‐one basis, over several sessions. We considered interventions provided in group formats if they met the above criteria. In some cases, CR was combined with other interventions delivered at the same time, such as cognitive training or physical exercise (Bahar‐Fuchs 2019). We excluded trials where this was the case, as it would not be possible to determine the distinct contribution of each intervention element to the outcomes of interest. However, we retained studies if the review authors judged that CR comprised at least 80% of the actual intervention time.
Comparators
Cognitive rehabilitation could be compared to inactive controls (treatment as usual, or a waiting‐list control condition), a non‐specific active control intervention, or an alternative treatment.
Treatment as usual. This may be described as standard treatment, usual treatment, or no treatment. In this review, usual treatment alone was compared to usual treatment plus cognitive rehabilitation. Usual treatment refers to the treatment usually available in the study locality, and might include memory clinic consultations, provision of medication, contact with a community mental health team, day care, or support from voluntary organisations.
Waiting‐list control. Participants allocated to the control group receive no intervention but are informed that they will be offered CR once the trial has ended.
Non‐specific active control. Participants allocated to the control group engage in a specified activity for an equivalent number of sessions and have similar levels of contact with the research team.
Alternative treatment. Participants in the comparator group receive another non‐pharmacological intervention, which is intended to influence the main outcomes of interest but via different components. We identified three categories of non‐pharmacological intervention that we intended to use to group alternative treatments: cognition‐focused (e.g. reminiscence therapy, cognitive stimulation therapy, cognitive training), exercise‐based (e.g. aerobic training, resistance training), or arts‐based (e.g. music therapy, drama therapy).
Use of different comparators was likely to constitute an important source of heterogeneity in the findings.
Types of outcome measures
We considered behavioural, cognitive, and psychosocial outcomes that were measured at the end of treatment or at follow‐up. Biomarker and economic outcomes were beyond the scope of this review.
Primary outcomes
Functional ability in targeted activities. The primary outcome of a CR intervention is the effect on participants’ functional ability to engage in and carry out the activities specifically targeted in the intervention (Wilson 2002). This may be assessed by means of ratings of performance on a standard scale made by the participant, caregiver, or practitioner (or a combination), or through direct observation and recording of performance on specific tasks. An example of a standard scale for rating the attainment of therapy goals is the Canadian Occupational Performance Measure (COPM, Law 2005). An example of an observational measure is the Direct Measure of Training (Thivierge 2014).
Secondary outcomes
General functional ability. A key secondary outcome is the effect on general functional ability, assessed by informant ratings on a standardised scale such as the Functional Activities Questionnaire (Martyr 2012b; Pfeffer 1982), or a reduction in dependence, assessed by informant ratings on a standardised scale such as the Dependence Scale (Brickman 2002; Stern 1994).
Other secondary outcomes for the person with dementia are:
Self‐efficacy
Mood
Quality of life
Cognition (global and domain‐specific)
Disease severity
Outcomes for care partners are:
Stress
Burden
Coping
Quality of life
We prioritised published and validated measures, and only accepted a non‐established measure if we found sufficient evidence to support its statistical properties. In classifying cognitive measures, we used well‐established classifications (e.g. Strauss 2006). Where there were multiple measures for the same outcome, we followed the principles described in Bahar‐Fuchs 2019.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register. The register is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment, and cognitive improvement. The studies are identified from:
monthly searches of several major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of the trial registers: the WHO International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, the Netherlands National Trials Register, plus others), and ClinicalTrials.gov;
quarterly searches of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of several grey literature sources from ISI Web of Science Core Collection.
See the Cochrane Dementia and Cognitive Improvement Group archived website: https://web.archive.org/web/20230322055531/https://dementia.cochrane.org/our-trials-registerfor details of the search strategies run in healthcare bibliographic databases and used for the retrieval of reports of dementia, cognitive improvement, and cognitive enhancement trials.
We ran additional searches in MEDLINE, Embase, PsycINFO, CINAHL, LILACS, ClinicalTrials.gov, and the WHO Portal/ICTRP to ensure that the searches for this review are as comprehensive and current as possible. See Appendix 1 for details of the search strategies used. We carried out the most recent search on 19 October 2022.
Searching other resources
We screened the reference lists of included trials, and of relevant systematic reviews and practice guidelines identified during the screening process.
Data collection and analysis
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'an RCT' or as 'not an RCT'; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs and, if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me webpage on the Cochrane Information Specialist’s portal: https://community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal. In addition, more detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2005; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2017.
We used Covidence to screen the remaining titles and abstracts and to manage full‐text review. Two review authors working independently reviewed each record and excluded those articles deemed ineligible by both review authors. We discussed any disagreements on eligibility, and where we could not reach consensus, we referred the abstract in question to a third review author. Where there was any doubt, we retained the abstract. We retrieved the full‐text articles for all abstracts retained at this stage, and two review authors working independently reviewed them. We discussed any disagreements on eligibility and, where we could not reach consensus, we referred the article in question to a third review author. We grouped multiple reports from the same trial under a single study identifier. We contacted study authors for further details where we required clarification. To prevent any conflicts of interest arising, review authors who have authored reports of studies being considered for inclusion at any stage of the selection process were not involved in decisions about the inclusion of those studies; instead, we referred the studies to other review authors for a decision. We confirmed the eligibility of the included studies by consensus of all review authors.
Data extraction and management
We prepared and used a structured proforma for data extraction, and then transferred data to Review Manager 5.
From each trial we extracted data including: detailed characteristics of the trial, its setting, design and outcomes; participant characteristics (diagnosis, age, sex, education, dementia severity, and medication use); and the experimental and comparator interventions (nature, intensity, frequency, and duration). For each outcome of interest, we extracted means and standard deviations of relevant measures from all available evaluations. Where available, we also extracted information about potential effect moderators: adherence and retention, intervention integrity and fidelity, and adverse events. Review authors did not extract data from any studies for which they are co‐authors; these studies were referred to other team members for data extraction.
Assessment of risk of bias in included studies
Two review authors, working independently, used the Cochrane risk of bias tool to assess bias in the domains of sequence generation, allocation concealment, blinding of participants and investigators, incomplete outcome data, and selective reporting of outcomes (Higgins 2017). We referred disagreements that we could not resolve through discussion to a third review author. We rated studies as low risk, high risk, or unclear risk in each of these domains. Review authors did not rate any studies for which they are co‐authors; these studies were referred to other team members for rating.
Measures of treatment effect
For continuous outcomes, we used the mean difference (MD) with 95% confidence interval (CI) when studies used the same rating scale to measure a particular outcome, and the standardised mean difference (SMD), which is the absolute mean difference divided by the pooled standard deviation, when the same outcome was assessed by different rating scales. We calculated effect estimates, with 95% CIs, using change‐from‐baseline scores. Baseline was defined as the latest available assessment prior to randomisation, undertaken not more than two months beforehand. Where change scores were not reported, we extracted the mean, standard deviation, and number of participants at each assessment point for each group and calculated the change scores. We based calculations of the standard deviation of change scores on an assumption that the correlation between measurements at baseline and those at subsequent time points was zero. This method overestimates the standard deviation of the change from baseline, but we considered it preferable in a meta‐analysis to take a conservative approach.
We decided whether to treat ordinal outcome data as continuous or to dichotomise after data extraction, depending on the number of categories. We treated outcome measures with more than 10 categories as continuous variables arising from a normal distribution (Bahar‐Fuchs 2019).
Unit of analysis issues
Cross‐over trials
We used data only from the first treatment period, prior to cross‐over.
Trials with multiple comparator conditions
For trials with more than one control condition (inactive and non‐specific active controls), we selected the condition most similar to the other comparator interventions in the analysis; in practice, this applied to two trials and we selected the inactive control condition (treatment as usual).
Duration of follow‐up
Our protocol specified grouping different durations of follow‐up for purposes of analysis into the following bands of time after the end of treatment: 3 to 6 months, 7 to 12 months, 13 to 18 months, 19 to 24 months, and > 24 months, using the latest assessment within any time band. In practice, due to the small number of included studies, we combined the 3 to 6 months and 7 to 12 months time bands and defined this as medium‐term follow‐up (see Differences between protocol and review). We noted any contact with the research team during the follow‐up period (for example, maintenance or ‘booster’ sessions).
Dealing with missing data
We identified the number of participants included in the final analysis as a proportion of all participants recruited and randomised.
Assessment of heterogeneity
In addition to visual inspection of forest plots, we assessed statistical heterogeneity using a standard Chi² statistic and the associated I² statistic (Higgins 2003). We considered heterogeneity to be substantial when the Chi² statistic was significant at the P = 0.1 level, or when the I² statistic suggested that more than 40% of the variability in effect estimate was due to heterogeneity (Deeks 2017).
Assessment of reporting biases
For the primary outcomes, we intended to evaluate the presence of reporting bias through a visual examination of funnel plots if 10 or more studies were included in a meta‐analysis (Egger 1997).
Data synthesis
We conducted data synthesis in Review Manager 5.
For each outcome of interest, where available data permitted, we undertook the following separate comparisons.
CR versus inactive control (treatment as usual) at the end of therapy
CR versus inactive control (treatment as usual) at subsequent follow‐up
CR versus alternative treatment at the end of therapy
CR versus alternative treatment at subsequent follow‐up
We intended to include data from trials using an inactive control condition (treatment as usual or waiting‐list) and a non‐specific active control condition in the same comparison with cognitive rehabilitation, using subgroup analysis to investigate these as potential sources of heterogeneity. However, there were no trials that used only a non‐specific active control condition. Where trials included both types of comparator, we selected the inactive control condition in order to keep the comparator condition as homogeneous as possible and to avoid splitting the CR group (see Differences between protocol and review).
For alternative treatment comparators, we intended to conduct separate analyses for the following categories of comparator: cognition‐focused, exercise‐based, and arts‐based interventions.
For multiple follow‐ups, we grouped comparable time points, and conducted separate analyses for each time point where possible.
Within each of the planned comparisons, we pooled data in relation to each outcome of interest when data from at least two trials were available. We conducted inverse‐variance, random‐effects meta‐analyses for all outcomes.
Subgroup analysis and investigation of heterogeneity
In relation to each outcome, we intended to carry out subgroup analyses if there was evidence of substantial heterogeneity and there were at least three studies per subgroup. These analyses were to evaluate the potential impact of the following factors that might modify observed treatment effects.
Intervention intensity (number of sessions and duration of intervention period)
Type of dementia
Type of practitioner (practitioner profession and qualification level)
Risk of bias (studies with high or unclear risk of bias in two or more domains versus studies with less risk of bias)
Registration status of the trial (registered versus not registered)
Type of control condition (inactive versus non‐specific active control)
Sensitivity analysis
Where indicated by the data we intended to use sensitivity analyses to clarify uncertainties relating to eligibility criteria, data, and analysis methods in the identified studies, following Cochrane guidelines. For example, in the presence of substantial heterogeneity, we would explore the effect of small studies by comparing fixed‐effect and random‐effects estimates; we would use a ‘trim and fill’ technique to address publication bias.
Summary of findings and assessment of the certainty of the evidence
We applied the GRADE framework to all primary and secondary outcomes in each comparison, classifying the certainty of evidence as high, moderate, low, or very low. We included this classification in the summary of findings (SoF) tables. See Schünemann 2019b for the details of how summary of findings tables are created. For each comparison, we used GRADEpro GDT software to generate SoF tables for the following primary and secondary outcomes.
Functional ability in targeted activities
General functional ability
Self‐efficacy
Mood
Quality of life
Cognition (global)
Quality of life (care partner)
Results
Description of studies
Results of the search
The flow of studies through the search and screening process is presented in Figure 1. We conducted four searches for this review: January 2020, November 2020, September 2021, and October 2022. The searches identified a total of 29,021 search results and one study was identified through other sources. After de‐duplication we were left with a total of 18,299 records. In assessing the search results for the initial search in January 2020, and for the most recent search in October 2022, we used the Cochrane Screen4Me workflow to help identify potential reports of randomised trials (Noel‐Storr 2021). After 9411 records were excluded in the Screen4Me process the Cochrane Dementia and Cognitive Improvement Group Information Specialist removed 4055 records based on the initial assessment of titles and abstracts and the author team removed 4722 records based on a full title and abstract assessment (4118 irrelevant studies and 604 duplicates). The full‐text review of the remaining 100 records identified six eligible studies.
1.
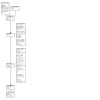
Included studies
A detailed description of the included studies is provided in Characteristics of included studies.
All included studies were published in English between 2010 and 2022. Four studies were conducted in the UK (Clare 2019; Clare 2010; Clarkson 2022; Hindle 2018), three of them by the same team (Clare 2019; Clare 2010; Hindle 2018), one was conducted in France (Amieva 2016), and one in Canada (Thivierge 2014). Five of the trials were registered in public trial registries (Amieva 2016; Clare 2010; Clare 2019; Clarkson 2022; Hindle 2018). There were no registration details provided for the Thivierge 2014 trial.
Three of the included studies were multicentre, parallel‐group RCTs (Amieva 2016; Clare 2019; Clarkson 2022), two were single‐site RCTs (Clare 2010; Hindle 2018), and there was one cross‐over trial (Thivierge 2014). The sample sizes ranged from 20 to 653 participants.
Amieva 2016 reported outcomes for three‐ and 24‐month follow‐up following randomisation, Clare 2010 and Hindle 2018 reported outcomes at two and six months, Clare 2019 reported outcomes at three and nine months, Clarkson 2022 reported outcomes at three and six months, and Thivierge 2014 was a cross‐over trial with outcomes assessed at one, two, and three months and then at four, five, and six months in a subgroup of participants following the cross‐over.
Clare 2019, Clarkson 2022, and Thivierge 2014 compared CR with inactive control conditions only. Clare 2010 and Hindle 2018 compared CR with two control conditions, inactive control (treatment as usual) and a non‐specific active control (individual relaxation therapy). Amieva 2016 included an inactive, treatment as usual control condition and two alternative treatment conditions (group cognitive training and group reminiscence therapy), but only directly compared CR with treatment as usual.
None of the analyses were based on data from all six included studies. Four of the trials included at least one measure of functional ability in targeted activities (Clare 2019; Clare 2010; Hindle 2018; Thivierge 2014), and five trials included a measure of participant quality of life (Clare 2019; Clare 2010; Clarkson 2022; Hindle 2018; Thivierge 2014). The other analyses were based on data from two or three studies only, mainly Clare 2019, Clare 2010, and Hindle 2018. Hindle 2018 was the study that contributed data to the highest number of analyses, while Amieva 2016 contributed data to only three analyses and Clarkson 2022 contributed data to eight analyses. See Table 3 for the list of outcomes and Table 4 an overview of which studies provided data for each analysis.
1. List of outcomes.
| List of outcomes | Subcategories | Measures used | End of therapy | Medium‐term follow‐up |
| PRIMARY OUTCOMES | ||||
| Person with dementia outcomes | ||||
| Functional ability in targeted activities | Personal goals – performance (participant self‐report)* | BGSI, COPM | X | X |
| Personal goals – performance (informant report of participant)* | BGSI, DMT | X | X | |
| Personal goals – satisfaction (participant self‐report) | BGSI, COPM | X | X | |
| SECONDARY OUTCOMES | ||||
| General functional ability (informant report of participant)* | DAD, FAQ, BADLS | X | X | |
| Self‐efficacy (participant self‐report)* | GSES | X | X | |
| Mood | Anxiety (participant self‐report) | HADS | X | X |
| Depression (participant self‐report)* | HADS | X | X | |
| Behavioural symptoms (informant report of participant) | NPI | X | – | |
| Quality of life (participant self‐report)* | QoL‐AD, DQoL, DEMQOL, WHOQOL‐BREF | X | X | |
| Cognition (performance based) | Memory ‐ overall | RBMT II | X | X |
| Memory ‐ immediate recall | RBMT II | X | X | |
| Memory ‐ delayed recall | RBMT II | X | X | |
| Sustained attention | TEA ‐ Elevator Counting | X | X | |
| Auditory selective attention/working memory | TEA ‐ Elevator Counting with Distraction | X | X | |
| Verbal letter fluency | COWA, DKEFS Letter Fluency | X | X | |
| Severity of condition | Survival without moderately severe to severe dementia | — | — | |
| Institutionalisation | — | — | ||
| Care partner outcomes | ||||
| Quality of life | Quality of life ‐ overall (informant self‐report) | EQ‐5D‐3L index | — | X |
| Quality of life ‐ physical health (informant self‐report) | WHOQOL‐BREF | X | X | |
| Quality of life ‐ psychological (informant self‐report)* | WHOQOL‐BREF | X | X | |
| Quality of life ‐ social (informant self‐report) | WHOQOL‐BREF | X | X | |
| Quality of life ‐ environmental (informant self‐report) | WHOQOL‐BREF | X | X | |
| Mood | Anxiety (informant self‐report) | HADS | X | X |
| Depression (informant self‐report) | HADS | X | X | |
| Psychological wellbeing | GHQ‐12 | X | X | |
| Stress (informant self‐report) | RSS | X | X | |
| Burden (informant self‐report) | ZBI, SSCQ | X | X | |
| Coping | — | — | — | |
BADLS – Bristol Activities of Daily Living Scale; BGSI – Bangor Goal‐Setting Interview; COPM – Canadian Occupational Performance Measure; DAD – Disability Assessment for Dementia; COWAT – Controlled Oral Word Association Test; DEMQOL – DEMentia Quality Of Life; D‐KEFS – Delis–Kaplan Executive Function System; DMT – Direct Measure of Training; DQoL – Dementia Quality of Life; EQ‐5D‐3L – EuroQol Questionnaire ‐ short; FAQ – Functional Activities Questionnaire; GHQ‐12 – General Health Questionnaire; GSES – Generalized Self‐Efficacy Scale, HADS – Hospital Anxiety and Depression Scale; NPI – Neuropsychiatric Inventory; RBMT‐II – Rivermead Behavioural Memory Test‐II; SSCQ – Short Sense of Competence Questionnaire; QoL‐AD – Quality of Life in Alzheimer’s Disease; RSS ‐ Relatives Stress Scale; TEA ‐ Test of Everyday Attention; WHOQOL‐BREF – World Health Organization’s Quality of Life Instrument (short version); ZBI – Zarit Burden Interview
*Measures included in the summary of findings tables.
2. Mapping outcomes.
| List of outcomes | Subcategories | End of therapy | Medium‐term follow‐up | ||||||||||
| Amieva 2016 | Clare 2010 | Clare 2019 | Clarkson 2022 | Hindle 2018 | Thivierge 2014 | Amieva 2016 | Clare 2010 | Clare 2019 | Clarkson 2022 | Hindle 2018 | Thivierge 2014 | ||
| PRIMARY OUTCOMES | |||||||||||||
| Person with dementia outcomes | |||||||||||||
| Functional ability in targeted activities | Personal goals – performance (participant self‐report)* | X | X | X | X | X | |||||||
| Personal goals – performance (informant report of participant)* | X | X | X | X | X | X | |||||||
| Personal goals – satisfaction (participant self‐report) | X | X | X | X | X | ||||||||
| SECONDARY OUTCOMES | |||||||||||||
| General functional ability (informant report of participant)* | X | X | X | X | X | X | |||||||
| Self‐efficacy (participant self‐report)* | X | X | X | X | |||||||||
| Mood | Anxiety (participant self‐report) | X | X | X | X | X | X | ||||||
| Depression (participant self‐report)* | X | X | X | X | X | X | |||||||
| Behavioural symptoms (informant report of participant)** | X | X | |||||||||||
| Quality of life (participant self‐report)*.** | X | X | X | X | X | X | X | X | X | X | |||
| Cognition (performance based) | Memory ‐ overall | X | X | X | X | ||||||||
| Memory ‐ immediate recall | X | X | X | X | |||||||||
| Memory ‐ delayed recall | X | X | X | X | |||||||||
| Sustained attention | X | X | X | X | |||||||||
| Auditory selective attention/working memory | X | X | X | X | X | ||||||||
| Verbal letter fluency | X | X | X | X | X | ||||||||
| Severity of condition | Survival without moderately severe to severe dementia | X | X | ||||||||||
| Institutionalisation | X | X | |||||||||||
| Care partner outcomes (self‐report) | |||||||||||||
| Quality of life | Quality of life ‐ overall | X | X | ||||||||||
| Quality of life ‐ physical health | X | X | X | X | X | X | |||||||
| Quality of life ‐ psychological* | X | X | X | X | X | X | |||||||
| Quality of life ‐ social | X | X | X | X | X | X | |||||||
| Quality of life ‐ environmental | X | X | X | X | X | X | |||||||
| Mood | Anxiety | X | X | X | X | ||||||||
| Depression | X | X | X | X | |||||||||
| Psychological wellbeing | X | X | X | X | |||||||||
| Stress | X | X | X | X | X | X | |||||||
| Burden** | X | X | X | X | X | ||||||||
| Coping | — | — | — | — | — | — | — | — | — | — | — | — | |
* Measures included in the summary of findings tables.
** All studies contributing data to this outcome analysis have an increased risk of bias resulting in a lower certainty of data (only at end of therapy time point for the general functional ability).
Characteristics of participants
In the six included studies there were 1702 participants overall. Participant characteristics are summarised in Table 5 (for the overall study samples and by comparison group).
3. Summary characteristics of participants.
| Study | Condition | Sample size at baseline | Age, mean (SD) |
Sex (% men) |
Ethnicity (% white) | Education, years of education, mean (SD), or level achieved, n (%) | Diagnosis | Dementia‐related medications use |
Baseline MMSE score |
Retention at medium‐term follow‐up, n (%) |
Adverse events |
| Amieva 2016* | Overall | 646 | 78.7 (6.7) | 40.4% | Not reported | 1) No formal education 96 (14.7%) 2) Primary school 224 (34.3%) 3) Secondary school 190 (29.1%) 4) Baccalaureate and more 131 (20.1%) |
AD (100%) | 576 (88.2%) | 21.6 (3.0) | At 3 months 586 (89.7%) | Not reported |
| Cognitive rehabilitation |
157 | 78.9 (6.2) | 64 (40.8%) | 1) No formal education 27 (17.2%) 2) Primary school 56 (35.7%) 3) Secondary school 42 (26.8%) 4) Baccalaureate and more 30 (19.1%) |
AD (100%) | 136 (86.6%) |
21.6 (3.0) | At 3 months 144 (91.7%) | |||
| TAU | 154 | 78.7 (6.5) | 63 (40.9%) | 1) 24 (15.6%) 2) 51 (33.1%) 3) 45 (29.2%) 4) 32 (20.8%) |
AD (100%) | 133 (86.4%) | 21.6 (3.3) | 141 (91.6%) | |||
| Cognitive training | 170 | 78.5 (7.2) | 69 (40.6%) | 1) 17 (10.0%) 2) 59 (34.7%) 3) 50 (29.4%) 4) 40 (23.5%) |
AD (100%) | 152 (89.4%) | 21.5 (3.2) | 151 (88.8%) | |||
| Reminiscence therapy | 172 | 78.7 (6.9) | 61 (35.5%) | 1) 28 (16.3%) 2) 58 (33.7%) 3) 53 (30.8%) 4) 29 (16.9%) |
AD (100%) | 155 (90.1%) |
21.1 (3.1) | 150 (87.2%) | |||
| Clare 2010 | Overall | Randomised: 69** (Analysed: 68) |
77.8 (6.32) | 28 (40.6%) | Not reported | 10.64 (SD 1.67) | AD (n = 55, 80.9%); mixed AD/VD (n = 13, 19.1%) | 68 (100%) | 23.0 (3.02) | 56 (81.16%) |
Not reported |
| Cognitive rehabilitation | Analysed: 22** |
76.32 (6.39) | 13 (59.1%) | 11.41 (2.81) | AD (n = 16, 72.2%); mixed AD/VD (n = 6, 27.3 %) | 22 (100%) | 23.14 (3.12) | 16 (69.6%) | |||
| TAU | Analysed: 22 | 78.18 (6.61) | 13 (59.1%) | 11.43 (2.99) | AD (n = 18, 81.8%); mixed AD/VD (n = 4, 18.2%) | 22 (100%) | 22.32 (3.05) | 20 (90.9%) | |||
| Relaxation therapy | Analysed: 24 | 77.92 (6.23) | 14 (58.3%) | 10.92 (2.52) | AD (n = 21, 87.5%); mixed AD/VD (n = 3, 12.5%) | 24 (100%) | 23.33 (2.88) | 20 (83.3%) | |||
| Clare 2019 | Overall | Analysed: 474*** (Randomised: 475) |
78.56 (7.07) | 52.3% | 457 (96.4%) | 12.57 (SD 3.37) | AD (n = 284, 59.5%); mixed AD/VD (n = 116, 24.5%), VD (n = 74, 15.6%) | 332 (75.8%) | 23.82 (3.02) | 426 (89.87%) | Details reported, no serious adverse reactions |
| Cognitive rehabilitation | Analysed: 238*** (Randomised: 239) |
78.25 (7.13) | 124 (52.1%) | 95.0% | 12.57 (3.33) | AD (n = 139, 58.4%); mixed AD/VD (n = 56, 23.5%), VD (n = 43, 18.1%) | 157 (73.0%) | 23.89 (3.04) | 209 (87.4%) | ||
| TAU | Analysed: 236 | 78.87 (7.01) | 124 (52.5%) | 97.9% | 12.58 (3.42) | AD (n = 145, 61.4%); mixed AD/VD (n = 60, 25.4%), VD (n = 31, 13.1%) | 175 (78.5%) | 23.75 (3.02) | 218 (92.4%) | ||
| Clarkson 2022 | Overall | 468 | 79.6 (6.95) | 220 (47%) | 427 (91.2%) | Not reported | Not reported | Not reported | S‐MMSE 22.4 (4.90) | 347 (74.1%) | Details reported, no serious adverse reactions |
| Cognitive rehabilitation | 234 | 79.6 (6.7) | 112 (48%) | 211 (90%) | S‐MMSE 22.38 (5.1) | 176 (75.2%) | |||||
| TAU | 234 | 79.5 (7.2) | 108 (46%) | 216 (92%) | S‐MMSE 22.60 (4.7) |
171 (73.1%) | |||||
| Hindle 2018 | Overall | 29 | 76.34 (6.42) | 22 (79.3%) | Not reported | 10.97 (SD 1.55) | PDD (25, 86.2%) DLB (4, 13.8%) |
None | ACE‐III 71.3 (7.5) | 25 (86.21%) | Not reported |
| Cognitive rehabilitation | 10 | 75.8 (6.61) | 8 (80%) | 10.9 (1.66) | PDD (9, 90%) DLB (1, 10%) |
ACE‐III 71.6 (6.74) | 7 (70%) | ||||
| TAU | 9 | 78.56 (5.77) | 7 (78%) | 11 (1.73) | PDD (7, 77.8%) DLB (2, 22.2%) |
ACE‐III 70.2 (9.38) | 9 (100%) | ||||
| Relaxation | 10 | 74.9 (6.87) | 7 (70%) | 11 (1.41) | PDD (9, 90%) DLB (1, 10%) |
ACE‐III 71.9 (7.19) | 9 (90%) | ||||
| Thivierge 2014 | Overall | Analysed: 17**** (Randomised: 20) |
80.0 (5.42) | 29.4% | Not reported | 11.30 (SD 3.87) | AD (100%) | 13 (76.5%) | 21.83 (2.38) |
17 (100%) | Not reported |
| Cognitive rehabilitation | 9 (Randomised: 10) |
80 (6.14) | 3 (33.3%) | 10.67 (3.91) | AD (100%) | 9 (100%) | 21.56 (2.51) | 9 (100%) | |||
| TAU | 8 (Randomised: 10) |
80 (4.90) | 2 (25%) | 12 (3.95) | AD (100%) | 4 (50%) | 22.13 (2.36) | 8 (100%) |
ACE‐III: Addenbrooke's Cognitive Examination; AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MMSE: Mini‐Mental State Examination; PDD: Parkinson's disease dementia; SD: standard deviation; TAU: treatment as usual; VD: vascular dementia
*Care partners received telephone contact (CR) or group support (TAU, cognitive training and reminiscence therapy) of frequency matching the therapy sessions.
**One person who was excluded from the analysis due to ineligible diagnosis contributed demographic details to the overall sample characteristics; subgroup characteristics were calculated by authors without that person included.
***One person was randomised and then excluded due to ineligible diagnosis; their data are not included in the demographic characteristics.
****Three people who withdrew are not included here as there were no data available.
The mean age ranged from 76 to 80 and was similar across the studies, with the youngest participants in Hindle 2018 and the oldest in Thivierge 2014. The male to female ratio ranged from 29.4% male participants in Thivierge 2014 to 79.3% male participants in Hindle 2018. The participants' profile in Hindle 2018 reflects the characteristics of people with Parkinson’s disease dementia and dementia with Lewy bodies (PDD/DLB), with typically earlier onset and higher prevalence among men. Ethnicity was reported in Clare 2019 and Clarkson 2022 only, with most participants (96.4% and 91.2% respectively) being of White British ethnicity.
Most participants were diagnosed with Alzheimer’s disease (n = 1002, 58.9% of the whole sample, 81.2% of participants with the specific diagnosis reported). Amieva 2016 and Thivierge 2014 included participants diagnosed with Alzheimer’s disease only, Clare 2010 included participants with Alzheimer’s disease and with mixed Alzheimer’s disease/vascular dementia, and Clare 2019 included participants with Alzheimer’s disease, mixed Alzheimer’s disease/vascular dementia and vascular dementia. Hindle 2018 included participants with PDD/DLB only. Clarkson 2022 included people with a confirmed diagnosis of mild‐to‐moderate dementia but did not report details of specific diagnoses. All participants in Clare 2010 and the majority in Amieva 2016, Clare 2019, and Thivierge 2014 were taking dementia‐related medications. As per the trial eligibility criteria, participants in Hindle 2018 were on stable doses of anti‐parkinsonian and dementia‐related medications. Clarkson 2022 did not report data on medication use. The mean MMSE scores ranged from 21.6 in Amieva 2016 to 23.82 in Clare 2019, with Hindle 2018 reporting an ACE‐III (Hsieh 2013) mean score of 71.3, where the dementia cut‐off score is 82 out of 100 (sensitivity = 0.93; specificity = 1.0). Participants in Clarkson 2022 had a mean score of 22.4 on the Standardised Mini‐Mental State Examination, with scores in the range 20 to 25 out of 30 interpreted as reflecting ‘mild’ impairment (Molloy 1997).
General characteristics of experimental interventions
A summary of the length, duration, and delivery mode of the interventions is provided in Table 6 and detailed characteristics of the interventions are presented in Table 7. In all the included studies, the intervention sessions were provided on a one‐to‐one basis, usually weekly (twice a week in Thivierge 2014), at the place of residence (settings not specified for Amieva 2016). The CR intervention in Amieva 2016 was the most intensive, involving approximately 45 hours of practitioner contact for the person with dementia overall (21 hours of therapy sessions and 24 hours of maintenance sessions) and telephone support for the care partner, while the other interventions involved 8 to 10 hours of practitioner contact. An unspecified number of participants in the CR intervention group in Amieva 2016 had individual reminiscence therapy instead of CR. The intervention in Clarkson 2022 was brief, with two in‐person visits and two optional follow‐up telephone calls. It focused on the use of personally meaningful memory aids.
4. Length, duration, and delivery mode of the interventions .
| Study | Intervention duration | Total number of sessions | Session frequency | Session duration | Total direct intervention | Session format | Intervention settings |
| Amieva 2016* | 3 months therapy and 21 months maintenance |
Therapy sessions: 14 Maintenance sessions: 16 |
Therapy sessions: weekly in the first 3 months Maintenance sessions: 6‐weekly over 21 months |
90 minutes | Therapy sessions: 21 hours Maintenance sessions: 24 hours** |
Individual | Not specified |
| Clare 2010 | 8 weeks | 8 | Weekly | 60 minutes | 8 hours | Individual | Home |
| Clare 2019 | 3 months therapy and 6 months maintenance | Therapy sessions: 10 Maintenance sessions: 4 |
Therapy sessions: weekly in the first 3 months Maintenance sessions: 6‐weekly over 6 months |
60 minutes | Therapy sessions: 10 hours Maintenance sessions: 4 hours |
Individual | Home |
| Clarkson 2022 | 4 weeks | 4 (2 optional) | 4 weekly sessions, 1st and 4th in person and 2nd and 3rd optional telephone follow‐ups | Protocol stipulated up to 6 hours of contact overall; a nested sub‐study reported an average of 3 hours | 2 to 4 hours (most participants opted for 4 hours) | Individual | Home/telephone |
| Hindle 2018 | 8 weeks | 8 | Weekly | 60 minutes | 8 hours | Individual | Home |
| Thivierge 2014 | 4 weeks | 8 | Twice a week | 45 to 60 minutes | 8 hours | Individual | Home |
*Care partners received telephone contact of frequency matching the CR sessions.
**Number of hours calculated based on authors’ description.
5. Intervention characteristics.
| Study | Personalisation and goal‐setting | CR techniques | Training and fidelity | Comments |
| Amieva 2016 | Meaningful activities identified in ‘made‐to‐measure program’ and the strategies matched individual goals; discussed in the first two sessions; ‘meaningful activities’ (daily living or leisure activities); at each session the psychologist evaluated the relevance of pursuing the selected activity; goals could be changed at any point to reflect changing priorities. Care partners received telephone contact of frequency matching the CR sessions. | Errorless learning procedure; avoiding failures where possible | Three days of training for therapists and a meeting with the co‐ordinating centre trainer; a manual detailing the guidelines for intervention provided; telephone support available; no fidelity measures reported | CR delivered by psychologists; goal performance not measured; no details on the nature of the goals; unknown proportion of participants in the CR group received individual reminiscence therapy |
| Clare 2010 | Each participant identified personally meaningful goals (up to five daily activities relating to self‐care, leisure, or productivity difficult to perform satisfactorily); elicited in a semi‐structured interview; goals reflected memory and other cognitive impairment difficulties in relation to everyday tasks | Individualised intervention supplemented by use of practical aids and strategies, techniques for learning new information, practice in maintaining attention, and techniques for stress management | No details of training and no reference to a manual; adherence to therapy protocol monitored through supervision and review of session and home‐practice records | CR delivered by an experienced Occupational Therapist; goal performance measured before and after therapy |
| Clare 2019 | The intervention addressed individual goals; goals reflected difficulties caused by memory and other cognitive impairment in relation to everyday tasks, activities and routines; engaging in pleasurable and meaningful activities, social contacts and relationships; expressed in behavioural terms using SMART principles | Environmental adaptations and prompts, use of compensatory memory aids, procedural learning of relevant skills, supported learning of important new information and restorative learning methods to reactivate prior knowledge; between‐session practice encouraged | CR intervention protocol published and a therapy handbook developed; adherence to therapy protocol monitored through monthly centralised supervision and therapy logs maintenance for each session | CR delivered by Occupational Therapists or Nurse; goal performance measured before and after therapy |
| Clarkson 2022 | Each participant set realistic goals to be achieved through the use of memory aids | Training in using personally relevant memory aids | Practitioners used a manual to guide each of the four sessions, and worksheets to facilitate and record delivery but no training other than the manual. Fidelity formally assessed and confirmed in a mixed‐method sub‐study. | Delivered by dementia support practitioners who did not need to have professional training, but four of the five practitioners were qualified occupational therapists in the process evaluation sub‐study. Goal attainment was not assessed as an intervention outcome. |
| Hindle 2018 | Each participant identified personally meaningful goals. Goals typically related to self‐management and orientation, medication adherence, learning new skills, and maintaining social and leisure activities | Strategies tailored to each person’s ability and goals; compensatory (e.g. using reminders, calendars, alarms) and/or restorative (e.g. spaced retrieval learning, mnemonics) approaches to circumvent difficulties relating to orientation, planning, the retention of learned information, and recall; between‐session practice encouraged | Ongoing training provided to the therapist; adherence to therapy protocol scrutinised through supervision and review of therapy logs detailing each session |
CR delivered by the occupational therapist; goal performance measured before and after therapy |
| Thivierge 2014 | An activity of daily living to be trained was chosen in collaboration with patient and care partner to reflect the patient’s needs and interests | Strategies adapted to each person’s ability and goals; decreasing degrees of assistance (as needed for each participant and task); expanding rehearsal; performance between‐session practice encouraged | Initial training and periodic monitoring provided, a manual detailing cognitive training procedures developed |
CR delivered by research assistants (Ph.D. candidates supervised by a registered neuropsychologist); goal performance measured before and after therapy with an observational instrument |
CR: cognitive rehabilitation
In line with the CR definition used in this review, the CR interventions in all the included studies had a direct focus on improving everyday functioning, were personalised, and utilised recognised CR techniques. Personalisation was achieved by identifying personal goals and addressing them with a tailored intervention built on CR principles, and/or particular techniques (e.g. expanding rehearsal).
General characteristics of inactive and non‐specific active comparison conditions
All of the included studies employed an inactive treatment as usual control condition. In Amieva 2016, participants in the comparison conditions received usual medical care (medication only) and care partners were invited to join support group sessions once a week during the first three months and every six weeks afterwards (care partners in the CR group received telephone support). In Clare 2010, Clare 2019, Clarkson 2022, and Hindle 2018 participants could access their usual support but had no contact with the research team between the assessments. Thivierge 2014 employed a control waiting‐list, with no contact with the research team between the assessments. Clare 2010 and Hindle 2018 included also a relaxation therapy (RT) condition that was described as a non‐specific active comparison condition. It involved the same number and duration of sessions as CR and was provided individually in the home setting by the same practitioner that provided CR. In this review, we only used the non‐active condition data to prioritise the homogeneity of the control condition and to avoid splitting the CR groups.
General characteristics of alternative treatment comparison conditions
Amieva 2016 included two alternative treatment conditions, group cognitive training and group reminiscence therapy; participants in these conditions had the same amount of contact time with the practitioner as the CR participants but in a group setting rather than one‐to‐one.
Excluded studies
Out of 94 excluded records, we excluded 42 as duplicate records or secondary publications of the studies already retrieved for full‐text screening, and 47 were excluded as not meeting the review criteria (27 did not meet intervention criteria, nine did not meet participant criteria, four had no appropriate comparator, four were not an RCT, and there was an insufficient amount of data to establish eligibility for one unpublished study). Two studies are awaiting classification and three are ongoing although unlikely to meet the review criteria. A full list of reasons for exclusion at the full‐text screening stage is presented in Characteristics of excluded studies.
Among the 10 studies not meeting participant criteria, some studies included a significant proportion of people with mild cognitive impairment (MCI) or with advanced dementia, and in other studies dementia did not seem to be diagnosed according to any established criteria (e.g. inclusion was based on a low MMSE score only). In the 27 studies not meeting intervention criteria, the reasons for exclusion were that the intervention offered no or limited personalisation, focused on the physical aspect of functioning, or represented cognitive stimulation or cognitive training (e.g. De Vreese 1998; Schecker 2013; Straubmeier 2017) or education‐based approaches (e.g. Koivisto 2013). We excluded several multicomponent interventions where CR elements were not distinguished or constituted less than 80% of the intervention (e.g. Kim 2015; Santos 2011). For some studies, we could not determine the extent to which the intervention goals and strategies targeted functional difficulties caused by cognitive rather than physical impairments (e.g. Brueggen 2017; Kurz 2012; Wenborn 2021). We did not have sufficient information to establish the eligibility of one unpublished study (Reuster 2010).
Risk of bias in included studies
We assessed the risk of bias for individual studies using the Cochrane risk of bias tool (Higgins 2017). The ratings and justifications are summarised in the Characteristics of included studies table. The risk of bias across studies for specific domains is presented in Figure 2 and Figure 3.
2.

3.

Allocation
All studies used a random sequence generation. In five studies randomisation was managed by a statistician and/or external trials unit (Amieva 2016; Clare 2010; Clare 2019; Clarkson 2022; Hindle 2018), and all studies used a remote computerised randomisation system. As allocation concealment is intrinsic to such an approach we rated all studies as low risk in this category.
Blinding
In all studies, the assessors were blinded to study group allocation and we rated the risks in relation to blinding of assessment as low in all the studies. Due to the nature of the interventions double‐blinding was not possible and therefore participants and practitioners were aware of the group allocation. This meant that the participants could have provided biased responses in the assessment and could have inadvertently unblinded the assessors, further increasing the risk of bias. Using an active control condition in Clare 2010 and Hindle 2018 and having several experimental conditions in Amieva 2016 could have limited the bias towards CR, but we rated the risk of bias in relation to blinding of participants and personnel as high in all studies.
Incomplete outcome data
We classified five out of six studies as low risk as there was no indication of attrition bias. In Thivierge 2014, three participants (out of 20) withdrew after the baseline evaluation and the authors excluded them from the analysis due to insufficient data being available. As there was no 'intention‐to‐treat' analysis undertaken we indicated the risk as high.
Selective reporting
We classified four out of six studies as low risk as there was no indication of reporting bias. In Amieva 2016, the participants were followed up at 3, 6, 12, 18, and 24 months, but the authors reported data for 3‐ and 24‐month time points only. There was no information about what measures were completed at 6, 12, and 18 months or why these were not reported, so we indicated the risk as high. In Clarkson 2022, some measures listed in the protocol were not reported in the main publication. As there was no explanation of why these measures were not reported, we indicated the risk as high. The outcome paper included descriptive statistics only for the primary outcomes measure, but the study authors provided the details for most measures on request.
Other potential sources of bias
Regarding other risks of bias, we considered whether the study used an intervention manual and/or provided formal training for the practitioners, as a proxy of implementation fidelity that could be a source of bias if the interventions were not delivered as initially planned. There were no high‐risk studies in that respect, although most studies provided limited information about the fidelity evaluation. Clarkson 2022 evaluated fidelity in a formal mixed method process evaluation. We noted a potential risk of intervention contamination for Amieva 2016 as an unspecified proportion of participants in the CR group received individual reminiscence therapy rather than CR.
Effects of interventions
See Table 1 for the main comparison: CR compared to an inactive control condition at the end of treatment; and Table 2 for CR compared to an inactive control condition after medium term follow‐up (3 to 12 months). See Schünemann 2019b for the details of how summary of findings tables are created.
For the comparison of CR with an inactive control condition, we were able to perform meta‐analyses for most outcomes stipulated in the protocol. We pooled data for three primary and 21 secondary outcomes at the end of therapy time point and for three primary and 21 secondary outcomes at the medium‐term (3 to 12 months) follow‐up time point. There were insufficient data on dementia severity and care partner coping. The list of outcome measures contributing to each analysis is presented in Table 3; Table 4 provides an overview of which studies provided data for each analysis. Hindle 2018 contributed data to the highest number of analyses (40 out of 48 analyses undertaken), with Clare 2019 contributing data to 37 analyses, Clare 2010 to 26 analyses, Thivierge 2014 to 11 analyses, Clarkson 2022 to eight, and Amieva 2016 to only three analyses.
Only one study compared CR to alternative treatments. Amieva 2016 included cognitive training and reminiscence therapy groups as well as a treatment as usual control group. We were able to compare both of these alternative treatments with CR for three outcomes at two time points and report these results narratively.
Time points
For our main comparison of CR with an inactive control, it was possible to undertake meta‐analyses for two time points only: at the end of treatment (the assessments were completed immediately post‐intervention between one and three months following the randomisation), and at a medium‐term follow‐up (we pooled the 3 to 6 months and 7 to 12 months categories into one category of assessments completed between three and 12 months following randomisation). Amieva 2016 and Clare 2019 both had a more intensive initial phase of treatment lasting three months and then a maintenance phase with less frequent sessions. We included data at the end of the more intensive phase in the end‐of‐treatment analyses. The time points represented in the included studies at the medium‐term follow up ranged from three to nine months (Clare 2010 at six months, Clare 2019 at nine months, Clarkson 2022 at six months, Hindle 2018 at six months, and Thivierge 2014 at four months). Clare 2010, Clare 2019, Hindle 2018, and Thivierge 2014 contributed assessment data to meta‐analyses at both time points. In Thivierge 2014, we included the final assessment before the cross‐over (at three months), in line with the protocol. Amieva 2016 also reported data at the end of their maintenance phase (24 months after randomisation) and we report these results narratively.
Imputations
Three studies addressed missing values by using imputation algorithms. Amieva 2016 reported two sets of analyses, based on data with and without imputation, and we used data with imputation as providing better correction for bias. Clare 2019 reported the no imputation data and a summary analysis demonstrating no significant impact of missing data on the study findings. Therefore, we used the no imputation data in our analyses. Clarkson 2022 undertook a sensitivity analysis comparing estimates with and without imputation for the primary outcome (Bristol Activities of Daily Living Scale) indicating no significant differences; we used no imputation data for the analysis that authors provided. Hindle 2018 presented data after imputation only, and we used those data.
Interpretation of effect sizes
When interpreting effect sizes (standardised mean difference, SMD), we used the following rule of thumb (Cohen 1988; Schünemann 2019a): < 0.20 = negligible effect, 0.20 to 0.49 = small effect, 0.50 to 0.79 = moderate effect, > 0.80 = large effect. Where mean differences (MDs) were used in the comparisons, we calculated the SMD to assist in the interpretation. We described results using guidelines from Ryan 2016. We identified findings as important when the certainty of evidence was high or moderate with medium or large effect sizes. We treated findings as less important where the certainty of evidence was high or moderate with small effect sizes (i.e. SMD 0.2 to 0.49) or the certainty of evidence was low with small, medium, or large effect sizes. We treated the remaining findings with very low certainty of evidence as not important. Where evidence existed for clinically significant differences these were considered in the discussion. As there were fewer than 10 studies in the comparisons, we did not use funnel plots to evaluate the possibility of publication bias.
Subgroup analyses
There were insufficient data for us to conduct any of our planned subgroup analyses. In particular, we did not undertake subgroup analyses for inactive and non‐specific active control conditions as only two studies included a non‐specific active control group (individual relaxation therapy in Clare 2010 and Hindle 2018) and both also included an inactive control group, which we selected for inclusion in the meta‐analyses. The authors of both studies reported that CR led to statistically significant improvements in functional ability in targeted activities at the end of treatment, relative to relaxation therapy.
Cognitive rehabilitation versus treatment as usual
Primary outcome: Functional ability in targeted activities
End of treatment
See Analysis 1.1, Analysis 1.2, and Analysis 1.3 and Figure 4, Figure 5, and Figure 6 for analyses comparing the functional ability in targeted activities of participants receiving CR or treatment as usual at the end of treatment.
1.1. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 1: Functional ability in targeted activities: personal goals ‐ performance (participant self‐report)
1.2. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 2: Functional ability in targeted activities: personal goals ‐ performance (informant report of participant)
1.3. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 3: Functional ability in targeted activities: personal goals ‐ satisfaction (participant self‐report)
4.

5.

6.

Included studies evaluated functional ability in targeted activities from three perspectives: as performance in relation to personal therapy goals (self‐reported by participants and rated by informants) and satisfaction with goal attainment (self‐reported by participants). We found large effects favouring CR over the inactive control condition in all three comparisons at the end of treatment. Relative to an inactive control condition, we found the following effects.
A large positive effect in functional ability in targeted activities as indicated by participant self‐ratings of goal attainment (SMD 1.46, 95% confidence interval (CI) 1.26 to 1.66; I2 = 0%; 3 RCTs, 501 participants; high‐certainty evidence).
A large positive effect in functional ability in targeted activities as indicated by informant ratings of goal attainment (SMD 1.61, 95% CI 1.01 to 2.21; I2 = 41%; 3 RCTs, 476 participants; high‐certainty evidence).
A large positive effect in functional ability in targeted activities as indicated by participant self‐ratings of satisfaction with goal attainment (SMD 1.31, 95% CI 1.09 to 1.54; I2 = 5%; 3 RCTs, 501 participants; high‐certainty evidence).
Medium‐term follow‐up
See Analysis 2.1, Analysis 2.2, and Analysis 2.3 for analyses comparing the functional ability in targeted activities of participants receiving CR or treatment as usual at medium‐term follow‐up.
2.1. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 1: Functional ability in targeted activities: personal goals ‐ performance (participant self‐report)
2.2. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 2: Functional ability in targeted activities: personal goals ‐ performance (informant report of participant)
2.3. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 3: Functional ability in targeted activities: personal goals ‐ satisfaction (participant self‐report)
Included studies again evaluated functional ability in targeted activities from three perspectives: as performance in relation to personal therapy goals (self‐reported by participants and rated by informants) and satisfaction with goal attainment (self‐reported by participants). We found large effects favouring CR over the inactive control condition in all three comparisons at the medium‐term follow‐up. Relative to an inactive control condition, we found the following effects.
A large positive effect in functional ability in targeted activities as indicated by participant self‐ratings of goal attainment (SMD 1.46, 95% CI 1.25 to 1.68; I2 = 0%; 2 RCTs, 432 participants; high‐certainty evidence).
A large positive effect in functional ability in targeted activities as indicated by informant ratings of goal attainment (SMD 1.25, 95% CI 0.78 to 1.72; I2 = 29%; 3 RCTs, 446 participants; high‐certainty evidence).
A large positive effect in functional ability in targeted activities as indicated by participant self‐ratings of satisfaction with goal‐attainment level (SMD 1.19, 95% CI 0.73 to 1.66; I2 = 28%; 2 RCTs, 432 participants; high‐certainty evidence).
Description of targeted activities
In Hindle 2018, the most common goals fell under categories of technology, maintenance of activities or pastimes, medication management, and self‐management and orientation.
In Clare 2010, the main categories of goals were: remembering (e.g. what someone has said or information and instructions), practical skills and activities (e.g. using a mobile phone or a computer), and concentration (e.g. keeping track of conversations or when cooking) (Clare 2011).
In Clare 2019, the therapy goals were set mainly in relation to engaging in activities and personal projects, using appliances, devices, and the Internet, and managing everyday activities, tasks, and situations, with several other categories represented.
In Clarkson 2022, the authors did not evaluate goal attainment post‐intervention though each participant was supported in identifying a realistic goal that could be achieved with personally relevant memory aids. Interviews with the practitioners indicated that orientation and misplacing items were two common areas of need.
In Thivierge 2014, the participants worked on goals relating to operating televisions, radios or music players, using a computer, and leisure activities.
Secondary outcomes for people living with dementia
End of treatment
See Analysis 1.4, Analysis 1.5, Analysis 1.6, Analysis 1.7, Analysis 1.8, Analysis 1.9, Analysis 1.10, Analysis 1.11, Analysis 1.12, Analysis 1.13, and Analysis 1.14 for details of secondary outcome measure analyses for the comparisons of CR and an inactive control condition at the end of treatment time point for people living with dementia.
1.4. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 4: General functional ability (informant report of participant)
1.5. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 5: Self‐efficacy (participant self‐report)
1.6. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 6: Mood: anxiety (participant self‐report)
1.7. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 7: Mood: depression (participant self‐report)
1.8. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 8: Behavioural symptoms (informant report of participant)
1.9. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 9: Quality of life (participant self‐report)
1.10. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 10: Cognition: memory, global score
1.11. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 11: Cognition: memory, immediate recall
1.12. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 12: Cognition: memory, delayed recall
1.13. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 13: Cognition: sustained attention
1.14. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 14: Cognition: auditory selective attention/working memory
We did not detect any important effects in secondary outcomes at the end of therapy time point. We classified the following outcomes as less important; relative to an inactive control condition, we found the following effects.
A small positive effect on participants’ self‐efficacy, as indicated by self‐ratings (MD 0.71, 95% CI 0.12 to 1.30; I2 = 0%; 2 RCTs, 456 participants; high‐certainty evidence).
A small positive effect on immediate recall, as indicated by a performance‐based measure (MD 0.27, 95% CI 0.02 to 0.52; I2 = 0%; 2 RCTs, 459 participants; high‐certainty evidence).
There were negligible effects on participants’ anxiety, quality of life, and sustained attention (moderate‐certainty), and on general functional ability, memory, and delayed recall (low‐certainty).
The remaining comparisons (depression, auditory selective attention/working memory, verbal letter fluency, and behavioural symptoms) were based on very low‐certainty evidence, and hence we were unable to determine whether CR was associated with any meaningful benefits in these outcomes for participants with dementia.
Medium‐term follow‐up
See Analysis 2.4, Analysis 2.5, Analysis 2.6, Analysis 2.7, Analysis 2.8, Analysis 2.9, Analysis 2.10, Analysis 2.11, Analysis 2.12, Analysis 2.13, and Analysis 2.14 for details of the secondary outcome measure analyses for the comparisons of CR and an inactive control condition at the medium‐term follow‐up time point for people living with dementia.
2.4. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 4: General functional ability (informant report of participant)
2.5. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 5: Self‐efficacy (participant self‐report)
2.6. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 6: Mood: anxiety (participant self‐report)
2.7. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 7: Mood: depression (participant self‐report)
2.8. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 8: Quality of life (participant self‐report)
2.9. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 9: Cognition: memory, global score
2.10. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 10: Cognition: memory, immediate recall
2.11. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 11: Cognition: memory, delayed recall
2.12. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 12: Cognition: sustained attention
2.13. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 13: Cognition: auditory selective attention/working memory
2.14. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 14: Cognition: verbal letter fluency
We did not detect any important effects in secondary outcomes at the medium‐term follow‐up. The following outcomes were classified as less important; relative to an inactive control condition, we found the following effects.
A small positive effect on auditory selective attention/working memory, as indicated by a performance‐based measure (MD 0.47, 95% CI 0.09 to 0.84; I2 = 0%; 2 RCTs, 386 participants; moderate‐certainty evidence).
A small negative effect on general functional ability, as indicated by self‐ratings (SMD ‐0.23, 95% CI ‐0.43 to ‐0.03; I2 = 0%; 3 RCTs, 380 participants; moderate‐certainty evidence).
A small negative effect on memory, as indicated by a performance‐based measure (SMD ‐0.43, 95% CI ‐1.24 to 0.38; I2 = 46%; 2 RCTs, 51 participants; low‐certainty evidence).
A small positive effect on sustained attention, as indicated by a performance‐based measure (MD 0.43, 95% CI ‐0.64 to 1.49; I2 = 66%; 2 RCTs, 413 participants; low‐certainty evidence).
A small negative effect on participants’ anxiety level, as indicated by self‐ratings (MD ‐0.49, 95% CI ‐1.56 to 0.58; I2 = 54%; 3 RCTs, 455 participants; low‐certainty evidence).
There were negligible effects on participants’ self‐efficacy, depression, quality of life, and immediate recall (moderate‐certainty), and on verbal fluency (low‐certainty). The remaining comparison (delayed recall) was based on very low‐certainty evidence, and hence we were unable to determine whether CR was associated with any meaningful benefits in delayed recall for participants with dementia.
Secondary outcomes for care partners
End of treatment
See Analysis 1.16, Analysis 1.17, Analysis 1.18, Analysis 1.19, Analysis 1.20, Analysis 1.21, Analysis 1.23, and Analysis 1.24 for details of secondary outcome measure analyses for the comparisons of CR and an inactive control condition at the end of treatment time point for care partners.
1.16. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 16: Quality of life: physical health (care partner self‐report)
1.17. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 17: Quality of life: psychological (care partner self‐report)
1.18. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 18: Quality of life: social (care partner self‐report)
1.19. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 19: Quality of life: environmental (care partner self‐report)
1.20. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 20: Mood: anxiety (informant self‐report)
1.21. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 21: Mood: depression (informant self‐report)
1.23. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 23: Stress (care partner self‐report)
1.24. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 24: Burden (care partner self‐report)
We did not detect any important effects in secondary outcomes at the end of therapy time point for care partners. We classified the following outcomes as less important; relative to an inactive control condition, we found the following effects.
A small negative effect on care partners’ depressive symptoms, as indicated by self‐ratings (MD ‐0.58, 95% CI ‐2.10 to 0.94; I2 = 61%; 2 RCTs, 32 participants; low‐certainty evidence).
A small negative effect on care partners’ psychological wellbeing, as indicated by self‐ratings (MD 1.11, 95% CI ‐1.81 to 4.04; I2 = 66%; 2 RCTs, 388 participants; low‐certainty evidence).
A small positive effect on the environmental aspect of care partners’ quality of life, as indicated by self‐ratings (MD 1.08, 95% CI ‐0.45 to 2.61; I2 = 65%; 3 RCTs, 465 participants; low‐certainty evidence).
There were negligible effects on the physical health, psychological and social aspects of quality of life, and on stress (moderate‐certainty), and on burden (low‐certainty).
The remaining comparison (anxiety) was based on very low‐certainty evidence, and hence we were unable to determine whether CR was associated with any meaningful benefits in relation to anxiety for care partners.
Medium‐term follow‐up
See: Analysis 2.15; Analysis 2.16; Analysis 2.17; Analysis 2.18; Analysis 2.19; Analysis 2.20; Analysis 2.21; Analysis 2.23 for details of the secondary outcome measure analyses for the comparisons of CR and an inactive control condition at the medium‐term follow‐up time point for care partners.
2.15. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 15: Quality of life: overall (care partner self‐report)
2.16. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 16: Quality of life: physical health (care partner self‐report)
2.17. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 17: Quality of life: psychological (care partner self‐report)
2.18. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 18: Quality of life: social (care partner self‐report)
2.19. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 19: Quality of life: environmental (care partner self‐report)
2.20. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 20: Mood: anxiety (informant self‐report)
2.21. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 21: Mood: depression (informant self‐report)
2.23. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 23: Stress (care partner self‐report)
We did not detect any important effects in secondary outcomes at medium‐term follow‐up for care partners. The following outcomes were classified as less important; relative to an inactive control condition, we found the following effects.
A small positive effect on the social aspect of care partners’ quality of life, as indicated by self‐ratings (MD 0.43, 95% CI 0.11 to 0.76; I2 = 0%; 3 RCTs, 436 participants; high‐certainty evidence).
A small positive effect on the psychological aspect of care partners’ quality of life, as indicated by self‐ratings (MD 0.40, 95% CI ‐0.24 to 1.05; I2 = 30%; 3 RCTs, 437 participants; moderate‐certainty evidence).
There was a negligible effect on the physical health aspect of care partners’ quality of life and psychological wellbeing (moderate‐certainty).
The remaining comparisons (overall quality of life, stress, burden, environmental aspect of quality of life, depression, and anxiety) were based on very low‐certainty evidence, and hence we were unable to determine whether CR was associated with any meaningful benefits in these outcomes for care partners.
We identified no outcomes relating to care partners’ coping in the included studies.
Long‐term follow‐up
Amieva 2016 was the only included study that provided data for long‐term follow up (24 months) and so we did not undertake meta‐analyses. Authors reported better functional ability and an average six‐month delay in institutionalisation at the 24‐month time point for CR in comparison to the inactive control group.
Cognitive rehabilitation versus alternative treatments
Amieva 2016 was the only study to evaluate CR alongside alternative treatments (cognitive training and reminiscence therapy) although the study did not directly compare outcomes for CR against outcomes for those alternative treatments. We calculated change‐from‐baseline scores for the three measures with sufficient data published (Disability Assessment for Dementia, Neuropsychiatric Inventory and Zarit Caregiver Burden) and then calculated SMDs for each of these measures at the three‐month and 24‐month follow‐ups, for CR versus cognitive training and CR versus reminiscence therapy. The observed effect sizes were mostly negligible. There were small benefits of cognitive training on the Neuropsychiatric Inventory at the 24‐month follow‐up, indicating that there might be less deterioration in behavioural symptoms in the CR group, relative to both the cognitive training and the reminiscence therapy groups (SMD 0.30, 95% CI 0.05 to 0.55 and SMD 0.37, 95% CI 0.11 to 0.62, respectively). The study reported no effects of cognitive training or reminiscence therapy on the primary or secondary outcome measures relative to the inactive control condition.
Discussion
Summary of main results
This review aimed to evaluate current evidence regarding the efficacy of cognitive rehabilitation (CR) for people with mild‐to‐moderate dementia and their care partners. Our primary outcome was the person with dementia's functional ability in the activities targeted by the rehabilitation intervention, assessed by a variety of methods. Six randomised controlled trials (RCTs) met our inclusion criteria, providing a sample of 1702 participants with dementia (mostly Alzheimer's disease, where the diagnosis was specified). For our main comparison of CR with usual care, we conducted meta‐analyses for three primary and 21 secondary outcome measures at the end of therapy time point and for three primary and 121 secondary outcome measures at the medium‐term (3 to 12 months) follow‐up time point.
We detected consistent large positive effects of CR relative to control in all measures of our primary outcome at both time points; these indicated with high certainty that people with mild or moderate dementia can make reliable improvements in functioning in relation to their personal rehabilitation goals, as rated by themselves and by the care partner or other informant.
Regarding participants with dementia at the end of treatment, there was high‐certainty evidence for a small positive effect of CR on participants’ self‐efficacy and immediate recall. There was also moderate‐certainty evidence indicating negligible effects on participants’ anxiety, quality of life, and sustained attention, and low‐certainty evidence indicating negligible effects on general functional ability, memory, and delayed recall.
At the medium‐term follow‐up, there was moderate‐certainty evidence for a small positive effect of CR on participants’ auditory selective attention and for a small negative effect on general functional ability, and low‐certainty evidence for a small positive effect on sustained attention and small negative effects on memory and on participants’ anxiety level. There was also moderate‐certainty evidence for negligible effects on self‐efficacy, depression, quality of life, and immediate recall, and low‐certainty evidence for negligible effects on verbal fluency.
Regarding care partners, at the end of treatment, we found moderate‐certainty evidence for negligible effects of CR on stress level and on the physical health, psychological, and social aspects of care partners’ quality of life. There was also low‐certainty evidence showing small negative effects on care partners’ depressive symptoms and psychological wellbeing, and a small positive effect on the environmental aspect of care partners’ quality of life. At the medium‐term follow‐up we found high‐certainty evidence showing a small positive effect of CR on the social aspect, and moderate‐certainty evidence showing a small positive effect on the psychological aspect of care partners’ quality of life. There was also moderate‐certainty evidence for negligible effects on the physical health aspect of care partners’ quality of life and psychological wellbeing.
Finally, there were also several comparisons for participants with dementia and care partners at both time points based on very low‐certainty evidence where we were unable to determine whether CR was associated with any meaningful effects.
Overall completeness and applicability of evidence
We were able to undertake meta‐analyses for most of the outcome categories specified in the protocol, except for overall severity of dementia and care partner coping. However, comparisons were possible only for the end of treatment and medium‐term follow‐up, and not for longer‐term effects. We could not conduct planned comparisons between CR and alternative treatments; there were very limited data from just one study, which compared CR with cognitive training and reminiscence therapy.
While the primary outcome comparisons are based on high‐quality data, they are strongly driven by one large RCT. Hindle 2018 was the smallest study, but contributed data to the highest number of analyses, while the two largest studies contributed data to only three comparisons each (Amieva 2016; Clarkson 2022). Given the relatively small sample sizes of Clare 2010, Hindle 2018, and Thivierge 2014, and the few contributions of Amieva 2016 and Clarkson 2022, the review findings are strongly driven by Clare 2019. Our confidence in these findings and their generalisability would be higher if more studies were included in the analyses.
The majority of participants in the studies that reported dementia types had a diagnosis of Alzheimer’s disease and so the review findings may not be equally applicable to all dementia types.
Outcome measures
The primary outcome in this review was functional ability in relation to the activities directly targeted in the intervention. Four out of the six eligible studies contributed a relevant measure to primary outcome analyses. Three of them (Clare 2010; Clare 2019; Hindle 2018) used ratings of performance on a simple 10‐point Likert‐style scale completed as part of semi‐structured interview protocols with the person with dementia and with the care partner (Bangor Goal‐Setting Interview; BGSI, Clare 2019; COPM, Law 2005). One study based its ratings on the practitioner’s direct observation of the person with dementia undertaking the target activity (Thivierge 2014). The comparisons provided consistent results showing large positive effects of CR on the primary outcome at the end of treatment, which were maintained at medium‐term follow‐up. Voigt‐Radloff 2017, in a study of CR that was excluded because the comparison was between two CR methods and not with a control condition, also demonstrated improvement in targeted activities whether via errorless or trial‐and‐error methods, and used direct observation with video‐recording to assess changes following training.
These improvements reflect gains in a range of areas, including learning to use memory aids to bypass memory difficulties, incorporating strategies to increase attention when completing tasks, learning to operate electronic devices, and acquiring skills relating to leisure activities and personal projects. Detailed descriptions of the process of eliciting therapy goals and more details about the nature of the identified goals have been published for four of the included studies (Clare 2010; Clare 2019; Clarkson 2022; Hindle 2018).
Increases in ratings of attainment in relation to the activities directly targeted in the intervention represent improvements in functioning, but it is important to note that these positive effects of CR on goal attainment are not accompanied by consistent gains on broader measures of functioning and wellbeing, as a large proportion of participant outcomes show only negligible effects. There were benefits for participants with dementia in some aspects of attention and recall, although the intervention did not target cognition specifically, and in self‐efficacy, which was proposed as a mechanism underlying the effects of CR (Clare 2019).
The discrepancy between the large effects in the primary outcomes and only a few important effects in the secondary outcomes may be due to a variety of reasons. The number of eligible studies was low overall and in many cases the low certainty of evidence meant we could not ascertain the effects of the intervention. There were several outcomes where we would expect to see an effect, such as anxiety and depression levels, quality of life, and general functional ability, where there were only negligible effects. It could be that some measures lack the sensitivity to capture the essence of the change associated with a personalised intervention and attaining a personal goal. For example, the Bristol Activities of Daily Living Scale used in Clarkson 2022 as the primary outcome comprises 20 questions with a range of scores between 0 and 60. More than half of the questions reflect physical ability that would not be a target of cognitive rehabilitation; to gain just one point in relation to orientation to time, a common area of need in that study, the person would need to demonstrate a shift between ‘Mixes up night and day (3 points)’, ‘Repeatedly asks the time/day/date (2 points)’, ‘Unaware of time/day etc. but seems unconcerned (1 point)’, and ‘Fully orientated to time/day/date etc. (0 points)’, which do not seem to distinguish more nuanced changes around using aides to being more oriented to time and/or asking fewer questions. There are no questions to reflect the difficulty around misplacing items, so the scale could not capture changes in this other common area of need in the Clarkson 2022 and other trials. Indeed, the reason often given for using personal goals and attainment scales was improving ecological validity and sensitivity to change (Clare 2010; Thivierge 2014). Another reason for the discrepancy could also be too little scope to demonstrate an improvement where the baseline ratings showed limited distress or dissatisfaction, as seems to be the case for the anxiety and depression levels. On the other hand, it could be that the gains in the very specific therapy goals did not have any major impact on people’s everyday life overall, either because they were not relevant or because the gains were not substantial enough to bring about a change. Other potential reasons for the discrepancy could be that the time and effort needed to attain the goals negated any positive impact, or that there have been some unintended effects of working on the selected goals, such as confrontation with one’s limitations.
The personalisation of CR with individual goals offers the person‐centredness that is advocated for by people with dementia, but it also brings questions about striking the balance between the person’s autonomy in deciding their goals and the need to potentially steer the person toward a goal that would be more likely to bring about a wider change in wellbeing and functional level. Relating to that is a question about the mechanisms by which the goals could bring about a wider change in a person’s life. While the origins of CR are in maximising independence in everyday life (Wilson 2002), it is possible that in dementia, the personal goals may need to go beyond everyday functioning and reflect the person’s need to protect the identity and self‐esteem threatened by the diagnosis of dementia. As such, the function of the goal may go beyond the increased capability to manage everyday tasks, more akin to the way that goals are used in Cognitive Behavioural Therapy or Acceptance Commitment Therapy (Cullen 2008). We did not come across such an approach in the included studies. Instead, we detected a small positive effect in self‐efficacy at the end of the therapy, strengthening the view that self‐efficacy could be a mechanism for change in CR. However, when considering the impact of individualised CR on the person’s functioning and wellbeing it is important to acknowledge the complexity of the factors that may be contributing to the observed effects in the outcome measures, including individual differences in people with dementia. More research might be needed to synthesise the process evaluation findings as the research evidence base grows.
There were two potentially relevant secondary outcomes that did not have sufficient data to allow us to conduct meta‐analyses. None of the included studies evaluated care partners’ coping, although we were able to consider other care partner outcomes. Dementia severity was evaluated in Amieva 2016 only, by analysing rates of progression to severe stages of dementia at two years and by comparing institutionalisation rates across the groups. Authors used MMSE scores and Global Deterioration Scale staging to calculate a rate of survival without progression to moderately severe or severe dementia at two years and found no statistically significant differences between the inactive control group and CR. However, CR was beneficial for reducing the rate of institutionalisation in the CR group relative to the inactive control group (and to the reminiscence and cognitive training groups), with the finding corroborated by better functional ability at two years as measured on two separate scales, Disability Assessment for Dementia and Grille d’Autonomie Gérontologique‐Groupes Iso‐Ressources (Gélinas 1999). CR may not affect general functional ability, but our certainty in the evidence is low.
Outcomes for care partners were equivocal. There is high‐ and moderate‐certainty evidence demonstrating small gains in some aspects of quality of life, but the benefits are not consistent across time points. There is some low‐certainty evidence based on a very small number of participants at the end of treatment time point indicating a worsening in the care partner’s depression in the CR group relative to controls. This raises a question about the value of providing specific support for care partners. Amieva 2016 provided weekly support for the care partners in the CR (individual telephone contact) and control (group sessions) conditions and reported a small improvement in care partners' burden in the CR group. However, when combined with other study data in this review the results translated into a negligible effect, based on low‐certainty data (end of treatment) and very low‐certainty data (follow‐up).
Hindle 2018 was the smallest study, but contributed data to the highest number of analyses, while the largest study contributed data to only three comparisons (Amieva 2016). Given the relatively small sample sizes of Clare 2010, Hindle 2018, and Thivierge 2014, and the few contributions of Amieva 2016, the review findings are strongly driven by Clare 2019.
It is worth emphasising that the positive outcomes of CR are achieved in relation to the individual goals worked on with the practitioner, and therefore a selection of meaningful goals that will make a difference in daily life is essential to realise the potential of the intervention. The limited gains in general functioning and wellbeing indicate the importance of eliciting therapy goals in a way that reflects an in‐depth understanding of what it means to ‘live well’ with dementia for each individual seeking CR and what constitutes a meaningful change (Clare 2022b).
The routinely reported participants' characteristics such as age, stage of dementia, and sex do not capture what may be crucial individual differences in relation to CR. In particular, the person’s personality and coping style may determine how well the person is likely to respond to a solution‐focused intervention and what magnitude of a change would be perceived as subjectively meaningful (Deci 2008). Subjectivity is essential in ascertaining that the intervention is fit for purpose from the service user's perspective. The arbitrary nature of the effect size interpretation may then be problematic. The individual therapy goals and goal attainment ratings used in some of the included studies address this to some extent, as it gives participants the voice to indicate areas of subjective importance and a means of indicating progress or the lack of it, as subjectively perceived. However, that approach is open to the criticism that the use of unblinded self‐ratings contravenes the gold standard of double‐blind assessment striven for in medical research.
Consideration of the minimally important difference is important for understanding the clinical significance of the observed changes in outcome measurements and hence for evaluating an intervention overall. A minimally important difference is defined as the least change in a measurement that is judged to matter to the service user (Cates 2015). There is little evidence to inform such a discussion in the context of psychosocial interventions for dementia, particularly in relation to the outcome measures in this review (Shabbir 2014). The performance self‐ratings (primary outcome) were all completed using 10‐point scales (Bangor Goal‐Setting Interview (BGSI), Clare 2019; Canadian Occupational Performance Measure (COPM), Law 2005) and the mean change score was 1.68 (confidence interval (CI) 1.29 to 2.07) at the end of treatment and 1.87 (CI 1.63 to 2.11) at the medium‐term follow‐up. The work on detecting clinically important changes in COPM indicated that what constitutes a minimally important difference varies depending on the population, target problems, and therapy context (Law 2005), and the suggested cut‐off values range from 0.9 in a mixed outpatient population (Eyssen 2011) to 4.3 points in physical rehabilitation for hand osteoarthritis (Raquel 2021). We are not aware of a dementia‐specific recommendation for the COPM, although some dementia studies adopted a two‐point cut‐off in line with the previous generic COPM recommendation (Clare 2019; Clare 2010). There were three high‐ or moderate‐certainty comparisons where all included studies used the same questionnaires, allowing examination of unstandardised values. Self‐efficacy was evaluated using the Generalized Self‐Efficacy Scale (Luszczynska 2005; Schwarzer 1995), with the scores ranging from 10 to 40. We recorded a mean difference of 0.71 at the end of therapy, favouring CR. This was interpreted as a small effect (SMD = 0.22). Care partners’ quality of life was assessed using the World Health Organization’s Quality of Life Instrument – brief version (WHOQOL‐BREF, Skevington 2004), with the scores in each domain ranging from four to 20. Mean differences in social and psychological aspects of care partners’ quality of life were 0.43 and 0.40 respectively, favouring CR. These were interpreted as small effects (SMD = 20 and SMD = 0.24, respectively). We did not identify any directly applicable studies to aid interpretation of these questionnaire‐based outcomes in the review. Some neuropsychological tests have limited ecological validity and the observed changes in scores do not translate easily into changes in functioning (Chaytor 2003).
A better understanding of minimally important difference is an important area for improvement in dementia research and practice (Cates 2015), and involving people with dementia and care partners in attaining such understanding is crucial. Application of qualitative methods can provide a richer understanding of what constitutes a meaningful goal and how achieving progress with that goal affects wellbeing and functioning to augment numerical data from standardised outcome measures. People with dementia involved in co‐producing self‐help resources based on the principles of CR commented on the value of CR in instilling hope following the diagnosis; they considered that belief in the possibility of ‘living well’ with dementia was needed to provide the motivation to engage in therapeutic work (Clare 2022a; Innovations in Dementia 2021). They reflected on the importance of hope for living well with dementia and captured the circularity in how hope can be both a prerequisite for engagement in, and an outcome of, CR work. Related to the importance of hope (Duggleby 2009) is self‐efficacy (Moraitou 2006), which was proposed as the mechanism for change in CR (Clare 2019). There is some qualitative evidence supporting this proposition (Clare 2019), and the finding of improved self‐efficacy in this review provides further support. Process evaluation research could help identify avenues through which to maximise the effects of CR and ensure wider impacts.
Economic evaluation was not a focus of this review, but we note the importance of considering the cost‐effectiveness and scalability and present the relevant findings from three eligible studies that provided relevant details.
In Amieva 2016, there was no statistically significant difference in sociomedical costs related to disease management, but the reduction of costs was approximately EUR 600 per month for the CR group in comparison to the control group and there was a six‐month delay in institutionalisation for CR participants at 24‐month follow‐up.
In Clare 2019, CR was not cost‐effective when gauged against a quality‐adjusted life‐year based on the Dementia Quality Of Life questionnaire for commissioning purposes. The cost‐effectiveness in relation to participant‐rated goal attainment, where the positive effect was observed, is dependent on the willingness to pay by decision‐makers. In Clare 2019, the average cost of the CR intervention was GBP 1736 per participant, and in the subsequent implementation study, the NHS organisations provided a six‐session intervention for GBP 349 per participant (excluding travel time).
Clarkson 2022 observed no overall benefits following their four‐session intervention and the economical evaluation indicated that the intervention was not cost‐effective.
The protocol for Hindle 2018 indicated cost‐effectiveness analysis, but the authors have not yet reported the results. Thivierge 2014 did not report a cost‐effectiveness analysis.
Only two out of six studies monitored adverse events (Clare 2019; Clarkson 2022), and both reported no serious adverse reactions linked to the intervention, despite large samples and relatively long follow‐ups. This suggests that serious negative side effects offsetting CR benefits are unlikely. The low level of studies monitoring serious adverse events in this review is consistent with the current practice in psychosocial interventions (Klatte 2022).
Not all of the included studies involved people with lived experience of dementia, which means their perspective may be overlooked, reducing the relevance of the findings. We did not find comments on involving people with lived experience of dementia in Amieva 2016, Hindle 2018, and Thivierge 2014. In Clare 2010, three people with lived experience were involved as advisors and two of them later contributed to trial development and delivery as Trial Steering Committee members. In Clarkson 2022, people with lived experience contributed to specifying research questions, selecting outcome domains and designing the intervention, developing recruitment and data collection procedures, and study materials. Care partners of people with dementia were formal members of the Data Monitoring and Ethics Committee and Programme Steering Committee.
Definitions of CR
Despite an increasing focus on psychosocial interventions in dementia research, our review identified only five new eligible CR studies since the most recent Cochrane Review on this topic was conducted by Bahar‐Fuchs 2013. One of the reasons for the low number of included studies is that psychosocial interventions represent a broad range of theoretical frameworks and utilise a multitude of delivery modes, with considerable inconsistencies in how the various types of interventions are defined (Sikkes 2021).
In order to reduce the heterogeneity in the included studies, we adopted a detailed definition of CR and operationalised eligibility criteria. That allowed us to be transparent about the type of studies we included in the review. We excluded studies where we did not have sufficient detail to ascertain whether an intervention met our CR criteria, in line with the protocol. That meant that several studies where there was some overlap between CR and the intervention, such as occupational therapy (OT) studies, were not included in the analyses.
Although there were numerous psychosocial intervention studies identified in the searches, including several labelled as CR, only a few met the CR criteria adopted in this review. Some of those had to be excluded due to insufficient methodological quality. Other studies prioritised CR provision for people with mild cognitive impairment (MCI) where it is often believed people are more likely to benefit, although impairment in functional ability has traditionally not been emphasised in MCI criteria. While manualised group interventions were prominent in the searches, we noted an increasing number of reablement interventions; however, these did not always offer the level of personalisation stipulated by our definition of CR. There were a few studies of occupational therapy that utilised goal‐setting and CR techniques within multicomponent interventions (Callahan 2017; Graff 2006; Wenborn 2021). Goal‐setting is an integral part of the occupational therapy (OT) approach and there is substantial overlap between occupational therapy and CR regarding the methods used. All OT studies addressing functional ability in people with dementia were carefully assessed against the eligibility criteria for this review; none met all the review criteria, with some excluded only after careful consideration by all review authors, as we could not establish to what extent the interventions addressed difficulties resulting from cognitive rather than physical impairments. While the distinction may seem artificial given that both types of impairments may contribute to difficulties in carrying out activities, therapy focusing on physical reablement would look very different to the work undertaken with people whose impairments are mainly cognitive.
The review of OT interventions for people with dementia completed by Bennett and colleagues focused on the studies where the OT intervention was delivered at home and addressed at least one activity of daily living and/or behavioural or psychological symptom of dementia (Bennett 2019). The review identified 15 studies and the meta‐analysis found positive effects of OT in people with dementia with regard to activities of daily living, number of behavioural and psychological symptoms, quality of life, and some care partner‐related outcomes. It is worth noting that the authors defined OT as any intervention delivered predominantly by or under the supervision of a qualified OT practitioner, irrespective of the intervention protocol. That meant that there was some variability in the interventions within that review. For example, the authors reported that most but not all focused on maximising a person's activities of daily living performance or management of behavioural or psychological symptoms. Unlike CR defined here, OT in the included studies could involve the care partner being coached or trained to support the person with dementia, without necessarily working directly with the person with dementia, and the review did not exclude studies with people with severe dementia. The differences mean that we cannot directly compare the results of Bennett 2019 and this review; nevertheless, the findings are worth noting, and it may be helpful to undertake a formal comparison of CR and OT in future research.
Other studies were excluded due to design and inclusion criteria, and among them were a few that met our CR intervention criteria. For example, Regan 2017 evaluated a CR intervention, but most of the sample had MCI rather than dementia (37 out of 40 participants). Voigt‐Radloff 2017 compared the effectiveness of two CR strategies (errorless versus trial‐and‐error learning) used to train activities of daily living, showing no differences between the two approaches, but this trial was excluded as there was no control condition.
Multicomponent interventions
Some studies included CR as one of several components (e.g. Brueggen 2017; Kim 2015; Kurz 2012; Santos 2011). While a multicomponent structure may be well suited to addressing the complexity of living with dementia, it creates a challenge for evidence synthesis. For example, Santos 2011 offered eight modules (CR, computer‐assisted cognitive training, speech therapy, occupational therapy, art therapy, physical training, physical therapy, and cognitive stimulation with reading and logic games) and any benefits of the intervention overall could not be attributable to an individual module or a combination of modules, making it impossible to determine the value of any particular approach. For that reason, we did not include multicomponent studies in the review where the CR element constituted less than 80% of the work, or the proportion could not be determined. It did not seem possible to categorise the multicomponent studies into meaningful groups relevant for this review, but with a growing number of studies, this may become feasible in the future.
Control conditions
The protocol stipulated that CR could be compared to two types of control conditions: inactive control (usual care or waiting‐list) and non‐specific active control; a subgroup analysis could be undertaken if we detected substantial heterogeneity and there were at least three studies per subgroup. We envisioned that the non‐specific active condition could be a specified activity for an equivalent number of sessions with similar levels of contact with the research team.
In this review, there were two included studies with a non‐specific active control condition, a manualised, structured relaxation therapy (RT) (Clare 2010; Hindle 2018). Both of these studies also had an inactive control group (usual treatment). In this review, we only used the inactive condition data to prioritise the homogeneity of the control condition and to avoid splitting the CR groups.
We note that while RT was listed in the protocol as an example of a non‐specific active condition, RT has been increasingly recognised as an intervention in its own right (McCallie 2006), and there is some evidence that RT may have a therapeutic effect on cognition and functioning in dementia (Ikemata 2017; Suhr 1999). Consequently, while RT is not normally used as an intervention aimed at improving a person’s functioning in dementia, it could be classified as an alternative treatment, especially if it has a structured format. The concept of a non‐specific active control condition in psychosocial RCTs is controversial as any time spent with participants in the control group could have some impact on their wellbeing or functioning, and so any non‐specific active condition with matched treatment time could be seen as an intervention (Mohr 2009).
We decided against reclassifying RT in Clare 2010 and Hindle 2018 as an alternative treatment, but the matter could be reconsidered in the future if more evidence of the therapeutic value of RT in dementia emerges.
Quality of the evidence
We considered the potential for bias in individual studies using the Cochrane risk of bias tool (Higgins 2017), and these ratings formed part of the subsequent GRADE evaluation of evidence certainty for the review outcomes across the studies.
The overall risk of bias in the individual studies was relatively low; we indicated a high risk of bias in relation to blinding of participants and practitioners for all included studies, as they all employed a single‐blind design. This seems to directly reflect the individual, personalised, and practitioner‐delivered nature of the intervention, which means that CR, like many other psychosocial and therapeutic interventions, does not lend itself to double‐blind evaluation. While not typically part of the risk of bias evaluation, we systematically reviewed practitioner training in the included studies to ascertain intervention fidelity. While most studies mentioned initial training for the practitioners and some ongoing supervision or ad hoc support, and there were a few references to detailed intervention manuals, only one trial reported formal verification of intervention fidelity (Clarkson 2022). However, it should be acknowledged that three of the included studies were small single‐site studies where in‐depth fidelity protocols are less relevant. In RCTs of treatments for mood disorders, psychotherapy sessions are routinely recorded for use in supervision and evaluation of adherence to protocol (Mowbray 2003), but this approach has not been adopted in any of the included studies. This may reflect the difference between recording one‐to‐one boundaried psychotherapy sessions in a clinical setting and recording more practically based sessions in the home setting that may involve moving around the house and interacting with family members or a practitioner accompanying the person on a trip to the shop. Nevertheless, it may be helpful to think more broadly about ways of capturing the relevant information in CR studies. For example, video recording of the person carrying out the goal‐related activity before and after the intervention could be used to demonstrate both how the therapy plan has been applied and the progress made.
Evidence certainty
To estimate our confidence in the review findings we used the GRADE approach to guide the ratings of inconsistency and imprecision in the results, directness of the evidence, and publication bias in the included studies (GRADE Handbook; GRADEpro GDT).
Risk of bias. As discussed above, the overall level of risk of bias in the included studies was low except for blinding of participants and practitioners. We decided not to downgrade the certainty of evidence in relation to the risk of bias solely on that basis. There might be strategies to mitigate expectation bias even if full blinding of participants is not possible, but it would seem out of context to apply standards developed for pharmacological trials, where blinding can usually be achieved, to trials of non‐pharmacological intervention, where blinding participants to the fact that they are receiving the intervention rather than being in the control group, and practitioners to the fact that they are providing a particular intervention, is difficult to achieve. The issue of applying standards and expectations that are commensurate with the nature of non‐pharmacological interventions has been raised before (Juul 2021). We downgraded certainty in this category by one point only in three comparisons: general functional ability, behavioural symptoms, and burden (at the end of therapy). This is where there were only two or three studies contributing data to the comparisons (Amieva 2016; Clarkson 2022; Thivierge 2014), which all had a high risk of bias in two out of five domains of risk assessed for individual studies.
Inconsistency. The consistency of the comparison data was generally good. We downgraded the certainty of assessment in relation to inconsistency by one point for two end of therapy outcomes where the statistical test of heterogeneity was significant at P < 0.05 and the l² was moderate (40% < l² < 75%). We downgraded two end of therapy outcomes and six follow‐up outcomes by two points as the l² was large (l² > 75%) and statistically significant.
Imprecision in the results. When assessing imprecision in the comparison data we considered mean effect sizes (SMD) and confidence intervals (CI) in relation to four interpretation categories: appreciable harm for SMD values below ‐0.5, negligible harm for values between ‐0.5 and 0, negligible benefits for values between 0 and 0.5, and appreciable benefits for values above 0.5. We downgraded certainty in relation to imprecision by two points if CIs were very broad and crossed three or all four interpretation categories (including both the benefit and harm categories) or if the CI crossed two interpretation categories (including both the benefit and harm categories) and the sample size for the analysis was fewer than 400. We downgraded certainty in relation to imprecision by one point if the CI crossed two interpretation categories and/or the sample size for the analysis was fewer than 400. We did not downgrade certainty in relation to imprecision if the CI was within one interpretation category.
Indirectness. Overall, measures in the included studies mapped well onto the outcomes of interest specified in the protocol. We had no concerns about the directness of the evidence and did not downgrade any comparisons under this category.
Publication bias. Our literature search identified a few registered trials that appeared not to have any published results and where possible authors were approached for data. As data were not forthcoming there is a possibility of positive publication bias, although one of the studies excluded due to an insufficient amount of data did report a positive effect of CR relative to controls (Reuster 2010). However, given the small number of included studies, we were unable to formally assess the risk and we did not downgrade the quality of evidence on that basis, which means the extent of concerns related to the presence of publication bias might be underestimated.
Finally, we note that three out of the six included studies were conducted by the same research team in the UK. They employed similar designs and outcome measures and conducted the studies in similar settings, and some of the comparisons rely exclusively on data from those studies. Amieva 2016 and Clarkson 2022 were two large RCTs that adopted a significantly different approach to CR evaluation than that used in those three UK‐based studies and both contributed no data for the primary outcomes of this review, providing data only for three and eight secondary outcome analyses respectively. Thivierge 2014 had significant overlap with the UK‐based studies, but the relatively small sample meant that its impact on the review conclusions was limited. While the similarities between the three UK‐based studies meant a reasonable sample size in the comparisons with good consistency, and strong methodology translated into the observed overall high certainty of the review comparisons, the results are driven effectively by a single RCT, albeit a high‐quality one with robust findings. The generalisability of the findings would be increased by including a more diverse sample of studies.
Potential biases in the review process
Our search strategy was based on detailed screening criteria and was conducted by two review authors independently, with disagreements resolved by a third review author or a vote by all review authors where necessary. The quality of evidence was also rated independently by two review authors, with disagreements resolved by discussion. The review authors include researchers at varying stages of their professional careers and with expertise in different aspects of dementia research, which lessens the risk of systematic bias in the review process. However, three of the included studies were co‐authored by one or two of our review team members, so we took steps to limit potential bias by ensuring that any screening, data extraction, and quality ratings were completed only by the review authors who were not involved in the delivery of the given trial. While this mitigates the likelihood of bias in the review process, we cannot rule out the possibility that there were some inadvertent biases in how decisions were made throughout the review process.
Agreements and disagreements with other studies or reviews
There is a limited amount of evidence about CR and, consequently, no other directly relevant reviews. There are two reviews with some overlap, although the comparison is complicated by differences in defining CR and classifying interventions.
Scott 2019 completed a systematic narrative review of non‐pharmacological intervention studies that included a measure of functional ability (e.g. activities of daily living scales, goal attainment rating). Authors classified two studies with a low risk of bias as investigating individualised CR; Amieva 2016, which was included in the present review, and Kurz 2012, which was excluded from the present review as it combined elements from neurorehabilitation, behavioural therapy, and reminiscence therapy. Scott 2019 concluded that individual CR was beneficial for functional ability in comparison to the control condition. In that review, CR was grouped under the cognition‐focused and reminiscence therapy category, with separate categories for occupational therapy and multicomponent interventions.
Authors' conclusions
Implications for practice.
People with mild‐to‐moderate dementia can make substantial improvements in their ability to carry out the tasks targeted in cognitive rehabilitation (CR), with the benefits remaining for up to nine months after the end of the intervention when a small number of maintenance sessions are provided. Results of Amieva 2016 demonstrated superiority of individualised CR over group cognitive training or reminiscence interventions in supporting functional ability and delaying institutionalisation, further strengthening the argument for improving access to CR for people with dementia. Given the size of the observed positive effects and the quality of the evidence, it seems justified that the intervention is considered for inclusion in post‐diagnostic care. CR can form a part of a clinical toolkit to assist people with dementia in overcoming some of the everyday barriers imposed by cognitive difficulties. It might be important to consider support for care partners to avoid the risk of deterioration in their mood.
The practicalities of implementing CR into healthcare settings need to be considered, especially since the intervention may appear expensive given the time and skill involved. Clare 2019 described an implementation study in the UK National Healthcare System, following from the GREAT CR programme. With a shorter six to eight session course of CR, the participants achieved a similar level of improvement in relation to personal goals to that seen in the randomised controlled trials (RCTs) for individual goals, and the intervention was well‐received by staff and by people living with dementia. Although the barriers were substantial and further complicated by the COVID pandemic, some of the research sites continued offering CR beyond the study (Clare 2019). The barriers to implementing CR in that study often reflected the level of service that was already in place in the local NHS sites, with more difficulty experienced in the services providing less formal post‐diagnostic support as standard. At a time when financial pressures see many healthcare systems struggling to provide even the essential treatments, the most worrying barrier to implementing CR into mainstream healthcare may be the widespread ageism that fuels clinical nihilism and other forms of bias, resulting in services for older people being chronically underfunded (de São José 2017; Hepple 2004). CR does not necessarily require more skill or time than the psychotherapy offered for mood disorders in working‐age adults, but perhaps the perception of the value is different when an older person is considered.
Implications for research.
Future research would benefit from adopting observational measures of activities targeted in CR to provide a more standardised approach to measuring change. It would also be beneficial to explore how to maximise the impact of CR and extend the benefits to securing improvements in wider aspects of quality of life and wellbeing, considering the kinds of outcomes that would be most relevant and worthwhile. Better understanding of minimally important differences in the utilised measures would assist in intervention planning and interpretation of results. Future research could aim to better understand the impact of CR on the care partner and possibly investigate the effect of complementing CR with specific support for care partners. Dementia severity was assessed in one study only, while care partner coping was not represented in the included studies at all; future studies may want to consider including these important factors. Given the promising results of providing CR over a longer period demonstrated at two‐year follow‐up in Amieva 2016, there would also be value in more research investigating effects of extended CR therapy and including longer‐term follow‐ups to explore the impact of CR over time.
History
Protocol first published: Issue 8, 2019
Acknowledgements
We would like to thank Sue Marcus, Jenny McCleery, and Anna Noel‐Storr of the Cochrane Dementia and Cognitive Improvement Group for their invaluable assistance.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Anna Noel‐Storr, Nicole Edworthy, Shirley Hall, Jamiu Aderonmu, Brady Catherine, Deirdre Beecher, Marta Rossignoli, Emmet Farragher, Deborah Jackson, Igor Svintsitskyi, Bernardo Costa, Nicole Askin, Auxiliadora Fraiz‐Padin, Farah Siddiqui, Aisling Armstrong, Hariklia Nguyen, Stefanie Rosumeck, Amanda Qiao Ying Yap, Klaus Luding, Karen Ma, Stella Maria O'Brien, Nikolaos Sideris, Abdul Shakoor, Aleksandra Pelczarska, Basavaraj Poojar, Nuno Fernandes, Leire Leache, Ricky Ravindra Fajar Adi Putra, Roberto Altamirano, Brian Duncan, Mohammad Shahbaz, Sunday Onagbiye, Benedict Davies, Muhammad Shukri Bin Yaakub, Lyle Croyle, Fazal Ghani, SarahJane Moll, Katja Matthias, Inga Agustsdottir, Kathryn Vela, Abhijit Dutta, Mohammed Deeb Zakkor, Ivan Belitsky, Abhijna Vithal Yergolkar, Chet Chaulagai, Georgina Johnstone, Hao‐Wen Sim, Mohammad Aloulou, Meenakumari Natarajan, Ana‐Marija Ljubenković, Junior Fandim, Hendrik Napierala, Shammas Mohammed, Yuan Chi, Vighnesh D, Ashutosh Kumar Singh, Muataz Kashbour, Neetu Bhadra, Aparna Rajaram, Amin Sharifan, Abu Emmil Qawarizmi Bin Abu Sofian, Dinah Amoah, Maike Scherhans, Shirley Hall, Nicole Askin, Therese Dalsbø, Nikolaos Sideris, Anna Resolver, Lai Ogunsola, Shammas Mohammed, Vighnesh D, Fernando Tortosa, Serina Cao, Ivan Perez‐Neri, Aldyla Raditya, Malak Ashraf Mohamed.
We would like to thank peer reviewers, Alex Kurz and Ralph Möhler, and consumer reviewer Kit Byatt for their comments and feedback.
We are also grateful to the authors who provided additional data or clarification regarding their studies and to Jenny Bellorini for copy‐editing the manuscript.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php (Date of most recent search: 19 October 2022) |
#1 MESH DESCRIPTOR Dementia EXPLODE ALL TREES 5656 #2 MESH DESCRIPTOR DELIRIUM EXPLODE ALL TREES 676 #3 MESH DESCRIPTOR Neurocognitive Disorders EXPLODE ALL TREES 10502 #4 MESH DESCRIPTOR Aphasia, Primary Progressive EXPLODE ALL TREES 43 #5 MESH DESCRIPTOR Wernicke Encephalopathy EXPLODE ALL TREES 4 #6 PDD:TI,AB,KY 343 #7 korsako*:TI,AB,KY 67 #8 huntington*:TI,AB,KY 672 #9 dement*:TI,AB,KY 12167 #10 deliri*:TI,AB,KY 3066 #11 binswanger*:TI,AB,KY 6 #12 alzheimer*:TI,AB,KY 10824 #13 (pick* adj2 disease):TI,AB,KY 66 #14 (lewy* adj2 bod*):TI,AB,KY 412 #15 (creutzfeldt or jcd or cjd):TI,AB,KY 64 #16 (chronic adj2 cerebrovascular):TI,AB,KY 110 #17 (cerebral* adj2 insufficient*):TI,AB,KY 1 #18 (cerebr* adj2 deteriorat*):TI,AB,KY 10 #19 ("normal pressure hydrocephalus" and "shunt*"):TI,AB,KY 77 #20 (primary progressive aphasia):TI,AB,KY 52 #21 (Parkinson* disease dementia):TI,AB,KY 17 #22 (organic brain syndrome):TI,AB,KY 114 #23 (organic brain disease):TI,AB,KY 22 #24 (major neurocognitive disorder*):TI,AB,KY 27 #25 (benign senescent forgetfulness):TI,AB,KY 2 #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 26259 #27 MESH DESCRIPTOR Cognitive Remediation EXPLODE ALL TREES 94 #28 MESH DESCRIPTOR Cognitive Therapy EXPLODE ALL TREES 855 #29 MESH DESCRIPTOR Rehabilitation Nursing EXPLODE ALL TREES 54 #30 (activities of daily living):TI,AB,KY 9088 #31 (Cog* retrain*):TI,AB,KY 43 #32 (cognitive intervention*):TI,AB,KY 555 #33 ("Cognitive skills" adj2 training):TI,AB,KY 41 #34 (cognitive support):TI,AB,KY 10 #35 (memory aid*):TI,AB,KY 102 #36 (memory function*):TI,AB,KY 923 #37 (memory group*):TI,AB,KY 34 #38 (memory management):TI,AB,KY 3 #39 (Memory rehabilitation):TI,AB,KY 74 #40 (memory retraining):TI,AB,KY 22 #41 (memory re‐training):TI,AB,KY 0 #42 (memory stimulation):TI,AB,KY 8 #43 (memory strateg*):TI,AB,KY 94 #44 (memory support):TI,AB,KY 27 #45 (memory training):TI,AB,KY 639 #46 (restorative care):TI,AB,KY 38 #47 (cognit* adj2 rehabilitation):TI,AB,KY 1270 #48 (cognit* adj2 retrain*):TI,AB,KY 50 #49 (cognit* adj2 stimulation):TI,AB,KY 366 #50 (cognit* adj2 training):TI,AB,KY 2928 #51 (memory adj2 rehabilitation):TI,AB,KY 191 #52 (memory adj2 therap*):TI,AB,KY 441 #53 (restorative care):TI,AB,KY 38 #54 reablement:TI,AB,KY 22 #55 ((rehab*) adj3 (activities of daily living or Attention or executive function or planning)):TI,AB,KY 270 #56 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 16174 #57 #26 AND #56 3253 |
Jan 2020: 3253 Nov 2020: 344 Sep 2021: 618 Oct 2022: 284 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) (Date of most recent search: 19 October 2022) |
1 exp Dementia/ 2 exp DELIRIUM/ 3 exp Neurocognitive Disorders/ 4 exp Aphasia, Primary Progressive/ 5 exp Wernicke Encephalopathy/ 6 PDD.ti,ab. 7 korsako*.ti,ab. 8 huntington*.ti,ab. 9 dement*.ti,ab. 10 deliri*.ti,ab. 11 binswanger*.ti,ab. 12 alzheimer*.ti,ab. 13 (pick* adj2 disease).ti,ab. 14 (lewy* adj2 bod*).ti,ab. 15 (creutzfeldt or jcd or cjd).ti,ab. 16 (chronic adj2 cerebrovascular).ti,ab. 17 (cerebral* adj2 insufficient*).ti,ab. 18 (cerebr* adj2 deteriorat*).ti,ab. 19 ("normal pressure hydrocephalus" and "shunt*").ti,ab. 20 "primary progressive aphasia".ti,ab. 21 "Parkinson* disease dementia".ti,ab. 22 "organic brain syndrome".ti,ab. 23 "organic brain disease".ti,ab. 24 "major neurocognitive disorder*".ti,ab. 25 "benign senescent forgetfulness".ti,ab. 26 or/1‐25 27 exp Cognitive Remediation/ 28 exp Cognitive Remediation/ 29 exp Cognitive Therapy/ 30 exp Rehabilitation Nursing/ 31 "activities of daily living".ti,ab. 32 "Cog* retrain*".ti,ab. 33 "cognitive intervention*".ti,ab. 34 ("Cognitive skills" adj2 training).ti,ab. 35 "cognitive support".ti,ab. 36 "memory aid*".ti,ab. 37 "memory function*".ti,ab. 38 "memory group*".ti,ab. 39 "memory management".ti,ab. 40 "Memory rehabilitation".ti,ab. 41 "memory retraining".ti,ab. 42 "memory re‐training".ti,ab. 43 "memory stimulation".ti,ab. 44 "memory strateg*".ti,ab. 45 "memory support".ti,ab. 46 "memory training".ti,ab. 47 "restorative care".ti,ab. 48 (cognit* adj2 rehabilitation).ti,ab. 49 (cognit* adj2 retrain*).ti,ab. 50 (cognit* adj2 stimulation).ti,ab. 51 (cognit* adj2 training).ti,ab. 52 (memory adj2 rehabilitation).ti,ab. 53 (memory adj2 therap*).ti,ab. 54 "restorative care".ti,ab. 55 reablement.ti,ab. 56 (rehabilitation/ or rehab*.ti,ab.) adj3 (activities of daily living/ or Attention/ or executive function/ or attention.ti,ab. or planning.ti,ab. or "activities of daily living".ti,ab. or "executive function".ti,ab.) 57 or/27‐56 58 26 and 57 59 randomized controlled trial.pt. 60 controlled clinical trial.pt. 61 randomized.ab. 62 placebo.ab. 63 drug therapy.fs. 64 randomly.ab. 65 trial.ab. 66 groups.ab. 67 or/59‐66 68 exp animals/ not humans.sh. 69 67 not 68 70 58 and 69 |
Jan 2020: 4470 Nov 2020: 299 Sep 2021: 704 Oct 2022: 806 |
| 3. Embase (Ovid SP) 1974 to present (Date of most recent search: 19 October 2022) |
1 Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 "major neurocognitive disorder".ti,ab. 6 exp primary progressive aphasia/ 7 PDD.ti,ab. 8 korsako*.ti,ab. 9 huntington*.ti,ab. 10 dement*.ti,ab. 11 deliri*.ti,ab. 12 binswanger*.ti,ab. 13 alzheimer*.ti,ab. 14 (pick* adj2 disease).ti,ab. 15 (lewy* adj2 bod*).ti,ab. 16 (creutzfeldt or jcd or cjd).ti,ab. 17 (chronic adj2 cerebrovascular).ti,ab. 18 (cerebral* adj2 insufficient*).ti,ab. 19 (cerebr* adj2 deteriorat*).ti,ab. 20 ("normal pressure hydrocephalus" and "shunt*").ti,ab. 21 "primary progressive aphasia".ti,ab. 22 "Parkinson* disease dementia".ti,ab. 23 "organic brain syndrome".ti,ab. 24 "organic brain disease".ti,ab. 25 "major neurocognitive disorder*".ti,ab. 26 "benign senescent forgetfulness".ti,ab. 27 or/1‐26 28 exp cognitive remediation therapy/ 29 exp cognitive therapy/ 30 exp rehabilitation nursing/ 31 "activities of daily living".ti,ab. 32 "Cog* retrain*".ti,ab. 33 "cognitive intervention*".ti,ab. 34 ("Cognitive skills" adj2 training).ti,ab. 35 "cognitive support".ti,ab. 36 "memory aid*".ti,ab. 37 "memory function*".ti,ab. 38 "memory group*".ti,ab. 39 "memory management".ti,ab. 40 "Memory rehabilitation".ti,ab. 41 "memory retraining".ti,ab. 42 "memory re‐training".ti,ab. 43 "memory stimulation".ti,ab. 44 "memory strateg*".ti,ab. 45 "memory support".ti,ab. 46 "memory training".ti,ab. 47 "restorative care".ti,ab. 48 (cognit* adj2 rehabilitation).ti,ab. 49 (cognit* adj2 retrain*).ti,ab. 50 (cognit* adj2 stimulation).ti,ab. 51 (cognit* adj2 training).ti,ab. 52 (memory adj2 rehabilitation).ti,ab. 53 (memory adj2 therap*).ti,ab. 54 "restorative care".ti,ab. 55 reablement.ti,ab. 56 (rehabilitation/ or rehab*.ti,ab.) adj3 (activities of daily living/ or Attention/ or executive function/ or attention.ti,ab. or planning.ti,ab. or "activities of daily living".ti,ab. or "executive function".ti,ab.) 57 or/28‐56 58 27 and 57 59 randomized controlled trial/ 60 controlled clinical trial/ 61 random$.ti,ab. 62 randomization/ 63 intermethod comparison/ 64 placebo.ti,ab. 65 (compare or compared or comparison).ti. 66 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 67 (open adj label).ti,ab. 68 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 69 double blind procedure/ 70 parallel group$1.ti,ab. 71 (crossover or cross over).ti,ab. 72 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 73 (assigned or allocated).ti,ab. 74 (controlled adj7 (study or design or trial)).ti,ab. 75 (volunteer or volunteers).ti,ab. 76 trial.ti. 77 or/59‐76 78 58 and 77 |
Jan 2020: 5894 Nov 2020: 580 Sep 2021: 1056 Oct 2022: 1340 |
| 4. PsycINFO (Ovid SP) (Date of most recent search: 19 October 2022) |
1 exp Dementia/ 2 exp Delirium/ 3 exp Huntingtons Disease/ 4 exp Kluver Bucy Syndrome/ 5 exp Wernickes Syndrome/ 6 exp Cognitive Impairment/ 7 dement*.mp. 8 alzheimer*.mp. 9 (lewy* adj2 bod*).mp. 10 deliri*.mp. 11 (chronic adj2 cerebrovascular).mp. 12 ("organic brain disease" or "organic brain syndrome").mp. 13 "supranuclear palsy".mp. 14 ("normal pressure hydrocephalus" and "shunt*").mp. 15 "benign senescent forgetfulness".mp. 16 (cerebr* adj2 deteriorat*).mp. 17 (cerebral* adj2 insufficient*).mp. 18 (pick* adj2 disease).mp. 19 (creutzfeldt or jcd or cjd).mp. 20 huntington*.mp. 21 binswanger*.mp. 22 korsako*.mp. 23 ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24 "major neurocognitive disorder".ti,ab. 25 exp Aphasia/ 26 "primary progressive aphasia".ti,ab. 27 or/1‐26 28 exp Cognitive Rehabilitation/ 29 exp Cognitive Therapy/ 30 "activities of daily living".ti,ab. 31 "Cog* retrain*".ti,ab. 32 "cognitive intervention*".ti,ab. 33 ("Cognitive skills" adj2 training).ti,ab. 34 "cognitive support".ti,ab. 35 "memory aid*".ti,ab. 36 "memory function*".ti,ab. 37 "memory group*".ti,ab. 38 "memory management".ti,ab. 39 "Memory rehabilitation".ti,ab. 40 "memory retraining".ti,ab. 41 "memory re‐training".ti,ab. 42 "memory stimulation".ti,ab. 43 "memory strateg*".ti,ab. 44 "memory support".ti,ab. 45 "memory training".ti,ab. 46 "restorative care".ti,ab. 47 (cognit* adj2 rehabilitation).ti,ab. 48 (cognit* adj2 retrain*).ti,ab. 49 (cognit* adj2 stimulation).ti,ab. 50 (cognit* adj2 training).ti,ab. 51 (memory adj2 rehabilitation).ti,ab. 52 (memory adj2 therap*).ti,ab. 53 "restorative care".ti,ab. 54 reablement.ti,ab. 55 (rehabilitation/ or rehab*.ti,ab.) adj3 (activities of daily living/ or Attention/ or executive function/ or attention.ti,ab. or planning.ti,ab. or "activities of daily living".ti,ab. or "executive function".ti,ab.) 56 or/28‐55 57 27 and 56 58 exp Clinical Trials/ 59 randomly.ab. 60 randomi?ed.ti,ab. 61 placebo.ti,ab. 62 groups.ab. 63 "double‐blind*".ti,ab. 64 "single‐blind*".ti,ab. 65 RCT.ti,ab. 66 or/58‐65 67 57 and 66 |
Jan 2020: 1892 Nov 2020: 121 Sep 2021: 241 Oct 2022: 280 |
| 5. CINAHL (EBSCOhost) (Date of most recent search: 19 October 2022) |
S67 S54 AND S66 S66 S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 S65 MH "Random Assignment" S64 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S63 MH "Crossover Design" S62 MH "Factorial Design" S61 MH "Placebos" S60 MH "Clinical Trials" S59 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S58 TX crossover OR "cross‐over" S57 AB placebo* S56 TX random* S55 TX "latin square" S54 S23 AND S53 S53 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 S52 TX (rehab*) N3 (activities of daily living or Attention or executive function or planning) S51 TX reablement S50 TX restorative care S49 TX memory N2 therap* S48 TX memory N2 rehabilitation S47 TX cognit* N2 training S46 TX cognit* N2 stimulation S45 TX cognit* N2 retrain* S44 TX cognit* N2 rehabilitation S43 TX restorative care S42 TX memory training S41 TX memory support S40 TX memory strateg* S39 TX memory stimulation S38 TX memory re‐training S37 TX memory retraining S36 TX Memory rehabilitation S35 TX memory management S34 TX memory group* S33 TX memory function* S32 TX memory aid* S31 TX cognitive support S30 TX "Cognitive skills" N2 training S29 TX cognitive intervention* S28 TX Cog* retrain* S27 TX activities of daily living S26 (MH "Rehabilitation Nursing") S25 (MH "Cognitive Therapy+") S24 (MH "Cognitive Remediation") S23 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 S22 TX "primary progressive aphasia" S21 (MH "Aphasia+") S20 TX "major neurocognitive disorder" S19 TX korsako* S18 TX binswanger* S17 TX huntington* S16 TX creutzfeldt or jcd or cjd S15 TX pick* N2 disease S14 TX cerebral* N2 insufficient* S13 TX cerebr* N2 deteriorat* S12 TX "benign senescent forgetfulness" S11 TX "normal pressure hydrocephalus" and "shunt*" S10 TX "organic brain disease" or "organic brain syndrome" S9 TX chronic N2 cerebrovascular S8 TX deliri* S7 TX lewy* N2 bod* S6 TX alzheimer* S5 TX dement* S4 MH "Wernicke's Encephalopathy" S3 MH "Delirium, Dementia, Amnestic, Cognitive Disorders" S2 MH "Delirium" S1 MH "Dementia+" |
Jan 2020: 1944 Nov 2020: 118 Sep 2021: 240 Oct 2022: 354 |
| 6. Web of Science – core collection (Clarivate) (Date of most recent search: 19 October 2022) |
TOPIC: (dement* OR alzheimer* OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL OR "cognit* impair*" OR FTD OF FTLD OR "cerebrovascular insufficienc*" OR AD OR VCI OR "major neurocognitive disorder" OR "primary progressive aphasia") AND TOPIC: ("Cognitive Remediation" OR "Cognitive Therapy" OR "Rehabilitation Nursing" OR "activities of daily living" OR "Cog* retrain*") AND TOPIC: (randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial) | Jan 2020: 1647 Nov 2020: 118 Sep 2021: 276 Oct 2022: 164 |
| 7. LILACS (BIREME) (Date of most recent search: 19 October 2022) |
alzheimer OR alzheimers OR alzheimer’s OR dementia OR demenc$ OR aphasia [Palavras] and randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind$" OR placebo [Palavras] and "Cognitive Remediation" OR "Cognitive Therapy" OR "Rehabilitation Nursing" OR "activities of daily living" OR "Cognitive retraining" [Palavras] | Jan 2020: 24 Nov 2020: 0 Sep 2021: 0 Oct 2022: 0 |
| 8. ClinicalTrials.gov (www.clinicaltrials.gov) (Date of most recent search: 19 October 2022) |
dementia OR alzheimers OR cognition OR cognitive or aphasia | "Cognitive Remediation" OR "Cognitive Therapy" OR "Rehabilitation Nursing" OR "activities of daily living" OR "Cognitive retraining" | Jan 2020: 241 Nov 2020: 25 Sep 2021: 49 Oct 2022: 75 |
| 9. ICTRP (Date of most recent search: 19 October 2022) |
dementia OR alzheimers OR cognition OR cognitive or aphasia | "Cognitive Remediation" OR "Cognitive Therapy" OR "Rehabilitation Nursing" OR "activities of daily living" OR "Cognitive retraining" OR "Cognitive rehabilitation" | Jan 2020: 191 Nov 2020: n/a Sep 2021: 88 Oct 2022: 21 |
| 10. CDCIG specialised register (CRS web) (Date of most recent search: 19 October 2022) |
Jan 2020: 656 Nov 2020: 99 Sep 2021: 311 Oct 2022: 198 |
|
| TOTAL before de‐duplication | Jan 2020: 20212 Nov 2020: 1704 Sep 2021: 3583 Oct 2022: 3522 TOTAL: 29,021 |
|
| TOTAL after de‐duplication | Jan 2020: 12297 Nov 2020: 1253 Sep 2021: 2339 Oct 2022: 2410 TOTAL: 18,299 |
|
| TOTAL after Cochrane's Screen4Me workflow | Jan 2020: 5468 Nov 2020: 1253 Sep 2021: 1101 Oct 2022: 1054 TOTAL: 8876 |
|
| TOTAL after first assessment by CDCIG Information Specialist | Jan 2020: 2038 Nov 2020: 1253 Sep 2021: 476 TOTAL: 3767 |
|
Data and analyses
Comparison 1. Cognitive rehabilitation versus inactive control at the end of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Functional ability in targeted activities: personal goals ‐ performance (participant self‐report) | 3 | 501 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [1.26, 1.66] |
| 1.2 Functional ability in targeted activities: personal goals ‐ performance (informant report of participant) | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | 1.61 [1.01, 2.21] |
| 1.3 Functional ability in targeted activities: personal goals ‐ satisfaction (participant self‐report) | 3 | 501 | Std. Mean Difference (IV, Random, 95% CI) | 1.31 [1.09, 1.54] |
| 1.4 General functional ability (informant report of participant) | 3 | 673 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.10, 0.20] |
| 1.5 Self‐efficacy (participant self‐report) | 2 | 456 | Mean Difference (IV, Random, 95% CI) | 0.71 [0.12, 1.30] |
| 1.6 Mood: anxiety (participant self‐report) | 3 | 500 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.65, 0.15] |
| 1.7 Mood: depression (participant self‐report) | 3 | 502 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐0.39, 3.29] |
| 1.8 Behavioural symptoms (informant report of participant) | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐2.98 [‐6.98, 1.02] |
| 1.9 Quality of life (participant self‐report) | 5 | 853 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.08] |
| 1.10 Cognition: memory, global score | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.67, 0.37] |
| 1.11 Cognition: memory, immediate recall | 2 | 459 | Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.52] |
| 1.12 Cognition: memory, delayed recall | 2 | 459 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.57, 0.75] |
| 1.13 Cognition: sustained attention | 2 | 446 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.12, 0.17] |
| 1.14 Cognition: auditory selective attention/working memory | 3 | 452 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.38, 2.19] |
| 1.15 Cognition: verbal letter fluency | 3 | 495 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.85, 0.45] |
| 1.16 Quality of life: physical health (care partner self‐report) | 3 | 464 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.15, 0.53] |
| 1.17 Quality of life: psychological (care partner self‐report) | 3 | 464 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.28, 0.71] |
| 1.18 Quality of life: social (care partner self‐report) | 3 | 463 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.73, 0.73] |
| 1.19 Quality of life: environmental (care partner self‐report) | 3 | 465 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐0.45, 2.61] |
| 1.20 Mood: anxiety (informant self‐report) | 2 | 32 | Mean Difference (IV, Random, 95% CI) | 2.49 [‐1.47, 6.45] |
| 1.21 Mood: depression (informant self‐report) | 2 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐2.10, 0.94] |
| 1.22 Psychological wellbeing (care partner self‐report) | 2 | 388 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐1.81, 4.04] |
| 1.23 Stress (care partner self‐report) | 3 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.39, 0.34] |
| 1.24 Burden (care partner self‐report) | 3 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.27, 0.06] |
1.15. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 15: Cognition: verbal letter fluency
1.22. Analysis.

Comparison 1: Cognitive rehabilitation versus inactive control at the end of therapy, Outcome 22: Psychological wellbeing (care partner self‐report)
Comparison 2. Cognitive rehabilitation versus inactive control at medium‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Functional ability in targeted activities: personal goals ‐ performance (participant self‐report) | 2 | 432 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [1.25, 1.68] |
| 2.2 Functional ability in targeted activities: personal goals ‐ performance (informant report of participant) | 3 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 1.25 [0.78, 1.72] |
| 2.3 Functional ability in targeted activities: personal goals ‐ satisfaction (participant self‐report) | 2 | 432 | Std. Mean Difference (IV, Random, 95% CI) | 1.19 [0.73, 1.66] |
| 2.4 General functional ability (informant report of participant) | 3 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.43, ‐0.03] |
| 2.5 Self‐efficacy (participant self‐report) | 2 | 417 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.05, 1.21] |
| 2.6 Mood: anxiety (participant self‐report) | 3 | 455 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.56, 0.58] |
| 2.7 Mood: depression (participant self‐report) | 3 | 456 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.49, 0.20] |
| 2.8 Quality of life (participant self‐report) | 5 | 783 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 2.9 Cognition: memory, global score | 2 | 51 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.24, 0.38] |
| 2.10 Cognition: memory, immediate recall | 2 | 427 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.12, 0.38] |
| 2.11 Cognition: memory, delayed recall | 2 | 426 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐1.14, 2.46] |
| 2.12 Cognition: sustained attention | 2 | 413 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.64, 1.49] |
| 2.13 Cognition: auditory selective attention/working memory | 2 | 386 | Mean Difference (IV, Random, 95% CI) | 0.47 [0.09, 0.84] |
| 2.14 Cognition: verbal letter fluency | 2 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.69, 0.63] |
| 2.15 Quality of life: overall (care partner self‐report) | 2 | 422 | Mean Difference (IV, Random, 95% CI) | 10.47 [‐5.97, 26.91] |
| 2.16 Quality of life: physical health (care partner self‐report) | 3 | 439 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.15, 0.56] |
| 2.17 Quality of life: psychological (care partner self‐report) | 3 | 437 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.24, 1.05] |
| 2.18 Quality of life: social (care partner self‐report) | 3 | 436 | Mean Difference (IV, Random, 95% CI) | 0.43 [0.11, 0.76] |
| 2.19 Quality of life: environmental (care partner self‐report) | 3 | 438 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐0.66, 2.50] |
| 2.20 Mood: anxiety (informant self‐report) | 2 | 28 | Mean Difference (IV, Random, 95% CI) | 1.25 [‐3.52, 6.01] |
| 2.21 Mood: depression (informant self‐report) | 2 | 30 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐3.07, 3.69] |
| 2.22 Psychological wellbeing (care partner self‐report) | 2 | 358 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.69, 0.67] |
| 2.23 Stress (care partner self‐report) | 3 | 440 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐2.39, 11.59] |
| 2.24 Burden (care partner self‐report) | 2 | 360 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.10] |
2.22. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 22: Psychological wellbeing (care partner self‐report)
2.24. Analysis.

Comparison 2: Cognitive rehabilitation versus inactive control at medium‐term follow‐up, Outcome 24: Burden (care partner self‐report)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amieva 2016.
| Study characteristics | ||
| Methods | A multicentre randomised, parallel‐group trial, with a two‐year follow‐up comparing group cognitive training, group reminiscence therapy, individual cognitive rehabilitation, and usual care | |
| Participants | 653 people (237 men) with mild‐to‐moderate AD (MMSE 16 to 26 and GDS 2 to 5), ≥ 50 years old, living at home, and with an identified care partner | |
| Interventions | Individualised cognitive rehabilitation (individual sessions): participants received individual sessions (settings not specified) where meaningful activities (instrumental ADL or leisure) were selected for training in line with personal goals and then the training strategy was adjusted to match the person’s cognitive ability with errorless learning principles used where possible; goals/trained activity could be changed during the programme and could include personalised reminiscence activities Cognitive training (group sessions): structured programme of a set of standard tasks relating to and tapping on a particular activity of daily life, with two levels of difficulty to match the level of ability Reminiscence therapy (group sessions): participants were engaged in group discussions on different personal themes Usual care: participants received usual medical care excluding non‐drug therapy, and care partners received support group sessions once a week during the first 3 months and every 6 weeks afterwards All interventions involved 43.5 hours with a therapist over 24 months, with 14 weekly 90‐minute sessions in the first 3 months and 16 6‐weekly 90‐minute sessions thereafter, with parallel care partner support group sessions for the cognitive training and reminiscence therapy groups, and telephone support contact in the cognitive rehabilitation group Only the individualised cognitive rehabilitation and usual care control conditions were compared for the purposes of this review |
|
| Outcomes | Outcomes were collected at 3, 6, 12, 18, and 24 months, but only data at the 3‐ and 24‐month time points were published The primary outcome was the rate of patients alive and without moderately severe to severe dementia (MMSE < 15 or GDS = 5 to 6) at 2 years. Secondary outcomes were: institutionalisation, cognitive ability (ADAScog), behavioural symptoms (Neuropsychiatric Inventory; NPI), functional abilities (Disability Assessment for Dementia; DAD, and Grille d’Autonomie Gérontologique‐Groupes Iso‐Ressources; AGGIR), apathy (Apathy Inventory; AI), depressive symptoms (Montgomery‐Asberg Depression Rating Scale; MADRS), quality of life (Quality of Life ‐ Alzheimer’s Disease scale; QoL‐AD), caregiver’s burden (Zarit Burden Interview), and resource utilisation (RUD Lite) No benefits were observed for the primary outcome in any of the groups. No benefits were observed in the secondary outcomes in cognitive training and reminiscence groups. In the individualised cognitive rehabilitation group, participants had better functional ability scores and a 6‐month delay in institutionalisation at 2 years was observed. |
|
| Notes | Not clear what proportion of participants in the individualised cognitive rehabilitation chose personalised reminiscence activities | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The list of randomization was prepared by a statistician using permuted blocks, stratified by site." |
| Allocation concealment (selection bias) | Low risk | Quote: "The list of randomization was prepared by a statistician using permuted blocks, stratified by site." No reason to assume that allocation concealment was ineffective as a centralised randomisation procedure was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapist were aware of their group allocation. There was a scope for potential bias in the self‐reported subjective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were administered by blinded researchers. There is no reason to assume blinding was not effective, although no measure of blinding efficiency was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors presented data with and without imputation of missing values (missing equals failure analysis vs analysis on available data), with both analyses providing comparable results. |
| Selective reporting (reporting bias) | High risk | Authors described that participants were followed at 3, 6, 12, 18, and 24 months, but reported data for 3 and 24 months time points only. There is no information about what measures were completed at 6, 12, and 18 months, and why these were not reported. |
| Other bias | Unclear risk | There is a potential risk of intervention contamination as it is unclear what proportion of participants in the CR group had instead received individual reminiscence therapy. No other significant sources of bias were identified. |
| Training of those delivering the intervention | Low risk | All therapists received a 3‐day training (no other details given) and were offered telephone support if needed. |
| Intervention manual | Unclear risk | A manual mentioned by authors but not made available to readers. |
Clare 2010.
| Study characteristics | ||
| Methods | Single‐blind randomised controlled trial comparing cognitive rehabilitation with relaxation therapy and with no treatment | |
| Participants | 69 people (28 men, 41 women) with mild‐to‐moderate AD (MMSE score ≥ 18) | |
| Interventions | Cognitive rehabilitation: 8 weekly sessions (1 hour) addressing patient‐derived personal goals with components addressing the use of practical aids and strategies, techniques for learning new information, practice in maintaining attention, and techniques for stress management Relaxation therapy: 8 weekly sessions where the therapist (same as CR group) used a structured treatment protocol to teach participants progressive muscle relaxation and breathing exercises No treatment: participants had no contact with the research team between the initial and post‐intervention assessment |
|
| Outcomes | Outcomes were reported at 8 weeks and 6 months. Cognitive outcomes for the person with dementia: memory (Rivermead Behavioural Memory Test‐II), language (verbal fluency), attention (Map Search, Elevator Counting, Elevator Counting With Distraction, from the Test of Everyday Attention), and perceived memory functioning (Memory Awareness Rating Scale, self‐ and carer‐rated) Non‐cognitive outcomes for the person with dementia: goal performance and satisfaction (Canadian Occupational Performance Measure), functional abilities (Independent Living Scale Health and Safety subset), mood (Hospital Anxiety and Depression Scale), and quality of life (QoL‐AD, self and care partner‐rated) fMRI was reported as a biomarker outcome for a subset of persons with dementia Outcomes of the care partner: quality of life (World Health Organization Quality of Life Scale‐Brief version, WHOQOL‐BREF), general health (General Health Questionnaire‐12), mood (Hospital Anxiety and Depression Scale) and stress (Relatives’ Stress Scale) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted by an indepen‐ dent trials unit using a computer algorithm and was stratified for gender, age (up to 69 years versus 70 years and older), and geographical location (western, central, or eastern district of the catchment area)." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is intrinsic to a remote computerised randomisation system used in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapist were aware of their group allocation. The ratings of performance and satisfaction were subjective, and this could therefore potentially be biased by lack of participant blinding. Not clear whether other research personnel, including the statistician, were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were administered by blinded researchers. There is no reason to assume blinding was not effective, although no measure of blinding efficiency was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants withdrew from the study or died (2 in CR group, 1 in TAU, and 1 in relaxation therapy group), resulting in missing data. Reasons for exclusion were reported. Given the low number of missing data the risk of bias in this category has been rated as low. |
| Selective reporting (reporting bias) | Low risk | Authors indicated that the COPM was not re‐administered at 6‐month follow‐up because there was no evidence regarding its reliability at long‐term follow‐up. The COPM test‐retest reliability at a 1‐week period was cited in support of conducting the 8‐week post‐intervention rating. |
| Other bias | Unclear risk | No other significant sources of bias were identified. |
| Training of those delivering the intervention | Low risk | There is no detail on training, but authors stated that therapy was provided by an experienced Occupational Therapist and that adherence to therapy protocols was monitored through supervision and review of session and home‐practice records. |
| Intervention manual | Unclear risk | There is no detail on training, but authors stated that therapy was provided by an experienced Occupational Therapist and that adherence to therapy protocols was monitored through supervision and review of session and home‐practice records. |
Clare 2019.
| Study characteristics | ||
| Methods | Parallel‐group, multicentre, single‐blind randomised controlled trial comparing cognitive rehabilitation added to usual treatment with usual treatment alone for people with dementia, mild‐to‐moderate cognitive impairment (MMSE score ≥ 18), living at home, and with a care partner willing to contribute | |
| Participants | 475 people (248 men) with an ICD‐10 diagnosis of Alzheimer's disease, vascular or mixed dementia | |
| Interventions | Cognitive rehabilitation: participants involved worked collaboratively on up to 3 rehabilitation goals chosen by the participant, using a problem‐solving approach, supplemented if needed by emotion regulation and behavioural activation strategies to address motivational and emotional difficulties, reviewing and optimising participants' existing use of strategies to manage cognitive difficulties, providing practice in maintaining attention and concentration, signposting to relevant services, and offering support for care partners (10 weekly sessions over 3 months and 4 maintenance sessions over 6 months) Treatment as usual: typically consisted of medication, monitoring, and general psychosocial support |
|
| Outcomes | Outcomes were reported at 3 and 9 months. The primary outcome was self‐rated goal attainment (at 3 months). Secondary outcomes for participants were: participant‐rated goal attainment (at 9 months), care partner‐rated goal attainment and self‐rated satisfaction with their goal attainment, self‐rated self‐efficacy (Generalised Self‐Efficacy Scale), mood (Hospital Anxiety and Depression Scale), dementia‐specific health‐related quality of life (DEMQOL), memory (story recall from the Rivermead Behavioural Memory Test‐II), attention (elevator counting and elevator counting with distraction subtests from the Test of Everyday Attention), and executive function (verbal letter fluency from the Delis‐Kaplan Executive Function System) Secondary outcomes for carers: self‐reported stress (Relatives' Stress Scale), quality of life (WHOQOL‐BREF), and health‐related quality of life (EQ‐5D). |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by remote location via an online webpage. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is intrinsic to a remote computerised randomisation system used in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All outcomes were administered by blinded researchers, but "In the majority of cases, researchers were able to correctly guess the participant’s allocation." "In 48.4% of cases, researchers acknowledged that their guesses were influenced by the presence or absence of change in the participant’s goal performance rating." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial researchers were blind to the participants’ group allocation. Owing to the nature of the intervention, it was not possible to blind participants and carers to group allocation. "Participants explicitly disclosed their group allocation to the researchers in 14.8% of cases". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The missing outcome measures at baseline were imputed using the centre‐level factors and the participant’s sex, age, and baseline MMSE scores. The missing outcome measure scores at the 3‐month and 9‐month assessments were estimated based on centre‐level factors, baseline characteristics, and scores for the same outcome at the earlier time point(s). |
| Selective reporting (reporting bias) | Low risk | There was no evidence for selected measures being reported in the report. |
| Other bias | Low risk | There was no evidence for other significant sources of bias. |
| Training of those delivering the intervention | Low risk | All therapists were trained by an experienced occupational therapist. "The intervention was delivered by trained therapists (nine occupational therapists and one nurse) who received regular individual and group supervision to ensure fidelity to the protocol." |
| Intervention manual | Low risk | "To support the implementation, we will develop materials, resources and training programmes." A manual has been subsequently released. |
Clarkson 2022.
| Study characteristics | ||
| Methods | Multicentre, pragmatic, single‐blind randomised controlled trial comparing the effects of introducing memory aids and guidance by dementia support practitioners against treatment as usual | |
| Participants | 469 people with mild‐to‐moderate dementia and their informal carers | |
| Interventions | DESCANT intervention: a manualised 4‐week intervention aiming to improve the abilities, functioning, and independence. A trained dementia support practitioner offered up to 6 hours of guidance and support in using memory aids. Treatment as usual: usual care from memory clinics that involved post‐diagnostic counselling and advice from the clinical team, with specialist follow‐up if needed |
|
| Outcomes | Outcomes were evaluated at 3 and 6 months after randomisation The primary outcome was the carer‐rated *Bristol Activities of Daily Living Scale (BADLS) at 6 months Secondary outcomes listed in the protocol for the person with dementia were: quality of life (self‐rated Control, Autonomy, Self‐realisation and Pleasure 19‐item, CASP‐19; either self‐rated or care partner Dementia Quality of Life, DEMQOL*; self‐rated Investigating Choice Experiments for the Capability of Older people, ICECAP‐O**), social engagement (self‐rated Lubben Social Network Scale‐Revised, LSNS‐R; self‐rated Practitioner Assessment of Network Type, PANT**), activities of daily living (care partner‐rated Revised Interview for Deterioration in Daily Living Activities in Dementia, R‐IDDD), cognition (Standardised Mini‐Mental State Examination, S‐MMSE; Clinical Dementia Rating scale, CDR) Outcomes completed by care partners listed in the protocol were: mental health status (self‐rated General Health Questionnaire, GHQ‐12), quality of life (self‐rated ICECAP‐O** and CASP‐19), and sense of competence in caring (self‐rated *Short Sense of Competence Questionnaire, SSCQ) The study included also economic measures There were no statistically significant differences detected between the groups at the end of therapy and follow‐up time points. Process evaluation sub‐study indicated appropriate fidelity of the intervention provision. *Included into the meta‐analysis; **Measure not reported in the main outcome paper or process evaluation paper |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Authors used a computerised system that was managed by a Clinical Trial Unit. Quote: "Allocation between groups used dynamic software to randomise participants in real time, thus preventing subversion while ensuring (stochastic) balance between groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "Trial managers coordinated recruitment and forwarded participants’ details to the trials unit’s email‐based randomisation service. After baseline interviews, the unmasked trial data manager oversaw randomisation, which allocated participants in equal proportions between intervention and comparator groups, stratified by Trust or Health Board (1 of 10); time since first attendance at memory clinic (more or less than 90 days); gender (male or female); age (more or less than 75 years); and living with primary carer or not." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists were not blinded. There was a scope for potential bias in the self‐reported subjective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. They were also asked to indicate which group they believed the participants was allocated to, at the end of each assessment. Quote: "Including masking status in covariate adjustment did not alter the treatment effect." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Descriptive data for outcome measures were not published in the main outcome paper and were not provided as supplementary data. Data were provided by the authors on request for most measures. Attrition was at 26% level, similar in both groups, and only slightly higher than anticipated. |
| Selective reporting (reporting bias) | High risk | Several measures that were included in the protocol and referenced in the trial findings paper did not have any data reported: ICECAP‐O, Practitioner Assessment of Network Type, Resource Utilisation in Dementia; Client Service Receipt Inventory data reported in supplementary materials. |
| Other bias | Low risk | There was no evidence for other significant sources of bias. |
| Training of those delivering the intervention | Unclear risk | No details provided regarding training for practitioners in relation to intervention provision. Online training was provided with regard to completing outcome measures. |
| Intervention manual | Low risk | A manual for practitioners has been developed and is available online. |
Hindle 2018.
| Study characteristics | ||
| Methods | Single‐blind randomised controlled trial comparing cognitive rehabilitation with relaxation therapy and with no treatment | |
| Participants | 29 people (23 men) diagnosed with Parkinson's disease, Parkinson's disease dementia, or dementia with Lewy bodies according to consensus criteria | |
| Interventions | Cognitive rehabilitation: 8 x 1‐hour weekly individualised sessions focusing on achieving personal goals using compensatory and enhanced learning techniques to circumvent cognitive impairments. The work was supported by practice in maintaining attention and techniques for stress management. They were encouraged to practise between the sessions where appropriate. Relaxation therapy: participants were taught progressive muscle relaxation and breathing exercises using a set protocol and were encouraged to implement these whenever they experienced anxiety. Participants received the same amount of therapist time and an equivalent level of between‐session practice as the cognitive rehabilitation group. No treatment: participants had no contact with the research team between the initial and post‐intervention assessment. |
|
| Outcomes | Outcomes were evaluated at 2 and 6 months. The primary outcome was goal attainment and satisfaction with goal attainment from the Bangor Goal‐Setting Interview (participant‐rated). Secondary outcomes for the participant were: mood (Hospital Anxiety and Depression Scale), health status (Parkinson's Disease Questionnaire‐8), quality of life (EuroQol Questionnaire‐short version, ED‐5D‐3L) and The WHOQOL‐BREF); Self‐Efficacy (Generalised Self‐Efficacy Scale); language (verbal letter fluency from the Delis‐Kaplan Executive Function System); executive function (Trail Making Test from the Delis‐Kaplan Executive Function System); memory (Story Recall from the Rivermead Behavioural Memory Test‐II); attention (Test of Everyday Attention, version A & C); medication prescription (the client services receipt inventory (CSRI) and Levodopa‐equivalent dose). The carer outcomes were: carer ratings for participants' goal attainment (Bangor Goal‐Setting Interview); mood (Hospital Anxiety and Depression), self‐efficacy (Generalised Self‐Efficacy Scale), quality of life (EuroQol Questionnaire‐short version (ED‐5D‐3L) and the WHOQOL‐BREF), and stress (the Neuropsychiatric Inventory Questionnaire (NPI‐Q) and the Relatives' Stress Scale). |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following the baseline visits, participants were randomised to 1 of the 3 treatment arms: CR, relaxation therapy (RT) or treatment‐as‐usual (TAU)." |
| Allocation concealment (selection bias) | Low risk | No reason to assume that allocation concealment was ineffective as a centralised randomisation procedure was used, but there is no explicit comment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapist were aware of the group allocation. Where the self‐reported outcomes were subjective, there was scope for potential bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher who collected follow‐up data (T.J.W.) was blinded to all randomisation outcomes for the duration of the data collection period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Given the small quantity of incomplete data and the relatively even spread across the study group, the results are not likely to have been biased by incomplete data. |
| Selective reporting (reporting bias) | Low risk | Outcome measures recorded in protocol/trial registration form and reported accordingly. No evidence suggested selective reporting of data. |
| Other bias | Low risk | No other significant sources of bias were identified. |
| Training of those delivering the intervention | Unclear risk | The therapist was described as "a qualified occupational therapist experienced in providing neurorehabilitation interventions", but there was no formal training mentioned. |
| Intervention manual | Unclear risk | Treatment protocol was available for the relaxation therapy, but no protocol was mentioned for cognitive rehabilitation. Authors shared examples of goals and techniques used in CR. |
Thivierge 2014.
| Study characteristics | ||
| Methods | Single‐blinded, block‐randomised, cross‐over, controlled study | |
| Participants | 20 participants with mild‐to‐moderate Alzheimer’s dementia (3 withdrew and no data available) | |
| Interventions | Cognitive training: a memory rehabilitation programme to re‐learn instrumental activities of daily living (IADL) chosen by each participant (twice‐weekly sessions over 4 weeks, each lasting 45 to 60 minutes, with evaluation) Controls waiting‐list: no intervention |
|
| Outcomes | Cross‐over The primary outcome was the performance on the trained IADL as assessed using an observational instrument (Direct Measure of Training). Secondary outcomes: global cognition (Dementia Rating Scale‐2) everyday memory functioning (Rivermead Behavioural Memory Test), quality of life (the self‐esteem and positive and negative affects subscales of the Dementia Quality of Life). Outcomes completed by care partners were: behavioural, mood, and psychotic symptoms (Neuropsychiatric Inventory), functional abilities (Disability Assessment for Dementia), and carer burden (Zarit Burden Interview). Direct Measure of Training measure indicated improvements in trained IADL in the active intervention group immediately following the intervention and at the 13‐week follow‐up. No benefits were observed in the secondary outcomes. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block‐randomised, cross‐over, controlled study |
| Allocation concealment (selection bias) | Low risk | Quote: "Following screening and baseline evaluations, five blocks of four participants were formed; each patient of each block was randomized to Group 1 or Group 2." It appears that participants were randomised in the blocks of 4 after all 4 completed baseline assessment, ensuring concealment, but there is no explicit statement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapist were aware of the group allocation due to nature of the intervention, although there were steps taken to reduce potential bias (i.e. test manuals and procedures for evaluations, with periodic monitoring). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blind to allocation outcomes, but effectiveness of blindness not evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no 'intention‐to‐treat' analyses undertaken despite 2 participants withdrawing after the baseline evaluation. Authors explained that this was due to insufficient data being available for those individuals. |
| Selective reporting (reporting bias) | Low risk | No evidence suggested selective reporting of data. |
| Other bias | Low risk | No other sources of bias were noted. |
| Training of those delivering the intervention | Low risk | Authors mentioned that therapists/research assistants received training and were monitored periodically, although there are no details given. |
| Intervention manual | Unclear risk | A manual has been produced, but it is not available to readers. |
AD: Alzheimer's disease ADL: activities of daily living ADAScog: Alzheimer's Disease Assessment Scale‐Cognitive COPM: Canadian Occupational Performance Measure CR: cognitive rehabilitation GDS: Global Deterioration Scale MMSE: Mini‐Mental State Examination fMRI: functional magnetic resonance imaging IADL: instrumental activities of daily living ICECAP‐O: ICEpop CAPability measure for Older people TAU: treatment as usual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amrani 2019 | No appropriate comparator |
| Andrade 2018 | Does not meet intervention criteria |
| Avila 2007 | No appropriate comparator |
| Baglio 2015 | Does not meet intervention criteria |
| Bourgeois 2016 | Does not meet intervention criteria |
| Brueggen 2017 | CR part of a multicomponent intervention (CR < 80%) |
| Buschert 2011 | Does not meet intervention criteria |
| Callahan 2017 | CR part of a multicomponent intervention (CR < 80%) |
| Chen 2020 | Does not meet intervention criteria |
| Ciro 2014 | Does not meet intervention criteria |
| Davis 2001 | Does not meet intervention criteria |
| De Vreese 1998 | Does not meet intervention criteria |
| Fortinsky 2016 | No dementia diagnosis according to established criteria |
| Galik 2014 | No dementia diagnosis according to established criteria |
| Gitlin 2001 | No dementia diagnosis according to established criteria |
| Gitlin 2010 | Inappropriate level of dementia severity |
| Gitlin 2021 | Does not meet participant criteria |
| Graff 2006 | CR part of a multicomponent intervention (CR < 80%) |
| Howard 2021 | Does not meet intervention criteria |
| Jeon 2019 | CR part of a multicomponent intervention (CR < 80%) |
| Jiménez Palomares 2021 | Does not meet intervention criteria |
| Juárez‐Cedillo 2020 | Does not meet intervention criteria |
| Kelly 2019 | Not RCT |
| Kim 2015 | CR part of a multicomponent intervention (CR < 80%) |
| Koivisto 2013 | No dementia diagnosis according to established criteria |
| Kumar 2013 | CR part of a multicomponent intervention (CR < 80%) |
| Kurz 2012 | CR part of a multicomponent intervention (CR < 80%) |
| Lam 2010 | No appropriate comparator |
| Liesk 2012 | Does not meet intervention criteria |
| Livelli 2015 | No dementia diagnosis according to established criteria |
| Moniz‐Cook 1998 | Does not meet intervention criteria |
| Mountain 2022 | CR part of a multicomponent intervention (CR < 80%) |
| O'Connor 2019 | Inappropriate level of dementia severity |
| Oliveira 2021 | Does not meet intervention criteria |
| Orgeta 2019 | Does not meet intervention criteria |
| Pedroso 2018 | Does not meet intervention criteria |
| Poon 2005 | No dementia diagnosis according to established criteria |
| Regan 2017 | No dementia diagnosis according to established criteria |
| Reuster 2010 | Other ‐ insufficient amount of data in the available unpublished sources and no intention to publish the results in full |
| Santos 2011 | CR not distinguished in a multicomponent intervention |
| Schecker 2013 | Does not meet intervention criteria |
| SeungHyun 2017 | Does not meet intervention criteria |
| Straubmeier 2017 | Does not meet intervention criteria |
| Tappen 1994 | No dementia diagnosis according to established criteria |
| Vanova 2018 | Does not meet intervention criteria |
| Voigt‐Radloff 2017 | No appropriate comparator |
| Wenborn 2021 | Does not meet intervention criteria |
CR: cognitive rehabilitation RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Zhang 2004.
| Methods | RCT |
| Participants | People with Alzheimer's disease |
| Interventions | Cognitive rehabilitation training that included ADL, intelligence, and physical training; usual treatment |
| Outcomes | Cognition (MMSE), ADL and health status questionnaires |
| Notes | Awaits translation |
ADL: activities of daily living MMSE: Mini Mental State Examination RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ciro 2016.
| Study name | High‐dose mass practice intervention to reduce ADL disability in dementia |
| Methods | RCT |
| Participants | Inclusion criteria: an adult aged 50 to 90 years old diagnosed with dementia (Mini‐Mental Status Examination score > 10 but ≤ 25), who lives in the community and speaks English, with someone who can provide consent to be in the study; the person needs to be able to understand and follow one‐step commands, can move one arm sufficiently for practising tasks and is able to participate in 3 hours of daily therapy in their home environment for 2 consecutive weeks; a participant or family member need to identify three goal areas related to self‐care or home management Exclusion criteria: Creutzfeldt‐Jakob dementia, delirium or a progressive neurological condition such as Parkinson's disease; receptive or global aphasia; uncorrected vision/hearing |
| Interventions | Skill‐building through Task‐Oriented Motor Practice (STOMP) |
| Outcomes | Primary outcome measures: 5‐point observation of activities of daily living Secondary outcome measures: frequency of behavioural responses during the trial; 10‐point caregiver perception of activities of daily living performance and satisfaction with performance; change in 10‐point caregiver perception of activities of daily living performance and satisfaction with performance; retention of 10‐point caregiver perception of activities of daily living performance and satisfaction with performance; change in observation of activities of daily living; retention of observed activities of daily living at 90 days |
| Starting date | October 2014 |
| Contact information | https://www.clinicaltrials.gov/ct2/show/study/NCT02356055 |
| Notes | Unlikely to meet inclusion criteria as no appropriate comparator group |
ISRCTN59155421.
| Study name | A person‐centred Multidimensional InterDisciplinary REhabilitation program for community dwelling older people with Dementia and their informal primary caregivers: a randomised controlled trial (MIDRED) |
| Methods | RCT |
| Participants | Community‐dwelling adults with dementia > 60 years old and their informal care partners if available |
| Interventions | A 16‐week multidisciplinary rehabilitation programme that includes physical exercise twice a week and other individually tailored goal‐oriented interventions based on the identified problems and the rehabilitation goals for the person with dementia, and education and support to primary caregivers |
| Outcomes | Persons with dementia: depressive symptoms, psychological well‐being, participation in society, physical activity, cognitive function, functional capacity, ADL performance, behavioural difficulties, nutritional status, oral health, inappropriate drug use, feasibility and cost‐effectiveness Care partners: burden, depressive symptoms, health‐related quality of life |
| Starting date | January 2014 |
| Contact information | https://www.isrctn.com/ISRCTN59155421 |
| Notes | — |
NCT02937883.
| Study name | NCT02937883 |
| Methods | RCT |
| Participants | Inclusion criteria: diagnosed with dementia before the age of 65, community‐dwelling Exclusion criteria: dementia is caused by Down's syndrome, Huntington's disease, HIV, or alcohol‐related dementia; limited contact between the person with dementia and the informal caregiver (< 3 times a week) |
| Interventions | Empowerment intervention for persons with young‐onset dementia Usual treatment |
| Outcomes | Primary outcomes: self‐management abilities Secondary outcomes: quality of life, neuropsychiatric symptoms, disability, apathy Caregiver measures: competence, emotional distress |
| Starting date | — |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02937883 |
| Notes | — |
NCT03430401.
| Study name | NCT03430401 |
| Methods | RCT |
| Participants | Selection criteria for people with MCI: no diagnosis of probable dementia, meets the diagnostic criteria for MCI, a Clinical Dementia Rating Score (CDR) of 0 indicating no dementia, able to provide voluntarily consent to participate in the study People with mild dementia: have a diagnosis of probable dementia; have a CDR score of 1 indicating mild dementia; have a carer or family members who are able to report functional performance; able to provide consent to participate in the study, or have a guardian to provide consent |
| Interventions | Perceptual‐based memory encoding, semantic‐based memory encoding, cognitive stimulation (active comparator) |
| Outcomes | Primary outcome measures: Disability Assessment for Dementia, Lawton and Brody Instrumental Activities of Daily Living Scale Secondary outcome measures: Colour Trails Test, Repeatable Battery for the Assessment of Neuropsychological Status, Behaviour Rating Inventory of Executive Function |
| Starting date | — |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03430401 |
| Notes | — |
ADL: activities of daily living MCI: mild cognitive impairment RCT: randomised controlled trial
Differences between protocol and review
The initial screening stage was generously supported by Screen4Me and the Cochrane Dementia and Cognitive Improvement Group Information Specialists, which was not stipulated by the protocol.
The protocol stipulated that cognitive rehabilitation (CR) could be compared to two types of control conditions: inactive and non‐specific active control. However, we did not identify any studies comparing CR only to a non‐specific active control. The two included studies that employed a non‐specific active control condition also had an inactive control group (usual treatment) (Clare 2010; Hindle 2018), as did the other four included studies. To prioritise the homogeneity of the control group and avoid splitting the CR group for the analysis, we decided to use only the inactive control data from Clare 2010 and Hindle 2018.
In the protocol, we envisioned grouping outcomes separately into three to six months and seven to 12 months categories. However, there were few studies included in the review, so we pooled these two categories into one category of assessments that were completed between three and 12 months following randomisation.
We made two changes to the summary of findings tables:
The primary outcome of goal attainment in relation to activities targeted in the intervention was evaluated from three perspectives (self‐rating of performance, informant rating of performance, and self‐rating of satisfaction with goal attainment). We included in the summary of findings tables two of them that seemed equally important (self‐rating of performance and informant rating of performance). To adhere to the limit of seven outcomes in the summary of findings table, we had to exclude an outcome. We excluded cognition as we were unable to undertake a comparison for the global measure of cognition stipulated in the protocol.
Data on quality of life (overall rating) were available only for the follow‐up time point and a small sample translated into very low‐certainty findings. Therefore, we decided instead to present the psychological aspect of quality of life comparisons that appeared to be particularly relevant in the context of this review.
Contributions of authors
Aleksandra Kudlicka screened and selected studies for inclusion, conducted the data extraction and data entry into RevMan, completed the relevant tables, rated studies for risk of bias, conducted analyses, graded the evidence, and wrote the manuscript.
Anthony Martyr screened and selected studies for inclusion, rated studies for risk of bias, conducted the data check, and contributed to writing the manuscript.
Alex Bahar‐Fuchs screened and selected studies for inclusion, rated studies for risk of bias, and contributed to writing the manuscript.
Julieta Sabates screened and selected studies for inclusion, rated studies for risk of bias, conducted the data check, graded the evidence, and contributed to writing the manuscript.
Bob Woods assisted in resolving issues related to study selection and risk of bias, and contributed to writing the manuscript.
Linda Clare drafted the protocol, assisted in resolving issues related to study selection and risk of bias, and contributed to writing the manuscript.
Review authors did not assess eligibility, extract data, or rate evidence quality for any studies for which they are co‐authors; these studies were referred to other team members for consideration.
Sources of support
Internal sources
-
No internal source of support, Other
None
External sources
-
NIHR, UK
This review was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health
-
NIHR , UK
Linda Clare acknowledges support from the NIHR Applied Research Collaboration South‐West Peninsula. The views expressed in this publication are those of the authors and not necessarily those of NIHR, the Department of Health and Social Care or the National Health Service.
-
NHMRC , Australia
Alex Bahar‐Fuchs acknowledges support from the National Health and Medical Research Council of Australia (NHMRC GNT: 1135605).
Declarations of interest
Review authors did not assess eligibility, extract data, or rate evidence quality for any studies for which they are co‐authors; these studies were referred to other team members for consideration.
Aleksandra Kudlicka: author of an eligible study.
Anthony Martyr: author of an eligible study.
Alex Bahar‐Fuchs: none known.
Bob Woods: author of an eligible study.
Linda Clare: author an eligible study.
Julieta M. Sabatés: none known.
New
References
References to studies included in this review
Amieva 2016 {published data only}
- Amieva H, Dartigues JF. ETNA3, a clinical randomized study assessing three cognitive-oriented therapies in dementia: Rationale and general design. Revue Neurologique 2013;169(10):752-6. [DOI: 10.1016/j.neurol.2013.07.011] [DOI] [PubMed] [Google Scholar]
- Amieva H, Robert PH, Grandoulier A-S, Meillon C, De Rotrou J, Andrieu S, et al. Group and individual cognitive therapies in Alzheimer's disease: the ETNA3 randomized trial. International Psychogeriatrics 2016;28(5):707-17. [DOI: 10.1017/S1041610215001830] [DOI] [PubMed] [Google Scholar]
Clare 2010 {published data only}
- Clare L, Evans S, Parkinson C, Woods R, Linden D. Goal-setting in cognitive rehabilitation for people with early-stage Alzheimer's disease. Clinical Gerontologist 2011;34(3):220-36. [DOI: 10.1080/07317115.2011.555937] [DOI] [Google Scholar]
- Clare L, Linden DEJ, Woods R, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. American Journal of Geriatric Psychiatry 2010;18(10):928-39. [DOI: 10.1097/JGP.0b013e3181d5792a] [DOI] [PubMed] [Google Scholar]
- Van Paasschen J, Clare L, Yuen KS, Woods RT, Evans SJ, Parkinson CH, et al. Cognitive rehabilitation changes memory-related brain activity in people with Alzheimer disease. Neurorehabilitation and Neural Repair 2013;27(5):448-59. [DOI: 10.1177/1545968312471902] [DOI] [PubMed] [Google Scholar]
Clare 2019 {published data only}
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Goal-oriented cognitive rehabilitation in early-stage Alzheimer’s and related dementias: a multi-centre single-blind randomized controlled trial (GREAT). Health Technology Assessment 2019;23(10):1-242. [DOI: 10.3310/hta23100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: a multicentre randomised controlled trial (the GREAT trial). International Journal of Geriatric Psychiatry 2019;34(5):709-21. [DOI: 10.1002/gps.5076] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Trimmer S, Kudlicka A, Warmoth K, Leroi I, Oyebode JR, Pool J, et al. Implementation processes in a cognitive rehabilitation intervention for people with dementia: a complexity-informed qualitative analysis. BMJ Open 2021;11(10):e051255. [DOI: 10.1136/bmjopen-2021-051255] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmoth K, Morgan-Trimmer S, Kudlicka A, Toms G, James IA, Woods B. GREAT trial team. Reflections on a personalized cognitive rehabilitation intervention: experiences of people living with dementia and their carers participating in the great trial. Neuropsychological Rehabilitation 2022;32(2):268-86. [DOI: 10.1080/09602011.2020.1820876] [DOI] [PubMed] [Google Scholar]
Clarkson 2022 {published and unpublished data}
- Chester H, Beresford R, Clarkson P, Entwistle C, Gillan V, Hughes J, et al. Implementing the Dementia Early Stage Cognitive Aids New Trial (DESCANT) intervention: mixed-method process evaluation alongside a pragmatic randomised trial. Aging & Mental Health 2022;26(4):667-78. [DOI] [PubMed] [Google Scholar]
- Chester H, Clarkson P, Davies L, Hughes J, Islam MS, Kapur N, et al. Cognitive aids for people with early stage dementia versus treatment as usual (Dementia Early Stage Cognitive Aids New Trial (DESCANT)): study protocol for a randomised controlled trial. Trials 2018;19(546 ):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson P, Challis D, Hughes J, Roe B, Davies L, Russell I, et al. Components, impacts and costs of dementia home support: a research programme including the DESCANT RCT. Programme Grants for Applied Research 2021;9(6):1-32. [DOI: 10.3310/pgfar09060] [DOI] [PubMed] [Google Scholar]
- Clarkson P, Pitts R, Islam S, Peconi J, Russell I, Fegan G, et al. Dementia Early-Stage Cognitive Aids New Trial (DESCANT) of memory aids and guidance for people with dementia: randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2022;93(9):1001-9. [DOI: 10.1136/jnnp-2021-326748] [DOI] [PubMed] [Google Scholar]
Hindle 2018 {published data only}
- Hindle JV, Watermeyer TJ, Roberts J, Brand A, Hoare Z, Martyr A, et al. Goal-orientated cognitive rehabilitation for dementias associated with Parkinson's disease-a pilot randomised controlled trial. International Journal of Geriatric Psychiatry 2018;33(5):718-8. [DOI: 10.1002/gps.4845] [DOI] [PubMed] [Google Scholar]
- Watermeyer TJ, Hindle JV, Roberts J, Lawrence CL, Martyr A, Lloyd-Williams H, et al. Goal setting for cognitive rehabilitation in mild to moderate Parkinson’s disease dementia and dementia with Lewy bodies. Parkinson’s Disease 2016;Article ID 8285041:1-8. [DOI: 10.1155/2016/8285041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thivierge 2014 {published data only}
- Thivierge S, Jean L, Simard M. A randomized cross-over controlled study on cognitive rehabilitation of instrumental activities of daily living in Alzheimer disease. American Journal of Geriatric Psychiatry 2014;22(11):1188-99. [DOI: 10.1016/j.jagp.2013.03.008] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amrani 2019 {published data only}
- El Amrani L, Benard C, Plourde M, Giguere-Rancourt A, Racine E, Simard M. Cognitive rehabilitation of instrumental activities of daily living in Alzheimer's disease. Alzheimer's & Dementia 2019;15(7):P1587. [DOI: 10.1016/j.jalz.2019.09.033] [DOI] [Google Scholar]
- El-Amrani L. Cognitive rehabilitation in Alzheimer's disease: effects on instrumental activities of daily living, quality of life, self-esteem and self-satisfaction [Réadaptation cognitive dans la maladie d’Alzheimer: effets sur les activités instrumentales de la vie quotidienne, la qualité de vie, l’estime de soi et la satisfaction de soi [thèse de doctorat]]. Université Laval 2020. [DOI: 10.1007/s11065-008-9052-3] [DOI]
Andrade 2018 {published data only}
- Andrade SM, Oliveira EA, Alves NT, Dos Santos ACG, Mendonca C, Sampaio DDA, et al. Neurostimulation combined with cognitive intervention in Alzheimer's disease (NeuroAD): study protocol of double-blind, randomized, factorial clinical trial. Frontiers in Aging Neuroscience 2018;10:1-14. [DOI: 10.3389/fnagi.2018.00334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Avila 2007 {published data only}
- Avila R, Carvalho IAM, Bottino CMC, Miotto EC. Neuropsychological rehabilitation in mild and moderate Alzheimer's disease patients. Behavioural Neurology 5469;18(4):225-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baglio 2015 {published data only}
- Baglio F, Griffanti L, Saibene FL, Ricci C, Alberoni M, Critelli R, et al. Multistimulation group therapy in Alzheimer's disease promotes changes in brain functioning. Neurorehabilitation and Neural Repair 2015;29(1):13-24. [DOI: 10.1177/1545968314532833] [DOI] [PubMed] [Google Scholar]
Bourgeois 2016 {published data only}
- Bourgeois J, Laye M, Lemaire J, Leone E, Deudon A, Darmon N, et al. Relearning of activities of daily living: a comparison of the effectiveness of three learning methods in patients with dementia of the Alzheimer type. Journal of Nutrition, Health and Aging 2016;20(1):48-55. [DOI: 10.1007/s12603-016-0675-4] [DOI] [PubMed] [Google Scholar]
Brueggen 2017 {published data only}
- Brueggen K, Kasper E, Ochmann S, Pfaff H, Webel S, Schneider W, et al. Cognitive rehabilitation in Alzheimer's disease: a controlled intervention trial. Journal of Alzheimer's Disease 2017;57(4):1315-24. [DOI: 10.3233/JAD-160771] [DOI] [PubMed] [Google Scholar]
Buschert 2011 {published data only}
- Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: a pilot study. Journal of Alzheimer's disease 2011;25(4):679-94. [DOI: 10.3233/JAD-2011-100999] [DOI] [PubMed] [Google Scholar]
Callahan 2017 {published data only}
- Callahan CM, Boustani MA, Schmid AA, LaMantia MA, Austrom MG, Miller DK, et al. Targeting functional decline in Alzheimer disease a randomized trial. Annals of Internal Medicine 2017;166(3):164-71. [DOI: 10.7326/M16-0830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020 {published data only}
- Chen M, Bai Y, Zhou X, Chen W, He D, Li Y . Effect of extended nursing on the behavioral and psychological symptoms and cognitive dysfunction of patients with moderate and severe Alzheimer's disease. Indian Journal of Pharmaceutical Sciences 2020;82(3):5-8. [DOI: 10.36468/pharmaceutical-sciences.spl.137] [DOI] [Google Scholar]
Ciro 2014 {published data only}
- Ciro CA, Poole JL, Skipper B, Hershey LA. Comparing differences in ADL outcomes for the STOMP Intervention for dementia in the natural home environment versus a clinic environment. Austin Alzheimer's and Parkinson's Disease 2014;1(1):1-7. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Davis 2001 {published data only}
- Davis RN, Massman PJ, Doody RS . Cognitive intervention in Alzheimer disease: a randomized placebo-controlled study. Alzheimer Disease and Associated Disorders 2001;15(1):1-9. [DOI: 10.1097/00002093-200101000-00001] [DOI] [PubMed] [Google Scholar]
De Vreese 1998 {published data only}
- De Vreese LP, Verlato C, Emiliani S, Schioppa S, Belloi L, Salvioli L, et al. Effect size of a three month drug treatment in AD when combined with individual cognitive retraining: preliminary results of a pilot study. Neurobiology of Aging 1998;19(Suppl 4):S123. [Google Scholar]
Fortinsky 2016 {published data only}
- Fortinsky RH, Gitlin LN, Pizzi LT, Piersol CV, Grady J, Robison JT, et al. Effectiveness of the care of persons with dementia in their environments intervention when embedded in a publicly funded home- and community-based service program. Innovation in Aging 2020;4(6):1–13. [DOI: 10.1093/geroni/igaa053] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortinsky RH, Gitlin LN, Pizzi LT, Piersol CV, Grady J, Robison JT, et al. Translation of the Care of Persons with Dementia in their Environments (COPE) intervention in a publicly-funded home care context: rationale and research design. Contemporary Clinical Trials 2016;49:155-65. [DOI: 10.1016/j.cct.2016.07.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galik 2014 {published data only}
- Galik E, Resnick B, Hammersla M, Brightwater J. Optimizing function and physical activity among nursing home residents with dementia: testing the impact of function-focused care. The Gerontologist 2014;54(6):930-43. [DOI: 10.1093/geront/gnt108] [DOI] [PubMed] [Google Scholar]
Gitlin 2001 {published data only}
- Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck WW. A randomized, controlled trial of a home environmental intervention: effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist 2001;41(1):4-14. [DOI: 10.1093/geront/41.1.4] [DOI] [PubMed] [Google Scholar]
Gitlin 2010 {published data only}
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. Jama 2010;304(9):983-91. [DOI: 10.1001/jama.2010.1253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2021 {published data only}
- Gitlin LN, Marx K, Piersol CV, Hodgson NA, Huang J, Roth DL, et al. Effects of the tailored activity program (TAP) on dementia-related symptoms, health events and caregiver wellbeing: a randomized controlled trial. BMC Geriatrics 2021 ;21(1):1-4. [DOI: 10.1186/s12877-021-02511-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Graff 2006 {published data only}
- Graff MJL, Vernooij-Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, Rikkert MGM. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006;333(7580):1196-9. [DOI: 10.1136/bmj.39001.688843.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2021 {published data only}
- Howard R, Gathercole R, Bradley R, Harper E, Davis L, Pank L, et al. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age and Ageing 2021;50(3):882-90. [DOI: 10.1093/ageing/afaa284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeon 2019 {published data only}
- Jeon Y-H, Krein L, Simpson JM, Szanton SL, Clemson L, Naismith SL, et al. Feasibility and potential effects of interdisciplinary home-based reablement program (i-harp) for people with cognitive and functional decline: a pilot trial. Aging & Mental Health 2019;24(11):1916-25. [DOI: 10.1080/13607863.2019.1642298] [DOI] [PubMed] [Google Scholar]
Jiménez Palomares 2021 {published data only}
- Jiménez Palomares M, González López-Arza MV, Garrido Ardila EM, Rodríguez Domínguez T, Rodríguez Mansilla J. Effects of a cognitive rehabilitation programme on the independence performing activities of daily living of persons with dementia - a pilot randomized controlled trial. Brain Sciences 2021;11(319):1-12. [DOI: 10.3390/brainsci11030319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juárez‐Cedillo 2020 {published data only}
- Juárez-Cedillo T, Gutiérrez-Gutiérrez L, Sánchez-Hurtado LA, Martínez-Rodríguez N, Juarez-Cedillo E . Randomized controlled trial of multi-component cognitive stimulation therapy (SADEM) in community-dwelling demented adults. Journal of Alzheimer's Disease 2020;78(3):1033-45. [DOI: 10.3233/JAD-200574] [DOI] [PubMed] [Google Scholar]
Kelly 2019 {published data only}
- Kelly ME, Lawlor BA, Coen RF, Robertson IH, Brennan S. Cognitive rehabilitation for early stage Alzheimer’s disease: a pilot study with an Irish population. Irish Journal of Psychological Medicine 2019;36(2):105-19. [DOI: 10.1017/ipm.2017.23] [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim, S. Cognitive rehabilitation for elderly people with early-stage Alzheimer's disease. Journal of Physical Therapy Science 2015;27(2):543-6. [DOI: 10.1589/jpts.27.543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koivisto 2013 {published data only}
- Martikainen JA, Kautiainen H, Väätäinen S, Välimäki T, Hongisto K, Hallikainen I, et al. Effectiveness of the early psychosocial intervention on institutionalization of patients with mild Alzheimer’s disease and caregivers’ quality of life – an ALSOVA study. International Journal of Geriatric Psychiatry 2013;16(7):PA618-9. [DOI: 10.1016/j.jval.2013.08.1803] [DOI] [Google Scholar]
Kumar 2013 {published data only}
- Kumar P, Tiwari SC, Sreenivas V, Kumar N, Tripathi RK, ABD. Profile of older adults in memory outpatients' clinic setting and effectiveness of novel occupational therapy intervention in patients with mild to moderate dementia. Indian Journal of Physiotherapy and Occupational Therapy 2013;7(3):297-302. [DOI: 10.5958/j.0973-5674.7.3.111] [DOI] [Google Scholar]
Kurz 2012 {published data only}
- Kurz A, Thöne-Otto A, Cramer B, Egert S, Frölich L, Gertz HJ, et al. CORDIAL: cognitive rehabilitation and cognitive-behavioral treatment for early dementia in Alzheimer disease: a multicenter, randomized, controlled trial. Alzheimer Disease & Associated Disorders 2012;26(3):246-53. [DOI: 10.1097/WAD.0b013e318231e46e] [DOI] [PubMed] [Google Scholar]
Lam 2010 {published data only}
- Lam LC, Lui VW, Luk DN, Chau R, So C, Poon V, et al. Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia--a randomized control trial. Journal of the Psychiatry of Late Life and Allied Sciences 2010;25(2):133-41. [DOI: 10.1002/gps.2309] [DOI] [PubMed] [Google Scholar]
Liesk 2012 {published data only}
- Liesk J, Petrelli A, Kaesberg S, Baller G, Kessler J, Kalbe E. Neuropsychological therapy and music geragogy for patients with mild-to-moderate dementia. Alzheimer's & Dementia 2012;8(4 Suppl 1):P393-4. [DOI: 10.1016/j.jalz.2012.05.1082] [DOI] [Google Scholar]
Livelli 2015 {published data only}
- Livelli A, Orofino GC, Calcagno A, Farenga M, Penoncelli D, Guastavigna M, et al. Evaluation of a cognitive rehabilitation protocol in HIV patients with associated neurocognitive disorders: efficacy and stability over time. Frontiers in Behavioral Neuroscience 2015;16(9):1-10. [DOI: 10.3389/fnbeh.2015.00306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moniz‐Cook 1998 {published data only}
- Moniz-Cook E, Agar S, Gibson G, Win T, Wang M. A preliminary study of the effects of early intervention with people with dementia and their families in a memory clinic. Aging & Mental Health 1998;2(3):199-211. [DOI: 10.1080/13607869856687] [DOI] [Google Scholar]
Mountain 2022 {published data only}
- Mountain GA, Cooper CL, Wright J, Walters SJ, Lee E, Craig C, et al. The journeying through dementia psychosocial intervention versus usual care study: a single-blind, parallel group, phase 3 trial. Lancet Healthy Longevity 2022;3(4):e276-85. [DOI: 10.1016/S2666-7568(22)00059-9] [DOI] [PubMed] [Google Scholar]
- Wright J, Foster A, Cooper C, Sprange K, Walters S, Berry K, et al. Study protocol for a randomised controlled trial assessing the clinical and cost-effectiveness of the Journeying through Dementia (JtD) intervention compared to usual care. BMJ Open 2019;9:e029207:1-11. [DOI: 10.1136/bmjopen-2019-029207] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2019 {published data only}
- O'Connor CM, Clemson L, Brodaty H, Low LF, Jeon YH, Gitlin LN, et al. The tailored activity program (TAP) to address behavioral disturbances in frontotemporal dementia: a feasibility and pilot study. Disability and Rehabilitation 2019;41(3):299-310. [DOI: 10.1080/09638288.2017.1387614] [DOI] [PubMed] [Google Scholar]
Oliveira 2021 {published data only}
- Oliveira J, Gamito P, Souto T, Conde R, Ferreira M, Corotnean T, et al. Virtual reality-based cognitive stimulation on people with mild to moderate dementia due to Alzheimer’s disease: a pilot randomized controlled trial. International Journal of Environmental Research and Public Health 2021;18(10):5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orgeta 2019 {published data only}
- Orgeta V, Tuijt R, Leung P, Verdaguer ES, Gould RL, Jones R, et al. Behavioral activation for promoting well-being in mild dementia: feasibility and outcomes of a pilot randomized controlled trial. Journal of Alzheimer's Disease 2019;72(2):563-74. [DOI] [PubMed] [Google Scholar]
Pedroso 2018 {published data only}
- Pedroso RV, Ayan C, Fraga FJ, da Silva TMV, Cancela JM, Santos-Galduroz RF. Effects of functional-task training on older adults with Alzheimer's disease. Journal of Aging and Physical Activity 2018;26(1):97-105. [DOI: 10.1123/japa.2016-0147] [DOI] [PubMed] [Google Scholar]
Poon 2005 {published data only}
- Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community-dwelling older persons with memory problems: telemedicine versus face-to-face treatment. International Journal of Geriatric Psychiatry 2005;20(3):285-6. [DOI: 10.1002/gps.1282] [DOI] [PubMed] [Google Scholar]
Regan 2017 {published data only}
- Regan B, Wells Y, Farrow M, O'Halloran P, Workman B. MAXCOG-maximizing cognition: a randomized controlled trial of the efficacy of goal-oriented cognitive rehabilitation for people with mild cognitive impairment and early Alzheimer disease. American Journal of Geriatric Psychiatry 2017;25(3):258-69. [DOI: 10.1016/j.jagp.2016.11.008] [DOI] [PubMed] [Google Scholar]
Reuster 2010 {published data only}
- Reuster T, Jurjanz L, Schutzwohl M, Holthoff V. A randomized controlled trial on occupational therapy for patients with dementia and their caregivers (ERGODEM) [German]. Zeitschrift für Gerontopsychologie & Psychiatrie 2010;21(3):185-9. [Google Scholar]
Santos 2011 {published data only}
- Santos G, Ortega L, Yassuda M, Forlenza O, Nunes P. The effects of a multiprofessional cognitive and functional rehabilitation program for patients with Alzheimer's disease and mild cognitive impairment. Alzheimer's & Dementia 2011:S800.
Schecker 2013 {published data only}
- Schecker M, Pirnay-Dummer P, Schmidtke K, Hentrich-Hesse T, Borchardt D. Cognitive interventions in mild Alzheimer's disease: a therapy-evaluation study on the interaction of medication and cognitive treatment. Dementia and Geriatric Cognitive Disorders Extra 2013;3(1):301-11. [DOI: 10.1159/000354190] [DOI] [PMC free article] [PubMed] [Google Scholar]
SeungHyun 2017 {published data only}
- Cho SH, Yang YA. The effect of occupational therapy based multimodal cognitive rehabilitation therapy on cognitive function in elderly people with mild dementia: a randomized controlled trial. The Journal of Korean Society of Occupational Therapy 2017;25(3):71-86. [Google Scholar]
Straubmeier 2017 {published data only}
- Straubmeier M, Behrndt EM, Seidl H, Özbe D, Luttenberger K, Graessel E. Non-pharmacological treatment in people with cognitive impairment - results from the randomized controlled German day care study. Deutsches Ärzteblatt International 2017;114(48):815-21. [DOI: 10.3238/arztebl.2017.0815] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappen 1994 {published data only}
- Tappen RM. The effect of skill training on functional abilities of nursing home residents with dementia. Research in Nursing and Health 1994;17(3):159-65. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanova 2018 {published data only}
- Vanova M, Irazoki E, García-Casal JA, Martínez-Abad F, Botella C, Shiells KR, et al. The effectiveness of ICT-based neurocognitive and psychosocial rehabilitation programmes in people with mild dementia and mild cognitive impairment using GRADIOR and ehcoBUTLER: study protocol for a randomised controlled trial. Trials 2018;19(100):1-15. [DOI: 10.1186/s13063-017-2371-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voigt‐Radloff 2017 {published data only}
- Voigt-Radloff S, Werd MM, Leonhart R, Boelen DH, Olde Rikkert MG, Fliessbach K, et al. Structured relearning of activities of daily living in dementia: the randomized controlled REDALI-DEM trial on errorless learning. Alzheimer's Research & Therapy 2017;9(1):1-11. [DOI: 10.1186/s13195-017-0247-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenborn 2021 {published data only}
- Wenborn J, Hynes S, Moniz-Cook E, Mountain G, Poland F, King M, et al. Community occupational therapy for people with dementia and family carers (COTiD-UK) versus treatment as usual (Valuing Active Life in Dementia [VALID] programme): study protocol for a randomised controlled trial. Trials 2016;17(65):1-10. [DOI: 10.1186/s13063-015-1150-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenborn J, O’Keeffe AG, Mountain G, Moniz-Cook E, King M, Omar RZ, et al. Community Occupational Therapy for people with dementia and family carers (COTiD-UK) versus treatment as usual (Valuing Active Life in Dementia [VALID]) study: a single-blind, randomised controlled trial. PLoS Medicine 2021;18(1):1-19. [DOI: 10.1371/journal.pmed.1003433] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhang 2004 {published data only}
- Zhang YH, Lu SP, Xu YN, Fu X, Huang Q. Effect of one-year rehabilitation training in patients with Alzheimer disease. Chinese Journal of Clinical Rehabilitation 2004;8(31):6859-61. [Google Scholar]
References to ongoing studies
Ciro 2016 {published data only}
- Ciro CA, Stoner J, Prodan C, Hershey L. Skill-building through Task-Oriented Motor Practice (STOMP) intervention for activities of daily living: study protocol for a randomized, single blinded clinical trial [Mass Practice of Activities of Daily Living in Dementia (STOMP)]. Clinical Trials in Degenerative Diseases 2016;1(2):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN59155421 {published data only}
- ISRCTN59155421. Evaluation of a person-centred multidimensional interdisciplinary rehabilitation program for community dwelling older people with dementia and their informal primary caregivers. https://www.isrctn.com/ISRCTN59155421 (first received 4 November 2015).
- Sondell A, Lampinen J, Conradsson M, Littbrand H, Englund U, Nilsson I, et al. Experiences of community-dwelling older people with dementia participating in a person-centred multidimensional interdisciplinary rehabilitation program. BMC Geriatrics 2021;21(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02937883 {published data only}
- NCT02937883. Empowerment intervention for persons with young onset dementia. https://clinicaltrials.gov/ct2/show/NCT02937883 (first received 19 October 2016).
NCT03430401 {published data only}
- NCT03430401. Computer-based cognitive rehabilitation program for older people with mild cognitive impairment and mild dementia. https://clinicaltrials.gov/ct2/show/NCT03430401 (first received 12 February 2018).
Additional references
ADI 2015
- Alzheimer’s Disease International. World Alzheimer Report 2019: The global impact of dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, 2015. [Google Scholar]
ADI 2023
- Alzheimer’s Disease International. From Plan to Impact VI. Making every step count. Alzheimer’s Disease International, 2023. [Google Scholar]
APA 1995
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders (DSM-4®). 4th edition. Washington, DC: American Psychiatric Association, 1995. [Google Scholar]
APA 2013
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). 5th edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Backman 1992
- Backman L. Memory training and memory improvement in Alzheimer's disease: rules and exceptions. Acta Neurologica Scandinavica. Supplementum 1992;85(S139):84-9. [DOI: 10.1111/j.1600-0404.1992.tb04461.x] [DOI] [PubMed] [Google Scholar]
Bahar‐Fuchs 2013
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD003260. [DOI: 10.1002/14651858.CD003260.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahar‐Fuchs 2019
- Bahar-Fuchs A, Martyr A, Goh AMY, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013069. [DOI: 10.1002/14651858.CD013069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2019
- Bennett S, Laver K, Voigt-Radloff S, Letts L, Clemson L, Graff M, et al. Occupational therapy for people with dementia and their family carers provided at home: a systematic review and meta-analysis. BMJ Open 2019;9(11):e026308. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosboom 2012
- Bosboom PR, Alfonso H, Eaton J, Almeida OP. Quality of life in Alzheimer's disease: different factors associated with complementary ratings by patients and family carers. International Psychogeriatrics 2012;24(5):708-21. [DOI: 10.1017/S1041610211002493] [DOI] [PubMed] [Google Scholar]
Boyle 2002
- Boyle PA, Cohen RA, Paul R, Moser D, Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. International Journal of Geriatric Psychiatry 2002;17(2):164-9. [DOI: 10.1002/gps.539] [DOI] [PubMed] [Google Scholar]
Brickman 2002
- Brickman AM, Riba A, Bell K, Marder K, Albert M, Brandt J, et al. Longitudinal assessment of patient dependence in Alzheimer disease. Archives of Neurology 2002;59(8):1304-8. [DOI: 10.1001/archneur.59.8.1304] [DOI] [PubMed] [Google Scholar]
Cates 2015
- Cates C, Karner C. Clinical importance cannot be ruled out using mean difference alone. BMJ 2015;351:1-5. [DOI: 10.1136/bmj.h5496] [DOI] [PubMed] [Google Scholar]
Chare 2014
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. Journal of Neurology, Neurosurgery & Psychiatry 2014;85(8):865-70. [DOI] [PubMed] [Google Scholar]
Chaytor 2003
- Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychology Review 2003;13(4):181-97. [DOI: 10.1023/B:NERV.0000009483.91468.fb] [DOI] [PubMed] [Google Scholar]
Clare 2008
- Clare L. Working with memory problems: cognitive rehabilitation in early dementia. In: Moniz-Cook E, Manthorpe J, editors(s). Early Psychosocial Interventions in Dementia. Evidence-based Practice. London, UK: Jessica Kingsley, 2008:73-80. [Google Scholar]
Clare 2017
- Clare L. Rehabilitation for people living with dementia: a practical framework of positive support. PLoS Medicine 2017;14(3):e1002245. [DOI: 10.1371/journal.pmed.1002245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clare 2022a
- Clare L, Kudlicka A, Collins R Evans S, Pool J, Henderson C, et al. Implementing a home-based personalised cognitive rehabilitation intervention for people with mild-to-moderate dementia: GREAT into Practice. BMC Geriatrics 2023;23:1-17. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clare 2022b
- Clare L, Gamble LD, Martyr A, Sabatini S, Nelis SM, Quinn C, et al, on behalf of the IDEAL study team. Longitudinal trajectories of quality of life among people with mild-to-moderate dementia: a latent growth model approach with IDEAL cohort study data. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences 2022;77(6):1037-50. [DOI: 10.1093/geronb/gbac022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences (2nd edition). Hillsdale (NJ): Lawrence Erlbaum Associates, Inc , 1988. [Google Scholar]
Covidence [Computer program]
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2022. [AVAILABLE FROM: www.covidence.org]
Cullen 2008
- Cullen C. Acceptance and commitment therapy (ACT): a third wave behaviour therapy. Behavioural and Cognitive Psychotherapy 2008;36(6):667-73. [DOI: 10.1017/S1352465808004797] [DOI] [Google Scholar]
de São José 2017
- São José JM, Amado CA. On studying ageism in long-term care: a systematic review of the literature. International psychogeriatrics. International Psychogeriatrics 2017;29(3):373-87. [DOI: 10.1017/S1041610216001915] [DOI] [PubMed] [Google Scholar]
Deci 2008
- Deci EL, Ryan RM. Self-determination theory: a macrotheory of human motivation, development, and health. Canadian Psychology/Psychologie Canadienne 2008;49(3):182-5. [DOI: 10.1037/a0012801] [DOI] [Google Scholar]
Deeks 2017
- Deeks J, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from handbook.cochrane.org.
Dourado 2016
- Dourado MC, Sousa MF, Santos RL, Simoes Neto JP, Nogueira ML, Belfort TT, et al. Quality of life in mild dementia: patterns of change in self and caregiver ratings over time. Revista Brasileira De Psiquiatria 2016;38(4):294-300. [DOI: 10.1590/1516-4446-2014-1642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duggleby 2009
- Duggleby W, Williams A, Wright K, Bollinger S. Renewing everyday hope: the hope experience of family caregivers of persons with dementia. Issues in Mental Health Nursing 2009;30(8):514-21. [DOI: 10.1080/01612840802641727] [DOI] [PubMed] [Google Scholar]
Dunn 2007
- Dunn J, Clare L. Learning face-name associations in early-stage dementia: comparing the effects of errorless learning and effortful processing. Neuropsychological Rehabilitation 2007;17(6):735-54. [DOI: 10.1080/09602010701218317] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
EIPAHA 2012
- European Innovation Partnership on Active and Healthy Ageing. Action plan on prevention and early diagnosis of frailty and functional decline, both physical and cognitive, in older people. Conference of Interested Partners, Brussels 2012;ec.europa.eu/eip/ageing/sites/eipaha/files/library/50acba37c1dff_A3-Action%20Plan%20Final%20v2.pdf.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Eyssen 2011
- Eyssen IC, Steultjens MP, Oud TA, Bolt EM, Maasdam A, Dekker J. Responsiveness of the Canadian occupational performance measure. Journal of Rehabilitation Research and Development 2011;48(5):517-28. [DOI: 10.1682/JRRD.2010.06.0110] [DOI] [PubMed] [Google Scholar]
Fernández‐Ballesteros 2003
- Fernández-Ballesteros R, Zamarrón MD, Tárraga L, Moya R, Iñiguez J. Cognitive plasticity in healthy, mild cognitive impairment (MCI) subjects and Alzheimer's disease patients: a research project in Spain. European Psychologist 2003;8(3):148-59. [DOI: 10.1027//1016-9040.8.3.148] [DOI] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI: 10.1016/0022-3956(75)90026-6] [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, GRADE Working Group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. https://gdt.gradepro.org/app/handbook/handbook.html 2013.
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 28 January 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
Gélinas 1999
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. American Journal of Occupational Therapy 1999;53(5):471-81. [DOI] [PubMed] [Google Scholar]
Hepple 2004
- Hepple J. Ageism in therapy and beyond. In: Cognitive Analytic Therapy and Later Life. Routledge, 2004:61-82. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2017:205-27. [Google Scholar]
Hodges 1992
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 1992;115(6):1783-806. [DOI: 10.1093/brain/115.6.1783] [DOI] [PubMed] [Google Scholar]
Hsieh 2013
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2013;36(3-4):242-50. [DOI] [PubMed] [Google Scholar]
Hughes 1982
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140(6):566-72. [DOI: 10.1192/bjp.140.6.566] [DOI] [PubMed] [Google Scholar]
Ikemata 2017
- Ikemata S, Momose Y. Effects of a progressive muscle relaxation intervention on dementia symptoms, activities of daily living, and immune function in group home residents with dementia in Japan. Japan Journal of Nursing Science 2017;14(2):135-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Innovations in Dementia 2021
- Innovations in Dementia. Living with dementia: my life, my goals. https://www.alzheimers.org.uk/blog/life-after-dementia-diagnosis-guide-setting-reaching-goals 2021.
Juul 2021
- Juul S, Gluud C, Simonsen S, Frandsen FW, Kirsch I, Jakobsen JC. Blinding in randomised clinical trials of psychological interventions: a retrospective study of published trial reports. BMJ Evidence-Based Medicine 2021 ;26(3):1-9. [DOI: http://dx.doi.org/ 10.1136/bmjebm-2020- 111407] [DOI] [PubMed] [Google Scholar]
Klatte 2022
Kudlicka 2011
- Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson's disease: systematic review and meta-analysis. Movement Disorders 2011;26(13):2305-15. [DOI: 10.1002/mds.23868] [DOI] [PubMed] [Google Scholar]
Kudlicka 2018
- Kudlicka A, Clare L. Cognitive rehabilitation in mild and moderate dementia. Oxford Research Encyclopedia of Psychology 2018;oxfordre.com/psychology/view/10.1093/acrefore/9780190236557.001.0001/acrefore-9780190236557-e-390. [DOI: 10.1093/acrefore/9780190236557.013.390] [DOI]
Law 2005
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 4th edition. Toronto (ON): Canadian Association of Occupational Therapists, 2005. [Google Scholar]
Little 1986
- Little AG, Volans PJ, Hemsley DR, Levy R. The retention of new information in senile dementia. British Journal of Clinical Psychology 1986;25(1):71-2. [DOI: 10.1111/j.2044-8260.1986.tb00673.x] [DOI] [PubMed] [Google Scholar]
Luszczynska 2005
- Luszczynska A, Gutierrez-Dona B, Schwarzer R. General self-efficacy in various domains of humanfunctioning: evidence from five countries. International Journal of Psychology 2005;40:80-9. [DOI: ] [Google Scholar]
Marshall 2005
- Marshall M. Perspectives on Rehabilitation and Dementia. London, UK: Jessica Kingsley, 2005. [Google Scholar]
Martyr 2012a
- Martyr A, Clare L. Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dementia and Geriatric Cognitive Disorders 2012;33(2-3):189-203. [DOI: 10.1159/000338233] [DOI] [PubMed] [Google Scholar]
Martyr 2012b
- Martyr A, Clare L, Nelis SM, Marková IS, Roth I, Woods RT, et al. Verbal fluency and awareness of functional deficits in early-stage dementia. The Clinical Neuropsychologist 2012;26(3):501-19. [DOI: 10.1080/13854046.2012.665482] [DOI] [PubMed] [Google Scholar]
Martyr 2014
- Martyr A, Nelis SM, Clare L. Predictors of perceived functional ability in early-stage dementia: self-ratings, informant ratings and discrepancy score. International Journal of Geriatric Psychiatry 2014;29(8):852-62. [DOI: 10.1002/gps.4071] [DOI] [PubMed] [Google Scholar]
Martyr 2018
- Martyr A, Nelis SM, Quinn C, Wu Y-T, Lamont RA, Henderson C, et al. Living well with dementia: a systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychological Medicine 2018;48(13):2130-9. [DOI: 10.1017/S0033291718000405] [DOI] [PubMed] [Google Scholar]
Matías‐Guiu 2018
- Matías-Guiu JA, Pytel V, Cortés-Martínez A, Valles-Salgado M, Rognoni T, Moreno-Ramos T, et al. Conversion between Addenbrooke's Cognitive Examination III and Mini-Mental State Examination. International Psychogeriatrics 2018;30(8):1227-33. [10.1017/S104161021700268X] [DOI] [PubMed] [Google Scholar]
McCallie 2006
- McCallie MS, Blum CM, Hood CJ. Progressive muscle relaxation. Journal of Human Behavior in the Social Environment 2006;13(3):51-66. [Google Scholar]
McKeith 1996
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47(5):1113-24. [DOI: 10.1212/WNL.47.5.1113] [DOI] [PubMed] [Google Scholar]
McKeith 2006
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Journal of Alzheimer's Disease 2006;9(3 Suppl):417-23. [DOI: 10.3233/JAD-2006-9S347] [DOI] [PubMed] [Google Scholar]
McKeith 2017
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89(1):88-100. [DOI: 10.1212/wnl.0000000000004058] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology 1984;34(7):939-44. [DOI: 10.1212/WNL.34.7.939] [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7(3):263-9. [DOI: 10.1016/j.jalz.2011.03.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLaughlin 2010
- McLaughlin T, Feldman H, Fillit H, Sano M, Schmitt F, Aisen P, et al. Dependence as a unifying construct in defining Alzheimer's disease severity. Alzheimer's & Dementia 2010;6(6):482-93. [DOI: 10.1016/j.jalz.2009.09.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLellan 1991
- McLellan DL. Functional recovery and the principles of disability medicine. In: Swash M, Oxbury J, editors(s). Clinical Neurology. Edinburgh, UK: Churchill Livingstone, 1991:768-90. [Google Scholar]
Mohr 2009
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics 2009;78(5):275-84. [DOI] [PubMed] [Google Scholar]
Molloy 1997
- Molloy DW, Standish TI. A guide to the Standardized Mini-Mental State Examination. International Psychogeriatrics 1997;9(S1):87-94. [DOI: 10.1017/S1041610297004754] [DOI] [PubMed] [Google Scholar]
Moraitou 2006
- Moraitou D, Kolovou C, Papasozomenou C, Paschoula C. Hope and adaptation to old age: their relationship with individual-demographic factors. Social Indicators Research 2006;76(1):71-93. [DOI: 10.1006/jrpe.1993.1011] [DOI] [Google Scholar]
Mowbray 2003
- Mowbray CT, Holter MC, Teague GB, Bybee D . Fidelity criteria: development, measurement, and validation. American Journal of Evaluation 2003;24(3):315-40. [Google Scholar]
Myshra 2016
- Myshra V, Barrett J. Reablement and older people. Final report of the International Federation on Aging Copenhagen Summit 2016. www.ifa-fiv.org/publication/health/copenhagen-summit-report-reablement-older-people/ (accessed 19 February 2019).
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53(4):695-9. [DOI: 10.1111/j.1532-5415.2005.53221.x] [DOI] [PubMed] [Google Scholar]
Njegovan 2001
- Njegovan V, Hing MM, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. The Journals of Gerontology. Series A: Biological Sciences and Medical Sciences 2001;56(10):M638-43. [DOI: 10.1093/gerona/56.10.M638] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Thomas J, Dooley G. Using crowdsourcing and machine learning for study identification: a quantitative and qualitative evaluation of Cochrane’s Screen4Me workflow. 27th Cochrane Colloquium 2020.
Noel‐Storr 2021
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021;133:130-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perneczky 2006
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. American Journal of Geriatric Psychiatry 2006;14(2):139-44. [DOI: 10.1097/01.JGP.0000192478.82189.a8] [DOI] [PubMed] [Google Scholar]
Pfeffer 1982
- Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology 1982;37(3):323-9. [DOI: 10.1093/geronj/37.3.323] [DOI] [PubMed] [Google Scholar]
Poulos 2017
- Poulos CJ, Bayer A, Beaupre L, Clare L, Poulos RG, Wang RH, et al. A comprehensive approach to reablement in dementia. Alzheimer's & Dementia 2017;3(3):450-8. [DOI: 10.1016/j.trci.2017.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2014
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK. Second edition. London (UK): Alzheimer’s Society, 2014. [Google Scholar]
Prince 2015
- Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed 28 July 2019).
Raquel 2021
- Raquel CT, Villafañe JH, Medina-Porqueres I, Garcia-Orza S, Valdes K. Convergent validity and responsiveness of the Canadian Occupational Performance Measure for the evaluation of therapeutic outcomes for patients with carpometacarpal osteoarthritis. Journal of Hand Therapy 2021;34(3):439-45. [DOI: 10.1016/j.jht.2020.03.011] [DOI] [PubMed] [Google Scholar]
Razani 2007
- Razani J, Kakos B, Orieta-Barbalace C, Wong JT, Casas R, Lu P, et al. Predicting caregiver burden from daily functional abilities of patients with mild dementia. Journal of the American Geriatrics Society 2007;55(9):1415-20. [DOI: 10.1111/j.1532-5415.2007.01307.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Reifler 1990
- Reifler BV, Larson E. Excess disability in dementia of the Alzheimer's type. In: Light E, Lebowitz BD, editors(s). Alzheimer's Disease Treatment and Family Stress. New York, NY: Hemisphere, 1990:363-82. [Google Scholar]
Review Manager 5 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roalf 2013
- Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimer's & Dementia 2013;9(5):529-37. [DOI: 10.1016/j.jalz.2012.10.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43(2):250-60. [DOI: 10.1212/WNL.43.2.250] [DOI] [PubMed] [Google Scholar]
Royall 2007
- Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. Journal of Neuropsychiatry and Clinical Neurosciences 2007;19(3):249-65. [DOI: 10.1176/appi.neuropsych.19.3.249] [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Synnot A, Hill S. Describing results. Cochrane Consumers and Communication Group. Version 1.0 June 2016. [AVAILABLE AT: http://cccrg.cochrane.org/author-resources]
Schwarzer 1995
- Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. In: Weinman J, Wright S, Johnston M, editors(s). Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. NFER-Nelson , 1995:35-7. [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al on behalf of Cochrane GRADEing Methods Group. Interpreting results and drawing conclusions. Cochrane handbook for systematic reviews of interventions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2019:403-31. [Google Scholar]
Schünemann 2019b
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2019. [Google Scholar]
Scott 2019
- Scott I, Cooper C, Leverton M, Burton A, Beresford‐Dent J, Rockwood K, et al. Effects of nonpharmacological interventions on functioning of people living with dementia at home: a systematic review of randomised controlled trials. International Journal of Geriatric Psychiatry 2019;34(10):1386-402. [DOI] [PubMed] [Google Scholar]
Shabbir 2014
- Shabbir SH, Sanders AE. Clinical significance in dementia research: a review of the literature. American Journal of Alzheimer's Disease & Other Dementias 2014;29(6):492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikkes 2021
- Sikkes SA, Tang Y, Jutten RJ, Wesselman LM, Turkstra LS, Brodaty H, et al. Toward a theory‐based specification of non‐pharmacological treatments in aging and dementia: focused reviews and methodological recommendations. Alzheimer's & Dementia 2021;17(2):255-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skevington 2004
- Skevington SM, Lotfy M, O’Connell KA, on behalf of WHOQOL Group. The World Health Organization’sWHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13:299–310. [DOI: ] [DOI] [PubMed] [Google Scholar]
Skrobot 2018
- Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer's & Dementia 2018;14(3):280-92. [DOI: 10.1016/j.jalz.2017.09.007] [DOI] [PubMed] [Google Scholar]
Squire 1995
- Squire LR, Knowlton BJ. Memory, hippocampus, and brain systems. In: Gazzaniga M, editors(s). The Cognitive Neurosciences. Boston, MA: The MIT Press, 1995:825-37. [Google Scholar]
Stern 1994
- Stern Y, Albert SM, Sano M, Richards M, Miller L, Folstein M, et al. Assessing patient dependence in Alzheimer's disease. Journal of Gerontology 1994;49(5):M216-22. [DOI: 10.1093/geronj/49.5.M216] [DOI] [PubMed] [Google Scholar]
Strauss 2006
- Strauss E, Sherman EM, Spreen O. A compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press, 2006. [Google Scholar]
Suhr 1999
- Suhr J. Progressive muscle relaxation in the management of behavioural disturbance in Alzheimer's disease. Neuropsychological Rehabilitation 1999;9(1):31-44. [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living Systematic Review Network. Living Systematic Reviews: 2. Combining Human and Machine Effort. Journal of Clinical Epidemiology 2017;91:23-30. [DOI: 10.1016/j.jclinepi.2017.08.010] [DOI] [PubMed] [Google Scholar]
Weintraub 2012
- Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine 2012;2(4):a006171. [DOI: 10.1101/cshperspect.a006171] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization (WHO). International Classification of Diseases, 11th Revision. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
Wilson 2002
- Wilson BA. Towards a comprehensive model of cognitive rehabilitation. Neuropsychological Rehabilitation 2002;12(2):97-110. [DOI: 10.1080/09602010244000020] [DOI] [Google Scholar]
Woods 2014
- Woods RT, Nelis SM, Martyr A, Roberts JL, Whitaker CJ, Marková IS, et al. What contributes to a good quality of life in early dementia? Awareness and the QoL-AD: a cross-sectional study. Health and Quality of Life Outcomes 2014;12:94. [DOI: 10.1186/1477-7525-12-94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2017
- Wu Y-T, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HC, et al. The changing prevalence and incidence of dementia over time — current evidence. Nature Reviews Neurology 2017;13(6):327-39. [DOI: 10.1038/nrneurol.2017.63] [DOI] [PubMed] [Google Scholar]


