Abstract
Zoonotic outbreaks of sporotrichosis are increasing in Brazil. We examined and described the emergence of cat-transmitted sporotrichosis (CTS) caused by the fungal pathogen Sporothrix brasiliensis. We calculated incidence and mapped geographic distribution of cases in Curitiba, Brazil, by reviewing medical records from 216 sporotrichosis cases diagnosed during 2011–May 2022. Proven sporotrichosis was established in 84 (39%) patients and probable sporotrichosis in 132 (61%). Incidence increased from 0.3 cases/100,000 outpatient visit-years in 2011 to 21.4 cases/100,000 outpatient visit-years in 2021; of the 216 cases, 58% (n = 126) were diagnosed during 2019–2021. The main clinical form of sporotrichosis was lymphocutaneous (63%), followed by localized cutaneous (24%), ocular (10%), multisite infections (3%), and cutaneous disseminated (<0.5%). Since the first report of CTS in Curitiba in 2011, sporotrichosis has increased substantially, indicating continuous disease transmission. Clinician and public awareness of CTS and efforts to prevent transmission are needed.
Keywords: sporotrichosis, zoonoses, mycoses, Sporothrix brasiliensis, cat-transmitted sporotrichosis, fungi, Brazil
Sporotrichosis, the most prevalent implantation mycosis worldwide, is caused by fungi of genus Sporothrix (1–4). In some regions of Brazil, sporotrichosis has been referred to as cat disease because of its zoonotic transmission from felines. Since 1990, a new Sporothrix species, S. brasiliensis, has emerged rapidly as an agent of cat-transmitted sporotrichosis (CTS) (5). Initially identified primarily in Rio de Janeiro, highly virulent S. brasiliensis causes a notable level of epizootic disease involving cats, dogs, and humans (5–12) that is emerging and expanding geographically across Brazil (7–9,11) and is now a major public health problem (12). Originally, CTS was reported primarily in the South and Southeast regions of Brazil, but by 2022, CTS was reported in 25 of its 26 states, as well as in neighboring Argentina, Chile, and Paraguay (Figure 1) (2,9,13–18). In 2022, a case of cutaneous CTS caused by S. brasiliensis was reported in a veterinarian in the United Kingdom who was infected by an imported cat with sporotrichosis (19,20).
Figure 1.
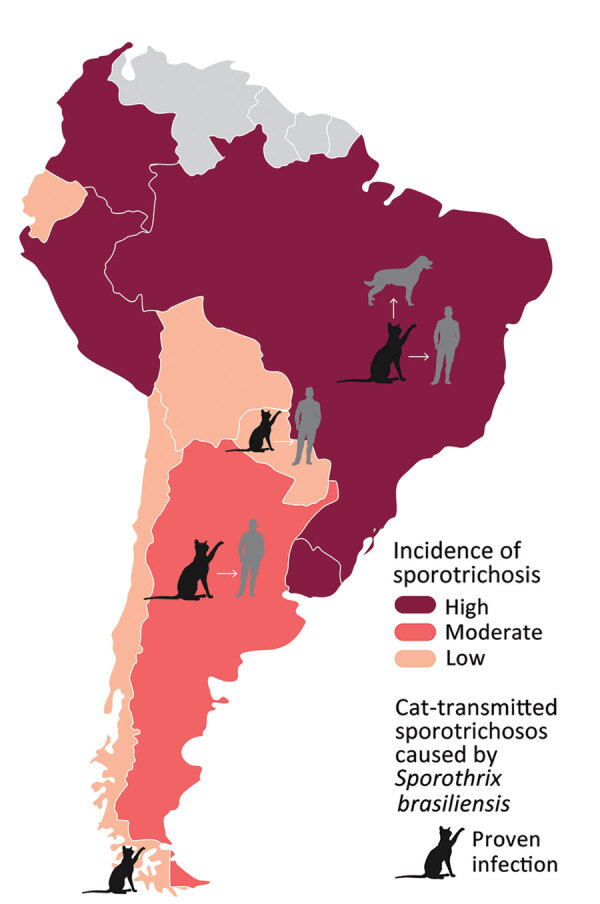
Burden of sporotrichosis in South America and distribution of cat-transmitted sporotrichosis in humans caused by Sporothrix brasiliensis, 2022. Cat icons indicate countries where cases of cat-transmitted sporotrichosis caused by S. brasiliensis have been reported; arrows indicate transmission from cats, humans, and dogs.
Rio de Janeiro state, in the Southeast region of Brazil, has the highest prevalence of CTS, >8,900 human cases reported since the beginning of the outbreak, followed by Rio Grande do Sul (South region) with 181 human cases (14,15,21). In Paraná state, also in the South region, public health officials and clinicians have been alarmed by the emergence of CTS, but epidemiologic and clinical data on this disease in this jurisdiction are lacking because reporting is not mandatory (11,22). Therefore, we performed a retrospective, descriptive study of human CTS to describe the characteristics of patients with sporotrichosis, based on a decade of experience in a single medical institution, the Hospital de Clínicas of the Federal University of Paraná (HC/UFPR), a tertiary referral hospital in Curitiba, Paraná’s largest city. Our study was approved by the HC/UFPR ethical committee (registration no. 12379819.4.0000.0096).
Methods
Study Design
We identified cases from the hospital database by using code B42 from the International Classification of Diseases, 10th Revision. Our study analyzed data from all proven and probable CTS cases from HC/UFPR during January 2011–May 2022.
Case Definition
We used case definitions approved by the Brazilian Ministry of Health (23–25). Proven human CTS was defined as microbiologic evidence (positive culture or histopathology) of sporotrichosis, presence of lesions or other symptoms compatible with sporotrichosis, and evidence of CTS transmission, including cat scratches or bites, contact with feline exudates, or exposure to sneezing by cats (Table 1) (23–26). Probable human CTS was defined as presence of compatible clinical manifestations after traumatic injury from or close contact with infected cats but lacking microbiologic evidence of sporotrichosis (Table 1) (23–25). We excluded patients with possible CTS or among whom CTS was ruled out.
Table 1. Cat-transmitted sporotrichosis case definitions used in study of human cases in Curitiba, Brazil, 2011–2022*.
| Definition | Epidemiology | Clinical | Laboratory |
|---|---|---|---|
| Proven |
History of trauma or contact with a cat with sporotrichosis |
Lesions compatible with sporotrichosis |
Positive culture and/or histopathology (microbiological evidence) |
| Probable |
History of trauma or contact with a cat with sporotrichosis |
Lesions compatible with sporotrichosis |
Human: Negative culture and/or histopathology.† Sick cat: A) sporotrichosis diagnosed by culture and/or histopathology in a veterinarian laboratory; B) cat resident in a region with confirmed presence of cats with sporotrichosis (data verified by public health authorities) |
| Possible |
History of trauma or contact with a cat with sporotrichosis |
Lesions compatible with sporotrichosis |
Absent |
| Non-CTS | History of trauma or contact with a cat with sporotrichosis | Lesions compatible with sporotrichosis | Negative culture and/or histopathology for Sporothrix spp.† Definition of other case of disease (infectious or noninfectious) |
Sporothrix Isolate Molecular Typing
We molecularly identified at the species level all Sporothrix isolates obtained from patients and extracted DNA from isolates using MasterPure Yeast DNA Purification Kit (Thermo Fisher Scientific). We amplified the calmodulin (CAL) locus region using the degenerate primers CL1 (5′-GAR TWC AAG GAG GCC TTC TC-3′) and CL2A (5′-TTT TTG CAT CAT GAG TTG GAC-3′), then sequenced amplicons using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). We visually inspected sequences using the BioEdit version 7.2.5 sequence alignment editor and used MAFFT version 7 to align sequences with reference strains from the National Center for Biotechnology Information databank and MEGA version 7.0.26 software with Tamura Nei method with 1,500 bootstraps to calculate evolutionary distance.
Data Collection and Statistical Analysis
From hospital clinical records we collected clinical, laboratory, and epidemiologic data from cases and stored them using a standardized Microsoft Excel form. We calculated descriptive statistics to record the characteristics of the cases. We also calculated the incidence rate per 100,000 outpatient all-reason visits per year (outpatient visit-years [OPVY]). We performed analyses using Medcalc version 19.0 statistical software and used Qgis version 3.28.3 to map distribution of CTS cases by residence coordinates (latitude and longitude). We obtained graphic sources of the vector layer from the Instituto de Pesquisa e Planejamento Urbano de Curitiba, Instituto Ambiental do Paraná, and Coordenação da Região Metropolitana de Curitiba. We created kernel density maps to show hotspot areas for sporotrichosis in Curitiba; for this analysis, we established a search radius of 750 m.
Results
We included 216 CTS cases in this analysis. Case-patients were more frequently female than male (ratio 1.8:1) except in the 11–17 year age group (Figure 2). Median age among CTS case-patients was 40 years (interquartile range 22.5–56.0 years) (Table 2); 29 (13%) patients were <18 years of age. Overall, 11% of patients had occupations with disease-related risk factors, including 9 veterinarians, 5 veterinary students, 3 pet sitters, and 2 gardeners. The most frequent clinical form of sporotrichosis was lymphocutaneous (n = 136, 63%), followed by fixed cutaneous (n = 53, 25%), ocular (n = 21, 10%), mixed forms (n = 5, 2%), and cutaneous disseminated (n = 1, <0.5%). We observed that some CTS case-patients had unusual clinical manifestations (Figure 3).
Figure 2.
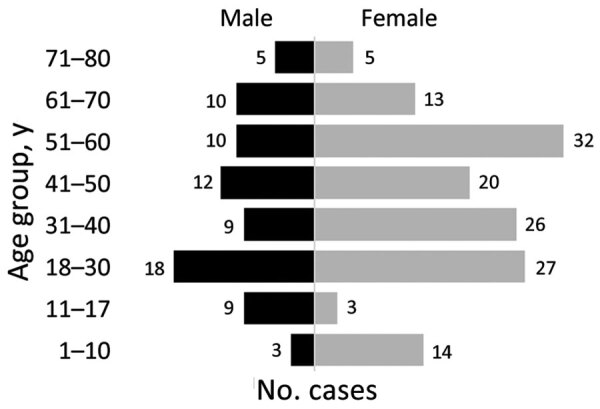
Age–sex pyramid showing the distribution of cat-transmitted sporotrichosis cases in patients treated at Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil, 2011–May 2022.
Table 2. Sociodemographic characteristics in study of human sporotrichosis cases in Curitiba, Brazil, 2011–2022.
| Characteristic | No. (%) patients |
|---|---|
| Sex | |
| F | 140 (65) |
| M |
76 (35) |
| Age range, y | |
| ≤10 | 17 (8) |
| 11–17 | 12 (6) |
| 18–30 | 45 (21) |
| 31–60 | 109 (50) |
| >60 |
33 (15) |
| Occupation | |
| Unemployed | 42 (19) |
| Student | 42 (19) |
| Retired | 23 (11) |
| Domestic worker | 17 (8) |
| Veterinarian or veterinary student | 17 (8) |
| Administrator | 11 (5) |
| Teacher | 3 (1) |
| Pet sitter | 3 (1) |
| Gardener | 2 (1) |
| Butcher | 2 (1) |
| Others | 54 (26) |
Figure 3.
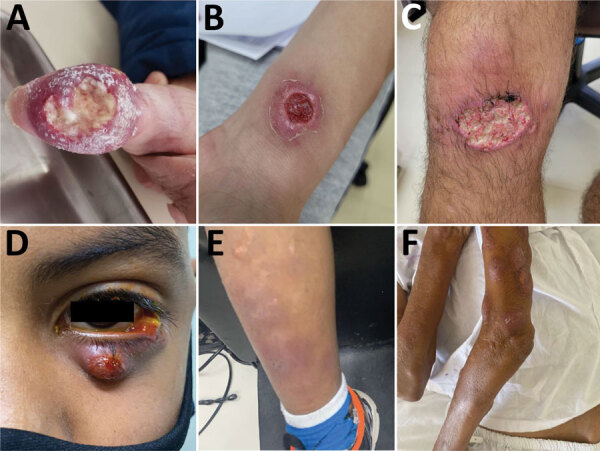
Unusual clinical manifestations of cat-transmitted sporotrichosis in patients treated at Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil, 2011–May 2022. A) Fixed cutaneous manifestation in the thumb with osteoarticular involvement. B) Fixed cutaneous manifestation in forearm with ulcer similar to primary cutaneous leishmaniasis. C) Lymphocutaneous manifestation in the knee with a large ulceration, mimicking cutaneous leishmaniasis. D) Ocular form with Parinaud ocular-glandular syndrome and dacryocystitis. E) Erythema nodosum resulting from immunoreactive sporotrichosis manifestation in the leg (same patient from panel D). F) Cutaneous disseminated manifestation in immunocompromised patient.
Overall, 84 (39%) patients had proven CTS and 132 (61%) had probable CTS. Among probable CTS cases, we tested 18 patients by microscopy and culture, and all tested negative. Laboratory testing was not requested for the 14 remaining probable CTS case-patients; the main causes for not testing were specimen unavailability for microbiology testing and previous initiation of antifungal treatment. Among the proven CTS cases, Sporothrix isolates were collected from direct swabbing of lesion secretions for 50 patients, skin biopsy for 29, and aspirate from abscesses for 5. Average times for positive Sporothrix culture results varied by specimen type: specimens collected using the swab method averaged 6 days (range 3–17 days), compared with 13 days (range 4–30 days) for specimens collected by biopsy (p<0.0001). On the basis of phylogenetic analysis of calmodulin sequence genes of 38 Sporothrix isolates from patients, we identified S. brasiliensis as the only etiologic agent (38/38 cases) of CTS during the study period (Figure 4).
Figure 4.

Phylogenetic analysis of Sporothrix spp. isolates from cat-transmitted sporotrichosis patients treated at Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil, 2011–May 2022. Tree shows analysis of clades based on calmodulin near-complete genes constructed with maximum-likelihood implemented in MEGA 7.0.26. Bootstrap values >80 from 1,500 resampled datasets are shown. Bold indicates strains isolated in this study; yellow shading represents S. schenckii isolates and S. brasiliensis isolates. GenBank accession numbers are shown in Appendix Table 1. Scale bar represents number of substitutions per site.
In 98% (212) of the 216 total CTS cases, infection was associated with zoonotic transmission; in those cases, the patient had direct or indirect contact with a cat with diagnosed sporotrichosis. In 4 (2%) sporadic cases, occurring in 2016, 2017, 2019, and 2021, infection was associated with saprozoic exposure with traumatic implantation from an environmental source. The first observed substantial increase in cases was from 1 in 2015 (0.3 cases/100,000 OPVY) to 12 in 2016 (3.5 cases/100,000 OPVY) (Figure 5), and incidence rates continued to increase thereafter. There were 34 cases (8.3 cases/100,000 OPVY) in 2018, almost 3 times as many as in 2016, and 61 cases (21.4 cases/100,000 OPVY) in 2021 (Figure 5). During January–May 2022, we identified 20 cases from 142,873 outpatient visits (14 cases/100,000 OPVY) (Figure 5).
Figure 5.
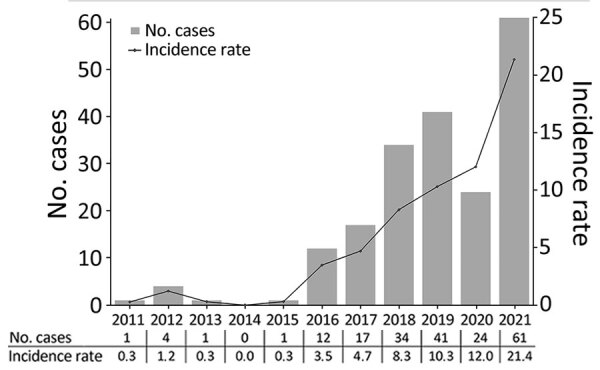
Epidemiologic curve and incidence rate (cases/100,000 outpatient visit-years) of cat-transmitted sporotrichosis patients treated at Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil, 2011–2021.
CTS cases were reported in 15 of the 399 municipalities in the state of Paraná (Figure 6, panels A, B) and in 11 of the 29 municipalities comprising the metropolitan region of Curitiba (Figure 6, panel C). Among the 216 CTS cases identified in the state of Paraná, the metropolitan region of Curitiba accounted for 205 (95%), the city of Curitiba alone for 170 (79%) (Figure 6, panel D). We also tracked triennial distribution of cases in the city of Curitiba during 2011–2013, 2014–2016, 2017–2019, and 2020–May 2022. The first case of CTS in Paraná was identified in 2011 in Campina Grande do Sul, a municipality located in the metropolitan region of Curitiba. The first case of CTS in the city of Curitiba was identified in 2012 in the Mercês neighborhood, located in the north-central part of the city (Figure 6, panel E). Since 2016, cases have spread across the city of Curitiba and been reported in 41 of 75 districts; most cases have been identified in the northwest region of the city, but during 2020–May 2022, incidence spread throughout the city (Figure 6, panels F, G, H). We also observed clusters of CTS cases involving members of the same household. Since 2011, a total of 10 clusters involving 21 cases were identified; 7/10 CTS clusters (15 cases) occurred after the COVID-19 pandemic began in 2020.
Figure 6.
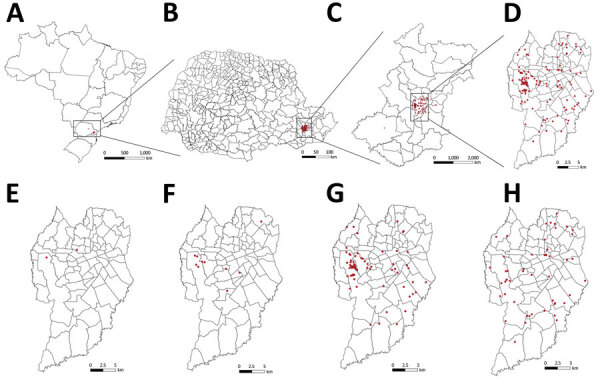
Locations of human cat-transmitted sporotrichosis cases (red dots) treated at Hospital de Clínicas, Federal University of Paraná, Curitiba, Brazil, during 2011–May 2022, and evolution of spatial distribution of cases in the city of Curitiba. A–D) Locations of all human cases in Brazil (A), Paraná state (B), Metropolitan Region of Curitiba (C), and Curitiba (D). E–H) Distribution of new human cases in Curitiba during 2011–2013 (E), 2014–2016 (F), 2017–2019 (G), and 2020–May 2022 (H).
Kernel density maps identified hotspots for sporotrichosis in the city of Curitiba (Figure 7). The most critical hotspots were identified in the western part, mainly the Cidade Industrial de Curitiba (CIC) neighborhood, and north-central (city center) areas of Curitiba. CIC has an area of 44,588 km2 and a population of 172,909 inhabitants (density 3.9 habitants/km2); it is largely residential, and most residents live in houses. CIC is economically characterized as a lower middle–income area, with an average daily income of USD $4.7 (27). The second hotspot, in downtown Curitiba, is a combination of commercial and residential areas, composed mostly of apartment buildings. Curitiba city center comprises 3,310 km2 and is the most densely populated (37,234 inhabitants; density 11.2 inhabitants/km2) part of the city. Average daily income among residents is USD $20 (27). The kernel density maps of Curitiba show additional medium-density hotspots for CTS throughout other parts of the city, especially in the south, southeast, and northeast parts.
Figure 7.
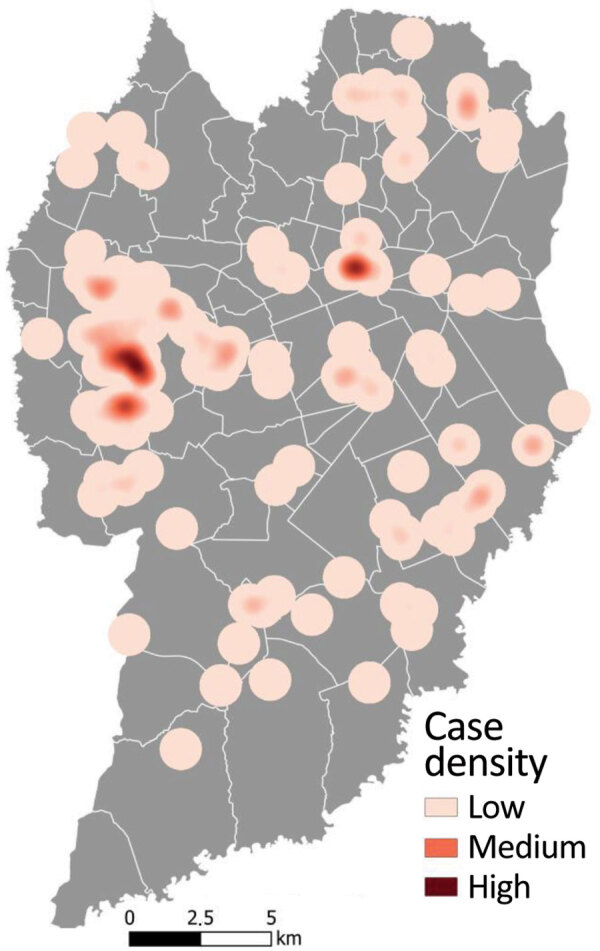
Heatmap of human cat-transmitted sporotrichosis cases using kernel density estimation in Curitiba, Brazil, 2011–May 2022. Kernel density map was created using a search radius of 750 m.
Discussion
During the 10-year study period, sporotrichosis incidence increased substantially, suggesting uncontrolled spread in the city of Curitiba. Brazil is facing the largest reported current zoonotic outbreak of CTS globally (9,11,13,28). CTS is a public health issue exacerbated by insufficient public health activities, such as case surveillance, animal control, and diagnostic capacity, and by lack of disease awareness among health professionals and the general population (9,13,25,29). Our study adds relevant epidemiologic information to promote better understanding of CTS in the state of Paraná, Brazil.
We found a higher prevalence of CTS among female patients, especially in adult women involved in domestic activities. This finding is similar to those of other previous reports and is likely related to close contact with infected domestic cats (30–33). In this study, we observed an increased proportion of pediatric CTS cases; 29 cases (13%) occurred in patients <18 years of age, likely reflecting the close nature of interactions between children and domestic pets, especially cats (23). In addition, we found that >10% of cases occurred in persons working in professional animal care disciplines (2,11,22), who are at increased risk for CTS because of close contact with and exposure to contaminated body fluids from infected animals. Those professionals would benefit from increased awareness that infection in humans can occur through direct contact with body fluids from cats contaminated with S. brasiliensis yeast (26).
Lymphocutaneous and fixed cutaneous forms were the most frequently identified clinical manifestations of sporotrichosis, consistent with findings in previous literature (1,22). However, since the sporotrichosis epidemic in Brazil began, reports of unusual clinical manifestations have been increasing (1,6,34). It should be highlighted that cutaneous disseminated sporotrichosis is a rare manifestation that mostly occurs in immunocompromised persons; it differs from cutaneous manifestations caused by multiple traumatic implantations, as also happens in CTS cases (35–37). We also found a relatively high frequency of ocular sporotrichosis, which occurred in 10% of cases. Ocular sporotrichosis occurs mainly because of direct contact of the ocular mucosa of a person with secretions from an infected cat, usually because of cutaneous exudates or respiratory droplets expelled during cat sneezing (5,12,26). Ocular sporotrichosis is characterized by acute or chronic conjunctivitis, including Parinaud ocular syndrome and dacryocystitis (2,12,38–41). We also observed that unusual clinical manifestations of CTS increased from 2018 onward, coinciding with the epidemic increase in the number of cases of the disease. On the basis of those findings, the current guidelines in Brazil recommend use of personal protective equipment, including glasses or a face shield, during contact with sick cats (24).
Proven cases of CTS in this study made up slightly more than one third (39%) of all cases, compared with 65.3% in other case-series studies of sporotrichosis (22,23). This difference may have resulted from using a case definition not based solely on culture and microscopy results, which are suboptimal assays for detecting sporotrichosis in humans. Cultures from pus or secretion from lesions could be a feasible alternative; that type of test shows good performance and generates results in half the time compared with testing specimens obtained by biopsy. In addition, that type of sample is noninvasive, simple, and inexpensive. HC/UFPR has a medical institute with extensive experience in the diagnosis of sporotrichosis, and its medical professionals consequently have a high index of suspicion for this disease, increasing the chances of early detection of cases in which culture and microscopy are generally negative. On the basis of this experience, we highlight the importance of the probable case classification, because recognizing probable CTS enables rapid initiation of treatment, resulting in a positive effect on the quality of life of patient with sporotrichosis (23,25).
Molecular typing identified a single species, S. brasiliensis, in all proven cases in our study. The isolates were related to the species that caused the CTS outbreak in the state of Rio de Janeiro in the early 2000s and is primarily causing outbreaks throughout Brazil (5,11,13,17,42–44). Those isolates were identified by phylogenetic analysis of the near-complete calmodulin gene sequencing, showing a high degree of similarity with previously sequenced isolates from Rio de Janeiro. Additional analyses, such as microsatellite typing, multilocus sequence typing, and amplified fragment-length polymorphic fingerprinting, can improve understanding of the expansion of cases in Paraná and the city of Curitiba (13,45). Moreover, analysis of whole-genome sequencing results is a tool relevant for elucidating central concerns about the differences in infectious potential between closely related species. Comparative genomic analysis of data on tissue invasion and transmission of pathogenic sibling species has been applied to highlight genes involved in fungal adaptation to an animal-associated lifestyle (11,46,47).
We observed an exponential increase in the incidence rate of sporotrichosis, from 0.3 OPVY in 2011 to 21.4 OPVY in 2021, a trend similar to that for data reported recently in a review of the burden of sporotrichosis in Brazil (9,15,22,29,30,48). In Brazil, the largest number of CTS cases is concentrated in the southwest and south of Brazil, including the states of Rio de Janeiro, Rio Grande do Sul, São Paulo, Espírito Santo, and Minas Gerais. However, cases of CTS have also been described in the Midwest, Federal District, and Northeast regions and in Pernambuco and Rio Grande do Norte states, clearly showing the spread of the disease throughout Brazil (22,32,49).
Most cases in this study occurred in the city of Curitiba. The first case identified at HC/UFPR was in the center-north part of the city in 2012, but the first substantial increase in the number of cases, a trend that continued, was observed in 2016. After the World Health Organization declared COVID-19 a pandemic in March 2020 and lockdowns started, we observed a 37% increase in cases and also an increase in CTS clusters. The increase may have been related to higher rates of pet adoption aimed at reducing anxiety and depression because of the lockdowns (23). We identified 2 areas of the city of Curitiba as CTS hotspots, one area characterized by high population density and the other by social inequalities. In addition, the kernel density estimate showed low or medium CTS expanded practically throughout the city, especially during 2020–2022; areas of medium density were located in the south, southeast, and northeast parts of the city.
The main limitation of our study was that data came from a single center; however, the data covered a decade of experience, and our findings add information based on actual case numbers rather than estimates. Since March 7, 2022, the State Department of Health of Paraná, has provided guidelines (resolution no. 093/2022) for mandatory notification of human and animal sporotrichosis cases in the state.
Because of the zoonotic transmission of this disease, a One Health approach is necessary to control the public health effects of CTS. Integrating human, animal, and environmental health approaches through coordinated actions among microbiologists, veterinarians, physicians, epidemiologists, and surveillance authorities could benefit control efforts. Potential actions include owners restricting cats from going outside homes and having contact with street cats, providing no-cost neutering of cats in regions where S. brasiliensis is endemic to prevent expansion of feral cat populations, widely establishing compulsory notification of CTS cases, increasing awareness of the disease among clinicians and the public, providing easy access to treatment in human and animal cases, and cremating cat remains (12,36,50). Additional interventions to control CTS outbreaks could include providing access to rapid, accurate diagnostic assays and antifungal drugs, developing vaccines specifically for cats, and evaluating novel treatment strategies, particularly for treating feline sporotrichosis. Enacting those and other prevention and control measures in a timely manner is urgently needed to repress the increased incidence and spread of this serious health condition.
Additional information from study of patients with cat-transmitted sporotrichosis Curitiba, Brazil.
Acknowledgments
We thank Lili Volochen Lopuch and Adriana de Fátima Gabriel for all support with collecting and preparing samples and Regiane Cognialli Born for the map design. We also thank Jeremy A.W. Gold for reviewing the manuscript. We also thank Jeremy Gold for reviewing the manuscript.
Biography
Ms. Cognialli is a mycologist at Hospital de Clínicas of Federal University of Paraná and a PhD student at Federal University of Paraná, Curitiba, Paraná, Brazil. Her primary interest is in neglected endemic mycosis.
Footnotes
Suggested citation for this article: Cognialli RCR, Cáceres DH, Bastos FAGD, Cavassin FB, Lustosa BPR, Vicente VA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011–2022. Emerg Infect Dis. 2023 Jul [date cited]. https://doi.org/10.3201/eid2907.230155
References
- 1.Orofino-Costa R, Macedo PM, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queiroz-Telles F, Bonifaz A, Rossow J, Chindamporn A. Sporothrix and sporotrichosis. In: Encyclopedia of infection and immunity. New York: Elsevier Science; 2021. [Google Scholar]
- 3.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. Neglected endemic mycoses. Lancet Infect Dis. 2017;17:e367–77. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. [DOI] [PubMed] [Google Scholar]
- 5.Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13:e1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schechtman RC, Falcão EMM, Carard M, García MSC, Mercado DS, Hay RJ. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barros MB, Schubach AO, do Valle AC, Gutierrez Galhardo MC, Conceição-Silva F, Schubach TM, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529–35. [DOI] [PubMed] [Google Scholar]
- 8.de Lima Barros MB, Schubach TM, Galhardo MC, de Oliviera Schubach A, Monteiro PC, Reis RS, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777–9. [DOI] [PubMed] [Google Scholar]
- 9.Gremião IDF, Oliveira MME, Monteiro de Miranda LH, Saraiva Freitas DF, Pereira SA. Geographic expansion of sporotrichosis, Brazil. Emerg Infect Dis. 2020;26:621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira SA, Gremião ID, Kitada AA, Boechat JS, Viana PG, Schubach TM. The epidemiological scenario of feline sporotrichosis in Rio de Janeiro, state of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2014;47:392–3. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues AM, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP. The threat of emerging and re-emerging pathogenic Sporothrix species. 2020;185:813–42. [DOI] [PubMed]
- 12.Rossow JA, Queiroz-Telles F, Caceres DH, Beer KD, Jackson BR, Pereira JG, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi (Basel). 2020;6:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues AM, Gonçalves SS, de Carvalho JA, Borba-Santos LP, Rozental S, Camargo ZP. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi (Basel). 2022;8:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandolt TM, Madrid IM, Poester VR, Sanchotene KO, Basso RP, Klafke GB, et al. Human sporotrichosis: a zoonotic outbreak in southern Brazil, 2012–2017. Med Mycol. 2018;57:myy082. [DOI] [PubMed] [Google Scholar]
- 15.Poester VR, Mattei AS, Madrid IM, Pereira JTB, Klafke GB, Sanchotene KO, et al. Sporotrichosis in southern Brazil, towards an epidemic? Zoonoses Public Health. 2018;65:815–21. [DOI] [PubMed] [Google Scholar]
- 16.Etchecopaz AN, Lanza N, Toscanini MA, Devoto TB, Pola SJ, Daneri GL, et al. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J Mycol Med. 2020;30:100908. [DOI] [PubMed] [Google Scholar]
- 17.Etchecopaz A, Toscanini MA, Gisbert A, Mas J, Scarpa M, Iovannitti CA, et al. Sporothrix brasiliensis: a review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J Fungi (Basel). 2021;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson P, González C, Blank O, Ramírez V, Río Cd, Santibáñez S, et al. Sporotrichosis outbreak due to Sporothrix brasiliensis in domestic cats in Magallanes, Chile: a One-Health-approach study. J Fungi (Basel). 2023;9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachman R, Ligaj M, Chinthapalli S, Serafino Wani R. Zoonotic acquisition of cutaneous Sporothrix braziliensis infection in the UK. BMJ Case Rep. 2022;15:e248418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnacle JR, Chow YJ, Borman AM, Wyllie S, Dominguez V, Russell K, et al. The first three reported cases of Sporothrix brasiliensis cat-transmitted sporotrichosis outside South America. Med Mycol Case Rep. 2022;39:14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinho Falcão EMM, Rocha Romão A, de Avelar Figueiredo Mafra Magalhães M, de Lima Filho JB, Francesconi do Valle AC, Bastos FI, et al. A spatial analysis of the spread of hyperendemic sporotrichosis in the state of Rio de Janeiro, Brazil. J Fungi (Basel). 2022;8:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabello VBS, Almeida MA, Bernardes-Engemann AR, Almeida-Paes R, de Macedo PM, Zancopé-Oliveira RM. The historical burden of sporotrichosis in Brazil: a systematic review of cases reported from 1907 to 2020. Braz J Microbiol. 2022;53:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Queiroz-Telles F, Bonifaz A, Cognialli R, Lustosa BPR, Aparecida Vicente V, Ramírez-Marín HA. Sporotrichosis in children: case series and narrative review. Curr Fungal Infec Rep. 2022;33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health of Brazil. Guide to health surveillance [in Portuguese]. 5th ed. Brasilia (Brazil): Ministry of Health of Brazil; 2022. [Google Scholar]
- 25.Cognialli R, Bloss K, Weiss I, Caceres DH, Davis R, Queiroz-Telles F. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Andrade Galliano Daros Bastos F, Raimundo Cognialli RC, Rodrigues de Farias M, Dos Santos Monti F, Wu K, Queiroz-Telles F. Spread of Sporothrix spp. through respiratory droplets from infected cats: a potential route of transmission. Med Mycol. 2022;60:myac079. [DOI] [PubMed]
- 27.Viezzer J, Moraes E, Biondi D, Martini A, Scarano F. Green areas, population and income in Curitiba, PR, Brasil [in Portuguese]. Rev Soc Bras Arb Urb. 2022;17:37–49. [Google Scholar]
- 28.Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi (Basel). 2019;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munhoz LS, Poester VR, Severo CB, Trápaga MR, Madrid IM, Benelli JL, et al. Update of the epidemiology of the sporotrichosis epidemic in the state of Rio Grande do Sul, Brazil. Mycoses. 2022;65:1112–8. [DOI] [PubMed] [Google Scholar]
- 30.Alzuguir CLC, Pereira SA, de Avelar Figueiredo Mafra Magalhães, Almeida-Paes R, Freitas DFS, Oliveira LFA, et al. Geo-epidemiology and socioeconomic aspects of human sporotrichosis in the municipality of Duque de Caxias, Rio de Janeiro, Brazil, between 2007 and 2016. Trans R Soc Trop Med Hyg. 2020;114:99–106. [DOI] [PubMed] [Google Scholar]
- 31.Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Oliveira Bento A, de Sena Costa AS, Lima SL, do Monte Alves M, de Azevedo Melo AS, Rodrigues AM, et al. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the northeast region. PLoS Negl Trop Dis. 2021;15:e0009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros MB, Schubach AO, Schubach TM, Wanke B, Lambert-Passos SR. An epidemic of sporotrichosis in Rio de Janeiro, Brazil: epidemiological aspects of a series of cases. Epidemiol Infect. 2008;136:1192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mialski R, de Almeida JN Jr, da Silva LH, Kono A, Pinheiro RL, Teixeira MJ, et al. Chronic meningitis and hydrocephalus due to Sporothrix brasiliensis in immunocompetent adults: a challenging entity. Open Forum Infect Dis. 2018;5:ofy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi (Basel). 2017;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orofino-Costa R, Freitas DFS, Bernardes-Engemann AR, Rodrigues AM, Talhari C, Ferraz CE, et al. Human sporotrichosis: recommendations from the Brazilian Society of Dermatology for the clinical, diagnostic and therapeutic management. An Bras Dermatol. 2022;97:757–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Queiroz-Telles F, Cognialli RC, Salvador GL, Moreira GA, Herkert PF, Hagen F. Cutaneous disseminated sporotrichosis in immunocompetent patient: case report and literature review. Med Mycol Case Rep. 2022;36:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arinelli A, Aleixo A, Freitas DFS, do Valle ACF, Almeida-Paes R, Gutierrez-Galhardo MC, et al. Ocular sporotrichosis: 26 cases with bulbar involvement in a hyperendemic area of zoonotic transmission. Ocul Immunol Inflamm. 2019;28:764–71. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira TA, Sodré CT, Costa JM, Setta CRP. Ramos-E-Silva M. Primary conjunctival sporotrichosis: an atypical presentation of the disease. JAAD Case Rep. 2018;4:497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacerda Filho AM, Cavalcante CM, Da Silva AB, Inácio CP, de Lima-Neto RG, Liete de Andrade MC, et al. High-virulence cat-transmitted ocular sporotrichosis. Mycopathologia. 2019;184:547–9. [DOI] [PubMed] [Google Scholar]
- 41.Aidar MN, Rebeschini BM, Salgado Silveira da Mata CT, Borges TC, Dos Santos Araújo MEX. The importance of considering the possibility of ocular sporotrichosis in areas with high incidence rates of sporotrichosis. Arq Bras Oftalmol. 2022;•••:S0004–27492022005006208. [DOI] [PubMed] [Google Scholar]
- 42.Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Della Terra PP, Rodrigues AM, Fernandes GF, Nishikaku AS, Burger E, Pires de Camargo Z. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Negl Trop Dis. 2017;11:e0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almeida-Paes R, de Oliveira MM, Freitas DF, do Valle AC, Zancopé-Oliveira RM, Gutierrez-Galhardo MC. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:e3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Carvalho JA, Hagen F, Fisher MC, de Camargo ZP, Rodrigues AM. Genome-wide mapping using new AFLP markers to explore intraspecific variation among pathogenic Sporothrix species. PLoS Negl Trop Dis. 2020;14:e0008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teixeira MM, Almeida-Paes R, Bernardes-Engemann AR, Nicola AM, de Macedo PM, Valle ACF, et al. Single nucleotide polymorphisms and chromosomal copy number variation may impact the Sporothrix brasiliensis antifungal susceptibility and sporotrichosis clinical outcomes. Fung Gen Biol. 2022;163:103743. [DOI] [PubMed] [Google Scholar]
- 47.Huang M, Ma Z, Zhou X. Comparative genomic data provide new insight on the evolution of pathogenicity in Sporothrix species. Front Microbiol. 2020;11:565439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchotene KO, Madrid IM, Klafke GB, Bergamashi M, Della Terra PP, Rodrigues AM, et al. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–8. [DOI] [PubMed] [Google Scholar]
- 49.Valeriano CAT, Ferraz CE, Marques Evangelista Oliveira M, Inácio CP, de Oliveira EP, Lacerda AM, et al. Cat-transmitted disseminated cutaneous sporotrichosis caused by Sporothrix brasiliensis in a new endemic area: case series in the northeast of Brazil. JAAD Case Rep. 2020;6:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrade EHP, Bastos CV, Silva AVD, Moreira SM, Costa TGA, Salvato LA, et al. Household outbreak of sporotrichosis: towards the One Health approach. Rev Soc Bras Med Trop. 2022;55:e0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information from study of patients with cat-transmitted sporotrichosis Curitiba, Brazil.


