Abstract
Stereotactic radiosurgery (SRS) is a mainstay treatment option for brain metastasis (BM). While guidelines for SRS use have been outlined by professional societies, consideration of these guidelines should be weighed in the context of emerging literature, novel technology platforms, and contemporary treatment paradigms. Here, we review recent advances in prognostic scale development for SRS-treated BM patients and survival outcomes as a function of the number of BM and cumulative intracranial tumor volume. Focus is placed on the role of stereotactic laser thermal ablation in the management of BM that recur after SRS and the management of radiation necrosis. Neoadjuvant SRS prior to surgical resection as a means of minimizing leptomeningeal spread is also discussed.
Keywords: brain metastases, stereotactic radiosurgery, cumulative intracranial tumor volume, stereotactic laser ablation
Introduction
Brain metastasis (BM) affects 20 to 40% of cancer patients, translating to approximately 170,000 cases in the U.S. each year. 1 While brain metastases can arise from virtually any cancer, they are most commonly found in patients afflicted with lung cancer (19.9%), melanoma (6.9%), renal cell carcinoma (RCC) (6.5%), breast cancer (5.1%), and colorectal cancer (1.8%). 2 Patients with HER2 breast cancer, triple negative breast cancer, melanoma, small cell lung cancer, and non-squamous/nonsmall cell lung cancer (NSCLC) have the highest risk of developing brain metastases. 3 The majority of BM patients present with oligometastatic disease, typically defined as 1 to 3 intracranial lesions. 4 Tumors such as melanoma and RCC have higher propensities for intracranial invasion relatively early during clinical course while breast and colorectal cancer invade the central nervous system after systemic metastases have been established. 5
In this review, we will provide an overview of stereotactic radiosurgery (SRS) as a treatment for BM. Emphasis will be placed on efficacy, prognostic variables, patient selection, and complications.
Diagnosis and Prognosis
Any cognitive decline or acute neurological symptom in a patient diagnosed with cancer merits prompt imaging workup. Approximately 90% of patients with brain metastases suffer neurocognitive decline prior to diagnosis. 6 For workup, the gold standard diagnostic tool is a thin axial magnetic resonance imaging (MRI) performed after administration of contrast material. On T1 imaging, brain metastases are typically solid, contrast-enhancing masses located at the gray-white junction. Magnetic resonance spectroscopy tends to demonstrate high choline/N-acetylaspartate and choline/creatinine ratios in the contrast-enhancing regions. 7 BM from melanomas, choriocarcinomas, germ cell tumors, thyroid cancer, and RCC are more likely to be hemorrhagic. 8
The prognosis of patients with brain metastases is poor, with a median survival of 4 months after whole brain radiation therapy (WBRT) and 1-year survival of 12%. 9 10 However, survivors beyond historical expectations are beginning to emerge as improved systemic therapy becomes available through targeted agents and immunotherapies. 11 12 Clinical variables that prognosticate survival can be divided into three categories: patient demographics and clinical condition (age, Karnofsky Performance Score [KPS] greater, systemic disease control), BM characteristics (cumulative intracranial tumor volume [CITV], number of BM), and the presence of targetable mutations (e.g., BRAF mutation). The relative importance of these prognostic factors varies as a function of the specific cancer type 13 and cancer-specific prognostic scales have been developed for BM patients. These scales aid in clinical decision making in terms of palliative versus curative intent. 14
Treatment Options
Because most systemic therapies poorly penetrate the blood–brain barrier, their application as therapies for BM require participation in pertinent clinical trials or the discretion of the treating oncologist. It is important to note that some BMs do respond to systemic therapy, particularly for smaller sized lesions. 15
Surgical resection is considered for patients with oligometastatic disease with symptomatic mass effect. Resection or biopsy is also warranted in cases of diagnostic uncertainty. 16 Additionally, randomized controlled trials demonstrate improved survival in patients with solitary BM who underwent surgical resection followed by radiation therapy relative to those treated with radiation therapy alone. 17 18 Importantly, surgical resection should be followed with radiation of the resection cavity, since approximately 50% of resected tumors recur locally without such treatment. 19
Radiation therapy remains a mainstay option for the treatment of BM and can be applied to the entire brain, as WBRT or only to the BM (SRS). In WBRT, radiation is delivered in small fractions on a daily basis. It is highly effective in achieving local control of tumor growth. 20 Most studies report local control rate of over 80%. 21 22 23 24 Because the entire cerebrum is radiated, WBRT “sterilizes” regions of the brain that are not affected by the macroscopic tumor. Since these regions may harbor micrometastatic foci that were invisible to the original MRI, WBRT minimizes the likelihood of new BM distant to the original tumor site (termed distant metastasis). This control of distant metastasis comes at the cost of injury to the cerebrum and neurocognitive decline following treatment. In two independent clinical trials, oligometastatic BM patients with WBRT exhibited worsened verbal memory capacity relative to those treated with SRS. 4 25 In general, current clinical practice employs WBRT for: prophylactic cranial irradiation for small cell lung cancers, treatment of miliary BM, or treatment with palliative intent. 8 26
SRS involves technology platforms that converge multiple, nonparallel beams to deliver a single, high radiation dose to a targeted region. 27 28 The radiation delivered through SRS is highly conformal to the lesion, with a rapid dose fall-off at the edge of the treatment volume. 5 With the exception of highly radiation resistant tumors, such as melanomas and sarcomas, SRS is highly efficacious as a means of controlling BM growth. 29 30 Since SRS spares cerebrum unaffected with BM, there is a decreased likelihood of posttreatment neurocognitive decline relative to WBRT. 4 25 31 Moreover, because higher doses can typically be delivered through SRS, local control is improved relative to WBRT. 29 30 32 However, repeat radiosurgery as treatment for distant recurrence is often required. 30 As such, continued imaging surveillance is required for patients who undergo SRS.
While WBRT and SRS differ in the control of local and distant BM, most studies indicate comparable survival after either treatment. 4 29 These observations are largely consistent with studies demonstrating uncontrolled systemic disease as the main cause of cancer death. 33 34 In this context, while there is an extended literature describing the effects of combining SRS with WBRT, 4 20 29 35 this practice is not routinely applied in the current clinical practice.
Platforms for SRS
The concept of SRS was first introduced by Leksell in 1951 with the use of several proton beams and later on the gamma beams. 36 Since this initial landmark development, multiple technology platforms have been developed to facilitate SRS. These technology platforms bear distinct commercial names, including Gammaknife, 37 Cyberknife, 38 Edge, 39 Hyperarc, 40 ZAP, 41 and proton beam radiosurgery. 42 While the mechanisms of radiation delivery differ between these platforms, the available literature suggests comparable clinical efficacy. 43
Considerations for SRS Treatment
Dose, Fraction, and Anatomic Considerations
Based on a landmark study by the Radiation Therapy Oncology Group (RTOG), the maximum tolerated SRS doses for tumors less than 20, 21 to 30, and 31 to 40 mm were 24, 18, and 15 Gy, respectively. 44 More current clinical applications utilize doses below the thresholds defined by this study. Dose deescalation when treating lesions in proximity of radiation sensitive structures, such as the optic nerve and the brainstem are routinely performed. 45
Historically, SRS requires headframe placement and is typically delivered in a single treatment. With improvement in methods for immobilization as well as time required for delivery, frameless SRS is now possible. For larger lesions, patients can undergo hypofractionated SRS, defined as up to five treatments of conformation radiation delivery. 46 An alternative approach for SRS of larger lesions involves staged SRS, where SRSs are separated by short time intervals or sequentially delivered to different regions of the lesion. 47 Efficacy of these treatment variations for larger lesions are largely comparable to single fraction radiosurgery for smaller lesions. Dose equivalent radiation delivered through fractionation has been shown to decrease posttreatment morbidity (Lau et al 48 ).
Prognostic Scales
Survival prognostication serves as a key foundation for tailoring therapy to BM patients. Several prognostic scales have been developed for SRS-treated BM patients ( Table 1 ). The earlier prognostic scales, including the recursive partitioning analysis (RPA), modified RPA, Score Index for Radiosurgery, Basic Score for Brain Metastasis, and Graded Prognosis Analysis (GPA), 48 49 50 51 52 53 treated BM as a single entity, irrespective of the original cancer diagnosis. These studies highlight the prognostic importance of patient demographics and clinical condition as well as BM characteristics. In recent studies, there is increased appreciation that BM derived from cancers of distinct histology exhibit differing clinical courses 54 and that prognostic scales need to be tailored to distinct tumor types. The disease-specific GPA was developed in this context ( Table 2 ). With the emergence of therapies targeting oncogenic mutations, such as BRAF and EGFR, modern prognostic scales now incorporate tumor mutation status as a prognostic factor. 55 56
Table 1. Summary of nontumor-specific prognostic scales.
| Year of publication | Number of patients | Prognostic variables | Resulting parameter | Median overall survival (mo) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | KPS | Primary tumor control | Tumor number | Non-brain metastases | Largest tumor volume (cm 3 ) | |||||
| RPA 48 | 1997 | 1,200 | < 65 ≥ 65 |
≥ 70 < 70 |
Yes No |
NA | NA | NA | Class I: Age < 65, KPS ≥ 70, and primary tumor control Class II: All others Class III: KPS < 70 |
Class I: 7.1 Class II: 4.2 Class III: 2.3 |
| Modified-RPA for class II 49 | 2012 | 3,753 | NA (All age ≥ 65) | 0: 90–100 1: 70–80 |
0: Yes 1: No |
0: Single 1: Multiple |
0: No 1: Yes |
NA | Class IIa: score of 0–1 Class IIb: score of 2 Class IIc: score of 3–4 |
Class IIa: 19.7–15.6 Class IIb: 8.4 Class IIc: 5.2–3.5 |
| Modified-RPA for class III 50 | 2002 | 916 | < 65 ≥ 65 |
NA (All KPS < 70) | Yes No |
Single Multiple |
NA | NA | Class IIIa: Age < 65, primary tumor control, single lesion Class IIIb: All others Class IIIc: Age ≥ 65, uncontrolled primary tumor, multiple lesions |
Class IIIa: 3.2 Class IIIb: 1.9 Class IIIc: 1.2 |
| SIR 51 | 2000 | 65 | 0: ≥ 60 1: 51–59 2: ≤ 50 |
0: ≤ 50 1: 50–70 2: ≥ 70 |
0: PD 1: PR-SD 2: CR-NED |
0: ≥ 3 1: 2 3: 1 |
NA | 0: > 13 1: 5–13 2: < 5 |
Score range of 0–10 | Score 1–3: 2.9 Score 4–7: 7.0 Score 8–10: 31.38 |
| BS-BM 52 | 2004 | 110 | NA | 0: 50–70 1: 80–100 |
0: No 1: Yes |
NA | 0: Yes 1: No |
NA | Score range of 0–3 | Score 0: 1.9 Score 1: 3.3 Score 2: 13.1 Score 3: Undefined |
| GPA 53 | 2008 | 1,960 | 0: > 60 0.5: 50–59 1: < 50 |
0: < 70 0.5: 70–80 1: 90–100 |
NA | 0: > 3 0.5: 2–3 1: 1 |
0: Present 1: None |
NA | Score range of 0–4 | Score 0–1: 2.6 Score 1.5–2.5: 3.8 Score 3: 6.9 Score 3.5–4: 11.0 |
Abbreviations: BS-BM, Basic Score for Brain Metastasis; CR, complete clinical remission; GPA, Graded Prognosis Analysis; KPS, Karnofsky Performance Score; NA, not applicable; NED, no evidence of disease; PD, progressive disease; PR, partial remission; RPA, recursive partitioning analysis, SD, stable disease; SIR, Score Index for Radiosurgery.
Table 2. Summary of tumor-specific prognostic scales.
| Year of publication | Number of patients | Prognostic variables | Resulting parameter | Median overall survival (mo) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | KPS | Tumor number | Non-brain metastases | Gene status | ||||||
| ds-GPA (lung - NSCLC and SCLC) a 13 | 2008 | 4,529 | 0: > 60 0.5: 50–59 1: < 50 |
0: < 70 0.5: 70–80 1: 90–100 |
0: > 3 0.5: 2–3 1: 1 |
0: Present 1: Absent |
NA | Score range of 0–4 | Score 0–1: 2.79–3.02 Score 1.5–2.5: 5.3–6.5 Score 3: 9.6–11.3 Score 3.5–4: 14.8–17.1 |
|
| ds-GPA (lung) 55 | 2017 | 2,186 | 0: ≥ 70 0.5: < 70 |
0: < 70 0.5: 70–80 1: 90–100 |
0: >4 0.5: 1–4 |
0: Present 1: Absent |
0: EGFR neg/unk and ALK neg/unk 1: EGFR pos or ALK pos |
Score range of 0–4 | AdenoCa Score 0–1.0: 6.9 Score 1.5–2.0: 13.7 Score 2.5–3.0: 26.5 Score 3.5–4.0: 46.8 |
Non-AdenoCa Score 0–1.0: 5.3 Score 1.5–2.0: 9.8 Score 2.5–3.0: 12.8. Score 3.5–4: Unavailable |
| ds-GPA (melanoma and RCC) 13 | 2008 | 4,529 | NA | 0: < 70 1: 70–80 2: 90–100 |
0: > 5 1: 2–3 2: 1 |
NA | NA | Score range of 0–4 | Score 0–1: 3.3–3.4 Score 1.5–2.5: 4.7–7.3 Score 3: 8.8–11.3 Score 3.5–4: 13.2–14.8 |
|
| ds-GPA (melanoma) 56 | 2017 | 823 | 0: ≥ 70 0.5: < 70 |
0: < 70 0.5: 70–80 1: 90–100 |
0: > 4 0.5: 2–4 1: 1 |
0: Present 1: Absent |
0: BRAF neg/unk 1: BRAF pos |
Score range of 0–4 | Score 0–1.0: 4.9 Score 1.5–2.0: 8.3 Score 2.5–3.0: 15.8 Score 3.5–4.0: 34.1 |
|
| ds-GPA (GI and breast) 13 | 2008 | 4,529 | NA | 0: < 70 1: 70 2: 80 3: 90 4: 100 |
NA | NA | NA | Score range of 0–4 | Score 0–1: 3.1–6.1 Score 1.5–2.5: 4.4–9.4 Score 3: 6.9–16.9 Score 3.5–4: 13.5–18.7 |
|
Abbreviations: AdenoCa, adenocarcinoma; ALK, anaplastic lymphoma kinase; ds-GPA, diagnosis-specific Graded Prognostic Assessment; EGFR, endothelial growth factor; GI, gastrointestinal; KPS, Karnofsky Performance Score; NA, not applicable; neg/unk, negative/unknown; NSCLC, nonsmall cell lung cancer; pos, positive; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
Same scale as original GPA.
Published studies suggest other variables that warrant consideration as prognostic factors. In one study of lung cancer BM, tumors that were more spherical in morphology were associated with better local control after SRS. 57 In another study, a pretreatment biological measure, the neutrophil-to-lymphocyte ratio, appeared to be predictive of local failure after SRS and poor survival. 58 Finally, radiomic features of NSCLC BM have also been associated with prognosis. A study analyzing 576 NSCLC brain metastases in 161 patients treated with SRS identified select radiomic features of BM on MRI that were associated with clinical survival. 59 Future prognostic scales should consider incorporation of these variables.
Number of Brain Metastases
The Congress of Neurological Surgeons (CNS) 2019 Guidelines for the use of SRS for the treatment of metastases in adults 60 provide level 3 recommendations for SRS as treatment of patients presenting with 2 to 4 BM.
This recommendation should be considered in the context of the study by Yamamoto et al, who conducted a prospective study of 1,194 SRS-treated BM patients. While the overall survival for patients with one BM (13.9 months) was improved relative to those with 2 to 10 BM (10.8 months), there were no significant differences in overall survival between those with 2 to 4 BM and those with 5 to 10 BM. 61 In the largest retrospective study to date, Ali et al 63 analyzed 5,750 patients treated with SRS for BM and recapitulated the findings reported by Yamamoto et al. 62 Other studies have reported similar findings. 30 63 64 Moreover, in patients with more than 4 lesions, there is level 3 evidence for the use of SRS to improve overall survival when the cumulative volume is less than 7 mL. 60 These studies support consideration for SRS in the treatment of more than 4 BM in select circumstances. The findings that lesion size, 63 64 patient KPS, 30 and tumor histology 13 63 influence local control following SRS treatment of multiple BM bear relevance to this decision.
Cumulative Intracranial Tumor Volume
CITV is defined as the sum of the volume of all BM detected at the time of diagnosis. It is an important prognostic factor for patients afflicted with SRS. In general, maximal radiation dose that can be safely delivered during SRS is inversely proportional to the volume of the BM. As such, dose deescalation is often required in the treatment of BM with larger CITV. 44 Moreover, BM with larger CITV is more likely to be associated with mass effect, which portends to poor prognostication. Finally, larger CITV may reflect an aggressive biology, which necessarily impacts survival prognostication. 65 66
The CNS 2019 Guidelines for the use of SRS for the treatment metastases in adults 60 provide level 3 recommendations for the use of SRS in the treatment of BM with cumulative volume of less than 7 mL. However, it is important to keep in mind that the prognostic threshold for CITV differs depending on the cancer histology. For instance, the prognostic CITV threshold for colorectal BM is 10 mL while the prognostic CITV threshold is 4 mL for lung cancer, melanoma, and RCC. In contrast, CITV does not influence survival in breast cancer who undergo SRS treatment for BM. 67 In this context, the 7-mL guideline is more of an “average” of the prognostic threshold for the various cancer types. It is important to bear this observation in mind when considering the CNS guidelines.
Additionally, it is essential to recognize that because of the heterogeneity in the volume of BMs, the number of BM does not always correlate with the CITV. In multiple studies, CITV and number of BM constitute independent prognostic factors. 45 65 68 69 Thoughtful consideration of these variables in the context of cancer histology, patient condition, and molecular profile of the BM is warranted.
Complications
Recurrent Brain Metastasis
Depending on tumor histology and CITV, up to 30% of SRS-treated BM recur. Although repeated SRS treatments of these lesions are feasible, they are associated with increased risk for radiation necrosis (RN) and treatment failure. 70 As such, stereotactic laser ablation (SLA) represents an alternative therapeutic option. SLA involves the insertion of a fiber-optic probe into the lesion, followed by laser activation to trigger thermocoagulation ( Fig. 1 ). As a stand-alone treatment for BM that recur after SRS, local control is largely influenced by the percent of tumor ablation. 71 Tumor recurrence is more likely when the ablation is incomplete. 72 In completed ablated BM, reported local control is consistently above 80%. 74 However, incomplete ablation can be safely combined with repeat, hypofractionated SRS to improve local control. 72 Importantly, SLA has been shown to improve quality of life for patients suffering from BM that recurred after SRS. 73
Fig. 1.
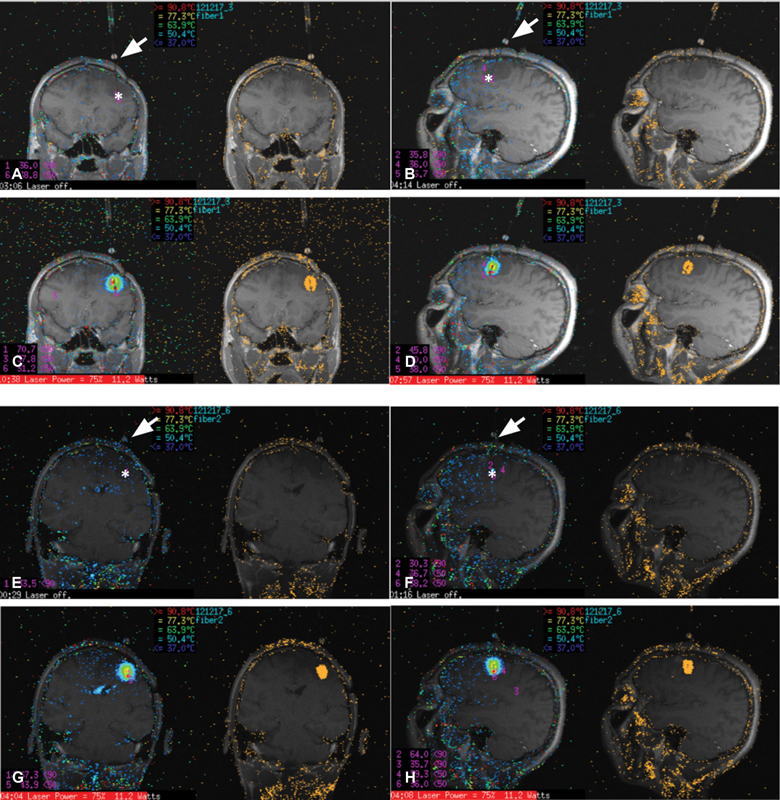
Thermometry of stereotactic laser ablation. Panels ( A ) (preablation magnetic resonance imaging [MRI]) and ( C ) (thermometry) represent coronal images; ( B ) (preablation MRI) and ( D ) (thermometry) demonstrate sagittal images. Panels ( E ) (preablation MRI) and ( G ) (thermometry) represent coronal images of the same patient in a second ablation; panels ( F ) (preablation MRI) and ( H ) (thermometry) demonstrate sagittal images. Temperature gradient demonstrated as heat maps. Orange pixels indicate regions of irreversible thermal damage. Arrows indicate insertion site. Asterisks indicate probe tip location.
Distant Recurrence
Although SRS provides good local tumor control, lesions distant to the original treated site can develop. In patients who suffer distant BM after initial SRS, the duration between the two treatments is approximately 6 months. 45 70 74 Additionally, approximately 20% of patients require more than one additional SRS as treatment of distant BM. The actuarial freedom from progression of the retreated tumors at 52 weeks is 92.4% 74 and local control rate at 6 months after salvage SRS is 90.7%. 70 Importantly, patients who undergo 1, 2, 3, 4 or greater repeat SRS exhibit comparable survival, suggesting efficacy of treatment. Notably, patients who received repeated SRS were more likely to be younger, have control of systemic disease, have metastases with smaller cumulative total volume, and suffer from melanoma, indicating a bias in patient selection for consideration of repeat SRS. 45 Repeat SRS of distant lesions improves quality of life for treated patients in terms of improving neurologic function as well as discontinuation of corticosteroids. 75
Multiple repeat SRS treatments, however, are associated with a risk for neurologic morbidity, including radionecrosis, nonspecific fluid-attenuated inversion recovery (FLAIR) signal abnormalities, cyst formation seizures, and hemorrhage. 63 76 77 78 79
Leptomeningeal Disease
Leptomeningeal disease involves the seeding of BM cells along the pia mater, arachnoid mater, and the cerebrospinal fluid (CSF)-filled subarachnoid space. It is a dreaded complication of cancer, with an extremely poor prognosis of survival that ranged 8 weeks to 6 months. 3 There has been no definitive conclusion as to whether SRS increases the risk of leptomeningeal disease. However, a recent systematic review suggests patients treated with SRS are at increased risk for developing leptomeningeal disease when the primary tumor histology was breast cancer. 80 Several studies have compared outcomes of SRS alone with resection surgery + SRS and identified an increased risk of leptomeningeal disease in the former. 81 82 83 The underlying premise is that surgical manipulation disperses cancer cells into the CSF space. Incidence of leptomeningeal disease after surgical resection with or without postresection SRS ranges 10 to 20%. 58 81 The type of leptomeningeal disease that occurs after surgery, however, appears to differ from that which develops without surgery. On MRI, leptomeningeal disease after surgical resection tends to appear nodular in contrast to the classic “sugar-coating.” Moreover, the nodular leptomeningeal disease is associated with improved overall survival. 58 84
There is an increasing number of studies suggesting that the risk of leptomeningeal after surgery can be mitigated by neoadjuvant SRS prior to surgical resection 85 86 87 88 ( Table 3 ). Reported incidence of leptomeningeal disease for patients treated with neoadjuvant SRS followed by surgery ranged from 5 to 10%. Further investigation into this novel paradigm is warranted.
Table 3. Neoadjuvant versus adjuvant SRS.
| Year of publication | Type of study and cohort size | Groups | BM characteristics | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Local recurrence | Overall survival | Radiation necrosis incidence | Leptomeningeal disease | |||||
| Prabhu et al 17 | 2017 | Retrospective, 213 (223 BM) |
1. SRS alone (
n
= 157)
2. Gross total resection + SRS ( n = 63 neoadjuvant, n = 94 adjuvant) |
Large BM (≥ 4 cm 3 ) | 1-year recurrence rate 1. 36.7% 2. 20.5% |
2 year OS rate 1. 19.8% 2. 38.9% |
1 year rate 1. 12.3% 2. Neoadjuvant: 5%, Adjuvant: 22.6% |
1 year rate 1. 1.9% 2. 5.8% a |
| Mahajan et al 18 | 2017 | Randomized controlled, 132 |
1. Resection alone (
n
= 68)
2. Gross total resection + adjuvant SRS ( n = 64) |
Resection of 1–3 BM, resection cavity diameter of ≤ 4 cm | 1-year recurrence-free rate 1. 43% 2. 72% |
Median OS time 1. 18 months 2. 17 months a |
NA | 1 year rate 1. 16% 2. 28% a |
| Johnson et al 83 | 2016 | Retrospective, 330 |
1. SRS alone (
n
= 218)
2. Gross total resection + adjuvant SRS ( n = 112) |
1–4 BM | NA | Median OS time 1. 10.6 months 2. 12.9 months a |
NA | 1 year rate 1. 5.2%2. 16.9% |
| Prabhu et al 87 | 2018 | Retrospective, 117 (125 BM) | Neoadjuvant SRS + gross total resection | 70% of patients with 1 BM | 1-year recurrence rate: 19.9% 2-year recurrence rate: 25.1% |
Median OS time: 17.2 months OS 2-year rate: 36.7% |
1 year rate 5.1% 2 year rate 8.1% |
1 and 2 year rate: 4.3% |
| Patel et al 88 | 2016 | Retrospective, 180 |
1. Neoadjuvant SRS + resection (
n
= 66)
2. Resection + adjuvant SRS ( n = 114) |
90% of patients had 1–3 BM | 1-year recurrence rate 1. 15.9% 2. 12.6% a |
Median OS time 1. 17.1 months 2. 13.5 months |
2 year rate 1. 4.9% 2. 16.4% |
2 year rate 1. 3.2% 2. 16.6% |
| Patel et al 89 | 2018 | Retrospective, 12 | Neoadjuvant SRS + surgical resection | Median tumor diameter: 3.66 cm | 6-month tumor control rate: 81.8% 1-year tumor control rate: 49.1% |
OS rate at 6 months: 83.3% OS rate at 1 year: 74.1% |
No evidence of RN | 1 year rate 16% ( n = 2) |
Abbreviations: BM, brain metastasis; NA, not applicable; OS, overall survival; RN, radiation necrosis; SRS, stereotactic radiosurgery.
Nonsignificant differences between groups.
Radiation Necrosis and Other Complication from SRS
RN is a poorly defined term that refers to MR changes occurring at or in proximity to a BM treated by SRS or WBRT. These findings can include new regions of contrast enhancement, increased FLAIR abnormality, or a combination of both. 31 89 Histological features of RN include coagulative and liquefactive necrosis of the white matter, thickened hyalinization of vessels, as well as a variable density of reactive cells and inflammatory cells. 90 While some tumor cells may be present, the threshold for determining active tumor versus RN is poorly defined and vary widely between pathologists.
The true incidence of RN following SRS for BM is difficult to estimate given the lack of a standardized definition. 89 90 Reported incidence ranges from 5 to 30%. 9 31 63 70 Most RN occur within 2 years of SRS, 44 though delayed RN decades after SRS has been reported. 91 Most RN are not associated with neurologic deterioration, though up to 54% of patients with RN may be symptomatic. 92 Risk factors for SRS-induced RN include radiation dose, 44 63 repeat SRS, 70 and tumor mutations. 92
Patients with asymptomatic RN are monitored with surveillance imaging. 90 Symptomatic RN are typically managed with corticosteroid treatment. 89 For patients whose RN symptoms are refractory to corticosteroid treatment, bevacizumab therapy, 93 hyperbaric oxygen, 94 95 surgical resection, 15 96 or SLA are considered. 97 Of these treatments, laser ablation shows tremendous promise in terms of efficacy. In independent studies involving retrospective and prospective design, SLA has significant steroid-sparing effects on symptomatic RN. 73 97 98 99 More than 85% of the RN treated with SLA resolves on subsequent MRI 75 100 101 102 103 104 105 ( Table 4 ).
Table 4. Treatment of radiation necrosis with SLA—summary of studies reporting outcomes for radiation necrosis due to SRS-treated brain tumors.
| Study | Year of publication | Cohort | Lesion description | Local control | Overall survival | RN response | Resolution of symptoms | Steroid use | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Ahluwalia et al 75 | 2018 | 42 | 19 patients with RN (confirmed with biopsy), median lesion volume 6.4 cm 3 | PFS of 74% at 26 weeks | 72% at 26 weeks (82.1% for RN subgroup) | 100% (4/4) of RN treated lesions (total SLA ablation) showed complete response | NA | 37% reduced or stopped steroid use at 12 weeks | NA |
| Rao et al 99 | 2014 | 15 | Intracranial recurrent enhancing lesions (BM or RN). Average lesion size was 3.7 cm 3 | PFS of 75.8% at 24 weeks | 57% at 37 weeks | NA | 5/7 symptomatic patients had resolution/decrease of symptoms | NA | 1 asymptomatic hemorrhage and 1 neurological deficit |
| Hong et al 101 | 2019 | 75 | 33 patients with RN, 18 treated with SLA, mean volume of 6.29 cm 3 , all lesions were 100% ablated | 87.8% local PFS at 1 year | 69% at 1 year | NA | 87% improved in symptoms | 34.8% weaned off steroids | NA |
| Rammo et al 102 | 2018 | 10 | All patients with biopsy confirmed RN, 86% lesion volume ablated | NA | 64.8% at 1 year | 69% with RN volume decrease at 6 months | NA | 7/10 weaned off steroids | 4 patients with neurological deficit |
| Smith et al 103 | 2016 | 25 | All patients with biopsy confirmed grade 3 and 4 RN | PFS time: 9.1 months | OS time: 39.2 months | 5/15 with mean 26.2% decrease in size at 6 months | NA | 3/7 weaned off steroids | NA |
| Chaunzwa et al 105 | 2018 | 30 | Intracranial recurrent enhancing lesions (BM or RN). 19 lesions were biopsy-confirmed RN | NA | 26.1% at 1 year | 77% decrease in FLAIR volume at 6 months | 32% with resolution of symptoms | 63% weaned off steroids | NA |
Abbreviations: BM, brain metastasis; FLAIR, fluid attenuated inversion recovery; NA, not available; OS, overall survival; PFS, progression-free survival; RN, radiation necrosis; SLA, stereotactic laser ablation; SRS, stereotactic radiosurgery.
Conclusion
BM is a frequent sequela of systemic cancer. For patients with oligometastatic BM, SRS is preferred to WBRT given the deleterious effects of the latter on neurocognition. Optimizing clinical decisions in SRS-treated BM patients requires reliable prognostication through synthesis of information pertaining to the condition of the patient, the characteristics of the tumor, and the availability of efficacious systemic therapy. Thoughtful consideration in terms of the number of metastasis and CITV is warranted when considering SRS. Local recurrence, distant recurrence, leptomeningeal disease, and RN present challenges in SRS-treated BM patients. Emerging literature suggests new technology platforms, including stereotactic laser thermal ablation, 72 show promise in navigating these challenges.
Footnotes
Conflict of Interest None declared.
References
- 1.Patchell R A. The treatment of brain metastases. Cancer Invest. 1996;14(02):169–177. doi: 10.3109/07357909609018892. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan J S, Sloan A E, Davis F G, Vigneau F D, Lai P, Sawaya R E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Valiente M, Ahluwalia M S, Boire A. The evolving landscape of brain metastasis. Trends Cancer. 2018;4(03):176–196. doi: 10.1016/j.trecan.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P D, Jaeckle K, Ballman K V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(04):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey P. Brain metastases: treatment options to improve outcomes. CNS Drugs. 2002;16(05):325–338. doi: 10.2165/00023210-200216050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Meyers C A, Smith J A, Bezjak A. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(01):157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg M S. 7 th ed. . New York: Thieme; 2006. Handbook of Neurosurgery. [Google Scholar]
- 8.Suh J H, Kotecha R, Chao S T, Ahluwalia M S, Sahgal A, Chang E L. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(05):279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 9.van den Bent M J. The diagnosis and management of brain metastases. Curr Opin Neurol. 2001;14(06):717–723. doi: 10.1097/00019052-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Langer C J, Mehta M P. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23(25):6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 11.Weaver B D, Goodman J R, Jensen R. Concurrent radiosurgery and systemic therapies for melanoma brain metastases: a systematic review. Cureus. 2019;11(11):e6147. doi: 10.7759/cureus.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y-L, Ahn M-J, Garassino M C. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36(26):2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto P W, Chao S T, Sneed P K. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(03):655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Jenkinson M D, Haylock B, Shenoy A, Husband D, Javadpour M. Management of cerebral metastasis: evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer. 2011;47(05):649–655. doi: 10.1016/j.ejca.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Lassman A B, DeAngelis L M.Brain metastases Neurol Clin 200321011–23., vii vii [DOI] [PubMed] [Google Scholar]
- 16.Gough M, Nielsen M, Coulter I C, Holliman D. Survival outcomes following craniotomy for intracranial metastases from an unknown primary. Int J Clin Oncol. 2020;25(08):1475–1482. doi: 10.1007/s10147-020-01687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhu R S, Press R H, Patel K R. Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(02):459–467. doi: 10.1016/j.ijrobp.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan A, Ahmed S, McAleer M F. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(08):1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill B P, Iturria N J, Link M J, Pollock B E, Ballman K V, O'Fallon J R. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(05):1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 20.Chidel M A, Suh J H, Reddy C A, Chao S T, Lundbeck M F, Barnett G H. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(04):993–999. doi: 10.1016/s0360-3016(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 21.Patchell R A, Tibbs P A, Regine W F. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 22.Patchell R A, Tibbs P A, Walsh J W. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(08):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong J G, Wronski M, Galicich J, Arbit E, Leibel S A, Burt M. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994;12(11):2340–2344. doi: 10.1200/JCO.1994.12.11.2340. [DOI] [PubMed] [Google Scholar]
- 24.Hagen N A, Cirrincione C, Thaler H T, DeAngelis L M. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40(01):158–160. doi: 10.1212/wnl.40.1.158. [DOI] [PubMed] [Google Scholar]
- 25.Verhaak E, Gehring K, Hanssens P EJ, Aaronson N K, Sitskoorn M M. Health-related quality of life in adult patients with brain metastases after stereotactic radiosurgery: a systematic, narrative review. Support Care Cancer. 2020;28(02):473–484. doi: 10.1007/s00520-019-05136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulvenna P, Nankivell M, Barton R.Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial Lancet 2016388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flickinger J C, Lunsford L D, Kondziolka D. Dose prescription and dose-volume effects in radiosurgery. Neurosurg Clin N Am. 1992;3(01):51–59. [PubMed] [Google Scholar]
- 28.Higuchi Y, Matsuda S, Serizawa T. Gamma knife radiosurgery in movement disorders: Indications and limitations. Mov Disord. 2017;32(01):28–35. doi: 10.1002/mds.26625. [DOI] [PubMed] [Google Scholar]
- 29.Andrews D W, Scott C B, Sperduto P W.Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial Lancet 2004363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 30.El Shafie R A, Celik A, Weber D. A matched-pair analysis comparing stereotactic radiosurgery with whole-brain radiotherapy for patients with multiple brain metastases. J Neurooncol. 2020;147(03):607–618. doi: 10.1007/s11060-020-03447-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Serizawa T, Higuchi Y. A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): irradiation-related complications and long-term maintenance of Mini-Mental State Examination scores. Int J Radiat Oncol Biol Phys. 2017;99(01):31–40. doi: 10.1016/j.ijrobp.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Gatterbauer B, Hirschmann D, Eberherr N. Toxicity and efficacy of Gamma Knife radiosurgery for brain metastases in melanoma patients treated with immunotherapy or targeted therapy-a retrospective cohort study. Cancer Med. 2020;9(11):4026–4036. doi: 10.1002/cam4.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoskin P J, Crow J, Ford H T. The influence of extent and local management on the outcome of radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 1990;19(01):111–115. doi: 10.1016/0360-3016(90)90142-7. [DOI] [PubMed] [Google Scholar]
- 34.Ebner D K, Gorovets D, Rava P. Patients with long-term control of systemic disease are a favorable prognostic group for treatment of brain metastases with stereotactic radiosurgery alone. World Neurosurg. 2017;98:266–272. doi: 10.1016/j.wneu.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Rades D, Nguyen T, Schild S E. Extra-cerebral metastasis - an independent predictor of survival in older patients with brain metastases receiving a local therapy plus whole-brain radiotherapy (WBRT) Anticancer Res. 2020;40(05):2841–2845. doi: 10.21873/anticanres.14258. [DOI] [PubMed] [Google Scholar]
- 36.Mehta M P. The physical, biologic, and clinical basis of radiosurgery. Curr Probl Cancer. 1995;19(05):265–329. [PubMed] [Google Scholar]
- 37.Bowden G N, Kim J O, Faramand A, Fallon K, Flickinger J, Lunsford L D. Clinical dose profile of Gamma Knife stereotactic radiosurgery for extensive brain metastases. J Neurosurg. 2020;134(05):1430–1434. doi: 10.3171/2020.3.JNS193369. [DOI] [PubMed] [Google Scholar]
- 38.Adler J R, Jr, Chang S D, Murphy M J, Doty J, Geis P, Hancock S L.The Cyberknife: a frameless robotic system for radiosurgery Stereotact Funct Neurosurg 199769(1-4 Pt 2):124–128. [DOI] [PubMed] [Google Scholar]
- 39.Wen N, Snyder K C, Scheib S G. Technical note: evaluation of the systematic accuracy of a frameless, multiple image modality guided, linear accelerator based stereotactic radiosurgery system. Med Phys. 2016;43(05):2527. doi: 10.1118/1.4947199. [DOI] [PubMed] [Google Scholar]
- 40.Thomas E M, Popple R A, Covington E. Initial experiences with first North American deployment of HyperArc radiosurgery treatment planning and delivery system on the edge platform. Int J Radiat Oncol Biol Phys. 2018;102:e519–e520. [Google Scholar]
- 41.Weidlich G A, Schneider M B, Adler J R. Characterization of a novel revolving radiation collimator. Cureus. 2018;10(02):e2146. doi: 10.7759/cureus.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker C, Lowe M, Radhakrishna G. An introduction to proton beam therapy. Br J Hosp Med (Lond) 2019;80(10):574–578. doi: 10.12968/hmed.2019.80.10.574. [DOI] [PubMed] [Google Scholar]
- 43.Meeks S L, Pukala J, Ramakrishna N, Willoughby T R, Bova F J. Radiosurgery technology development and use. J Radiosurg SBRT. 2011;1(01):21–29. [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw E, Scott C, Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(02):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 45.Marshall D C, Marcus L P, Kim T E. Management patterns of patients with cerebral metastases who underwent multiple stereotactic radiosurgeries. J Neurooncol. 2016;128(01):119–128. doi: 10.1007/s11060-016-2084-2. [DOI] [PubMed] [Google Scholar]
- 46.Loo M, Pin Y, Thierry A, Clavier J B. Single-fraction radiosurgery versus fractionated stereotactic radiotherapy in patients with brain metastases: a comparative study. Clin Exp Metastasis. 2020;37(03):425–434. doi: 10.1007/s10585-020-10031-5. [DOI] [PubMed] [Google Scholar]
- 47.Ito D, Aoyagi K, Nagano O, Serizawa T, Iwadate Y, Higuchi Y. Comparison of two-stage Gamma Knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol. 2020;147(01):237–246. doi: 10.1007/s11060-020-03421-y. [DOI] [PubMed] [Google Scholar]
- 48.Lau S KM, Patel K, Kim T. Clinical efficacy and safety of surface imaging guided radiosurgery (SIG-RS) in the treatment of benign skull base tumors. J Neurooncol. 2017;132(02):307–312. doi: 10.1007/s11060-017-2370-7. [DOI] [PubMed] [Google Scholar]
- 49.Gaspar L, Scott C, Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(04):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto M, Sato Y, Serizawa T. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83(05):1399–1405. doi: 10.1016/j.ijrobp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Lutterbach J, Bartelt S, Stancu E, Guttenberger R. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol. 2002;63(03):339–345. doi: 10.1016/s0167-8140(02)00119-6. [DOI] [PubMed] [Google Scholar]
- 52.Weltman E, Salvajoli J V, Brandt R A. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46(05):1155–1161. doi: 10.1016/s0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 53.Lorenzoni J, Devriendt D, Massager N. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60(01):218–224. doi: 10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Sperduto P W, Berkey B, Gaspar L E, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(02):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 55.Sperduto P W, Kased N, Roberge D. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(04):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieder C, Hintz M, Bilger A, Oehlke O, Grosu A L. Validation of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA) J Clin Med Res. 2018;10(03):178–181. doi: 10.14740/jocmr3248w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sperduto P W, Jiang W, Brown P D. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA) Int J Radiat Oncol Biol Phys. 2017;99(04):812–816. doi: 10.1016/j.ijrobp.2017.06.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko P H, Kim H J, Lee J S, Kim W C. Tumor volume and sphericity as predictors of local control after stereotactic radiosurgery for limited number (1-4) brain metastases from nonsmall cell lung cancer. Asia Pac J Clin Oncol. 2020;16(03):165–171. doi: 10.1111/ajco.13309. [DOI] [PubMed] [Google Scholar]
- 59.Shi S, Sandhu N, Jin M C. Stereotactic radiosurgery for resected brain metastases: single-institutional experience of over 500 cavities. Int J Radiat Oncol Biol Phys. 2020;106(04):764–771. doi: 10.1016/j.ijrobp.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 60.Huang C-Y, Lee C-C, Yang H-C. Radiomics as prognostic factor in brain metastases treated with Gamma Knife radiosurgery. J Neurooncol. 2020;146(03):439–449. doi: 10.1007/s11060-019-03343-4. [DOI] [PubMed] [Google Scholar]
- 61.Graber J J, Cobbs C S, Olson J J. Congress of neurological surgeons systematic review and evidence-based guidelines on the use of stereotactic radiosurgery in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84(03):E168–E170. doi: 10.1093/neuros/nyy543. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto M, Serizawa T, Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(04):387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 63.Ali M A, Hirshman B R, Wilson B. Survival patterns of 5750 stereotactic radiosurgery-treated patients with brain metastasis as a function of the number of lesions. World Neurosurg. 2017;107:944–9510. doi: 10.1016/j.wneu.2017.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minniti G, Capone L, Nardiello B. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol. 2020;148(01):47–55. doi: 10.1007/s11060-020-03442-7. [DOI] [PubMed] [Google Scholar]
- 65.Palmer J D, Sebastian N T, Chu J. Single-isocenter multitarget stereotactic radiosurgery is safe and effective in the treatment of multiple brain metastases. Adv Radiat Oncol. 2019;5(01):70–76. doi: 10.1016/j.adro.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirshman B R, Wilson B, Ali M A. Superior prognostic value of cumulative intracranial tumor volume relative to largest intracranial tumor volume for stereotactic radiosurgery-treated brain metastasis patients. Neurosurgery. 2018;82(04):473–480. doi: 10.1093/neuros/nyx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14(03):198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Ali M A, Hirshman B R, Wilson B. Improving the prognostic value of disease-specific graded prognostic assessment model for renal cell carcinoma by incorporation of cumulative intracranial tumor volume. World Neurosurg. 2017;108:151–156. doi: 10.1016/j.wneu.2017.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma M, Jia X, Ahluwalia M. Cumulative intracranial tumor volume and number of brain metastasis as predictors of developing new lesions after stereotactic radiosurgery for brain metastasis. World Neurosurg. 2017;106:666–675. doi: 10.1016/j.wneu.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 70.Joshi R S, Hirshman B R, Ali M A. Prognostic importance of cumulative intracranial tumor volume in patients with gastrointestinal brain metastasis treated with stereotactic radiosurgery. World Neurosurg. 2019;121:e747–e754. doi: 10.1016/j.wneu.2018.09.209. [DOI] [PubMed] [Google Scholar]
- 71.Kwon K-Y, Kong D-S, Lee J-I, Nam D H, Park K, Kim J H. Outcome of repeated radiosurgery for recurrent metastatic brain tumors. Clin Neurol Neurosurg. 2007;109(02):132–137. doi: 10.1016/j.clineuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Carpentier A, McNichols R J, Stafford R J. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950. doi: 10.1002/lsm.21138. [DOI] [PubMed] [Google Scholar]
- 73.Ali M A, Carroll K T, Rennert R C. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(04):E11. doi: 10.3171/2016.7.FOCUS16227. [DOI] [PubMed] [Google Scholar]
- 74.Alattar A A, Bartek J, Jr, Chiang V L. Stereotactic laser ablation as treatment of brain metastases recurring after stereotactic radiosurgery: a systematic literature review. World Neurosurg. 2019;128:134–142. doi: 10.1016/j.wneu.2019.04.200. [DOI] [PubMed] [Google Scholar]
- 75.Ahluwalia M, Barnett G H, Deng D. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(03):804–811. doi: 10.3171/2017.11.JNS171273. [DOI] [PubMed] [Google Scholar]
- 76.Chen J C, Petrovich Z, Giannotta S L, Yu C, Apuzzo M L.Radiosurgical salvage therapy for patients presenting with recurrence of metastatic disease to the brain Neurosurgery 20004604860–866., discussion 866–867 [DOI] [PubMed] [Google Scholar]
- 77.Yomo S, Hayashi M. Salvage stereotactic radiosurgery with adjuvant use of bevacizumab for heavily treated recurrent brain metastases: a preliminary report. J Neurooncol. 2016;127(01):119–126. doi: 10.1007/s11060-015-2019-3. [DOI] [PubMed] [Google Scholar]
- 78.Minniti G, Scaringi C, Paolini S. Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol. 2016;126(01):91–97. doi: 10.1007/s11060-015-1937-4. [DOI] [PubMed] [Google Scholar]
- 79.Fritz C, Borsky K, Stark L S. Repeated courses of radiosurgery for new brain metastases to defer whole brain radiotherapy: feasibility and outcome with validation of the new prognostic metric brain metastasis velocity. Front Oncol. 2018;8:551. doi: 10.3389/fonc.2018.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balermpas P, Stera S, Müller von der Grün J. Repeated in-field radiosurgery for locally recurrent brain metastases: Feasibility, results and survival in a heavily treated patient cohort. PLoS One. 2018;13(06):e0198692. doi: 10.1371/journal.pone.0198692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreau J, Khalil T, Dupic G. Second course of stereotactic radiosurgery for locally recurrent brain metastases: safety and efficacy. PLoS One. 2018;13(04):e0195608. doi: 10.1371/journal.pone.0195608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown D A, Lu V M, Himes B T. Breast brain metastases are associated with increased risk of leptomeningeal disease after stereotactic radiosurgery: a systematic review and meta-analysis. Clin Exp Metastasis. 2020;37(02):341–352. doi: 10.1007/s10585-020-10019-1. [DOI] [PubMed] [Google Scholar]
- 83.Johnson M D, Avkshtol V, Baschnagel A M. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(03):537–543. doi: 10.1016/j.ijrobp.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen T K, Sahgal A, Detsky J. Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neuro-oncol. 2020;22(01):84–93. doi: 10.1093/neuonc/noz144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cagney D N, Lamba N, Sinha S. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019;5(05):703–709. doi: 10.1001/jamaoncol.2018.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prabhu R S, Turner B E, Asher A L. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-oncol. 2019;21(08):1049–1059. doi: 10.1093/neuonc/noz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prabhu R S, Miller K R, Asher A L. Preoperative stereotactic radiosurgery before planned resection of brain metastases: updated analysis of efficacy and toxicity of a novel treatment paradigm. J Neurosurg. 2018 doi: 10.3171/2018.7.JNS181293. [DOI] [PubMed] [Google Scholar]
- 88.Patel K R, Burri S H, Asher A L. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016;79(02):279–285. doi: 10.1227/NEU.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 89.Patel A R, Nedzi L, Lau S. Neoadjuvant stereotactic radiosurgery before surgical resection of cerebral metastases. World Neurosurg. 2018;120:e480–e487. doi: 10.1016/j.wneu.2018.08.107. [DOI] [PubMed] [Google Scholar]
- 90.Vetlova E, Golbin D A, Golanov A V. Preoperative stereotactic radiosurgery of brain metastases: preliminary results. Cureus. 2017;9(12):e1987. doi: 10.7759/cureus.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber P E, Hawighorst H, Fuss M, van Kaick G, Wannenmacher M F, Debus J. Transient enlargement of contrast uptake on MRI after linear accelerator (linac) stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2001;49(05):1339–1349. doi: 10.1016/s0360-3016(00)01511-x. [DOI] [PubMed] [Google Scholar]
- 92.Chao S T, Ahluwalia M S, Barnett G H. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(03):449–457. doi: 10.1016/j.ijrobp.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Babu R, Huang P P, Epstein F, Budzilovich G N. Late radiation necrosis of the brain: case report. J Neurooncol. 1993;17(01):37–42. doi: 10.1007/BF01054272. [DOI] [PubMed] [Google Scholar]
- 94.Miller J A, Bennett E E, Xiao R. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96(05):1060–1069. doi: 10.1016/j.ijrobp.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 95.Levin V A, Bidaut L, Hou P. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(05):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H.Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy J Neurol Sci 2003209(1-2):115–117. [DOI] [PubMed] [Google Scholar]
- 97.Ohguri T, Imada H, Kohshi K. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(01):248–255. doi: 10.1016/j.ijrobp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Alexander E, III, Moriarty T M, Loeffler J S. Radiosurgery for metastases. J Neurooncol. 1996;27(03):279–285. doi: 10.1007/BF00165485. [DOI] [PubMed] [Google Scholar]
- 99.Rao M S, Hargreaves E L, Khan A J, Haffty B G, Danish S F.Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis Neurosurgery 20147406658–667., discussion 667 [DOI] [PubMed] [Google Scholar]
- 100.Luther E, McCarthy D, Shah A. Radical laser interstitial thermal therapy ablation volumes increase progression-free survival in biopsy-proven radiation necrosis. World Neurosurg. 2020;136:e646–e659. doi: 10.1016/j.wneu.2020.01.116. [DOI] [PubMed] [Google Scholar]
- 101.Hong C S, Deng D, Vera A, Chiang V L. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142(02):309–317. doi: 10.1007/s11060-019-03097-z. [DOI] [PubMed] [Google Scholar]
- 102.Rammo R, Asmaro K, Schultz L. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neurooncol. 2018;138(03):609–617. doi: 10.1007/s11060-018-2828-2. [DOI] [PubMed] [Google Scholar]
- 103.Smith C J, Myers C S, Chapple K M, Smith K A. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79 01:S59–S72. doi: 10.1227/NEU.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 104.Rammo R, Scarpace L, Nagaraja T, Lee I. MR-guided laser interstitial thermal therapy in the treatment of recurrent intracranial meningiomas. Lasers Surg Med. 2019;51(03):245–250. doi: 10.1002/lsm.23045. [DOI] [PubMed] [Google Scholar]
- 105.Chaunzwa T L, Deng D, Leuthardt E C. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(01):56–63. doi: 10.1093/neuros/nyx142. [DOI] [PubMed] [Google Scholar]


