ABSTRACT
Background
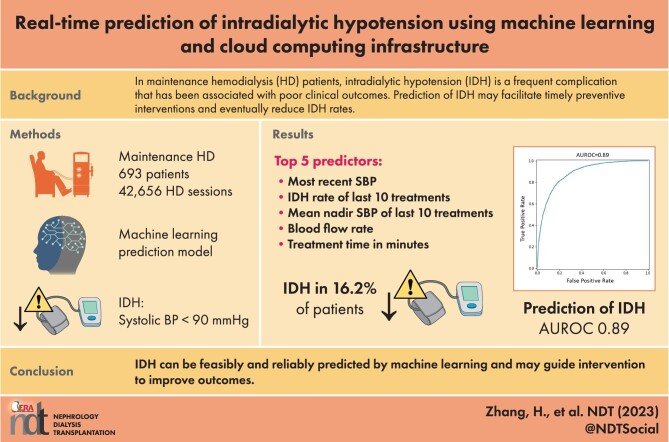
In maintenance hemodialysis patients, intradialytic hypotension (IDH) is a frequent complication that has been associated with poor clinical outcomes. Prediction of IDH may facilitate timely interventions and eventually reduce IDH rates.
Methods
We developed a machine learning model to predict IDH in in-center hemodialysis patients 15–75 min in advance. IDH was defined as systolic blood pressure (SBP) <90 mmHg. Demographic, clinical, treatment-related and laboratory data were retrieved from electronic health records and merged with intradialytic machine data that were sent in real-time to the cloud. For model development, dialysis sessions were randomly split into training (80%) and testing (20%) sets. The area under the receiver operating characteristic curve (AUROC) was used as a measure of the model's predictive performance.
Results
We utilized data from 693 patients who contributed 42 656 hemodialysis sessions and 355 693 intradialytic SBP measurements. IDH occurred in 16.2% of hemodialysis treatments. Our model predicted IDH 15–75 min in advance with an AUROC of 0.89. Top IDH predictors were the most recent intradialytic SBP and IDH rate, as well as mean nadir SBP of the previous 10 dialysis sessions.
Conclusions
Real-time prediction of IDH during an ongoing hemodialysis session is feasible and has a clinically actionable predictive performance. If and to what degree this predictive information facilitates the timely deployment of preventive interventions and translates into lower IDH rates and improved patient outcomes warrants prospective studies.
Keywords: end-stage kidney disease, intradialytic hypotension, machine learning, real-time prediction
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Intradialytic hypotension (IDH) occurs in over 10% of hemodialysis treatments.
Timely interventions may prevent IDH.
What this study adds?
In this manuscript, we developed a machine learning model for real-time (i.e. intradialytic) prediction of IDH, utilizing electronic health records comprising intradialytic blood pressure measurements and multiple treatment- and patient-level variables from 42 656 hemodialysis sessions in 693 in-center hemodialysis patients.
In the training cohort, the model was optimized to generate an alert between 15 and 75 min before an IDH event. In the validation cohort, the model achieved an area under the receiver operating characteristic curve of 0.89.
What impact this may have on practice or policy?
Based on this performance, the model could alert clinicians and trigger the timely deployment of interventions to prevent IDH.
INTRODUCTION
Intradialytic hypotension (IDH) is a common complication of hemodialysis (HD), occurring in 8%–40% of HD sessions [1–4]. IDH reduces quality of life and is associated with morbidity and mortality [1, 4–8]. The management of IDH requires staff interventions, such as patient evaluation, monitoring and adjustment of ultrafiltration rates. It is reasonable to assume that prediction of IDH followed by appropriate preventive measures may lower IDH rates.
Risk factors for IDH are diabetes, cardiovascular disease, autonomic dysfunction and high interdialytic weight gain, to name a few [4, 9, 10]. Because these risk factors are prevalent in many HD patients, IDH prediction is challenging.
Artificial intelligence (AI) and machine learning (ML), and cloud infrastructures enable the evaluation of large data volumes in a secure, reliable and efficient way. This affords an opportunity to develop and leverage models for real-time prediction of IDH among individual patients while undergoing HD. AI and ML have been used to develop models to predict IDH [11–14]. One model developed to predict IDH [defined as systolic blood pressure (SBP) <90 mmHg] based on a relatively limited number of parameters (e.g. body and dialysate temperature, ultrafiltration rate) predicted IDH with a sensitivity of 86% and a specificity of 81% [13]. It is expected that real-time models that consider a larger number of factors may result in improved IDH predictive ability.
Robust data are routinely captured before and during HD, including patient characteristics, clinical status, HD treatment parameters and intradialytic vital signs. These variables can be considered for cloud computing, advanced modeling and near real-time reporting. In this quality improvement project (QIP), we retrospectively assessed data from several HD clinics that used a secure cloud-based computer infrastructure to develop and deploy an ML prediction model that can predict IDH prior to an IDH event in real time. Clinically, prediction is only useful if it predicts an IDH event for a given patient during an ongoing dialysis treatment.
MATERIALS AND METHODS
Participants
We conducted a post hoc analysis of data from a convenience sample comprising patients who received in-center HD in six US Fresenius Kidney Care clinics between January 2020 and November 2020. Four clinics are located in Waltham, MA, and two clinics in New York City, NY. These clinics transfer data from dialysis machines to a secure Internet of Things (IoT) private server via Amazon Web Services (AWS) (Amazon Web Services, Inc., Seattle, WA, USA) using IoT software [15–17]. In total, data from 42 656 dialysis treatments in 693 patients were included. This project was conducted as an internal QIP and therefore was not submitted for Institutional Review Board review.
Data characteristics and cloud architecture
IDH was defined as any intradialytic SBP <90 mm Hg [18]. The outcome (i.e. dependent variable) was a binary classification of patients at risk for IDH within 15–75 min of the given prediction time point. Variables included in the predictive model comprised demographic, clinical and laboratory data obtained from electronic health records and dialysis machine data. Dialysis machine data included variables such as sitting SBP and diastolic blood pressure (DBP), pulse, blood flow rate, dialysate flow rate, ultrafiltration rate, ultrafiltration volume removed and time on dialysis. Additional variables were derived from these data, including changes in SBP, DBP and heart rate during HD, DBP and SBP nadirs over the last 10 treatments, and IDH event rates over the last 10 treatments. Although some of these variables were assessed as frequently as every 10 s (e.g. blood flow rate, dialysate flow rate, ultrafiltration rate, ultrafiltration volume removed, time on dialysis), data points that aligned with SBP measurements were used for analysis. Therefore, whenever intradialytic SBP was measured (approximately every 30 min), a patient's IDH risk within the next 15–75 min was computed in real time. The prediction process took less than a minute.
Patients’ demographics were extracted from electronic health records. Prescribed treatment time, vascular access type, pre- and post-dialysis weight, and the dialysate sodium concentration were also evaluated. Laboratory variables were captured monthly; hemoglobin was measured weekly. In total, 99 variables were evaluated in the present model (Supplementary data, Table S1).
Supplementary data, Fig. S1 shows the cloud-based infrastructure used for this QIP. Dialysis machine data were sent in real-time to the cloud-based platform. The data stream was enriched with patient and historical treatment data, flowed through streaming analytical processes, and the data were segmented into windows of time. Features were calculated for each time window and passed to the ML model. The model results were then visualized on a dashboard, plotting intradialytic SBP and predicted probability of IDH over treatment time. This dashboard could be accessed by the clinical staff and provide insights into patients who are predicted to experience IDH during the ongoing treatment. All streamed machine data and model results could be stored and monitored.
Model design and development
Model design, development and evaluation were substantially guided by inputs from healthcare professionals (HCP) who are experienced in the care of HD patients. HCP requested an actionable 15–75 min prediction time window preceding IDH. Data from the 15 min before an IDH were not used, because an alert within such a short timeframe would not provide sufficient lead time for HCP to respond to a predicted IDH event. Post-IDH data were not considered by the model. HCP indicated that a 10% rate of false IDH alarms (=false positive rate) is clinically and operationally acceptable.
The model was built using the AWS SageMaker development platform and was trained and tested on data collected in the dialysis clinics [19].
To develop the model, we created training (80%) and test (20%) datasets in two ways, depending on the unit of randomization. In the session randomization design, we randomly assigned HD sessions to the respective training and test sets. In the patient randomization design, we randomly assigned patients to training and test sets.
We used Python version 3.7.7 (Python Software Foundation, Fredericksburg, VA, USA) to build the ML model using the XGBoost (Extreme Gradient Boosting) package [20]. Model-specific internal parameters were set to predetermined default values to cover general use cases and were tuned for the specific task to obtain optimal performance using a Bayesian strategy [21]. The number of weak learners was 200, the learning rate was 0.05 and the maximal depth was 7 for the final model. The XGBoost used input variables from the training dataset to construct multiple decision trees, giving each a random sample, and established a series of thresholds that split variables to maximize the information gain. Decision trees were constructed iteratively, and new decision trees were added to predict prior errors. XGBoost decision trees handle missing values without imputation, by including their presence when determining the splits. To show the integrity of the dataset, the percentage of missing values for each variable was summarized in Supplementary data, Table S2.
Evaluation of model performance
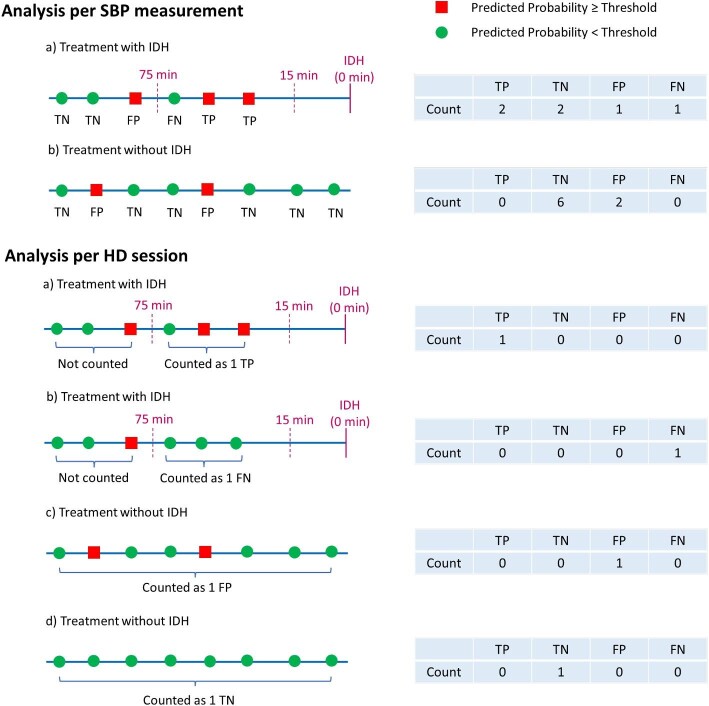
Evaluation of model performance was based on the definitions of true positive (TP), false positive (FP), true negative (TN) and false negative (FN) as defined in Fig. 1. The performance analysis comprised two aspects, one per SBP measurement (usually multiple SBP measurements per HD session) and one that considered the entire HD session. This approach allowed us to look at the model performance from different angles. For both analyses we assessed sensitivity (also termed recall) as [TP/(TP+FN)], and specificity as [TN/(TN+FP)]. For the model performance based on SBP measurements, we also calculated the area under the receiver operating characteristics curve (AUROC). To that end, the rates of TP and FP were computed by the prediction model across the entire IDH probability spectrum (i.e. zero to one). Selected IDH prediction thresholds are presented in the Results section. We considered an AUROC of at least 0.85 as clinically meaningful. We also calculated the area under the precision-recall curve (AUPRC). Precision (also called positive predictive value) is defined as TP/(TP + FP).
Figure 1:
Evaluation of model performance. The performance analysis comprised two aspects, one per SBP measurement (usually multiple SBP measurements per HD session) and one that considered the entire HD session. Evaluation of model performance was based on the definitions of TP, FP, TN and FN.
We also conducted a patient-level analysis of the model's performance. For the respective analysis of model sensitivity, only patients with IDH events were included, because only they contribute TP and FN predictions. Conversely, for the analysis of model specificity, patients who experienced IDH during all treatments had to be excluded because they did not contribute TN and FP results.
In addition to these performance indices, we constructed calibration plots. To that end, we plotted the probability of IDH as predicted by the model versus the true IDH event rate. To increase insights from the calibration plots, we used two binning methods, one with 10 equal bin widths and one with equal sample size per bin (200 bins).
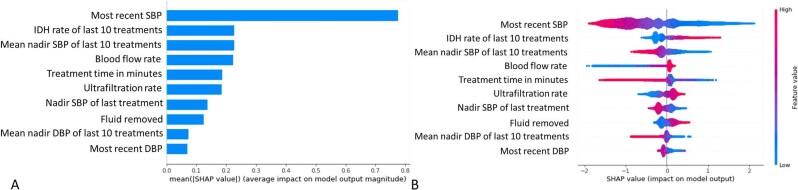
Shapley values were calculated using the SHAP Python package (Python Software Foundation) to determine a particular feature's contribution to the outcome [22, 23]. The SHAP (SHapley Additive exPlanations) value method assigns each feature an importance value for a particular prediction. Shapley values are calculated for each variable and each observation, representing a measure of effect (positive or negative value) of the observed value on each individual prediction. SHAP methods withhold and include individual inputs in all possible combinations, and compare differences between withheld and included data, to compute the mean value of all possible differences for attributing the feature importance. SHAP values are output as log-odds, meaning they are additive explanations of feature importance. Therefore, positive SHAP values increase the predicted probability of IDH, whereas negative SHAP values decrease the probability of IDH. We calculated the overall feature importance for individual variables in the model with the SHAP value method using the mean absolute values for each variable across all observations.
RESULTS
Patient characteristics
We studied 693 in-center HD patients during 42 656 dialysis sessions. Their mean age was 60.5 ± 16.1 years. Most patients were males (58.2%), 47.8% were white and 40.1% had diabetes (Table 1). Of the 693 patients, approximately 80% experienced at least one IDH episode (Supplementary data, Fig. S2). About 16.2% of all HD sessions were complicated by IDH. A total of 355 693 intradialytic SBP were recorded. About 4% of all SBP measurements fell within the window of 75–15 min before an IDH event (Table 2).
Table 1:
Patient baseline demographics (N = 693 patients).
| Variable | ||
|---|---|---|
| Age, years [mean (SD)] | 60.5 (16.1) | |
| Dialysis vintage, years [mean (SD)] | 3.8 (4.6) | |
| Male [N (%)] | 403 (58.2) | |
| Race [N (%)] | ||
| Black | 140 (20.2) | |
| White | 331 (47.8) | |
| Other | 222 (32.0) | |
| Hispanic [N (%)] | 101 (15.9) | |
| Comorbidities [N (%)] | ||
| Chronic obstructive pulmonary disease | 44 (6.3) | |
| Diabetes | 278 (40.1) | |
| HIV/AIDS | 27 (3.9) | |
| Hyperparathyroidism | 648 (93.5) | |
| Myocardial infarction | 34 (4.9) | |
| Peripheral vascular disease | 38 (5.5) | |
| Ischaemic heart disease | 4 (0.5) | |
| Hepatitis | 55 (7.9) | |
| Vascular access type (%) | ||
| Arteriovenous fistula | 57.5 | |
| Arteriovenous graft | 10.7 | |
| Catheter | 31.8 | |
SD, standard deviation; HIV/AIDS, human immunodeficiency virus/acquired immuno-deficiency syndrome.
Table 2:
Characteristics of HD sessions with respect to IDH and BP.
| Variable | All | Training data | Test data |
|---|---|---|---|
| HD sessions [N (% of total)] | 42 656 (100) | 34 124 (80) | 8532 (20) |
| HD sessions with IDH [N (% of HD sessions)] | 6922 (16.2) | 5548 (16.3) | 1374 (16.1) |
| BP measurements [N (% of total)] | 355 693 (100) | 284 268 (79.9) | 71 425 (20.1) |
| BP measurements in the window of 75–15 min before IDH [N (% of BP measurements)] | 14 452 (4.06) | 11 544 (4.06) | 2908 (4.07) |
Model development, performance and top features
All variables used in the prediction model and percentage of missing values for each variable are shown in Supplementary data, Tables S1 and S2, respectively.
Session randomization design
Here we randomized 42 656 HD sessions to training (34 124 sessions) and test (8532 sessions) sets. Supplementary data, Table S3 shows descriptive characteristics of selected model input variables for the entire patient population (N = 42 656), the training (N = 34 124) and the test datasets (N = 8532), respectively. In the test sample, an AUROC of 0.887 [95% confidence interval (CI) 0.881–0.892] was achieved (Fig. 2A). The AUPRC was 0.334 (Supplementary data, Fig. S3).
Figure 2:
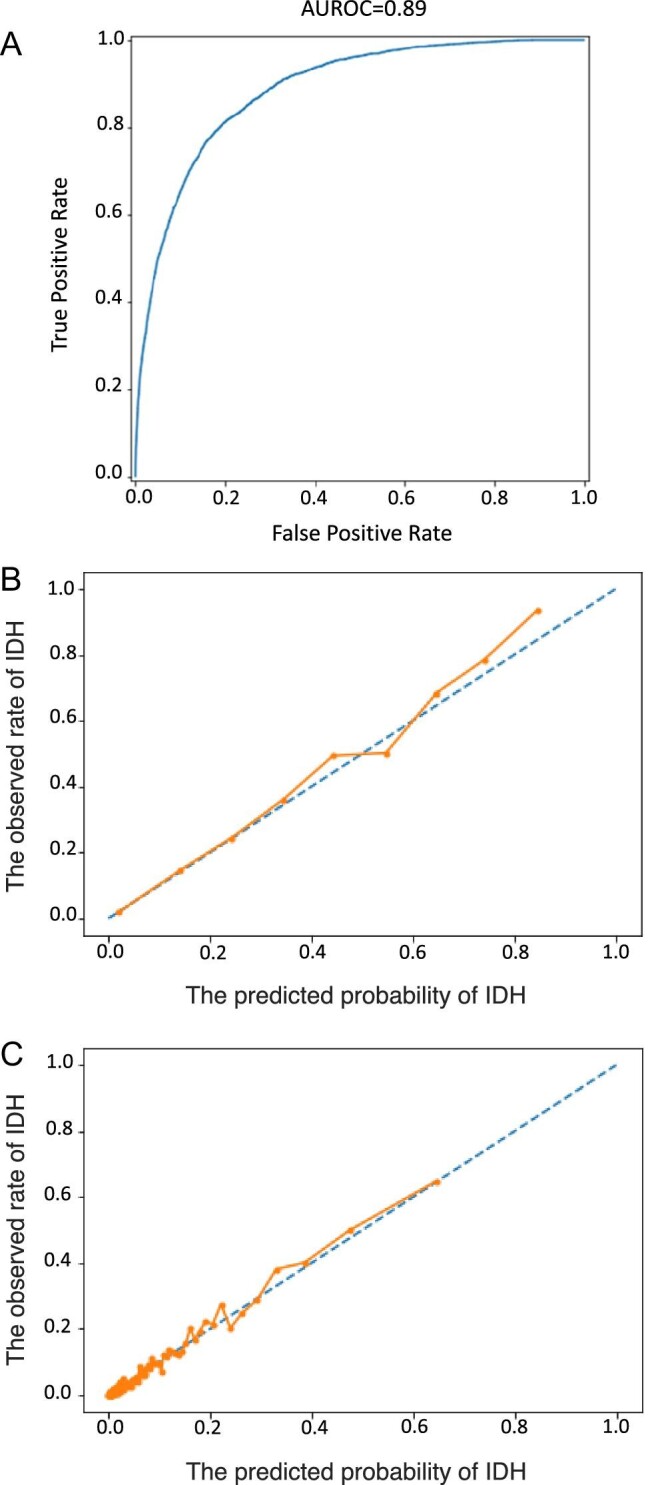
(A) AUROC. The true positive rate is shown on the y-axis, equal to sensitivity. The false positive rate on the x-axis is calculated as 1 – specificity. The 95% CIs of the AUROC are 0.881–0.892. (B) Calibration plot with equal bin width. Probability calibration plot shows the predicted probability against observed events. (C) Calibration plot with equal number of samples per bin (200 bins in total). Here, instead of equal bin-width, we set the bin width based on the number of samples to account for the data distribution (most probabilities are under 0.5).
The performance analysis per SBP measurement is shown in Table 3. Based on discussions with HCP, a false positive rate of 10% was deemed acceptable. Therefore, we choose an IHD probability of ≥0.09 as the threshold for classifying predictions. With this probability threshold, a sensitivity and specificity of 0.65 and 0.90, respectively, were attained (Table 3). Model performance was also assessed considering the entire HD session. The resulting sensitivities and specificities with respect to three selected IDH probability thresholds (0.09; 0.15; 0.2) are shown in Table 4. Using a threshold of ≥0.09, sensitivity and specificity were 0.734 and 0.780, respectively.
Table 3:
Sensitivity, specificity and precision as a function of IDH probability thresholds.
| IDH probability | |||
|---|---|---|---|
| threshold | Sensitivity | Specificity | Precision |
| 0.00 | 1.00 | 0.00 | 0.05 |
| 0.05 | 0.79 | 0.82 | 0.16 |
| 0.09 | 0.65 | 0.90 | 0.22 |
| 0.1 | 0.62 | 0.91 | 0.23 |
| 0.15 | 0.50 | 0.95 | 0.30 |
| 0.2 | 0.38 | 0.97 | 0.35 |
| 0.3 | 0.25 | 0.99 | 0.47 |
| 0.4 | 0.16 | 0.99 | 0.56 |
| 0.5 | 0.10 | 1.00 | 0.62 |
| 0.6 | 0.06 | 1.00 | 0.73 |
| 0.7 | 0.02 | 1.00 | 0.81 |
| 0.8 | 0.01 | 1.00 | 0.94 |
This analysis considered individual SBP measurements.
Table 4:
Descriptive statistics and sensitivity, specificity and precision as a function of IDH probability thresholds.
| Descriptives | IDH probability thresholds | |||
|---|---|---|---|---|
| N | 0.09 | 0.15 | 0.20 | |
| Patients | 648 | |||
| HD sessions | 8532 | |||
| HD sessions with IDH | 1374 | |||
| HD sessions without IDH | 7158 | |||
| Correctly predicted sessions with IDH (TP) | 1009 | 797 | 646 | |
| Falsely predicted sessions with IDH (FP) | 1574 | 901 | 550 | |
| Correctly predicted sessions without IDH (TN) | 5584 | 6257 | 6608 | |
| Falsely predicted sessions without IDH (FN) | 365 | 577 | 728 | |
| Sensitivity | 0.734 | 0.580 | 0.470 | |
| Specificity | 0.780 | 0.874 | 0.923 | |
| Precision | 0.391 | 0.469 | 0.540 | |
This analysis considered entire HD sessions.
We also assessed the model performance at the patient level. For the patient-level sensitivity analysis, we had to exclude 288 patients (44%) from the test dataset because these patients did not experience any IDH and thus contributed neither TP nor FN results. The mean patient-level sensitivity in the remaining 360 patients was 0.48. Only 2 of the 648 patients in the test dataset experienced IDH during every session. These two patients were not considered for the computation of specificity, as they contributed—by definition—neither TN nor FP results. The mean specificity calculated from the remainder of 646 patients is 0.86. In addition, a subgroup analysis in the 288 patients without any IDH resulted in a mean specificity of 0.96.
Figure 2B and C shows calibration plots. Our model is reliable and very likely to return the true probability of an IDH event. The Shapley values (Fig. 3) indicate that the input variables most predictive of IDH within the next 15–75 min were the “most recent SBP,” “IDH rate during the last 10 treatments,” “mean nadir SBP during the last 10 treatments,” “blood flow rate,” “treatment time,” “ultrafiltration rate,” “lowest SBP during the previous treatment,” “fluid removed,” “mean nadir DBP during the last 10 treatments,” and “most recent DBP”.
Figure 3:
Top 10 predictors for IDH in the ML model. (A) Mean absolute SHAP values. (B) The SHAP summary plot shows the degree of each measurement's positive or negative effect on the prediction (x-axis). Warmer colors represent higher observed values for that measurement; cooler colors indicate lower values. For example, the higher (warmer color) the “most recent SBP,” the more the negative impact it has on the model (less chance of IDH).
Patient randomization design
Here we randomized patients to training (554 patients; 33 816 sessions) and test (139 patients; 8840 sessions) sets. The model performance was materially identical to the session randomization design [AUROC of 0.876 (95% CI 0.870–0.882)] (Supplementary data, Fig. S4).
Prediction examples
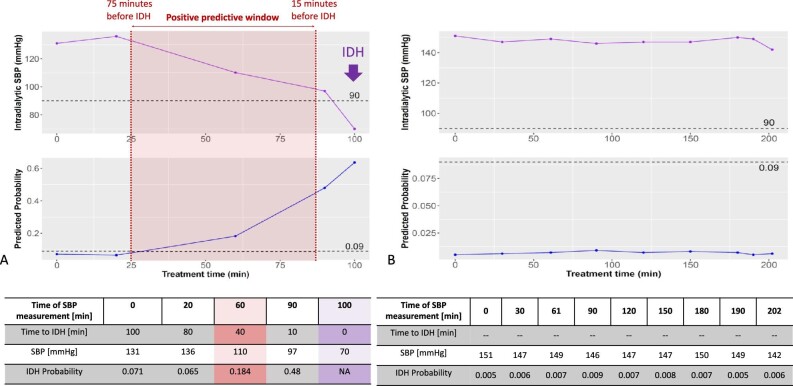
Figure 4A depicts an example dashboard for a patient having IDH, with an SBP of 70 mmHg, at 100 min into treatment. This was the fifth assessment of BP during the session. The dashboard displays a low prediction probability for the first two SBP measurements, then a higher-than-threshold prediction probability at both SBP measurements before the occurrence of IDH.
Figure 4:
Two examples demonstrating a patient with (A; left panel) and without (B; right panel) IDH. (A) An example of SBP (top row) and IDH probability (bottom row) in a patient who experienced IDH. The model predicted the probability of IDH throughout HD with each SBP measurement (approximately every 30 min during a regular HD treatment); the model was trained to predict IDH 15–75 min before the event. An IDH probability ≥0.09 was set as IDH alert threshold (dashed horizonal lines in the lower panels). Data after the IDH event were not considered. (B) An example of SBP and predicted IDH probability in a patient who did not experience IDH; the IDH probability remained below the IDH alert threshold throughout the treatment.
Figure 4B shows a patient with stable SBP and without IDH. The prediction probability associated was well below the threshold of ≥0.09.
DISCUSSION
Predicting IDH in HD patients is challenging due to the numerous patient- and treatment-related factors that affect IDH risk. Clinically, prediction is only useful if it predicts an IDH event for a given patient during an ongoing dialysis treatment. Our analysis shows that IDH can be predicted using real-time dialysis data and ML algorithms during an ongoing dialysis session. Our model was developed and validated on 42 656 HD sessions from a large and diverse patient cohort. It achieved AUROCs of 0.887 (95% CI 0.881–0.892) (session randomization design) and 0.876 (95% CI 0.870–0.882) (patient randomization design), both numbers are significantly greater than 0.85. The model's high performance in the testing dataset suggests that the tool may potentially assist clinicians in intervening proactively and in real-time in patients at risk for IDH.
Although previous studies have evaluated the ability of ML models to predict IDH, most did not consider the impact of time-varying factors, such as blood pressure (BP) [11–14]. For example, Barbieri et al. developed an AI model to guide the management of BP, fluid volume and dialysis dose in chronic HD patients [11]. Their multiple endpoint model predicted Kt/V, fluid volume removal, BP and heart rate, based on patient characteristics, prior hemodynamic responses and dialysis prescriptions. While the model predicted post-dialysis weight and Kt/V accurately, the authors reported that modeling minimum SBP was more difficult, possibly due to measurement errors by machine sensors or to the natural variations in the physiology of hemodynamic reactions.
A more recent deep learning (recurrent neural network) model developed by Lee et al. to predict the real-time risk of IDH using a timestamp-bearing dataset appeared to perform better than previous models [12]. The model predicted IDH, defined as intradialytic SBP <90 mmHg within 1 h, with an AUROC of 0.94 (95% CI 0.94–0.94). According to the authors, the improved performance of their model could be attributed to the fact that it considered the impact of patients’ vital signs and HD settings, which vary over time and are known to affect the risk of IDH [12, 24, 25]. In addition, the results of the analysis’ feature set–ablation analysis and feature ranking analysis, both of which confirmed the value of considering real-time changes in vital signs and HD settings, supported the inclusion of these data in the IDH prediction model [12].
For any real-time prediction, a stable and sophisticated IT infrastructure is essential. A proof-of-concept analysis of real-time data on intradialytic relative blood volume (RBV) using ML confirmed that a cloud-based framework can be used to predict intradialytic RBV changes [26].
The XGBoost model is a robust choice well suited to general ML problems. The model is typically excellent at identifying and modeling complex feature interactions and performs well on tabular data. Although the given model is very accurate, experimentation with other algorithms that natively model time lag dependencies (e.g. recurrent neural network, long short-term memory networks, gated recurrent units) may provide some benefit in the future. In addition, although modeling as a binary classifier simplifies the problem, we may also consider predicting IDH risk directly. In the present model, whereas SBP during the current and previous treatments had the highest SHAP values, the relative importance of blood flow rate as an important contributor to the model was somewhat unexpected. Recent literature showed no relation between blood flow rate and hemodynamics during HD [27, 28]. In a sub-analysis of the Hemodialysis (HEMO) study, an increased incidence of IDH was observed among patients randomized to higher Kt/V targets resulting from increased blood flow, dialysate flow, dialyzer surface area and, if needed, session length [29]. The authors of this study hypothesized that rapid shifts in osmolality explain this observation. Whereas it should be recognized that ML models cannot provide mechanistic explanations for observed associations, the results of our program might provide rationale for future intervention studies assessing the effects of lowering blood flow rate in patients with impending IDH.
The true value of IDH prediction tools depends on the ability of clinicians to promptly intervene to prevent IDH. After evaluating our results, we had extensive discussions with clinical practitioners (i.e. nephrologists and nursing staff) who indicated that a 10% false positive rate (90% specificity) is an operationally reasonable number while still having a decent sensitivity of 65%. The probability threshold was set to ≥0.09 to avoid too many false alarms. Our prediction tool records data in real time and sends them to the cloud, where the ML algorithm computes the probability of IDH. Based on the IDH risk, alerts are generated and reported to the dashboard. Our system and other similar tools that may provide real-time prediction represent a valuable advance in the preemptive management of IDH. Preventive interventions include, e.g. adjustments of the dialysis prescription (such as lowering ultrafiltration rate), the patient's position and fluid administration [6].
While predicting IDH during an ongoing HD session is highly relevant clinically, there is also interest in analyzing the model's performance on a patient level. For the patient-level analysis of model sensitivity, note that we had to exclude 288 patients (44%) because they did not experience an IDH and thus—by definition—contributed neither TP nor FN results. To better understand and quantitate the model sensitivity on a patient level, a larger population needs to be studied. This would also inform an important aspect of the model's patient-level usefulness in clinical practice. In contrast, for the patient-level specificity analysis only the two patients (0.3%) who experienced IDH during all sessions had to be excluded, so the patient number does not impact this analysis.
One limitation of our approach is the post hoc analysis of routinely collected data. Also, we did not calculate a specific sample size for our research; instead, we used a large convenience sample. An additional limitation relates to general drawbacks of ML models, including the fact that it is difficult to understand how the model arrives at its prediction or how any individual factor influences its results [12]. Because the model was created based on a specific US dataset, its design may need to be adjusted to populations with different demographic, clinical or dialysis treatment characteristics. Another shortcoming is the lack of oral medication information in our dataset.
In conclusion, cloud-based ML enables real-time intradialytic IDH prediction. If and to what degree this predictive information facilitates the timely deployment of preventive interventions and translates into lower IDH rates and improved patient outcomes warrants prospective studies.
Supplementary Material
Contributor Information
Hanjie Zhang, Renal Research Institute, New York, NY, USA.
Lin-Chun Wang, Renal Research Institute, New York, NY, USA.
Sheetal Chaudhuri, Fresenius Medical Care, Global Medical Office, Waltham, MA, USA; Maastricht University Medical Center, Maastricht, The Netherlands.
Aaron Pickering, Fresenius Medical Care, Data Solutions, Berlin, Germany.
Len Usvyat, Fresenius Medical Care, Global Medical Office, Waltham, MA, USA.
John Larkin, Fresenius Medical Care, Global Medical Office, Waltham, MA, USA.
Pete Waguespack, Fresenius Medical Care, Digital Technology & Innovation, Waltham, MA, USA.
Zuwen Kuang, Fresenius Medical Care, Digital Technology & Innovation, Waltham, MA, USA.
Jeroen P Kooman, Maastricht University Medical Center, Maastricht, The Netherlands.
Franklin W Maddux, Fresenius Medical Care, Global Medical Office, Waltham, MA, USA.
Peter Kotanko, Renal Research Institute, New York, NY, USA; Icahn School of Medicine at Mount Sinai, New York, NY, USA.
FUNDING
No external funding was provided for the conduct of the program. Analysis was supported by Fresenius Medical Care employee salaries and company infrastructure.
AUTHORS’ CONTRIBUTIONS
Design was performed by H.Z., P.K., P.W. and Z.K. The interpretation, drafting and revision of this manuscript was conducted by all authors. The decision to submit this manuscript for publication was jointly made by all authors, and the manuscript was confirmed to be accurate and approved by all authors.
DATA AVAILABILITY STATEMENT
As this was an internal quality improvement project, data will not be shared publicly.
CONFLICT OF INTEREST STATEMENT
S.C., A.P., L.U., J.L., P.W., Z.K. and F.W.M. are employees of Fresenius Medical Care. P.K., H.Z. and L.-C.W. are employees of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care. J.P.K. is an employee of Maastricht University Medical Center. L.U., S.C., F.W.M. and P.K. have share options/ownership in Fresenius Medical Care. S.C., J.L., L.U., P.K., H.Z. and F.W.M. are inventors on patent(s) in the field of dialysis. P.K. receives honorarium from Up-To-Date, is on the Editorial Board of Blood Purification and Kidney and Blood Pressure Research. J.L. is a guest editor on the Editorial Board of Frontiers in Physiology. F.W.M. has directorships in Fresenius Medical Care Management Board, Goldfinch Bio and Vifor Fresenius Medical Care Renal Pharma.
REFERENCES
- 1. Keane DF, Raimann JG, Zhang Het al. . The time of onset of intradialytic hypotension during a hemodialysis session associates with clinical parameters and mortality. Kidney Int 2021;99:1408–17. 10.1016/j.kint.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuipers J, Verboom LM, Ipema KJRet al. . The prevalence of intradialytic hypotension in patients on conventional hemodialysis: a systematic review with meta-analysis. Am J Nephrol 2019;49:497–506. 10.1159/000500877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sands JJ, Usvyat LA, Sullivan Tet al. . Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 2014;18:415–22. 10.1111/hdi.12138 [DOI] [PubMed] [Google Scholar]
- 4. Kanbay M, Ertuglu LA, Afsar Bet al. . An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J 2020;13:981–93. 10.1093/ckj/sfaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caplin B, Kumar S, Davenport A.. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant 2011;26:2656–63. 10.1093/ndt/gfq763 [DOI] [PubMed] [Google Scholar]
- 6. Chou JA, Streja E, Nguyen DVet al. . Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant 2018;33:149–59. 10.1093/ndt/gfx037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jansen MA, Hart AA, Korevaar JCet al. . Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002;62:1046–53. 10.1046/j.1523-1755.2002.00505.x [DOI] [PubMed] [Google Scholar]
- 8. Stefansson BV, Brunelli SM, Cabrera Cet al. . Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 2014;9:2124–32. 10.2215/CJN.02680314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho A, Lee YK, Oh Jet al. . The relationship between intradialytic hypotension and vascular calcification in hemodialysis patients. PLoS One 2017;12:e0185846. 10.1371/journal.pone.0185846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gul A, Miskulin D, Harford Aet al. . Intradialytic hypotension. Curr Opin Nephrol Hypertens 2016;25:545–50. 10.1097/MNH.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 11. Barbieri C, Cattinelli I, Neri Let al. . Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: proof of concept and first clinical assessment. Kidney Dis 2019;5:28–33. 10.1159/000493479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H, Yun D, Yoo Jet al. . Deep learning model for real-time prediction of intradialytic hypotension. Clin J Am Soc Nephrol 2021;16:396–406. 10.2215/CJN.09280620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin CJ, Chen CY, Wu PCet al. . Intelligent system to predict intradialytic hypotension in chronic hemodialysis. J Formos Med Assoc 2018;117:888–93. 10.1016/j.jfma.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 14. Thakur SS, Abdul SS, Chiu HSet al. . Artificial-intelligence-based prediction of clinical events among hemodialysis patients using non-contact sensor data. Sensors 2018;18:2833. 10.3390/s18092833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amazon . What is AWS https://aws.amazon.com/what-is-aws/ (21 March 2023, date last accessed) [Google Scholar]
- 16. Amazon . AWS HIPAA eligible services https://aws.amazon.com/compliance/hipaa-eligible-services-reference/ (21 March 2023, date last accessed) [Google Scholar]
- 17. HP Enterprise . Edge device https://www.hpe.com/us/en/servers/edgeline-systems.html (21 March 2023, date last accessed) [Google Scholar]
- 18. Flythe JE, Xue H, Lynch KEet al. . Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 2015;26:724–34. 10.1681/ASN.2014020222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amazon . SageMaker. 2020. https://aws.amazon.com/sagemaker/ (21 March 2023, date last accessed).
- 20. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016;785–94. 10.1145/2939672.2939785 [DOI]
- 21. Dernoncourt F, Nemati S, Kassis EBet al. . Hyperparameter selection. In: MIT Critical Data (ed.), Secondary analysis of electronic health records. Cham (CH): Springer, 2016, 419–27. [PubMed] [Google Scholar]
- 22. Lundberg SM, Erion G, Chen Het al. . from local explanations to global understanding with explainable AI for trees. Nat Mach Intell 2020;2:56–67. 10.1038/s42256-019-0138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundberg SMLS. A unified approach to interpreting model predictions. Adv Neural Inf Process Syst 2017;30. NeurlPS Proceedings. [Google Scholar]
- 24. Park J, Rhee CM, Sim JJet al. . A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int 2013;84:795–802. 10.1038/ki.2013.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang F, Wang Y, Tian Yet al. . Pattern recognition and prognostic analysis of longitudinal blood pressure records in hemodialysis treatment based on a convolutional neural network. J Biomed Inform 2019;98:103271. 10.1016/j.jbi.2019.103271 [DOI] [PubMed] [Google Scholar]
- 26. Chaudhuri S, Han H, Monaghan Cet al. . Real-time prediction of intradialytic relative blood volume: a proof-of-concept for integrated cloud computing infrastructure. BMC Nephrol 2021;22:274. 10.1186/s12882-021-02481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schytz PA, Mace ML, Soja AMet al. . Impact of extracorporeal blood flow rate on blood pressure, pulse rate and cardiac output during haemodialysis. Nephrol Dial Transplant 2015;30:2075–9. 10.1093/ndt/gfv316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sherman RA. We lower blood flow for intradialytic hypotension. Semin Dial 2016;29:295–6. 10.1111/sdi.12486 [DOI] [PubMed] [Google Scholar]
- 29. Mc Causland FR, Brunelli SM, Waikar SS.. Dialysis dose and intradialytic hypotension: results from the HEMO study. Am J Nephrol 2013;38:388–96. 10.1159/000355958 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this was an internal quality improvement project, data will not be shared publicly.