Abstract
Osteoarthritis (OA) is a chronic debilitating joint disease, characterized by degeneration of the cartilage and loss of the cartilage matrix, and it is clinically manifested as joint pain. Osteopontin (OPN) is a glycoprotein that is abnormally expressed in the bone and cartilage tissues and plays a vital role in various pathological processes such as the osteoarthritic inflammatory response and endochondral ossification. The focus of our study is to investigate the therapeutic potential and specific role of OPN in OA. Using morphological comparisons, we found that the cartilage was severely worn-out and there was a significant loss of the cartilage matrix in OA. OPN, CD44, and hyaluronic acid (HA) synthase 1 (HAS1) were highly expressed, and the anabolism of HA was significantly higher in the OA chondrocytes than in the control chondrocytes. Additionally, we treated the OA chondrocytes with small interfering RNA (siRNA) targeting OPN, recombinant human OPN (rhOPN), and a combination of rhOPN and anti-CD44 antibodies. Furthermore, in vivo experiments were performed in mice. We found that OPN upregulated the expression of downstream HAS1 and increased the anabolism of HA through CD44 protein expression in OA mice compared with those in control mice. Moreover, intra-articular injection of OPN in mice with OA significantly inhibited OA progression. In summary, OPN initiates an intracellular cascade via CD44 which results in an anabolic increase in HA levels, thereby inhibiting OA progression. Therefore, OPN is a promising therapeutic agent in precision treatment of OA.
Keywords: Cartilage degeneration, CD44, Hyaluronic acid, Osteoarthritis, Osteopontin
Introduction
Osteoarthritis (OA) is a chronic crippling degenerative joint disease. It is characterized by the degeneration and destruction of articular cartilage, subchondral osteosclerosis or cystic change, hyperosteogeny of the joint margins, synovitis, contracture of the joint capsule, and relaxation or contraction of ligaments.1,2 OA clinically manifests as joint pain and may lead to joint deformity and movement dysfunction, which increases the incidence of cardiovascular events and all-cause mortality. OA is highly prevalent in middle-aged and elderly population, and more than 50% of people above 65 years are affected by OA.3,4 OA is characterized by high morbidity and disability rates, ineffective drug treatments, and expensive treatment costs. This incurs a financial burden on the society, particularly on the families of patients, in addition to the physical and mental suffering that the older patients undergo.5,6 Therefore, there is an urgent need for further research on OA to elucidate its pathological mechanism, formulate effective preventive measures, develop effective treatments, block or delay its pathological development, and alleviate suffering or cure the disease. The main pathological feature of OA is the degeneration of the joint caused by wear and tear of the articular cartilage, and the core mechanism involved in the progression of OA is the apoptosis of chondrocytes and loss of the cartilage matrix. However, the specific molecular mechanisms involved are not fully understood yet. Therefore, the focus of our study was to identify mechanisms to effectively inhibit the apoptosis of chondrocytes and degradation of cartilage.
Osteopontin (OPN) is a phosphorylated glycoprotein, which is widely present in bone tissues and is secreted by cells such as chondrocytes and synovial cells.7, 8, 9 OPN binds to cell surface receptors at the tripeptide Arg-Gly-Asp (RGD) sequence in the extracellular matrix (ECM), and OPN is involved in the development, repair, and homeostasis of normal articular cartilage.10,11 OPN also regulates hyaluronic acid (HA), type II collagen, proteoglycan, and other cartilage matrix components, thereby preventing the development and deterioration because of OA.12,13 OPN transduces intracellular signals by binding to integrins and adhesion glycoproteins on the surface of chondrocytes, thereby affecting anabolic processes and regulating cell growth and movement. CD44, a member of the adhesion receptor family, is involved in cell adhesion, migration, proliferation, differentiation, and intracellular signal transduction. CD44 is necessary to maintain cartilage homeostasis, and elevated CD44 levels in vivo contribute to OA pathogenesis.14,15 The upregulation of CD44 in OA cartilage is consistent with the severity of OA and positively correlated with the role of OPN in OA. However, the internal regulatory mechanism of OPN and CD44 in OA requires further exploration.
HA is a glycosaminoglycan that is present extensively in tissues, including the articular cartilage, lens, and skin dermis, and is the main component of joint synovial fluid. HA directly affects cell behavior by influencing the formation of the ECM, and it is involved in the maintenance of intra-articular homeostasis and joint lubrication.16, 17, 18 Several clinical studies have shown that intra-articular injection of HA effectively relieves joint pain and inhibits the progression of OA.19,20 Because of the preventive effects, HA has attracted considerable attention in the prevention and treatment of OA. OPN upregulates the expression of HA, proteoglycan, and type II collagen in OA affected human knee chondrocytes, thereby maintaining the stability of the cartilage matrix.18 However, the mechanisms of OPN-induced upregulation of HA remain unknown. The regulation of HA mainly depends on its metabolism by HA synthase (HAS), and three types of HASs mediate the synthesis and regulation of HA of different molecular weights, among which HAS1 is the main enzyme involved in chondrocytes. Therefore, the regulation of HA by OPN may involve mechanism that is related to HAS activity.
In this study, we hypothesized that OPN secreted by chondrocytes activates intracellular signaling by binding to the membrane receptor molecule CD44, and upregulates the anabolism of HA to maintain a stable concentration of the ECM in the chondrocytes, thereby inhibiting the progression of OA. Using in vitro and in vivo experiments, we explored the role of the OPN/CD44/HAS1 activity in the pathological process of OA cartilage degeneration, elucidated the specific regulatory mechanism of OPN on HA, and confirmed the protective role of HA in OA.
Materials and methods
Specimen acquisition and ethical considerations
Primary OA cartilage tissue samples were obtained from 34 patients with OA (Kellgren–Lawrence grades III–IV) who underwent total knee arthroplasty, and healthy knee joint cartilage tissue samples were obtained from patients with osteosarcoma who underwent hinge knee arthroplasty as shown in Table 1. The study obtained approval from the Ethics Committee of the Xiangya Hospital and Animal Research Center of the Central South University (Changsha, China). Written informed consent was obtained from the patients or their legal guardians.
Table 1.
Patients information.
| Group | Average age (year) | Gender (F/M) | Diagnosis |
|---|---|---|---|
| OA | 66.8 | F:20 | OAa |
| M:14 | |||
| Normal | 18.2 | F:8 | Osteosarcoma |
| M:7 |
All patients with K-L grade III or IV.
Chondrocyte isolation
Surgically obtained cartilage tissues were promptly placed in phosphate buffer and digested within 6 h. The type II collagenase digestion method was used for the isolation of human knee joint chondrocytes as previously described.21 Chondrocytes from the second generation were used in all experiments.
Limb culture in vitro
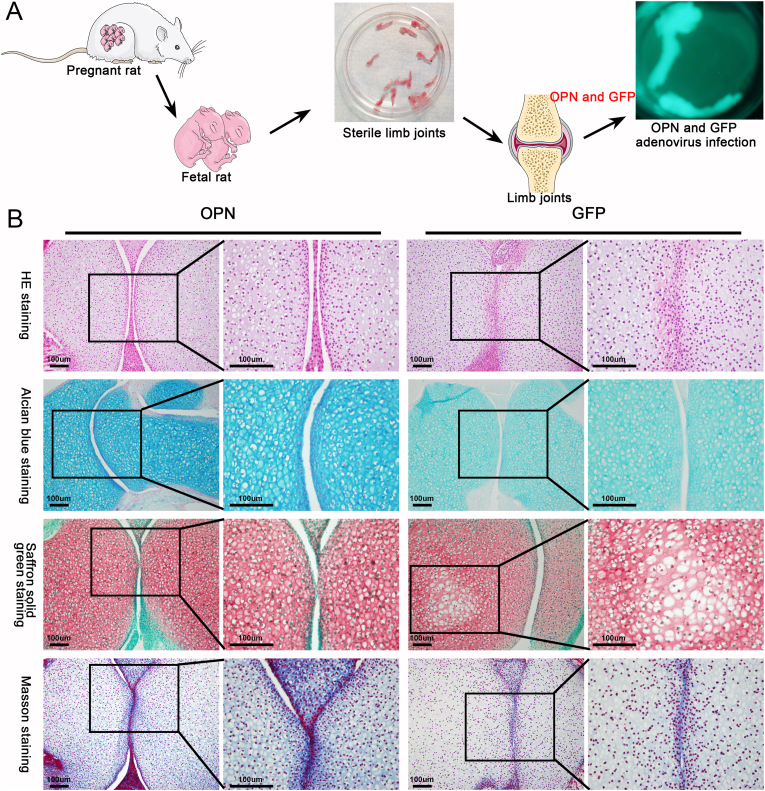
The limb joints of premature or newborn fetal mouse purchased from the SJA Laboratory Animal Co., Ltd (Hunan, China) were dissected on a sterile table and washed thrice using phosphate-buffered saline containing 1% penicillin streptomycin. The limbs were then placed into 12-well plates and 2 mL of Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) was added for in vitro culture. After 12 h, the appropriate titer of OPN or green fluorescent protein (GFP) adenovirus, which was packed and verified by our laboratory,22 was added for infection for 12 h. The media was then replaced with fresh DMEM and culturing continued for three to five days. Finally, the cultured limb tissue was collected and embedded into paraffin blocks for morphological staining.
Histology and morphology
Cartilage tissue samples and fetal mice limb tissue cultured in vitro, were immersed in 4% paraformaldehyde for at least 24 h, decalcified in 10% ethylenediaminetetraacetic acid, dehydrated using an alcohol gradient, embedded in paraffin tissue blocks, and then 5 mm-thick sections were prepared. Hematoxylin-eosin (HE), toluidine blue, Alcian blue, safranin solid green, Masson, and alizarin red staining techniques were performed, to evaluate the overall morphology of the cartilage, composition of the cartilage matrix, thickness of the subchondral bone, and calcium deposition in the cartilage matrix. Toluidine blue and alizarin red staining techniques were also performed on chondrocytes in vitro.
Cell proliferation
Primary chondrocytes were resuspended and plated in 96-well plates. The proliferation of the chondrocytes was analyzed at 24, 48, 72, 96, and 120 h after plating. Cell counting kit-8 (CCK-8) reagent (7Sea Pharmaceutical, Shanghai, China) was added to each well, and the cells were further incubated for 1 h. The absorbance was measured at 450 nm.
Immunohistochemistry
Immunohistochemical staining of the sections was performed as previously described.23,24 The expression of OPN in cartilage and chondrocytes was detected using primary antibodies targeting OPN (ab69498; Abcam, Cambridge, UK). A horseradish peroxidase-conjugated secondary antibody (AS064, ABclonal, Woburn, MA, USA) was used to bind to the primary antibody, and 3,3′-diaminobenzidine tetrahydrochloride was used for signal detection.
Immunofluorescence (IF)
Differences in the expression of OPN and CD44 (using an anti-CD44 antibody, D261338; Sangon Biotech, Shanghai, China) in OA and healthy chondrocytes were detected using IF as previously described.25,26 Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Beyotime, Hangzhou, China) and a confocal laser scanning microscope (TCS SP8, Leica, Wetzlar, Germany) was used to capture the images.
Enzyme-linked immunosorbent assay (ELISA)
Chondrocytes were plated uniformly on 24-well plates and incubated in complete medium containing 10% FBS for 120 h. The supernatant was then collected and was used to detect HA using an ELISA kit (Enzyme-linked Biotechnology, Shanghai, China) according to the manufacturer's instructions.
Western blot analysis
Total protein was extracted from the cartilage tissues and chondrocytes using radioimmunoprecipitation with lysis buffer containing benzenesulfonyl fluoride and protease inhibitors. Quantification of the protein levels was performed using a protein analysis kit (ab102536; Abcam). Total protein (20 μg per sample) was loaded onto a 12% gel, separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electro transferred onto a polyvinylidene difluoride membrane (MilliporeSigma, Burlington, MA, USA). Non-specific antigen sites were blocked using 5% bovine serum albumin. The blots were incubated with specific primary antibodies against OPN, CD44, HAS1 (ab75329; Abcam, Cambridge, UK), and β-actin (#8457, Cell Signaling Technology, Danvers, MA, USA) overnight. The blots were then incubated with the corresponding secondary antibody (AS064, ABclonal) for 1 h. The intensity signals were visualized using an enhanced chemiluminescence substrate (NCM Biotech, Suzhou, China) and the gray scale of the target bands was analyzed using the Image J software.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cartilage tissues and chondrocytes as previously reported.23,27 mRNA expression was detected using the SYBR Green PCR Master Mix (Takara Bio, Kusatsu, Shiga, Japan), with β-actin as the internal control. The 2−ΔΔCt method was used to analyze gene expression. The primer sequences shown in Table 2 were obtained by using Invitrogen (Waltham, MA, USA).
Table 2.
Primer sequences used in this study.
| Primer | 5ʹ–3ʹ sequence |
|---|---|
| β-actin | F:CACCCAGCACAATGAAGATCAAGAT |
| R:CCAGTTTTTAAATCCTGAGTCAAGC | |
| OPN | F:AGTTTCGCAGACCTGACATCC |
| R:TTCCTGACTATCAATCACATCGG | |
| CD44 | F:TGACAACGCAGCAGAGTAATTC |
| R:TTCCACCTGTGACATCATTCCT | |
| HAS1 | F:GCGATACTGGGTAGCCTTCA |
| R:GGTTGTACCAGGCCTCAAGA |
Cell transfection and treatment
An OA chondrocyte model was constructed using a small interfering RNA (siRNA) plasmid targeting OPN (GenePharma, Shanghai, China), to silence the OPN gene. The primer sequences are shown in Table 3. Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to transfect the chondrocytes following the instruction guide of the manufacturer. OPN was overexpressed using recombinant human OPN (rhOPN; r&D Systems, Minneapolis, MN, USA), at the recommended concentration of 1500 pg/mL. The ligand-receptor response between OPN and CD44 was blocked using a CD44-specific antagonist (LS-C179404, LifeSpan Biosciences, Seattle, WA, USA).
Table 3.
Designed OPN-siRNA sequence.
| Gene | 5ʹ–3ʹ siRNA sequence | |
|---|---|---|
| OPN | siRNA1 | F:CAGGUUGUUAUAGGUCUCGAGUUUA |
| R:UAAACUCGAGACCUAUAACAACCUG | ||
| siRNA2 | F:GACGCAGCAUCCAUUUCCUACUUUA | |
| R:UAAAGUAGGAAAUGGAUGCUGCGUC | ||
| siRNA3 | F:GAACUCCUUGCAGGCUUGAACAAUA | |
| R:UAUUGUUCAAGCCUGCAAGGAGUUC | ||
Intra-articular injection and establishment of the OA model in vivo
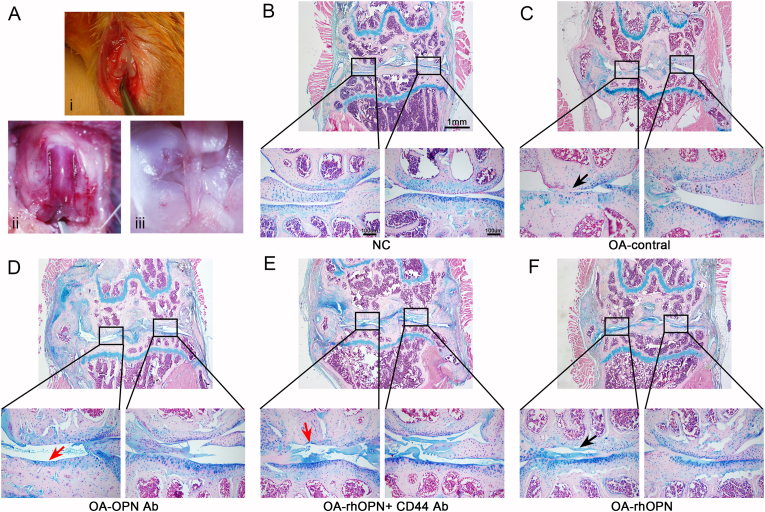
Forty-eight-week-old male mice were obtained from the SJA Laboratory Animal Co., Ltd. Destabilization of the medial meniscus (DMM) was performed to obtain an OA model (Fig. 5A). The mice were anesthetized via intraperitoneal injection of 2% pentobarbital sodium solution, and DMM was performed in the right hindlimb to induce OA as previously described.28 The mice were divided into groups (eight mice/group) as follows: healthy control group, OA group, OPN antibody group, rhOPN group, and rhOPN + CD44 antibody combination intervention group, according to different intra-articular injections of drugs. The injections were administered two weeks after surgery and continued for 4 weeks at a weekly frequency. One week after the final injection, the mice were sacrificed and knee joints of their right hindlimbs were dissected. The samples were fixed, decalcified, dehydrated, prepared for tissue sectioning, and stained using Alcian blue, to observe the morphological changes.
Figure 5.
Alcian blue staining of mouse joint sections showed that OPN mediated inhibition of OA progression through CD44 in mice (n = 6 in each group). (A) The OA model was established by DMM, and the general structure of mouse joint, cruciate ligament and meniscus can be clearly revealed. (B) Normal mouse femoral joint surface is smooth and flat with a thick cartilage layer (C) Articular surface of the femur in OA is fissured, and the cartilage layer is thin. (D) OPN antibody intervention led to further aggravation of OA, more severe cartilage wear, and complete exfoliation of the cartilage layer. (E) rhOPN combined with CD44 antibody did not improve the symptoms of OA but increased cartilage wear. (F) Articular cartilage surface in the rhOPN intervention group is complete and smooth, and the cartilage layer is thicker than that in the OA control group (the black arrow indicates the worn cartilage layer, and the red arrow indicates the subchondral bone after cartilage loss).
Statistical analysis
Data are presented as mean ± standard deviation and were analyzed using the GraphPad Prism software (version 8.0). A Student's t-test was used to analyze the statistical significance between the groups, and statistical significance was set at P < 0.05.
Results
Histological and basic metabolic differences in OA cartilage
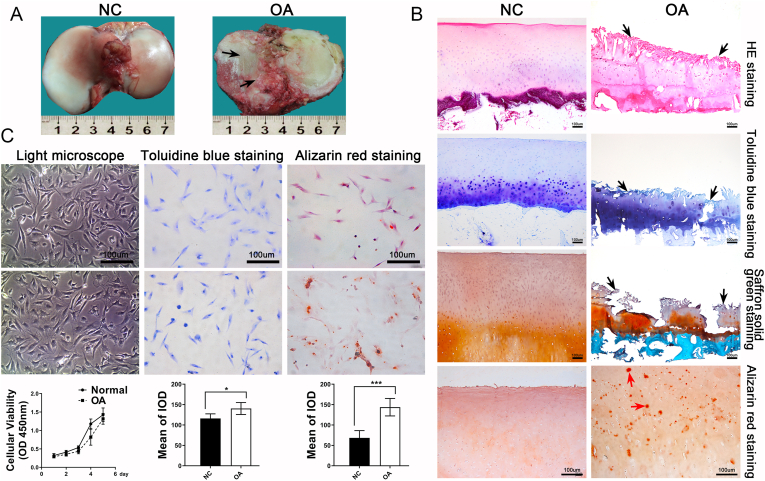
The tibial plateau of the knee joint was obtained from patients with OA and osteosarcoma who voluntarily participated in this study. A clear visual contrast was observed between the samples from the two groups. The OA cartilage revealed an extremely abraded surface, villous changes, pale color, and few areas completely missing in the cartilage (Fig. 1A). Paraffin sections were prepared from the cartilage and stained. HE staining revealed that the surface of the OA cartilage was longitudinally grooved with flocculent cartilage matrices. Furthermore, the cartilage matrix was uneven in thickness and poorly layered. The number of cartilage lacunae were limited and the ECM was deeply stained. In contrast, the surface of the healthy cartilage was smooth, cartilage layer was thick and uniform, and cartilage matrix was clearly stratified (Fig. 1A). Toluidine blue staining revealed light coloration in the horizontal layer of the OA cartilage, with dark blue changes in the matrix below the transplanted layer. Additionally, the tidal line was disordered, indicating that the content of glycosaminoglycan in the superficial layer of the OA cartilage was lower than that in the deep layer (Fig. 1B). The superficial layer of the OA cartilage was lightly stained or unstained with safranin solid green, while the deep matrix layer was deeply stained. This indicates a massive loss of proteoglycans in the superficial matrix and an increased synthesis of proteoglycans in the deep matrix layer when compared with those in the control (Fig. 1B). Alizarin red staining showed large amounts of calcium salt deposits in the OA cartilage matrix, which was a clear manifestation of cartilage degeneration (Fig. 1B). Through multiple comparisons, the results revealed that the destruction of cartilage integrity and loss of matrix components were the most evident changes in OA cartilage. However, there were no significant morphological differences between primary OA and control chondrocytes isolated from cartilage tissue, and also no significant differences in the proliferative capacity of OA and control chondrocytes as determined by the CCK-8 assay (Fig. 1C). Toluidine blue staining of chondrocytes demonstrated that OA chondrocytes stained deeper than control chondrocytes. Furthermore, the synthesis of glycosaminoglycan in OA chondrocytes was significantly upregulated compared with that in the control (Fig. 1C). Finally, alizarin red staining revealed scattered calcium salt deposits in the OA chondrocytes, which is representative of cartilage degeneration (Fig. 1C).
Figure 1.
Histologic and cytological differences between normal human cartilage and OA cartilage. (A) Normal human articular cartilage has a smooth and flat surface and thick texture and elasticity, and its surface is bright white. OA cartilage has a rough and cracked surface, slightly hard texture, lack of flexibility, and pale color. (B) Histological staining of cartilage sections was used to compare the morphological differences. The results showed that the surface of OA cartilage was crisscross and seriously worn. Toluidine blue and safranine solid green staining showed significant loss of cartilage matrix. Alizarin red staining showed a large amount of calcium deposition in OA cartilage matrix (the black arrow indicates the location of OA cartilage wear, and the red arrow indicates calcium deposition) (C) The proliferative capacity of OA chondrocytes did not different from normal chondrocytes, but toluidine blue staining found higher levels of glycan synthesis in OA cells than normal chondrocytes (P = 0.018), and alizarin red staining showed scattered calcium deposition in OA chondrocytes (P = 0.0003).
OPN promotes ECM synthesis in cartilage
Alcian blue staining showed that OPN effectively promoted the anabolism of cartilage ECM, specifically through the synthesis of HA in the cartilage matrix (Fig. 2B). Safranin solid green staining showed that OPN promotes the synthesis of proteoglycan in cartilage, because the staining in the OPN intervention group was significantly higher than that in the GFP group (Fig. 2B). Furthermore, Masson staining suggested that OPN also promoted the synthesis of collagen fibers in the cartilage (Fig. 2B). The staining analysis of fetal mouse joint tissue after OPN intervention revealed that OPN played a positive role in the anabolism of the cartilage ECM, and promoted the synthesis of HA, proteoglycan, and collagen fibers in the cartilage matrix. These results suggest that OPN adenovirus intervention may significantly enhance the anabolism of articular cartilage and levels of ECM components including collagen fibers, proteoglycans, and HA in cartilage compared with those in the GFP intervention group. Therefore, OPN may play a positive role in the repair of articular cartilage.
Figure 2.
Effect of OPN on metabolism of fetal mouse limb cartilage in vitro. (A) Limbs of upcoming or newborn fetal mouse were obtained for in vitro culture, followed by intervention with OPN and GFP adenovirus, and after 3–5 days of continuous intervention, the limbs were prepared as paraffin sections (B) Alcian blue staining showed that the ECM components of articular chondrocytes increased significantly after OPN intervention, indicating that the synthesis of HA in chondrocytes increased. Similarly, the synthesis of proteoglycan and collagen fibers in cartilage matrix also increased significantly, indicating that OPN can effectively promote the synthesis and metabolism of cartilage ECM.
Upregulation of OPN, CD44, and HA in OA cartilage tissue and chondrocytes
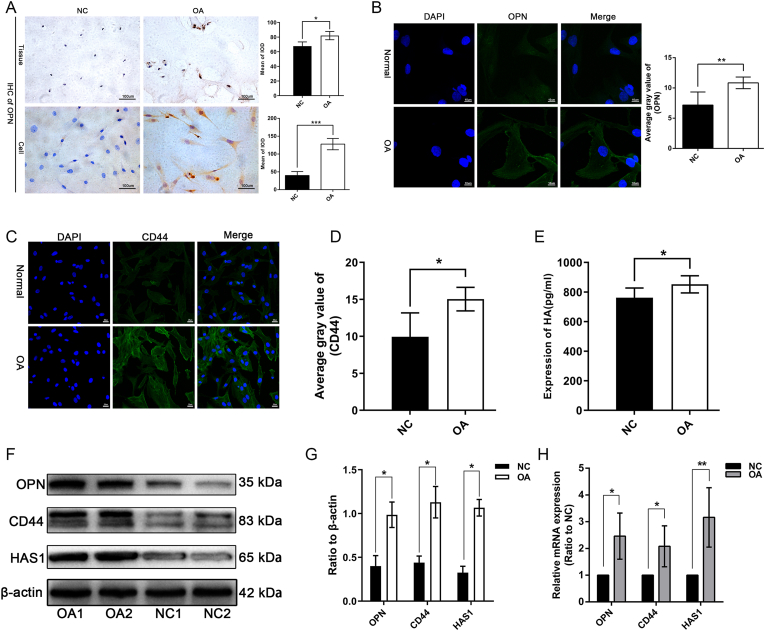
Immunohistochemical examination and semi-quantitative analysis revealed that OPN expression was significantly upregulated (P < 0.001) in OA cartilage tissues and chondrocytes compared with that in the control (Fig. 3A). Furthermore, IF analysis revealed that OPN expression was upregulated in OA chondrocytes compared with that in control cells (Fig. 3B). The IF results also showed that CD44 expression was upregulated in the OA chondrocytes and was significantly higher (P < 0.05) than that in the control chondrocytes (Fig. 3C, D). The ELISA analysis of HA levels in the supernatants from the chondrocytes revealed that HA anabolism was more active in OA chondrocytes than in the control cells (Fig. 3E). Considering that the expression of HA mainly depends on metabolism by HAS1, the expression of HAS1 is related to the anabolism of HA. Furthermore, quantification of the protein and mRNA expression levels of OPN, CD44, and HAS1 revealed that their expressions were significantly upregulated (P < 0.05) in OA chondrocytes compared with that in control cells (Fig. 3F, H).
Figure 3.
Upregulation of OPN, CD44, and HAS1 expression in OA chondrocytes. (A, B) The results of immunohistochemistry showed that OPN was highly expressed in OA cartilage, and the expression of OPN in OA chondrocytes was also significantly higher than that in normal cartilage (P = 0.0002). Immunofluorescence detection of OPN showed that only scattered bright green fluorescence could be seen in normal chondrocytes, but it was significantly enhanced in OA chondrocytes (P = 0.0054). (C, D) The fluorescence signal of CD44 in normal chondrocytes was significantly lower than that in OA chondrocytes (P = 0.013). (E) The ELISA results of HA also confirmed that anabolic level of HA in OA chondrocytes is significantly higher than that in normal chondrocytes (P = 0.011). (F–H) mRNA and protein expression levels of OPN, CD44, and HAS1 in OA chondrocytes is upregulated compared to those in normal chondrocytes. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
In vitro analysis of the OPN/CD44/HAS1 signal axis
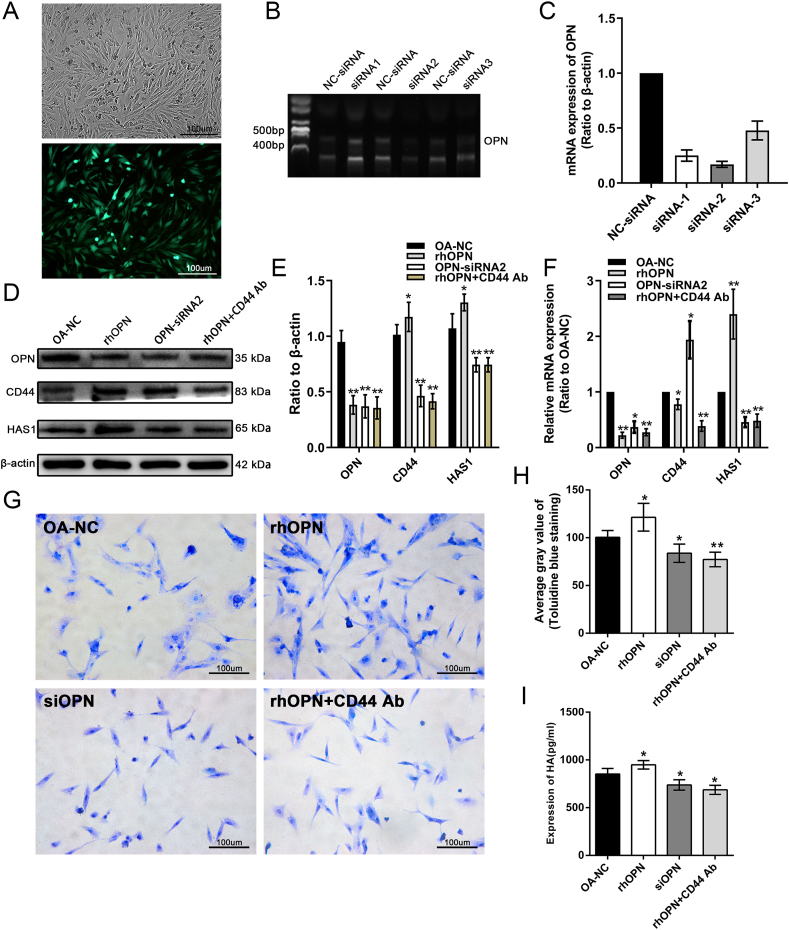
To silence the expression of OPN, siRNAs were used and OPN-siRNA2 was found to have the highest knockdown efficiency of up to 80% (Fig. 4A–C). Therefore, OPN-siRNA2 was used to silence OPN expression in the subsequent experiments. OA chondrocytes were treated with OPN-siRNA, rhOPN, or CD44 antagonists, followed by detection of changes in protein and mRNA expression. Following treatment with rhOPN, the protein and mRNA expression levels of CD44 and HAS1 were upregulated in OA chondrocytes, while after OPN-siRNA2 intervention, their expression levels were significantly downregulated compared with that in the control cells. These results indicate that OPN has a positive regulatory effect on the expression of CD44 and HAS1. Immediately after combined treatment with rhOPN and CD44 antagonists, the protein and mRNA expression of HAS1 decreased compared with that in the control cells, which also indicate that OPN regulated the expression of HAS1 through CD44 antagonist (Fig. 4D–F). The results of toluidine blue staining of the chondrocytes were consistent with those in the previous molecular studies. rhOPN antagonist increased the level of glycosaminoglycan synthesis in OA chondrocytes, whereas OPN-siRNA2 and CD44 antagonists significantly inhibited glycosaminoglycan synthesis levels compared with those in control cells. Furthermore, the level of HA in the cellular supernatant was analyzed using an HA ELISA kit. We found that rhOPN antagonist increased the HA content in the supernatant compared with that in the healthy control group, whereas OPN-siRNA2 and CD44 antagonists decreased the HA content in the supernatant when compared with that in the control cells (Fig. 4G–I).
Figure 4.
OPN mediates upregulation of HAS1 expression through CD44 resulting in increased HA anabolism. (A–C) Validation of OPN-short interfering RNA (siRNA) silencing efficiency showing OPN-siRNA2 silencing efficiency of up to 80%. (D–F) OPN-siRNA2, recombinant human OPN (rhOPN), and CD44 antagonists were used to treat OA chondrocytes. rhOPN could increase the mRNA and protein expression of CD44 and HAS1. However, OPN siRNA intervention had the opposite effect, that is, the mRNA and protein expression levels of CD44 and HAS1 were down-regulated. After further intervention with rhopn + CD44 antagonists, the expression level of HAS1 was also down regulated, indicating that the role of OPN on HAS1 was mediated by CD44. (G–I) The results of toluidine blue staining of chondrocytes after intervention showed that exogenous rhopn can promote anabolic levels of glycosaminoglycans in OA chondrocytes (P = 0.019), while OPN siRNA2 and CD44 antagonists can inhibit glycosaminoglycan biosynthesis, and the inhibitory effect of CD44 antagonist (P = 0.014) is more significant than OPN siRNA2 (P = 0.0011). The ELISA results of HA among different groups were basically consistent with the staining results. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Analysis of the OPN/CD44/HAS1 signal axis in mice
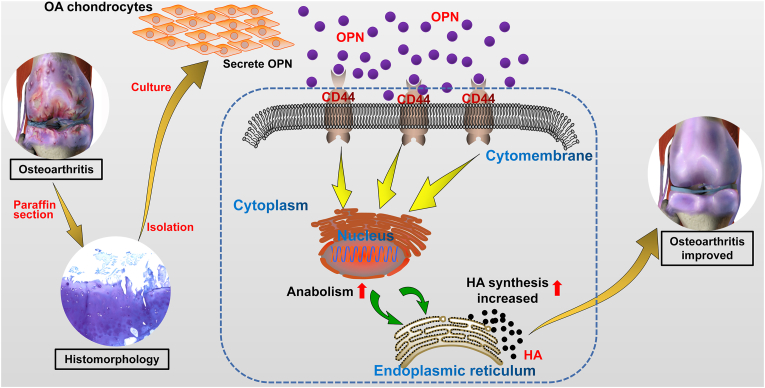
The knee joint tissue specimens from mice in the different intervention groups were collected and stained using Alcian blue. The results revealed that the cartilage layer was thin and the integrity of the cartilage surface was compromised in the OA group compared with that in the healthy control group (Fig. 5C). Furthermore, the knee joint cartilage was severely worn and the cartilage matrix was largely missing following intervention with OPN or rhOPN + CD44 antagonists compared with the cartilage conditions in the OA group (Fig. 5D, E). In severe cases, the cartilage layer was completely absent. However, the wear and tear of the knee joint, and thickness and integrity of the cartilage layer were significantly improved in the rhOPN intervention group compared with that in the OA group (Fig. 5F); however, inferior to the conditions in the healthy control group (Fig. 5B). From the results of the in vivo experiments in mice, we found that the severity of joint degeneration was not consistent in the double compartments of the knee joint, and degeneration of the medial articular surface of most experimental animals was more severe than that of the lateral articular surface. These observations in our experimental results resemble the degeneration observed in clinical patients with OA. Clinically, in most patients, the degeneration degree of the medial articular surface is severe compared with that of the lateral articular surface. This difference can be explained by the theory of uneven settlement of the knee joint surface. These results demonstrate that rhOPN ameliorates cartilage degeneration and inhibits the progression of OA, and the protective effect of OPN on cartilage is mediated by CD44 (Fig. 6).
Figure 6.
Mechanism of osteopontin (OPN)-mediated inhibition of OA progression involves increased HA anabolism mediated through CD44.
Discussion
OA is a chronic degenerative joint disease characterized by the progressive wear and tear of the cartilage, massive loss of the cartilage matrix, joint pain, and dysfunction. Because of its unidentified pathogenesis, OA is considered an incurable disease.29, 30, 31, 32 With the acceleration of the social aging process, rising life expectancy, and increase in physical activity, the prevalence of OA is expected to gradually increase, which poses a severe financial burden to families and society. Therefore, it is of great clinical significance and practical value to analyze the histomorphological changes and pathological mechanisms associated with OA, which can provide new insight on the prevention, diagnosis, and treatment of OA.
In the present study, morphological analysis of patient tissue specimens and primary chondrocytes was conducted, and the results were further validated with the in vitro results using an OA mouse model. The present study revealed the following important findings: (1) the main morphological changes in OA include severe wear and tear of the cartilage surface and massive loss of the cartilage matrix, accompanied by enhanced anabolism of OA chondrocytes; (2) the secretion of HA increases along with the upregulation of OPN, CD44, and HAS1 expression in OA chondrocytes; (3) the upregulation of OPN expression leads to an increase in HA secretion in the OA chondrocytes, mediated by the cell membrane molecule CD44; (4) in vivo studies in mice suggest a positive role of the OPN/CD44/HAS1 signal axis in delaying the progression of OA; and (5) the upregulation of OPN expression is mediated by CD44 to increase the synthesis of HA in OA chondrocytes, thereby inhibiting the occurrence and development of OA. Based on the results of the current and previous studies, we propose a specific mechanism of OPN-mediated prevention in OA (Fig. 5). Although the preventive effect of OPN in OA has been reported, studies on the specific pathophysiological mechanism are limited. Here, using in vitro and in vivo experiments, we demonstrate that OPN directly affects the CD44-mediated synthesis of HA, which is an important component of the cartilage matrix, at the molecular level, thus affecting the prognosis of OA.
OPN, a multifunctional phosphorylated protein secreted by osteoclasts, macrophages, lymphocytes, epithelial cells, and vascular smooth muscle cells, is present in the ECM of mineralized tissues and extracellular fluid of inflammatory sites.13,33 OPN binds to cell surface receptors mainly through RGD and non-RGD sequences, promoting cell surface adhesion and migration.34 OPN is involved in cell-to-matrix and cell-to-cell interactions, and it is involved in the processes of bone mineralization, cell migration, and pathophysiology of chronic infectious diseases.35 The role of OPN in OA has gradually become an attractive research topic. Gao et al10 found that OPN in the plasma and synovial fluid contributes to progressive joint injury in knee OA. Thus, OPN may serve as a biochemical marker to estimate the severity of OA and predict its prognosis. Jiang et al36 found that the −443C/T and −66 T/G polymorphisms in the OPN gene are significantly associated with the risk and severity of OA. This suggests that OPN gene polymorphisms may be used as molecular markers of severity and susceptibility to OA. Calcium deposition, as a pathological manifestation, has been reported in OA, and was also observed in this study. Rosenthal et al37 demonstrated that OPN plays an important role in promoting calcium pyrophosphate dihydrate crystal formation in articular cartilage. This indicates that OPN may be a risk factor for OA and is involved in promoting OA. However, other studies have reported that OPN prevents OA progression.7, 8, 9 OPN inhibits the loss of ECM, accelerates the structural reconstruction of the cartilage matrix network,38 inhibits the release of inflammatory factors such as interleukin (IL)-1, nitric oxide, and prostaglandin E2 (PGE2) from chondrocytes,39 and upregulates the anabolism of proteoglycans and type II collagen in OA chondrocytes.8 In this study, we show that upregulation of OPN expression leads to increased HA secretion in OA chondrocytes which is mediated by the cell membrane molecule CD44.
As a member of the membrane surface receptor integrin family, CD44 is involved in the regulation of intracellular and extracellular signal transmission, and also in cell proliferation, differentiation, adhesion, and migration.15,40 Zhang et al15 reported that the expression of CD44 in the articular cartilage of knee OA was positively correlated with the severity of the disease, which was consistent with the expression of OPN. Yang et al41 showed that OPN in combination with CD44 is a promising independent predictor of tumor recurrence and survival in patients with hepatocellular carcinoma. Moreover, Cai et al42 demonstrated that an HA hydrogel inhibits the release of oligonucleotides and regulates the progression of OA via a binding interaction between CD44 and chondrocytes. In addition, intra-articular injection of HA not only inhibits synovial neovascularization and fibrosis, but also maintains the integrity of articular cartilage through CD44-dependent mechanisms.43, 44, 45 In the current study, through OPN and CD44 interventions, we found that OPN regulates the expression of CD44 and further affects the expression and phenotypic changes of downstream molecules. Although the OPN-CD44 ligand-receptor reaction is critical in the occurrence and development of OA, the specific changes involved in downstream signaling need to be studied.
HA is a main component of the cartilage matrix and participates in the flexibility and elasticity of articular cartilage, and also in maintaining the viscoelasticity and lubricity of synovial fluid.16,46 HA affects the behavior of cells by influencing the formation of the ECM, and directly acting on cells to maintain joint homeostasis and lubrication. Additionally, HA prevents cartilage degradation and degeneration by forming a protective membrane on the articular cartilage surface.17,42,47 Furthermore, HA intervention may be used as a method to relieve the pain due to OA, and regulate OA-related inflammatory factors and metabolism of the cartilage matrix.17,42,48 Intra-articular injection of HA regulates nociceptors, reduces fibrosis of the synovium and capsule, increases endogenous production of high molecular weight HA in synovium cells, reduces inflammatory transmitters including PGE2, IL-1, and IL-6, and reduces the matrix of articular cartilage in animal models of OA.17 Our results demonstrate that OPN influences the anabolism of HA in chondrocytes through CD44. Upregulation of OPN expression increases the expression of HAS1 in chondrocytes, inducing the synthesis and secretion of HA. In vivo experiments in mice also show that upregulated OPN expression inhibits the progression of OA by increasing the synthesis of the cartilage matrix mediated by CD44.
Conclusions
In summary, the expression levels of OPN, CD44, and HAS1 are elevated in OA chondrocytes; upregulated OPN expression increases the expression of HAS1 via CD44, thereby enhancing the anabolism of HA in chondrocytes. In vivo studies in mice reveal that OPN enhances the anabolism of the cartilage matrix mediated by CD44, and ultimately inhibits the progression of OA. Therefore, OPN represents a promising target for the prevention and treatment of OA.
Ethics declaration
This study has been approved by the Animal Research Center of Central South University and the Ethics Committee of Xiangya Hospital.
Author contributions
Wei Luo conceptualized and designed the study, performed the experiment, collected and analyzed the data, prepared the manuscript, and approved final version of the manuscript. Zili Lin and Yuhao Yuan performed the experiment, analyzed the data, critical revision the manuscript, and approved final version of the manuscript. Ziyi Wu and Wei Zhong analyzed the data, critical revision the manuscript, and approved final version of the manuscript. Qing Liu conceptualized and designed the study, drafted and revised the manuscript, and approved final version of the manuscript.
Conflict of interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the Special funds for the construction of innovative provinces in Hunan Province (No. 2020RC3058), the Natural Science Foundation of Hunan (No.2022JJ40802 and 2022JJ30075), and the China Postdoctoral Science Foundation (No. 2022M713525).
Acknowledgements
We would like to give our sincere thanks to Dr. Zhixi Duan, Dr. Xiaopeng Tong, and Dr. Peng Huang for their assistance in the implementation of the experiment.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Abbreviations
- DMM
destabilization of the medial meniscus
- ECM
extracellular matrix
- HA
hyaluronic acid
- HAS
hyaluronic acid synthase
- IF
immunofluorescence
- IHC
immunohistochemistry
- IL-1
interleukin-1
- NO
nitric oxide
- OA
osteoarthritis
- OPN
osteopontin
- PGE2
prostaglandin E2
- rhOPN
recombinant human osteopontin
- siRNA
interfering RNA
References
- 1.Song Y., Hao D., Jiang H., et al. Nrf 2 regulates CHI3L1 to suppress inflammation and improve post-traumatic osteoarthritis. J Inflamm Res. 2021;14:4079–4088. doi: 10.2147/JIR.S310831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain M.A., Adithan A., Alam M.J., et al. IGF-1 facilitates cartilage reconstruction by regulating PI3K/AKT, MAPK, and NF-kB signaling in rabbit osteoarthritis. J Inflamm Res. 2021;14:3555–3568. doi: 10.2147/JIR.S316756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice S.J., Beier F., Young D.A., Loughlin J. Interplay between genetics and epigenetics in osteoarthritis. Nat Rev Rheumatol. 2020;16(5):268–281. doi: 10.1038/s41584-020-0407-3. [DOI] [PubMed] [Google Scholar]
- 5.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos E.M., Arden N.K. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12(2):92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 7.Li L., Lv G., Wang B., Kuang L. XIST/miR-376c-5p/OPN axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J Cell Physiol. 2020;235(1):281–293. doi: 10.1002/jcp.28968. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Jiang W., Wang H., et al. Osteopontin promotes expression of matrix metalloproteinase 13 through NF- κ B signaling in osteoarthritis. BioMed Res Int. 2016;2016 doi: 10.1155/2016/6345656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Xiao W., Sun M., et al. The expression of osteopontin and Wnt5a in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. BioMed Res Int. 2016;2016 doi: 10.1155/2016/9561058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao S.G., Li K.H., Zeng K.B., Tu M., Xu M., Lei G.H. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage. 2010;18(1):82–87. doi: 10.1016/j.joca.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z., Tian X.Y., Huang X.X., He L.L., Xu F. microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. J Cell Physiol. 2019;234(5):6042–6053. doi: 10.1002/jcp.27225. [DOI] [PubMed] [Google Scholar]
- 12.Li Y.S., Zhang F.J., Zeng C., et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143–148. doi: 10.1016/j.jbspin.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C., Gao S., Lei G. Association of osteopontin with osteoarthritis. Rheumatol Int. 2014;34(12):1627–1631. doi: 10.1007/s00296-014-3036-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F.J., Luo W., Gao S.G., et al. Expression of CD44 in articular cartilage is associated with disease severity in knee osteoarthritis. Mod Rheumatol. 2013;23(6):1186–1191. doi: 10.1007/s10165-012-0818-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F.J., Gao S.G., Cheng L., et al. The effect of hyaluronic acid on osteopontin and CD44 mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int. 2013;33(1):79–83. doi: 10.1007/s00296-011-2339-3. [DOI] [PubMed] [Google Scholar]
- 16.Miller L.E. Towards reaching consensus on hyaluronic acid efficacy in knee osteoarthritis. Clin Rheumatol. 2019;38(10):2881–2883. doi: 10.1007/s10067-019-04597-z. [DOI] [PubMed] [Google Scholar]
- 17.Faust H.J., Sommerfeld S.D., Rathod S., et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials. 2018;183:93–101. doi: 10.1016/j.biomaterials.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Xu M., Zhang L., Zhao L., et al. Phosphorylation of osteopontin in osteoarthritis degenerative cartilage and its effect on matrix metalloprotease 13. Rheumatol Int. 2013;33(5):1313–1319. doi: 10.1007/s00296-012-2548-4. [DOI] [PubMed] [Google Scholar]
- 19.Belk J.W., Kraeutler M.J., Houck D.A., Goodrich J.A., Dragoo J.L., McCarty E.C. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 20.de Lucia O., Murgo A., Pregnolato F., et al. Hyaluronic acid injections in the treatment of osteoarthritis secondary to primary inflammatory rheumatic diseases: a systematic review and qualitative synthesis. Adv Ther. 2020;37(4):1347–1359. doi: 10.1007/s12325-020-01256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan Z.X., Tu C., Liu Q., et al. Adiponectin receptor agonist AdipoRon attenuates calcification of osteoarthritis chondrocytes by promoting autophagy. J Cell Biochem. 2020;121(5–6):3333–3344. doi: 10.1002/jcb.29605. [DOI] [PubMed] [Google Scholar]
- 22.Wei Q., Fan J., Liao J., et al. Engineering the rapid adenovirus production and amplification (RAPA) cell line to expedite the generation of recombinant adenoviruses. Cell Physiol Biochem. 2017;41(6):2383–2398. doi: 10.1159/000475909. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q., Wang Z., Zhou X., et al. miR-342-5p inhibits osteosarcoma cell growth, migration, invasion, and sensitivity to Doxorubicin through targeting Wnt7b. Cell Cycle. 2019;18(23):3325–3336. doi: 10.1080/15384101.2019.1676087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Y., Wang K., Wan W., Cheng Y., Pu X., Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018;5(3):245–255. doi: 10.1016/j.gendis.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q., He H., Yuan Y., Zeng H., Wang Z., Luo W. Novel expression of EGFL7 in osteosarcoma and sensitivity to cisplatin. Front Oncol. 2020;10:74. doi: 10.3389/fonc.2020.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi W.E.I., Tang X.L., Zhou Y., et al. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018;5(3):263–274. doi: 10.1016/j.gendis.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie W., Zheng W., Liu M., et al. BRF1 ameliorates LPS-induced inflammation through autophagy crosstalking with MAPK/ERK signaling. Genes Dis. 2018;5(3):226–234. doi: 10.1016/j.gendis.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Shu B., Xie R., et al. Deletion of Axin 1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of β-catenin and FGF signaling. J Cell Physiol. 2019;234(2):1720–1729. doi: 10.1002/jcp.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAlindon T.E., Bannuru R.R. Osteoarthritis in 2017: latest advances in the management of knee OA. Nat Rev Rheumatol. 2018;14(2):73–74. doi: 10.1038/nrrheum.2017.219. [DOI] [PubMed] [Google Scholar]
- 30.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., van der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13(5):302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Wang Y., Tang Y., et al. Quercetin alleviates osteoarthritis progression in rats by suppressing inflammation and apoptosis via inhibition of IRAK1/NLRP3 signaling. J Inflamm Res. 2021;14:3393–3403. doi: 10.2147/JIR.S311924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Jiang Z., Pang Z., et al. Engeletin protects against TNF-α-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J Inflamm Res. 2021;14:745–760. doi: 10.2147/JIR.S297166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shevde L.A., Samant R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–141. doi: 10.1016/j.matbio.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Tanani M.K. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276–4284. doi: 10.2741/3004. [DOI] [PubMed] [Google Scholar]
- 35.Rangaswami H., Bulbule A., Kundu G.C. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16(2):79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y., Yao M., Liu Q., Zhou C. OPN gene polymorphisms influence the risk of knee OA and OPN levels in synovial fluid in a Chinese population. Arthritis Res Ther. 2013;15(1):R3. doi: 10.1186/ar4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenthal A.K., Gohr C.M., Uzuki M., Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol. 2007;26(2):96–105. doi: 10.1016/j.matbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui Y., Iwasaki N., Kon S., et al. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009;60(8):2362–2371. doi: 10.1002/art.24705. [DOI] [PubMed] [Google Scholar]
- 39.Attur M.G., Dave M.N., Stuchin S., et al. Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum. 2001;44(3):578–584. doi: 10.1002/1529-0131(200103)44:3<578::AID-ANR106>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Jain Singhai N., Ramteke S. CNTs mediated CD44 targeting; a paradigm shift in drug delivery for breast cancer. Genes Dis. 2019;7(2):205–216. doi: 10.1016/j.gendis.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang G.H., Fan J., Xu Y., et al. Osteopontin combined with CD44, a novel prognostic biomarker for patients with hepatocellular carcinoma undergoing curative resection. Oncol. 2008;13(11):1155–1165. doi: 10.1634/theoncologist.2008-0081. [DOI] [PubMed] [Google Scholar]
- 42.Cai Y., López-Ruiz E., Wengel J., Creemers L.B., Howard K.A. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J Contr Release. 2017;253:153–159. doi: 10.1016/j.jconrel.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Li J., Gorski D.J., Anemaet W., et al. Hyaluronan injection in murine osteoarthritis prevents TGFbeta 1-induced synovial neovascularization and fibrosis and maintains articular cartilage integrity by a CD44-dependent mechanism. Arthritis Res Ther. 2012;14(3):R151. doi: 10.1186/ar3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Julovi S.M., Ito H., Nishitani K., Jackson C.J., Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011;29(2):258–264. doi: 10.1002/jor.21216. [DOI] [PubMed] [Google Scholar]
- 45.Dunn S., Kolomytkin O.V., Waddell D.D., Marino A.A. Hyaluronan-binding receptors: possible involvement in osteoarthritis. Mod Rheumatol. 2009;19(2):151–155. doi: 10.1007/s10165-008-0136-y. [DOI] [PubMed] [Google Scholar]
- 46.Hermans J., Bierma-Zeinstra S.M.A., Bos P.K., Niesten D.D., Verhaar J.A.N., Reijman M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: a randomised controlled trial. BMC Muscoskel Disord. 2019;20(1):196. doi: 10.1186/s12891-019-2546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maheu E., Bannuru R.R., Herrero-Beaumont G., Allali F., Bard H., Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48(4):563–572. doi: 10.1016/j.semarthrit.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Jimbo S., Terashima Y., Teramoto A., et al. Antinociceptive effects of hyaluronic acid on monoiodoacetate-induced ankle osteoarthritis in rats. J Pain Res. 2019;12:191–200. doi: 10.2147/JPR.S186413. [DOI] [PMC free article] [PubMed] [Google Scholar]