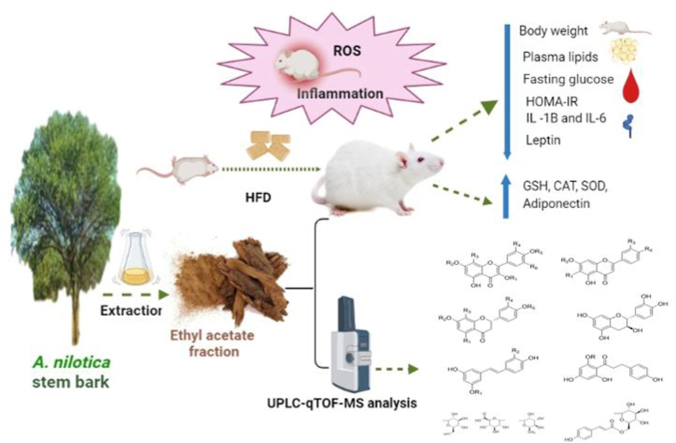
Abstract
Background and aim
Acacia nilotica (A. nilotica) is an imperative plant with many medicinal uses. The current study aimed to investigate the protective effects of the stem bark of A. nilotica and its fractions in a high fat diet (HFD) rat model.
Experimental procedure
Seventy-two male albino rats were randomly divided into 9 groups, 8 rats per each. Group 1 was the normal control and received standard balanced diet. All the remaining groups were fed HFD for 8 weeks to induce obesity. Group 2 served as the HFD control group, group 3 received orlistat (5 mg/kg/day), groups 4 and 5 received total extract of A. nilotica stem bark (250 and 500 mg/kg). Groups 6 and 7 received A. nilotica ethyl acetate fraction (250 and 500 mg/kg), while groups 8 and 9 received butanol fraction (250 and 500 mg/kg).
Results and conclusion
Both doses of the ethyl acetate fraction of the stem bark of A. nilotica significantly decreased the body weight, blood glucose, lipid profile and improved insulin sensitivity. Levels of MDA, leptin and inflammatory cytokines were significantly decreased by the ethyl acetate fraction while adiponectin and HDL-C were significantly increased relative to the HFD control group. Both doses of the ethyl acetate fraction significantly abolished HDF induced oxidative stress and normalized the values of antioxidant enzymes. Furthermore, metabolic profiling of the ethyl acetate fraction was performed by UHPLC/Q-TOF-MS. In conclusion, the ethyl acetate fraction of A. nilotica stem bark possessed antioxidant, anti-inflammatory and insulin sensitizing properties in HFD rat model.
Keywords: A. nilotica, Obesity, Inflammatory cytokines, Oxidative stress, Insulin resistance
Graphical abstract
Highlights of the finding and novelties
-
•
The extract of A. nilotica stem bark ameliorated oxidative stress in HFD rats.
-
•
The extract of A. nilotica stem bark reduced the levels of inflammatory cytokines.
-
•
The extract of A. nilotica stem bark improved lipid profile and insulin sensitivity.
List of abbreviations
- A. nilotica
Acacia nilotica
- COX-2
Cyclooxygenase-2
- FFA
Free fatty acids
- GSH
Reduced glutathione
- H&E
Hematoxylin and Eosin
- HDL-C:
High density lipoprotein-cholesterol
- HFD
High fat diet
- HOMA-IR
Homoeostasis Model Assessment Insulin Resistance
- HPLC
High performance liquid chromatography
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- LDL-C:
Low density lipoprotein-cholesterol
- MAPK
Mitogen-activated protein kinase
- MDA
Malondialdehyde
- NF-κB
Nuclear factor kappa B
- PPARγ
Peroxisome proliferator-activated receptor γ
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglycerides
- TNF
Tumor necrosis factor
- UHPLC/Q-TOF-MS
Ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry
- WHO
World Health Organization
1. Introduction
The incidence of obesity and overweight has increased globally.1 Insulin resistance, chronic low-grade inflammation, and specific adipokines such as adiponectin, leptin, and resistin have been identified as essential contributors to the development and outcomes of obesity.2
The use of therapeutic plants and their bioactive constituents in the management and prevention of chronic diseases has gained more attention.3 Numerous studies have reported the capability of medicinal plants to manage obesity; examples are lingonberry, ginger, omija fruits, aloe vera and red cabbages.4 Trigonella foenum-graecum (Fabaceae) was claimed to have anti-diabetes, antioxidant, and anti-hyperlipidemic activities.5 Wolfberry, the fruit of Lycium barbarum (Solanaceae) had protective effects against HFD induced liver oxidative stress injury.6
In addition, the anti-obesity effects of some plant-derived bioactive compounds; such as anthocyanin, dioscin, capsaicin, quercetin, and kaempferol; have been enormously reported.7 Also, dihydromyricetin rich herbal mixture extracts were used in treatment of hepatitis, hypertension, and hyperglycemia, where they improved blood glucose, lipid profile, body fat deposition, and hepatic lipid accumulation in HFD rat.8
A. nilotica is a member of the Fabaceae family with many therapeutic properties. It is widely distributed in tropical and subtropical regions, and contains a number of powerful chemicals that have antioxidant, anti-hypertensive, anti-inflammatory, antispasmodic, and anti-platelet aggregatory activities.9
The therapeutic value of the plant may change depending on the part of the plant. The stem barks from A. nilotica have been found to have more flavonoids and polyphenols when compared to roots and leaves. An In vitro study showed that the leaf extract of A. nilotica contains antioxidant chemicals that help to protect DNA from oxidative damage. Polyphenols which present in green pod extracts can chelate metals and scavenge free radicals.10
It is thought that the bark, pods and leaves of A. nilotica are effective against cancer, fever, diarrhea, and menstruation issues.11 The bark of the plant has been used to treat viral, bacterial, amoeboid, fungal, bleeding piles, and leucodermal disorders because it contains a lot of tannins, catechin, epicatechin, and epigallocatechin gallate. Previous studies have shown that the bark of A. nilotica has high contents of kaempferol, umbelliferon, gallic acid, ellagic acid, which have strong antioxidant, antimutagenic and cytotoxic properties.9
The purpose of the present research was to investigate the potential anti-obesity, antioxidant, and anti-inflammatory effects of the total extract of the stem bark of A. nilotica and its ethyl acetate (EA) and butanol fractions in a high fat diet (HFD) rat model, and to use ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS) analysis technique for chemical profiling of the most active fraction of the plant.
2. Materials and methods
2.1. Plant collection and preparation
A. nilotica stem bark was collected in February 2018 from El Dakhla Oasis, New Valley Governorate, Egypt. The plant sample was authenticated by Botany Department, Faculty of Science, Cairo University, Egypt.
2.2. Preparation of plant extracts
The stem bark of air-dried A. nilotica plant was turned into a fine powder. At room temperature, the powder (2 kg) was extracted by percolation with 70% ethanol (3 × 6L EtOH, each 48 h). The combined extracts were concentrated using rotary evaporator at 45 °C to afford the total dried extract (123 g). This extract was chromatographically fractionated by dissolving in approximately 600 mL distilled water (H2O) in a separating funnel and then successively extracted with ethyl acetate and n-butanol by liquid-liquid fractionation. The resulted fractions were concentrated by evaporation till dryness using rotary evaporator to give 45, 40 and 38 g of ethyl acetate, n-butanol and remaining aqueous (H2O) fractions, respectively. The extracts were stored at 4 °C for biological investigation. For the chemical profile of the active fraction, a solution (100 μg/mL) of ethyl acetate stem bark fraction of A. nilotica was prepared in high performance liquid chromatography (HPLC) analytical grade solvent of methanol, filtered using a membrane disc filter (0.2 μm) then subjected to UPLC-qTOF-MS analysis.
2.3. Experimental animals and study design
The present study was carried out on a total number of 72 healthy male Albino rats, weighing 150–200 g, purchased from the Animal House, Research Institute of Ophthalmology. Rats were kept at the animal House, Research Institute of Ophthalmology (Cairo, Egypt). They were housed in separate metal cages under controlled room temperature (22 ± 2 °C) and humidity (55 ± 5%). Rats were allowed one week for acclimatization and handled in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The Ethics committee of the Faculty of Pharmacy, Suez Canal University approved the research protocol (Code 201804MA1).
The rats were randomly divided into 9 groups, each containing 8 rats. Group 1 served as the normal group, in which rats were fed standard balanced diet for throughout the total period of the study (12 weeks). The standard chow diet consisted of carbohydrate 48.8%, protein 21%, and fat 3%, calcium 0.8%, phosphorus 0.4%, fiber 5%, moisture 13%, and ash 8%.12 All the remaining groups were fed HFD for 8 weeks to induce obesity. The HFD was composed of 87.7% standard diet, 10% pork fat, 0.3% bile salts (w/w) and 2% cholesterol.13 The HFD was freshly prepared every day by warming the components of the diet together with the basal diet. After induction of obesity, group 2 served as the HFD control group, while groups (3–9) were treated for 4 weeks as follows:
Group 3: received orlistat (Sigma-Aldrich, Egypt) (5 mg/kg/day) as a standard drug for 4 weeks.
Groups 4 and 5: received total 70% ethanol extract of A. nilotica stem bark at doses of 250 mg/kg and 500 mg/kg, respectively.
Groups 6 and 7: received ethyl acetate fraction of A. nilotica stem bark at doses of 250 mg/kg and 500 mg/kg, respectively.
Groups 8 and 9: received butanol fraction of A. nilotica stem bark at doses of 250 mg/kg and 500 mg/kg, respectively.
All treatments were given daily per oral for a total treatment period of 4 weeks. The weight of rats after induction of obesity and before the start of treatment was recorded, and their weight at the end of the treatment period was also recorded. Percentage weight reduction was calculated for each rat according to the formula:
| [Weight before treatment (g) – weight after treatment (g)] ∗ 100 |
2.4. Collection of samples
At the end of the treatment period, overnight fasting rats were anesthetized with thiopental sodium (50 mg/kg) and blood samples were withdrawn from the orbital sinus. Blood was obtained in part using EDTA anticoagulant tubes to separate plasma by centrifugation at 4000 rpm for 10 min at 4 °C. Plasma was aspirated, and the erythrocytes were washed four times with 0.9% NaCl solution. Each wash was followed by centrifugation at 4000 rpm for 10 min. After the last wash, the washed centrifuged erythrocytes were made up to 2 mL with cold redistilled water, mixed and left to stand at 4ᴼC for 15 min. The other blood portion was collected in plain tubes to separate serum by centrifugation at 3000 rpm for 15 min at 4 °C. Plasma, serum and erythrocyte lysate samples were stored at −80 °C.
Anesthetized rats were killed by cervical dislocation. White adipose tissue was dissected from rats, washed with ice cold phosphate buffered saline (pH 7.4), and fixed in 10% formalin for further histopathological examination.
2.5. Assessment of the biochemical markers
Fasting plasma glucose was determined by enzymatic colorimetric kit (Cat. No. GL 1320) (Biodiagnostic, Egypt). Fasting plasma insulin was detected by Rat Inulin ELISA Kit (Cat. No. MBS724709) (MyBioSource, USA), according to the manufacturer's instructions. Homoeostasis Model Assessment Insulin Resistance (HOMA-IR) was calculated for each rat by the equation: [fasting plasma glucose (mg/dL) ∗ fasting plasma insulin (μIU/mL)]/405.14
Lipid profile was assessed in plasma of the experimental rats. Levels of total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C) and triglycerides (TG) were determined by colorimetric kits (Cat. No. TR2030, CH1202, CH1231, and CH1230, respectively) (Biodiagnostic, Egypt).
Serum levels of malondialdehyde (MDA) and reduced glutathione (GSH) were determined by colorimetric kits (Cat. No. MD2529 and GR2511, respectively) (Biodiagnostic, Egypt). Activities of catalase and superoxide dismutase (SOD) antioxidant enzymes in erythrocyte lysates were assessed by colorimetric kits (Cat. No. CA2517 and SD2521, respectively) (Biodiagnostic, Egypt).
Serum levels of the inflammatory cytokines interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) were determined by ELISA kits (Cat. No. MBS825017, and MBS269892, respectively) (MyBioSource, USA). Similarly, serum levels of the adipocytokines leptin and adiponectin were determined by ELISA kits (Cat. No. MBS730855, and MBS068220, respectively) (MyBioSource, USA), following the manufacturer's instructions.
2.6. Histopathological examination of the adipose tissue
Paraffin slices from adipose tissue were routinely stained with hematoxylin and eosin (H&E). The diameter of 100 adipocyte spaces in two random microscopic fields was determined using the image analysis system [ImageJ 1.45F] (National Institute of Health, USA) following the previously described procedure.15 The mean diameter for each slide was then calculated.
2.7. UPLC-qTOF-MS analysis of the most active fraction
Based on the finding of the current in vivo study, the bioactive ethyl acetate extract fractionated from 70% ethanol extract of A. nilotica stem bark was chosen for further chemical profiling using UPLC-qTOF-MS.16 Chromatographic separation was carried out using Sciex Exion LC coupled with TripleTOF 5600+ equipped with a Xbridge C18 column (3.5 μm, 2.1 × 50 mm; Waters). The elution binary gradient was applied at a flow rate of 0.3 mL min−1: 0–1 min, isocratic 90% A (5 mM ammonium formate buffer pH = 8 containing 1% methanol [v/v]), 10% B (100% acetonitrile); 1–21 min, linear from 10 to 90% B; 21–25 min, isocratic 90% B; 25–28 min, isocratic 10% B. The injection volume from ethyl acetate extract was 10 μL. Eluted compounds from UPLC were detected using MicroTOFQ hybrid quadrupole time-of-flight mass spectrometer equipped with an Apollo-II electrospray ion source in negative ion mode from m/z 100 to 1000 and the instrument settings were: dry gas, nitrogen, 190 °C; nebulizer gas, nitrogen, 1.6 bar; end plate offset, 500 V; capillary, 5500 V (+4000 V); funnel 1 RF, 200 Vpp; funnel 2 RF, 200 Vpp. For MS/MS fragmentation collision-induced dissociation energy 30 eV was used. Wiff file conversion was processed by Reifycs Abf (Analysis Base File) Converter software and Data analysis using MS-DIAL (RIKEN) software. Peaks and spectra were tentatively recognized by comparing mass spectra, retention times, and their fragmentation pattern with data the phytochemical dictionary of natural products (DNP) database as well as the information published in the literature.
2.8. Statistical analysis
The results were expressed as mean ± standard deviation (SD). Statistical analysis was performed using the statistical package for social science (SPSS Inc, Chicago, USA), version 19. One-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test was used to test the significance of the difference between quantitative variables in different groups. P < 0.05 was considered to be statistically significant.
3. Results
Weight gain was developed in the rats that received HFD, where the mean ± SD of the body weight of the rats in all groups (except the normal group) before treatment was 340 ± 22 g. Increased weight was accompanied with insulin resistance where values of fasting plasma glucose, fasting plasma insulin and HOMA-IR were significantly increased in the HFD control group in comparison to the normal group (Table 1). Insulin sensitivity and glucose homeostasis were significantly improved by treatment in the groups that received orlistat, butanol extract of the stem bark of A. nilotica (500 mg/kg), and both doses of the ethyl acetate fraction (250 and 500 mg/kg). The body weight of rats in all treated groups was decreased. The percentage of weight loss after treatment in all treated groups was significantly higher than that in the HFD control group. It was noticed that ethyl acetate fraction was the most active in decreasing body weight and diminishing insulin resistance. No significant difference was detected between both groups treated with the ethyl acetate fraction (250 and 500 mg/kg) and the group the received orlistat. Moreover, the group that received ethyl acetate fraction at the dose of 500 mg/kg showed no significant difference in comparison to the normal group in terms of fasting plasma glucose, fasting plasma insulin and HOMA-IR values (Table 1).
Table 1.
Effect of A. nilotica extracts on body weight, fasting plasma glucose, fasting plasma insulin, and HOMA-IR index and in the experimental rats.
| Weight loss (%) | Glucose (mg/dL) | Insulin (μIU/mL) | HOMA-IR | |
|---|---|---|---|---|
| Normal | 2.7 ± 2.2 | 62 ± 11 | 2.9 ± 0.7 | 0.4 ± 0.2 |
| HFD Control | 1.4 ± 1.1 | 105 ± 18∗ | 13.3 ± 2.3∗ | 3.5 ± 1.1∗ |
| Orlistat | 28 ± 2∗# | 68 ± 14# | 4.9 ± 1.0∗# | 0.8 ± 0.3∗# |
| Total (250 mg/kg) | 4.2 ± 1.5∗#ˆ | 103 ± 14∗ˆ | 12.1 ± 2.5∗ˆ | 3.1 ± 1∗ˆ |
| Total (500 mg/kg) | 10 ± 3.3∗#ˆ | 92 ± 10∗ˆ | 11.6 ± 2.3∗ˆ | 2.7 ± 0.8∗ˆ |
| EA (250 mg/kg) | 27.8 ± 2.1∗# | 72 ± 7# | 4.5 ± 0.6∗# | 0.8 ± 0.2∗# |
| EA (500 mg/kg) | 31.6 ± 2.3∗# | 64 ± 7# | 3.4 ± 0.6# | 0.5 ± 0.1# |
| Bu (250 mg/kg) | 14.3 ± 1.9∗#ˆ | 92 ± 12∗ˆ | 10.6 ± 1.6∗ˆ | 2.4 ± 0.6∗ˆ |
| Bu (500 mg/kg) | 16 ± 2.6∗#ˆ | 82 ± 11∗#ˆ | 9.1 ± 1.3∗#ˆ | 1.9 ± 0.5∗#ˆ |
EA, ethyl acetate fraction; Bu, butanol fraction. Data are represented as mean ± SD and analyzed by ANOVA followed by Tuckey's post hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05.
As expected, the plasma lipid profile of the rats in the HFD control group showed significantly higher TG, TC, and LDL-C, and significantly lower HDL-C levels compared to the normal rats (Table 2). Levels of TG and LDL-C were significantly lowered in all treated groups except the groups that received 250 mg/kg of the total extract of the stem bark of A. nilotica. Levels of TC were significantly decreased in the groups that received orlistat, both doses of the ethyl acetate fraction and the high dose (500 mg/kg) of the butanol fraction, but only orlistat and the ethyl acetate fraction at both doses resulted in a significant increase in the levels of HDL-C (Table 2).
Table 2.
Effect of A. nilotica extracts on lipid profile in the experimental rats.
| TG (mg/dL) | TC (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | |
|---|---|---|---|---|
| Normal | 99 ± 11 | 144 ± 22 | 79 ± 14 | 45 ± 6 |
| HFD Control | 171 ± 32∗ | 210 ± 34∗ | 149 ± 19∗ | 27 ± 9∗ |
| Orlistat | 112 ± 14# | 172 ± 28∗# | 114 ± 19∗# | 36 ± 6∗# |
| Total (250 mg/kg) | 155 ± 24∗ˆ | 190 ± 30∗ | 131 ± 15∗ˆ | 28 ± 10∗ˆ |
| Total (500 mg/kg) | 151 ± 21∗#ˆ | 182 ± 28∗ | 123 ± 14∗# | 29 ± 10∗ˆ |
| EA (250 mg/kg) | 120 ± 12∗# | 167 ± 18∗# | 104 ± 8∗# | 39 ± 8∗# |
| EA (500 mg/kg) | 118 ± 12∗# | 165 ± 13∗# | 101 ± 7∗# | 40 ± 8# |
| Bu (250 mg/kg) | 150 ± 22∗#ˆ | 183 ± 25∗ | 122 ± 16∗# | 31 ± 5∗ |
| Bu (500 mg/kg) | 143 ± 17∗#ˆ | 179 ± 18∗# | 120 ± 9∗# | 30 ± 6∗ |
EA, ethyl acetate fraction; Bu, butanol fraction; TG, triglycerides; TC, total cholesterol; LDL-C, low density lipoprotein-cholesterol, HDL-C, high density lipoprotein-cholesterol. Data are represented as mean ± SD and analyzed by ANOVA followed by Tuckey's post hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05.
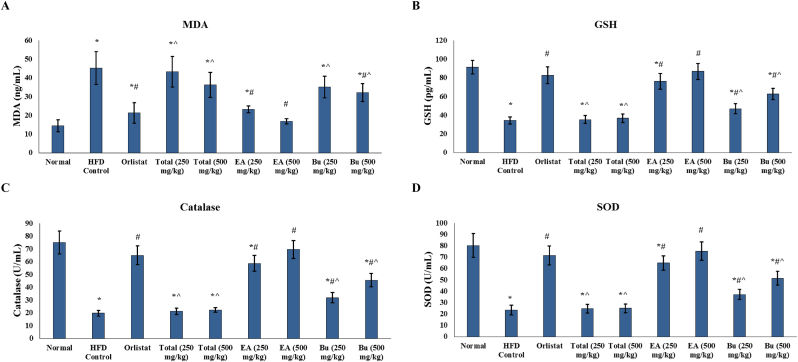
Markers of oxidative stress and antioxidant enzymes were determined in the study groups. Serum levels of MDA were significantly increased in the HFD control rats compared to the normal group. Serum levels of GSH, along with the levels of catalase and SOD in erythrocyte lysate, were significantly decreased in the HFD control rats relative to the normal group (Fig. 1). Levels of MDA were significantly decreased in serum in the groups that received orlistat, A. nilotica butanol fraction (500 mg/kg), and A. nilotica ethyl acetate fraction (250 and 500 mg/kg) (Fig. 1A). Serum levels of GSH as well as the activity of catalase and SOD enzymes in erythrocyte lysate were significantly increased by treatment with orlistat and both doses (250 and 500 mg/kg) of the butanol and ethyl acetate fractions of the stem bark of A. nilotica (Fig. 1B–D). Values of MDA, GSH, catalase and SOD in rats treated with both ethyl acetate fraction doses (250 and 500 mg/kg) were comparable to their values in the orlistat treated group. The higher dose of the ethyl acetate fraction (500 mg/kg) of A. nilotica abolished HDF induced oxidative stress and normalized the values of those markers (Fig. 1).
Fig. 1.
Antioxidant effects of A. nilotica stem bark extracts in the experimental rats. Serum levels of (A) MDA, (B) GSH in the study groups. Activity of (C) catalase, and (D) SOD in erythrocyte lysates of the experimental rats. EA, ethyl acetate fraction; Bu, butanol fraction; MDA, malondialdehyde; GSH, reduced glutathione; SOD, superoxide dismutase. Data are represented as mean ± SD and analyzed by ANOVA and Bonferroni's post-hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05.
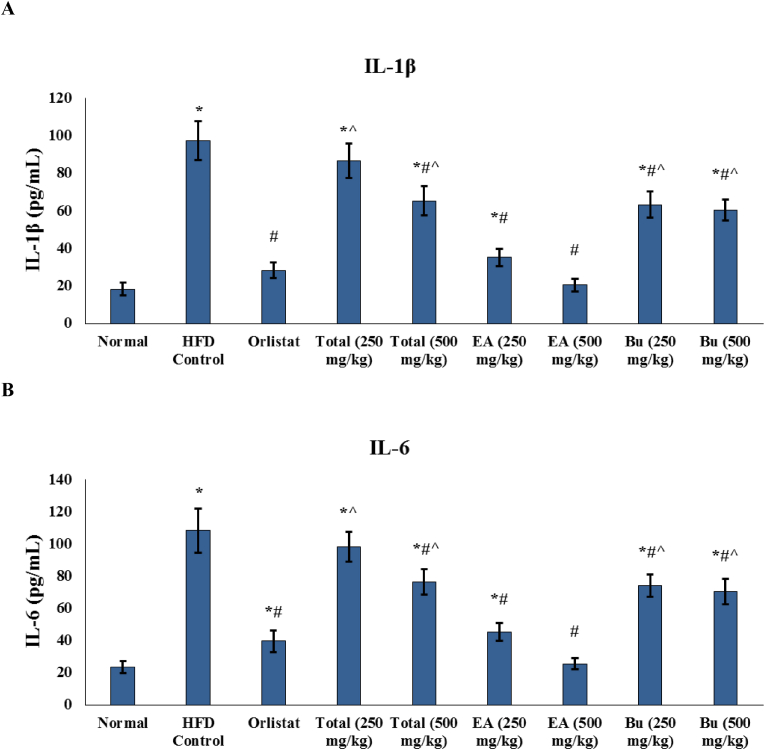
Development of oxidative stress in HFD rats was accompanied by significant increase in the serum levels of the inflammatory cytokines IL-1β and IL-6 (Fig. 2). Both markers were significantly decreased in all treated groups except the 250 mg/kg total extract receiving group. Serum levels of IL-1β and IL-6 in the group that received the higher dose (500 mg/kg) of ethyl acetate fraction of A. nilotica were not significantly different than their levels in the normal group (Fig. 2).
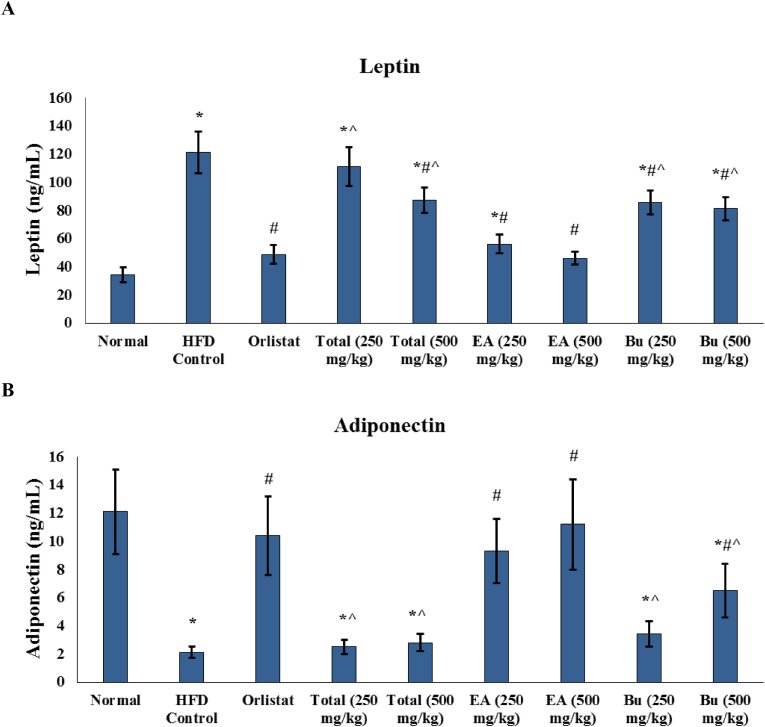
Fig. 2.
Effect of A. nilotica stem bark extracts on the serum levels of adipocytokines in the experimental rats. (A) Leptin, and (B) adiponectin. EA, ethyl acetate fraction; Bu, butanol fraction. Data are represented as mean ± SD and analyzed by ANOVA and Bonferroni's post-hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05.
Serum levels of leptin were significantly increased, while serum levels of adiponectin were significantly decreased in the HFD control group compared to the normal group (Fig. 3). Leptin levels were significantly decreased in all treated groups except the group that received 250 mg/kg of the total ethanaolic extract of A. nilotica stem bark, while levels of adiponectin were significantly increased in the groups that were treated with orlistat, higher dose (500 mg/kg) of the butanol fraction, and both doses (250 and 500 mg/kg) of ethyl acetate fraction of A. nilotica. Levels of both leptin and adiponectin in the ethyl acetate fraction treated rats at both doses were not significantly different compared to the orlistat treated group. Interestingly, serum levels of leptin in the ethyl acetate fraction (500 mg/kg) group and serum levels of adiponectin in both 250 and 500 mg/kg ethyl acetate fraction receiving rats were not significantly different compared to the normal group (Fig. 3).
Fig. 3.
Effect of A. nilotica stem bark extracts on the serum levels of inflammatory cytokines in the experimental rats. (A) IL-1β, and (B) IL-6. EA, ethyl acetate fraction; Bu, butanol fraction; IL-1β, interleukin-1 beta; IL-6, interleukin-6. Data are represented as mean ± SD and analyzed by ANOVA and Bonferroni's post-hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05.
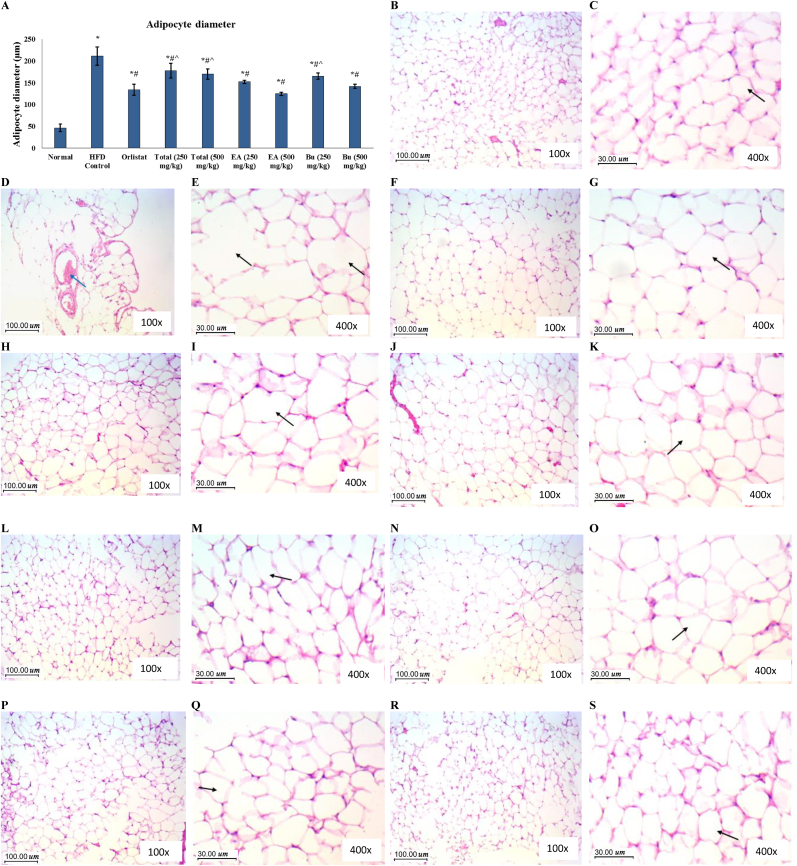
Histopathological examination of adipose tissue showed small regular adipocytes with thin cell membranes and small regular peripheral nuclei in the normal rats, while the adipocytes appeared distorted with irregular shapes, increased size and scattered congested vessels in the HFD control rats. Adipocyte diameter was significantly increased in the HFD control rats compared to the normal rats. The features of adipocytes were enhanced in all treated groups, and adipocyte diameter was significantly decreased in all treated groups, especially the groups that received ethyl acetate fraction of A. nilotica stem bark at both doses (250 and 500 mg/kg) and the group that received butanol fraction at 500 mg/kg dose, where adipocyte diameters in the three groups were not significantly different compared to the orlistat treated rats (Fig. 4).
Fig. 4.
Histopathological examination of the adipose tissue of the experimental rats. (A) Adipocyte diameter represented as mean ± SD and analyzed by ANOVA and Bonferroni's post-hoc test. ∗ significantly different compared to the normal group; # significantly different compared to the HFD control group; ˆ significantly different compared to the orlistat treated group. Differences were considered significantly different at P < 0.05. (B) & (C) Normal group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are of small average size with regular arrangement. (D) & (E) HFD Control group (100x and 400x, respectively); section in adipose tissue showing adipocytes (black arrow) with distorted irregular shapes and increased size and irregular contours, with scattered congested vessels (blue arrow). (F) & (G) Orlistat group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are of smaller size than obese group. (H) & (I) Total extract group (250 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and slight distortion of cells contours and increased cell size. (J) & (K) Total extract group (500 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes with thin cell membranes and small regular peripheral nuclei. Adipocytes are predominantly regularly arranged with few cells showing distortion and increased size (arrow). (L) & (M) Ethyl acetate group (250 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are arranged more regularly with average size. (N) & (O) Ethyl acetate group (500 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are arranged regularly with average size. (P) & (Q) Butanol group (250 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are arranged regularly with average size. (R) & (S) Butanol group (500 mg/kg) group (100x and 400x, respectively); section in adipose tissue showing many adipocytes (arrow) with thin cell membranes and small regular peripheral nuclei. Adipocytes are arranged regularly with average size.
Metabolic profiling of the bioactive ethyl acetate extract fractionated from 70% ethanol extract of A. nilotica stem bark was carried out using UPLC-qTOF-MS in negative ionization mode using DNP databases as well as by comparing with the reported data in the literature. LC-MS of ethyl acetate extract revealed the annotation of 31 secondary metabolites (Suppl. Table 1 and Suppl. Fig. 1) according to their molecular weights and mass fragmentation patterns. These annotated phytochemicals were divided into 30 flavonoids and one phenolic acid. The basic structures of all the tentative identified compounds are listed in Suppl. Table 1 and illustrated in Suppl. Fig 2. In the studied extract, just one phenolic acid was characterized as caffeic acid (12).17 Meanwhile, the flavonoid compounds provided the presence of diverse flavonoid (flavonoids and/or flavonoid glycosides) subclasses including, twelve flavonols (4–6, 8, 15, 21–23, 25–26 and 30–31),10,15,18, 19, 20, 21 five flavones (1, 9, 11 and 19–20),10,15,18,22 five flavanones (3, 10, 18 and 27–28),23, 24, 25 two flavanols (16–17),10,24 a biflavanol (13),24 a flavanonol (2),10 an isoflavone (7),26 a dihydrochaclcone (14),17 and two stilbenes (24 and 29).25,27
In MS analysis, the type of the sugar substructure of the tentatively identified plant flavonoid glycosides were determined by the neutral loss of 146, 162, and 176 amu corresponding to deoxyhexose (rhamnose), hexose (glucose) and hexouronic acid (glucuronic acid) moieties, respectively as shown in compounds 1, 6–7, 9–10, 14–15, 18, 20–21, 23–26 and 30–31. Also, the p-coumaroyl and p-coumaroylhexoside moieties which present in compound 25 could be observed by a neutral loss of 146 and 308 amu, respectively.21 Numerous of these annotated phenolic compounds in the ethyl acetate fraction of A. nilotica stem bark (e.g. taxifolin, quercetin, myricetin, catechin, epicatechin, naringenin, naringenin-7-O-glucopyranoside, kaempferol-7-O-neohesperidoside and caffeic acid) have been previously reported in this plant.10,28
4. Discussion
A. nilotica, a member of the Fabaceae family, is a well-known medicinal plant. The main types of phytochemical components in this plant are alkaloids, flavonoids, tannins, fatty acids and polysaccharides.28 The antibacterial, antioxidant and anticancer activities of A. nilotica leaf extracts have been previously established.29,30 Also, the therapeutic value of A. nilotica pods extract on diabetic nephropathy induced by streptozotocin in rats31 and the protective impact of the polyphenolic extract of A. nilotica on alloxan monohydrate-induced diabetes32 were previously investigated.
Despite its extensive use in traditional medicine and in management of a variety of metabolic problems for decades, the mechanisms contributing to the therapeutic impact of A. nilotica stem bark in preventing body weight gain, hyperlipidemia, and insulin resistance were not fully discussed. So, the present work aimed to provide an updated study of the anti-obesity, antioxidant, and anti-inflammatory effects of the stem bark extracts of A. nilotica in a HFD rat model.
A. nilotica contains a lot of phenolic chemicals especially flavonoids that act as potent antioxidants and have a variety of biological outcomes, such as anti-inflammatory, anti-cancer, anti-diabetic, and anti-aging effects.10,28 Accordingly, one of the main targets in the current research was to study the poly-phenolic (flavonoid) rich fractions of A. nilotica stem bark in a bio-assay guided identification. Thus, we have initially extracted the total extract of this plant stem bark using 70% ethanol. After that, the total extract was successively fractionated by liquid-liquid partition chromatography using ethyl acetate, and then n-butanol. We have particularly used these two solvents because ethyl acetate and n-butanol are considered as two of the favorable and suitable solvents usually used for successively extract and/or fractionate both of less and more polar flavonoids.
Long-term consumption of HFD causes hyperlipidemia, hyperglycemia, and insulin resistance in experimental animals.33 Additionally, the activity of autophagy is generally reduced in HFD models. Autophagy is important in the process of adipocyte differentiation. Lipophagy, the autophagy mediated disposal of lipid droplets, plays an important role in lipid homeostasis and its stimulation by natural polyphenols prevents abnormal lipid accumulation in the tissues.34,35 Insulin resistance in obese mice is increased by defective autophagy, which increases endoplasmic reticulum stress.36 Also, Autophagy affects many cellular processes including oxidative stress, inflammation, and innate and acquired immune response, autophagy modifies the inflammation status of adipose tissue by regulating the synthesis of proinflammatory cytokines including IL-6, IL-8, and IL-1β. The adipose tissue secretes more proinflammatory cytokines when autophagy is inhibited.37
Our findings showed that HFD group become significantly larger during the trial period when compared to their starting body weights and the normal group. Additionally, a considerable drop was noticed in body weight after treatment with ethyl acetate fraction of A. nilotica.
The potent physiological impact of the ethyl acetate fraction that was observed in the current study might be due to the existence of predominant bioactive flavonoids such as taxifolin, quercetin, myricetin, catechin, epicatechin, naringenin, naringenin-7-O-glucopyranoside, kaempferol-7-O-neohesperidoside in A. nilotica as described previously10,28 as well as other identified known compounds like gossypin and baicalin.
The positive effects of flavonoids, particularly epicatechin against obesity are supported by a lot of evidence. It interacts with digestive enzymes and nutrients in the gastrointestinal tract; this gives epicatechin the chance to reduce obesity by decreasing the absorption and digestion of calorie-rich nutrients.38 Furthermore, it increases fat oxidation, prevents fat synthesis, and stimulates energy wasting.39
Increased fasting blood sugar and serum insulin levels in our study clearly indicate that insulin's ability to control blood sugar was impaired in HFD-fed control rats. According to the HOMA-IR index, the level of insulin resistance was high. Treatment of HFD-fed rats with the ethyl acetate fraction of A. nilotica (either 250 or 500 mg/kg) resulted in lowering fasting blood sugar, serum insulin, and the HOMA-IR index. These findings are consistent with many studies suggesting that A. nilotica might contribute to the treatment of diabetes and insulin resistance,40,41 which can be linked to its active ingredients that were revealed by the metabolic profiling that was conducted in the current work (Suppl. Table 1).
Epicatechin and epicatechin-rich foods have a potent effect in improving glucose homeostasis and insulin sensitivity in humans and animal models of obesity and T2DM.42 The favorable effects of epicatechin on glucose metabolism are further supported by in vitro investigations in hepatocytes and adipocytes; these studies found that epicatechin can directly affect glucose homeostasis in cells by increasing insulin receptor substrate-1, activating tyrosine phosphorylation and insulin receptor in response to insulin stimulation, reducing NADPH oxidase upregulation and inhibiting gluconeogenesis through the phosphatidylinositol-3-kinase and 5′-AMP-activated protein kinase pathways.43 Also, Baicalin was suggested to reduce gluconeogenic activity and hepatic insulin resistance via inhibition of p38 mitogen-activated protein kinases/peroxisome proliferator-activated receptor gamma pathway.44
Numerous studies have demonstrated that the flavonoid taxifolin had anti-hyperglycemic action through modulating gastric enzymes; taxifolin exhibited significant inhibiting action against pancreatic lipase, α-glucosidase and α-amylase, furthermore taxifolin has anti-inflammatory and antioxidant activities in alloxan diabetic rat model.45,46 Kaempferol is another A. nilotica flavonoid that exhibited anti-obesity benefits through decreasing adipogenesis and boosting lipolysis in 3T3-L1 cells.7 Furthermore, it ameliorated dyslipidemia and hyperglycemia in HFD induced obesity through altering imbalance of intestinal microbiota and intestinal inflammation, and counteracting Toll-like receptor 4/nuclear factor-kappa B (NF-κB) pathway activation response.47
Dyslipidemia, characterized by elevated levels of TG and LDL-C with decreased HDL-C levels, is a critical risk element for non-alcoholic fatty liver, cardiovascular disease and insulin resistance.48 In our study, Four-week administration of ethyl acetate fractions of A. nilotica or (250 and 500 mg/kg) considerably enhanced lipid profiles in HFD-fed rats as evidenced by decreased TG, TC, LDL levels, and raised serum HDL levels, toward a healthy index.
It has been previously reported that A. nilotica leaf extract decreased levels of blood lipids and blood sugar levels in alloxan-induced diabetic rats.40 Epicatechin, the major A. nilotica constituent, decreases absorption of lipids by suppressing the activity of pancreatic lipase in the gastrointestinal lumen, in addition to controlling lipid absorption, epicatechin also regulates the transcription factors that expressed during cholesterol and TG synthesis, such as peroxisome proliferator-activated receptor gamma (PPARγ) and sterol regulatory element-binding protein.49 Furthermore, epicatechin was able to reduce circulating free fatty acids (FFA) by inhibiting white adipose tissue inflammation and, as a result, lipolysis.50 Epicatechin may also target miRNAs which have been implicated as lipid metabolic regulators in adipose tissue and the liver.51
Baicalin therapy reduced TG content and lipid accumulation in human hepatoma cells treated with palmitic acid. Increased expression of solute carrier family 2 member 1 and the downregulation of NF-κB subunit, sterol regulatory element-binding transcription factor 1, tumor necrosis factor (TNF), and PPARγ were probably the mechanisms responsible for the baicalin-induced decrease in lipid and TG accumulation.52 Taxifolin was also reported to have anti-hyperlipidemic properties through inhibiting cellular cholesterol, TG, and phospholipid synthesis from esterification. Furthermore, taxifolin inhibited hepatic lipid production by altering apolipoprotein B and apolipoprotein A-I secretion.53
The level of oxidative stress in the obese population is another established factor that influences the impact of obesity on insulin resistance and the cardiovascular system.54 Consumption of HFD has been linked to a pronounced rise in oxidative stress.55 In the current study, the ethyl acetate fraction of A. nilotica demonstrated considerable reduction in MDA serum levels and increase in SOD, GSH and catalase activity. The ethyl acetate extract of the stem bark of A. nilotica has considerable hydroxyl free radical scavenging potential due to existence of polyphenolic chemicals in the extract. The observed results confirm the in vitro antioxidant activity of A. nilotica bark that was previously reported.56,57
A. nilotica possesses important anti-free radical capabilities due to a high number of antioxidants like curcumin, flavonoids, terpenoids, phenolics and tannins. They have the capacity to scavenge oxygen nitrogen-derived free radicals by giving an electron or hydrogen atoms, chelating metal catalysts, inhibiting oxidases and activating antioxidant enzymes. As a result, they can lessen the interaction of oxidants and other toxic compounds.58
It has been reported that epicatechin is one of the most potent antioxidants59,60; mainly due to the presence of ortho-hydroxyl groups that are accountable and necessary for their direct detoxifying effects when they react with hydrogen peroxide and superoxide.61 Epicatechin also enhances antioxidant defenses by inhibiting enzymes involved in reactive oxygen species (ROS) production such as mitochondrial succinoxidase, NADH oxidase and microsomal monooxygenase.62
The A. nilotica flavone gossypin had an antioxidant effect in mice with femur fracture; where in gossypin-treated groups, higher levels of SOD, GSH, and low MDA levels were detected and this is consistent with our results. Free radical scavenging properties and specific signals by genes of antioxidant and anti-inflammatory compounds are two aspects that contribute to gossypin's antioxidant effect.63 Taxifolin, another flavonoid detected in the ethyl acetate fraction of A. nilotica, has been found to prevent stress-induced apoptosis, particularly oxidative stress, and endoplasmic reticulum stress through the phosphatidylinositol-3-kinase/Protein kinase B pathway.64
Leptin is a peptide hormone that controls appetite, body weight, proinflammatory immunological responses, angiogenesis, and lipolysis. The pleiotropic actions of leptin are important in regulating the body mass through a negative feedback process between the hypothalamus and the adipose tissue.65 In the current study, levels of leptin in the both groups that received the ethyl acetate fraction at 250 and 500 mg/kg were considerably lower than the obese control group; this is in line with prior research that found a link between body weight loss and a lower leptin level.66
Interestingly, the reduction of leptin levels was accompanied with the reversal of insulin resistance. This effect might result from the suppression of cytokines that cause leptin resistance; this is backed up by other studies that demonstrated that hypothalamic oxidative stress causes leptin resistance which triggers the development of insulin resistance.67 Epicatechin therapies were reported to reduce serum leptin levels, which lessens the risk of body weight gain and adipose tissue accumulation.68
Conversely, our results revealed that low levels of adiponectin in HFD induced obesity were significantly increased by treatment with both doses of A. nilotica stem bark ethyl acetate fraction. Adiponectin has anti-inflammatory, antioxidant, and cardiovascular regulating properties in addition to its insulin-sensitizing properties.69 The A. nilotica active compound Kaempferol had a protective effect aganist obesity and T2DM in HFD mice, it also boosted adiponectin expression while decreasing leptin expression.70
Since obesity is the main contributor to chronic low-grade inflammation, adipose tissue can upregulate pro-inflammatory interleukins by accumulating excess lipids. These interleukins may facilitate immune cell infiltration into adipose tissue, leading to low-grade inflammation and abnormal adipocyte function.12 The primary source of local and systemic inflammatory mediators including IL-6, IL-1β, and TNF-α is thought to be adipose tissue macrophages. These cytokines produce insulin resistance via triggering cytokine suppressors signaling proteins.71
IL-1 is a master cytokine because of its involvement in local and systemic inflammation. Overexpression of IL-1β in adipose tissues leads to infiltrating of immune cells which results in low-grade inflammation12 Circulating levels of IL-6 could be an indicator of how severe the systemic and ongoing chronic inflammation that results from severe obesity. Pro-inflammatory cytokine IL-6 suppresses lipoprotein lipase activity and increases endothelium lipase's lipolytic activity, both have been linked to low HDL-C values during acute or chronic inflammatory conditions.72 In the current study, high levels of IL-6 and IL-1β in obese rats were significantly reduced upon treatment with either A. nilotica stem barks extracts, especially the ethyl acetate fractions. The anti-inflammatory effect was supported by improvements in the histopathological findings among the groups that received treatment. In a mouse model of obesity-induced by HFD, epicatechin supplementation reduced the activation of pro-inflammatory signaling pathways such as c-Jun N-terminal kinase, NF-κB, as well as macrophage recruitment.70 Epicatechin reduced cypermethrin-induced inflammation in rats by significantly lowering plasma TNF-α and IL-6 levels, possibly through inactivating the NF-κB and mitogen-activated protein kinase (MAPK) pathways.59,73,74
Gossypin is another A. nilotica flavonoid with potent immunomodulatory and anti-inflammatory effects; increased IL-1, IL-6 and TNF levels were lowered after gossypin administration.75 Gossypin suppresses the inflammatory pathway by decreasing NF-κB immunopositivity.76,77 It was found to inhibit the production of IL-6, and expression of cyclooxygenase-2 COX-2 in bone marrow-derived mast cells, supporting taxifolin's anti-inflammatory properties.13
Kaempferol was suggested to reduce chronic inflammation and oxidative stress, according to previous studies.78 Furthermore, kaempferol significantly reduced neutrophil and macrophage infiltration, this was followed by a decline in cytokines such as IL-6 and TNF-α indicating an anti-inflammatory effect.47
5. Conclusion
In conclusion, this study emphasizes the potent anti-obesity, antioxidant, and anti-inflammatory effects of the ethyl acetate fraction of the stem bark of A. nilotica in a HFD rat model. The current work provided evidence that the ethyl acetate fraction of A. nilotica was effective in preventing and decreasing obesity, insulin resistance and hyperlipidemia in diet-induced obesity in rats. These effects may be due to reduced cytokine production, inhibition of leptin resistance and boosting adiponectin, which functions as an anti-adipogenic and anti-obesity agent. However, more investigation is needed to examine the clinical safety and efficacy data with a variety of doses to detect its efficacy in the treatment of obesity and insulin resistance. Also, further qualitative and quantitative verification of the definite pure constituents of the promising A. nilotica stem bark ethyl acetate fraction is strongly recommended.
CRediT authorship contribution statement
ARH and MKE designed the study. OAS, ARH and MKE performed the laboratory experiments. OAS, SSK and ETM analyzed the data. OAS, SSK and ETM wrote the original manuscript. SSK and ETM revised the manuscript and prepared the final version. All authors read and approved the final manuscript.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The research data is available upon request from the corresponding author.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge the support of Prof. Azza El Hadidy, Professor of Plant Taxonomy and Flora, Faculty of Science, Cairo University, for the excellent plant handling and maintenance. We also are grateful to Prof. Dina Abo-elmatty, Professor of Biochemistry, Faculty of Pharmacy, Suez Canal University, and Prof. Noha Mesbah, Professor of Biochemistry, Faculty of Pharmacy, Suez Canal University, for their kind guidance and support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2023.03.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Nimptsch K., Konigorski S., Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. doi: 10.1016/j.metabol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Shang A., Cao S.Y., Xu X.Y., et al. Bioactive compounds and biological functions of garlic (Allium sativum L.) Foods. 2019;8(7):246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abduljawad E.A. Review of some evidenced medicinal activities of Acacia Nilotica. Arch Pharm Pract. 2020;11(4):20–25. [Google Scholar]
- 5.Nagamma T., Konuri A., Bhat K.M., Udupa P., Rao G., Nayak Y. Prophylactic effect of Trigonella foenum-graecum L. seed extract on inflammatory markers and histopathological changes in high-fat-fed ovariectomized rats. J Tradit Complement Med. 2021;12(2):131–140. doi: 10.1016/j.jtcme.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao J., Fai So K., Liong E.C., Tipoe G.L. Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J Tradit Complement Med. 2013;3(2):88–94. doi: 10.4103/2225-4110.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Villarreal D., Camacho A., Castro H., Ortiz-Lopez R., de la Garza A.L. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J Physiol Biochem. 2019;75(1):83–88. doi: 10.1007/s13105-018-0659-4. [DOI] [PubMed] [Google Scholar]
- 8.Chien M.Y., Yang C.M., Lin Y.T., Chen C.H. Dihydromyricetin-rich herbal mixture extracts as a potential prescription for treatment of metabolic syndrome in rats fed a high-fat diet and subacute toxicity assessment in rats. J Tradit Complement Med. 2018;9(3):221–226. doi: 10.1016/j.jtcme.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur P., Arora S., Singh R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci Rep. 2022;7:12(1):9370. doi: 10.1038/s41598-022-13338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadiq M.B., Hanpithakpong W., Tarning J., Anal A.K. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind Crop Prod. 2015;77:873–882. [Google Scholar]
- 11.Sadiq M.B., Tharaphan P., Chotivanich K., Tarning J., Anal A.K. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Compl Alternative Med. 2017;17(1):372–378. doi: 10.1186/s12906-017-1878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aljuboury R.A., Al-Shawi N.N. Anti-obesity effect of simvastatin and omega-3 and its combination on obese model male Wistar rats. Iraqi Journal of Pharmaceutical Sciences. 2022;31(2):101–112. [Google Scholar]
- 13.Pan M., Song Y.L., Xu J.M., Gan H.Z. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J Pineal Res. 2006;41(1):79–84. doi: 10.1111/j.1600-079X.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 14.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Zaitone S.A., Essawy S. Addition of a low dose of rimonabant to orlistat therapy decreases weight gain and reduces adiposity in dietary obese rats. Clin Exp Pharmacol Physiol. 2012;39(6) doi: 10.1111/j.1440-1681.2012.05717.x. 551-519. [DOI] [PubMed] [Google Scholar]
- 16.El-Kousy S.M., Emam S.S., Hassan A.R., Sanad I.M. Metabolites profiling of Limonium tubiflorum (Delile) Kuntze var tubiflorum via UPLC-qTOF-MS technique in relation to its cytotoxic activity. Jordan J Biol Sci. 2021;14(4):663–669. [Google Scholar]
- 17.Hassan A.R. Chemical profile and cytotoxic activity of a polyphenolic-rich fraction from Euphorbia dendroides aerial parts. South Afr J Bot. 2022;147:332–339. [Google Scholar]
- 18.El-Hawary S.S., Hammam W.E., El-Tantawi M.E.M., et al. Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: evidence from in vivo and in silico studies. Arab J Chem. 2021;14(6) [Google Scholar]
- 19.Mari A., Lyon D., Fragner L., et al. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics. 2013;9(3):599–607. doi: 10.1007/s11306-012-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezende F.M., Ferreira M.J.P., Clausen M.H., Rossi M., Furlan C.M. Acylated flavonoid glycosides are the main pigments that determine the flower colour of the Brazilian native tree tibouchina pulchra (cham. Cogn. Molecules. 2019;24(4):718. doi: 10.3390/molecules24040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreeja P.S., Arunachalam K., Martins D.T.O., et al. Sphenodesme involucrata var. paniculata (C.B. Clarke) Munir.: chemical characterization, anti-nociceptive and anti-inflammatory activities of methanol extract of leaves. J Ethnopharmacol. 2018;225:71–80. doi: 10.1016/j.jep.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Lu C.M., Lin L.C., Tsai T.H. Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin, and swertiamarin in Chinese herbal formulae after oral administration in rats by LC-MS/MS. Molecules. 2014;19(12):21560–21578. doi: 10.3390/molecules191221560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Huang C., Fu W., et al. Screening of the active fractions from the Coreopsis tinctoria Nutt. Flower on diabetic endothelial protection and determination of the underlying mechanism. J Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112645. [DOI] [PubMed] [Google Scholar]
- 24.Lyu Q., Kuo T.H., Sun C., Chen K., Hsu C.C., Li X. Comprehensive structural characterization of phenolics in litchi pulp using tandem mass spectral molecular networking. Food Chem. 2019;282:9–17. doi: 10.1016/j.foodchem.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Presta M.A., Bruyneel B., Zanella R., Kool J., Krabbe J.G., Lingeman H. Determination of flavonoids and resveratrol in wine by turbulent-flow chromatography-LC-MS. Chromatographia. 2009;69:167–173. doi: 10.1365/s10337-009-1132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Xu Q., Zhang X., Chen J., Liang X., Kettrup A. High-performance liquid chromatography-tandem mass spectrometry for identification of isoflavones and description of the biotransformation of kudzu root. Anal Bioanal Chem. 2005;383(5):787–796. doi: 10.1007/s00216-005-0068-8. [DOI] [PubMed] [Google Scholar]
- 27.Gabaston J., Richard T., Biais B., et al. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola) Ind Crop Prod. 2017;103:267–273. [Google Scholar]
- 28.Rather L., Mohammad F. Acacia nilotica (L.): a review of its traditional uses, phytochemistry, and pharmacology. Sustainable Chem Pharm. 2015;2:12–30. [Google Scholar]
- 29.Sampath G., Shyu D., Rameshkumar N., Krishnan M., Kayalvizhi N. In vitro anti-Helicobacter pylori and anti-gastric cancer activities of Acacia nilotica aqueous leaf extract and its validation using in silico molecular docking approach. Mater Today Chem. 2022;51(4):1675–1684. [Google Scholar]
- 30.Revathi S., Govindarajan R.K., Rameshkumar N., et al. Anti-cancer, anti-microbial and antioxidant properties of Acacia nilotica and their chemical profiling. Biocatal Agric Biotechnol. 2017;11:322–329. [Google Scholar]
- 31.Omara E.A., Nada S.A., Farrag A.R., Sharaf W.M., El-Toumy S.A. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine. 2012;19(12):1059–1067. doi: 10.1016/j.phymed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Wafa M., Bilal A., Asra I., et al. Acacia nilotica polyphenol extract restores glucose homeostasis by upregulating the insulin secretion and lowering the oxidative stress through down regulation of c-Jun N-terminal kinase (JNK) signaling cascade. J King Saud Univ Sci. 2021;23(5) [Google Scholar]
- 33.Srinivasan K., Viswanad B., Asrat L., Kaul C.L., Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Parafati M., Lascala A., Morittu V.M., et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J Nutr Biochem. 2015;26(9):938–948. doi: 10.1016/j.jnutbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Shin D.W. Lipophagy: molecular mechanisms and implications in metabolic disorders. Mol Cell. 2020;43(8):686–693. doi: 10.14348/molcells.2020.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kounakis K., Chaniotakis M., Markaki M., Tavernarakis N. Emerging roles of lipophagy in Health and disease. Front Cell Dev Biol. 2019;7:185. doi: 10.3389/fcell.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen H.J., Van Essen P., Koenen T., et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153(12):5866–5874. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 38.Cires M.J., Wong X., Carrasco-Pozo C., Gotteland M. The gastrointestinal tract as a key target organ for the health-promoting effects of dietary proanthocyanidins. Front Nutr. 2017;3:57. doi: 10.3389/fnut.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhlaghi M., Kohanmoo A. Mechanisms of anti-obesity effects of catechins: a review. Int J Nutr Sci. 2018;3(3):127–132. [Google Scholar]
- 40.Asad M., Munir T.A., Farid S., Aslam M., Shah S.S. Duration effect of Acacia nilotica leaves extract and glibenclamide as hypolipidaemic and hypoglycaemic activity in alloxan induced diabetic rats. J Pakistan Med Assoc. 2015;65(12):1266–1270. [PubMed] [Google Scholar]
- 41.Roozbeh N., Darvish L., Abdi F. Hypoglycemic effects of Acacia nilotica in type II diabetes: a research proposal. BMC Res Notes. 2017;10(1):331. doi: 10.1186/s13104-017-2646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cremonini E., Fraga C.G., Oteiza P.I. (-)-Epicatechin in the control of glucose homeostasis: involvement of redox-regulated mechanisms. Free Radic Biol Med. 2019;130:478–488. doi: 10.1016/j.freeradbiomed.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Cremonini E., Oteiza P.I. (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells. Arch Biochem Biophys. 2018;646:55–63. doi: 10.1016/j.abb.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 44.Fang P., Sun Y., Gu X., et al. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine. 2019;64 doi: 10.1016/j.phymed.2019.153074. [DOI] [PubMed] [Google Scholar]
- 45.Rehman K., Chohan T.A., Waheed I., Gilani Z., Akash M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: evidence from an in vivo and in silico studies. J Cell Biochem. 2019;120(1):425–438. doi: 10.1002/jcb.27398. [DOI] [PubMed] [Google Scholar]
- 46.Su H., Ruan Y.T., Li Y., Chen J.G., Yin Z.P., Zhang Q.F. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. Int J Biol Macromol. 2020;150:31–37. doi: 10.1016/j.ijbiomac.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Bian Y., Lei J., Zhong J., et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J Nutr Biochem. 2022;99 doi: 10.1016/j.jnutbio.2021.108840. [DOI] [PubMed] [Google Scholar]
- 48.Matsuzaka T., Shimano H. New perspective on type 2 diabetes, dyslipidemia and non-alcoholic fatty liver disease. J Diabetes Investig. 2020;11(3):532–534. doi: 10.1111/jdi.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanley T.H., Van Buiten C.B., Baker S.A., Elias R.J., Anantheswaran R.C., Lambert J.D. Impact of roasting on the flavan-3-ol composition, sensory-related chemistry, and in vitro pancreatic lipase inhibitory activity of cocoa beans. Food Chem. 2018;255:414–420. doi: 10.1016/j.foodchem.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cremonini E., Bettaieb A., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch Biochem Biophys. 2016;599:13–21. doi: 10.1016/j.abb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Huang Y., Shao H., Bi Q., Chen J., Ye Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor γ with miR-483-5p involved mechanism. Biomed Pharmacother. 2017;86:292–296. doi: 10.1016/j.biopha.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z.Y., Jiang Z.M., Xiao P.T., Jiang Y.Q., Liu W.J., Liu E.H. The mechanisms of baicalin ameliorate obesity and hyperlipidemia through a network pharmacology approach. Eur J Pharmacol. 2020;878 doi: 10.1016/j.ejphar.2020.173103. [DOI] [PubMed] [Google Scholar]
- 53.Theriault A., Wang Q., Van Iderstine S.C., Chen B., Franke A.A., Adeli K. Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid 1. JLR (J Lipid Res) 2000;41(12):1969–1979. [PubMed] [Google Scholar]
- 54.Levy E., Saenger A.K., Steffes M.W., Delvin E. Pediatric obesity and cardiometabolic disorders: risk factors and biomarkers. EJIFCC. 2017;28(1):6–24. [PMC free article] [PubMed] [Google Scholar]
- 55.Lasker S., Rahman M.M., Parvez F., et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gowri S., Pavitha S., Vasantha K. Free radical scavenging capacity and antioxidant activity of young leaves and barks of Acacia nilotica (L.), Del. Int J Pharm Pharmaceut Sci. 2011;3:160–164. [Google Scholar]
- 57.Malviya S., Rawat S., Anil Kharia A., MeenaVerma M. Medicinal attributes of Acacia nilotica Linn. -A comprehensive review on ethnopharmacological claims. Int J Pharm Life Sci. 2011:2830–2837. [Google Scholar]
- 58.Maldini M., Montoro P., Hamed A.I., et al. Strong antioxidant phenolics from Acacia nilotica: profiling by ESI-MS and qualitative-quantitative determination by LC-ESI-MS. J Pharm Biomed Anal. 2011;56(2):228–239. doi: 10.1016/j.jpba.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 59.Afolabi O.K., Aderibigbe F.A., Folarin D.T., Arinola A., Wusu A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: mitigating potential of epicatechin. Heliyon. 2019;5(8) doi: 10.1016/j.heliyon.2019.e02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos L.F.S., Stolfo A., Calloni C., Salvador M. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. J arrhythmia. 2017;33(3):220–225. doi: 10.1016/j.joa.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shay J., Elbaz H.A., Lee I., Zielske S.P., Malek M.H., Hüttemann M. Molecular mechanisms and therapeutic effects of (-)-Epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/181260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yildiz K., Turalioglu M.F., Tahiroglu V., Fatih B.O.Y., Yigit S. Effects of gossypin on fracture healing in experimental femur fractured mouse mechano-bioregulatory model. Kafkas Univ Vet Fak Derg. 2021;27(4):445–454. [Google Scholar]
- 64.Shu Z., Yang Y., Yang L., Jiang H., Yu X., Wang Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019;10(1):203–215. doi: 10.1039/c8fo01256c. [DOI] [PubMed] [Google Scholar]
- 65.Obradovic M., Sudar-Milovanovic E., Soskic S., et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arabpour-Dahoue M., Mohammadzadeh E., Avan A., et al. Leptin level decreases after treatment with the combination of Radiofrequency and Ultrasound cavitation in response to the reduction in adiposity. Diabetes Metabol Syndr. 2019;13(2):1137–1140. doi: 10.1016/j.dsx.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 67.Yagishita Y., Uruno A., Fukutomi T., et al. Nrf 2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 2017;18(8):2030–2044. doi: 10.1016/j.celrep.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 68.Rabadan-Chávez G., Quevedo-Corona L., Garcia A.M., Reyes-Maldonado E., Jaramillo-Flores M.E. Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. J Funct Foods. 2016;20:54–67. [Google Scholar]
- 69.Gariballa S., Alkaabi J., Yasin J., Al Essa A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr Disord. 2019;19(1):1–6. doi: 10.1186/s12902-019-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi E.H., Chun Y.S., Kim J., et al. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J Funct Foods. 2020;72 [Google Scholar]
- 71.Khodabandehloo H., Gorgani-Firuzjaee S., Panahi G., Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167(1):228–256. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 72.El-Mikkawy D.M.E., El-Sadek M.A., El-Badawy M.A., et al. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt Rheumatol Rehabil. 2020;47:7. [Google Scholar]
- 73.Bettaieb A., Cremonini E., Kang H., Kang J., Haj F.G., Oteiza P.I. Anti-inflammatory actions of (-)-epicatechin in the adipose tissue of obese mice. Int J Biochem Cell Biol. 2016;81(Pt B):383–392. doi: 10.1016/j.biocel.2016.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang D.J., Liu S.C., Chen Y.C., Hsu S.H., Chang Y.P., Lin J.T. Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage RAW 264.7 Cells. J Food Biochem. 2015;39(3):334–343. [Google Scholar]
- 75.Cinar I., Sirin B., Aydin P., et al. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sci. 2019;221:327–334. doi: 10.1016/j.lfs.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 76.Katary M., Salahuddin A. Ameliorative effect of gossypin against gentamicin-induced nephrotoxicity in rats. Life Sci. 2017;176:75–81. doi: 10.1016/j.lfs.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Tanyeli A., Eraslan E., Güler M.C., Nezahat K.U., Akaras N. Gossypin protects against renal Ischemia-Reperfusion Injury in rats. Kafkas Univ Vet Fak Derg. 2020;26(1):89–96. [Google Scholar]
- 78.Aa L.X., Fei F., Qi Q., et al. Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacol Sin. 2020;41(1):73–81. doi: 10.1038/s41401-019-0279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research data is available upon request from the corresponding author.