Abstract
Introduction
The aim of this study was to investigate the effects of adding whole-plant ensiled corn stalks (WECS) to the diet of Holdorbagy geese on their growth performance, serum parameters, and cecal microbiota. Geese farming is an important agricultural practice, and optimizing their diet can contribute to better growth and health outcomes. However, there is limited research on the utilization of WECS as a feed source for geese. Understanding the potential effects of WECS on growth, blood parameters, and cecal microbiota can provide valuable insights into its feasibility and impact on geese farming practices.
Methods
A total of 144 six-week-old Holdorbagy geese were randomly assigned to one of three groups: a control group (0% WECS), a group fed 15% WECS and 85% concentrated feed (15% WECS), and a group fed 30% WECS and 70% concentrated feed (30% WECS). The trial period lasted for three weeks, during which the growth performance, serum parameters, and cecal microbiota were assessed.
Results
The results revealed significant findings in different aspects. Firstly, the feed-to-gain ratio (F/G ratio) of the 15% WECS group was significantly higher than that of the control group (p<0.05), indicating potential challenges in feed efficiency. Additionally, the average daily feed intake (ADFI) of both the 15% and 30% WECS groups was significantly higher than that of the control group (p<0.05), suggesting increased appetite or palatability of the diet containing WECS. In terms of serum parameters, the level of lactate dehydrogenase (LDH) in the 30% WECS group was significantly lower than that in the control group (p<0.05). Moreover, there was a tendency for increasing Fe levels and decreasing Zn levels with higher levels of WECS supplementation, although the differences were not statistically significant (p<0.05). Furthermore, the principal coordinate analysis showed significant differences in the composition of cecal microbiota among the three groups (p < 0.01). The observed_species, Shannon, and Pielou_e indices of the 30% WECS group were significantly higher than those of the 0% and 15% WECS groups (p<0.05), while the Simpson index of the 15% WECS group was significantly lower than that of the control group (p<0.05).
Discussion
The results indicate that the addition of WECS to the geese diet has both positive and negative effects. The study suggests that WECS can be a long-term stable feed source for geese, which can contribute to reducing feeding costs. However, it is important to monitor the amount of WECS added as it can affect the absorption of Zn by geese. Supplementation of Zn in the diet might be necessary to meet the needs of geese. Notably, adding 30% WECS to the diet can increase the richness, evenness, and diversity of the cecal microbiota, indicating potential benefits to gut health. In conclusion, this study highlights the potential of WECS as a feed source for geese. It provides valuable insights into the effects of WECS on growth performance, serum parameters, and cecal microbiota. These findings contribute to optimizing geese farming practices, improving feed utilization, and enhancing overall productivity and well-being of geese. Further research is needed to determine the optimal inclusion level of WECS and to explore strategies for mitigating any negative effects.
Keywords: whole-plant ensiled corn stalks, Holdobagy geese, growth performance, alpha diversity, principal coordinate analysis, Spearman’s analysis
Introduction
Geese are herbivorous waterfowl, especially in wild flocks. However, in modern intensive production systems, high-energy and high-protein concentrate feed has become the main component of meat goose diets. Although concentrate feed has high nutritional value, it is expensive. Solely feeding concentrate may bring uncontrollable nutritional diseases to goslings, thereby limiting meat goose production (1).
Therefore, people are increasingly interested in exploring unconventional feeds that can replace meat goose concentrate, reduce reliance on traditional feeds, and enhance sustainability (2). Whole-plant ensiled corn stalks (WECS) are one such alternative feed source. Compared to ordinary corn and ensiled straw, WECS have more complete and balanced nutritional components and potential benefits for animal health and welfare.
The digestive structure of geese has innate advantages in digesting high-fiber foods, such as WECS. Geese have a relatively independent and developed caecum, similar to the rumen of ruminants, which allows insufficiently digested food to stay inside for sufficient fermentation (3, 4). The caecum of geese contains cellulose-digesting microorganisms lacking in other avian digestive tracts, which convert cellulose into short-chain fatty acids (SCFAs) and provide energy to geese (5). In addition, the digestive tract of geese has strong motility and rotational ability, and the gizzard accounts for about 8% of their body weight (6). This helps to fully grind plant cell walls, promote the decomposition and utilization of cellulose (7).
Therefore, adding high-yield and low-cost WECS as an additional dietary supplement may reduce production costs and improve the intestinal health of geese. Although recent studies on using ensiled corn in meat goose production (8–10) have yielded encouraging results, the use of traditional ensiled corn stalks is mainly suitable for ruminants such as cows, beef cattle, etc., and their application in monogastric animals such as meat geese needs further research and confirmation.
Therefore, we conducted a study to explore the effects of adding different levels of WECS on meat goose growth performance and caecal microorganisms. The results of this study will help us understand the potential benefits and limitations of using WECS as meat goose feed and provide reference for their application in meat goose production.
Materials and methods
The experimental procedures were conducted in compliance with the guidelines established by the Chinese Animal Care and Protocol Committee and approved by the Animal Care and Use Committee of the Shanghai Academy of Agricultural Sciences.
Grouping and treatment of geese
A total of 144 six-week-old Holdorbagy geese were purchased from (Xiangtiange Poultry, Maanshan, Anhui, China). The geese were randomly divided into three treatment groups based on body weight, with each group having 8 replicates and 6 geese in each replicate. Specifically, the 0 WECS group was the control group, which was fed 100% commercial feed, while the 15 WECS group was fed a diet consisting of 15% whole-plant ensiled corn stover and 85% commercial feed, and the 30 WECS group was fed a diet consisting of 30% whole-plant ensiled corn stover and 70% commercial feed. The geese were raised in a net fence with dimensions of 2 meters in length, 2 meters in width, and 0.6 meters in height, and each fence was equipped with a drinking waterer and a feeding trough. The experiment lasted for 3 weeks.
Nutritional composition of diets and silage corn
TheWECS and concentrate feed required for the experiment were purchased from (Shengwang Feed Co., Ltd., Fengxian District, Shanghai, China). After air-drying, the dry matter, crude protein (CP), crude fiber (CF), ether extract (EE) and metabolizable energy (ME) of the WECS were measured according to the AOAC (1998) test method. Based on the WECS addition level, the daily feed of each group of test geese was designed according to the standards of the National Research Council (11). These feeds met the minimum nutritional requirements of geese during the growth period. The feed formula is shown in Table 1, and the nutritional level of the WECS is shown in Table 2.
Table 1.
Ingredients and nutrient compositions of experimental diets (air dry basis).
| Items | Diet | ||
|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | |
| Ingredient, % | |||
| Corn | 66.07 | 58.25 | 57.18 |
| Soybean meal (43% CP) | 26.42 | 26.99 | 27.22 |
| Soybean oil | 0.00 | 2.45 | 4.00 |
| Premix2 | 3.00 | 3.00 | 3.00 |
| Corn gluren | 4.51 | 4.51 | 5.00 |
| Silage corn (air dry basis) | 0.00 | 4.8 | 9.6 |
| Total | 100 | 100 | 100 |
| Calculated nutrient concentration1 | |||
| ME MJ/Kg | 11.77 | 11.71 | 11.48 |
| CP, % | 18.00 | 18.00 | 18.00 |
| EE, % | 3.00 | 2.89 | 2.82 |
| CF, % | 2.82 | 4.94 | 6.22 |
| Lysine, % | 1.01 | 1.01 | 1.01 |
| Methionine, % | 0.45 | 0.45 | 0.45 |
| Cystine, % | 0.40 | 0.40 | 0.40 |
| Calcium, % | 1.52 | 1.52 | 1.52 |
| Non-phytate, % | 0.40 | 0.40 | 0.40 |
| Arginine, % | 1.20 | 1.20 | 1.20 |
1Calculated nutrient concentration: Dietary nutrient composition are calculated values.
2Provided per kg of diet:Vitamin A 233000 IU, Vitamin B1 26.7 mg, Vitamin B2 (riboflavin) 150 mg, Pantothenic acid 5 mg, Vitamin B6 60 mg, Vitamin B12 0.3 mg, Vitamin D3 50,000 IU, Vitamin E 433.14 IU, Vitamin K3 16.7 mg, biotin 2.5 mg, folic acid 20 mg, nicotinamide 800 mg, choline chloride 10 g; Cu 0.3 mg, Fe 2.0 g, Mn 2.0 g, Zn 2.0 g, I 16.7 mg, Se 3.3 mg, Ca 4%, P 3.3%, salt 10 g. 0SC control group, 15SC 15% silage corn +85% concentrate feed, 30SC 30% silage corn +70% concentrate feed.
Table 2.
Nutrient composition of WECS (air dry basis).
| Items | Nutrient concentration1 |
|---|---|
| Dry matter, % | 32 |
| CP, % | 8.61 |
| ME, MJ/kg | 15.91 |
| Lysine, % | 0.90 |
| Methionine+ Cystine, % | 0.66 |
| Threonine, % | 0.63 |
| CF, % | 22.56 |
| EE, % | 0.5 |
1Nutrient concentration: Silage corn nutrient composition is measured.
Sample collection
In the third week of the experiment, all geese were weighed after an 8-h fast. One male goose with a weight close to the average weight was selected for each replicate group, and its brachial vein blood was collected. The collected blood was left to stand at room temperature for 2 h, then centrifuged at 3000 rpm for 10 min to separate the serum. The serum was collected and used for subsequent indicator measurements. After blood collection, the geese were intravenously injected with 5 mL of 3% pentobarbital solution, then slaughtered by bleeding from the jugular vein. The abdominal cavity of the goose was opened, and the cecum was cut open to collect its contents in a 2 mL EP tube. Finally, the collected cecal contents were rapidly frozen in liquid nitrogen and stored at −80°C for subsequent indicator measurements.
Growth performance determination
On the day before the experiment began and ended, geese were fasted for 8 h overnight, and then weighed the next morning. The Initial Body Weight (IBW) and Final Body Weight (FBW) of each goose were recorded. The feed consumption of each replicate group was recorded during the entire experimental period, and the Average Daily Feed Intake (ADFI), Average Daily Gain (ADG), and Feed Gain ratio (F/G) were calculated.
The calculation methods of growth performance indicators were as follows:
Determination of blood parameters
The serum, which was stored at −80°C after centrifugation, was sent to (Ingle Testing Technology Service Co., Ltd., Shanghai, China) for the detection of serum calcium (Ca), phosphorus (P), zinc (Zn), and iron (Fe). At the same time, the serum stored at −80°C was also sent to (Shanghai Pinyi Biotechnology Co., Ltd., Shanghai, China) for the detection of serum ghrelin (GHRL), insulin-like growth factor-1 (IGF-1), lactate dehydrogenase (LDH), and serum amylase (AMY).
16S rRNA sequencing
The contents of the cecum were sent to Passino Biotechnology Co., Ltd. in Shanghai, China for 16S rRNA analysis and data processing. To put it simply, first, fecal genomic DNA extraction kits (Tiagen Biotech, Beijing, China) were used to extract DNA from the cecal contents. After assessing the quality and quantity of the extracted DNA, universal primers (F:ACTCCTACGGGAGGCAGCA and R:GGACTACHVGGTWTCTAATPCR) were used to amplify the V3-V4 region of the 16S rDNA, and a library was constructed. These libraries were then subjected to paired-end sequencing on the Illumina platform (Illumina, San Diego, California, United States) (12). The QIIME DADA2 DENOISE-PAIRED tools were used for quality control, denoising, stitching, and chimera filtering. After denoising all libraries, the ASVs (AMPLICON Sequence Variants) and ASV tables were combined, and singleton ASVs were removed.
Statistics and analysis
All data were initially processed using Microsoft Excel 2003 (Redmond, Washington, United States) and subsequently analyzed using SPSS 17.5 (International Business Machines Corporation, Armonk, New York, United States) software, which performed one-way ANOVA and Duncan multiple comparison tests to determine the differences between the experimental groups. All data were presented as mean ± standard error of the mean (SEM). Differences with p < 0.05 were considered significant, while differences with p < 0.01 were considered highly significant. To annotate species from the phylum to genus level, we employed the classify-sklearn algorithm (13) in QIIME2 (14) software, utilizing a pre-trained Naive Bayes classifier. Furthermore, we calculated the alpha diversity index using the ggplot2 package in the R language of QIIME2 software and generated dilution and abundance grade curves using an R script. To visualize the flattened ASV table, we utilized the ape package in the R language of QIIME2 software to calculate the Bray-Curtis distance (15) and created a two-dimensional scatter plot using an R script.
Result
Growth performance
Table 3 indicates that there were no significant distinctions (p > 0.05) in the ADG, IBW, and FBW of the experimental geese in the different treatment groups. However, the F/G of the geese in the 15 WECS group was significantly higher than that of the 0 WECS group (p < 0.05). Moreover, the ADFI of the geese in the 15 WECS and 30 WECS groups were significantly higher than that of the 0 WECS group (p < 0.01).
Table 3.
Effects of feeding WECS on the growth performance of geese.
| Item | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| IBW, g | 2513.71 ± 29.37 | 2530.97 ± 29.41 | 2532.45 ± 30.07 | 0.884 |
| FBW, g | 4239.61 ± 69.39 | 4367.77 ± 84.61 | 4344.61 ± 44.31 | 0.458 |
| ADG, g | 82.19 ± 2.49 | 87.46 ± 2.97 | 86.29 ± 3.05 | 0.392 |
| F/G | 5.24 ± 0.25a | 6.39 ± 0.37b | 6.22 ± 0.11ab | 0.027 |
| ADFI, g | 437.44 ± 13.19A | 556.83 ± 18.50B | 536.84 ± 19.28B | p<0.001 |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
a–b Means with different superscript letters in the same row differ significantly (p < 0.05).
A–B Means with different superscript letters in the same row differ extremely significantly (p < 0.01).
Serum minerals
Based on Table 4, there were no statistically significant differences in the levels of Ca, P, Fe and Zn in the serum of geese across the different treatment groups (p>0.05). However, there was a tendency toward an increase in Fe levels and a decrease in Zn levels in the serum of geese in the experimental group as the proportion of WECS increased, although these trends were not statistically significant (p > 0.05).
Table 4.
Effects of feeding WECS on serum mineral levels of geese.
| Items | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| Ca, mmol/L | 1.64 ± 0.25 | 1.86 ± 0.17 | 1.81 ± 0.17 | 0.720 |
| P, mmol/L | 1.57 ± 0.21 | 1.85 ± 0.15 | 1.72 ± 0.17 | 0.545 |
| Fe, μmol/L | 53.41 ± 7.73 | 69.19 ± 5.00 | 76.28 ± 8.52 | 0.096 |
| Zn, μmol/L | 122.33 ± 24.75 | 103.55 ± 13.52 | 77.91 ± 16.68 | 0.275 |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
Serum hormones and enzymes
As shown in Table 5, there were no significant differences in the levels of GHRL, LGF-1, and AMY in the serum of the experimental geese across the treatment groups (p > 0.05). Nevertheless, the feeding of WECS led to a reduction in the serum LDH level of the experimental geese, with a significant difference observed at 30% WECS addition (p < 0.05).
Table 5.
Effects of feeding WECS on serum hormones and enzymes levels of geese.
| Items | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| GHRL, ng/mL | 90.51 ± 11.73 | 104.57 ± 11.75 | 100.36 ± 26.90 | 0.855 |
| IGF-1, ng/mL | 99.99 ± 8.59 | 96.52 ± 8.12 | 96.26 ± 23.07 | 0.981 |
| LDH, ng/mL | 65.68 ± 7.02a | 40.27 ± 7.47ab | 29.67 ± 12.54b | 0.044 |
| AMY, μg/mL | 13.40 ± 0.46 | 11.78 ± 0.55 | 10.94 ± 2.39 | 0.639 |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
a–b Means with different superscript letters in the same row differ extremely significantly (p < 0.05).
Sequence quality assessment
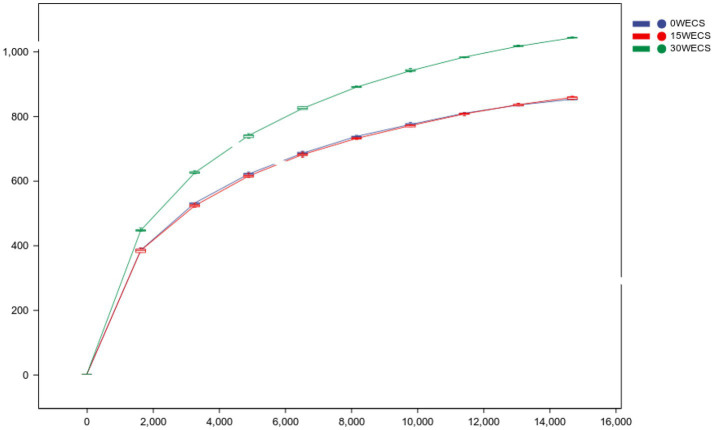
Table 6 illustrates that the original data contained 911,642 ASVs with matching forward and reverse primers. After quality control, denoising, splicing, chimera removal, and removal of singletons, a total of 460,877 high-quality ASVs were retained, with an effective ratio of 50.55%. Figure 1 depicts the length and percentage distribution of high-quality sequences in the samples. The length of high-quality sequences ranges from 405–432 bp, with 39.77% of sequences having a length of 425 bp, and 0.25% of sequences having a length of 409 bp, which is the lowest percentage. Notably, no abnormal sequence lengths were detected.
Table 6.
Sample sequencing volume statistics.
| SampleID | Input | Filtered | Denoised | Merged | Non-chimeric | Non-singleton |
|---|---|---|---|---|---|---|
| 0 WECS 1 | 33,538 | 28,677 | 26,942 | 19,945 | 16,895 | 16,710 |
| 0 WECS 2 | 33,578 | 29,169 | 28,031 | 23,123 | 18,501 | 18,385 |
| 0 WECS 3 | 38,856 | 34,113 | 32,744 | 24,367 | 19,668 | 19,418 |
| 0 WECS 4 | 39,960 | 34,238 | 32,291 | 24,952 | 22,130 | 21,963 |
| 0 WECS 5 | 38,104 | 33,337 | 31,865 | 25,528 | 20,717 | 20,471 |
| 0 WECS 6 | 34,625 | 29,069 | 27,300 | 19,077 | 17,113 | 16,912 |
| 0 WECS 7 | 44,965 | 37,540 | 35,156 | 26,221 | 21,088 | 20,712 |
| 0 WECS 8 | 34,808 | 29,545 | 28,184 | 21,439 | 17,210 | 16,999 |
| 15 WECS 1 | 36,690 | 32,005 | 30,337 | 23,243 | 20,446 | 20,272 |
| 15 WECS 2 | 37,922 | 32,662 | 31,051 | 24,079 | 19,554 | 19,241 |
| 15 WECS 3 | 37,072 | 32,544 | 31,421 | 24,967 | 21,371 | 21,205 |
| 15 WECS 4 | 37,691 | 32,757 | 31,426 | 24,865 | 20,846 | 20,625 |
| 15 WECS 5 | 36,976 | 32,361 | 31,052 | 26,055 | 22,361 | 22,247 |
| 15 WECS 6 | 37,266 | 32,666 | 31,047 | 23,700 | 19,985 | 19,723 |
| 15 WECS 7 | 39,791 | 33,769 | 31,609 | 23,130 | 20,534 | 20,319 |
| 15 WECS 8 | 45,359 | 39,179 | 37,526 | 29,990 | 25,670 | 25,388 |
| 30 WECS 1 | 36,613 | 30,755 | 28,998 | 22,792 | 17,491 | 17,189 |
| 30 WECS 2 | 40,590 | 34,004 | 31,898 | 22,485 | 18,422 | 18,080 |
| 30 WECS 3 | 37,109 | 32,481 | 30,706 | 23,207 | 18,451 | 18,133 |
| 30 WECS 4 | 36,721 | 30,747 | 29,171 | 23,503 | 18,224 | 17,878 |
| 30 WECS 5 | 42,928 | 37,316 | 34,803 | 23,881 | 18,732 | 18,267 |
| 30 WECS 6 | 38,232 | 33,188 | 30,989 | 22,441 | 17,722 | 17,231 |
| 30 WECS 7 | 34,688 | 29,468 | 27,609 | 21,337 | 15,676 | 15,464 |
| 30 WECS 8 | 37,560 | 32,671 | 30,684 | 22,955 | 18,500 | 18,045 |
The second column indicates the number of sequences that matched the forward and reverse primers in the original data. The third column displays the amount of data after removal of low-quality sequences. The fourth column shows the sequence data after denoising. The fifth column represents the sequence amount after splicing. The sixth column displays the sequence amount after removing chimeras. The seventh column represents the sequence amount after removing singleton.
Figure 1.
Rarefaction Curve. The abscissa is the leveling depth, and the ordinate is the median value and boxplot of the alpha diversity index calculated 10 times. 0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
Alpha diversity
Table 7 shows the alpha diversity indices between groups, and the Goods_coverage for all test groups is >98%, indicating that the sequencing results can accurately represent the real situation of the samples. The observed_species, Shannon, and Pielou_e indices in group 30 WECS were significantly higher than those in groups 0 WECS and 15 WECS (p < 0.05), while the Simpson index in group 15 WECS was significantly lower than that in groups 0 WECS and 30 WECS (p < 0.05).
Table 7.
Effects of WECS on α-diversity of cecal contents bacterial communities (n = 8).
| Items | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| Observed_species | 853.34 ± 53.63b | 857.35 ± 57.12b | 1042.70 ± 56.37a | 0.040 |
| Shannon | 7.15 ± 0.23b | 7.09 ± 0.21b | 7.89 ± 0.15a | 0.016 |
| Simpson | 0.96 ± 0.01b | 0.95 ± 0.01a | 0.98 ± 0.00b | 0.042 |
| Chao1 | 944.52 ± 61.39 | 989.27 ± 76.01 | 1138.89 ± 63.53 | 0.126 |
| Pielou_e | 0.74 ± 0.02b | 0.73 ± 0.01b | 0.79 ± 0.01a | 0.019 |
| Faith_pd | 42.57 ± 1.23 | 41.99 ± 1.80 | 46.75 ± 1.51 | 0.079 |
| Goods_coverage | >98% | >98% | >98% | / |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
a–b Means with different superscript letters in the same row differ significantly (p < 0.05).
Rarefaction curve
Figure 1 shows that, at the same sequencing depth, the dilution curves of the 0 WECS and 15 WECS groups are relatively flat. This suggests that the diversity and uniformity of microbial species in these two groups are similar. On the other hand, the dilution curve of the 30 WECS group is relatively steep and higher, indicating a greater diversity of community composition in the 30 WECS group.
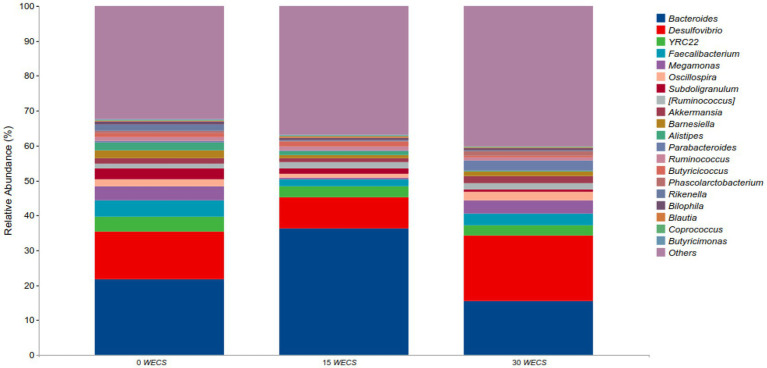
Composition of cecal contents at the level of phyla
Figure 2 shows the relative abundance at phylum level in each group, and it can be found that the dominant bacteria groups in the cecum of geese in each group are Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria. Table 8 shows the phyla with differences between groups, with a significant increase in Bacteroidetes abundance in the 15 WECS group compared to the 0 WECS and 30WECS groups (p < 0.01).
Figure 2.
Analysis of the composition of cecal contents at the level of phyla (n = 8). 0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed. In the figure, the abscissa is the name of the grouping scheme, and the ordinate is the relative abundance of each taxonomic at the phylum taxonomic level.
Table 8.
Analysis of the composition of cecal contents at the level of genus (n = 8).
| Items | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| Bacteroidetes, (%) | 41.79 ± 3.21B | 54.94 ± 2.41A | 36.78 ± 2.15B | p<0.001 |
| Firmicutes, (%) | 39.88 ± 3.15 | 35.14 ± 1.10 | 37.13 ± 3.00 | 0.477 |
| Proteobacteria, (%) | 15.39 ± 2.33 | 9.96 ± 2.53 | 21.50 ± 5.08 | 0.094 |
| Verrucomicrobia, (%) | 0.25 ± 0.13 | 1.25 ± 0.61 | 0.63 ± 0.36 | 0.284 |
| Actinobacteria, (%) | 0.27 ± 0.07 | 0.24 ± 0.07 | 0.27 ± 0.06 | 0.938 |
| Tenericutes, (%) | 0.45 ± 0.15 | 0.24 ± 0.04 | 0.41 ± 0.10 | 0.332 |
| Cyanobacteria, (%) | 0.05 ± 0.02 | 0.13 ± 0.05 | 0.31 ± 0.12 | 0.090 |
| Elusimicrobia, (%) | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.12 ± 0.05 | 0.229 |
| Others, (%) | 0.52 ± 0.07 | 0.40 ± 0.03 | 0.40 ± 0.06 | 0.200 |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
A–B Means with different superscript letters in the same row differ extremely significantly (p < 0.01).
Composition of cecal contents at the level of genus
Figure 3 displays the relative abundance of each group at the genus level, with Bacteroides, Desulfovibrio, YRC22, and Faecalibacterium as the dominant genera. Table 9 highlights some genera with significant differences between groups. Compared to group 0 WECS, the abundance of Rikenella and Alistipes in groups 15 WECS and 30 WECS was significantly lower (p < 0.01). The abundance of Bacteroides in group 15 WECS was significantly higher than in groups 0WECS and 30 WECS whereas the abundance of Butyricicoccus was significantly higher in group 15 WECS (p < 0.01). Moreover, the abundance of Parabacteroides in the 30 WECS group was significantly higher than that in the 0 WECS and 15 WECS groups (p < 0.01).
Figure 3.
Analysis of the composition of cecal contents at the level of genus (n = 8), 0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed. In the figure, the abscissa is the name of the grouping scheme, and the ordinate is the relative abundance of each taxonomic at the genus taxonomic level.
Table 9.
Analysis of the composition of cecal contents at the level of genus (n = 8).
| Item | Diet treatment | value of p | ||
|---|---|---|---|---|
| 0 WECS | 15 WECS | 30 WECS | ||
| Bacteroides, (%) | 21.67 ± 4.11B | 36.37 ± 2.43A | 15.48 ± 2.83B | p<0.001 |
| Desulfovibrio, (%) | 13.62 ± 2.52 | 8.94 ± 2.63 | 18.68 ± 4.71 | 0.159 |
| YRC22, (%) | 4.32 ± 1.24 | 3.01 ± 0.56 | 3.01 ± 0.76 | 0.501 |
| Faecalibacterium, (%) | 4.66 ± 1.12 | 1.35 ± 0.70 | 3.28 ± 0.73 | 0.149 |
| Megamonas, (%) | 1.58 ± 0.91 | 0.42 ± 0.38 | 1.54 ± 0.88 | 0.431 |
| Oscillospira, (%) | 2.02 ± 0.35 | 1.24 ± 0.30 | 2.60 ± 0.55 | 0.089 |
| Subdoligranulum, (%) | 3.22 ± 1.39 | 1.50 ± 0.59 | 0.61 ± 0.15 | 0.665 |
| [Ruminococcus], (%) | 0.92 ± 0.20 | 1.74 ± 0.49 | 1.49 ± 0.22 | 0.383 |
| Akkermansia, (%) | 0.25 ± 0.13 | 1.25 ± 0.61 | 0.52 ± 0.37 | 0.338 |
| Barnesiella, (%) | 2.55 ± 1.48 | 0.79 ± 0.26 | 1.34 ± 0.44 | 0.497 |
| Alistipes, (%) | 2.21 ± 0.47A | 1.18 ± 0.10B | 0.35 ± 0.05C | p<0.001 |
| Parabacteroides, (%) | 0.50 ± 0.12B | 0.23 ± 0.06B | 2.88 ± 0.93A | 0.003 |
| Ruminococcus, (%) | 1.10 ± 0.29 | 1.12 ± 0.13 | 0.92 ± 0.16 | 0.251 |
| Butyricicoccus, (%) | 1.13 ± 0.32 | 0.89 ± 0.17 | 0.47 ± 0.04 | 0.149 |
| Phascolarctobacterium, (%) | 0.77 ± 0.18 | 0.30 ± 0.07 | 1.25 ± 0.62 | 0.237 |
| Rikenella, (%) | 1.68 ± 0.44B | 0.17 ± 0.05A | 0.31 ± 0.13A | 0.003 |
| Bilophila, (%) | 0.17 ± 0.05 | 0.55 ± 0.24 | 0.61 ± 0.40 | 0.299 |
| Blautia, (%) | 0.32 ± 0.07 | 0.41 ± 0.27 | 0.15 ± 0.03 | 0.058 |
| Coprococcus, (%) | 0.13 ± 0.07 | 0.29 ± 0.12 | 0.21 ± 0.03 | 0.134 |
| Butyricimonas, (%) | 0.20 ± 0.02AB | 0.25 ± 0.05A | 0.12 ± 0.02B | 0.007 |
| Others, (%) | 32.43 ± 1.47 | 36.90 ± 2.53 | 36.50 ± 1.47 | 0.094 |
0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed.
a–b Means with different superscript letters in the same row differ significantly (p < 0.05).
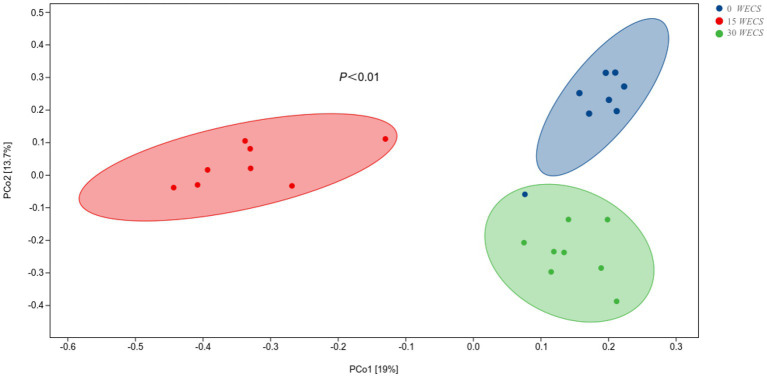
Analysis of The distance matrix and PCoA
The results of Principal coordinates analysis (PCoA) based on Bray-Curtis algorithm are shown in Figure 4, where Pco1 value is 19% and Pco2 value is 13.7%. The projection of inter-group samples on the matrix is far away, while intra-group samples are relatively clustered together, indicating that the community composition of each group of samples in the corresponding dimension is not similar.
Figure 4.
Principal coordinate analysis (PCoA) on community structures of the cecal microbiota after silage corn treatment (n = 8), 0 WECS control group, 15 WECS 15% whole-plant ensiled corn stalks +85% concentrate feed, 30 WECS 30% whole-plant ensiled corn stalks +70% concentrate feed. Each point in the figure represents a sample, and different colored points indicate different groupings. The percentages in the brackets on the axes represent the proportion of the sample difference data (the distance matrix) that can be explained by the corresponding axes.
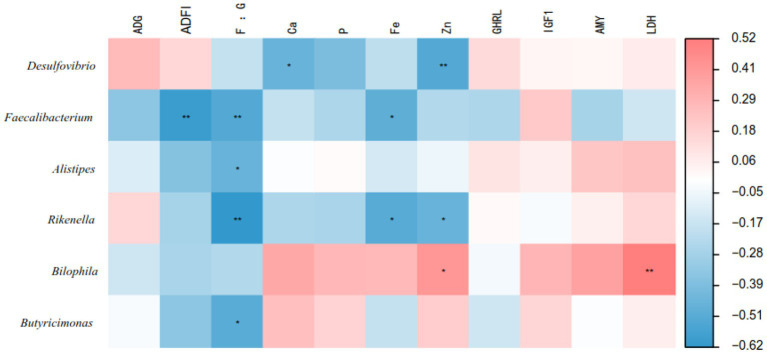
Correlation analysis
Figure 5 illustrates the correlation analysis between the bacterial genera of the cecal microbiota of geese and their growth performance and serum parameters. The results showed that Faecalibacterium was negatively correlated with ADFI (p < 0.05). In addition, Faecalibacterium, Alistipes, Rikenella and Butyricimonas were negatively correlated with the F/G ratio (p < 0.05). Faecalibacterium and Rikenella were negatively correlated with serum Fe levels (p < 0.05). Desulfovibrio and Rikenella were negatively correlated with serum Zn levels (p < 0.05), while Bilophila was positively correlated with serum Zn levels (p < 0.05). Bilophila was positively correlated with serum LDH levels (p < 0.05).
Figure 5.
Relationships between growth performance, blood parameters and the top 20 genera in the cecum. Red represents positive correlation, blue represents negative correlation, and *p < 0.05 and **p < 0.01 represent significant correlations.
Discussion
Whole-plant ensiled corn stover (WECS) is produced by chopping and ensiling the entire corn plant, including the stover, with the addition of a fermentation inoculant in a sealed container to allow for anaerobic fermentation (16). This process creates a low pH environment that promotes the growth of beneficial bacteria while inhibiting the proliferation of spoilage organisms (17). Geese fed on WECS not only directly absorb and utilize existing organic acids, but also promote the colonization of beneficial bacteria in their gastrointestinal tract. Moreover, WECS is rich in crude fiber, which can be partially digested and utilized, thereby increasing feed conversion rate and improving intestinal health. During the season of low availability of fresh green forage, WECS can provide animals with a stable source of nutrients and microbial biomass, and its high quality as a silage feed has been widely applied in animal production. Zhang et al. (18) showed that feeding WECS to fattening bulls increased their average daily weight gain, reduced production costs, and improved rumen degradable protein and fermentation efficiency. Wu et al. (19) found that compared to whole-plant sorghum silage, feeding WECS significantly improved lamb growth performance and carcass traits. He et al. (20) found that compared to other crop straws, WECS could increase the activity of proteases in the gastrointestinal tract of geese, indicating that WECS can improve protein digestion in geese. The results of this experiment showed that feeding WECS significantly increased the average daily feed intake of Holdobagy, and adding 15% WECS increased the F/G of geese, indicating that WECS has good palatability for geese, and its acid aroma can enhance their appetite. Although the digestibility and utilization of WECS by geese is not high, resulting in an increase in F/G, feeding WECS does not affect the FBW and ADG of geese, which is consistent with the results of (21). From an economic point of view, although adding WECS may increase feed consumption in the goose farm, it will not have a negative impact on the growth of meat geese, and the price of WECS is less than half that of ordinary corn. Therefore, feeding WECS can improve the economic benefits of the farm, and the amount of WECS added should not be too small.
Fe is a major component of hemoglobin and myoglobin in the body, as well as an important component of various enzymes involved in physiological processes such as oxygen transport, immunity, and metabolism (22). Zn is an important cofactor for various enzymes and is involved in various metabolic and physiological processes such as protein synthesis, DNA synthesis, and maintenance of the immune system (23). Our research results show that feeding WECS affects the levels of Fe and Zn in goose serum, with the level of Fe in goose serum positively correlated with the addition level of WECS, and the level of Zn in goose serum negatively correlated with the addition level of WECS. This may be due to the stimulation of high levels of fiber and lignin in WECS, which continuously stimulate the proventriculus to secrete gastric acid, which aids in the digestion of iron, while possibly inhibiting the absorption of Zn, leading to an increase in Fe levels and a decrease in Zn levels in goose serum (24). In addition, the cellulose and anti-nutritional factor phytic acid in WECS may form complexes with zinc, reducing its bioavailability. Lactate dehydrogenase (LDH) is an enzyme that catalyzes reactions in animals and is widely present in the cytoplasm. LDH can catalyze the oxidative decomposition of lactate to provide energy for the body (25). The results of this experiment show that after feeding WECS, the level of LDH in goose serum significantly decreased and was negatively correlated with the addition level of WECS. The reason for the decrease in LDH may be due to the rapid synthesis and consumption of lactate. There are few nutrients in WECS feed that can be directly digested and absorbed by geese, and structural polysaccharides such as cellulose and hemicellulose need to be fermented and metabolized by microorganisms to produce organic acids such as lactate. The lactate is then directly provided to intestinal microorganisms and animal cells for oxidative decomposition to produce pyruvate and ATP, and the resulting pyruvate continues to participate in the tricarboxylic acid cycle to provide energy for the body, compensating for the low nutrient concentration in WECS feed to maintain normal goose life activities (26). In addition, the decrease in Zn levels in goose serum after feeding WECS may also be one of the reasons for the decrease in LDH levels. Zn is an important component of LDH, maintaining its stability and catalytic activity, and the decrease in serum Zn levels may also lead to a decrease in LDH levels (27). The decrease in LDH levels indicates that geese have a considerable digestive ability for WECS, but it should be noted that in diets with high proportions of WECS, additional Zn should be added to meet the needs of geese.
Principal Coordinates Analysis (PCoA) results showed two-dimensional projected distances indicating differences in bacterial community between samples with different WECS ratios. This suggests that varying WECS addition ratios could have an impact on the microbial composition of the goose cecum. Alpha diversity analysis indicated that the cecal microorganisms of geese in the 30WECS group had higher observed species, Shannon, and Pielou’s evenness indexes, suggesting that increasing the proportion of WECS could enhance the richness, uniformity, and diversity of goose cecal microorganisms. These findings are consistent with the results of Chao et al. (28).
Similar to previous studies, this experiment found that the phyla Bacteroidetes, Firmicutes, and Proteobacteria were the dominant bacterial phyla in the cecum of geese. Although adding WECS did not change the dominant bacterial phyla, it did affect their relative abundance (29, 30). Bacteroidetes is a gram-positive bacterial phylum associated with weight gain and feed intake (31, 32). The results of this experiment showed that the abundance of Bacteroidetes increased and then decreased with the increasing proportion of WECS addition. This may be due to the nutritional concentration of the feed and the digestive characteristics of the goose. Adding WECS can reduce the nutritional concentration of the feed, forcing geese to increase their feed intake to maintain their energy levels. At low addition proportions, the feed intake of geese increases, causing the food renewal rate in their intestines to accelerate. However, the cecum is not sufficiently adapted to the cellulose in WECS, which reduces the efficiency of digestion and absorption of feed nutrients and slows down weight gain. An increase in feed intake can increase the abundance of Bacteroidetes (33). However, as the proportion of WECS increases, more organic acids enter the goose’s intestines, which can be directly digested and utilized. The renewal rate of food in the intestine slows down, and the cecum becomes more adapted to the cellulose in WECS (34). This leads to an increase in the digestion and absorption rate of cellulose and feed, a decrease in feed intake, and a decrease in the abundance of Bacteroidetes (35). Additionally, the study found a positive correlation between the relative abundance of Cyanobacteria and the proportion of WECS addition, but the difference was not significant. The increase in abundance of Cyanobacteria may be due to the residual cyanobacteria on WECS entering the cecum of the digestive tract (7).
Alistipes is a genus of bacteria belonging to the family Bacteroidetes, which is widely found in the cecum of geese (10, 36). Our experimental results indicate that the abundance of Alistipes is negatively correlated with the proportion of WECS in the feed, suggesting that this bacterium avoids a diet rich in plant-based food and grows well in a low-fiber diet (37). Kong et al. (38) found that the abundance of Alistipes in the cecum of mice fed a high-fat, high-sugar diet increased significantly, which is consistent with our results. Studies suggest that Alistipes may play an important role in the breakdown of complex carbohydrates and the production of SCFAs, which can be used as a source of energy for the host. The abundance of Alistipes may be related to the concentration of starch in the cecum (39). Unlike geese fed concentrated feed, the starch concentration in the cecum contents of geese fed WECS is lower, resulting in a decrease in the abundance of Alistipes. Rikenella is a gram-negative rod-shaped bacterium and a member of the Rikenellaceae family, found in the cecum and feces of birds. Our research results indicate that the level of Rikenella in goose cecum was significantly reduced after feeding with WECS, consistent with the findings of (18). The decrease in Rikenella abundance may be attributed to its narrow metabolic capability. The main carbon source for Rikenella growth is glucose, and it can hardly hydrolyze the fiber polysaccharides in WECS, which may be the reason for its reduced abundance in the cecum (40). Parabacteroides is a genus of bacteria belonging to the family Bacteroidaceae, which is a common component of the human and animal gut microbiota. Currently, there is limited research on the role of Parabacteroides in the goose gut, but it has been shown to be one of the main species involved in the digestion of dietary fiber in the human gut, producing SCFAs that maintain gut health (41, 42). Our experimental results show that adding 30% WECS to the feed significantly increases the abundance of Parabacteroides in the goose cecum. Therefore, the abundance of Parabacteroides is positively correlated with the dietary fiber level in the feed. Butyricimonas is a Gram-negative anaerobic bacterium belonging to the family Lachnospiraceae, which ferments dietary fiber to produce butyric acid, providing energy to intestinal wall cells and maintaining a healthy gut microbiota, preventing enteritis (43). Our experimental results indicate that adding 15% ensiled corn significantly increases the abundance of Butyricimonas in the goose cecum. However, when the addition amount reaches 30%, the abundance of Butyricimonas decreases. This may be related to the pH of the ensiled corn feed, as high addition amounts can lower the pH of the feed and have an adverse effect on the cell wall of Butyricimonas (44).
Conclusion
In summary, adding WECS to the diet may increase feed consumption in geese, but it does not have a negative impact on their growth and can increase economic benefits. However, it is important to pay attention to the amount of WECS added and supplement the diet with Zn to meet the geese’s normal needs. The proportion of WECS in the diet does not appear to affect the major bacterial phyla in the goose cecum, but it does impact the overall microbial composition and abundance of certain bacterial phyla and genera. Increasing the proportion of WECS in the feed can enhance the richness, evenness, and diversity of the cecal microbiota. As the amount of WECS added to the diet increases, the abundance of Alistipes decreases. On the other hand, the abundance of Parabacteroides increases with an increasing proportion of WECS, while the abundance of Butyricimonas increases when 15% WECS is added and decreases when 30% WECS is added to ensiled corn.
Methods
All animal protocols were approved by the Shanghai Science and Technology Committee (STCSM) with a license number of SYXK (HU): 2015–0007, and carried out in accordance with approved guidelines and regulations. Every effort was made to minimise the suffering of the geese in the current study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject; PRJNA961934.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Shanghai Academy of Agricultural Sciences (SAASPZ0522046), Shanghai Academy of Agricultural Sciences.
Author contributions
XW completed the experimental manuscript’s initial draft writing and inspection. HW and DH carried out the experimental design and paper editing. YY, GL, CW, and YL performed the data software analysis and sample collection. SG participated the on-site breeding of geese. All authors mentioned above have reviewed and approved the final manuscript.
Funding
This study was financially supported by the Climbing plan of Shanghai Academy of Agricultural Sciences [PG23171], the Earmarked Fund for China Agriculture Research System [CARS-42-35] and the SAAS Program for Excellent Research Team (SPERT), and the Special Project of Modern Agriculture [ZY221702].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Ma W, Zhou L, Li Y, Xia D, Chen J, Chen J, et al., Persistent purine metabolic abnormality induces the aggravation of visceral inflammation and intestinal microbiota Dysbiosis in Magang goose. Front Vet Sci. (2021) 8:737160. doi: 10.3389/fvets.2021.737160, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng M, Mao P, Tian X, Guo Q, Meng L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult Sci. (2019) 98:2250–2259. doi: 10.3382/ps/pey550, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Jamroz D, Jakobsen K, Orda J, Skorupinska J, Wiliczkiewicz A. Development of the gastrointestinal tract and digestibility of dietary fibre and amino acids in young chickens, ducks and geese fed diets with high amounts of barley. Comp Biochem Physiol A. (2001) 130:643–652. doi: 10.1016/s1095-6433(01)00386-5, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Li M, Zhou H, Xu T, Zi X. Effect of cassava foliage on the performance, carcass characteristics and gastrointestinal tract development of geese. Poult Sci. (2019) 98:2133–2138. doi: 10.3382/ps/pey567, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Xi Y, Huang Y, Li Y, Huang Y, Yan J, Shi Z. The effects of dietary protein and fiber levels on growth performance, gout occurrence, intestinal microbial communities, and immunoregulation in the gut-kidney axis of goslings. Poult Sci. (2022) 101:101780. doi: 10.1016/j.psj.2022.101780, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Zheng LY, Yang L, Li A, Gao YY. Effect of dietary fibre and grit on performance, gastrointestinal tract development, and grit pattern of goose. Br Poult Sci. (2020) 61:408–413. doi: 10.1080/00071668.2020.1736267, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Li X, Zheng Z, Pan J, Jiang D, Tian Y, Fang L, et al., Impacts of colored light-emitting diode illumination on the growth performance and fecal microbiota in goose. Poult Sci. (2020) 99:1805–1812. doi: 10.1016/j.psj.2019.12.034, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan R, Öztürk E. Effects of maize silage feeding on growth performance, carcass characteristics, digestive system length, chemical composition, and meat quality of domestic geese. Trop Anim Health Prod. (2022) 54:325. doi: 10.1007/s11250-022-03313-5, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Kokoszyński D, Bernacki Z, Grabowicz M, Stańczak K. Effect of corn silage and quantitative feed restriction on growth performance, body measurements, and carcass tissue composition in white Kołuda W31 geese. Poult Sci. (2014) 93:1993–99. doi: 10.3382/ps.2013-03833, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Liu B Y, Wang Z Y, Wang H R, Hu P, Xu D, Wang Q. Molecular profiling of bacterial species in the caecum of geese. Czech J Anim Sci. (2011) 56: 192–203. doi: 10.17221/1433-CJAS [DOI] [Google Scholar]
- 11.National Research Council . Nutrient requirements of poultry (9th Edn.). Washington, DC: National Academy Press. (1994). chapter Ten [Google Scholar]
- 12.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al., The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41, D590–D596. doi: 10.1093/nar/gks1219, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al., Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. (2018) 6:90. doi: 10.1186/s40168-018-0470-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, al-Ghalith GA, et al., Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–857. doi: 10.1038/s41587-019-0209-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. (1957) 27:325–349. doi: 10.2307/1942268 [DOI] [Google Scholar]
- 16.Lu J, Kong XL, Wang ZY, Yang HM, Zhang KN, Zou JM. Influence of whole corn feeding on the performance, digestive tract development, and nutrient retention of geese. Poult Sci. (2011) 90:587–594. doi: 10.3382/ps.2010-01054, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Johnson LM, Harrison JH, Davidson D, Hunt C, Mahanna WC, Shinners K, et al., Corn silage management: effects of hybrid, maturity, chop length, and mechanical processing on rate and extent of digestion. J Dairy Sci. (2003) 86:3271–299. doi: 10.3168/jds.S0022-0302(03)73930-7 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhao X, Chen W, Zhou Z, Meng Q, Wu H. Effects of adding various silage additives to whole corn crops at ensiling on performance, rumen fermentation, and serum physiological characteristics of growing-finishing cattle. Animals (2019) 9:695. doi: 10.3390/ani9090695, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu P, Fu X, Wang H, Hou M, Shang Z. Effect of silage diet (sweet Sorghum vs. whole-crop corn) and breed on growth performance, carcass traits, and meat quality of lambs. Animals (2021) 11:3120. doi: 10.3390/ani11113120, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He LW, Meng QX, Li DY, Zhang YW, Ren LP. Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing Greylag geese. Poult Sci. (2015) 94:2464–2471. doi: 10.3382/ps/pev238, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Karwowska M, Grabowicz M, Stadnik J, Szterk P, Bernacki Z, Dolatowski ZJ. The effect of corn or beet pulp silage supplemented diet on production parameters, oxidative stability of muscles and fatty acid composition of abdominal fat in geese. Ann Anim Sci. (2017) 17:887–902. doi: 10.1515/aoas-2016-0075 [DOI] [Google Scholar]
- 22.Zhang B, Sui F, Wang B, Wang Y, Li W. Dietary combined supplementation of iron and Bacillus subtilis enhances reproductive performance, eggshell quality, nutrient digestibility, antioxidant capacity, and hematopoietic function in breeder geese. Poult Sci. (2020) 99:6119–6127. doi: 10.1016/j.psj.2020.06.077, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan W, Shi J, Wang B, Zhang M, Kong M, Li W. Effects of zinc and Bacillus subtilis on the reproductive performance, egg quality, nutrient digestion, intestinal morphology, and serum antioxidant capacity of geese breeders. Poult Sci. (2022) 101:101677. doi: 10.1016/j.psj.2021.101677, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Li X, Lu K, Wang L, Ma X, Song K, et al., Effects of dietary iron levels on growth performance, iron metabolism and antioxidant status in spotted seabass (Lateolabrax maculatus) reared at two temperatures. Aquaculture (2023) 562:738717. doi: 10.1016/j.aquaculture.2022.738717 [DOI] [Google Scholar]
- 25.Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase a: therapeutic potential in brain tumor. Brain Pathol. (2016) 26:3–17. doi: 10.1111/bpa.12299, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and diagnostic significance of lactate dehydrogenase and its Isoenzymes in animals. Vet Med Int. (2020) 2020:5346411–5346483. doi: 10.1155/2020/5346483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem. (2010) 17:672–697. doi: 10.2174/092986710790416263 [DOI] [PubMed] [Google Scholar]
- 28.Chao R, Xia C, Pei C, Huo W, Liu Q, Zhang C, et al., Comparison of the microbial communities of alpacas and sheep fed diets with three different ratios of corn stalk to concentrate. J Anim Physiol Anim Nutr. (2021) 105:26–34. doi: 10.1111/jpn.13442, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Liu X, Du Y, Wang B, Zhao N, Geng Z. Green forage and fattening duration differentially modulate cecal microbiome of Wanxi white geese. PLoS One (2018) 13:e0204210. doi: 10.1371/journal.pone.0204210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia F, Guo W, Liu Y, Zhang T, Xu B, Teng Z, et al., Effects of dietary fibre on intestinal microbiota in geese evaluated by 16SrRNA gene sequencing. J Appl Microbiol. (2022) 132:4440–4451. doi: 10.1111/jam.15536, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature (2006) 444:1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 32.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al., The Firmicutes/Bacteroidetes ratio: a relevant marker of gut Dysbiosis in obese patients? Nutrients (2020) 12:1474. doi: 10.3390/nu12051474, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo B, Li D, Zhou B, Jiang Y, Bai H, Zhang Y, et al., Comparative characterization of bacterial communities in geese consuming of different proportions of ryegrass. PLoS One (2019) 14:e0223445. doi: 10.1371/journal.pone.0223445, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyu W, Liu X, Lu L, Dai B, Wang W, Yang H, et al., Cecal microbiota modulates fat deposition in Muscovy ducks. Front Vet Sci. (2021) 8:609348. doi: 10.3389/fvets.2021.609348, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Luo X, Zhao X, Zhang A, Jiang N, Yang L, et al., Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult Sci. (2018) 97:3899–3909. doi: 10.3382/ps/pey249, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Yan J, Zhou B, Xi Y, Huan H, Li M, Yu J, et al., Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult Sci. (2019) 98:4673–4684. doi: 10.3382/ps/pez169, PMID: [DOI] [PubMed] [Google Scholar]
- 37.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al., Diet rapidly and reproducibly alters the human gut microbiome. Nature (2014) 505:559–563. doi: 10.1038/nature12820, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition (2019) 60:175–184. doi: 10.1016/j.nut.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, Yuan X, Gu T, Li Y, Dai W, Shen X, et al., Comparative characterization of bacterial communities in geese fed all-grass or high-grain diets. PLoS One (2017) 12:e0185590. doi: 10.1371/journal.pone.0185590, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Board TE. Rikenella. John Wiley & Sons, Ltd. (2015). Available at: 10.1002/9781118960608.gbm00252 [DOI] [Google Scholar]
- 41.Cui Y, Zhang L, Wang X, Yi Y, Shan Y, Liu B, et al., Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol Lett. (2022) 369:fnac072. doi: 10.1093/femsle/fnac072 [DOI] [PubMed] [Google Scholar]
- 42.Li G, Wang X, Liu Y, Wang C, Yang Y, Gong S, et al., Supplementation with honeysuckle extract improves growth performance, immune performance, gut morphology, and cecal microbes in geese. Front Vet Sci. (2022) 9:1006318. doi: 10.3389/fvets.2022.1006318, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau SKP, Teng JLL, Chiu TH, Chan E, Tsang AKL, Panagiotou G, et al., Differential microbial communities of omnivorous and herbivorous cattle in southern China. Comput Struct Biotechnol J. (2018) 16:54–60. doi: 10.1016/j.csbj.2018.02.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H, An J, Kim J, Choi D, Song Y, Lee CK, et al., A novel bacterium, Butyricimonas virosa, preventing HFD-induced diabetes and metabolic disorders in mice via GLP-1 receptor. Front Microbiol. (2022) 13:858192. doi: 10.3389/fmicb.2022.858192, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject; PRJNA961934.