Abstract
Background/Aims:
To assess the long-term variability of macular optical coherence tomography (OCT)/OCT angiography (OCTA) and visual field (VF) parameters.
Methods:
Healthy and glaucoma eyes with ≥1-year follow-up were included. 24–2 VF and macular OCT/OCTA parameters, including VF mean deviation (MD), whole-image vessel density (wiVD) and ganglion cell complex thickness (wiGCC) were analyzed. Intra-class correlation co-efficient (ICC), root mean squared error (RMSE), within-subject test-retest standard deviation (Sw), and test-retest variability were calculated for stable eye cohort (max follow-up=1.5 years). Rates of change and RMSE were evaluated in the extended cohort including all eyes (unlimited follow-up).
Results:
From a total of 230 eyes (150 participants; age=67.7 years), 86 eyes (37%, 62 participants) were stable. In stable eyes, OCT parameters showed the highest mean(95%) ICC (wiGCC=0.99[0.99, 0.99]), followed by VF (VF MD=0.91[0.88, 0.93]) and OCTA (wiVD=0.82[0.75, 0.87]). RMSE and SW for VF MD were 0.92dB and 0.81dB, respectively, for wiVD were 1.64% and 1.48%, respectively, and for wiGCC, 0.91μm and 0.78μm, respectively. The long-term test-rest variability of VF MD, wiVD, and wiGCC was 2.2dB, 4.1%, and 2.2μm, respectively. In the extended cohort (mean follow-up=3.0 years), all parameters had significant rates of change (P<0.001), and compared to the stable cohort, only slightly higher RMSE (VF MD=1.07dB; wiGCC=2.03μm; wiVD=2.57%) were found.
Conclusions:
VF and macular OCT/OCTA, particularly OCT parameters, showed small long-term variability in all eyes, including stable ones, supporting the use of these instruments in glaucoma follow-up. Changes in macular VD and GCC greater than 4–5% and 2μm, respectively, indicate possible progression.
Keywords: glaucoma, OCT, OCTA, visual field, reproducibility, variability
INTRODUCTION
Glaucoma is characterized by irreversible, progressive retinal ganglion cells loss.[1] In current practice, clinical monitoring of glaucoma relies on both functional and structural evaluation, with the visual field (VF) test and optical coherence tomography (OCT) considered part of the routine examinations. OCT angiography (OCTA) has also emerged as a new imaging modality with the potentials to refine glaucoma management through its ability to evaluate alterations in retinal microvasculature.[2]
With a chronic and progressive course, the evaluation of true glaucomatous progression relies heavily on the long-term variability of clinical examinations. However, studies investigating the long-term variability of measurements pertinent to glaucoma management, which required a follow-up period of at least several months, were very limited,[3–5] with the majority performed on only stable cases. In addition, most prior works examining macular structural parameters were short-term, thus could not inform clinicians on the longitudinal trend of measurement variability that should be considered during long-term follow-up.
Among the few available studies on macular thickness measured by OCT, Miraftabi et al. revealed a low short-term variability,[6] and Kim et al. showed a small long-term variability in non-progressive glaucoma eyes.[7] As for OCTA, a few studies have shown a satisfactory short-term variability of superficial macular vessel density (VD) in healthy and glaucoma subjects.[8–10] Our prior study examined the long-term reproducibility of macular OCT/OCTA; nevertheless, only a smaller number of stable cases with a mean follow-up of 1 year were included.[11] Of note, assessment of the long-term variability of both VF and macular OCT/OCTA in the same study remains lacking.
To accurately differentiate true glaucomatous changes from measurement variability, it is important to investigate the long-term variability of these functional and structural parameters. Moreover, to better simulate clinical settings, the variability of these parameters should also be examined on an extended cohort consisting of both stable and non-stable cases. In this study, the long-term variability of macular OCT/OCTA and VF parameters were assessed in both stable eyes and all eyes.
METHODS
Participants
The study adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act, and was approved by the University of California San Diego (UCSD) Human Research Protection Program (NCT00221897). Written informed consent was obtained from all participants.
This retrospective cohort study included healthy, glaucoma suspect, and primary open angle glaucoma patients (definitions provided in Supplemental Method 1) from the Diagnostic Innovations in Glaucoma Study (DIGS)[12 13] meeting the inclusion criteria. All DIGS participants underwent the examinations described in Supplemental Method 2. Demographic information and systemic medical history were also collected. The inclusion criteria for DIGS were: (1)older than 18 years of age; (2)open angles on gonioscopy; (3)best-corrected visual acuity of 20/40 or better; (4)refraction within ±5.0 diopters spherical and within ±3.0-diopters cylinder at study entry.
For the current study, only DIGS subjects with at least 3 visits of VF/OCT/OCTA examinations during the same period (<3-month difference) and 1-year follow-up were included. Further exclusion criteria included: (1)history of trauma; (2)uveitis; (3)axial length≥27mm; (4)coexisting retinal disease including diabetic retinopathy; (5)non-glaucomatous optic neuropathy; (6)history of Parkinson’s disease, clinical dementia, or stroke.
OCTA and Spectral-Domain OCT
This study included 3mm × 3mm macula OCT/OCTA scans acquired using the Avanti Angiovue system (Optovue, Inc. Fremont, CA, software version 2018.1.1.63).[14] The OCT/OCTA images were acquired simultaneously. Ganglion cell complex (GCC) thickness and VD, the percentage of measured area occupied by flowing blood vessels, were analyzed from the same scan slab. Whole-image superficial VD (wiVD) and GCC thickness (wiGCC) were measured from the entire macula scan, and parafoveal superficial VD (pfVD) and GCC thickness (pfGCC) were measured from the fovea-centered annular region within the 1–3mm ring diameter zone. OCT/OCTA image quality review was performed by trained graders, and poor-quality images were excluded based on criteria described in Supplemental Method 3.
Visual Field Examination
VF examination was performed using Swedish Interactive Thresholding Algorithm standard 24–2 threshold test (Humphrey Field Analyzer, Carl Zeiss Meditec, Dublin, California, USA). Quality review of 24–2 VF was performed by the VF Assessment Center staffs at UCSD. VF results were considered unreliable and excluded if the following artifacts were present: (1)inattention or fatigue effects; (2)evidence of rim and eyelid artifacts; (3)VF damage caused by diseases other than glaucoma. All VF tests were performed within 3 months of the OCT/OCTA exam date, and the numbers of visits/tests of VF and OCT/OCTA included for analysis were comparable.
Assessment of Glaucoma Stability
The long-term variability of OCT/OCTA and VF parameters was assessed under two scenarios. One scenario, the “stable eye cohort” included only stable eyes with a maximum follow-up of 1.5 years to eliminate the effect of aging on the long-term measurement variability. The other scenario, the “extended cohort” simulated clinical settings and included all eyes, with no limitation on the follow-up duration and stability status. The stability of glaucoma was determined using both serial color stereophotographs and VF. A case was considered stable only when both structural progression and functional progression were not detected.
Color stereophotographs (Nidek Stereo Camera Model 3-DX, Nidek, Palo Alto, California, USA) were taken after pupil dilation and used to determine structural stability. Glaucomatous structural progression was defined as the presence of progressive optic disc changes, focal or diffuse neuroretinal rim narrowing or notching, adjacent vascular position shift, or optic disc hemorrhage, or the presence of progressive RNFL changes. A structurally stable case was confirmed by the consensus of two independent masked observers (TN, JHW), with the third observer (SM) being the adjudicator when there was disagreement.
Glaucomatous functional progression was evaluated using serial VF obtained from a minimum of 5-year follow-up before the first OCT/OCTA visit included in this study, and determined based on both event-based and trend-based analysis. VF obtained later were not evaluated to prevent from affecting the variability measurement. Functional stability was determined when neither trend-based nor event-based analysis showed signs of progression based on definition described in Supplemental Method 4.
Statistical analysis
Demographic and clinical characteristic data were presented as mean (95% confidence interval [CI]) for continuous variables and count (%) for categorical variables. For primary metrics demonstrating the long-term variability, a linear random effects analysis of variance model was used to calculate the intraclass correlation coefficient (ICC), root mean squared error (RMSE), within-subject test–retest standard deviation (Sw), and test-retest variability (TRV) (details of metrics provided in Supplemental Method 5).[5 10 11 15–17] The long-term TRV was calculated by Sw × 2.77. The coefficient of variation (CV, %) was calculated by 100×(Sw/overall mean) and was presented supplementary. To account for the within-subject variability, comparison of rates of change of VF and OCT/OCTA parameters was performed using a linear mixed model. The time-dependent effects of age, IOP, and central corneal thickness (CCT) on the measurement of these parameters were also calculated using linear mixed effects models. Analyses of variability were performed on both the stable eye cohort and the extended cohort including all eyes. For the stable eye cohort, ICC, RMSE, Sw, and CV were calculated. For the extended cohort, the rates of change and RMSE were calculated. Statistical analyses were performed using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). A P-value≤0.05 was considered statistically significant.
RESULTS
A total of 230 eyes of 150 patients (mean [95% CI] age = 67.7[65.8, 69.6] years) were included, and 86 eyes (37%) of 62 patients (age = 65.2[62.0, 68.5] years) were determined stable. Demographic and clinical characteristics of the extended cohort including all eyes (unlimited follow-up) and the stable eye cohort (max follow-up=1.5 years) are summarized in Table 1. The mean (95% CI) follow-up durations of the extended cohort and stable eye cohort were 3.0(2.8, 3.2) years and 1.2(1.1, 1.2) years, respectively.
Table 1.
Demographics and Baseline Clinical Characteristics of the Subjects
| Extended cohort including all eyes (Unlimited follow-up) | Stable eye cohort (Max follow-up = 1.5 years) | |
|---|---|---|
|
| ||
| Characteristic | n = 230 eyes (150 subjects) | n = 86 eyes (62 subjects) |
|
| ||
| Age (years) | 67.7 (65.8, 69.6) | 65.2 (62.0, 68.5) |
|
| ||
| Sex (Female/ Male) | 80/70 | 37/25 |
|
| ||
| Race (African American/ non-African American) | 39/111 | 19/43 |
|
| ||
| Self-reported hypertension, n(%) | 87 (58%) | 32 (52%) |
|
| ||
| Self-reported diabetes, n(%) | 22 (15%) | 9 (15%) |
|
| ||
| Eye related variables | ||
|
| ||
| Diagnosis (%) | ||
| Healthy | 28 (12%) | 18 (21%) |
| Glaucoma suspect | 65 (28%) | 28 (33%) |
| POAG | 137 (60%) | 40 (47%) |
|
| ||
| Mean IOP during follow up (mmHg) | 15.0 (14.4, 15.5) | 15.4 (14.5, 16.3) |
|
| ||
| Axial length (mm) | 24.4 (24.2, 24.5) | 24.5 (24.2, 24.7) |
|
| ||
| CCT (μm) | 541.1 (536, 546.3) | 538.6 (530.2, 547.1) |
|
| ||
| Baseline 24-2 VF MD (dB) | −3.0 (−3.6, −2.4) | −2.0 (−2.7, −1.3) |
|
| ||
| Baseline 24-2 VF PSD (dB) | 4.1 (3.6, 4.5) | 3.3 (2.7, 4.0) |
|
| ||
| Baseline wiGCC thickness (μm) | 92.2 (90.5, 93.9) | 94.5 (91.9, 97.0) |
|
| ||
| Baseline pfGCC thickness (μm) | 97.2 (95.3, 99.0) | 99.8 (97.0, 102.5) |
|
| ||
| Baseline wiVD (%) | 44.9 (44.3, 45.5) | 45.4 (44.5, 46.4) |
|
| ||
| Baseline pfVD (%) | 47.8 (47.1, 48.4) | 48.3 (47.3, 49.3) |
|
| ||
| OCT/OCTA SSI | 67.7 (66.8, 68.7) | 68.7 (67.1, 70.2) |
|
| ||
| Follow-up duration (years) | 3.0 (2.8, 3.2) | 1.2 (1.1, 1.2) |
|
| ||
| Number of follow-up visits | 5.1 (4.9, 5.3) | 4.3 (3.8, 4.8) |
Values are shown in mean (95% confidence interval), unless otherwise indicated.
Abbreviations: CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; pfGCC =parafoveal ganglion cell complex; pfVD = parafoveal vessel density; POAG = primary open angle glaucoma; PSD = pattern standard deviation; SSI = signal strength index; VF = visual field; wiGCC = whole-image ganglion cell complex; wiVD = whole-image vessel density
The long-term variability of 24–2 VF and macular OCT/OCTA parameters in the stable eye cohort (max follow-up=1.5 years) are summarized in Table 2. The macular OCT parameters showed the highest ICC (95% CI) (wiGCC=0.99[0.99, 0.99]) followed by VF parameters (VF MD=0.91[0.88, 0.93]), and OCTA parameters (wiVD=0.82[0.75, 0.87]). The RMSE were <1dB for VF parameters, between 1.0–2.0% for macular OCTA parameters, and <1μm for macular OCT parameters. Similar values were observed for Sw, which were <1dB for VF parameters, between 1.0–2.0% for macular OCTA parameters, and <1μm for OCT parameters. As for the long-term measurement variability, VF MD and pattern standard deviation (PSD) showed a TRV of 2.24 dB and 1.45 dB, respectively. The TRV of macular VD ranged between 4.1–4.5%, and that of macular GCC ranged between 2.1–2.2μm. The CV were shown in Supplemental Table 1. For both macular OCT and OCTA, no significant difference in variability was observed between whole-image and parafoveal measurements.
Table 2.
ICC, RMSE, Sw, and TRV of VF and OCT/OCTA parameters in the stable eye cohort (max follow-up = 1.5 years)
| 24-2 VF | ICC (95% CI) | RMSE (dB) | Sw (dB) | TRV (dB) |
| MD | 0.91 (0.88, 0.93) | 0.92 | 0.81 | 2.24 |
| PSD | 0.93 (0.90, 0.95) | 0.75 | 0.53 | 1.45 |
| OCTA | ICC (95% CI) | RMSE (%) | Sw (%) | TRV (%) |
| wiVD | 0.82 (0.75, 0.87) | 1.64 | 1.48 | 4.10 |
| pfVD | 0.80 (0.73, 0.85) | 1.80 | 1.61 | 4.46 |
| OCT | ICC (95% CI) | RMSE (μm) | Sw (μm) | TRV (μm) |
| wiGCC | 0.99 (0.99, 0.99) | 0.91 | 0.78 | 2.16 |
| pfGCC | 0.99 (0.99, 1.00) | 0.91 | 0.75 | 2.08 |
Abbreviations: ICC = intra-class correlation; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; pfGCC =parafoveal ganglion cell complex; pfVD = parafoveal vessel density; PSD = pattern standard deviation; RMSE = root mean squared error; Sw, within-subject test–retest standard deviation; TRV = test-retest variability; VF = visual field; wiGCC = whole-image ganglion cell complex; wiVD = whole-image vessel density
For the variability of VF and macular OCT/OCTA parameters in the extended cohort including all eyes (unlimited follow-up), the rates of change over the follow-up period and RMSE are presented in Table 3. Significant rates of change over time were observed for all measurement (P<0.001). As compared to the stable eye cohort, a slightly higher RMSE was observed for all parameters in the extended cohort, although the difference was small. The RMSE was 1.07 dB for VF MD, 2.57% for wiVD, and 2.03μm for wiGCC, respectively.
Table 3.
Rates of change and RMSE of VF and OCT/OCTA parameters in the extended cohort including all eyes (unlimited follow-up)
| 24-2 VF | Rate of change (95% CI) (dB/year) | P-value | RMSE (dB) |
| MD | −0.22 (−0.27, −0.16) | <0.001 | 1.07 |
| PSD | 0.10 (0.06, 0.14) | <0.001 | 0.79 |
| OCTA | Rate of change (95% CI) (%/year) | P-value | RMSE (%) |
| wiVD | −1.17 (−1.26, −1.08) | <0.001 | 2.57 |
| pfVD | −1.07 (−1.16, −0.97) | <0.001 | 2.58 |
| OCT | Rate of change (95% CI) (μm /year) | P-value | RMSE (μm) |
| wiGCC | −0.81 (−0.89, −0.72) | <0.001 | 2.03 |
| pfGCC | −0.84 (−0.92, −0.76) | <0.001 | 2.08 |
Statistically significant p value is shown in bold representing a rate of change different from zero.
Abbreviations: MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; pfGCC =parafoveal ganglion cell complex; pfVD = parafoveal vessel density; PSD = pattern standard deviation; RMSE = rootmean squared error; VF = visual field; wiGCC = whole-image ganglion cell complex; wiVD = whole-image vessel density
Figure 1 demonstrates the distribution of Sw of all parameters in the stable eye cohort (lower panel) and the extended cohort (upper panel). In both cohorts, the distribution of Sw was generally narrowed and centered, indicating a satisfactory variability. When comparing the two cohorts, for almost all parameters, a wider range of total distribution was observed in the extended cohort. In addition, a higher percentage of eyes in the extended cohort was observed to have Sw distributed at the ends of the histograms for all parameters. This suggests that, as compared to the stable eye cohort, the extended cohort including all eyes still possessed a slightly greater within-subject test–retest measurement variability.
Figure 1.
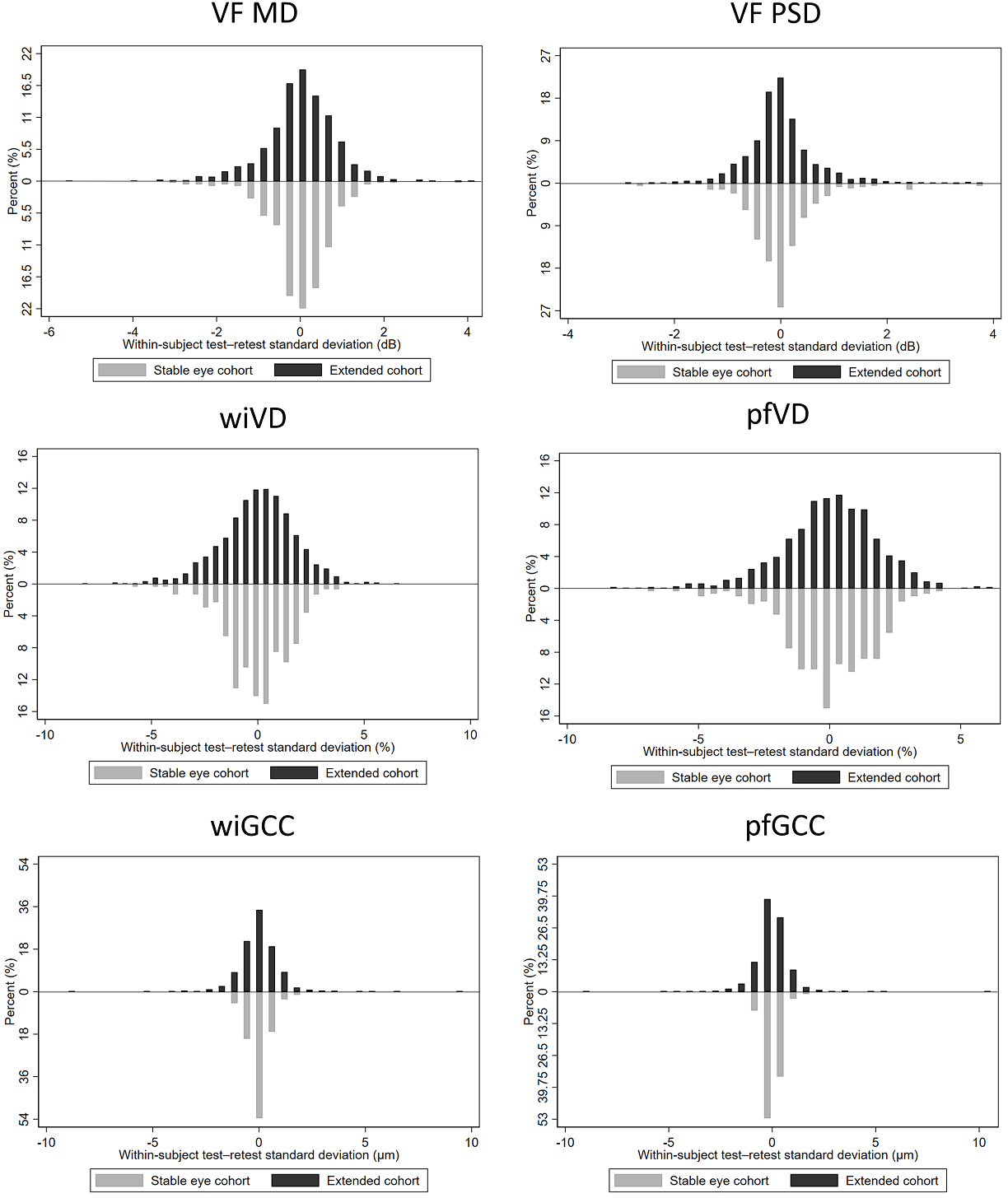
Distribution of the within-subject test–retest standard deviation of VF and OCT/OCTA parameters in the stable eye cohort (lower panel) and the extended cohort (upper panel) (MD = mean deviation; PSD = pattern standard deviation; VF = visual field; pfGCC = parafoveal ganglion cell complex; pfVD = parafoveal vessel density; wiGCC = whole-image ganglion cell complex; wiVD = whole-image vessel density)
The main effects of age, intraocular pressure (IOP), and CCT on the variability of VF and macular OCT/OCTA parameters are summarized in Supplemental Tables. In the stable eye cohort (Supplemental Table 2), older age was associated with increased variability of macular GCC thickness (β:0.25–0.28, R2=0.61–0.64) and VD (β:0.13, R2=0.22–0.25), while thicker CCT was associated with decrease in variability of both macular GCC (β:−0.10~−0.09, R2=0.66–0.69) and VD (β:−0.03, R2=0.12–0.14) in the repeat scans (P<0.05). In the extended cohort including all eyes (Supplemental Table 3), older age was associated with a significant increase in macular VD (β:0.12–0.13, R2=0.14) variability, while higher IOP was linked to a decreased variability in VF measurements (β=−0.33 for VF MD, R2=0.35; 0.29 for VF PSD, R2=0.44) and macular VD (β:−0.17~−0.14, R2=0.02–0.03) in repeated visits, although effects on the latter was weak (P<0.05).
DISCUSSION
This study examined the long-term variability of 24–2 VF and macular OCT/OCTA parameters. In stable eyes with a maximum follow-up of 1.5 years, all parameters demonstrated small variability; macular GCC showed the best results. In the extended cohort including all eyes, the RMSE of all parameters were similar to that in the stable eye cohort, and showed a comparable result.
When comparing the reproducibility of different devices, ICC is the most relevant indicator, since its calculation is unaffected by data unit and independent from sample mean, unlike RMSE, Sw and CV. In our study, both macular OCT and OCTA showed satisfactory and small long-term variability, with macular GCC demonstrating the lowest variability. Overall, the current results are consistent with our previous estimates of long-term variability of macular OCT and OCTA in 49 stable cases that showed both instruments were reliable for long-term clinical evaluation, with OCT showing lower variability.[11] A possible explanation for the better OCT results was the known greater influence of image quality on OCTA measurements,[18] which is supported by studies directly showing the effect of signal strength index on the short-term and long-term variability of VD.[10 11] To examine this possibility, we also performed a sensitivity analysis on selected OCTA images with the best quality (data not shown), and a slight improvement was found for macular VD in all eyes (RMSE:1.6–1.7%). Another explanation is the potentially differential effects of aging on macular VD and GCC.[19] Although information about age-related VD loss is sparse, if OCTA possesses a steeper slope of change, it may also cause greater variability. In addition, it may be that the technique for detecting motion (of flowing blood vessels) is inherently more variable than that for determining thickness.
A particularly informative finding of this study is the long-term TRV of each measurement in stable eye cohort. To clinicians, the TRV in stable eyes can be seen as the cut-off when determining disease progression, and any measurement change exceeding it should be considered clinically significant. Based on our results, the long-term TRV of global macular VD and macular GCC ranged between 4.1–4.5% and 2.1–2.2μm, respectively. This means that changes observed between visits that are greater than the aforementioned values are indicative of true glaucoma progression, which may warrant further evaluation. The long-term TRV of sectoral OCT/OCTA parameters were additionally provided in Supplemental Table 4. The TRV of sectoral macular VD and GCC ranged between 4.2–5.6% and 2.0–2.8μm, respectively, with the temporal sector showing the smallest variability. Overall, there has not been a consensus on the cut-offs for determining glaucoma progression, and future studies are needed to confirm our results. However, for macular GCC, a longitudinal thinning rate worse than −1.3μm/year was previously endorsed as strongly suggestive of uncontrolled glaucoma progression.[20–23] As for macular VD, a short-term TRV ranged around 4–5% was reported in a prior study,[10] which was similar to our long-term findings using AngioVue.
Regarding 24–2 VF, only a small variability was found in the stable eye cohort. Consistent with our findings, Tattersall et al. found a long-term VF MD fluctuation (99% CI) of <1dB in stable eyes.[24] Since VF was evaluated when determining glaucoma stability, an overestimation of VF performance in stable eyes was expected. Nevertheless, the results in the extended cohort including all eyes also suggested a small variability, which somewhat contradicts our past impression. Nevertheless, it should be noted that global VF metrics were tested in this study, instead of individual visual field test point metrics. In clinical practice, greater variability is mostly found for point-level VF measurements.[18 25] While in global metrics, these fluctuations are averaged out, thus resulting in a better reproducibility. Of note, to examine the possible discrepancy in the variability of global and point metrics, the average ICC of point-level VF total deviation (TD) was further analyzed (data not shown). The mean ICC (0.81) of the point-level VF TD was lower than that of the global VF MD, with a wide distribution (0.27–0.96) across different VF points, agreeing with the hypothesis of a greater long-term variability of point-level metrics.
Similar to our findings, prior studies also suggested a small long-term variability of global 24–2 VF parameters. A long-term, large-scaled study including both progressive and non-progressive glaucoma eyes reported a median RMSE of 0.97 dB for VF MD, while that for pointwise analyses was 2.57 dB.[26] Another study examining glaucoma eyes over 4 years also reported a small Sw (1.05 dB) for VF MD.[5] One explanation for such result is that since DIGS subjects underwent VF examination regularly, they may be better test takers as compared to other patients. Considering the subject performance directly affects VF outcome, the long-term variability of global VF parameters in the clinic is likely greater than the current results.
One major strength of this study is the comparison of the long-term variability of each measurement in stable eyes with limited follow-up and all eyes with longer follow-up (extended cohort). Results of the latter is of clinical interest, as it simulated clinical settings where stable and non-stable cases are all present. In general, our results suggest a small long-term variability of VF and OCT/OCTA measurements in this scenario, indicating these parameters may also be helpful when evaluating long-term glaucoma progression in real-world practice. Still, with the inclusion of non-stable cases in the extended cohort, variability greater than that of the stable eye cohort was anticipated, which is supported by the wider distribution of Sw and the slightly higher RMSE. Nonetheless, the observed differences were small and clinically insignificant. Among the three devices, OCT consistently demonstrated the smallest variability across the two cohorts, suggesting that it performed more reliably than OCTA when used to detect structural loss. However, it should be noted that macular GCC reached the measurement floor (around VF MD of −14 dB) earlier than macular VD (around VF MD of −26 dB),[27] making it less suitable for evaluating advanced glaucoma. Thus, in addition to the measurement variability, the dynamic range should also be considered when applying OCT and OCTA in clinical practice.
In the supplemental analysis, a few clinical factors were found with potential effects on the variability of these parameters, including age, CCT, and IOP. The aging effect on retinal structures has been well recognized, thus the significant result was not surprising.[20 28 29] The positive effect of IOP in the extended cohort including all eyes may also be explained by the greater sample size and the potentially more intense management administered on patients with a higher IOP. The most inexplicable finding was perhaps the effect of CCT found in the stable eye cohort but not the extended cohort, which was not previously reported and difficult to explain. The association of variability with CCT in stable eyes requires further confirmation.
This study has several limitations. First, as aforementioned, a stable VF was one of the criteria for determining glaucoma stability, and DIGS subjects are likely better VF test takers. Therefore, selection bias and underestimated variability might be present for VF parameters. Additionally, poor-quality OCT/OCTA images were excluded prior to the analysis. In clinical settings, a worse real-world variability of macular OCTA may be expected.[30] Second, some other factors potentially affecting measurement variability were not examined, including the severity/pattern of VF defects and participant race for VF variability,[31 32] and the differing scan sizes for OCT/OCTA variability.[33 34] Third, while the design of the extended cohort can better simulate the clinical scenario,[5 26] the proportion of progressing eyes may affect the results. Last, as participants generally had early-moderate glaucoma, caution should be taken when generalizing our findings to cases at later stages of glaucoma.
In conclusion, 24–2 global VF and macular OCT/OCTA parameters showed small long-term variability in all eyes, including stable ones, supporting the use of these instruments in the long-term follow-up for glaucoma. Demonstrating the smallest variability, is it likely that OCT is more reliable for evaluating glaucomatous change. Further, true glaucomatous progression should be suspected when the change between visits in macular VD and GCC is greater than 4–5% and 2μm, respectively. .
Supplementary Material
SYNOPSIS.
In both stable and all eyes, 24–2 VF and macular OCT/OCTA showed small long-term variability suitable for glaucoma follow-up. Progression is suggested when macular VD change exceeds 4–5% and/or GCC thickness change exceeds 2μm.
KEY MESSAGE.
What is already known on this topic – Evaluation of glaucomatous progression relies on the long-term variability of clinical examinations. However, studies on the long-term variability of measurements pertinent to glaucoma were limited.
What this study adds – 24–2 global VF parameters, OCT-measured macular GCC thickness, and OCTA-measured macular VD showed small long-term variability in both stable eyes with 1–1.5 years of follow-up and all eyes with extended follow-up, with macular GCC showing the best results.
How this study might affect research, practice or policy – With a small long-term variability, 24–2 VF and macular OCT/OCTA, particularly OCT parameters, are reliable for glaucoma follow-up and progression detection.
Grant information/Funding/Support:
This work is supported by National Institutes of Health/National Eye Institute Grants (R01EY029058, R01EY011008, R01EY019869, R01EY027510, R01EY026574, R01EY018926 P30EY022589); University of California Tobacco Related Disease Research Program (T31IP1511), and an unrestricted grant from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Commercial Disclosures: Linda Zangwill reported grants from the National Eye Institute; grants from Heidelberg Engineering and nonfinancial support from Carl Zeiss Meditec, Optovue, Heidelberg Engineering, and Topcon. consultant of Abbvie and patents from Carl Zeiss Meditec. Robert N. Weinreb is a consultant of Abbvie, Aerie Pharmaceuticals, Allergan, Amydis, Equinox, Eyenovia, Iantrek, IOPtic, Implandata, Nicox, and Topcon. Robert N. Weinreb reported nonfinancial support from Heidelberg Engineering, Carl Zeiss Meditec, Konan Medical, Optovue, Centervue, and Topcon; grants from the National Eye Institute;, patents from Toromedes, Carl Zeiss Meditec; all outside the submitted work. No other disclosures were reported.
Ethic Statement
The study adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act, and was approved by the University of California San Diego (UCSD) Human Research Protection Program (NCT00221897). Written informed consent was obtained from all participants.
Data availability:
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCE
- 1.Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. Jama 2021;325(2):164–74 doi: 10.1001/jama.2020.21899[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Shoji T, Zangwill LM, Akagi T, et al. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. American Journal of Ophthalmology 2017;182:107–17 doi: 10.1016/j.ajo.2017.07.011[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Moraes CG, Paula JS, Blumberg DM, et al. Detection of Progression With 10–2 Standard Automated Perimetry: Development and Validation of an Event-Based Algorithm. American Journal of Ophthalmology 2020;216:37–43 doi: 10.1016/j.ajo.2020.03.046[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Artes PH, O’Leary N, Nicolela MT, et al. Visual Field Progression in Glaucoma: What Is the Specificity of the Guided Progression Analysis? Ophthalmology 2014;121(10):2023–27 doi: 10.1016/j.ophtha.2014.04.015[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Urata CN, Mariottoni EB, Jammal AA, et al. Comparison of Short- And Long-Term Variability in Standard Perimetry and Spectral Domain Optical Coherence Tomography in Glaucoma. American Journal of Ophthalmology 2020;210:19–25 doi: 10.1016/j.ajo.2019.10.034[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miraftabi A, Amini N, Gornbein J, et al. Local Variability of Macular Thickness Measurements With SD-OCT and Influencing Factors. Translational Vision Science & Technology 2016;5(4):5–5 doi: 10.1167/tvst.5.4.5[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KE, Yoo BW, Jeoung JW, et al. Long-Term Reproducibility of Macular Ganglion Cell Analysis in Clinically Stable Glaucoma Patients. Investigative Ophthalmology & Visual Science 2015;56(8):4857–64 doi: 10.1167/iovs.14-16350[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma 2017;26(10):851–59 doi: 10.1097/ijg.0000000000000768[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim CW, Cheng J, Tay ELT, et al. Optical coherence tomography angiography of the macula and optic nerve head: microvascular density and test-retest repeatability in normal subjects. BMC Ophthalmol 2018;18(1):315 doi: 10.1186/s12886-018-0976-y[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. British Journal of Ophthalmology 2018;102(3):352–57 [DOI] [PubMed] [Google Scholar]
- 11.Nishida T, Moghimi S, Hou H, et al. Long-term reproducibility of optical coherence tomography angiography in healthy and stable glaucomatous eyes. British Journal of Ophthalmology 2021:bjophthalmol-2021–320034 doi: 10.1136/bjophthalmol-2021-320034[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45 doi: 10.1001/archophthalmol.2009.187[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–50 doi: 10.1001/archophthalmol.2010.49[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710–25 doi: 10.1364/OE.20.004710[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KE, Yoo BW, Jeoung JW, et al. Long-Term Reproducibility of Macular Ganglion Cell Analysis in Clinically Stable Glaucoma Patients. Invest Ophthalmol Vis Sci 2015;56(8):4857–64 doi: 10.1167/iovs.14-16350[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.De Vet HC, Terwee CB, Mokkink LB, et al. Measurement in medicine: a practical guide: Cambridge university press, 2011. [Google Scholar]
- 17.Srebotnjak T, Mokdad AH, Murray CJL. A novel framework for validating and applying standardized small area measurement strategies. Population Health Metrics 2010;8(1):26 doi: 10.1186/1478-7954-8-26[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Sheikh M, Ghasemi Falavarjani K, Akil H, et al. Impact of image quality on OCT angiography based quantitative measurements. International Journal of Retina and Vitreous 2017;3(1):13 doi: 10.1186/s40942-017-0068-9[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Jiang H, Shi Y, et al. Age-Related Alterations in the Retinal Microvasculature, Microcirculation, and Microstructure. Invest Ophthalmol Vis Sci 2017;58(9):3804–17 doi: 10.1167/iovs.17-21460[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatham AJ, Medeiros FA. Detecting Structural Progression in Glaucoma with Optical Coherence Tomography. Ophthalmology 2017;124(12, Supplement):S57–S65 doi: 10.1016/j.ophtha.2017.07.015[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holló G, Zhou Q. Evaluation of Retinal Nerve Fiber Layer Thickness and Ganglion Cell Complex Progression Rates in Healthy, Ocular Hypertensive, and Glaucoma Eyes With the Avanti RTVue-XR Optical Coherence Tomograph Based on 5-Year Follow-up. J Glaucoma 2016;25(10):e905–e09 doi: 10.1097/ijg.0000000000000410[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Francis BA, Dastiridou A, et al. Longitudinal and Cross-Sectional Analyses of Age Effects on Retinal Nerve Fiber Layer and Ganglion Cell Complex Thickness by Fourier-Domain OCT. Transl Vis Sci Technol 2016;5(2):1 doi: 10.1167/tvst.5.2.1[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iverson SM, Feuer WJ, Shi W, et al. Frequency of abnormal retinal nerve fibre layer and ganglion cell layer SDOCT scans in healthy eyes and glaucoma suspects in a prospective longitudinal study. British Journal of Ophthalmology 2014;98(7):920–25 doi: 10.1136/bjophthalmol-2013-303877[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Tattersall CL, Vernon SA, Menon GJ. Mean deviation fluctuation in eyes with stable Humphrey 24–2 visual fields. Eye 2007;21(3):362–66 doi: 10.1038/sj.eye.6702206[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 25.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol 1989;108(2):130–5 doi: 10.1016/0002-9394(89)90006-8[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Rabiolo A, Morales E, Kim JH, et al. Predictors of Long-Term Visual Field Fluctuation in Glaucoma Patients. Ophthalmology 2020;127(6):739–47 doi: 10.1016/j.ophtha.2019.11.021[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126(7):980–88 doi: 10.1016/j.ophtha.2019.03.003[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song WK, Lee SC, Lee ES, et al. Macular Thickness Variations with Sex, Age, and Axial Length in Healthy Subjects: A Spectral Domain–Optical Coherence Tomography Study. Investigative Ophthalmology & Visual Science 2010;51(8):3913–18 doi: 10.1167/iovs.09-4189[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 29.Pujari A, Chawla R, Markan A, et al. Age-related changes in macular vessels and their perfusion densities on optical coherence tomography angiography. Indian Journal of Ophthalmology 2020;68(3):494–99 doi: 10.4103/ijo.IJO_521_19[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmen IC, Konda SM, Pak JW, et al. Prevalence and Severity of Artifacts in Optical Coherence Tomographic Angiograms. JAMA Ophthalmol 2020;138(2):119–26 doi: 10.1001/jamaophthalmol.2019.4971[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardiner SK. Differences in the Relation Between Perimetric Sensitivity and Variability Between Locations Across the Visual Field. Invest Ophthalmol Vis Sci 2018;59(8):3667–74 doi: 10.1167/iovs.18-24303[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gracitelli CPB, Zangwill LM, Diniz-Filho A, et al. Detection of Glaucoma Progression in Individuals of African Descent Compared With Those of European Descent. JAMA Ophthalmol 2018;136(4):329–35 doi: 10.1001/jamaophthalmol.2017.6836[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penteado RC, Bowd C, Proudfoot JA, et al. Diagnostic Ability of Optical Coherence Tomography Angiography Macula Vessel Density for the Diagnosis of Glaucoma Using Difference Scan Sizes. J Glaucoma 2020;29(4):245–51 doi: 10.1097/ijg.0000000000001447[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.You QS, Tan O, Pi S, et al. Effect of algorithms and covariates in glaucoma diagnosis with optical coherence tomography angiography. British Journal of Ophthalmology 2021:bjophthalmol-2020–318677 doi: 10.1136/bjophthalmol-2020-318677[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


