ABSTRACT
Background
Short and long sleep durations are associated with cognitive dysfunction. Given the increased prevalence of sleep abnormalities in the chronic kidney disease (CKD) population, we tested whether the association between sleep duration and cognitive function differed between older adults with and without CKD.
Methods
This was a study of 3215 older adults (age ≥60 years) enrolled in the National Health and Nutrition Examination Survey (2011–14) evaluating sleep duration, cognitive function (immediate recall, delayed recall, verbal fluency, executive function and processing speed and global cognition) and kidney function. We quantified the association between sleep duration and cognitive function using linear regression and tested whether the associations differed among those with CKD and without using a Wald test for interaction.
Results
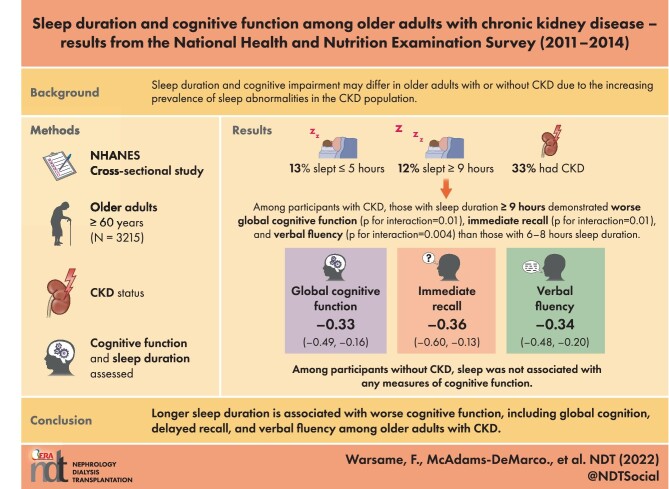
Among 3215 participants, 13.3% reported 2–5 hours of sleep/day, 75.2% reported 6–8 hours, and 11.5% reported ≥9 hours. Persons with CKD were more likely to sleep ≥9 hours [odds ratio 1.73 (95% confidence interval 1.22–2.46)]. Among participants with CKD, those with a sleep duration ≥9 hours demonstrated worse global cognitive function (P for interaction = .01), immediate recall (P for interaction = .01) and verbal fluency (P for interaction = .004) than those with a sleep duration of 6–8 h; no differences were observed for participants with CKD who slept 2–5 hours. Among participants without CKD, sleep was not associated with any measures of cognitive function.
Conclusions
Longer sleep duration is associated with worse cognitive function only among persons with CKD, and global cognition, delayed recall and verbal fluency are particularly affected. Studies should identify interventions to improve sleep patterns and quality in this population.
Keywords: cognitive function, cognitive impairment, chronic kidney disease, sleep duration
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Sleep duration is an extensively studied component of sleep quality and has been found to have a U-shaped association with cognitive decline. Given that shorter and longer sleep durations are associated with cognitive dysfunction, we sought to explore whether this association differs among persons with CKD.
What this study adds?
Long sleep duration is more common among persons with CKD. There were no significant associations between cognitive function and sleep duration among those without CKD. Among participants with CKD, those with long sleep duration demonstrated worse global cognitive function, immediate recall and verbal fluency compared with those with shorter sleep duration.
What impact this may have on practice or policy?
Sleep duration may be a salient factor in the relationship between kidney function and cognitive decline. Primary care physicians and nephrologists should consider counseling patients with CKD about excessive sleep and further studies should identify any underlying sleep disturbances in this patient population that may mediate cognitive decline.
INTRODUCTION
Individuals with chronic kidney disease (CKD) have a higher risk of developing cognitive impairment compared with the general population and cognitive impairment increases in severity as kidney function declines [1–4]. Lower kidney function is associated with worse cognitive functioning across multiple cognitive domains, including global cognition, verbal memory, visual-spatial organization, attention, naming and executive function [4–6]. Cognitive dysfunction across these domains is significant because of its impact on health behaviors and downstream effects on morbidity and mortality [7]. For instance, impaired executive function and verbal memory are associated with medication regimen and dialysis nonadherence [8,9]. Among patients with CKD, cognitive dysfunction is associated with a lower chance of transplant listing and increased waitlist mortality [10]. Potential mechanisms underlying the pathophysiological relationship between brain and kidney function include vascular injury, neuronal injury from uremic toxins, glymphatic dysfunction and endothelial dysfunction [2,11]. Mitigating the risk factors of cognitive dysfunction in CKD is important to potentially curtail cognitive decline and limit downstream effects.
Sleep-related problems, including abnormal sleep duration and poor sleep quality, are prevalent among people with CKD [12]. Appropriate durations of sleep promote memory consolidation, procedural memory, executive function and attention [13]. Among older adults in the general population, sleep duration has been identified as a modifiable risk factor for dementia and the literature suggests a U-shaped associated between cognition and sleep [14–16]. Both extremes of sleep duration have been linked to a greater likelihood of developing dementia in later life [17]. Further, longitudinal studies conducted over decades suggest inadequate sleep may precede changes in cognitive function [16]. It is unclear whether the relationship between sleep duration and cognitive impairment differs among individuals with CKD, however, the association is likely stronger in this population given more rapid cognitive declines and illness-related sleep abnormalities.
Given the association between sleep duration and cognitive impairment among older adults, we sought to test whether the relationship between sleep duration and cognitive function differed among older adults with and without CKD. We utilized the National Health and Nutrition Examination Survey (NHANES, 2011–14) to examine whether the relationship between sleep and cognitive function differs among older adults with and without CKD.
MATERIALS AND METHODS
Study design
The NHANES is a cross-sectional survey of noninstitutionalized US residents conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) [18]. The NHANES sampling model is not random, but instead uses a multistage probability sampling design in order to select participants representative of the US population. The participant selection process also oversamples certain subgroups to increase reliability estimates for these groups. We used sampling weights for all analyses to account for the differential probabilities of study selection as recommended by the NHANES. Data collection for the NHANES includes a brief household screening interview, an in-depth household interview and a medical examination conducted in a mobile examination center. The NHANES survey and consent form were approved by the NCHS Research Ethics Review Board.
Among NHANES participants from the 2011–12 and 2013–14 cycles, 3215 participants were adults ≥60 years of age with measured serum creatine and at least one assessment of cognitive function. Participants’ health information was ascertained via direct measurement or self-report, including hypertension [systolic blood pressure (SBP) ≥130 mmHg, diastolic blood pressure (DBP) ≥80 mmHg, or current use of antihypertensive medication], diabetes (fasting blood glucose level ≥126 mg/dl, non-fasting blood glucose level ≥200 mg/dl, reported history of diabetes and/or current use of medications for diabetes or high blood sugar), anemia (hemoglobin <12 g/dl in males and <11 g/dl in females or reported taking treatment for anemia in the past 3 months), body mass index (kg/m2), clinically significant depressive symptoms [Patient Health Questionnaire (PHQ-9) ≥10] and physical activity (collected using the Global Physical Activity Questionnaire and converted to MET-min/week) [19]. Participants with ≥600 MET-min/week were categorized as ‘physically active’ based on the US physical activity guidelines [20]. Histories of coronary heart disease (CHD), myocardial infarction (MI), stroke and smoking (smoked at least 100 cigarettes over a lifetime) were also self-reported.
Additionally, NHANES participants were asked if they took prescription medications in the previous 30 days. Medication names were identified by inspecting medication containers or by self-report and then matched to a standard generic drug name. A therapeutic classification was assigned to each drug and each ingredient of the drug. Participants were classified as taking sleep or cognitive function–affecting medications if they had taken one or more types of medication with the following therapeutic classification: central nervous system (CNS) agents (anxiolytics, sedatives and hypnotics; analgesics; anticonvulsants; CNS stimulants); psychotherapeutic agents (psychotherapeutic combinations; antidepressants; antipsychotics; antimanic agents) and respiratory agents (antihistamines).
CKD and sleep duration
Serum creatinine (mg/dl) and albuminuria [albumin:creatinine ratio (ACR), mg/g] were measured during each study cycle. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate estimated glomerular filtration rate (eGFR) [21]. We categorized CKD as no CKD (eGFR ≥60 ml/min/1.73 m2 and ACR <30 mg/g) and CKD (eGFR <60 ml/min/1.73 m2 and/or ACR ≥30 mg/g) [22]. Participants on dialysis were excluded from the study, as the sample was insufficient. Sleep duration was assessed with the question ‘How much sleep do you get (hours)?’ and participants’ responses were the number of hours ranging from 2–11 or ≥12, refuse to answer or don't know. Normal sleep duration was defined as 6–8 hours, short sleep duration as <6 hours, and long sleep duration as ≥9 hours, based on common cutoffs in previous studies investigating sleep duration in the CKD population [23, 24]. Additionally, the NHANES further assessed participants’ sleep using the questions, ‘Have you ever told a doctor or other health professional that you have trouble sleeping?’ and ‘Have you ever been told by a doctor or other health professional that have a sleep disorder’ (yes/no).
Cognitive function
In the 2011–12 and 2013–14 NHANES cycles, a series of cognitive function assessments were administered, including word learning modules from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuropsychological battery [25], the Animal Fluency (AF) Test and the Digit Symbol Substitution test (DSST). All participants who were ≥60 years of age were eligible for cognitive testing and assessments were presented to participants in the language of their choosing.
The CERAD neuropsychological battery is used to detect cognitive decline in older adults at risk for Alzheimer's disease [26]. The CERAD word learning modules assess both immediate (CERAD-WL) and delayed memory (CERAD-DL) of novel verbal information. Participants are presented with 10 unrelated words and are then asked to recall the words during three consecutive learning trials [27]. The number of words correctly identified across three trials comprises the immediate recall score (CERAD-WL). Delayed recall (CERAD-DL) is tested after participants complete both the AF and DSST assessments and the score is the number of words recalled out of 10. Diminished recall is an early sign of dementia, and the word learning tests have been found to efficiently distinguish older adults with dementia from those with normal cognition [25, 28, 29].
In the AF test, participants are asked to name as many animals as they can in 1 minute, with a point given per named animal. Naming animals from memory allows for participants to identify common animals across cultural backgrounds. This task relies on verbal fluency and having adequate semantic memory storage as well as the executive functioning to explore memory stores in a time-efficient manner [30]. The AF test is also able to sensitively discriminate patients with normal cognition from those with cognitive impairment and dementia [31].
In the DSST, participants are given a key with a series of nine numbers and corresponding symbols. Participants are then instructed to match numbers with their corresponding symbol in a 2-minute time span. The DSST score is the count of correctly matched numbers and symbols. This test assesses a range of executive functions, including attention, scanning and processing speed, and has been validated to sensitively capture cognitive dysfunction [32]. In each of the above four tests, participants were given 1 point for each correct response, with higher scores reflecting better cognitive performance [27, 33]. A composite measure of global cognition function was assessed based on the four objective tests. Each domain score was standardized to a mean of 0 and standard deviation (SD) of 1 and then averaged into a global cognitive function score, as described previously [34].
Descriptive statistics
The characteristics of the study sample were summarized by the proportion of the overall sample for categorical variables, by means and SDs for normally distributed continuous variables or by medians and interquartile ranges (IQRs) for nonnormally distributed continuous variables. The data were summarized and presented in groups of participants with reported sleep durations of 2–5 hours, 6–8 hours or ≥9 hours (Table 1). The NHANES sample weights were accounted for in the analysis to calculate nationally representative estimates that are appropriately adjusted for survey nonresponse [35].
Table 1:
Characteristics of participants ≥60 years of age with and without CKD from the NHANES (2011–2014; N = 3215).
| Sleep (hours) | ||||
|---|---|---|---|---|
| Characteristics | Values | 2–5 | 6–8 | ≥9 |
| Overall, n | 3215 | 427 | 2418 | 370 |
| Non-CKDa, n | 2052 | 258 | 1606 | 178 |
| CKD, n | 1163 | 169 | 802 | 192 |
| Age (years) | ||||
| 60–69 | 53.6 | 58.9 | 55.1 | 40.0 |
| 70–79 | 29.5 | 24.2 | 29.5 | 33.7 |
| ≥80 | 16.9 | 16.9 | 15.4 | 26.3 |
| Race | ||||
| Mexican American | 3.8 | 5.2 | 3.6 | 3.5 |
| Other Hispanic | 3.7 | 6.7 | 3.5 | 2.8 |
| Non-Hispanic White | 78.4 | 60.3 | 79.9 | 83.0 |
| Non-Hispanic Black | 8.4 | 18.7 | 7.5 | 6.6 |
| Non-Hispanic Asian | 4.0 | 6.0 | 3.9 | 2.6 |
| Other | 1.7 | 3.1 | 1.6 | 1.6 |
| Female | 54.2 | 57.3 | 53.8 | 54.8 |
| Education ≥12 years | 81.5 | 74.1 | 82.7 | 79.8 |
| CKD | 33.0 | 37.9 | 30.1 | 48.4 |
| Hypertension | 75.4 | 79.9 | 74.4 | 78.5 |
| Diabetes | 23.1 | 30.4 | 21.8 | 26.5 |
| CHD | 9.9 | 7.3 | 10.0 | 11.5 |
| MI | 8.8 | 8.1 | 8.8 | 9.7 |
| Stroke | 7.6 | 11.8 | 6.8 | 9.8 |
| Anemia | 7.0 | 10.6 | 5.8 | 11.9 |
| Physically active | 49.4 | 43.9 | 52.3 | 34.5 |
| Depressive symptoms | 7.6 | 21.4 | 5.9 | 7.7 |
| Prescription medication use | 35.8 | 40.2 | 33.8 | 45.3 |
| Ever smoker | 50.1 | 53.0 | 50.1 | 48.3 |
| BMI (kg/m2), median (IQR) | 27.9 (7.3) | 30.4 (9.3) | 27.7 (7.1) | 28.0 (7.4) |
Values are presented as proportions (%) unless stated otherwise, accounting for NHANES sampling weights. eGFR was calculated using serum creatinine and the CKD-EPI equation. CKD was defined as an eGFR <60 ml/min/1.73 m2 or ACR ≥30 mg/g. Depressive symptoms were defined as a PHQ-9 score >9. Physical activity was collected using the Global Physical Activity Questionnaire and converted to MET-min/week. Physically active was defined as ≥600 MET-min/week. Prescription medication use was defined as taking prescription medicine that potentially affected sleep or cognitive function in the past 30 days.
aIndicates preweighted sample sizes of participants with and without CKD.
BMI, body mass index.
CKD, sleep duration and cognitive function
Using a linear regression model, differences in cognitive function by sleep duration were estimated using Cohen's d. Cohen's d, or the standardized mean difference, measures the difference between two means, even when the dependent variables are measured using different scales [36]. A Cohen's d of 1 indicates that two groups differ by 1 SD; a d of 0.2, 0.5 or 0.8 suggests small, medium or large effect sizes, respectively [37]. To test whether the association between cognitive function and sleep duration differed by CKD status, we tested this interaction using a Wald test. Models were adjusted for potential confounders of sleep duration and cognitive impairment (age, sex, race, education, hypertension, diabetes, CHD, MI, stroke, anemia, physical activity, depressive symptoms and smoking). The above were covariates that satisfied criteria for potential confounding: (1) associated with the outcome (cognitive function), (2) unequally distributed between exposure (sleep duration) groups (Table 1) and (3) must not be an effect of the exposure. We calculated variance inflation factors (VIFs) and did not find multicollinearity.
Sensitivity analysis
A sensitivity analysis was conducted to test whether the associations between CKD, sleep duration and cognitive function were robust to the exclusion of participants with depressive symptoms. The presence of depressive symptoms could introduce uncertainty in the true effect sizes given the association with CKD, sleep duration and cognitive function [38, 39]. Participants reported depressive symptoms on the PHQ-9 (a score ≥10 was indicative of clinically significant depressive symptoms), which has been validated against Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for clinical depression in the dialysis and primary care settings [40, 41].
We also assessed whether inferences remained robust after accounting for the use of prescription medications that can influence sleep or cognitive function.
Statistical analysis
For all analyses, P-values <.05 were used as the cutoff for statistical significance. All analyses were performed using Stata 16.0 (StataCorp, College Station, TX, USA).
RESULTS
Participant characteristics
In this weighted sample of 3215 participants, 53.6% were 60–69 years old, 29.5% were 70–79 years and 16.9% were >80 years (Table 1). A total of 78.4% of participants identified as White, 8.4% as Black, 3.7% as Hispanic and 4.0% as Asian. Additionally, 54.2% of participants were female and 81.5% attained greater than a high school education. Using the CKD-EPI equation, 33% of participants were identified as having CKD. Medical comorbidities in the sample included hypertension (75.4%), diabetes (23.1%), coronary heart disease (9.9%), myocardial infarction (8.8%), stroke (7.6%) and anemia (7.0%). A total of 7.6% of participants screened positively for clinically significant depressive symptoms and 50.1% reported ever smoking (at least 100 cigarettes over their lifetime). Among the participants, 13.3% reported sleeping for 2–5 hours, 75.2% for 6–8 hours and 11.5% for ≥9 hours (Table 1).
Sleep duration and cognitive function
After adjustment, participants with a sleep duration of ≥9 hours had significantly worse global cognitive function than those with a sleep duration of 6–8 hours {Cohen's d = −0.20 [95% confidence interval (CI) −0.31 to −0.08]}. Overall, a sleep duration of ≥9 hours was also associated with worse performance on three of four domain-specific tests, including delayed recall [Cohen's d = −0.28 (95% CI −0.46 to −0.11)], immediate recall [Cohen's d = −0.16 (95% CI −0.31 to −0.02)] and executive function and processing [Cohen's d = −0.14 (95% CI −0.25 to −0.03)] compared with participants who slept 6–8 hours (Table 2). Additionally, those with a sleep duration of 2–5 hours had higher odds of reporting sleep problems compared with those with a sleep duration of 6–8 hours (P < .05). Participants with a sleep duration of ≥9 hours were as likely as those sleeping 6–8 hours to report trouble sleeping (P > .05).
Table 2:
Association between sleep duration, CKD and global and domain-specific cognitive function among participants ≥60 years of age from the NHANES (2011–2014; N = 3215)
| Sleep (hours) | Overall, mean (95% CI) | No CKD, mean (95% CI) | CKD, mean (95% CI) | P for interaction |
|---|---|---|---|---|
| Global function | ||||
| 2–5 | −0.02 (−0.11–0.07) | 0.01 (−0.10–0.12) | −0.07 (−0.21–0.08) | 0.38 |
| 6–8 | 0 (ref) | 0 (ref) | 0 (ref) | |
| ≥9 | −0.20 (−0.31 to −0.08) | −0.07 (−0.21–0.06) | −0.33 (−0.49 to −0.16) | 0.01 |
| Immediate recall (CERAD-WL) | ||||
| 2–5 | −0.06 (−0.17–0.04) | −0.03 (−0.15–0.09) | −0.13 (−0.31–0.05) | 0.35 |
| 6–8 | 0 (ref) | 0 (ref) | 0 (ref) | |
| ≥9 | −0.16 (−0.31 to −0.02) | 0.01 (−0.15–0.17) | −0.36 (−0.60 to −0.13) | 0.01 |
| Delayed recall (CERAD-DL) | ||||
| 2–5 | −0.00 (−0.13–0.13) | 0.08 (−0.07–0.23) | −0.15 (−0.37–0.07) | 0.09 |
| 6–8 | 0 (ref) | 0 (ref) | 0 (ref) | |
| ≥9+ | −0.28 (−0.46 to −0.11) | −0.19 (−0.41–0.03) | −0.41 (−0.64 to −0.17) | 0.13 |
| Verbal fluency (AF) | ||||
| 2–5 | −0.02 (−0.15–0.11) | −0.09 (−0.24–0.07) | 0.08 (−0.15–0.30) | 0.23 |
| 6–8 | 0 (ref) | 0 (ref) | 0 (ref) | |
| ≥9 | −0.13 (−0.29–0.02) | 0.06 (−0.17–0.29) | −0.34 (−0.48 to −0.20) | 0.004 |
| Executive function (DSST) | ||||
| 2–5 | −0.02 (−0.13–0.10) | −0.01 (−0.17–0.14) | −0.01 (−0.23–0.21) | 0.99 |
| 6–8 | 0 (ref) | 0 (ref) | 0 (ref) | |
| ≥9 | −0.14 (−0.25 to −0.03) | −0.09 (−0.22–0.04) | −0.18 (−0.35 to −0.01) | 0.35 |
Cognitive test scores were standardized to a mean of 0 and SD of 1. Global cognitive function was defined as an average score of all four objective cognitive tests. Models were adjusted for age, sex, race, education, hypertension, diabetes, CHD, MI, stroke, anemia, physical activity, depressive symptoms and smoking. NHANES sampling weights were accounted for in linear regression analyses to obtain nationally representative estimates. eGFR was calculated using serum creatinine and the CKD-EPI equation. CKD was defined as an eGFR <60 ml/min/1.73 m2 or ACR ≥30 mg/g.
Sleep duration, CKD and cognitive function
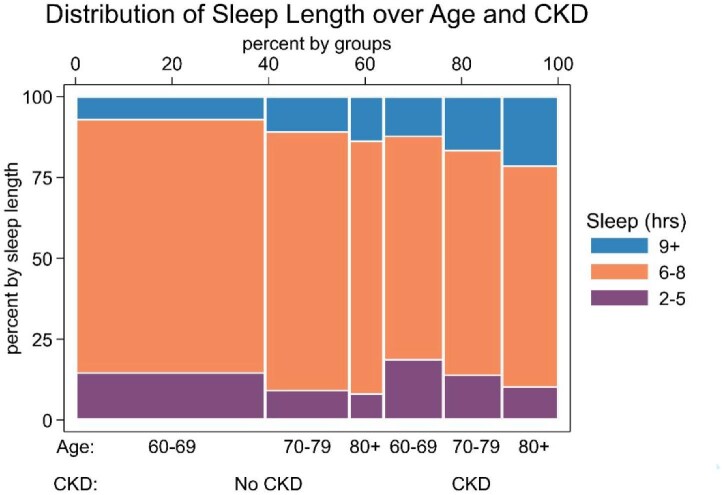
Nonoptimal sleep durations were more common in participants with CKD (Fig. 1). Participants with CKD were significantly more likely to sleep longer [≥9 hours/day; OR 1.73 (95% CI 1.22–2.46)] compared with those without CKD. The association between sleep duration and cognition differed between those with and without CKD. Among participants without CKD, there was no association found between sleep duration and the measured markers of cognitive function (Table 2). However, among participants with CKD, sleep duration of ≥9 hours was associated with worse global cognitive function [Cohen's d = −0.33 (95% CI −0.49 to −0.16), P for interaction = .01], immediate recall [Cohen's d = −0.36 (95% CI −0.60 to −0.13), P for interaction = .01)] and verbal fluency [Cohen's d = −0.34 (95% CI −0.48 to −0.20), P for interaction = .004] compared with those with sleep durations of 6–8 hours.
Figure 1:
Distribution of sleep length of participants ≥60 years of age with and without CKD from the NHANES (2011–2014; N = 3215). Spline plot displaying stacked frequencies of sleep duration (2–5, 6–8 and >9 hours). Participants with and without CKD were categorized into three age groups: 60–69, 70–79 and >80 years. Sleep duration was assessed by self-report on the NHANES household interview. No CKD was defined as eGFR >60 ml/min/1.73 m2 or ACR <30 mg/g and CKD was defined as eGFR <60 ml/min/1.73 m2 or ACR ≥30 mg/g.
Among those with CKD, sleep duration ≥9 hours was associated with worse delayed recall [Cohen's d = −0.41 (95% CI −0.64 to −0.17)] and executive functioning and processing [Cohen's d = −0.18 (95% CI −0.35 to −0.01)] among those with CKD; however, these domains did not significantly differ from participants without CKD (P for interaction = .13 and .35, respectively). There were no significant differences in cognitive test scores in all participants sleeping <6 hours compared with 6–8 hours, regardless of CKD status.
Sensitivity analysis
The results above were robust to exclusion of participants with clinically significant depressive symptoms (Supplementary Table 1). After exclusion of participants with depressive symptoms, sleep duration ≥9 hours remained associated with worse performance on global cognitive function [Cohen's d = −0.30 (95% CI −0.50 to −0.10)] and three of four domain-specific tests among those with CKD. The association between sleep duration ≥9 hours and immediate recall (P for interaction = .03) and verbal fluency (P for interaction = .02) differed between those with and without CKD. Similar to previous analyses, there were no significant differences in cognitive performance on global or domain-specific tests between those with a sleep duration <6 hours versus 6–8 hours.
After accounting for neurotropic and psychotropic medications that have the potential to impact sleep or cognitive function, we found results remained similar after adjustment for prescription medication use (Supplementary Table 2). Among participants with CKD, sleep duration ≥9 hours was associated with worse performance in global cognitive function [Cohen's d = −0.32 (95% CI −0.48 to −0.16)] and in four domain-specific tests. The association between sleep duration ≥9 hours and global cognitive function (P for interaction = .008), immediate recall (P for interaction = .008) and verbal fluency (P for interaction = .003) differed between those with and without CKD.
DISCUSSION
We characterized the association between sleep duration and cognitive function among people with and without CKD. Using a nationally representative survey of 3215 NHANES participants, CKD was present in 33% and was independently associated with 1.7 times higher odds of sleep duration ≥9 hours. Overall, long sleep duration was associated with delayed recall [Cohen's d = −0.28 (95% CI −0.46 to −0.11)] and executive functioning and processing [Cohen's d = −0.14 (95% CI −0.25 to −0.03)]; these associations did not differ by CKD. Among participants with CKD, there was an association between sleep duration ≥9 hours and immediate recall (P for interaction = .01), verbal fluency (P for interaction = .004) and global cognitive performance (P for interaction = .01). When participants who reported clinically significant depressive symptoms were excluded in a sensitivity analysis, the effect sizes remained consistent. Additionally, results were robust in a sensitivity analysis accounting for medications impacting sleep.
Our finding of 1.7 times higher odds of long sleep duration among CKD participants is in line with findings in other cross-sectional studies. Using the National Health Interview Survey, participants reporting a sleep duration ≥8 hours had nearly 2-fold higher odds of reporting a CKD diagnosis compared with those with an average sleep duration [42]. This finding was also comparable to a cohort study that found long sleep duration was associated with 2.31-fold higher odds of renal insufficiency compared with those with an average sleep duration [43]. Additionally, a previous national study reported a higher prevalence of short sleep duration among participants with CKD stage 1 or 2; however, those with CKD stage 3 or 4 were more likely to sleep >7 hours. This is in line with findings in our study that focused on a population with CKD stage 3 and 4 (eGFR <60 ml/min/1.73 m2) [12]. Sleep disorders are likely highly prevalent in CKD due to the physiological multifaceted and bidirectional relationship between sleep and CKD [44]. Altered sleep may affect the dampening of sympathetic activity overnight, resulting in a nocturnal nadir of systolic and diastolic blood pressure [45]. Elevated nocturnal BP has been associated with a higher risk of developing microalbuminuria, which may explain the association between sleep and CKD progression [46].
Our study extended prior findings by focusing on the potential role of sleep duration in cognitive impairment among older adults with and without CKD. Nonoptimal sleep duration has been extensively linked with cognitive impairment in community-dwelling older adults [17, 47, 48]. An NHANES of older adults found long sleep durations were an independent risk factor for worse performance on immediate recall, delayed recall, verbal fluency and executive function [49]. In CKD and ESRD populations, lower eGFR was associated with worse performance on global cognitive function, recall and executive function domains, leading to subsequent dementia [5, 50–53]. Our study suggests that older adults with CKD and long sleep durations may be more vulnerable to cognitive decline. Interestingly, participants with CKD and short sleep durations did not demonstrate significantly worse cognitive function across these domains compared with non-CKD participants. Potential explanations include that in patients with CKD, buildup of uremic toxins is correlated with somnolence and daytime sleepiness and has been shown to cause uremic neurotoxicity in experimental models [11]. In the general population, long sleep duration rather than short sleep duration is linked to higher inflammatory markers [54]. Additionally, long (versus short) sleep duration may be an indicator of sleep disordered breathing, leading to chronic hypoxic episodes that may have worse neurocognitive effects [55].
In this novel nationally representative study, the interaction between long sleep duration and CKD was most notable in specific cognitive domains, namely immediate recall, verbal fluency and global cognition. Previously, recall on the CERAD and other similar 10-word learning trials were found to be a sensitive measure for mild cognitive impairment and early Alzheimer's disease [56, 57]. Additionally, verbal fluency is useful for detection of mild cognitive impairment and vascular cognitive impairment without dementia [58]. While our study did not find a significant interaction on the executive function domain, verbal fluency tasks also require some executive control functions, such as tracking of working memory and inhibition of responses [59]. Verbal memory and executive functioning appear to have a significant degree of overlap and it is suggested that executive function impacts both the storage and retrieval of information [60]. Several mechanisms may explain these findings and the impact of sleep duration and CKD on these cognitive domains. A meta-analysis synthesizing 44 studies showed CKD patients with eGFR ≤60 ml/min/1.73 m2 appear to experience cognitive changes in the domains of orientation, attention, memory, executive function and global cognition [6]. Long sleep duration may reflect worse renal function as a result of buildup of uremic toxins, nocturnal hypoxia secondary to obstructive sleep apnea and fatigue in this patient population [61, 62]. Additionally, abnormal sleep duration may independently mediate cognitive declines due to sleep fragmentation, higher sympathetic tone and increased inflammatory cytokines [44, 63, 64].
This study has several important strengths, including capturing a national sample of older adults with objective measures of cognitive and renal function. Given that depression may be an important confounder in an association between sleep duration and CKD, we conducted a sensitivity analysis excluding participants with clinically significant depressive symptoms and findings remained robust. We also leveraged data on medication use and demonstrated that findings remained robust after accounting for the use of sleep aids and other medications that can impact sleep.
The main limitation was the cross-sectional nature of the study, which limits insight into the directionality or causality in the association between sleep duration, CKD and cognitive function. Additionally, prospective studies may be able to better assess for bidirectional associations between sleep and cognitive function. While sleep duration was a self-reported measure, it is possible that the use of large categories (short, normal, long sleep durations) decreased the impact of self-report bias. Further studies using objective assessments of sleep quality, home-based actigraphy or laboratory-based sleep studies could be a future research direction. While the presence of obstructive sleep apnea was not ascertained in the NHANES 2011–14, it may be an important mechanism in the association between long sleep duration and cognitive performance [65, 66].
Additionally, the Cohen's d effect sizes suggested small to moderate effects. One potential explanation is that sleep duration does not capture the full scope of sleep disturbances among patients with CKD. Sleep problems in this population also include sleep apnea, fragmented sleep, daytime somnolence and poor sleep quality [67, 68]. Sleep disordered breathing as measured by actigraphy is associated with cognitive dysfunction in CKD [55]. Future studies could investigate the combined impact of sleep duration, quality and sleep-related disorders on cognitive function.
In conclusion, our findings support a relationship between sleep duration and cognitive function among older persons with CKD. Older adults with CKD and long sleep durations demonstrated worse performance in the domains of memory and verbal fluency compared with those without CKD. Providers may need to screen patients with CKD for long sleep duration, particularly as these patients are less likely to inform a provider they have trouble sleeping. Patients who endorse long sleep duration may need to be counseled regarding the potential impact on cognitive function. Cognitive screening initiatives and sleep hygiene training in patients with CKD may mitigate cognitive decline in later life among this highly vulnerable population.
Supplementary Material
Contributor Information
Fatima Warsame, Division of Biology and Medicine, Warren Alpert Medical School of Brown University, Providence, RI, USA.
Nadia M Chu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jingyao Hong, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Aarti Mathur, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Deidra C Crews, Division of Nephrology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
George Bayliss, Division of Biology and Medicine, Warren Alpert Medical School of Brown University, Providence, RI, USA; Division of Kidney Disease and Hypertension, Brown Medicine, Warren Alpert Medical School of Brown University, Providence, RI, USA.
Dorry L Segev, Department of Surgery, NYU Grossman School of Medicine and NYU Langone Health, NY, NY, USA.
Mara A McAdams-DeMarco, Department of Surgery, NYU Grossman School of Medicine and NYU Langone Health, NY, NY, USA.
FUNDING
This study was supported by the National Institute of Diabetes and Digestive and Kidney Disease, the National Institute of Allergy and Infectious Disease and the National Institute on Aging [grants K01AG064040 [principal investigator (PI): N.M.C.], K24AI144954 (PI: D.L.S.), R01AG055781 (PI: M.A.M.-D.), R01DK120518 (PI: M.A.M-D.) and R01DK114074 (PI: M.A.M.-D.).
AUTHORS’ CONTRIBUTIONS
F.W. participated in the concept design, interpretation of data, drafting, critical revision and approval of the article. N.M.C. and M.A.M.-D participated in the concept design, analysis and interpretation of data, drafting, critical revision and approval of the article. J.H. participated in analysis and interpretation of data, drafting, critical revision and approval of the article. A.M., D.C.C. and G.B. participated in critical revision and approval of the article. D.L.S. participated in concept design, critical revision and approval of the article.
DATA AVAILABILITY STATEMENT
The data underlying this article are available from the NHANES.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Zammit AR, Katz MJ, Bitzer Met al. Cognitive impairment and dementia in older adults with chronic kidney disease: a review. Alzheimer Dis Assoc Disord 2016;30:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bugnicourt JM, Godefroy O, Chillon JMet al. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013;24:353–63. [DOI] [PubMed] [Google Scholar]
- 3. Kurella M, Chertow GM, Luan Jet al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 2004;52:1863–9. [DOI] [PubMed] [Google Scholar]
- 4. Elias MF, Elias PK, Seliger SLet al. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 2009;24:2446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaffe K, Ackerson L, Kurella Tamura Met al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2010;58:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berger I, Wu S, Masson Pet al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall PA, Crossley M, D'Arcy C. Executive function and survival in the context of chronic illness. Ann Behav Med 2010;39:119–27. [DOI] [PubMed] [Google Scholar]
- 8. Insel K, Morrow D, Brewer Bet al. Executive function, working memory, and medication adherence among older adults. J Gerontol B Psychol Sci Soc Sci 2006;61:P102–7. [DOI] [PubMed] [Google Scholar]
- 9. Hain DJ. Cognitive function and adherence of older adults undergoing hemodialysis. Nephrol Nurs J 2008;35:23–9. [PubMed] [Google Scholar]
- 10. Chu NM, Shi Z, Haugen CEet al. Cognitive function, access to kidney transplantation, and waitlist mortality among kidney transplant candidates with or without diabetes. Am J Kidney Dis 2020;76:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viggiano D, Wagner CA, Martino Get al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 2020;16:452–69. [DOI] [PubMed] [Google Scholar]
- 12. Plantinga L, Lee K, Inker LAet al. Association of sleep-related problems with CKD in the United States, 2005–2008. Am J Kidney Dis 2011;58:554–64. [DOI] [PubMed] [Google Scholar]
- 13. Rasch B, Born J.. About sleep's role in memory. Physiol Rev 2013;93:681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider AC, Moon C, Whitaker KMet al. Cross-sectional and prospective associations between self-reported sleep characteristics and cognitive function in men and women: the Midlife in the United States study. J Sleep Res 2021;31:e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scarlett S, Kenny RA, O'Connell MDet al. Associations between cognitive function, actigraphy-based and self-reported sleep in older community-dwelling adults: findings from the Irish Longitudinal Study on Ageing. Int J Geriatr Psychiatry 2021;36:731–42. [DOI] [PubMed] [Google Scholar]
- 16. Sabia S, Fayosse A, Dumurgier Jet al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun 2021;12:2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kondo R, Miyano I, Lee Set al. Association between self-reported night sleep duration and cognitive function among older adults with intact global cognition. Int J Geriatr Psychiatry 2021;36:766–74. [DOI] [PubMed] [Google Scholar]
- 18. Johnson CL, Dohrmann SM, Burt VLet al. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat 2 2014;162:1–33. [PubMed] [Google Scholar]
- 19. National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies: Physical Activity (PAQ_G). Atlanta: Centers for Disease Control and Prevention, National Center for Health, 2013. [Google Scholar]
- 20. US Department of Health and Human Services . Physical activity guidelines for Americans. Washington, DC: US Department of Health and Human Services, 2008. [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 23. Yamamoto R, Shinzawa M, Isaka Yet al. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin J Am Soc Nephrol 2018;13:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee HJ, Kwak N, Kim YCet al. Impact of sleep duration on mortality and quality of life in chronic kidney disease: results from the 2007–2015 KNHANES. Am J Nephrol 2021;52:396–403. [DOI] [PubMed] [Google Scholar]
- 25. Fillenbaum GG, van Belle G, Morris JCet al. Consortium to Establish a Registry for Alzheimer's Disease (CERAD): the first twenty years. Alzheimers Dement 2008;4:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossetti HC, Munro Cullum C, Hynan LSet al. The CERAD neuropsychologic battery total score and the progression of Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leng SX, Cappola AR, Andersen REet al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 2004;16:153–7. [DOI] [PubMed] [Google Scholar]
- 28. Wolfsgruber S, Jessen F, Wiese Bet al. The CERAD neuropsychological assessment battery total score detects and predicts Alzheimer disease dementia with high diagnostic accuracy. Am J Geriatr Psychiatry 2014;22:1017–28. [DOI] [PubMed] [Google Scholar]
- 29. Chandler MJ, Lacritz LH, Hynan LSet al. A total score for the CERAD neuropsychological battery. Neurology 2005;65:102–6. [DOI] [PubMed] [Google Scholar]
- 30. Reverberi C, Cherubini P, Baldinelli Set al. Semantic fluency: cognitive basis and diagnostic performance in focal dementias and Alzheimer's disease. Cortex 2014;54:150–64. [DOI] [PubMed] [Google Scholar]
- 31. Canning SJ, Leach L, Stuss Det al. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology 2004;62:556–62. [DOI] [PubMed] [Google Scholar]
- 32. Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 2018;38:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leng SX, Hung W, Cappola ARet al. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 2009;64:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson RS, Mendes De Leon CF, Barnes LLet al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–8. [DOI] [PubMed] [Google Scholar]
- 35. Chen TC, Parker JD, Clark Jet al. National Health and Nutrition Examination Survey: estimation procedures, 2011–2014. Vital Health Stat 2 2018;177:1–26. [PubMed] [Google Scholar]
- 36. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen J. Statistical power analysis for behavioral sciences, rev. ed. Mahwah, NJ: Lawrence Erlbaum, 1977. [Google Scholar]
- 38. Hedayati SS, Finkelstein FO.. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis 2009;54:741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhai L, Zhang H, Zhang D.. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety 2015;32:664–70. [DOI] [PubMed] [Google Scholar]
- 40. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watnick S, Wang PL, Demadura Tet al. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 2005;46:919–24. [DOI] [PubMed] [Google Scholar]
- 42. Salifu I, Tedla F, Pandey Aet al. Sleep duration and chronic kidney disease: analysis of the national health interview survey. Cardiorenal Med 2014;4:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan NYQ, Chan J, Cheng CYet al. Sleep duration and diabetic kidney disease. Front Endocrinol 2018;9:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turek NF, Ricardo AC, Lash JP.. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 2012;60:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Degaute JP, van de Borne P, Linkowski Pet al. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991;18:199–210. [DOI] [PubMed] [Google Scholar]
- 46. Lurbe E, Redon J, Kesani Aet al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- 47. Hua J, Zhuang S, Shen Yet al. Exploring the bidirectional associations between short or long sleep duration and lower cognitive function: a 7-year cohort study in China. Front Aging Neurosci 2021;13:727763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fu Y, Wang ZT, Qu Yet al. Sleep characteristics and cognitive function in older adults without dementia: the CABLE study. J Alzheimers Dis 2021;84:1029–38. [DOI] [PubMed] [Google Scholar]
- 49. Izci-Balserak B, Zhu B, Wang Het al. Independent associations between sleep duration, gamma gap, and cognitive function among older adults: results from the NHANES 2013–2014. Geriatr Nurs 2022;44:1–7. [DOI] [PubMed] [Google Scholar]
- 50. Drew DA, Weiner DE, Sarnak MJ.. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis 2019;74:782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McAdams-DeMarco MA, Bae S, Chu Net al. Dementia and Alzheimer's disease among older kidney transplant recipients. J Am Soc Nephrol 2017;28:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McAdams-DeMarco MA, Daubresse M, Bae Set al. Dementia, Alzheimer's disease, and mortality after hemodialysis initiation. Clin J Am Soc Nephrol 2018;13:1339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas AG, Ruck JM, Shaffer AAet al. Kidney transplant outcomes in recipients with cognitive impairment: a national registry and prospective cohort study. Transplantation 2019;103:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Irwin MR, Olmstead R, Carroll JE.. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang EW, Abdel-Kader K, Yabes Jet al. Association of sleep-disordered breathing with cognitive dysfunction in CKD stages 4–5. Am J Kidney Dis 2012;60:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shankle WR, Romney AK, Hara Jet al. Methods to improve the detection of mild cognitive impairment. Proc Natl Acad Sci USA 2005;102:4919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dierckx E, Engelborghs S, De Raedt Ret al. The 10-word learning task in the differential diagnosis of early Alzheimer's disease and elderly depression: a cross-sectional pilot study. Aging Ment Health 2011;15:113–21. [DOI] [PubMed] [Google Scholar]
- 58. Zhao Q, Guo Q, Hong Z.. Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neurosci Bull 2013;29:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shao Z, Janse E, Visser Ket al. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duff K, Schoenberg MR, Scott JGet al. The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol 2005;20:111–22. [DOI] [PubMed] [Google Scholar]
- 61. Perl J, Unruh ML, Chan CT.. Sleep disorders in end-stage renal disease: ‘Markers of inadequate dialysis’? Kidney Int 2006;70:1687–93. [DOI] [PubMed] [Google Scholar]
- 62. Gregg LP, Bossola M, Ostrosky-Frid Met al. Fatigue in CKD: epidemiology, pathophysiology, and treatment. Clin J Am Soc Nephrol 2021;16:1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel SR, Zhu X, Storfer-Isser Aet al. Sleep duration and biomarkers of inflammation. Sleep 2009;32:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Somers VK, Dyken ME, Mark ALet al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303–7. [DOI] [PubMed] [Google Scholar]
- 65. Liguori C, Mercuri NB, Izzi Fet al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer's disease biomarkers changes. Sleep 2017;40:zsx011. [DOI] [PubMed] [Google Scholar]
- 66. Jackson CL, Umesi C, Gaston SAet al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: findings from the multi-ethnic study of atherosclerosis. Thorax 2021;76:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ricardo AC, Knutson K, Chen Jet al. The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 2017;28:3708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Knutson KL, Lash J, Ricardo ACet al. Habitual sleep and kidney function in chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Sleep Res 2018;27:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from the NHANES.