Abstract
Background
Periodontal inflammation are inflammation of supporting tissues of periodontium. The microbial factor can cause infection which is polymicrobial in origin and causes dysbiosis and shift in oxidative stress with compromised antioxidant capacity. This study focused at determination of the effect of nonsurgical periodontal therapy (NSPT) and vitamin C supplementation on total antioxidant capacity (TAOC) in chronic periodontitis patients (ChP).
Material & method
A total of 70 ChPand 35 periodontally healthy subjects (control) were recruited in this study. Further, ChP group was subdivided into ChP1 group (n = 35) which received NSPT only and ChP 2 group (n = 35) which received NSPT with vitamin C 500 mg once daily for 3 months. Serum and saliva samples were taken at baseline and at 3 months postNSPT for measurement of TAOC. Clinical parameters measured were measured at 1-, 3-, 6- and 12-month interval.
Results
Lower levels of serum and salivary TAOC levels were observed in ChP patients than healthy subjects (p < 0.05). Improvement in Clinical parameters was observed in both the groups ChP1 and ChP 2 group post therapy (p < 0.05). The periodontal treatment showed insignificant changes in serum and salivary TAOC levels (p > 0.05). The supplemental dose of vitamin C didn't have any additional benefits (p > 0.05).
Conclusion
There lies an association of oxidative stress with periodontitis, low serum and salivary TAOC levels were seen in chronic periodontitis patients. NSPT improved the periodontal inflammatory status. However, benefits of vitamin C as an adjunct to NSPT remains inconclusive and needs to be further explored by multicentre longitudinal studies.
Keywords: Antioxidant, Oxidative stress, Periodontal therapy, Periodontitis, Saliva, Total antioxidant capacity, Vitamin C
Graphical abstract
Contribution details
Nisha Swet: Concepts, Design, Literature search, Clinical studies, Experimental studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review, Guarantor, Shivamallu Bettahalli: Concepts, Design, Definition of intellectual content, Literature search, Data acquisition, Manuscript preparation, Manuscript review, Prashant Akila: Design, Literature search, Data analysis, Manuscript editing, Manuscript review, Shashikumar Pratibha: Concepts, Design, Definition of intellectual content, Manuscript editing, Manuscript review.
1. Introduction
Periodontal disease is multifactorial in origin, it is caused as a result of interaction between host defence cells and environmental factors like periodontal pathogens.1 Risk factors like diabetes mellitus, cardiovascular disease, smoking attributes to the disease process.2 A meta-analysis showed more than 51% of Indian population has periodontitis and there is a lack in continuoussurveillance of oral diseases.3
The systemic association, complex disease progression, host immune response, possibly makes periodontitis as a disease for early diagnosis and intervention.4 However, in developing countries wherepeople lack awareness, preference given to systemic health than oral health and financial constraints makesit difficult to halt the disease process.3 Education of individuals regarding association of systemic health and oral health diseases like periodontitis, gives patients an idea and renders importance of periodontal health.5
Subgingival biofilm plays an important role is disease progression.6 The alteration in host response to microorganisms leads to imbalance in oxidative stress. The imbalance in antioxidant and oxidant level, results in oxidative damage i.e., release of free radicals which causes tissue injury.7
Role of antioxidant in periodontal disease cannot be overlooked. Studies on antioxidant micronutrient and periodontitis have conflicting results, and a possible association cannot be drawn yet.8 Though, deficiency of a micronutrient affects the overall immunity but its individual impact on oral health is difficult to analyse as antioxidants work in concert rather than as individuals.8
Measuring individual species will be costly, time consuming, also sum of the individual antioxidant concentrations, will not yield the total antioxidant capacity (TAOC)as uncharacterized antioxidant, also exits in nature and their biological significance may be unknown.9 Assessment of TAOC gives us an overall antioxidant capacity of an individual in various biological fluids.9
The antioxidant system consists of preventive and scavenging antioxidants based on mode of action. Scavenging antioxidants consists of vitamin C, vitamin E, Vitamin A, bilirubin, reduced glutathione and several thiols.10 Among all these, the most studied yet with inconclusive results are with Vitamin C. There lies positive association between periodontal health and vitamin C, though clinical improvements were seen in many studies but statistically significant difference of vitamin C supplementation in periodontal health remains doubtful.11 This study evaluated the effect of nonsurgical periodontal therapy (NSPT)and Vitamin C supplementation on TAOC in patients with chronic generalised Periodontitis.
2. Materials & methods
2.1. Study participants
This was a parallel arm single-blind prospective randomised controlled clinical trial, 70 Chronic periodontitis (ChP)patients and 35 periodontally healthy subjects, age group between 35 and 55 yearswith no systemic disease were recruited from department of Periodontology, over a period of 2 years (January 2015 to February 2017). Written informed consent were obtained from all the participants. The study was approved by the Institutional Ethics committee and registered at Clinical Trial Registry-India (CTRI) trial no. (CTRI/2017/10/010,185).
Subjects diagnosed as ChP with probing depths (PD) >5 mm in 2 or more non-adjacent sites per quadrant, bleeding on probing >10% and radiographic bone loss >30% of the root lengthwere included in the study. According to recent classification patients with Stage II Grade B periodontitis were recruited in this study.12
Patients on antimicrobial drugs in past 3 months, pregnant or lactating, use of any vitamin supplements or mouthwashes, anti-inflammatory drugs in past 3 months, subjects with special dietary requirements because of underlying systemic disease were excluded from the study.
Healthy control group presented with probing depths <3 mm, no loss of attachment, bleeding on probing< 10% and no evidence of radiographic alveolar bone loss.
2.2. Sample size determination
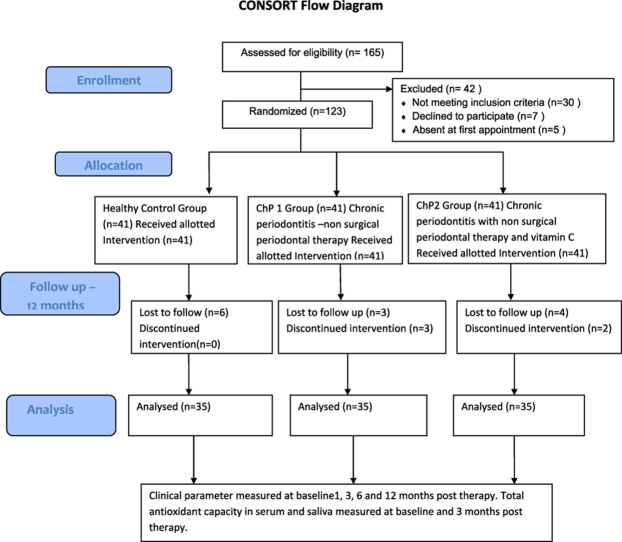
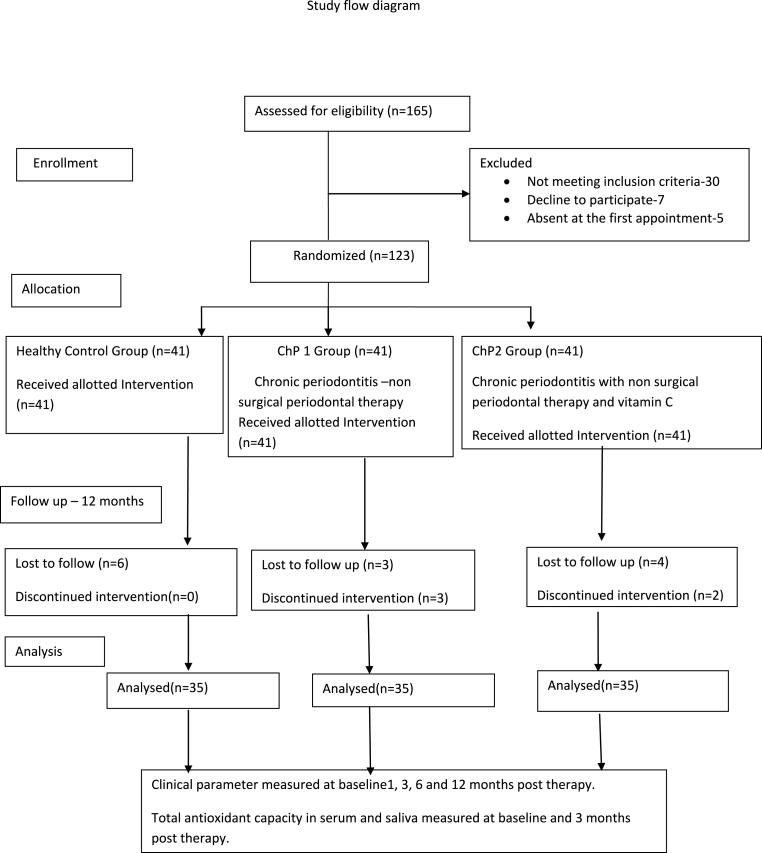
The sample size was calculated based on the primary outcome of the study. Two tailed unpaired t-test with effect size of 0.5, power of the study was 0.8 and the allocation ratio 1:1. The calculated sample size was 102 (34 subjects in each group). Attrition rate of 20% was expected, so the final sample size was 123 (41 subjects in each group). After the drop outs in each group 35 subjects were assessed.
2.3. Clinical parameter measurement
Subjects were instructed about collection of baseline saliva and blood samples after an overnight fasting. Then the clinical measurement involving assessment of Plaque index (PI),13 gingival index (GI),14 clinical attachment level (CAL), probing depth (PD), sulcular bleeding index (SBI)15 was done using Mouth mirror and Florida probe (Florida Probe Corp., USA).
All teeth present including third molars were probed at six sites per tooth. All the clinical measurements were taken by a single examiner (SN). The intra-examiner reliability ranged between 0.825 (95% CI: 0.71–0.88).
2.4. Randomisation
Randomisation was done based on computer-based method, Prism 4.0 software package (GraphPad, La Jolla, CA, USA) and the ChP group was randomly divided into two groups.
In each group 35 subjects were allotted.
2.5. ChP 1 group underwent nonsurgical periodontal treatment only
ChP2 group received non-surgical therapy with vitamin C 500 mg (Celin 500 mg, Glaxo SmithKline Pharmaceuticals Ltd.) once daily for 3 months by examiner (ABS) who was not involved in any clinical or laboratory procedures.
No placebo arm was introduced in this study. The patients were informed about medications, its direction of use and in case of any adverse effects to call investigator (PS) or report to the hospital immediately.
2.6. Blinding
Only the clinician recording the clinical parameters was blinded and. The vitamin C tablets were distributed by (PS) who was further not involved in any clinical measurement or treatment. Biochemical tests results and statistical interpretation of the results were also blinded on the treatment performed.
2.7. Treatment plan
The NSPT involved ultrasonic scaling and root planing using Gracey curets (Hu-friedy) under local analgesia. No post-treatment complications were noted. ChP patients recruited in both the groups were recalled after 3 months to recollect saliva and venous blood sample (serum sample). Clinical measurement was taken at baseline, 1,3,6 and 12 months interval. Serum and saliva samples were taken at baseline and at 3 months interval post nonsurgical periodontal treatment.
Questionnaire and personal interview were conducted for demographic data collection.
2.8. Dietary instructions
Daily nutrition of the subjects was assessed by a dietary record. All the subjects in both the ChP groups were instructed to maintain a daily diary for 3 months and bring the diary at all the following appointments. No change in diet plans were instructed in both the groups and subjects were encouraged to have their normal diet. Diet was assessed by a self-administered dietary record. All subjects were instructed to keep a record of their dietary consumption 3 days prior to the hospital visit and they were enquired about their dietary habits as well as the use of food supplements at subsequent visits.
2.9. Sample collection method
2.9.1. Serum sample collection
Venous blood samples from antecubital vein were collected in tubes. Each sample was allowed to stand for at least 30 min and then centrifuged at 3000 g for 10 min. Further analysis, samples were stored at −80 °C.
2.9.2. Saliva sample collection
Unstimulated whole saliva was collected by spitting method in a sterile saliva collection vial. The sample was collected after overnight 8 h fasting and the subjects were instructed to avoid intake of any food or liquid 1 h before the saliva sample collection. The sample was centrifuged at 3000 rotations per minute (rpm) for 15 min and the supernatant was stored at −20 °C until further analysis.
2.10. Total antioxidant capacity measurement
Antioxidant capacity measurement in serum and saliva was performed using the ferric reducing ability of plasma (FRAP) assay adapted for a microplate reader for control group at baseline only and for groups ChP 1 and ChP 2 at baseline, 3 months and 6 months).16 To avoid blood contamination of saliva, saliva collection was done before the clinical examination as bleeding on probing might lead to saliva contamination. However still there was a possibility of blood contamination as sometime patients with periodontitis might have blood contaminated saliva itself from highly inflamed sites. The advantage of FRAP assay is it is very sensitive to contamination with haemoglobin and any saliva sample with blood contamination can be detected by this assay.
2.11. Statistical analysis
Statistical program was carried out by means of SPSS Statistical Software Package (SPSS for Windows Version 16.0, SPSS, Inc., Chicago, IL). The Kolmogorov–Smirnov test was used to test the normality of the data and the data followed normal distribution. For serum and saliva total antioxidant capacity measurement the data was non-normal distributed and so non-parametric test was done. The difference between age and sex in between the study groups were assessed by Chi-square test. Intragroup differences in clinical parameters were analysed using repeated measure ANOVA and one way ANOVA was used for intergroup differences. Intergroup differences in serum and salivary TAOC levels were assessed using Mann-Whitney U test. Wilcoxon test was used to measure serum and saliva TAOC intragroup differences. Spearman rank correlation test was used to measure the levels of serum TAOC and clinical parameters relationship. Statistical significance was considered at p < 0.05.
3. Results
A total of 70 chronic periodontitis patients were enrolled in this study which was further subdivided as 35 in each group (Fig. 1). The demographic details of subjects are presented in Table 1.
Fig. 1.
Participants study flowchart.
Table 1.
Demographic characteristics of the groups.
| Variable | Control group | ChP1 | ChP2 | p* value |
|---|---|---|---|---|
| Age in years (mean ± S.D) | 46 ± 0.72 | 44 ± 0.84 | 45 ± 0.88 | >0.05 |
| Gender | 35 | 35 | 35 | >0.05 |
| Male | 17 | 16 | 18 | |
| Female | 18 | 19 | 17 |
ChP- chronic periodontitis, S.D - standard deviation, p-probability,*Chi -square test, Statistically significant at p < 0.05.
The mean and standard deviation of all the clinical parameters at baseline – 12 months post-treatment for ChP1 and ChP2 groups are presented in Table 2.
Table 2.
Table Serum total antioxidant levels of the study groups.
| Serum TAOC | Control group | ChP1 | ChP2 | p* value |
|---|---|---|---|---|
| baseline | 845.05 ± 86.66 | 587.78 ± 79.34 | 570.08 ± 88.33 | <0.05 |
| 3 months | – | 615.22 ± 67.59 | 661.91 ± 65.39 | >0.05 |
TAOC-Total antioxidant capacity, ChP- Chronic periodontitis, p-probability,* Mann-Whitney U test, Statistically significant at p < 0.05.
When we consider the clinical measurements PD, CAL, GI, and BOP scores significant reduction was seen post therapy at 3 months when compared to baseline in both the ChP1 and ChP2 groups (p < 0.001). Furthermore, at the 6 and 12 months recall both the groups showed significant improvement in all clinical measures (p < 0.001). However, Intergroup comparison in relation to clinical parameters between ChP1 and ChP2 group (administration of Vitamin C as an adjunct to NSPT) did not offer additional effect (p > 0.05) (Table 2).
The mean serum and saliva TAOC baseline levels from patients with ChP1 and ChP2 group was less as compared to healthy controls (p < 0.05).
TAOC level in serum was increased post therapy at 3 months interval in both the groups when compared to baseline (p < 0.05) but intergroup comparison showed insignificant differences (p > 0.05). Saliva TAOC levels post therapy at 3 months increased significantly in both the groups when compared to their baseline (p < 0.05). Intergroup comparison between both the groups showed insignificant changes (p > 0.05) (Table 3, Table 4).
Table 3.
Salivary total antioxidant levels of the study groups.
| Salivary TAOC | Control group | ChP1 | ChP2 | p* value |
|---|---|---|---|---|
| baseline | 743.14 ± 45.04 | 556.05 ± 28.20 | 551.14 ± 31.94 | <0.05 |
| 3 months | – | 654.68 ± 36.86 | 650.40 ± 47.61 | >0.05 |
TAOC-Total antioxidant capacity, ChP- Chronic periodontitis, p-probability,* Mann-Whitney U test, Statistically significant at p < 0.05.
Table 4.
-Mean Clinical Indices in ChP Groups at different time interval post therapy.
| Clinical parameter | Group | Baseline mean ± S.D | 1 month | p* value | 3 months | p* value | 6 months | p* value | 12 months | p* value |
|---|---|---|---|---|---|---|---|---|---|---|
| PD (mm) | ChP1 | 3.42 ± 0.56 | 2.87 ± 0.52 | 0.353 | 2.53 ± 0.38 | 0.417 | 2.25 ± 0.33 | 0.32 | 2.18 ± 0.42 | 0.313 |
| ChP2 | 3.56 ± 0.62 | 2.85 ± 0.45 | 2.67 ± 0.48 | 2.21 ± 0.41 | 2.19 ± 0.44 | |||||
| CAL (mm) | ChP1 | 5.18 ± 0.32 | 4.21 ± 0.28 | 0.331 | 3.56 ± 0.53 | 0.473 | 3.32 ± 0.58 | 0.59 | 3.29 ± 0.34 | 0.552 |
| ChP2 | 5.16 ± 0.41 | 4.24 ± 0.27 | 3.82 ± 0.56 | 3.28 ± 0.62 | 3.18 ± 0.23 | |||||
| BOP (%) | ChP1 | 57 ± 0.53 | 29.12 ± 17.21 | 0.432 | 27.15 ± 18.11 | 26.13 ± 17.31 | 0.22 | 27.14 ± 15.66 | 0.232 | |
| ChP2 | 59 ± 0.55 | 34.14 ± 16.71 | 32.17 ± 16.51 | 31.12 ± 15.51 | 28.14 ± 16.63 | |||||
| PI | ChP1 | 2.43 ± 0.11 | 0.66 ± 0.13 | 0.363 | 0.62 ± 0.14 | 0.334 | 0.58 ± 0.11 | 0.38 | 0.54 ± 0.13 | 0.343 |
| ChP2 | 2.46 ± 0.13 | 0.67 ± 0.12 | 0.61 ± 0.16 | 0.56 ± 0.09 | 0.55 ± 0.12 | |||||
| GI | ChP1 | 2.33 ± 0.13 | 1.58 ± 0.11 | 0.151 | 1.23 ± 0.09 | 0.181 | 1.15 ± 0.11 | 0.114 | 1.14 ± 0.12 | 0.125 |
| ChP2 | 2.38 ± 0.16 | 1.55 ± 0.14 | 1.16 ± 0.16 | 1.13 ± 0.17 | 1.12 ± 0.18 |
S.D - standard deviation, PD = probing depth; CAL = clinical attachment level; BOP = bleeding on probing; PI = plaque index; GI = gingival index, * Anova, Statistically significant at p < 0.05.
No significant difference was seen in TAOC levels between ChP1, ChP2 and control groups at the 3 months post-treatment interval (p > 0.05).
4. Discussion
Oxidative stress has significant contribution in the pathogenesis of periodontitis.17 The reactive oxygen species (ROS)causes host tissue damage and imbalance in oxidant-antioxidant levels. This highlights the possible antioxidant intervention for restoration of periodontal health.18 Vitamin C is a potent antioxidant and association of low levels of vitamin C and risk for periodontitis is observedwith odds ratio [OR] = 1.19; 95% CI: 1.05 to 1.33).19 However, role of vitamin C as antioxidant in periodontitis remains inconclusive.20
We hypothesized that an inverse association exist between serum and salivary TAOC levels and chronic periodontitis and intake of Vitamin C will restore the TAOC levels and periodontal health. The rationale behind such hypothesis is the mechanism of periodontal destruction which causes oxidative stress. Studies have confirmed that in periodontitis hyperactivity of polymorphonuclear neutrophils (PMNs) releasesROS which sets host-immune reaction.21,22 This causes changes in local and systemic antioxidant activity. Brock et al. suggested positive association between serum TAOC levels and periodontitis, suggesting possible role of supplements to increase the TAOC levels and reduce periodontal inflammation.23 Randa et al. showed decrease level of salivary TAOC levels (40%)was associated with periodontitis.24 Novakovic et al. suggested that non-enzymatic antioxidants like catalase, superoxidase dismutase, glutathione peroxidaseare associated with periodontitis and salivary TAOC levels (0.40 ± 0.23)decreases in periodontitis which improved to 0.65 ± 0.35 after NSPT.25 This gave us the rationale behind use of antioxidant supplements as an adjunct to conventional periodontal therapy.
There is an increased trend in measuring total oxidant status or oxidative stress of different molecules and using it as biomarkers in detecting periodontitis. However, our study focused on antioxidant (Vitamin C) supplement and evaluating its possible role in reducing periodontal inflammation and increasing the TAOC levels.
Sulaiman et al. evaluated plasma TAOC levels in ChP patients with 2000 mg daily supplementation of Vitamin C for four weeks and found that lower levels of plasma TAOC was significantly associated with ChP i.e. 559 – 53.2 compared to 625–88.7 mmTeq in healthy controls and NSPT reduced the oxidative stress.26 The result is in accordance with our study, showing lower levels of TAOC in serum and saliva and after periodontal treatment increase levels of TAOC was obtained. Also, vitamin C supplement 500 mg daily at 3 months interval didn't show any additional benefits in group ChP2. Vogel et al. administered mega doses of vitamin C 1500 mg/day supplementationfor 90 days and the subjects underwent experimental gingivitis for a period of 4 weeks. No statistical significance was obtained on terms of clinical parameters –plaque index, gingival index, bleeding index between the experimental and placebo group.27 Our results contradict, study by Staudte et al. where grape fruit consumption for 2 weeks showed significant reduction in sulcular bleeding index (1.68 ± 0.6 to 1.05 ± 0.6, p < 0.001), however no changes were seen in plaque index and probing depth.28 This might be due to short term changes induced by vitamin C supplementation as the study duration was very short. Leggott et al. showed decrease percentage of gingival bleeding sites with vitamin C supplementation. This might be due the difference in study design as this study had short term vitamin C depleted and repleted conditions and small sample size.11
Nutrition based studies should be interpreted cautiously, as the dosage of supplements varies in different studies. It is difficult to perform detailed dose-response studies. Also, in trial-based studies sometimes it's difficult to replicate similar results which we get in vitro or in animal-based studies. This might hold true for antioxidant capacity of Vitamin C. It might have a weak antioxidant in vivo with small or no physiological role, it might be acting on specific locations. In the present study, vitamin C was consumed 500 mg once daily, this dosage was based on reabsorption saturation levels and threshold dose for excretion i.e., at doses 500 mg and above, the entire absorbed dose is excreted. The plasma completely saturates at doses of 400 mg and higher, producing a steady plasma concentration.29
We found a significant improvement in intragroup comparison of PI, GI, SBI, PD, CAL at 3 months post therapy in both ChP1 and ChP 2 group. Raghuvendra et al. found similar findings with vitamin c supplementation in Indian population failed to show additional benefits in clinical parameters like PI,PD, CAL reduction(P < 0.001) except for gingival bleeding index.30 However, the present study result didn't show any significant changes in gingival index after administration of Vitamin C in ChP2 group at any follow up intervals. This might be due to the change in dose of vitamin C administration and also its duration which might have a significant impact on clinical indices like gingival index. In Raghuvendra et al. study 500 mg of Vitamin C tablets were given thrice daily for two months whereas as in the present study Vitamin C −500 mg was given once daily for 3 months.30 This dose was decided based on peak plasma concentration of the Vitamin C.
Another study by Shimabukuro et al. found reduced gingival inflammation (P = 0.01)after regular application of a dentifrice containing Vitamin C with antioxidant properties.31
In the present study both serum and salivary TAOC levels were reduced in chronic periodontitis subjects when compared to healthy controls. This result is similar to study by Brock et al. which showed systemic and local antioxidant capacity reduced in periodontitis.23 No effect of NSPT on serum and salivary TAOC levels were seen post 3 months therapy and intergroup comparison did not show any statistically significant results. This result was similar to Sulaiman et al. study on plasma TAOC levels where they failed to show any significant changes in plasma TAOC levels post 1 month therapy.26 This might be due to short duration of TAOC evaluation done in the study.
Javed et al. evaluated the impact of diet on antioxidant capacity and periodontal indices in periodontitis and found significantly increased plasma TAOC but no significant changes were shown on periodontal indices post 3 and 6 months of dietary intervention.32 They had a follow up of 2 months duration which again is a short period for evaluation of effects of an antioxidant on TAOC levels. However, they found that vitamin C intake was approximately 50 mg day higher in the dietary intervention group compared to the control group (P = 0.008). A systematic review byMuniz et al.concluded that administration of antioxidants has no definite pattern on oxidative stress.33 Factor like antioxidants quantity, administration duration, study design can affect the result. Wei et al. showed significant decrease in total oxidant status (TOS) in serum, saliva and gingival crevicular fluid in ChP patients compared to baseline post periodontal therapy (p < 0.05).34
Measurement of total antioxidant capacity in periodontal disease assessment can be performed in biological fluids like serum, plasma, saliva and gingival crevicular fluid (GCF). Deciding which fluid to be taken into consideration for TAOC levels measurement depends on various factors like ease of collection, patient consent, clinical skills and availability of measuring devices and reagents and method of TAOC measurement. There are no conclusive results on any particular biological fluid rendering accurate TAOC levels as compared to other.9 In the present study serum and salivary TAOC levels were measured as a systemic and local marker representative respectively. Saliva collection can be by stimulated or unstimulated method and is comparatively quicker, easier and less armamentarium required as compared to GCF collection.25Toczewska et al.compared GCF and both stimulated and non-stimulated saliva with TOS in periodontitis patients. They stated that no correlations exits between the two biomarkers. In stimulated saliva, mean TOS values were the highest as parotid gland secreting stimulated saliva is the most important source of free radicals among all salivary glands.35 Zhang et al. suggested that patient selection is a critical parameter influencing salivary levels of TAOC, limiting its use TAOC measurement.36 However, which biological fluid -GCF, saliva, serum or plasma is a better reflector of periodontal antioxidant status remain inconclusive.
In the present study TAOC levels were taken as they represent the overall antioxidant levels of an individual, as it is cumulative of all antioxidant levels, instead of calculating individual antioxidants which would be expensive and time consuming.9 We used FRAP method to calculate the TAOC levels as it is sensitive to blood and any contamination of samples can be discarded.37
5. Limitations
Vitamin C levels were not assessed at any point of the study. We considered decrease vitamin C levels are associated with periodontitis based on previous studies.38 However measuring serum or salivary Vitamin C levels would have given better clarity to its role as antioxidant in periodontal inflammation. Another, drawback is the short study duration, this was due to compliance related issues by the patients, their dietary habits regulation. Also, this was a single centre study in south India, multicentre studies are suggested to evaluate role of vitamin C in ChP.
6. Conclusion
This study showed that NSPT reduced periodontal inflammation and improved clinical parameters, however no significant change was seen in TAOC levels of serum or saliva in ChP 2 group with Vitamin C supplementation. Further, the study concludes that vitamin C supplementation had no significant additional benefits in terms of either clinical parameters or TAOC levels. Nevertheless, levels of Vitamin C in other fluids like GCF may throw light in its potential antioxidant activity.
References
- 1.Bartold P.M., Van Dyke T.E. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol. 2000;62:203–217. doi: 10.1111/j.1600-0757.2012.00450.x. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G., Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janakiram C., Mehta A., Venkitachalam R. Prevalence of periodontal disease among adults in India: a systematic review and meta-analysis. J Oral BiolCraniofac Res. 2020;10(4):800–806. doi: 10.1016/j.jobcr.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., Li M., Zhu F., et al. The effects of oral health education regarding periodontal health on non-dental undergraduates in southwestern China—exploring the feasibility of an e-learning course for oral health promotion. BMC Oral Health. 2021;21(119):1–10. doi: 10.1186/s12903-021-01476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston W., Rosier B.T., Artacho A., et al. Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci Rep. 2021;11:9796. doi: 10.1038/s41598-021-89002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battino M., Bullon P., Wilson M., Newman H. Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–476. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- 8.Chapple I.L.C., Matthews J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini N., Vitaglione P., Granato D., Fogliano V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: merits and limitations. J Sci Food Agric. 2020;100(14):5064–5078. doi: 10.1002/jsfa.9550. [DOI] [PubMed] [Google Scholar]
- 10.Parcheta M., Świsłocka R., Orzechowska S., Akimowicz M., Choińska R., Lewandowski W. Recent developments in effective antioxidants: the structure and antioxidant properties. Materials. 2021 Apr 15;14(8):1984. doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leggott P.J., Robertson P.B., Jacob R.A., Zambon J.J., Walsh M., Armitage G.C. Effects of ascorbic acid depletion and supplementation on periodontal health and subgingival microflora in humans. J Dent Res. 1991;70:1531–1536. doi: 10.1177/00220345910700121101. [DOI] [PubMed] [Google Scholar]
- 12.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018 Jun;89(1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 13.Loe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 14.Silness J., Loe H. Periodontal Disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 15.Muhlemann H.R., Son S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–113. [PubMed] [Google Scholar]
- 16.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 17.Sczepanik F.S.C., Grossi M.L., Casati M., et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. 2020;84(1):45–68. doi: 10.1111/prd.12342. 2000. [DOI] [PubMed] [Google Scholar]
- 18.Tóthová L., Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. 2017 Dec 14;8:1055. doi: 10.3389/fphys.2017.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida M., Grossi S.G., Dunford R.G., Ho A.W., Trevisan M., Genco R.J. Dietary vitamin C and the risk for periodontal disease. J Periodontol. 2000;71:1215–1223. doi: 10.1902/jop.2000.71.8.1215. [DOI] [PubMed] [Google Scholar]
- 20.Fageeh H.N., Fageeh H.I., Prabhu A., Bhandi S., Khan S., Patil S. Efficacy of vitamin C supplementation as an adjunct in the non-surgical management of periodontitis: a systematic review. Syst Rev. 2021 Jan 4;10(1):5. doi: 10.1186/s13643-020-01554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicu E.A., Rijkschroeff P., Wartewig E., Nazmi K., Loos B.G. Characterization of oral polymorphonuclear neutrophils in periodontitis patients: a case-control study. BMC Oral Health. 2018;18:149. doi: 10.1186/s12903-018-0615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussbaum G., Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 23.Brock G.R., Butterworth C.J., Matthews J.B., Chapple I.L. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 24.Diab-Ladki R., Pellat B., Chahine R. Decrease in the total antioxidant activity of saliva in patients with periodontal diseases. Clin Oral Invest. 2003;7:103–107. doi: 10.1007/s00784-003-0208-5. [DOI] [PubMed] [Google Scholar]
- 25.Novakovic N., Todorovic T., Rakic M., et al. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment out come. J Periodontal Res. 2014;49:129–136. doi: 10.1111/jre.12088. [DOI] [PubMed] [Google Scholar]
- 26.Abou Sulaiman A.E., Shehadeh R.M. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010;81:1547–1554. doi: 10.1902/jop.2010.100173. [DOI] [PubMed] [Google Scholar]
- 27.Vogel R.I., Lamster I.B., Wechsler S.A., Macedo B., Hartley L.J., Macedo J.A. The effects of megadosesof ascorbic acid on PMN chemotaxis and experimental gingivitis. J Periodontol. 1986;57:472–479. doi: 10.1902/jop.1986.57.8.472. [DOI] [PubMed] [Google Scholar]
- 28.Staudte H., Sigusch B.W., Glockmann E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J. 2005;199:2113–2217. doi: 10.1038/sj.bdj.4812613. [DOI] [PubMed] [Google Scholar]
- 29.Padayatty S.J., Katz A., Wang Y., et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 30.Raghavendra U., Rao A., Kashyap S.R., D'Souza J., Kumar V., Kalal B.S. Vitamin C supplementation as an adjunct to nonsurgical therapy in the treatment of chronic periodontitis: a clinical and biochemical study. J Int Oral Health. 2018;10:256–261. [Google Scholar]
- 31.Shimabukuro Y., Nakayama Y., Ogata Y., et al. Effects of an ascorbic acid–derivative dentifrice in patients with gingivitis: a double-masked, randomized, controlled clinical trial. J Periodontol. 2015;86:27–35. doi: 10.1902/jop.2014.140138. [DOI] [PubMed] [Google Scholar]
- 32.Zare Javid A., Seal C.J., Heasman P., Moynihan P.J. Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J Hum Nutr Diet. 2013;27:523–532. doi: 10.1111/jhn.12184. [DOI] [PubMed] [Google Scholar]
- 33.Muniz F.W., Nogueira S.B., Mendes F.L., et al. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: a systematic review. Arch Oral Biol. 2015;60:1203–1214. doi: 10.1016/j.archoralbio.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.WeiD, Zhang X.L., Wang Y.Z., Yang C.X., Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–78. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 35.Toczewska J., Maciejczyk M., Konopka T., Zalewska A. Total oxidant and antioxidant capacity of gingival crevicular fluid and saliva in patients with periodontitis: review and clinical study. Antioxidants. 2020;9(5):450. doi: 10.3390/antiox9050450. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T., Andrukhov O., Haririan H., et al. Total antioxidant capacity and total oxidant status in saliva of periodontitis patients in relation to bacterial load. Front Cell Infect Microbiol. 2016;6(5):97. doi: 10.3389/fcimb.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. 2021 25;22(7):3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tada A., Miura H. The relationship between vitamin C and periodontal diseases: a systematic review. Int J Environ Res Publ Health. 2019;16:1–15. doi: 10.3390/ijerph16142472. [DOI] [PMC free article] [PubMed] [Google Scholar]