Abstract
A history of abortion is associated with cervical dysfunction during pregnancy, but there remains uncertainty about whether risk can be stratified by the abortion type, the abortion procedure, or number of previous abortions. The objective of this study was to verify the relationship between cervical dysfunction measures in pregnancies with and without a history of termination. Embase and Medline databases were searched from 01 January 1960 to 01 March 2022 resulting in a full-text review of 28 studies. The Newcastle–Ottawa Scale (NOS) was used to assess the quality and risk of bias for non-randomized studies. The meta-analysis consisted of 6 studies that met all inclusion and exclusion criteria and included a combined total of 2,513,044 pregnancies. Cervical dysfunction was defined as either cervical insufficiency/incompetence in 4 of the studies and as short cervix in the others. Results from a random-effects model using reported adjusted odds ratios (aOR) estimated an increase in the odds of 2.71 (95% CI 1.76, 4.16) for cervical dysfunction in the current pregnancy related to a history of induced or spontaneous abortion. Subgroup analyses with only induced abortions (surgical/medical) estimated an aOR of 2.54 (95% CI 1.41, 4.57), while studies limited to surgical abortions had an aOR of 4.08 (95% CI 2.84, 5.86). The risk of cervical dysfunction in the current pregnancy was also found to be dependent on the number of previous abortions. In this meta-analysis, a prior history of abortion, and specifically induced abortions, was associated with cervical dysfunction. The protocol was registered in PROSPERO (CRD42020209723).
Keywords: Cervical length, Cervical insufficiency, Cervical incompetence, Abortion, Pregnancy, Preterm birth
Introduction
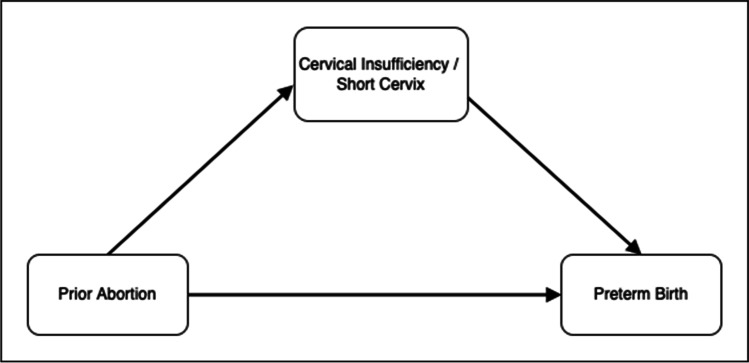
The cervical insufficiency syndrome affects 1% of the obstetric population and is characterized by recurrent spontaneous preterm births and/or spontaneous abortions in the second trimester of pregnancy [1]. A short cervix measured by transvaginal ultrasound is used as a diagnostic criterion for cervical insufficiency [2] and for the prediction of preterm birth [3]. A history of spontaneous and induced abortions has been shown to correlate with both a short cervix/cervical insufficiency [4] and preterm birth [5, 6]. The possibility that the influence of a prior abortion on the gestational age at birth is mediated, in part, through an insufficient cervix has been suggested (Fig. 1) [5, 7].
Fig. 1.
Conceptual model illustrating the mediation of cervical dysfunction on the relationship between prior abortion and preterm birth
Cervical insufficiency is defined by a transvaginal ultrasound cervical measurement of < 25 mm before 24 weeks gestational age in singleton pregnancies with a history of at least one pregnancy loss, preterm birth, or the presence of cervical changes detected by physical exam before 24 weeks [2, 8]. A diagnosis of cervical insufficiency has also been suggested by the presence of a short cervix and the observation of significant risk factors other than previous pregnancy loss [8]. While demographic factors have been associated with a short cervix [9, 10], there is limited understanding of pathophysiological mechanisms contributing to cervical dysfunction during pregnancy. One intriguing question is whether mechanical trauma to the cervical tissue or infection related to invasive procedures, such as cone biopsies and surgical and medical abortions abortions, may also contribute to risk for cervical dysfunction in subsequent pregnancies [7]. A better pathophysiological understanding of factors that may complicate the biomechanical transition the cervix undergoes between maintaining the fetus in the uterus to cervical remodeling leading to delivery is needed [11].
A short cervix is a risk factor for spontaneous preterm birth [4], while cervical insufficiency is a clinical diagnosis, but both reflect overlapping aspects of the condition of the cervix during pregnancy. For example, sonographic cervical length measurement approximates cervical effacement [12], a necessary condition for cervical ripening, and if occurring in the mid-trimester is a criterion for cervical insufficiency. Indeed, sonographic cervical length has been shown to indirectly measure cervical competence, thus providing a rationale for reconsidering this clinical diagnosis as a continuous trait [13]. Thus, the development of a short cervix and cervical insufficiency can both be influenced by congenital factors, loss of cervical tissue due to surgical procedures, intrauterine infection, and primary cervical disease [12]. Although both these measures cannot be equated, they provide an index of cervical health and potential complications during pregnancy.
The results of two related systematic reviews and meta-analyses that considers 49 unique studies conclude there is a significant relationship between a prior abortion and preterm birth [5, 6]. These studies are relevant considering the possible relationship between abortion, cervical dysfunction, and preterm birth (Fig. 1). More specifically, Lemmers et al. report an OR for preterm birth of 1.29 (95% CI 1.17, 1.42) for women with a history of dilatation and curettage compared to women absent a history of these procedures [5]. Saccone et al. report an OR for preterm birth of 1.44 (95% CI 1.09, 1.90) for women with a history of prior uterine evacuation versus women without a history [6]. Both studies stress the importance of conditional factors that could modify the primary relationship of interest and perform a series of sub-analyses to clarify clinical findings. Lemmers et al. report a slightly higher risk of preterm birth for a history of D&C compared to medically managed abortion (OR 1.19, 95% CI 1.10, 1.28). Saccone et al. report an OR for preterm birth of 1.52 (95% CI 1.08, 2.16) restricting to surgical induced abortions and an OR of 1.50 (95% CI 1.00, 2.25) when restricting to medically managed abortion. Both meta-analyses confirm an increasing risk of preterm birth with a history of multiple surgical induced abortions which supports a causal interpretation [5].
The aim of this systematic review and meta-analysis was to identify and report on the cumulative evidence for the relationship between a prior spontaneous or induced abortion and the risk of cervical dysfunction. Sub-analyses were conceived to test this relationship considering the type of abortion procedure, by induced or spontaneous abortions, based on the number previous abortions, and by definition of cervical health measure used by the study (i.e., short cervix or cervical insufficiency).
Methods
Search Strategy and Sources
The systematic review identified research studies published over the last 60 years, beginning in January of 1960 to 01 March 2022, to capture the span of time whereby both surgical and medical abortion procedures were widely adopted. Databases queried included Medline and Embase using the OVID interface. The search strategy for identifying qualified studies was developed using a controlled vocabulary and related keywords connecting to previous cervical trauma and cervical health. Initial concepts were developed in accordance with the Population, Exposure, Outcomes (PEO) framework [14], with assistance from a research librarian, and included female, human, pregnancy, abortion, induced/adverse effects, pregnancy reduction, multifetal pregnancy reduction, dilatation and curettage, loop electrosurgical procedure, dilatation and evacuation, endocervical curettage, cone biopsy, punch biopsy, spontaneous abortion, cervix uteri/pathology, uterine cervical, incompetence, ultrasonography, prenatal/methods, cervical, cervical insufficiency, cervical shortening, uterine cervical incompetence, and cervical length measurement. Initial concepts were translated for use in each database (MeSH for Medline and EmTree for Embase) and included related keywords. The PROSPERO repository was searched to identify other registered studies with a similar focus [15]. The Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) was used as a guide to report methods and results [16].
Study Selection
Eligible studies included peer-reviewed publications and excluded dissertations, case studies, and unpublished literature. Eligibility was extended regardless of cohort location, nationality, or racial group identification or upon original publication language, if translated to English. Studies were included that investigated the relationship between previous abortion status and cervical insufficiency (formerly called cervical incompetence) during pregnancy as the primary outcome. The relationship between cervical insufficiency and prior history of cervical trauma was included to ensure that all eligible studies related to abortion and cervical health were identified. Eligible studies also included cervical length, as long as information regarding prior history of abortion and the relationship between both variables were reported. A short cervix was initially defined in pregnant women as a cervical length shorter than 25 mm between 18 and 24 weeks of gestation. The definitions of cervical insufficiency and cervical incompetence were evaluated within each study to assess homogeneity of results. An additional outcome included a dose–response relationship between the number of previous abortions and cervical insufficiency/shortening.
Prior abortion history was the primary exposure examined and included but was not limited to oral mifepristone (Mifeprex) and oral misoprostol (Cytotec), aspiration abortion, dilation and curettage (D&C), dilation and evacuation (D&E) abortion, and spontaneous abortion. Cervical procedures such as LEEP, endocervical curettage (ECC), cone biopsy, and punch biopsy were considered as indicators of possible cervical trauma. A pap smear was not considered as a cervical procedure, and multifetal pregnancy reductions were not considered as an eligible exposure.
Data Extraction and Risk of Bias Assessment
Literature identified by database searches was annotated electronically by two independent reviewers. Variables collected included author and publication year, overall format of study, period of sample collection, location of research study, number of pregnancies included, definition of the cervical health measure, indication of abortion method, gestational age of abortion, presence of covariates in statistical models, and crude/adjusted effect size summary statistics. Any differences were resolved by a third reviewer. Study authors were contacted for missing information.
The quality of individual studies was evaluated using the Newcastle–Ottawa Scale (NOS) [17] by two independent reviewers. NOS is used for evaluating the quality of non-randomized studies in meta-analyses based across the domains of selection, comparability, and exposure. Each of the domains is divided into more specific categories, and each category, except comparability, can be awarded a maximum of one point. Each study could receive a maximum of 9 points, and studies that receive less than 5 points indicate a high risk of bias.
Data Analysis
The meta-analysis was conducted using the R metafor package version 3.0–2 [18]. Models were fit separately using the effect sizes reported by the crude and/or adjusted odds ratios and associated standard errors, as applicable. Sampling variances were estimated by back-calculation of the 95% confidence intervals if required. Linear mixed-effects models were utilized when the test for heterogeneity using the Cochran’s Q test [19] was significant at P value of < 0.05; otherwise, a fixed effect model was considered. Random/mixed-effect models were specified using the restricted maximum-likelihood estimator and inverse variance weights. For the primary relationship of interest, the prediction interval, which presents the expected range of true effects in future studies, was reported [20]. A series of sensitivity analyses were performed to gauge the robustness of the primary result which tested the pooled association of a history of a previous abortion with cervical health during pregnancy. Planned sensitivity analyses were contingent on the breadth of studies identified from the systematic review.
Results
Study Selection
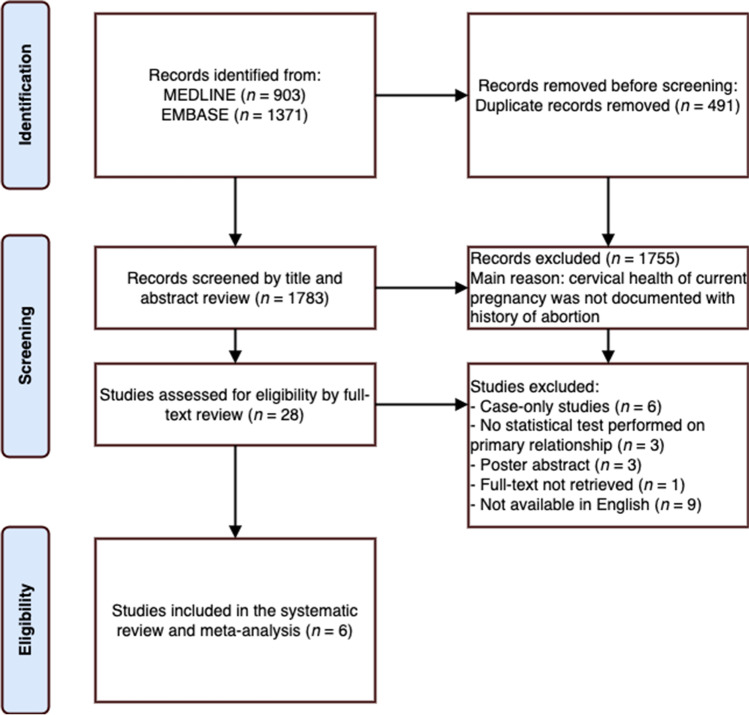
The literature database search identified 2198 studies, of which 1715 were unique citations (Fig. 2). A total of 1687 studies were omitted after title and abstract screening since the majority did not contain cervical health measures in the current pregnancy and/or abortion history status. A full-text review was conducted on the 28 remaining studies, which identified 6 studies that met all criteria for the meta-analysis. Of the 22 articles excluded at this stage, 6 were case-only studies, 3 studies lacked a statistical analysis of the relationship of interest, 9 articles were not available in English, and the full text of 1 study was not retrievable. There were 3 poster abstracts identified, of which 2 corresponded to full-text articles present in this list. The retained studies included 2 prospective [9, 21] and 4 retrospective [7, 22–24] cohorts represented by 2,513,044 pregnancies (Table 1).
Fig. 2.
PRISMA flow diagram of search procedure and number of studies identified
Table 1.
Characteristics of the included studies on prior termination of pregnancy and cervical health
| Author | Period | Location | Study type | Number of pregnancies assessed | Cervical health measure | Pregnancy abortion indicator | Gestational age at abortion | Covariates adjusted |
|---|---|---|---|---|---|---|---|---|
| Boelig (2018) | 2012–2014 | USA | Retrospective cohort | 2672 | Short cervix (≤ 20 mm) between 18–26 weeks 6 days | Prior uterine evacuation1 | Any | Race, BMI, smoking status, prior full-term delivery |
| Tanner (2018) | 2012 | USA | Retrospective cohort | 34,137 | Cervical insufficiency | Previous pregnancy termination2 | Not indicated | Maternal age, race/ethnic group, nulliparity, diabetes, hypertension, autoimmune disorders, any prior cervical procedure, any prior preterm delivery |
| Scholten (2013) | 2000–2007 | Netherlands | Retrospective cohort | 1,357,894 | Cervical incompetence treated by cerclage | Previous pregnancy termination3 | Not indicated | Maternal age, ethnicity, socio-economic status, parity, smoking, drug dependence, pyelitis, polyhydramnios, history of spontaneous PTB, history of cervical incompetence or Shirodkar procedure, history of uterine myoma, or history of cervical surgery |
| Anum (2010) | 2005 | USA | Retrospective cohort | 1,115,541 | Cervical incompetence as indicated by birth certificates | Previous pregnancy termination4 | Not indicated | Number of live births now living, number of live births now dead, history of previous preterm birth |
| Vyas (2006) | 2003–2005 | USA | Prospective case–control | 98 | Cervical insufficiency | Previous curettage procedure5 | First trimester | Previous precipitous delivery, prolonged second stage of labor, interdelivery interval |
| Heath (1998) | 1997–1998 | UK | Prospective cohort | 2702 | Short cervix (≤ 15 mm) at 23 weeks | Previous pregnancy termination6 | 16–23 weeks | None |
1Medical chart review indicating at least one D&C or D&E of a spontaneous (< 20 weeks) or induced abortion (any gestational age)
2Abstracted from electronic medical records system
3Based on self-report responses by the pregnant woman in a predefined pregnancy intake questionnaire. Surgical abortion was estimated for 90-95% of cases
4Recorded as a history of a single pregnancy termination from birth certificate records
5For the management of spontaneous abortion or voluntary termination of pregnancy
6Obstetric and medical history obtained from patients at their first antenatal visit
Data Extraction and Quality Assessment
The included studies documented cervical health during pregnancy using variable but commonly accepted vocabulary which included cervical insufficiency, cervical incompetence, and cervical length. There were 2 studies that endorsed the outcome of interest as cervical insufficiency, as defined in one study using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code [24], while the other study documented cases from a history of mid-trimester pregnancy loss with painless cervical dilation, no history of contraction, bleeding, or infection [21]. The latter study also tracked clinical symptoms to diagnose cervical insufficiency if a mid-trimester examination had complete cervical effacement or cervical dilation > 1 cm with protrusion of the membranes. Cervical incompetence was defined for subjects for those patients treated with cervical cerclage [22] and from US natality records indicating cervical incompetency [23]. The remaining studies utilized a definition of short cervix with slightly different thresholds including a cervix of ≤ 20 mm between 18 and ≤ 27 weeks [7] or a length ≤ 15 mm at 23 weeks [9].
There were 4 studies that defined the exposure of interest as a previous pregnancy termination, and these were documented by medical records from either the first antenatal visit [9], as at least a single pregnancy termination from birth certificate [23] or as self-reports from an intake questionnaire [22, 24]. No study was restricted to only medical abortion procedures, but surgical methods were primarily used in three studies where at least one D&C or D&E procedure was reported [7, 21] or from a cohort estimated to utilize surgical abortion in 90–95% of cases [22].
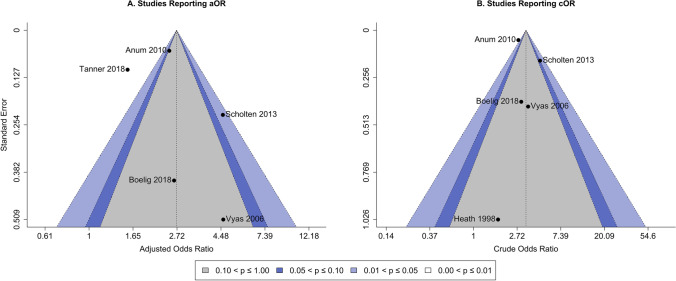
A quality and bias assessment was conducted for each study using the Newcastle–Ottawa Scale (NOS) for cohort studies [17]. The NOS procedure assesses studies on 3 domains including (1) the selection of the cohort and ascertainment of the exposure; (2) on the comparability of cohorts by controlling for important factors and; and (3) by the assessment of the outcome. NOS scores are calculated by summing across domains with the maximum obtainable score being 9 where a score of ≥ 7 is generally considered to be of high quality. All retained studies provided sufficient methodological detail to describe the experimental design in each of these domains. The overall quality assessment was consistent across studies with a median NOS score of 8 and a range of 6 to 9. The Egger’s regression test for funnel plot asymmetry was not significant for either the adjusted OR (β = 1.14, P = 0.256) or crude OR (β = − 0.26, P = 0.796). The individual study points in the respective funnel plots appeared symmetrical and evenly distributed across confidence contours indicating a lack of evidence for publication bias (Fig. 3).
Fig. 3.
Funnel plot for observed study A adjusted and B crude odds ratios versus their standard errors. The vertical dashed line is the summary effect size and the shaded areas indicate the confidence interval regions. No asymmetry is visually observed nor an enrichment of studies within either significance contour indicating a lack of publication bias
Meta-analysis
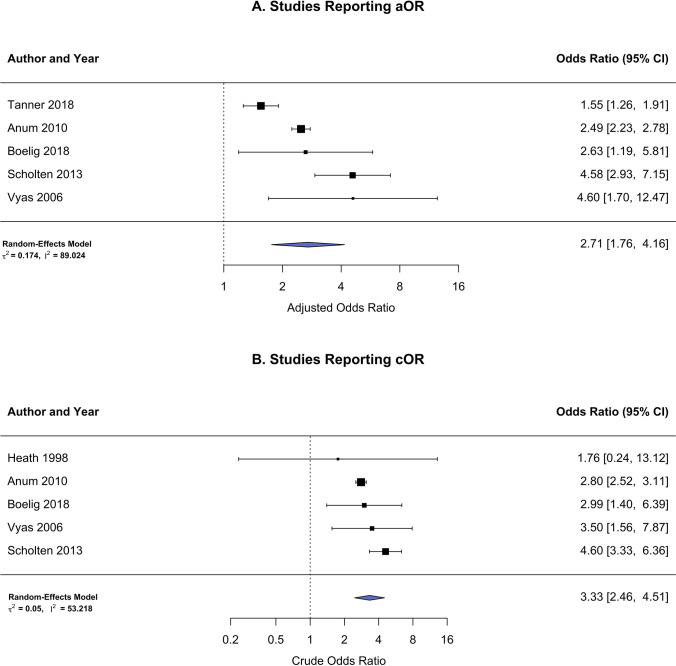
Results from a random-effects model using the reported adjusted odds ratios (aOR) estimated an increase in the odds of 2.71 (95% CI 1.76, 4.16) for cervical dysfunction in the current pregnancy related to a history of induced or spontaneous abortion and 3.33 (95% CI 2.46, 4.51) for unadjusted/crude odds ratios (cOR). The calculated 95% prediction interval for the adjusted model was 1.07 to 6.82 which is the expected range of true effects in future studies. A moderator analysis showed no differences in effect size due to NOS score (β = − 0.21, 95% CI − 0.79–0.37, P = 0.475). Both the aOR and cOR were reported in four of six studies, while only the aOR was provided in Tanner et al., and only the cOR was reported in Heath et al. limiting a pooled analysis of all 6 studies. For these reasons, the complete set of analyses was reported separately for aOR and cOR statistics which included 2,510,342 and 2,478,907 recorded pregnancies, respectively (Fig. 4A–B). Model summaries for all meta-analyses are listed in Table 2.
Fig. 4.
Forest plot for primary outcome risk (i.e., short cervix/cervical insufficiency) in women with a history of prior spontaneous or induced abortions. A Studies reporting adjusted odds ratios. B Studies reporting crude odds ratios
Table 2.
Summary of meta-analyses for risk of cervical dysfunction
| Model results | Heterogeneity | Prediction interval | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure / control | Pregnancies | OR (95% CI) | Z val (P val) | Tau2 (SE) | I2 | H2 | Lower | Upper |
| Adjusted | ||||||||
| Prior abortion/no prior abortion | 2,510,342 |
2.71 (1.76, 4.16) |
4.53 (0.000) |
0.174 (0.168) |
89.02 | 9.11 | 1.07 | 6.82 |
| Prior surgical abortion/no prior abortion | 1,360,664 |
4.08 (2.84, 5.86) |
7.61 (0.000) |
0.000 (0.127) |
0.00 | 1.00 | 2.84 | 5.86 |
| Prior induced abortion/no prior abortion | 2,507,572 |
2.54 (1.41, 4.57) |
3.10 (0.002) |
0.251 (0.272) |
95.53 | 22.36 | 0.81 | 7.97 |
| History of 1 abortion/no prior abortion | 1,118,213 |
2.48 (2.23, 2.77) |
16.56 (0.000) |
0.000 (0.166) |
0.00 | 1.00 | 2.23 | 2.77 |
| History of 2 abortions/no prior abortion | 1,118,213 |
4.64 (4.06, 5.31) |
22.49 (0.000) |
0.000 (0.218) |
0.00 | 1.00 | 4.06 | 5.31 |
| Prior abortion: cervical incompetence/no prior abortion | 2,507,670 |
2.76 (1.64, 4.65) |
3.81 (0.000) |
0.229 (0.230) |
93.08 | 14.44 | 0.94 | 8.07 |
| Crude | ||||||||
| Prior abortion/no prior abortion | 2,478,907 |
3.33 (2.46, 4.51) |
7.81 (0.000) |
0.050 (0.079) |
53.22 | 2.14 | 1.95 | 5.68 |
| Prior surgical abortion/no prior abortion | 1,360,664 |
4.20 (3.18, 5.56) |
10.07 (0.000) |
0.000 (0.092) |
0.00 | 1.00 | 3.18 | 5.56 |
| Prior induced abortion/no prior abortion | 2,476,137 |
3.38 (2.16, 5.29) |
5.34 (0.000) |
0.095 (0.155) |
76.48 | 4.25 | 1.59 | 7.19 |
| History of 1 abortion/no prior abortion | 1,118,213 |
2.79 (2.52, 3.10) |
19.34 (0.000) |
0.000 (0.160) |
0.00 | 1.00 | 2.52 | 3.10 |
| History of 2 abortions/no prior abortion | 1,118,213 |
6.00 (5.28, 6.83) |
27.28 (0.000) |
0.000 (0.204) |
0.00 | 1.00 | 5.28 | 6.83 |
| Prior abortion: cervical incompetence/no prior abortion | 2,473,533 |
3.47 (2.40, 5.02) |
6.60 (0.000) |
0.070 (0.109) |
72.89 | 3.69 | 1.84 | 6.55 |
| Prior abortion: short cervix/no prior abortion | 5374 |
2.80 (1.37, 5.70) |
2.84 (0.005) |
0.000 (0.850) |
0.00 | 1.00 | 1.37 | 5.70 |
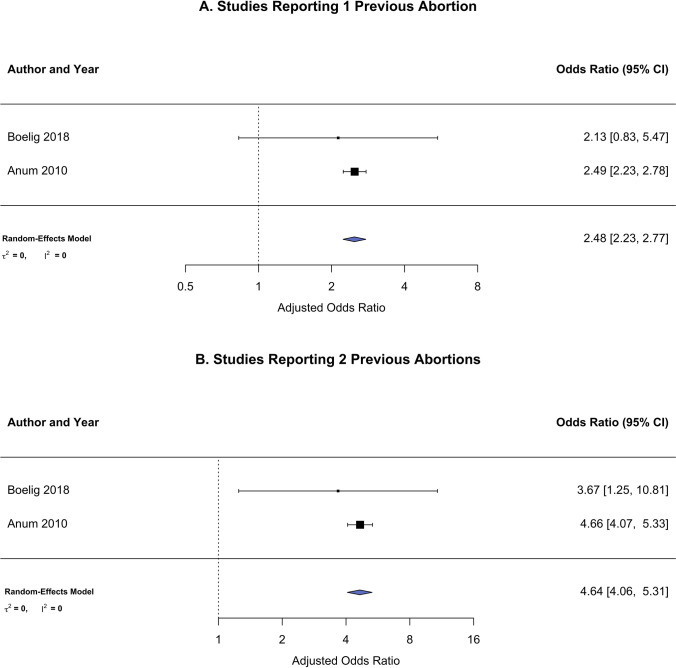
There were two published studies that both demonstrate a dose–response relationship by comparing women without a history of abortion to women with multiple abortions from a combined total of 1,118,213 pregnancies. In one study [23], increasing odds for cervical incompetence was seen after one abortion (aOR 2.49, 95% CI 2.23–2.77), after two abortions (aOR 4.66, 95% CI 4.07–5.33), after three abortions (aOR 8.07, 95% CI 6.77–9.61), and for four or more abortions (aOR 12.36, 95% CI 10.19–15.00). The other study [7] reports an increase in odds after one abortion (aOR 2.13, 95% CI 0.83–5.48), after two abortions (aOR 3.67, 95% CI 1.25–10.84), and for two or more abortions (aOR 3.52, 95% CI 1.33–9.33). The pooled analysis combined levels in common used by both studies and was found to be dependent on the number of previous abortions which increased from an aOR of 2.48 (95% CI 2.23, 2.77) for one reported abortion to an aOR of 4.64 (95% CI 4.06, 5.31) for two reported abortions (Fig. 5).
Fig. 5.
Forest plot for primary outcome risk (i.e., short cervix/cervical insufficiency) stratified by number of prior spontaneous or induced abortions. A Women reporting a history of 1 previous abortion. B Women reporting a history of 2 previous abortion
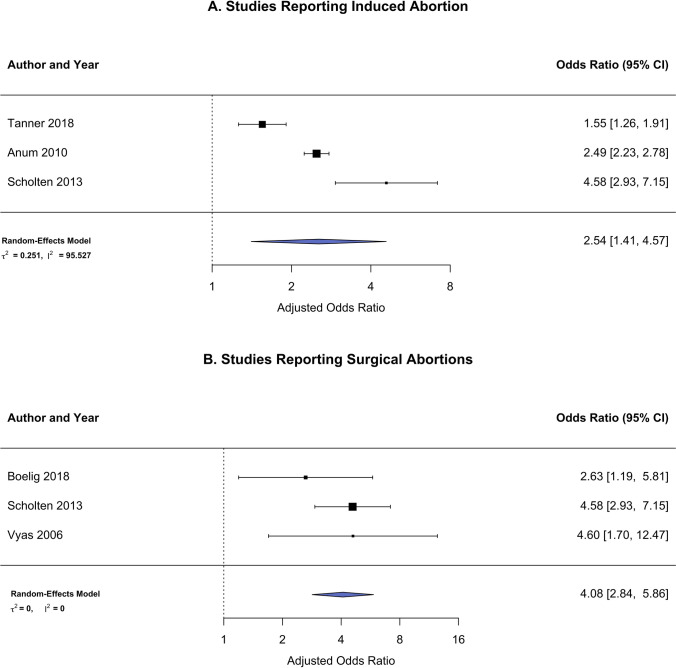
There were four studies [9, 22–24] that include only induced abortions and show a pooled increased odds for cervical dysfunction in the current pregnancy: aOR 2.54 (95% CI 1.41, 4.57) (Fig. 6A). In the study by Heath et al., induced abortions are classified as distinct from miscarriages. The study by Anum et al. include abortions that are likely all induced as typically listed in US natality records and verified by personal communication with the author. In the large Netherlands Prenatal Registry (NPR) accessed by Scholten et al. study, different terminologies are used for pregnancy terminations and miscarriages. Finally, Tanner et al. indicate abortions as prior pregnancy terminations. Surgical abortion methods could be inferred from three of the retained studies [7, 21, 22], and pooled analysis showed an aOR of 4.08 (95% CI 2.84, 5.86) with cervical health complications (Fig. 6B). Scholten et al. restricted their investigation to pregnancy records from January 2000 to December 2007 to mitigate bias since medical terminations were not commonly performed during this time. The studies by Boelig et al. and Vyas et al. select only pregnancies with a history of operative abortion procedures.
Fig. 6.
Forest plot for primary outcome risk (i.e., short cervix/cervical insufficiency) in women with a history of prior A induced abortions or B surgical abortions
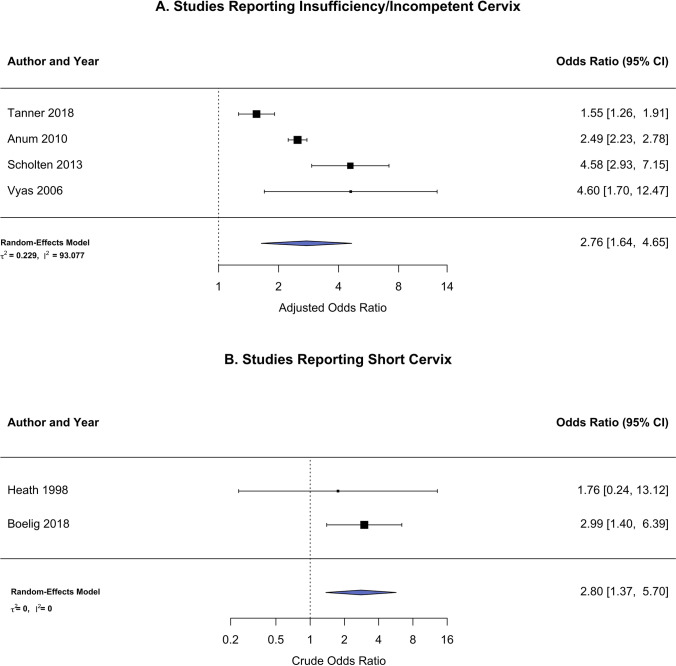
Previous abortion was found to have increased odds for the four studies with the outcome defined as cervical insufficiency/incompetence (aOR 2.76, 95% CI 1.64, 4.65) and an increased odds for the two studies with short cervix as the endpoint (cOR 2.80, 95% CI 1.37, 5.70) (Fig. 7A–B). A moderator analysis showed no differences in effect size due to the definition of cervical health (β = − 0.44, 95% CI − 1.38–1.28, P = 0.943).
Fig. 7.
Forest plot for primary outcome risk in women with a history of prior spontaneous or induced abortions reported as either A short cervix/cervical insufficiency or B short cervix
Discussion
Summary of Main Findings
The results of the systematic review and meta-analysis confirmed that pregnant women with a history of a spontaneous or induced abortion, compared to those without, were at 2.71 times increased risk for cervical dysfunction during pregnancy, defined as either short cervix or cervical insufficiency/incompetence. Women reporting a single prior abortion had significantly higher risk for cervical dysfunction compared to women without a history of abortion. This risk was nearly twice as high for women who reported having two previous abortions compared to women without a history of abortion (OR = 2.48 versus 4.64), suggesting a dose–response relationship.
Sensitivity analyses restricted to studies that report only induced abortions revealed a higher risk of cervical dysfunction (OR = 2.54). While no study reported data from only medical abortions, those studies where surgical-only abortions were reported indicated a strong relationship between abortion and cervical dysfunction (OR = 4.08). The increased risk observed was similar whether the outcome was recorded as either short cervix or cervical insufficiency (OR = 2.80 and 2.76, respectively), and the results of a moderator analysis did not find evidence that these relationships differed by the definition of cervical health used.
Interpretation of Findings and Clinical Implications
These findings are congruent with those from two recent systematic reviews and meta-analyses directed at evaluating the effect of prior abortion and risk for preterm birth [5, 6]. Namely, these studies are characterized by a heightened risk of preterm birth with a history of abortion procedures and an observed dose–response relationship. Interestingly, the odds ratio reported for the relationship between prior abortion and cervical dysfunction in this meta-analysis (OR = 2.71) was nearly twice as high as those reported for in Lemmers et al. (OR = 1.29) and Saccone et al. (OR = 1.44) that tested the relationship between preterm birth and prior dilatation and curettage or uterine evacuation, respectively. All three meta-analyses demonstrate an increased risk of reproductive health complications with increased number of prior abortions. Specifically, the dose–response relationship of prior abortion with both cervical dysfunction and preterm birth supports a causal interpretation [5] and the larger effect of prior abortion on cervical dysfunction versus preterm birth potentially positions cervical dysfunction as a mediating variable.
The closed cervix functions during pregnancy to support the developing fetus and as a barrier to protect the intrauterine environment from external pathogens [25]. For these reasons, the length of the cervix in the second trimester likely serves as one of the strongest predictors of preterm birth risk in the current pregnancy [4, 26]. The robust association of prior abortion with both cervical dysfunction and preterm birth provides a common cause to explain this observed risk. While a prior abortion could have other consequences for reproductive tissue, for instance, curettage damage of the endometrial tissue [5, 27], collectively, these findings are consistent with the conceptual model presented in Fig. 1. Considering the strong prima facie case for the interrelation among these clinical measures, a mediational analysis conducted from prospectively collected epidemiological data can test the extent in which changes in cervical tissue/length during pregnancy can account for the association between prior abortion and preterm birth [28]. Such an analysis would provide valuable insight into the pathophysiological mechanism behind the association between prior abortion and preterm birth.
The structural integrity of the cervix, determined by the cellular components and extracellular matrix network, is essential for carrying a pregnancy to term [29, 30]. The remodeling of the cervical stroma is a complex process that begins early in pregnancy and is necessary to transition from physically maintaining the pregnancy in the uterus to allowing the baby passage through the cervical canal during labor through cervical softening, effacement, and dilation [3]. In contrast to normal labor where the cervix is dilated slowly, the more rapid mechanical stretching of the cervix required by surgical abortion and later-term medical abortions can result in long-term cervical damage [5, 31]. The full scope of how surgical and medical abortion-driven complications affect the balance of cervical function in subsequent pregnancies is not well understood and may require an engineering framework to assess how the presence of an abnormal material structure of the cervix and/or abnormal anatomical changes throughout pregnancy can lead to birth complications [11]. A candidate mechanism could be related to disrupted changes in turnover of the extracellular matrix composition [30, 32, 33], but whether this could be due to cervical trauma has yet to be investigated.
Besides a history of multiple abortions, the next largest risk estimated in this meta-analysis was for studies that reported on surgical-only abortions. Whether an argument could be made that medical management would present a safer alternative to surgical methods is not possible from the data collected from this systematic review. Relative to increased preterm birth risk, the meta-analysis by Lemmers et al. show only a slight increase due to surgical versus medical management (OR = 1.19, 95% CI 1.10, 1.28), but critically no comparison could be made between women with prior medical management of abortion compared to women without a history of abortion. The meta-analysis of Saccone et al. was able to make this comparison, and results show a borderline significant risk due to medical management (OR = 1.50, 95% CI 1.00, 2.25) yet at the same order of magnitude as surgical methods from the same meta-analysis (OR = 1.45, 95% CI 1.27, 1.65). More studies are needed to evaluate the true risk of medical management considering this estimate was based on two relatively small studies compared to their estimate of surgical methods based on 27 studies, many of which contain sample sizes several orders of magnitude greater.
The increased risk of cervical dysfunction due to a history of abortion, and especially multiple abortions, raises the question of whether more intensive cervical health surveillance in pregnancy is warranted to initiate early interventions. Future research is needed to evaluate the scope of possible assessments of cervical health but may include the monitoring of intra-amniotic inflammation [34, 35], infection status [36–38], or less widely used modalities such as elastography [39, 40]. Enhanced monitoring of cervical length by serial measurements across pregnancy has been shown to more accurately predict preterm birth in twin gestations [41, 42] but not in singleton pregnancies [43].
Limitations and Strengths
To the best of our knowledge, this is the first systematic review and meta-analysis of cervical dysfunction risk relative to prior abortion status. The limited number of studies meeting the systematic review selection criteria represents the state of research in this field, contains low levels of bias according to the NOS guideline, and includes over 2.5 million pregnancies assessed primarily from two studies [22, 23]. Studies identified are from Western European countries and the USA and include only prospective and retrospective cohorts which may limit the relevance of study conclusions to certain populations. For example, population-specific genetic factors may have influenced outcomes [44, 45]. While the search strategy was comprehensive and the calculated prediction intervals do not suggest additional studies would alter the conclusions, there is always a possibility that relevant studies were not identified.
The estimated risk of cervical dysfunction during pregnancy associated with prior abortion was nearly twice as large compared to contemporary meta-analyses where preterm birth was the outcome [5, 6]. Similar to these studies, data on medical abortion was limited, and sensitivity analyses were contingent on the available data. Although the cervical dysfunction outcome differed slightly among studies, they were clinically related, and moderation analysis did not show differences in association by definition used.
All but one study considered covariates in analyses [9], yet for those that did, the set of variables differed widely across studies. One study only reported adjusted effects [24]. For this reason, both the crude and adjusted odds ratios were reported as applicable, although adjusted values were preferred, and results show only small differences between estimate types. The testing of the dose–response effect was limited to a history of one and two prior abortions since only these frequencies were common across studies that considered this relationship.
Conclusion
In comparison to recent systematic reviews and meta-analyses, the risk of cervical dysfunction was 1.9–2.1 times higher than the risk of preterm birth [5, 6] in women with a history of abortion compared to women without a history. The consistency of effects, the observed dose–response relationship, pathophysiological plausibility, and temporal ordering of measures supports a causal explanation for the relationship between prior abortion and cervical health dysfunction. The possibility that cervical health dysfunction serves as a mediating variable to, in part, account for the relationship between prior abortion and preterm birth is intriguing and should be explored in future studies. The lack of data on the medical management of abortion in this study and the unclear picture of similar effects in the other meta-analyses mentioned preclude a full assessment of the risk of this procedure. This study provides an updated assessment of the risk associated with prior abortion on cervical health that practitioners should review with women of reproductive age.
Author Contribution
JJB and TPY were responsible for designing the study. SEW and JWC provided expertise on systematic review best practices and SEW performed the literature search. JJB, KM, and TPY performed the data abstraction and TPY performed the statistical analysis. HMW, RR, and JFS provided expertise on clinical outcomes. The first draft of the manuscript was written by TPY and JJB. All authors critically reviewed the manuscript and approved the final version.
Funding
This research is supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal–Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Data and Code Availability
Data available on request.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown R, Gagnon R, Delisle M-F. No. 373-cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233–247. doi: 10.1016/j.jogc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Roman A, Suhag A, Berghella V. Overview of cervical insufficiency: diagnosis, etiologies, and risk factors. Clin Obstet Gynecol. 2016;59:237–240. doi: 10.1097/GRF.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 3.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 4.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–573. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 5.Lemmers M, Verschoor MAC, Hooker AB, Opmeer BC, Limpens J, Huirne JAF, et al. Dilatation and curettage increases the risk of subsequent preterm birth: a systematic review and meta-analysis. Hum Reprod. 2016;31:34–45. doi: 10.1093/humrep/dev274. [DOI] [PubMed] [Google Scholar]
- 6.Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214:572–591. doi: 10.1016/j.ajog.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Boelig RC, Villani M, Jiang E, Orzechowski KM, Berghella V. Prior uterine evacuation and the risk of short cervical length: a retrospective cohort study. J Ultrasound Med. 2018;37:1763–1769. doi: 10.1002/jum.14529. [DOI] [PubMed] [Google Scholar]
- 8.Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557–567. doi: 10.1097/GRF.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 9.Heath VC, Southall TR, Souka AP, Novakov A, Nicolaides KH. Cervical length at 23 weeks of gestation: relation to demographic characteristics and previous obstetric history. Ultrasound Obstet Gynecol. 1998;12:304–311. doi: 10.1046/j.1469-0705.1998.12050304.x. [DOI] [PubMed] [Google Scholar]
- 10.Harville EW, Miller KS, Knoepp LR. Racial and social predictors of longitudinal cervical measures: the cervical ultrasound study. J Perinatol. 2017;37:335–339. doi: 10.1038/jp.2016.240. [DOI] [PubMed] [Google Scholar]
- 11.Myers KM, Feltovich H, Mazza E, Vink J, Bajka M, Wapner RJ, et al. The mechanical role of the cervix in pregnancy. J Biomech. 2015;48:1511–1523. doi: 10.1016/j.jbiomech.2015.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 14.Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute’s approach. Int J Evid Based Healthc. 2015;13:163–169. doi: 10.1097/XEB.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Booth A, Stewart L. How to reduce unnecessary duplication: use PROSPERO. BJOG. 2014;121(7):784–6. 10.1111/1471-0528.12657. [DOI] [PubMed]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z. [DOI] [PubMed]
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 19.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 20.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyas NA, Vink JS, Ghidini A, Pezzullo JC, Korker V, Landy HJ, et al. Risk factors for cervical insufficiency after term delivery. Am J Obstet Gynecol. 2006;195:787–791. doi: 10.1016/j.ajog.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 22.Scholten BL, Page-Christiaens GC, Franx A, Hukkelhoven CW, Koster MP. The influence of pregnancy termination on the outcome of subsequent pregnancies: a retrospective cohort study. BMJ Open. 2013;3(5):e002803. 10.1136/bmjopen-2013-002803. [DOI] [PMC free article] [PubMed]
- 23.Anum EA, Brown HL, Strauss JF., 3rd Health disparities in risk for cervical insufficiency. Hum Reprod. 2010;25:2894–2900. doi: 10.1093/humrep/deq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner LD, Tucker L-Y, Postlethwaite D, Greenberg M. Maternal race/ethnicity as a risk factor for cervical insufficiency. Eur J Obstet Gynecol Reprod Biol. 2018;221:156–159. doi: 10.1016/j.ejogrb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Nott JP, Bonney EA, Pickering JD, Simpson NAB. The structure and function of the cervix during pregnancy. Trans Res Anatomy. 2016;2:1–7. doi: 10.1016/j.tria.2016.02.001. [DOI] [Google Scholar]
- 26.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-P. [DOI] [PubMed] [Google Scholar]
- 27.Watson LF, Rayner J-A, King J, Jolley D, Forster D. Intracervical procedures and the risk of subsequent very preterm birth: a case-control study. Acta Obstet Gynecol Scand. 2012;91:204–210. doi: 10.1111/j.1600-0412.2011.01322.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolf HM, Romero R, Strauss JF, Hassan SS, Latendresse SJ, Webb BT, Tarca AL, Gomez-Lopez N, Hsu CD, York TP. Study protocol to quantify the genetic architecture of sonographic cervical length and its relationship to spontaneous preterm birth. BMJ Open. 2022;12(3):e053631. 10.1136/bmjopen-2021-053631. [DOI] [PMC free article] [PubMed]
- 29.Yoshida K, Jayyosi C, Lee N, Mahendroo M, Myers KM. Mechanics of cervical remodelling: insights from rodent models of pregnancy. Interface Focus. 2019;9:20190026. doi: 10.1098/rsfs.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nallasamy S, Akins M, Tetreault B, Luby-Phelps K, Mahendroo M. Distinct reorganization of collagen architecture in lipopolysaccharide-mediated premature cervical remodeling. Biol Reprod. 2018;98:63–74. doi: 10.1093/biolre/iox155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renner R-M, Brahmi D, Kapp N. Who can provide effective and safe termination of pregnancy care? A systematic review BJOG. 2013;120:23–31. doi: 10.1111/j.1471-0528.2012.03464.x. [DOI] [PubMed] [Google Scholar]
- 32.Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012;153:3493–3503. doi: 10.1210/en.2011-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Word RA, Li X-H, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 34.Lee SE, Romero R, Park C-W, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(633):e1–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Mönckeberg M, Valdés R, Kusanovic JP, Schepeler M, Nien JK, Pertossi E, et al. Patients with acute cervical insufficiency without intra-amniotic infection/inflammation treated with cerclage have a good prognosis. J Perinat Med. 2019;47:500–509. doi: 10.1515/jpm-2018-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/jpm.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, Yoon BH. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221(2):140.e1–140.e18. 10.1016/j.ajog.2019.03.017. [DOI] [PMC free article] [PubMed]
- 38.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. doi: 10.1016/S0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 39.Patberg ET, Wells M, Vahanian SA, Zavala J, Bhattacharya S, Richmond D, Akerman M, Demishev M, Kinzler WL, Chavez MR, Vintzileos AM. Use of cervical elastography at 18 to 22 weeks' gestation in the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2021;225(5):525.e1–525.e9. 10.1016/j.ajog.2021.05.017. [DOI] [PubMed]
- 40.Wozniak S, Czuczwar P, Szkodziak P, Milart P, Wozniakowska E, Paszkowski T. Elastography in predicting preterm delivery in asymptomatic, low-risk women: a prospective observational study. BMC Pregnancy Childbirth. 2014;14:238. doi: 10.1186/1471-2393-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melamed N, Pittini A, Hiersch L, Yogev Y, Korzeniewski SJ, Romero R, et al. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? Am J Obstet Gynecol. 2016;215(616):e1–616.e14. doi: 10.1016/j.ajog.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melamed N, Pittini A, Hiersch L, Yogev Y, Korzeniewski SS, Romero R, et al. Serial cervical length determination in twin pregnancies reveals 4 distinct patterns with prognostic significance for preterm birth. Am J Obstet Gynecol. 2016;215(476):e1–476.e11. doi: 10.1016/j.ajog.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conde-Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2015;213:789–801. doi: 10.1016/j.ajog.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alves APVD, Freitas AB, Levi JE, Amorim Filho AG, Franco LAM, Hoshida MS, et al. COL1A1, COL4A3, TIMP2 and TGFB1 polymorphisms in cervical insufficiency. J Perinat Med. 2021;49:553–558. doi: 10.1515/jpm-2020-0320. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.