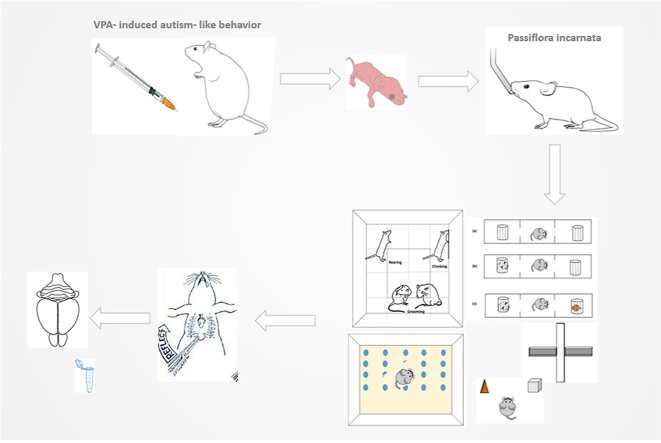
Abstract
Experimental autism in rodents can be caused by prenatal valproic acid (VPA) exposure. Some diseases, such as attention-deficit hyperactivity disorder (ADHD), insomnia, opiate withdrawal, and generalized anxiety disorder can be treated by consuming Passiflora incarnata, due to the possession of bioactive compounds like alkaloids, phenols, and flavonoids.
The present study aims to investigate the role of the hydroalcoholic extract of Passiflora incarnata in behavioral and oxidative stress aberrations induced by VPA.
On the gestational day (GD), 12.5, pregnant Wistar rats received VPA (600 mg/kg subcutaneously). Male pups were treated with the extract (30,100, and 300 mg/kg) from postnatal day 35 to the end of the experiment, and underwent behavioral testing to evaluate locomotion, repetitive, and stereotyped movements, anxiety, and social and cognitive behaviors. After behavioral testing, the blood sample was taken from the left ventricle to determine serum catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (TAC). Then the animals were euthanized and their brains were taken out for histological assays of the prefrontal cortex (PFC) and CA1 hippocampus with hematoxylin/eosin. The total phenol and flavonoid content and antioxidant activity of the extract were also measured. A significant improvement was observed in behavioral disturbances, particularly with 300 mg/kg of Passiflora. Moreover, the formation of oxidative stress markers significantly decreased at this dose. The extract also reduced the percentage of damaged cells in the CA1 and PFC. The results indicated that Passiflora extract could ameliorate VPA-induced behavioral aberrations possibly due to the antioxidant actions of its bioactive compounds.
Keywords: Passiflora, Extract, Autism, Oxidative stress, Behavior, Valproic acid
Graphical abstract
Abbreviations
- ADHD
Attention-Deficit Hyperactivity Disorder
- ASD
Autistic Spectrum Disorder
- CAT
Catalase
- EPM
Elevated Plus Maze
- E.D.
Embryonic Day
- G.D.
Gestational Day
- H&E
Hematoxylin and Eosin
- MDA
Malondialdehyde
- MBR
Marble Burying Test
- MAO
Monoamine Oxidase
- NORT
Novel Object Recognition Test
- OFT
Open Field Test
- P.F.
Passion Flower
- PND
Postnatal Day
- PFC
Prefrontal Cortex
- SI
Sociability Index
- SPI
Social Preference Index
- SAL
Saline
- S.C
Subcutaneous injection
- SOD
Superoxide Dismutase
- TCT
Three Chamber Test
- TAC
Total Antioxidant Capacity
- VPA
Valproic Acid
- XOD
Xanthine Oxidase
1. Introduction
Children diagnosed with autistic spectrum disorder (ASD) frequently exhibit impaired communication skills and social interactions. They display repetitive and stereotypical patterns of behavior, and additionally, their executive function is impaired. Given that the number of these children is rising and is now estimated to be as high as 1 in 59 children,1 the challenges regarding the best and most effective treatments continue. Both genetic and environmental factors seem to contribute to the risk of developing ASD although the etiology of the disease is unknown.2 Exposure to some environmental pollutants (oxidizing agents, heavy metals, herbicides, and pesticides) and drugs such as valproic acid (VPA) and thalidomide in early embryonic days are recognized as causes of autism. Accordingly, the gestational administration of VPA, a teratogenic drug, exhibits a similar behavioral phenotype as ASD patients, and it is known as a high-validity autism animal model in this field.3
Also, literature associates ASD neurobiology with redox imbalance and oxidative stress, and it is hypothesized that environmental factors exert their detrimental effects by increasing oxidative activity.4 Studies have shown that medicinal plants possessing antioxidant and neuroprotective effects can have protective and therapeutic effects on neurodevelopmental diseases.5
The current study was done to investigate the Passiflora incarnata behavioral and histological effects on the VPA-induced autism model. The passionflower is a medicinal plant that is claimed to have sedative, hypnotic, anticonvulsant, and anxiolytic effects.6 It has been traditionally used in Europe and America (USA) to treat patients with anxiety and sleep disorders.7,8 A growing body of evidence has established that Passiflora incarnata showed a prominent and potent antioxidant activity. This plant contains high flavonoids, alkaloids, phenolic compounds, and cyanogenic glycosides, and due to its action as a monoamine oxidase (MAO) inhibitor, it can have antidepressant properties.9, 10, 11, 12 Also, the cognition-enhancing activity of the plant extract in Parkinson's and Alzheimer's disease and the possible association of these conditions with the antioxidant potential of the extract have been reported.13
Considering that plant-based drug alternatives have minimal side effects, the study researchers aimed to evaluate the effect of Passiflora incarnate on autistic-like behaviors and the possible underlying redox-based and neuroprotective mechanisms in the VPA-autistic rat model.
2. Materials and methods
2.1. Animal groups and source of plant material
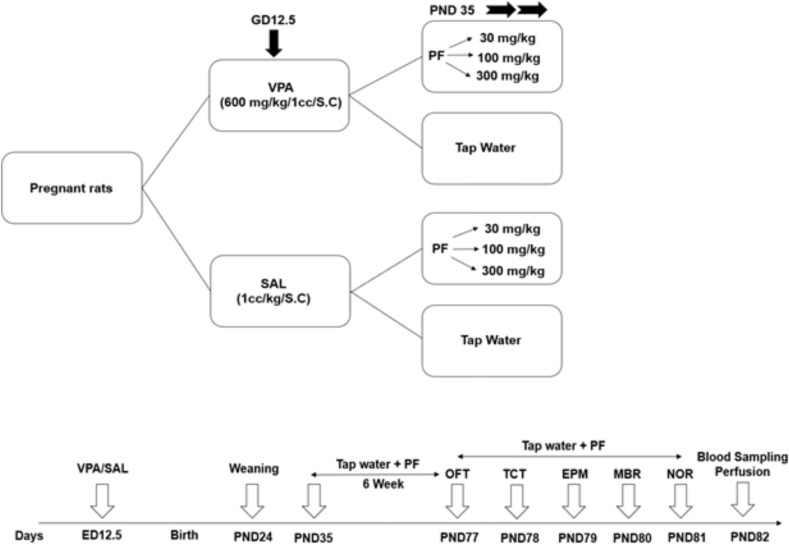
Male and female Wistar rats (200–250 g) were prepared from the Animal Care Center (Shahrekord, Iran) and housed under standard laboratory conditions (22 ± 2 °C, 25 ± 5% humidity, 12 h light/dark cycle, accessible food and water). The rats with a controlled fertility cycle were mated overnight, and the next day, the vaginal smear was performed (in case of the presence of sperm, was considered as the gestational day (GD 0.5)). Pregnant animals were randomly classified into two groups on GD 12.5 and subjected to VPA (dissolved in saline, 600 mg/kg, subcutaneously) or saline (1 mL/kg). Following pregnancy, on postnatal day 21, the pups were weaned (PND 21), and male pups were divided into eight subgroups (n = 8), including group I, II, III, IV (saline groups), and Group V, VI, VII, VIII (VPA groups). I and V subgroups were treated with tap water, and the others received Passionflower extract 30, 100, and 300 mg/kg in adolescence (PND 35–81).1,14,15
A hydroalcoholic extract of Passiflora incarnate L. (Passiflora edulis Sims) was prepared from Iran Darouk Co (Batch No: I.EE.013). The extract was dissolved in drinking water. The dose according to the mean intake of water by a rat was about 20 ± 2 mL/day. For more reassurance, the doses of the extract were calculated daily. Also, the total phenol and flavonoid content and antioxidant activity of the extract were measured according to the following methods.
2.1.1. Total phenolic content determination
Folin-Ciocalteu reagent assay was used to determine the total phenolic content of Passiflora hydroalcoholic extract according to a previously described method with slight modification.16 Briefly, 100 μL hydroalcoholic extract of Passiflora or a standard solution of gallic acid was mixed with 500 μL of diluted Folin-Ciocalteu reagent (1:10 v/v), and the resulting mixture was slightly shaken for a minute. Afterward, 400 μL of an aqueous solution of Na2CO3 (7.5% w/v) was added, and the obtained mixture was incubated for 30 min in the darkness at room temperature. Finally, the absorbance, relative to that of a blank prepared using distilled water, was measured at 765 nm using a multi-mode microplate reader (BioTek, USA).
The concentration of total phenolic compounds was determined as mg of gallic acid/g of the dry weight of the extract by using a regression equation obtained from the calibration curve of the gallic acid.
2.1.2. Total flavonoids determination
The colorimetric method was used to measure the amounts of total flavonoids in the extract.17 To this end, different rutin and quercetin standards (250-31 μg/mL) were prepared in methanol 60%. One mL of the Passiflora hydroalcoholic extract or methanolic solution of rutin or quercetin standard was mixed with 1 mL distilled water in a test tube. Then 100 μL sodium nitrate 5% was added to each test tube and shacked for 6 min; afterward, 200 μL aluminum chloride 10% was added to each sample. After 5 min incubation at room temperature, 1 mL aqueous solution of NaOH (1 N) was added, and the absorbance, relative to that of a blank prepared using methanol 60%, was determined at 510 nm using a multi-mode microplate reader (BioTek, USA).
2.1.3. Antioxidant activity assay
The free radical-scavenging activity of the extract was assessed by using the DPPH method.18 The different concentrations of extracts (50 μl) or vitamin C as a standard antioxidant agent were added to 0.004% (w/v) methanol solution of DPPH (150 μl). Then, the absorbance of color changes of samples and blank (extract + methanol) was measured at 517 nm using a multi-mode microplate reader (BioTek, USA), and % inhibition was calculated for each evaluated concentration of extract.
| % Inhibition = [A0−A] / A0 × 100, where (A0) is the absorbance of the control and (A) sample |
All experiments were carried out in triplicate (Table 1).
Table 1.
Total phenolic and flavonoid content of Passiflora hydroalcoholic extract.
| Parameters | mg gallic acid/g Dried extract (Mean ± SEM) | mg quercetin/g Dried extract (Mean ± SEM) | mg rutin/g Dried extract (Mean ± SEM) | mg hyperoside/g Dried extract (Mean ± SEM) |
|---|---|---|---|---|
| Passiflora extract | 69.12 ± 1.49 | 66.46 ± 1.81 | 112.54 ± 3.63 | 33.72 ± 1.23 |
2.1.4. Behavioral assessments
Behavioral tests were done on PND 77–81 with 100–110 g body weight. The treatment groups and schedules are briefly mentioned in Fig. 1.
Fig. 1.
Treatment groups (a) and schedules (b). Valproic acid (VPA), Saline (SAL), Subcutaneous injection (S.C), Passion Flower (P.F.), Gestational Day (G.D.), Postnatal Day (PND), Embryonic Day (E.D.), Open Field Test (OFT), Three Chamber Test (TCT), Elevated Plus Maze (EPM), Marble Burying Test (MBR), Novel object recognition test (NORT).
2.1.5. Open field test
An open-field test was used to assess motor activity and repetitive behaviors. This task was performed in a plexiglass box (30 × 100 × 100), the floor of which was divided into 16 squares by hypothetical lines. First, the rat was placed in the center to move freely in the box for 5 min. A camera was used to record the motor activity including the total traveled distance and speed of movement. Then, the number of grooming (rubbing the head with paws and rubbing the body with paws or mouth) and vertical activities (rearing and climbing) were noted. The stereotypical and monotonous behaviors in the animal model of autism were confirmed by the high number of these movements.1,19
2.1.6. Social interaction test (three-chamber test)
The social interaction test has been developed to evaluate rodent social behaviors quantitatively and was designed based on the rodent instinct to discover new objects or animals. This behavior is disturbed in autistic animal models.20 To this end, a rectangular plexiglass box was placed under standard room-lighting conditions. The box was divided into three chambers, including two side chambers and a middle chamber. The rat could explore the box freely in all the stages, including habituation, sociability, and social preference. The habituation stage was performed by placing the animal in the middle chamber to move around the chambers to become familiar with the environment. Two cylindrical wire cages were placed in the two side chambers to perform the sociability stage. The researcher randomly sited a same-sex and same-age animal with no previous contact with the subject in one wire cage (the novel rat 1). Then the exploring time in each chamber was noted by two observers for 10 min. In the social preference stage, a new rat (novel rat 2, similar to the characteristics of novel rat 1) was placed in the empty wire cage in another side chamber. Time spent in each chamber was recorded for 10 min. Note that, in the second stage, novel rat 1 was named “the known cage”. Finally, the following formulae were used to calculate the sociability index (SI) and social novelty preference index (SPI)20:
The SI and SPI scores range from −1 to +1, where values closer to +1 indicate that the animal is more social and prefers the novel situation. Negative values display no sociability and social preference.
2.1.7. Elevated plus maze test
Anxiety is common in autistic populations, and anxiety-related tests were well-characterized for rodents. The elevated plus-maze (EPM) is probably the most popular of them, and designed based on rodents’ spontaneous activity and studied the relationship between exploration and fear.21,22 EPM apparatus is a plus-shaped plexiglass with two open arms (30 × 5 cm), two closed arms (30 × 5, with 15 cm high walls), and a central square area (5 × 5 cm) elevated above the floor (39 cm high). The rat was placed in the center square and allowed to explore the apparatus for 5 min freely. Ethovision tracking software (version 7, Noldus Information Technology) was used to measure the time spent in each arm and the number of entries into them.23
2.1.8. Marble burying test
To evaluate repetitive behaviors in rodents, the marble burying test (MBR) was used. The subject was placed in a propylene cage (26.5 × 42 × 15 cm) containing a thick sawdust bed (5 cm thick) and 20 marbles (1 cm in diameter) in the four rows on the bed surface. Burying behavior was recorded after 30 min by a camera. The number of marbles that covered more than half of their surface area was counted and analyzed.24,25
2.1.8.1. Novel object recognition test
To evaluate the learning, memory, and limited interests in autism animal models, the novel object recognition test (NORT) was used. A wooden box consisting of two identical objects (A and B) with different shapes, colors, and textures was used to perform the NORT. The test procedure had three steps inclding habituation, training, and retention.
The habituation step was performed for 5 min in the box a day before training, without any objects. The same objects (A1 and A2) were, then, placed on the opposite side of the box, and the animal was allowed to explore the box. The exploring behaviors were recorded for 5 min. After 45 min, the animal was placed in the box with A and B objects for 3 min in the retention step.26
The preference index was defined in the training step as dividing the time spent exploring object A1 (the object replaced by the novel object B in the retention step) by the total time investigating both objects (objects A1 and A2). A recognition index was also calculated from the retention step as a ratio of the time spent interacting with the novel object (object B) over the total time spent examining both the objects (object A and B).27
2.2. Biochemical analysis
2.2.1. Malondialdehyde (MDA) measurement
MDA activity was almost performed based on Yagi's method. Briefly, 125 μL of serum was mixed with 1.5 mL of phosphoric acid in a tube and shacked. Then, 0.5 mL of thiobarbituric acid was added, and the tube was sited in boiling water for 45 min. When the mixture temperature was reduced, 1 mL of n-butanol was added and centrifuged for 10 min at 7000 rpm. Finally, the researcher separated the pink phase and assessed it at 532 nm. By the standard curve of tetra ethoxy propane, the amount of MDA in the sample was calculated and analyzed.28
2.2.2. Superoxide dismutase (SOD) activity assessment
The Randox kit protocol (U.K.; Cat NO.RS504) was used to carry out this procedure. SOD acted as a catalyst in converting the superoxide radical (O2 -) into elemental oxygen (O2) and hydrogen peroxide (H2O2). Xanthine oxidase (XOD) produced superoxide ions (O2 -) in the Randox assay kit or they were produced by altering xanthine to hydrogen peroxide and uric acid, leading to the conversion of NBT to NBT-diformazan. Light at 560 nm was absorbed by NBT-diformazan. The superoxide ions concentrations decreased due to the slowing down of NBT-diformazan formation by SOD. Therefore, the reduction rate of superoxide ions that occurred in the presence of NBT-diformazan in an experimental sample was used to measure the SOD activity.
2.2.3. Catalase activity assessment
Catalase (CAT) activity was measured by Sinha modified method. At first, 30 mM H2O2, phosphate buffer (50 mM; pH 7.4), and the dichromate/acetic acid solution (5% aqueous solution of potassium dichromate in distilled water + 150 mL of Glacial (98–100%) acetic acid) were mixed and then a boiling water bath was used to heat the mixture for 10 min. To evaluate sample absorbance, a spectrophotometer at 570 nm was used.29
2.2.4. Serum level measurement of total antioxidant capacity (TAC)
This procedure was displayed by Benzie and Strain in 1996 to assess the ferric-reducing ability of plasma. Generally, at low pH, ferric tripyridyltriazine (Fe III–TPTZ) complex can be reduced by plasma to an intense blue-colored ferrous (Fe II) form. The antioxidant capacity of the sample indicates the intensity of blue color. At first, 5 μL of plasma was mixed with 70 μL of FRAP reagent, and distilled water was utilized as a blank. Then the mixture was incubated at 37 °C for 5 min, and the sample absorbance was measured at 593 nm. The FRAP values were stated as micromolar (μM).30
2.2.5. Histological studies
Four rats in each group were selected for histological study. In this way, the animals were anesthetized and the brains extracted. Then the brain tissues were fixed in 10% formalin. After fixation, tissues were processed in tissue processing, embedded in paraffin, and sectioned on a rotary microtome at the thickness of 5 μm using the sagittal plane. The routine hematoxylin and eosin (H&E) method was used to stain tissue sections.31 Intact (light cytoplasm and normal-appearing nucleus) and degenerated (eosinophilic cytoplasm and shrunken, pyknotic, or karyorrhectic nuclei) neurons in CA1 and prefrontal cortex (PFC) were recognized and counted using a light field microscope (Olympus, CX31, Tokyo, Japan) and camera connected to the microscope in 40 x images. Cell count was performed on three slides per rat and three rats from each group and reported the percentage of damaged cells by field.
3. Ethics statement
The study tests and animal care protocols were accepted by the Animal Experiment Committee at Kerman University of Medical Sciences (Ethics code: IR.KMU.AH.REC.1399.134) and performed in line with the NIH Guide for the Care and Use of Laboratory Animals.
4. Statistical analysis
The data were tested for normality using the Shapiro-Wilk test and analyzed by GraphPad Prism 9 (San Diego, USA) software using a two-way ANOVA test followed by a Tukey test. Independent variables (fixed factors) were group (saline vs. VPA) and treatment (water or Passiflora extract). P < 0.05 was considered statistically significant, and data were expressed as the mean ± standard error of the mean (SEM).
5. Results
The total phenolic and flavonoid content of Passiflora extract is shown in Table 1. According to the findings, rutin is the highest flavonoid composition of Passiflora extract among determined flavonoids. Moreover, the hydroalcoholic extract of Passiflora showed scavenging activity with an IC50 value of 293.5 ± 24.2 μg/mL, which is significantly lesser than vitamin C (IC50 value of 52.5 ± 4.3 μg/mL) due to the main flavonoid contents of hydroalcoholic extract.
5.1. Effects of Passiflora extract on spontaneous motor activity
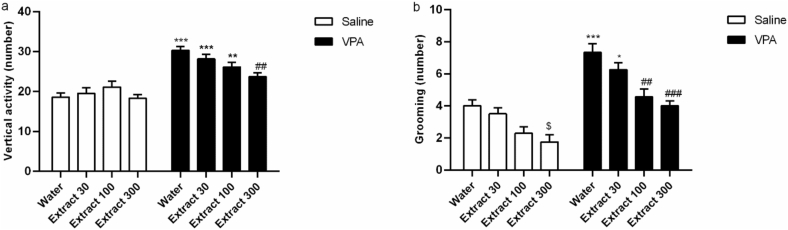
Two-way ANOVA for the number of vertical activity and grooming revealed the main effect of prenatal VPA administration [vertical activity (Fig. 2a; F (1,50) = 82.31, p < 0.001); grooming (Fig. 2b; F (1,50) = 75.07, p < 0.001)], and Passiflora extract treatment [vertical activity (Fig. 2a; F (3,50) 3.18, p < 0.05); grooming (Fig. 2b; F (3,50) = 17.20, p < 0.001)]. A significant interaction effect was observed between the treatment and group in rearing [F (3,50) = 3.35, p > 0.05]; however, no significant interaction was observed between these factors in grooming.
Fig. 2.
The effects of Passiflora extract on the stereotypic behaviors in the VPA-exposed rats. Vertical activities (a, n = 8), Self-grooming test (b, n = 8). ∗, ∗∗, ∗∗∗, p < 0.05, 0.01, 0.001 vs. prenatal saline/postnatal water group (group I), ##, ###, 0.01, 0.001 vs. prenatal VPA/postnatal water group (group V). The data are expressed as mean ± SEM.
Tuckey Post hoc analysis indicated that prenatal administration of VPA (along with postnatal tap water: group V) increased the number of vertical activity (p < 0.001) and grooming (p < 0.001) compared to the prenatal saline group (group I).
The increase in these motor activities induced by VPA was dose-dependently reversed by Passiflora. VPA rats treated with a 300 mg/kg dose of Passiflora represented a significant reduction in vertical activity (p < 0.001) and grooming compared to its levels in VPA-Water rats. The treatment with 100 mg/kg of Passiflora significantly decreased only grooming numbers compared to VPA-Water rats (p < 0.01).
No significant difference was observed in the total travel distance and speed of movement between groups.
5.2. Effects of Passiflora extract on social impairment in the VPA-exposed rats
The three-chamber test was employed to confirm the presence of social impairments in the VPA-ASD offspring and the consequences of Passiflora extract treatment.
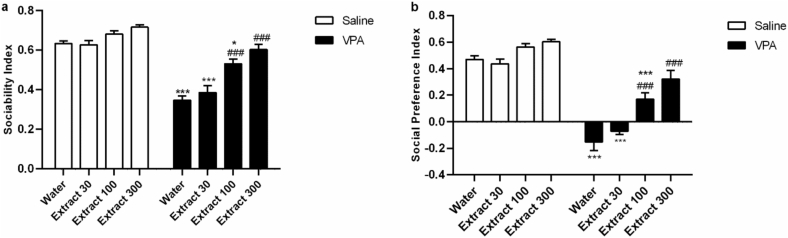
Based on the methods, the sociability index and social preference index were calculated. Two-way ANOVA indicated a significant alter by group effect [ SI: (Fig. 3a; F (1,50) = 152.4, p < 0.001); SPI (Fig. 3b; F (1,50) = 237.4, p < 0.001)] and treatment effect [SI: (Fig. 3a; F (3,50) = 25.13, p < 0.001); SPI (Fig. 3b; F (3,50) = 24.79, p < 0.001)] and group × treatment interaction [SI:(Fig. 3a; F (3,50) = 6.08, p < 0.01); SPI (Fig. 3b; F (1,50) = 152.4, p < 0.01)].
Fig. 3.
The Passiflora extract restored social impairment in the VPA rats. The sociability and social preference tests were calculated based on the time that each rat spent in each chamber (a, b). All the data were expressed as mean ± SEM (n = 8). ∗p < 0.05, ∗∗∗p < 0.001 vs. group I (saline/water); ###p < 0.001 vs. VPA-water group (group V).
Tukey's multiple comparison test indicated that male prenatal VPA/postnatal water rats (group V) spent less exploring time with the animal and more with an empty cage, thus representing a reduced sociability index (SI) compared to saline-water rats (p < 0.001, Fig. 3a). Moreover, they preferred interaction with the familiar animal compared to the novel one and had a lower social preference index (SPI) compared to saline-water rats (p < 0.001, Fig. 3b). Interestingly, Passiflora extract produced a dose-dependent increase in SI and SPI; the 300 mg/kg extract significantly prevented VPA-induced deficit in SI and SPI (p < 0.001 compared to the VPA-Water group, Fig. 3a and b).
5.3. Effects of Passiflora extract on anxiety in the VPA-exposed rats
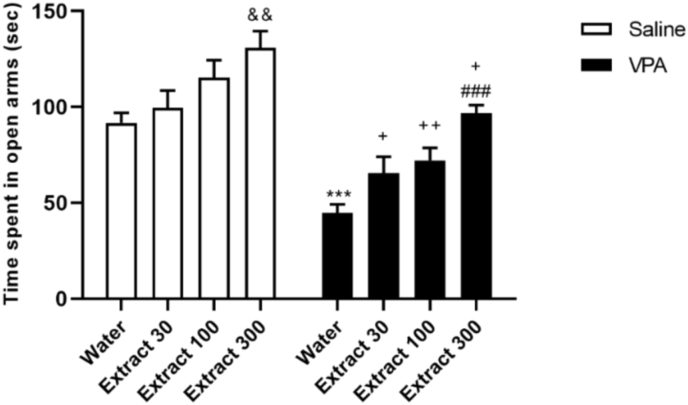
The two-way ANOVA revealed the main effect of VPA injection (Fig. 4; F (1,50) = 58.76, p < 0.001) and treatment (Fig. 4; F (3,50) = 25.13, p < 0.001). However, no significant interaction effect of group and treatment was observed on time spent in the open arm (Fig. 4; F (3,50) = 0.4, p > 0.05). Based on post hoc analyses, prenatal VPA induced anxiety-like behavior as observed by a significant decrease in time sent in the open arm of EPM (p < 0.001, VPA/Water compared to Saline/Water). Moreover, 300 mg/kg Passiflora extract significantly increased exploration time in open arms ((Fig. 4; p < 0.001 VPA + Extract300 compared to VPA + Water).
Fig. 4.
The Passiflora extract (dose 300) decreased anxiety-like behaviors in the elevated plus-maze test and increased the spent time in open-arm. ∗∗p < 0.01, ∗∗∗p < 0.001 compared to the saline/water group; ###p < 0.001 compared to the VPA/water group. (n = 8 in each group).
5.4. Effects of Passiflora extract on repetitive behavior (MBR) in the VPA-exposed rats
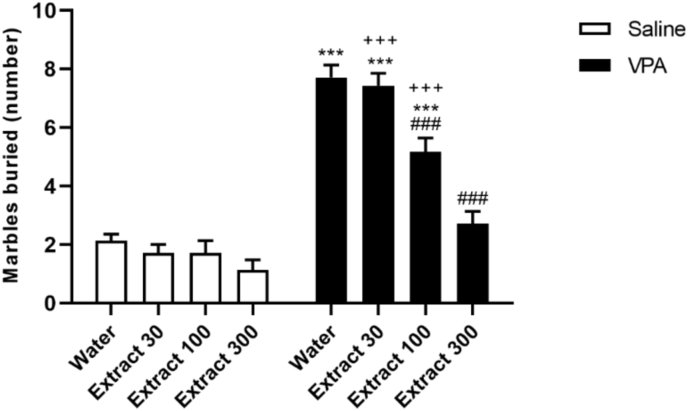
In the MBR activity, prenatal VPA rats buried significantly more marbles than saline rats (Fig. 5; F (1,50) = 231.5, p < 0.001), in accordance with an increase in repetitive behavior. The number of marbles buried in VPA-exposed rats treated with Passiflora extract (dose 300) significantly reduced compared to VPA animals that received only saline (Fig. 5, p < 0.001, Tukey post-test), suggesting the effect of Passiflora on repetitive behaviors.
Fig. 5.
VPA rats demonstrated elevated stereotyped and repetitive behaviors in the MBR that were significantly reduced by Passiflora extract (dose 300). Figure shows mean ± SEM (n = 8). ∗∗∗P < 0.001 compared to the saline/water group; ###p < 0.001 compared to the VPA/water group.
5.5. Effects of Passiflora extract on learning and memory impairment the VPA-exposed rats
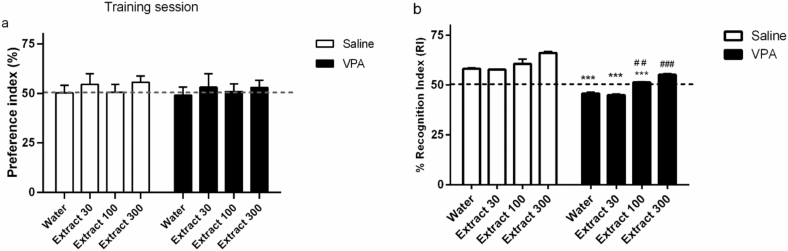
Based on the two-way ANOVA, there was no significant effect of group, treatment, and interaction between them on the Preference index in the training session of the NORT. Animals had no preference for the place of object A during the training phase. In Fig. 6a, the dotted line at 50% indicates no preference.
Fig. 6.
The effect of Passiflora extract on VPA-induced cognitive impairment in a NORT in male rats. All rats showed similar performances in training sessions (a). VPA rats were impaired at recognition memory (b). The deleterious effects of VPA were completely reversed by Passiflora extract (300). Values were represented as mean ± SE (n = 8). ∗∗∗p < 0.05 vs. saline/water, ##, ###, 0.01, 0.001 vs. VPA/water.
In the retention session, the recognition index was measured for each animal: T2/(T1+ T2) × 100, where T2 is time spent exploring the novel object (B) and T2 is time spent exploring the familiar object (A). A significant effect of treatment [F (3, 50 = 36, p < 0.001] and group [F (1, 50 = 256, p < 0.001] for recognition index was observed according to the two-way ANOVA. No significant difference was found in the interaction between the treatment and group [F (3, 50) = 1.3, p > 0.05].
According to the post hoc Tukey test, during the retention sessions, VPA significantly decreased the recognition index compared to saline (p < 0.001, Fig. 6b), and Passiflora (dose 100 and 300) treatment increased the recognition index [Extract (dose 100 and 300) p > 0.001 compared to VPA- Water, and Extract (dose 100 and 300), p > 0.001 compared to Saline- Water].
5.6. The effect of treatments on oxidative stress and anti-oxidative status parameters
The effect of different doses of Passiflora extract on serum CAT, SOD, MDA, and TAC of animals treated with VPA-induced autism are presented in Table 2. Serum CAT, SOD, and TAC significantly reduced (p < 0.001), and serum MDA significantly increased (p < 0.001) in the group treated with VPA, while animals treated with Passiflora (dose 300) were more or less like Saline/Water group. By treating autistic rats with Passiflora, a significant reduction was observed in the anti-oxidative stress-response machinery. Autistic rats treated with a higher dose of Passiflora showed a significant improvement in serum level of anti-oxidative enzymes in comparison to other groups [CAT and SOD (P < 0.05), TAC (P < 0.01), MDA (P < 0.001) compared to rats with VPA exposed].
Table 2.
Effect of Passiflora extract on serum level of catalase, SOD, MDA, and TAC in rats treated with VPA-induced autism.
| Saline |
VPA |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Water | Extract 30 | Extract 100 | Extract 300 | water | Extract 30 | Extract 100 | Extract 300 |
| Catalase (KU/L) | 115.67 ± 3.22a | 117.38 ± 2.40a | 115.82 ± 2.12a | 117.98 ± 2.21a | 86.23 ± 1.70b | 91.70 ± 1.04b | 95.80 ± 1.73b | 96.60 ± 2.1c |
| SOD (U/mL) | 63.99 ± 4.67a | 64.81 ± 2.83a | 64.14 ± 5.64a | 67.57 ± 2.00a | 42.01 ± 2.04b | 45.80 ± 2.29b | 48.76 ± 1.20b | 57.45 ± 3.26a |
| MDA (nmol/mL) | 1.75 ± 0.02a | 1.69 ± 0.04a | 1.65 ± 0.02a | 1.60 ± 0.01a | 2.32 ± 0.03b | 2.08 ± 0.02c | 1.99 ± 0.03c | 1.96 ± 0.05c |
| TAC (nmol/mL) | 225.00 ± 4.11a | 236 ± 3.47a | 245 ± 10.20a | 246.54 ± 3.92a | 176.22 ± 5.54b | 190.65 ± 3.64b | 197.56 ± 2.13b | 208.54 ± 2.95c, a |
SOD: superoxide dismutase; MDA: malondialdehyde; TAC: total anti-oxidative capacity; VPA: valproic acid; Values are represented as mean ± SEM (n = 6). Within each row, means superscripts with different letters are significantly different (P ⩽ 0.05).
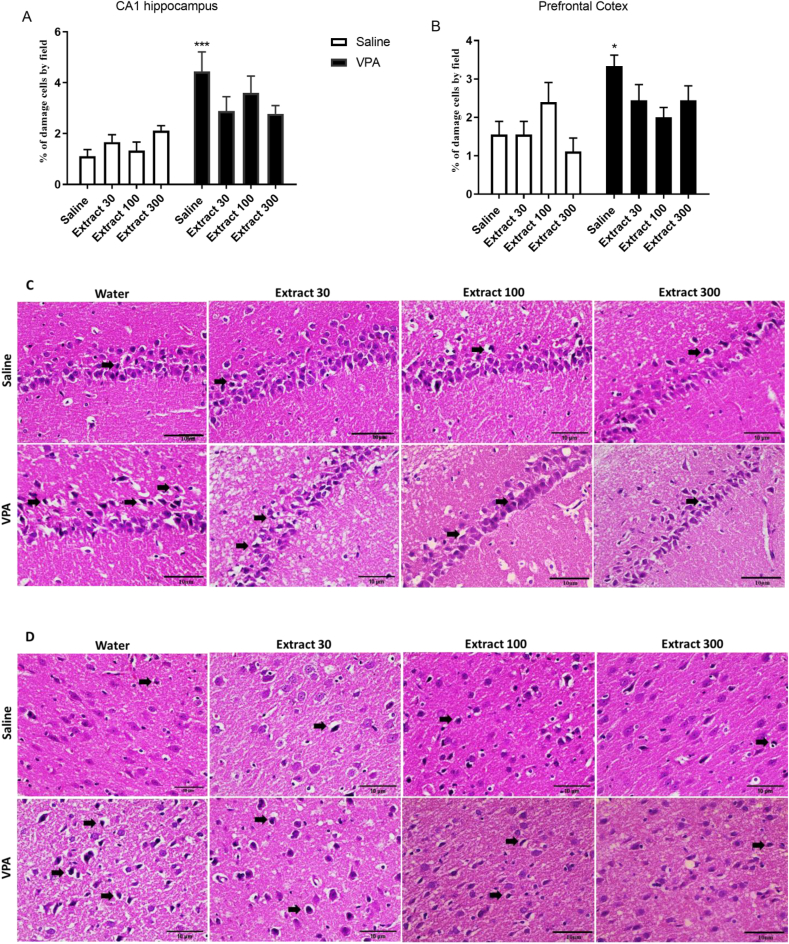
5.7. The effect of treatments on the number of damaged neurons in CA1 area and frontal cortex
Fig. 7 (A) and 7 (B) show a representative photograph of the hematoxylin-eosin-stained CA1 hippocampus and PFC damage neurons, respectively, in each treatment group. The data analysis revealed a significant increase of the damaged cells (a shrunken appearance with eosinophilic cytoplasm, and pyknotic staining nuclei) in these areas in the prenatal VPA/postnatal water compared to prenatal saline/postnatal water rats [ PFC (p < 0.05), CA1 (p < 0.001)]. Fig. 7 (A) and 7 (B) also reveal that Passiflora extract treatment decreased the neuronal damage associated with VPA treatment (p > 0.05 compared to prenatal VPA/postnatal saline and prenatal saline/postnatal water) compared to other groups, including prenatal VPA/postnatal water group [PFC (3.33 ± 0.28), CA1(4.44 ± 0.76)], prenatal VPA/postnatal Passiflora extract dose 30 [PFC (2.4 ± 0.41), CA1(2.8 ± 0.56)], dose 100 [PFC (2.00 ± 0.25), CA1(3.6 ± 0.66)], and dose 300 [PFC (2.4 ± 0.37), CA1(2.7 ± 0.32)].
Fig. 7.
Percentage of cellular damage (above) and representative photomicrographs of the hematoxylin-eosin stained (below) CA1 hippocampus (A and C) and PFC (B and D) (40x). The damaged cells exhibited a shrunken appearance with eosinophilic cytoplasm, and pyknotic staining nuclei (black arrow). Scale bar 10 μm. Values are expressed as the percentage of damaged cells counted in three slides per rat and three rats from each group. ∗P < 0.05, ∗∗∗P < 0.001 vs. Saline- Water group.
6. Discussion
In the present study, it was observed that prenatal VPA exposure resulted in increased exploratory and spontaneous activity, decreased social interaction, and induced anxiety-like behavior along with learning and memory impairment and an increase in oxidative stress. The potential therapeutic effect of hydroalcoholic extract of Passiflora incarnata in rescuing the ASD-like behaviors of VPA rats was also demonstrated in this study. It was found that VPA increased, and Passiflora partially decreased the number of damaged neurons in the PFC and CA1 region of the hippocampus. The contents of MDA and antioxidant enzymes (SOD, CAT) activities and TAC in plasma were different between VPA-treated and saline animals, and chronic administration of Passiflora to VPA-induced rats significantly increased antioxidant enzymes levels and decreased oxidative stress markers levels. Akhondzadeh et al. (2001) reported that an effective drug for patients with a generalized anxiety disorder is Passiflora extract.32 In the present study, animals treated with Passiflora had lower anxiety-like behavior in the EPM test. There is growing evidence that anxiety disorders may be associated with oxidative-antioxidant imbalance. Antioxidants are recommended, as adjuvant therapy, for patients with anxiety disorders; classical antidepressants can modify the oxidative stress processes. On the other hand, oxidative stress dysregulates physiological signaling mechanisms and leads to neuroinflammation, and in this way increases the risk of anxiety and depression.33,34
Poor sleep in autistic patients is very common and may have harmful effects on children's attention, learning, and memory in addition to their social and emotional development.35 It is well documented that Passiflora can help treat insomnia and depression36 and improves spatial learning and memory.14,37
Alkaloids and flavonoids are the most important bioactive ingredients in plant extract, and the pharmacological action of Passiflora is attributed to these substances. Here, this study used hydroalcoholic as a solvent for extraction, since maximum chemical compositions are soluble in this mixture. Flavonoids act as powerful antioxidants or scavengers of various oxidizing species.38,39
Flavonoid compounds in the plant extract are chlorogenic acid, hyperosid, isovitexin, caffeic acid, quercitin, orientin, rutin, chrysin, and vitexin. The present study measured the amount of quercetin, rutin, and hyperoside in the hydroalcoholic extract of P. incarnata. Masteikova et al. results showed that the quantity of quercetin is lower than rutin in a hydroalcoholic extract of P. incarnate,40 which is in line with the current study. Hyperoside is another flavonoid compound found in plants, which has been reported to have good antioxidant effects in many studies. Ahmadi et al. reported that hyperoside has antioxidant effects on the human body by inhibiting the oxidation of low-density lipoprotein41. In another study that investigated the flavonoid compounds of the hydroalcoholic extract of P. incarnata, it has been shown that the amount of hyperoside is lower than that of rutin and quercetin, which is similar to the results of the current study.40 Moreover, hyperoside in 0.1–0.16 mg/mL concentration showed antioxidant activity in cancerous cells.42 The concentration of hyperoside (33.72 ± 1.23 mg/g) in the present study is more than the mentioned concentration, so that the hydroalcoholic extract of P. incarnata has shown good antioxidant activity.
Accumulating evidence suggests increased oxidative stress-related biomarkers in autism that may be associated with the development of this disease.4,43 In line with the recent study, previous studies have reported oxidative imbalance in the VPA model of the disease.44 It is postulated that these biomarkers are a major cause of neural tube defects (NTDs).45 The study results showed that the hydroalcoholic extract of Passiflora reliably improves several serum stress markers (MDA, SOD, CAT, TAC).
It is known that Passiflora incarnata possesses antipsychotic activity and inhibits amphetamine-induced stereotyped behavior in rats through its dopamine D2 and 5-HT2 receptor-blocking effects.38 Due to the effectiveness of dopamine blocker drugs, such as antipsychotics in reducing hyperactivity, stereotyped behaviors, aggression, and self-injury behavior, hyperactivity of dopaminergic neurons has been implicated in the pathophysiology of autism.46 In the present study, the extract decreased repetitive and stereotyped behaviors in the MBR and open field test. The study results indicated the association between the beneficial effects of Passiflora with its antioxidative activity and modulation of redox homeostasis in the brain. Similarly, Zhang et al. showed that repetitive/stereotypic behavior in autism ameliorates due to the antioxidant properties of N-acetylcysteine and Sulindac.47,48 Further studies are required to elucidate the underlying physiological mechanisms of Passiflora incarnata on the repetitive/stereotypic behavior in autism.
7. Conclusion
The study results confirmed that Passiflora incarnata has the potential to alleviate some autistic-like behaviors in the animal model of the disease. Furthermore, it suggested that the extract may be considered as a neuroprotective agent and a potential antioxidant resource which can encourage further clinical research in using Passiflora incarnata in treating autism or other neurodegenerative diseases. However, it is more important to notice that although the VPA animal model of autism has both face and predictive validity and is an appropriate tool to investigate novel behavioral and pharmacological therapies, like any preclinical animal model, there are limitations to its translation to the clinical setting. Future studies in genetic and environmental animal models of ASD are necessary to clarify the possible therapeutic role of Passiflora in the ASD population.
Funding statement
This study was supported by the Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran [grant number 99000615].
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declaration of competing interest
The authors declare that there is no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
The plant name has been checked with http://www.theplantlist.org.
References
- 1.Mohammadi S., Asadi-Shekaari M., Basiri M., Parvan M., Shabani M., Nozari M. Improvement of autistic-like behaviors in adult rats prenatally exposed to valproic acid through early suppression of NMDA receptor function. Psychopharmacology (Berl) 2020;237:199–208. doi: 10.1007/s00213-019-05357-2. [DOI] [PubMed] [Google Scholar]
- 2.Taylor M.J., Rosenqvist M.A., Larsson H., et al. Etiology of Autism Spectrum Disorders and Autistic Traits Over Time. 2021;77:936–943. doi: 10.1001/jamapsychiatry.2020.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabrucker A.M. Environmental factors in autism. Front Psychiatr. 2013;3:1–13. doi: 10.3389/fpsyt.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjørklund G., Meguid N.A., El-bana M.A., et al. Oxidative stress in autism spectrum disorder. Mol Neurobiol. 2020;57:2314–2332. doi: 10.1007/s12035-019-01742-2. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt I.D., Rawat S., Rawal R.S. Antioxidants in medicinal plants. 2011. [DOI]
- 6.Janda K., Wojtkowska K., Jakubczyk K., Antoniewicz J., Zydecka K.S. 2020. Passiflora Incarnata in Neuropsychiatric; pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca L.R.d., Rodrigues R. de A., Ramos A. de S., et al. Herbal medicinal products from passiflora for anxiety: an unexploited potential. Sci World J. 2020 doi: 10.1155/2020/6598434. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M., Lim H.-S., Lee H.-H., Kim T.-H. Role identification of passiflora incarnata linnaeus : a mini review. J. Menopausal Med. 2017;23:156. doi: 10.6118/jmm.2017.23.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avelino da Silva J., De Carvalho da Costa M.J., Rodrigues da Alves M. da C., Fernandes da Braga J.E., Bezerra Luna da Lima C.M., De Morais da Pordeus L.C. Effects of the single supplementation and multiple doses of Passiflora incarnata L. on human anxiety: a Clinical Trial, Double-blind, Placebo-controlled, Randomized. Int Arch Med. 2017;10 doi: 10.3823/2276. [DOI] [Google Scholar]
- 10.Michael H.S.R., Mohammed N.B., Ponnusamy S., Edward Gnanaraj W. A folk medicine: passiflora incarnata L. Phytochemical profile with antioxidant potency. Turkish J. Pharm. Sci. 2022;19 doi: 10.4274/tjps.galenos.2021.88886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B., Singh D., Goel R.K. Dual protective effect of Passiflora incarnata in epilepsy and associated post-ictal depression. J Ethnopharmacol. 2012;139:273–279. doi: 10.1016/j.jep.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Trial A.A.C., Rodrigues C. Effects of the single supplementation and multiple doses of passiflora incarnata L. 2017. on Human 0, 1–9. [DOI]
- 13.Ingale S.P., Kasture S.B. Protective effect of standardized extract of passiflora incarnata flower in Parkinson's and alzheimer's disease. Ancient Sci Life. 2017;36:200. doi: 10.4103/ASL.ASL_231_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jawna-Zboińska K., Blecharz-Klin K., Joniec-Maciejak I., et al. Passiflora incarnata L. Improves spatial memory, reduces stress, and affects neurotransmission in rats. Phyther. Res. 2016;30:781–789. doi: 10.1002/ptr.5578. [DOI] [PubMed] [Google Scholar]
- 15.Kim K.C., Lee D.K., Go H.S., et al. Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol. 2014;49:512–528. doi: 10.1007/s12035-013-8535-2. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimpour N., Khazaneha M., Mehrbani M., Rayegan P., Raeiszadeh M. Efficacy of Herbal Based Syrup on male sexual experiences: a double-blind randomized clinical trial. J. Tradit. Complement. Med. 2021;11:103–108. doi: 10.1016/j.jtcme.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rameshk M., Sharififar F., Farsinejad A., et al. In vitro proliferation and wound healing effects of Narcissus tazetta L. bulb on primary human dermal fibroblasts. researchgate.net. 2018;20:1–13. doi: 10.9734/JPRI/2017/39090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raeiszadeh M., Pardakhty A., Sharififar F., Mehrabani Mehrnaz, Nejatmehrab-Kermani H., Mehrabani Mitra. Phytoniosome: a novel drug delivery for myrtle extract. Iran. J Pharm Res. 2018;17:804–817. [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider T., Przewłocki R., Tomasz Schneider R.P., others, Schneider T., Przewłocki R., Tomasz Schneider R.P., Schneider T., Przewłocki R. Behavioral alterations in rats prenatally to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. others. [DOI] [PubMed] [Google Scholar]
- 20.Haratizadeh S., Parvan M., Mohammadi S., Shabani M., Nozari M. An overview of modeling and behavioral assessment of autism in the rodent. Int J Dev Neurosci. 2021;81:221–228. doi: 10.1002/jdn.10096. [DOI] [PubMed] [Google Scholar]
- 21.Crawley J.N. Mouse behavioral assays relevant to the symptoms of autism. in: Brain Pathol. 2007:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plus-maze. in: Neurosci Biobehav Rev. 1997:801–810. doi: 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 23.Ghotbi Ravandi S., Shabani M., Bashiri H., Saeedi Goraghani M., Khodamoradi M., Nozari M. Ameliorating effects of berberine on MK-801-induced cognitive and motor impairments in a neonatal rat model of schizophrenia. Neurosci Lett. 2019;706:151–157. doi: 10.1016/j.neulet.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Haratizadeh S., Ranjbar M., Darvishzadeh-Mahani F., Basiri M., Nozari M. The effects of postnatal erythropoietin and nano-erythropoietin on behavioral alterations by mediating K-Cl co-transporter 2 in the valproic acid-induced rat model of autism. Dev Psychobiol. 2023;65 doi: 10.1002/DEV.22353. [DOI] [PubMed] [Google Scholar]
- 25.Pasciuto E., Borrie S.C.C., Kanellopoulos A.K.K., et al. Autism Spectrum Disorders: translating human deficits into mouse behavior. Neurobiol Learn Mem. 2015;124:71–87. doi: 10.1016/j.nlm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Li K., Li L., Cui B., et al. Early postnatal exposure to airborne fine particulate matter induces autism-like phenotypes in male rats. Toxicol Sci. 2018;162:189–199. doi: 10.1093/toxsci/kfx240. [DOI] [PubMed] [Google Scholar]
- 27.Faatehi M., Basiri M., Nezhadi A., et al. Early enriched environment prevents cognitive impairment in an animal model of schizophrenia induced by MK-801: role of hippocampal BDNF. Brain Res. 2019;1711:115–119. doi: 10.1016/j.brainres.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Buege J.A., Aust S.D. Biomembranes - Part C: biological oxidations. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 30.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘“Antioxidant power”’: the FRAP assay Iris. Anal Methods. 1996;7:70–76. doi: 10.1039/c6ay01739h. [DOI] [PubMed] [Google Scholar]
- 31.Cardiff R.D., Miller C.H., Munn R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014:655–658. doi: 10.1101/pdb.prot073411. 2014. [DOI] [PubMed] [Google Scholar]
- 32.Naghavi H.R., Vazirian M., Shayeganpour A., Rashidi H., Khani M., Akhondzadeh S. Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial with oxazepam. J Clin Pharm Therapeut. 2001;26:363–367. doi: 10.1046/j.1365-2710.2001.00367.x. [DOI] [PubMed] [Google Scholar]
- 33.Fedoce A., das G., Ferreira F., et al. The role of oxidative stress in anxiety disorder: cause or consequence? Free Radic Res. 2018 doi: 10.1080/10715762.2018.1475733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y., Wang C., Klabnik J., O’ Donnell J. Novel therapeutic targets in depression and anxiety: antioxidants as a candidate treatment. Curr Neuropharmacol. 2014;12:108–119. doi: 10.2174/1570159x11666131120231448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elrod M.G., Nylund C.M., Susi A.L., et al. Prevalence of diagnosed sleep disorders and related diagnostic and surgical procedures in children with autism spectrum disorders. J Dev Behav Pediatr. 2016;37:377–384. doi: 10.1097/DBP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 36.Kim G.H., Kim Y., Yoon S., Kim S.J., Yi S.S. Sleep-inducing effect of Passiflora incarnata L. extract by single and repeated oral administration in rodent animals. Food Sci Nutr. 2020;8:557–566. doi: 10.1002/fsn3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G.H., Lim K., Yang H.S., et al. Improvement in neurogenesis and memory function by administration of Passiflora incarnata L. extract applied to sleep disorder in rodent models. J Chem Neuroanat. 2019;98:27–40. doi: 10.1016/j.jchemneu.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Ingale S.P., Kasture S.B. International journal of phytopharmacology psychopharmacological profile of passiflora incarnata linn in mice. Int J Phytopharm. 2012;3:263. [Google Scholar]
- 39.Ożarowski M., Karpiński T.M. Extracts and flavonoids of passiflora species as promising anti-inflammatory and antioxidant substances. Curr Pharmaceut Des. 2020;27:2582–2604. doi: 10.2174/1381612826666200526150113. [DOI] [PubMed] [Google Scholar]
- 40.Masteikova R., Bernatoniene J., Bernatoniene R., Velziene S. Antiradical activities of the extract of Passiflora incarnata. Acta Pol. Pharm. - Drug Res. 2008;65:577–583. [PubMed] [Google Scholar]
- 41.Liu Z., Tao X., Zhang C., Lu Y., Wei D. Protective effects of hyperoside (quercetin-3-o-galactoside) to PC12 cells against cytotoxicity induced by hydrogen peroxide and tert-butyl hydroperoxide. Biomed Pharmacother. 2005;59:481–490. doi: 10.1016/j.biopha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74 doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damodaran L.P.M., Arumugam G. Urinary oxidative stress markers in children with autism. Redox Rep. 2011;16:216–222. doi: 10.1179/1351000211Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y., Zhao S., Liu X., Zheng Y., Li L., Meng S. Biomedicine & Pharmacotherapy Oxytocin improves animal behaviors and ameliorates oxidative stress and in fl ammation in autistic mice. Biomed Pharmacother. 2018;107:262–269. doi: 10.1016/j.biopha.2018.07.148. [DOI] [PubMed] [Google Scholar]
- 45.Mabunga D.F.N., Gonzales E.L.T., Kim J., Kim K.C., Shin C.Y. Exploring the validity of valproic acid animal model of autism. Exp. Neurobiol. 2015;24:285–300. doi: 10.5607/en.2015.24.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandhi T., Lee C.C. Neural mechanisms underlying repetitive behaviors in rodent models of autism spectrum disorders. Front Cell Neurosci. 2021;14:1–44. doi: 10.3389/fncel.2020.592710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Cui W., Zhai Q., Zhang T., Wen X. N-acetylcysteine ameliorates repetitive/stereotypic behavior due to its antioxidant properties without activation of the canonical Wnt pathway in a valproic acid-induced rat model of autism. Mol Med Rep. 2017;16:2233–2240. doi: 10.3892/mmr.2017.6787. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Yang C., Yuan G., Wang Z., Cui W., Li R. Sulindac attenuates valproic acid-induced oxidative stress levels in primary cultured cortical neurons and ameliorates repetitive/stereotypic-like movement disorders in Wistar rats prenatally exposed to valproic acid. Int J Mol Med. 2015;35:263–270. doi: 10.3892/ijmm.2014.1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.