Abstract
Myokines, which are recently identified cytokines secreted by skeletal muscle in response to stimulation, are crucial for the maintenance of liver function. Fulminant hepatitis (FH) is a life-threatening pathological condition with severe hepatic dysfunction. In this study, we investigated the role of meteorin-like (METRNL), a new myokine, in the pathogenesis of FH. We compared serum samples and liver tissues from FH patients and healthy controls and found that hepatic and serum METRNL levels were significantly increased in FH patients, and serum METRNL levels were related to disease severity in FH patients. We then established a concanavalin A-induced FH model in METRNL-overexpressing and control mice. We found that hepatic METRNL levels in FH mice were significantly increased, and METRNL in the liver was mainly derived from macrophages. In the cultured mouse macrophage line (RAW264.7 cells) and mouse primary peritoneal macrophages (PMs), METRNL overexpression significantly inhibited the release of the proinflammatory cytokines TNF and IL-1β. In METRNL-overexpressing mice, concanavalin A-induced liver injury was significantly ameliorated. Moreover, METRNL overexpression significantly reduced chemokine-dependent inflammatory cell infiltration into the liver. METRNL overexpression also suppressed liver CD4+ T cell differentiation into Th 1 cells and inhibited the secretion of Th 1 cytokines. Taken together, these data suggest that METRNL overexpression effectively ameliorates FH. Therefore, METRNL may serve as a potential biomarker and therapeutic target for FH.
Keywords: fulminant hepatitis, meteorin-like, macrophages, kupffer cells, chemokines, Th 1 cells
Introduction
Fulminant hepatitis (FH) is a life-threatening pathological condition characterized by severe hepatic dysfunction followed by hepatic encephalopathy, coagulopathy, and systemic inflammatory response syndrome [1]. Circumstantial evidence suggests that an excessive inflammatory response is the major cause of FH [2]. Many pathological immune cells are activated and recruited to the liver, where these cells produce numerous proinflammatory mediators that induce apoptosis and necrosis in hepatocytes [3]. Despite advances in the management of FH, liver transplantation is the only proven therapy for those who develop severe FH [4]. Therefore, a deeper understanding of the molecular mechanisms of disease progression is needed to develop effective alternative therapeutic approaches.
Patients with chronic liver diseases often suffer from sarcopenia [5], which is an independent predictor of death in patients with liver cirrhosis [6]. Moreover, sarcopenia is related to acute-on-chronic liver failure development and systemic inflammation n [7]. These results indicate the close relationship between liver diseases and muscle. Muscle was recently identified as a secretory organ [8]. Myokines, which are peptides or cytokines produced by skeletal muscle, are crucial for the maintenance of liver function and other systemic actions [9]. A previous study showed that increased levels of the myokine irisin were associated with the presence and severity of nonalcoholic fatty liver disease in humans [10]. The myokine fibroblast growth factor (FGF) 21 is an early predictor of acute-on-chronic liver failure in patients with severe cirrhosis [11]. These findings provide compelling evidence for the role of myokines as promising targets for liver diseases.
Meteorin-like (METRNL) protein, which is also known as Meteorin-β protein (Metrnβ) or IL-41, is a recently identified myokine secreted from skeletal muscle in response to stimulation [12]. In 2014, Spiegelman’s laboratory reported for the first time that METRNL was produced by skeletal muscle after physical activity and attenuated inflammation in white adipose tissue [13]. Further research showed that METRNL levels were increased in patients with the autoimmune disease psoriasis [14]. METRNL was strongly induced in alternatively activated macrophages [15]. In injured skeletal muscle, METRNL coordinated muscle repair by promoting macrophage differentiation into anti-inflammatory phenotypes [16]. In cardiomyocytes with ischaemia‒reperfusion injury, METRNL alleviated apoptosis by activating the AMPK-PAK2 signaling pathway [17]. Thus, METRNL is crucial in immune homeostasis and tissue repair. However, the role of METRNL and its specific mechanism in liver diseases, including FH, has not been fully elucidated.
In the current study, serum and hepatic METRNL expression was significantly increased in mice with concanavalin A (ConA)-induced FH. METRNL overexpression ameliorated FH by inhibiting chemokine-dependent immune cell infiltration. Consistent with the findings in mice, serum and hepatic METRNL expression was significantly increased in FH patients, which was related to disease severity and prognosis. Thus, our study identified METRNL as a new biomarker and a potential therapeutic target for FH.
Materials and methods
Animals and treatments
Male C57BL/6 mice (4–5 weeks old) received a single intravenous injection (2 × 1011 viral genome per mouse) of an adeno-associated virus serotype 2/8 (AAV2/8; Obio Technology, Shanghai, China) to overexpress METRNL or the negative control AAV2/8 (NC). Four weeks after AAV2/8 injection, the mice were intravenously injected with ConA (15 mg/kg) to induce FH and then sacrificed for functional and molecular analyses.
Patients and samples
Thirty patients with FH and 15 patients with CHB who were hospitalized in the Department of Infectious Diseases, Ruijin Hospital, Shanghai, China between June 2018 and May 2019 were enroled, as well as 10 healthy controls (HCs). All CHB patients were positive for hepatitis B surface antigen for more than 6 months. The clinical data are presented in Table 1. According to the Asian Pacific Association for the Study of the Liver criteria, FH patients had a total bilirubin (TBil) ≥ 5 mg/dL and prothrombin activity < 40%, which were complicated with clinical ascites and/or encephalopathy within 4 weeks. The study was approved by the Human Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Table 1.
Baseline demographic and clinical characteristics of the enroled participants.
| HC | CHB | FH | P valuea | P valueb | |
|---|---|---|---|---|---|
| Case | 10 | 15 | 30 | ||
| Male (%) | 5 (50%) | 9 (60%) | 17 (56%) | NS | NS |
| Age (years) | 44.00 (32.25, 50.25) | 42.00 (37.00, 48.00) | 48.50 (40.00, 58.25) | NS | NS |
| ALT (IU/L) | 17.50 (11.00, 22.50) | 46.00 (39.00, 79.00) | 210.50 (77.00, 710.50) | <0.001 | <0.05 |
| AST (IU/L) | 20.00 (18.75, 23.25) | 44.00 (27.00, 65.00) | 147.50 (95.25, 593.50) | <0.001 | <0.01 |
| TBil (μmol/L) | 14.15 (12.30, 16.15) | 14.40 (11.30, 23.80) | 433.50 (293.00, 587.30) | <0.001 | <0.001 |
| PT (s) | ND | 11.70 (10.90, 13.20) | 20.15 (16.90, 28.70) | - | <0.001 |
| INR | ND | 0.990 (0.920, 1.120) | 1.670 (1.448, 2.443) | - | <0.001 |
| Albumin (g/L) | 45.50 (44.00, 46.25) | 44.00 (36.00, 45.00) | 27.50 (25.00, 31.00) | <0.001 | <0.001 |
| Creatinine (μmol/L) | 76.00 (65.00, 86.75) | 77.00 (64.00, 84.00) | 81.00 (70.00, 113.3) | NS | NS |
| HBV DNA (Log IU/mL) | ND | 5.754 (4.201, 7.253) | 3.633 (2.699, 6.055) | - | NS |
| HBsAg positive (%) | 0 (0%) | 15 (100%) | 30 (100%) | - | NS |
| MELD | ND | 5.11 (2.60, 7.37) | 24.50 (20.00, 30.25) | - | <0.001 |
| MELD-Na | ND | ND | 26.50 (21.44, 33.64) | - | - |
Quantitative variables were expressed as the median (interquartile range). Categorical variables were expressed as numbers (percentage).
ALT alanine aminotransferase, AST aspartate aminotransferase, HC healthy control, CHB chronic hepatitis B, FH fulminant hepatitis, HBsAg hepatitis B surface antigen, INR international normalized ratio, MELD Model for End-stage Liver Disease, MELD-Na Model for End-stage Liver Disease and sodium, ND not determined, NS no significance, PT prothrombin time, TBil total bilirubin.
aHC vs. CHB.
bHC vs. FH.
Statistical analysis
All results were analysed using SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as the median (range), and categorical variables are presented as numbers (percentages). Differences between two groups were evaluated by Student’s t test, while differences among three or more groups were compared using one-way analysis of variance (ANOVA). Correlations between two variables were assessed using Spearman’s correlation test. Survival data were analysed by the Kaplan‒Meier method. Two-sided P < 0.05 was considered statistically significant.
For detailed methods and materials, please refer to online supplementary information.
Results
The increase in METRNL expression in the FH mouse model was derived from macrophages
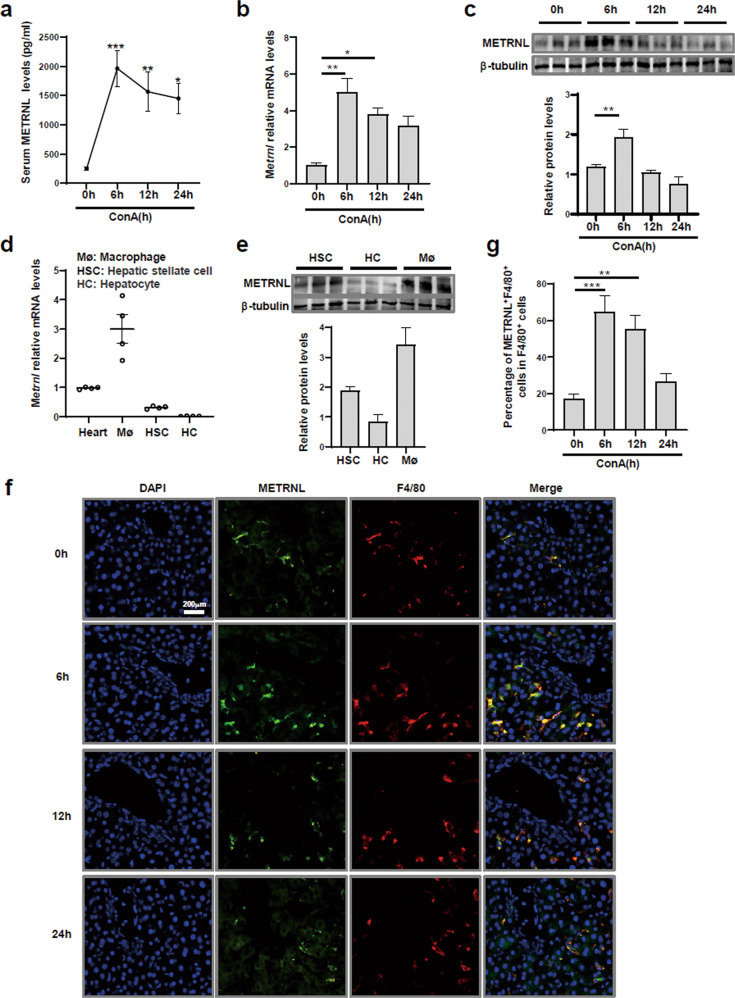
A ConA-induced FH mouse model was established to investigate the role of METRNL in FH. The characteristics of the FH model were analysed. The levels of secreted proinflammatory cytokines in liver tissues peaked at 6 h after ConA injection (Supplementary Fig. 1a). Serum levels of ALT and AST peaked at 12 h postinjection (Supplementary Fig. 1b). Liver injury started to repair at 24 h postinjection (Supplementary Fig. 1c). Serum METRNL levels increased rapidly during the first 6 h postinjection and then gradually declined over time (Fig. 1a). Then, the relative levels of Metrnl transcripts in various mouse tissues were analysed. In heart, skeletal muscle and adipose tissues, relative Metrnl mRNA levels were decreased at 6 h after ConA injection. However, there were no significant differences among the groups at 6, 12 or 24 h (Supplementary Fig. 1d). In contrast, the protein and mRNA levels of METRNL increased at 6 h, which was the peak time of proinflammatory cytokine secretion (Fig. 1b, c).
Fig. 1. The increase in METRNL expression in the FH mouse model was derived from macrophages.
a C57BL/6 mice were intravenously injected with ConA (15 mg/kg), and sera were obtained at 0, 6, 12 and 24 h. Serum METRNL levels were measured. b Relative Metrnl mRNA levels in the liver tissues of mice were measured by Q-PCR at the indicated times after ConA injection. c METRNL protein levels in the liver tissues of mice were analysed at the indicated times after ConA injection. d Relative Metrnl mRNA levels in hepatocytes and nonparenchymal cells from healthy mouse livers were measured. e METRNL protein levels in hepatocytes and nonparenchymal cells from healthy mouse livers were analysed. f Representative immunofluorescence images showing costaining of F4/80 and METRNL in the liver tissue of ConA-treated mice. g Percentages of F4/80 and METRNL double-staining in the liver tissues of ConA-treated mice. n = 4–6 per group. The data are presented as the mean ± SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
We then assessed the expression of METRNL in different liver cell populations in healthy mice: hepatocytes, hepatic stellate cells (HSCs), and macrophages. As shown in Fig. 1d, e, the highest expression level was found in macrophages, indicating that METRNL was preferentially expressed by macrophages rather than hepatocytes or HSCs. Next, whether METRNL was primarily expressed in macrophages in ConA-treated mice was determined. Immunofluorescence staining showed double-positive signals for F4/80 (red)/METRNL (green) in the liver. As expected, the increase in the F4/80/METRNL double-positive signal was consistent with METRNL expression in liver tissues (Fig. 1f, g). Collectively, these results indicated that serum and hepatic METRNL levels were increased in the FH mouse model and that the main cell source of METRNL was macrophages.
METRNL inhibited the secretion of proinflammatory cytokines by macrophages
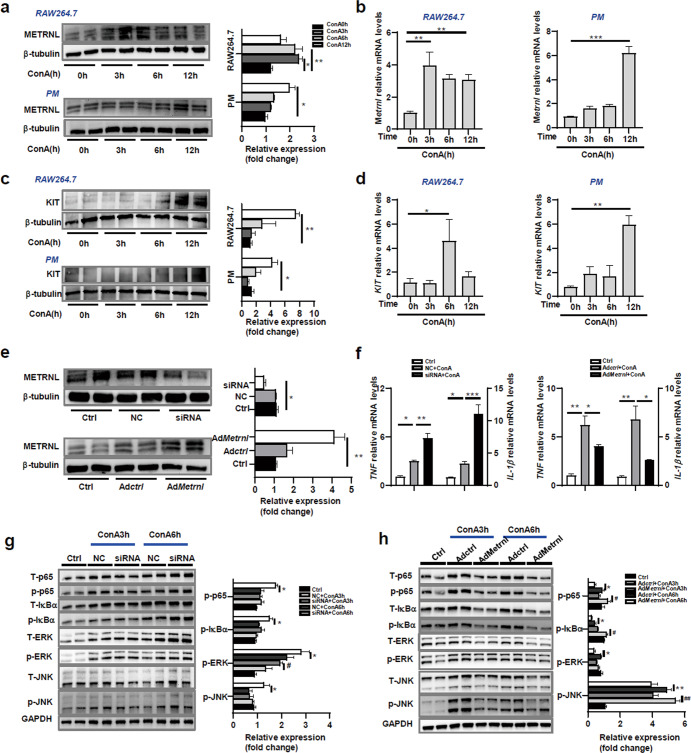
Given our observations that macrophages were the main source of METRNL in the liver, we next analysed the role of METRNL in macrophages using the cultured mouse macrophage line RAW264.7 and mouse primary peritoneal macrophages (PMs). The mRNA and protein levels of METRNL increased in these cells after ConA treatment (Fig. 2a, b). A study by Reboll MR confirmed that METRNL bound to the extracellular domain of KIT and mediated angiogenic effects via KIT. The researchers first identified METRNL as a high-affinity ligand for the stem cell factor receptor KIT [18]. Therefore, we measured the expression of KIT in RAW264.7 cells and PMs. As expected, the mRNA and protein levels of KIT increased in these cells after ConA treatment (Fig. 2c, d).
Fig. 2. METRNL inhibited the secretion of proinflammatory factors by macrophages.
a Protein expression levels of METRNL in the mouse macrophage cell line RAW264.7 and mouse primary peritoneal macrophages (PMs) after ConA treatment for 0, 3, 6 and 12 h. b Relative Metrnl mRNA levels in RAW264.7 cells and mouse primary PMs were measured by Q-PCR at the indicated times after ConA treatment. c Protein expression levels of KIT in RAW264.7 cells and PMs were measured at the indicated times after ConA treatment. d Relative KIT mRNA levels in RAW264.7 cells and mouse primary PMs were measured by Q-PCR at the indicated times after ConA treatment. e RAW264.7 cells were pretreated with negative control (NC) or METRNL siRNA for 48 h or infected with control adenovirus (AdCtrl) or adenovirus carrying Metrnl (AdMetrnl) at a viral titre of 1 × 108 PFU/ml for 48 h. The protein expression levels of METRNL in cells after the different treatments were measured by Western blotting. f Relative TNF and IL-1β mRNA levels in the different groups were measured by Q-PCR. g The activation of the MAPK and NF-κB pathways was measured in RAW264.7 cells transfected with NC or siRNA after stimulation with ConA (10 ng/mL) for 3 and 6 h. h The MAPK and NF-κB pathways were measured in RAW264.7 cells infected with AdCtrl or AdMetrnl after stimulation with ConA (10 ng/mL) for 3 and 6 h. The results are representative of 3 independent experiments. The data are presented as the mean ± SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01.
To determine the effect of METRNL on inflammation in macrophages, the secretion of the inflammatory cytokines TNF and IL-1β by macrophages was examined after METRNL knockdown or overexpression. The knockdown efficiency of small interfering RNA (siRNA) transfection and overexpression efficiency of adenovirus carrying Metrnl (AdMetrnl) infection were confirmed by Western blotting (Fig. 2e). Unsurprisingly, the relative mRNA levels of TNF and IL-1β in the siRNA+ConA group were significantly higher than those in the NC + ConA group. The relative mRNA levels in the AdMetrnl + ConA group were significantly lower than those in the Adctrl + ConA group (Fig. 2f). These results suggested that METRNL inhibited the secretion of proinflammatory cytokines by macrophages.
Previous studies have shown that the release of proinflammatory cytokines by macrophages is regulated by the MAPK and NF-κB signaling pathways [19]. Therefore, the MAPK and NF-κB signaling pathways were examined in METRNL-knockdown or -overexpressing RAW264.7 cells after ConA treatment. We found that the phosphorylation levels of JNK, ERK, p65 and IκB-α in the siRNA+ConA group were significantly higher than those in the NC + ConA group (Fig. 2g). Moreover, the phosphorylation levels of JNK, ERK, p65 and IκB-α in the AdMetrnl + ConA group were significantly lower than those in the Adctrl + ConA group (Fig. 2h). These findings demonstrated that METRNL suppressed inflammatory signaling pathways.
METRNL overexpression alleviated FH in vivo
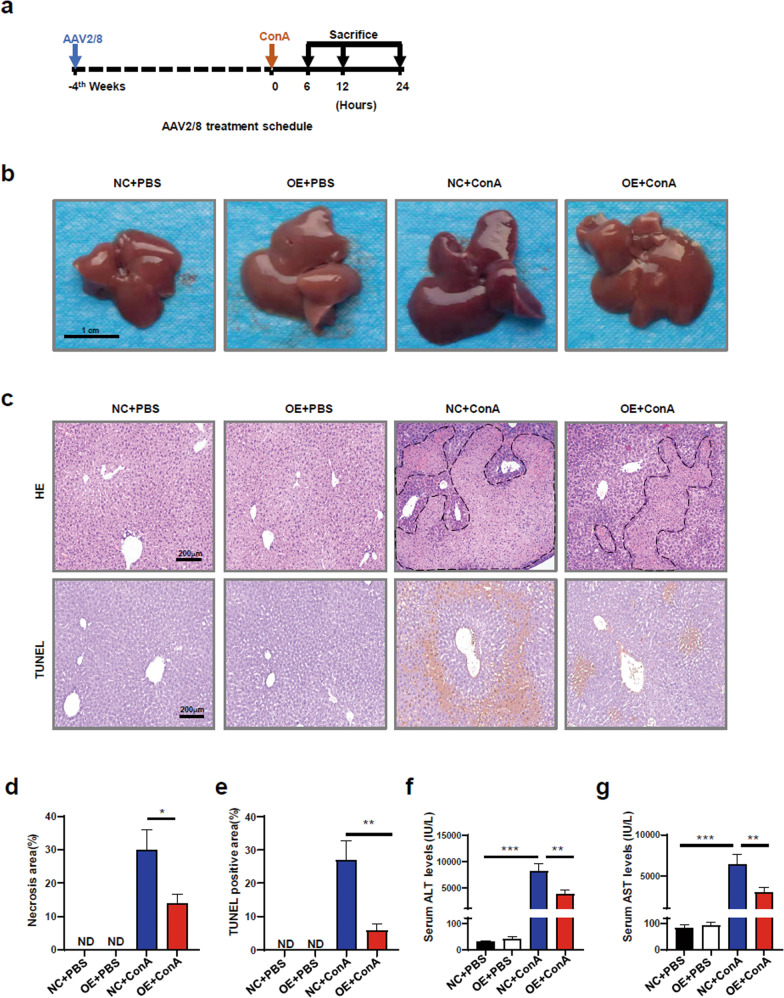
FH may be driven by a systemic hyperinflammatory state. Because of the anti-inflammatory effect of METRNL on macrophages, we hypothesized that METRNL played a protective role in ConA-induced FH by suppressing the hyperinflammatory response. Mice that were injected with AAV2/8 carrying Metrnl or a negative control vector were exposed to ConA to mimic clinical FH. The high transduction efficiency of AAV2/8 was determined (Supplementary Fig. 2a). As shown in Supplementary Fig. 2b, d, AAV2/8 injection caused robust and persistent expression of METRNL in adult mouse serum and liver tissue. There was no difference in weight between the overexpression (OE) group and the normal control (NC) group (Supplementary Fig. 2e). Gross anatomy showed that the liver was swollen and congested after ConA injection, with a darker colour, bleeding spots and superficial lesions. Compared with those in the NC + ConA group, the liver lesions in the OE + ConA group were significantly improved (Fig. 3b). HE and TUNEL staining showed that the liver necrosis area in the OE + ConA group was significantly smaller than that in the NC + ConA group (Fig. 3c–e). Consistently, there were dramatic reductions in ALT and AST levels in the serum of the OE + ConA group (Fig. 3f, g). Taken together, these data indicated that METRNL overexpression alleviated ConA-induced FH in vivo.
Fig. 3. METRNL overexpression alleviated FH in vivo.
a Schematic protocol for AAV2/8 and ConA injection. b Representative gross anatomical images showing livers from normal control (NC) and overexpression (OE) mice treated with PBS or ConA. c Haematoxylin-eosin (HE) and TUNEL staining of livers from NC and OE mice treated with PBS or ConA. d Quantification of necrosis area percentages in HE-stained tissue from the different treatment groups. e Quantification of TUNEL-positive percentages in the different treatment groups. f Serum ALT levels in NC and OE mice treated with PBS or ConA. g Serum AST levels in NC and OE mice treated with PBS or ConA. n = 4–6 per group. The data are presented as the mean ± SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
Macrophage-derived METRNL attenuated liver injury by inhibiting immune cell infiltration
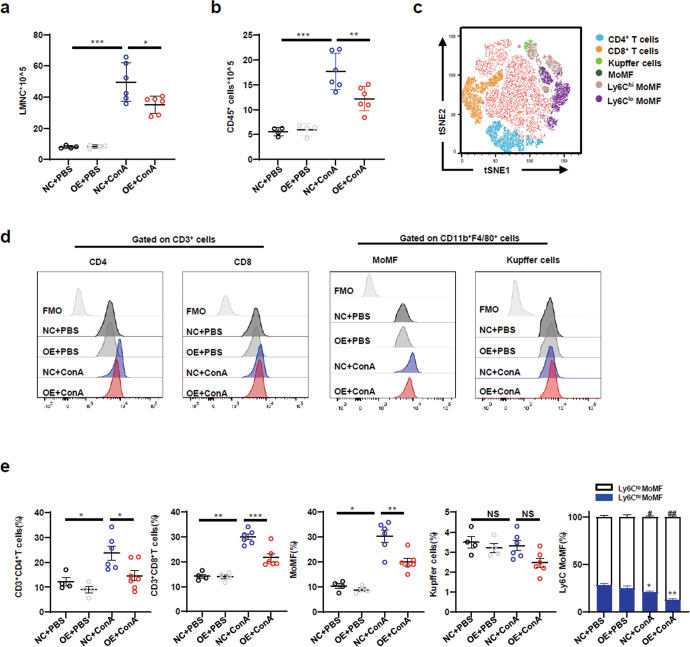
The inflammatory response mediated by infiltrating immune cells plays a critical role in ConA-induced FH [20]. To confirm this, the total numbers of LMNCs and CD45+ immune cells were analysed. The total numbers of LMNCs and CD45+ immune cells were markedly lower in the OE + ConA group than in the NC + ConA group (Fig. 4a, b). To determine the main subsets contributing to the effects on our model, CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), monocyte-derived macrophages (MoMFs, CD11b+F4/80int), Kupffer cells (CD11bintF4/80+), and neutrophils (CD11b+Ly6G+) in the liver were detected after ConA injection. Compared with those in the NC + ConA group, the percentages of CD4+ T cells, CD8+ T cells and MoMFs in LMNCs in the OE + ConA group were significantly decreased (Fig. 4d, e). However, there was no marked difference in the proportions of Kupffer cells (Fig. 4d, e) or neutrophils (data not shown) between the OE + ConA group and the NC + ConA group.
Fig. 4. Macrophage-derived METRNL attenuated liver injury by inhibiting immune cell infiltration.
a Absolute numbers of total liver mononuclear cells (LMNCs) in NC and OE mice treated with PBS or ConA were determined by flow cytometry. b Absolute numbers of CD45+ immune cells in the liver were determined by flow cytometry. c The proportions of cell subclusters of LMNCs were analysed using t-distributed stochastic neighbour embedding (tSNE) analysis. d The frequencies of CD3+CD4+ T cells, CD3+CD8+ T cells, CD11bintF4/80+ Kupffer cells and CD11b+F4/80int monocyte-derived macrophages (MoMFs) in LMNCs were assessed by flow cytometry. e The frequencies of CD3+CD4+ T cells, CD3+CD8+ T cells, CD11bintF4/80+ Kupffer cells and CD11b+F4/80int MoMFs (Ly6Chi and Ly6Clo) in LMNCs were quantified. n = 4–6 per group. The data are presented as the mean ± SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
MoMFs were divided into two subsets according to the expression levels of Ly6C (Ly6Chi and Ly6Clo). Ly6Chi MoMFs contribute to liver inflammation, and Ly6Clo MoMFs contribute to liver resolution/repair. Therefore, the proportions of Ly6Chi and Ly6Clo MoMFs were analysed. There was a decrease in the percentage of Ly6Chi MoMFs and an increase in the percentage of Ly6Clo MoMFs in the OE + ConA group compared with the NC + ConA group (Fig. 4e). These findings indicated that METRNL protected against liver injury by inhibiting immune cell infiltration.
Spleen MNCs and CD4+ T cells were also analysed. Gross anatomical images of the spleen showed that there was no difference between the NC + ConA and OE + ConA groups (Supplementary Fig. 3a). Next, we found that the total number of spleen MNCs increased significantly after ConA injection, but there was no significant difference between the OE + ConA group and the NC + ConA group (Supplementary Fig. 3b). The percentage of spleen CD4+ T cells was significantly reduced after ConA injection. METRNL overexpression restored the reduction in CD4+ T cells in the spleen (Supplementary Fig. 3c). Furthermore, the percentage of blood CD4+ T cells was significantly reduced after ConA injection. There was no significant difference between the OE + ConA group and the NC + ConA group (Supplementary Fig. 3c). Collectively, these data suggested that METRNL inhibited the migration of CD4+ T cells from the spleen to the liver.
Macrophage-derived METRNL attenuated liver inflammation by suppressing T helper 1 cells
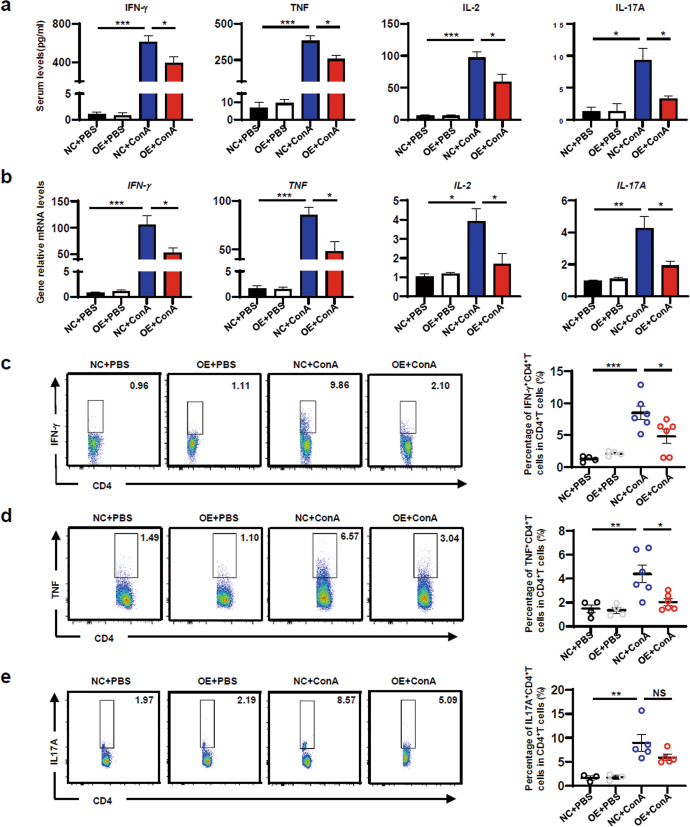
It is well known that T cells, especially CD4+ T cells, are the major pathogenic contributors to ConA-induced liver injury. Serum levels of the Th1 cytokines IFN-γ, TNF and IL-2, the Th2 cytokine IL-4, the Th17 cytokine IL-17A and the immunosuppressive cytokine IL-10 were determined. METRNL overexpression significantly reduced serum levels of IFN-γ, TNF, IL-2 and IL-17A (Fig. 5a) but had no effect on IL-4 or IL-10 production (Supplementary Fig. 4a). Hepatic mRNA levels of these cytokines was examined and further confirmed the reduction in IFN-γ, TNF, IL-2 and IL-17A expression (Fig. 5b). There were no significant differences in the mRNA levels of IL-4 or IL-10 (Supplementary Fig. 4b).
Fig. 5. Macrophage-derived METRNL attenuated liver inflammation by suppressing Th1 cells.
aSerum IFN-γ, TNF, IL-2 and IL-17A levels in NC and OE mice treated with PBS or Con A. b Hepatic IFN-γ, TNF, IL-2 and IL-17A mRNA levels in NC and OE mice treated with PBS or Con A. c The percentages of IFN-γ+CD4+ T cells in LMNCs were analysed by flow cytometry. d The percentages of TNF+CD4+ T cells in LMNCs were analysed by flow cytometry. e The percentages of IL-17A+CD4+ T cells in LMNCs were analysed by flow cytometry. n = 3–6 per group. The data are presented as the mean±SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
To further investigate the effect of METRNL overexpression on Th1 and Th17 cells, Th1 and Th17 cells in the liver were analysed. The percentages of IFN-γ+CD3+CD4+ T cells significantly increased after ConA injection. Compared with those in the NC + ConA group, the percentages of IFN-γ+CD3+CD4+ T cells in the OE + ConA group were significantly lower (Fig. 5c). Consistently, the percentages of TNF+CD3+CD4+ T cells in the OE + ConA group were significantly lower than those in the NC + ConA group (Fig. 5d). Furthermore, the percentages of IL-17A+CD3+CD4+ T cells significantly increased after ConA injection, but there was no significant difference between the OE + ConA group and the NC + ConA group (Fig. 5e). These results demonstrated that METRNL attenuated liver inflammation by suppressing Th1 cells.
In addition, Th1 and Th17 cells in the spleen were analysed. The percentages of IFN-γ+CD3+CD4+ T cells in the spleen increased after ConA injection, but there was no significant difference (Supplementary Fig. 3d). The percentages of TNF+CD3+CD4+ T cells and IL-17A+CD3+CD4+ T cells in the spleen significantly increased (Supplementary Fig. 3d). However, there was no significant difference in the percentages of IFN-γ+CD3+CD4+ T cells, TNF+CD3+CD4+ T cells or IL-17A+CD3+CD4+ T cells between the OE + ConA group and the NC + ConA group. These results indicated that METRNL overexpression had no effect on the differentiation of CD4+ T cells in the spleen.
METRNL-mediated suppression of liver inflammation was chemokine‑dependent
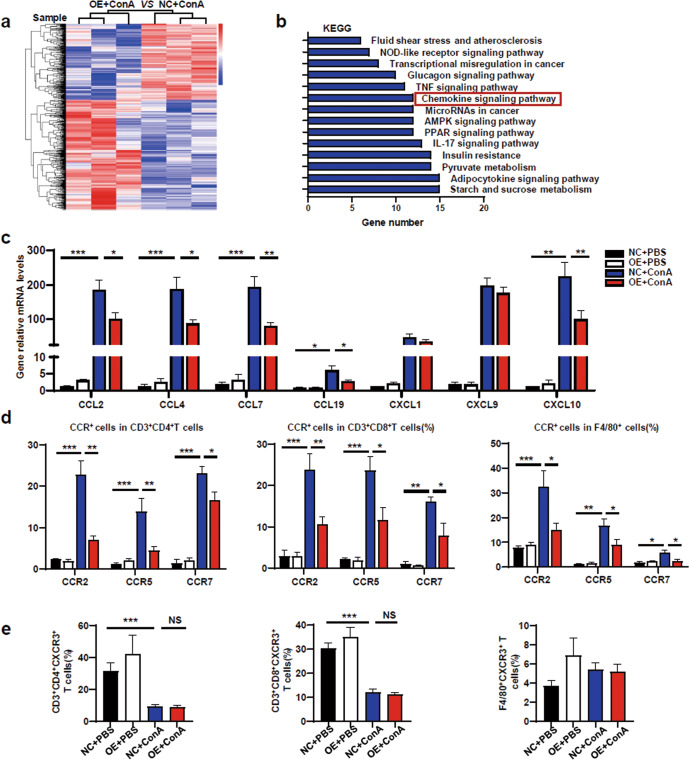
RNA-seq was performed to study gene expression profiles in the different treatment groups. The hierarchical clustering of differentially expressed genes (DEGs) between the NC + ConA and OE + ConA groups was different (Fig. 6a). The KEGG results displayed the top 14 pathways by sorting the number of altered genes in each pathway (Fig. 6b). The infiltration of inflammatory cells into the liver requires recruitment by chemokines. As expected, the chemokine signaling pathway was one of the top 14 pathways. Some of the related chemokine genes were selected and verified. The relative mRNA levels of CCL2, CCL4, CCL7, CCL19 and CXCL10 were significantly decreased in the OE + ConA group compared with the NC + ConA group (Fig. 6c). No marked differences in the mRNA levels of CXCL1 or CXCL9 were found between the OE + ConA group and the NC + ConA group (Fig. 6c).
Fig. 6. METRNL-mediated suppression of liver inflammation was chemokine‑dependent.
aHierarchical clustering of differentially expressed genes (DEGs) in the NC + ConA and OE + ConA groups were analysed, and gene expression profiles are shown in the heatmap (n = 3 per group). b The DEGs of each pair were subjected to KEGG analysis, and the top 14 annotations enriched in biological functional pathways are shown (FC ≥ 2, P ≤ 0.05, n = 3 per group). c The mRNA levels of the hepatic chemokines CCL2, CCL4, CCL7, CCL19, CXCL1, CXCL9 and CXCL10 in NC and OE mice treated with PBS or Con A were analysed. d The levels of CCR2, CCR5, and CCR7 in CD4+ T cells, CD8+ T cells and F4/80+ cells derived from livers were assessed by flow cytometry. e The levels of CXCR3 in CD4+ T cells, CD8+ T cells and F4/80+ cells derived from livers were assessed by flow cytometry. n = 4–6 per group. The data are presented as the mean±SEM and were analysed by one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
Chemokines bind to their corresponding chemokine receptor (CCR) to exert their corresponding biological effects. Compared with that in the NC + ConA group, reduced expression of CCR2, CCR5, CCR7 and CXCR3 on LMNCs (CD4+ T cells, CD8+ T cells, F4/80+ cells) was observed in the OE + ConA group (Fig. 6d), but CXCR3 showed no difference between the two groups (Fig. 6e). These results indicated that METRNL overexpression suppressed the chemotaxis of pathogenic immune cells into the liver.
Serum and hepatic METRNL levels were upregulated in FH patients
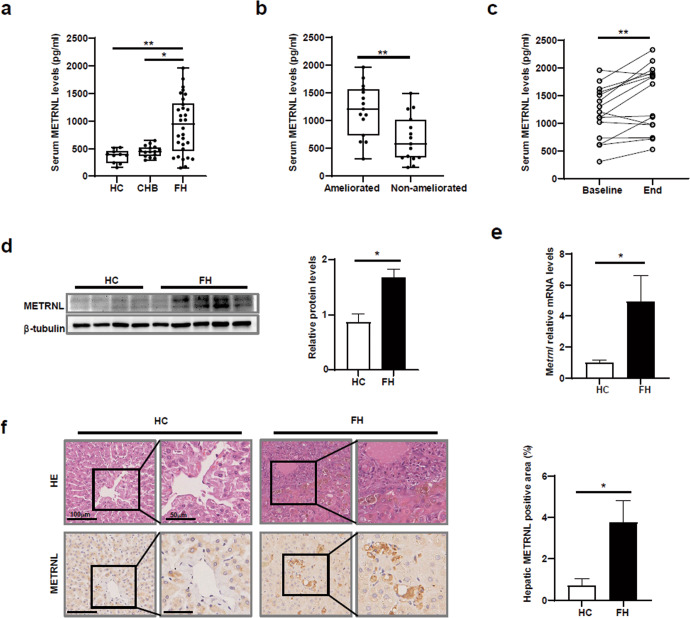
To investigate whether METRNL contributes to FH progression, serum levels of METRNL in patients with FH, patients with CHB and HCs were analysed. Serum METRNL levels in FH patients were significantly higher than those in HCs and CHB patients (Fig. 7a). Moreover, serum METRNL levels in ameliorated FH patients were significantly higher than those in non-ameliorated patients (Fig. 7b). Among the ameliorated FH patients, serum METRNL levels were increased at the end point compared with baseline levels (Fig. 7c).
Fig. 7. Serum and hepatic METRNL levels were upregulated in FH patients.
a Baseline serum levels of METRNL were compared among healthy controls (HCs, n = 10), chronic hepatitis B (CHB) patients (n = 15), and fulminant hepatitis (FH) patients (n = 30). b Baseline serum levels of METRNL were compared between ameliorated FH patients (n = 15) and non-ameliorated FH patients (n = 15). c The kinetic changes in serum METRNL levels in ameliorated FH patients (n = 15) during follow-up are shown. d METRNL protein levels in liver tissues from HCs (n = 4) and patients with FH (n = 6) were measured by Western blotting. e Relative Metrnl mRNA levels in the liver tissues of HCs (n = 4) and FH patients (n = 6) were measured by Q-PCR. f Representative HE staining and METRNL immunohistochemical analysis of livers from HCs and FH patients are shown. The results are presented as medians with interquartile ranges and were analysed by the Kruskal‒Wallis test, Mann‒Whitney U test, and Wilcoxon test. *P < 0.05, **P < 0.01.
Given these findings, hepatic METRNL levels in FH patients and healthy controls were examined. Hepatic METRNL protein and mRNA levels were significantly increased in FH patients (Fig. 7d, e). The increases in hepatic METRNL levels were also confirmed by immunohistochemical (IHC) staining, which showed a higher percentage of METRNL-positive area in FH patients than in HCs (Fig. 7f).
INR, MELD and MELD-Na are commonly used parameters with good accuracy in predicting prognosis for FH patients [21]. Serum METRNL levels at baseline significantly correlated with the INR, MELD score, and MELD-Na score (Supplementary Fig. 5a–c). An optimal cut-off value of 1017 pg/mL for serum METRNL levels was used to discriminate the prognosis of FH patients. The sensitivity, specificity, positive predictive value, and negative predictive value were 80.00%, 73.33%, 78.57% and 75.00%, respectively. The area under the receiver operating characteristic curve of serum METRNL was 0.791 (P < 0.01) (Supplementary Fig. 5d). The survival rate of the baseline METRNL ≥1017 pg/mL group was significantly higher than that of the baseline METRNL <1017 pg/mL group (Supplementary Fig. 5e).
Discussion
Our findings demonstrated that serum and hepatic METRNL levels in FH mice were significantly increased. The main cell source of METRNL in the liver was macrophages rather than hepatocytes or HSCs. METRNL inhibited the release of proinflammatory cytokines by macrophages via the MAPK and NF-κB signaling pathways in vitro. Functional experiments showed that METRNL overexpression significantly ameliorated FH. METRNL overexpression reduced chemokine-dependent inflammatory cell infiltration into the liver and suppressed CD4+ T cell differentiation into Th1 cells. METRNL overexpression inhibited the secretion of Th1 cytokines. Consistently, serum and hepatic METRNL levels were increased in FH patients. Moreover, serum METRNL levels were related to the severity and prognosis of FH.
METRNL is essential in both innate and adaptive immunity and is widely expressed in various tissues [12]. Our data confirmed that METRNL was expressed in heart, adipose, skeletal muscle and liver tissues. It has been reported that METRNL plays a key role in ulcerative colitis, sepsis and other inflammatory diseases [22, 23]. Consistently, our data showed increased expression of METRNL in FH, which was characterized by an excessive inflammatory response. Single-cell sequencing showed that METRNL was mainly expressed in macrophages, with only a small amount of expression in monocytes, neutrophils and natural killer cells [16]. Our study determined that the main cell source of METRNL in the liver was macrophages. Thus, the increase in METRNL expression in macrophages may participate in the pathogenesis of FH.
The MAPK and NF-κB signaling pathways are classic inflammatory signaling pathways [24, 25]. A study by Reboll MR first confirmed that METRNL was a high-affinity ligand for the stem cell factor receptor KIT [18]. In our study, the expression of KIT was increased in macrophages after ConA treatment. KIT may promote the survival and migration of melanocytes during development, and excessive KIT activity can hyperactivate the RAS/MAPK pathway and drive the formation of melanomas [26]. In addition, KIT can bind to the promoter of NF-κB inhibitor beta (NF-κBIκB) and enhance IκB protein expression in GIST cells [27]. Our data showed that JNK, ERK, p65 and IκB-α were activated after ConA treatment. Furthermore, activation was partially inhibited after METRNL overexpression and was enhanced when METRNL was knocked down by siRNA. Therefore, we concluded that METRNL mediated the release of proinflammatory cytokines in macrophages via the KIT/MAPK and KIT/NF-κB signaling pathways. However, whether METRNL alleviated FH through the abovementioned signaling pathways remains to be further explored.
Immune cells and inflammatory cytokine networks are important factors that cause liver damage. It has been verified that METRNL regulates the production of different cytokines [28]. Serum METRNL levels are negatively correlated with sensitivity C-reactive protein, IL-1β, and TNF levels in patients with coronary heart disease [29]. Consistent with previous studies, our study demonstrated that METRNL overexpression suppressed the production of proinflammatory cytokines, thereby attenuating liver injury. It has been shown that IL-4 and IL-17A induce the production of METRNL in macrophages, and IFN-γ inhibits the secretion of METRNL [28], indicating a close relationship between METRNL and Th cytokines. Therefore, it is thought that METRNL may play a role in the differentiation of Th1, Th2 and Th17 cells. Our data showed that METRNL overexpression inhibited the differentiation of CD4+ T cells into Th1 cells, confirming the essential regulatory role of METRNL in the differentiation of Th1 cells. Moreover, in our study, IL-17A levels decreased with METRNL overexpression. However, METRNL overexpression had no regulatory effect on the differentiation of Th17 cells. This inconsistency may be because a small amount of IL-17A is secreted by other cells in addition to Th17 cells. There was no difference in IL-4 levels after METRNL overexpression, indicating that METRNL has no regulatory effect on the differentiation of Th2 cells.
Studies have shown that METRNL promotes the secretion of IL-10 by macrophages [28]. In our study, there was no significant difference in IL-10 between the OE + ConA group and the NC + ConA group. In cultured macrophages in vitro, METRNL promoted IL-10 production, but the effect of METRNL on IL-10 in vivo in the pathological context of FH is still unclear. Our study revealed that METRNL did not induce IL-10 levels in the FH mouse model. Recombinant METRNL promoted the secretion of chemokines, including CCL2 and CXCL1 by macrophages [28]. However, our data showed that liver CCL2 levels in the OE + ConA group were lower than those in the NC + ConA group, while CXCL1 levels showed no significant differences. This inconsistency may be caused by the different effects of METRNL under physiological and pathological conditions. This hypothesis is supported by the results of Zuo’s study [30], which showed that in mice with spontaneous enteritis, recombinant METRNL significantly reduced the infiltration of macrophages, which were mainly recruited by CCL2. Therefore, METRNL may play different biological roles in physiological and pathological conditions.
Overall, our research investigated whether elevated METRNL participated in the pathogenesis of FH. In vitro experiments showed that METRNL partially mediated the release of proinflammatory cytokines by macrophages through the MAPK and NF-κB signaling pathways. METRNL overexpression reduced chemokine-dependent immune cell infiltration into the liver and inhibited the differentiation of CD4+ T cells into Th1 cells. METRNL overexpression suppressed the secretion of Th1 cytokines, thereby reducing immune-mediated liver injury. Moreover, serum METRNL levels were related to disease severity. This is the first study to provide direct experimental evidence of the role of METRNL in FH, which supports the potential clinical applicability of pharmacologically targeting epigenetic modifications for the treatment of FH.
Supplementary information
Acknowledgements
This study was funded by the Natural Science Foundation of Shanghai (20ZR1433500), the Major Project of National Thirteenth Five-year Plan (2017ZX09304016), and the National Natural Science Foundation of China (8210030899).
Author contributions
YND performed the experiments and wrote the manuscript. JMT and BYD made the clinical diagnoses and collected the clinical samples. THZ performed the data analysis. WC designed and supervised the project and revised the manuscript for important content.
Data availability
The dataset (excluding personal identifiers) will be available to proper academic parties upon request from the corresponding author in accordance with the data sharing policies of Shanghai Jiao Tong University School of Medicine.
Competing interests
The authors declare no competing interests.
Consent to participate
Written consent was obtained from each subject.
Ethics approval
The study was approved by the Human Ethics Committee, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Footnotes
These authors contributed equally: Ya-nan Du, Jia-ming Teng
Contributor Information
Bing-ying Du, Email: dabenyu@126.com.
Wei Cai, Email: carieyc@hotmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-01049-4.
References
- 1.Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl. 2005;11:594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–61. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869–81. doi: 10.1016/S0140-6736(19)31894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. Electronic address eee, Clinical practice guidelines p. Wendon J, Panel M, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Moon JH, Koo BK, Kim W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2021;12:964–72. [DOI] [PMC free article] [PubMed]
- 6.Li T, Xu M, Kong M, Song W, Duan Z, Chen Y. Use of skeletal muscle index as a predictor of short-term mortality in patients with acute-on-chronic liver failure. Sci Rep. 2021;11:12593. doi: 10.1038/s41598-021-92087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, et al. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10:e00025. doi: 10.14309/ctg.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bay ML, Pedersen BK. Muscle-organ crosstalk: focus on immunometabolism. Front Physiol. 2020;11:567881. doi: 10.3389/fphys.2020.567881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitham M, Febbraio MA. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov. 2016;15:719–29. doi: 10.1038/nrd.2016.153. [DOI] [PubMed] [Google Scholar]
- 10.Monserrat-Mesquida M, Quetglas-Llabres M, Abbate M, Montemayor S, Mascaro CM, Casares M, et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants (Basel). 2020;9:759. [DOI] [PMC free article] [PubMed]
- 11.Ruiz-Margain A, Pohlmann A, Ryan P, Schierwagen R, Chi-Cervera LA, Jansen C, et al. Fibroblast growth factor 21 is an early predictor of acute-on-chronic liver failure in critically ill patients with cirrhosis. Liver Transpl. 2018;24:595–605. doi: 10.1002/lt.25041. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SL, Li ZY, Song J, Liu JM, Miao CY. Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin. 2016;37:571–9. doi: 10.1038/aps.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridgewood C, Russell T, Weedon H, Baboolal T, Watad A, Sharif K, et al. The novel cytokine Metrnl/IL-41 is elevated in Psoriatic Arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin Immunol. 2019;208:108253. doi: 10.1016/j.clim.2019.108253. [DOI] [PubMed] [Google Scholar]
- 15.Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol. 2015;156:119–27. doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab. 2020;2:278–89. doi: 10.1038/s42255-020-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) attenuates myocardial ischemia/reperfusion injury-induced cardiomyocytes apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 signaling in H9C2 cells. Med Sci Monit. 2020;26:e924564. doi: 10.12659/MSM.924564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reboll MR, Klede S, Taft MH, Cai CL, Field LJ, Lavine KJ, et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science. 2022;376:1343–7. doi: 10.1126/science.abn3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan H, Huang W, Wang Z, Ren F, Luo L, Zhou J, et al. The ACE2-Ang-(17)-Mas axis modulates M1/M2 macrophage polarization to relieve CLP-induced inflammation via TLR4-mediated NF-кb, Cyrillicb and MAPK pathways. J Inflamm Res. 2021;14:2045–60. doi: 10.2147/JIR.S307801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012;18:119–25. doi: 10.3748/wjg.v18.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan H, Fan J, Chen S, Chen Y, Gao H, Shan L, et al. Prognostic significance of end-stage liver diseases, respiratory tract infection, and chronic kidney diseases in symptomatic acute hepatitis E. Front Cell Infect Microbiol. 2020;10:593674. doi: 10.3389/fcimb.2020.593674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao ZW, Hu WJ, Li ZY, Miao CY. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41:1525–30. doi: 10.1038/s41401-020-00529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gholamrezayi A, Mohamadinarab M, Rahbarinejad P, Fallah S, Barez SR, Setayesh L, et al. Characterization of the serum levels of Meteorin-like in patients with inflammatory bowel disease and its association with inflammatory cytokines. Lipids Health Dis. 2020;19:230. doi: 10.1186/s12944-020-01404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Sun X, Zhao J, Xia L, Li J, Xu M, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17:753–64. doi: 10.1038/s41423-019-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 26.Neiswender JV, Kortum RL, Bourque C, Kasheta M, Zon LI, Morrison DK, et al. KIT suppresses BRAF(V600E)-mutant melanoma by attenuating oncogenic RAS/MAPK signaling. Cancer Res. 2017;77:5820–30. doi: 10.1158/0008-5472.CAN-17-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsueh YS, Chang HH, Shan YS, Sun HS, Fletcher JA, Li CF, et al. Nuclear KIT induces a NFKBIB-RELA-KIT autoregulatory loop in imatinib-resistant gastrointestinal stromal tumors. Oncogene. 2019;38:6550–65. doi: 10.1038/s41388-019-0900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ushach I, Arrevillaga-Boni G, Heller GN, Pone E, Hernandez-Ruiz M, Catalan-Dibene J, et al. Meteorin-like/Meteorin-beta is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201:3669–76. doi: 10.4049/jimmunol.1800435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZX, Ji HH, Yao MP, Wang L, Wang Y, Zhou P, et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J Cell Mol Med. 2019;23:271–80. doi: 10.1111/jcmm.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo L, Ge S, Ge Y, Li J, Zhu B, Zhang Z, et al. The adipokine metrnl ameliorates chronic colitis in Il-10-/- mice by attenuating mesenteric adipose tissue lesions during spontaneous colitis. J Crohns Colitis. 2019;13:931–41. doi: 10.1093/ecco-jcc/jjz001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset (excluding personal identifiers) will be available to proper academic parties upon request from the corresponding author in accordance with the data sharing policies of Shanghai Jiao Tong University School of Medicine.