ABSTRACT
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common primary glomerulonephritis worldwide and it is characterized by mesangial IgA deposition. Asymptomatic hematuria with various degrees of proteinuria is the most common clinical presentation and up to 20%–40% of patients develop end-stage kidney disease within 20 years after disease onset. The pathogenesis of IgAN involves four sequential processes known as the “four-hit hypothesis” which starts with the production of a galactose-deficient IgA1 (gd-IgA1), followed by the formation of anti-gd-IgA1 IgG or IgA1 autoantibodies and immune complexes that ultimately deposit in the glomerular mesangium, leading to inflammation and injury. Although several key questions about the production of gd-IgA1 and the formation of anti-gd-IgA1 antibodies remain unanswered, a growing body of evidence is shedding light on the innate and adaptive immune mechanisms involved in this complex pathogenic process. Herein, we will focus on these mechanisms that, along with genetic and environmental factors, are thought to play a key role in disease pathogenesis.
Keywords: adaptive immunity, galactose-deficient IgA1, innate immunity, IgA nephropathy
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Immunoglobulin A (IgA) nephropathy (IgAN) is characterized by mesangial deposition of aberrantly glycosylated IgA, and is now recognized as the most common primary glomerulonephritis globally [1]. IgAN was first described by Jean Berger in 1969 [2], and has an annual incidence of 2–10 cases per 100 000 people with most of the cases occurring during the second and third decades of life [3]. The prevalence varies widely from West-to-East, ranging from 12% to 25% of all native kidney biopsies in North America and Europe, and up to 40% in Japan [4]. Although variability in screening policies may account for some of this difference, genetic and environmental factors are thought to play a role as well [5].
Clinical presentation is heterogeneous and varies widely among different age groups. The classical clinical phenotype includes hematuria intercurrent with gastrointestinal or upper respiratory tract infections. Gross hematuria is more commonly seen in younger patients, while asymptomatic hematuria with various degrees of proteinuria is the most common presentation in older adults [1]. Nephrotic syndrome is observed in about 5% of cases [6, 7]. Disease progression is usually slow, but up to 20%–40% of patients develop end-stage kidney disease (ESKD) within 20 years after diagnosis [1]. The severity and time-average of proteinuria are considered major predictors of kidney function loss [8] along with histopathologic features listed in the Oxford Classification [9].
The “four-hit hypothesis” in IgAN offers a concise account of what is currently known about the pathogenesis of the disease (Fig. 1). This hypothesis postulates that the development of IgAN starts with increased circulating levels of an aberrantly glycosylated galactose-deficient IgA (gd-IgA1), followed by the formation of immune complexes with anti-gd-IgA1 antibodies that ultimately deposit in the glomerular mesangium, leading to kidney injury [1]. The origin of gd-IgA1 is still unclear, but data converge to indicate that the gut-associated lymphoid tissue (GALT) plays a key pathogenic role. Interactions between microbiota and intestinal mucosa have been deemed essential for the development of IgA in the gut, but it is not clear how changes to the microbiota and dysbiosis affect the production of aberrant gd-IgA1 [10]. However, the presence of gd-IgA1 in circulation is by itself not sufficient to cause IgAN. The fact that gd-IgA1 is also found in healthy subjects [11] underlines the presence of additional abnormalities that have to take place before the disease develops [12]. Herein, we will review the immune alterations that, along with genetic and environmental factors, play a key role both in the onset and progression of IgAN.
Figure 1:
Representation of four hit hypothesis involved in the pathogenesis of IgA nephropathy.
THE FOUR-HIT HYPOTHESIS AND THE ROLE OF GALACTOSE-DEFICIENT I A
A
Hit 1: increased production and circulation of gd-IgA1
IgA is the predominant antibody subtype in external secretions and plays an important role in mucosal immunity (Fig. 2) [13]. In humans and higher primates, IgA exists in two subclasses: IgA1 which is found in mucosal surfaces and systemic circulation, and IgA2 which is mostly limited to the mucosal surfaces [14]. The two isotypes differ in the numbers of N-linked carbohydrates in the heavy chain and in their hinge region (HR): compared with IgA2, IgA1 contains a unique extended HR characterized by nine serine and threonine residues and usually three to six of them have O-linked glycans attached. These O-glycans are formed by an N-acetylgalactosamine (GalNac) with β1,3-linked galactose that can be sialylated with one molecule of sialic acid [12]. Both the total number and the structure of attached O-glycans contribute to the heterogeneity of IgA1 [15]. Variations in O-glycosylation impact the biological functions of the immunoglobulin, including its ability to nonspecifically bind to bacteria which enables IgA to participate in innate immunity [16].
Figure 2:
IgA forms and subclasses (A and B, modified from ‘Perše M, Večerić-Haler Ž. The role of IgA in the pathogenesis of IgA nephropathy. Int J Mol Sci 2019;20:6199’). Schematic representation of the nine serine and threonine residues in the hinge region that serve as potential O-glycosylations (up to six are O-glycosylated). Six different type of O-glycans can be found in the IgA1 HR. The residues highlighted in yellow are the frequent sites with galactose-deficient O-glycan (C).
Circulating levels of gd-IgA1 have been found to be consistently higher in patients with IgAN compared with healthy controls and other immune- and nonimmune-mediated kidney diseases. Furthermore, the levels of gd-IgA1 correlate with IgAN disease progression in some series [17–19]. The detailed structure of gd-IgA1 with site-specific attachment of Gal-deficient O-glycans specific for the disease has not been identified [15]. The available assays [20–22] used to detect the gd-IgA1 in the serum cannot discriminate among different O-glycoforms, and quantitative analyses using liquid chromatography–mass spectrometry is required [11].
The high levels of gd-IgA1 seen in patients with IgAN may be the result of post-translational modifications of IgA1 [23] or altered expression of key glycosyltransferases involved in the galactosylation process. Premature sialylation by ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase 2 (ST6GALNAC2) prevents addition of galactose to GalNac. Gd-IgA1 cells have been found to have elevated expression of ST6GALNAC2 [24]. Decreased expression of core 1 β1,3-galactosyltransferase (C1GALT1), controlled by chaperone 1 (C1GALT1C1) have also been found in gd-IgA producing cells.
The site of gd-IgA1 production is still unclear, although there is evidence on the involvement of Peyer's patches and mesenteric lymph nodes. [25] The IgA produced at the mucosal surface (secretory IgA) is exclusively polymeric as opposed to the monomeric isoforms of IgA normally found in circulation [14]. The presence of secretory IgA in the mesangial deposits in 15%–30% of IgAN patients [26, 27] suggests the mucosal involvement in the pathogenesis of the disease, although the mechanisms that lead to the deposition of secretory IgA remain unclear. Circulating levels of secretory IgA have also been found to be higher in patients with IgAN compared with healthy controls with a positive correlation to disease activity [28]. Serum levels of gd-IgA1 directed against mucosal pathogens are increased in IgAN compared with healthy controls [29].
Hit 2: antiglycan antibodies target gd-IgA1
High levels of serum gd-IgA1 are not sufficient per se to induce glomerular injury. The disease requires the formation of IgG or IgA1 autoantibodies that recognize the terminal GalNac of gd-IgA1 as a neoepitope [17, 30]. These autoantibodies share an unusual sequence in the variable region of their heavy chains that enhances binding to galactose-deficient glycans of gd-IgA1 [31], probably due to a somatic mutation [32]. These autoantibodies are mainly IgG isotype, and serum levels of anti-gd-IgA1 IgG correlate with proteinuria [30] and with serum levels of gd-IgA1 [31, 33]. Several studies have shown a clear association between anti-gd-IgA1 IgG, disease progression and absolute risk for ESKD [34, 35].
The mechanisms that lead to the formation of anti-glycan antibodies are not completely understood. It is conceivable that in patients with IgAN an infection with bacteria that express the GalNac molecule on their surface facilitates production of glycan-specific antibodies that cross-react with gd-IgA1. This molecular mimicry between bacteria and gd-IgA1 might explain the fact that hematuria is often concomitant with mucosal infections [12, 36].
Hit 3 and hit 4: formation of immune complexes and mesangial deposition
Circulating immune complexes can be detected in patients with IgAN. These circulating immune complexes are composed of gd-IgA1, anti-gd-IgA1 antibodies and complement C3 [1, 37]. A recent study [38] showed that patients with IgAN have significantly higher levels of poly-IgA immune complexes compared with healthy controls, consistent with previous evidence [39] of increased polymer/monomer IgA ratio in immune complexes in IgAN patients (0.64 ± 0.13 vs 0.39 ± 0.01, in controls; P < .001). These complexes are not efficiently cleared from circulation so they tend to deposit in the renal mesangium [12]. Immunofluorescence microscopy often fails to detect IgG colocalizing with IgA, but the presence of IgG has been confirmed using confocal microscopy with an IgG-specific nanobody in a recent study by Rizk et al. [40]. These investigators also corroborated the presence of IgG-specific antibodies against gd-IgA1 by extracting immune deposits from frozen kidney biopsy specimens of patients with IgAN. These specific antibodies were found even in cases where regular immunofluorescence microscopy was negative for IgG [40, 41]. The results of this study highlight the role of IgG–gd-IgA1 immunocomplexes in the pathogenesis of IgAN.
Deposition of immune complexes in the glomeruli provokes activation of mesangial cells and release of aldosterone, angiotensin II, pro-inflammatory cytokines [interleukin (IL)-6] [42] and growth factors [transforming growth factor (TGF)-β] [43]. The result is mesangial cell proliferation and complement pathway activation which ultimately cause glomerular injury and interstitial fibrosis [1, 44].
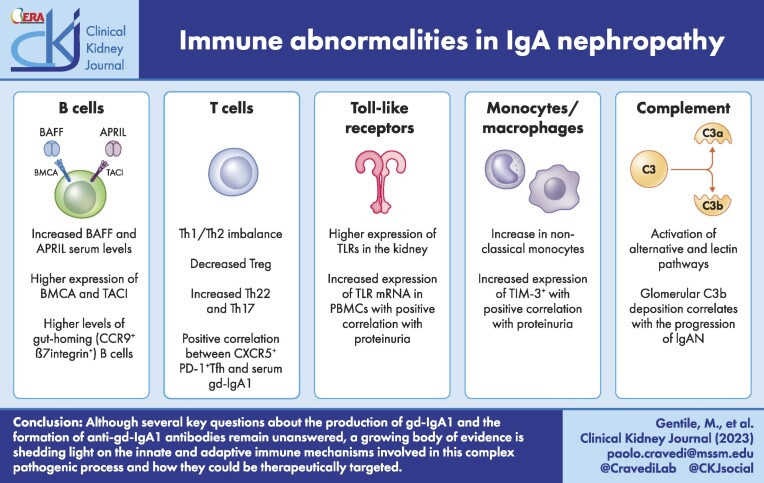
BAFF AND APRIL
B-cell-activating factor (BAFF) and A proliferation-inducing ligand (APRIL) [1], produced by antigen-exposed dendritic cells and intestinal epithelial cells [45], are critical factors in the maintenance of the B cell pool and in humoral immunity and are involved in the pathogenesis of several human autoimmune diseases [46]. BAFF maintains B cell homeostasis while APRIL modulates the function and survival of antigen-experienced B cells.
BAFF overexpression in mice induces autoimmune diseases similar to human systemic lupus erythematosus and IgAN. McCarthy et al. [47] showed that BAFF transgenic (BAFF-Tg) mice have increased levels of total IgA, aberrantly glycosylated IgA and mesangial IgA deposits with the development of proteinuria. The presence of commensal bacteria was essential to produce the IgAN phenotype and commensal-bacteria-reactive IgA antibodies were found in the blood of these mice, suggesting that overreactive B cells and mucosal microbiota both play a very important role in the pathogenesis of IgAN.
Several human studies (Table 1) have reported increased serum levels of BAFF and APRIL in patients with IgAN that correlate with gd-IgA1 levels and disease severity [48–51]. Ex vivo stimulation with APRIL of lymphocytes from IgAN patients led to an increased production of gd-IgA1 compared with lymphocytes from healthy controls [50]. Also, an enhanced expression of BCMA and TACI mRNA and protein was observed in B cells derived from IgAN patients [50]. Recently, Sallustio et al. [51] compared BAFF and APRIL levels in 44 patients with IgAN with 23 healthy controls and 22 patients with non-IgA glomerulonephritis, and reported that patients with IgAN had higher serum levels of BAFF, APRIL and gd-IgA1 along with higher circulating levels of gut-homing (CCR9+, β7 integrin+) B cells. A recent study has confirmed increased of expression of gut homing receptors in B cells in IgAN patients compared with controls [52]. The relationship between commensal bacteria, dietary antigens and high BAFF levels is not clear, but elevated BAFF levels have been associated with specific fecal metabolites, especially phenols, in individuals consuming high beef diet who had increased populations of phenol-producing anaerobic Bacteroides [51].
Table 1:
Human studies about abnormalities of adaptatitve immunity in IgAN.
| Authors | Classes of subjects | Principal findings in IgAN patients | |
|---|---|---|---|
| B cells and BAFF-APRIL axis | Xin et al. 2013 [48] | • 153 IgAN patients • 55 healthy controls • 20 disease controls |
• Higher BAFF levels compared with controls • Levels were associated with severity of histologic damage |
| Li et al. 2014 [49] | • 30 IgAN patients • 30 healthy controls • 30 minimal change disease |
• Positive correlation between serum levels of BAFF, TLR9 and IgA1 levels and mesangial IgA deposition density | |
| Zhai et al. 2016 [50] | • 166 IgAN patients • 77 healthy controls |
• Increased plasma APRIL levels • Positive correlation between plasma APRIL levels and gd-IgA1 levels • Higher expression of BCMA and TACI |
|
| Sallustio et al. 2021 [51] | • 44 IgA patients • 23 healthy controls • 22 non-IgA glomerulonephritis |
• Increased serum BAFF levels with positive correlation with specific microbiota metabolites • Increased serum APRIL levels • Higher levels of gut-homing (CCR9+ β7 integrin+) regulatory B cells. Memory B cells and IgA+ memory B cells |
|
| Zachova et al. 2022 [52] | • 30 IgAN patients • 30 healthy controls • 18 membranous nephropathy controls |
• Gd-IgA1 cells from IgAN patients express predominantly λ chains compared with controls • IgAN patient's blood was enriched with λ+ gd-IgA1, CCR10+ and CCR9 + cells, which preferentially home to the upper respiratory and digestive tracts |
|
| T cells | Sallustio et al. 2016 [68] | • 24 IgA patients • 24 healthy controls |
• Aberrant methylations in three regions involved in the response of CD4+ T cells • Higher IL-2/IL-5 ratio with Th1/Th2 imbalance |
| Yang et al. 2017 [69] | • 60 IgAN patients • 25 healthy controls |
• Lower levels of Th1 and Treg (with reduced levels of IFN-γ and IL-10) • Higher levels of Th2 and Th17 (with higher levels of IL-5 and IL-17) |
|
| Zhang et al. 2014 [70] | • 24 IgAN patients • 12 healthy controls |
• Higher % of CD4+CXCR5+, CD4+CXCR5+ICOS+, CD4+CXCR5+PD-1+ Tfh • Increased levels of IL-17A, IFN-γ, IL-2, IL-10, IL-4, IL-21 • Positive correlation between CD4+CXCR5+PD-1+ Tfh and serum IL-21, gd-IgA1 and 24-h urinary proteins |
|
| Peng et al. 2013 [74] | • 32 IgAN patients • 32 healthy controls • 16 MPGN patients |
• Higher Th22, Th17 and plasma IL-22 • Positive correlation between Th22 and proteinuria |
|
| Lin et al. 2012 [75] | • 63 IgAN patients • 36 healthy controls |
• Decreased CD45−FoxP3high Treg • Increased Th17 and decreased Treg/Th17 • Positive correlation between Th17 and proteinuria • Increased IL-17A, IL-21, IL-23, IL-1β, IL-6 and decreased IL-10 |
|
| Gan et al. 2018 [76] | • 44 IgAN patients • 16 healthy controls • 5 renal carcinoma patients |
• Increased Th22 • Positive correlation between Th22 and MESTc score |
|
| Yang et al. 2015 [77] | • 20 IgAN patients • 20 healthy controls |
• Decreased iTreg • Reduced serum levels of IL-10, TGF-β, increased IL-17 |
|
| Huang et al. 2014 [79] | • 35 IgAN patients • 35 healthy controls |
• Decreased CD4+CD25+ Treg with negative correlation with IL-4 and proteinuria and positively with eGFR • Increased IL-2, IL-4, IL-6 |
IFN: interferon; MPGN: membranoproliferative glomerulonephritis.
Altogether, these data support the hypothesis of a strong link between gut mucosal hyperresponsiveness and the activation of specific subtypes of B cells through BAFF and APRIL, resulting in an increased production of gd-IgA1. Evidence on the pathogenic role of BAFF/APRIL in IgAN led to the hypothesis that drugs initially developed for other immune disease [53, 54] can serve as a disease-modifying agents also in IgAN. Ongoing phase II and III clinical trials are testing the efficacy of BION-1301, an anti-APRIL monoclonal antibody (NCT03945318), and blisibimod, a selective inhibitor of BAFF (NCT02052219). The role of atacicept, a humanized recombinant TACI-IgG Fc fusion protein with anti-BAFF and anti-APRIL activity, is being explored in another phase II trial (NCT04716231). However, given the role of these cytokines in the maintenance of the B cell pool and humoral immunity, these drugs will likely not selectively inhibit IgA production.
TARGETING B CELLS AND PLASMA CELLS AS A THERAPEUTIC STRATEGY FOR I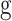 AN
AN
Despite the evidence suggesting that B cells have an important role in the pathogenesis of IgAN and the successful use of B cell depleting therapy in case reports [55–57], mainly of IgA vasculitis [58, 59], a randomized controlled trial comparing rituximab with conventional therapy in IgAN did not show any benefits despite effective B cell depletion [60]. Moreover, there were no differences in serum levels of gd-IgA1 and anti-gd-IgA1 between the two groups. Possible explanations to the lack of efficacy on B cell depleting agents may be due to the production of anti-gd-IgA1 IgG or IgA by plasma cells in the bone marrow or the reduced effects of rituximab in depleting mucosal B cells [60]. The stable presence of CD20−CD19+CD27high IgA-secreting cells of mucosal origin has been reported in patients treated with rituximab [61]. B cell precursors resident in the mucosa may be self-sufficient in adults and not replenished by CD20 B cells immigrating from systemic circulation [62].
A targeted-release formulation of the corticosteroid budesonide has been engineered to target the Peyer's patches in the distal ileum. This medication has been approved by the Food and Drug Administration to treat patients with IgAN based on the results from a randomized controlled trial showing a significant reduction of proteinuria in treated patients compared with the placebo group [63]. Although the mechanisms of action are not clearly understood, it is thought that budesonide inhibits the local activation of B cells and thereby attenuates the gd-IgA1 production, with less systemic steroid absorption [64, 65].
The proteasome inhibitor bortezomib has also been tested in IgAN to clear plasma cells. In a single-center open-label pilot trial in eight patients with IgAN, three patients had complete remission of proteinuria (<300 mg/day) [66]. Though further studies are needed, it is possible that bortezomib could be useful in decreasing production of gd-IgA1 antibodies and inhibiting nuclear factor kappa B (NF-κB) expression.
T CELLS
Th1 and Th2
Abnormalities in T helper 1 (Th1) and Th2 cell numbers and function have been reported in IgAN (Table 1), but the data are not clear. A murine model of IgAN showed more Th1 in animals with signs of illness [67], and an in vitro study [68] in humans found a higher IL-2/IL-5 ratio in patients with IgAN compared with controls, suggesting a Th1 shift. In contrast, other studies revealed an increase of Th2 and IL-4 in patients with IgAN [69, 70] and a correlation between Th2 cytokines and reduced glycosylation of IgA1 [71]. In particular, IL-4 has been associated with downregulation of 1 β1,3-galactosyltransferase (Cosmc) gene, chaperone, that may lead to reduced glycosylation of IgA1 in IgAN patients [72].
Th17 and Th22
Th17 and Th22 may also play a pathogenic role in IgAN, although the mechanisms remain unknown [73]. Th17 cells are increased in patients with IgAN compared with healthy controls [74] as well as serum levels of Th17 cytokines IL-17A and IL-21. A positive correlation between IL-17A levels and proteinuria has also been observed [75]. The number of Th22 cells and plasma levels of IL-22 are higher in patients with IgAN compared with healthy controls and non-IgAN controls. Furthermore, the number of Th22 cells is higher in patients with IgAN with proteinuria and high grade histological lesions defined in the Oxford Classification of IgA nephropathy (MESTc score) [74, 76].
Tfh
Studies on the role of Tfh are limited [73], but evidence exists showing an increased percentage of circulating Tfh cells and higher serum levels of IL-2, IL-4, IL-10, IFN-γ, IL-17A and IL-21 in IgAN patients compared with healthy controls. Tfh cell percentages negatively correlate with estimated glomerular filtration rate (eGFR), but positively correlate with gd-IgA1 and proteinuria [70].
Treg
IgAN patients display abnormalities in Treg number and function. Yang et al. [77] showed that the number of Treg in patients with IgAN was lower compared with healthy controls, and other studies found lower mRNA expression of TGF-β1 and FoxP3 genes in IgAN patients [78], as well as increased serum levels of IgA, IL-2, IL-4 and IL-6 [79], overall suggesting an imbalance between Treg and T effector cells.
INNATE IMMUNITY AND TOLL-LIKE RECEPTORS
Innate immune cells express pattern recognition receptors (PRRs) including Toll-like receptors (TLRs) which are responsible for the detection of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [80, 81]. Increased expression of TLR-4 on PBMCs has been associated with higher disease activity in IgAN patients [82] (Table 2). Patients with IgAN also express up-regulation of TLR-4, TLR-7, TLR-8 and TLR-9 in the kidney [83] and increased mRNA expression of TLR-2, TLR-3, TLR-4, TLR-5, TLR-7 and TLR-9 in PBMCs, with a positive correlation with proteinuria [84]. In vitro, TLR-9 activation enhances the production of APRIL and IL-6 in human IgA1-secreting B cells [85]. Therefore, activation of TLRs by infections may exacerbate IgAN by activating cells of the innate immune system that, in turn, affect glomerular function, either directly, or through increased autoimmune response. TLR-4 has also been involved in the activation of NF-κB [86] and in B cell proliferation [87]. Animal studies have showed a relationship between high TLR-4 levels and conversion of B cells from IgM+ to IgA+ [84].
Table 2:
Human studies about abnormalities of innate immunity in IgAN.
| Authors | Classes of subjects | Principal findings in IgAN patients | |
|---|---|---|---|
| TLRs | Coppo et al. 2010 [82] | • 47 IgAN patients • 40 healthy controls |
• Higher expression of TLR-4 in mononuclear cells with positive correlation with proteinuria |
| Saito et al. 2016 [84] | • 49 IgAN patients • 20 IgA vasculitis patients • 20 basement membrane nephropathy |
• Increased mRNA expression of TLR-2, TLR-3, TLR-4, TLR-5, TLR-7, TLR-9 with positive correlation with proteinuria | |
| Monocytes | Hou et al. 2021 [90] | • 48 IgAN patients • 18 healthy controls |
• Increased expression of TIM-3+ on CD14+ monocytes with positive correlation between proteinuria and negative correlation with eGFR |
| Esteve Cols et al. 2020 [91] | • 22 IgAN patients • 12 healthy controls |
• Reduced of classical monocytes • Reduced expression levels of CD89 on non-classical monocytes with positive correlation with poor renal function |
|
| Sendic et al. 2021 [92] | • 13 IgAN patients • 13 healthy controls • 13 ADPKD patients |
• Higher proportion of non-classical monocytes • Positive correlation between MCP-1 and UACR |
ADPKD: autosomal dominant polycystic kidney disease; UACR: urine albumin-creatinine ratio.
Intriguingly, TLR-4 is also constitutively expressed by podocytes and mesangial cells [88] and in vitro studies have shown that stimulation of mouse mesangial cells with human secretory IgA increases TLR-4 mRNA and protein levels, suggesting a direct role of TLR-4 in mesangial cell injury [89].
MONOCYTES AND MACROPHAGES
Monocytes and macrophages play a role in mediating kidney injury in IgAN [90] (Table 2). CD89, the main receptor for IgA expressed on the surface of myeloid cells, has been found as a component of immune complexes in patients with IgAN [91]. A study comparing 22 patients with IgAN with 12 healthy controls showed a reduction in circulating classical monocytes (CD14+CD16−) in patients with IgAN but no differences in intermediate monocytes (CD14+CD16+) or non-classical monocytes (CD14lowCD16+) [91]. Consistently, a prospective study [92] found a higher proportion of non-classical monocytes in 13 IgAN patients compared with 13 healthy controls and 13 patients with polycystic kidney disease, accompanied by increased level of IL-6 and a positive correlation between monocyte chemoattractant protein-1 (MCP-1; a chemotactic factor for monocytes) levels and albuminuria in IgAN.
The infiltration of macrophages into the tubulointerstitial compartment of the kidney correlates with fibrosis and unfavorable kidney outcomes in patients with IgAN [93]. A recent paper showed a higher expression of TIM-3+ (T-cell immunoglobulin and mucin-domain-containing protein-3, an immunoregulatory molecule) on CD14+ monocytes in 48 patients with IgAN compared with 18 healthy controls with a positive correlation with proteinuria and a negative correlation with eGFR [90]. Lastly, CD68+TIM-3+ cells are abundant in kidney immune infiltrates in IgAN patients. These data suggest that Tim3+ monocytes may play a role in IgAN pathogenesis and could be tested as a biomarker for disease activity.
COMPLEMENT
The complement system is an important component of innate immunity that can be activated through three pathways. The classical pathway (CP) is triggered by cross-linking, cell-bound IgM or IgG antibodies, while activation of the alternative pathway (AP) occurs spontaneously by the association of C3 with a water molecule. Activation of the lectin pathway (LP) is triggered by carbohydrates present on bacteria surface and follows a route similar to that of the classical pathway [94]. These three pathways converge in the formation of a terminal membrane attack complex (MAC, C5b9) that directly lyses pathogens or damaged self-cells [95, 96].
Several studies have provided evidence of the activation of the AP and LP as effector mechanisms of kidney injury in IgAN [97]. Components of AP are found in renal biopsies of patients with IgAN, especially C3 (90%), complement factor H (CFH, 30%–90%) and properdin (75%–100%), and regulators such as CFH-related proteins (CFHR) [97]. Glomerular C3 deposition correlates with the progression of IgAN and the presence of complement components may distinguish between IgAN and IgA depositions that can be found in healthy subjects [98, 99]. A factor B inhibitor, iptacopan (LNP023), is being studied in the placebo-controlled APPLAUSE-IgA study (NCT04578834) [100] to test the hypothesis that AP inhibition reduces disease severity.
A genome-wide association study has identified a deletion of the gene encoding the CFHR [101], which appears protective against IgAN [102]. It is unclear how loss of function of CFHRs may be protective in IgAN but it is believed that it could enhance the regulating capacity of CFH, thus reducing AP activity [97].
The identification of C4d in the absence of C1q in kidney biopsies of patients with IgAN strongly suggests that the LP is also an effector mechanism of injury in this disease [97]. Polymeric IgA has strong mannan-binding lectin (MBL) binding and gd-IgA1 may trigger LP activation due to interaction with ficolin [97]. A study on 323 patients with IgAN found increased circulating levels of ficolin, MBL-associated protease (MASP-1) and MBL-associated protein (Map-19) compared with healthy controls [103]. Based on the pathogenic role of LP in IgAN, LP inhibitors have been tested in clinical trials. Lafayette et al. [104] showed an improvement in proteinuria and stability of eGFR in high-risk patients with advanced IgAN treated with narsoplimab (OMS71), a humanized monoclonal antibody targeting MASP-2. Based on these data, a randomized, double-blind, placebo-controlled trial of narsoplimab is ongoing for patients with IgAN and persistent proteinuria (ARTEMISAN-IGAN, NTC030608033).
The activation of AP and LP converge to the activation of the terminal pathway (TP). An animal model of IgAN showed a possible role of C5a/C5aR signaling in the pathogenesis of IgAN. C5aR knockout mice had less proteinuria, and C3 and IgA deposition in the glomeruli [105]. In humans, MAC glomerular deposition has a positive correlation with the degree of glomerulosclerosis, tubular atrophy and interstitial inflammation in IgAN [106]. However, terminal complement inhibition with the humanized recombinant monoclonal anti-C5 antibody eculizumab has not shown efficacy in patients with IgAN [107–109], suggesting that major IgAN effector mechanisms of complement cascade are upstream C5.
IgAN RECURRENCE AFTER KIDNEY TRANSPLANTATION
IgAN has a high rate of recurrence (20%–40% clinical but 60% on biopsy) [110, 111] but its impact on allograft survival is small [112], possibly because the immunosuppressive drugs used to prevent rejection may also inhibit the IgAN. In the largest study (TANGO consortium) involving over 500 patients with IgAN that underwent kidney transplantation, the 10-year death-censored graft survival was 76% in patients with recurrence compared with 89% in those without recurrence [112]. The mean time to IgA recurrence was 3.4 years after transplantation [112]. While initial smaller studies suggested that maintenance steroids was associated with lower recurrence rate, the TANGO study did not show any association between steroid withdrawal and higher risk of recurrence [112]. The role of gd-IgA1 in recurrence is controversial, since they remain elevated only in some recipients regardless of the immunosuppressive therapy [113, 114]. The successful use of budesonide in IgA recurrence has been reported only in six patients so far [115, 116]. The fact that kidneys from donors with subclinical IgAN are clear of IgA deposits shortly after transplantation into recipients with non-IgAN renal diseases [117] points to the importance of persistent IgA immune complex production and deposition in the pathogenesis of IgAN.
The role of BAFF and APRIL has been investigated in transplant recipients with IgAN relapse. Penagos et al. have shown increased serum levels of APRIL at 6 months after transplant up to 3 years post-transplant in patients in with IgAN recurrence [110], while BAFF levels were reduced. The authors speculate that TACI, a positive regulator for APRIL and a negative regulator of BAFF, may be implicated in IgAN recurrence. Intriguingly, genetic mutations that inactivate TACI cause selective IgA deficiency [118]. Although additional studies are needed to understand the pathogenesis of IgAN relapse, these data suggest that targeting APRIL and TACI may have a therapeutic role in kidney transplant patients with IgAN recurrence.
CONCLUSIONS
The immune abnormalities that lead to the accumulation of aberrant forms of gd-IgA1 and to the formation of immune complexes that mediate glomerular injury in IgAN are not fully understood. However, a growing knowledge of these pathogenic mechanisms, together with the availability of small molecules and biologics targeting innate and adaptive immunity, has resulted in a dramatic increase in the number of ongoing clinical trials in IgAN that are expected to provide new tools to improve patients’ outcomes. We believe that it will be important to accompany clinical studies with mechanistic analyses to better understand disease pathophysiology, which could be obtained also from negative trials. Sharing clinical samples across investigators will be instrumental to further deepen our understanding of disease pathophysiology and to design hypothesis-driven studies.
Contributor Information
Micaela Gentile, Translational Transplant Research Center and Department of Medicine, Icahn School of Medicine at Mount Sinai, NY, USA; UO Nefrologia, Dipartimento di Medicina e Chirurgia, Università di Parma, Parma, Italy.
Luis Sanchez-Russo, Translational Transplant Research Center and Department of Medicine, Icahn School of Medicine at Mount Sinai, NY, USA.
Leonardo V Riella, Division of Nephrology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Alberto Verlato, Translational Transplant Research Center and Department of Medicine, Icahn School of Medicine at Mount Sinai, NY, USA.
Joaquin Manrique, Nephrology Service, Complejo Hospitalario de Navarra, Pamplona, Spain.
Simona Granata, Nephrology, Dialysis and Transplantation Unit, University of Foggia, Foggia, Italy.
Enrico Fiaccadori, UO Nefrologia, Dipartimento di Medicina e Chirurgia, Università di Parma, Parma, Italy.
Francesco Pesce, Nephrology, Dialysis and Transplantation Unit, Department of Emergency and Organ Transplantation, University of Bari “A. Moro”, Bari, Italy.
Gianluigi Zaza, Nephrology, Dialysis and Transplantation Unit, University of Foggia, Foggia, Italy.
Paolo Cravedi, Translational Transplant Research Center and Department of Medicine, Icahn School of Medicine at Mount Sinai, NY, USA.
CONFLICT OF INTEREST STATEMENT
Paolo Cravedi is an advisor for Chinook Therapeutics and Calliditas Therapeutics. The other authors have no conflict of interest to declare.
REFERENCES
- 1. Pattrapornpisut P, Avila-Casado C, Reich HN.. IgA nephropathy: core curriculum 2021. Am J Kidney Dis 2021;78:429–41. 10.1053/j.ajkd.2021.01.024 [DOI] [PubMed] [Google Scholar]
- 2. Berger J. IgA glomerular deposits in renal disease. Transpl Proc 1969;1:939–44. [PubMed] [Google Scholar]
- 3. Rajasekaran A, Julian BA, Rizk DV.. IgA nephropathy: an interesting autoimmune kidney disease: IgA nephropathy. Am J Med Sci 2021;361:176–94. 10.1016/j.amjms.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woo KT, Chan CM, Chin YMet al. Global evolutionary trend of the prevalence of primary glomerulonephritis over the past three decades. Nephron Clin Pract 2010;116:c337–46. 10.1159/000319594 [DOI] [PubMed] [Google Scholar]
- 5. McGrogan A, Franssen CFM, De Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 6. Seikrit C, Rauen T, Floege J.. IgA nephropathy. Der Nephrologe 2020;15:336–42. 10.1007/s11560-020-00452-4 [DOI] [Google Scholar]
- 7. Hassler JR. IgA nephropathy: a brief review. Semin Diagn Pathol 2020;37:143–7. 10.1053/j.semdp.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 8. Thompson A, Carroll K, Inker LAet al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 2019;14:469–81. 10.2215/CJN.08600718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trimarchi H, Barratt J, Cattran DCet al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017;91:1014–21. 10.1016/j.kint.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 10. Sanchez-Russo L, Rajasekaran A, Bin S. et al. The gut and kidney cross talk in immunoglobulin-A nephropathy. Kidney360 2022;3:1630–9. 10.34067/KID.0002382022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi K, Smith AD, Poulsen Ket al. Naturally occurring structural isomers in serum IgA1 o-glycosylation. J Proteome Res 2012;11:692–702. 10.1021/pr200608q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novak J, Julian BA, Mestecky Jet al. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 2012;34:365–82. 10.1007/s00281-012-0306-z [DOI] [PubMed] [Google Scholar]
- 13. Woof JM, Ken MA.. The function of immunoglobulin A in immunity. J Pathol 2006;208:270–82. 10.1002/path.1877 [DOI] [PubMed] [Google Scholar]
- 14. Al Hussain T, Hussein MH, Al Mana Het al. Pathophysiology of IgA nephropathy. Adv Anat Pathol 2017;24:56–62. 10.1097/PAP.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 15. Ohyama Y, Renfrow MB, Novak Jet al. Aberrantly glycosylated IgA1 in IgA nephropathy: what we know and what we don't know. J Clin Med 2021;10:3467. 10.3390/jcm10163467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Royle L, Roos A, Harvey DJet al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem 2003;278:20140–53. 10.1074/jbc.M301436200 [DOI] [PubMed] [Google Scholar]
- 17. Moldoveanu Z, Wyatt RJ, Lee JYet al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007;71:1148–54. 10.1038/sj.ki.5002185 [DOI] [PubMed] [Google Scholar]
- 18. Berthoux F, Suzuki H, Thibaudin Let al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 2012;23:1579–87. 10.1681/ASN.2012010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao N, Hou P, Lv Jet al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 2012;82:790. 10.1038/ki.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore JS, Kulhavy R, Tomana Met al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 2007;44:2598–604. 10.1016/j.molimm.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yasutake J, Suzuki Y, Suzuki Het al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 2015;30:1315–21. 10.1093/ndt/gfv221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiki Y, Hori H, Yamamoto Ket al. Specificity of two monoclonal antibodies against a synthetic glycopeptide, an analogue to the hypo-galactosylated IgA1 hinge region. J Nephrol 2015;28:181–6. 10.1007/s40620-014-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeo SC, Cheung CK, Barratt J.. New insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol 2018;33:763–77. 10.1007/s00467-017-3699-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruszkowski J, Lisowska KA, Pindel Met al. T cells in IgA nephropathy: role in pathogenesis, clinical significance and potential therapeutic target. Clin Exp Nephrol 2019;23:291–303. 10.1007/s10157-018-1665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brandtzaeg P, Kiyono H, Pabst Ret al. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol 2008;1:31–7. 10.1038/mi.2007.9 [DOI] [PubMed] [Google Scholar]
- 26. Oortwijn BD, Rastaldi MP, Roos Aet al. Demonstration of secretory IgA in kidneys of patients with IgA nephropathy. Nephrol Dial Transplant 2007;22:3191–5. 10.1093/ndt/gfm346 [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Zhou R, Mi Yet al. Role of human mesangial-tubular crosstalk in secretory IgA-induced IgA nephropathy. Kidney Blood Press Res 2021;46:286–97. 10.1159/000514183 [DOI] [PubMed] [Google Scholar]
- 28. Zhang JJ, Xu LX, Liu Get al. The level of serum secretory IgA of patients with IgA nephropathy is elevated and associated with pathological phenotypes. Nephrol Dial Transplant 2008;23:207–12. 10.1093/ndt/gfm492 [DOI] [PubMed] [Google Scholar]
- 29. Smith AC, Molyneux K, Feehally Jet al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol 2006;17:3520–8. 10.1681/ASN.2006060658 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki H, Fan R, Zhang Zet al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 2009;119:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki H, Fan R, Zhang Zet al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 2009;119:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang ZQ, Raska M, Stewart TJet al. Somatic mutations modulate autoantibodies against galactose-deficient IgA1 in IgA nephropathy. J Am Soc Nephrol 2016;27:3278–84. 10.1681/ASN.2014101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Placzek WJ, Yanagawa H, Makita Yet al. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS One 2018;13:e0190967. 10.1371/journal.pone.0190967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maixnerova D, Ling C, Hall Set al. Correction: galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS One 2019;14:e0219947. Erratum for: PLoS One 2019;14:e0212254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berthoux F, Suzuki H, Thibaudin Let al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 2012;23:1579–87. 10.1681/ASN.2012010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novak J, Julian BA, Tomana Met al. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 2008;28:78–87. 10.1016/j.semnephrol.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knoppova B, Reily C, Glenn King Ret al. Pathogenesis of IgA nephropathy: current understanding and implications for development of disease-specific treatment. J Clin Med 2021;10:4501. 10.3390/jcm10194501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, Lv J, Liu Pet al. Poly-IgA complexes and disease severity in IgA nephropathy. Clin J Am Soc Nephrol 2021;16:1652–64. 10.2215/CJN.01300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valentijn RM, Radl J, Haaijman JJet al. Circulating and mesangial secretory component-binding IgA-1 in primary IgA nephropathy. Kidney Int 1984;26:760–6. 10.1038/ki.1984.213 [DOI] [PubMed] [Google Scholar]
- 40. Rizk D V., Saha MK, Hall Set al. Glomerular immunodeposits of patients with IgA nephropathy are enriched for IgG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol 2019;30:2017–26. 10.1681/ASN.2018111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki H, Novak J.. IgA glycosylation and immune complex formation in IgAN. Semin Immunopathol 2021;43:669–78. 10.1007/s00281-021-00883-8 [DOI] [PubMed] [Google Scholar]
- 42. Lai KN, Tang SCW, Schena FPet al. IgA nephropathy. Nat Rev Dis Prim 2016;2:16001. [DOI] [PubMed] [Google Scholar]
- 43. Amore A, Conti G, Cirina Pet al. Aberrantly glycosylated IgA molecules downregulate the synthesis and secretion of vascular endothelial growth factor in human mesangial cells. Am J Kidney Dis 2000;36:1242–52. 10.1053/ajkd.2000.19840 [DOI] [PubMed] [Google Scholar]
- 44. Wyatt RJ, Julian BA.. IgA nephropathy. N Engl J Med 2013;368:2402–14. 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 45. Haniuda K, Gommerman JL, Reich HN.. The microbiome and IgA nephropathy. Semin Immunopathol 2021;43:649–56. 10.1007/s00281-021-00893-6 [DOI] [PubMed] [Google Scholar]
- 46. Samy E, Wax S, Huard Bet al. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int Rev Immunol 2017;36:3–19. 10.1080/08830185.2016.1276903 [DOI] [PubMed] [Google Scholar]
- 47. McCarthy DD, Kujawa J, Wilson Cet al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 2011;121:3991–4002. 10.1172/JCI45563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xin G, Shi W, Xu LXet al. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol 2013;26:683–90. 10.5301/jn.5000218 [DOI] [PubMed] [Google Scholar]
- 49. Li WW, Peng X, Liu Yet al. TLR9 and BAFF: their expression in patients with IgA nephropathy. Mol Med Rep 2014;10:1469–74. 10.3892/mmr.2014.2359 [DOI] [PubMed] [Google Scholar]
- 50. Zhai YL, Zhu L, Shi SFet al. Increased APRIL expression induces IgA1 aberrant glycosylation in IgA nephropathy. Medicine (Baltimore) 2016;95:e3099. 10.1097/MD.0000000000003099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sallustio F, Curci C, Chaoul Net al. High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol Dial Transplant 2021;36:1765. 10.1093/ndt/gfaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zachova K, Jemelkova J, Kosztyu Pet al. Galactose-deficient IgA1 B cells in the circulation of IgA nephropathy patients carry preferentially lambda light chains and mucosal homing receptors. J Am Soc Nephrol 2022;33:908–17. 10.1681/ASN.2021081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaegi C, Steiner UC, Wuest Bet al. Systematic review of safety and efficacy of atacicept in treating immune-mediated disorders. Front Immunol 2020;11:433. 10.3389/fimmu.2020.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Merrill JT, Shanahan WR, Scheinberg Met al. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2018;77:883–9. 10.1136/annrheumdis-2018-213032 [DOI] [PubMed] [Google Scholar]
- 55. Lundberg S, Westergren E, Smolander Jet al. B cell-depleting therapy with rituximab or ofatumumab in immunoglobulin A nephropathy or vasculitis with nephritis. Clin Kidney J 2017;10:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hunjan MK, Bardhan A, Harper Net al. Successful use of rituximab, an anti-CD20 monoclonal antibody, to treat IgA nephropathy in a patient with recessive dystrophic epidermolysis bullosa. Clin Exp Dermatol 2022;47:1588–90. 10.1111/ced.15228 [DOI] [PubMed] [Google Scholar]
- 57. Tan SL, Potezny T, Li JY.. The successful use of rituximab in crescentic IgA nephropathy with concurrent ANCA positivity. Nephrology 2022;27:216–7. 10.1111/nep.13950 [DOI] [PubMed] [Google Scholar]
- 58. Maritati F, Fenoglio R, Pillebout Eet al. Brief report: rituximab for the treatment of adult-onset IgA vasculitis (Henoch-Schönlein). Arthritis Rheumatol 2018;70:109–14. 10.1002/art.40339 [DOI] [PubMed] [Google Scholar]
- 59. Fenoglio R, Naretto C, Basolo Bet al. Rituximab therapy for IgA-vasculitis with nephritis: a case series and review of the literature. Immunol Res 2017;65:186–92. 10.1007/s12026-016-8827-5 [DOI] [PubMed] [Google Scholar]
- 60. Lafayette RA, Canetta PA, Rovin BHet al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 2017;28:1306–13. 10.1681/ASN.2016060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang YM, Zhang H.. Insights into the role of mucosal immunity in IgA nephropathy. Clin J Am Soc Nephrol 2018;13:1584–6. 10.2215/CJN.04370418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mei HE, Frölich D, Giesecke Cet al. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood 2010;116:5181–90. 10.1182/blood-2010-01-266536 [DOI] [PubMed] [Google Scholar]
- 63. Barratt J, Lafayette R, Kristensen Jet al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int 2023;103:391–402. [DOI] [PubMed] [Google Scholar]
- 64. Fellström BC, Barratt J, Cook Het al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet North Am Ed 2017;389:2117–27. 10.1016/S0140-6736(17)30550-0 [DOI] [PubMed] [Google Scholar]
- 65. Barratt J, Stone A, Kristensen J.. POS-830 Nefecon for the treatment of IgA nephropathy in patients at risk of progressing to end-stage renal disease: the NefIgArd phase 3 trial results. Kidney Int Rep 2021;6:S361. 10.1016/j.ekir.2021.03.868 [DOI] [Google Scholar]
- 66. Hartono C, Chung M, Perlman ASet al. Bortezomib for reduction of proteinuria in IgA nephropathy. Kidney Int Rep 2018;3:861–6. 10.1016/j.ekir.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suzuki H, Suzuki Y.. Murine models of human IgA nephropathy. Semin Nephrol 2018;38:513–20. 10.1016/j.semnephrol.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 68. Sallustio F, Serino G, Cox SNet al. Aberrantly methylated DNA regions lead to low activation of CD4+ T-cells in IgA nephropathy. Clin Sci (Colch) 2016;130:733–46. 10.1042/CS20150711 [DOI] [PubMed] [Google Scholar]
- 69. Yang L, Zhang XY, Peng Wet al. MicroRNA-155-induced T lymphocyte subgroup drifting in IgA nephropathy. Int Urol Nephrol 2017;49:353–61. 10.1007/s11255-016-1444-3 [DOI] [PubMed] [Google Scholar]
- 70. Zhang L, Wang Y, Shi Xet al. A higher frequency of CD4+CXCR5+ T follicular helper cells in patients with newly diagnosed IgA nephropathy. Immunol Lett 2014;158:101–8. 10.1016/j.imlet.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 71. Chintalacharuvu SR, Yamashita M, Bagheri Net al. T cell cytokine polarity as a determinant of immunoglobulin A (IgA) glycosylation and the severity of experimental IgA nephropathy. Clin Exp Immunol 2008;153:456–62. 10.1111/j.1365-2249.2008.03703.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun Q, Zhang J, Zhou Net al. DNA methylation in Cosmc promoter region and aberrantly glycosylated IgA1 associated with pediatric IgA nephropathy. PLoS One 2015;10:e0112305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tang Y, He H, Hu Pet al. T lymphocytes in IgA nephropathy. Exp Ther Med 2020;20:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peng Z, Tian J, Cui Xet al. Increased number of Th22 cells and correlation with Th17 cells in peripheral blood of patients with IgA nephropathy. Hum Immunol 2013;74:1586–91. 10.1016/j.humimm.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 75. Lin FJ, Jiang GR, Shan JPet al. Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand J Clin Lab Invest 2012;72:221–9. 10.3109/00365513.2011.652158 [DOI] [PubMed] [Google Scholar]
- 76. Gan L, Zhu M, Li Xet al. Tonsillitis exacerbates renal injury in IgA nephropathy through promoting Th22 cells chemotaxis. Int Urol Nephrol 2018;50:1285–92. 10.1007/s11255-018-1792-2 [DOI] [PubMed] [Google Scholar]
- 77. Yang S, Chen B, Shi Jet al. Analysis of regulatory T cell subsets in the peripheral blood of immunoglobulin A nephropathy (IgAN) patients. Genet Mol Res 2015;14:14088–92. 10.4238/2015.October.29.28 [DOI] [PubMed] [Google Scholar]
- 78. Donadio ME, Loiacono E, Peruzzi Let al. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch-Schönlein purpura and primary IgA nephropathy. Pediatr Nephrol 2014;29:1545–51. 10.1007/s00467-014-2807-6 [DOI] [PubMed] [Google Scholar]
- 79. Huang H, Sun W, Liang Yet al. CD4 (+)CD 25 (+)Treg cells and IgA nephropathy patients with tonsillectomy: a clinical and pathological study. Int Urol Nephrol 2014;46:2361–9. 10.1007/s11255-014-0851-6 [DOI] [PubMed] [Google Scholar]
- 80. Chang S, Li XK.. The role of immune modulation in pathogenesis of IgA nephropathy. Front Med 2020;7:92. 10.3389/fmed.2020.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol 2018;59:391–412. 10.1016/j.intimp.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coppo R, Camilla R, Amore Aet al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol 2010;159:73–81. 10.1111/j.1365-2249.2009.04045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ciferska H, Honsova E, Lodererova Aet al. Does the renal expression of Toll-like receptors play a role in patients with IgA nephropathy? J Nephrol 2020;33:307–16. 10.1007/s40620-019-00640-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saito A, Komatsuda A, Kaga Het al. Different expression patterns of Toll-like receptor mRNAs in blood mononuclear cells of IgA nephropathy and IgA vasculitis with nephritis. Tohoku J Exp Med 2016;240:199–208. 10.1620/tjem.240.199 [DOI] [PubMed] [Google Scholar]
- 85. Makita Y, Suzuki H, Kano Tet al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int 2020;97:340–9. 10.1016/j.kint.2019.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen X, Peng S, Zeng Het al. Toll-like receptor 4 is involved in a protective effect of rhein on immunoglobulin A nephropathy. Indian J Pharmacol 2015;47:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McCarthy DD, Chiu S, Gao Yet al. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol 2006;241:85–94. 10.1016/j.cellimm.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 88. Banas MC, Banas B, Hudkins KLet al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 2008;19:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lim BJ, Lee D, Hong SWet al. Toll-like receptor 4 signaling is involved in IgA-stimulated mesangial cell activation. Yonsei Med J 2011;52:610–5. 10.3349/ymj.2011.52.4.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hou J, Zhang L, Wu Het al. Increased Tim-3+ monocytes/macrophages are associated with disease severity in patients with IgA nephropathy. Int Immunopharmacol 2021;97:107666. 10.1016/j.intimp.2021.107666 [DOI] [PubMed] [Google Scholar]
- 91. Esteve Cols C, Graterol Torres FA, Quirant Sánchez Bet al. Immunological pattern in IgA nephropathy. Int J Mol Sci 2020;21:1389. 10.3390/ijms21041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sendic S, Mansouri L, Lundberg Set al. B cell and monocyte phenotyping: a quick asset to investigate the immune status in patients with IgA nephropathy. PLoS One 2021;16:e0248056. 10.1371/journal.pone.0248056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Silva GEB, Costa RS, Ravina RCet al. Renal macrophage infiltration is associated with a poor outcome in IgA nephropathy. Clinics 2012;67:697–703. 10.6061/clinics/2012(07)01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cravedi P, Heeger PS.. Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest 2014;124:2348–54. 10.1172/JCI72273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Walport MJ. Complement. First of two parts. N Engl J Med 2001;344:1058–66. 10.1056/NEJM200104053441406 [DOI] [PubMed] [Google Scholar]
- 96. Walport MJ. Complement. Second of two parts. N Engl J Med 2001;344:1140–4. 10.1056/NEJM200104123441506 [DOI] [PubMed] [Google Scholar]
- 97. Tortajada A, Gutierrez E, Pickering MCet al. The role of complement in IgA nephropathy. Mol Immunol 2019;114:123–32. 10.1016/j.molimm.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 98. Suzuki K, Honda K, Tanabe Ket al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 2003;63:2286–94. 10.1046/j.1523-1755.63.6s.2.x [DOI] [PubMed] [Google Scholar]
- 99. Li M, Yu XQ.. genetic determinants of IgA nephropathy: Eastern perspective. Semin Nephrol 2018;38:455–60. 10.1016/j.semnephrol.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 100. Reich HN, Floege J. How I treat IgA nephropathy. Clin J Am Soc Nephrol 2022;17:1243–6. 10.2215/CJN.02710322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gharavi AG, Kiryluk K, Choi Met al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 2011;43:321–7. 10.1038/ng.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhu L, Zhai YL, Wang FMet al. Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol 2015;26:1195–204. 10.1681/ASN.2014010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Medjeral-Thomas NR, Troldborg A, Constantinou Net al. Progressive IgA nephropathy is associated with low circulating mannan-binding lectin-associated serine protease-3 (MASP-3) and increased glomerular factor H-related protein-5 (FHR5) deposition. Kidney Int Rep 2018;3:426–38. 10.1016/j.ekir.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lafayette RA, Rovin BH, Reich HNet al. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep 2020;5:2032–41. 10.1016/j.ekir.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang Y, Yan X, Zhao Tet al. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol 2017;189:60–70. 10.1111/cei.12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stangou M, Alexopoulos E, Pantzaki Aet al. C5b-9 glomerular deposition and tubular alpha3beta1-integrin expression are implicated in the development of chronic lesions and predict renal function outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol 2008;42:373–80. 10.1080/00365590801943241 [DOI] [PubMed] [Google Scholar]
- 107. Ring T, Pedersen BB, Salkus Get al. Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J 2015;8:489–91. 10.1093/ckj/sfv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rosenblad T, Rebetz J, Johansson Met al. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr Nephrol 2014;29:2225–8. 10.1007/s00467-014-2863-y [DOI] [PubMed] [Google Scholar]
- 109. Herzog AL, Wanner C, Amann Ket al. First treatment of relapsing rapidly progressive IgA nephropathy with eculizumab after living kidney donation: a case report. Transplant Proc 2017;49:1574–7. 10.1016/j.transproceed.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 110. Martín-Penagos L, Benito-Hernández A, San Segundo Det al. A proliferation-inducing ligand increase precedes IgA nephropathy recurrence in kidney transplant recipients. Clin Transplant 2019;33:e13502. 10.1111/ctr.13502 [DOI] [PubMed] [Google Scholar]
- 111. Wyld ML, Chadban SJ.. Recurrent IgA nephropathy after kidney transplantation. Transplantation 2016;100:1827–32. 10.1097/TP.0000000000001093 [DOI] [PubMed] [Google Scholar]
- 112. Uffing A, Pérez-Saéz MJ, Jouve Tet al. Recurrence of IgA nephropathy after kidney transplantation in adults. Clin J Am Soc Nephrol 2021;16:1247–55. 10.2215/CJN.00910121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Berthoux F, Suzuki H, Mohey Het al. Prognostic value of serum biomarkers of autoimmunity for recurrence of IgA nephropathy after kidney transplantation. J Am Soc Nephrol 2017;28:1943–50. 10.1681/ASN.2016060670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Coppo R, Amore A, Chiesa Met al. Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant 2007;21:728–37. [DOI] [PubMed] [Google Scholar]
- 115. Lingaraj U, Aralapuram K, Chikkanayakanhalli Set al. Successful treatment of a patient with posttransplant IgA nephropathy with targeted release formulation of budesonide. Saudi J Kidney Dis Transplant 2020;31:521–3. 10.4103/1319-2442.284029 [DOI] [PubMed] [Google Scholar]
- 116. Lopez-Martinez M, Torres I, Bermejo Set al. Enteric budesonide in transplant and native IgA nephropathy: real-world clinical practice. Transpl Int 2022;35:10693. 10.3389/ti.2022.10693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Silva FG, Chander P, Pirani CLet al. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation 1982;33:241–6. [PubMed] [Google Scholar]
- 118. Castigli E, Geha RS.. Molecular basis of common variable immunodeficiency. J Allergy Clin Immunol 2006;117:740–6. 10.1016/j.jaci.2006.01.038 [DOI] [PubMed] [Google Scholar]