Abstract
Light is one of the most important factors for photosynthetic organisms to grow. Historically, the amount of light in plant sciences has been referred to as light intensity, irradiance, photosynthetic active radiation, photon flux, photon flux density, etc. On occasion, all these terms are used interchangeably, yet they refer to different physical units and each metric offers distinct information. Even for experts in the fields of plant photobiology, the use of these terms is confusing, and there is a loose implementation of each concept. This makes the use of radiometric units even more confusing to non-experts when looking for ways to measure light, since they could easily feel overwhelmed by the specialized literature. The use of scientific concepts must be accurate, as ambiguity in the use of radiometric quantities can lead to inconsistencies in analysis, thus decreasing the comparability between experiments and to the formulation of incorrect experimental designs. In this review, we provide a simple yet comprehensive view of the use of radiometric quantities in an effort to clarify their meaning and applications. To facilitate understanding, we adopt a minimum amount of mathematical expressions and provide a historical summary of the use of radiometry (with emphasis on plant sciences), examples of uses, and a review of the available instrumentation for radiometric measurements.
Keywords: Photon flux density, Photosynthetically active radiation, Irradiance, Light meter, Spectroradiometer
Introduction
Since photoautotrophic organisms are frequently optimizing light use, either for photosynthetic reactions or to control their development, light is one of the most important environmental factors (Blonquist and Bugbee 2020). Small numbers of photons can lead to suboptimum growth (Hurd 1968), while excessive photons can lead to the inactivation of photosynthesis (photoinhibition) (Vass 2012; Tyystjärvi 2013; Zavafer et al. 2019). A recent meta-analysis revealed that several traits, ranging from molecules to a whole plant, respond to light dose (Poorter et al. 2019).
Quantitative aspects of light in a given environment are frequently referred to as light intensity in plant sciences. This name, however, is broad and unspecific, since light could be measured in several units, such as power (W), watts per steradian (W sr−1), energy (J), and the number of photons. The concept of intensity has several possible formal characterizations depending on the field of application (Paschotta 2008). Nevertheless, the terms that refer to the amount of light are often confusing to researchers not directly involved in the field of radiometry.
Researchers working in plant sciences often use micro-moles of photons per square meter per second (μmol m−2 s−1) as units, and they call this quantity light intensity, irradiance, photosynthetically active radiation (PAR), or photon flux density (PFD, Fig. 1A) (Blonquist and Bugbee 2020). These terms are not the same, since they refer to different metrics (quantities). A haphazard adoption of radiometric units readily leads to measurement errors, inconsistencies in analysis, and experimental comparability issues.
Fig. 1.
Basics in the terminology of light intensity. A Comparison of the most common terms that refer to the quantitative aspects of light in relation to photosynthesis. A search was performed on the 17th of January 2020 that covers all years in the database published in research articles and reviews (English only) using Scopus: the number alongside each label is the total number of publications. All terms were explored using the following searches: [“Irradiance” AND “photosynthesis”], [“Light Intensity” AND “photosynthesis”], [“Photon Flux Density” OR “PFD” AND “photosynthesis”], [“Photosynthetic Photon Flux Density” OR “PPFD” AND “photosynthesis”], and [[“Photosynthetically Active Radiation” AND “Photosynthetic Active Radiation”] OR “PAR” AND “photosynthesis”]. B The diagram that describes the concept of light intensity using a point source as an example. If the light intensity is measured over the integrated area of the sphere, it is called optical intensity. If the radiant power is measured only in a small area of the sphere determined by the solid angle (Ω), it is called radiant intensity. If the light power is measured in an integrated area, it is called irradiance. R represents the sphere. Figure 1B was modeled after Andy Anderson (Amherst College, Amherst MA, USA)
Just as radiometric terminology is laxly used in biology, most plant scientists are unaware there is a cohesive, consensual theoretical framework in radiometry that rigorously defines each quantity. This consensual use of quantities is usually recorded in industry standards such as the ISOs (Standards of the International Organization for Standardization) or the definitions of the IUPAC (International Union of Pure and Applied Chemistry) (IUPAC 2019; ISO 1992, 1990). While it is true that terminology in radiometry has been evolving, there is a well-defined usage of each term, and this brief review aims to illustrate the convention in an effort to prevent incorrect use of the terminology. Even though the importance of terminology has been repeatedly stressed by plant scientists (e.g., Bell and Rose 1981), the ambiguous use of radiometric terms is quite common in plant and algal sciences (here on referred to as plant sciences). To reach a commonly shared consensus among plant scientists, it is necessary to address this issue periodically and to discuss the progress in the implementation of new metrics. For this reason, we will examine the four most used quantities and terms, light intensity, irradiance, PAR, and PFD, as well as other derivatives (Table 1), revisit their mathematical formulations and the type of instruments that allow measuring them, and discuss the appropriate use of biological meaningful units for plant research.
Table 1.
Most important radiometric quantities and derivatives for plant biology
| Quantity | Abbr | Sym | Unit | Concept | Uses | Ref. |
|---|---|---|---|---|---|---|
| Irradiance | W m−2 | Amount of light power transferred to a given area in a collection angle of 180° | Meteorology, controlled environments, light source efficiency | IUPAC (2019) | ||
| Photon flux density | PFD | μmol m−2 s−1 | Incident photons flux per unit of area in a collection angle of 180° | Amount of light received at a given surface, such as a leaf | ISO (2009, 1998); IUPAC (2019) | |
| Photon fluence rate | PFR | μmol m−2 s−1 | Incident photons flux per unit of area in a collection angle of 360° | Algal cultures, solutions and water applications | IUPAC (2019) | |
| Photosynthe-tic photon flux density | PPFD | - | μmol m−2 s−1 | PFD in the range between 400 and 700 nm | Photosynthesis research | McCree (1981); Ryther (1956); McCree (1973, 1972, 1971) |
| Absorbed photosynthetically active radiation by vegetation | APAR | - | µmol m−1 s−1 | The product of incident PAR and vegetative light interception | Gas exchange models, remote sensing, biophysics | Hilker et al. (2008) Field et al. (1995) |
| Photosynthe-tically active radiation absorbed by PS II | PARII | - | s−1 PSII−1 | The number of photons absorbed by Photosystem II | Gas exchange models and photosynthesis research | Schreiber and Klughammer (2013); Schreiber et al. (2012) |
| Daily light integral | DLI | - | mol m−2 d1 | Value calculated by integrating PPFD over a day and the unit | Horticulture | Faust and Logan (2018) |
| Phytochrome photostatio-nary state | - | - | - | A ratio of active form phytochrome to total phytochrome | Ecology, physiology, horticulture | Sager et al. (1988); Morgan and Smith (1979) |
| Morphogene-tically active radiation | MAR | - | W m−2 or μmol m−2 s−1 | Amount of PFD absorbed by a given photoreceptor | Horticulture, developmental biology, epigenetics | Kittas et al. (1999) Butler et al. (1964); Shinomura et al. (1996); Christie et al. (1999) |
| Ultra-violet radiation | UVR | - | μmol m−2 s−1 | Amount of PFD of ultraviolet light | Ecology, remote sensing, and photobiology | Strid et al. (1994a); Cockell and Knowland (1999); Rizzini et al. (2011); Wu et al. (2012) |
Quantity refers to the name; Abbr., abbreviation; Sym., symbol; Concept, a simplified definition; Uses, major research fields that use the quantity and derivatives; and Ref., reference
Photometric and radiometric quantities
Before discussing the use of light intensity, we must define two sub-disciplines of spectroscopy that focus on the quantification of light, radiometry, and photometry. Often non-specialists confuse radiometry with photometry, which can be defined in analogy to radiometry but focuses on the brightness perceived by the human eye instead of the radiometric power (McCluney 2014). In plant sciences, photometric units are effective only in a few cases—e.g., studies on the psychological effects of ornamental plants or post-harvest visual quality of products. For the field of photobiology of photosynthetic organisms, only radiometric quantities are relevant. Nevertheless, a brief explanation will be given about photometric quantities, since there is often confusion on whether these quantities can be used in plant sciences.
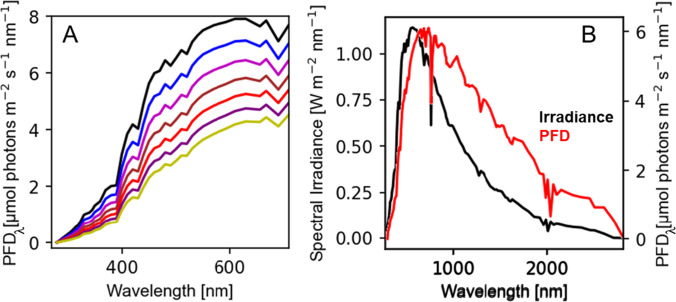
Three photometric quantities occasionally found in plant sciences are luminous power (measured in lumens, lm), luminous intensity (measured in candelas, cd = lm sr), and illuminance (measured in lux, lx = lm s−1). All these units are based on the lumen, which is meant to evaluate light in terms of the sensitivity of human vision (McCluney 2014). Photometric quantities are not usefully applied to plant photobiology, then, since the absorption occurs through photosynthetic pigment complexes (Schreiber and Klughammer 2013), which have an absorption spectrum that differs from the spectral sensitivity of the human eye. For these reasons, researchers working with phototrophic organisms are discouraged from using luxmeters—light meters that measure photometric quantities. Inconveniently for plant scientists, luxmeters are far more common and usually have lower costs (due to the larger market size). There are several conversion factors for different light sources (sun, white LED, incandescent, etc.) (Singh et al. 2019; Ahn et al. 2017) that allow an approximate conversion from lux values to radiometric units. This conversion is reasonably accurate only if the spectrum of light closely matches the one used to calculate the conversion factor. This is troublesome for sunlight (Fig. 2A), for example, since its light spectrum is often changing due to atmospheric conditions (Lee and Hernandez-Andres 2005).
Fig. 2.
Comparison of ground-level sun spectra in different atmospheric conditions or radiometric quantities. A The effect of turbidity (concentration of aerosols) in the sun spectrum illustrates how atmospheric conditions affect the sunlight spectrum. Spectra simulated using SOLCORE (Alonso-Álvarez et al. 2018) and turbidity values from 0 (clear sky; top) to 0.35 (hazed; bottom) are shown. B Comparison of spectral irradiance and PFD of the sun at turbidity of 0.15. Both panels were simulated using the SPECTRL2 model under the following conditions: latitude: 52° 23′ 24″ N; longitude: 1° 33′ 36″; date and time 2011/6/21 at 12:16 PM; aerosol optical depth model: “rural,” pressure: 103,000.0 Pa; relative humidity: 30%; precipitable water: 0.00142 cm; ozone thickness: 34 mm
Concepts of energy- and photon-basis quantities important for plant sciences
The concepts of light-, optical-, and radiant-intensity
Even though we found that the concept of “light intensity” was the second most frequently used in plant sciences to describe the light environment (Fig. 1A), this term does not specify its quantity and unit. In this sense, instead of light intensity, we recommend using quantities with a broad consensus, such as irradiance and PFD. In this section, we briefly explain optical intensity, which may be referred to as light intensity and related concepts.
Formally, the only explicit SI unit of light is candela, which is used to quantify the luminous intensity and does not represent a radiometric quantity (see section “Photometric and radiometric quantities”). However, another relevant quantity in radiometry is energy (E), measured in J. For a given particle, such as a photon, E is proportional to its frequency (v, in Hz or s–1) and the Planck constant (h = 6.62607015 10−34 J s), as shown in Eq. 1:
| 1 |
Since in radiometry light is more commonly expressed in terms of wavelength (λ, in m) rather than frequency, we can rewrite Eq. 1 into:
| 2 |
where E is the energy of a photon with wavelength λ, and c is the speed of light in vacuum (2.99792458 108 m s−1). If photons are emitted across time, it is possible to define the net flux of energy emitted as optical power of a light source as follows:
| 3 |
where t is the time in seconds and, thus, the unit for optical power is J s−1 or W.
It is then possible to define a quantity called optical intensity (Paschotta 2008) of a light source (IOp) that can be defined as the amount of optical power transfer to a unit of area (A, in m2), as it is formally defined in Eq. 4:
| 4 |
In physics, optical intensity is what is usually referred to as light intensity and is expressed in watts per square meter (W m−2). The criterion to define the area unit is the plane perpendicular to the direction of the propagation of energy (Singer et al. 2005). While optical intensity considers a surface illuminated by a light source, an emitter-side quantity called radiant intensity (IR) is defined as the radiant flux transferred to a given interval of the solid angle (van Dijk et al. 2010), which is formally expressed as
| 5 |
where Φ is the radiant flux in W and Ω is the solid angle in steradians (sr). The radiant flux is the rate of electrometric flow of energy in the form of electromagnetic waves (radiant energy, in J). While optical intensity, a quantity commonly used in optics and photonics (Paschotta 2008), could be defined as any given unit of a solid angle, radiant intensities are narrowly defined by a given field of view (McCluney 2014).
The best way to define the solid angle is in analogy to an ordinary angle but in three dimensions (Eriksson 2018). In Fig. 1B, the edge of the yellow disk is projected to the center of a sphere. The projection intersects the sphere and forms a surface area A. The solid angle is the area A on the surface of the sphere divided by the total area of the sphere multiplied by 4π. Ω is measured in steradians (sr), which is the ratio between two area units (1 sr = 1 m2 m−2). A steradian is in fact dimensionless, since it corresponds to one unit of area on the sphere surrounding the point source (Jaffey 1954). Therefore, the radiant intensity is expressed as W sr−1 and is the basis of all radiometric quantities.
Basic radiometric quantities
In plant sciences, the most frequently used radiometric quantity is irradiance (Fig. 1A). Irradiance, in W m–2, can be defined as the amount of energy of any wavelength received by a surface usually within a field of view of 2π (i.e., a hemisphere). Therefore, as a counterpart of radiant intensity (Eq. 5), irradiance (Ee) must be understood as the radiant flux received by a surface per unit area as follows:
| 6 |
Although photon-number-based quantities are less used than energy-based quantities (Fig. 1A), those are essential to describe the physiological responses of plants to the light environment. The photon-based equivalent of Ee (the received number of photons per unit area per second) at a wavelength of λ (in nm), Ep,λ, an incident from all upward directions upon the area of an object (e.g., detector) can be defined as follows:
| 7 |
where Lλ is the number of photons at a given wavelength per time interval passing through a unit of surface in a given direction from the source (also known as photon radiance) (IUPAC 2019). Concomitantly, Eq. 7 limits its scope to photons not scattered or reflected by the target or its surroundings. To convert units from irradiance and photon irradiance to photon flux density (PFD in mol m−2 s−1), it suffices to use the following geometrical conversion:
| 8 |
where NA is Avogadro’s number (NA). It is worth noting that Eq. 8 is constructed by multiplying Eq. 2 by the irradiance value at a given wavelength (which converts the irradiance from energy fluxes to the total number of photons) and dividing it by the NA.
Although irradiance usually refers to power units (with units W m−2) in plant sciences, it possibly refers to photon-number units as photon irradiance (). We do not discourage the use of photon irradiance. It is worth noticing that PFD is far more common in plant science works and is a better term to avoid misunderstanding. However, we discourage the use of irradiance and PFD as synonyms. Since both are conceptually distinct quantities (ISO 2009, 1998; IUPAC 2019), they will provide a different spectrum of the light source, as shown in Fig. 2B. Several authors have used the concepts of radiant intensity and irradiance interchangeably (Sundby et al. 1993; Evans and Poorter 2001b). While radiant intensity describes power per steradian (i.e., the quantity for the light source), irradiance refers to the power per unit of area (i.e., the quantity for the irradiated surface). Finally, it is worth mentioning that mol m−2 s−1 is used with the µ- prefix in plant sciences simply because plants and algae survive in the μmol photon range.
According to our research, the earliest mention of the concept of photon flux density (photons per unit of time and area) occurred as early as 1949 (Makowski 1949; Meyerott and Breit 1949) and the concept of photon flux, as early as 1916 (Levine 1916). The unit of PFD has suffered little change since its inception from mol cm−2 s−1 to mol m−2 s−1.
If the number of photons coming from all directions is of interest, one can measure light as photon fluence rate (PFR). Formally, PFR (Ep,o) is defined as the number of incident photons from all directions per unit of time on a small sphere divided by the cross-section area of the sphere. Mathematically, in analogy to Eq. 7, Ep,o is defined as
| 9 |
where subindex “o” denotes a spherical collection. In a similar fashion to PFD, if PFR is expressed in moles, then one estimates the “photon fluence rate, amount basis” (En,p,o).
It is worth noting that the solar constant is defined as an irradiance incident on a horizontal area at the top of the atmosphere and fluctuates around 1361 W m−2 (Kopp and Lean 2011). Since solar radiation attenuates through the atmosphere, land plants receive light at an irradiance of about 1,000 W m−2, depending on the solar angle, site elevation, and atmospheric conditions (Kopp and Lean 2011).
Radiometric units defined on a photobiological basis
Concerning the photosynthetic effect of photons, the irradiance of a certain waveband corresponding to that of photon absorption of photosynthetic pigments could be considered equal to the photosynthetically active radiation (PAR) (McCree 1981). Note that this term does not specify the unit because it literally refers to a concept and not a quantity. Nevertheless, PAR is frequently referred to as a quantity. In the literature, PAR is used as an acronym for either “photosynthetically active radiation” or “photosynthetic active radiation.” Only the first is grammatically correct, though, for only that syntagm splits into the core element “radiation” and the determiner “photosynthetically active,” while the second splits into the core element “active radiation” and the determiner “photosynthetic.”
We found that the concept of PAR had been used as early as the 1950s (Ryther 1956). Studies revealed that a more appropriate unit for PAR was the moles of photons per unit of area and time, not the sum of the total power contained in them (Blonquist and Bugbee 2020; McCree 1971, 1972). However, we discourage the use of PAR as a quantity and advocate instead for the use of photosynthetic PFD (PPFD) to prevent redundancy in terminology (discussion in the section “State of the field of radiometry and its relevance to photophysiology”).
PPFD (coined by Muel and Malpiece 1969) is the appropriate quantification of photons of a waveband that have a photosynthetic effect. In addition to the unit, McCree proposed that the photosynthetically active waveband is 400 to 700 nm (McCree 1973, 1972, 1971). This range was experimentally determined by investigating the wavelength dependency of the number of fixed CO2 per photon (i.e., action spectra) of a wide range of plants. PPFD can be defined as the integration of the PFD in the waveband of the absorption spectrum of photosynthetic pigments:
| 10 |
In analogy to PPFD, some authors have used photosynthetic PFR (PPFR) as an important metric for plants and algae. Rupert (1974) pointed out the relevance of PFR for photobiological problems, since light from all directions can significantly contribute to the net light dose and, consequently, require the use of a spherical detector (Jones 2013). PFR is an appropriate quantity to evaluate the light environments of objects irradiated from various directions, such as chloroplasts in a leaf or photosynthetic microorganisms in water. As PFR became of wider use during the mid-1980s in plant science works (Beer and Levy 1983; Morgan and Smith 1981; Raven and Beardall 1982), this rapidly evolved, and by 1985, the concept of PPFR was being used by several groups (Britz et al. 1985; Browse and Slack 1985).
Although a conservative demarcation of the photosynthetically active waveband has been delimited as 400 to 700 nm (McCree 1971, 1972, 1973), it should be noted that it is well known now that photosystem I and II may have significant activity in the far-red (Thapper et al. 2009; Pettai et al. 2005). In plant sciences, light in 700–750 nm (i.e., near-infrared light) is conventionally called far-red light. Although far-red photons contribute significantly to photosynthesis, they are considered to be photosynthetically inactive. Our search in the Scopus database revealed that PPFD is the least used concept in plant research (Fig. 1A) among the terms related to light intensity.
Other radiometric derivatives focus on specific aspects of photosynthesis, such as APAR, PARII, and DLI.
In remote sensing studies, absorbed PAR by vegetation (APAR) is considered the product of incident PAR and vegetative light interception, both available via satellite measurements (Hilker et al. 2008). By multiplying spatial distributions of APAR and a coefficient of photosynthetic light utilization (i.e., light use efficiency), we could estimate vegetative CO2 assimilation at the landscape and regional levels (e.g., Field et al. 1995). The light use efficiency is determined in situ or remotely sensed by monitoring optical signals such as chlorophyll fluorescence (Porcar-Castell et al. 2014). Note that researchers employing APAR use both energy and photon-based units for this quantity.
Photosynthetically active radiation absorbed by photosystem II (PARII) has been proposed as a means to estimate the absolute photosynthetic electron transport rates of PSII in controlled environments by using monochromatic light, such as LEDs. PARII refers to the total amount of photons used in the photosystem II turnover (s−1 per PSII) (Schreiber and Klughammer 2013; Schreiber et al. 2012; Szabo et al. 2014). In algal research, for instance, the absorption spectrum is very diverse across taxa. Since not every incident photon is absorbed and not every absorbed photon is used in photosynthetic electron transport, PARII allows normalizing light to the net function of PSII.
Daily light integral (DLI) is a widely used quantity, mainly in applicational horticultural studies. DLI is calculated by integrating PPFD over a day and its unit is mol m−2 d−1. While this parameter ignores diurnal light fluctuation, it represents the total amount of photons delivered to a given unit area. DLI is a helpful measure to analyze the biomass accumulation and yield of plants cultivated for a long term. A typical application of this measure is the assessment of suitable places for plant production through regional climatic normal values (e.g., Faust and Logan 2018).
There is also great interest in quantifying light that has photobiological effects on photosynthetic organisms beyond photosynthesis, such as morphogenetic effects and photodamage thresholds. A typical index is the phytochrome photostationary state (e.g., Sager et al. 1988). This index is calculated from the spectral photon flux density of incident light and absorption spectra of two interconvertible forms of phytochrome and reflects the ratio of active phytochrome to total phytochrome. The phytochrome photostationary index is correlated to various developmental characteristics of plants, including stem elongation rate, leaf-to-stem biomass allocation ratio, and total chlorophyll content (Morgan and Smith 1979).
Another metric called morphogenetically active radiation (MAR) is the net amount of PFD or irradiance within a certain waveband that can activate photoreceptors (Fig. 3A) such as phytochromes, cryptochromes, and phototropins involved in processes of morphogenesis and the circadian rhythm of plants. The range of MAR depends on the literature, extending from between 350–400 to 500 nm for cryptochromes (Kittas et al. 1999), 500 to 800 nm for the phytochrome (Butler et al. 1964; Shinomura et al. 1996), and 350 to 500 nm for phototropins (Christie et al. 1999). Kittas et al. (1999) indicate that MAR originated from the work of Varlet-Grancher et al. (1993), but we could not have access to this report. The first indexed report we found that explicitly refers to MAR, however, is the work of Combes et al. (2000). Although MAR has been seldom used in plant sciences, it can become a suitable metric to determine the light dose for activating specific photoreceptors and separate responses mediated by photoreceptors and PPFD.
Fig. 3.

Spectral responses of different photoreceptors involved in morphogenetic effects. Absorption spectra of different photoreceptor proteins: UVR8 (purple), cryptochrome (blue), phototropins and zeitluples (green), phytochrome in red form (Pr in red), and far-red form (Pfr in black). All spectra formalized to their highest peak. Bars above the spectra indicate reported wavebands of morphogenetically active radiation for the respective photoreceptors. Spectra re-digitized from (Galvão and Fankhauser 2015)
The effects of UV light have been measured under the umbrella of Ultra-Violet Radiation (UVR). This metric is calculated by integrating photon flux density within UV-A (315–400 nm), UV-B (280–315 nm), UV-C (100–280 nm), or some of them. This radiometric quantity is useful for the fields of photoinhibition (Hideg and Vass 1996), DNA-photodamage (Strid et al. 1994a), and photoprotection (Cockell and Knowland 1999). Some studies have used UVR as a quantitative measure of morphogenesis mediated by photoreceptors sensitive to UV (Rizzini et al. 2011; Wu et al. 2012), similar to MAR.
Types of light meters
A light meter can be defined as an instrument to measure a quantitative aspect of light. Since there are several types of sensors used for this purpose in plant sciences, we here review the most modern light meters with two types of detector elements: thermopiles and silicon photodiodes. Other light sensors, however, such as bolometers, phototransistors, and photoresistors, could be implemented for particular purposes. Based on their detector elements, all light meters could be integrated into two types: actinometers and spectroradiometers. These instruments offer different advantages and are ideal for specific uses, which mostly depend on their spectral response. It seems convenient to state here that we will not recommend or describe specific equipment of a particular brand, since we intend to provide the reader with enough knowledge to make an informed decision on which light measuring system to buy. The spectral response (also named sensitivity) is the parameter that determines how active a detector element is at a given wavelength (Smith 2008).
Actinometer-based instruments
Dating from 1825, actinometers are the oldest type of light meters (Dulin and Mill 1982; Kidwell 1981). Pyranometers are the most common type of actinometers (ISO 2018) and have been widely used in the field of plant sciences since as early as the 1930s (Shirley 1931). These instruments were designed to measure irradiance (W m−2), and their general structure consists of a detector element enclosed in a case and protected by a hemispherical dome (Fig. 4A). The hemispherical dome allows a 2π field of view, protects the sensor from the environment, and acts as a filter that only allows light from 300 to 2800 nm (ISO 1993). Pyranometers, based on thermopiles (a set of thermocouples connected in series), have a broad detection range with a mostly homogenous spectral response (Fig. 4B) (van Herwaarden et al. 1990). The spectral response of the thermopiles is determined by the black light-absorbing coating on the top of the thermopile (Hou et al. 2020), which permits a strong, uniform absorption over a broad wavelength band (ISO 1993). As their name suggests, they measure the temperature difference between sectors that are exposed to light and sectors that are not. The temperature difference generates an electromotive force, which is converted into power and then transformed into irradiance (ISO 1993). Because it takes a certain time to reach thermal equilibrium (typically < 0.1 s), thermopiles are not suitable for tracking rapid light fluctuation in micro- to milli-seconds (e.g., single turnover flashes to measure photosystem I or II activity). These instruments are employed when one is interested in the shortwave radiation of the sun and not just the photosynthetically active waveband, so they are mostly used in meteorological observation and direct solar energy applications (Reda 2011).
Fig. 4.
Types of light meters and detector elements. A Diagram of a thermopile-based pyranometer. The instrument is composed of two glass domes, a thermopile detector, a desiccant to protect the detector element and the electronics, and a solar shield to protect the electronics against UV. B Comparison of the spectral response of commonly used detector elements: a thermopile (TD2X, Thorlabs Inc.), silicon diode A (FDS100, Thorlabs Inc.), and silicon diode B (FD11A long-range, Thorlabs Inc.). Note that not all silicon diodes have the same spectral response and thermopiles have a steadily even response. C Comparison of the spectral response of commercial light meters. Three examples were chosen to compare contrasting sensors: LI-190R (LI-COR Inc.), SQ-110 (Apogee Instruments Inc.), SKP215 (Skye Instruments Ltd.), plus an ideal PAR detector (LI-COR-Biosciences 2018). If the response is above the ideal curve, it overestimates photons on those wavelengths and, if the contrary, it underestimates them. D Diagram of the basic components and function of a spectroradiometer. Light is captured at the entry port and diffused through a cosine corrector. Light crosses a slit to then be projected into a diffraction grating to obtain monochromatic light. The slit size determines the spectral resolution, the narrower the slot, the higher the resolution. Light is then corrected and projected onto a diode array to assess the PFD at each wavelength. Note that this comparison does not intend to favor one brand over another but just to illustrate the spectral response differences. Readers are encouraged to consult two technical reports of different companies: LI-COR Biosciences (2018) and Blonquist & Johns (2019)
Some newer models of the pyranometers employ silicon photodiodes as a detector element that spots narrower portions of the light spectrum (ISO 2018). Still, most photodiodes often show uneven spectral responses (Fig. 4B) (Paschotta 2008). As a consequence, in most cases, these instruments are calibrated by the manufacturer to correct this issue (ISO 2018). Their anatomy is somewhat similar to the other thermopile-based pyranometers. They are equipped with a cosine corrector diffuser (see section “State of the field of radiometry and its relevance to photophysiology”) instead of a hemispherical dome. They work on the principle of the photoelectric effect that occurs when photons are absorbed by a semiconductor, which generates a small current (McKagan et al. 2009)—this current is amplified through a transimpedance amplifier circuit to generate a voltage that is captured by a data acquisition device (Bates et al. 2019). This type of pyranometers is the basis of quantum meters (ISO 2018).
As their name suggests, quantum meters measure the number of photons in various regions of the light spectrum, UV, visible, and near-infrared (IEC 2019). Quantum meters designed to respond only to the photosynthetically active waveband for PPFD measurements (examples in Fig. 4C) are sometimes referred to as PAR meters. These meters are useful for most light sources, but they provide no information on the quantity of UV and far-red photons (LI-COR-Biosciences 2018). For this reason, quantum meters are often used to measure light with a specific waveband (IEC 2019). There are, however, broad-range quantum sensors equipped with adaptors for optics that allow installing filters to customize the detection range.
Some commercial quantum sensors are interchangeable with others in the brand of instruments if they allow to change the compensation point (voltage difference) of each sensor (LI-COR-Biosciences 2018). This useful feature allows upgrading a given light quantum sensor kit to be used in other procedures. Another important aspect to mention on the quantum meters is that some manufacturers have recently improved uniformity in the spectral response to obtain accurate estimates (Fig. 4C). Older instruments usually underestimate either blue, red, or both wavebands (Blonquist and Johns 2019; LI-COR-Biosciences 2018). This is a critical aspect since some of the measurements done in earlier years could have underestimated the actual PFD values. This is particularly important to the fields interested in photodamage, photoprotection, and the action spectrum of photosynthesis.
There are other types of actinometers, such as pyrheliometers and net radiometers: both are similar to the thermopile-based pyranometers, but the former measures the direct solar irradiance by excluding an effect of scattered light and the latter measures the incident (downward) and reflected (upward) irradiance (ISO 1990).
Spectroradiometer-based instruments
More recently, spectroradiometers have earned popularity in plant sciences. As their name suggests, these instruments allow the spectral characterization of light sources to resolve the irradiance or PFD per unit of wavelength (ISO 2017). Spectroradiometers are simply modified spectrophotometers (Fig. 4D), the light input port of which is equipped with a cosine corrector (Zong et al. 2006). The light is shined into a dispersive element, most of the time a diffraction grating that diffracts it into beams of monochromatic light (Wuttke et al. 2006). These beams of monochromatic light are then captured by a linear diode array (a detector element similar to a digital camera), which records the irradiance or PFD values per unit of wavelength (Zong et al. 2006). Since the area under the light spectrum of a light source is the irradiance or PFD, spectroradiometers help measure the PFD of monochromatic light, such as that of LEDs, for which the method based on some older models of light meters cannot offer reliable estimates. Note that the accuracy of the estimates depends on the spectral resolution of the spectrometer because high-resolution devices are required to detect sharp peaks of some artificial light sources (e.g., high-pressure sodium lamps and fluorescent lamps). When working with monochromatic light sources, MAR, UVR, or specific spectral ranges, a better option is the use of spectroradiometers. In recent years, the cost of spectroradiometers has decreased significantly. The retail price for some spectroradiometers could be as low as 500–1000 USD—based on a web search in March 2022. While for most “white light” applications a recent quantum meter is enough for researchers, it has been reported that photomorphogenesis and biomass accumulation of plants cultivated under three “white” LED lights are distinct (Cope and Bugbee 2013). It is recommended to measure the light spectrum to ensure research reproducibility, particularly in experiments with artificial light sources and/or light that transmits certain substrates such as leaf layer, culture medium, and covering materials.
2π and 4π cosine correctors, and incident and total irradiance
An important component of light meters is the cosine corrector. Virtually all light meters used for plant research have a dome-like diffusor at the top of the input port, properly called the Lambertian cosine corrector (Fig. 5A) (Bendig et al. 2018). This optical component is installed in order to diffuse incoming light before it reaches the detector by utilizing Lambert’s cosine law—generally referred to in optics just as cosine law (Bendig et al. 2018). According to this law, the radiant intensity from an object radiating light diffusely is directly proportional to the cosine of the angle between the incident light and a surface that is perpendicular to the source (Fig. 5B and Eq. 11) (Worthing 1912; Celestini and Mortessagne 2008), so
| 11 |
where Iθ is the light quantity (either in PFD, PFR, Irradiance, etc.) at a given incident angle (θ) and is the intensity at zero degrees.
Fig. 5.
Quantum meters and cosine correctors. A Quantum meter anatomy. B Lambert’s cosine law, light collection, and function of the cosine corrector. C Light captured by a quantum meter equipped with either a 2π (hemispherical) cosine corrector or a 4π (spherical) cosine corrector
Because in light meters the light diffuser follows the cosine law, the amount of light reaching the corrector is proportional to the cosine of the light beam incident angle. For example, at θ = 60°, the radiant intensity is only half of what it would be if measured at θ = 0°.
Most instruments have a 2π cosine corrector, and they consequently allow a hemispherical field of view, so they act as PFD meters (see Fig. 5C). 4π cosine correctors allow measuring the number of photons from all directions, which is the sum of the incident plus the reflected light and, as such, they are PFR meters (Fig. 5C). This second type is not as commonly used but it has clear advantages, since reflected light can significantly contribute to the light environment of plants. One example of the use of 4π correctors is inside incubators: if the measurement were made with a 4π quantum sensor, we would observe higher moles of photons per square meter per second than with a 2π quantum sensor, which is consistent with Chenu et al. (2008). This gap arises mainly from the fact that the reflection of incubator walls can lead to inconsistency between laboratory and field data. Another example is the analysis of the light profile in the plant canopy, since the upward light reflected from lower leaf layers contributes to the light environment (e.g., Suits 1971). Furthermore, advances in 3D canopy modeling and ray-tracing technique allow us to analyze directional light profiles on leaf surfaces (Vos et al. 2010), which suggests the necessity of utilizing 4π quantum sensors.
Examples of applications of quantities
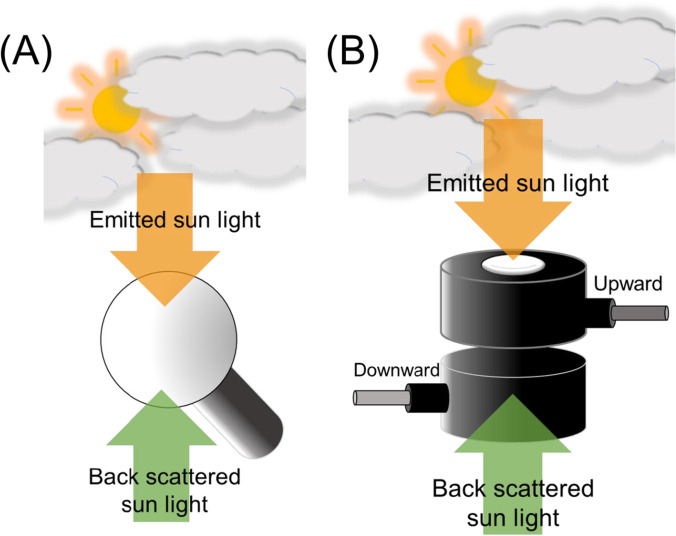
To exemplify the uses of the reviewed parameters, we will discuss some common research scenarios. The first one must determine whether a 2π or a 4π measurement is appropriate. For example, if the light is provided from the top of a leaf enclosed in a chamber, such as measurements with a chlorophyll fluorometer, oxygen electrode, or gas exchange system, a 2π based metric is sufficient. However, for photosynthesis in leaves or cells in solution (such as algal culture, photobioreactor, waterbody), the absorbed PAR does not originate only from the upper hemisphere, but in light from all directions. For example, light scattering and reflection from the ground, soil, or growth incubator can contribute significantly to the net light absorbed by the photosynthetic pigments. Thus, in the case of plants in the field or an algal culture, a combined approach using 2π and 4π sensors provides a complete figure of the light environment. If both types of sensors are employed, the difference between the 4π and 2π measurements provides an estimation of the backscattered light that a photosynthetic sample receives. As 4π sensors are not very common in laboratories, an approximation to a 4π measurement can be done by summing the magnitude of a 2π sensor facing the light source and one placed against it (Fig. 6).
Fig. 6.
Application example of how to assess the PFR using either 2π or 4π sensors in the field. In orange, the emitted sunlight is displayed as an arrow traveling at a quasi-right angle towards the sensors. In green, the backscattered (scattered and reflected light) reaches the sensors
It is recommended to avoid the comparison of experiments done with 2π and 4π sensors, as they are measured in a different manner—numerically, 4π yielding has a higher magnitude. For example, it would be incorrect to compare the light response curve of photosynthesis obtained using the PFD (2π measurement) and the PFR (4π measurement).
Based on units, values on a molar-basis (either PFD or PFR) are more useful for studies in which dose–response is measured, as in photosynthesis research, because the rates of oxygen evolution (Gilbert et al. 2000; Zhang et al. 2018), electron transport (Flexas et al. 1999; Szabó et al. 2017), and CO2 intake (Evans and Poorter 2001a; Moualeu-Ngangue et al. 2017, Farquhar et al. 1980) are all calculated based on the number of photons. The term PFD could be used to quantify other wavebands outside the PAR, such as UV, far-red, and the NIR, since photons in these wavebands have important physiological effects on photoinhibition (Zavafer et al. 2015; Oguchi et al. 2021; Strid et al. 1994b) and morphogenesis via responses of photoreceptors (e.g., Briggs and Olney 2001). Thus, quantities based on moles of photons are handy to determine the action spectra of diverse reactions and the quantum yield of several photochemical events.
Energy-based measurements (in J) are more suitable for light flashes of monochromatic light, such as single turnover flashes to measure photosystem I or II activity. Power-based measurements (in W) are ideal to assess the efficiency of energy conversion of light sources and fixtures (such as LEDs in growth chambers), as electric power consumption (W · h) turned into irradiance (in W m−2) and for works interested in the energy balance of plants (e.g., transpiration and thermal analysis).
Spectral-based measurements, either in molar-, energy-, or power-basis, are particularly useful when dealing with monochromatic light sources (such as LEDs) or when one wants to compare the photosynthetically utilized, morphogenically active, or photodamaging radiation using different types of light sources. For example, it is known that, at equal values of PFD (on a molar basis), photosystem II photodamage is two to ten times stronger in the blue than in the red region of the visible spectrum.
State of the field of radiometry and its relevance to photophysiology
In this review, we have discussed the main radiometric quantities and measurements used in the field of plant sciences. In addition, we have tracked down part of the history behind the evolution and integration of radiometric quantities in plant sciences. Radiometry offers powerful tools for the planning of adequate experiments for the assessment of environmental conditions, plant growth, and photophysiology. A major difficulty for new users of radiometric tools is that the terminology of radiometry is not used consistently and often the designation does not match the formal definition (the mathematical expression to measure it). Terms such as light intensity, irradiance, and PAR have been used interchangeably and incorrectly, and creating awareness of the correct formal definition has been the main motivation of this article. In our opinion, the loose use of scientific terminology will logically lead to imprecise science.
Every quantity mentioned in this work has a specific definition and this offers advantages, so we advocate for the proper use of terms. We think that, while terms like PAR, PPFR, and PPFD are helpful concepts in referring to the photons with an actinic effect of photosynthesis, they are also misleading for certain experimental designs. For example, whereas photosynthetic light response curves can use PPFD as the x-axis variable, far-red PFD is not controlled in most experiments. It has been repeatedly reported that far-red photons drive photosynthetic electron transport and the sensitivity to far-red photons is dependent on the growth environments (e.g., Chow et al. 1990; Murakami et al. 2016). Therefore, when PPFD just refers to values between 400 and 700 nm, it must be used exclusively in experiments that focus on this waveband. If one is interested in studying the effects of photosynthetically “inactive” light beyond 400–700 nm, such as far-red and UV, the use of the simpler PFD is a more helpful quantity, as well as less restrictive since it can be applied to far-red and UV. It is also strongly recommended to evaluate and report the spectral distribution of light by using a spectroradiometer.
There is a notion that there is no consensus on the use of radiometry terminology and that some radiometric quantities could be used interchangeably, which is not entirely true for most radiometric quantities. Based on a thorough review of the literature, we believe the inconsistencies lay in the fact that several terms have been used loosely in some circles of biological research—especially between 1960 and 1985. This gave rise to an “anything that means light intensity” phenomenon, in which the quantity measured in μmol m−2 s−1 could be called many different names. A typical example is the term PAR, a Janus-faced parameter having a different unit W m−2 or µmol m−2 s−1 depending on the context. Although researchers focusing on large-scale sciences (e.g., ecosystem modeling and agricultural meteorology) tend to use it with a unit of W m−2 and those focusing on small-scale sciences (e.g., biology, physiology, and horticulture) tend to use μmol m−2 s−1, this segregation seems ambiguous. Despite this term having been conventionally used for over 50 years, in our opinion, there is no legitimate reason to use PAR beyond its conceptual meaning. This is because PAR depends on the organism studied, light spectra used, and specific activity assessed. It creates unnecessary confusion concerning other metrics such as PPFD or PPFR and is not in agreement with scientific consensuses such as the IUPAC, the SI conventions, and all international standardizations. This makes it difficult to translate what we generate in our fields of plant science research into other disciplines such as physics, chemistry, or engineering. We should use PFD or irradiance instead of PAR or light intensity to discuss the effects of quantitative aspects of light and should define the photosynthetically active waveband explicitly in each report if necessary.
Acknowledgements
All figures used in this work were made by the authors using PowerPoint, SketchUp 2019, and Python 3.
Alonso Zavafer is a pseudonym of Alonso Zavaleta Fernández de Córdova.
The authors would like to thank Eric Prince and Kristin Feese (LI-COR Biosciences) for providing information about the response of the detectors.
AZ would like to thank Prof. Wah Soon Chow for the insightful discussions on the definition of “photon irradiance.”
Author contribution
AZ: investigation, software, conceptualization, methodology, validation, visualization, data curation, writing—original draft, formal analysis, writing—review and editing, resources, administration. CM: conceptualization, methodology, validation, writing—original draft, visualization, writing—review and editing, supervision, resources. GJ: validation, formal analysis, writing—review and editing, supervision. KM: investigation, conceptualization, validation, writing—original draft, writing—review and editing, supervision, resources.
Data availability
Data are available under reasonable request. Please contact AZ through alonso.zavaleta@anu.edu.au.
Code availability
No code is available in this work.
Declarations
Ethics approval
No ethical concerns are involved in this article.
Consent to participate
All authors participate willingly in the writing of this work.
Consent for publication
The authors agree to publish this work.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn YD, Bae S, Kang SJ. Power controllable LED system with increased energy efficiency using multi-sensors for plant cultivation. Energies. 2017;10(10):1607. doi: 10.3390/en10101607. [DOI] [Google Scholar]
- Alonso-Álvarez D, Wilson T, Pearce P, Führer M, Farrell D, Ekins-Daukes N. Solcore: a multi-scale, Python-based library for modelling solar cells and semiconductor materials. J Comput Electron. 2018;17(3):1099–1123. doi: 10.1007/s10825-018-1171-3. [DOI] [Google Scholar]
- Bates H, Zavafer A, Szabo M, Ralph PJ. A guide to Open-JIP, a low-cost open-source chlorophyll fluorometer. Photosynth Res. 2019;142(3):361–368. doi: 10.1007/s11120-019-00673-2. [DOI] [PubMed] [Google Scholar]
- Beer S, Levy I. Effects of photon fluence rate and light spectrum composition on growth, photosynthesis and pigment relations in Gracilaria Sp. J Phycol. 1983;19(4):516–522. doi: 10.1111/j.0022-3646.1983.00516.x. [DOI] [Google Scholar]
- Bell CJ, Rose DA. Light measurement and the terminology of flow. Plant Cell Environ. 1981;4(2):89–96. doi: 10.1111/j.1365-3040.1981.tb01043.x. [DOI] [Google Scholar]
- Bendig J, Gautam D, Malenovský Z, Lucieer A (2018) Influence of cosine corrector and UAS platform dynamics on airborne spectral irradiance measurements. In: IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium. Valencia 8822–8825. 10.1109/IGARSS.2018.8518864
- Blonquist J, Bugbee B (2020) Solar, net, and photosynthetic radiation. In: Hatfield JL, Sivakumar MVK and Prueger JH (eds) Agroclimatology. 10.2134/agronmonogr60.2016.0001
- Blonquist JM, Johns J (2019) Accurate PAR measurement: comparison of eight quantum sensor models. Technical Report. Apogee Instruments, Inc., Utah.
- Briggs WR, Olney MA. Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 2001;125(1):85–88. doi: 10.1104/pp.125.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz SJ, Hungerford WE, Lee DR. Photosynthate partitioning into digitaria-decumbens leaf starch varies rhythmically with respect to the duration of prior incubation in continuous dim light. Photochem Photobiol. 1985;42(6):741–744. doi: 10.1111/j.1751-1097.1985.tb01641.x. [DOI] [Google Scholar]
- Browse J, Slack CR. Fatty-acid synthesis in plastids from maturing safflower and linseed cotyledons. Planta. 1985;166(1):74–80. doi: 10.1007/BF00397388. [DOI] [PubMed] [Google Scholar]
- Butler WL, Hendricks SB, Siegelman HW. Actton spectra of phytochrome in vitro. Photochem Photobiol. 1964;3(4):521–528. doi: 10.1111/j.1751-1097.1964.tb08171.x. [DOI] [Google Scholar]
- Celestini F, Mortessagne F. Cosine law at the atomic scale: toward realistic simulations of Knudsen diffusion. Phys Rev E. 2008;77(2 Pt 1):021202. doi: 10.1103/PhysRevE.77.021202. [DOI] [PubMed] [Google Scholar]
- Chenu K, Rey H, Dauzat J, Lydie G, Lecoeur J. Estimation of light interception in research environments: a joint approach using directional light sensors and 3D virtual plants applied to sunflower (Helianthus annuus) and Arabidopsis thaliana in natural and artificial conditions. Funct Plant Biol. 2008;35(10):850–866. doi: 10.1071/FP08057. [DOI] [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci U S A. 1990;87(19):7502–7506. doi: 10.1073/pnas.87.19.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA. 1999;96(15):8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell CS, Knowland J. Ultraviolet radiation screening compounds. Biol Rev Camb Philos Soc. 1999;74(3):311–345. doi: 10.1017/s0006323199005356. [DOI] [PubMed] [Google Scholar]
- Combes D, Sinoquet H, Varlet-Grancher C (2000) Preliminary measurement and simulation of the spatial distribution of the Morphogenetically Active Radiation (MAR) within an isolated tree canopy. Ann For Sci 57:497–511. 10.1051/forest:2000137
- Cope KR, Bugbee B. Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience. 2013;48(4):504–509. doi: 10.21273/Hortsci.48.4.504. [DOI] [Google Scholar]
- Dulin D, Mill T. Development and evaluation of sunlight actinometers. Environ Sci Technol. 1982;16(11):815–820. doi: 10.1021/es00105a017. [DOI] [PubMed] [Google Scholar]
- Eriksson F. On the measure of solid angles. Math Mag. 2018;63(3):184–187. doi: 10.1080/0025570x.1990.11977515. [DOI] [Google Scholar]
- Evans J, Poorter H. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell Environ. 2001;24(8):755–767. doi: 10.1046/j.1365-3040.2001.00724.x. [DOI] [Google Scholar]
- Evans JR, Poorter H. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001;24(8):755–767. doi: 10.1046/j.1365-3040.2001.00724.x. [DOI] [Google Scholar]
- Farquhar GD, von Caemmerer SV, Berry JA. A biochemical model of photosynthetic CO 2 assimilation in leaves of C 3 species. Planta. 1980;149(1):78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Faust JE, Logan J. Daily light integral: a research review and high-resolution maps of the United States. HortScience. 2018;53(9):1250–1257. doi: 10.21273/Hortsci13144-18. [DOI] [Google Scholar]
- Field CB, Randerson JT, Malmstrom CM. Global net primary production - combining ecology and remote-sensing. Remote Sens Environ. 1995;51(1):74–88. doi: 10.1016/0034-4257(94)00066-V. [DOI] [Google Scholar]
- Flexas J, Escalona JM, Medrano H. Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines. Plant Cell Environ. 1999;22(1):39–48. doi: 10.1046/j.1365-3040.1999.00371.x. [DOI] [Google Scholar]
- Galvão VC, Fankhauser C. Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol. 2015;34:46–53. doi: 10.1016/j.conb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Wilhelm C, Richter M. Bio-optical modelling of oxygen evolution using in vivo fluorescence: comparison of measured and calculated photosynthesis/irradiance (PI) curves in four representative phytoplankton species. J Plant Physiol. 2000;157(3):307–314. doi: 10.1016/S0176-1617(00)80052-8. [DOI] [Google Scholar]
- Gold V (2019) IUPAC. Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. 10.1351/goldbook
- Hideg E, Vass I. UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Sci. 1996;115(2):251–260. doi: 10.1016/0168-9452(96)04364-6. [DOI] [Google Scholar]
- Hilker T, Coops NC, Wulder MA, Black TA, Guy RD. The use of remote sensing in light use efficiency based models of gross primary production: a review of current status and future requirements. Sci Total Environ. 2008;404(2–3):411–423. doi: 10.1016/j.scitotenv.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Hou HG, Huang QW, Liu JL, Liu GW, Qiao GJ. Si3N4-TiN loaded carbon coating with porous structure as broadband light superabsorber for uncooled IR sensors. Infrared Phys Techn. 2020;105:103240. doi: 10.1016/j.infrared.2020.103240. [DOI] [Google Scholar]
- Hurd RG. Effects of CO2-enrichment on the growth of young tomato plants in low light. Ann Bot. 1968;32(3):531–542. doi: 10.1093/oxfordjournals.aob.a084227. [DOI] [Google Scholar]
- IEC (2019) IEC 60904–4:2019 Photovoltaic devices - Part 4: Photovoltaic reference devices - Procedures for establishing calibration traceability. Edited by International Electrotechnical Commission. 2 edn. International Standard, edited by TC 82 - Solar photovoltaic energy systems. International Electrotechnical Commission. Switzerland
- ISO (1990) ISO 9060:1990 Solar energy-specification and classification of instruments for measuring hemispherical solar and direct solar radiation. Edited by International Organization for Standardization. 1 ed.ISO Norm, edited by ISO/TC 180/SC 1 Climate - Measurement and data. International Organization for Standardization. Switzerland
- ISO (1992) Solar energy-calibration of field pyranometers by comparison to a reference pyranometer. Edited by International Organization for Standardization. ISO Norm. International Organization for Standardization. Switzerland
- ISO (1993) ISO 9846:1993 Calibration of a pyranometer using a pyrheliometer. Edited by International Organization for Standardization. 1 ed. Vol. International Organization for StandardizationISO Norm, edited by ISO/TC 180/SC 1 Climate - Measurement and data. International Organization for Standardization. Switzerland
- ISO (1998) ISO 7726:1998 Ergonomics of the thermal environment—instruments for measuring physical quantities. Edited by International Organization for Standardization.ISO Norm, edited by ISO/TC 159/SC 5 Ergonomics of the physical environment. International Organization for Standardization. Switzerland
- ISO (2009) 80000–1. Quantities and Units-Part 1: General. Edited by International Organization for Standardization. ISO Norm, edited by International Organization for Standardization. International Organization for Standardization. Switzerland
- ISO (2017) ISO 13655:2017 Graphic technology — Spectral measurement and colorimetric computation for graphic arts images. Edited by International Organization for Standardization. 49 ed. ISO Norm, edited by ISO/TC 130 Graphic technology. International Organization for Standardization. Switzerland
- ISO (2018) ISO 9060:2018 Solar energy — Specification and classification of instruments for measuring hemispherical solar and direct solar radiation. Edited by International Organization for Standardization. ISO Norm, edited by ISO/TC 180/SC 1 Climate - Measurement and data. International Organization for Standardization. Switzerland
- Jaffey AH. Solid angle subtended by a circular aperture at point and spread sources - formulas and some tables. Rev Sci Instrum. 1954;25(4):349–354. doi: 10.1063/1.1771061. [DOI] [Google Scholar]
- Jones HG (2013) Radiation. In Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. Cambridge: Cambridge University Press pp 9-46. 10.1017/CBO9780511845727.003
- Kidwell PA. Prelude to solar-energy - pouillet, herschel forbes and the solar-constant. Ann Sci. 1981;38(4):457–476. doi: 10.1080/00033798100200321. [DOI] [Google Scholar]
- Kittas C, Baille A, Giaglaras P. Influence of covering material and shading on the spectral distribution of light in greenhouses. J Agr Eng Res. 1999;73(4):341–351. doi: 10.1006/jaer.1999.0420. [DOI] [Google Scholar]
- Kopp G, Lean JL (2011) A new, lower value of total solar irradiance: evidence and climate significance. Geophys Res Lett 38:L01706. 10.1029/2010gl045777
- Lee RL, Jr, Hernandez-Andres J. Colors of the daytime overcast sky. Appl Opt. 2005;44(27):5712–5722. doi: 10.1364/ao.44.005712. [DOI] [PubMed] [Google Scholar]
- Levine WI (1916) Measurement of spin density matrix elements in the reaction γp→ K+Λ (1520) using CLAS at Jefferson Lab. Jefferson Lab
- LI-COR-Biosciences (2018) Comparison of quantum sensors with different spectral sensitivities. United Sates of America: LI-COR Biosciences Ltd. LI-COR Biosciences Ltd, Nebraska
- Makowski MW. A slide rule for radiation calculations. Rev Sci Instrum. 1949;20(12):876–884. doi: 10.1063/1.1741420. [DOI] [PubMed] [Google Scholar]
- McCluney WR (2014) Introduction to radiometry and photometry. Artech House, 2nd edn. United States, Norwood, MA
- McCree KJ. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol. 1971;9:191–216. doi: 10.1016/0002-1571(71)90022-7. [DOI] [Google Scholar]
- Mccree KJ. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric Meteorol. 1972;10(6):443–0. doi: 10.1016/0002-1571(72)90045-3. [DOI] [Google Scholar]
- McCree KJ. The measurement of photosynthetically active radiation. Sol Energy. 1973;15(1):83–87. doi: 10.1016/0038-092x(73)90010-8. [DOI] [Google Scholar]
- McCree KJ (1981) Photosynthetically active radiation. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological Plant Ecology, vol. 42. Springer, Berlin, Heidelberg, pp 41–55. 10.1007/978-3-642-68090-8_3
- McKagan SB, Handley W, Perkins KK, Wieman CE. A research-based curriculum for teaching the photoelectric effect. Am J Phys. 2009;77(1):87–94. doi: 10.1119/1.2978181. [DOI] [Google Scholar]
- Meyerott RE, Breit G. A small differential analyzer with ball carriage integrators and selsyn coupling. Rev Sci Instrum. 1949;20(12):874–876. doi: 10.1063/1.1741419. [DOI] [Google Scholar]
- Morgan D, Smith H. A systematic relationship between phytochrome-controlled development and species habitat, for plants grown in simulated natural radiation. Planta. 1979;145(3):253–258. doi: 10.1007/BF00454449. [DOI] [PubMed] [Google Scholar]
- Morgan DC, Smith H. Control of development in chenopodium-album L by shadelight - the effect of light quantity (total fluence rate) and light quality (red - far-red ratio) New Phytol. 1981;88(2):239–248. doi: 10.1111/j.1469-8137.1981.tb01720.x. [DOI] [Google Scholar]
- Moualeu-Ngangue DP, Chen TW, Stutzel H. A new method to estimate photosynthetic parameters through net assimilation rate-intercellular space CO2 concentration (A-Ci ) curve and chlorophyll fluorescence measurements. New Phytol. 2017;213(3):1543–1554. doi: 10.1111/nph.14260. [DOI] [PubMed] [Google Scholar]
- Muel B, Malpiece C. Chemical filters for narrow band U.V. irradiation between 235 and 300 nm. Photochem Photobiol. 1969;10(4):283–291. doi: 10.1111/j.1751-1097.1969.tb05692.x. [DOI] [PubMed] [Google Scholar]
- Murakami K, Matsuda R, Fujiwara K. Interaction between the spectral photon flux density distributions of light during growth and for measurements in net photosynthetic rates of cucumber leaves. Physiol Plantarum. 2016;158(2):213–224. doi: 10.1111/ppl.12421. [DOI] [PubMed] [Google Scholar]
- Oguchi R, Terashima I, Chow WS (2021) The effect of different spectral light quality on the photoinhibition of photosystem I in intact leaves. Photosynth Res 149:83–92. 10.1007/s11120-020-00805-z [DOI] [PubMed]
- Paschotta R (2008) Encyclopedia of laser physics and technology, vol 1 and 2\. Wiley Online Library, p 856
- Pettai H, Oja V, Freiberg A, Laisk A. Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta. 2005;1708(3):311–321. doi: 10.1016/j.bbabio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets U, Ntagkas N, Siebenkas A, Maenpaa M, Matsubara S, Pons T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019;223(3):1073–1105. doi: 10.1111/nph.15754. [DOI] [PubMed] [Google Scholar]
- Porcar-Castell A, Tyystjarvi E, Atherton J, van der Tol C, Flexas J, Pfundel EE, Moreno J, Frankenberg C, Berry JA. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: mechanisms and challenges. J Exp Bot. 2014;65(15):4065–4095. doi: 10.1093/jxb/eru191. [DOI] [PubMed] [Google Scholar]
- Raven JA, Beardall J. The lower limit of photon fluence rate for phototrophic growth: the significance of ‘slippage’ reactions. Plant Cell Environ. 1982;5(2):117–124. doi: 10.1111/1365-3040.ep11571479. [DOI] [Google Scholar]
- Reda I (2011) Method to calculate uncertainty estimate of measuring shortwave solar irradiance using thermopile and semiconductor solar radiometers. National Renewable Energy Lab.(NREL), Golden, CO (United States). 10.2172/1021250, https://www.osti.gov/servlets/purl/1021250
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O'Hara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI, Ulm R. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332(6025):103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Ryther JH. Photosynthesis in the ocean as a function of light intensity. Limnol Oceanogr. 1956;1(1):61–70. doi: 10.4319/lo.1956.1.1.0061. [DOI] [Google Scholar]
- Sager J, Smith W, Edwards J, Cyr K. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Transactions of the ASAE. 1988;31(6):1882–1889. doi: 10.13031/2013.30952. [DOI] [Google Scholar]
- Schreiber U, Klughammer C. Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res. 2013;114(3):165–177. doi: 10.1007/s11120-013-9801-x. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C, Kolbowski J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res. 2012;113(1–3):127–144. doi: 10.1007/s11120-012-9758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93(15):8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley HL. Light sources and light measurements. Plant Physiol. 1931;6(3):447–466. doi: 10.1104/pp.6.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Totzeck M, Gross H (2005) Handbook of Optical Systems. Physical Image Formation, October 2005. (Series Editor Gross H ISBN 3-527-40378-7), vol 2. Wiley-VCH, p 714
- Singh H, Dunn BL, Payton M, Brandenberger L. Selection of fertilizer and cultivar of sweet pepper and eggplant for hydroponic production. Agronomy-Basel. 2019;9(8):433. doi: 10.3390/agronomy9080433. [DOI] [Google Scholar]
- Smith WJ (2008) Modern optical engineering. Tata McGraw-Hill Education, 4th edn. United States of America, p 764
- Strid A, Chow WS, Anderson JM. UV-B damage and protection at the molecular level in plants. Photosynth Res. 1994;39(3):475–489. doi: 10.1007/BF00014600. [DOI] [PubMed] [Google Scholar]
- Suits GH. The calculation of the directional reflectance of a vegetative canopy. Remote Sens Environ. 1971;2:117–125. doi: 10.1016/0034-4257(71)90085-X. [DOI] [Google Scholar]
- Sundby C, Chow WS, Anderson JM. Effects on photosystem II function, photoinhibition, and plant performance of the spontaneous mutation of serine-264 in the photosystem II reaction center D1 protein in triazine-resistant Brassica napus L. Plant Physiol. 1993;103(1):105–113. doi: 10.1104/pp.103.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó M, Larkum AWD, Suggett DJ, Vass I, Sass L, Osmond B, Zavafer A, Ralph PJ, Chow WS (2017) Non-intrusive assessment of photosystem II and photosystem I in whole coral tissues. Frontiers in Marine Science 4:(269). 10.3389/fmars.2017.00269, https://www.frontiersin.org/article/10.3389/fmars.2017.00269
- Szabo M, Parker K, Guruprasad S, Kuzhiumparambil U, Lilley RM, Tamburic B, Schliep M, Larkum AW, Schreiber U, Raven JA, Ralph PJ. Photosynthetic acclimation of Nannochloropsis oculata investigated by multi-wavelength chlorophyll fluorescence analysis. Bioresour Technol. 2014;167:521–529. doi: 10.1016/j.biortech.2014.06.046. [DOI] [PubMed] [Google Scholar]
- Thapper A, Mamedov F, Mokvist F, Hammarstrom L, Styring S. Defining the far-red limit of photosystem II in spinach. Plant Cell. 2009;21(8):2391–2401. doi: 10.1105/tpc.108.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E. Photoinhibition of photosystem II. Int Rev Cell Mol Biol. 2013;300:243–303. doi: 10.1016/B978-0-12-405210-9.00007-2. [DOI] [PubMed] [Google Scholar]
- van Dijk T, Fischer DG, Visser TD, Wolf E. Effects of spatial coherence on the angular distribution of radiant intensity generated by scattering on a sphere. Phys Rev Lett. 2010;104(17):173902. doi: 10.1103/PhysRevLett.104.173902. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AW, van Duyn DC, van Oudheusden BW, Sarro PM. Integrated thermopile sensors. Sens Actuators, A. 1990;22(1–3):621–630. doi: 10.1016/0924-4247(89)80046-9. [DOI] [Google Scholar]
- Varlet-Grancher C, Moulia B, Sinoquet H, Russell G (1993) Spectral modification of light within plant canopies : how to quantity its effects on the architecture of the plant stand. Crop structure and light microclimate: characterization and applications, INRA (National Research Institute for Agriculture), 2-7380-0448-2. ⟨hal-02847162⟩, France
- Vass I (2012) Molecular mechanisms of photodamage in the Photosystem II complex. Biochim et Biophys Acta (BBA)-Bioenerg 1817 (1):209–217. 10.1016/j.bbabio.2011.04.014 [DOI] [PubMed]
- Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, De Visser PH. Functional–structural plant modelling: a new versatile tool in crop science. J Exp Bot. 2010;61(8):2101–2115. doi: 10.1093/jxb/erp345. [DOI] [PubMed] [Google Scholar]
- Worthing AG. On the deviation from Lambert’s cosine law of the emission from tungsten and carbon at glowing temperatures. Astrophys J. 1912;36(5):345–361. doi: 10.1086/141969. [DOI] [Google Scholar]
- Wu D, Hu Q, Yan Z, Chen W, Yan C, Huang X, Zhang J, Yang P, Deng H, Wang J, Deng X, Shi Y. Structural basis of ultraviolet-B perception by UVR8. Nature. 2012;484(7393):214–219. doi: 10.1038/nature10931. [DOI] [PubMed] [Google Scholar]
- Wuttke S, Seckmeyer G, Bernhard G, Ehramjian J, McKenzie R, Johnston P, O'Neill M. New spectroradiometers complying with the NDSC standards. J Atmos Ocean Tech. 2006;23(2):241–251. doi: 10.1175/Jtech1826.1. [DOI] [Google Scholar]
- Zavafer A, Chow WS, Cheah MH. The action spectrum of photosystem II photoinactivation in visible light. J Photochem Photobiol B. 2015;152(Pt B):247–260. doi: 10.1016/j.jphotobiol.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Zavafer A, Iermak I, Cheah MH, Chow WS. Two quenchers formed during photodamage of phostosystem II and the role of one quencher in preemptive photoprotection. Sci Rep. 2019;9(1):17275. doi: 10.1038/s41598-019-53030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Fan DY, Sun GY, Chow WS. Optimising the linear electron transport rate measured by chlorophyll a fluorescence to empirically match the gross rate of oxygen evolution in white light: towards improved estimation of the cyclic electron flux around photosystem I in leaves. Funct Plant Biol. 2018;45(11):1138–1148. doi: 10.1071/FP18039. [DOI] [PubMed] [Google Scholar]
- Zong Y, Brown SW, Johnson BC, Lykke KR, Ohno Y. Simple spectral stray light correction method for array spectroradiometers. Appl Opt. 2006;45(6):1111–1119. doi: 10.1364/ao.45.001111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available under reasonable request. Please contact AZ through alonso.zavaleta@anu.edu.au.
No code is available in this work.