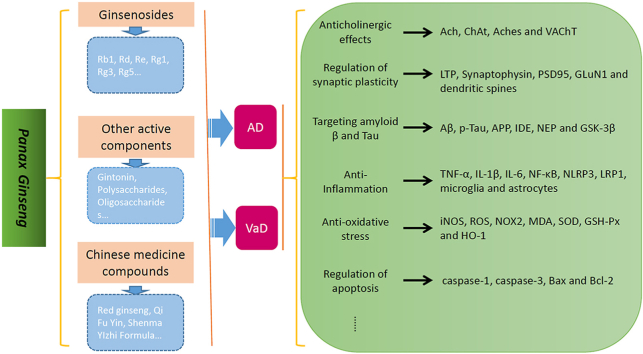
Abstract
Dementia has become one of the most important diseases threatening human health. Alzheimer's disease (AD) and vascular dementia (VaD) have the highest incidence rates among the types of dementia, but until now, therapeutic methods have been limited. Panax ginseng has been used in China for thousands of years to treat dementia, and modern medical studies have found that it contains multiple active components, such as ginsenosides, polysaccharides, amino acids, volatile oils and polyacetylenes, many of which have therapeutic effects in treating AD and VaD. Studies have found that ginsenosides have multitarget therapeutic effects in treating dementia, such as regulation of synaptic plasticity and the cholinergic system, inhibition of Aβ aggravation and tau hyperphosphorylation, anti-neuroinflammation, anti-oxidation effects and anti-apoptosis effects. Other active components of Panax ginseng, such as gintonin, oligosaccharides, polysaccharides and ginseng proteins, also have therapeutic effects on AD and VaD. The effectiveness of ginseng-containing Chinese medicine compounds has also been confirmed by clinical and basic investigations in treating AD and VaD. In this review, we summarized the potential therapeutic effects and related mechanisms of Panax ginseng in treating AD and VaD to provide some examples for further studies.
Keywords: Panax ginseng, Ginsenosides, Alzheimer's disease, Vascular dementia, Chinese medicine
Graphical abstract
1. Introduction
With the aging population and the increase in the average life expectancy, the incidence of dementia has dramatically increased. In 2015, there were approximately 47 million dementia patients worldwide, and this number is expected to triple by 2050 [1]. Patients with dementia experience impairments in memory, executive function, and other cognitive functions, accompanied by a decline in their daily living abilities, imposing a heavy burden on families and society. Alzheimer's disease (AD) and vascular dementia (VaD) are the most common types of dementia, accounting for approximately 80% of all dementia patients [2]. In China, the estimated prevalence of dementia is 6% in people aged 60 years or older, 3.9% for AD, and 1.6% for VaD [3]. Despite the high incidence and serious social impact, until now, the treatment methods and curative effects have been very limited, and new treatment strategies need to be urgently addressed [4].
Panax ginseng, considered “The Lord of Herbs”, is a perennial herb that belongs to the Araliaceae family and has been used for more than 4000 years in China, Korea and Japan. It was first described as a Chinese herbal medicine in China's earliest pharmacy work “Sheng Nong's herbal classic” and has the effects of calming the spirit and soul and enhancing happiness and intelligence, and it can be used in dementia treatment [5]. Modern pharmacological studies have identified nearly 200 active components of ginseng, including ginsenosides, polysaccharides and monosaccharides, vitamins, amino acids, organic acids and nonsaponin water-soluble glycosides [6]. Active components of Panax ginseng have a variety of pharmacological activities in many diseases, such as heart failure, myocardial ischemia, type II diabetes, Parkinson's disease, depression, cancer, and dementia [7]. Chinese herbs can be used in combination, so as to form traditional Chinese medicine compounds to treat diseases, and they have multi-ingredient and multi-target therapy synergistic effects, making them appropriate for individualized treatment schemes [8]. Many ginseng-containing Chinese medicinal compounds have been developed to treat dementia, such as the Huannao Yicong formula, Dengzhan Shengmai capsule and Shenma Yizhi formula [[9], [10], [11]].
In this review, we focused on the pharmacological effects and clinical efficacy of the active components of Panax ginseng and Panax ginseng-containing Chinese medicine compounds in the treatment of AD and VaD.
2. Ginsenosides
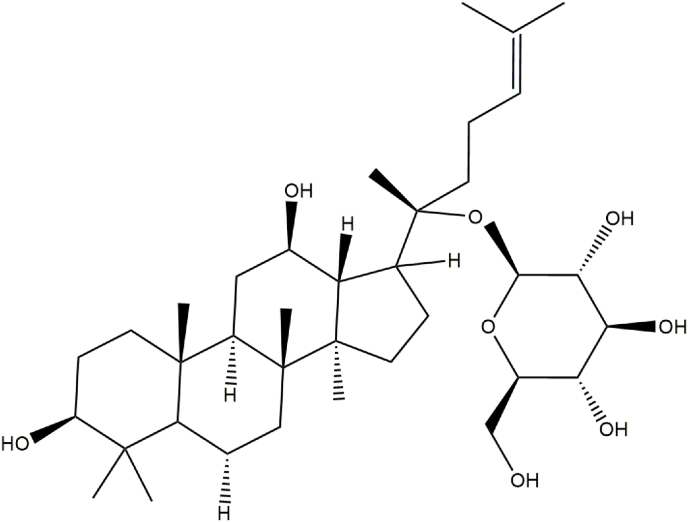
Ginseng saponins, namely, ginsenosides, are the most important active components of Panax ginseng, a type of triterpene glycoside. To date, approximately 30 types of ginsenosides have been identified from ginseng root and processed ginseng products, which are usually classified into three categories: protopanaxadiol, such as Rb1, Rb2, Rc, Rd, Rg3 and Rh2; protopanaxtriol, such as Re, Rf, Rg1, Rg2 and Rh1; and oleanolic acid (OA), such as Ro and Ri. All share a similar basic structure, but the first two belong to the dammarane family, consisting of a four-ring structure, and the latter belongs to the oleanane family, consisting of a five-ring structure (Table 1) [[12], [13], [14]]. Many ginsenosides produce AD and VaD treatment effects through various mechanisms. In this literature review, these mechanisms are summarized (Table 2).
Table 1.
Chemical structural formula of ginsenosides
| Ginsenoside | Molecular formula | Structural formula |
|---|---|---|
| Rb1 | C54H92O23 | 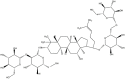 |
| Rd | C48H82O18 | 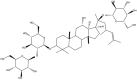 |
| Re | C48H82O18 | 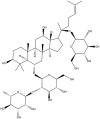 |
| F1 | C36H62O9 | 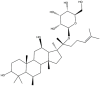 |
| Rg1 | C42H72O14 | 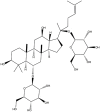 |
| Rg3 | C42H72O13 | 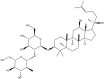 |
| Rg5 | C42H70O12 | 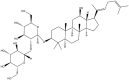 |
| Ro | C48H76O19 | 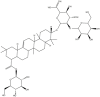 |
Table 2.
Ginsenosides in the treatment of AD and VaD
| Ginsenoside | Subject | Targets | Actions | Reference |
|---|---|---|---|---|
| Rb1 | Wistar rats | Ach, ChAt, AChes | Anticholinergic | [19] |
| C57BL/6 mice | Synaptophysin | Synaptic regulation | [30] | |
| SAMP8 mice | TNF-α, ASC and caspase-1, iNOS | Anti-inflammation | [47] | |
| SD rats | Bax, bcl-2, caspase-3 | Anti-apoptosis | [53] | |
| PC12 cells | LC3Ⅱ/Ⅰ, p62 | Regulate autophagy | [57] | |
| C57BL/6N mice | Blood glucose, NMDAR1, IDE, Cdk5/p35 | Alleviate insulin resistance | [61] | |
| Rd | Neuro-2a cells | ChAT, VAChT, ACh | Anticholinergic | [21] |
| APP mice | p-Tau, GSK-3β | Targeting tau | [40] | |
| Re | Neuro-2a cells, SD rats | ChAT, VAChT, ACh | Anticholinergic | [21,22] |
| Rg1 | C57BL/6J mice | LTP, dendritic spines | Synaptic regulation | [29] |
| C57BL/6 mice | Synaptophysin, PSD95, GLuN1, CaMKIIα | Synaptic regulation | [30,31] | |
| APP/PS1 mice | APP, Aβ, p-Tau | Targeting Aβ and tau | [35] | |
| HT22 cells, SAMP8 mice | ROS, iNOS, NOX2, p22phox, p47phox, NLRP1, caspase-1, IL-1β, NF-κB and p–NF–κB | Anti-inflammation and anti-oxidation | [46,47] | |
| C57BL/6J mice | Bcl-2/Bax | Anti-apoptosis | [54] | |
| Tree shrews | Gut microbiota | Regulate the gut microbiota | [59,60] | |
| Rg3 | Nuro-2a cells,HM06 microglial cells, BALB/c mice | Aβ, sAPPα, NEP, IDE | Targeting Aβ | [37,38] |
| Rg5 | Wistar rats | ChAt, AChes | Anticholinergic | [20] |
| F1 | N2a and SH-SY5Y cells, APP/PS1 mice | Aβ, IDE, NEP | Targeting Aβ | [36] |
| Oleanolic acid | ICR mice | Ach | Anticholinergic | [23] |
| N2a/APP695swe cells | ROS, MDA, Aβ, Bax, Bcl-2, caspase-3 | Anti-inflammation and anti-apoptosis | [50] | |
| SD rats, primary rat neurons, DI-TNC1, SH-SY5Y cells | IL-6, TNF-α, and IL-1β | Anti-inflammation | [51] | |
| Compound K | 2VO-SD rats | Aβ, pSer9-GSK-3β, IDE | Targeting Aβ | [39] |
| db/db mice | TNF-α, IL-6, IL-1β, NLRP3, MDA, SOD, GSH-Px, BiP, CHOP, p-PERK, p-IRE1α, ATF6 | Anti-inflammation and anti-oxidation | [48] | |
| BV2 cells | NF-κB, LRP1, IL-6, TNF-α | Anti-inflammation | [49] | |
| Primary astrocytes (C57 mice) | LC3, mTOR, P70S6K, P62, ULK1, Aβ | Regulate autophagy | [56] |
Note: Ach: acetylcholine; ChAt: cholineacetyltransferase; Aches: acetylcholinesterases; TNF-α: tumor necrosis factor-α; ASC: apoptosis-associated speck-like protein containing a CARD; iNOS: inducible nitric oxide synthase; LC3: Microtubule-associated protein 1A/1B-light chain 3; IDE: insulin-degrading enzyme; NMDAR1: N-methyl-daspartate receptor type 1; CDK5: Cyclin-dependent kinase; IL-1β: interleukin-1β; IL-6: interleukin-6; NF-κB: nuclear factor-κB; VAChT: vesicular acetylcholine transporter; GSK: Glycogen synthase kinase; LTP: long-term potentiation; PSD95: postsynaptic density-95; GLuN1: N-methyl-d-aspartate receptor subunit 1; CaMKIIα: calcium/calmodulin-dependent protein kinase II alpha; APP: amyloid precursor protein; Aβ: amyloid β; ROS: reactive oxygen species; NOX2: NADPH oxidase 2; NLRP1: NLR family, pyrin domain containing 1; NEP: neprilysin; IDE: insulin-degrading enzyme; MDA: malonaldehyde; NLRP3: NOD-like receptor protein 3; SOD: superoxide dismutases; GSH-Px: glutathione peroxidases; PERK: proteins kinase R-liked endoplasmic reticulum kinases; IRE1: inositol-requiring kinases 1; ATF6: activation of transcription factors 6; BiP: binding immunoglobulin proteins; CHOP: transcription factors C/EBP homologous proteins; LRP1: low-density lipoprotein receptor-related protein 1; mTOR: mammalian target of rapamycin; ULK1: Unc-51 Like Autophagy Activating Kinase 1.
2.1. Anticholinergic effects
Cholinergic neurons exist widely in the brain, especially in the thalamus, striatum, limbic system, and neocortex, and are closely related to cognition and other higher brain functions [15]. The cholinergic hypothesis is one of the most important hypotheses related to the pathogenesis of AD. Cholinergic lesions emerge in the early stages of AD, and cholinesterase inhibitors such as donepezil and galantamine are recommended for mild-to-moderate AD [15,16]. In VaD, ischemic infarction may directly damage cholinergic neurons or interrupt the lateral cholinergic pathway, and a meta-analysis showed that cholinesterase inhibitors may have beneficial effects in VaD patients, improve the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) score, and increase the clinical global impression scale [17,18].
Some ginsenosides have anticholinergic activity, thus improving cognitive function. Ginsenoside Rb1 elevates acetylcholine (Ach) levels, increases choline acetyltransferase (ChAT) activity, reduces acetylcholinesterase (AChE) activity in the hippocampus of rats with cognitive impairment, and rescues cisplatin-induced memory impairment; ginsenoside Rg5 could also improve cognitive functions by regulating AChE and ChAT activities in streptozotocin-induced memory-impaired rats [19,20]. Ginsenoside Rd and Re can enhance ChAT and vesicular Ach transporter (VAChT) expression and increase ACh production in neuro-2a (N2a) cells. Ginsenoside Re also shows a dose-dependent increase in extracellular levels of ACh in the hippocampus and medial prefrontal cortex of SD rats [21,22]. Another ginsenoside, OA, could increase ACh levels in mice with cholinergic blockade-induced cognitive deficits [23].
2.2. Regulation of synaptic plasticity
Neuroplasticity is considered the internal mechanism of learning, memory, thoughts, feelings and other behaviors, and synaptic plasticity is thought to play key roles in this process [24,25]. Synaptic damage in the hippocampus and cerebral cortex has been established as an early event and a major brain structural change in AD and VaD, which is directly related to cognitive impairments [25,26]. Restoring synaptic function in AD and VaD may be a viable therapeutic method [27].
Long-term potentiation (LTP) is a persistent strengthening of signal transmission between two neurons and is closely related to learning, memory and behavior [28]. Synaptic proteins and dendritic spines are common indicators of synaptic plasticity [25]. Ginsenoside Rg1 can facilitate LTP and increase the density of dendritic spines in the hippocampus of aged mice, thus increasing memory function [29]. Other studies found that ginsenosides Rg1 and Rb1 could promote the expression of synaptic vesicles and postsynaptic membrane-associated proteins such as postsynaptic density protein 95 (PSD-95), thus improving the learning and memory abilities of dementia mice [30,31].
2.3. Targeting amyloid β (Aβ) and tau
The deposition of amyloid plaques by Aβ and the assembly of neurofibrillary tangles by hyperphosphorylated tau are the most important pathological changes in brains with AD [32]. Antiamyloid therapy and anti-tau therapy for AD have been studied for years, including secretase inhibitors, Aβ aggregation inhibitors, Aβ immunotherapy, phosphatase modifiers, tau-aggregation inhibitors, microtubule stabilizers and others, which are expected to become a new breakthrough in AD treatment [33]. Cerebral amyloid angiopathy is an important pathological feature of VaD [34].
Ginsenoside Rg1 decreases amyloid precursor protein (APP) expression, Aβ deposition and hyperphosphorylated Tau levels, alleviates neuronal damage in the hippocampus and cortex of AD model mice, and improves cognitive functions tested by the Morris water maze test and open field experiments [35]. Ginsenoside F1, a metabolite of Re and Rg1 deglycosylated by intestinal microflora, can protect against Aβ1–42-induced cytotoxicity, reduce the secretion of Aβ1–42 in mouse neuroblastoma N2a and human neuroblastoma SH-SY5Y cells and decrease Aβ plaque formation in APP/PS1 mice. The mechanisms may be related to the increase in the levels of insulin-degrading enzyme (IDE) and neprilysin (NEP), which play important roles in Aβ catabolism [36]. Ginsenoside Rg3 promotes microglial activity and Aβ uptake by promoting the expression of scavenger receptor class A (SRA), clathrin and caveolin, accelerating the degradation of Aβ by increasing the levels of NEP and IDE, and further increasing the nonamyloid degradation mode of amyloid precursor protein (APP) in APP-Swe-transfected N2a cells [37,38]. Ginsenoside Compound K (CK) (Fig. 1), the main metabolite and final absorption form of protopanaxadiol-type ginsenosides in the human intestine by gut microbiota, induced a decrease in Aβ1-42 deposition in the hippocampus of VaD rats and protected against learning and memory impairments by enhancing the phosphorylation of GSK-3β and IDE expression [39]. Ginsenoside Rd decreased the levels of hyperphosphorylated tau in the olfactory bulb, telencephalon and spinal cord of AD model mice by inhibiting the activities of GSK 3β and cyclin-dependent kinase 5, thereby ameliorating cognitive impairment [40].
Fig. 1.
Chemical structure of compound K.
2.4. Anti-inflammation and antioxidative stress
Inflammation and oxidative stress play critical roles in the initiation and development of AD and VaD. Inflammation is usually essential for the repair process; however, once it is prolonged or overactivated, it may cause detrimental effects. In AD and VaD, risk factors and pathological products may activate microglia, astrocytes and lymphocytes, leading to the production and release of inflammatory cytokines and inflammasomes such as interleukin-1 (IL-1), IL-8, tumor necrosis factor-α (TNF-α) and nucleotide-binding oligomerization domain-like receptors (NLRs), which in turn aggravate Aβ and tau pathologies, promote endothelial damage and vascular dysfunction, and ultimately accelerate cognitive decline [[41], [42], [43]]. Excessive oxidative stress has been detected in the brains of patients with AD and VaD and may promote the production of Aβ, induce mitochondrial and neural cell damage, synaptic loss and hyperphosphorylation of tau, reduce nitric oxide bioavailability, and damage endothelial function [42,44,45].
Ginsenoside Rg1 significantly decreased the levels of ROS, NADPH oxidase 2 (NOX2), p47-phox, NLRP1, IL-1β and NF-κB in lipopolysaccharide (LPS)-induced HT22 cells [46]. Ginsenoside Rg1 and Rb1 could decrease the levels of TNF-α, caspase-1, inducible nitric oxide synthase (iNOS), and other indices in the cerebral cortex and peripheral blood; inhibit the activity of microglia and astrocytes; and reduce nerve cell loss in SAMP8 mice, alleviating spatial learning and memory deficits [47]. CK could target both neuroinflammation and oxidative stress, improve cognitive function, promote the expression of low-density lipoprotein receptor-related protein 1 and inhibit the NF-κB pathway in Aβ42 oligomer-damaged BV2 cells. It can also decrease the levels of TNF-α, IL-1β, IL-6, the NOD-like receptor protein 3 (NLRP3) inflammasome and malonaldehyde (MDA) and enhance the activities of superoxide dismutases (SOD), glutathione peroxidases (GSH-Px), and endoplasmic reticulum (ER) stress in the hippocampus of diabetic model mice [48,49]. OA inhibited caspase-3 activity, decreased ROS, MDA, Bax and Aβ levels, and increased Bcl2 levels in N2a/APP695swe cells via the regulation of stanniocalcin-1 (STC-1) and uncoupling protein-2 (UCP2) signaling [50]. OA can also decrease neuroinflammation by regulating the activity of astrocytes and decreasing the levels of IL-1β, IL-6 and TNF-α in AD model rats and cell lines [51].
2.5. Other effects
Apoptosis is one of the important mechanisms in maintaining homeostasis of the internal environment in the body and in the development of neurodegenerative diseases, and ginsenosides can regulate these processes [52]. Ginsenoside Rb1 targets apoptosis-associated Bax, caspase-3 and bcl-2 in the hippocampus of Aβ1-40-induced rats and prevents cognitive deficits [53]. Ginsenoside Rg1 increased the ratio of Bcl-2/Bax expression and decreased neuronal loss in the hippocampus of VaD model mice [54]. Autophagy is a critical cellular process that is responsible for the disintegration of misfolded proteins and damaged organelles by lysosomes and has been shown to be involved in the pathological changes of neurodegenerative diseases [55]. Studies have found that CK and ginsenoside Rb1 have therapeutic potential for AD by regulating autophagy. CK could enhance autophagy in primary astrocytes by promoting the expression of LC3 proteins and inhibiting the mTOR signal pathway, which promote Aβ clearance [56]. Ginsenoside Rb1 could improve the LC3Ⅱ/Ⅰratio and decrease p62 protein expression by activating the PINK1/parkin signaling pathway in Aβ-damaged PC12 cells [57].
Changes in the gut microbiota are associated with various diseases, including dementia [58]. Ginsenoside Rg1 could regulate the gut microbiota to achieve a neuroprotective effect by altering the abundance of Bacteroidetes, Proteobacteria and Verrucomicrobia in the gut and increasing the energy requirement in the hippocampus [59,60]. Diabetes mellitus (DM), which is characterized by impairment in insulin signaling, is thought to be strongly associated with cognitive dysfunction. A study found that ginsenoside Rb1 could improve the memory and cognition of streptozotocin (STZ)-lesioned mice by improving glucose tolerance and alleviating insulin resistance. These mechanisms may be related to the suppression of Cdk5/p35 activity and upregulation of N-methyl-D-aspartate receptor type 1 (NMDAR1) and IDE expression [61,62].
3. Other active components of Panax ginseng
In addition to ginsenosides, other active components of Panax ginseng have also been found to have potential in the treatment of dementia (Table 3). Gintonin, a glycolipoprotein derived from ginseng, contains G-protein-coupled lysophosphatidic acids and has the potential to treat AD and other cognitive impairment-related diseases [63]. Studies have found that gintonin can treat dementia through multiple mechanisms, including antioxidant and anti-inflammatory effects, decrease β-amyloid deposition, ameliorate functional damage to the blood‒brain barrier (BBB), promote hippocampal neurogenesis, decrease ACh levels and ChAT activity, increase AChE activity and regulate synaptic function [[64], [65], [66], [67]].
Table 3.
Other active components of Panax ginseng in the treatment of AD and VaD
| Component | Subject | Targets | Actions | Reference |
|---|---|---|---|---|
| Gintonin | C57BL/6J mice, HT22 cells | LPO, ROS, NRF-2, HO-1, PARP-1, NF-κB, TNF-α, APP, Aβ, BACE-1, ADAM-10, PSD95, syntaxin, SNAP-25, SNAP-23 | Anti-inflammation, anti-oxidation and anticholinergic | [64] |
| APPswe/PSEN-1 mice | Aβ, BBB integrity, occludin, claudin-5, claudin-3, ZO-1, ICAM-1, VCAM-1 | Targeting Aβ and microvascular protection | [65] | |
| APPswe/PSEN-1 mice, primary cortical astrocytes | glial fibrillary acidic protein, NeuN, LPA1 receptor | Promotion of hippocampal neurogenesis | [66] | |
| AβPPswe/PSEN1dE9 mice, hippocampal neural progenitor cell | Acetylcholine, ChAT, AChE | Anticholinergic | [67] | |
| Oligosaccharides | ICR mice | IL-1β, IL-6 | Anti-inflammation | [68] |
| Polysaccharides | 5XFAD mice, HT22 cells | Aβ, microglia | Targeting Aβ and anti-inflammation | [69] |
| Ginseng protein | Wistar rats | Bcl-2/Bax, PI3K, Akt | Targeting Aβ and tau | [70] |
| primary cortical neurons; Wistar rats | Bcl-2, Bax, caspase-3, Aβ, p-Tau, SOD, MDA, T-NOS, iNOS, NO, CREB | Anti-oxidation | [71] | |
| Polyacetylene | Biochemical methods | Ache, butyrylcholinesterase, β-secretase | Anticholinergic | [72] |
Note: LPO: lipid peroxidation; ROS: reactive oxygen species; NRF-2: nuclear factor erythroid-2 related factor-2; HO-1: heme oxygenase-1; PARP-1: poly(ADP-ribose) polymerase-1; NF-κB: nuclear factor-κB; TNF-α: tumor necrosis factor-α; APP: amyloid precursor protein; Aβ: amyloid β; BACE-1: beta-amyloid cleaving enzyme-1; ADAM-10: a disintegrin and metalloproteinase domain-containing protein 10; PSD95: postsynaptic density protein-95; SNAP: synaptosomal-associated protein; BBB: brain-blood barrier; ICAM: intercellular cell adhesion molecule; VACM: vascular cell adhesion molecule; NeuN: neuronal nuclear protein; LPA1: lysophosphatidic acid-1; ChAT: choline acetyltransferase; AChE: Acetylcholinesterase; IL-1β: interleukin-1β; IL-6: interleukin-6; PI3K: phosphatidylinositol-3 kinase; AKT: serine/threonine protein kinase B; SOD: superoxide dismutases; MDA: malonaldehyde; T-NOS: total nitric oxide synthase; iNOS: inducible nitric oxide synthase; CREB: cAMP response element binding protein.
Ginseng oligosaccharides, a class of active components extracted from ginseng, comprise polymers of 2-14 D-glucose molecules, which can inhibit the expression of IL-1β and IL-6 and the activity of astrocytes in the hippocampus of scopolamine-treated mice and protect cognitive function, as demonstrated by the Morris water maze task and novel object recognition tasks [68]. Shin and colleagues [69] identified the curative effect of a nonsaponin fraction with rich polysaccharides (NFP) in the treatment of AD and found that NFP could inhibit Aβ accumulation and microglial activity, improve mitochondrial function, neurogenesis and neuron proliferation in the brains of 5XFAD mice and HT22 cells, and alleviate cognitive impairment in AD model mice.
Ginseng protein, which is a protein isolated from ginseng, also improves cognitive functions. Li and colleagues [70,71] found that ginseng protein could increase the expression of Bcl-2 and the activity of SOD, decrease the expression of Bax and levels of MDA, NO, total nitric oxide synthase and iNOS, decrease the levels of Aβ1-42 and p-tau in D-galactose/AlCl3-induced rats, and alleviate Aβ and H2O2-induced primary cortical neuron damage; the mechanisms may be related to the activation of the PI3K/Akt and CREB signaling pathways. Polyacetylene, another active component of ginseng, can inhibit the activities of AChE, butyrylcholinesterase and β-secretase [72].
4. Panax ginseng-containing Chinese medicine compounds
Chinese medicine compounds are a unique feature of traditional Chinese medicine in treating diseases. Through the combination of different drugs, multitarget therapeutic effects can be exerted, and side effects can be significantly reduced [73]. Based on syndrome differentiation and treatment, ginseng alone or in combination with other Chinese herbal medicines to form a Chinese medicine compound has been used in China for thousands of years for the treatment of dementia [74]. Using modern medical technology, the mechanisms underlying the treatment of dementia have been partially analyzed (Table 4).
Table 4.
Panax ginseng-containing Chinese medicine compounds in the treatment of AD and VaD
| Compound | Subject | Targets | Actions | Reference |
|---|---|---|---|---|
| Red ginseng | HT22 Cells, 5XFAD mice | Aβ, astrocytes, microglia, neurogenesis | Anti-inflammation | [75] |
| Tg2576 mice | Aβ, Iba-1, Claudin-5, Occludin, Laminin, CD13, gut microbiota | Regulation of gut-brain axis | [76] | |
| White Ginseng | ICR mice | Iba-1, Synaptophysin, ChAT | Anticholinergic | [77] |
| Qi Fu Yin | AD and VaD patients | MMSE, HDS | Cognition improving | [78] |
| BV-2 cells | iNOS | Anti-inflammation | [79] | |
| Shenqi Yizhi granules | 5XFAD mice | Iba-1, 2-DE, GFAP | Anti-inflammation, energy metabolism, synaptic transmission and so on | [81] |
| Huannao Yicong Formula | mild to moderate AD patients | ADAS-Cog, CM-SS, MMSE, MoCA | Cognition improving | [82] |
| SD rats, APP/PS1 mice, APP695V717I mice | IL-1, TNF-α, APP, Aβ, caspase-3, -8, -9, -12, Bcl-2/Bax, γ-secretase, p-Tau, TTBK1, GSK-3β, CDK-5, PKC, TrkA | Anti-inflammation, anti-apoptosis, targeting Aβ and tau | [[83], [84], [85], [86]] | |
| Shenzhi Jiannao formula | 2VO-SD rats, PC12 cells, wistar rats, | Clathrin, RAB5B, NMDAR1, calcium, ROS, superoxide, INS, pAKT1, caspase-3 | Synaptic protection, anti-apoptosis and anti-oxidation | [[87], [88], [89]] |
| Sailuotong | mild-to-moderate VaD patients | VaDAS-cog, ADCS-CGIC, MMSE, ADCS-ADLs, | Cognition improving | [90] |
| SD rats, hCMEC/D3 cells, EA.hy926 Cells | IL-1α, IL-6, IL-12, CXCL10, LCN2, p-STAT3, p-JAK2, GFAP, Claudin-1, Occludin, Nrf2, HO-1, ROS, SOD, Bax/Bcl-2, caspase-3 | Anti-inflammation, anti-oxidation and anti-apoptosis | [[91], [92], [93]] | |
| Shenma YIzhi Formula | mild-to-moderate VaD patients | MMSE, NIHSS, CM-SS | Cognition improving | [94] |
| SD rats | SOD, GSH-Px, GSH, MDA, ATP5A, ChAT, AChE | Anti-oxidation and anticholinergic | [95,96] |
Note: Aβ: amyloid β; ChAT: choline acetyltransferase; MMSE: mini-mental state examination; HDS: Hastgawa Dementia Scale; iNOS: inducible nitric oxide synthase; 2-DE: two-dimensional gel electrophoresis; GFAP: glial fibrillary acidic protein, the astrocyte marker; ADAS-Cog: Alzheimer's Disease Assessment Scale- Cognitive Subscale; CM-SS: Chinese Medicine Symptom Scale; MoCA: Montreal Cognitive Assessment; IL-1: interleukin-1; TNF-α: tumor necrosis factor-α; APP: amyloid precursor protein; TTBK1: total tau protein kinase; GSK-3β: glycogen synthase kinase-3β; CDK-5: cyclin-dependent kinase-5; PKC: protein kinase C; TrkA: tyrosine amyloid protein kinase; RAB5B: member RAS oncogene family; NMDAR1: N-methyl-d-aspartic acid receptor 1; ROS: reactive oxygen species; INS: insulin; pAKT1: protein kinase B; VaDAS-cog: vascular dementia assessment scale–cognitive subscale; ADCS-CGIC: Alzheimer's disease cooperative study-clinical global impression of change; ADCS-ADLs: Alzheimer's Disease Cooperative Study ADL Scale; CXCL10: C-X-C motif chemokine ligand 10; LCN2: lipocalin-2; p-STAT3: phosphorylated signal transducer and activator of transcription 3; p-JAK2: Janus kinase-2; Nrf2: nuclear factor erythroid 2–related factor 2; HO-1: anti–heme oxygenase-1; SOD: superoxide dismutases; NIHSS: National Institutes of Health Stroke Scale; GSH-Px: glutathione peroxidases; GSH: glutathione; MDA: malonaldehyde; ATP5A: a mitochondrial marker; AChE: Acetylcholinesterase.
A single Chinese herbal medicine is a unique form of Chinese medicine, and red ginseng or white ginseng alone have therapeutic effects on dementia. Red ginseng, a processed form of ginseng obtained by steaming and drying, reduces Aβ deposition, inhibits the activity of astrocytes and microglia, improves neurogenesis in 5XFAD mice, and promotes mitochondrial function in HT22 cells [75]. Another study found that red ginseng could increase the expression of the BBB tight-junction proteins claudin-5 and occludin, restore the diversity of the gut microbiota, increase the population of Lactobacillus species, reduce Aβ accumulation and microglial activation, and improve the cognitive function of Tg2576 transgenic mice [76]. White ginseng, which is dried ginseng without steam, was reported to have anti-AD effects by alleviating neuronal damage, inhibiting microglial activity and synaptic loss, and increasing ChAT-positive cells in the Aβ1–42-injected mouse hippocampus [77].
Qi Fu Yin, a classic prescription of the Ming Dynasty, consists of Panax ginseng, Rehmannia glutinosa, Angelica sinensis, Glycyrrhiza uralensis, Atractylodes macrocephala, Polygala tenuifolia and Semen ziziphi spinosae. A meta-analysis of 697 AD and VaD patients found that Qi Fu Yin could increase the scores of cognitive function assessment scales such as the Hasegawa Dementia Scale (HDS) and Mini-Mental State Examination (MMSE) [78]. Basic experimental studies found that Qi Fu Yin could downregulate iNOS expression in LPS-challenged BV-2 cells, and network pharmacology analysis found that Qi Fu Yin may have an AD therapeutic effect by alleviating tau hyperphosphorylation by inhibiting GSK3β [79,80]. Shenqi Yizhi granules consist of Panax ginseng, Radix astragali, and Radix scutellariae, which can inhibit astrocyte and microglia activities, and the cognitive improvement function may be related to the regulation of energy metabolism, stress response, amino acid metabolism and other pathological processes in 5XFAD mice [81]. The Huannao Yicong formula (HYF) consists of Radix ginseng, Rhizoma chuanxiong, Radix polygoni multiflora, Rhizoma coptidis and Rhizoma acori tatarinowii, could increase the scores of the Montreal Cognitive Assessment (MoCA) and MMSE and could decrease the scores of the Chinese Medicine Symptom Scale (CM-SS) and ADAS-Cog of mild-to-moderate AD patients, which may be related to the reduction of the serum levels of AchE and Aβ42, anti-inflammation and anti-apoptosis functions, regulation of γ-secretase activity, inhibition of Aβ aggravation and tau hyperphosphorylation [[82], [83], [84], [85], [86]].
Shenzhi Jiannao (SZJN) formula consists of Panax ginseng, Rhizoma Anemarrhenae, and Radix Paeoniae Rubra and can treat VaD by promoting clathrin-mediated endocytosis and cell proliferation and inhibiting apoptosis and oxidative stress. The SZJN formula could increase clathrin and RAB5B expression, reduce NMDAR1 expression, increase the proportion of G0/G1 and G2/M phase cells, reduce Ca2+, ROS and superoxide expression, increase the expression of insulin and phosphorylated-AKT1 (pAKT1), and inhibit caspase-3 expression in the hippocampus of VaD model rats and PC12 cells [[87], [88], [89]].
Sailuotong consists of Panax ginseng, Ginkgo biloba, and Crocus sativus, and clinical studies found that Sailuotong could significantly decrease the VaD Assessment Scale–cognitive subscale scores (VaDAS-cog), increase the scores of Alzheimer's disease cooperative study-clinical global impression of change (ADCS-CGIC), MMSE and Alzheimer's Disease cooperative study-activities of daily living (ADCS-ADLs), improve the cognitive function and daily life ability of mild-to-moderate VaD patients, and prevent some amount of adverse events [90]. The mechanisms of Sailuotong in treating VaD may be related to its antineuroinflammation, antioxidation, antiapoptosis and brain microvascular endothelial cell (BMEC) protection effects [[91], [92], [93]]. The Shenma YIzhi formula (SYF) consists of Panax ginseng, Ramulus euonymi, Rhizoma chuanxiong and Rhizoma gastrodiae. A clinical trial found that SYF could significantly improve the MMSE, National Institutes of Health Stroke Scale (NIHSS), and CM-SS scores in VaD patients, which may be related to the improvement of vascular endothelial functions, mitochondrial structure, energy metabolism and hippocampal cholinergic dysfunction [[94], [95], [96]].
5. Conclusion and perspective
As the most common types of dementia, AD and VaD have aroused widespread concern worldwide. Although there are many hypotheses regarding their pathogenesis, their therapeutic methods need to be further explored. The use of Panax ginseng in AD and VaD treatment has been studied for years. Panax ginseng alone, ginsenosides and other active components of Panax ginseng and Panax ginseng-containing Chinese medicine compounds have therapeutic effects on AD and VaD through multiple mechanisms, such as anti-neuroinflammation, antioxidation and anti-apoptosis; inhibition of Aβ aggravation and tau hyperphosphorylation, targeting the cholinergic system; and regulation of gut microflora, synaptic plasticity and autophagy. Most of these compounds target multiple therapeutic effects, and as there has been no breakthrough in the research of single target drugs in the treatment of dementia, Panax ginseng could be a potential treatment. The therapeutic effects of Rb1, Rg1 and Compound K on AD and VaD have been widely studied and deserve more attention. Although the compatibility law and mechanism of Chinese medicine compounds need to be further clarified, many randomized controlled clinical studies have confirmed the effectiveness and safety of Panax ginseng-containing Chinese medicine compounds in the treatment of AD and VaD. Considering the complexity of the pathogenesis of dementia, Chinese medicine compounds may be a feasible treatment approach. Based on the above analysis and summary, we believe that Panax ginseng has great potential in AD and VaD treatment and warrants further research and development.
Declaration of competing interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (NO: 82004434), Special training program for outstanding young scientific and technological talents (innovation) of China Academy of Chinese Medical Sciences (NO: ZZ-14-YQ-001), “Miao Pu” Foundation Project of Xiyuan Hospital, China Academy of Chinese Medical Sciences (NO: 2019XYMP--40), and cultivation project of Xiyuan Hospital for the National Natural Science Foundation project (XY20--08).
References
- 1.Livingston G., Sommerlad A., Orgeta V., Costafreda S., Huntley J., Ames D., et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 2.Cao Q., Tan C.C., Xu W., Hu H., Cao X.P., Dong Q., et al. The prevalence of dementia: a systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2020;73(3):1157–1166. doi: 10.3233/JAD-191092. [DOI] [PubMed] [Google Scholar]
- 3.Jia L., Du Y., Chu L., Zhang Z., Li F., Lyu D., Li Y., Li Y., Zhu M., Jiao H., et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. The Lancet Public Health. 2020;5(12):e661–e671. doi: 10.1016/S2468-2667(20)30185-7. [DOI] [PubMed] [Google Scholar]
- 4.Tisher A., Salardini A. A comprehensive update on treatment of dementia. Seminars in Neurology. 2019;39(2):167–178. doi: 10.1055/s-0039-1683408. [DOI] [PubMed] [Google Scholar]
- 5.Xu W., Choi H., Huang L. State of Panax ginseng research: a global analysis. Molecules. 2017;22(9):1518. doi: 10.3390/molecules22091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H., Lu X., Hu Y., Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacological Research. 2020;161 doi: 10.1016/j.phrs.2020.105263. [DOI] [PubMed] [Google Scholar]
- 7.Flagg A. Traditional and current use of ginseng. The Nursing Clinics of North America. 2021;56(1):109–121. doi: 10.1016/j.cnur.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Luan X., Zhang L.J., Li X.Q., Rahman K., Zhang H., Chen H.Z., Zhang W.D. Compound-based Chinese medicine formula: from discovery to compatibility mechanism. Journal of Ethnopharmacol. 2020;254 doi: 10.1016/j.jep.2020.112687. [DOI] [PubMed] [Google Scholar]
- 9.Huang P., He X., Xu M. Dengzhan shengmai capsule combined with donepezil hydrochloride in the treatment of Alzheimer's disease: preliminary findings, randomized and controlled clinical trial. Revista da Associacao Medica Brasileira. 2021;67(2):190–194. doi: 10.1590/1806-9282.67.02.20200378. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y., Liu J., Fang J., Wang H., Wei Y., Cao Y., Liu J., Liu L., Li H. Effect and safety of Huannao Yicong formula in patients with mild-to-moderate Alzheimer's disease: a randomized, double-blinded, donepezil-controlled trial. Chinese Journal of Integrative Medicine. 2019;25(8):574–581. doi: 10.1007/s11655-018-3054-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Cao Y., Pei H., Wang H., Ma L., Wang Z., et al. Shenmayizhi formula combined with Ginkgo extract tablets for the treatment of vascular dementia: a randomized, double-blind, controlled trial. Evidence-Based complementary and alternative medicine. 2020;2020 doi: 10.1155/2020/8312347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancuso C., Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2017;107:362–372. doi: 10.1016/j.fct.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. Journal of Ginseng Research. 2018;42(3):239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen L. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Advances in Food and Nutrition Research. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain : A Journal of Neurology. 2018;141(7):1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joe E., Ringman J.M. Cognitive symptoms of Alzheimer's disease: clinical management and prevention. BMJ. 2019;367:l6217. doi: 10.1136/bmj.l6217. [DOI] [PubMed] [Google Scholar]
- 17.Battle C.E., Abdul-Rahim A.H., Shenkin S.D., Hewitt J., Quinn T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. The Cochrane Database of Systematic Reviews. 2021;2(2) doi: 10.1002/14651858.CD013306.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Román G., Kalaria R. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27(12):1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Zhang H., Xu H., Zheng Y., Wu T., Lian Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. Journal of Ginseng Research. 2019;43(4):499–507. doi: 10.1016/j.jgr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu S., Gu J., Feng L., Liu J., Zhang M., Jia X., Liu M., Yao D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. International Immunopharmacology. 2014;19(2):317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.S., Yu J.M., Kim H.J., Kim H.B., Kim S.T., Jang S.K., Choi Y.W., Lee D.I., Joo S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biological & Pharmaceutical Bulletin. 2014;37(5):826–833. doi: 10.1248/bpb.b14-00011. [DOI] [PubMed] [Google Scholar]
- 22.Shi J., Xue W., Zhao W.J., Li K.X. Pharmacokinetics and dopamine/acetylcholine releasing effects of ginsenoside Re in hippocampus and mPFC of freely moving rats. Acta Pharmacologica Sinica. 2013;34(2):214–220. doi: 10.1038/aps.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon S.J., Lee H.J., Lee H.E., Park S.J., Gwon Y., Kim H., Zhang J., Shin C.Y., Kim D.H., Ryu J.H. Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology. 2017;113:100–109. doi: 10.1016/j.neuropharm.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Citri A., Malenka R.C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 25.Skaper S.D., Facci L., Zusso M., Giusti P. Synaptic plasticity, dementia and alzheimer disease. CNS & Neurological Disorders Drug Targets. 2017;16(3):220–233. doi: 10.2174/1871527316666170113120853. [DOI] [PubMed] [Google Scholar]
- 26.Kozubski W., Ong K., Waleszczyk W., Zabel M., Dorszewska J. Molecular factors mediating neural cell plasticity changes in dementia brain diseases. Neural Plasticity. 2021;2021 doi: 10.1155/2021/8834645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M.-K. Potential therapeutics for vascular cognitive impairment and dementia. Current Neuropharmacology. 2018;16(7):1036–1044. doi: 10.2174/1570159X15666171016164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweatt J.D. Neural plasticity and behavior - sixty years of conceptual advances. Journal of Neurochemistry. 2016;139(Suppl 2):179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- 29.Zhu G., Wang Y., Li J., Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015;292:81–89. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Mook-Jung I., Hong H.S., Boo J.H., Lee K.H., Yun S.H., Cheong M.Y., Joo I., Huh K., Jung M.W. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. Journal of Neuroscience Research. 2001;63(6):509–515. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Zhang J., Zheng K., Shen H., Chen X. Long-term ginsenoside Rg1 supplementation improves age-related cognitive decline by promoting synaptic plasticity associated protein expression in C57BL/6J mice. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2014;69(3):282–294. doi: 10.1093/gerona/glt091. [DOI] [PubMed] [Google Scholar]
- 32.Ju Y., Tam K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer's disease. Neural Regen Res. 2022;17(3):543–549. doi: 10.4103/1673-5374.320970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T.W., Lane H.Y., Lin C.H. Novel therapeutic approaches for Alzheimer's disease: an updated review. International Journal of Molecular Sciences. 2021;22(15) doi: 10.3390/ijms22158208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan A., Kalaria R., Corbett A., Ballard C. Update on vascular dementia. Journal of Geriatric Psychiatry and Neurology. 2016;29(5):281–301. doi: 10.1177/0891988716654987. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Su Y., Sun Z., Chen M., Han Y., Li Y., Dong X., Ding S., Fang Z., Li W., et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J Ginseng Res. 2021;45(6):665–675. doi: 10.1016/j.jgr.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun Y.-J., Park B.-H., Hou J., Oh J.-P., Han J.-H., Kim S.-C. Ginsenoside F1 protects the brain against amyloid beta-induced toxicity by regulating IDE and NEP. Life (Basel) 2022;12(1):58. doi: 10.3390/life12010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn J.W., Jang S.K., Jo B.R., Kim H.S., Park J.Y., Park H.Y., Yoo Y.M., Joo S.S. A therapeutic intervention for Alzheimer's disease using ginsenoside Rg3: its role in M2 microglial activation and non-amyloidogenesis. Journal of Physiology and Pharmacology. 2021;72(2) doi: 10.26402/jpp.2021.2.04. [DOI] [PubMed] [Google Scholar]
- 38.Jang S.K., Yu J.M., Kim S.T., Kim G.H., Park D.W., Lee D.I., Joo S.S. An Aβ42 uptake and degradation via Rg3 requires an activation of caveolin, clathrin and Aβ-degrading enzymes in microglia. European Journal of Pharmacology. 2015;758:1–10. doi: 10.1016/j.ejphar.2015.03.071. [DOI] [PubMed] [Google Scholar]
- 39.Zong W., Zeng X., Chen S., Chen L., Zhou L., Wang X., Gao Q., Zeng G., Hu K., Ouyang D. Ginsenoside compound K attenuates cognitive deficits in vascular dementia rats by reducing the Aβ deposition. Journal of Pharmacological Sciences. 2019;139(3):223–230. doi: 10.1016/j.jphs.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Li T., Tian X., Zhao L. Ginsenoside Rd attenuates tau phosphorylation in olfactory bulb, spinal cord, and telencephalon by regulating glycogen synthase kinase 3β and cyclin-dependent kinase 5. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/4485957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozben T., Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer's disease. Clinical Biochemistry. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y.J., Han S.B., Nam S.Y., Oh K.W., Hong J.T. Inflammation and Alzheimer's disease. Archives of Pharmacal Research. 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 43.Wang X.X., Zhang B., Xia R., Jia Q.Y. Inflammation, apoptosis and autophagy as critical players in vascular dementia. European Review for Medical and Pharmacological Sciences. 2020;24(18):9601–9614. doi: 10.26355/eurrev_202009_23048. [DOI] [PubMed] [Google Scholar]
- 44.Bennett S., Grant M.M., Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. Journal of Alzheimer's Disease. 2009;17:245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 45.Aliev G., Priyadarshini M., Reddy V., Grieg N., Kaminsky Y., Cacabelos R., Ashraf G., Jabir N., Kamal M., Nikolenko V., et al. Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Current Medicinal Chemistry. 2014;21(19):2208–2217. doi: 10.2174/0929867321666131227161303. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Ding S., Chen Y., Sun Z., Zhang J., Han Y., Dong X., Fang Z., Li W. Ginsenoside Rg1 alleviates lipopolysaccharide-induced neuronal damage by inhibiting NLRP1 inflammasomes in HT22 cells. Exp Ther Med. 2021;22(1):782. doi: 10.3892/etm.2021.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y., Li S., Huang H., Lv J., Chen S., Pires Dias A.C., Li Y., Liu X., Wang Q. Comparison of the protective effects of ginsenosides Rb1 and Rg1 on improving cognitive deficits in SAMP8 mice based on anti-neuroinflammation mechanism. Frontiers in Pharmacology. 2020;11:834. doi: 10.3389/fphar.2020.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C., Deng M., Gao Z., Dang Y., Zheng G., Yang X., Chao Y., Cai Y., Wu X. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food & Function. 2020;11(5):4416–4427. doi: 10.1039/c9fo02602a. [DOI] [PubMed] [Google Scholar]
- 49.Jiao H., Jia J. Ginsenoside compound K acts via LRP1 to alleviate Amyloid β(42)-induced neuroinflammation in microglia by suppressing NF-κB. Biochemical and Biophysical Research Communications. 2022;590:14–19. doi: 10.1016/j.bbrc.2021.12.071. [DOI] [PubMed] [Google Scholar]
- 50.Guo Q., He J., Zhang H., Yao L., Li H. Oleanolic acid alleviates oxidative stress in Alzheimer's disease by regulating stanniocalcin-1 and uncoupling protein-2 signalling. Clinical and Experimental Pharmacology & Physiology. 2020;47(7):1263–1271. doi: 10.1111/1440-1681.13292. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L., Xia R., Jia J., Wang L., Li K., Li Y., Zhang J. Oleanolic acid protects against cognitive decline and neuroinflammation-mediated neurotoxicity by blocking secretory phospholipase A2 IIA-activated calcium signals. Molecular Immunology. 2018;99:95–103. doi: 10.1016/j.molimm.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Mattson M.P. Apoptosis in neurodegenerative disorders. Nature Reviews Molecular Cell Biology. 2000;1(2):120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Li Y., Yang W., Gao S., Lin J., Wang T., Zhou K., Hu H. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer's disease induced by Aβ(1-40) American Journal of Translational Research. 2018;10(3):796–805. [PMC free article] [PubMed] [Google Scholar]
- 54.Shen F., Wang J., Gao F., Wang J., Zhu G. Ginsenoside Rg1 prevents cognitive impairment and hippocampal neuronal apoptosis in experimental vascular dementia mice by promoting GPR30 expression. Neural Plasticity. 2021;2021 doi: 10.1155/2021/2412220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alirezaei M., Kemball C.C., Whitton J.L. Autophagy, inflammation and neurodegenerative disease. Eur J Neurosci. 2011;33(2):197–204. doi: 10.1111/j.1460-9568.2010.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo J., Chang L., Zhang X., Pei S., Yu M., Gao J. Ginsenoside compound K promotes β-amyloid peptide clearance in primary astrocytes via autophagy enhancement. Exp Ther Med. 2014;8(4):1271–1274. doi: 10.3892/etm.2014.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Y., Xie X., Huang P., Li B., Li H., Xia X. Mechanism of ginsenoside Rb1 activating mitophagy in PC12 cells injured by Aβ. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2021;23(7):2319–2326. [Google Scholar]
- 58.Alkasir R., Li J., Li X., Jin M., Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Lu J., Zeng Y., Guo Y., Wu C., Zhao H., Zheng H., Jiao J. Improving Alzheimer's disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiology Letters. 2020;367(4) doi: 10.1093/femsle/fnaa011. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y., Wang L., Lu J., Jiao J., Yang Y., Zhao H., Liang Z., Zheng H. Ginsenoside Rg1 improves cognitive capability and affects the microbiota of large intestine of tree shrew model for Alzheimer's disease. Molecular Medicine Reports. 2021;23(4) doi: 10.3892/mmr.2021.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang R., Jiang X., He X., Liang D., Sun S., Zhou G. Ginsenoside Rb1 improves cognitive impairment induced by insulin resistance through cdk5/p35-NMDAR-IDE pathway. Biomed Res Int. 2020;2020 doi: 10.1155/2020/3905719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gudala K., Bansal D., Schifano F., Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakaria M., Azam S., Go E.A., Uddin M.S., Jo S.H., Choi D.K. Biological evidence of gintonin efficacy in memory disorders. Pharmacological Research. 2021;163 doi: 10.1016/j.phrs.2020.105221. [DOI] [PubMed] [Google Scholar]
- 64.Ikram M., Jo M.G., Park T.J., Kim M.W., Khan I., Jo M.H., Kim M.O. Oral administration of gintonin protects the brains of mice against aβ-induced alzheimer disease pathology: antioxidant and anti-inflammatory effects. Oxidative Medicine and Cellular Longevity. 2021;2021 doi: 10.1155/2021/6635552. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Jang M., Choi S.-H., Choi J.H., Oh J., Lee R.M., Lee N.-E., Cho Y.-J., Rhim H., Kim H.-C., Cho I.-H., et al. Ginseng gintonin attenuates the disruptions of brain microvascular permeability and microvascular endothelium junctional proteins in an APPswe/PSEN-1 double-transgenic mouse model of Αlzheimer's disease. Exp Ther Med. 2021;21(4):310. doi: 10.3892/etm.2021.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H.J., Kim D.J., Shin E.J., Lee B.H., Choi S.H., Hwang S.H., Rhim H., Cho I.H., Kim H.C., Nah S.Y. Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer's disease. Neurochemistry International. 2016;101:56–65. doi: 10.1016/j.neuint.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Kim H.J., Shin E.J., Lee B.H., Choi S.H., Jung S.W., Cho I.H., Hwang S.H., Kim J.Y., Han J.S., Chung C., et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Molecules and Cells. 2015;38(9):796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu T., Shen X., Yu H., Sun L., Lin W., Zhang C. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J Ginseng Res. 2016;40(3):211–219. doi: 10.1016/j.jgr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin S.J., Nam Y., Park Y.H., Kim M.J., Lee E., Jeon S.G., Bae B.S., Seo J., Shim S.L., Kim J.S., et al. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer's disease. Free Radical Biology & Medicine. 2021;164:233–248. doi: 10.1016/j.freeradbiomed.2020.12.454. [DOI] [PubMed] [Google Scholar]
- 70.Li H., Kang T., Qi B., Kong L., Jiao Y., Cao Y., Zhang J., Yang J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer's disease. Journal of Ethnopharmacology. 2016;179:162–169. doi: 10.1016/j.jep.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Li H., Song J., Zhang J., Wang T., Yan Y., Tao Z., Li S., Zhang H., Kang T., Yang J. Ginseng protein reverses amyloid beta peptide and H(2) O(2) cytotoxicity in neurons, and ameliorates cognitive impairment in AD rats induced by a combination of D-galactose and AlCl(3) Phytotherapy Research. 2017;31(2):284–295. doi: 10.1002/ptr.5747. [DOI] [PubMed] [Google Scholar]
- 72.Murata K., Iida D., Ueno Y., Samukawa K., Ishizaka T., Kotake T., Matsuda H. Novel polyacetylene derivatives and their inhibitory activities on acetylcholinesterase obtained from Panax ginseng roots. Journal of Natural Medicines. 2017;71(1):114–122. doi: 10.1007/s11418-016-1036-7. [DOI] [PubMed] [Google Scholar]
- 73.Dong J. The relationship between traditional Chinese medicine and modern medicine. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y., Li X., Li Z., Lu M. Analysis of the prescription law of Chinese patent medicine to treat dementia. Chinese Journal of Modern Applied Pharmacy. 2020;37(23):2883–2887. [Google Scholar]
- 75.Shin S.J., Jeon S.G., Kim J.-I., Jeong Y.-O., Kim S., Park Y.H., Lee S.-K., Park H.H., Hong S.B., Oh S., et al. Red ginseng attenuates aβ-induced mitochondrial dysfunction and aβ-mediated pathology in an animal model of Alzheimer's disease. International Journal of Molecular Sciences. 2019;20(12):3030. doi: 10.3390/ijms20123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee M., Lee S.-H., Kim M.-S., Ahn K.-S., Kim M. Effect of Lactobacillus dominance modified by Korean Red Ginseng on the improvement of Alzheimer's disease in mice. J Ginseng Res. 2022;46(3):464–472. doi: 10.1016/j.jgr.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi J.G., Kim N., Huh E., Lee H., Oh M.H., Park J.D., Pyo M.K., Oh M.S. White ginseng protects mouse hippocampal cells against amyloid-beta oligomer toxicity. Phytotherapy Research. 2017;31(3):497–506. doi: 10.1002/ptr.5776. [DOI] [PubMed] [Google Scholar]
- 78.Wang L., Qiao P., Yue L., Sun R. Is Qi Fu Yin effective in clinical treatment of dementia?: a meta-analysis of 697 patients. Medicine (Baltimore) 2021;100(5):e24526–e. doi: 10.1097/MD.0000000000024526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngo F.Y., Wang W., Chen Q., Zhao J., Chen H., Gao J.M., Rong J. Network pharmacology analysis and molecular characterization of the herbal medicine formulation qi-fu-yin for the inhibition of the neuroinflammatory biomarker iNOS in microglial BV-2 cells: implication for the treatment of Alzheimer's disease. Oxidative Medicine and Cellular Longevity. 2020;2020 doi: 10.1155/2020/5780703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao Q.Y., Ye T.Y., Wang X.L., Han L., Wang T.X., Qi D.M., Cheng X.R., Wang S.Q. A network pharmacology-based study on key pharmacological pathways and targets of Qi Fu Yin acting on Alzheimer's disease. Experimental Gerontology. 2021;149 doi: 10.1016/j.exger.2021.111336. [DOI] [PubMed] [Google Scholar]
- 81.Ren J., Wei D., An H., Zhang J., Zhang Z. Shenqi Yizhi granules protect hippocampus of AD transgenic mice by modulating on multiple pathological processes. Journal of Ethnopharmacology. 2020;263 doi: 10.1016/j.jep.2020.112869. [DOI] [PubMed] [Google Scholar]
- 82.Yang Y., Liu J.P., Fang J.Y., Wang H.C., Wei Y., Cao Y., Liu J.G., Liu L.T., Li H. Effect and safety of Huannao Yicong formula ( ) in patients with mild-to-moderate Alzheimer's disease: a randomized, double-blinded, donepezil-controlled trial. Chinese Journal of Integrative Medicine. 2019;25(8):574–581. doi: 10.1007/s11655-018-3054-7. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q., Li H., Wang F.X., Gao L., Qin J.C., Liu J.G., Wei Y., Liu M.X. Huannao Yicong Decoction extract reduces inflammation and cell apoptosis in Aβ(1-42)-induced Alzheimer's disease model of rats. Chinese Journal of Integrative Medicine. 2017;23(9):672–680. doi: 10.1007/s11655-016-2255-1. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z.Y., Liu J.G., Wei Y., Liu M.X., Wang Q., Liang L., Yang H.M., Li H. Huannao Yicong Formula ( ) regulates γ-secretase activity through APH-1 and PEN-2 gene ragulation pathways in hippocampus of APP/PS1 double transgenic mice. Chinese Journal of Integrative Medicine. 2017;23(4):270–278. doi: 10.1007/s11655-017-2402-3. [DOI] [PubMed] [Google Scholar]
- 85.Cao Y., Jia X., Wei Y., Liu M., Liu J., Li H. Traditional Chinese medicine Huannao Yicong decoction extract decreases tau hyperphosphorylation in the brain of Alzheimer's disease model rats induced by aβ(1-42) Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/6840432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H., Liu M.F., Liu J.G., Liu L.T., Guan J., Cai L.L., Hu J., Wei Y. Effect of Huannao Yicong prescription [See Text] extract on β-amyloid precursor protein metabolic signal transduction-related protein in brain tissue of dementia model transgenic mouse. Chinese Journal of Integrative Medicine. 2012;18(9):683–689. doi: 10.1007/s11655-012-1204-x. [DOI] [PubMed] [Google Scholar]
- 87.Tian D., Guo Y., Zhang D., Gao Q., Liu G., Lin J., Chang Z., Wang Y., Su R., Han Z. Shenzhi Jiannao formula ameliorates vascular dementia in vivo and in vitro by inhibition glutamate neurotoxicity via promoting clathrin-mediated endocytosis. Chin Med. 2021;16(1):65. doi: 10.1186/s13020-021-00477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian D., Gao Q., Lin J., Chang Z., Wang Y., Shi Y., Su R., Han Z., Ma D. Uncovering the mechanism of the Shenzhi Jiannao formula against vascular dementia using a combined network pharmacology approach and molecular biology. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2021;90 doi: 10.1016/j.phymed.2021.153637. [DOI] [PubMed] [Google Scholar]
- 89.Tian D., Gao Q., Chang Z., Lin J., Ma D., Han Z. Network pharmacology and in vitro studies reveal the pharmacological effects and molecular mechanisms of Shenzhi Jiannao prescription against vascular dementia. BMC Complementary Medicine and Therapies. 2022;22(1):33. doi: 10.1186/s12906-021-03465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jia J., Wei C., Chen S., Li F., Tang Y., Qin W., Shi L., Gong M., Xu H., Li F., et al. Efficacy and safety of the compound Chinese medicine SaiLuoTong in vascular dementia: a randomized clinical trial. Alzheimers Dement. 2018;4:108–117. doi: 10.1016/j.trci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y., Liu J., Yao M., Song W., Zheng Y., Xu L., Sun M., Yang B., Bensoussan A., Chang D., et al. Sailuotong capsule prevents the cerebral ischaemia-induced neuroinflammation and impairment of recognition memory through inhibition of LCN2 expression. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8416105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan X.D., Yao M.J., Yang B., Han X., Zhang Y.H., Wang G.R., Li P., Xu L., Liu J.X. Chinese herbal preparation SaiLuoTong alleviates brain ischemia via Nrf2 antioxidation pathway-dependent cerebral microvascular protection. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.748568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seto S., Chang D., Ko W., Zhou X., Kiat H., Bensoussan A., Lee S., Hoi M., Steiner G., Liu J. Sailuotong prevents hydrogen peroxide (H₂O₂)-Induced injury in EA.hy926 cells. International Journal of Molecular Sciences. 2017;18(1) doi: 10.3390/ijms18010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H., Cao Y., Pei H., Wang H., Ma L., Wang Z., Diao X., Yang Y., Liu N., Wei Y., et al. Shenmayizhi formula combined with Ginkgo extract tablets for the treatment of vascular dementia: a randomized, double-blind, controlled trial. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/8312347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun C., Liu M., Liu J., Zhang T., Zhang L., Li H., Luo Z. ShenmaYizhi decoction improves the mitochondrial structure in the brain and ameliorates cognitive impairment in VCI rats via the AMPK/UCP2 signaling pathway. Neuropsychiatric Disease and Treatment. 2021;17:1937–1951. doi: 10.2147/NDT.S302355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Q., Cao Y., Liu M., Liu F., Brantner A.H., Yang Y., Wei Y., Zhou Y., Wang Z., Ma L., et al. Traditional Chinese medicine shenmayizhi decoction ameliorates memory and cognitive impairment induced by scopolamine via preventing hippocampal cholinergic dysfunction in rats. Neuropsychiatric Disease and Treatment. 2019;15:3167–3176. doi: 10.2147/NDT.S214976. [DOI] [PMC free article] [PubMed] [Google Scholar]