Abstract
Inflammatory bowel disease (IBD) is a chronic relapsing gastrointestinal disorder, while the treatment effect is not satisfactory. Immune responsive gene 1 (IRG1) is a highly expressed gene in macrophage in response to inflammatory response and catalyzes the production of itaconate. Studies have reported that IRG1/itaconate has a significant antioxidant effect. This study aimed to investigate the effect and mechanism of IRG1/itaconate on dextran sulfate sodium (DSS)-induced colitis in vivo and in vitro. In vivo experiments, we found IRG1/itaconate exerted protective effects against acute colitis by increasing mice weight, the length of colon, reducing disease activity index and colonic inflammation. Meanwhile, IRG1 deletion aggravated the macrophages/CD4+/CD8+ T-cell accumulation, and increased the release of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), IL-6, the activation of nuclear factor-κB (NF-κB)/mitogen-activated protein kinase (MAPK) signaling pathway, and gasdermin D (GSDMD) mediated pyroptosis. Four-octyl itaconate (4-OI), a derivative of itaconate, attenuated these changes, therefore relieved DSS-induced colitis. In vitro experiment, we found 4-OI inhibited the reactive oxygen species production, thereby inhibiting the activation of MAPK/NF-κB signaling pathway in RAW264.7 and murine bone-marrow-derived macrophages. Simultaneously, we found 4-OI inhibited caspase1/GSDMD-mediated pyroptosis to reduce the release of cytokines. Finally, we found anti-TNF-α agent reduced the severity of DSS-induced colitis and inhibited gasdermin E (GSDME)-mediated pyroptosis in vivo. Meanwhile, our study revealed that 4-OI inhibited caspase3/GSDME-mediated pyroptosis induced by TNF-α in vitro. Taken together, IRG1/itaconate exerted a protective role in DSS-induced colitis by inhibiting inflammatory response and GSDMD/GSDME-mediated pyroptosis, which could be a promising candidate for IBD therapy.
Keywords: Colitis, Gasdermin D, Gasdermin E, Inflammation, IRG1
Introduction
Inflammatory bowel disease (IBD) is a chronic relapsing gastrointestinal disorder that mainly includes ulcerative colitis and Crohn's disease.1, 2, 3 Ulcerative colitis mainly affects the colon, and the typically clinical symptoms are mucopurulent bloody stools, abdominal pain, weight loss, and limb weakness.4,5 In recent years, the incidence of IBD has been increasing year by year. However, the exact pathogenesis of IBD has not yet been clarified, and genetic and environmental factors are recognized as the main pathogenic factors, and are closely related to intestinal immunoregulatory disorders.6,7 At the same time, chronic IBD is highly correlated with the occurrence of colorectal cancer, and inflammation–abnormal crypt–adenoma–cancer is the basic path of colitis-related colorectal cancer.8,9 IBD that are not effectively treated will increase the risk of colorectal cancer. Therefore, IBD treatment is one of the current research hotspots and is significant for the prevention and treatment of colorectal cancer.
Intestinal epithelial cells play a crucial role in mucosal immune response and are also the main components of the intestinal barrier, which prevent bacteria and other antigens from entering the circulatory system from the intestinal lumen.10,11 When IBD persists, it disrupts the intestinal barrier, culminating in erosion of the intestinal epithelium, ulceration, and decreased defensin production, resulting in massive cellular (neutrophils, macrophages, dendritic cells, and natural killer cells) infiltration of the lamina propria.12 Meanwhile, studies have shown that the expressions of nuclear factor-κB (NF-κB) is significantly increased in macrophages of patients with IBD.13,14 NF-κB is an important transcription factor involved in inflammation and immune response. Many molecules are regulated by NF-κB, such as tumor necrosis factor-α (TNF-α), interleukin (IL)- 1β, IL-6, and iNOS.15,16 Elevated expression of NF-κB in macrophages promotes the increased expression of proinflammatory cytokines in colonic epithelial cells, which in turn enhances the intestinal inflammatory response.
In recent years, many studies have demonstrated that pyroptosis plays an important role in the development of IBD.17, 18, 19 Pyroptosis is an inflammation-inducing programmed cell death, characterized by rapid membrane rupture and release of inflammation-inducing cellular contents. NOD-like receptor family pyrin domain containing 3 (NLRP3) and caspase-1 activities are increased in macrophages of patients with active IBD, and the production of IL-1β and IL-18 is increased.20 NLRP3 inflammasome can initiate pyroptosis by cleaving gasdermin D (GSDMD) to form N-terminal domain of GSDMD (GSDMD-N). GSDMD-N can cause cell membranes to rupture and inflammatory factors (IL-1β and IL-18) to be released, which can further exacerbate the inflammatory response and ultimately lead to intestinal epithelial damage.21,22 In addition, GSDME, another member of the gasdemin family, can also cause pyroptosis. Unlike GSDMD, GSDME is specifically cleaved by caspase-3, and caspase-3 can be activated by TNF-α.23,24 Currently, TNF-α has been used as a classical biological therapeutic target in the clinical treatment of Crohn's disease.25,26 Owing to the high levels of TNF-α produced in DSS model, we speculate GSDME-mediated pyroptosis play a critical role in this model.
Immune responsive gene 1 (IRG1) is a highly expressed gene in macrophage mitochondria in a pro-inflammatory state, which catalyzes the production of itaconate. IRG1/itaconate has a significant antioxidant effect. Itaconate is electrophilic, easily binds to the cysteine residues of KEAP1 and promotes its alkylation, so that nuclear factor E2-related factor 2 (Nrf2) dissociates from Keap1 and is activated. Nrf2 continues to activate and initiate some column downstream pathways, such as inhibiting reactive oxygen species (ROS) production, further inhibiting the NF-κB pathway, and reducing oxidative stress.27 It has also been reported that the itaconate derivative 4-octyl-itaconate (4-OI) has an inhibitory effect on macrophage pyroptosis and reduces the release of inflammatory factors such as IL-1β.28 In addition, our previous study has showed 4-OI significantly reduced the release of TNF-α.29 Whether 4-OI can inhibit GSDME-mediated pyroptosis to alleviate DSS-colitis remains undetermined. In this study, we therefore used DSS to create an IBD model to investigate the protective effect of IRG1/itaconate.
Materials and methods
Drugs and chemicals
Dextran sulfate sodium salt (DSS, 36,000–50,000 Da) was purchased from MP Biomedicals (Irving, CA, USA). 4-OI, etanercept (TNF-α inhibitor), and nigericin (NG) were purchased from Med Chem Express (New Jersey, USA). Lipopolysaccharide (LPS) was purchased from Sigma–Aldrich (St. Louis, MO). Z-DEVD-FMK was purchased from Selleck.cn (Houston, UAS). Murine TNF-α and G-CSF were purchased from PeproTech (Rocky Hill, UAS). Myeloperoxidase (MPO) assay kit (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China) was used to detect serum MPO. Enzyme-linked immunosorbent assay (ELISA) kits (DAKEWE Bioengineering, Shenzhen, China) was used to detect levels of serum IL-1β, TNF-α, and IL-6. Antibodies against Nrf2 (12721), NF-κB p65 (8242), phospho–NF–κB p65 (3033), ERK1/2 (4695), phospho- ERK1/2 (8544), p38 (8690), phospho-p38 (9216), cleaved-caspase-3 (9664), and GSDMD (36,425) were purchased from cell signaling Technology (Beverly, MA, USA). Antibodies against F4/80 (ab6640) and DFNA5/GSDME (ab215191) were purchased from Abcam (USA). Antibodies against heme oxygenase-1 (HO-1, 27,282–1) and GAPDH (10,494–1) were purchased from Proteintech (Wuhan, China). Antibodies against JNK (A4867), phospho-JNK (AP0631), and NLRP3 (A5652) were purchased from ABclonal (Wuhan, China). PE anti-mouse F4/80 flow antibodies was purchased from Biolegend (California, USA).
Animals and experimental procedures
Male 6–10-week-old C57BL/6J wild-type (WT) and IRG1−/− mice on the C57BL/6J background were purchased from China Three Gorges University (Hubei, China) and Cyagen company, respectively. Animals were housed in cages at around 22 °C under a 12-h light/dark cycle, and they had free access to food and water. The experimental procedures were approved by the Ethical Committee for the use of experimental animals of the Huazhong University of Science and Technology. To explore the effect of IRG1/itaconate in protecting against DSS-induced IBD, mice were divided into the following four groups (n = 6 each group): (1) WT: WT mice were administrated with normal water; (2) DSS+WT: WT mice were administrated with 3% DSS via drinking water; (3) DSS+IRG1−/−; (4) DSS + IRG1−/−+4-OI: IRG1−/− mice were administrated with 3% DSS and 4-OI (50 mg/kg) via intraperitoneal injection. To further explore the protective role of anti-TNF-α agent, IRG1−/− mice were random divided two groups (n = 5 each group): (1) DSS; (2) DSS + etanercept: Etanercept (10 mg/kg) was administered to mice via intraperitoneal injection. The body weight and disease activity index (DAI) of mice were recorded every day for 7 days. At last, the mice were anesthetized, the blood of the eyeball were collected. Their intestines and spleens were also collected after being sacrificed.
Histological analysis and inflammation scores
Colon tissue were immediately fixed in 4% formaldehyde solution and made into wax blocks. Then wax blocks were stained with hematoxylin and eosin (H&E) and Alcian blue-periodic acid schiff (AB-PAS). Histopathological inflammation scores were determined by loss of epithelial surface, infiltration of immunocytes, and destruction of crypt.
Immunofluorescence staining
Colon tissue sections were treated accordingly as described previously.30 Anti-F4/80 was used and DAPI staining solution was added to label the nucleus.
MPO assay
MPO assay kit was used to detect MPO activity in serum according to the manufacturer's instruction.
ELISA assay
The serum levels of IL-1β, TNF-α, and IL-6 were detected by ELISA kits. Briefly, 100 μL of cytokine standard and (or) diluted serum were added to the wells, then 50 μL of biotinylated antibody was added, and plates were incubated at 37 °C for 90 min. After washing for four times, 100 μL of streptavidin-HRP was added, and plates were incubated at 37 °C for 30 min. After washing for four times again, 100 μL of TMB was added. After incubation at 37 °C for 10–15 min, 100 μL of stop solution was added. The absorbance was test at a wavelength of 450 nm within 10 min.
ROS assay
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) (Beyotime, Wuhan, China) was used as a fluorescent probe to measure the level of ROS production.
Murine bone-marrow-derived macrophages (BMDM) isolation and differentiation
C57BL/6 mice were sacrificed and disinfected with 75% alcohol. The mouse femur was taken and placed in PBS containing penicillin-streptomycin. The two segments of femur were subtracted with ophthalmic scissors, and then rinsed with a syringe. After centrifugation, the supernatant was discarded. Red blood cell lysate was added, and the supernatant was centrifuged again. RPMI-1640 medium containing double penicillin-streptomycin and 20 ng/mL G-CSF was added and cells were planted on 6-well plates. On the 7th day, the transformation degree of BMDMs was detected by flow cytometry.
Cell culture
RAW264.7 and NCM460 cells were cultured in DMEM with 10% FBS obtained from Gibco-BRL (Grand Island, NY) in a humidified incubator at 37 °C with 5% CO2.
Lactate dehydrogenase (LDH) release assay
The LDH release was detected by LDH Cytotoxicity Assay Kit (Beyotime, Wuhan, China). The methods were followed by previous study.31
Flow cytometry
Cell apoptosis was performed using APC-Annexin V/7-AAD Apoptosis Detection Kit (BioLegend, California, USA). Briefly, cells were washed in PBS for three times, then stained with 5 μL APC-Annexin V and 10 μL 7-AAD for 25 min at room temperature in the dark. The data were analyzed by FlowJo software.
Quantitative real-time PCR (qRT-PCR)
RNA from colon tissue and cells were taken following the instructions, then reverse transcribed into cDNA. qRT-PCR was conducted on a StepOne Plus Real-Time PCR System (Thermo Fisher Scientific) to detect the mRNA level. GAPDH was used as an internal reference, and the fold change was calculated using the 2−ΔΔCt method. The primers used are listed as follows: GAPDH F: CATCACTGCCACCCAGAAGACTG; R: ATGCCAGTGAGCTTCCCGTTCAG; IL-1β F: GGGCCTCAAAGGAAAGAATC; R: TACCAGTTGGGGAACTCTGC; TNF-α F: CCTGTAGCCCACGTCGTAG, R: GGGAGTAGACAAGGTACAACCC; IL-6 F: AGTTGCCTTCTTGGGACTGA, R: TCCACGATTTCCCAGAGAAC; 16sRNA F: GTGGTGCATGGTTGTCGTCA, R: ACGTCGTCCCCACCTTCCTC.
Western blot analysis
The proteins of tissue and cells were extracted, and the protein concentration was determined using a BCA kit (Beyotime, Wuhan, China). Electrophoresis and membrane transfer were performed as previously described.32 The membrane was incubated with the primary and secondary antibody. The membrane was imaged with ECL reagents.
Statistical analysis
The results were expressed as mean ± SD, and two-tailed Student's t-test or one-way ANOVA followed by post-hoc test was used to compare differences. P values less than 0.05 were considered statistically significant; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Results
IRG1 deletion aggravated the severity of DSS-induced acute colitis
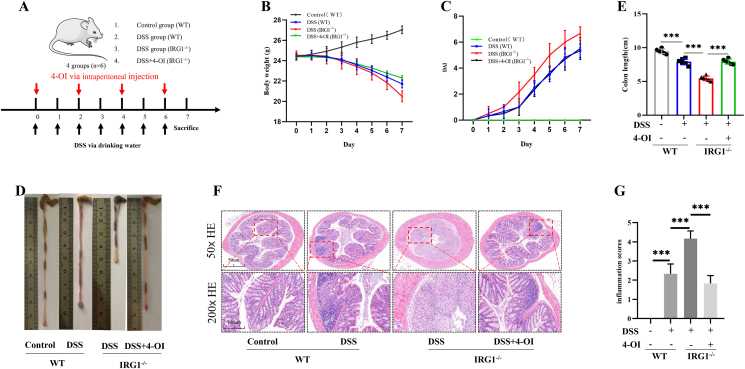
In order to explore the effect of IRG1 deletion on DDS-induced colitis in mice, WT mice and IRG1−/− mice were given different treatments. The treatment methods were shown in Figure 1A. The weight and DAI score of each mouse were measured every day. The weight loss of IRG1−/− mice was more obvious than that of WT mice, and the weight loss of IRG1−/− mice significantly reduced after receiving 4-OI treatment (Fig. 1B). At the same time, as shown in Figure 1C, the mean DAI score of the WT group was significantly lower than that of the IRG1−/− mice group. After receiving 4-OI treatment, the DAI score of the IRG1−/− mice significantly reduced. After treating with DSS for 7 days, the colon was taken. The colon length of the WT mice group was shorter than that of the control group, but it was significantly longer than the IRG1−/− mice group. When mice in the IRG1−/− group received 4-OI treatment, the colon length became significantly longer (Fig. 1D, E). The results of immunohistochemistry also showed that the WT mice group was more infiltrated with inflammatory cells than the control group, while the inflammatory cell infiltration in the IRG1−/− mice group were more obvious. Furthermore, 4-OI significantly alleviated these changes (Fig. 1F). In addition, the inflammation score of the IRG1−/− mice was significantly higher than that of the WT group, and the inflammation score of the IRG1−/− mice significantly reduced after receiving 4-OI treatment (Fig. 1G).
Figure 1.
IRG1 deletion aggravated the severity of DSS-induced acute colitis. (A) The treatment methods. (B) Mice body weight. (C) The mean DAI score. After treating with DSS for 7 days, the colon was taken. (D, E) The colon length of the WT mice and IRG1−/− mice group. (F) The immunohistochemistry of colon. (G) The inflammation scores. Data are shown as the mean ± SD, ∗∗∗P < 0.001.
IRG1 deletion aggravated the macrophages/T-cell accumulation and bacterial translocation in DSS-induced colitis
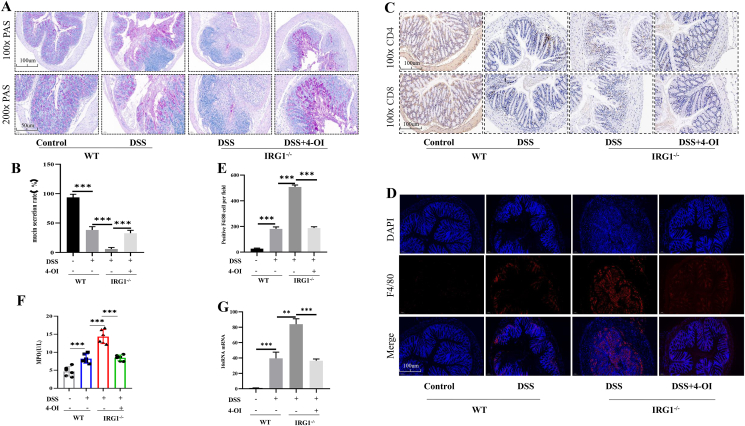
PAS stain showed that DSS obviously destroyed the mucus layer and goblet cells. The mucus layer of IRG1−/− mice was damaged more obviously than that in the WT group, and after 4-OI treatment, the positive rate of PAS staining significantly increased in IRG1−/− mice (Fig. 2A, B). Immunohistochemical staining showed that the infiltration of CD4+/CD8+ T cells in WT mice receiving DSS treatment was more obvious than that of control group. And the CD4+/CD8+ T cells of IRG1−/− mice were more obvious than that of WT group, which were significantly reduced in 4-OI treated mice (Fig. 2C). Next, F4/80 was used to label the intestinal macrophages, and immunofluorescence staining showed that the degree of macrophages infiltration in IRG1−/− group was significantly higher than that of WT mice group. When the IRG1−/− group received 4-OI treatment, the degree of macrophage infiltration significantly reduced (Fig. 2D, E). MPO, a heme protein, is rich in neutrophils. We detected the MPO content in the serum of mice, and the results showed that the level of serum MPO significantly increased with DSS, which was more obvious in the IRG1−/− mice group. When IRG1−/− mice received 4-OI treatment, the serum MPO significantly reduced (Fig. 2F). 16sRNA is present in all bacteria. We used RT-PCR to detect the bacterial content in the spleen. The results showed that DSS caused bacterial translocation, and the bacterial translocation was more obvious in IRG1−/− mice, which decreased after receiving 4-OI treatment (Fig. 2G).
Figure 2.
IRG1 deletion aggravated the macrophages/T-cell accumulation and bacterial translocation. (A, B) PAS stain showed the mucus layer of IRG1−/− mice was damaged more obviously than that in the WT group. (C) Immunohistochemical staining showed the CD4+ and CD8+ T cells of IRG1−/− mice were more obvious than those of WT group. (D, E) Immunofluorescence staining showed that the infiltration of macrophages in IRG1−/− group was significantly higher than that of WT mice group. When the IRG1−/− group received 4-OI treatment, the degree of macrophage infiltration was significantly reduced. (F) The level of serum MPO. (G) The level of 16sRNA in the spleen was detected by RT-PCR. Data are shown as the mean ± SD, ∗∗P < 0.01; ∗∗∗P < 0.001.
IRG1/4-OI inhibited the release of proinflammatory cytokines and the activation of NF-κB signaling pathway
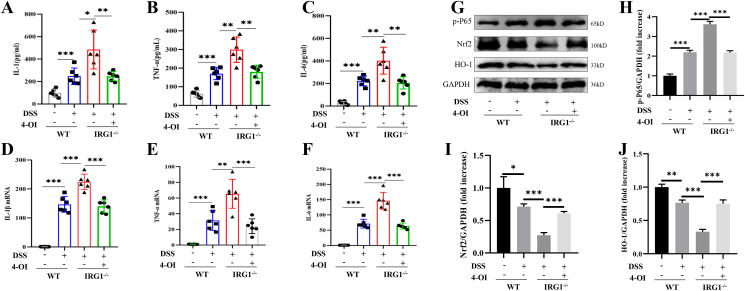
DSS destroys the intestinal epithelium and causes bacterial translocation, which causes obvious oxidative stress in the body, and promotes the release of pro-inflammatory factors. We tested the serum levels of IL-1β, TNF-α, and IL-6, and the results showed that the serum levels of pro-inflammatory factors significantly increased after the mice were treated with DSS. The level of serum IL-1β, TNF-α, and IL-6 in the IRG1−/− mice group were significantly higher than those of the WT group, which significantly decreased after receiving 4-OI treatment (Fig. 3A–C). Next, we extracted the intestinal tissue RNA, and the RT-PCT results also showed that the expressions of IL-1β, TNF-α, and IL-6 mRNA in the IRG1−/− group were significantly higher than those of the WT group, which significantly decreased with 4-OI treatment (Fig. 3D–F). Nrf2/HO-1 and NF-κB signaling pathway are closely related to the occurrence and development of inflammation. Nrf2/HO-1 has the effect of anti-oxidative stress and inhibits inflammation. We extracted intestinal tissue proteins, and the results showed that DSS obviously activated the NF-κB signaling pathway and inhibited the expression of Nrf2/HO-1. Furthermore, the expression of p-p65 in the IRG1−/− group was significantly increased, and the expression of Nrf2/HO-1 protein significantly reduced compared with the WT group (Fig. 3G–J).
Figure 3.
IRG1/4-OI inhibited inflammatory response. (A–C) The serum levels of IL-1β, TNF-α, and IL-6 were detected by ELISA. (D–F) The expressions of IL-1β, TNF-α, and IL-6 mRNA of colon tissue were detected by RT-PCR. (G–J) The expression of p-p65 in the IRG1−/− group was significantly increased, and the expression of Nrf2/HO-1 protein was significantly reduced compared with the WT group, and these changes were significantly reduced after IRG1−/− mice received 4-OI treatment. Data are shown as the mean ± SD, ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
4-OI inhibited the ROS production, thereby inhibiting the activation of MAPK/NF-κB signaling pathway
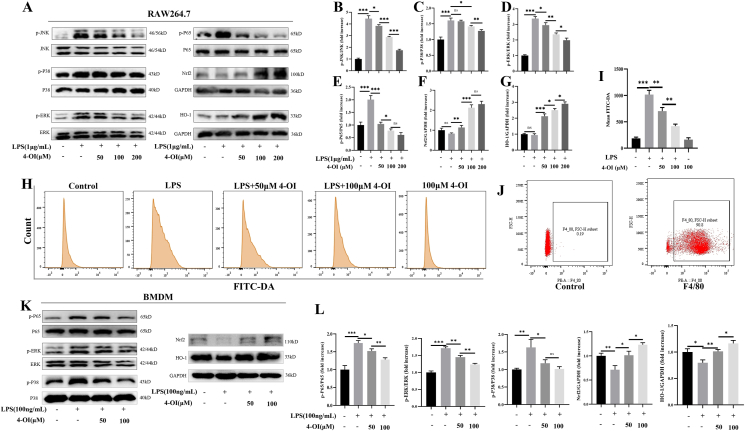
Stimuli such as LPS can obviously activate MAPK/NF-κB signaling pathway. To explore the anti-inflammatory action of 4-OI, RAW264.7 cells were pretreated with 4-OI for 1 h, and then LPS was added for 24 h. Western blot showed LPS significantly activated the MAPK/NF-κB signaling pathway, while 4-OI significantly reduced the expression levels of p-JNK, p-P38, p-ERK, and p-P65, and promoting the expression of Nrf2/HO-1 (Fig. 4A–G). LPS can cause a sharp increase in the level of ROS in the body, which can cause serious damage to the cell structure. Then, we investigated the effects of LPS and 4-OI on ROS. RAW264.7 cells were treated with LPS and 4-OI for 8 h. The results showed that LPS promoted ROS production of macrophage in vitro. After 4-OI treatment, the level of ROS significantly reduced (Fig. 4H, I). These suggested that 4-OI might inhibit the production of ROS by promoting the expression of NRF2/HO-1, thereby inhibiting the MAPK/NF-κB signaling pathway. In order to further verify, we extracted and successfully induced BMDM cells, and the flow cytometry showed that the macrophage purity reached 90% (Fig. 4J). Then we used the same treatment for BMDMs. Western blot showed 4-OI significantly inhibited the expression of p-P38, p-ERK, and p-P65, and promoted the expression of Nrf2/HO-1 (Fig. 4K and L).
Figure 4.
4-OI inhibited the ROS production, thereby inhibiting the activation of MAPK/NF-κB signaling pathway. (A–G) 4-OI significantly reduced the expression levels of p-JNK, p-P38, p-ERK, and p-P65, and promoting the expression of Nrf2/HO-1 in RAW264.7 cells. (H, I) LPS promoted macrophage ROS production and 4-OI inhibited the ROS production in RAW264.7 cells. (J) Flow cytometry results showed that the macrophage purity reached 90%. (K, L) 4-OI significantly inhibited the expression of p-P38, p-ERK, and p-P65, and promote the expression of NRF2/HO-1 in BMDMs. Data are shown as the mean ± SD, ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
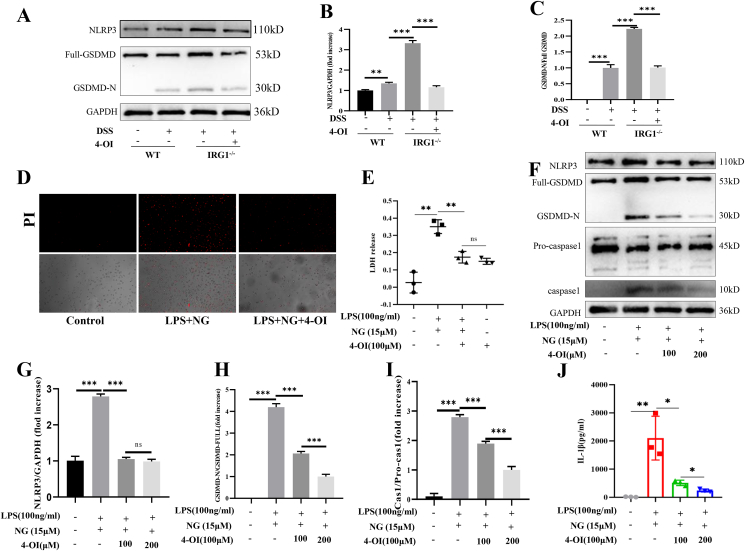
4-OI inhibited GSDMD medicated-pyroptosis in macrophages
GSDMD, one member of the gasdermin family, can cause pyroptosis. During the infection process, the body can activate the inflammasome by cleaving caspase-1. The activated caspase-1 cleaves GSDMD to form holes on the cell membrane. Simultaneously, activated caspase-1 can cleave the IL-1β precursor to produce mature IL-1β, thereby causing inflammation. Therefore, we further explored whether 4-OI can inhibit the production of inflammatory factors by inhibiting macrophage pyroptosis, thereby attenuating DSS-induced colitis. First, we extracted intestinal tissue proteins, and the results showed that the expression of GSDMD-N and NLRP3 were significantly increased after the mice receiving DSS, which was more obvious in the IRG1−/− group. When IRG1−/− mice received 4-OI treatment, the expression of NLRP3 and GSDMD-N was significantly reduced (Fig. 5A–C). Then, we treated BMDM cells with LPS (100 ng/ml), changed the medium and added 4-OI for 45 min before adding NG (15 μM). PI assays showed that 4-OI reduced the PI-positive cells (Fig. 5D). LDH release assay showed that 4-OI inhibited cell death (Fig. 5E). Western blot results showed that 4-OI significantly reduced the expression of caspase-1 and GSDMD-N (Fig. 5F–I). ELISA results showed that 4-OI significantly reduced IL-1β release (Fig. 5J).
Figure 5.
4-OI inhibited GSDMD medicated-pyroptosis in vivo and in vitro.(A–C) 4-OI treatment significantly reduced the expression of NLRP3 and GSDME-N in vivo. (D) PI assays showed that 4-OI reduced the PI-positive cells. (E) The LDH release assay. (F–I) 4-OI significantly reduced the expression of caspase-1 and GSDMD-N. (J) ELISA results showed that 4-OI significantly reduced IL-1β release. Data are shown as the mean ± SD, ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
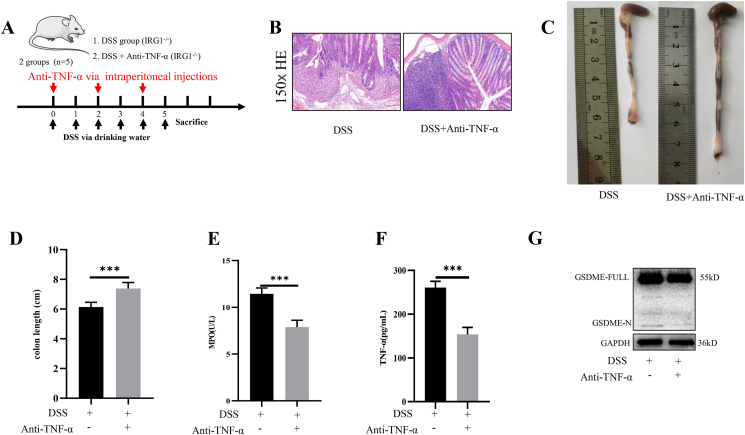
Anti-TNF-α therapy relieved the severity of DSS-induced colitis
The treatment methods were shown in Figure 6A. Immunohistochemical staining showed that etanercept significantly decreased the severity of DSS-induced colitis in IRG1−/− mice (Fig. 6B). The colon length of DSS group was shorter than that of the DSS + anti-TNF-α group (Fig. 6C, D). The level of serum MPO in DSS + anti-TNF-α group was lower than that of DSS group (Fig. 6E). The ELISA assay showed that the level of serum TNF-α significantly decreased with anti-TNF-α therapy (Fig. 6F). Western blot showed that etanercept decreased the expression of GSDME-N (Fig. 6G).
Figure 6.
Anti-TNF-α therapy relieved the severity of DSS - induced colitis. (A) The treatment methods. (B) Anti-TNF-α therapy significantly decreased the severity of DSS-induced colitis in IRG1−/− mice. (C, D) The colon length of DSS group was shorter than that of the DSS + anti-TNF-α group. (E) The level of serum MPO in DSS + anti-TNF-α group was lower than DSS group. (F) The level of serum TNF-α significantly decreased with anti-TNF-α therapy. (G) Etanercept decreased the expression of cleaved-GSDME. Data are shown as the mean ± SD, ∗∗∗P < 0.001.
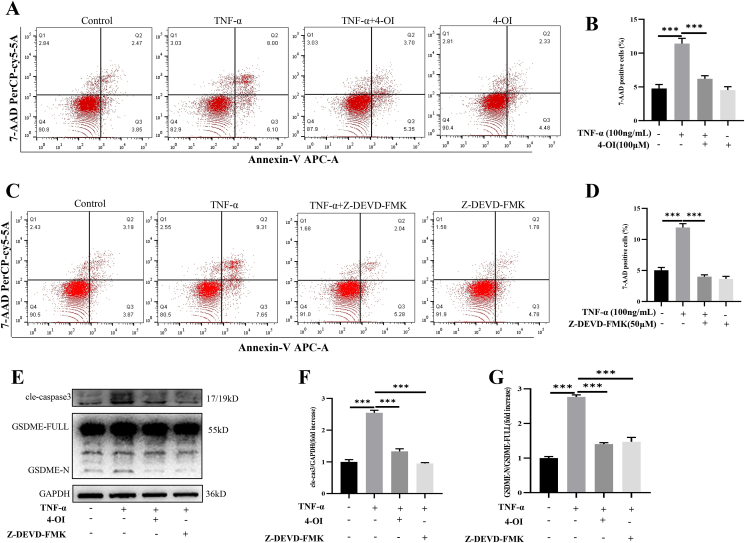
4-OI inhibited caspase3/GSDME medicated-pyroptosis induced by TNF-α
Then we treated NCM460 cells with TNF-α (50 ng/ml) and 4-OI for 36 h. The flow cytometry showed that TNF-α significantly increased 7-AAD-positive cell percentages, while 4-OI attenuated this change (Fig. 7A, B). Next, we used caspase-3 special inhibitors (Z-DEVD-FMK, 50 μM) and TNF-α (50 ng/ml) to treat NCM460 cells. The flow cytometry showed that Z-DEVD-FMK reduced the 7-AAD-positive cells (Fig. 7C, D). To further explore the underlying mechanism, we extracted cell proteins, and we found 4-OI significantly reduced the expression of cleaved-caspase-3 and GSDME-N (Fig. 7E–G). This showed that 4-OI might inhibit GSDME activation by inhibiting caspase-3 activation, thereby inhibiting GSDME mediated-pyroptosis.
Figure 7.
4-OI inhibited caspase3/GSDME medicated-pyroptosis induced by TNF-α. (A, B) TNF-α significantly increased 7-AAD-positive cell percentages, while 4-OI attenuated this change. (C, D) Z-DEVD-FMK reduced the 7-AAD-positive cells. (E–G) 4-OI significantly reduced the expression of cleaved-caspase-3 and GSDME-N. Data are shown as the mean ± SD, ∗∗∗P < 0.001.
Discussion
In recent years, with the development of society and the changes of human diet, the incidence of IBD is increasing which seriously affects people's daily life. Therefore, it is very important to find a targeted treatment strategy for IBD. This study was to explore the therapeutic effect of IRG/4-OI on DSS-induced colitis. The results showed that IRG/4-OI significantly alleviated DSS-induced colitis by inhibiting inflammatory response and gasdermins-mediated pyroptosis. This provides some promising prospects for the treatment of IBD.
The IRG1 encodes the aconitic acid decarboxylase in mitochondria, which catalyzes decarboxylation of cis-aconitic acid, an intermediate product of the tricarboxylic acid cycle (TAC), to itaconate.33 IRG1 can change the metabolic environment of macrophages by affecting the mitochondrial TAC. IRG1 and its metabolites play an anti-inflammatory and antioxidant effect through a variety of pathways.34,35 4-OI, a derivative of itaconate, can activate Nrf2 and drive Nrf2 dependent gene expression in macrophages. Nrf2 is considered to be a key control factor in the process of infection and inflammation. Nrf2 activation can increase the activation of HO-1 and enzymes involved in the synthesis of glutathione, which is the main protective agent against oxidative stress.36 In addition, Nrf2 activation can inhibit IL-1β generation.37 This may be due to the direct inhibition of IL-1β gene transcription by Nrf2 or the indirect inhibition of ROS production, ultimately inhibiting IL-1β production.
Since the first reported in 1985 that a hamster model of ulcerative colitis was prepared with DSS, a large number studies have proved that DSS-induced colitis model was similar to that of human ulcerative colitis.38, 39, 40 Our results also showed that DSS caused intestinal epithelial barrier damage, extensive infiltration of inflammatory cells and destruction of intestinal tissue, especially in IRG1−/− mice. This indicated that IRG1 had a protective effect on DSS-induced colitis. After IRG1−/− mice received 4-OI treatment, the body weight loss, DAI score, and inflammation score significantly decreased. Kim et al41 also reported that 4-OI administration alleviated the severity of colitis, and Wang et al42 reported that dimethyl itaconate (DI), another itaconate derivative, suppressed DSS-induced colitis-associated colorectal cancer. However, one study showed that unmodified itaconate aggravated DSS-induced colitis.43 Maybe the function of unmodified itaconate is different from that of its derivatives. DI and 4-OI can induce a strong electrophilic stress response, in contrast to unmodified itaconate. DI and 4-OI can inhibit cytokine production and play an anti-inflammatory effect, while itaconate treatment suppressed IL-1β secretion but not pro-IL-1β levels.44 Therefore, the anti-inflammatory effects of exogenous unmodified itaconate and its derivatives are not exactly the same, and it also prompts us speculate that itaconate is an immunoregulatory as opposed to purely immunosuppressive metabolite.
The destruction of intestinal epithelial barrier by DSS can cause bacteria to enter blood and induce bacterial translocation. Bacterial translocation and LPS can cause inflammatory reaction, and NF-κB/MAPK signaling pathway plays important roles as the body fights infection.45 In the present study, we found that the level of 16sRNA in spleen of IRG1−/− mice group was significantly higher. Furthermore, the in vivo experiments showed that the NF-κB/MAPK signaling pathways significantly activated in the IRG1 deficiency group. In vitro experiments showed that 4-OI significantly inhibited the activation of NF-κB/MAPK pathways in RAW264.7 and BMDM cells. Studies have reported that LPS could cause a sharp increase in the level of ROS, which caused severe inflammatory response.46,47 To further investigate the underlying mechanisms of 4-OI in inhibiting the NF-κB/MAPK signaling pathway, we tested the effect of 4-OI on ROS. The results showed that 4-OI significantly decreased the ROS production and activated Nrf2/HO-1 pathway, which suggested that 4-OI might inhibit MAPK/NF-κB signaling pathway by inhibiting the production of ROS through promoting the expression of Nrf2/HO-1.
Over the years, many studies have tried to understand the roles of pyroptosis in colitis.19,48 Pyroptosis mainly relies on inflammasomes to activate some proteins of the caspase family to cut and activate gasdermin protein. The activated gasdermin protein is translocated to the membrane to form holes, and IL-1β and IL-18 are released. The classical pathway of pyroptosis mainly involves caspase-1 cutting the N-terminal sequence of GSDMD, activating IL-18 and IL-1β. IL-1β is an important proinflammatory cytokine, which can further cause macrophage pyroptosis.49,50 Thus, we speculated that the role of pyroptosis was further expanding the inflammatory response in macrophages, forming a vicious circle in the DSS model. Furthermore, studies reported that the production of IL-1β and IL-18 in the patients with active IBD increased, and the activity of caspase-1 in macrophages increased. Our study revealed that the expression of GSDMD-N in the DSS group significantly increased, which indicated that GSDMD-mediated pyroptosis played a crucial role in DSS-induced colitis. Meanwhile, the expression of GSDMD-N was significantly higher in the IRG1−/− group than that of the WT group, which decreased after receiving 4-OI treatment. Therefore, we hypothesized that 4-OI reduced the degree of colitis by inhibiting the macrophage pyroptosis. To further test our hypothesis, we used LPS and NG to induce pyroptosis in vitro, and the results showed that 4-OI significantly reduced the activation of GSDMD, caspase-1 and the release of IL-1β. Hooftman et al28 also reported 4-OI could reduce the macrophage pyroptosis. Therefore, IRG1/4-OI might play an important role in inhibiting the GSDMD-mediated pyroptosis of macrophages, reducing inflammatory response and alleviating colitis. Interestingly, two studies used GSDMD−/− mice to explore the effect of GSDMD on DSS-induced colitis and reported conflicting roles of GSDMD.19,51 The effect of GSDMD in regulating inflammatory response may not be unilateral. GSDMD can induce pyroptosis to promote cytokine production and aggravate inflammatory response; it may also reduce inflammation through pathways such as cyclic GMP–AMP synthase. Ultimately, GSDMD exerts its effect through the sum of multiple pathways. Our study was to explore the effect of IRG1 deficiency on DSS-induced colitis, and in vivo and in vitro experiments showed that IRG1/4-OI significantly inhibited GSDMD-N production and cytokine release, therefore reduce inflammation, which was different from directly knocking out of GSDMD.
In recent years, Shao's team discovered that GSDME, another member of the gasdemin family, can be cleaved and activated by caspase-3 to cause pyroptosis, which can act as an inhibitory factor in tumors.23 Zhai et al24 also reported inhibiting caspase3/GSDME-mediated pyroptosis induced by TNF-α attenuated rheumatoid arthritis. Previous results showed DSS produced a large amount of TNF-α through activation of MAPK/NF-κB signaling pathway and GSDMD-mediated pyroptosis in macrophages, so we speculated that TNF-α might activate caspase-3 to cause GSDME-mediated pyroptosis. Our study showed that etanercept, a TNF-α inhibitor, significantly decreased the expression of GSDME-N and the severity of colitis. In vitro experiments, we also found that 4-OI inhibited the formation of GSDME-N by inhibiting the activation of caspase-3 caused by TNF-α. Indeed, TNF inhibitors have been clinically important target drugs for the treatment of IBD. Our findings provided another possible mechanism for IRG1/4-OI through the inhibition of the caspase3/GSDME pathway induced by TNF-α.
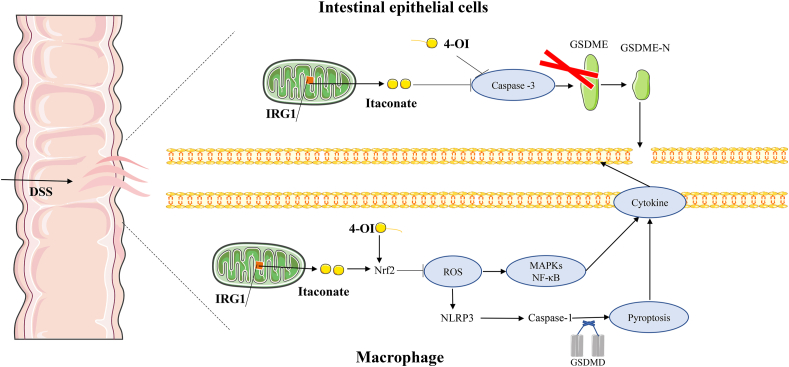
To sum up, our research results revealed that IRG1/4-OI alleviated DSS-induced colitis by inhibiting ROS-medicated MAPK/NF-κB signaling pathway and GSDMD/GSDME-mediated pyroptosis (Fig. 8). Taken together, these data show that IRG1/itaconate may be a potential target for treating IBD.
Figure 8.
Proposed mechanism and pathway of IRG1/4-OI protect against DSS-induced IBD.
Author contributions
All authors helped to perform the research. R.D.L. and K.X.T.: Funding acquisition, Project administration, Supervision, Validation, Visualization, Writing-review & editing; W.C.Y. and Y.X.W.: Conceptualization, Data curation, Roles/Writing - original draft; T.W. and C.G.L.: Formal analysis; P.Z., L.S., and Y.P. Y.: Methodology, Resources, Software.
Conflict of interests
The author declares no conflict of interests.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81701883, 82072736 and 82172171).
Data availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Kaixiong Tao, Email: kaixiongtao@hust.edu.cn.
Ruidong Li, Email: liruidong@hust.edu.cn.
References
- 1.Hodson R. Inflammatory bowel disease. Nature. 2016;540(7634):S97. doi: 10.1038/540S97a. [DOI] [PubMed] [Google Scholar]
- 2.Le Berre C., Peyrin-Biroulet L., SPIRIT-IOIBD study group Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology. 2021;160(5):1452–1460. doi: 10.1053/j.gastro.2020.10.065. [DOI] [PubMed] [Google Scholar]
- 3.Sun J., Chang E.B. Exploring gut microbes in human health and disease: pushing the envelope. Genes Dis. 2014;1(2):132–139. doi: 10.1016/j.gendis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 5.Kaenkumchorn T., Wahbeh G. Ulcerative colitis: making the diagnosis. Gastroenterol Clin N Am. 2020;49(4):655–669. doi: 10.1016/j.gtc.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Glassner K.L., Abraham B.P., Quigley E.M.M. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019 doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J., Liang W., Wang T., et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol Res. 2019;144:66–72. doi: 10.1016/j.phrs.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 9.James J.P., Riis L.B., Malham M., Høgdall E., Langholz E., Nielsen B.S. MicroRNA biomarkers in IBD-differential diagnosis and prediction of colitis-associated cancer. Int J Mol Sci. 2020;21(21):7893. doi: 10.3390/ijms21217893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado-Contreras A.L., McCormick B.A. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 2011;343(1):5–12. doi: 10.1007/s00441-010-1082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao S., Wang C., Yan J., Li X., Wen J., Hu C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic Biol Med. 2020;147:8–22. doi: 10.1016/j.freeradbiomed.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Parikh K., Antanaviciute A., Fawkner-Corbett D., et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567(7746):49–55. doi: 10.1038/s41586-019-0992-y. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Liu G., Yuan Y., Wu G., Wang S., Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019;10(12):906. doi: 10.1038/s41419-019-2157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens M., von der Weid P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microb. 2020;11(3):421–432. doi: 10.1080/19490976.2019.1629235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinheiro D.M.L., de Oliveira A.H.S., Coutinho L.G., et al. Resveratrol decreases the expression of genes involved in inflammation through transcriptional regulation. Free Radic Biol Med. 2019;130:8–22. doi: 10.1016/j.freeradbiomed.2018.10.432. [DOI] [PubMed] [Google Scholar]
- 16.Shen X., Weng C., Wang Y., et al. Lipopolysaccharide-induced podocyte injury is regulated by calcineurin/NFAT and TLR4/MyD88/NF-κB signaling pathways through angiopoietin-like protein 4. Genes Dis. 2022;9(2):443–455. doi: 10.1016/j.gendis.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulek K., Zhao J., Liao Y., et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J Clin Invest. 2020;130(8):4218–4234. doi: 10.1172/JCI138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhou X., Zou K., et al. Monocarboxylate transporter 4 triggered cell pyroptosis to aggravate intestinal inflammation in inflammatory bowel disease. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.644862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C., Yang D., Wang B., et al. Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation. Sci Adv. 2020;6(21):eaaz6717. doi: 10.1126/sciadv.aaz6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei-Leston A.C., Murphy A.G., Maloy K.J. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front Immunol. 2017;8:1168. doi: 10.3389/fimmu.2017.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmakar M., Minns M., Greenberg E.N., et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11(1):2212. doi: 10.1038/s41467-020-16043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider K.S., Groß C.J., Dreier R.F., et al. The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 2017;21(13):3846–3859. doi: 10.1016/j.celrep.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Gao W., Shi X., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 24.Zhai Z., Yang F., Xu W., et al. Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-induced caspase 3/gasdermin E-mediated pyroptosis. Arthritis Rheumatol. 2022;74(3):427–440. doi: 10.1002/art.41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West N.R., Hegazy A.N., Owens B.M.J., et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23(5):579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandborn W.J., Vermeire S., Tyrrell H., et al. Etrolizumab for the treatment of ulcerative colitis and Crohn's disease: an overview of the phase 3 clinical program. Adv Ther. 2020;37(7):3417–3431. doi: 10.1007/s12325-020-01366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xian Y., Su Y., Liang J., et al. Oroxylin A reduces osteoclast formation and bone resorption via suppressing RANKL-induced ROS and NFATc1 activation. Biochem Pharmacol. 2021;193 doi: 10.1016/j.bcp.2021.114761. [DOI] [PubMed] [Google Scholar]
- 28.Hooftman A., Angiari S., Hester S., et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metabol. 2020;32(3):468–478. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R., Yang W., Yin Y., Ma X., Zhang P., Tao K. 4-OI attenuates carbon tetrachloride-induced hepatic injury via regulating oxidative stress and the inflammatory response. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.651444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W., Wang Y., Zhang P., et al. Immune-responsive gene 1 protects against liver injury caused by concanavalin A via the activation Nrf2/HO-1 pathway and inhibition of ROS activation pathways. Free Radic Biol Med. 2022;182:108–118. doi: 10.1016/j.freeradbiomed.2022.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Yang W., Tao K., Zhang P., Chen X., Sun X., Li R. Maresin 1 protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting macrophage pyroptosis and inflammatory response. Biochem Pharmacol. 2022;195 doi: 10.1016/j.bcp.2021.114863. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P., Yin Y., Wang T., et al. Maresin 1 mitigates concanavalin A-induced acute liver injury in mice by inhibiting ROS-mediated activation of NF-κB signaling. Free Radic Biol Med. 2020;147:23–36. doi: 10.1016/j.freeradbiomed.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 33.Lampropoulou V., Sergushichev A., Bambouskova M., et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabol. 2016;24(1):158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills E.L., Ryan D.G., Prag H.A., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi Z., Deng M., Scott M.J., et al. Immune-responsive gene 1/itaconate activates nuclear factor erythroid 2-related factor 2 in hepatocytes to protect against liver ischemia-reperfusion injury. Hepatology. 2020;72(4):1394–1411. doi: 10.1002/hep.31147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Y., Chen Z., She C., et al. Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury. Cell Death Dis. 2020;11(9):772. doi: 10.1038/s41419-020-02987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo Early, Menon D., Wyse C.A., et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115(36):E8460–E8468. doi: 10.1073/pnas.1800431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D.G., Lee M.R., Yoo J.M., Park K.I., Ma J.Y. Fermented herbal formula KIOM-MA-128 protects against acute colitis induced by dextran sodium sulfate in mice. BMC Compl Alternative Med. 2017;17(1):354. doi: 10.1186/s12906-017-1855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman R.W., Sillery J., Fontana D.D., Matthys C., Saunders D.R. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489–493. doi: 10.1016/0016-5085(85)90441-x. [DOI] [PubMed] [Google Scholar]
- 40.Bauer C., Duewell P., Mayer C., et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59(9):1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 41.Kim H.W., Yu A.R., Lee J.W., et al. Aconitate decarboxylase 1 deficiency exacerbates mouse colitis induced by dextran sodium sulfate. Int J Mol Sci. 2022;23(8):4392. doi: 10.3390/ijms23084392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q., Li X.L., Mei Y., et al. The anti-inflammatory drug dimethyl itaconate protects against colitis-associated colorectal cancer. J Mol Med. 2020;98(10):1457–1466. doi: 10.1007/s00109-020-01963-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang H.G., Zhang M.N., Wen X., Yang X.Z. Itaconate aggravates experimental colitis. Clin Res Hepatol Gastroenterol. 2021;45(2) doi: 10.1016/j.clinre.2021.101629. [DOI] [PubMed] [Google Scholar]
- 44.Swain A., Bambouskova M., Kim H., et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2(7):594–602. doi: 10.1038/s42255-020-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangaran P.G., Ibrahim Z.A., Chik Z., Mohamed Z., Ahmadiani A. LPS preconditioning attenuates apoptosis mechanism by inhibiting NF-κB and caspase-3 activity: TLR4 pre-activation in the signaling pathway of LPS-induced neuroprotection. Mol Neurobiol. 2021;58(5):2407–2422. doi: 10.1007/s12035-020-02227-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Liu P., Feng X., Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21(12):3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tur J., Pereira-Lopes S., Vico T., et al. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Rep. 2020;32(8) doi: 10.1016/j.celrep.2020.108079. [DOI] [PubMed] [Google Scholar]
- 48.Chao L., Li Z., Zhou J., et al. Shen-Ling-Bai-Zhu-San improves dextran sodium sulfate-induced colitis by inhibiting caspase-1/caspase-11-mediated pyroptosis. Front Pharmacol. 2020;11:814. doi: 10.3389/fphar.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K., Sun Q., Zhong X., et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941–955. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z., Wang C., Yang J., et al. Caspase-1 engages full-length gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity. 2020;53(1):106–114. doi: 10.1016/j.immuni.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao H., Cao M., Yao Y., et al. Dysregulated microbiota-driven gasdermin D activation promotes colitis development by mediating IL-18 release. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.