Abstract
Objectives:
Approximately 1% of individuals have a hereditary cancer predisposition syndrome, however, the majority are not aware. Collecting a cancer family history (CFH) can triage patients to receive genetic testing. To rigorously assess different methods of CFH collection, we compared a web-based tool (WBT) to usual care (clinician collects CFH) in a randomized controlled trial.
Methods:
New gynecologic oncology patients (seen 9/2019–9/2021) were randomized to one of three arms in a 2:2:1 allocation ratio: 1) usual care clinician CFH collection, 2) WBT completed at home, or 3) WBT completed in office. The WBT generated a cancer-focused pedigree and scores on eight validated cancer risk models. The primary outcome was collection of an adequate CFH (based on established guidelines) with usual care versus the WBT.
Results:
We enrolled 250 participants (usual care - 110; WBT home - 105; WBT office - 35 [closed early due to COVID-19]). Within WBT arms, 109 (78%) participants completed the tool, with higher completion for office versus home (33 [94%] vs. 76 [72%], P=0.008). Among participants completing the WBT, 63 (58%) had an adequate CFH versus 5 (5%) for usual care (P<0.001). Participants completing the WBT were significantly more likely to complete genetic counseling (34 [31%] vs. 15 [14%], P=0.002) and genetic testing (20 [18%] vs. 9 [8%], P=0.029). Participant and provider WBT experience was favorable.
Conclusions:
WBTs for CFH collection are a promising application of health information technology, resulting in more comprehensive CFH and a significantly greater percentage of participants completing genetic counseling and testing.
Introduction
Approximately 1% of individuals harbor a germline pathogenic variant (mutation) that increases their lifetime risk for cancer [1]. For those at risk, genetically targeted screening, chemoprevention and/or risk-reducing surgery can decrease cancer incidence and mortality [2–4]. Population-based screening for hereditary cancer syndromes and referral of those who screen positive for genetic testing is a cost effective, evidence-based, national health priority endorsed by several organizations including the Centers for Disease Control and Prevention (Tier 1 recommendation), United States Preventative Task Force, National Academy of Medicine, National Comprehensive Cancer Center (NCCN), the American Society of Clinical Oncology (ASCO), Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) [5–26].
Under-recognition of hereditary cancer syndromes remains a critical concern, with fewer than 20% of individuals with hereditary breast and ovarian cancer and Lynch syndrome identified [1, 27–30]. Cancer family history (CFH) collection is an essential first step to identify those who may be at increased risk for hereditary cancer syndromes and triage them to genetic counseling and testing; however, CFH collection has historically been difficult to execute [31–37]. Our group and others have found that most participants do not undergo adequate CFH collection, with commonly cited barriers including lack of clinician training, limited appointment time, and limited patient knowledge of family history [38–47]. Health information technology has successfully improved clinical documentation, clinical workflows, quality of care, communication, and clinical decision support, and recent studies demonstrate the utility of health information technology in cancer genetics and CFH collection [48–50]. To our knowledge, no randomized controlled trials have evaluated outcomes associated with different mechanisms of CFH collection.
We evaluated a web-based tool (WBT) versus clinician-directed usual care for CFH collection in a prospective randomized controlled trial. We hypothesized that use of the WBT would result in improved quality of the CFH compared to usual care whereby the clinician interviews the patient to collect CFH.
Methods
Participants
Participants were eligible for this trial if they were at least 18 years, had a new patient appointment at a single academic institution’s gynecologic oncology clinic—Weill Cornell Medicine’s Gynecologic Oncology Clinic—and could read English (as the WBT was only available in English). Participants who were adopted with no knowledge of their maternal and paternal family history were excluded as this trial focused on CFH collection.
Cancer family history collection tool
The health information technology tool piloted in this study was the system known as Progeny FHQ (https://www.progenygenetics.com/). Progeny FHQ is an online application, available in English language, that allows participants to complete a questionnaire on a tablet or computer and uses the collected information to generate a cancer-focused pedigree as well as conduct eight validated cancer risk assessments (Claus, Gail, BRCAPRO, MMRPRO, MELAPRO, PANCPRO, PREMM, Tyrer-Cuzick).
Trial design
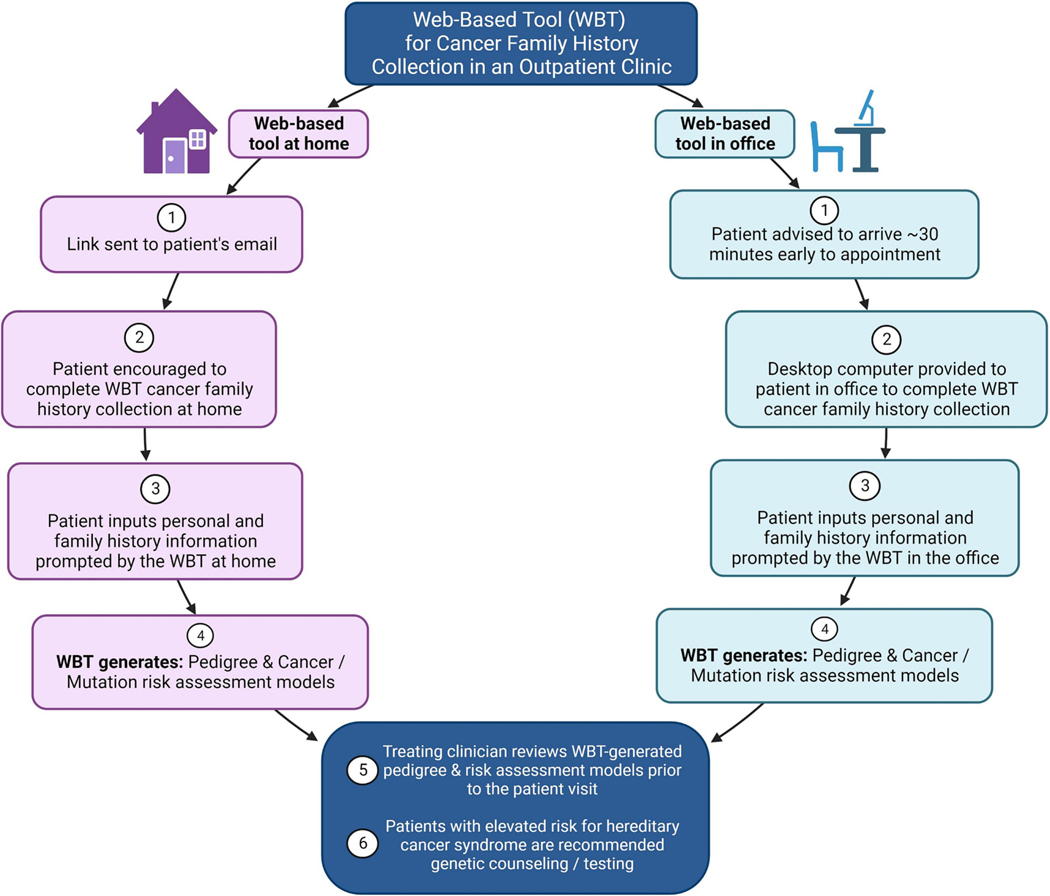
This prospective study was approved by the Weill Cornell Medical College’s Institutional Review Board and registered on ClinicalTrials.gov (NCT04890327). Participants scheduled for new patient appointments at the Weill Cornell Gynecologic Oncology Clinic were contacted by telephone prior to their scheduled appointments and offered participation in the trial. Once consented and enrolled, participants were randomized with permuted blocked randomization to one of three arms, in a 2:2:1 allocation ratio: 1) usual care – CFH collection by the clinician during the in-person clinician-patient interview; 2) WBT at home – the patient was advised by telephone that an email link to the WBT would be sent prior to the visit and encouraged to complete the tool at home; 3) WBT completed in the office – the patient was advised to arrive 30 minutes early for the scheduled appointment and provided a desktop computer in the Weill Cornell Gynecologic Oncology Clinic to complete the WBT. For participants randomized to the WBT arms, treating clinicians were provided with the WBT-generated pedigree and risk assessment models in advance of seeing the patient. Participants found to have a risk of a cancer-associated pathogenic variant greater than or equal to 2.5%, as determined by any of the validated cancer risk assessments, were recommended genetic testing per National Comprehensive Cancer Network (NCCN) guidelines [51, 52] (Figure 1). At the conclusion of the visit, participants and clinicians were provided with a survey to complete on their experience with CFH collection, adapted from a prior study of a patient-facing family health history collection tool [53].
Figure 1.
Web-based tool for cancer family history collection
Outcomes
The primary outcome was collection of an adequate CFH—based on the ASCO and ACOG guidelines—with usual care versus with the WBT [26, 54]. ASCO/ACOG recommend inclusion of the following key elements for minimum adequate collection of CFH: 1) first-degree relatives; 2) second-degree relatives; 3) both maternal and paternal sides; 4) ethnicity; 5) for each cancer case in the family, must establish age at cancer diagnosis and type of primary cancer. Adequacy was defined as a CFH that included all five of the above listed key elements (> 1 first-degree and second-degree relatives, ≥ 1 relative from each of the maternal and paternal sides), or measures 1–4 for those participants without a cancer case in the family. The electronic medical records and, for those participants randomized to the WBT arms, the WBT-generated pedigree, were independently evaluated by two reviewers and disagreements were discussed with a third reviewer to assess for inclusion of the key elements of CFH.
Secondary outcomes included: evaluating specific components of the collected CFH (e.g., numbers of included relatives and pedigrees) and success completing the WBT for each study arm; participant and clinician experience with the WBT; and completion of genetic counseling and genetic testing. At 6-months post initial patient visit, the electronic medical record was reviewed to determine if genetic counselling and testing had been completed for enrolled participants. Rates of WBT completion, genetic counseling, and genetic testing were additionally evaluated and stratified by patient age, race, and ethnicity.
Statistical Methods
The distribution of continuous variables was tested for normality via the Shapiro-Wilk normality test. To evaluate if certain CFH elements were associated with sociodemographic or clinical factors, univariate tests were applied based on whether the variable of interest was distributed normally (i.e., t-test, analysis of variance) or not normally (i.e., Mann–Whitney U test, Kruskal-Wallace test). Associations between categorical variables were evaluated using the chi-square test or Fisher’s exact test, as appropriate for category size. Multivariable linear regression analysis was explored to evaluate the independent effect of age, race, personal cancer history, family cancer history, and prior genetic testing on the number of relatives included in the CFH. Statistical significance was evaluated at the 0.05 alpha level, and 95% confidence intervals were calculated for all obtained estimates. Data were analyzed using Stata Version 16.0 (StataCorp, College Station, TX) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Power Analysis
The power analysis was performed for the primary outcome of collection of an adequate CFH based on the ASCO/ACOG guidelines with usual care versus with completion of the WBT. We assumed that collection of an adequate CFH would occur for approximately 4% of the population with usual care versus approximately ≥25% of the population using the WBT. With 50 participants assigned to usual care and 75 participants to complete the WBT (i.e., 50 at home, 25 at office), we had ≥ 95% power to detect the difference noted above in adequate CFH collection between the two groups, using a two-group chi-square test with a 0.05 two-sided significance level [43, 46]. Additionally, enrolling 50 and 25 participants to their respective arms would allow for an exploratory assessment of potential differences in collection of an adequate CFH based on location of WBT administration (i.e., home vs. office). The trial was approved to enroll 250 total participants to provide additional information for evaluation of secondary outcomes, resulting in doubling of initially projected sample sizes (100 usual care, 100 WBT at home, 50 WBT at office).
Results
Patient demographics
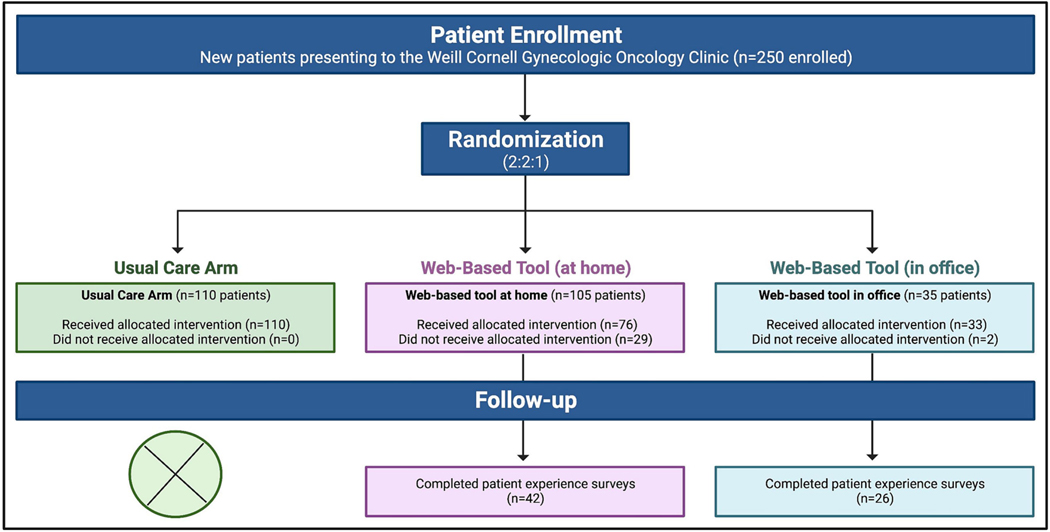
Two hundred and fifty participants were enrolled in the prospective randomized controlled trial. One hundred and ten participants were randomized to usual care, 105 to home completion of the WBT, and 35 to office completion of the WBT. The office completion arm was closed early due to the COVID-19 pandemic with intentional minimization of in-office time and exposure to additional medical personnel. The 15 randomization slots originally meant to be assigned to the WBT at office group were instead randomized, via a random number generator, to usual care (N=10) or WBT at home (N=5). The median patient age was 57 years (range 20–100 years).
Among the 250 participants, 141 (56%) self-identified as White, 27 (11%) as Black, 18 (7%) as Asian, 16 (6%) as Other, 47 (19%) declined to answer, and 1 (0.4%) as American Indian/Alaska Native. There were no significant differences in any of the evaluated patient demographics between study arms. (Figure 2 and Table 1)
Figure 2.
CONSORT Flow Diagram
Table 1.
Patient demographics (N=250)
| Combined cohort (250) | Usual care (110) | Web-based tool (140) | P Value | |
|---|---|---|---|---|
| Age (median, range) | 57 (20–100) | 58 (29–100) | 55.5 (20–88) | 0.226 |
| Race | 0.849 | |||
| White | 141 (56.4%) | 59 (53.6%) | 82 (58.6%) | |
| Black | 27 (10.8%) | 14 (12.7%) | 13 (9.3%) | |
| Asian | 18 (7.2%) | 9 (8.2%) | 9 (6.4%) | |
| Other | 16 (6.4%) | 7 (6.4%) | 9 (6.4%) | |
| Decline | 47 (18.8%) | 21 (19.1%) | 26 (18.6%) | |
| American Indian/Alaska Native | 1 (0.4%) | 0 (0%) | 1 (0.7%) | |
| Ethnicity | 0.289 | |||
| No answer | 59 (23.6%) | 31 (28.2%) | 29 (20.0%) | |
| Hispanic/Latino | 14 (5.6%) | 5 (4.5%) | 9 (6.4%) | |
| Not Hispanic/Latino | 177 (70.8%) | 74 (67.3%) | 103 (73.6%) |
Use of the web-based tool at home and in the office
Among 140 participants randomized to the WBT arms, 109 (78%) completed the tool. Among 105 participants randomized to home completion of the WBT, 76 (72%) completed the tool, and among 35 participants randomized to office completion of the WBT, 33 (94%) completed the tool (P = 0.008). (Figure 2 and Table 3) Patients completing the WBT presented to the gynecologic oncology clinic for several indications including invasive cancer (32), pre-cancer including cervical/vulvar dysplasia and endometrial hyperplasia (33), hereditary cancer syndrome (26), pelvic mass (24), postmenopausal bleeding and/or thickened endometrium (17), and benign gynecologic disease (8). Invasive cancer diagnoses included endometrial cancer (19), ovarian cancer (7), vulvar cancer (2), cervical cancer (1), gestational trophoblastic neoplasia (1), and non-gynecologic cancer (2). There was no significant difference in completion of the WBT based on indication for the new patient appointment.
Table 3.
Quality of the cancer family history for the web-based tool completed at home versus in the office
| Home web-based tool (76) | Office web-based tool (33) | P value | |
|---|---|---|---|
| Number of relatives in the pedigree | 29 (6–121) | 25 (0–85) | 0.343 |
| First-degree relatives | 5 (2–14) | 4 (0–12) | 0.028 |
| Second-degree relatives | 13 (4–35) | 12 (0–38) | 0.637 |
| Third-degree relatives | 8.5 (0–79) | 7 (0–40) | 0.442 |
| Number of generations in the pedigree | 4 (3–4) | 4 (0–4) | 0.071 |
| Recommended key elements for minimum adequate cancer family history | |||
| First-degree relatives | 76 (100%) | 32 (97.0%) | 0.303 |
| Second degree-relatives | 76 (100%) | 31 (93.9%) | 0.09 |
| Both maternal and Paternal sides | 76 (100%) | 32 (97.0%) | 0.303 |
| Ethnicity | 66 (86.8%) | 32 (97.0%) | 0.167 |
| Proband ≥ 1 relative with cancer | 59 (77.6%) | 26 (78.8%) | 1 |
| Relative’s age at cancer diagnosis | 39 (66.1%) | 14 (53.8%) | 0.335 |
| Relative’s primary type of cancer | 56 (94.9%) | 18 (69.2%) | 0.003 |
| Adequate cancer family history | 48 (63.2%) | 15 (45.5%) | 0.096 |
Quality of cancer family history
Participants completing the WBT compared to usual care resulted in a CFH with a significantly greater number of total relatives, first-degree relatives, second-degree relatives, third-degree relatives, and number of generations. Participants completing the WBT compared to usual care were significantly more likely to have the following elements of CFH collected: 1) First-degree relatives; 2) Second-degree relatives; 3) Both maternal and paternal sides; 4) Ethnicity; 5) Age at cancer diagnosis for each cancer case in the family. Based on the ASCO/ACOG definition, 5 (5%) participants in the usual care arm, as compared to 63 (58%) participants who completed the WBT, were found to have adequate collection of CFH (P<0.001). (Table 2)
Table 2.
Quality of the collected cancer family history
| Usual Care (110) | Web-based tool (109) | P value | |
|---|---|---|---|
| Relatives (#) | 3 (0–21) | 28 (0–121) | <0.001 |
| First-degree relatives (#) | 2 (0–7) | 5 (0–14) | <0.001 |
| Second-degree relatives (#) | 0 (0–14) | 12 (0–38) | <0.001 |
| Third-degree relatives (#) | 0 (0–4) | 8 (0–79) | <0.001 |
| Generations (#) | 2 (0–4) | 4 (0–4) | <0.001 |
| Recommended key elements for minimum adequate cancer family history | |||
| First-degree relatives | 90 (81.8%) | 108 (99.1%) | <0.001 |
| Second-degree relatives | 54 (49.1%) | 107 (98.2%) | <0.001 |
| Both maternal and Paternal sides | 72 (65.5%) | 108 (99.1%) | <0.001 |
| Ethnicity | 80 (72.7%) | 98 (89.9%) | 0.002 |
| Proband ≥ 1 relative with cancer | 78 (70.9%) | 85 (78.0%) | 0.279 |
| Relative’s age at cancer diagnosis | 17 (21.8%) | 53 (62.4%) | <0.001 |
| Relative’s primary type of cancer | 70 (89.7%) | 74 (87.1%) | 0.633 |
| Adequate cancer family history | 5 (4.5%) | 63 (57.8%) | <0.001 |
We compared the collected CFH among participants completing the WBT at home versus in the office (Table 3). Completion of most of the key elements of the CFH was similar whether the tool was completed at home versus in the office. However, participants completing the WBT at home had significantly greater number of first-degree relatives in their CFH (5, range 2–14, vs. 4, range 0–12, P=0.028) and were more likely to report the type of primary cancer for relatives with a cancer diagnosis (56 [95%] vs. 18 [69%], P=0.003). Among participants completing the WBT at home, 48 (63%) had an adequate CFH versus 15 (46%) for participants completing the WBT in the office (P=0.096).
Cancer risk modeling
Among 109 participants who completed the WBT, 31 (28%) were identified by the WBT as meeting criteria for genetic counseling/testing. Of these 31 participants, 17 had not had prior genetic testing. All 17 (100%) participants underwent genetic counseling and 8 (47%) completed genetic testing. As per standard of care in this Gynecologic Oncology clinic, genetic testing is offered as point of care testing for those patients interested following genetic counseling. The WBT also identified 20 (18%) participants with a lifetime risk of breast cancer greater than 20%. The American Cancer Society and the US Preventive Services Task Force recommend magnetic resonance imaging screening for women with an approximately 20–25% or greater lifetime risk of breast cancer [55]. Among these 20 participants who met such criteria, 10 were eligible for enhanced breast screening starting at their current age and all 10 (100%) were referred to a high-risk breast specialty clinic. No participants from the usual care arm underwent assessment with cancer risk models or for lifetime breast cancer risk and none were referred to the high-risk breast specialty clinic.
Genetic counseling and genetic testing
Among the 110 participants receiving usual care, 15 (13.6%) completed genetic counseling vs. 34 (31.2%) of the 109 participants completing the WBT (P = 0.002). Among the participants receiving usual care, 9 (8.2%) completed genetic testing vs. 20 (18.3%) of the participants completing the WBT (P = 0.029).
Age, race, and ethnicity
We evaluated for any associations between patient age (less than 65 years versus 65 years and older), race, and ethnicity, and completion of the WBT. Participant age and ethnicity did not affect likelihood of completing the WBT. Black participants were significantly less likely to complete the WBT compared to White participants (8 [62%] vs. 73 [89%], P = 0.022). Participants who declined to provide information on race were significantly less likely to complete the WBT compared to participants identifying as White (14 [54%] vs. 73 [89%], P < 0.001). (Supplementary Tables 1–2)
Among participants receiving usual care, age, race, and ethnicity were not significantly associated with collection of an adequate CFH. Among participants completing the WBT, those 65 years and older were less likely to have an adequate CFH compared to those who were less than 65 years (14 [40%] vs. 49 [66%], P = 0.013). Participants who declined to provide ethnicity information were significantly less likely to have an adequate CFH compared to Not Hispanic/Latino participants (7 [33%] vs. 50 [63%], P ≤ 0.025).
Patient experience with the web-based tool
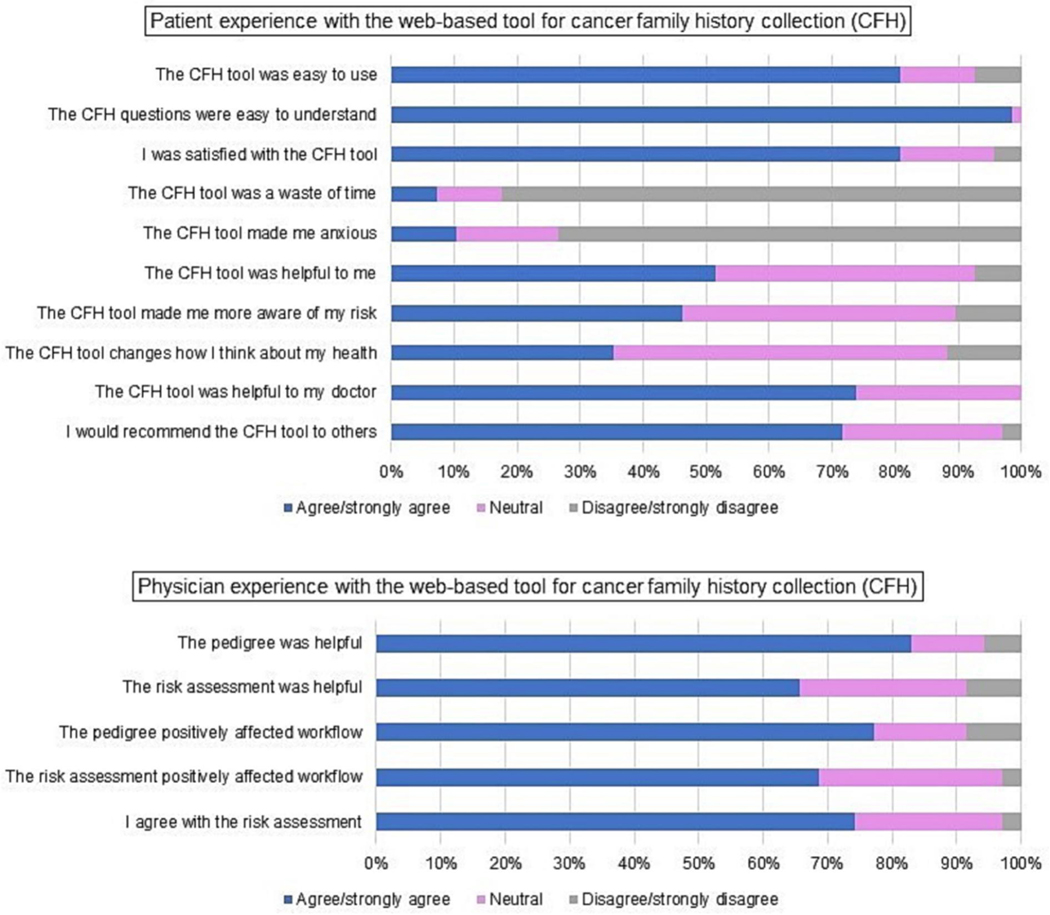
Of the 109 participants completing the WBT, 68 (62%) completed surveys at the conclusion of their office visit (42 [40%] vs. 26 [74%] in the WBT at home vs. in office arms, respectively). There were no significant differences in patient age, race, or ethnicity among those completing and not completing follow-up surveys. Seventeen participants (25%) reported technical difficulties with the tool and 7 (10%) stated that lack of smartphone accessibility was an important barrier. Twenty (29%) participants contacted at least one relative to gather additional family information while using the tool. Participants reported contacting a median of one relative (range 1–8) to gather information. The median amount of time participants reported spending on the WBT was 15 minutes (range 5–180 minutes). The median number of times participants reported opening the website to use the WBT was one (range 1–10) (Figure 3). The patient that spent 180 minutes on the WBT reported contacting 8 relatives and opening the tool to add information 10 separate times prior completing the tool. This patient did not report any technical difficulty with the tool.
Figure 3.
Patient and physician experience with the web-based tool for cancer family history collection (CFH). Patient experience (Top); physician experience (Bottom).
Among participants that completed the WBT at home versus in office, who then completed follow-up surveys, 15 (36%) and 5 (19%) reported contacting relatives to assist with learning about CFH, respectively (P=0.179). There were no significant differences in reported number of relatives contacted or in the amount of time to complete the WBT between study arms.
The majority of participants agreed or strongly agreed that the CFH tool was easy to use (81%), that the CFH questions were easy to understand (99%), and that they would recommend the CFH tool to others (72%). Fifty-two percent of participants agreed or strongly agreed that the CFH tool was helpful to them, 46% that the CFH tool made them more aware of their risk, and 35% that the CFH tool changed the way they thought about their health (Figure 3).
Physician experience with the web-based tool
The four physicians at the gynecologic oncology office completed feedback surveys for a convenience sample of the first 35 participants completing the WBT. Physicians agreed or strongly agreed with the following statements: the pedigree generated by the WBT was helpful – 29 (83%), the risk assessment was helpful – 23 (66%), the pedigree positively affected workflow – 27 (77%), the risk assessment positively affected workflow – 24 (69%), I agree with the risk assessment – 26 (74%) (Figure 3).
Discussion
Our study demonstrates that incorporating health information technology to facilitate CFH collection allows an outpatient gynecologic oncology practice to capture a more comprehensive CFH compared to usual care whereby the process is completed by the physician during the patient interview. The WBT resulted in collection of CFH with a significantly greater number of included relatives and generations and a higher likelihood of including information on first-degree relatives, second-degree relatives, maternal and paternal family members, ethnicity, and cancers among family members, all key components of an adequate CFH based on national guidelines. Further, participants completing the WBT as compared to those receiving usual care were significantly more likely to undergo genetic counseling (34 [31%] vs. 15 [14%], P=0.002) and complete genetic testing (20 [18%] vs. 9 [8%], P=0.029). Evidence suggests that a major barrier to CFH and cancer genetic risk assessment is clinician under-recognition of those at risk; our data suggest that health information technology in the form of a WBT for CFH collection may be a scalable solution [17, 56–59].
With the emergence of the COVID-19 pandemic seven months following study launch and intentional minimization of patient time in medical facilities, the WBT office completion arm was closed following enrollment of only 35 of the planned 50 participants. However, the premature closure did not materially affect our ability to compare the two settings of WBT administration. Participants offered the WBT in the office were significantly more likely to complete the tool compared to home administration (33 [94%] vs. 76 [72%], P = 0.008). This finding was not surprising given that participants in the office were prompted by the study team and provided with an office computer to complete the tool, minimizing barriers to tool usability as well as access to a computer/smartphone. While both the home and office WBT administration arms were superior to usual care for collecting a high quality CFH, home completion of the tool resulted in collection of a more comprehensive CFH, with significantly greater numbers of first-degree relatives and reporting of the primary site of cancer for relatives, with an overall trend towards higher rates of ASCO/ACOG adequate CFH collection. Our protocol did not include a reminder telephone call for those participants that did not complete the WBT at home. Future studies could include a telephone call or automatic email/text message reminder which may improve patient participation at home. Additionally, several participants reported lack of smartphone accessibility for the WBT as an important barrier to utilization. Therefore, future studies and practices should consider prompting patients to complete the CFH at home prior to the visit but include an in-office option to capture participants unable to complete electronic tools at home.
Among tools offering comprehensive CFH collection, non-randomized data support the acceptability and usefulness of CFH collection tools. Nazareth et al. retrospectively evaluated a chatbot tool and found a completion rate of 89% and average satisfaction rating of 4.6 on a scale of 1 to 5, with 5 indicating the highest satisfaction score [60]. ItRunsInMyFamily, a family health history tool with output that includes recommendation for genetic consultation for participants deemed to be at risk for inherited cancers, was noted to have promising uptake in a non-clinical population-level setting, similarly supporting the usability of a CFH tool [61, 62]. Implementation of HughesRiskApps, a family history and cancer genetic risk assessment software, was associated with improvement in utilization of genetic referral and genetic testing in an observational study [63]. While these non-randomized data support the use of patient facing CFH collection tools, our randomized controlled trial demonstrates the clinical impact of a CFH tool on the collection of an adequate CFH as well as its capacity to consequently facilitate appropriate downstream genetic services. Furthermore, unlike our tool, many of the current patient-facing CFH collection tools do not incorporate national guideline-based cancer risk modeling for the purpose of identifying participants who qualify for cancer genetic testing [64–73].
The American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute, all cite a critical need to improve genetic cancer risk assessment and testing for underrepresented populations [74]. Research suggests that minority participants are less likely to be identified as high-risk for hereditary cancer, less likely to be recommended genetic testing, and less likely to complete genetic testing when referred [36, 75–77]. Furthermore, minority participants are more likely to utilize genetics services only after a cancer diagnosis and not based on family history, suggesting a missed opportunity for detection of pathogenic mutations and potentially life-saving cancer prevention in certain vulnerable populations [76]. In this study, Black participants were less likely to complete the WBT as compared to White participants, suggesting that future work must strive to create electronic tools that are equally accessible to diverse patient populations. However, this study was limited by minimal racial and ethnic diversity, and thus was not powered to address the effects of patient demographics on CFH collection or cancer genetic risk assessment. Focus groups are needed to comprehensively examine the user experience with the WBT and better define potential issues faced by participants with this instrument in addition to large prospective trials of CFH collection and cancer genetic risk assessment in diverse patient populations.
In addition to the premature closure of the office WBT tool arm, this study has several limitations. This study was piloted in a gynecologic oncology clinic and therefore was enriched for participants at increased risk for hereditary cancer. Future studies should explore similar platforms of health information technology in primary care and general gynecology settings with the goal to reach participants prior to a cancer diagnosis and extend cancer genetic risk assessment to medical clinicians who are less comfortable with hereditary cancer and NCCN guidelines for genetic testing. The WBT utilized in this study was available only in English, limiting the diversity of our patient population and scalability of this model. This is an important limitation to identify and address in future work, whereby, we will need to identify more inclusive health information technology tools and thoroughly evaluate contributors to disparities in use of such tools. These future initiatives will help design tools that ultimately decrease rather than accentuate healthcare disparities.
Currently in the US, the majority of individuals meeting guideline-based criteria for genetic testing have not been identified [1, 3, 4]. This is a critical problem as, for those with a cancer-associated mutation, lack of identification prevents cancer-risk reduction. Health information technology like the WBT evaluated in this study may help to overcome commonly cited barriers to cancer genetic risk assessment including limited clinician knowledge and time, lack of training for clinicians, and complex and dynamic criteria for genetic assessment [78–86]. The WBT in this randomized prospective trial resulted in collection of an adequate CFH for a significantly greater number of participants compared to usual care face-to-face clinician interview. Future work is needed to optimize methods of electronic patient communication to improve equitable utilization of these tools with integrated links to receive genetic testing. These measures will help provide those individuals identified as high-risk for a hereditary cancer syndrome with access to genetic testing prior to ever receiving a cancer diagnosis.
Supplementary Material
Supplementary Table 1. Age, race, and ethnicity and completion of the web-based tool based
Supplementary Table 2. Age, race, and ethnicity and collection of an adequate cancer family history
Research Highlights.
A web-based tool for cancer family history collection was offered to new gynecologic oncology patients compared to clinician collection.
Among 140 participants offered the web-based tool, 78% completed it.
The web-based tool resulted in collection of higher quality cancer family history compared to clinician collection.
Participants completing the web-based tool (vs. usual care) were more likely to complete genetic counseling and testing.
Acknowledgements:
Melissa K Frey was supported by the following grant: NIH/NCATS Grant # KL2-TR-002385. Ravi Sharaf was supported by the following grants: National Cancer Institute Grant # K07CA216326 and R01CA211723 and Patient Centered Outcomes Research Institute Grant # IHS-2017C3-9211. Charlene Thomas and Paul J. Christos were both partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Footnotes
Disclosures:
Progeny Genetics provided use of the web-based tool for this clinical trial free of charge.
Conflict of Interest:
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Offit K, Tkachuk KA, Stadler ZK, Walsh MF, Diaz-Zabala H, Levin JD, et al. Cascading After Peridiagnostic Cancer Genetic Testing: An Alternative to Population-Based Screening. J Clin Oncol. 2020:JCO1902010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG. 2019. [DOI] [PubMed] [Google Scholar]
- [3].Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–7. [DOI] [PubMed] [Google Scholar]
- [4].Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. 2017;146:217–24. [DOI] [PubMed] [Google Scholar]
- [5]. https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm.
- [6].Force UPST. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–65. [DOI] [PubMed] [Google Scholar]
- [7].Rubenstein JH, Enns R, Heidelbaugh J, Barkun A. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015;149:777–82; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- [8].Ladabaum U, Ford JM, Martel M, Barkun AN. American Gastroenterological Association Technical Review on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015;149:783–813.e20. [DOI] [PubMed] [Google Scholar]
- [9]. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
- [10]. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf.
- [11].Nelson HD, Pappas M, Cantor A, Haney E, Holmes R. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019;322:666–85. [DOI] [PubMed] [Google Scholar]
- [12].Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. https://www.nap.edu/catalog/11971/cancer-related-genetic-testing-and-counseling-workshop-proceedings.
- [14]. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/hereditary-cancers.
- [15]. https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increaseproportion-people-colorectal-cancer-who-get-tested-lynch-syndrome-c-r03.
- [16]. https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-females-increased-risk-who-get-genetic-counseling-breast-andor-ovarian-cancer-c-d01.
- [17].Green RF, Ari M, Kolor K, Dotson WD, Bowen S, Habarta N, et al. Evaluating the role of public health in implementation of genomics-related recommendations: a case study of hereditary cancers using the CDC Science Impact Framework. Genet Med. 2019;21:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Centers for Disease Control and Prevention. Family Health History Still an Important Tool for Clinical and Public Health Practice, but Knowledge Gaps Remain. [Google Scholar]
- [19].US Preventive Services Task Force aUSDoHaHS. BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. 2013. [Google Scholar]
- [20].Io Medicine. Cancer-Related Genetic Testing and Counseling. Workshop Proceedings - See more at: http://www.nationalacademies.org/hmd/Reports/2007/Cancer-Related-Genetic-Testing-and-Counseling-Workshop-Proceedings.aspx#sthash.rcWiR1kW.dpuf. 2007.
- [21].Network NCC. Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 2.2015. [Google Scholar]
- [22].Network NCC. Genetic/Familial High-Risk Assessment: Colorectal p. Clinical Practice Guidelines. [Google Scholar]
- [23].Lu KH, Wood ME, Daniels M, Burke C, Ford J, Kauff ND, et al. American Society of Clinical Oncology Expert Statement: Collection and se of a Cancer Family History for Oncology Providers. J Clin Oncol. 2014;32:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. Journal of Clinical Oncology. 2015;33:3660–7. [DOI] [PubMed] [Google Scholar]
- [25].Lancaster JM, Powell CB, Chen LM, Richardson DL, Committee SCP. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136:3–7. [DOI] [PubMed] [Google Scholar]
- [26].Hereditary Cancer Syndromes and Risk Assessment: ACOG COMMITTEE OPINION SUMMARY, Number 793. Obstet Gynecol. 2019;134:1366–7. [DOI] [PubMed] [Google Scholar]
- [27].Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila). 2011;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. Journal of Clinical Oncology. 2017;35:3800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cross DS, Rahm AK, Kauffman TL, Webster J, Le AQ, Spencer Feigelson H, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Allen CG, Roberts M, Guan Y. Exploring Predictors of Genetic Counseling and Testing for Hereditary Breast and Ovarian Cancer: Findings from the 2015 U.S. National Health Interview Survey. Journal of Personalized Medicine. 2019;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guttmacher AE, Collins FS, Carmona RH. The family history--more important than ever. N Engl J Med. 2004;351:2333–6. [DOI] [PubMed] [Google Scholar]
- [32].Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379:2495–505. [DOI] [PubMed] [Google Scholar]
- [34].González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381:2391–402. [DOI] [PubMed] [Google Scholar]
- [35].Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med. 2019;381:2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meyer LA, Anderson ME, Lacour RA, Suri A, Daniels MS, Urbauer DL, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Biller LH, Syngal S, Yurgelun MB. Recent advances in Lynch syndrome. Fam Cancer. 2019;18:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin J, Wolfe I, Ahsan MD, Krinsky H, Lackner AI, Pelt J, et al. Room for improvement in capturing cancer family history in a gynecologic oncology outpatient setting. Gynecol Oncol Rep. 2022;40:100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Murff HJ, Byrne D, Syngal S. Cancer risk assessment: quality and impact of the family history interview. Am J Prev Med. 2004;27:239–45. [DOI] [PubMed] [Google Scholar]
- [40].Murff HJ, Greevy RA, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10:174–80. [DOI] [PubMed] [Google Scholar]
- [41].Acton RT, Burst NM, Casebeer L, Ferguson SM, Greene P, Laird BL, et al. Knowledge, attitudes, and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med. 2000;75:850–2. [DOI] [PubMed] [Google Scholar]
- [42].Summerton N, Garrood PV. The family history in family practice: a questionnaire study. Fam Pract. 1997;14:285–8. [DOI] [PubMed] [Google Scholar]
- [43].Powell KP, Christianson CA, Hahn SE, Dave G, Evans LR, Blanton SH, et al. Collection of family health history for assessment of chronic disease risk in primary care. N C Med J. 2013;74:279–86. [PubMed] [Google Scholar]
- [44].Wu RR, Himmel TL, Buchanan AH, Powell KP, Hauser ER, Ginsburg GS, et al. Quality of family history collection with use of a patient facing family history assessment tool. BMC Fam Pract. 2014;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Berg AO, Baird MA, Botkin JR, Driscoll DA, Fishman PA, Guarino PD, et al. National Institutes of Health State-of-the-Science Conference Statement: Family History and Improving Health. Ann Intern Med. 2009;151:872–7. [DOI] [PubMed] [Google Scholar]
- [46].Qureshi N, Wilson B, Santaguida P, Little J, Carroll J, Allanson J, et al. Family history and improving health. Evid Rep Technol Assess (Full Rep). 2009:1–135. [PMC free article] [PubMed] [Google Scholar]
- [47].Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: implications for genetic screening. Genet Med. 2000;2:180–5. [DOI] [PubMed] [Google Scholar]
- [48].Ritchie JB, Allen CG, Morrison H, Nichols M, Lauzon SD, Schiffman JD, et al. Utilization of health information technology among cancer genetic counselors. Mol Genet Genomic Med. 2020:e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li X, Kahn RM, Wing N, Zhou ZN, Lackner AI, Krinsky H, et al. Leveraging Health Information Technology to Collect Family Cancer History: A Systematic Review and Meta-Analysis. JCO Clin Cancer Inform. 2021;5:775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Qureshi N, Carroll JC, Wilson B, Santaguida P, Allanson J, Brouwers M, et al. The current state of cancer family history collection tools in primary care: a systematic review. Genet Med. 2009;11:495–506. [DOI] [PubMed] [Google Scholar]
- [51].National Comprehensive Cancer Network Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2021. 2021.
- [52].NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines Version 1.2022): Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. 2022.
- [53].Wu RR, Orlando LA, Himmel TL, Buchanan AH, Powell KP, Hauser ER, et al. Patient and primary care provider experience using a family health history collection, risk stratification, and clinical decision support tool: a type 2 hybrid controlled implementation-effectiveness trial. BMC Fam Pract. 2013;14:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lu KH, Wood ME, Daniels M, Burke C, Ford J, Kauff ND, et al. American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol. 2014;32:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. [DOI] [PubMed] [Google Scholar]
- [56].Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding TSt, Goldstein K. Racial/Ethnic Disparities in BRCA Counseling and Testing: a Narrative Review. J Racial Ethn Health Disparities. 2019;6:570–83. [DOI] [PubMed] [Google Scholar]
- [57].Health NIo. NIH State-of-the-Science Conference Statement on Family History and Improving Health [PDF]. https://consensus.nih.gov/2009/Fhx%20images/familyhistory_final_stmt.pdf.A.
- [58].Lu KH, Wood ME, Daniels M, et al. American Society of Clinical Oncology Expert Statement: Collection and se of a Cancer Family History for Oncology Providers. J Clin Oncol. 2014;32(8):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rolf H. Sijmons M Identifying Patients with Familial Cancer Syndromes. https://www.ncbi.nlm.nih.gov/books/NBK45295/. . [PubMed]
- [60].Nazareth S, Hayward L, Simmons E, Snir M, Hatchell KE, Rojahn S, et al. Hereditary Cancer Risk Using a Genetic Chatbot Before Routine Care Visits. Obstet Gynecol. 2021;138:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Welch BM, Allen CG, Ritchie JB, Morrison H, Hughes-Halbert C, Schiffman JD. Using a Chatbot to Assess Hereditary Cancer Risk. JCO Clin Cancer Inform. 2020;4:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ritchie JB, Bellcross C, Allen CG, Frey L, Morrison H, Schiffman JD, et al. Evaluation and comparison of hereditary Cancer guidelines in the population. Hered Cancer Clin Pract. 2021;19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ozanne EM, Loberg A, Hughes S, Lawrence C, Drohan B, Semine A, et al. Identification and management of women at high risk for hereditary breast/ovarian cancer syndrome. Breast J. 2009;15:155–62. [DOI] [PubMed] [Google Scholar]
- [64].Welch BM, Wiley K, Pflieger L, Achiangia R, Baker K, Hughes-Halbert C, et al. Review and Comparison of Electronic Patient-Facing Family Health History Tools. J Genet Couns. 2018;27:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Orlando LA, Buchanan AH, Hahn SE, Christianson CA, Powell KP, Skinner CS, et al. Development and validation of a primary care-based family health history and decision support program (MeTree). N C Med J. 2013;74:287–96. [PMC free article] [PubMed] [Google Scholar]
- [66].Facio FM, Feero WG, Linn A, Oden N, Manickam K, Biesecker LG. Validation of My Family Health Portrait for six common heritable conditions. Genet Med. 2010;12:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Doerr M, Edelman E, Gabitzsch E, Eng C, Teng K. Formative evaluation of clinician experience with integrating family history-based clinical decision support into clinical practice. J Pers Med. 2014;4:115–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hulse NC, Ranade-Kharkar P, Post H, Wood GM, Williams MS, Haug PJ. Development and early usage patterns of a consumer-facing family health history tool. AMIA Annu Symp Proc. 2011;2011:578–87. [PMC free article] [PubMed] [Google Scholar]
- [69].Wang C, Bickmore T, Bowen DJ, Norkunas T, Campion M, Cabral H, et al. Acceptability and feasibility of a virtual counselor (VICKY) to collect family health histories. Genet Med. 2015;17:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rubinstein WS, Acheson LS, O’Neill SM, Ruffin MTt, Wang C, Beaumont JL, et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genet Med. 2011;13:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Braithwaite D, Sutton S, Mackay J, Stein J, Emery J. Development of a risk assessment tool for women with a family history of breast cancer. Cancer Detect Prev. 2005;29:433–9. [DOI] [PubMed] [Google Scholar]
- [72].Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32:218–27. [DOI] [PubMed] [Google Scholar]
- [73].Heald B, Keel E, Marquard J, Burke CA, Kalady MF, Church JM, et al. Using chatbots to screen for heritable cancer syndromes in patients undergoing routine colonoscopy. J Med Genet. 2021;58:807–14. [DOI] [PubMed] [Google Scholar]
- [74].Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, et al. Charting the Future of Cancer Health Disparities Research: A Position Statement From the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol. 2017;35:3075–82. [DOI] [PubMed] [Google Scholar]
- [75].Lin J, Sharaf RN, Saganty R, Ahsan D, Feit J, Khoury A, et al. Achieving universal genetic assessment for women with ovarian cancer: Are we there yet? A systematic review and meta-analysis. Gynecol Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chapman-Davis E, Zhou ZN, Fields JC, Frey MK, Jordan B, Sapra KJ, et al. Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J Gen Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Frey MK, Finch A, Kulkarni A, Akbari MR, Chapman-Davis E. Genetic Testing for All: Overcoming Disparities in Ovarian Cancer Genetic Testing. Am Soc Clin Oncol Educ Book. 2022;42:1–12. [DOI] [PubMed] [Google Scholar]
- [78].Nikolaidis C, Duquette D, Mendelsohn-Victor KE, Anderson B, Copeland G, Milliron KJ, et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med. 2019;21:1363–70. [DOI] [PubMed] [Google Scholar]
- [79].Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns. 2010;19:618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gammon AD, Rothwell E, Simmons R, Lowery JT, Ballinger L, Hill DA, et al. Awareness and preferences regarding BRCA1/2 genetic counseling and testing among Latinas and non-Latina white women at increased risk for hereditary breast and ovarian cancer. J Genet Couns. 2011;20:625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hamilton JG, Shuk E, Arniella G, González CJ, Gold GS, Gany F, et al. Genetic Testing Awareness and Attitudes among Latinos: Exploring Shared Perceptions and Gender-Based Differences. Public Health Genomics. 2016;19:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rajpal N, Muñoz J, Peshkin BN, Graves KD. Insights into BRCA1/2 Genetic Counseling from Ethnically Diverse Latina Breast Cancer Survivors. J Genet Couns. 2017;26:1221–37. [DOI] [PubMed] [Google Scholar]
- [83].Sheppard VB, Graves KD, Christopher J, Hurtado-de-Mendoza A, Talley C, Williams KP. African American women’s limited knowledge and experiences with genetic counseling for hereditary breast cancer. J Genet Couns. 2014;23:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Adams I, Christopher J, Williams KP, Sheppard VB. What Black Women Know and Want to Know About Counseling and Testing for BRCA1/2. J Cancer Educ. 2015;30:344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hurtado-de-Mendoza A, Graves KD, Gómez-Trillos S, Song M, Anderson L, Campos C, et al. Developing a culturally targeted video to enhance the use of genetic counseling in Latina women at increased risk for hereditary breast and ovarian cancer. J Community Genet. 2020;11:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding TS, Goldstein K. Racial/Ethnic Disparities in BRCA Counseling and Testing: a Narrative Review. J Racial Ethn Health Disparities. 2019;6:570–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Age, race, and ethnicity and completion of the web-based tool based
Supplementary Table 2. Age, race, and ethnicity and collection of an adequate cancer family history