Abstract
Wnt signaling executes an indispensable performance in osteoblast differentiation, bone development, homeostasis, and remodeling. Wnt signals trigger the intracellular Wnt signaling cascade to initiate regulating the implication of β-catenin in the bone environment. Going through the novel discoveries done via high-throughput sequencing technologies on genetic mouse models, we highlighted the significant contribution of Wnt ligands, co-receptors, inhibitors, their related skeletal phenotypes in mouse models and the similar bone disorders clinically observed in human beings. Moreover, the crosstalk between Wnt signaling pathway and BMP, TGF-β, FGF, Hippo, Hedgehog, Notch and PDGF signaling pathways is thoroughly demonstrated to be the underlying gene regulatory network that orchestrates osteoblast differentiation and bone development. We also introspected the significance of Wnt signaling transduction in the reorganization of cellular metabolism by stimulating glycolysis, glutamine catabolism, and fatty acid oxidation in osteoblast-lineage cells that display an important regulatory arbor in the cellular bioenergetics of the bone. Throughout this evaluation, most to date therapeutical approaches towards osteoporosis and other bone maladies found in human beings, are formulated with an aspiration to holistically revamp the present clinical applications involving various monoclonal antibodies therapies that lack specificity, efficacy, and safety into more requisite advanced therapeutics that satisfy these three requirements for further clinical considerations. Conclusively, our review provides comprehensive scientific findings related to the fundamental significance of Wnt signaling cascades in skeletal system and the underlying gene regulatory network with other signaling pathways enlightening researchers with the possibility to further integrate the identified target molecules into therapeutic strategies for skeletal disorders treatment in the clinic.
Keywords: Bone development, Bone homeostasis, Osteoblast differentiation, Skeletal disorders, Wnt signaling
Introduction
Bone is an exceptionally vigorous organ that goes through frequent resorption by osteoclasts and a brand-new development by osteoblasts during the remodeling process. The four types of cells that make up bone, the stiff tissue that constitutes the skeleton in vertebrates, are osteoblasts, bone lining cells, osteocytes, and osteoclasts.1 It has significant roles in the body, including motility, resistance, and soft tissue protection, as well as Ca2+ and PO4 storage and bone marrow nurturing.2, 3, 4 Evidence also shows that osteocytes can sense mechanical stimuli and orchestrate the bone remodeling process by modifying their environment.5, 6, 7 The old bone is replenished through a composite process of bone remodeling that includes three phases1: the resorption of bone tissue by osteoclasts,2 the growth progression to new bone creation, and3 the completed bone production by osteoblasts.8,9 This process occurs as a result of the coordinated actions of osteoblasts, osteoclasts, osteocytes, and bone lining cells, within the bone cavities, which form the fundamental multicellular unit (BMU).9,10 Besides its self-mending abilities, there are some deficiencies that bone is unable to provide regeneration for, therefore various signaling pathways actively respond to these deficiencies and one of them is the Wnt signaling pathway.11
The Wnt pathway is a developmentally conserved route that contains a series of signaling pathways that regulate a variety of biological activities ranging from embryonic growth to tissue regeneration.12, 13, 14, 15 In both human beings and mice mammals, there are 5 genes in C. elegans and 7 genes in Drosophila that encode for the large family of Wnt secreted protein growth factors and regulate the behavior, adhesion, and polarity of the cells. Wnt is an abbreviation for wingless, the Drosophila segment polarity gene, and integrated (int-1), the vertebrate analog. This signaling pathway controls a plethora of functions with the help of Wnt ligands, R-spondin proteins, norrin and other receptors found on the cell surface that trigger downstream gene expression and determine the specificity of Wnt pathway.12,16 In addition, the Wnt signaling pathway may be separated into the canonical mechanism that depends on β-catenin's activity and the non-canonical mechanism that doesn't depend on β-catenin's activity. These two major divisions are subdivided into the Wnt/planar cell polarity (PCP) and the Wnt/Ca2+ pathways and are required for osteocyte cell migration and patterning throughout embryonic maturation, for tissue homeostasis and regeneration and most notably during bone remodeling and homeostasis in bone.17,18 Consequently, dysregulations of Wnt signaling have been associated with a variety of human skeletal diseases. Further studies are required to understand the underlying issues that lead to a cavity in targeted therapy of Wnt-linked diseases.12,19
Canonical and non-canonical Wnt signaling pathways
Wnt/β-catenin signaling: canonical Wnt pathway
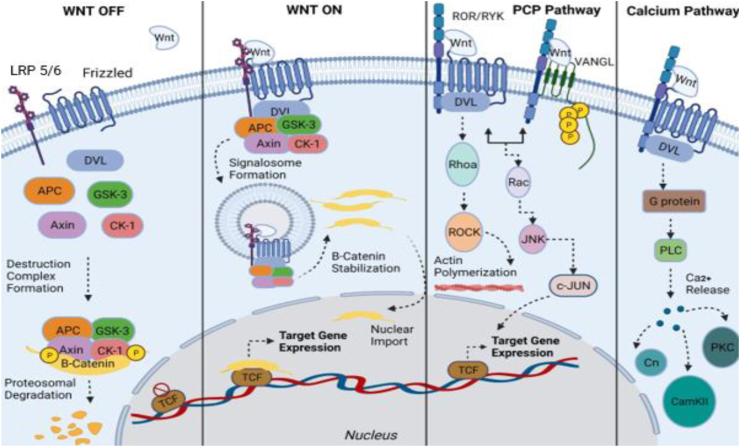
Canonical Wnt signaling pathway promotes the production of β-catenin protein in the cytoplasm and is a requisite process for fetal maturity and metabolic equilibrium.12 When the canonical Wnt ligand is missing in this specific pathway, it is a destructive complex of proteins, that includes serine–threonine kinases glycogen synthase kinase 3β (GSK-3β) and casein kinase 1α (CK1α), the protein Axin, the cytoplasmic phosphoproteins Dishevelled (DVL) and Adenomatous Polyposis Coli (APC), that calls for β-catenin's phosphorylation and eventually its destruction via the degrading actions of ubiquitin-proteasome system (UPS).20,21 On the other side, when the canonical Wnt ligand is not missing, its cell surface receptor frizzled (FZD) and co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6) attach themselves to it causing DVL and Axin proteins to assemble near the plasma membrane, thus deconstructing the destructive complex of proteins. This process results into the accumulation of β-catenin in the cytoplasm and eventually its entrance into the nucleus thus triggering gene expression upon its molecular connection with T-cell factor/lymphoid enhancer factor (TCF/LEF)16,22, 23, 24, 25 (Fig. 1).
Figure 1.
Canonical and non-canonical Wnt signaling pathways. Canonical Signaling- Wnt-Off: At the plasma membrane, both the Frizzled receptor (FZD) and LRP5/6 receptor stand inoperative, when in lack of Wnt ligand. In the cytoplasm, the destruction complex (consisted of GSK-3β, APC, CK-1 and Axin) bind and phosphorylate β-catenin, followed by its proteasomal degradation. In the nucleus, Wnt target gene expression remains off. Canonical signaling- Wnt-On: When Wnt ligand binds to the Frizzled receptor, an active receptor complex is formed by sequestering LRP5/6 receptor. This brings the inactivation destruction complex and signalosome formation mediated by Dishevelled (DVL). Thus β-catenin, after translocating into the nucleus, binds to T cell factor gene region and forms a transcriptional protein-activator complex in order for Wnt target genes to become expressable. Non-canonical Wnt/PCP pathway: Wnt ligand binds to the Frizzled receptor thus recruiting Dishevelled. They form a complex, with co-receptors such as ROR and RYK included, in order for RhoA/Rock pathway to be activated and actin to become polymerized. This particular pathway can instigate JNK pathway's operation via Frizzled-Dishevelled-ROR/RYK system or VANGL planar cell polarity protein-ROR/RYK system. Non-canonical Wnt/Ca2+pathway: Frizzled receptor sequesters ROR&RYK receptors when Wnt ligand binds and trigger the membrane proteins Dishevelled and Guanine nucleotide-binding proteins to operate and consequently activate Plc enzyme to release Ca2+ from within the cell. High amounts of Ca2+ trigger other pathways to initiate their activity assisted by activating receptor molecules like CaN (calcium-dependent serine–threonine phosphatase), CaM-kinase II (Ca2+/calmodulin-dependent protein kinase II) and PKC (protein kinase C).
Wnt/PCP and Wnt/Ca2+ signaling: non-canonical Wnt pathways
The non-canonical Wnt/PCP pathway operates separately from β-catenin's activity. It combines cell mobility with tissue polarity via GTPase RhoA and/or c-Jun N-terminal kinase catalysis (JNK).26 PCP pathway initiates functioning only when ligands like WNT5A, WNT5B, and/or WNT11, bind to their receptors Frizzled 3 or Frizzled 6 and their co-receptors ROR1/2 (Receptor tyrosine kinase-like orphan receptor 1 and 2), or PTK7 (Protein tyrosine kinase 7).27 Other proteins such as VANG (11/12), Prickle (1/2), Cadherin EGF LAG (CELSR 1/2/3), DVL (1/2/3), and Ankyrin repeat domain 6 (ANKRD6) are major participants in this signaling catalyzation28 (Fig. 1). Since this pathway is dependent on the Dishevelled protein's activity, it is exactly these group of proteins DAAM1 and DAAM2 (Dishevelled-associated activator of morphogenesis proteins) as well as MAP kinases (Mitogen activated protein) that trigger the Rho-associated protein kinase to initiate the RhoA-ROCK pathway or the RAC-JNK signaling pathway, sequentially. Contrastingly, in the situation of non-canonical Wnt pathway's independence from the activities of the Dishevelled protein, it is RTKs (Receptor tyrosine kinases), G proteins and PLC (Phospholipase C) that trigger the serine/threonine protein phosphatase calcineurin and cause the NFAT (Nuclear factor of activated T cells), via calcium release, to assemble inside the nucleus.26,29,30 Other pathways, such as NLK (Nemo-like kinase) signaling can initiate functioning in the presence of high level of calcium released in the cytoplasm, via CaMKII (Calcium/calmodulin dependent kinase II)'s activity.
Mutations in Wnt components generate deficient skeletal phenotypes in mouse models and humans
Wnt ligands mutation in osteoblasts
WNT1. WNT1 plays a crucial function during osteoblastogenesis. Given that WNT1 is found in mesenchymal stem cells (MSCs), osteoblasts, and osteocytes, it activates the canonical pathway and suppresses MSCs differentiation into adipocytes causing the production of osteoblasts to increase.31,32 Studies have shown that WNT1 plays a principal role in the early stages of fetal growth. For instance, purebred Wnt 1-KO mice emerged with infant mortality whilst hybrid Wnt1-KO mice underwent lenient low bone density without a weakened bone development and ossification.31 At a later stage, WNT1 activates mTORC1 pathway promoting osteoblasts' differentiation and mineralization as studies have shown that the inhibition of mTORC1 in the Wnt1 gain-of-function mouse model was associated with reduced mineralization and bone formation.33 Due to WNT1's strong linkage with bone growth and formation, loss-of-function mutations associated with WNT1 are responsible for diseases like premature osteoporosis and osteogenesis imperfecta in humans.34, 35, 36
WNT3A. WNT3A ligand has a major contribution to differentiating mesenchymal stem cells into osteoblastic cells via the canonical Wnt pathway as well as the non-canonical Wnt/Protein kinase C pathway.37,38 It may also prevent the process of programmed cell death in both mature and immature osteoblastic cells39, 40, 41 as well as mesenchymal stem cells’ further progression to transform into chondrocytic cells.42 Reported experiments have resulted in defective fetal development to non-surviving Wnt3a-KO mouse models.43 Moreover, a patient diagnosed with skeletal dysplasia was found with a wild-type alteration on WNT3A gene.44 Other findings have shown that the treatment of ESCs (embryonic stem cells) in mouse with recombinant Wnt3a, which positively affects the activity of β-catenin, downregulated the osteogenic expression.45
WNT4. WNT4 contributes to osteoblastic differentiation via activating the non-canonical Wnt pathway and hinders both the inflammation of bone as well as bone remodeling.46,47 Wnt 4-KO mice have displayed a delay in chondrocyte maturation and an early postnatal death while Wnt4-cKO female mice showed decreased femoral BMD.48,49 WNT4 can hinder RANK to attach to the TRAF6 in osteoclastic cells (TNF receptor-associated factor 6) suppressing the RANKL-induced osteoclast differentiation.47 Experimenting with mouse models has shown that WNT4 ligand functions as a protective element against bone resorption as well as against bone destruction. Via in vitro reports, it was demonstrated that the main alterations of Wnt4 gene as well as the knockdown of a protein coding gene named Zinc Finger and BTB/POZ Domain Containing 40 (ZBTB40) occurring in osteoblastic cells, resulted in a suspended osteoblastic development and ossification.49, 50, 51
WNT5A. WNT5A has a distinct role in stimulating the differentiation of both osteoblastic and osteoclastic cells by enhancing or hindering the canonical Wnt pathway in bone.52, 53, 54,55 This ligand is responsible for independent-GSK-phosphorylation-β-catenin-deterioration.58 It can also hinder the genes for β-catenin's adjustment to transcript via the non-canonical Wnt signaling pathway and T-cell factor (TCF) intervention.54 Moreover, the WNT5A expression in osteoblasts is promoted by sphingosine-1-phosphate (S1p).59 Experiments have shown Wnt5a knockout mouse models to display a diversity of developmental anomalies, a delay in chondrocyte hypertrophy and skeletal ossification, and perinatal death.57,60 Meanwhile, the Wnt5a conditional knockout mouse models have displayed under-expressed RANK levels in osteoclast progenitors that led to breakdown of old bone, and a damaged osteoblast development.57 In addition to that, the excessive signaling activity of WNT5A ligand and neurotrophic tyrosine kinase, receptor-related 2 (ROR2) has shown to cause bone depletion in rheumatoid arthritis.56,57 Opposite to Wnt3a, the treatment of ESCs in mouse models with a recombinant Wnt5a upregulated various kinases' activity such as PKC, CaMKII and JNK while antagonizing the activity of β-catenin thus increasing the osteogenic proliferation and displaying a potential application in tissue engineering.45 Loss of function alterations occurring in WNT5A gene have clinically shown to be a cause for Acral dysostosis with facial and genital abnormalities (Robinow syndrome).53,61
WNT5B. WNT5B is associated with MSC differentiation and chondrocyte proliferation during bone development.60,62 Furthermore, WNT5B gene has been found to be overexpressed in female osteoarthritis (OA) patients.63,64 Wnt5b-KO mice displayed a delayed bone ossification due to the overexpressed Wnt5b in chondrocytes.60
WNT7A. WNT7A is necessary in the development of cranium, face, and limbs during the formation process of the embryo.65,66 Upregulation of WNT7A reduced RUNX2 expression thus reducing the osteogenic cell proliferation, while the downregulation of WNT7A in MSCs increased RUNX2 expression thus promoting osteogenic cell proliferation.67 Wnt7a-KO mice models display impaired limb development.68 Alterations like loss of functions for the WNT7A gene was shown to be a great cause for the Fuhrmann malady, the Al-Awadi-Raas-Rothschild disease (AARRS) and lastly the Santos affliction in human.65,69,70 These diseases have the ectodermal dysplasia physical composition accompanied by acute limb deficiencies.65,69
WNT7B. WNT7B ligand functions as an enhancing factor for osteoblast differentiation in mice, prenatally and postnatally41 through the non-canonical activation of mTORC1 and PKC δ signaling.37,41 Wnt7b knockout mouse models were shown perinatally dead.71 In occasions where particular parts of Wnt7b gene in osteogenic cells were missing, a delayed chrondocytic development and a deficient osteoblastic differentiation mediated by the osterix transcription factor were displayed.37,41 Overexpression of Wnt7b gene, in osteoblasts and chondrocytes of mice, resulted in accelerated bone formation and denser bones.33,41 In humans, increased WNT7B expression has been linked to osteoarthritis (OA) patients and rheumatoid arthritis (RA) patients.72,73
WNT9A. WNT9A arranges appropriately chondrocyte development prior to ossification and maintains the joint stability.48,74 During osteoblastic differentiation, Wnt9a expression decreases.40 Wnt9a knockout mouse models have displayed bone malformations like, incomplete arthrodesis, low levels of bone ossification, indications of skeletal dysplasia, metaplastic cartilage and lastly, neonatal death.48
WNT10A. WNT10A favors ostosis by hindering the formation of adipocytes during osteoblastogenic process, via the Wnt-canonical signaling pathway.56,75 Wnt 10a knockout mouse models have displayed reduced bone ossification, bone marrow adipose tissue development and tooth development.76, 77, 78 Due to this ligand's significant role in dentinoblast differentiation, WNT10A gene alterations have displayed abnormal development of ectodermally-derived organs (also known as ectodermal dysplasia) in humans, causing absence of teeth (also known as anodontia) as well as the schöpf-schulz-passarge syndrome (also known as the odontoonycho-dermal dysplasia, OODD).79, 80, 81, 82
WNT10B. WNT10B ligand functions as a regulator in the process of osteoblastic cells formation via the Wnt canonical pathway. It hinders PPARγ and C/EBPα (also known as the family of transcription factors during adipogenesis) from expressing accurately and enhances RUNX2 to express properly.83 WNT10B maintains and renews osteoblastic mesenchymal stem cells in the bone marrow84 due to its highly expressed level in numerous progenitor cellular compartments.84 In Wnt 10b knockout mouse models, the mineralized bone volume (BV/TV), bone density level (BMD) and the number of trabeculae (Tb.N) were all shown to be substantially reduced, when contrasted with homozygous mice.32 WNT10B has been implied in various human disorders.85 In humans, homozygous missense mutations in WNT10B cause split limb malformation with autosomal recessive inheritance, dental anomalies including here oligodontia and osteosarcoma.53,86, 87, 88
WNT16. WNT16 is involved in the maintenance of postnatal bone homeostasis by regulating both canonical and non-canonical Wnt targets in osteoblasts.89 Wnt 16 is found to be more highly expressed in cortical bone than in trabecular bone90 as both Wnt16 knockout and the conditional knockout mouse models displayed reduced bone density level, low density in cortex, weakened bones and increased impermeability in cortex, while the trabeculated bone tissue's volume remained unaltered.90,91 Alternatively, mouse models with a high expression of Wnt16 genes, in both their osteoblastic and osteocytic cells of trabeculae and cortex compartments, displayed an increased bone density level as well as a more resistant bone.92, 93, 94 WNT16 gene, has long been linked to with various bone physical compositions such as bone density level (BMD), the resistance of bone, bone linear framework, the density of cortex and lastly bone rupture possibility.95, 96, 97, 98 Researchers assume for WNT16 ligand to contribute to hindering the process of bone formation in two ways, by increasing the osteoprotegerin (also known as the osteoclastogenesis inhibitory factor, OCIF) or activating the osteoclastic hematopoietic progenitors via ostoblastic mediation.90,99
Wnt-co-receptors in osteoblasts
LRP. LRP stands for low-density-lipoprotein-receptor-related-protein and belongs to the low density lipoprotein receptor family, recognized in the cell surface,100 with endocytic and signaling functions.101 These LRP integral polypeptides are composed of an outer-cell compartment which binds itself to proteins that are present in the extra-cellular matrix, and an inner-cell compartment that functions as an interceder in signaling mechanism transductions.102 In experimental phases, both Lrp5-KOs and cKOs mice in skeletal cells display a low bone density level postnatally, caused by a decreased skeletal development processing.103, 104, 105, 106, 107, 108 Point-mutations occurring in Lrp6 gene are linked to anodontia, the disorder related to congenital absence of more than six teeth (also known as ogliodontia) and lastly high bone mass phenotype.109, 110, 111 The Lrp6 knockout mice, during the development of embryo, display lost non-proximal limb appendage as well as shortened transverse bones.105 Moreover, cKO mouse models have also confirmed the involvement of Lrp6 in postnatal bone metabolism.106,108,112 Various researchers tend to acknowledge the Lrp5-mediated regulation of Wnt-signaling pathway in mature osteoblast cells found in the cortex as well as the Lrp5-mediated regulation of Wnt-signaling pathway in premature osteoblast cells found in trabeculae, to enhance the bone mass density's well-development.105,106,108,113 Lrp4 is necessary to enhance the inhibitory function of sclerostin inhibitor in the canonical Wnt pathway114, 115, 116, 117 amongst other inhibitors such as DKK1 and SOSTdc1.116,118 LRP4 ligand is also known to promote DKK1 and SOSTdc1's inhibiting activity in the Wnt-signaling pathway.116,118 In addition, mutations in Lrp4 cause Cenani–Lenz syndrome (CLS), sclerosteosis-2 and bilateral syndactyly.114,119, 120, 121, 122 Lrp4-KO mutant mouse models absent neuromuscular functions and the inability to breathe115 whilst, Lrp4-cKO mouse models do not.115,117 Lrp4 is also shown to apply its functions in hindering the canonical pathway and eventually affect the osteoblastic development process, during in-vitro and in-vivo animal experimental phases.
In humans, alterations related to LRP5 gene appear linked to two types of skeletal physical compositions such as, osteogenesis perfecta (OPPG) and increased bone mineral density (HBM). OPPG is highly linked to loss of function mutations in LRP5 gene that cause the Wnt-canonical pathway to become inoperative, while gain of function mutations are linked to the reduction of propinquity for other inhibiting elements found in the cell's surface such as SOSTdc1 and DKK1, an event that eventually generated the increased bone mineral density phenotype to display.123, 124, 125, 126, 127, 128
ROR. ROR1/2 are type I transmembrane protein tyrosine kinases that belong to the ROR family of kinases. Ror1/2 genes are highly expressed in most main skeletal compartments during the formation of embryo but significantly reduced in mature tissue compartments.129 Ror2 gene, especially, is distinctly expressed in fully-developed osteoclastic cells130 and has been outlined to modulate bone length, via interacting with two other Wnt ligands such as WNT5A and WNT9A.129,131 The Ror2 knockout mouse prototypes have shown traits of nanism characterized by shortened or misshaped bones, anomalous face typology, congenital defective ventricle, and lastly blue skin disorder (also known as cyanosis).57,132, 133, 134 ROR2-WNT5A moderate signaling controls the osteoclastic development and ossification therewith preserving proper amounts of BMD while being initiated by the constitutively active form of RhoA.57,130,134 Inactive loss of function ROR2 gene modification is clinically shown to be linked to the less common type of robinow syndrome (RRS). On the other hand, gain of function alterations are associated with BDB (also known as brachydactyly type B), a condition characterized hypoplasia or aplasia, also in human beings.130,135, 136, 137, 138
KRM. KRM1/2 are single-spanning trans-membrane receptors that display excessive binding interaction for Wnt inhibitors like Dickkorpf 1 and 2 (DKK1 and DKK2).139,140 However, Kremen 1 is shown to have a more extensive expression habit while Kremen 2 is mainly highly expressed in bone cells [109]. Therefore, when the inhibitor DKK1 is not present, KRM 1/2 attaches to receptor LRP6 thus activating the Wnt signaling pathway.141,142 Krm1 knockout mouse models and premature Krm2 knockout mouse models appear alive with no malformations and a normalized BMD in general,143 while mature Krm2 knockout mouse models show an increased BMD.141 In humans, KRM1 alterations have been associated with ectrodactyly and the congenital absence of more than six teeth (also known as oligodontia).144 Putting these data altogether, KRM 1 and KRM 2 appear superfluously present in amount during skeletal development with especially, KRM 2, playing a significant role during bone remodeling process.
LGR. G-protein-coupled receptors rich in leucine (LGRs) subfamily consists of three specific types 4, 5 and 6.145 Specifically, LGR4 is broadly expressed in various body compartments, like bone cells, adipocytic cells and myocytic cells since premature stages of embryo's formation and development until the maturity stages.146, 147, 148 LGR4 has also demonstrated to inhibit the development of osteoclasts mediated by a type II membrane known as RANKL.149 Lgr4 fully knockout mouse models are shown at risk of dying in their early stages of life exhibiting an abnormal light and small body as well as shortened bones.147, 148, 149, 150 Lgr4 knockout mouse models appear to have a reduced bone mineral density caused by a diminished process of ossification and bone mineralization thus obstructing the regular process of osteoblastogenesis in the early stages of embryogenesis and reducing the uncalcified bone matrix (also known as the osteoid) development.147,150 Lgr5 is expressed in osteoblastic progenitors during bone development and formation. Lgr5 knockout mouse models display traits of tongue-tie disease (also known as ankyloglossia) a congenital oral anomaly that reduces the mobility of the tongue tip accompanied by acute respiratory distress syndrome (ARDS) and lastly death in their newly days of being born.151 LGR6 enhances Wnt pathway downstream of the BMP signaling pathway.152 Although Lgr6 knockout mouse models have displayed a normal pattern of development, LGR6 is compulsory for multi-tissue restoration (also known as the digit tip regeneration) of bone in mouse stereotypes.153
Wnt inhibitors in osteoblasts
DKK. Dickkopf (DKK) is comprised of four genes that code for the Dickkopf-related proteins.1, 2, 3, 4 Members DKK1, DKK2 and DKK4 have a similar structure and act through the canonical Wnt pathway, while DKK3 has no effect through the canonical Wnt pathway154 as DKK1, DKK2 and DKK4 can interact with LRP5/6 while DKK3 does not.155, 156, 157 DKK1 binds to LRP5/6 co-receptors and inhibits the canonical Wnt pathway and is mostly found in osteoprogenitors, osteoblasts, osteocytes, and adipocytes.158,159 DKK1 is known to hinder osteoblastic and chondrocytic differentiation, promote adipocytic development and prompt osteoclastic development thereby enhancing the osteoprotegerin-RANKL binding.158, 159, 160, 161, 162 Dkk 1 knockout mouse models manifest a deficiency in bone development, like head and limb abnormalities and ending up dying in a short time, while the hybrid Dkk1 knockout mice and those characterized by a specialized lineage of ostoblastic cells appeared to have a high bone mineral density as well as bone composition.159,160,163, 164, 165 Deregulated expression levels of Dkk1 are linked to different skeletal afflictions, characterized by the death of osteocytes and bone marrow (also known as femoral head avascular necrosis/osteonecrosis (ONFH)),166 bone deformity (also known as osteitis deformans/Paget disease) as well as rheumatoid arthritis, osteoarthritis, and multiple myeloma.162,167,168 DKK2 attaches strongly to Kremen 1/2 and LRP5/6 to prevent Wnt to attach to the respective cell-surface molecules. However, when Kremen 2 is not present, LRP 6 gets activated without any DVL-mediation activity. Consequently, the Dkk 2 knockout mice display a reduced level of osteoblastogenesis and a high level of osteclastogenesis mediated by the osteoprotegerin-RANKL activity, causing low bone mass (osteopenia).140,169
SOST. SOST is a glycoprotein rich in cysteine that acts as an inhibitor of the Wnt pathway, by blocking LRP5/6 co-receptors’ activity.170,171 SOST transcripts are found in high levels in osteocytes as well as in bone marrow and cartilage.172 High BMD and bone strength were seen in Sost-KO mice, as well as an increase in the number of osteoblasts that recapitulate the phenotypic of sclerosteosis or van buchem disease.173,174 In addition, the mice with specific overexpression in osteocytes have shown increased levels of RANKL.175,176 In humans, alterations of SOST gene are linked to diseases such as sclerosteosis, van buchem disease and craniodiaphyseal dysplasia, an extremely rare sclerosing bone dysplasia, that is caused by missense mutations in SOST gene and decreases its extracellular secretion.177 SOST inhibits osteoblast function and stimulates osteocyte function by detaching bone formation and resorption. In addition, explorations done in vitro and in vivo have shown SOST to increase and promote adipogenesis by inhibiting the Wnt pathway.178, 179, 180 As a result, the findings show that SOST inhibits bone growth while also stimulating bone resorption.
SFRPS. SFRPS are secreted glycoproteins that share similarity with FZD receptors. They can bind to Wnt ligands and FZD receptors and eventually block both canonical and non-canonical Wnt pathways.181 SFRP1 and 2, amongst others, attach themselves to WNT5A whilst SFRP3 only prevents WNT1 and 8.182 In bone, during ossification process the Sfrp1 gene is overexpressed thus deactivating the canonical Wnt pathway and boosting both the osteoblast and osteocyte cell death.183,184 Sfrp1-KO mice have resulted with an increased bone trabecular area, a decrease in osteoblast apoptosis and enhancement of osteogenesis and mineralization.183,184 On the other side, when Sfrp2 gene was overly expressed, the mice resulted with an augmented production of mesenchymal stem cells and an abatement in apoptosis, thus inhibiting the accurate procession of osteogenesis and chondrogenesis. SFRP3 is also linked to mature osteoblastogenesis and programmed cell death (also known as apoptosis) promotion in mesenchymal stem cells.182,185 Both osteoblasts and osteoclasts hold an over-expressed level of SFRP4 in embryogenesis and postpartum periods, displaying the significance of this glycoprotein in the growth of bone and the loss of bone linked to stress oxidation and age.186,187 Gene alterations in SFRP4 are known to cause bone disorders such as metaphyseal chondrodysplasia (also known as the Pyle's disease), characterized flaring of the ends of long bones amongst other abnormalities.188 Upraised SFRP4 levels are linked to osteomalacia, characterized by a soft and more flexible bone.189
WIF-1. Wnt inhibitory factor-1 is an excreted Wnt inhibitor that acts as a negative feedback regulator of the Wnt canonical pathway. WIF-1 gene expression is increased during osteoblast differentiation as well as in the treatment of MSCs with Bmp 2.185,190,191 Alterations of WIF-1 gene are closely related to its methylation and this fact has been used to propose, Gossypol, for treatment against osteoporosis as it promotes bone proliferation through methylation and inhibition of the WIF-1 promoter.192 However, WIF-1 methylation is associated with bone tumors although it is epigenetically silenced in human osteosarcoma cell lines.190
Ultimately, a documentation of the roles of most Wnt signaling pathway constituents during osteoblastogenesis, osteclastogenesis and osteocytogenesis are outlined in Table 1. Gene variations of Wnt elements are linked to their respective physical compositions in mouse stereotypes. Their related human disorder is also presented in detail. These encompassing interpretations provided from both past and current research highlight the importance of Wnt signaling in assisting the differentiation of MSCs precursors into osteoblastogenesis as well as in impeding the bone development process.
Table 1.
Genetic alterations of Wnt signaling molecular components in mouse phenotype and their related human disease.
| Gene | Method | Cre line (Tissue) | Phenotype(s) | Reference | Related human skeletal disorder |
|---|---|---|---|---|---|
| Wnt 1 | KO | Germline | Homozygous resulted in perinatal death. Heterozygous resulted with mild osteopenia. | 31 | LOF mutations can cause early-onset of osteoporosis (OMIM: 166710) or osteogenesis imperfecta (OI) (OMIM: 166200) |
| Wnt 3 | cKO | RARβ-Cre (Forelimb Ectoderm) or MSX2-Cre (Hindlimb Ectoderm) | Resulted in the loss of forelimbs (RARβ-Cre) or hindlimbs (Msx2-Cre). | 193 | N/A |
| Wnt3a | KO | Germline | Heterozygous resulted in low amounts of BMD, BV/TV, Tb N, and a high amount of Tb Sp. | 43,44 | May be linked to Skeletal Dysplasia (OMIM: 618870) |
| Wnt 4 | KO | Germline | Homozygotes resulted in delayed chondrocyte maturation and early postnatal death. | 48,49 | N/A |
| Wnt 4 | cKO | Germline | Female mice resulted in a decreased femoral BMD. | 48,49 | N/A |
| Wnt5a | KO | Germline | Homozygotes resulted in delay in chondrocyte hypertrophy and skeletal ossification as well as perinatal death. Heterozygotes resulted in low BMD, BV/TV, Tb N, and a high Tb Sp. | 57,61 | Robinow Syndrome (OMIM: 180700) |
| Wnt5a | cKO | Germline | Osteoclast and bone formation as well as bone resorption. | 57 | N/A |
| Wnt5b | OE | Chondro-osteo progenitors | Resulted in a delayed bone ossification due to the overexpressed Wnt5b in chondrocytes. | 60,63 | Overexpressed Osteoarthritis in female patients (OMIM: 165720). |
| Wnt7a | KO | Germline | Frequent impaired limb development. | 65,69,70 | Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome (OMIM:276820); Santos syndrome (OMIM: 613005). |
| Wnt7b | cKO | Dermo1-Cre (Mesoderm) | Homozygotes resulted alive with a low ossification and anomalous chondrocytes and osteoblasts. | 37 | N/A |
| Wnt7b | KO | Germline | Resulted in delay in chondrocyte maturation, accelerated bone formation and perinatal death. | 41,71,73 | Linked to osteoarthritis (OMIM: 165720); Rheumatoid arthritis patients (OMIM: 180300). |
| Wnt9a | KO | Germline | Resulted in skeletal abnormalities, reduced mineralization and chondroid metaplasia and probable prenatal death. | 48 | N/A |
| Wnt 10a | KO | Germline | Resulted in decrease in bone mineralization, adipose tissue, bone marrow and dental formation. | 77,80 | Linked to odontoonychodermal dysplasia OODD (OMIM: 257980) |
| Wnt 10b | KO | Germline | Resulted in decrease in bone volume fraction, BMD, and tb.N. | 32,86,88 | Homozygous missense mutations are linked to split limb malformation, oligodontia (OMIM: 106600) as well as osteosarcoma (OMIM: 165660). |
| Wnt 16 | KO & cKO | Germline | Resulted in a decreased BMD level, cortex density, skeletal strength and an increased porous cortex. | 93,96 | N/A |
| Wls | cKO | OC-Cre (Osteoblasts) | Resulted in low BMD, trabeculae, calvaria and BV/TV. | 194 | N/A |
| Fzd2 | KO-LacZ | Germline | Homozygotes resulted less viable with smaller sized skeletal elements. | 195 | N/A |
| Fzd9 | KO | Germline | Resulted in low vertebral Tb N, osteoblastic surface, osteoid apposition, and mechanical strength in long bones. | 196 | LoF mutations may be linked to Williams-Beuren syndrome (OMIM: 194050) |
| Lrp4 | KO | Germline | Homozygotes resulted in perinatal death. | 197 | Cenani-Lenz Syndactyly Syndrome (OMIM: 212780) |
| Lrp5 | KO-Genetrap | Germline | Decreased BMC, BMD, as well as cortical and trabecular bone mass. | 103 | Osteoporosis-Pseudoglioma Syndrome (OMIM: 259770) |
| CGOF | DMP1-Cre (Osteocytes), PRX1-Cre (Limb mesenchyme) | Resulted in high bone mass with DMP1-Cre and high bone mass with PRX1-Cre. | 107 | Osteosclerosis, autosomal dominant (OMIM: 144750) | |
| GOF | Germline | Resulted in high in cortical bone mass, BV/TV, Tb N and very thick trabeculae with a low Tb Sp. | 107,198 | Osteopetrosis, autosomal dominant 1 (OMIM: 607634) | |
| CGOF | DMP1-Cre (Osteocytes), PRX1-Cre (Limb mesenchyme), COL1A1-Cre (Mature osteoblasts) | Resulted in high bone mass with DMP1-Cre, high bone mass with Prx1-Cre and no enhancement of bone mass with COL1A1-Cre. | 107,199 | High bone mass (OMIM: 601884) | |
| Lrp6 | KO-LacZ | Germline | Homozygotes resulted in close to perinatal death, shortening of the axial skeleton and limb defects. Heterozygotes resulted in a low BV/TV. | 113,200 | Associated with low bone mass (OMIM: 610947) in CAD. |
| Dkk 1 | KO | Germline | Homozygotes resulted in perinatal death, severe craniofacial malformation, and ectopic digits. Heterozygotes resulted in high BV/TV, mineral apposition rate, osteoblast surface, and mechanical strength. | 159,163 | Associated with rheumatoid arthritis (OMIM: 180300). |
| Dkk 2 | KO | Germline | Homozygotes were viable. Resulted in osteopenia with defective mineralization level and high osteoclast numbers. | 201 | N/A |
| Sost | KO-LacZ | Germline | Resulted in high bone mass. | 173,198,202 | Sclerosteosis 1 (OMIM: 269500) |
| cKO-Hypomorphic | COL1A1-Cre (Mature osteoblasts) | Resulted in increased BV/TV and osteoblast surface. | 203 | Van Buchem disease (OMIM: 239100) and Craniodiaphyseal dysplasia (OMIM: 112860) | |
| OE | Osteoblasts and osteocytes | Resulted in osteopenia with disarranged bone construction, thin cortex, low Tb.N and osteochondrodysplasia. | 204 | N/A | |
| Sfrp1 | KO-LacZ | Germline | Resulted in reduced age-related trabecular bone loss mostly in female mice. | 183 | N/A |
| Sfrp2 | KO | Germline | Resulted in truncated metacarpus and phalanges with a retarded ossification and a low rate of chondrocytic cells. | 205 | N/A |
| Sfrp3 | cKO | E2A-Cre (Germline) | Resulted in high BMD level, and cortex density. | 206 | N/A |
| Sfrp4 | OE | Various | Resulted in decreased rate of bone acquisition, BV/TV, and Tb size. | 207 | N/A |
| Dvl2 | KO | Germline | Resulted in viable mice with twisted tails, anomalous vertebras, and fusion in ribs. | 208 | N/A |
| Axin 1 | KO (Fu-TG1) | Germline | Homozygotes died at days 9.5. Heterozygotes had tail bisections and fused ribs. | 209 | Caudal duplication anomaly (OMIM: 607864) |
| Axin 2 | KO-LacZ | Germline | Resulted in skull bone fusion from high mineralization rate and bone ossification, and low chondrocyte growth zone. | 210, 211, 212 | Oligodontia-colorectal cancer syndrome (OMIM: 608615) |
| Apc | cKO | COL2A1-Cre (Chondro-osteo progenitors) | Homozygotes resulted in PNM, truncated limbs, abnormal craniofacial construction, highly abnormal vertebras, and immature chondrocyte and osteoblast cells. | 213 | N/A |
| Gsk3β | Germline | Resulted in nanism with truncated limbs and vertebras, and a COL2A1 under-expression. | 214 | N/A | |
| Gsk3β | Germline | Homozygotes resulted in neonatal mortality. Resulted in low skull ossification and craniofacial features. Heterozygotes resulted in high density of cortex and trabeculae bones. | 215, 216, 217 | N/A | |
| β-catenin | COL2A1-Cre (Chondro-osteo progenitors) | Homozygotes resulted in neonatal mortality with bone deficiencies with truncated limbs, loss of transverse tarsal joint leading to a fused bone and a dome-like skull. | 218 | N/A | |
| Tcfl (Tcf 7) | Germline | Homozygotes displayed viability. Resulted in low bone mass. | 219 | N/A | |
Notes: BMD, Bone mineral density; CAD, Coronary artery disease; CGOF, Conditional gain of function; COL1A1/COL2A1, Collagen, type I/type II, alpha 1; DMP1, Dentin matrix acidic phosphoprotein 1; E2a/Tcf 3, Transcription factor 3/E2A immunoglobulin enhancer-binding factors E12/E47; GOF, Gain of function; LOF, Loss of function; MSX2, Homeobox protein MSX-2; NA, Not applicable; OC, Osteocalcin; OE, Overexpression; OMIM, Online Mendelian Inheritance in Man; PNM, Perinatal mortality; PRX1, Peroxiredoxin 1; RARβ, Retinoic acid receptor beta; Tb.N, Trabecular number; Tb.Sp, Trabecular separation; TMD, Tissue mineral density.
Crosstalk between Wnt pathways and other signaling pathways in osteoblasts differentiation and bone metabolism
Multiple former and recent publications have investigated the integration of Wnt signaling pathway with other signaling pathways such as BMP, TGF-β, FGF, Notch, Hedgehog, and PDGF in osteoblast differentiation. This cross–functional interaction serves as a gene regulatory network that coordinates bone orientation, development, and flexibility, which are an attribute to the skeletal well-development (Fig. 2 and Table 2).
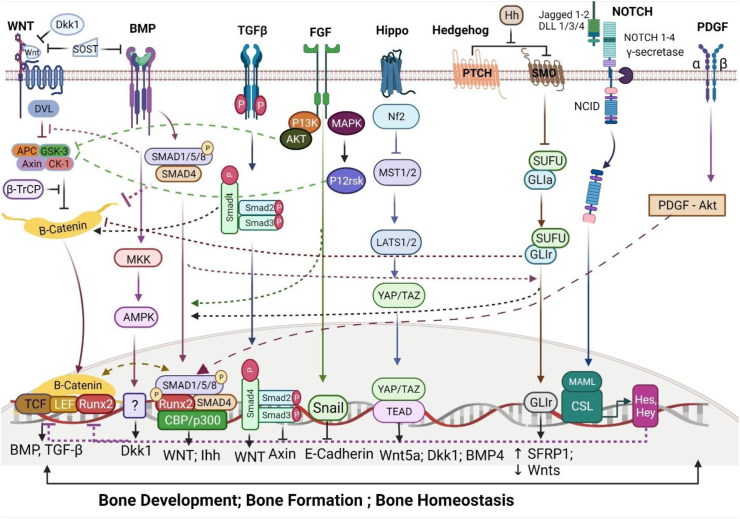
Figure 2.
Cross-functional signaling between Wnt and other signaling pathways in osteoblasts. Wnt and Bmp: Wnt/β-catenin signaling is stimulated by Bmp signaling activity by creating β-catenin/TCF/LEF/RUNX2 complex thus increasing the Wnt expression, inhibiting DVL and decreasing BTRC expression, oppositely Wnt signaling is inhibited when Bmp activates Wnt antagonist Dkk 1 and Sost expression thus preventing β-catenin translocation in the nucleus. Wnt and TGF-β: TGF-β stimulates Wnt ligands gene expression to increase and Axin protein gene expression to decrease via Wnt signaling activation. Wnt, BMP and FGF: FGF expression is increased by Bmp signaling and vice versa, thus indirectly increasing and decreasing DKK levels, respectively. The Fgf-mediated activation of P13K-AKT pathway or MAP kinase pathway causes the GSK-3β to phosphorylate and further translocate β-catenin into the nuclei while the inhibition of SNAIL phosphorylation hinders the E-cadherin-mediated translocation of β-catenin into the nuclei. Wnt and Hippo: In the nucleus, YAP forms transcriptional complexes with TEADs (Transcriptional enhanced associate domains), inducing downstream gene expression. Wnt and Hh: Smoothened (SMO) catalyzes a series of reactions in order to release glioma-associated oncogene (GLI) from the deactivated compound and translocate it to the nucleus to operate target gene expression. During the post-translational modification (PTM) phase, GLI and Suppressor of fused homolog (SUFU) negatively regulate the Hh signaling and hinder β-catenin to translocate to the nuclei, while Smoothened positively promotes β-catenin production. Wnt and Notch: Notch proteins initiate the expression of Hes and Hey genes thus hindering the well-functioning of Wnt signaling and restricting β-catenin-mediated transcription. Wnt and PDGF: PDGF signaling pathway can stimulate the SMAD1/5/8/RUNX2 complex to induce gene production and guide the differentiation of bone.
Table 2.
Cross-functional signaling between Wnt and other signaling pathways in osteoblasts.
| Molecules of respective pathway | Crosstalk signaling target molecules | Molecular mechanism's results | Reference |
|---|---|---|---|
| BMP and WNT | SMAD complex with TCF/LEF/β-catenin | Increased osteoblast differentiation | 256,257 |
| TGFβ1 | WNTs increased, Axin 1/2 decreased | Increased chondrocyte differentiation, Decreased adipocyte differentiation | 258,259 |
| TGF-β1 stimulation | Increased BMP2 | Stimulates ectopic bone formation | 260 |
| Decreased level of SMAD4 | β-catenin activity increased | Increased bone formation | 261,262 |
| BMPR1A stimulation | SOST increased, increased DKK1 stimulates β-catenin to decrease | Decreased bone mass | 263 |
| BMP2 | Decreased β-TRCP stimulates β-catenin to increase. | Increased osteoblast differentiation, Increased chondrocyte hypertrophy. | 264,265 |
| FGF-FGFR3 | TGF-β | Mediates embryonic bone formation | 266 |
| FGFR stimulation | Increased BMP2 and TGF-β1 | Increased osteogenic expression | 267 |
| FGR2 stimulation | Increased Bmp-induced osteogenesis | Increased osteoblast differentiation | 268,269 |
| BMP2 stimulation | Increased Fgf-induced osteogenesis | Increased osteoblast differentiation | 270 |
| Notch | Increased Bmp-induced ALP | Stimulated SMAD and Notch | 271 |
| Ihh stimulation | Increased Bmp-induced osteogenesis | Bone formation | 272 |
| Ihh and Bmp stimulation | Increased ALP; Increased Ihh | Long bone development | 272 |
| YAP | β-catenin | Increased β-catenin transcriptional activity and downstream genes | 273,274 |
| TAZ | β-catenin, DVL | Knockdown of TAZ significantly increased DVL phosphorylation and β-catenin nuclear levels | 275 |
| MST1/2 | β-catenin | Deletion of MST1/2 led to activation of β-catenin | 276 |
| GLI stimulation | Increased BMP2 | Normal osteoblast differentiation | 277 |
| PDGF | Increased MSCs | Highly mitogenic factor and chemotactic for MSCs, osteoblasts and perivascular cells | 278 |
Notes: BMP, bone morphogenetic protein; FGF, fibroblast growth factor; GLI, glioma-associated oncogene; Ihh, Indian hedgehog; MSCs, meristematic stem cells; MST1/2, macrophage-stimulating protein; TGF-β, transforming growth factor-β; PDGF, platelet-derived growth factor; TAZ, transcription adaptor putative zinc finger; YAP, Yes-associated protein.
Wnt and Bmp signaling in osteoblasts
Wnt and BMP pathway interact in mesenchymal cells during chondrocyte or osteoblast differentiation. For instance, BMP2 can promote differentiation of chondrogenic cells while β-catenin has no effect or even inhibits the mechanism.220,221 Moreover, BMP2 blocks ALP (Alkaline phosphatase) induction while, the Wnt/β-catenin pathway plays a direct role in upregulating ALP induction in mesenchymal cells. β-Catenin interacts with SOX9, that mediates the transcription of collagen, and causes ubiquitination-mediated degradation in chondrocytes.222, 223, 224 However, BMP2 inhibits β-catenin–SOX9 interaction by activating p38 MAPK factor suggesting that BMP signaling indirectly promotes chondrogenesis by blocking Wnt/β-catenin signaling. Wnt signaling can repress BMP-induced activation by over-expressing Dkk1 gene that automatically enhance BMP4-triggered apoptosis in vertebrate limb development thus indicating that Wnt signaling inhibits apoptosis induced by BMP signaling.225 Other results confirm the cooperative interaction between SMAD1/4 and β-catenin in gene expression during mesenchymal cell differentiation.226 Also, the loss of bone morphogenetic protein receptor type 1a (Bmpr1a) gene in both osteoblastic and osteocytic cells has shown to block the proper expression of various metabolic-promoting Wnt genes in the cortex and bring about an overly-expressed Wnt7b gene that displayed an effective operation in completely rescuing the deficiency in periosteum's growth.227,228 These data illustrate the functional role of Wnt signaling in BMP2-regulated mesenchymal cell differentiation.
Wnt and TGF-β signaling in osteoblasts
β-Catenin signaling pathway is a process through which TGF-β regulates osteoblastogenesis in hMSCs (human mesenchymal stem cells). Through RNAi approaches, was shown that TGF-β1 uplifted the amounts of β-catenin and augmented β-catenin- T-cell factor/lymphoid enhancer factor transcription rates in human mesenchymal stem cells through both SMADand non-SMAD pathways. Moreover, it was shown that after knockdown of β-catenin with siRNA, transforming growth factor beta 1 (TGF-β1), activin receptor-like kinase 5 (ALK-5), protein kinase A (PKA) and Jun N-terminal Kinase (JNK), hinder osteoblastogenesis in hMSCs in vitro. In recent findings it was demonstrated that when mesenchymal progenitor cells are treated with TGF-β, a β-catenin-responsive firefly luciferase reporter plasmid (also known as TOPflash), is activated and eventually β-catenin is translocated into the nucleus without the activation of Wnt signaling pathway.229 Also, cooperation between TGF-β and WNT3A led to high amounts of nucleic β-catenin. Transcriptionally, SMADs and β-catenin can form a complex that is imported into the nucleus to trigger osteoblast-dependent gene expressions however, further investigation is needed to better-understand this interaction.
Wnt, Fgf and Bmp signaling in osteoblasts
During limb development, a regulatory system displayed by FGF, Wnt and BMP signaling interaction is observed to manage the cell proliferation and development.230 This regulatory system induces cell death in the anterior margin of the limb by either inhibiting Wnt or FGF signaling or by activating Bmp signaling through failing in regulating Dkk, Fgf 8 or Bmp 4 expression.231 Through experiments, the repression of FGF signaling is considered crucial in inducing DKK gene expression, by hindering its expression. Similarly, DKK together with BMP4 hinder Fgf8 gene expression, playing a dominative role in apoptosis.231 Glycogen synthase kinase 3 (GSK-3β) also functions as an intermediator in assisting the Wnt-FGF signaling amalgamation.232 Moreover, osteoblast differentiation during bone development can be stimulated by Fgf 2 via Wnt pathway where Fgf2 deletion reduced the expression of WNT10B, LRP5 and β-catenin genes.233 During exogenous Fgf2 administration in marrow stromal cells of Fgf2-KO mice, β-catenin was accumulated in nucleus thus increasing the effect of mineralization.233 In the absence of endogenous Fgf2, SOST mRNA and SOST protein expression were increased thus reducing osteoblast differentiation and bone development in Fgf2-KO mice.234
Wnt and Hippo signaling in osteoblasts
Wnt and Hippo signaling cooperate to regulate each other's activity for precise regulation of biological phenomenon. YAP/TAZ (Yes-associated protein 1/Transcription adaptor putative zinc finger) interact with β-catenin and DVL and regulate their levels and activities.235 Apparently, there is a combinational interaction of YAP/TAZ co-activators in promoting skeletal formation. The absence of genes coding for the transcriptional YAP/TAZ proteins has shown to decrease bone size therefore causing a condition similar to osteogenesis imperfecta in Osx-Cre mouse line.236 Yap and/or Taz gene deletion in Osx-Cre mouse line has also shown to produce a damaged thickness in periosteum by negatively affecting the process of differentiation in osteo-chondro-progenitor cells.237 Other transgenic mouse lines with a Yap/Taz deletion, such as Prx-Cre and Dmp-Cre, have resulted in fetal death and a disbalance in the osteoblast/osteoclast cells ratio with a low number of osteoblastic cells and a high number of osteoclastic cells, respectively.238 This phenomenon occurs due to the suppressive effect of YAP/TAZ co-activators in osteo-chondro-progenitor cells that cause the osteoblasts number to decrease and a promotive effect in adult osteoblastic cells that cause the osteoblasts number to increase and positively enhance the skeletal development and fracture repair.238,239 Altogether, the given data identify YAP/TAZ as key transcriptional regulators in osteoblast, osteoclast, and osteocyte mediated bone remodeling.236,240
Wnt and hedgehog signaling in osteoblasts
Wnt and Hh signaling integration is pivotal for the development and differentiation of embryogenesis. During the limb regeneration process, Hh pathway inhibits the activation of Wnt signaling241 and hinders the expression of β-catenin in order to cause cartilaginous disorders.242 The Ihh signaling activation during bone repair is another important evidence that demonstrates the synergetic interaction of these two pathways.243 While Wnt signaling pathway's activity is enhanced in mature osteoblast cells, the Ihh signaling pathway's activity is enhanced in early phases of osteoblast cells differentiation in order to promote bone repair and formation.243 The Ihh signaling is also highly operative in skeletal cancerous cells and the deletion of PTCH1 gene (protein patched homolog 1) causing the benign bone tumor of enchondroma and the malignant neoplasm known as osteosarcoma, demonstrates that. This phenomenon occurs while PTCH1 deletion enhances the WNT5A and WNT6 ligands expression causing the β-catenin's activity to increase and originate bone abnormalities.244
Wnt and Notch signaling in osteoblasts
Wnt and Notch signaling pathways are two highly-cooperative pathways that well-manage cell fate proliferation especially the bone cell formation process.245 In human cells, both inactive and active form of Notch signaling pathway when limited to the membrane fraction and nucleus can inhibit Wnt/β-catenin signaling by seizing β-catenin through endocytosis to prevent its interactivity with T cell factor/lymphoid enhancer factor family (TCF/LEF). The nuclear-limited Notch binds to β-catenin forming a composite that interrupts Wnt/β-catenin signaling and restricts β-catenin-induced transcription through the expression of downstream target genes HES (Hairy and enhancer of split-1) and HEY (Hairy/enhancer-of-split related with YRPW motif protein) repressors.246 When the activation of HES and HEY repressors is blocked, there is no displayed modification in the function of Notch intracellular domain (NICD), in order to inhibit Wnt signaling.246 In bone cells, the link between these two pathways is represented by JAG1, one of the five surface-interactive ligands of Notch pathway,247 and osteocytes with JAG1 deletion containing a higher amount of other Notch ligands and receptors, demonstrates this cooperation. Also, Notch stimulates β-catenin's activity by hindering the GSK-3β activity in order to stabilize the β-catenin-activator SNAIL (Zinc finger protein SNAI1).248,249 Commonly, Wnt and Notch have opposite functions and whenever one of them is obstructed, the other pathway reacts to the Wnt–Notch interaction.250 Among other factors, HIF-1α (hypoxia-inducible factor-1α) is one that upregulates the NICD domain levels and promotes Notch pathway but, on the other side, it obstructs the activity of Wnt pathway therefore modifying the growth of osteoblast cells.251
Wnt and PDGF signaling in osteoblasts
Platelet-derived growth factor receptor alpha (PDGF-Rα) is necessary for a normal development of skull and face bones.252 In general, PDGF-Rβ can potentially regulate the role of MSCs however and reduce mitogenesis and enhances osteogenesis.253 PDGF-R is involved in osteoblatogenesis process mediated by WNT3A in MC3T3-E1 cells (subclone 4 pre-osteoblasts) with PDGF-R reducing osteoblast proliferation induced by the WNT3A ligand.254 PDGF-R is transactivated by WNT3A ligand via SRC (non-receptor tyrosine kinase) anchoring to DVL2 and stimulating the activity of its tyrosine kinases.255 Moreover, WNT3A binds to LRP5/6-FZD receptors and activates DVL, which stimulates β-catenin-independent pathways via the transactivation of PDGF-R in osteoprogenitors and osteoblastic cells and therefore result in bone formation increment.254
Osteoblastic utilization of glucose, glutamine and fatty acids via Wnt-stimulation
Wnt stimulates β-oxidation in osteoblasts
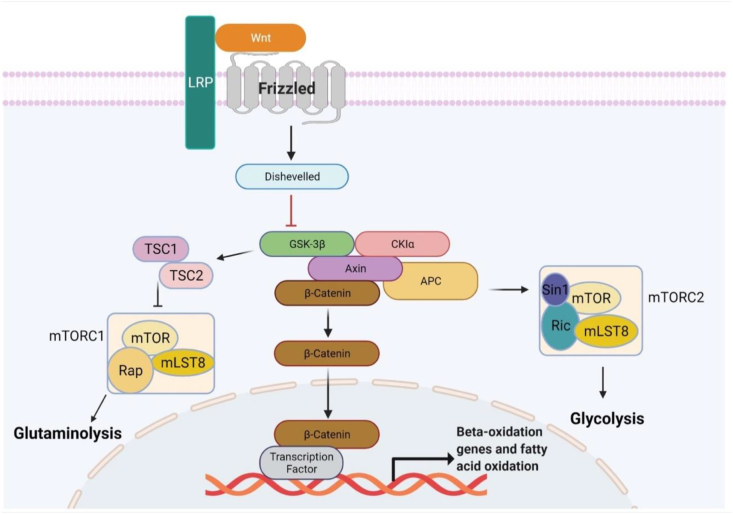
Wnt-mediated mechanisms of metabolism in osteoblastic cells were observed to maintain LRP5 and LRP6 effectors' intersecting activities.279 LRP5 variants developed modifications in body configuration not seen in the LRP6 variants such as white adipose tissue depots increased in size and whole-body energy expenditure reduced. The genetic fingerprinting of osteoblastic cells containing defective LRP5 genes displayed physical traits of varied triglyceride catabolic reaction caused by an under-expressed gene activity taking place in mitochondria in deviant osteoblasts during the saturated fats oxidation.279 Frey et al280 explored if the operation of this mitochondrial type of oxidation executed by LRP5 gene was associated with β-catenin's stimulation. Treatments of primary osteoblasts in vitro demonstrated that only WNT3A, WNT10B and WNT16 ligands were shown to be linked to β-catenin activation, suggesting the canonical mechanism to be the cause of alteration induction. On the other side, mature osteoblastic cells that were missing the activation of β-catenin, encountered a hindering of osteoblastic cells' further development as well as a significant depletion in oxidative reactions and adenosine triphosphate's accumulation, in spite of glycolysis activity being enhanced. Anyhow, the manipulated β-catenin in vivo, resulted in loss of function for LRP5 as well as an increase in adipose tissue mass and serum fatty acids (Fig. 3).280
Figure 3.
Glucose, glutamine and fatty acids utilization in osteoblasts via Wnt signaling regulation. Wnt ligand binds to FZD and LRP5/6 receptors which leads to degradation compound's deactivation, which is composed of Dishevelled, Axin, GSK-3β, APC's and CKlα (casein kinase 1). Through this mechanism, in mature osteoblastic cells, β-catenin's amount increases in the cytoplasm then translocates to the nuclei and assisted by the TF (also known as sequence-specific DNA-binding factor), the mitochondrial fatty-acid β-oxidation gene expression initiates. In osteogenic cells, mTORC1 signaling pathway is stimulated by Wnt pathway and causes the amount of glutamine amidohydrolase, the initiative enzyme in the glutaminolytic pathway, to increase. Wnt pathway also stimulates mTORC2 signaling pathway in order to adjust the proper number of proteins engaged in the glycolytic pathway. Notes: MLST8, mTOR associated protein, LST8 homolog; RAP, RAS/RAP GTP-binding protein; RIC, resistance to inhibitors of cholinesterase; SIN1, stress activated protein kinase interacting protein 1; TSC1/2, tuberous sclerosis proteins 1 and 2.
Kim et al281 examined the skeletal and metabolic phenotypes of a mouse model to investigate the requirements and contributions of lipid homeostasis in bone development and vice versa and eventually, the study demonstrated the disruption of the expression of carnitine palmitoyl transferase 2 (CPT2), a highly required enzyme for mitochondrial fatty-acid β-oxidation in osteoblasts and osteocytes being caused by CPT2 deficiency. However, the seriousness of the matter was determined by the sex of the mutant. Cpt2-male-mouse-variants demonstrated an impermanent low mechanical competence in trabeculae or Tbvf (also known as the trabecular bone volume fraction). On the other side, Cpt2-female-mouse-variants showed a low mechanical competence in trabeculae in thighbones (distal femur) and the fifth lumbar spine vertebrae (L5) all the time. It is important to point out that Cpt2-female-mouse-variants cumulated osteoid with an enhanced adjustment period (lag period) during the mineralizing process. The sex dimorphic phenotype is linked to whether the estrogen hormone adjusts fuel selection according to both in vivo and in vitro studies. In male mutants' CPT2 deficient osteoblasts, cultured in vitro, the glucose uptake was increased, which was not shown in female mutants’ CPT2 deficient osteoblast cultures treated with exogenic estrogen.281 Similarly important is the fact that the abnormal amount of lipids (dyslipidemia) has shown significant alterations in bone metabolism equilibrium and caused trabeculae to decline in hyperlipidemic and high cholesterol diet mice.282 Altogether, these studies illustrate the importance of fatty acid catabolism in normal osteoblast function and bone mass development which is highly influenced by both sex and food diet.
Wnt stimulates glycolysis in osteoblasts
Glucose is the vitality origin for most mammalian cell types and here it is required to normalize osteoblast function. Wnt signaling pathway is intrinsically linked to carbohydrate metabolism, therefore, Esen and colleagues283 illustrated the increase of carbohydrate attainment via the stimulation of various gene expressions, such as glucose transporter 1 (GLUT1), HK2 (known as hexokinase II), PDC kinase (known as pyruvate dehydrogenase complex kinase) and lactic acid dehydrogenase (LDH) via WNT3A and WNT10B ligands. This was shown to be more efficacious than the usage of insulin-cellosaurus cell line (ST2) as a stimulant. Interestingly enough, it was shown that the Wnt3a stimulated lactate production suggesting that Wnt signaling activated aerobic glycolysis instead of oxidative phosphorylation.284
Esen et al283 also demonstrated via gene knockdown studies, that in both lrp5−/− mice and those in which Lrp5 expression was eradicated by Osterix-Cre, displayed a reduction in glycolytic enzyme expression in bone which did not continue via alterations in the activity of either GSK3β or β-catenin activity because these effectors were altered as inhibitors and did not impact glucose utilization. Alternatively, the mammalian target of rapamycin complex 2 (mTORC2) was promoted by the activity of WNT3A ligand assisted by the RAC Family Small GTPase 1 (RAC1) and consequently enhancing the carbohydrate utilization via gene overexpression (Fig. 3).
Through RNA-Seq technique, it was demonstrated that the increased number of genes caused by Wnt3a-stimulated cellosaurus cells (ST2) was exceeded by the number of genes with a decreasing effect in gene expression.285 Gene downregulation is linked to chondrocytic and adipocytic differentiation identified via CCAAT and CEBPA detectors. To conclude, Wnt signaling controls the genomic landscape and fate-specification of osteoprogenitors by hindering carbohydrate to enter the Krebs or citric acid cycle whereby both citric acid and acetyl coenzyme A catalyzation are decreased.
Wnt stimulates glutaminolysis in osteoblasts
Glutamine is the most abundant free amino acid in circulation and is not only an important oxidative fuel, but also a precursor for the synthesis of non-essential amino acids, nucleotides, and the antioxidant. Experimental research has put forward the necessity for GLN (glutamine) addition in order to assist the process of osteoblast-mediated ossification.286
The stimulation of the ST2 cell is caused by Wnt factors during osteoblast differentiation and bone associated with glutamine metabolism.287 This stimulation increased glutamine content. Glutamine catabolism, resulted from toxicological hindrance of glutaminase catalyzation, was considered necessary for stimulating osteoblastogenesis in an artificial environment and enhancing BMD via the Hemoglobin subunit mu (Hbm) gene well-expression found in the LRP5 cell surface receptor in the living body of an Hbm-variant. Simultaneously, the “fight or flight” reaction and the over-expression of particular genes encoding for protein utilization and their three-dimensional structure acquisition were initiated by glutaminolysis. Furthermore, research on this mechanism, stipulate that the mTORC1 (mechanistic target of rapamycin complex 1) pathway stimulates glutamine usage, therefore, glutamine attainment and consumption in response to Wnt signaling constitutes a molecular resistance mechanism that sustains metabolism, ER (endoplasmic reticulum) status287 and oxidoreduction equilibrium.288
The association of Wnt signals with various human skeletal disorders and their forms of treatment
Taking into consideration to date therapeutical advancements related to Wnt signaling pathway, we elaborate the association of numerous muscular-skeletal maladies with Wnt ligands, recent and innovative prospective forms of treatment, pre-symptomatic observations acquired by both human and animal samples, potential novel therapies, and lastly the aspects at issue that should be taken into consideration when performing clinical interventions (Table 3).
Table 3.
The association of Wnt signals with various human skeletal disorders and their forms of treatment.
| Human skeletal disease | Wnt-related molecules involved | Results of mutations | Existing therapies | Potential novel therapies | Clinical issues | Reference |
|---|---|---|---|---|---|---|
| Osteoporosis (OR) | LRP5, WNT3Aa upregulation, SOST overexpression. | High bone resorption and low bone formation caused by agedness and reduced amount of estrogen. | Drugs like Raloxifene (also known as SERM), diphosphonates, and denosumab (monoclonal antibody therapy) hinder bone resorption. | Anti-Sost monoclonal antibody (Romosuzab, BPS804, etc) stimulating bone formation and suppressing bone resorption by enhancing osteoblast differentiation and OCIF/OPG production, treating HPP and OI. | Romosozumab treatment is linked to cardiovascular incidents. | 289, 290, 291, 292, 293, 294, 295, 296, 297, 298, 299, 300, 301, 302 |
| Teriparatide (parathyroid hormone PTH) an effective anabolic agent promotes bone remodeling. | Caveolin-3 serving as a biological indicator by enhancing ossification and inhibiting OR. | A mixture of antibodies that attack both DKK1 and SOST has shown great possibilities to be effective in the enhancement of bone-rupture healing. | ||||
| Osteoarthritis (OA) | β-catenin activation, WNT7B overexpression and SOST expression. | Joint-related (Articular) cartilaginous tissue impairment, Development of bone spur (osteophyte), and solidification of the under-cartilaginous-bone (subchondral). | Treatment with P.O NSAIDs and HA joint shot (injection). | Sirolimus (Rapamycin) decreasing MMP13 gene expression and reducing cartilaginous demolition. | The systematic hindering of Wnt signaling pathway has shown possibilities of affecting the bone remodeling process non-positively. | 73,303, 304, 305, 306, 307, 308, 309 |
| Small Wnt pathway inhibitor molecules (like SM04690) protecting cartilaginous tissues from demolition. | ||||||
| Mianserin, an anti-R-spondin 2 agent, inhibiting the abnormal functioning of Wnt pathway in OA. | ||||||
| Rheumatoid Arthritis (RA) | TNF-α induction of DKK1 expression and Sost expression; Increased WNT5A expression; | Bone resorption stimulation; Erosive Arthritis (Bone erosion); Pro-inflammatory cytokine expression enhancement; Cartilaginous demolition via MMPs production stimulation by Wnt5a ligand. | MTX treatment (a traditionally synthesized DMARD) and other biological DMARDs functioning as anti-inflammatory cytokines factors. | Possible treatment of OCD (easing the pain caused by cartilaginous demolition and inflammation) by hindering Wnt5A gene expression via the WNT5A/ROR2 signaling pathway. | The monoclonal antibody treatment with Denosumab have no apparent premises on giving positive results in cartilaginous demolition. | 310, 311, 312, 313, 314, 315, 316, 317, 318, 319, 320, 321, 322, 323 |
| Decreased SFRP5 expression. | ||||||
| Osteosarcoma/OS (Primary Bone Tumors) | WIF-1; DKK3; SFRP2. | Metastatic cell proliferation facilitation by both Wnt ligands and inhibitors. | Standardized treatment using MMT, combination CTx, RTx, and surgery. | GSK-3β treatment has shown great premises in suppressing the further-production of osteogenic sarcoma cells by activating the Wnt signaling pathway. | The inhibitory function of GSK-3Β is done through a distinct Wnt-independent mechanism that calls for additional research in order for actual application to take place. | 324, 325, 326, 327, 328, 329, 330, 331 |
| Virus-like-particle-based anti-HER2/neu vaccine as well as CAR-T cells also show great premises for the future. | ||||||
| Multiple Myeloma (MM) | SOST overexpression. | Osteolytic lesions from the high concentration of SOST. | Treatment of osteolytic lesions with Zoledronic acid (also known as Zometa) and Denosumab (monoclonal antibody treatment). | Antibodies targeting Sost and suppressers targeting a protein complex (also known as proteasome inhibitor) integrated has displayed an acceptable method for treating osteolytic lesions and decrease the proliferation of metastatic cells. | There are concerns whether the usage of antibodies targeting SOST in MM patients aggravate the MM disease by increasing the proliferation of metastatic cells. | 332, 333, 334, 335 |
| Bone Metastasis | DKK1 overexpression | Osseous metastatic disease (bone metastasis) stimulation caused by osteoclastic cells high-differentiation. | Usage of Zoledronic acid and Denosumab to treat bone lesions. | The expression of DKK1 gene serving as biological indicator to predict and prevent the osseous metastatic disease in the upcoming years. | Further research is needed when considering if bone metastasis and other tumors are biologically affined or not. | 336,337 |
Notes: CAR-T, chimeric antigen receptor T cells; CTx, chemotherapy; DMARDs, disease-modifying antirheumatic drugs; HA, hyaluronic acid; HPP, hypophosphatasia; MMP13, matrix metallopeptidase 13; MMPs, matrix metalloproteinases; MMT, multi-modal therapy; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; OCD, osteochondritis dissecans; OCIF, osteoclastogenesis inhibitory factor; OI, osteogenesis imperfecta, P.O, per Os (oral administration); OPG: osteoprotegerin; RA, rheumatoid arthritis; RANKL, receptor activator of nuclear factor kappa-Β ligand; SERM, selective estrogen receptor modulators; RTx, radiotherapy; HER2/neu, human epidermal growth factor receptor 2.
Therapeutic targeting of Wnt signaling components in clinical trials
Wnt molecules are implicated to well-maintain bone mass, repair bone fractures, and provide remission for tumor treatments during separate phases.30,338 ETC1922159 and WNT-974 (selective molecule inhibitors of O-acyltransferase porcupine) reach the first phase of clinical trial and palmitoleate Wnt ligands to moderate the β-catenin productivity and maintain the uncontrolled cell-growth,339, 340, 341 Ipafricept (IPA) (transgenic integrating protein containing an extracellular human frizzled 8 receptor) engages with a Cr-domain (Cysteine-rich) of Wnt ligand and eventually hinders the activation of the Wnt signaling,342, 343, 344 Tabituximab barzuxetan (monoclonal immunoglobulin G1 targeting frizzled 10 receptor), reaching the first phase of clinical trial, experiments have shown a provision of potent remission for synovial sarcoma in mice stereotype,345,346 Vantictumab (monoclonal immunoglobulin G2, anti-frizzled receptor) having completed the first phase of clinical trial, hinders the Wnt signaling by blocking the binding of WNT3A ligand to frizzled receptors (FZD1, FZD2, FZD5, FZD7, FZD8),347, 348, 349 Rosmantuzumab (monoclonal immunoglobulin G1 anti-human R-spondin 3) being on its clinical trial, targets Rspo3 to hinder its binding to the leucine-rich repeat-containing G-coupled receptors (LGRs) and prevent the activation of RSPO-LGR pathway thus inhibiting the proliferation of tumor cells in acute myeloid leukemia.30,350 Foscenvivint/PRI724 (inhibiting receptor that targets β-catenin and hinders the transcriptive activity of cAMP response element-binding protein) effectively inhibits the Wnt signaling pathway by decreasing the level of β-catenin in pluripotent human embryonal carcinoma cisplatin-resistant germ cell tumor line,351, 352, 353 and Zilovertamab/Cirmtuzumab (monoclonal immunoglobulin G1, anti-receptor tyrosine kinase like orphan receptor Ror-1) blocks the activity of WNT5A ligand and hinders cell-proliferation in chronic lymphocytic leukemia354,355 amongst others, are overly suggested to be utilized in the clinic.
In skeletal disorders treatment, targeting the Wnt signaling pathway is validated to be clinically sufficient. Gene alterations encoding for porcupine inhibitors such as WNT974 and WNT-C59 (inhibitor for Wnt3a-mediated activation of T-cell factor binding site) are documented for their detrimental outcomes in decreasing bone ossification and increasing bone remodeling activity thereby causing various skeletal disorders356 but with an efficacy of alendronate used in patients reported.
It is reported, through clinical observations, on the development of sclerostin moAbs (monoclonal antibodies) targeting and counterpoising sclerostin as an approving pharmacological method due to its abilities to enhance BMD, reduce bone-rupture risks and promote metabolic activity in mouse models.293,357 It is intriguing that antisclerostin compounds increase bone formation while decreasing bone resorption in contrast to teriparatide, the only osteoanabolic agent currently approved in the USA for treating osteoporosis. Antisclerostin therapy has led to improved clinical outcomes with a favorable balance of benefits and risks, therefore this approach to osteoporosis treatment is now a welcomed addition to current options.
Utilizing other agents in targeting the Wnt signaling cascade has also shown effectiveness.
A flavonol found aplenty in herbs, named Quercetin (nonspecific protein kinase enzyme inhibitor), was shown in a recent study, functioning as an antioxidant agent enhancing the production of osteocytes and hindering the death of osteoblast cells amongst other functions. However, there are a few underlying negative results in bone coming from its utilization which require further investigation.358 Recent report has demonstrated, Polydatin, also known as Piecid, a stilbenoid glucoside derived found in grape juice, to enhance the production of human bone marrow mesenchymal stem cells in osteogenic cells via the crosstalk between Wnt pathway and BMP2 pathway.330 Another new report has shown the significance of a WIOTM (Wnt-induced osteogenic tissue model) “band-aid” capable of being transplanted in maintaining human skeletal stem cells and repairing bone defects in mice thus demonstrating the therapeutical significance of Wnt signaling manipulation in clinic.359
Summary and perspective
Bone well-development and homeostasis is vital for health maintenance, and it is highly regulated by the Wnt signaling pathway known to orchestrate the osteoblast differentiation, bone growth and repair. This review coordinates the studies on the critical role of Wnt signaling pathway for the attainment of normal bone mass and structure and their significance in generating the energy in osteoblasts-lineage cells. The identification of specific and well-conserved regulatory proteins and different signaling pathways involved at various steps of this pathway designate that the Wnt-related processes are as tightly regulated as they are highly predisposed to gain alterations and be linked to pathological processes and development of numerous abnormal skeletal conditions. Supplementary research is highly necessary to further inquest the contributions of Wnt signaling pathway on osteoblast metabolism, bone homeostasis and the energy balance of the body. The compelling evidence emerging from newly reported studies will be highly embraced and utilized for novel therapeutics design to treat or alleviate skeletal disorders or bone diseases in the clinic. In the upcoming years, researchers dedicated to carcinogenic therapies and bone disorder operation should gain a better understanding on bone-related-affairs and further supply with relevant and applicable pre-emptive inventions. In spite of all that, aspiration, and expectation for the expansion of accurate understanding and execution in this discipline and especially on Wnt signaling pathway, will always remain increasingly serviceable to human beings.
Author contributions
RV: data analysis and literature formation. GC, MW, and XZ: supervision and grant holder.
Conflict of interests
The authors declare no conflict of interest.
Funding
This work was supported by grants by Zhejiang Qianjiang Talent Program (No. 21040040-E), the Department of Sci-Tech of Zhejiang Province (No. LGF19H140002), a startup grant from Zhejiang Sci-Tech University (No. 18042290-Y, and 2021Q031), funds from National Natural Science Foundation of China (No. 81900806, and 81400489), the basic Public Welfare Planning Project of Zhejiang Province (No. LGD20C040001) and Jiaxing Science Technology Foundation (No. 2020AY10001) and Jiaxing Key Laboratory of Animal Model Generation and Precise Synthesis of New Drug Leads.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Mengrui Wu, Email: mengruiwu@zju.edu.cn.
Guiqian Chen, Email: gqchen@zstu.edu.cn.
Abbreviations
- BMD
Bone mineral density
- BMP
Bone morphogenetic protein
- CAD
Coronary artery disease
- CAR-T
Chimeric antigen receptor T cells
- COL1A1/COL2A1
Collagen, type I/type II, alpha 1
- CGOF
Conditional gain of function
- CTx
Chemotherapy
- DMARDs
Disease-modifying antirheumatic drugs
- DMP1
Dentin matrix acidic phosphoprotein 1
- E2A/TCF3
Transcription factor 3/ E2A immunoglobulin enhancer-binding factors E12/E47
- FGF
Fibroblast growth factor
- GLI
Glioma-associated oncogene
- GOF
Gain of function
- HA
Hyaluronic acid
- HER2/neu
Human epidermal growth factor receptor 2
- HPP
Hypophosphatasia
- Ihh
Indian hedgehog
- LOF
Loss of function
- MLST8
mTOR associated protein, LST8 homolog
- MMPs
Matrix metalloproteinases
- MMP13
Matrix metallopeptidase 13
- MMT
Multi-modal therapy
- MSCs
Meristematic stem cells
- MST1/2
Macrophage-stimulating protein
- MSX2
Homeobox protein MSX-2
- MTX
Methotrexate
- NSAIDs
Non-steroidal anti-inflammatory drugs
- OC
Osteocalcin
- OCD
Osteochondritis dissecans
- OCIF
Osteoclastogenesis inhibitory factor
- OE
Overexpression
- OI
Osteogenesis imperfecta
- OMIM
Online Mendelian inheritance in man
- OPG
Osteoprotegerin
- PDGF
Platelet-derived growth factor
- PNM
Perinatal mortality
- P.O
Per Os (oral administration)
- PRX1
Peroxiredoxin 1
- RA
Rheumatoid arthritis
- RANKL
Receptor activator of nuclear factor kappa-Β ligand
- RAP
RAS/RAP GTP-binding protein
- RIC
Resistance to inhibitors of cholinesterase
- RARβ
Retinoic acid receptor beta
- RTx
Radiotherapy
- SERM
Selective estrogen receptor modulators
- SIN1
Stress activated protein kinase interacting protein 1
- TAZ
Transcription adaptor putative zinc finger
- Tb.N
Trabecular number
- Tb.Sp
Trabecular separation
- TGF-β
Transforming growth factor-β
- TMD
Tissue mineral density
- TSC1/2
Tuberous sclerosis proteins 1 and 2
- YAP
Yes-associated protein
References
- 1.Downey P.A., Siegel M.I. Bone biology and the clinical implications for osteoporosis. Phys Ther. 2006;86(1):77–91. doi: 10.1093/ptj/86.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Datta H.K., Ng W.F., Walker J.A., Tuck S.P., Varanasi S.S. The cell biology of bone metabolism. J Clin Pathol. 2008;61(5):577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 3.Florencio-Silva R., Sasso G.R., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D., Xie R., Shu B., et al. Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann N Y Acad Sci. 2019;1442(1):48–60. doi: 10.1111/nyas.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karsenty G., Kronenberg H.M., Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 6.Bonewald L.F. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robling A.G., Bonewald L.F. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. doi: 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims N.A., Gooi J.H. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19(5):444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo K., Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473(2):231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majidinia M., Sadeghpour A., Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233(4):2937–2948. doi: 10.1002/jcp.26042. [DOI] [PubMed] [Google Scholar]
- 12.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Aman A.J., Fulbright A.N., Parichy D.M. Wnt/β-catenin regulates an ancient signaling network during zebrafish scale development. Elife. 2018;7 doi: 10.7554/eLife.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11):dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- 15.Liao J., Huang Y., Wang Q., et al. Gene regulatory network from cranial neural crest cells to osteoblast differentiation and calvarial bone development. Cell Mol Life Sci. 2022;79(3):158. doi: 10.1007/s00018-022-04208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 17.Du J.H., Lin S.X., Wu X.L., et al. The function of Wnt ligands on osteocyte and bone remodeling. J Dent Res. 2019;98(8):930–938. doi: 10.1177/0022034519854704. [DOI] [PubMed] [Google Scholar]
- 18.Lojk J., Marc J. Roles of non-canonical Wnt signalling pathways in bone biology. Int J Mol Sci. 2021;22(19):10840. doi: 10.3390/ijms221910840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimelman D., Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25(57):7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 23.Taketo M.M. Shutting down Wnt signal-activated cancer. Nat Genet. 2004;36(4):320–322. doi: 10.1038/ng0404-320. [DOI] [PubMed] [Google Scholar]
- 24.Angers S., Moon R.T. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14(6):1583–1588. [PubMed] [Google Scholar]
- 27.Humphries A.C., Mlodzik M. From instruction to output: wnt/PCP signaling in development and cancer. Curr Opin Cell Biol. 2018;51:110–116. doi: 10.1016/j.ceb.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azbazdar Y., Karabicici M., Erdal E., Ozhan G. Regulation of Wnt signaling pathways at the plasma membrane and their misregulation in cancer. Front Cell Dev Biol. 2021;9:631623. doi: 10.3389/fcell.2021.631623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn A.D., Moon R.T. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Katoh M., Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review) Int J Mol Med. 2017;40(3):587–606. doi: 10.3892/ijmm.2017.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F., Tarkkonen K., Nieminen-Pihala V., et al. Mesenchymal cell-derived juxtacrine Wnt1 signaling regulates osteoblast activity and osteoclast differentiation. J Bone Miner Res. 2019;34(6):1129–1142. doi: 10.1002/jbmr.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett C.N., Longo K.A., Wright W.S., et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joeng K.S., Lee Y.C., Lim J., et al. Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest. 2017;127(7):2678–2688. doi: 10.1172/JCI92617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keupp K., Beleggia F., Kayserili H., et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet. 2013;92(4):565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50(5):345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 36.Collet C., Ostertag A., Ricquebourg M., et al. Primary osteoporosis in young adults: genetic basis and identification of novel variants in causal genes. JBMR Plus. 2017;2(1):12–21. doi: 10.1002/jbm4.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu X., Joeng K.S., Nakayama K.I., et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12(1):113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jullien N., Maudinet A., Leloutre B., Ringe J., Häupl T., Marie P.J. Downregulation of ErbB3 by Wnt3a contributes to wnt-induced osteoblast differentiation in mesenchymal cells. J Cell Biochem. 2012;113(6):2047–2056. doi: 10.1002/jcb.24076. [DOI] [PubMed] [Google Scholar]
- 39.Almeida M., Han L., Bellido T., Manolagas S.C., Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and-independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280(50):41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 40.Boland G.M., Perkins G., Hall D.J., Tuan R.S. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Tu X., Esen E., et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10(1) doi: 10.1371/journal.pgen.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu F., Wang J., Xu N., et al. WNT3A modulates chondrogenesis via canonical and non-canonical Wnt pathways in MSCs. Front Biosci (Landmark Ed). 2013;18(2):493–503. doi: 10.2741/4116. [DOI] [PubMed] [Google Scholar]
- 43.Shang Y.C., Wang S.H., Xiong F., et al. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin. 2007;28(11):1761–1774. doi: 10.1111/j.1745-7254.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 44.Maddirevula S., Alsahli S., Alhabeeb L., et al. Expanding the phenome and variome of skeletal dysplasia. Genet Med. 2018;20(12):1609–1616. doi: 10.1038/gim.2018.50. [DOI] [PubMed] [Google Scholar]
- 45.Keller K.C., Ding H., Tieu R., Sparks N.R., Ehnes D.D., Zur Nieden N.I. Wnt5a supports osteogenic lineage decisions in embryonic stem cells. Stem Cell Dev. 2016;25(13):1020–1032. doi: 10.1089/scd.2015.0367. [DOI] [PubMed] [Google Scholar]
- 46.Wu H.Y., Bi R., Sun T., Xie F. Deletion of Dicer blocks osteogenic differentiation via the inhibition of Wnt signalling. Mol Med Rep. 2019;19(4):2897–2905. doi: 10.3892/mmr.2019.9941. [DOI] [PubMed] [Google Scholar]
- 47.Yu B., Chang J., Liu Y., et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat Med. 2014;20(9):1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Später D., Hill T.P., O'sullivan R.J., Gruber M., Conner D.A., Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133(15):3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 49.Doolittle M.L., Calabrese G.M., Mesner L.D., et al. Genetic analysis of osteoblast activity identifies Zbtb40 as a regulator of osteoblast activity and bone mass. PLoS Genet. 2020;16(6) doi: 10.1371/journal.pgen.1008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina-Gomez C., Kemp J.P., Trajanoska K., et al. Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am J Hum Genet. 2018;102(1):88–102. doi: 10.1016/j.ajhg.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp J.P., Medina-Gomez C., Estrada K., et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet. 2014;10(6) doi: 10.1371/journal.pgen.1004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Amerongen R., Fuerer C., Mizutani M., Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369(1):101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudin E., Fijalkowski I., Piters E., Van Hul W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum. 2013;43(2):220–240. doi: 10.1016/j.semarthrit.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Mikels A.J., Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolzoni M., Donofrio G., Storti P., et al. Myeloma cells inhibit non-canonical Wnt co-receptor ror2 expression in human bone marrow osteoprogenitor cells: effect of wnt5a/ror2 pathway activation on the osteogenic differentiation impairment induced by myeloma cells. Leukemia. 2013;27(2):451–463. doi: 10.1038/leu.2012.190. [DOI] [PubMed] [Google Scholar]
- 56.Maeda K., Kobayashi Y., Koide M., et al. The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 2019;20(22):5525. doi: 10.3390/ijms20225525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maeda K., Kobayashi Y., Udagawa N., et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 58.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P.J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weske S., Vaidya M., Reese A., et al. Targeting sphingosine-1-phosphate lyase as an anabolic therapy for bone loss. Nat Med. 2018;24(5):667–678. doi: 10.1038/s41591-018-0005-y. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., Topol L., Lee H., Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130(5):1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 61.Person A.D., Beiraghi S., Sieben C.M., et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dynam. 2010;239(1):327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fazzi R., Pacini S., Carnicelli V., et al. Mesodermal progenitor cells (MPCs) differentiate into mesenchymal stromal cells (MSCs) by activation of Wnt 5/calmodulin signalling pathway. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurson C.J., Butler J.S., Keating D.T., et al. Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Muscoskel Disord. 2007;8:12. doi: 10.1186/1471-2474-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hopwood B., Tsykin A., Findlay D.M., Fazzalari N.L. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res Ther. 2007;9(5):R100. doi: 10.1186/ar2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Qattan M.M. Molecular basis of the clinical features of Al-Awadi-Raas-Rothschild (limb/pelvis/uterus-hypoplasia/aplasia) syndrome (AARRS) and Fuhrmann syndrome. Am J Med Genet. 2013;161A(9):2274–2280. doi: 10.1002/ajmg.a.35437. [DOI] [PubMed] [Google Scholar]
- 66.Cheng S.L., Shao J.S., Cai J., et al. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283(29):20505–20522. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L., Li Q., Zhang J., et al. Wnt7a promotes the osteogenic differentiation of human mesenchymal stem cells. Int J Mol Med. 2021;47(6):94. doi: 10.3892/ijmm.2021.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adamska M., MacDonald B.T., Sarmast Z.H., et al. En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Dev Biol. 2004;272(1):134–144. doi: 10.1016/j.ydbio.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 69.Alves L.U., Santos S., Musso C.M., et al. Santos syndrome is caused by mutation in the WNT7A gene. J Hum Genet. 2017;62(12):1073–1078. doi: 10.1038/jhg.2017.86. [DOI] [PubMed] [Google Scholar]
- 70.Woods C.G., Stricker S., Seemann P., et al. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006;79(2):402–408. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turgeon B., Meloche S. Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol Rev. 2009;89(1):1–26. doi: 10.1152/physrev.00040.2007. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura Y., Nawata M., Wakitani S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol. 2005;167(1):97–105. doi: 10.1016/S0002-9440(10)62957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Usami Y., Gunawardena A.T., Iwamoto M., et al. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest. 2016;96(2):186–196. doi: 10.1038/labinvest.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ling I.T., Rochard L., Liao E.C. Distinct requirements of wls, wnt9a, wnt5b and gpc4 in regulating chondrocyte maturation and timing of endochondral ossification. Dev Biol. 2017;421(2):219–232. doi: 10.1016/j.ydbio.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cawthorn W.P., Bree A.J., Yao Y., et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50(2):477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsukamoto M., Wang K.Y., Tasaki T., et al. Findings as a starting point to unravel the underlying mechanisms of in vivo interactions involving Wnt10a in bone, fat and muscle. Bone. 2019;120:75–84. doi: 10.1016/j.bone.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Wang K.Y., Yamada S., Izumi H., et al. Critical in vivo roles of WNT10A in wound healing by regulating collagen expression/synthesis in WNT10A-deficient mice. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J., Wang S.K., Choi M., et al. Taurodontism, variations in tooth number, and misshapened crowns in Wnt10a null mice and human kindreds. Mol Genet Genomic Med. 2015;3(1):40–58. doi: 10.1002/mgg3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bohring A., Stamm T., Spaich C., et al. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet. 2009;85(1):97–105. doi: 10.1016/j.ajhg.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adaimy L., Chouery E., Megarbane H., et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81(4):821–828. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shungin D., Haworth S., Divaris K., et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat Commun. 2019;10(1):2773. doi: 10.1038/s41467-019-10630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jonsson L., Magnusson T.E., Thordarson A., et al. Rare and common variants conferring risk of tooth agenesis. J Dent Res. 2018;97(5):515–522. doi: 10.1177/0022034517750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu H., Zhang N., Liu L., et al. Correction to: Effect of human Wnt10b transgene overexpression on peri-implant osteogenesis in ovariectomized rats. Hum Gene Ther. 2018;29(12):1416–1427. doi: 10.1089/hum.2018.003. [DOI] [PubMed] [Google Scholar]
- 84.Stevens J.R., Miranda-Carboni G.A., Singer M.A., Brugger S.M., Lyons K.M., Lane T.F. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010;25(10):2138–2147. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wend P., Wend K., Krum S.A., Miranda-Carboni G.A. The role of WNT10B in physiology and disease. Acta Physiol. 2012;204(1):34–51. doi: 10.1111/j.1748-1716.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- 86.Yu P., Yang W., Han D., et al. Mutations in WNT10B are identified in individuals with oligodontia. Am J Hum Genet. 2016;99(1):195–201. doi: 10.1016/j.ajhg.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kantaputra P.N., Hutsadaloi A., Kaewgahya M., et al. WNT10B mutations associated with isolated dental anomalies. Clin Genet. 2018;93(5):992–999. doi: 10.1111/cge.13218. [DOI] [PubMed] [Google Scholar]
- 88.Chen K., Fallen S., Abaan H.O., et al. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51(3):349–355. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- 89.Sebastian A., Hum N.R., Morfin C., Murugesh D.K., Loots G.G. Global gene expression analysis identifies Mef2c as a potential player in Wnt16-mediated transcriptional regulation. Gene. 2018;675:312–321. doi: 10.1016/j.gene.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 90.Movérare-Skrtic S., Henning P., Liu X., et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279–1288. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wergedal J.E., Kesavan C., Brommage R., Das S., Mohan S. Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology. 2015;156(3):1023–1032. doi: 10.1210/en.2014-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Movérare-Skrtic S., Wu J., Henning P., et al. The bone-sparing effects of estrogen and WNT16 are independent of each other. Proc Natl Acad Sci U S A. 2015;112(48):14972–14977. doi: 10.1073/pnas.1520408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alam I., Alkhouli M., Gerard-O'Riley R.L., et al. Osteoblast-specific overexpression of human WNT16 increases both cortical and trabecular bone mass and structure in mice. Endocrinology. 2016;157(2):722–736. doi: 10.1210/en.2015-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alam I., Reilly A.M., Alkhouli M., et al. Bone mass and strength are significantly improved in mice overexpressing human WNT16 in osteocytes. Calcif Tissue Int. 2017;100(4):361–373. doi: 10.1007/s00223-016-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medina-Gomez C., Kemp J.P., Estrada K., et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7) doi: 10.1371/journal.pgen.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng H.F., Tobias J.H., Duncan E., et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8(7) doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng H.F., Forgetta V., Hsu Y.H., et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–117. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullin B.H., Zhao J.H., Brown S.J., et al. Genome-wide association study meta-analysis for quantitative ultrasound parameters of bone identifies five novel loci for broadband ultrasound attenuation. Hum Mol Genet. 2017;26(14):2791–2802. doi: 10.1093/hmg/ddx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi Y., Thirukonda G.J., Nakamura Y., et al. Wnt16 regulates osteoclast differentiation in conjunction with Wnt5a. Biochem Biophys Res Commun. 2015;463(4):1278–1283. doi: 10.1016/j.bbrc.2015.06.102. [DOI] [PubMed] [Google Scholar]
- 100.May P., Woldt E., Matz R.L., Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39(3):219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 101.Herz J., Chen Y., Masiulis I., Zhou L. Expanding functions of lipoprotein receptors. J Lipid Res. 2009;50(Suppl):S287–S292. doi: 10.1194/jlr.R800077-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rey J.P., Ellies D.L. Wnt modulators in the biotech pipeline. Dev Dynam. 2010;239(1):102–114. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwaniec U.T., Wronski T.J., Liu J., et al. PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res. 2007;22(3):394–402. doi: 10.1359/jbmr.061118. [DOI] [PubMed] [Google Scholar]
- 104.Sawakami K., Robling A.G., Ai M., et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 105.Joeng K.S., Schumacher C.A., Zylstra-Diegel C.R., Long F., Williams B.O. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359(2):222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riddle R.C., Diegel C.R., Leslie J.M., et al. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cui Y., Niziolek P.J., MacDonald B.T., et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schumacher C.A., Joiner D.M., Less K.D., Drewry M.O., Williams B.O. Characterization of genetically engineered mouse models carrying Col2a1-cre-induced deletions of Lrp5 and/or Lrp6. Bone Res. 2016;4:15042. doi: 10.1038/boneres.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sarrión P., Mellibovsky L., Urreizti R., et al. Genetic analysis of high bone mass cases from the BARCOS cohort of Spanish postmenopausal women. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Massink M.P., Créton M.A., Spanevello F., et al. Loss-of-function mutations in the WNT co-receptor LRP6 cause autosomal-dominant oligodontia. Am J Hum Genet. 2015;97(4):621–626. doi: 10.1016/j.ajhg.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ockeloen C.W., Khandelwal K.D., Dreesen K., et al. Novel mutations in LRP6 highlight the role of WNT signaling in tooth agenesis. Genet Med. 2016;18(11):1158–1162. doi: 10.1038/gim.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kubota T., Michigami T., Sakaguchi N., et al. Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J Bone Miner Res. 2008;23(10):1661–1671. doi: 10.1359/jbmr.080512. [DOI] [PubMed] [Google Scholar]
- 113.Holmen S.L., Giambernardi T.A., Zylstra C.R., et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19(12):2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 114.Leupin O., Piters E., Halleux C., et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286(22):19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiong L., Jung J.U., Wu H., et al. Lrp4 in osteoblasts suppresses bone formation and promotes osteoclastogenesis and bone resorption. Proc Natl Acad Sci U S A. 2015;112(11):3487–3492. doi: 10.1073/pnas.1419714112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi H.Y., Dieckmann M., Herz J., Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang M.K., Kramer I., Huber T., et al. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc Natl Acad Sci U S A. 2014;111(48):E5187–E5195. doi: 10.1073/pnas.1413828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohazama A., Johnson E.B., Ota M.S., et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3(12) doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y., Pawlik B., Elcioglu N., et al. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet. 2010;86(5):696–706. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fijalkowski I., Geets E., Steenackers E., et al. A novel domain-specific mutation in a sclerosteosis patient suggests a role of LRP4 as an anchor for sclerostin in human bone. J Bone Miner Res. 2016;31(4):874–881. doi: 10.1002/jbmr.2782. [DOI] [PubMed] [Google Scholar]
- 121.Sukenik Halevy R., Chien H.C., Heinz B., et al. Mutations in the fourth β-propeller domain of LRP4 are associated with isolated syndactyly with fusion of the third and fourth fingers. Hum Mutat. 2018;39(6):811–815. doi: 10.1002/humu.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hettiaracchchi D., Bonnard C., Jayawardana S.M.A., et al. Cenani-Lenz syndactyly syndrome - a case report of a family with isolated syndactyly. BMC Med Genet. 2018;19(1):125. doi: 10.1186/s12881-018-0646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ai M., Holmen S.L., Van Hul W., Williams B.O., Warman M.L. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005;25(12):4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Wang Y., Li X., et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24(11):4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ellies D.L., Viviano B., McCarthy J., et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21(11):1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 126.Bhat B.M., Allen K.M., Liu W., et al. Structure-based mutation analysis shows the importance of LRP5 beta-propeller 1 in modulating Dkk1-mediated inhibition of Wnt signaling. Gene. 2007;391(1–2):103–112. doi: 10.1016/j.gene.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 127.Semenov M.V., He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281(50):38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- 128.Balemans W., Piters E., Cleiren E., et al. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int. 2008;82(6):445–453. doi: 10.1007/s00223-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 129.Kamizaki K., Endo M., Minami Y., Kobayashi Y. Role of noncanonical Wnt ligands and Ror-family receptor tyrosine kinases in the development, regeneration, and diseases of the musculoskeletal system. Dev Dynam. 2021;250(1):27–38. doi: 10.1002/dvdy.151. [DOI] [PubMed] [Google Scholar]
- 130.Uehara S., Udagawa N., Mukai H., et al. Protein kinase N3 promotes bone resorption by osteoclasts in response to Wnt5a-Ror2 signaling. Sci Signal. 2017;10(494) doi: 10.1126/scisignal.aan0023. [DOI] [PubMed] [Google Scholar]
- 131.Weissenböck M., Latham R., Nishita M., et al. Genetic interactions between Ror2 and Wnt9a, Ror1 and Wnt9a and Ror2 and Ror1:phenotypic analysis of the limb skeleton and palate in compound mutants. Gene Cell. 2019;24(4):307–317. doi: 10.1111/gtc.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oishi I., Suzuki H., Onishi N., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Gene Cell. 2003;8(7):645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 133.Takeuchi S., Takeda K., Oishi I., et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Gene Cell. 2000;5(1):71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 134.Chen K., Ng P.Y., Chen R., et al. Sfrp4 repression of the Ror2/Jnk cascade in osteoclasts protects cortical bone from excessive endosteal resorption. Proc Natl Acad Sci U S A. 2019;116(28):14138–14143. doi: 10.1073/pnas.1900881116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Bokhoven H., Celli J., Kayserili H., et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet. 2000;25(4):423–426. doi: 10.1038/78113. [DOI] [PubMed] [Google Scholar]
- 136.Afzal A.R., Rajab A., Fenske C.D., et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet. 2000;25(4):419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 137.Schwabe G.C., Tinschert S., Buschow C., et al. Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. Am J Hum Genet. 2000;67(4):822–831. doi: 10.1086/303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patton M.A., Afzal A.R. Robinow syndrome. J Med Genet. 2002;39(5):305–310. doi: 10.1136/jmg.39.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mao B., Wu W., Davidson G., et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 140.Mao B., Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302(1–2):179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 141.Schulze J., Seitz S., Saito H., et al. Negative regulation of bone formation by the transmembrane Wnt antagonist Kremen-2. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cselenyi C.S., Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1(8):pe10. doi: 10.1126/stke.18pe10. [DOI] [PubMed] [Google Scholar]
- 143.Ellwanger K., Saito H., Clément-Lacroix P., et al. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28(15):4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Intarak N., Theerapanon T., Srijunbarl A., Suphapeetiporn K., Porntaveetus T., Shotelersuk V. Novel compound heterozygous mutations in KREMEN1 confirm it as a disease gene for ectodermal dysplasia. Br J Dermatol. 2018;179(3):758–760. doi: 10.1111/bjd.16541. [DOI] [PubMed] [Google Scholar]
- 145.Vass M., Kooistra A.J., Yang D., Stevens R.C., Wang M.W., de Graaf C. Chemical diversity in the G protein-coupled receptor superfamily. Trends Pharmacol Sci. 2018;39(5):494–512. doi: 10.1016/j.tips.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 146.Hirose K., Shimoda N., Kikuchi Y. Expression patterns of lgr4 and lgr6 during zebrafish development. Gene Expr Patterns. 2011;11(7):378–383. doi: 10.1016/j.gep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 147.Luo J., Zhou W., Zhou X., et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136(16):2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mazerbourg S., Bouley D.M., Sudo S., et al. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol. 2004;18(9):2241–2254. doi: 10.1210/me.2004-0133. [DOI] [PubMed] [Google Scholar]
- 149.Luo J., Yang Z., Ma Y., et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22(5):539–546. doi: 10.1038/nm.4076. [DOI] [PubMed] [Google Scholar]
- 150.Sun P., Jia K., Zheng C., et al. Loss of Lgr4 inhibits differentiation, migration and apoptosis, and promotes proliferation in bone mesenchymal stem cells. J Cell Physiol. 2019;234(7):10855–10867. doi: 10.1002/jcp.27927. [DOI] [PubMed] [Google Scholar]
- 151.Morita H., Mazerbourg S., Bouley D.M., et al. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24(22):9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Y.M., Yang Y.Y., Jing Y.X., et al. BMP9 reduces bone loss in ovariectomized mice by dual regulation of bone remodeling. J Bone Miner Res. 2020;35(5):978–993. doi: 10.1002/jbmr.3957. [DOI] [PubMed] [Google Scholar]
- 153.Lehoczky J.A., Tabin C.J. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci U S A. 2015;112(43):13249–13254. doi: 10.1073/pnas.1518874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 155.Mao B., Wu W., Li Y., et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 156.Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 157.Brott B.K., Sokol S.Y. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22(17):6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Christodoulides C., Laudes M., Cawthorn W.P., et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119(Pt 12):2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morvan F., Boulukos K., Clément-Lacroix P., et al. Deletion of a single allele of the Dkk 1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 160.Colditz J., Thiele S., Baschant U., et al. Postnatal skeletal deletion of dickkopf-1 increases bone formation and bone volume in male and female mice, despite increased sclerostin expression. J Bone Miner Res. 2018;33(9):1698–1707. doi: 10.1002/jbmr.3463. [DOI] [PubMed] [Google Scholar]
- 161.Li J., Sarosi I., Cattley R.C., et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 162.Pinzone J.J., Hall B.M., Thudi N.K., et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mukhopadhyay M., Shtrom S., Rodriguez-Esteban C., et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1(3):423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 164.MacDonald B.T., Joiner D.M., Oyserman S.M., et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41(3):331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Witcher P.C., Miner S.E., Horan D.J., et al. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight. 2018;3(11):98673. doi: 10.1172/jci.insight.98673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ko J.Y., Wang F.S., Wang C.J., Wong T., Chou W.Y., Tseng S.L. Increased Dickkopf-1 expression accelerates bone cell apoptosis in femoral head osteonecrosis. Bone. 2010;46(3):584–591. doi: 10.1016/j.bone.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 167.Daoussis D., Andonopoulos A.P. The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. 2011;41(2):170–177. doi: 10.1016/j.semarthrit.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 168.Ray S., Khassawna T.E., Sommer U., et al. Differences in expression of Wnt antagonist Dkk1 in healthy versus pathological bone samples. J Microsc. 2017;265(1):111–120. doi: 10.1111/jmi.12469. [DOI] [PubMed] [Google Scholar]
- 169.Li L., Mao J., Sun L., Liu W., Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem. 2002;277(8):5977–5981. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- 170.Li X., Zhang Y., Kang H., et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 171.Semënov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 172.Delgado-Calle J., Sato A.Y., Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li X., Ominsky M.S., Niu Q.T., et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 174.Krause C., Korchynskyi O., de Rooij K., et al. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285(53):41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rhee Y., Allen M.R., Condon K., et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011;26(5):1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tu X., Rhee Y., Condon K.W., et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim S.J., Bieganski T., Sohn Y.B., et al. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Hum Genet. 2011;129(5):497–502. doi: 10.1007/s00439-011-0947-3. [DOI] [PubMed] [Google Scholar]
- 178.Ukita M., Yamaguchi T., Ohata N., Tamura M. Sclerostin enhances adipocyte differentiation in 3T3-L1 cells. J Cell Biochem. 2016;117(6):1419–1428. doi: 10.1002/jcb.25432. [DOI] [PubMed] [Google Scholar]
- 179.You L., Chen L., Pan L., Peng Y., Chen J. SOST gene inhibits osteogenesis from adipose-derived mesenchymal stem cells by inducing Th17 cell differentiation. Cell Physiol Biochem. 2018;48(3):1030–1040. doi: 10.1159/000491971. [DOI] [PubMed] [Google Scholar]
- 180.Kim S.P., Frey J.L., Li Z., et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114(52):E11238–E11247. doi: 10.1073/pnas.1707876115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cruciat C.M., Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harbor Perspect Biol. 2013;5(3):a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cho S.W., Her S.J., Sun H.J., et al. Differential effects of secreted frizzled-related proteins (sFRPs) on osteoblastic differentiation of mouse mesenchymal cells and apoptosis of osteoblasts. Biochem Biophys Res Commun. 2008;367(2):399–405. doi: 10.1016/j.bbrc.2007.12.128. [DOI] [PubMed] [Google Scholar]
- 183.Bodine P.V., Zhao W., Kharode Y.P., et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 184.Bodine P.V., Seestaller-Wehr L., Kharode Y.P., Bex F.J., Komm B.S. Bone anabolic effects of parathyroid hormone are blunted by deletion of the Wnt antagonist secreted frizzled-related protein-1. J Cell Physiol. 2007;210(2):352–357. doi: 10.1002/jcp.20834. [DOI] [PubMed] [Google Scholar]
- 185.Vaes B.L., Dechering K.J., van Someren E.P., et al. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone. 2005;36(5):803–811. doi: 10.1016/j.bone.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 186.Haraguchi R., Kitazawa R., Mori K., et al. sFRP4-dependent Wnt signal modulation is critical for bone remodeling during postnatal development and age-related bone loss. Sci Rep. 2016;6:25198. doi: 10.1038/srep25198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nakanishi R., Akiyama H., Kimura H., et al. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23(2):271–277. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- 188.Kiper P.O.S., Saito H., Gori F., et al. Cortical-bone fragility--insights from sFRP4 deficiency in Pyle's disease. N Engl J Med. 2016;374(26):2553–2562. doi: 10.1056/NEJMoa1509342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bovolenta P., Esteve P., Ruiz J.M., Cisneros E., Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 190.Kansara M., Tsang M., Kodjabachian L., et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119(4):837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cho S.W., Yang J.Y., Sun H.J., et al. Wnt inhibitory factor (WIF)-1 inhibits osteoblastic differentiation in mouse embryonic mesenchymal cells. Bone. 2009;44(6):1069–1077. doi: 10.1016/j.bone.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 192.Liang J., Chen C., Liu H., Liu X., Zhao H., Hu J. Gossypol promotes Wnt/β-catenin signaling through WIF1 in ovariectomy-induced osteoporosis. BioMed Res Int. 2019:8745487. doi: 10.1155/2019/8745487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barrow J.R., Thomas K.R., Boussadia-Zahui O., et al. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17(3):394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhong Z., Zylstra-Diegel C.R., Schumacher C.A., et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109(33):E2197–E2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yu H., Smallwood P.M., Wang Y., Vidaltamayo R., Reed R., Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137(21):3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Albers J., Schulze J., Beil F.T., et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192(6):1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karner C.M., Dietrich M.F., Johnson E.B., et al. Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation: a mouse model for Cenani-Lenz syndrome. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Niziolek P.J., Farmer T.L., Cui Y., Turner C.H., Warman M.L., Robling A.G. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011;49(5):1010–1019. doi: 10.1016/j.bone.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yadav V.K., Ryu J.H., Suda N., et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pinson K.I., Brennan J., Monkley S., Avery B.J., Skarnes W.C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 201.Li X., Liu P., Liu W., et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37(9):945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 202.Ominsky M.S., Li C., Li X., et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26(5):1012–1021. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 203.Collette N.M., Genetos D.C., Economides A.N., et al. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci U S A. 2012;109(35):14092–14097. doi: 10.1073/pnas.1207188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Winkler D.G., Sutherland M.K., Geoghegan J.C., et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morello R., Bertin T.K., Schlaubitz S., et al. Brachy-syndactyly caused by loss of Sfrp2 function. J Cell Physiol. 2008;217(1):127–137. doi: 10.1002/jcp.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lories R.J., Peeters J., Bakker A., et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56(12):4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 207.Cho H.Y., Choi H.J., Sun H.J., et al. Transgenic mice overexpressing secreted frizzled-related proteins (sFRP)4 under the control of serum amyloid P promoter exhibit low bone mass but did not result in disturbed phosphate homeostasis. Bone. 2010;47(2):263–271. doi: 10.1016/j.bone.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 208.Hamblet N.S., Lijam N., Ruiz-Lozano P., et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129(24):5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 209.Xie R., Jiang R., Chen D. Generation of Axin1 conditional mutant mice. Genesis. 2011;49(2):98–102. doi: 10.1002/dvg.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dao D.Y., Yang X., Flick L.M., Chen D., Hilton M.J., O'Keefe R.J. Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res. 2010;28(1):89–95. doi: 10.1002/jor.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yan Y., Tang D., Chen M., et al. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J Cell Sci. 2009;122(Pt 19):3566–3578. doi: 10.1242/jcs.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yu H.M., Jerchow B., Sheu T.J., et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132(8):1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miclea R.L., Karperien M., Bosch C.A., et al. Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev Biol. 2009;9:26. doi: 10.1186/1471-213X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Itoh S., Saito T., Hirata M., et al. GSK-3α and GSK-3β proteins are involved in early stages of chondrocyte differentiation with functional redundancy through RelA protein phosphorylation. J Biol Chem. 2012;287(35):29227–29236. doi: 10.1074/jbc.M112.372086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hoeflich K.P., Luo J., Rubie E.A., Tsao M.S., Jin O., Woodgett J.R. Requirement for glycogen synthase kinase-3 beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 216.Kugimiya F., Kawaguchi H., Ohba S., et al. GSK-3 beta controls osteogenesis through regulating Runx2 activity. PLoS One. 2007;2(9):e837. doi: 10.1371/journal.pone.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nelson E.R., Levi B., Sorkin M., et al. Role of GSK-3β in the osteogenic differentiation of palatal mesenchyme. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guo X., Day T.F., Jiang X., Garrett-Beal L., Topol L., Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18(19):2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Glass D.A., Bialek P., Ahn J.D., et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 220.Fischer L., Boland G., Tuan R.S. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J Cell Biochem. 2002;84(4):816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]
- 221.Bain G., Müller T., Wang X., Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301(1):84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 222.Akiyama H., Chaboissier M.C., Martin J.F., Schedl A., de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Akiyama H., Lyons J.P., Mori-Akiyama Y., et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jin E.J., Lee S.Y., Choi Y.A., Jung J.C., Bang O.S., Kang S.S. BMP-2-enhanced chondrogenesis involves p38 MAPK-mediated down-regulation of Wnt-7a pathway. Mol Cell. 2006;22(3):353–359. [PubMed] [Google Scholar]
- 225.Grotewold L., Rüther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21(5):966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Haegele L., Ingold B., Naumann H., Tabatabai G., Ledermann B., Brandner S. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 2003;24(3):696–708. doi: 10.1016/s1044-7431(03)00232-x. [DOI] [PubMed] [Google Scholar]
- 227.He G., Shi Y., Lim J., Bellido T., Ni J., Long F. Differential involvement of Wnt signaling in Bmp regulation of cancellous versus periosteal bone growth. Bone Res. 2017;5:17016. doi: 10.1038/boneres.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Song D., He G., Shi Y., Ni J., Long F. Functional interaction between Wnt and Bmp signaling in periosteal bone growth. Sci Rep. 2021;11(1):10782. doi: 10.1038/s41598-021-90324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tuli R., Tuli S., Nandi S., et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 230.Mariani F.V., Fernandez-Teran M., Ros M.A. Ectoderm-mesoderm crosstalk in the embryonic limb: the role of fibroblast growth factor signaling. Dev Dynam. 2017;246(4):208–216. doi: 10.1002/dvdy.24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Díaz-Hernández M.E., Galván-Hernández C.I., Marín-Llera J.C., et al. Activation of the WNT-BMP-FGF regulatory network induces the onset of cell death in anterior mesodermal cells to establish the ANZ. Front Cell Dev Biol. 2021;9:703836. doi: 10.3389/fcell.2021.703836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jope R.S., Johnson G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 233.Fei Y., Xiao L., Doetschman T., Coffin D.J., Hurley M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286(47):40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bellido T., Ali A.A., Gubrij I., et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 235.Varelas X., Miller B.W., Sopko R., et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 236.Kegelman C.D., Mason D.E., Dawahare J.H., et al. Skeletal cell YAP and TAZ combinatorially promote bone development. Faseb J. 2018;32(5):2706–2721. doi: 10.1096/fj.201700872R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kegelman C.D., Nijsure M.P., Moharrer Y., et al. YAP and TAZ promote periosteal osteoblast precursor expansion and differentiation for fracture repair. J Bone Miner Res. 2021;36(1):143–157. doi: 10.1002/jbmr.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Xiong J., Almeida M., O'Brien C.A. The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation. Bone. 2018;112:1–9. doi: 10.1016/j.bone.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kegelman C.D., Collins J.M., Nijsure M.P., Eastburn E.A., Boerckel J.D. Gone caving: roles of the transcriptional regulators YAP and TAZ in skeletal development. Curr Osteoporos Rep. 2020;18(5):526–540. doi: 10.1007/s11914-020-00605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kegelman C.D., Coulombe J.C., Jordan K.M., et al. YAP and TAZ mediate osteocyte perilacunar/canalicular remodeling. J Bone Miner Res. 2020;35(1):196–210. doi: 10.1002/jbmr.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Singh B.N., Doyle M.J., Weaver C.V., Koyano-Nakagawa N., Garry D.J. Hedgehog and Wnt coordinate signaling in myogenic progenitors and regulate limb regeneration. Dev Biol. 2012;371(1):23–34. doi: 10.1016/j.ydbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rockel J.S., Yu C., Whetstone H., et al. Hedgehog inhibits β-catenin activity in synovial joint development and osteoarthritis. J Clin Invest. 2016;126(5):1649–1663. doi: 10.1172/JCI80205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Day T.F., Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 244.Deng Q., Li P., Che M., et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. Elife. 2019;8 doi: 10.7554/eLife.50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hayward P., Kalmar T., Arias A.M. Wnt/Notch signalling and information processing during development. Development. 2008;135(3):411–424. doi: 10.1242/dev.000505. [DOI] [PubMed] [Google Scholar]
- 246.Acar A., Hidalgo-Sastre A., Leverentz M.K., et al. Inhibition of Wnt signalling by Notch via two distinct mechanisms. Sci Rep. 2021;11(1):9096. doi: 10.1038/s41598-021-88618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tu X., Delgado-Calle J., Condon K.W., et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112(5):E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sieiro D., Rios A.C., Hirst C.E., Marcelle C. Cytoplasmic NOTCH and membrane-derived β-catenin link cell fate choice to epithelial-mesenchymal transition during myogenesis. Elife. 2016;5 doi: 10.7554/eLife.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ballhause T.M., Jiang S., Baranowsky A., et al. Relevance of Notch signaling for bone metabolism and regeneration. Int J Mol Sci. 2021;22(3):1325. doi: 10.3390/ijms22031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Abdallah B.M., Jafari A., Zaher W., Qiu W., Kassem M. Skeletal (stromal) stem cells: an update on intracellular signaling pathways controlling osteoblast differentiation. Bone. 2015;70:28–36. doi: 10.1016/j.bone.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 251.Li C.T., Liu J.X., Yu B., Liu R., Dong C., Li S.J. Notch signaling represses hypoxia-inducible factor-1α-induced activation of Wnt/β-catenin signaling in osteoblasts under cobalt-mimicked hypoxia. Mol Med Rep. 2016;14(1):689–696. doi: 10.3892/mmr.2016.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tallquist M.D., Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130(3):507–518. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- 253.Tokunaga A., Oya T., Ishii Y., et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23(9):1519–1528. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 254.Caverzasio J., Biver E., Thouverey C. Predominant role of PDGF receptor transactivation in Wnt3a-induced osteoblastic cell proliferation. J Bone Miner Res. 2013;28(2):260–270. doi: 10.1002/jbmr.1748. [DOI] [PubMed] [Google Scholar]
- 255.Kim S.E., Choi K.Y. EGF receptor is involved in WNT3a-mediated proliferation and motility of NIH3T3 cells via ERK pathway activation. Cell Signal. 2007;19(7):1554–1564. doi: 10.1016/j.cellsig.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 256.Tang N., Song W.X., Luo J., et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13(8B):2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rodríguez-Carballo E., Ulsamer A., Susperregui A.R., et al. Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J Bone Miner Res. 2011;26(4):718–729. doi: 10.1002/jbmr.260. [DOI] [PubMed] [Google Scholar]
- 258.Zhou S., Eid K., Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19(3):463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 259.Dao D.Y., Yang X., Chen D., Zuscik M., O'Keefe R.J. Axin1 and Axin2 are regulated by TGF- and mediate cross-talk between TGF- and Wnt signaling pathways. Ann N Y Acad Sci. 2007;1116:82–99. doi: 10.1196/annals.1402.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tachi K., Takami M., Sato H., et al. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1. Tissue Eng. 2011;17(5–6):597–606. doi: 10.1089/ten.TEA.2010.0094. [DOI] [PubMed] [Google Scholar]
- 261.Salazar V.S., Zarkadis N., Huang L., et al. Postnatal ablation of osteoblast Smad4 enhances proliferative responses to canonical Wnt signaling through interactions with β-catenin. J Cell Sci. 2013;126(Pt 24):5598–5609. doi: 10.1242/jcs.132233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.van de Laar I.M., Oldenburg R.A., Pals G., et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43(2):121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 263.Qi H., Jin M., Duan Y., et al. FGFR3 induces degradation of BMP type I receptor to regulate skeletal development. Biochim Biophys Acta. 2014;1843(7):1237–1247. doi: 10.1016/j.bbamcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang M., Yan Y., Lim Y.B., et al. BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. J Cell Biochem. 2009;108(4):896–905. doi: 10.1002/jcb.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Papathanasiou I., Malizos K.N., Tsezou A. Bone morphogenetic protein-2-induced Wnt/β-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes. Arthritis Res Ther. 2012;14(2):R82. doi: 10.1186/ar3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sasaki T., Ito Y., Bringas P., et al. TGFbeta-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development. 2006;133(2):371–381. doi: 10.1242/dev.02200. [DOI] [PubMed] [Google Scholar]
- 267.Fakhry A., Ratisoontorn C., Vedhachalam C., et al. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36(2):254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 268.Naganawa T., Xiao L., Coffin J.D., et al. Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. J Cell Biochem. 2008;103(6):1975–1988. doi: 10.1002/jcb.21589. [DOI] [PubMed] [Google Scholar]
- 269.Agas D., Sabbieti M.G., Marchetti L., Xiao L., Hurley M.M. FGF-2 enhances Runx-2/Smads nuclear localization in BMP-2 canonical signaling in osteoblasts. J Cell Physiol. 2013;228(11):2149–2158. doi: 10.1002/jcp.24382. [DOI] [PubMed] [Google Scholar]
- 270.Jiang T., Ge S., Shim Y.H., Zhang C., Cao D. Bone morphogenetic protein is required for fibroblast growth factor 2-dependent later-stage osteoblastic differentiation in cranial suture cells. Int J Clin Exp Pathol. 2015;8(3):2946–2954. [PMC free article] [PubMed] [Google Scholar]
- 271.Luo Z., Shang X., Zhang H., et al. Notch signaling in osteogenesis, osteoclastogenesis, and angiogenesis. Am J Pathol. 2019;189(8):1495–1500. doi: 10.1016/j.ajpath.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Murgai A., Altmeyer S., Wiegand S., Tylzanowski P., Stricker S. Cooperation of BMP and IHH signaling in interdigital cell fate determination. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Glinka A., Dolde C., Kirsch N., et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12(10):1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chiurillo M.A. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5(2):84–102. doi: 10.5493/wjem.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morgan R.G., Mortensson E., Williams A.C. Targeting LGR5 in Colorectal Cancer: therapeutic gold or too plastic? Br J Cancer. 2018;118(11):1410–1418. doi: 10.1038/s41416-018-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Müller M.F., Ibrahim A.E., Arends M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469(2):125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hojo H., Ohba S., Taniguchi K., et al. Hedgehog-Gli activators direct osteo-chondrogenic function of bone morphogenetic protein toward osteogenesis in the perichondrium. J Biol Chem. 2013;288(14):9924–9932. doi: 10.1074/jbc.M112.409342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Phipps M.C., Xu Y., Bellis S.L. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Frey J.L., Li Z., Ellis J.M., et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–1991. doi: 10.1128/MCB.01343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Frey J.L., Kim S.P., Li Z., Wolfgang M.J., Riddle R.C. β-catenin directs long-chain fatty acid catabolism in the osteoblasts of male mice. Endocrinology. 2018;159(1):272–284. doi: 10.1210/en.2017-00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim S.P., Li Z., Zoch M.L., et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2(16):92704. doi: 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Alekos N.S., Moorer M.C., Riddle R.C. Dual effects of lipid metabolism on osteoblast function. Front Endocrinol. 2020;11:578194. doi: 10.3389/fendo.2020.578194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Esen E., Chen J., Karner C.M., Okunade A.L., Patterson B.W., Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metabol. 2013;17(5):745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Karner C.M., Esen E., Chen J., Hsu F.F., Turk J., Long F. Wnt protein signaling reduces nuclear acetyl-CoA levels to suppress gene expression during osteoblast differentiation. J Biol Chem. 2016;291(25):13028–13039. doi: 10.1074/jbc.M115.708578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Brown P.M., Hutchison J.D., Crockett J.C. Absence of glutamine supplementation prevents differentiation of murine calvarial osteoblasts to a mineralizing phenotype. Calcif Tissue Int. 2011;89(6):472–482. doi: 10.1007/s00223-011-9537-6. [DOI] [PubMed] [Google Scholar]
- 287.Karner C.M., Esen E., Okunade A.L., Patterson B.W., Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125(2):551–562. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Stegen S., van Gastel N., Eelen G., et al. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metabol. 2016;23(2):265–279. doi: 10.1016/j.cmet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kondoh S., Inoue K., Igarashi K., et al. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone. 2014;60:68–77. doi: 10.1016/j.bone.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gao Y., Huang E., Zhang H., et al. Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hay E., Bouaziz W., Funck-Brentano T., Cohen-Solal M. Sclerostin and bone aging: a mini-review. Gerontology. 2016;62(6):618–623. doi: 10.1159/000446278. [DOI] [PubMed] [Google Scholar]
- 292.Reid I.R. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11(7):418–428. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 293.MacNabb C., Patton D., Hayes J.S. Sclerostin antibody therapy for the treatment of osteoporosis: clinical prospects and challenges. J Osteoporos. 2016;2016:6217286. doi: 10.1155/2016/6217286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Appelman-Dijkstra N.M., Papapoulos S.E. Clinical advantages and disadvantages of anabolic bone therapies targeting the WNT pathway. Nat Rev Endocrinol. 2018;14(10):605–623. doi: 10.1038/s41574-018-0087-0. [DOI] [PubMed] [Google Scholar]
- 295.Lewiecki E.M., Blicharski T., Goemaere S., et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183–3193. doi: 10.1210/jc.2017-02163. [DOI] [PubMed] [Google Scholar]
- 296.Sølling A.S.K., Harsløf T., Langdahl B. The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis. 2018;10(5–6):105–115. doi: 10.1177/1759720X18775936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Florio M., Gunasekaran K., Stolina M., et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Glorieux F.H., Devogelaer J.P., Durigova M., et al. BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J Bone Miner Res. 2017;32(7):1496–1504. doi: 10.1002/jbmr.3143. [DOI] [PubMed] [Google Scholar]
- 299.Seefried L., Baumann J., Hemsley S., et al. Efficacy of anti-sclerostin monoclonal antibody BPS804 in adult patients with hypophosphatasia. J Clin Invest. 2017;127(6):2148–2158. doi: 10.1172/JCI83731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ominsky M.S., Boyce R.W., Li X., Ke H.Z. Effects of sclerostin antibodies in animal models of osteoporosis. Bone. 2017;96:63–75. doi: 10.1016/j.bone.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 301.Baron R., Gori F. Targeting WNT signaling in the treatment of osteoporosis. Curr Opin Pharmacol. 2018;40:134–141. doi: 10.1016/j.coph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 302.Yang R.B., Lin F.F., Yang J., et al. Overexpression of CAV3 facilitates bone formation via the Wnt signaling pathway in osteoporotic rats. Endocrine. 2019;63(3):639–650. doi: 10.1007/s12020-018-1803-1. [DOI] [PubMed] [Google Scholar]
- 303.Zhu M., Tang D., Wu Q., et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang Y., Vasheghani F., Li Y.H., et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 305.Bouaziz W., Funck-Brentano T., Lin H., et al. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. doi: 10.1186/s13075-015-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lietman C., Wu B., Lechner S., et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight. 2018;3(3):96308. doi: 10.1172/jci.insight.96308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Deshmukh V., Hu H., Barroga C., et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2018;26(1):18–27. doi: 10.1016/j.joca.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 308.Okura T., Ohkawara B., Takegami Y., et al. Mianserin suppresses R-spondin 2-induced activation of Wnt/β-catenin signaling in chondrocytes and prevents cartilage degradation in a rat model of osteoarthritis. Sci Rep. 2019;9(1):2808. doi: 10.1038/s41598-019-39393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lian Q., Chi B., Zhang L., Tian F. The role of Wnt signaling pathway in osteoarthritis via the dual-targeted regulation of cartilage and subchondral bone. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34(6):797–803. doi: 10.7507/1002-1892.201909088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Świerkot J., Gruszecka K., Matuszewska A., Wiland P. Assessment of the effect of methotrexate therapy on bone metabolism in patients with rheumatoid arthritis. Arch Immunol Ther Exp. 2015;63(5):397–404. doi: 10.1007/s00005-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Miao C.G., Yang Y.Y., He X., et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25(10):2069–2078. doi: 10.1016/j.cellsig.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 312.Wehmeyer C., Frank S., Beckmann D., et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8(330):330ra35. doi: 10.1126/scitranslmed.aac4351. [DOI] [PubMed] [Google Scholar]
- 313.Marenzana M., Vugler A., Moore A., Robinson M. Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study. Arthritis Res Ther. 2013;15(5):R125. doi: 10.1186/ar4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Miao P., Zhou X.W., Wang P., et al. Regulatory effect of anti-gp130 functional mAb on IL-6 mediated RANKL and Wnt5a expression through JAK-STAT3 signaling pathway in FLS. Oncotarget. 2018;9(29):20366–20376. doi: 10.18632/oncotarget.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kwon Y.J., Lee S.W., Park Y.B., Lee S.K., Park M.C. Secreted frizzled-related protein 5 suppresses inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes through down-regulation of c-Jun N-terminal kinase. Rheumatology. 2014;53(9):1704–1711. doi: 10.1093/rheumatology/keu167. [DOI] [PubMed] [Google Scholar]
- 316.Smolen J.S., Landewé R., Bijlsma J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 317.MacLauchlan S., Zuriaga M.A., Fuster J.J., et al. Genetic deficiency of Wnt5a diminishes disease severity in a murine model of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):166. doi: 10.1186/s13075-017-1375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cao W., Niu M., Tong Y., et al. Depleting the carboxy-terminus of human Wnt5a attenuates collagen-induced arthritis in DBA/1 mice. Biochem Biophys Res Commun. 2018;504(4):679–685. doi: 10.1016/j.bbrc.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 319.Tanaka S. RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.17296.1. Faculty Rev-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Fukuyo S., Yamaoka K., Sonomoto K., et al. IL-6-accelerated calcification by induction of ROR2 in human adipose tissue-derived mesenchymal stem cells is STAT3 dependent. Rheumatology. 2014;53(7):1282–1290. doi: 10.1093/rheumatology/ket496. [DOI] [PubMed] [Google Scholar]
- 321.Sonomoto K., Yamaoka K., Oshita K., et al. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012;64(10):3355–3363. doi: 10.1002/art.34555. [DOI] [PubMed] [Google Scholar]
- 322.Chen X.X., Baum W., Dwyer D., et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72(10):1732–1736. doi: 10.1136/annrheumdis-2013-203345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tanaka Y. Clinical immunity in bone and joints. J Bone Miner Metabol. 2019;37(1):2–8. doi: 10.1007/s00774-018-0965-5. [DOI] [PubMed] [Google Scholar]
- 324.Pridgeon M.G., Grohar P.J., Steensma M.R., Williams B.O. Wnt signaling in Ewing sarcoma, osteosarcoma, and malignant peripheral nerve sheath tumors. Curr Osteoporos Rep. 2017;15(4):239–246. doi: 10.1007/s11914-017-0377-9. [DOI] [PubMed] [Google Scholar]
- 325.Baker E.K., Taylor S., Gupte A., et al. Wnt inhibitory factor 1 (WIF1) is a marker of osteoblastic differentiation stage and is not silenced by DNA methylation in osteosarcoma. Bone. 2015;73:223–232. doi: 10.1016/j.bone.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 326.Lin C.H., Guo Y., Ghaffar S., et al. Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma. 2013;2013:147541. doi: 10.1155/2013/147541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Techavichit P., Gao Y., Kurenbekova L., Shuck R., Donehower L.A., Yustein J.T. Secreted Frizzled-Related Protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential. BMC Cancer. 2016;16(1):869. doi: 10.1186/s12885-016-2909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Danieau G., Morice S., Rédini F., Verrecchia F., Royer B.B. New insights about the Wnt/β-catenin signaling pathway in primary bone tumors and their microenvironment: a promising target to develop therapeutic strategies? Int J Mol Sci. 2019;20(15):3751. doi: 10.3390/ijms20153751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shimozaki S., Yamamoto N., Domoto T., et al. Efficacy of glycogen synthase kinase-3β targeting against osteosarcoma via activation of β-catenin. Oncotarget. 2016;7(47):77038–77051. doi: 10.18632/oncotarget.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Xiao H., Jensen P.E., Chen X. Elimination of osteosarcoma by necroptosis with graphene oxide-associated anti-HER2 antibodies. Int J Mol Sci. 2019;20(18):4360. doi: 10.3390/ijms20184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Crompton J.G., Ogura K., Bernthal N.M., Kawai A., Eilber F.C. Local control of soft tissue and bone sarcomas. J Clin Oncol. 2018;36(2):111–117. doi: 10.1200/JCO.2017.75.2717. [DOI] [PubMed] [Google Scholar]
- 332.Marino S., Roodman G.D. Multiple myeloma and bone: the fatal interaction. Cold Spring Harb Perspect Med. 2018;8(8):a031286. doi: 10.1101/cshperspect.a031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Eda H., Santo L., Wein M.N., et al. Regulation of sclerostin expression in multiple myeloma by Dkk-1: a potential therapeutic strategy for myeloma bone disease. J Bone Miner Res. 2016;31(6):1225–1234. doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Delgado-Calle J., Anderson J., Cregor M.D., et al. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia. 2017;31(12):2686–2694. doi: 10.1038/leu.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.McDonald M.M., Reagan M.R., Youlten S.E., et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129(26):3452–3464. doi: 10.1182/blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhuang X., Zhang H., Li X., et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. 2017;19(10):1274–1285. doi: 10.1038/ncb3613. [DOI] [PubMed] [Google Scholar]
- 337.D'Oronzo S., Coleman R., Brown J., Silvestris F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. 2019;15:4. doi: 10.1016/j.jbo.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pai S.G., Carneiro B.A., Mota J.M., et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Madan B., Ke Z., Harmston N., et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35(17):2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Liu J., Pan S., Hsieh M.H., et al. Targeting wnt-driven cancer through the inhibition of porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110(50):20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Shah K., Panchal S., Patel B. Porcupine inhibitors: novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway. Pharmacol Res. 2021;167:105532. doi: 10.1016/j.phrs.2021.105532. [DOI] [PubMed] [Google Scholar]
- 342.Le P.N., McDermott J.D., Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Jimeno A., Gordon M., Chugh R., et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(24):7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 344.Moore K.N., Gunderson C.C., Sabbatini P., et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301. doi: 10.1016/j.ygyno.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Giraudet A.L., Cassier P.A., Iwao-Fukukawa C., et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18(1):646. doi: 10.1186/s12885-018-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sudo H., Tsuji A.B., et al. FZD10-targeted α-radioimmunotherapy with 225Ac-labeled OTSA101 achieves complete remission in a synovial sarcoma model. Cancer Sci. 2022;113(2):721–732. doi: 10.1111/cas.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gurney A., Axelrod F., Bond C.J., et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Katoh M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review) Int J Mol Med. 2018;42(2):713–725. doi: 10.3892/ijmm.2018.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Salik B., Yi H., Hassan N., et al. Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication in acute myeloid leukemia. Cancer Cell. 2020;38(2):263–278. doi: 10.1016/j.ccell.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 351.Lenz H.J., Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105(9):1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Manegold P., Lai K.K.Y., Wu Y., et al. Differentiation therapy targeting the β-catenin/CBP interaction in pancreatic cancer. Cancers. 2018;10(4):95. doi: 10.3390/cancers10040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Schmidtova S., Kalavska K., Liskova V., et al. Targeting of deregulated Wnt/β-catenin signaling by PRI-724 and LGK974 inhibitors in germ cell tumor cell lines. Int J Mol Sci. 2021;22(8):4263. doi: 10.3390/ijms22084263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yu J., Chen L., Cui B., et al. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2016;126(2):585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Choi M.Y., Widhopf G.F., 2nd, Ghia E.M., et al. Phase I trial: cirmtuzumab inhibits ROR1 signaling and stemness signatures in patients with chronic lymphocytic leukemia. Cell Stem Cell. 2018;22(6):951–959. doi: 10.1016/j.stem.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Funck-Brentano T., Nilsson K.H., Brommage R., et al. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J Endocrinol. 2018;238(1):13–23. doi: 10.1530/JOE-18-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Li X., Ominsky M.S., Villasenor K.S., et al. Sclerostin antibody reverses bone loss by increasing bone formation and decreasing bone resorption in a rat model of male osteoporosis. Endocrinology. 2018;159(1):260–271. doi: 10.1210/en.2017-00794. [DOI] [PubMed] [Google Scholar]
- 358.Wong S.K., Chin K.Y., Ima-Nirwana S. Quercetin as an agent for protecting the bone: a review of the current evidence. Int J Mol Sci. 2020;21(17):6448. doi: 10.3390/ijms21176448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Okuchi Y., Reeves J., Ng S.S., et al. Wnt-modified materials mediate asymmetric stem cell division to direct human osteogenic tissue formation for bone repair. Nat Mater. 2021;20(1):108–118. doi: 10.1038/s41563-020-0786-5. [DOI] [PubMed] [Google Scholar]