Abstract
Introduction
Inconvenient administration and side effects of some disease-modifying therapies (DMTs) for relapsing multiple sclerosis (RMS) can deter adherence. We evaluated treatment satisfaction with cladribine tablets (CladT) for RMS in the Arabian Gulf.
Methods
This was a non-interventional, multicentre, prospective observational study in non-pregnant/lactating adults (aged ≥ 18 years) with RMS eligible for 1st treatment with CladT (EU labelling). The primary outcome was overall treatment satisfaction at 6 months (Treatment Satisfaction Questionnaire for Medication [TSQM]-14, v. 1.4), Global Satisfaction subscale. Secondary endpoints were TSQM-14 scores for convenience, satisfaction with side effects and satisfaction with effectiveness. Patients provided written informed consent.
Results
Of 63 patients screened, 58 received CladT and 55 completed the study. Mean age was 33 ± 9 years; mean weight 73 ± 17 kg; 31% male/69% female; mostly from the United Arab Emirates (52%) or Kuwait (30%). All had RMS (mean 0.9 ± 1.1 relapses in the past year), mean Expanded Disability Status Scale (EDSS) 1.4 ± 1.2; 36% were DMT-naïve. Mean [95% CI] score was high for overall treatment satisfaction (77.8 [73.0–82.6]), ease of use (87.4 [83.7–91.0]), tolerability (94.2 [91.0–97.3]) and effectiveness (76.2 [71.6–80.7]). Scores were similar irrespective of DMT history, age, gender, relapse history or EDSS. No relapses or serious treatment-emergent adverse events (TEAE) occurred. Two severe TEAE occurred (fatigue, headache) and 16% reported lymphopenia (two cases of grade 3 lymphopenia). Absolute lymphocyte counts at baseline and 6 months were 2.2 ± 0.8 × 109/L and 1.3 ± 0.3 × 109/L, respectively.
Conclusions
Treatment satisfaction, ease of use, tolerability and patient-perceived effectiveness for CladT were high, irrespective of baseline demographics, disease characteristics and prior treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00497-2.
Keywords: Multiple sclerosis, Cladribine tablets, Patient-reported outcomes, Disease-modifying therapy
Key Summary Points
| We evaluated treatment satisfaction with cladribine tablets (CladT) for relapsing multiple sclerosis (RMS) in the Arabian Gulf in an observational study. |
| The Treatment Satisfaction Questionnaire for Medication (TSQM)-14, v. 1.4 was administered 6 months after the year 1 course of CladT. |
| Of 63 subjects screened, 58 received CladT, and 55 completed the study. |
| Mean TSQM scores for CladT were high for overall treatment satisfaction, ease of use, tolerability and effectiveness, irrespective of DMT history, age, gender, relapse history or disability status. |
Introduction
Treatment with disease-modifying therapies (DMTs) represents the cornerstone of management of relapsing multiple sclerosis (RMS) [1]. Long-term management of RMS with DMTs has been shown to reduce the frequency of clinical MS relapses, as well as the appearance of new demyelinating lesions in the central nervous system (CNS) and the progression of disability related to RMS, especially when high-efficacy treatment is given early [1–3]. Treatment with cladribine tablets (CladT), a high-efficacy DMT believed to act via a mechanism related to immune reconstitution, is indicated as monotherapy for the management of RMS in patients with high MS disease activity [4–7].
The approval of CladT was based largely on the results of the 2-year, randomised, placebo-controlled, phase III CLARITY study, together with long-term follow-up in an extension to this trial and in registries of patients with MS [4, 7, 8]. The measurement of patient-reported outcomes is also important in assessing the overall impact of a treatment on the patient’s life, and no such data are available from countries of the Arabian Gulf. We report the results of a 6-month, prospective, observational evaluation of the effects of CladT on patient satisfaction in people with RMS, using the Treatment Satisfaction Questionnaire for Medication (TSQM), a validated instrument for this purpose.
Methods
Objectives and Outcomes
This study used the TSQM under an appropriate licence. The TSQM version 1.4 includes 14 questions that address four distinct domains of treatment satisfaction (overall satisfaction, convenience, perceived effectiveness, and side effects). Questions elicit responses on a Likert scale ranging from 1 (lowest satisfaction) to 5 or 7 (highest satisfaction), apart from one question on the presence or absence of side effects, which requires an answer of “yes” or “no” [9, 10]. Individual scores are pooled to provide overall scores for the four domains and are transformed to a scale of 0 (least satisfaction) to 100 (most satisfaction) [9, 10]. Accordingly, higher TSQM scores indicate better patient satisfaction with treatment.
The primary outcome of this study was overall patient satisfaction with CladT treatment 6 months after starting treatment, as measured using the TSQM. Secondary objectives included patient-reported outcomes based on subscales of the TSQM (ease of use of CladT [convenience subscale], tolerability of CladT [satisfaction with side effects subscale] and the subject-perceived effectiveness of CladT [satisfaction with effectiveness subscale]). Other secondary outcomes were exploration of the relationships between treatment satisfaction and the characteristics of subjects at baseline, the nature of previous DMT (identity of prior DMTs, reasons for discontinuation, and washout period required before starting CladT), tolerability (the proportion of subjects who experienced grade 3 or 4 lymphopenia) and an analysis of MS relapses during the first 6 months of CladT.
Patients
Eligible subjects were adult (at least 18 years of age) men or women with RMS who were indicated (in the opinion of the investigator) for treatment with CladT according to its European labelling (Summary of Product Characteristics); Table 1 summarises the details of this indication, which is consistent with the presence of “highly active” MS disease activity, as defined in the EU label. Additionally, patients were required to be receiving their first treatment with CladT, to be not pregnant or breastfeeding, to be free of contraindications to CladT (Table 1) and to have provided informed consent (see below). Patients received CladT at the discretion of the investigator, who was also the patient’s usual-care neurologist. Inclusion in the study occurred at the point of administration of the first dose of CladT.
Table 1.
Therapeutic indications and contraindications for cladribine tablets in the management of RMS
| Therapeutic indication | Treatment of adult patients with highly active RMS as defined by clinical or imaging features |
| Contraindications | Infection with human immunodeficiency virus (HIV) |
| Active chronic infection (tuberculosis or hepatitis) | |
| Immunocompromised patients, including patients currently receiving immunosuppressive or myelosuppressive therapy | |
| Active malignancy | |
| Moderate or severe renal impairment (creatinine clearance < 60 mL/min) | |
| Pregnancy and breastfeeding | |
| Hypersensitivity to the active substance or to any of the excipients |
Compiled from information presented in the European Summary of Product Characteristics
All patients who had received at least one dose of CladT and who were enrolled in the study were included in analyses of safety and tolerability (safety population). Subjects who completed the study were included in the full analysis set (FAS).
On the basis of a sample size of 55 subjects, the Global Satisfaction subscale score of the TSQM questionnaire was estimated with a precision (half-width of the 95% CI around the estimate) of 5.3 based on a two-sided 95% confidence for the mean and an SD of 20. Assuming a 10% dropout rate, approximately 60 subjects were needed to achieve the targeted precision on the primary endpoint.
Study Design
This was a prospective, non-interventional, observational study. The TSQM was administered 6 months after the first course of CladT. Where subjects withdrew from the study before the 6-month time point, the reason for discontinuation was recorded, along with details of lymphopenia, MS relapses, and any other tolerability/safety findings.
Statistics
Data were analysed using descriptive statistics (means and SD and/or 95% confidence intervals [95%CI] for continuous variables and number [%] for categorical variables). No imputation of missing data was performed. Formal testing for statistical significance was not performed.
Ethics
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients provided written informed consent before being enrolled into the study. Prior to commencement of the study at a given site, the protocol and associated documents were submitted to the institutional review board (IRB) or independent ethics committee (IEC) responsible for approval at each site. The study could only proceed at any site after IEC/IRB approval. Online Supplementary Table 1 provides a list of IECs/IRBs that provided approval at each study site.
Results
Patients
Of 63 patients screened for inclusion, 58 were enrolled (safety population). Reasons for non-inclusion were failure to meet inclusion/exclusion criteria (n = 2), lost to follow-up (n = 1) and other reasons (n = 2). Fifty-five patients completed the study (FAS; one was lost to follow-up and two withdrew for other reasons) (Table 2). All subjects had a diagnosis of RMS. There were about twice as many women vs. men in the study population. Patients had active RMS, as shown by the presence of clinical relapses and/or new or enlarged MRI lesions in the past year. Physical status was good on average, with a mean Expanded Disability Status Scale (EDSS) of 1.4.
Table 2.
Demographic and disease characteristics at baseline (full analysis set, n = 55)
| Mean age (years) | 33.9 ± 9.3 |
| Gender [n (%) male/female] | 17 (31)/38 (69) |
| Mean number of MS relapses in year before enrolment | 0.9 ± 1.1 |
| Mean EDSSa | 1.4 ± 1.2 |
| MRI parametersa | |
| Mean no. of Gd+ lesions | 0.9 ± 3.1 |
| Mean no. of new or enlarged T2 hyperintense lesions | 3.6 ± 5.0 |
| Mean no. of T1 hypointense lesions | 2.2 ± 3.8 |
| Absolute lymphocyte countb | |
| Cells/mm3 | 2200 ± 800 |
| Cells/L × 109 | 2.2 ± 0.8 |
| Previous DMT therapy [yes/no, n (%)] | 35 (64)/20 (36) |
| > 1 prior DMT [n (%)] | 19 (35) |
| Individual DMTs received | |
| Interferon-β | 7 (13) |
| Teriflunomide | 7 (13) |
| Dimethyl fumarate | 7 (13) |
| Fingolimod | 6 (11) |
| Natalizumab | 8 (15) |
EDSS Expanded Disability Status Scale
aEDSS or MRI had never been measured in 7 subjects (i.e. n = 48 for this parameter)
bData available from 54/55 subjects. Data are means ± SD for continuous variables or [n (%)] for categorical variables
The majority of patients (64%) had received prior DMT-based therapy for MS (interferon-β, dimethyl fumarate, teriflunomide, fingolimod or natalizumab). The mean duration of prior DMT was 779 ± 816 days and the mean washout period before starting treatment with CladT was 108 ± 175 days.
Patient Satisfaction with CladT Treatment
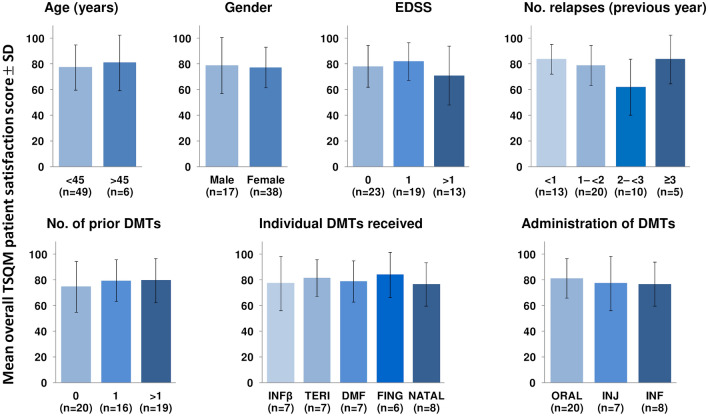
TSQM scores and their subscores indicated a high level of satisfaction with CladT treatment (Table 3), with a score for overall treatment satisfaction (the primary endpoint) of 77.8 (73.0–82.6). Figure 1 shows that overall treatment scores were similar between subgroups of patients stratified for age (cutoff 45 years), gender, number of prior DMTs received (0, 1 or > 1), individual prior DMTs (interferon-β, dimethyl fumarate, teriflunomide, fingolimod or natalizumab), route of administration of DMTs (oral, injectable, infusional), number of MS relapses in the previous year (0, 1 or > 1), or EDSS at baseline (< 1, 1 to < 2, 2 to < 3, ≥ 3). Mean scores for the three subscales were also similar between these subgroups (Online Supplementary Figs. 1–3).
Table 3.
Mean scores from the Treatment Satisfaction Questionnaire for Medication (TSQM)
| TSQM scale | Mean score (95% CI) |
|---|---|
| Overall treatment satisfaction (primary endpoint) | 77.8 (73.0–82.6) |
| Convenience | 87.4 (83.7–91.0) |
| Side effects | 94.2 (91.0–97.3) |
| Effectiveness | 76.2 (71.6–80.7) |
Higher TSQM scores indicate better patient satisfaction with treatment
Fig. 1.
Overall Treatment Satisfaction Questionnaire for Medication (TSQM) scores according to subject characteristics at baseline. DMT disease-modifying therapy, EDSS Expanded Disability Status Scale, INFβ interferon-β, TERI teriflunomide, DMF dimethyl fumarate, FING fingolimod, NATAL natalizumab, INJ injectable, INF infusion. Derived from the full analysis set (n = 55). Higher TSQM scores indicate better patient satisfaction with treatment
Safety and Tolerability
These analyses were based on the safety population (all 58 enrolled subjects). Half of the population (n = 29; 50%) reported at least one treatment-emergent adverse event (TEAE). The most common all-cause TEAE (occurring in at least 5% of the safety population) were lymphopenia (n = 9, 16%), fatigue (n = 6, 10%) and headache (n = 3, 5%). AE were mild in severity except for moderate events of lymphopenia, oropharyngeal pain, productive cough, and rhinorrhoea (n = 1, 1.7% for each) and severe events of fatigue, headache, and dyspnoea (n = 1, 1.7% for each); these AE were considered by investigators to be related to CladT. There were no deaths, serious AE or AE that led to discontinuation of treatment with CladT.
Grade 3–4 lymphopenia occurred in two subjects (3.4%). Mean ALC was 2.243 × 109/L (SD 0.8) at baseline and 1.265 × 109/L (SD 0.4) at month 6 (equivalent to 2243 ± 800 and 1265 ± 400 cells/mm3, respectively).
No MS relapses occurred during the study period.
Discussion
Our data showed that TSQM scores for overall patient satisfaction, and its subscales of convenience, side effects and effectiveness, were high among patients with “highly active” RMS (as described in the EU label for CladT) who had received their first course of CladT for RMS. There were no clear or consistent differences in patient satisfaction scores when the patient population was stratified for age, gender, recent MS relapse history, EDSS, or number and type of prior DMTs. Importantly, prior receipt of an oral vs. injectable vs. infusional DMT, or a high-efficacy vs. first-line DMT, did not alter patients’ perceptions of CladT. Previous data on treatment satisfaction with CladT are available from a 6-month planned interim analysis from the CLARIFY-MS cohort, conducted in a larger (N = 482) but otherwise comparable group of people with RMS in Europe or Australia [11]. Comparable mean TSQM scores were found in CLARIFY-MS and the present study for overall satisfaction (70.4 vs. 77.8, respectively), tolerability (91.9 vs. 94.2), ease of use (86.6 vs. 87.4) and perceived effectiveness (65.8 vs. 76.2). Other evaluations of high-efficacy DMTs (alemtuzumab, ocrelizumab and fingolimod) have demonstrated mean global TSQM scores in the range 64–74 after treatment [12–16]. Patient satisfaction with CladT in the present study therefore compares well with these agents, although caution must be observed when comparing results across different clinical trials, especially when injectable and oral treatments are involved. Ensuring high acceptance of treatments by patients is important, as suboptimal satisfaction with treatment is a strong predictor of poor adherence to long-term therapies for MS and other conditions [17–20].
Lymphopenia was a common TEAE reported with CladT in this study, consistent with previous clinical trial data [21]. However, only 3.4% developed grade 3–4 lymphopenia in the present study (and were monitored as lymphocytes recovered, in line with the EU label for CladT), compared with 25.6% in the randomised, 2-year phase III CLARITY trial and its 2-year extension [8]. The nadir in B and T lymphocytes occurs about 13 and 24 weeks, respectively, after treatment with CladT [22]. It is unlikely, therefore, that the present study missed cases of severe lymphopenia arising from the single course given. The year 2 course of CladT was given irrespective of the lymphocyte count in CLARITY, and most cases of severe lymphopenia in that study arose from administration of CladT to patients who still had markedly suppressed lymphocyte levels [8, 23]. This led to a change in the labelling for CladT so that a delay of up to 6 months in the year 2 course is permitted to allow lymphocytes to recover. Accordingly, the low rate of severe lymphopenia due to CladT in the present study reflects actual clinical practice and is consistent with the results of other real-world studies [4, 24–26]. CladT was generally well tolerated in this study, with no new safety issues or withdrawal for AE, and this likely contributed to the high TSQM scores for patient satisfaction related to tolerability.
The use of an instrument for measuring patient satisfaction that has been validated extensively, including in populations with MS [9], was a strength of this study. Another strength was the broad inclusion/exclusion criteria (any patient indicated for CladT according to European labelling and without contraindications to the treatment could be included) that led to the recruitment of a relatively unselected population of adults receiving CladT for RMS. The gender balance of the population in our study was broadly similar to that of the global population with MS, with an approximate 2:1 preponderance of women vs. men [27]. The low average EDSS in our population (mean 1.4 at baseline) is consistent with an absence of disability, with minor signs in one or more functional systems [28]. This may reflect a bias arising from an unwillingness of some physicians to prescribe CladT for patients with already established disability [7]. The low average EDSS score does suggest that our patients were generally early in the clinical course of RMS, and this may reflect differing practices regarding the use of highly active DMTs in the Gulf and elsewhere [29].
The main limitation of the study was its observational design and lack of a control group. Treatment with CladT was at the discretion of investigators and it is possible in theory that inclusion in the study could have influenced the choice of DMT for some patients. However, it should be noted that all enrolled patients were indicated for treatment with CladT (according to EU labelling), physicians chose treatment for RMS under usual care conditions and that inclusion in the study did not occur until the time of actual treatment with CladT. Accordingly, we consider it unlikely that the existence of the study influenced patients’ treatment, or introduced bias into its results. Finally, the follow-up duration was relatively short (6 months). Given that CladT is an immune reconstitution therapy, patients received no treatment for almost all of the last 5 months of the study period, which may influence their recall of the treatment process. Also, 6 months is sufficient to include the post-treatment nadir in lymphocyte counts for most patients. Overall, we believe that 6 months was a reasonable compromise between capturing patients’ impressions of the treatment and its main tolerability/safety issue.
Conclusions
Overall treatment satisfaction with CladT was high in patients with highly active RMS in the Arabian Gulf who had received the first treatment course of this agent. Scores for treatment satisfaction related to convenience, side effects and effectiveness of CladT were also high. Stratification of the population for age, gender, prior use of DMTs or the level of disability had little or no effect on these findings. This high level of treatment satisfaction is likely to be important in maintaining adherence to treatment with CladT.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Merck Serono Middle East FZ-Ltd (CrossRef Funder ID https://doi.org/10.13039/100009945). Merck Serono Middle East FZ-Ltd also funded the Rapid Service Fee and editorial assistance (see below) through an independent medical writing grant.
Medical Writing and/or Editorial Assistance
Dr Mike Gwilt (GT Communications) provided editorial assistance, funded by Merck Serono Middle East FZ-Ltd (CrossRef Funder ID https://doi.org/10.13039/100009945).
Author Contributions
Jihad Inshasi and Raed Alroughani led development of the initial draft of the article. Jihad Inshasi, Samar Farouk, Ahmed Shatila, Ali Hassan, Miklos Szolics, Mona Thakre, Derk Krieger, Taoufik Alsaadi, Abubaker Almadani, Victoria Mifsud, Anu Jacob, Deeb Kayed, Beatrice Benedetti, Shatha Sayegh and Raed Alroughani identified eligible patients from their local MS registries and contributed to development and finalisation of the article. Amir Boshra provided coordination on behalf of the study sponsor, contributed to the inception and design of the study and contributed to development and finalisation of the article.
Prior Publication
An abstract/poster based on data from this study was presented at the 18th Pan Arab Union of Neurological Societies congress, Jeddah, Kingdom of Saudi Arabia, Jan 12–14, 2023.
Disclosures
Ahmed Shatila received honoraria for lectures (Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Boehringeringer Ingelheim, Biologix) and for advisory boards (Sanofi-Genzyme, Roche, Novartis, Pfizer, Biologix); educational conferences travel and registration and hotel accommodation has been sponsored by Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Biologix. Raed Alroughani received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Taoufik Alsaadi Alsaadi received consulting fees, honoraria, and research grants from Novartis, GlaxoSmithKline, Merck, Pfizer, and Hekma. Jihad Inshasi and Samar Farouk received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Victoria Mifsud received honoraria for participation in Advisory boards from Merck, Roche and Novartis. Amir Boshra and Shatha Sayegh are employees of Merck Serono Middle East FZ-Ltd. Mona Thakre, Abubaker Almadani, Beatrice Benedetti, Deeb Kayed, Derk Krieger, Miklos Szolics, Anu Jacob and Ali Hassan have nothing to disclose beyond the current work.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients provided written informed consent before being enrolled into the study. Prior to commencement of the study at a given site, the protocol and associated documents were submitted to the institutional review board (IRB) or independent ethics committee (IEC) responsible for approval at each site. The study could only proceed at any site after IEC/IRB approval. Online Supplementary Table 1 provides a list of IECs/IRBs that provided approval at each study site.
Data Availability
All data generated or analyzed during this study are included in this published article. No data repository is available beyond the online supplementary data supplied with this article.
Footnotes
Merck Serono Middle East FZ Ltd is an affiliate of Merck KGaA, Darmstadt, Germany.
References
- 1.Practice guideline recommendations summary Disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019;92:112. doi: 10.1212/WNL.0000000000006722. [DOI] [PubMed] [Google Scholar]
- 2.Iaffaldano P, Lucisano G, Caputo F, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. doi: 10.1177/17562864211019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fyfe I. Multiple sclerosis: real-world long-term benefits of disease-modifying MS therapy. Nat Rev Neurol. 2016;12:372. doi: 10.1038/nrneurol.2016.83. [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician's review. Neurol Ther. 2022;11:571–595. doi: 10.1007/s40120-022-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther. 2020;9:55–66. doi: 10.1007/s40120-020-00187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol Ther. 2019;8:13–23. doi: 10.1007/s40120-019-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sèze J, Suchet L, Mekies C, et al. The place of immune reconstitution therapy in the management of relapsing multiple sclerosis in France: an expert consensus. Neurol Ther. 2022 doi: 10.1007/s40120-022-00430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 9.Vermersch P, Hobart J, Dive-Pouletty C, Bozzi S, Hass S, Coyle PK. Measuring treatment satisfaction in MS: is the treatment satisfaction questionnaire for medication fit for purpose? Mult Scler. 2017;23:604–613. doi: 10.1177/1352458516657441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brochet B, Hupperts R, Langdon D, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord. 2022;57:103385. doi: 10.1016/j.msard.2021.103385. [DOI] [PubMed] [Google Scholar]
- 12.Mékiès C, Heinzlef O, Jenny B, Ramelli AL, Clavelou P. Treatment satisfaction and quality of life in patients treated with fingolimod. Patient Prefer Adherence. 2018;12:899–907. doi: 10.2147/PPA.S144021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wray S, Jacques F, Miller TA, et al. Satisfaction with alemtuzumab in relapsing multiple sclerosis patients: results from the real-world PRO-ACT study. Mult Scler J Exp Transl Clin. 2022;8:20552173221135888. doi: 10.1177/20552173221135888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manchon E, Laplaud D, Vukusic S, et al. Efficacy, safety and patient reported outcomes in patients with active relapsing multiple sclerosis treated with ocrelizumab: final results from the PRO-MSACTIVE study. Mult Scler Relat Disord. 2022;68:104109. doi: 10.1016/j.msard.2022.104109. [DOI] [PubMed] [Google Scholar]
- 15.Räuber S, Pawlitzki M, Korsen M, et al. A national, multi-center study in Germany to assess implementation of infusion management, treatment satisfaction and quality of life in MS patients receiving alemtuzumab. Mult Scler Relat Disord. 2022;59:103670. doi: 10.1016/j.msard.2022.103670. [DOI] [PubMed] [Google Scholar]
- 16.Hanson KA, Agashivala N, Stringer SM, Balantac Z, Brandes DW. A cross-sectional survey of patient satisfaction and subjective experiences of treatment with fingolimod. Patient Prefer Adherence. 2013;7:309–318. doi: 10.2147/PPA.S41992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl 1):S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 18.Glanz BI, Musallam A, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Treatment satisfaction in multiple sclerosis. Int J MS Care. 2014;16:68–75. doi: 10.7224/1537-2073.2013-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kołtuniuk A, Chojdak-Łukasiewicz J. Adherence to therapy in patients with multiple sclerosis-review. Int J Environ Res Public Health. 2022;19:2203. doi: 10.3390/ijerph19042203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washington F, Langdon D. Factors affecting adherence to disease-modifying therapies in multiple sclerosis: systematic review. J Neurol. 2022;269:1861–1872. doi: 10.1007/s00415-021-10850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29:157–167. doi: 10.1016/j.msard.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986. doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook S, Comi C, Giovannoni G, et al. Rates of lymphopenia in years 1–4 in patients with relapsing multiple sclerosis treated annually with cladribine tablets. Abstract 039 at the Australian & New Zealand Association of Neurologists (ANZAN) 2018 meeting, May 29th–June 1st, Darwin, Australia. J Neurol Neurosurg Psychiatr 2018;89Abstract 039.
- 24.Oreja-Guevara C, Brownlee W, Celius EG, et al. Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: systematic literature review of real-world evidence. Mult Scler Relat Disord. 2023;69:104459. doi: 10.1016/j.msard.2022.104459. [DOI] [PubMed] [Google Scholar]
- 25.Nasir M, Rabvukwa P, Fuller S, Chaudhuri A, Mattoscio M. Real world data evaluating efficacy and safety of oral cladribine for multiple sclerosis. J Neurol Neurosurg Psychiatr. 2022;93:e2. doi: 10.1136/jnnp-2022-abn2.169. [DOI] [Google Scholar]
- 26.Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler. 2022;28:257–268. doi: 10.1177/13524585211012227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Multiple Sclerosis International Federation. Atlas of MS 3rd edition (2020). https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf. Accessed Feb 2023.
- 28.Multiple Sclerosis Trust. Expanded Disability Status Scale (EDSS). https://www.mstrust.org.uk/a-z/expanded-disability-status-scale-edss. Accessed Feb 2023.
- 29.Alroughani R, Inshasi J, Al-Asmi A, et al. Expert consensus from the Arabian Gulf on selecting disease-modifying treatment for people with multiple sclerosis according to disease activity. Postgrad Med. 2020;132:368–376. doi: 10.1080/00325481.2020.1734394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. No data repository is available beyond the online supplementary data supplied with this article.