Abstract
The idea that eye movements can reflect certain aspects of brain function and inform on the presence of neurodegeneration is not a new one. Indeed, a growing body of research has shown that several neurodegenerative disorders, such as Alzheimer’s and Parkinson’s Disease, present characteristic eye movement anomalies and that specific gaze and eye movement parameters correlate with disease severity. The use of detailed eye movement recordings in research and clinical settings, however, has been limited due to the expensive nature and limited scalability of the required equipment. Here we test a novel technology that can track and measure eye movement parameters using the embedded camera of a mobile tablet. We show that using this technology can replicate several well-known findings regarding oculomotor anomalies in Parkinson’s disease (PD), and furthermore show that several parameters significantly correlate with disease severity as assessed with the MDS-UPDRS motor subscale. A logistic regression classifier was able to accurately distinguish PD patients from healthy controls on the basis of six eye movement parameters with a sensitivity of 0.93 and specificity of 0.86. This tablet-based tool has the potential to accelerate eye movement research via affordable and scalable eye-tracking and aid with the identification of disease status and monitoring of disease progression in clinical settings.
Keywords: eye-tracking, biomarker, Parkinson’s disease, UPDRS (unified Parkinson’s disease rating scale), gaze mapping, neurodegenerative disorders
Introduction
While we have long known that the eyes are our windows to the world, a growing body of research suggests a bi-directional relationship whereby the eyes –and particularly how they move– can also serve as a window into the brain. Although eye movements have previously been linked to certain cognitive processes like attention and decision-making, recent work has unequivocally shown that eye movements can reflect certain aspects of brain function and inform on the presence of neurodegeneration and cognitive impairment (1–5). The link between eye movements and brain health should not be too surprising given that eye movements are controlled by a diverse network of cortical and subcortical structures (4, 6) that are susceptible to a variety of degenerative processes (1, 7, 8). Moreover, the analysis of gaze patterns and visual tasks that measure cognitive inhibition can provide insights into the integrity of various cognitive processes (2, 9, 10).
For instance, two consistent impairments have emerged from Alzheimer’s disease (AD) oculomotor research: a high frequency of saccadic intrusions during attempted fixation and visual capture by the target in the anti-saccade paradigm (11, 12). Furthermore, microsaccades, tiny horizontal rapid eye movements that interrupt periods of fixation tend to be uniquely obliquely oriented (13) and occur at an elevated rate in Alzheimer’s (14). Parkinson’s Disease (PD) is generally associated with hypometric and multi-step saccades in all types of oculomotor tasks (15, 16), in addition to a high rate of saccadic intrusions during smooth pursuit (17). Multiple sclerosis (MS) is particularly associated with internuclear ophthalmoparesis (INO)—a slowing of the adducting eye during horizontal saccades—and saccadic intrusions during fixation (8). Furthermore, smooth pursuit metrics (low pursuit gain and increased saccadic amplitudes) have been proposed as a marker of early MS (18).
Not only do these oculomotor anomalies characterize these neurodegenerative disorders, but a growing body of research shows that they can serve as markers of disease severity and cognitive impairment. In AD, oculomotor signatures of disease severity have been identified via correlations between specific eye movement characteristics and the Mini-Mental State Examination (MMSE) (19–22). Similarly, in PD, several oculomotor metrics have been shown to correlate with the Unified Parkinson’s Disease Rating Scale (UPDRS) or some of its subscales (23–26), or with measures of general cognition such as the MMSE (27, 28) or the MoCA (29, 30). In MS similar relationships have been observed between such metrics and the Expanded Disability Status Scale (EDSS) or the Symbol Digit Modalities Test (SDMT) (31–35).
Although a clinical oculomotor examination is usually sufficient to aid clinicians in the differential diagnosis of advanced neurological disorders, these exams do not typically capture subtle changes such as those highlighted in the aforementioned studies. Indeed, many have proposed that laboratory eye movement recordings can be extremely useful for objective and precise identification of disease status and monitoring of disease progression (1) and assist with differential diagnoses (36–38) though there is hope that the precise quantification of eye movements could also eventually lead to early diagnoses in individuals with less pronounced oculomotor symptoms. Unfortunately, the use of detailed eye movement recordings in clinical settings has been limited due to the expensive nature and limited scalability of the required equipment, such as infrared eye-tracking cameras. Although several mobile tablet-based (or smartphone-based) gaze-tracking systems have been developed to provide more accessible and affordable solutions (39–42), to our knowledge, they have not been used to capture precise oculomotor parameters on a millisecond timescale such as those evoked during saccade and anti-saccade tasks. They have instead been primarily used to analyze gross eye movements, such as those required to study gaze search patterns or to determine the on-screen location of an individual’s fixation point.
Eye-Tracking Neurological Assessment (ETNA™) is a recently developed technology that can reliably and accurately track eye movements without the need for infrared cameras, using the iPad Pro embedded camera. This technology allows for the precise quantification of several eye movement parameters currently only available with specialized and costly research-grade infrared eye tracking devices, such as the latency, velocity, and amplitude of saccades, and the presence of saccadic intrusions during fixation. In this paper, we show using the ETNA™ with a standard tablet mobile camera that we can measure and replicate eye movement anomalies and replicate findings from the literature on PD and eye movement, further demonstrating how eye movement parameters can reflect disease status and severity.
Methods
Study design and subject population
This cross-sectional study included 121 participants and was approved by both the Veritas and the Montreal University Health Center (MUHC) research ethics boards. Fifty-nine (59) PD participants (age 63.76 ± 8.23, range 45–79, 32.2% females) took part in this study. All were recruited by the Quebec Parkinson Network. Inclusion criteria were confirmed diagnosis of PD and sufficient corrected visual acuity to allow for the accurate reading of the on-screen visual task instructions. Exclusion criteria were the presence of comorbid neurological or psychiatric conditions to avoid eye movement anomaly confounds. To assess clinical status, all PD patients underwent the motor subscale (part III) of the MDS-UPDRS (43, 44), which was developed to evaluate various aspects of Parkinson’s Disease. Note that because the MDS-UPDRS was performed in a research setting with time constraints and not as part of the standard of care, not all patients underwent the full MDS-UPDRS evaluation. As a result, only part III scores were used in analyses presented herein.
Sixty-two (62) healthy control (HC) participants (age 56.64 ± 8.56, range 45–77, 46.7% females) took part in this study. All were recruited from the Montreal community. The inclusion criterion was sufficient visual acuity to perform the tablet-based visual tasks. Exclusion criteria were evidence or history of other significant neurological or psychiatric disorders. Summary patient demographics are shown in Table 1.
Table 1.
Group demographics.
| PD patients | Healthy controls | |
|---|---|---|
| n | 59 | 62 |
| Age mean ± sd (median, range) | 63.76 ± 8.23 (64, 45–79) | 56.64 ± 8.56 (55; 45–77) |
| UPDRS part III | 27.56 ± 13.8 (7–65) |
Gaze-tracking experimental setup
All tests were performed using a 12.9-inch iPad Pro tablet with the ETNA™ software installed, which enables simultaneous video recordings of the eyes at 60 frames per second using the embedded front-facing camera and the presentation of visual stimuli on the screen. All participants performed three oculomotor tasks (fixation task, pro-saccade task, and anti-saccade task; see Figure 1), which was preceded by a calibration step, where participants were instructed to follow a slowly moving target across the screen. All tablet-based oculomotor tasks were completed in under 6 min.
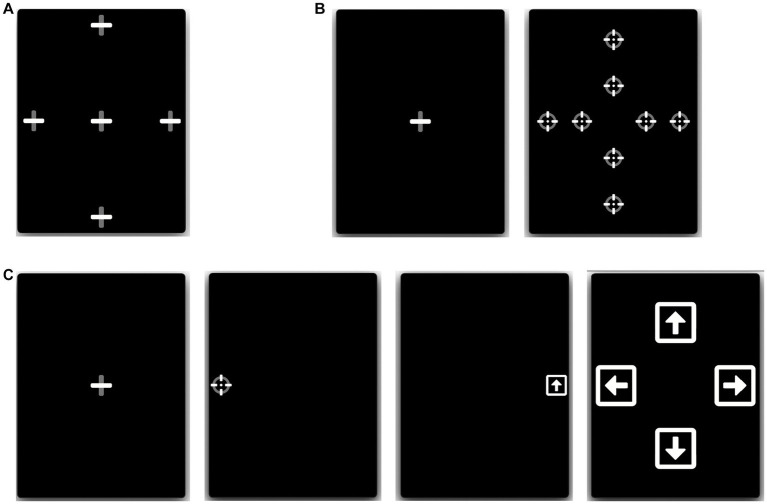
Figure 1.
Eye-tracking tasks. (A) Fixation: participants fixated a stationary target for 7 s, at one of 5 locations. (B) Pro-saccades: participants initially fixated a central fixation point, which disappeared after 1.0–3.5 s, after which a different target appeared at one of 8 eccentric locations for 1.5 s. (C) Anti-saccades: participants initially fixated a central fixation point, which disappeared after 1.0–3.5 s, after which a round target appeared at 10° to the left or right from the center. Participants were instructed to move their gaze in the opposite direction to the round target, where after 1,200 ms they were shown a square with an arrow inside that pointed in one of 4 random directions (left, right, up, or down; shown during 400 ms). The users then had to direct their gaze towards the arrow orientation corresponding to the arrow they saw in the preceding step.
All tasks were performed with the tablets placed vertically, camera side up, and secured at eye level using a tablet pole mount. Participants were positioned approximately 45 cm from the tablet screen and were allowed to use their best-spectacle correction (proportion of participants wearing glasses: PD = 39%, HC = 29%, X2(1) = 1.33, p = 0.24). Safeguards within the gaze-tracking software ensured the participant’s head was properly positioned and visible, at an acceptable angle and distance from the screen.
Fixation task: Participants had to fixate a stationary target for 7 s, at five different locations (one central and 4 eccentric locations). The eccentric positions were located 10 degrees of visual angle left and right from the center and 14 degrees of visual angle up and down from the center (Figure 1A).
Pro-saccade task: Participants had to initially fixate a central fixation point, which disappeared after a random period of 1.0–3.5 s, after which a different target reappeared at an eccentric location for 1.5 s either to the left or right, above, or below the central fixation point. Participants were instructed to move their gaze as quickly as possible to the new target location. Both short (5o horizontal, 6o vertical) and large (10o horizontal, 12o vertical) eccentric target distances were used, and each target location was sampled 3 times, for a total of 24 trials (Figure 1B).
Anti-saccade task: Participants had to initially fixate a central fixation target, which disappeared after a random period of 1.0–3.5 s, after which a different target reappeared at an eccentric location (10o) to the left or right from the center. Participants were instructed to move their gaze as quickly as possible in the opposite direction to the new target location. After being displayed for only 100 ms, the target disappeared and the screen was left blank for a predetermined duration of time. Following the blank screen, a symbol appeared in the opposite location of where the initial stimulus appeared (i.e., where the participant should be looking). This symbol consisted of a white square with an arrow inside oriented in one of 4 random directions: either left, right, up, or down. The blank screen period lasted 1,200 ms and the arrow symbol duration of 400 ms. After each trial, a screen was displayed for 5 s prompting the user to answer which symbol they saw by directing their gaze towards the arrow orientation corresponding to what they believe is the correct answer (Figure 1C). This task was inspired by an anti-saccade task used in a previous study (45), whereby participants could only identify the second symbol had they performed the anti-saccade task correctly (i.e., looked in the opposite direction of the initial target).
Parameter extraction and analysis
Offline analysis was performed using ETNA™‘s proprietary analysis pipeline to automatically extract the eye movement parameters reported for each task. Before parameter extraction, all gaze signals were denoised and non-saccadic artifacts (e.g., blinks) were removed by the software’s analysis pipeline.
The following parameters were extracted from the fixation task gaze recordings (parameters were averaged across the five fixation trials): (1) 68% bivariate contour ellipse area (BCEA) of fixation – a measure of fixation stability which encompasses an ellipse that covers the 68% of fixation points that are closest to target, (2) 95% BCEA, (3) Horizontal gaze SD – standard deviation of the horizontal gaze position, (4) Vertical gaze SD – standard deviation of the vertical gaze position, and (5) the rate of saccadic intrusions (at least 0.5 deg. in amplitude) during fixation.
The following parameters were extracted from the pro-saccade task gaze recordings (averaged across all short-eccentricity targets and all large-eccentricity targets): (1) average saccade latency, (2) average total time to reach the target, (3) average mean saccade velocity, (4) average peak saccade velocity, (5) average saccade amplitude gain (amplitude of the saccade relative to the eccentricity of the target; a measure of saccade accuracy), (6) average saccade amplitude error (average distance separating the saccade from the target; a measure of saccade precision), and (7) the average number of saccades required to reach the target.
The following parameters were extracted from the anti-saccade task gaze recordings: (1) direction error rate, (2) direction corrected rate (proportion of trials where participants directed their gaze in the correct direction following an initial saccade in the wrong direction), (3) target (arrow) recognition rate, (4) correct direction latency, and (5) incorrect direction latency.
Group comparisons were performed using multivariate analysis of covariance to simultaneously test statistical differences for multiple response variables (eye-tracking parameters) by one grouping variable (PD or HC). As age and sex were significantly different between study groups (t(1) = 4.66, p < 0.001), these grouping variables were used as covariates for between-group comparisons. F-statistics with degrees of freedom and p-values are reported. For correlation analyses with MDS-UPDRS-part III (motor) scores, data normality was assessed with the Shapiro–Wilk test to determine the appropriate correlation coefficient for each eye-movement parameter (i.e., Pearson’s R or Spearman’s ρ). Data analyses and visualization were conducted using R 4.2.1 in RStudio (build 554), packages dplyr, tidyverse, ggplot2, ggpubr, and rstatix. Although the main purpose of the present paper is to replicate well-known findings in the literature using a novel device, and not to make novel scientific claims, we opted for transparency to present corrected p-values for correlations and post-hoc between-group comparisons to adjust for the false discovery rate using the Benjamini-Hochberg procedure evaluated at an alpha level of 0.05 (46).
Finally, a logistic regression with ridge regularization and random subsampling (1,000 samples) is used to assess the strength of six eye-tracking parameters [the fixation saccadic intrusion rate (1), for short amplitude pro-saccades: the first gain (2), mean velocity (3), mean latency (4) and the average number of saccades (5), and the first gain error for large amplitude pro-saccades (6)] as predictors of PD diagnosis (PD vs. HC). Receiver Operating Characteristics (ROC) analysis and a confusion matrix were used to assess the performance of the classifier. Training, classification and visualization of the logistic regression classifier was conducted using scikit-learn 1.2.2 and matplotlib 3.7.1 in Python 3.9.6.
Results
Correlations with MDS-UPDRS – part III
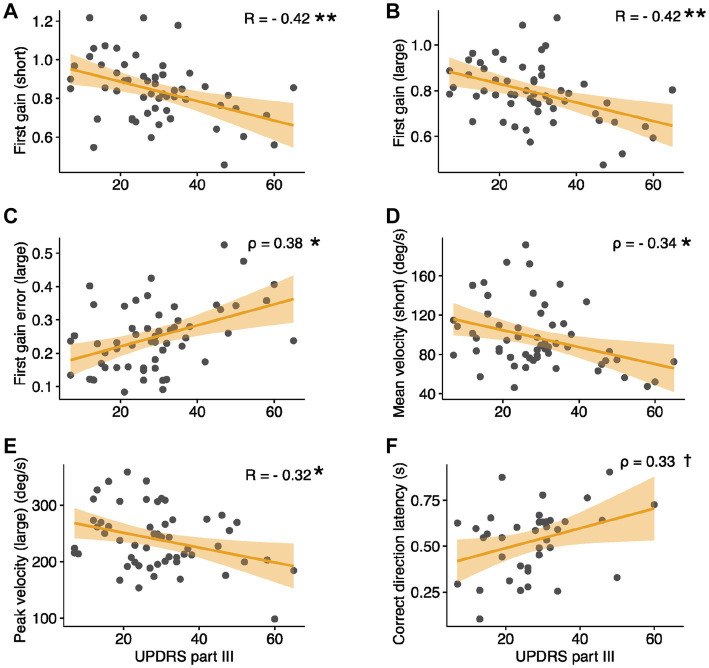
No fixation parameters were found to correlate with the UPDRS motor score (all ρ ≤ 0.187, p ≥ 0.542; see Table 2). In contrast, most pro-saccade parameters were found to correlate with it, particularly for large eccentricity targets (see Table 3; Figures 2A–E), six of which survived the value of p correction for multiple comparisons (|R| = 0.315–0.419, |ρ| = 0.334–377, p ≤ 0.028). A single anti-saccade parameter, correct direction latency, was found to correlate with the UPDRS motor score (ρ = 0.331, Figure 2F), however, the corrected value of p was greater than 0.05.
Table 2.
Fixation task.
| UPDRS part III correlations | PD/HC comparisons | |||
|---|---|---|---|---|
| Eye-tracking parameter | Correlation coefficient | p (corrected) | F-statistic | p (corrected) |
| BCEA 68 | ρ = 0.107 | 0.425 (0.708) | 3.075 | 0.082 (0.137) |
| BCEA 95 | ρ = 0.165 | 0.217 (0.542) | 2.143 | 0.146 (0.182) |
| Horizontal gaze SD | ρ = 0.187 | 0.161 (0.542) | 3.704 | 0.056 (0.137) |
| Vertical gaze SD | ρ = 0.02 | 0.882 (0.978) | 0.002 | 0.964 (0.964) |
| Saccadic intrusion rate | ρ = 0.004 | 0.978 (0.978) | 20.878 | 1.32 × 10−5 (6.6 × 10−5) |
For each eye-tracking parameter, parameter-UPDRS motor score correlations are shown on the left side of the table (UPDRS part III correlations). Between-group (PD vs. HC) comparisons are shown on the right side of the table (PD/HC comparisons). ρ, Spearman’s rho. F-statistic and p-values for post-hoc analyses are reported. Raw p-values are presented, followed by their corrected value in parentheses (Benjamini-Hochberg procedure, α = 0.05).
Table 3.
Prosaccade task.
| UPDRS part III correlations | PD/HC comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| Eye-tracking parameter | Short amplitude | Large amplitude | Short amplitude | Large amplitude | ||||
| Correlation coefficient | p (corrected) | Correlation coefficient | p (corrected) | F-statistic | p (corrected) | F-statistic | p (corrected) | |
| Latency (mean) | R = 0.263 | 0.048 (0.061) | R = 0.251 | 0.059 (0.068) | 25.308 | 1.83 × 10−6, (8.5 × 10−6) | 9.35 | 0.002 (0.005) |
| Time to target (mean) | R = 0.269 | 0.042 (0.058) | R = 0.341 | 0.009 (0.02) | 6.843 | 0.01 (0.015) | 0.002 | 0.962 (0.962) |
| Saccades to target (mean) | ρ = 0.153 | 0.253 (0.253) | ρ = 0.196 | 0.142 (0.152) | 28.834 | 4.21 × 10−7 (2.94 × 10−6) | 45.519 | 6.54 × 10−10 (9.23 × 10−9) |
| First gain (mean) | R = −0.416 | 0.001 (0.007) | R = −0.419 | 0.001 (0.007) | 19.278 | 2.53 × 10−5 (8.88 × 10−5) | 9.829 | 0.002 (0.005) |
| First gain (mean error) | ρ = 0.371 | 0.004 (0.014) | ρ = 0.377 | 0.003 (0.014) | 2.619 | 0.108 (0.142) | 7.987 | 0.005 (0.009) |
| Velocity (mean) | ρ = −0.339 | 0.01 (0.02) | ρ = −0.334 | 0.01 (0.02) | 11.376 | 0.001 (0.002) | 0.009 | 0.924 (0.962) |
| Peak velocity (mean) | R = −0.271 | 0.04 (0.058) | R = −0.315 | 0.016 (0.028) | 2.565 | 0.112 (0.142) | 0.056 | 0.813 (0.948) |
For each eye-tracking parameter, parameter-UPDRS motor score correlations are shown on the left side of the table (UPDRS part III correlations). Between-group (PD vs. HC) comparisons are shown on the right side of the table (PD/HC comparisons). ρ, Spearman’s rho. R, Pearson’s R. F-statistic and p-values for post-hoc analyses are reported. Raw p-values are presented, followed by their corrected value in parentheses (Benjamini-Hochberg procedure, α = 0.05).
Figure 2.
Correlations between select eye-tracking parameters and UPDRS Part III scores. (A,B) Pro-Saccades: first gain, (C) first gain error, (D) mean velocity, (E) peak velocity. (F) Anti-Saccades: correct direction latency. (C,D,F) depict Spearman’s rho values; trend lines are shown for reference only. Large, large amplitude pro-saccades; short, short amplitude pro-saccades. *p < 0.05, **p < 0.01 (corrected for multiple comparisons), †p = 0.039 (0.19 corrected).
Group comparisons
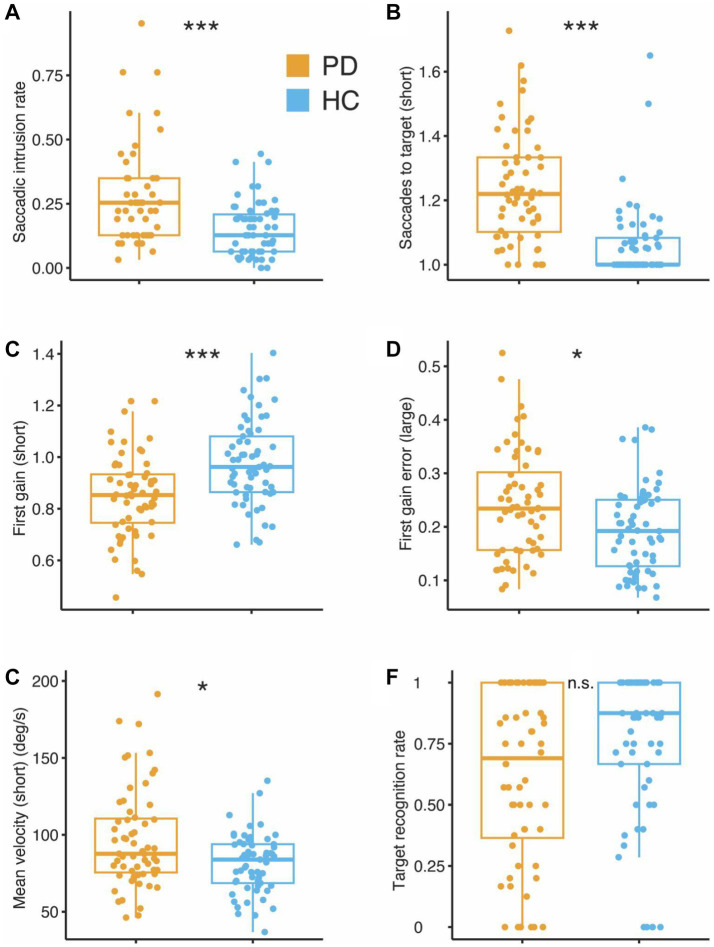
For the fixation task, the group effect was significant but the effects of age and sex were not (group: F(5) = 5.34, p < 0.001; age and sex: both F(5) ≤ 1.40, p ≥ 0.22, MANCOVA). Only one significant group difference was observed among the fixation parameters, where PD patients displayed a higher saccadic intrusion rate (88.7% average increase in PD, F(1) = 20.87, p < 0.001; see also Table 2 and Figure 3A).
Figure 3.
Group differences in eye-tracking parameters between patients with Parkinson’s Disease (PD) and Healthy Controls (HC). (A) Fixation: saccadic intrusion rate. (B) Pro-Saccades: saccades to target, (C) first gain, (D) first gain error, (E) mean velocity, and (F) Anti-Saccades: target recognition rate. Large, large amplitude pro-saccades; short, short amplitude pro-saccades. *p < 0.05, ***p < 0.001 (corrected for multiple comparisons).
The effects of age and group were both significant for the pro-saccade task (F(5) ≥ 1.90, p ≤ 0.034; sex: F(5) = 0.62, p = 0.84, MANCOVA). Post-hoc analyses yielded several significant group differences (Table 3), particularly those relating to the number of saccades required to reach the target (14.8 and 23% increase in PD, for short and long eccentricities, respectively; Figure 3B), latency (9.7 and 7.2% average decrease in PD, for short and long eccentricities, respectively), saccade precision (13.1 and 9.1% average gain decrease in PD, short and long eccentricities, respectively; Figures 3C,D), and mean saccade velocity (20.6% increase in PD, short eccentricity only; Figure 3E) (all F(1) ≥ 6.84, p ≤ 0.015).
Finally, we found no significant effects for group, age, or sex for anti-saccade parameters (all F(5) ≤ 2.13, p ≥ 0.072, MANCOVA; e.g., Figure 3F). Between-group comparisons are reported in Table 4 for transparency, although correcting for multiple comparisons was not deemed necessary in the absence of potential false positives (Type I errors).
Table 4.
Anti-saccade task.
| UPDRS part III correlations | PD/HC comparisons | |||
|---|---|---|---|---|
| Eye-tracking parameter | Correlation coefficient | p (corrected) | F-statistic | p |
| Correct saccade (%) | ρ = −0.083 | 0.537 (0.621) | 2.448 | 0.124 |
| Corrected saccade (%) | ρ = 0.07 | 0.621 (0.621) | 1.254 | 0.267 |
| Target recognition rate | ρ = −0.189 | 0.158 (0.365) | 2.195 | 0.145 |
| Correct direction latency (mean) | ρ = 0.331 | 0.039 (0.195) | 0.045 | 0.832 |
| Incorrect direction latency (mean) | ρ = 0.173 | 0.219 (0.365) | 0.521 | 0.47 |
For each eye-tracking parameter, parameter-UPDRS motor score correlations are shown on the left side of the table (UPDRS part III correlations). Between-group (PD vs. HC) comparisons are shown on the right side of the table (PD/HC comparisons). ρ, Spearman’s rho. F-statistic and p-values for post-hoc analyses are reported.
Logistic regression classification
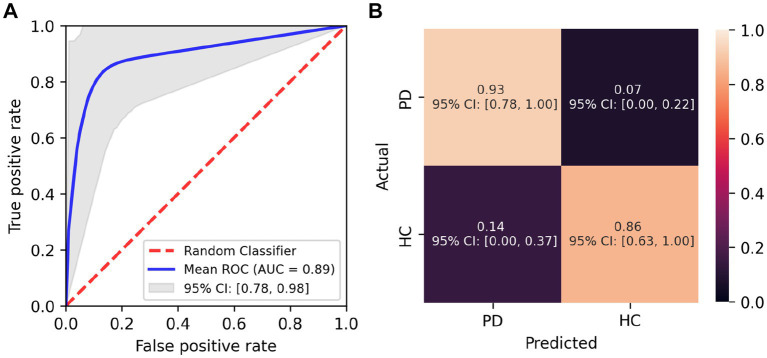
The average receiver operating characteristic (ROC) curve for the logistic regression classifier was computed and had an area under the curve (AUC) of 0.89 (95% CI [0.78,0.98]; Figure 4A). The classifier has a sensitivity of 0.93 (95% CI [0.78, 1.00]) and specificity of 0.86 (95% CI [0.63–1.00]; Figure 4B). Adding age to the logistic regression classifier as an additional parameter did not improve the sensitivity (0.93) or specificity (0.86) of the classifier.
Figure 4.
Performance of the logistic regression classifier. (A) Mean ROC curve for the logistic regression classifier across random subsamples with 95% confidence interval. (B) Confusion matrix for classification of eye tracking parameters as Parkinson’s Disease (PD) and Healthy Controls (HC).
Discussion
The purpose of the present paper was to demonstrate the potential and usefulness of a novel tablet-based software (currently designed for use with iPad Pros) for the assessment of gaze and eye-movement parameters both in research and clinical practice settings. Not only are our oculomotor findings in line with those previously reported (discussed in greater detail below), but by performing a logistic regression classification, we were able to reliably differentiate individuals with PD from healthy participants based on a subset of the collected eye movement data for which there were statistically significant differences between groups. The AUC (0.89), sensitivity (0.93), and specificity (0.86) metrics obtained are highly comparable to previously published studies on oculomotor anomalies in PD using eye movement parameters extracted with conventional eye-tracking equipment (25, 28, 47, 48). These findings highlight the potential for broader application of eye-movement-based monitoring and early diagnosis technologies.
Individual oculomotor findings are very much in line with those previously reported in the scientific literature on oculomotor anomalies specific to PD. Our finding of an increased rate of saccadic intrusions during fixation confirms previous reports (26, 28, 49, 50). With regards to measures of gaze stability, to our knowledge, only one study reported BCEA and horizontal/vertical gaze SD measures in PD patients and found no significant differences with healthy controls (28).
Pro-saccades have been more extensively studied in PD than measures of fixation instability. Findings regarding saccade latency have been mixed to date, with several studies finding either no differences between PD patients and controls (47, 51–53), shorter latencies in PD (54) and longer latencies in PD (25, 26, 55). It’s unclear why this discrepancy across studies exists, but it may have to do with the type of eye-tracking technology used; the majority of the studies cited above that found either no difference or shorter latencies in PD used infrared eye-trackers from manufacturers such as Eyelink and Tobii, whereas the majority of studies cited above finding increased latencies used devices from other manufacturers such as Micromedical Technologies and Interacoustics, and EyeBrain. To make a more definite statement as to why this would be the case, however, would require a more in-depth investigation that is beyond the scope of the paper.
Similarly, findings regarding peak velocity have been mixed, with a few studies finding faster velocities in PD (24, 56), whereas most other studies found no differences between PD and healthy control (47, 51, 54, 57, 58). In the present study, we find peak and mean velocities to be significantly increased in PD patients (only uncorrected value of p for peak velocity) for short eccentricity targets only. Combined with the available literature, this finding suggests that peak velocity might normalize in PD with increasing eccentricity.
In contrast to latency and velocity parameters, the literature is quite rich with strong evidence of PD patients requiring multi-step saccades to reach the target (47, 54, 57, 59), which is in line with the present findings reported here, where the largest group difference amongst all parameters investigated concerned the average number of saccades required to reach the pro-saccade targets. In addition, our findings here indicate that the first saccade towards the target (for both short and large eccentricity targets) was closer to the target in HC.
With regards to anti-saccade parameters, although several studies report a reduced proportion of correct initial direction (or an increase in error rate) in PD patients (25, 52, 60–62), several other studies found no such difference (63–65). Similarly, while several studies have identified slower latencies for correct (52, 61, 63), others found no group differences (60, 62, 66).
Taking a closer look at the reported findings in the literature, it can be observed that many of those studies that identified a difference in the correction direction rate found no differences regarding latency (60, 62, 66), and vice-versa (25, 63, 65), indicating either variability in the PD population or that the differences measured could be specific to the anti-saccadic task parameters (e.g., eccentricity of the targets or inter-trial interval). A recent meta-analysis on antisaccade parameters in PD confirmed that, although both antisaccade latency and error rate are significantly increased in PD, these effects are strongly moderated by disease duration and disease severity – as assessed by UPDRS score and H&Y stages (67). This likely explains the absence of significant findings regarding the antisaccade latency and error rate in the present study, as the majority of our PD participants would fall in the mild or moderate category based on their MDS-UPDRS score part III (68).
Few studies to date, to our knowledge, have investigated the relationship between disease severity in PD, such as measured by the MDS-UPDRS motor score and the magnitude of gaze and eye movement parameters. These have primarily observed a relationship between the motor score and pro-saccade latency (26, 48), prosaccade gain (25), anti-saccade latency (24) and anti-saccade direction rate (23). However, (65) found no significant correlation between anti-saccade latency or pro-saccade latency and the UPDRS motor score.
In the present study, we only found a significant UPDRS motor score correlation with pro-saccade gain (large eccentricities) and the number of saccades to reach the target (large eccentricities). We also found a significant correlation between the UPDRS motor score and the pro-saccade time-to-target parameter (large eccentricities), which in many ways represents a composite measure of the latency and the mean velocity of the saccade. With regards to the anti-saccade task parameters, it is unclear why the discrepancies between the cited literature and our study exist. One obvious difference between our PD patient sample is that the error rate was significantly larger in our study [61% vs. 15% in (23)] despite anti-saccade targets being positioned at a similar eccentricity. Finally, a limitation of the present study, however, is the absence of cognitive measures (e.g., MMSE of MoCA) that could have allowed us to further quantify disease severity and investigate associated oculomotor anomalies such as previously done (27–30).
Despite the promise of eye tracking for both research and clinical settings, applications have been limited by the high cost of eye trackers and their inability to scale due to the use of specialized hardware. Being able to make use of the embedded cameras of mobile devices allows us to overcome these cost and scalability barriers by democratizing access to eye-tracking assessment tools. In particular, we believe tablet-based tools have the potential to aid with disease progression monitoring via the assessment of the integrity of the oculomotor system, as demonstrated by the strong relationships found between various eye-movement parameters and clinical status. Such tools could help clinicians monitor changes to disease status, disease progress, or response to treatment remotely without the need for an in-clinic visit until a change in associated eye movement parameters is detected by the software. This approach would be akin to current alternative strategies being developed to remotely monitor motor function & dysfunction with gyroscope/accelerometer-based wearable technologies (69, 70) and speech analysis using machine learning techniques (71, 72).
An advantage of eye-movement-based monitoring technologies, as opposed to wearable technologies, for example, is that they could potentially be more easily scaled to other neurodegenerative disorders. Indeed, as highlighted earlier, several eye-movement anomalies have been tied to AD (11, 14) and MS (8, 18), for instance, and several measured parameters have been shown to highly correlate with their respective cognitive (20–22) or clinical disease scales (31–35).
To conclude, in this study we show that a novel tablet-based eye-tracking technology can reliably identify differences in subtle eye movement abnormalities in PD, and that specific oculomotor parameters were found to significantly correlate with the disease severity stage. Moreover, we were able to reliably differentiate individuals with PD from healthy participants based on a subset of the collected eye movement data. These findings suggest the potential for broader application of eye-movement-based monitoring technologies in neurodegenerative disorders, such as MS and AD, holding promise for their future role in facilitating early diagnosis and monitoring of disease progression. Next steps include validating the technology within a distinct neurodegenerative disorder with known oculomotor impairments and to establish links between oculomotor parameters and clinical measures of cognition. This tablet-based tool has the potential to rapidly scale eye-tracking use and usefulness in both research and clinical settings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Veritas Independent Review Board and the Montreal University Health Center (MUHC) Research Ethics Board gave ethical approval of this work. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ÉV-S and SD: conceptualization and supervision. PV, JC-F, and NK: formal analysis. ÉV-S: funding acquisition. JC-F and SD: investigation. ÉV-S, PV, and DG: methodology. JC-F and NK: visualization. PV: writing – original draft preparation. ÉV-S, PV, DG, JC-F, and SD: writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by Natural Science and Engineering Research Council of Canada, grant RGPIN-2019-04761.
Conflict of interest
ÉV-S is a co-founder of Innodem Neurosciences, which developed the Eye-Tracking Neurological Assessment (ETNA™) technology used in this study. PV has ownership options in Innodem Neurosciences. JC-F is a part-time employee of Innodem Neurosciences and NK is a research intern at Innodem Neurosciences. SD has previously served as an advisor to Innodem Neurosciences.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express our sincere gratitude to Edward Fon, Clotilde Degroot, and Roozbeh Sattari from the Quebec Parkinson Network for their valuable contributions to this research project. They also thank Jimmy Lai, Redouane Allache, Sarah Fon, Lydia Ouellet, Fama Tounkara, and Thedora Yaneva for their help with data acquisition.
References
- 1.Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. (2013) 9:74–85. doi: 10.1038/nrneurol.2012.273.23338283 [DOI] [PubMed] [Google Scholar]
- 2.Bueno APA, Sato JR, Hornberger M. Eye tracking – the overlooked method to measure cognition in neurodegeneration? Neuropsychologia. (2019) 133:107191. doi: 10.1016/j.neuropsychologia.2019.107191, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Crotty GF, Chwalisz BK. Ocular motor manifestations of movement disorders. Curr Opin Ophthalmol. (2019) 30:443–8. doi: 10.1097/ICU.0000000000000605, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York, NY, USA: Oxford University Press; (2006). [Google Scholar]
- 5.Terao Y, Fukuda H, Hikosaka O. What do eye movements tell us about patients with neurological disorders?- an introduction to saccade recording in the clinical setting. Proc Jpn Acad Ser B Phys Biol Sci. (2017) 93:772–801. doi: 10.2183/pjab.93.049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goffart L, Bourrelly C, Quinton JC. Neurophysiology of visually guided eye movements: critical review and alternative viewpoint. J Neurophysiol. (2018) 120:3234–45. doi: 10.1152/jn.00402.2018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorges M, Pinkhardt EH, Kassubek J. Alterations of eye movement control in neurodegenerative movement disorders. J Ophthalmol. (2014) 2014:658243: 1–11. doi: 10.1155/2014/658243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serra A, Chisari CG, Matta M. Eye movement abnormalities in multiple sclerosis: pathogenesis, modeling, and treatment. Front Neurol. (2018) 9:31. doi: 10.3389/fneur.2018.00031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding J, Clough M, Beh S, Millist L, Sears D, Frohman AN, et al. Ocular motor signatures of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. (2015) 11:637–45. doi: 10.1038/nrneurol.2015.174, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Yang Z, Gu Y, Liu H, Wang P. The effectiveness of eye tracking in the diagnosis of cognitive disorders: a systematic review and meta-analysis. PLoS One. (2021) 16:e0254059. doi: 10.1371/journal.pone.0254059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniades CA, Kennard C. Ocular motor abnormalities in neurodegenerative disorders. Eye (Lond). (2014) 29:200–7. doi: 10.1038/eye.2014.276, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. (2008) 131:1268–81. doi: 10.1093/brain/awn047, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoula Z, Yang Q, Otero-Millan J, Xiao S, Macknik SL, Lang A, et al. Distinctive features of microsaccades in Alzheimer’s disease and in mild cognitive impairment. Age. (2014) 36:535–43. doi: 10.1007/s11357-013-9582-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakespeare TJ, Kaski D, Yong KX, Paterson RW, Slattery CF, Ryan NS, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. (2015) 138:1976–91. doi: 10.1093/brain/awv103, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorges M, Müller HP, Lulé D, LANDSCAPE Consortium, Pinkhardt EH, Ludolph AC, Kassubek J . The association between alterations of eye movement control and cerebral intrinsic functional connectivity in Parkinson’s disease. Brain Imaging Behav. (2016) 10:79–91. doi: 10.1007/s11682-015-9367-7 [DOI] [PubMed] [Google Scholar]
- 16.Terao Y, Fukuda H, Ugawa Y, Hikosaka O. New perspectives on the pathophysiology of Parkinson's disease as assessed by saccade performance: a clinical review. Clin Neurophysiol. (2013) 124:1491–506. doi: 10.1016/j.clinph.2013.01.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frei K. Abnormalities of smooth pursuit in Parkinson's disease: a systematic review. Clin Park Relat Disord. (2020) 4:100085. doi: 10.1016/j.prdoa.2020.100085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lizak N, Clough M, Millist L, Kalincik T, White OB, Fielding J. Impairment of smooth pursuit as a marker of early multiple sclerosis. Front Neurol. (2016) 7:206. doi: 10.3389/fneur.2016.00206, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford TJ, Higham S, Renvoize T, Patel J, Dale M, Suriya A, et al. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer's disease. Biol Psychiatry. (2005) 57:1052–60. doi: 10.1016/j.biopsych.2005.01.017, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Noiret N, Carvalho N, Laurent É, Chopard G, Binetruy M, Nicolier M, et al. Saccadic eye movements and attentional control in Alzheimer's disease. Arch Clin Neuropsychol. (2018) 33:1–13. doi: 10.1093/arclin/acx044, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Simó-Servat O, Ciudin A, Ortiz-Zúñiga ÁM, Hernández C, Simó R. Usefulness of eye fixation assessment for identifying type 2 diabetic subjects at risk of dementia. J Clin Med. (2019) 8:59. doi: 10.3390/jcm8010059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Wang T, Su N, Xiao S, Kapoula Z. Specific saccade deficits in patients with Alzheimer's disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age (Dordr). (2013) 35:1287–98. doi: 10.1007/s11357-012-9420-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT. Antisaccades and executive dysfunction in early drug-naive Parkinson's disease: the discovery study. Mov Disord. (2015) 30:843–7. doi: 10.1002/mds.26134, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Buchanan T, Kennard C, Fitz Gerald JJ, Antoniades CA. The effect of levodopa on saccades – Oxford quantification in parkinsonism study. Parkinsonism Relat Disord. (2019) 68:49–56. doi: 10.1016/j.parkreldis.2019.09.029, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Waldthaler J, Tsitsi P, Svenningsson P. Vertical saccades and antisaccades: complementary markers for motor and cognitive impairment in Parkinson's disease. NPJ Parkinsons Dis. (2019) 5:11. doi: 10.1038/s41531-019-0083-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang B, Ren Q, Zhong Q, Li Y, Liu G, et al. Eye movement especially vertical oculomotor impairment as an aid to assess Parkinson's disease. Neurol Sci. (2021) 42:2337–45. doi: 10.1007/s10072-020-04796-6, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M. Delayed saccade to perceptually demanding locations in Parkinson's disease: analysis from the perspective of the speed-accuracy trade-off. Neurol Sci. (2016) 37:1841–8. doi: 10.1007/s10072-016-2678-7, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Tsitsi P, Benfatto MN, Seimyr GÖ, Larsson O, Svenningsson P, Markaki I. Fixation duration and pupil size as diagnostic tools in Parkinson's disease. J Parkinsons Dis. (2021) 11:865–75. doi: 10.3233/JPD-202427, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macaskill MR, Graham CF, Pitcher TL, Myall DJ, Livingston L, van Stockum S, et al. The influence of motor and cognitive impairment upon visually-guided saccades in Parkinson’s disease. Neuropsychologia. (2012) 50:3338–47. doi: 10.1016/j.neuropsychologia.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 30.Waldthaler J, Stock L, Sommerkorn J, Krüger-Zechlin C, Timmermann L. Antisaccade latency is sensitive to longitudinal change of motor and cognitive symptoms in Parkinson’s disease. Mov Disord. (2021b) 36:266–8. doi: 10.1002/mds.28374, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Gajamange S, Shelton A, Clough M, White O, Fielding J, Kolbe S. Functional correlates of cognitive dysfunction in clinically isolated syndromes. PLoS One. (2019) 14:e0219590. doi: 10.1371/journal.pone.0219590, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolbe SC, Kilpatrick TJ, Mitchell PJ, White O, Egan GF, Fielding J. Inhibitory saccadic dysfunction is associated with cerebellar injury in multiple sclerosis. Hum Brain Mapp. (2014) 35:2310–9. doi: 10.1002/hbm.22329, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nygaard GO, de Rodez Benavent SA, Harbo HF, Laeng B, Sowa P, Damangir S, et al. Eye and hand motor interactions with the symbol digit modalities test in early multiple sclerosis. Mult Scler Relat Disord. (2015) 4:585–9. doi: 10.1016/j.msard.2015.08.003, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Polet K, Hesse S, Cohen M, Morisot A, Joly H, Kullmann B, et al. Video-oculography in multiple sclerosis: links between oculomotor disorders and brain magnetic resonance imaging (MRI). Mult Scler Relat Disord. (2020) 40:101969. doi: 10.1016/j.msard.2020.101969 [DOI] [PubMed] [Google Scholar]
- 35.Sheehy CK, Bensinger ES, Romeo A, Rani L, Stepien-Bernabe N, Shi B, et al. Fixational microsaccades: a quantitative and objective measure of disability in multiple sclerosis. Mult Scler. (2020) 26:343–53. doi: 10.1177/1352458519894712, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Armstrong RA. Oculo-visual dysfunction in Parkinson's disease. J Parkinsons Dis. (2015) 5:715–26. doi: 10.3233/JPD-150686, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalkias E, Topouzis F, Tegos T, Tsolaki M. The contribution of ocular biomarkers in the differential diagnosis of Alzheimer's disease versus other types of dementia and future prospects. J Alzheimers Dis. (2021) 80:493–504. doi: 10.3233/JAD-201516, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Kassavetis P, Kaski D, Anderson T, Hallett M. Eye movement disorders in movement disorders. Mov Disord Clin Pract. (2022) 9:284–95. doi: 10.1002/mdc3.13413, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bott N, Madero EN, Glenn J, Lange A, Anderson J, Newton D, et al. Device-embedded cameras for eye tracking-based cognitive assessment: validation with paper-pencil and computerized cognitive composites. J Med Internet Res. (2018) 20:e11143. doi: 10.2196/11143, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bott NT, Lange A, Rentz D, Buffalo E, Clopton P, Zola S. Web camera based eye tracking to assess visual memory on a visual paired comparison task. Front Neurosci. (2017) 11:370. doi: 10.3389/fnins.2017.00370, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haque RU, Pongos AL, Manzanares CM, Lah JJ, Levey AI, Clifford GD. Deep convolutional neural networks and transfer learning for measuring cognitive impairment using eye-tracking in a distributed tablet-based environment. IEEE Trans Biomed Eng. (2021) 68:11–8. doi: 10.1109/TBME.2020.2990734, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas-Cuentas NI, Roman-Gonzalez A, Gilman RH, Barrientos F, Ting J, Hidalgo D, et al. Developing an eye-tracking algorithm as a potential tool for early diagnosis of autism spectrum disorder in children. PLoS One. (2017) 12:e0188826. doi: 10.1371/journal.pone.0188826, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. (2007) 22:41–7. doi: 10.1002/mds.21198, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 45.Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. (1985) 58:455–72. doi: 10.1007/BF00235863, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 47.Blekher T, Weaver M, Rupp J, Nichols WC, Hui SL, Gray J, et al. Multiple step pattern as a biomarker in Parkinson disease. Parkinsonism Relat Disord. (2009) 15:506–10. doi: 10.1016/j.parkreldis.2009.01.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Yan A, Liu B, Wan Y, Zhao Y, Liu Y, et al. Oculomotor performances are associated with motor and non-motor symptoms in Parkinson's disease. Front Neurol. (2018) 9:960. doi: 10.3389/fneur.2018.00960, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altiparmak UE, Eggenberger E, Coleman A, Condon K. The ratio of square wave jerk rates to blink rates distinguishes progressive supranuclear palsy from Parkinson disease. J Neuroophthalmol. (2006) 26:257–9. doi: 10.1097/01.wno.0000249326.65227.2e, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Otero-Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez-Conde S. Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: from microsaccades to square-wave jerks. PLoS One. (2013) 8:e58535. doi: 10.1371/journal.pone.0058535, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gitchel GT, Wetzel PA, Baron MS. Pervasive ocular tremor in patients with Parkinson disease. Arch Neurol. (2012) 69:1011–7. doi: 10.1001/archneurol.2012.70 [DOI] [PubMed] [Google Scholar]
- 52.Nagai K, Kaneko Y, Suzuki M, Teramoto H, Morita A, Kamei S, et al. Multimodal visual exploration disturbances in Parkinson's disease detected with an infrared eye-movement assessment system. Neurosci Res. (2020) 160:50–6. doi: 10.1016/j.neures.2019.11.003, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Perkins JE, Janzen A, Bernhard FP, Wilhelm K, Brien DC, Huang J, et al. Saccade, pupil, and blink responses in rapid eye movement sleep behavior disorder. Mov Disord. (2021) 36:1720–6. doi: 10.1002/mds.28585, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Wang X, Ma D, Jiang Y, Li F, Sun Y, et al. The differential diagnostic value of a battery of oculomotor evaluation in Parkinson's disease and multiple system atrophy. Brain Behav. (2021) 11:e02184. doi: 10.1002/brb3.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L, Wang Q, Zhao L, Jiang CY, Xu Q, Wu SC, et al. Clinical and oculomotor correlates with freezing of gait in a Chinese cohort of Parkinson's disease patients. Front Aging Neurosci. (2020) 12:237. doi: 10.3389/fnagi.2020.00237, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grillini A, Renken RJ, Vrijling ACL, Heutink J, Cornelissen FW. Eye movement evaluation in multiple sclerosis and Parkinson's disease using a standardized oculomotor and neuro-ophthalmic disorder assessment (SONDA). Front Neurol. (2020) 11:971. doi: 10.3389/fneur.2020.00971, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorges M, Müller HP, Lulé D, Ludolph AC, Pinkhardt EH, Kassubek J. Functional connectivity within the default mode network is associated with saccadic accuracy in Parkinson's disease: a resting-state FMRI and videooculographic study. Brain Connect. (2013) 3:265–72. doi: 10.1089/brain.2013.0146, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Stuart S, Lawson RA, Yarnall AJ, Nell J, Alcock L, Duncan GW, et al. Pro-Saccades Predict Cognitive Decline in Parkinson’s Disease: ICICLE-PD. Mov Disord. (2019) 34:1690–1698. doi: 10.1002/mds.27813 [DOI] [PubMed] [Google Scholar]
- 59.Shaikh AG, Ghasia FF. Saccades in Parkinson's disease: hypometric, slow, and maladaptive. Prog Brain Res. (2019) 249:81–94. doi: 10.1016/bs.pbr.2019.05.001, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Barbosa P, Kaski D, Castro P, Lees AJ, Warner TT, Djamshidian A. Saccadic direction errors are associated with impulsive compulsive behaviours in Parkinson's disease patients. J Parkinsons Dis. (2019) 9:625–30. doi: 10.3233/JPD-181460, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Terao Y, Tokushige SI, Inomata-Terada S, Fukuda H, Yugeta A, Ugawa Y. Differentiating early Parkinson's disease and multiple system atrophy with parkinsonism by saccade velocity profiles. Clin Neurophysiol. (2019) 130:2203–15. doi: 10.1016/j.clinph.2019.09.004, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Gorges M, Maier MN, Rosskopf J, Vintonyak O, Pinkhardt EH, Ludolph AC, et al. Regional microstructural damage and patterns of eye movement impairment: a DTI and video-oculography study in neurodegenerative parkinsonian syndromes. J Neurol. (2017) 264:1919–1928. doi: 10.1007/s00415-017-8579-8 [DOI] [PubMed] [Google Scholar]
- 63.Ewenczyk C, Mesmoudi S, Gallea C, Welter ML, Gaymard B, Demain A, et al. Antisaccades in Parkinson disease: a new marker of postural control? Neurology. (2017) 88:853–61. doi: 10.1212/WNL.0000000000003658 [DOI] [PubMed] [Google Scholar]
- 64.Pagonabarraga J, Horta-Barba A, Busteed L, Bejr-Kasem H, Illán-Gala I, Aracil-Bolaños I, et al. Quantitative evaluation of oculomotor disturbances in progressive supranuclear palsy. Parkinsonism Relat Disord. (2021) 85:63–8. doi: 10.1016/j.parkreldis.2021.03.002, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Visser F, Bour LJ, Lee YX, Ten Brinke TR, van Rootselaar AF. Eye movement abnormalities in essential tremor versus tremor dominant Parkinson's disease. Clin Neurophysiol. (2019) 130:683–91. doi: 10.1016/j.clinph.2019.01.026, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Ouerfelli-Ethier J, Elsaeid B, Desgroseilliers J, Munoz DP, Blohm G, Khan AZ. Anti-saccades predict cognitive functions in older adults and patients with Parkinson’s disease. PLoS One. (2018) 13:e0207589. doi: 10.1371/journal.pone.0207589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldthaler J, Stock L, Student J, Sommerkorn J, Dowiasch S, Timmermann L. Antisaccades in Parkinson's disease: a meta-analysis. Neuropsychol Rev. (2021a) 31:628–42. doi: 10.1007/s11065-021-09489-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martínez-Martín P, Rodríguez-Blázquez C, Alvarez M, Arakaki T, Arillo VC, Chaná P, et al. Parkinson's disease severity levels and MDS-unified Parkinson's disease rating scale. Parkinsonism Relat Disord. (2015) 21:50–4. doi: 10.1016/j.parkreldis.2014.10.026, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez-Molinero A, Pérez-López C, Samà A, de Mingo E, Rodríguez-Martín D, Hernández-Vara J, et al. A kinematic sensor and algorithm to detect motor fluctuations in Parkinson disease: validation study under real conditions of use. JMIR Rehabil Assist Technol. (2018) 5:e8. doi: 10.2196/rehab.8335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tripoliti EE, Tzallas A, Tsipouras MG, Rigas G, Bougia P, Leontiou M, et al. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput Methods Prog Biomed. (2013) 110:12–26. doi: 10.1016/j.cmpb.2012.10.016, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Almeida JS, Filho PPR, Carneiro T, Wei W, Damaševičius R, Maskeliunas R, et al. Detecting Parkinson’s disease with sustained phonation and speech signals using machine learning techniques. Pattern Recogn Lett. (2019) 125:55–62. doi: 10.1016/j.patrec.2019.04.005 [DOI] [Google Scholar]
- 72.Rahman A, Rizvi SS, Khan A, Abbasi AA, Khan SU, Chung T-S. Parkinson’s disease diagnosis in Cepstral domain using MFCC and dimensionality reduction with SVM classifier. Mob Inf Syst. (2021) 2021:1–10. doi: 10.1155/2021/8822069 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.