Abstract
Hospitalizations involving fungal infections increased 8.5% each year in the United States during 2019–2021. During 2020–2021, patients hospitalized with COVID-19–associated fungal infections had higher (48.5%) in-hospital mortality rates than those with non–COVID-19–associated fungal infections (12.3%). Improved fungal disease surveillance is needed, particularly during respiratory virus pandemics.
Keywords: fungi, invasive fungal infections, mycoses, hospitalizations, aspergillosis, candidiasis, blastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, mucormycosis, pneumonia, Pneumocystis, international classification of diseases, COVID-19, respiratory infections, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, SARS, coronavirus disease, zoonoses, viruses, coronavirus, United States
In the United States, fungal infections impose considerable healthcare costs (≈$6.7 billion during 2018) and cause substantial illness and death (>7,000 deaths during 2021) (1,2). Fungi causing serious infections include yeasts, such as Candida, Cryptococcus, and Pneumocystis spp.; molds, such as Aspergillus spp. and Mucorales; and dimorphic fungi, such as Blastomycoses, Coccidioides, and Histoplasma spp. (2). Risk factors vary but generally are associated with environmental exposures; underlying immunocompromising conditions, such as solid organ or stem cell transplantation, cancer, and immunosuppressive medications; and critical illness (2–4).
COVID-19 infection is a substantial risk factor for certain fungal infections, particularly those caused by invasive molds, likely because of COVID-19–related immune system dysregulation and immunosuppressive therapies, such as corticosteroids or other immunomodulatory medications (3). US vital statistics data showed that deaths from fungal infections increased during the COVID-19 pandemic (2). However, additional data on fungal infections during the pandemic, including hospitalization rates and healthcare utilization, are lacking because many fungal diseases are not reportable in the United States (https://www.cdc.gov/fungal/fungal-disease-reporting-table.html). Those data might help inform public health planning and clinical practice. Therefore, we analyzed a large healthcare services database to determine rates, patient demographic features, and healthcare utilization for fungal infection–related hospitalizations during the COVID-19 pandemic.
The Study
The Premier Healthcare Database, Special COVID-19 Release (PHD-SR), is a US, hospital-based, all-payer database used by the Centers for Disease Control and Prevention to inform COVID-19 response activities (5,6). The database contains deidentified records from >1,000 nongovernment, community, and teaching hospitals that contributed inpatient data during the analytic period. We used diagnosis codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), listed for each hospitalization and identified hospitalizations involving fungal infections (fungal hospitalizations) and COVID-19 (COVID-19 hospitalizations) during January 1, 2019–December 31, 2021 (Appendix Table 1). We defined COVID-19–associated fungal hospitalizations as those in which both a COVID-19 and fungal infection diagnosis were listed during the same hospitalization.
We estimated annual hospitalization rates (per 10,000 population) by fungal infection type and calculated average annual percentage change during 2019–2021. For COVID-19–associated fungal hospitalizations (2020–2021 only), we calculated hospitalization rates per 10,000 COVID-19 hospitalizations. We stratified 2020–2021 fungal hospitalizations by COVID-19 association and fungal infection type and compared patient demographics, US hospital census regions and urban–rural classifications (https://www.cdc.gov/nchs/data_access/urban_rural.htm), lengths of hospital stays, intensive care unit (ICU) admissions, invasive mechanical ventilation (IMV) receipt, and in-hospital deaths. We assessed annual trends in fungal hospitalizations by using Cochran-Armitage tests and compared fungal hospitalizations according to COVID-19 status by using χ2 tests.
During 2019–2021, a total of 59,212 fungal hospitalizations were identified in the PHD-SR. Rates of fungal hospitalizations (per 10,000 hospitalizations) increased from 22.3 in 2019 to 25.0 in 2020 and 26.8 in 2021 (p<0.01), representing an average annual percentage change of 8.5% (Table 1). Average annual rates of hospitalization significantly increased for each fungal infection, except for those caused by Pneumocystis spp., Cryptococcus spp., and other specified fungi (Table 1).
Table 1. Hospitalization rates for invasive fungal infections associated with COVID-19 in study of increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021*.
| Fungal pathogen | All fungal hospitalizations, n = 59,212 |
COVID-19–associated hospitalizations,† n = 5,288 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | p value‡ | % Change§ | 2020 | 2021 | p value‡ | % Change¶ | |
| Pathogenic fungi | 22.3 | 25.0 | 26.8 | <0.01 | 8.5 | 43.1 | 57.4 | <0.01 | 24.9 |
| Candida | 4.2 | 5.3 | 5.6 | <0.01 | 12.4 | 11.2 | 10.9 | 0.69 | −2.4 |
| Aspergillus | 4.3 | 4.2 | 5.3 | <0.01 | 9.4 | 7.9 | 18.9 | <0.01 | 58.2 |
| Coccidioides | 3.2 | 4.0 | 4.3 | <0.01 | 13.0 | 6.6 | 7.4 | 0.15 | 10.4 |
| Pneumocystis | 2.6 | 2.4 | 2.5 | 0.08 | −2.7 | 1.9 | 2.6 | 0.03 | 25.4 |
| Histoplasma | 1.4 | 1.6 | 1.6 | <0.01 | 5.6 | 1.1 | 1.6 | 0.03 | 32.1 |
| Cryptococcus | 1.3 | 1.4 | 1.2 | 0.46 | −2.0 | 1.2 | 1.4 | 0.48 | 11.6 |
| Blastomyces | 0.3 | 0.3 | 0.4 | <0.01 | 11.7 | 0.2 | 0.5 | <0.01 | 65.6 |
| Mucorales species | 0.3 | 0.3 | 0.4 | <0.01 | 17.9 | 0.7 | 1.1 | 0.02 | 39.8 |
| Other specified fungi | 1.5 | 1.6 | 1.5 | 0.38 | −1.9 | 1.7 | 2.5 | <0.01 | 32.9 |
| Unspecified | 4.0 | 4.9 | 5.1 | <0.01 | 10.8 | 12.2 | 12.7 | 0.47 | 4.1 |
*Rates are per 10,000 hospitalizations. The total number of hospitalizations per year was 8,884,472 in 2019, 7,640,470 in 2020 (424,475 COVID-19–associated), and 7,559,882 in 2021 (601,831 COVID-19–associated). Patients could have >1 fungal infection in a given hospitalization, which occurred for <5% of hospitalizations. †Hospitalizations of patients with COVID-19–associated fungal infections. ‡Calculated by using Cochran-Armitage tests. §Average annual percentage change during 2019–2021. ¶Annual percentage change during 2020–2021.
During 2020–2021, a total of 5,288 (13.4%) of 39,423 fungal hospitalizations were COVID-19–associated. Rates of COVID-19–associated fungal hospitalizations (per 10,000 COVID-19 hospitalizations) increased by 24.9% (43.1% to 57.4%; p<0.01). Annual rates increased significantly for COVID-19–associated fungal hospitalizations involving blastomycosis (0.2 to 0.5 [65.6% change]; p<0.01), aspergillosis (7.9 to 18.9 [58.2% change]; p<0.01), mucormycosis (0.7 to 1.1 [39.8% change]; p = 0.02), histoplasmosis (1.1 to 1.6 [32.1% change]; p = 0.03), pneumocystosis (1.9 to 2.6 [25.4% change]; p = 0.03), and other specified mycoses (1.7 to 2.5 [32.9% change]; p<0.01). Compared with non–COVID-19–associated fungal hospitalizations, COVID-19–associated fungal hospitalizations more frequently involved aspergillosis (27.8% vs. 16.9%; p<0.01), mucormycosis (1.8% vs. 1.4%; p = 0.03), and unspecified mycoses (24.3% vs. 18.5%; p<0.01) and, in general, less frequently involved other fungal infection types (Table 2).
Table 2. Hospitalizations for fungal infections according to COVID-19 status in study of increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021*.
| Fungal pathogen | Any fungal infection | COVID-19–associated | Non–COVID-19–associated | p value |
|---|---|---|---|---|
| Total no. | 39,423 | 5,288 | 34,135 | NA |
| Candida | 8,289 (21.0) | 1,135 (21.5) | 7,154 (21.0) | 0.40 |
| Aspergillus | 7,248 (18.4) | 1,471 (27.8) | 5,777 (16.9) | <0.01 |
| Coccidioides | 6,278 (15.9) | 723 (13.7) | 5,555 (16.3) | <0.01 |
| Pneumocystis | 3,718 (9.4) | 235 (4.4) | 3,483 (10.2) | <0.01 |
| Histoplasma | 2,386 (6.1) | 139 (2.6) | 2,247 (6.6) | <0.01 |
| Cryptococcus | 2,022 (5.1) | 138 (2.6) | 1,884 (5.5) | <0.01 |
| Blastomyces | 543 (1.4) | 41 (0.8) | 502 (1.5) | <0.01 |
| Mucorales species | 569 (1.4) | 94 (1.8) | 475 (1.4) | 0.03 |
| Other specified fungi | 2,300 (5.8) | 221 (4.2) | 2,079 (6.1) | <0.01 |
| Unspecified fungi | 7,599 (19.3) | 1,286 (24.3) | 6,313 (18.5) | <0.01 |
*Values are no. (%) hospitalizations except as indicated. Patients could have >1 fungal infection in a given hospitalization, which occurred for <5% of hospitalizations. p values were calculated by using χ2 tests comparing COVID-19–associated and non–COVID-19–associated fungal hospitalizations. NA, not applicable.
Median patient age was 63 (interquartile range [IQR] 52–72) years for COVID-19–associated hospitalizations versus 61 (IQR 46–72) years for non–COVID-19–associated hospitalizations (p<0.01) (Figure). Compared with hospitalizations of patients with non–COVID-19–associated fungal infections, hospitalizations of patients with COVID-19–associated fungal infections more frequently involved those who were male (59.9% vs. 57.5%; p<0.01) and Hispanic/Latino (18.8% vs. 11.7%; p<0.01); occurred in hospitals located in the western United States (29.1% vs. 27.5%; p<0.01); involved longer hospital stays (21 [IQR 11–35] days vs. 9 [IQR 4–17] days; p<0.01); and involved ICU-level care (70.0% vs. 35.5%; p<0.01), IMV receipt (64.4% vs. 22.5%; p<0.01), and increased in-hospital deaths (48.5% vs. 12.3%; p<0.01) (Table 3).
Figure.
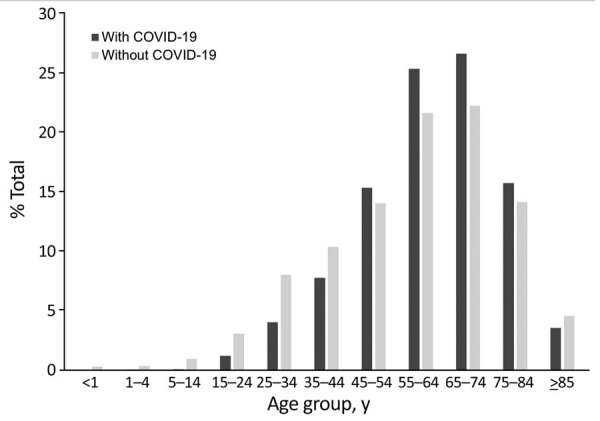
Age distribution of patients in study of increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021. Hospitalizations for fungal infections were COVID-19–associated (n = 5,288) or non–COVID-19–associated (n = 34,135).
Table 3. Demographic features and outcomes of patients with fungal infections by COVID-19 status in study of increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021*.
| Characteristics | Any fungal infection | COVID-19–associated | Non–COVID-19–associated | p value† |
|---|---|---|---|---|
| Total no. hospitalizations |
39,423 |
5,288 |
34,135 |
NA |
| Median age, y (range) |
61 (47–72) |
63 (52–72) |
61 (46–72) |
<0.01 |
| Sex‡ | ||||
| M | 22,779 (57.8) | 3,166 (59.9) | 19,613 (57.5) | <0.01 |
| F |
16,634 (42.2) |
2,121 (40.1) |
14,513 (42.5) |
<0.01 |
| Race/ethnicity | ||||
| White, NH | 18,359 (46.6) | 2,131 (40.3) | 16,228 (47.5) | <0.01 |
| Black, NH | 5,907 (15.0) | 771 (14.6) | 5,136 (15.0) | 0.38 |
| Hispanic or Latino | 5,002 (12.7) | 993 (18.8) | 4,009 (11.7) | <0.01 |
| Asian, NH | 878 (2.2) | 106 (2.0) | 772 (2.3) | 0.24 |
| Other, NH | 1,331 (3.4) | 210 (4.0) | 1,121 (3.3) | 0.01 |
| Unknown |
7,946 (20.2) |
1,077 (20.4) |
6,869 (20.1) |
0.68 |
| US census region | ||||
| South | 16,241 (41.2) | 2,141 (40.5) | 14,100 (41.3) | 0.26 |
| West | 10,914 (27.7) | 1,538 (29.1) | 9,376 (27.5) | 0.01 |
| Midwest | 7,385 (18.7) | 943 (17.8) | 6,442 (18.9) | 0.07 |
| Northeast |
4,883 (12.4) |
666 (12.6) |
4,217 (12.4) |
0.62 |
| Urban location |
35,938 (91.2) |
4,801 (90.8) |
31,137 (91.2) |
0.31 |
| Healthcare utilization and outcomes | ||||
| Median length of stay, d (range) | 10 (5–20) | 21 (11–35) | 9 (4–17) | <0.01 |
| ICU-level care | 15,808 (40.1) | 3,703 (70.0) | 12,105 (35.5) | <0.01 |
| IMV receipt | 11,076 (28.1) | 3,407 (64.4) | 7,669 (22.5) | <0.01 |
| In-hospital death | 6,758 (17.1) | 2,566 (48.5) | 4,192 (12.3) | <0.01 |
*Values are no. (%) patients except as indicated. ICU, intensive care unit; IMV, invasive mechanical ventilation; NA, not applicable; NH, not Hispanic or Latino. †p values were calculated by using χ2 tests for categorical variables or Wilcoxon rank-sum tests for continuous variables, comparing COVID-19–associated and non-COVID-19–associated hospitalizations for fungal infections. Given the heterogeneous nature of fungal infections and their associated risk factors, underlying medical conditions were not analyzed. ‡Sex was unknown for 10 hospitalizations (1 COVID-19–associated, 9 non-COVID-19–associated). Those data were excluded from the χ2 test calculation.
Longer hospital stays, higher ICU admission rates, more IMV receipts, and more deaths were generally observed for hospitalizations caused by COVID-19–associated fungal infections than for non–COVID-19–associated fungal infections, regardless of the specific fungal pathogens involved (Appendix Tables 2, 3). COVID-19–associated fungal hospitalizations with the highest percentages of deaths involved aspergillosis (57.6%), invasive candidiasis (55.4%), mucormycosis (44.7%), and unspecified mycoses (59.0%).
Conclusions
Analysis of a large US healthcare services database indicated that hospitalization rates involving fungal infections increased significantly during 2019–2021, primarily driven by hospitalizations of patients with COVID-19–associated fungal infections. During 2020–2021, a total of 13.4% of fungal hospitalizations were COVID-19–associated, and COVID-19–associated fungal infections were associated with ≈2-fold increase in ICU admission rates and ≈4-fold increase in in-hospital death rates compared with non–COVID-19–associated fungal hospitalizations. Consistent with national mortality data, hospitalizations of patients with COVID-19–associated (compared with non–COVID-19–associated) fungal infections most often involved invasive candidiasis and aspergillosis and disproportionately occurred among non-White male patients in the western United States (2). Racial or ethnic disparities observed for fungal infection–associated hospitalization rates might relate to longstanding inequities in social health determinants, such as lack of access to medical care or occupational exposures, and prevalence of underlying conditions, such as diabetes, that might increase fungal and COVID-19 infection risk among certain minority groups (2,7–9). Also consistent with national mortality data, hospitalization rates for COVID-19–associated aspergillosis and mucormycosis increased from 2020 to 2021 (2), likely reflecting a greater burden of COVID-19 during 2021 than 2020 (https://gis.cdc.gov/grasp/covidnet/covid19_5.html), increased clinician awareness and testing for COVID-19–associated mold infections (10,11), and increased use of corticosteroids for COVID-19 treatment, a major risk factor for aspergillosis and mucormycosis (4). Our findings emphasize the importance of maintaining a high index of clinical suspicion for fungal infections in patients at high risk, including those with COVID-19, and the need for increased fungal disease surveillance to detect and evaluate emerging trends.
The first limitation of our study is that, although ICD-10-CM codes for COVID-19 correlate well with SARS-CoV-2 test results in PHD-SR data (12), fungal ICD-10-CM codes might be associated with underreporting, misclassification, and nonspecific coding of pathogenic fungi, particularly those causing candidemia and invasive mold disease (13–15). Second, PHD-SR data are broadly representative of US hospitals, and hospital types remained relatively consistent during the analytic period. However, data might overrepresent certain regions of the country, particularly the South, and participating hospitals can vary over time. Finally, we suspect that most COVID-19–associated fungal infections were secondary complications of COVID-19 because of the natural history of fungal disease in patients with respiratory infections (3), but we could not verify this supposition by using PHD-SR data.
Our analysis underscores the substantial burden of patient hospitalizations with fungal infections in the United States and indicates that increased hospitalizations involving fungal infections occurred during the COVID-19 pandemic. As the COVID-19 pandemic evolves, and to increase preparedness for future infectious diseases outbreaks, comprehensive public health surveillance for fungal diseases is needed to characterize disease epidemiology and guide efforts to prevent illness and death.
Additional information for increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021.
Acknowledgments
We thank Tegan Boehmer, Lara Bull, Cheryl Cornwell, and Carol Yen-Chin Lin for their guidance and assistance in developing this analytic project.
This activity was reviewed by the Centers for Disease Control and Prevention and conducted in accordance with its policies and applicable federal law (45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Biography
Dr. Gold is a medical epidemiologist in the Mycotic Diseases Branch, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. His research interests include the epidemiology and prevention of fungal infections.
Footnotes
Suggested citation for this article: Gold JAW, Adjei S, Gundlapalli AV, Huang YLA, Chiller T, Benedict K, et al. Increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021. Emerg Infect Dis. 2023 Jul [date cited]. https://doi.org/10.3201/eid2907.221771
References:
- 1.Rayens E, Norris KA. Prevalence and healthcare burden of fungal infections in the United States, 2018. Open Forum Infect Dis. 2022;9:ofab593. [DOI] [PMC free article] [PubMed]
- 2.Gold JAW, Ahmad FB, Cisewski JA, Rossen LM, Montero AJ, Benedict K, et al. Increased deaths from fungal infections during the coronavirus disease 2019 pandemic—National Vital Statistics System, United States, January 2020–December 2021. Clin Infect Dis. 2023;76:e255–62. 10.1093/cid/ciac489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddley JW, Thompson GR 3rd, Chen SCA, White PL, Johnson MD, Nguyen MH, et al. Coronavirus disease 2019–associated invasive fungal infection. Open Forum Infect Dis. 2021;8:ofab510. [DOI] [PMC free article] [PubMed]
- 4.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16–22. 10.1093/cid/cir865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PINC AI Applied Sciences. PINC AI healthcare data special release: COVID-19. October 2021. [2022 Dec 1]. https://offers.premierinc.com/rs/381-NBB-525/images/PHD_COVID-19_White_Paper.pdf
- 6.Lawandi A, Warner S, Sun J, Demirkale CY, Danner RL, Klompas M, et al. Suspected severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) reinfections: incidence, predictors, and healthcare use among patients at 238 US healthcare facilities, 1 June 2020 to 28 February 2021. Clin Infect Dis. 2022;74:1489–92. 10.1093/cid/ciab671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bui DP, McCaffrey K, Friedrichs M, LaCross N, Lewis NM, Sage K, et al. Racial and ethnic disparities among COVID-19 cases in workplace outbreaks by industry sector—Utah, March 6–June 5, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1133–8. 10.15585/mmwr.mm6933e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–7. 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayens E, Rayens MK, Norris KA. Demographic and socioeconomic factors associated with fungal infection risk, United States, 2019. Emerg Infect Dis. 2022;28:1955–69. 10.3201/eid2810.220391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger M, Bussini L, Hoenigl M, Bartoletti M. Prevalence of COVID-19-associated pulmonary aspergillosis: critical review and conclusions. J Fungi (Basel). 2022;8:390. 10.3390/jof8040390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. ; ECMM and ISHAM collaborators. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543–52. 10.1016/S2666-5247(21)00237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadri SS, Gundrum J, Warner S, Cao Z, Babiker A, Klompas M, et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA. 2020;324:2553–4. 10.1001/jama.2020.20323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold JAW, Revis A, Thomas S, Perry L, Blakney RA, Chambers T, et al. Clinical characteristics, health care utilization, and outcomes among patients in a pilot surveillance system for invasive mold disease—Georgia, United States, 2017–2019. Open Forum Infect Dis. 2022;9:ofac215. [DOI] [PMC free article] [PubMed]
- 14.Benedict K, Gold JAW, Jenkins EN, Roland J, Barter D, Czaja CA, et al. Low sensitivity of International Classification of Diseases, tenth revision coding for culture-confirmed candidemia cases in an active surveillance system: United States, 2019–2020. Open Forum Infect Dis. 2022;9:ofac461. [DOI] [PMC free article] [PubMed]
- 15.Benedict, K, Baggs, J, Wolford J, Jackson, BR, Gold, JAW. Hospitalizations for unspecified mycoses in a large administrative data set and implications for fungal disease burden estimates, United States, 2019–2021. Open Forum Infect Dis. 2023;10:ofad100. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information for increased hospitalizations involving fungal infections during COVID-19 pandemic, United States, January 2020–December 2021.


