ABSTRACT
Background
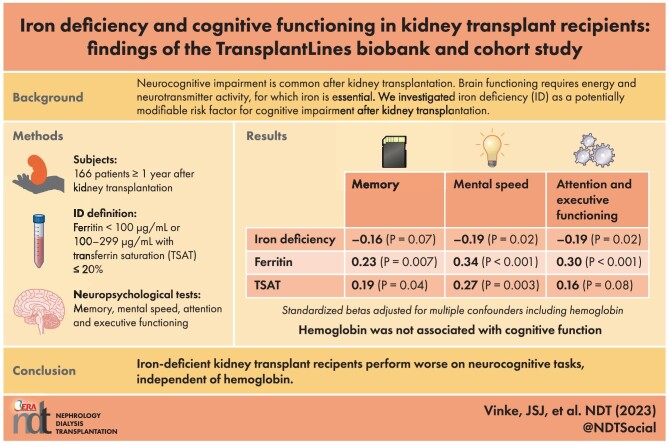
Neurocognitive impairment is common in kidney transplant recipients (KTRs). Adequate brain functioning requires energy and neurotransmitter activity, for which iron is essential. We aimed to investigate iron deficiency (ID) as a potentially modifiable risk factor for cognitive impairment in KTRs.
Methods
We analyzed stable KTRs participating in the TransplantLines Biobank and Cohort study. Participants underwent neuropsychological tests for memory, mental speed, and attention and executive functioning. ID was defined as ferritin <100 µg/mL or 100–299 µg/mL with transferrin saturation (TSAT) ≤20%. Associations between iron status and norm scores of neurocognitive outcomes, corrected for age, sex and education, were assessed using multivariable linear regression analyses adjusted for potential confounders including hemoglobin.
Results
We included 166 KTRs [median (IQR) age 57 (45–65) years, 59% male, estimated glomerular filtration rate 51±18 mL/min/1.73 m2]. Time since transplantation was 5.8 (1.0–12.0) years. Prevalence of ID was 65%. ID was independently associated with lower scores for mental speed (std.β = –0.19, P = .02) and attention and executive functioning (std.β = –0.19, P = .02), and tended to be associated with worse memory (std.β = –0.16, P = .07). Lower plasma ferritin levels were associated with worse memory (std.β = 0.23, P = .007), mental speed (std.β = 0.34, P < .001), and attention and executive functioning (std.β = 0.30, P = .001). Lower TSAT was associated with worse memory (std.β = 0.19, P = .04) and mental speed (std.β = 0.27, P = .003), and tended to be associated with worse attention and executive functioning (std.β = 0.16, P = .08).
Conclusions
Iron-deficient KTRs performed worse on neurocognitive tasks measuring memory, mental speed, and attention and executive functioning. These findings set the stage for prospective studies addressing whether ID correction restores cognitive function after kidney transplantation.
Keywords: cognitive function, iron, kidney transplant recipients
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about the subject?
Kidney transplant recipients have an increased risk of cognitive deficits.
Iron deficiency is highly prevalent after kidney transplantation.
Iron is essential for nucleotide synthesis, neurotransmitter metabolism, neuron myelination and cellular energy.
What this study adds?
In the the TransplantLines Biobank and Cohort Study, extensive neuropsychological testing has been performed systematically in 166 stable kidney transplant recipients.
Results show that iron deficiency and lower plasma ferritin and transferrin saturation are linked to worse cognitive performance.
What impact this may have on practice or policy?
This study shows that iron deficiency is a potentially modifiable risk factor for cognitive deficits in kidney transplant recipients and sets the stage for prospective studies addressing whether iron supplementation restores cognitive function after kidney transplantation.
INTRODUCTION
Over the past decades, surgical and medical improvements have led to longer graft and patient survival after kidney transplantation [1]. However, kidney transplant recipients (KTRs) remain exposed to an increased risk of various health problems including cardiovascular diseases, infections and cognitive impairment [2]. In patients with advanced chronic kidney disease, memory, mental speed, and attention and executive functioning are impaired [3, 4]. After kidney transplantation, cognitive function may improve [5–8] but often remains impaired compared with healthy controls with similar age, sex distribution and educational level [8–10]. These neurocognitive impairments may result in challenges in everyday life situations requiring concentration, storage of important information in memory, and planning and organizing relevant tasks, including medication use. Accordingly, preservation of cognitive function is important for societal participation and overall well-being [9]. Although some possible risk factors for cognitive impairment in KTRs have been proposed, including immunosuppressive medication, vascular senescence and fatigue [11–13], the potential to modify this risk remains limited.
Iron deficiency (ID) is highly prevalent among KTRs, with reported percentages up to 47%, depending on the definition [14–19]. Potential mechanisms contributing to ID in KTRs include blood loss, increased erythropoiesis driven by erythropoietin production in the kidney graft, a hepcidin-mediated shift of iron to the reticuloendothelial system, and impaired gastrointestinal uptake resulting from chronic inflammation [20]. ID after kidney transplantation has been associated with adverse outcomes, including worse graft function and a higher mortality risk [14, 21, 22], independent of co-existing anemia [14]. Furthermore, ID in premenopausal women [23–25] and in older individuals [26, 27] has been associated with impaired cognitive functioning, while iron supplementation might improve memory [28], attention and mental speed [23, 29], and impulse control [29]. The importance of iron in nucleotide synthesis, neurotransmitter metabolism and cellular energy metabolism theoretically supports a relationship between ID and cognitive deficits [30].
These prior findings position ID as a potential modifiable biological risk factor for impaired cognitive function in KTRs. Therefore, the aim of the present study was to investigate the relationship between iron status and cognitive functioning, independent of potential confounders.
MATERIALS AND METHODS
Study population
This study was conducted as a part of the TransplantLines Cohort study (ClinicalTrials.gov Identifier: NCT03272841), a prospective single-center study aimed at finding modifiable risk factors after transplantation. The study design has previously been described in detail [31]. The study protocol was approved by the medical ethical committee of the University Medical Center Groningen (METc 2014/077), conducted in accordance with the principles of the Declaration of Helsinki and consistent with the Good Clinical Practice guidelines provided by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. All KTRs who visited the outpatient clinic from June 2015 onwards were considered for enrollment. Exclusion criteria for participation in the TransplantLines Cohort Study are inability to understand the Dutch language or to intellectually comprehend the questionnaires or tests. All eligible patients are asked informed consent prior to enrollment. Eligible patients who give consent to participate are given the option to participate in either the extensive protocol, comprising body material collection for a biobank, data collection from the clinical file and extensive testing, or in a restricted protocol, comprising only collection of body material for the biobank and collection of data from the clinical file. To limit the effort required from the participants willing to participate in the extensive protocol, they are randomized with a 1:1 ratio to a subgroup involving more physical function tests or a subgroup focused on cognitive assessment.
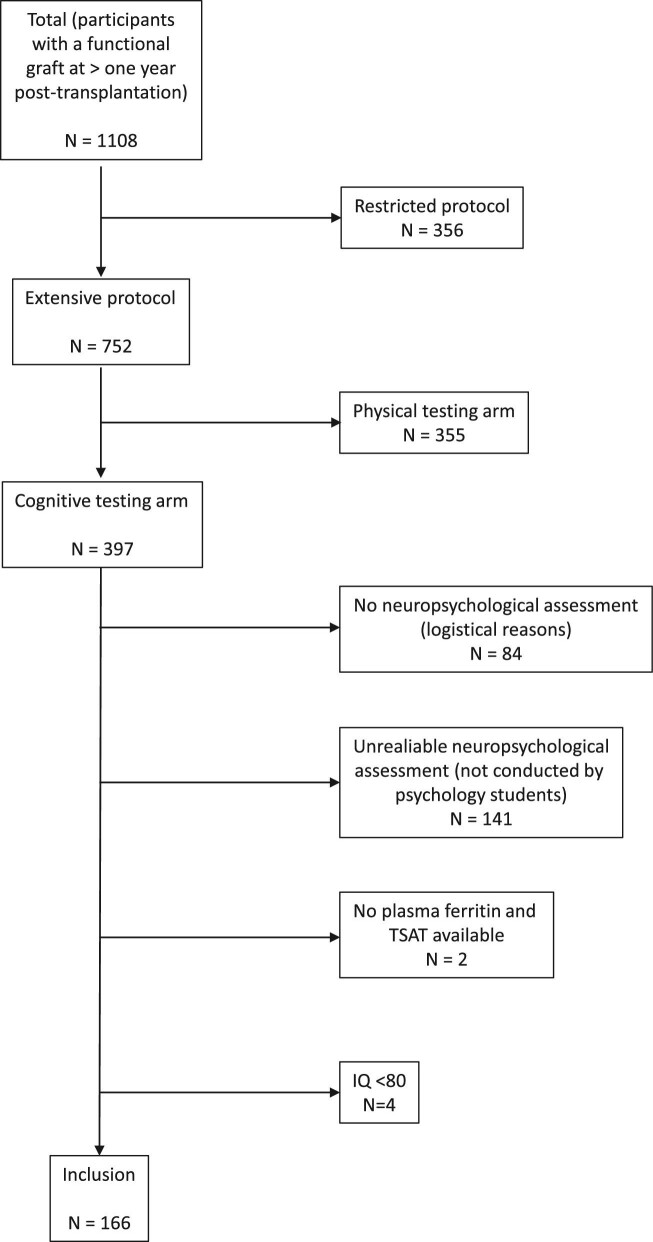
For the current study, stable KTRs with a functional graft at more than 1 year after transplantation with data on plasma iron status and a reliable neurocognitive assessment available were included (Fig. 1). Participants who met criteria for a neurodegenerative disorder based on the cognitive screening test [32], who had an estimated intelligence quotient (IQ) of <80 based on the Dutch version of the National Adult Reading Test (Nederlandse Leestest voor Volwassenen) [33], or who had insufficient comprehension of the Dutch language, were excluded.
Figure 1:
Flowchart of included participants. None of the included participants had a Cognitive Screening Test score below the cut-off.
Data collection
Blood was drawn in the morning according to a strict protocol [31]. Information on age, sex, body height and weight, medical history and medication use was obtained from the patients’ records. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured seven times using a Dinamap pressure meter, of which the median was calculated. Alcohol intake and smoking status were questioned systematically.
The Checklist Individual Strength (CIS), a questionnaire comprising 20 items, was used to assess subjective fatigue in the preceding 2 weeks, which was considered to be a potential mediator [34]. The CIS was completed at or around the time of neuropsychological assessment. Higher scores indicate more severe subjective fatigue. Cardiovascular disease was defined as myocardial infarction, cerebrovascular accident, thromboembolism or peripheral arterial vascular disease.
Laboratory parameters and definitions
The estimated glomerular filtration rate (eGFR) was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation, omitting the Black race coefficient [35]. Plasma iron, ferritin and transferrin were measured using colorimetric assay, immunoassay or immunoturbidimetric assay (Roche Diagnostics, Mannheim, Germany), respectively. Transferrin saturation (TSAT, %) was calculated as 100 × plasma iron (μmol/L) ÷ [25 × transferrin (g/L)]. ID was defined as ferritin <100 µg/mL or 100–299 µg/mL with TSAT ≤20%. Other laboratory parameters were determined using routine techniques.
Neuropsychological assessment
The neuropsychological assessment was performed by a neuropsychologist or by one of the neuropsychology students who had been specifically trained for conduction of this assessment.
Memory
The Digit Span Forward (Digit Span FW; subtest of the Wechsler Adult Intelligence Scale IV) measures immediate memory span [36]. The participant is asked to repeat a series of numbers after the examiner has read them out loud. The score is calculated as the total number of strings repeated correctly, with a maximum of 16. The 15 Words Test (15WT; Dutch version of the Rey Auditory Verbal Learning Test) measures verbal memory [37]. Fifteen unrelated words are presented to the participant in five trials. He or she is asked to recall as many as possible after each trial (immediate recall; 15WTIR). The score is calculated as the total number of words recalled, with a maximum of 75. After 20 min, participants are again asked to recall as many of the 15 words as possible (delayed recall; 15WTDR), with a maximum score of 15. Both raw test scores as well as norm scores (corrected for age, sex and/or education) are collected. The combined score for memory is calculated as the mean of the norm scores of Digit Span FW, 15WTIR and 15WTDR (corrected for 15WTIR).
Mental speed
The Symbol Digit Modalities Test (SDMT) measures information processing speed [38]. Participants are shown nine symbols which are each assigned to a number. Subsequently, they are given a list of those symbols in a random order and are asked to write as many matching numbers below the symbols. The score is the total correct matches within 90 s, with a maximum of 110. The Trail Making Test part A (TMT-A) measures visuomotor and mental speed [39]. Participants have to connect a series of randomly distributed numbers from 1 to 25 in ascending order as fast as possible. Completion of the test is timed in seconds. Both raw test scores as well as norm scores (corrected for age, sex and/or education) are collected. The combined score for mental speed is calculated as the mean of the norm scores of SDMT and TMT-A.
Attention and executive functioning
The Trail Making Test part B (TMT-B) measures cognitive flexibility [39]. Numbers and letters have to be connected as fast as possible in an alternating ascending order. The total score is time (in seconds) to complete the test. The Controlled Oral Word Association Test (COWAT) measures executive control [40]. Participants have to produce as many words as possible that start with a specific letter within 1 min. Total scores from three different starting letters (D-A-T) are added up. The Digit Span Backward (Digit Span BW; subtest of the Wechsler Adult Intelligence Test) measures working memory, which is considered an aspect of executive functioning [36]. The participant has to repeat a series of numbers in the opposite order as the examiner has read them out. The score is the total number of strings repeated correctly, with a maximum of 16. Both raw test scores as well as norm scores (corrected for age, sex and/or education) are used. The combined score for attention and executive functioning is calculated as the mean of the norm scores of TMT-B, COWAT and Digit Span BW.
Statistical analyses
IBM SPSS Statistics version 23.0 (IBM, Chicago, IL, USA) was used to analyze the data. Normally distributed data are presented as mean ± standard deviation (SD), while data with a skewed distribution are presented as median [interquartile range (IQR)]. Categorical data are expressed as number (percentage). We analyzed differences between iron-deficient and non-iron-deficient patients with independent T-tests for normally distributed parameters and Mann–Whitney tests for parameters with a skewed distribution.
Linear regression was used to address associations between parameters reflecting iron status, namely the presence of ID, plasma ferritin level and TSAT, and hemoglobin, as independent variables, and the combined norm scores (corrected for age, sex and/or education) measuring memory, mental speed and attention and executive functioning as dependent variables. Analyses by the combined definition of ID using ferritin and TSAT and analyses using ferritin or TSAT individually were performed separately. Regression analyses were based on univariable (model 1) and multivariable models, to account for potential confounders. In model 2, we adjusted for factors known to be related to iron parameters [BMI, eGFR, high-sensitive C-reactive protein (hs-CRP), total cholesterol level, history of dialysis, history of cardiovascular disease, smoking status and alcohol use]. In model 3, we additionally adjusted for transplantation-related factors [transplant vintage, donor type (living vs post-mortal) and use of calcineurin inhibitors, antiproliferative agents and systemic corticosteroids] and for CIS total score as a potential mediator. Finally, to assess whether associations between iron status and cognitive performance were explained by hemoglobin level as another potential mediator, we additionally adjusted for hemoglobin in model 4.
In all regression analyses, skewed variables (i.e. plasma ferritin, hs-CRP, CIS score and time since transplantation) were naturally log-transformed. Missing data (specified in the Table 1 footnote) were imputed using regressive switching. In none of the variables was more than 10% of the data was missing. Five datasets were multiple-imputed, and results were pooled according to Rubin's rules [41]. In all analyses, a P-value of ≤.05 was considered significant.
Table 1:
Baseline characteristics.
| All (N = 166a) | ID (N = 107) | Non-ID (N = 50) | |
|---|---|---|---|
| Demographics and lifestyle parameters | |||
| Age, years | 57 (45–65) | 56 (45–64) | 59 (47–66) |
| Men, n (%) | 98 (59) | 57 (53) | 37 (74) |
| Educational level, n (%) | |||
| Primary or secondary education not completed | 10 (6) | 6 (6) | 3 (6) |
| Finished low or intermediate level secondary education | 102 (61) | 75 (68) | 26 (52) |
| Finished high level secondary education or university degree | 54 (33) | 28 (26) | 21 (42) |
| Alcohol intake, units/week, n (%) | |||
| None | 62 (38) | 42 (40) | 17 (35) |
| 0–7 units per week | 60 (37) | 42 (40) | 15 (31) |
| >7 units per week | 41 (25) | 22 (21) | 16 (33) |
| Smoking, n (%) | 17 (11) | 11 (11) | 4 (9) |
| Clinical parameters | |||
| BMI, kg/m2 | 27.1 ± 4.1 | 27.0 ± 4.1 | 27.1 ± 4.1 |
| Systolic blood pressure, mmHg | 136 ± 16 | 135 ± 16 | 136 ± 17 |
| History of cardiovascular disease | 45 (27) | 30 (28) | 11 (22) |
| CIS Fatigue score | 60 (43–83) | 67 (43–86) | 54 (44–66) |
| Transplantation parameters | |||
| Time since transplantation, years | 5.8 (1.0–12.0) | 5.0 (1.0–11.3) | 10.0 (2.6–12.3) |
| Pre-emptive transplantation, n (%) | 58 (35) | 45 (42) | 12 (24) |
| Type of donor | |||
| Living, n (%) | 86 (52) | 60 (56) | 21 (42) |
| Postmortal, n (%) | 80 (48) | 47 (44) | 29 (58) |
| Laboratory parameters | |||
| eGFR mL/min/1.73 m2 | 51 ± 18 | 50 ± 17 | 52 ± 21 |
| Hemoglobin, g/dL | 13.5 ± 1.8 | 13.5 ± 1.9 | 13.4 ± 1.8 |
| MCV, fL | 90 ± 5 | 88 ± 5 | 92 ± 5 |
| Plasma ferritin, µg/L | 87 (37–170) | 44 (28–85) | 223 (143–333) |
| Transferrin saturation | 23.1 ± 9.7 | 19.7 ± 9.1 | 30.0 ± 6.8 |
| hs-CRP, mg/L | 1.9 (0.7–4.0) | 1.9 (0.7–3.9) | 2.2 (0.8–4.9) |
| Total cholesterol, mg/dL | 179 ± 41 | 181 ± 39 | 170 ± 40 |
| Medication use, n (%) | |||
| Calcineurin inhibitor | 125 (75) | 88 (82) | 31 (62) |
| Antiproliferative agent | 147 (89) | 93 (87) | 46 (92) |
| Prednisolone | 162 (98) | 105 (98) | 48 (96) |
| Benzodiazepines | 14 (9) | 10 (9) | 4 (8) |
| Antipsychotic agents | 1 (1) | 0 (0) | 1 (2) |
| Erythropoetin-stimulating agents | 3 (2) | 2 (1) | 1 (2) |
Data are presented as mean ± SD, median (IQR) or number (n) with percentage (%).
aIn nine patients, TSAT was missing and therefore it was unknown whether they had ID.
TSAT was available for 151 KTRs. Data on smoking status were available for 155 KTRs. CIS Fatigue score was available in 153 KTRs.
MCV, mean corpuscular volume.
Additionally, several sensitivity analyses were performed. First, we compared parameters of cognitive function in subjects in quintiles of ferritin or TSAT, with the fourth quintile as a reference, since all participants in this quintile had ferritin levels of 100–299 µg/mL. Second, we repeated analyses with ID based on four alternative definitions: (i) TSAT ≤20% [42], (ii) ferritin <300 µg/mL with TSAT ≤20% [14], (iii) ferritin <100 µg/mL, often considered to reflect absolute ID in patients with chronic disease, or (iv) ferritin 100–299 µg/mL, considered to reflect functional ID in patients with chronic disease.
RESULTS
Baseline characteristics
As shown in Table 1, 166 KTRs [median age 57 (IQR 45–65) years, 59% men] were included at a median of 5.8 (1.0–12.0) years after transplantation. Mean eGFR was 51±18 mL/min/1.73 m2 and mean plasma hemoglobin level was 13.5 ± 1.8 g/dL with a median plasma ferritin concentration of 87 (37–170) μg/L and a mean TSAT of 23 ± 10%. ID was present in 107 (65%) subjects. Among iron-deficient KTRs there were more women, more calcineurin inhibitor users and more participants with a pre-emptive transplantation.
Iron status and neurocognitive outcomes
Memory
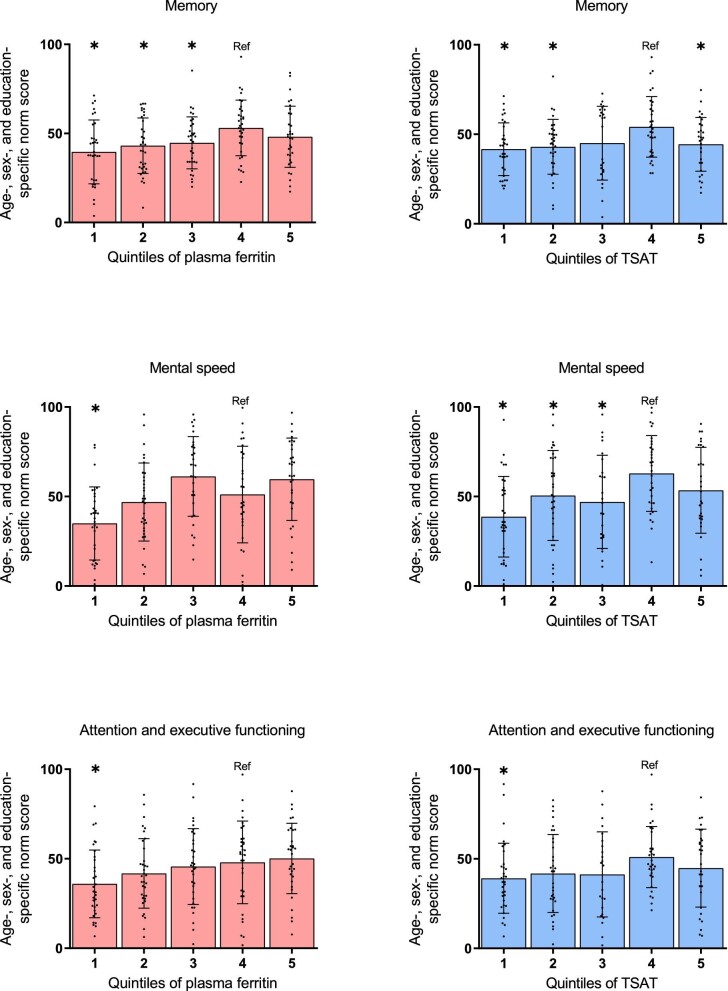
KTRs with ID had significantly lower norm scores on the Digit Span FW (44 ± 28) compared with KTRs without ID (55 ± 32, P = .02, Table 2). Mean memory norm score was 44 ± 16 in KTRs with ID, compared with 49 ± 17 in KTRs without ID (P = .06). In univariable analysis, the presence of ID was associated with a trend towards a worse score on memory, which remained highly similar after multivariable adjustment for potential confounders including hemoglobin (Table 3). Lower plasma ferritin levels were significantly associated with worse memory scores and lower TSAT showed a similar trend (Table 3, Supplementary data, Tables S1 and S2, Fig. 2). The association between plasma hemoglobin and memory did not reach statistical significance.
Table 2:
Different parameters of cognitive function in kidney transplant recipients with versus without ID.
| Neuropsychological parameters | ID (N = 107) | Non-ID (N = 50) | |
|---|---|---|---|
| Memory | Mean of norm scores of the individual tests | 44 ± 16 | 49 ± 17 |
| Digit Span Forward Test | Norm score | 44 ± 28 | 55 ± 32 |
| Raw score | 8 ± 2 | 9 ± 2 | |
| 15 Word Test, immediate recall | Norm score | 30 ± 26 | 36 ± 27 |
| Raw score | 40 ± 10 | 42 ± 10 | |
| 15 Word Test, delayed recall | Norm score | 58 ± 30 | 57 ± 27 |
| Raw score | 9 ± 3 | 9 ± 3 | |
| Mental speed | Mean of norm scores of the individual tests | 47 ± 24 | 57 ± 24 |
| Symbol Digit Modalities Test | Norm score | 50 ± 27 | 59 ± 29 |
| Raw score | 45 ± 10 | 48 ± 12 | |
| Trail Making Test (A) | Norm score | 42 (16–69) | 66 (27–85) |
| Raw score | 33 (26–49) | 31 (24–41) | |
| Attention and executive functioning | Mean of norm scores of the individual tests | 42 ± 21 | 49 ± 19 |
| Trail Making Test (B) | Norm score | 54 (24–73) | 58 (29–81) |
| Raw score | 72 (56–99) | 68 (51–95) | |
| Digit Span Backward Test | Norm score | 37 ± 27 | 52 ± 31 |
| Raw score | 8 ± 2 | 8 ± 2 | |
| Controlled Oral Word Association Test | Norm score | 38 ± 29 | 39 ± 29 |
| Raw score | 33 ± 11 | 35 ± 11 |
Data are presented as mean ± SD or median (IQR). Norm scores are percentile scores adjusted for age, sex and/or educational level.
Table 3:
Linear regression of the association between iron status parameters or hemoglobin level and age-, sex- and/or educational level-specific norm scores for different domains of cognitive function.
| Memory | Mental speed | Attention and executive functioning | ||||
|---|---|---|---|---|---|---|
| St.β | P | St.β | P | St.β | P | |
| Iron deficiency | ||||||
| Model 1 | –0.15 | .06 | –0.19 | .02 | –0.16 | .04 |
| Model 2 | –0.15 | .07 | –0.19 | .02 | –0.19 | .02 |
| Model 3 | –0.17 | .05 | –0.20 | .02 | –0.20 | .02 |
| Model 4 | –0.16 | .07 | –0.19 | .02 | –0.19 | .02 |
| Plasma ferritina | ||||||
| Model 1 | 0.20 | .01 | 0.30 | <.001 | 0.20 | .01 |
| Model 2 | 0.21 | .02 | 0.34 | <.001 | 0.27 | .001 |
| Model 3 | 0.23 | .01 | 0.34 | <.001 | 0.29 | <.001 |
| Model 4 | 0.23 | .007 | 0.34 | <.001 | 0.30 | <.001 |
| TSAT | ||||||
| Model 1 | 0.14 | .09 | 0.22 | .006 | 0.12 | .15 |
| Model 2 | 0.13 | .13 | 0.24 | .004 | 0.12 | .17 |
| Model 3 | 0.17 | .07 | 0.27 | .003 | 0.14 | .12 |
| Model 4 | 0.19 | .04 | 0.27 | .003 | 0.16 | .08 |
| Plasma hemoglobin | ||||||
| Model 1 | –0.15 | .06 | –0.02 | .82 | –0.07 | .35 |
| Model 2 | –0.16 | .09 | –0.04 | .68 | –0.15 | .10 |
| Model 3 | –0.17 | .08 | –0.05 | .64 | –0.14 | .13 |
aln-transformed.
Model 1: crude analysis.
Model 2: adjusted for BMI, eGFR, hs-CRP, total cholesterol level, history of dialysis, history of cardiovascular disease, smoking status, alcohol use.
Model 3: adjusted for model 2 + transplantation-related factors [transplant vintage, donor type (living vs post-mortal) and use of calcineurin inhibitors, antiproliferative agents and systemic corticosteroids] and CIS total score.
Model 4: adjusted for model 3 + plasma hemoglobin level.
Figure 2:
Cross-sectional associations of iron status parameters with the combined scores on the domains of cognitive functioning in KTRs. Scatter dot plots were used to show the relation between ferritin (A–C) or TSAT (D–F), both divided into quintiles, and memory (A, D), mental speed (B, E), and attention and executive functioning (C, F). Data are presented as mean ± SD. Norm scores are percentile scores adjusted for age, sex and/or educational level. *P < .05 vs quartile 4 (reference)
Mental speed
KTRs with ID had significantly lower norm scores on the TMT-A [42 (16–69)], compared with KTRs without ID [66 (27–85), P = .02, Table 2]. Mean norm scores of mental speed was 47 ± 24 in KTRs with ID, compared with 57 ± 24 in KTRs without ID (P = .02, Table 2). The presence of ID was associated with a worse score on mental speed upon multivariable regression analysis (Table 3). Similarly, both lower plasma ferritin levels and lower TSAT were associated with lower mental speed (Table 3, Supplementary data, Tables S1 and S2, Fig. 2). Plasma hemoglobin was not associated with mental speed.
Attention and executive functioning
KTRs with ID had significantly lower norm scores on the Digit Span BW (37 ± 27), compared with KTRs without ID (52 ± 31, P = .003, Table 2). The mean norm score for attention and executive functioning was 41 ± 21 in KTRs with ID, compared with 49 ± 19 in KTRs without ID (P = .04, Table 2). The presence of ID was associated with a worse score on attention and executive functioning upon multivariable regression analysis (Table 3). Lower plasma ferritin levels were associated with worse attention and executive functioning, while there was a trend towards worse attention and executive functioning with lower TSAT (Table 3, Supplementary data, Tables S1 and S2, Fig. 2). Plasma hemoglobin was not associated with attention and executive functioning.
Alternative definitions of ID and neurocognitive function
We subsequently performed sensitivity analyses with several alternative ID definitions. In participants with TSAT ≤20 or participants with plasma ferritin <300 µg/mL combined with TSAT ≤20%, ID was significantly associated with worse memory and mental speed, while the association with worse attention and executive functioning was slightly weaker and borderline significant (Supplementary data, Table S3). The presence of absolute ID was stronger and more significantly associated with worse outcomes on all domains of cognitive function, compared with the presence of ID as defined in the main analyses. There was no significant association of functional ID with neurocognitive outcomes.
DISCUSSION
In this study, we show for the first time that the presence of ID and lower iron indices are independently associated with worse performance on neurocognitive tasks measuring memory, mental speed, and attention and executive functioning in KTRs. The relation between iron status and memory seemed to be mostly driven by immediate memory span, while the association between iron status and attention and executive functioning was driven by working memory (Table 2). Our study suggests that ID is a new modifiable risk factor for impaired cognitive performance after kidney transplantation, in addition to known causes of cognitive decline such as vascular aging, prevalent cerebrovascular disease and immunosuppressive therapy [9, 43]. These findings are in line with prior studies connecting ID with neurocognitive deficits in other populations [23, 25–29].
Iron is a component of hemoglobin and is therefore involved in oxygen transport to all organs including the brain [44]. Interestingly, our finding that the associations between parameters of iron status and cognitive function were independent of hemoglobin might imply that the pathophysiological mechanisms explaining this relation lie beyond iron-dependent erythropoiesis. Also, lower hemoglobin levels were not significantly associated with cognitive function, in line with a previous study in older adults [27]. Iron is crucial to cellular energy metabolism since it is a cofactor of enzymes such as aconitase and succinate dehydrogenase, both involved in the Krebs cycle, and since it is a component of the mitochondrial respiratory chain complexes. [44] Mechanistically, ID might impair neuronal energy metabolism and may thereby affect cognitive performance. Alternatively, disordered neurotransmitter metabolism might explain the relation between ID and cognitive performance. Iron is involved in synthesis of dopamine, noradrenalin, adrenalin and serotonin [30]. Furthermore, ID impairs the neuronal postsynaptic uptake of neurotransmitters including dopamine [45, 46] and norepinephrine [47]. Baumgartner et al. showed that in rats, systemic ID led to a lower brain iron content and changes in concentrations of dopamine and serotonin in different parts of the brain, which were both related to impaired performance on a task measuring working memory [48]. This is in agreement with our results, showing a significant difference in working memory between iron-deficient and iron-sufficient KTRs.
Notably, subjects in the highest ranges of ferritin and TSAT also performed worse on cognitive tests. Ferritin is an acute-phase reactant and is increased in individuals with inflammation, which might also affect cognitive function. Higher TSAT might indicate iron overload, which may induce oxidative stress and thereby negatively affect cerebral function.
At a group level, cognitive function in our cohort was relatively good with median percentile scores ranging between 32 and 58. Nevertheless, there was considerable inter-individual variation among participants. Since we excluded participants with signs of neurodegeneration or a low IQ, our cohort is not fully representative of the KTR outpatient population. Mean hemoglobin levels in our full cohort as well as in the iron-deficient patients were within the normal range. We hypothesize that erythropoiesis is not limited until the availability of iron is severely decreased, because iron availability in the bone marrow may be rescued at the expense of its abundance in other organs such as the brain. The prevalence of ID in our cohort was 64%, which is much higher than the prevalence of 43% reported in a previous study by Eisenga et al. [14] This can be explained by a difference in the definition of ID, which was more strict and more dependent on TSAT in the study by Eisenga et al. We found that associations of ferritin levels with cognitive outcomes are more pronounced than associations of TSAT with the same parameters. Particularly absolute ID, defined as plasma ferritin <100 µg/mL, rather than ID based on a definition with an emphasis on TSAT, had a stronger relationship with worse scores on memory, mental speed and attention and executive functioning (Supplementary data, Table S3).
This study has several strengths and limitations. Neuropsychological assessment was performed systematically by well-trained neuropsychology students, using an extensive set of tests measuring memory, mental speed, and attention and executive functioning. Limitations include the fact that the cross-sectional and observational design of this study do not permit to draw any conclusions about causality. Residual confounding cannot be excluded, although we adjusted for multiple confounders including cardiovascular history, subjective fatigue and hemoglobin levels. Second, although neuropsychological assessment was performed according to a standardized protocol by a team of specifically trained neuropsychology students, a certain degree of inter-observer variation in scoring cannot be excluded.
In conclusion, this study demonstrated that lower iron availability is independently associated with impaired memory, mental speed, and attention and executive functioning in KTRs. These results provide a rationale to prospectively investigate whether iron supplementation improves cognitive function after kidney transplantation, as is one of the objectives of the currently ongoing EFFECT-KTx randomized controlled trial (ClinicalTrials.gov Identifier: NCT03769441), addressing the effect of intravenous iron supplementation versus placebo on several clinical outcomes including cognitive function in iron-deficient KTRs.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the neuropsychology student team for performing neuropsychological examinations.
Contributor Information
Joanna Sophia J Vinke, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
Aaltje L Ziengs, Department of Neuropsychology, University Medical Center Groningen, Groningen, The Netherlands.
Anne M Buunk, Department of Neuropsychology, University Medical Center Groningen, Groningen, The Netherlands.
Lisanne van Sonderen, Department of Neuropsychology, University Medical Center Groningen, Groningen, The Netherlands.
Antonio W Gomes-Neto, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
TransplantLines Investigators, Groningen Transplant Center, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Stefan P Berger, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
Stephan J L Bakker, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
Michele F Eisenga, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
Jacoba M Spikman, Department of Neuropsychology, University Medical Center Groningen, Groningen, The Netherlands.
Martin H De Borst, Department of Nephrology, University Medical Center Groningen, Groningen, The Netherlands.
FUNDING
This work is supported by the Dutch Kidney Foundation (grant no 17OKG18 to M.H.d.B.).
AUTHORS’ CONTRIBUTIONS
J.S.J.V and M.H.d.B conceptualized the study; A.L.Z, L.v.S and A.W.G.-N were responsible for data collection; A.L.Z and J.S.J.V performed data analysis and wrote the first concept of the manuscript; A.M.B, S.P.B, S.J.L.B, M.F.E, J.M.S and M.H.d.B supervised the project, reviewed and edited the manuscript and provided their expertise on the topic.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
J.S.J.V. received a speaker fee from Vifor Pharma (to employer). M.F.E. has declared receiving consultant fees from Vifor Pharma; serving on the Advisory Board for Cablon Medical; and receiving speakers bureaus from Vifor Pharma, Cablon Medical and Astellas (all to employer). M.H.d.B. has consultancy agreements with Amgen, Astellas, AstraZeneca, Bayer, Kyowa Kirin, Vifor Fresenius Medical Care Renal Pharma and Sanofi Genzyme, and received grant support from Sanofi Genzyme and Vifor Pharma (all to employer).
REFERENCES
- 1. Wolfe RA, Ashby VB, Milford ELet al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. [DOI] [PubMed] [Google Scholar]
- 2. Legendre C, Canaud G, Martinez F.. Factors influencing long-term outcome after kidney transplantation. Transpl Int 2014;27:19–27. [DOI] [PubMed] [Google Scholar]
- 3. Pliskin NH, Yurk HM, Ho LTet al. Neurocognitive function in chronic hemodialysis patients. Kidney Int 1996;49:1435–40. [DOI] [PubMed] [Google Scholar]
- 4. Kramer L, Madl C, Stockenhuber Fet al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int 1996;49:833–8. [DOI] [PubMed] [Google Scholar]
- 5. Griva K, Thompson D, Jayasena Det al. Cognitive functioning pre- to post-kidney transplantation—a prospective study. Nephrol Dial Transplant 2006;21:3275–82. [DOI] [PubMed] [Google Scholar]
- 6. Kaya Y, Ozturkeri OA, Benli US CT. Evaluation of the cognitive functions in patients with chronic renal failure before and after renal transplantation. Acta Neurol Belg 2013;113:147–55. [DOI] [PubMed] [Google Scholar]
- 7. Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko Met al. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int 2011;79:1353–60. [DOI] [PubMed] [Google Scholar]
- 8. Joshee P, Wood AG, Wood ERet al. Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrol Dial Transplant 2018;33:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziengs AL, Buunk AM, van Sonderen Let al. Long-term cognitive impairments in kidney transplant recipients: impact on participation and quality of life. Nephrol Dial Transplant 2023;38:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, Mahnken JD, Johnson DKet al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol 2017;18:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dixon BS, VanBuren JM, Rodrigue JRet al. Cognitive changes associated with switching to frequent nocturnal hemodialysis or renal transplantation. BMC Nephrol 2016;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madero M, Gul A, Sarnak MJ.. Cognitive function in chronic kidney disease. Semin Dial 2007;21:29–37. [DOI] [PubMed] [Google Scholar]
- 13. Zheng G, Wen J, Zhang Let al. Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functionalMR imaging study. Metab Brain Dis 2014;29:777–86. [DOI] [PubMed] [Google Scholar]
- 14. Eisenga MF, Minović I, Berger SP.. Iron deficiency, anemia, and mortality in renal transplant recipients. Transpl Int 2016;11:1176–83. [DOI] [PubMed] [Google Scholar]
- 15. Lorenz M, Kletzmayr J, Perschl Aet al. Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 2002;3:794–7. [DOI] [PubMed] [Google Scholar]
- 16. Molnar MZ, Czira M, Ambrus Cet al. Anemia is associated with mortality in kidney-transplanted patients - a prospective cohort study. Am J Transplant 2007;4:818–24. [DOI] [PubMed] [Google Scholar]
- 17. Przybylowski P, Malyszko J, Glowinska Iet al. Prevalence of iron deficiency in heart and kidney allograft recipients. Transplant Proc 2011;10:3885–7. [DOI] [PubMed] [Google Scholar]
- 18. Molnar MZ, Mucsi I, Macdougall ICet al. Prevalence and management of anaemia in renal transplant recipients: data from ten European centres. Nephron Clin Pract 2011;117:c127–34. [DOI] [PubMed] [Google Scholar]
- 19. Allegra V, Mengozzi G, Martimbianco Let al. Long-term monitoring of iron stores in renal transplant recipients. Nephron 1990;55:440–1. [DOI] [PubMed] [Google Scholar]
- 20. Vinke JSJ, Francke MI, Eisenga MFet al. Iron deficiency after kidney transplantation. Nephrol Dial Transplant 2020;11:1976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winkelmayer WC, Lorenz M, Kramar Ret al. Percentage of hypochromic red blood cells is an independent risk factor for mortality in kidney transplant recipients. Am J Transplant 2004;12:2075–81. [DOI] [PubMed] [Google Scholar]
- 22. Vaugier C, Amano MT, Chemouny JMet al. Serum iron protects from renal postischemic injury. J Am Soc Nephrol 2017;12:3605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanton CA, Green MW, Kretsch MJ.. Body iron is associated with cognitive executive planning function in college women. Br J Nutr 2013;5:906–13. [DOI] [PubMed] [Google Scholar]
- 24. Murray-Kolb LE, Beard JL.. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;3:778–87. [DOI] [PubMed] [Google Scholar]
- 25. Dziembowska I, Kwapisz J, Izdebski Pet al. Mild iron deficiency may affect female endurance and behavior. Physiol Behav 2018;1:44–50. [DOI] [PubMed] [Google Scholar]
- 26. Gong Z, Song W, Gu Met al. Association between serum iron concentrations and cognitive impairment in older adults aged 60 years and older: a dose-response analysis of National Health and Nutrition Examination Survey. PLoS One 2021;16:e0255595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portugal-Nunes C, Castanho TC, Amorim Let al. Iron status is associated with mood, cognition, and functional ability in older adults: a cross-sectional study. Nutrients 2020;12:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruner AB, Joffe A, Duggan AKet al. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet North Am Ed 1996;9033:992–6. [DOI] [PubMed] [Google Scholar]
- 29. Leonard AJ, Chalmers KA, Collins CE PA. A study of the effects of latent iron deficiency on measures of cognition: a pilot randomised controlled trial of iron supplementation in young women. Nutrients 2014;6:2419–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hare D, Ayton S, Bush Aet al. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 2013;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisenga MF, Gomes-Neto AW, Van Londen Met al. Rationale and design of TransplantLines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018;8:e024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deelman BG, de Graaf A.. Gognitieve Screening Test. Handleiding. Swets & Zeitlinger, Lisse, the Netherlands. 1991. [Google Scholar]
- 33. Schmand B, Lindeboom J.. Nederlandse leestest voor volwassenen: handleiding. Pearson Assessment and Information BV, Amsterdam, the Netherlands, 1992. [Google Scholar]
- 34. Worm-Smeitink M, Gielissen M, Bloot Let al. The assessment of fatigue: psychometric qualities and norms for the Checklist Individual Strength. J Psychosom Res 2017;98:40–6. [DOI] [PubMed] [Google Scholar]
- 35. Inker LA, Eneanya ND, Coresh Jet al. New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stinissen J, Willems PJ, Coetsier Pet al. Handleiding bij de Nerderlandse bewerking van de Wechsler Adult Intelligence Scale (W.A. I. S.). [Manual of the Dutch edition of the Wechsler Adult Intelligence Scale]. Lisse, Switzerland: Swets & Zeitlinger. [Google Scholar]
- 37. Saan RJ, Deelman BG. De 15-Woorden Tests A en B. (Een voorlopige handleiding). Academisch Ziekenhuis Groningen, Groningen, the Netherlands, 1986. [Google Scholar]
- 38. Smith A. Symbol Digit Modalities Test (SDMT) Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- 39. Reitan R, Wolfson D.. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. South Tucson, AZ: Neuropsychology Press,1985. [Google Scholar]
- 40. Schmand B, Groenink SC, den Dungen M.. Letter fluency: Psychometrische eigenschappen en Nederlandse normen. GEEG 2008;39:64–74. [DOI] [PubMed] [Google Scholar]
- 41. Rubin DB. An overview of multiple imputation. Harvard University, Cambridge, Massachusetts, 1988. [Google Scholar]
- 42. van der Wal HH, Grote Beverborg N, Dickstein Ket al. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Hear J 2019;40:3616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viggiano D, Wagner CA, Martino Get al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 2020;16:452–69 [DOI] [PubMed] [Google Scholar]
- 44. Camaschella C. Iron deficiency. Blood 2019;133:30–9. [DOI] [PubMed] [Google Scholar]
- 45. Erikson KM, Jones BC, Hess EJet al. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 2001;3:409–18. [DOI] [PubMed] [Google Scholar]
- 46. Ashkenazi R, Ben-Shachar D, Youdim MBH.. Nutritional iron and dopamine binding sites in the rat brain. Pharmacol Biochem Behav 1982;17:43–7. [DOI] [PubMed] [Google Scholar]
- 47. Beard JL, Chen Q, Connor Jet al. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav 1994;3:621–4. [DOI] [PubMed] [Google Scholar]
- 48. Baumgartner J, Smuts CM, Malan Let al. Combined deficiency of iron and (n-3) fatty acids in male rats disrupts brain monoamine metabolism and produces greater memory deficits than iron deficiency or (n-3) fatty acid deficiency alone. J Nutr 2012;8:1463–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.