Abstract
Transmission of dermatophytes, especially Trichophyton mentagrophytes genotype VII, during sexual intercourse has been recently reported. We report 13 such cases in France. All patients were male; 12 were men who have sex with men. Our findings suggest sexual transmission of this pathogen within a specific population, men who have sex with men.
Keywords: Tinea, sexually transmitted infections, Trichophyton mentagrophytes, monkeypox, HIV, terbinafine, dermatophytosis, fungi, viruses, France
Dermatophytes are keratinophilic fungi responsible for frequent skin infections. They are transmitted either by direct contact from an infected host (human or animal) to a receptive host or from the environment. In 2002, two surveys reported cases of tinea cruris infection in sex workers, raising the hypothesis of sexual transmission of dermatophytes (1,2). In 2009, transmission of Trichophyton mentagrophytes, responsible for tinea genitalis, between a heterosexual couple was reported (3). Subsequently, a specific internal transcribed spacer (ITS) genotype of T. mentagrophytes, genotype VII (TMVII), was reported for cases of suspected sexual transmission, most frequently tinea genitalis (4–7). In some cases, a temporal association was demonstrated between the appearance of lesions and sexual intercourse between occasional partners, especially sex workers in Southeast Asia (4,8). Moreover, similar lesions were repeatedly documented in sex partners of infected patients (4,6). Unlike other T. mentagrophytes genotypes, TMVII has not been reported in association with dermatophytosis in children in contact with animals (9). We report 13 cases of TMVII infections, highly suspected of being sexually transmitted, diagnosed in 3 large tertiary care hospitals in Paris, France, in men who have sex with men.
The Study
During January 2021–September 2022, for all strains that could correspond to T. mentagrophytes or T. indotineae that were isolated at La Pitié-Salpêtrière and Saint-Antoine Hospitals in Paris, we sequenced the ITS1–5.8S-ITS2 region for species identification and genotype determination (10). At Saint-Louis Hospital in Paris, sequencing was limited to the isolates responsible for widespread dermatophytosis. For all cases of confirmed TMVII infection, we retrieved the medical records.
Of the 13 cases of TMVII infection, the first was detected in March 2021, and 9 were diagnosed during June–September 2022. All patients were male; median age was 39 (22–59) years (Table 1). Five patients had a single skin lesion, and others had multiple lesions. One patient had inguinal papules and nodules suggestive of Majocchi granulomas, 2 had highly inflammatory folliculitis of the beard (kerion), and the others had typical erythemato-squamous lesions with an active border (Figure).
Table 1. Main epidemiologic and clinical features of 13 cases of Trichophyton mentagrophytes genotype VII infections diagnosed in Paris, France, 2021–2022*.
| Pt no. | Age, y | HIV+ | PrEP | STI history |
Travel | Tinea genitalis |
Tinea glutealis |
Tinea corporis | Tinea faciei/barbae | Prior treatment | T. mentagrophytes treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1† |
45 |
No |
Yes |
Ng, Ct, Mg |
No |
No |
Yes |
Yes |
Yes |
No |
TRB 1 mo |
| 2 |
34 |
No |
Yes |
Ng |
EE |
No |
Yes |
Yes |
Yes |
ECZ, TS |
TRB 5 d, then ITR 200 mg 1 mo, then ITR 100 mg 1 mo |
| 3 |
28 |
No |
No |
ND |
ND |
Yes |
No |
Yes |
No |
No |
TRB 4 mo + BFZ 1 mo |
| 4 |
59 |
Yes |
NA |
Ng, Ct, Mg, Tp, HCV |
No |
Yes |
Yes |
Yes |
Yes |
No |
TRB 2 mo + ECZ |
| 5‡ |
39 |
Yes |
NA |
Tp |
ND |
Yes |
Yes |
Yes |
Yes |
No |
TRB + CPX 3 wk |
| 6‡ |
41 |
Yes |
NA |
Tp |
ND |
No |
Yes |
Yes |
Yes |
No |
TRB + CPX 3 wk |
| 7 |
40 |
No |
Yes |
Ng, Ct, Tp |
No |
No |
No |
No |
Yes |
PRI + MPC |
TRB 6 wk |
| 8 |
48 |
Yes |
NA |
Ng, Ct, Tp, Ss |
No |
No |
No |
Yes |
No |
No |
CPX 4 wk |
| 9‡ |
26 |
Yes |
NA |
Ng, Ct, Tp |
ND |
Yes |
Yes |
Yes |
No |
No |
ECZ 6 wk |
| 10ठ|
35 |
No |
Yes |
Tp |
ND |
No |
No |
Yes |
No |
No |
ECZ 6 wk |
| 11§ |
22 |
No |
Yes |
Ng, Ct, Tp |
DE |
No |
Yes |
Yes |
Yes |
AMX then FLC |
TRB 4 wk |
| 12 |
35 |
Yes |
NA |
Ng,Tp |
IN |
No |
No |
Yes |
No |
TS then CPX |
BFZ 4 wk |
| 13 | 46 | Yes | NA | Ng, Ct, Tp, Ss | ES | No | No | No | Yes | FCD + TS then FCD alone then PRI then AMX/CLAV |
ITR 100 mg 2 d, then IV VRC 10 d, then TRB |
*AMX, amoxicillin; BFZ, bifonazole; CLAV, clavulanic acid; CPX, ciclopirox olamine; Ct, Chlamydia trachomatis; DE, German; ECZ, econazole; EE, Estonia; ES, Spain; FCD, fucidin; FLC, fluconazole; HCV, hepatitis C virus; IN, India; ITR, itraconazole; Mg, Mycoplama genitalium; MPC, mupirocin; NA, not applicable; ND, no data; Ng; Neisseria gonorrheae; PrEP, pre-exposure prophylaxis; PRI, pristinamycin; Pt, patient; Ss, Sarcoptes scabiei; Tp, Treponema pallidum; TRB, oral terbinafine (250 mg 1×/d); TS, topical steroids; VRC, voriconazole. †Patient 1 strain was included in a previous survey (11). ‡Patients 5 and 6 and patients 9 and 10 were partners. §Patients 10 and 11 were co-infected with monkeypox virus.
Figure.
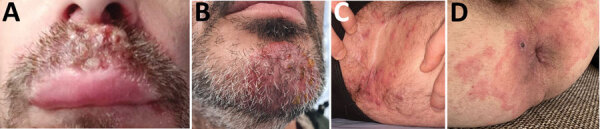
Clinical appearance of Trichohpyton mentagrophytes genotype VII infections in men in France, 2022. A, B) Swollen lesions of the mustache (A) and beard (kerions) (B). C) Papular and nodular inguinal lesions. D) Peri-anal mpox lesions with associated papules and pustules with central umbilication and a large lesion with a central necrotic crust, surrounded by extensive erythemato-squamous circinate lesions caused by TMVII infection.
Of the 13 patients, 11 reported having sexual relations exclusively with men and 1 reported having sexual intercourse with men and women. At least 9 had multiple sex partners in the month before lesion onset. Seven patients were HIV positive, and 5 were taking HIV preexposure prophylaxis. Apart from 1 patient who had recently discontinued treatment, all HIV-positive patients were receiving long-term effective antiretroviral treatment. Twelve patients had previously experienced sexually transmitted infections (STIs) other than HIV. STI testing was performed for 8 patients, leading to detection of Chlamydia trachomatis DNA in an anal sample for 1 patient and a syphilis diagnosis for 1 patient. Two patients were co-infected with monkeypox virus, and mpox developed in another patient 1 month after the dermatophytosis diagnosis. For 1 patient, TMVII infection and mpox lesions appeared concurrently in the peri-anal region, suggesting transmission of both agents at the same time (Figure).
Four patients denied any travel outside of France, 1 patient was infected in Germany (Munich) where he lived, and for 3 others, lesions developed after they had returned from travel (to Slovenia, Spain, and India). Only 3 patients reported having had contact with animals (cats or dogs). For 2 patients, both sex partners had documented TMVII infection; for 2 others, contamination from sex partners who had similar skin lesions was suspected. Likewise, 2 patients reported secondary appearance of lesions on the skin of sex partners. However, infection was not medically confirmed.
The median time between lesion appearance and hospital consultation was 28 (range 7–102) days. Five patients had previously received antibiotic, antifungal, or topical steroid treatments. Nine patients received systemic antifungal treatment (terbinafine, itraconazole, or voriconazole) for 3 weeks to 4 months; the others received only topical treatment. One patient with beard kerion and bacterial superinfection (Klebsiella aerogenes) was hospitalized. Among the 13 patients, 10 recovered while receiving antifungal therapy, 1 was still receiving treatment at most recent follow-up, and the 2 others were not available for further follow-up. At least 3 patients experienced postinflammatory pigmentation; 2 others had scars or beard hair loss.
During the study period, of 70 strains sequenced, the predominant identified agent was T. indotineae (n = 53), followed by TMVII (n = 13) (GenBank accession no. OK632215, ON740661, OP876812–22). The remaining strains corresponded to T. mentagrophytes genotype II*.
Conclusions
For most patients in this series, sexually transmitted dermatophytosis was likely. This hypothesis is supported by the sites of the infection (external genitalia, buttocks, face), the high-risk STI profile of the patients, and consistent identification of TMVII. Lack of animal contact for most patients also suggests human-to-human transmission. Moreover, although T. mentagrophytes is considered to be a zoophilic species, no animal-to-human transmission has been documented for TMVII and only 1 strain has been isolated from an animal (cat) (6). All 13 patients were male, 12 of whom were men who have sex with men. Even if we cannot exclude recruitment bias, the study suggests active circulation of the pathogen within this population. Previous studies reported TMVII infections in men and women whose sexual practices were either heterosexual or not mentioned. Our series demonstrates that TMVII infected the same population of patients as did the monkeypox virus during the 2022 outbreak (12). Contrary to previous reports (4–6), intimate shaving was not associated with TMVII infection in our case series.
Patients might have acquired TMVII infections in France or internationally, supporting the hypothesis of active circulation of TMVII in Europe, in line with the 51 cases reported since 2014 (Table 2). Southeast Asia might have been the starting point of pathogen spread, as suggested by the first reported cases in Europe being associated with travel to that region (4–6,8). Of 37 cases of TMVII infection reported in Berlin, Germany, over 18 months (January 2016–July 2017), only a small proportion of documented cases was associated with travel outside Germany, suggesting that TMVII was already circulating in Europe (6).
Table 2. Cases of Trichophyton mentagrophytes genotype VII infections in Europe, 2014–2019*.
| Reference | Year | Location | Age, y/sex | No. cases | Tinea genitalis |
Tinea corporis/ inguinalis | Tinea faciei/ barbae |
Tinea glutealis |
Comments |
|---|---|---|---|---|---|---|---|---|---|
| (4) |
2014 | Zurich, Switzerland |
27/F | NA | Yes | Yes | No | No | Sexual intercourse 1–2 wk before symptom onset, Southeast Asia; similar lesions in 1 sex partner |
| 2014 | 24/M | NA | Yes | Yes | No | No | |||
| 2014 |
31/M |
NA |
Yes |
No |
No |
No |
|||
| (13) |
2014 |
St. Petersburg, Russia |
ND |
NA |
No |
Yes |
No |
No |
None |
| (5) |
ND |
Germany |
ND/F |
NA |
Yes |
Yes |
No |
No |
Lesions appeared after return from travel in Egypt; indirect transmission was suspected |
| (8) |
ND |
Germany |
ND/M |
NA |
No |
No |
Yes |
No |
Sexual intercourse with female sex workers in Thailand; secondary case in wife’s patient
(tinea faciei) |
| (6) |
2016– 2017 |
Berlin, Germany |
20–40/ mostly young men |
37 |
Most frequent presentation
was tinea genitalis |
4/18 persons traveled in Southeast Asia 5/18 persons had sex partner with similar lesions |
|||
| (9) |
2016 | Zurich, Switzerland |
21/M | NA | Yes | Yes | Yes | No | None |
| 2016 | 33/F | NA | Yes | Yes | No | No | |||
| 2018 | 65/M | NA | No | No | Yes | No | |||
| 2018 |
50/M |
NA |
No |
No |
Yes |
No |
|||
| (7) | 2010– 2019 | Athens, Greece |
ND | 4 | Yes | No | No | No | None |
*NA, not applicable; ND, no data.
Our case series is characterized by a substantial delay in diagnosis. Patients with the most inflammatory lesions were initially mistakenly believed to have had bacterial infections (4,8). Moreover, prolonged systemic antifungal treatments and patient hospitalizations highlight how severe TMVII infections can be (4–6,8). Therefore, healthcare professionals should be aware of the various features of TMVII infection and perform targeted mycological samplings. In contrast to findings for T. indotineae (14), no terbinafine resistance has been reported for TMVII. Of note, during the study period, T. indotineae, which was recently described as an emerging species of dermatophyte in Paris, responsible for difficult-to-treat tinea (14), was the main agent of skin dermatophytosis caused by T. mentagrophytes complex species.
Transmission of dermatophytes during sexual intercourse is an example of direct human-to-human transmission, as previously described for combat sports (tinea gladiatorum) (15). Sexual transmission should be suspected for patients with STI risk factors and tinea corporis, tinea genitalis, tinea glutealis, or tinea faciei. Diagnosis of sexually transmitted dermatophytosis should lead to exhaustive STI screening of patients and their sex partners.
Biography
Dr. Jabet is a medical mycologist with a specific research interest in emerging fungal infections and dermatophytosis.
Footnotes
Suggested citation for this article: Jabet A, Dellière S, Seang S, Chermak A, Schneider L, Chiarabini T, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023 Jul [date cited]. https://doi.org/10.3201/eid2907.230025
References
- 1.Bakare RA, Oni AA, Umar US, Adewole IF, Shokunbi WA, Fayemiwo SA, et al. Pattern of sexually transmitted diseases among commercial sex workers (CSWs) in Ibadan, Nigeria. Afr J Med Med Sci. 2002;31:243–7. [PubMed] [Google Scholar]
- 2.Otero L, Palacio V, Vázquez F. Tinea cruris in female prostitutes. Mycopathologia. 2002;153:29–31. 10.1023/A:1015257320824 [DOI] [PubMed] [Google Scholar]
- 3.Mølenberg D, Deleuran M, Sommerlund M. Connubial tinea gladiatorum due to Trichophyton mentagrophytes. Mycoses. 2010;53:533–4. 10.1111/j.1439-0507.2009.01734.x [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger I, Bosshard PP, Kasper RS, Reinhardt D, Lautenschlager S. Tinea genitalis: a new entity of sexually transmitted infection? Case series and review of the literature. Sex Transm Infect. 2015;91:493–6. 10.1136/sextrans-2015-052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nenoff P, Schubert K, Jarsumbeck R, Uhrlaß S, Krüger C. Tinea genitalis profunda durch Trichophyton mentagrophytes nach Ägypten-Reise. Akt Dermatol. 2017;43:146–53. 10.1055/s-0043-106149 [DOI] [Google Scholar]
- 6.Kupsch C, Czaika V, Deutsch C, Gräser Y. Trichophyton mentagrophytes—a new genotype of zoophilic dermatophyte causes sexually transmitted infections. J Dtsch Dermatol Ges. 2019;17:493–501. 10.1111/ddg.13776 [DOI] [PubMed] [Google Scholar]
- 7.Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J Fungi (Basel). 2021;7:419. 10.3390/jof7060419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendrock-Shiga G, Mechtel D, Uhrlaß S, Koch D, Krüger C, Nenoff P. [Tinea barbae profunda due to Trichophyton mentagrophytes after journey to Thailand : Case report and review] [in German]. Hautarzt. 2017;68:639–48. 10.1007/s00105-017-4008-2 [DOI] [PubMed] [Google Scholar]
- 9.Klinger M, Theiler M, Bosshard PP. Epidemiological and clinical aspects of Trichophyton mentagrophytes/Trichophyton interdigitale infections in the Zurich area: a retrospective study using genotyping. J Eur Acad Dermatol Venereol. 2021;35:1017–25. 10.1111/jdv.17106 [DOI] [PubMed] [Google Scholar]
- 10.Taghipour S, Pchelin IM, Zarei Mahmoudabadi A, Ansari S, Katiraee F, Rafiei A, et al. Trichophyton mentagrophytes and T interdigitale genotypes are associated with particular geographic areas and clinical manifestations. Mycoses. 2019;62:1084–91. 10.1111/myc.12993 [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Sabater A, Normand AC, Bidaud AL, Cremer G, Foulet F, Brun S, et al. Terbinafine resistance in dermatophytes: a French multicenter prospective study. J Fungi (Basel). 2022;8:220. 10.3390/jof8030220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. ; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–91. 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
- 13.Pchelin IM, Zlatogursky VV, Rudneva MV, Chilina GA, Rezaei-Matehkolaei A, Lavnikevich DM, et al. Reconstruction of phylogenetic relationships in dermatomycete genus Trichophyton Malmsten 1848 based on ribosomal internal transcribed spacer region, partial 28S rRNA and beta-tubulin genes sequences. Mycoses. 2016;59:566–75. 10.1111/myc.12505 [DOI] [PubMed] [Google Scholar]
- 14.Jabet A, Brun S, Normand AC, Imbert S, Akhoundi M, Dannaoui E, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229–33. 10.3201/eid2801.210883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kermani F, Moosazadeh M, Hosseini SA, Bandalizadeh Z, Barzegari S, Shokohi T. Tinea gladiatorum and dermatophyte contamination among wrestlers and in wrestling halls: a systematic review and meta-analysis. Curr Microbiol. 2020;77:602–11. 10.1007/s00284-019-01816-3 [DOI] [PubMed] [Google Scholar]


