ABSTRACT
The relationship between socioeconomic deprivation and health is inequitable. Chronic kidney disease (CKD) is an archetypal disease of inequality, being more common amongst those living in deprivation. The prevalence of CKD is rising driven by an increase in lifestyle-related conditions. This narrative review describes deprivation and its association with adverse outcomes in adults with non-dialysis-dependent CKD including disease progression, end-stage kidney disease, cardiovascular disease and all-cause mortality. We explore the social determinants of health and individual lifestyle factors to address whether patients with CKD who are socioeconomically deprived have poorer outcomes than those of higher socioeconomic status. We describe whether observed differences in outcomes are associated with income, employment, educational attainment, health literacy, access to healthcare, housing, air pollution, cigarette smoking, alcohol use or aerobic exercise. The impact of socioeconomic deprivation in adults with non-dialysis-dependent CKD is complex, multi-faceted and frequently under-explored within the literature. There is evidence that patients with CKD who are socioeconomically deprived have faster disease progression, higher risk of cardiovascular disease and premature mortality. This appears to be the result of both socioeconomic and individual lifestyle factors. However, there is a paucity of studies and methodological limitations. Extrapolation of findings to different societies and healthcare systems is challenging, however, the disproportionate effect of deprivation in patients with CKD necessitates a call to action. Further empirical study is warranted to establish the true cost of deprivation in CKD to patients and societies.
Keywords: chronic renal failure, chronic renal insufficiency, CKD, exercise, prognosis
INTRODUCTION
The relationship between socioeconomic deprivation and health is both inequitable and morally unjustifiable. Deprivation can be defined by measures at both an individual and population level. The former includes household income, educational attainment and employment, whereas the latter frequently extrapolates findings from a person's postcode which may or may not reflect their individual circumstances [1]. However measured, the economic cost of inequities in health on the whole population are substantial [2].
Chronic kidney disease (CKD) can be considered to be an archetypal disease of inequality, being more common amongst those living in deprivation [3]. CKD is estimated to affect 850 million people worldwide and is predicted to be the fifth leading cause of death globally by 2050 [4, 5]. It may progress to end-stage kidney disease (ESKD) and is associated with a significantly increased risk of morbidity and mortality from cardiovascular disease (CVD) [6]. The prevalence of CKD is rising driven by an increase in lifestyle-related conditions including hypertension and diabetes [4].
This narrative review describes deprivation and its association with adverse outcomes in adults with non-dialysis-dependent CKD including disease progression, ESKD, CVD and all-cause mortality. We utilize a social determinants of health model to explore the following factors: income, employment, education, health literacy, access to healthcare, housing and air pollution. Exploring the social determinants of health and individual lifestyle factors, the following questions are addressed:
Do patients with CKD who are socioeconomically deprived have poorer outcomes than those of higher socioeconomic status?
Are any observed differences in health-related outcomes explained by specific socioeconomic factors?
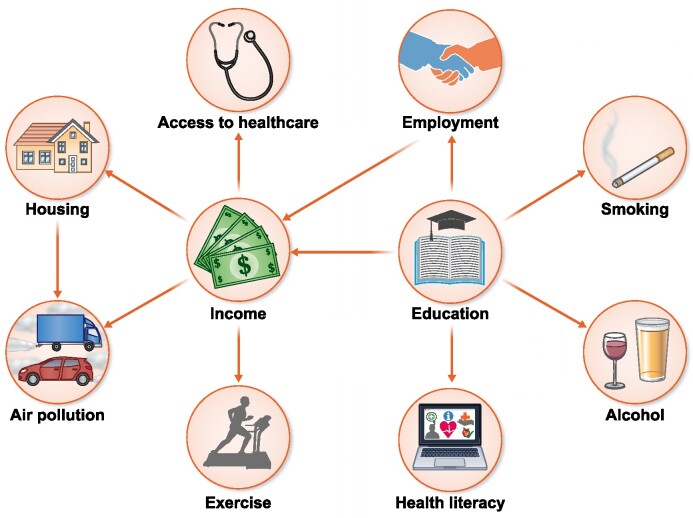
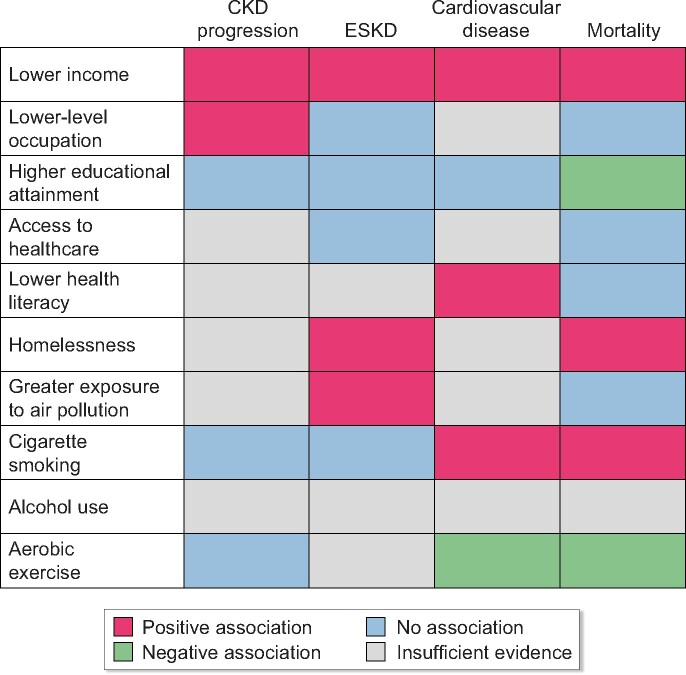
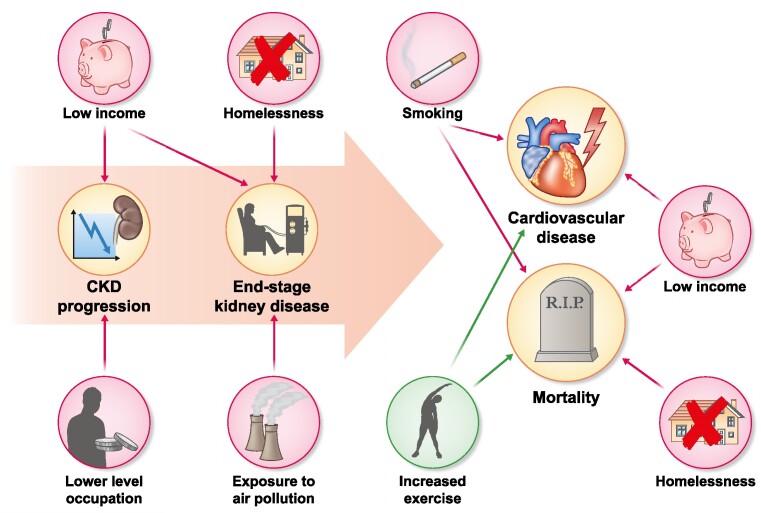
The factors examined are frequently inter-dependent, thereby making any analysis of the impact of such individual components challenging (see Fig. 1). The impact of ethnicity on adverse outcomes in CKD was deemed beyond the scope of our review and so has not been included. Table 1 and Fig. 2 summarize the strength of available evidence and primary studies included related to social determinants of health and individual lifestyle factors. Figure 3 highlights the factors associated with adverse renal outcomes and mortality.
Figure 1:
Schematic of the social determinants of health and individual lifestyle factors.
Table 1:
Summary of primary studies assessing the social determinants of health or individual lifestyle factors with relevant outcomes in adults with CKD.
| Author [ref.] (year) | Country | Population | Relevant exposures | Outcomes |
|---|---|---|---|---|
| Norris et al. [9] (2006) | USA | CKD 2–4 | Income | CVD, mortality |
| Alves et al. [10] (2010) | USA | CKD 2–4 | Income, education | ESKD, CVD, mortality |
| Fedewa et al. [11] (2014) | USA | CKD 3–4 | Income, education | ESKD, mortality |
| Hossain et al. [13] (2012) | UK | CKD 1–5 | Education, occupation, smoking, alcohol | CKD progression, ESKD, mortality |
| Couchoud et al. [15] (2012) | France | General population | Occupation | ESKD |
| Morton et al. [18] (2016) | International | CKD 3–ESKD | Education, smoking, alcohol | CKD progression, ESKD, CVD, mortality |
| Winitzki et al. [19] (2022) | Germany | CKD 1–3 | Income, education, smoking | ESKD, CVD, mortality |
| Bello et al. [21] (2012) | Canada | CKD 3–4 | Income, geographical remoteness | ESKD, mortality |
| Jurkovitz et al. [22] (2013) | USA | ‘At risk’ general population (HTN, DM or family history) | Education, health insurance status, smoking | ESKD, mortality |
| Ward et al. [23] (2007) | USA | Incident ESKD 2nd to lupus nephritis | Health insurance status | ESKD |
| Devraj et al. [27] (2015) | USA | CKD 1–4 | Education, income, health insurance, health literacy | CKD progression |
| Gurgel et al. [32] (2021) | Netherlands | CKD 1–5 | Income, education, health literacy | CVD, mortality |
| Hall et al. [33] (2012) | USA | CKD 3–5 | Income, health insurance, homelessness | ESKD, mortality |
| Lin et al. [37] (2020) | Taiwan | CKD 3–4 | Education, air pollution, smoking, alcohol | ESKD, mortality |
| Ran et al. [38] (2020) | Hong Kong | CKD 1–5 | Income, air pollution, exercise, smoking, alcohol | Mortality |
| Wu et al. [39] (2022) | Taiwan | CKD 3b–5 | Air pollution, smoking, alcohol | CKD progression |
| Ran et al. [43] (2020) | Hong Kong | CKD 1–5 | Income, air pollution, exercise, smoking, alcohol | Mortality |
| Bundy et al. [46] (2018) | USA | CKD 2–4 | Smoking, alcohol, exercise | CKD progression, mortality |
| Grams et al. [47] (2012) | USA | General population | Income, smoking, alcohol, exercise | CKD progression, ESKD |
| Ricardo et al. [48] (2015) | USA | CKD 2–4 | Education, smoking, exercise | CKD progression, CVD |
| Staplin et al. [49] (2016) | USA | CKD 3–5 including HD or PD | Education, smoking, alcohol | CKD progression, ESKD, CVD, mortality |
| Hall et al. [50] (2016) | USA | General population | Smoking, alcohol, exercise | CKD progression |
| Joo et al. [56] (2020) | South Korea | CKD 2–4 | Income, education, smoking, alcohol | CKD progression, ESKD |
| Robinson-Cohen et al. [58] (2014) | USA | CKD 1–5 | Education, smoking, alcohol, exercise | ESKD, mortality |
| Chen et al. [59] (2008) | USA | CKD 3-4 | Education, exercise | Mortality |
| Kuo et al. [62] (2022) | Taiwan | CKD 1–3A with proteinuria or CKD 3b–5 | Exercise | ESKD, CVD, mortality |
| Beddhu et al. [63] (2009) | USA | CKD 3–5 | Exercise, smoking | Mortality |
| Beddhu et al. [64] (2015) | USA | CKD 3–5 | Exercise, smoking, alcohol | Mortality |
DM, diabetes mellitus; HD, haemodialysis; HTN, hypertension; PD, peritoneal dialysis.
Figure 2:
Summary of evidence assessing the social determinants of health and individual lifestyle factors with relevant outcomes in adults with CKD. *Red: positive association; green: negative association; blue: no association; grey: insufficient evidence.
Figure 3:
Schematic of factors associated with renal outcomes and mortality. *Red: positive association; green: negative association.
THE SOCIAL DETERMINANTS OF HEALTH
Income
The impact of income on health is multifaceted, affecting food security, and access to affordable housing and healthcare services. Evidence of adverse renal, cardiovascular and mortality outcomes according to income in adults with CKD is limited.
Many studies are based in the USA which does not have universal health coverage, limiting the generalizability to non-US populations. Several papers assessing renal outcomes are based on general population cohorts and are not limited to adults with CKD. These shortcomings were highlighted in two recent review articles [7, 8].
Morton et al. systematically reviewed the literature for evidence of a social gradient in adults with moderate to severe CKD, identifying only five studies wherein individual income was reported [7]. The sparse data related to income was effectively limited to the findings of a single randomized controlled trial from the USA.
In the African American Study of Kidney Disease and Hypertension (AASK) trial, Norris et al. randomized 1095 participants with CKD 2–4 to different anti-hypertensive agents and blood pressure targets to assess the impact on cardiovascular outcomes (a composite of cardiac death, myocardial infarction, stroke and heart failure). In a multivariable adjusted analysis, the authors reported that an annual income of <$15 000 at baseline was associated with a near 2-fold increased risk of adverse cardiovascular outcomes {adjusted hazard ratio (aHR) 1.94 [95% confidence interval (CI) 1.27–2.98]}. However, half of the cohort had a history of heart disease at baseline (determined by self-report, medical note review or electrocardiogram). Therefore, it is possible that these individuals had low income due to pre-existing heart disease (i.e. reverse causality) [9].
The only paper cited to have assessed the impact of income on CKD progression, all-cause and cardiovascular mortality was a subsequent study of longitudinal outcomes in AASK trial participants. The authors reported incident rate ratios (IRR) for each outcome, unadjusted for potential confounders, revealing modest increases in ESKD (4 vs 3.8/100 person years), cardiovascular composite event (3.7 vs 2.7/100 person years) and all-cause mortality (2.3 vs 2/100 person years) between those earning less than or greater than $15 000 per year, respectively. The trial was limited to patients with hypertensive nephrosclerosis, therefore results may not be generalizable to other CKD patients. Furthermore, the unadjusted nature of the analysis limits utility as the apparent relationship between income and adverse outcomes may simply reflect unmeasured confounders known to be associated with ESKD, cardiovascular events and mortality (e.g. smoking). Moreover, the possibility of comorbid illness leading to low income cannot be excluded [9, 10].
A 2018 meta-analysis highlighted seven cohort studies that assessed the impact of income on CKD progression. CKD progression was significantly associated with lower income [relative risk (RR) 1.24 (95% CI 1.12–1.37), P < 0.001; I2 = 66.6%, P = .006], however, several limitations were apparent. Six of the seven studies were based in the USA; only one adjusted for the presence of comorbid conditions; and there was variation in study recruitment, exposure and outcome definition. Furthermore, the parameters used to quantify high and low income in the meta-regression were not defined, thereby significantly limiting the utility of the findings and necessitating further study [8].
One other pertinent study was identified: Reasons for Geographic and Racial Differences in Stroke (REGARDS) was a retrospective cohort study of 2761 patients with stage 3–4 CKD in the USA, carried out between 2003 and 2007 to assess the impact of income and race on a composite outcome (incident ESKD and all-cause mortality). In a multivariable adjusted analysis, low income (annual household income <$20 000) was associated with a near one third increase in ESKD or mortality [aHR 1.32 (95% CI 1.06–1.65)]. The impact was more pronounced when comparing those in the lowest versus the highest (>$75 000) income categories [aHR 1.85 (95% CI 1.31–2.55)]. Again there were several limitations: measurement bias [defining CKD with a single glomerular filtration rate measure (GFR)], confounding (lack of adjustment for medication use) and selection bias [11].
Employment
Employment impacts morbidity and mortality in the general population through direct and indirect effects [12]. Data on adults with CKD is comparatively sparse and of low quality with variable definitions of exposure status (e.g. employed vs unemployed or skilled vs unskilled). In the systematic review by Morton et al., few studies were identified that reported employment status [7]. Only one assessed its impact on cardiorenal outcomes or mortality [13].
In a retrospective cohort study from the UK, participants completed a questionnaire to identify occupation at baseline, and were followed for a median 3-year period. Occupation was categorized as skilled (47%) or unskilled (53%), and further delineated according to nine major subgroups. There was no significant association between occupation and ESKD or mortality. However, lack of variation in occupational class across deprivation quintiles suggests that both non-response and social desirability bias may have contributed to misclassification of employment status. None of the participants was reported as being unemployed and thus the findings cannot be extrapolated to a contemporary cohort of patients with CKD [13]. Unemployment has been reported to be as high as 74% in non-dialysis-dependent advanced CKD [14].
In the 2018 meta-analysis by Zeng et al., lower level occupation was associated with a small increase in the risk of CKD progression [RR 1.05 (95% CI 1.01–1.09), P = .012; I2 = 0.0%, P = .796] [8]. This was based on two observational studies: the UK study [13] and a prospective cohort study from France assessing spatial variation in kidney replacement therapy (KRT) incidence rates. The appropriateness of presenting aggregated findings in the meta-analysis is debatable: these studies were based on general population cohorts and not limited to those with CKD [8, 15].
Educational attainment
Educational attainment may indirectly lead to poor health outcomes by affecting an individual's employability and income, and health-related behaviours [16].
In a longitudinal study of a subgroup of 14 631 Kidney Early Evaluation Programme (KEEP) participants with CKD, educational attainment was self-reported and categorized into four groups: less than high school, high school, college and professional degree attainment. In a Cox proportional hazards model adjusted for cardiovascular risk factors including hypertension and baseline estimated GFR (eGFR), educational attainment was not associated with risk of ESKD. Higher educational attainment (high school graduate or greater) was associated with lower mortality risk [aHR 0.72 (95% CI 0.62–0.83)]. Notably, the original KEEP programme was a free screening schedule which by design leads to selection bias. Medication use, which may have confounded the results, was not available. The 2097 exclusions may have been differentially excluded according to educational attainment once more contributing to selection bias [17].
In a meta-analysis, Zeng et al. found no association between education and CKD progression [n = 7 studies: RR 1.11 (95% CI 0.94–1.30) I2 = 71.3%, P = .002]. Five of the seven included studies were conducted in the USA. In a restricted analysis including two European studies, lower educational attainment was associated with higher risk of CKD progression [RR 1.23 (95% CI 1.17–1.30), I2 = 0.0%, P = .861]. However, the aggregated results were not limited to participants with CKD [8]. One study reported only unadjusted IRR for each outcome, limiting interpretability [10]. Hossain et al. found no association between educational attainment—classified as low (0 to 11 years of study) and normal/high (>11 years study)—and ESKD [HR 1.38 (95% CI 0.38–5.01)] or death [HR 1.15 (95% CI 0.63–2.10)], likely due to adjustment for area level socioeconomic deprivation [13].
The two most compelling contemporary studies show no independent association between educational attainment and ESKD or mortality [18, 19]. Morton et al. followed 9270 adults from 18 countries with moderate to severe non-dialysis and dialysis-requiring CKD and without established vascular disease for a median of 4.9 years. Among 6245 individuals with non-dialysis CKD, highest educational attainment was not associated with a composite renal outcome (ESKD or doubling of serum creatinine) or mortality following adjustment for lifestyle factors, comorbidity and CKD stage [18]. Winitzki et al. conducted a multi-centre, prospective cohort study in Germany, including 5095 adults with CKD over a median follow-up of 6.5 years. Low educational attainment was associated with increased risk of mortality, major adverse cardiovascular events and ESKD, but not after adjustment for baseline eGFR, albuminuria and other potential confounders. Mediation analysis identified prevalent smoking, cardiovascular disease and a number of biochemical markers that mediated the apparent association between low educational achievement and all-cause mortality [19]. It is, therefore, plausible that the relationship between educational attainment and adverse renal outcomes or mortality may be due to residual confounding or unmeasured effect modifiers.
Access to healthcare
Access to healthcare has been defined as the timely use of health services to achieve the best possible outcomes [20]. Relevant studies exploring the impact of healthcare access in patients with CKD were limited to North America [21–23].
Geographical remoteness, as a proxy indicator of healthcare access, was not significantly associated with increased mortality in a cohort of 31 337 patients with diabetes and non-dialysis CKD in Canada. However, remote dwellers living over 100 km away from the nearest nephrology centre were more likely to progress to GFR <10 mL/min/1.73 m2 during follow-up [21]. Geographical remoteness may not in isolation equate to access to medical services.
In a systematic review, lack of insurance was associated with delayed referral of patients with CKD to nephrology services [24].
In a subset of KEEP study participants (n = 86 588), 27.8% of participants had no form of insurance. Among 12 998 participants with CKD and compared with those with private health insurance, lack of insurance was associated with all-cause mortality in CKD 1–2 [aHR1.60 (95% CI 1.13–2.26)], but not in CKD 3 or above following adjustment for traditional atherosclerotic risk factors, ethnicity and education. There was no relationship between insurance status and progression to ESKD. The findings may have been affected by the small number of participants with CKD stage 4–5 (n = 383). Moreover, misclassification bias likely impacted the results as a diagnosis of CKD was based upon a single serum creatinine measurement while insurance status was only measured at baseline [22].
Conversely, in a cross-sectional study of 7971 adults with ESKD attributed to lupus nephritis, those with private health insurance developed ESKD at an older age than those without. Results were not fully adjusted and thus residual confounding cannot be discounted [23].
Health literacy
In the general population, inadequate health literacy has been associated with adverse health outcomes including mortality [25].
In people with CKD, health literacy is of particular relevance given the importance of dietary modification, medication adherence, glycaemic and blood pressure control to slow disease progression. These treatment goals require active self-management for which functional health literacy is a pre-requisite. Numerous studies have sought to capture the prevalence of inadequate health literacy in CKD with prevalence estimates varying from 18% to 63% [26–30]. There are comparatively few which examine the effect of health literacy on outcomes [31].
In a cross-sectional study of 150 adults with non-dialysis CKD from the USA, limited health literacy was associated with GFR when adjusted for multiple demographic factors, though age was a more important determinant of GFR than health literacy [27].
In the only cohort study identified, 2742 patients were followed for a median of 4.2 years to assess the impact of health literacy on self-reported CVD outcomes and mortality. After adjustment for age and sex, low health literacy was associated with CVD. This relationship was mediated by uncontrolled diabetes and obesity [32].
Poor health literacy per se may not be associated with adverse outcomes, but the lack of evidence denotes the need for further study.
Housing
Adequacy of housing is inextricably linked to health. There is little empirical research on the impact of housing or homelessness on outcomes in patients with CKD; the latter reflects that such patients represent a hard-to-reach population of the most socioeconomically deprived. We identified one single-centre, retrospective cohort study of 15 343 adults with CKD in the USA who were receiving ambulatory nephrology care. Homelessness (n = 858; 6%) was associated with nearly one-third increased risk of ESKD or mortality after adjustment for sociodemographic, clinical and biochemical covariates [aHR 1.28 (95% CI 1.04–1.58)]. In subgroup analyses, this relationship was only evident in those without a history of substance misuse. The results may underestimate the true effect size in the population: there was selection bias in the requirement for included participants to be attending ambulatory care and hence engaging with treatment [33].
Air pollution
Air pollution is a mix of solid, liquid and gas components suspended in atmosphere which varies within and between geographical locations. Particulate matter (PM) represents solid particles and is the major component of air pollution associated with most adverse health outcomes [34, 35]. Exposure to air pollution is often greatest among those living in areas of socioeconomic deprivation [36].
There is compelling evidence of adverse outcomes associated with air pollution in adults with CKD [37–39]. Furthermore, three recent systematic reviews suggested an association between exposure to outdoor air pollution, CKD incidence and lower GFR in the general population [40–42].
The largest individual study was a prospective cohort study of 6628 adults with CKD, conducted from 2003 to 2015 in Taiwan. Satellite-based spatiotemporal models were utilized to estimate each participant's annual PM2.5 exposure at enrolment. Increased PM2.5 exposure was independently associated with a near one-fifth increase risk of progression to KRT [n = 941 events; aHR 1.19 (95% CI 1.08–1.31)] with evidence of a dose–response relationship with increased exposure. No association was identified with all-cause mortality.
Misclassification bias is likely to have occurred as individuals’ outdoor activity patterns were not available to researchers and hence their recorded level of PM2.5 exposure may have been inaccurate [37]. The findings were vindicated by a recent retrospective study of adults with CKD in Taiwan, which found that after adjustment for traditional risk factors for CKD progression, PM2.5 and nitric oxide exposure was associated with increased risk of GFR decline over 2 years of follow up [39].
Two analyses of 66 820 elderly patients with CKD from Hong Kong did not demonstrate an association between PM2.5 exposure and all-cause mortality [38, 43]. However, there was a near 2-fold increase in cardiovascular mortality associated with a 4.0 mg/m3 increase in PM2.5 [aHR 1.97 (95% CI 1.34–2.91)]. Although the findings were based on a large cohort with 10 years of follow-up data, there were a number of unmeasured confounders—including baseline GFR, albuminuria and prevalent CVD—which may have been implicated in the causal pathway [38].
INDIVIDUAL LIFESTYLE FACTORS
Cigarette smoking
Cigarette smoking is more prevalent amongst those living in greater socioeconomic deprivation [44]. A recent meta-analysis suggested that cigarette smoking is an independent risk factor for incident CKD [45].
Among adults with CKD, current or past smoking has consistently been shown to be independently associated with all-cause mortality [46–49]. As in the general population, smoking is associated with cardiovascular events in CKD populations [48, 49]. A few observational studies conducted in the USA have assessed the association between smoking status and CKD progression. In a secondary analysis of the Study of Heart and Renal Protection (SHARP) trial of lipid lowering therapy in patients with CKD or on dialysis (n = 9270 participants; median follow-up 4 years), there was no significant difference in CKD progression (ESKD or doubling of serum creatinine) according to smoking status [49]. In the Chronic Renal Insufficiency Cohort (CRIC) study—a longitudinal study of 3939 participants followed for 5 years—the results were consistent, showing no association between smoking status and CKD progression [46].
Conversely, the Jackson Heart Study included 5301 African American volunteers in a prospective cohort study to assess adverse renal outcomes between a baseline (2000–04) and follow-up visit (2009–12). Current smokers had a higher IRR for eGFR decline compared with never or past smokers [IRR 1.83 (95% CI 1.31–2.56)]. There was also a dose-dependent increase in CKD progression events depending upon the number of cigarettes smoked [50]. The conflicting results among these three studies may reflect differences in the outcome definitions: Hall et al. defined their CKD progression event as a drop in GFR of 30% between study visits whereas SHARP and CRIC studies specified GFR decline of 50% or ESKD as the outcomes of interest [46, 49, 50].
Alcohol use
Alcohol use is not more prevalent in those living in deprivation, however, the adverse effects of consumption are disproportionately experienced [51]. Excessive alcohol use is associated with hypertension, liver disease and malignancy in the general population. There is extensive evidence to suggest that alcohol use, particularly light or moderate consumption, is associated with a reduction in the incidence of CKD compared with abstinence amongst the general population [52–55]. However, few studies have quantified the impact of alcohol use on adverse outcomes in patients with established CKD. Two observational studies from the USA and South Korea reached different conclusions regarding the impact of alcohol use on CKD progression [46, 56]. In 3933 participants from the CRIC study, there was no difference in CKD progression events between those who reported persistent alcohol use compared with non-drinkers. There was a modest reduction in all-cause mortality amongst drinkers [aHR 0.73 (95% CI 0.58–0.91)], which may be explained by the exclusion of patients with liver cirrhosis and the measurement of alcohol consumption in a binary rather than graded manner [46]. Conversely, in the Korean Cohort Study for Outcome in Patients with CKD (KNOW-CKD), Joo et al. followed 1883 patients with CKD for a renal composite endpoint (>50% eGFR decline or ESKD) according to alcohol use. Over a median follow-up of 3.0 years, regular [HR 2.20 (95% CI 1.38–3.46)] and occasional binge [HR 2.00 (95% CI 1.33–2.98)] drinking were associated with a 2-fold increase in CKD progression [56].
The paucity and inconsistency of evidence necessitates further study.
Aerobic exercise
The benefits of aerobic exercise are well established [57]. In adults with CKD, socioeconomic deprivation is associated with reduced physical activity [58, 59]. People living in deprivation often have poorer access to green spaces and fewer opportunities for regular physical activity. The effect of this is compounded in patients with CKD who are frequently overweight and experience greater comorbidity and physical deconditioning with disease progression [60].
There is little evidence to suggest that aerobic exercise directly influences disease progression in people with CKD. A systematic review and meta-analysis of 15 trials found that aerobic exercise had no significant impact on GFR trajectory [61]. Similar results were reported in the CRIC observational study [48].
There is evidence that aerobic exercise reduces both adverse cardiovascular outcomes and all-cause mortality [62–65]. In the largest observational study including 906 adults with CKD over 8.8 years of follow-up, a higher level of self-reported physical activity was associated with lower hazards of all-cause mortality in insufficiently active [HR 0.58 (95% CI 0.42–0.79)] and active [HR 0.44 (95% CI 0.33–0.58)] compared with inactive participants [63].
SOCIOECONOMIC DEPRIVATION AND OUTCOMES IN CHRONIC KIDNEY DISEASE
Although many of the studies mentioned adjusted for sex in their analyses, none stratified their results according to sex. It is difficult to comment upon any interaction between sex and socioeconomic deprivation.
Progressive CKD and ESKD
Studies of socioeconomic disadvantage in adults with CKD have yielded conflicting results with regards to adverse renal outcomes. A 2018 meta-analysis of observational studies assessing indices of socioeconomic status and CKD progression included 12 cohort studies that predominantly assessed income or education at baseline. Pooled analyses demonstrated that lower combined socioeconomic status was associated with a one-third increase risk of CKD progression [RR 1.39 (95% CI 1.09–1.79), P = .009; I2 = 74.2%, P = .009]. However, the results varied according to geographic location. There was also considerable heterogeneity in the classification of socioeconomic indicators, definition of CKD and subject recruitment. Furthermore, many studies assessed socioeconomic exposures only at baseline rather than accounting for the potential effect of social mobility during follow-up, potentially leading to misclassification bias [8].
The findings were supported by a retrospective cohort study from the UK, which also found that area level socioeconomic deprivation was associated with CKD progression, but not ESKD. The authors followed 918 of 1427 patients attending regular renal clinic follow up for a median of 3 years. On multivariate logistic regression, compared with the least deprived quintile (Q5) those living in greatest deprivation (Q1) were at a 2-fold risk of both CKD progression, i.e. >2 mL/min/1.73 m2/year loss [aHR 2.17 (95% CI 1.14–4.51)], and rapid progression, i.e. >5 mL/min/1.73 m2/year loss [aHR 2.07 (95% CI 1.05–4.36)] [13].
The results were inconsistent with another retrospective cohort study from Italy that examined 715 consecutive patients with CKD undergoing nephrology care for at least 12 months. Patients were followed for a median of 10 years for adverse renal and cardiovascular outcomes and mortality after 1 year of evidence-based, goal-directed therapy. Participants were delineated according to an area measure of socioeconomic attributes determined by census including education, employment, home ownership, single parent family and overcrowding. Among this cohort of older-aged adults with predominantly mild–moderate CKD, deprivation was not associated with CKD progression or ESKD. Although compelling, the findings may have been affected by both survival and measurement bias. Patients were required to attend for 12 months of renal follow-up to be included which may have failed to capture those less likely to attend, amongst whom deprivation may have been more prevalent. Socioeconomic status was deduced from the 2001 census despite follow-up to 2018. Misclassification may have occurred whereby participants relocated or achieved greater material wealth over the course of follow-up. Both of these biases would have under estimated the effect of disadvantage on adverse outcomes [66].
In the only prospective cohort study identified, Solbu et al. followed 2950 unselected adults with CKD from first attendance at a renal clinic in the UK for a median of under 2 years. In adjusted analyses, residing in an area of greatest socioeconomic deprivation was not associated with ESKD [67].
Patients living in areas of greater socioeconomic deprivation present later to nephrology services. In a cross sectional study of 1657 patients with CKD in the UK, living in the lowest quintile of socioeconomic status was independently associated with a lower eGFR at first nephrology assessment [68]. In a systematic review, lower socioeconomic status was associated with late referral to nephrology services [24]. Late presentation in CKD is associated with adverse outcomes, including mortality [69]. In a population in France with broadly equitable access to healthcare provision, the incidence of KRT for ESKD has been shown to increase along a social gradient according to a multi-dimensional index of deprivation [70].
Patients with CKD from socioeconomically deprived areas have also been shown to be at greater risk of hospitalization than more affluent counterparts even after adjusting for individual lifestyle factors and comorbidity [71].
Cardiovascular outcomes and mortality
Socioeconomic deprivation is associated with all-cause mortality in CKD.
In Scotland, living in the most deprived area was independently associated with all-cause mortality [aHR 1.60 (95% CI 1.10–2.34)]. However, the findings must be interpreted cautiously: cause of death was unknown in 33% of cases. Furthermore, the authors were unable to capture data pertaining to baseline comorbidity and smoking which may have confounded any relationship between deprivation and mortality [67]. A larger retrospective cohort study of 13 400 participants with CKD in the USA found that socioeconomic advantage—assessed by a composite of income, employment, education, marital status and substance misuse—was positively associated with decreased mortality [aHR 0.88 (95% CI 0.86–0.89)] [72]. There was no adjustment for access to treatment by means of healthcare insurance coverage and so extrapolating these results to other countries with universal health coverage is difficult. Neither study above found an association with cardiovascular mortality [66, 67].
In a 2016 systematic review, low educational attainment, income and lack of home ownership were significantly associated with adverse cardiovascular outcomes and increased mortality [7].
The only discordant finding for the impact of socioeconomic deprivation on all-cause mortality was in the UK study by Hossain et al.; however, non-response bias may have contributed to an under estimate of adverse outcomes, with those living in areas of socioeconomic deprivation less likely to participate [13].
CONCLUSION
CKD can be considered to be an archetypal disease of deprivation, with inequitable differences in outcomes. The impact of socioeconomic deprivation in adults with non-dialysis-dependent CKD is complex, multi-faceted and frequently under-explored within the literature.
This review summarizes the evidence in this patient cohort with reference to the wider structural influences on health including selected individual lifestyle risk factors. There is evidence that patients with non-dialysis-dependent CKD who are socioeconomically deprived have faster disease progression and higher risk of both CVD and premature mortality. This seems to be the result of both socioeconomic and individual lifestyle factors. However, delineating the relatively contribution of individual socioeconomic factors that mediate this complex relationship is challenging given the inter-dependent nature of the social determinants of health (Fig. 1). The difficulty in isolating any one factor (e.g. education from employment) is likely to at least in part explain the significant paucity of studies in many areas.
The major gaps within the literature to date pertain to relative contributions of income, employment, healthcare access, health literacy, housing, air pollution and alcohol use (Fig. 2). In many cases, these gaps simply reflect a lack of empirical study. This may represent a perception amongst researchers that the generalizability of findings from any one centre or region to other societies with different and complex healthcare systems is of dubious importance. Undoubtedly, a limitation within the evidence to date is the number of studies from countries without universal health coverage. Diagnosis and management of CKD requires laboratory testing and hence (timely) access to medical services. Other key limitations include: the retrospective nature of many studies, relatively short follow-up, variable definitions of socioeconomic exposures and significant under-representation of individuals at the highest risk of poor outcomes (e.g. advanced CKD, homeless populations, etc.). The latter is likely to represent that these patients are often the hardest to reach and hence capture in health research. They are, however, likely to be in the greatest need of targeted interventions to improve health. Our narrative review is important as it highlights both the scarcity of research and low-quality evidence underpinning our understanding of the complex relationship between deprivation and CKD.
It is clear that the disproportionate effect of deprivation in patients with CKD necessitates a call to action: that patients from more deprived areas present later and with lower GFR is of major concern and highlights a cohort that could be identified for early intervention, yielding health and economic benefits. Further empirical study is warranted to establish the true cost of deprivation in CKD to patients, societies and healthcare systems.
Contributor Information
Christopher H Grant, The Glasgow Renal & Transplant Unit, Queen Elizabeth University Hospital, Govan, Glasgow, UK; College of Medical, Veterinary & Life Sciences, The University of Glasgow, Glasgow, UK.
Ehsan Salim, College of Medical, Veterinary & Life Sciences, The University of Glasgow, Glasgow, UK.
Jennifer S Lees, The Glasgow Renal & Transplant Unit, Queen Elizabeth University Hospital, Govan, Glasgow, UK; College of Medical, Veterinary & Life Sciences, The University of Glasgow, Glasgow, UK.
Kate I Stevens, The Glasgow Renal & Transplant Unit, Queen Elizabeth University Hospital, Govan, Glasgow, UK; College of Medical, Veterinary & Life Sciences, The University of Glasgow, Glasgow, UK.
AUTHORS’ CONTRIBUTIONS
J.S.L. and K.I.S. conceived the work. C.H.G., J.S.L. and K.I.S. designed the plan for the narrative review. C.H.G. and E.S. performed the literature search and review. C.H.G. drafted the initial manuscript. C.H.G., E.S., J.S.L. and K.I.S. drafted and revised the work critically for important intellectual content and approved the final submitted version for publication. All authors are mutually accountable for the accuracy and integrity of the work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Unrelated to this work, K.I.S. has received personal lectureship honorarium from Bayer and Boehringer Ingelheim. K.I.S. is member of the CKJ editorial board. J.S.L. has received personal lectureship honorarium from Pfizer, AstraZeneca and Bristol Myers Squibb. The results presented in this paper have not been published previously in whole or part.
REFERENCES
- 1. Robinson WS. Ecological correlations and the behavior of individuals. Int J Epidemiol 2009;38:337–41. 10.1093/ije/dyn357. [DOI] [PubMed] [Google Scholar]
- 2. Mackenbach JP, Meerding WJ, Kunst AE. Economic costs of health inequalities in the European Union. J Epidemiol Community Health 2011;65:412–9. 10.1136/jech.2010.112680. [DOI] [PubMed] [Google Scholar]
- 3. So BH, Methven S, Hair MDet al. Socio-economic status influences chronic kidney disease prevalence in primary care: a community-based cross-sectional analysis. Nephrol Dial Transplant 2015;30:1010–7. 10.1093/ndt/gfu408. [DOI] [PubMed] [Google Scholar]
- 4. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019;34:1803–5. 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 5. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet North Am Ed 2015;385:117–71. 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen CP, Cheng TY, Tsai MKet al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet North Am Ed 2008;371:2173–82. 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 7. Morton RL, Schlackow I, Mihaylova Bet al. The impact of social disadvantage in moderate-to-severe chronic kidney disease: an equity-focused systematic review. Nephrol Dial Transplant 2016;31:46–56. 10.1093/ndt/gfu394. [DOI] [PubMed] [Google Scholar]
- 8. Zeng X, Liu J, Tao Set al. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health 2018;72:270–9. 10.1136/jech-2017-209815. [DOI] [PubMed] [Google Scholar]
- 9. Norris K, Bourgoigne J, Gassman Jet al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 2006;48:739–51. 10.1053/j.ajkd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10. Alves TP, Wang X, Wright JT Jret al. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol 2010;21:1361–9. 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedewa SA, McClellan WM, Judd Set al. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol 2014;15:136. 10.1186/1471-2369-15-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marmot MG, Smith GD, Stansfeld Set al. Health inequalities among British civil servants: the Whitehall II study. Lancet North Am Ed 1991;337:1387–93. 10.1016/0140-6736(91)93068-K. [DOI] [PubMed] [Google Scholar]
- 13. Hossain MP, Palmer D, Goyder Eet al. Association of deprivation with worse outcomes in chronic kidney disease: findings from a hospital-based cohort in the United Kingdom. Nephron Clin Pract 2012;120:c59–70. 10.1159/000334998. [DOI] [PubMed] [Google Scholar]
- 14. Nie Y, Witten B, Schatell Det al. Changes in employment status prior to initiation of maintenance hemodialysis in the USA from 2006 to 2015. Clin Kidney J 2019;13:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couchoud C, Guihenneuc C, Bayer Fet al. Medical practice patterns and socio-economic factors may explain geographical variation of end-stage renal disease incidence. Nephrol Dial Transplant 2012;27:2312–22. 10.1093/ndt/gfr639. [DOI] [PubMed] [Google Scholar]
- 16. Green JA, Cavanaugh KL. Understanding the influence of educational attainment on kidney health and opportunities for improved care. Adv Chronic Kidney Dis 2015;22:24–30. 10.1053/j.ackd.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 17. Babayev R, Whaley-Connell A, Kshirsagar Aet al. Association of race and body mass index with ESRD and mortality in CKD stages 3-4: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013;61:404–12. 10.1053/j.ajkd.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 18. Morton RL, Schlackow I, Staplin Net al. Impact of educational attainment on health outcomes in moderate to severe CKD. Am J Kidney Dis 2016;67:31–9. 10.1053/j.ajkd.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winitzki D, Zacharias HU, Nadal Jet al. Educational attainment is associated with kidney and cardiovascular outcomes in the German CKD (GCKD) cohort. Kidney Int Rep 2022;7:1004–15. 10.1016/j.ekir.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulliford M, Figueroa-Munoz J, Morgan Met al. What does ‘access to health care’ mean? J Health Serv Res Policy 2002;7:186–8. 10.1258/135581902760082517. [DOI] [PubMed] [Google Scholar]
- 21. Bello AK, Hemmelgarn B, Lin Met al. Impact of remote location on quality care delivery and relationships to adverse health outcomes in patients with diabetes and chronic kidney disease. Nephrol Dial Transplant 2012;27:3849–55. 10.1093/ndt/gfs267. [DOI] [PubMed] [Google Scholar]
- 22. Jurkovitz CT, Li S, Norris KCet al. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013;61:S24–32. 10.1053/j.ajkd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol 2007;34:2024–7. [PubMed] [Google Scholar]
- 24. Navaneethan SD, Aloudat S, Singh S. A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol 2008;9:3. 10.1186/1471-2369-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berkman ND, Sheridan SL, Donahue KEet al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107. 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 26. Wright JA, Wallston KA, Elasy TAet al. Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis 2011;57:387–95. 10.1053/j.ajkd.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devraj R, Borrego M, Vilay AMet al. Relationship between health literacy and kidney function. Nephrology 2015;20:360–7. 10.1111/nep.12425. [DOI] [PubMed] [Google Scholar]
- 28. Boyer A, Begin Y, Dupont Jet al. Health literacy level in a various nephrology population from Québec: predialysis clinic, in-centre hemodialysis and home dialysis; a transversal monocentric observational study. BMC Nephrol 2021;22:259. 10.1186/s12882-021-02464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine R, Javalkar K, Nazareth Met al. Disparities in health literacy and healthcare utilization among adolescents and young adults with chronic or end-stage kidney disease. J Pediatr Nurs 2018;38:57–61. 10.1016/j.pedn.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 30. Moraes KL, Brasil VV, Oliveira GFet al. Functional health literacy and knowledge of renal patients on pre-dialytic treatment. Rev Bras Enferm 2017;70:155–62. 10.1590/0034-7167-2015-0169. [DOI] [PubMed] [Google Scholar]
- 31. Dageforde LA, Cavanaugh KL. Health literacy: emerging evidence and applications in kidney disease care. Adv Chronic Kidney Dis 2013;20:311–9. 10.1053/j.ackd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gurgel do Amaral M, Reijneveld SA, Almansa Jet al. Do uncontrolled hypertension, diabetes, dyslipidemia, and obesity mediate the relationship between health literacy and chronic kidney disease complications? Int J Environ Res Public Health 2021;18:5235. 10.3390/ijerph18105235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall YN, Choi AI, Himmelfarb Jet al. Homelessness and CKD: a cohort study. Clin J Am Soc Nephrol 2012;7:1094–102. 10.2215/CJN.00060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afsar B, Elsurer Afsar R, Kanbay Aet al. Air pollution and kidney disease: review of current evidence. Clin Kidney J 2019;12:19–32. 10.1093/ckj/sfy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu X, Nie S, Ding Het al. Environmental pollution and kidney diseases. Nat Rev Nephrol 2018;14:313–24. 10.1038/nrneph.2018.11. [DOI] [PubMed] [Google Scholar]
- 36. Brunt H, Barnes J, Jones SJet al. Air pollution, deprivation and health: understanding relationships to add value to local air quality management policy and practice in Wales, UK. J Public Health 2016;39:485–97. [DOI] [PubMed] [Google Scholar]
- 37. Lin YT, Lo YC, Chiang HYet al. particulate air pollution and progression to kidney failure with replacement therapy: an advanced CKD registry-based cohort study in Taiwan. Am J Kidney Dis 2020;76:645–57.e1. 10.1053/j.ajkd.2020.02.447. [DOI] [PubMed] [Google Scholar]
- 38. Ran J, Sun S, Han Let al. Fine particulate matter and cause-specific mortality in the Hong Kong elder patients with chronic kidney disease. Chemosphere 2020;247:125913. 10.1016/j.chemosphere.2020.125913. [DOI] [PubMed] [Google Scholar]
- 39. Wu YH, Wu CD, Chung MCet al. Long-term exposure to fine particulate matter and the deterioration of estimated glomerular filtration rate: a cohort study in patients with pre-end-stage renal disease. Front Public Health 2022;10:858655. 10.3389/fpubh.2022.858655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu B, Fan D, Huang F. Relationship of chronic kidney disease with major air pollutants - A systematic review and meta-analysis of observational studies. Environ Toxicol Pharmacol 2020;76:103355. 10.1016/j.etap.2020.103355. [DOI] [PubMed] [Google Scholar]
- 41. Okoye OC, Carnegie E, Mora L. Air pollution and chronic kidney disease risk in oil and gas-situated communities: a systematic review and meta-analysis. Int J Public Health 2022;67:1604522. 10.3389/ijph.2022.1604522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu MY, Lo WC, Chao CTet al. Association between air pollutants and development of chronic kidney disease: a systematic review and meta-analysis. Sci Total Environ 2020;706:135522. 10.1016/j.scitotenv.2019.135522. [DOI] [PubMed] [Google Scholar]
- 43. Ran J, Yang A, Sun Set al. Long-term exposure to ambient fine particulate matter and mortality from renal failure: a retrospective cohort study in Hong Kong, China. Am J Epidemiol 2020;189:602–12. 10.1093/aje/kwz282. [DOI] [PubMed] [Google Scholar]
- 44. Sharma A, Lewis S, Szatkowski L. Insights into social disparities in smoking prevalence using Mosaic, a novel measure of socioeconomic status: an analysis using a large primary care dataset. BMC Public Health 2010;10:755. 10.1186/1471-2458-10-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xia J, Wang L, Ma Zet al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 2017;32:475–87. 10.1093/ndt/gfw452. [DOI] [PubMed] [Google Scholar]
- 46. Bundy JD, Bazzano LA, Xie Det al. Self-reported tobacco, alcohol, and illicit drug use and progression of chronic kidney disease. Clin J Am Soc Nephrol 2018;13:993–1001. 10.2215/CJN.11121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grams ME, Coresh J, Segev DLet al. Vascular disease, ESRD, and death: interpreting competing risk analyses. Clin J Am Soc Nephrol 2012;7:1606–14. 10.2215/CJN.03460412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ricardo AC, Anderson CA, Yang Wet al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2015;65:412–24. 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Staplin N, Haynes R, Herrington WGet al. Smoking and adverse outcomes in patients with CKD: the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2016;68:371–80. 10.1053/j.ajkd.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hall ME, Wang W, Okhomina Vet al. Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. J Am Heart Assoc 2016;5:e003280. 10.1161/JAHA.116.003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Katikireddi SV, Whitley E, Lewsey Jet al. Socioeconomic status as an effect modifier of alcohol consumption and harm: analysis of linked cohort data. Lancet Public Health 2017;2:e267–76. 10.1016/S2468-2667(17)30078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai Wet al. High alcohol consumption and the risk of renal damage: a systematic review and meta-analysis. QJM 2015;108:539–48. 10.1093/qjmed/hcu247. [DOI] [PubMed] [Google Scholar]
- 53. Kelly JT, Su G, Zhang Let al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol 2021;32:239–53. 10.1681/ASN.2020030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park S, Lee S, Kim Yet al. Causal effect of alcohol use on the risk of end-stage kidney disease and related comorbidities: a Mendelian randomization study. Kidney Res Clin Pract 2021;40:282. 10.23876/j.krcp.20.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuan H, Yu Q, Bai Het al. Alcohol intake and the risk of chronic kidney disease: results from a systematic review and dose–response meta-analysis. Eur J Clin Nutr 2021;75:1555–67. 10.1038/s41430-021-00873-x. [DOI] [PubMed] [Google Scholar]
- 56. Joo YS, Koh H, Nam KHet al. Alcohol consumption and progression of chronic kidney disease: results from the Korean cohort study for outcome in patients with chronic kidney disease. Mayo Clin Proc 2020;95:293–305. 10.1016/j.mayocp.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 57. Posadzki P, Pieper D, Bajpai Ret al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 2020;20:1724. 10.1186/s12889-020-09855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robinson-Cohen C, Littman AJ, Duncan GEet al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 2014;25:399–406. 10.1681/ASN.2013040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen JL, Lerner D, Ruthazer Ret al. Association of physical activity with mortality in chronic kidney disease. J Nephrol 2008;21:243–52. [PubMed] [Google Scholar]
- 60. Fraser SD, Roderick PJ, May CRet al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol 2015;16:193. 10.1186/s12882-015-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamamoto R, Ito T, Nagasawa Yet al. Efficacy of aerobic exercise on the cardiometabolic and renal outcomes in patients with chronic kidney disease: a systematic review of randomized controlled trials. J Nephrol 2021;34:155–64. 10.1007/s40620-020-00865-3. [DOI] [PubMed] [Google Scholar]
- 62. Kuo CP, Tsai MT, Lee KHet al. Dose-response effects of physical activity on all-cause mortality and major cardiorenal outcomes in chronic kidney disease. Eur J Prev Cardiol 2022;29:452–61. 10.1093/eurjpc/zwaa162. [DOI] [PubMed] [Google Scholar]
- 63. Beddhu S, Baird BC, Zitterkoph Jet al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009;4:1901–6. 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beddhu S, Wei G, Marcus RLet al. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol 2015;10:1145–53. 10.2215/CJN.08410814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zelle DM, Klaassen G, van Adrichem Eet al. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol 2017;13:152–68. 10.1038/nrneph.2016.187. [DOI] [PubMed] [Google Scholar]
- 66. Borrelli S, Chiodini P, Caranci Net al. Area deprivation and risk of death and CKD progression: long-term cohort study in patients under unrestricted nephrology care. Nephron 2020;144:488–97. 10.1159/000509351. [DOI] [PubMed] [Google Scholar]
- 67. Solbu MD, Thomson PC, Macpherson Set al. Serum phosphate and social deprivation independently predict all-cause mortality in chronic kidney disease. BMC Nephrol 2015;16:194. 10.1186/s12882-015-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bello AK, Peters J, Rigby Jet al. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol 2008;3:1316–23. 10.2215/CJN.00680208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Udayaraj UP, Haynes R, Winearls CG. Late presentation of patients with end-stage renal disease for renal replacement therapy—is it always avoidable? Nephrol Dial Transplant 2011;26:3646–51. 10.1093/ndt/gfr164. [DOI] [PubMed] [Google Scholar]
- 70. Occelli F, Deram A, Génin Met al. Mapping end-stage renal disease (ESRD): spatial variations on small area level in northern France, and association with deprivation. PLoS One 2014;9:e110132. 10.1371/journal.pone.0110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saunders MR, Ricardo AC, Chen Jet al. Neighborhood socioeconomic status and risk of hospitalization in patients with chronic kidney disease: a chronic renal insufficiency cohort study. Medicine (Baltimore) 2020;99:e21028. 10.1097/MD.0000000000021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goldfarb-Rumyantzev AS, Rout P, Sandhu GSet al. Association between social adaptability index and survival of patients with chronic kidney disease. Nephrol Dial Transplant 2010;25:3672–81. 10.1093/ndt/gfq177. [DOI] [PubMed] [Google Scholar]