Abstract
All cells release extracellular vesicles (EVs) as part of their normal physiology. As one of the subtypes, exosomes (EXOs) have an average size range of approximately 40 nm–160 nm in diameter. Benefiting from their inherent immunogenicity and biocompatibility, the utility of autologous EXOs has the potential for both disease diagnosis/treatment. EXOs are generally employed as “bioscaffolds” and the whole diagnostic and therapeutic effects are mainly ascribed to exogenous cargos on the EXOs, such as proteins, nucleic acids, and chemotherapeutic agents and fluorophores delivered into specific cells or tissues. Surface engineering of EXOs for cargo loadings is one of the prerequisites for EXO-mediated diagnosis/treatment. After revisiting EXO-mediated diagnosis/treatment, the most popular strategies to directly undertake loadings of exogenous cargos on EXOs include genetic and chemical engineering. Generally, genetically-engineered EXOs can be merely produced by living organisms and intrinsically face some drawbacks. However, chemical methodologies for engineered EXOs diversify cargos and extend the functions of EXOs in the diagnosis/treatment. In this review, we would like to elucidate different chemical advances on the molecular level of EXOs along with the critical design required for diagnosis/treatment. Besides, the prospects of chemical engineering on the EXOs were critically addressed. Nevertheless, the superiority of EXO-mediated diagnosis/treatment via chemical engineering remains a challenge in clinical translation and trials. Furthermore, more chemical crosslinking on the EXOs is expected to be explored. Despite substantial claims in the literature, there is currently no review to exclusively summarize the chemical engineering to EXOs for diagnosis/treatment. We envision chemical engineering of EXOs will encourage more scientists to explore more novel technologies for a wider range of biomedical applications and accelerate the successful translation of EXO-based drug “bioscaffolds” from bench to bedside.
Keywords: Chemical engineering, Diagnostics, Exosomes, Extracellular vesicles, Theranostics, Therapeutics
Introduction
Exosomes (EXOs), with an average diameter of ∼100 nm, are a subset of extracellular vesicles (EVs) shed from eukaryotic cells. Since the first discovery of EXOs in 1987,1 they always grasp wide attention in clinics. Their biogenesis involves the origin of endosomes and interactions with other intracellular vesicles and organelles; this process mainly includes four steps: budding, invagination, the formation of intracellular multivesicular bodies (MVBs), and secretion.2,3 They play an essential role in the “crosstalk” of cell-to-cell/cell-to-tissue communications. EXOs contain different mother cell-type-specific signatures such as membrane proteins, cytosolic and nuclear proteins, phospholipids, and nucleic acids.4 Since the discovery that EXOs as “crosstalk” tools to transfer their messaging cargos to recipient cells or specific tissues,5 they have garnered wide interest across areas of theranostics. Although natїve EXOs have been reported in antitumor immunotherapy6, 7, 8 and organ regenerations,9,10 most of them have been extensively explored as “bioscaffolds” for the delivery of medicines to specific cells or tissues, benefiting from excellent biocompatibility, prominent solubility, metabolic stability, and long circulating half-life. As one of the natural routes for drug transport, innate protein-decorated EXOs contain specific barcodes to possess a unique “homing” effect by receptor-mediated pathway, i.e., the intrinsic ability to target mother cells, and might be expected to avoid the immunological reactions in the human body, such as anaphylaxis, cytokine release syndrome, neutralization of biological activity, etc.11,12 Meanwhile, they can escape from immunophagocytosis to cross stringent biological barriers, such as vascular endothelium and blood–brain barrier, in distinctly different manners.12, 13, 14 All of these characteristics make EXOs have several distinct advantages over conventional synthetic carriers in theranostics. However, the straightforward utility of natїve EXO-mediated theranostics remains restricted by their inability to track themselves in vivo or low loading capacities. By a variety of orchestrated tailoring on them, EXOs enriched for foreign cargos endow them with better orientation, visual tracking, and excellent performance in biomedical applications, and have already shed light on the next-generation theranostics for various diseases.
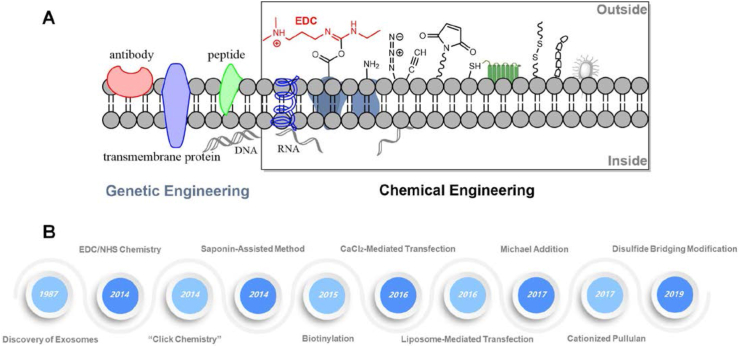
With enrichments of exogenous cargos, tailored EXOs as biological entities have captured the imagination of scientists for their clinical performance. If not all, the most commonly used methodologies by membrane rearrangements to enrich exogenous cargos on the EXOs include sonication,15, 16, 17 electroporation,18, 19, 20 freeze-thaw,21, 22, 23 extrusion,24, 25, 26 and passive incubation.15,27, 28, 29 However, the stability of cargos and integrity of membranes by taking these physical approaches across EXOs are still unsatisfactory, extremely restricting their clinical applications in theranostics. To some extent, the surface tailoring of EXOs is essential to determine their possible interactions with conjugates or recipient cells while preserving their natural integrity. The genetic manipulation of mother cells ensures that EXOs shed by such cells are able to express desired cargos of interest and improve their interactions with recipient cells. Besides, genetically engineered-mother cells secrete fluorescent protein-labeled EXOs, which can be directly applied for noninvasive in vivo imaging.30 What's more, EXOs obtained by genetic engineering can efficiently deliver proteins or siRNA/miRNA as agents for disease therapeutics.18,31, 32, 33 Nevertheless, accumulating evidence indicates that the reproducibility of genetically engineered-EXOs remains controversial, such as inherent biological variability and heterogeneity. Even worse, genetic engineering can only enclose transcriptional proteins or RNAs as theranostic agents in the EXOs and is impotent for other special payloads, e.g., synthetic imaging and therapeutic agents. Nowadays, endowing a diversity of payloads on the EXOs mainly ventures into chemical engineering, which has evolved into a highly sophisticated and versatile methodology. From the discovery of EXOs until 2014, there was a huge blank in chemical engineering on the EXOs. This promising strategy is capable of not only directly tailoring natїve EXOs but also indirectly modifying their mother cells before EXO generation. Firstly, the presence of transmembrane proteins on the EXOs provides plentiful functional moieties, such as carboxylic (-COOH), amine (-NH2), and sulfhydryl (SH) groups, accessible to crosslink reactions by chemical engineering for sorts of payloads34; and secondly, an embedding of other foreign groups such as alkyne, azide, amino, maleimide (Mal) and many others into cytomembrane of mother cells by metabolic labeling increases the scalability of cargos on their secreted EXOs, as evidently shown in Figure 1. By employing chemical engineering, EXO-mediated payload transfer could be put into widespread applications in diagnostic readouts (e.g., fluorescent imaging, magnetic resonance imaging, and computed tomography) and the transport of medicines for treatment.35, 36, 37
Figure 1.
Overview and history of chemical engineering on the EXOs. (A) Classification of chemical engineering on the EXOs. Apart from genetic engineering, theranostic agents for diagnosis/treatment can be selectively conjugated on the surface of EXOs by many kinds of chemical molecules or functional groups such as saponin, carboxyl, amino, azide, alkyne, maleimide, thiol, disulfide, biotin, and many others. The chemical engineering of surface modification on the EXOs includes the uses of original groups and foreign moieties. Usually, the foreign moieties are intentionally embedded into membranes by metabolic engineering. (B) The history and evolution of chemical engineering on the EXOs. Since the discovery of EXOs in 1987, there was an enormous gap in chemical engineering on the EXOs until 2014.
Over the past years, growing evidence suggests that emerging chemical engineered-EXOs herald a new era of theranostics in diseases. There has been explosive growth in publications describing the wealth of their applications. By chemical engineering, EXOs can piggyback on cargos into regions of interest for early detection, in vivo imaging, and targeted drug delivery. A recent review highlights the emerging areas of chemical engineering to EXOs as delivery vehicles for diagnosis/treatment. These chemical strategies of EXOs have been classified into five major categories: (i) chemical transfection, (ii) 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxy succinimide (EDC/NHS) coupling, (iii) azide–alkyne cycloaddition, (iv) thiol-maleimide addition, and (v) other new-style chemical methodologies; their merits have been also compared with each other, as briefly summarized in Table 1. By laying out different chemical techniques on the EXOs, we seek to provide an overview for gradually knowing more about brief history and recent advances, with perspectives on guidelines for choosing suitable techniques for EXO-based theranostics. However, some critical factors of these chemical approaches on the EXOs remain matters of debate, such as specificity, integrity, morphology and the density of intrinsic cargos, and many others after chemical modifications. Importantly, we have outlined the key challenges and future perspectives of these chemical engineered-EXOs. We also hope that such knowledge will not only make new research insight into the progress of chemical engineering on the EXOs but also spur more chemical protocols applied in EXO-based drug “bioscaffolds” and their translations for various diseases from bench to clinical reality, including rheumatoid arthritis,38, 39, 40 cardiovascular dysfunction,41, 42, 43 neurodegenerative diseases,44, 45, 46 inflammatory,28,47, 48, 49 and even cancer.50, 51, 52, 53
Table 1.
Emerging chemical engineering of EXOs in diagnostics and therapeutics as mentioned in this review.
| Source of EXOs | Chemical strategies | Cargos on EXOs | Applications | Reference | Merits |
|---|---|---|---|---|---|
| Serum | CaCl2-mediated transfection | siRNA | Lung inflammation | 46 |
|
| OSCC cells | CaCl2-mediated transfection | miRNA | Therapy of oral cancer | 57 | |
| MSCs | Lipo-mediated transfection | Photosensitizer | Tracking drug delivery | 59 | |
| Fibroblasts | Lipo-mediated transfection | Docetaxel | Metastatic peritoneal carcinoma | 61 | |
| Breast cancer cells | Saponin-assisted method | Porphyrins | Photodynamic therapy | 64 |
|
| Macrophage | Saponin-assisted method | Catalase | Parkinson's disease | 24 | |
| HepG2 cells | EDC/NHS | Polyarginine | Anticancer therapy | 70 |
|
| MSCs | EDC/NHS | Aptamers | Central nervous system | 71 | |
| Colostrum | EDC/NHS | Folic acids | Therapy of lung cancer | 74 | |
| HEK-293T | NHS | Folic acids | Therapy of breast cancer | 75 | |
| HEK-293T | NHS | Cy5.5 dye | Bioimaging and immunotherapy | 77 | |
| Macrophage | NHS | Cy5.5 dye | Bioimaging and wound healing | 79 | |
| Macrophage | EDC/NHS and “click chemistry” | Peptides | Therapy of glioma | 80 | The same to EDC/NHS chemistry |
| Dendritic cell | NHS and “click chemistry” | Glycopeptide and Cy5.5 | Antitumor vaccine | 81 | |
| MSCs | NHS and “click chemistry” | Cy5.5 dye | Imaging and cerebral ischemia therapy | 82 | |
| Breast cancer cells | “click chemistry” | Fluorescent tags | Bioimaging and tracking | 94 |
|
| 549 cells | “click chemistry" | Cy5 dye | Bioimaging and tracking | 87 | |
| Breast cancer cells | “click chemistry” | Fluorescent tags | Tracking and organotropic uptakes | 97 | |
| Macrophage | “click chemistry” | Antibodies | Cancer immunotherapy | 96 | |
| Macrophage | “click chemistry” | Mannose | Intracellular infection | 100 | |
| 4T1 cells | “click chemistry” | Fluorescent tags | Bioimaging and tracking | 69 | |
| MSCs | “click chemistry” | Peptide and miRNA | Cerebral ischemia | 102 | |
| Prostate cancer cells | Thiol-Michael addition | Fluorescent tags | Bioimaging and tracking | 104 |
|
| Macrophage | Thiol-Michael addition | Quantum dots | Bioimaging and tracking | 106 | |
| HEK293T | Thiol-Michael addition | Aptamer | Targeted therapeutics | 107 | |
| BM-MSCs | Thiol-Michael addition | Oxaliplatin | Cancer therapy | 110 | |
| Dendritic cells | Thiol-Michael addition | aCTLA antibody | Cancer immunotherapy | 111 | |
| Prostate cancer cells | Disulfide bridge | – | Detection of intact EXOs | 114 |
|
| MSCs | Electrostatic interaction | Pullulan | Therapy of liver injury | 115 | |
| Milk | PEGylation | – | Preventing the degradation of EXOs | 116 |
|
| Neuro2A cells | PEGylation | Nanobodies | Targeting tumor cells | 117 | |
| Colostrum | Polyethyleneimine | siRNA | Therapy of lung cancer | 74 | |
| A549 cells | Polyelectrolytes | DOX | Carrier for DOX loading | 118 |
Loading of theranostic agents on exosomes by chemical transfection
miRNA in EXOs derived from tumor cells extensively acts as a biomarker, commonly providing clinical diagnosis for tumors. Reversely, normal RNA-functionalized EXOs are able to afford reliable tools for gene therapy of diseases by restoring their biological functions to trigger genotypic and phenotypic responses.54 For the functionalization of EXOs by nucleic acids such as siRNAs and miRNAs, chemical transfection has emerged as a common choice. By traditional chemical transfection, many RNA-based drugs have entered clinical trials. The conventional approach of nucleic acid-functionalized EXOs by chemical engineering is calcium chloride (CaCl2)-mediated transfection. In this fashion, desired RNAs as interference agents are firstly mixed with EXOs in phosphate buffer saline (PBS) containing a low concentration of CaCl2. Then, the mixture is managed by freeze–thaw cycles, thereby ensuring the coordinated complexes of RNA and CaCl2 enter into EXOs by membrane rearrangements. After that, RNA-loaded EXOs will be harvested from PBS solution by ultracentrifugation. Ultimately, the EXOs containing desired nucleic acids for diagnosis/treatment of human diseases are delivered to regions of interest. Of note, the EXOs shed from tumor cells are not suggested to be adopted, owing to their roles in tumorigenesis and angiogenesis.55 So far, a large cohort of studies have developed protocols to load RNAs onto EXOs by CaCl2-mediated transfection for gene therapy of human disease.47,56 In 2016, Zhang et al reported a CaCl2-mediated transfection method to directly integrate miRNA mimics onto the isolated EXOs for the first time, instead of transfecting their mother cells, by which a robustly more amount of miRNA could be delivered to recipient cells compared with electroporation.57 As recently studied, small RNA-loaded EXOs by CaCl2-mediated transfection have been employed to recognize lung macrophages, by which it successfully modulates lipopolysaccharide (LPS)-induced lung inflammation in vivo.47 What's more, EXOs derived from serum were able to successfully circumvent lung immune response. Besides, the feasibility of miRNA-loaded EXOs by CaCl2-mediated transfection has gotten concerns to improve cisplatin resistance during cancer therapy. Sayyed et al have currently reported that miR-155-loaded EXOs by this simple way of transfection reverse cisplatin-resistance for the management of refractory oral cancer to prolong the survival of chemoresistant tumor-bearing mice.58 Upon administering miR-155-loaded EXOs on the mouse xenograft model, miR-155 was able to directly target forkhead-box protein O3a (FOXO3a), as one of tumorigenesis suggestive when its downregulation.59 Furthermore, miR-155-laden EXOs simultaneously upregulated the expression of FOXO3a and induced mesenchymal-to-epithelial transition in oral cancer, by which it greatly improved the chemosensitization toward cisplatin during the treatment of oral cancer. Unfortunately, by CaCl2-mediated protocol, the efficiency of RNA loading on the EXOs is still not satisfactory. Even worse, the repeated freeze–thaw cycle can possibly damage the surface proteins of natїve EXOs.
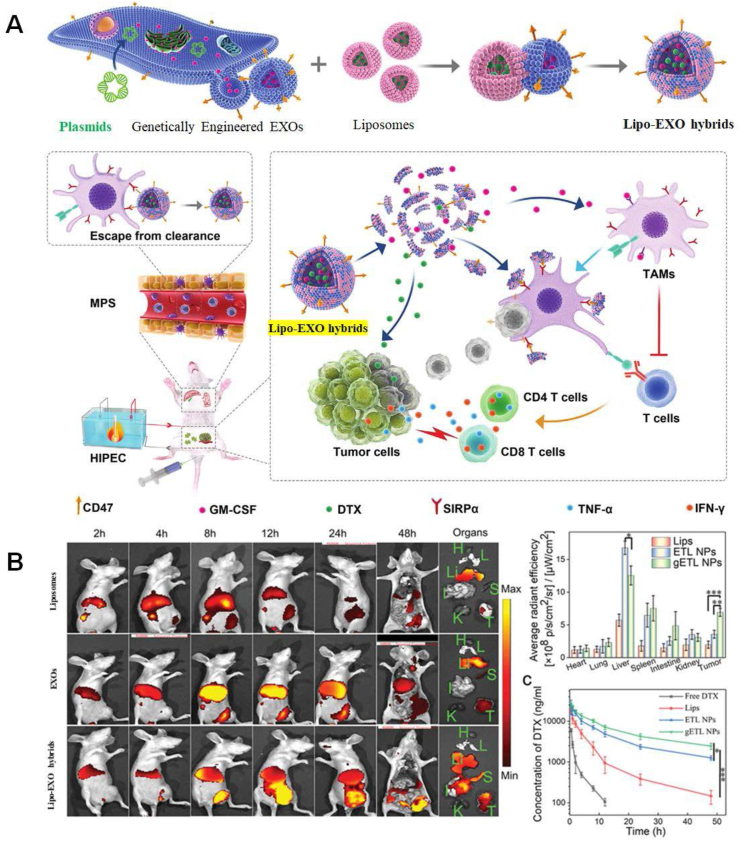
Along with the use of CaCl2 as a medium in EXO engineering, liposome-mediated functionalization of EXOs have expanded their biomedical applications by fusing the membrane of EXOs with liposomes (Fig. 2A).22 By employing this engineering strategy, the surface of EXOs can be easily modified via liposomes those embedded with exogenous cargos of interest, e.g., peptides or antibodies as well as poly (ethylene glycol) (i.e., PEG), to form Lipo-EXO hybrids, endowing natїve EXOs with exogenous peptides or antibodies.60, 61, 62 Lipo-mediated modification of EXOs substantially relies on PEG-triggered fusion between liposomes and EXOs to design Lipo-EXO hybrids with tunable composition and properties. The fusion efficiency and critical parameters are generally monitored by fluorescent resonance energy transfer, such as liposome-to-EXOs ratio and bilayer composition.22 What first intrigued us is that Lipo-EXO hybrids have been directed toward bioimaging agents. A proof-of-concept paradigm has described the point-for-point Lipo-EXO hybrids-based bioimaging for in vivo tracking drug delivery. In this paradigm, liposomes were initially labeled by meta (tetrahydroxyphenyl) chlorin (mTHPC), a clinically approved fluorescent anti-tumor photosensitizer; and then, mTHPC-labeled liposomes could fuse with murine mesenchymal stem cells (MSC)-derived EXOs that is under clinical assessment for future use in regenerative medicine. As a result, it is of no surprise that, mTHPC-labeled Lipo-EXO hybrids performed much better than liposomes in the drug delivery to colon cancer cells, and precisely tracked themselves by a fluorescent signal from mTHPC.60 Except for bioimaging in vivo, the versatile Lipo-EXO hybrids have drawn significant attention in cancer therapy. More recently, Lv et al have constructed thermosensitive Lipo-EXOs hybrids for the delivery of chemoimmunotherapeutic agents to combat metastatic peritoneal carcinoma (mPC) when receiving hyperthermic intraperitoneal chemotherapy, a deadly disease without effective treatments.62 In this study, they started with genetic engineering of fibroblasts to produce granulocyte-macrophage colony-stimulating factor (GM-CSF)/CD47-overexpressed EXOs, which were subsequently fused with thermosensitive docetaxel (DTX)-loaded liposomes to form Lipo-EXOs hybrids. By interaction of CD47 and signal regulatory protein alpha receptor (SIRPa) on macrophages, acting as a “don't eat me” signal, Lipo-EXO hybrids escaped from phagocytosis by macrophages to reinforce drug delivery.63 After administration, Lipo-EXO hybrids efficiently penetrated mPC tumors and released GM-CSF and DTX under hyperthermic conditions, by which they were able to induce repolarization of macrophages towards M1-like phenotypes and apoptosis of tumor cells, respectively, as shown in Figure 2A for clarity. Moreover, they found distribution pattern of Lipo-EXO hybrids on mPC-bearing mice was 2–3 times greater than those of liposomes or EXOs in the tumors at 48 h post-injection, by monitoring the fluorescent signal of encapsulated DiR dyes, as shown in Figure 2B. The clearance half-time (t1/2b) of DTX delivered by liposomes and EXOs were 3.5 h and 6.2 h, respectively, considerably shorter than that (8.1 h) of Lipo-EXO hybrids. Likewise, it has been reported that chemically transfected-Lipo-EXO hybrids could successfully deliver CRISPR/Cas9 expression vectors in MSCs to potentially cure various genetic diseases such as cancers and inherited disorders by repairing, deleting, or silencing certain genetic mutations.61 By taking this approach to deliver the CRISPR/Cas9 system, it effectively avoided possible cytotoxicity, immunogenic response, long-term expression, and off-target effects. Although the composition of Lipo-EXO hybrids can be tuned by varying liposome-to-EXO ratio, the morphology of natїve EXOs will be transformed after liposome-mediated transfection. Along with that, the size homogeneity of the EXO population will be disrupted. It seems to be worse that a rearrangement and dilution of intrinsic cargos on the surface of natїve EXOs will occur, which results in a distortion of natїve EXO's characteristics and a loss of their recessive functions.
Figure 2.
Lipo-mediated EXO functionalization for therapeutics of diseases. (A) Schematic diagram of Lipo-mediated EXO functionalization for metastatic peritoneal carcinoma (mPC) treatment. Genetically engineered fibroblasts by transduction with lentiviral vectors produced GM-CSF/CD47-overexpressed EXOs, which then were fused with thermosensitive liposomes encapsulating DTX to form Lipo-EXO hybrids with the loading of GM-CSF and DTX. CD47-overexpressed Lipo-EXO hybrids with the loading of therapeutic agents would escape from phagocytosis of phagocytes, by interaction with SIRPa in phagocytes. Under hypothermia condition of hyperthermic intraperitoneal chemotherapy, GM-CSF and DTX releasing from Lipo-EXO hybrids were able to induce repolarization of macrophages towards M1 and apoptosis of tumor cells, respectively, in the treatment of metastatic mPC. (B) Real-time images of liposomes engineered EXOs and Lipo-EXO hybrids with the loading of therapeutic agents and encapsulation of DiR dye in tumor-bearing mice. After 48 h, the DiR in major organs and DTX in plasma were quantitively analyzed. Information is acquired from Wiley Open Access.
A little more early than chemical transfection, saponin-assisted drug loading on the EXOs has caught attention since it was first reported. Making use of this way, saponins specifically react with cholesterol to form small pores in the bilayer membrane of EXOs, making it convenient for the uptake of exogenous cargos. Dating back to 2015, pioneering work has initiated saponin-assisted methods to incorporate porphyrin into EXOs shed from breast cancer cells. The porphyrin-incorporated EXOs exhibited excellent photodynamic performance in a breast cancer cell model. Their findings rendered a firm basis for the development of EXOs as drug “bioscaffolds”.64 Almost at the same time, Haney et al have fabricated catalase-loaded EXOs by saponin's assistance to provide significant neuroprotective effects in the models of Parkinson's disease.24 Primarily due to capsulation of catalase by EXOs derived from macrophages, the therapeutic drugs can escape from phagocytosis of macrophages, and their delivery to neuronal cells is greatly increased. As such, chemical transfection or saponin-assisted drug loading on the EXOs can destroy the integrity of EXOs and cause a rearrangement of their membrane, thus affecting the drug delivery function of EXOs. Besides, the scalability and stability of cargos loaded by these methodologies are still open to question.
Loading of theranostic agents on exosomes by EDC/NHS mediated amide reaction
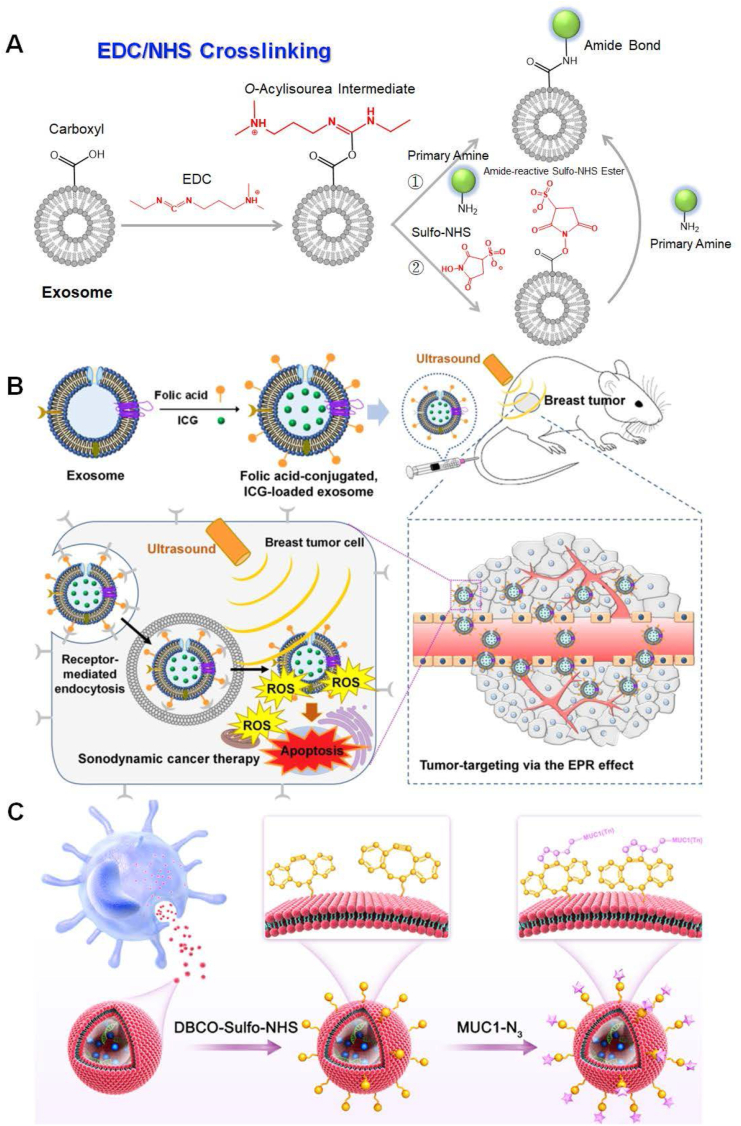
Owing to the presence of proteins and phospholipids, carboxyl (-COOH)/amine (-NH2)-terminated membrane protein and phospholipid moieties of EXOs endows their surface tunability. In this regard, crosslinking primary amine-labeled cargos to terminal carboxylic acids by strong covalent bonds or vice versa is a feasible approach for bioconjugations of oligonucleotides, peptides, proteins, and biomolecular probes on the EXOs, which raises the possibility of accurate diagnosis and effective treatment for various diseases. However, to the best of our knowledge, extremely few chemical groups are known to provide specific conjugation to carboxylic acids or amines. The advent of carbodiimide chemistry has led to an influx of new ideas in EXO-based theranostics, by which the terminal carboxylic acids of proteins or phospholipids are activated for direct conjugation to primary amines via amide bonds (e.g., a crosslinking of two proteins).65 Among carbodiimide species, water-soluble 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and water-insoluble dicyclohexylcarbodiimide (DCC) are commonly used reagents for aqueous and non-aqueous cross-linking of carboxylic acids and primary amines, respectively.66 Considering the physiological restriction, direct crosslink of primary amines to residue-specific carboxylic acids on the EXOs usually adopts EDC-mediated amide reaction rather than DCC. EDC-mediated amide reactions of protein carboxyl groups have the highest efficiency in acidic conditions (pH = 4.5) to form O-acylisourea active intermediates, which subsequently tend to react with necleophilic ligands such as primary amine groups.66 To facilitate this type of reaction at physiological pH (pH = 7.2), the assistance of N-hydroxysuccinimide (NHS) is needed to improve the chemical yields of amide bonds,67 as shown in Figure 3A. Devoid of EDC adducts, direct NHS-mediated conjugations of cargos on the terminal carboxylic acids of membrane proteins or phospholipids for in vivo imaging, antitumor, vaccines, and so on, have also been widely verified to be feasible.68 Despite originating only less than a decade ago, a marriage between EDC/NHS-mediated amine/carboxyl coupling reaction and tunability of EXO's surface, as a milestone in EXO-based drug delivery, has received extensive attention since its first proposal.69
Figure 3.
Chemical engineering on the EXOs by EDC/NHS crosslinking. (A) Schematic diagram of EDC/NHS crosslinking on the EXOs. (i) EDC reacts with a carboxyl group on the EXOs to form an O-acylisourea intermediate that is easily substituted by nucleophilic attack from the primary amino group for the conjugation of theranostic agents. The primary amine forms an amide bond with the carboxyl group on the EXOs, and the EDC-by product is released as a soluble urea derivative. EDC crosslinking is most efficient in acidic (pH = 4.5) conditions. Physiological pH conditions are compatible with this crosslinking, albeit with lower efficiency. (ii) NHS or its water-soluble analog (Sulfo-NHS) is commonly adopted in EDC coupling protocols to improve efficiency. EDC makes NHS couple to carboxyl on the EXOs, forming an NHS ester that is more stable than the O-acylisourea intermediate. The NHS ester allows for efficient conjugation to primary amine at physiological pH. (B) Schematic illustration of sonodynamic therapeutics by FA-conjugated and ICG-loaded EXOs against breast cancer. ICG was successfully loaded on the EXOs by incubating HEK-293T-derived EXOs with ICG solution. Under the irradiation of ultrasound, ICG generated ROS to kill tumor cells. FA as a targeting ligand was covalently conjugated to proteins on the surface of EXOs by NHS-mediated amide reaction. Referenced from American Chemical Society (Copyright 2021). (C) Dendritic cell-derived EXOs as “bioscaffolds” for the antitumor vaccine. Terminal amine groups on the EXOs were conjugated with DBCO-Sulfo-NHS via amide reaction, subsequently allowing for crosslinking of MUC1 glycopeptides. The MUC1 glycopeptides were able to induce MUC1-specific IgG antibodies, which had strong affinities with MUC1-positive tumor cells. In this way, the EXO-based vaccines increased the killing activity of T cells. Referenced with permission from Elsevier.
Intense efforts have been dedicated to cargo loading on the EXOs by EDC/NHS chemistry. Recently, Xu et al have constructed a novel peptide-equipped EXOs platform, by which polyarginine peptide (R9) was directly conjugated to carboxyls on the surface of the HepG2 cell-derived EXOs via an EDC/NHS-mediated amide reaction.70 Before the conjugation of peptides, the terminal carboxyl on the HepG2 cell-derived EXOs was initially activated by EDC/NHS chemistry. As one the of cell-penetrating peptides, the presence of R9 peptides could not only enhance the penetrating capacity of EXOs but also correlate EXOs with loading oligonucleotides by static interaction for genetic therapy. Surprisingly, together with these two functionalities, it was noteworthy that R9 peptides on the EXOs by EDC/NHS crosslinking also rendered EXOs with targeting ability to cancer cells. Nowadays, affinity ligand-guided drug delivery presages a new era of targeted therapeutics in diseases. If taking the same strategy, conjugations of affinity ligands, e.g., aptamers and antibodies, to the surface of EXOs may fulfill a specific accumulation of EXOs on the target cells. Inversely, one also can proceed with the conjugation of amine-reactive payloads to terminal amines of membrane proteins or phospholipids on the EXOs by EDC/NHS chemistry. An early report of EDC/NHS chemistry described that carboxylic acid-functionalized aptamers were directly conjugated on the primary amines of the MSC-derived EXOs.48 The goal of particular aptamer decorated on EXOs not only endowed targeting ability of delivery but also suppressed inflammatory response in central nervous system (CNS) disease. Likewise, MSC-derived EXOs cross-linked with aptamers by EDC/NHS chemistry, which has the potential to actively target mucin-1 glycopeptide highly expressed on colorectal tumor cells, were able to safely and specifically deliver Doxorubicin (DOX) to the region of interest for cancer chemotherapy.71 By far, folate receptor is typically well-known to overexpress on cancer cells.72,73 Folic acids (FA) have been originally attached to EXOs by EDC/NHS crosslinking, as an effective “platform” for targeted delivery of therapeutic siRNA to treat lung cancer.74 To this end, FA could guide the endocytosis of whole siRNA-loaded EXOs system by cancer cells to achieve the purpose of genetic therapy. Recently, as one of the emerging treatments, sonodynamic therapy (SDT) that utilizes cytotoxic reactive oxygen species (ROS) generated from indocyanine green (ICG) under ultrasound exposure has received tremendous attention. Chemically FA-modified EXOs through NHS mediation were readily utilized for cancer-targeted delivery of sonosensitizer ICG. An integration of FA on the EXOs greatly improved cellular uptake of ICG, resulting in a significant increase of cytotoxic ROS in breast cancer cells by subjecting them to ultrasound irradiation. By targeted delivery of ICG via FA-modified EXOs, tumor growth within mice was notably suppressed without systemic toxicity,75 as illustrated in Figure 3B. Current studies of EXO-based delivery not only concentrate on the targeted therapy of diseases but also on bioimaging in living subjects.37,76, 77, 78, 79 Compared with the non-covalent incorporation of lipophilic dyes, the conjugated dyes on the EXOs by EDC/NHS-mediated covalent coupling are difficult to release from “bioscaffolds” in the process of in vivo trafficking and provide precise tracking information. For example, Koh et al have employed NHS-mediated amide reaction to fluorescently label signal regulatory protein a (SIRPa) highly expressed EXOs derived from HEK293T cells. The amine-reactive fluorophore Cy5.5-NHS was successfully conjugated on the EXOs to observe the biodistribution of EXOs under the guide of Cy5.5. Simultaneously, by the interaction between SIRPa of EXOs and CD47 of phagocytes, it was able to restore potent immune responses against tumor cells and mediate tumor phagocytosis by macrophages for cancer immunotherapy.77 Therewith, a Cy5.5-NHS probe-labeled EXOs derived from anti-inflammatory M2 macrophage was used for in vivo fluorescently tracking delivery of EXOs. Besides, the EXOs from host cells could reprogram proinflammatory M1 to anti-inflammatory M2 macrophage for cutaneous wound healing.79 Due to the accessibility and flexibility of EDC/NHS chemistry in the membrane proteins on the surface of EXOs, it has paved the way for secondary chemical reactions on the EXOs for loading exogenous cargos.
In the virtue of EDC/NHS chemistry, a previous study originally conjugated 4-pentynoic acid terminated with alkyne (-C C) group to murine macrophage cell-derived EXOs, aiming to further crosslink targeted peptide (i.e. neuropilin-1) on the EXOs by emerging “click chemistry”, by which EXOs were able to specifically carry superparamagnetic Fe3O4 NPs and curcumin to the region of interest for in vivo imaging and therapy of glioma, respectively.80 Almost by the corresponding steps, dendritic cell-derived EXOs were firstly functionalized with Dibenzylcycloocctyne-Sulfo-NHS (DBCO-Sulfo-NHS) by reacting with amine-active EXOs, then allowing for crosslink of azide-modified mucin-1 glycopeptide and Cy5.5 by “click chemistry” to induce mucin-1-specific IgG antibody and track drug delivery, respectively (Fig. 3C). The induced mucin-1-specific IgG antibody by mucin-1 glycopeptide was able to specifically bind to mucin-1-expressing tumor cells, by which it eventually triggered cytotoxic T lymphocyte response and inhibited tumor growth in animal models.81 This conjugation strategy on the EXOs has been also adopted for cerebral ischemia therapy. After functionalization of DBCO-Sulfo-NHS on the MSC-derived EXOs, the cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyk)] together with Cy5.5 fluorophore were successfully crosslinked on the EXOs for actively targeting to the ischemic region through specific binding of c(RGDyk) to integrin avb3 and bioimaging, respectively.82 Taken together, EDC/NHS chemistry can overcome the scalability limitation of chemical transfection. Moreover, amine/carboxyl coupling reaction by EDC/NHS mediation primarily takes place on the surface proteins or phospholipids of EXOs and its simplicity equips EXOs with more tunable surface properties, facilitating the development of “click chemistry” as an effective technique for more cargo loading on the EXOs. Unfortunately, a straightforward modification on the original moieties of EXOs by EDC/NHS chemistry to cause their denaturation, especially their membrane proteins, is also fraught with problems by constraints on functions of proteins that can affect intrinsic properties of EXO, e.g., “homing” propensity. Despite of high stability of cargo loading by EDC/NHS chemistry, the kind of chemical technique on the EXOs does not possess high selectivity or specificity because of the complexity of surface proteins and phospholipids on the EXOs.
Imaging probes and medicines loaded on exosomes by “click chemistry”
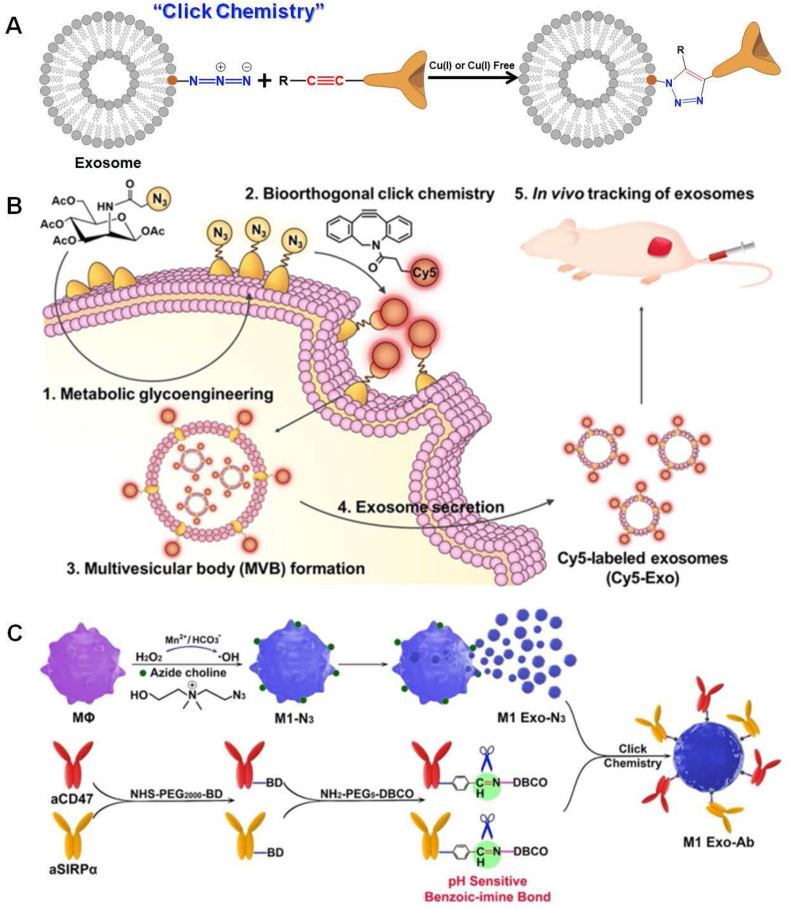
In recent years, orthogonal chemistry has emerged as a versatile method of choice within the frontier science of biology and nanotechnology. Azide–alkyne cycloaddition, as one canonical subtype of “click chemistry”, is an easy-to-adapt and modular methodology for bioconjugation of theranostic agents to the surface of EXOs by Cu(I)-catalyzed reaction, benefiting from short reaction time, high specificity, and compatibility in aqueous buffers.69 Owing to a degree of cytotoxicity and DNA damage of Cu(I) ions, Cu(I)-free azide–alkyne cycloaddition by strain promotion will be particularly attractive in the modifications of EXOs, in which the choice of DBCO derivatives is crucial to achieving an efficient reaction.83,84 Usually, “click chemistry” proceeds under mild conditions and features compatibility with various functional groups, yielding covalent product 1, 5-disubstituted 1, 2, 3-trazole hinge species, as shown in Figure 4A for clarity.85,86 As one of the efficient and “green” tools, advances of “click chemistry” is able to load not only fluorescent,87,88 radioactive,89,90 and MRI agents80,91 for in vivo tracking injected EXOs, but also proteins, nucleic acids, and chemotherapeutic agents for therapeutics. Moreover, this chemical technique is also commonly used for conjugations of the signal transducer, e.g., metalloenzymes92 and Raman repoter,93 to ultrasensitively detect cancer cell-derived EXOs themselves in the human serum. By taking the approach of “click chemistry”, the surface of EXOs initially needs to label with chemically active alkyne (-C C) or azide (-N3) moieties by embedding functional groups to the surface of host cells, e.g., EDC/NHS chemistry,69,80, 81, 82 glycometabolism,87,94,95 lipometabolism,96, 97, 98 protein labeling99,100 and many others. Representative examples of azide-crosslinked EXOs by EDC/NHS chemistry have been described previously. As one of the alternative strategies, integration of alkyne-reactive azide moieties onto host cells by azide-labeled saccharide has been strongly recommended, so as to ultimately conjugate fluorescent probes onto EXOs derived from host cells by “click chemistry”. Lee et al have provided a proof-of-concept of “click chemistry” to integrate fluorescent tag onto the EXOs and firstly implanted azide groups into cytomembranes of breast cancer cells by incubating these cells with tetraacetylated N-azidoacetyl-d-mannosamine (Ac4ManNAz) solution. Afterward, the EXOs shed from these cells contained azide ingredients on the surface, thereby allowing for the conjugation of DBCO-functionalized fluorescent tags by Cu(I)-free “click chemistry”. The fluorescent tag-integrated EXOs were finally applied to infer “homing” propensity, along with their distribution within different organs in breast tumor-bearing mice.94 Another example of fluorescently labeled EXOs by glycometabolism together with “click chemistry” has been directly operated on their host cells. First of all, EXO-secreted host 549 cells were incubated with Ac4ManNAz to anchor azide moieties on their surface by glycometabolism. Taking the use of “click chemistry” paradigm, the azide groups on the cells were easy to specifically label by DBCO-Cy5. Eventually, Cy5-labelled EXOs shed from 549 cells had the potential to serve as delivery “bioscaffolds” to in vivo track the distributions of therapeutic drugs, as shown in Figure 4B.87 Apart from glycometabolism, lipometabolism has been also utilized to integrate azide groups into host cells, conferring their secreted EXOs with azide moieties on the surface. Zhang and coworkers have incubated breast cancer cells with newly-synthesized azide chorine, by which it endowed their cytomembranes with azide moieties by metabolic incorporation of azide chorine into phospholipids. Afterward, azide-integrated EXOs from host cells were specifically conjugated with DBCO-functionalized fluorescent dyes for in vivo tracking of their biodistributions and organotropic uptakes.97 By employing the same way, Nie et al have embedded azide chorine into the membrane of antitumoral M1 macrophages through their intrinsic lipometabolism, by which the EXOs derived from M1 macrophages contained azide functional groups on the surface. Then, both anti-CD47 (aCD47) and anti-SIRPa (aSIRPa) antibodies were simultaneously labeled with DBCO by pH-sensitive benzoic-imine bonds, facilitating their conjugation onto the azide-modified EXOs by “click chemistry” paradigm. Upon accumulation of the aCD47 and aSIRPa-conjugated EXOs in the tumor regions, selective cleavage of benzoic-imine bonds within the tumor microenvironment resulted in a release of aCD47 and aSIRPa, both of which individually blockade the immune checkpoint CD47 on tumor cells and SIRPa on macrophages, as demonstrated in Figure 4C. The two immune “brake” antibodies were able to abolish the “don't eat me” signaling and restore a potent immune response against tumor cells. On the other hand, the M1 EXOs effectively reeducated the protumoral M2 toward M1.96 In this study, administration of aCD47 and aSIRPa-conjugated EXOs will be a great way to kill two birds with one stone for tumor immunotherapy. Meanwhile, a pioneering study has proposed l-azidohomoalanine (AHA), an analogue of methionine containing azide moiety, to label proteins by cellular metabolism on the murine melanoma cells. After incubation of murine melanoma cells and AHA solution, these cells would produce azide-integrated EXOs for clicking variable cargos onto EXOs, including theranostic agents and bioimaging probes.99 Inspired by this work, Yang et al have also adopted almost the same way to integrate mannose onto EXOs shed from macrophages. The mannose-integrated EXOs could be preferentially phagocytized by macrophages to deliver lysostaphin and vancomycin for eradicating intracellular methicillin-resistant Staphylococcus Aureus (MRSA).100 Notably, chemically-engineered EXOs are also used as a delivery platform of antibiotics to combat the infection occurrence.
Figure 4.
Chemical engineering on the EXOs by “click chemistry”. (A) Schematic diagram of “click chemistry” on the EXOs. An azide-functionalized EXOs specifically reacted with alkyne-labeled payloads by azide–alkyne cycloaddition to carry probes and biomolecules for the diagnosis/treatment of diseases. The azide–alkyne cycloaddition reaction selectively gives 1,2,3-triazoles linkage. (B) Conjugation of fluorescent probes by “click chemistry” onto EXOs for bioimaging. The azide groups were embedded on the surface of A549 cells via glycometabolism. The azide groups were then bioorthogonally labeled with DBCO-Cy5 via “click chemistry”. Cy5-labeled EXOs were successfully harvested from parental cells, which were finally used for real-time in vitro and in vivo imaging and tracking. Referenced from American Chemical Society (Copyright 2020). (C) Conjugation of antibodies by “click chemistry” onto EXOs for cancer immunotherapy. The cell membranes secreted from M1 macrophages were modified with azide-functionalized phospholipids by metabolic incorporation. Then, EXOs from M1 macrophages could be conjugated with DBCO-modified anti-CD47 and anti-SIRPa antibodies by “click chemistry”. The releases of anti-CD47 and anti-SIRPa in the tumor microenvironment individually blocked interaction with SIRPa on macrophages and CD47 on tumor cells, by which it abolished the “don't eat me” signaling and improved the phagocytosis of tumor cells by macrophages. Meanwhile, the EXOs from M1 macrophages were able to reeducate protumoral M2 toward antitumoral M1 macrophages for cancer therapy. Referenced with permission from Wiley-VCH.
Actually, chemically engineered EXOs by robust “click chemistry” firstly track back from the alkyne-modified surface. Starting with the alkyne-modified surface, Smyth et al have carried out 4-pentynoic acid (containing –C C group) to chemically modify membrane proteins and phospholipids on the surface of EXOs shed from 4T1 cells for ultimate bioconjugation of azide-fluor 545, which initiates to modify EXOs by “click chemistry”.69 By “click chemistry” for the first time, they successfully labeled EXOs by the fluorescent probe for in vivo bioimaging. The publication of this paper occasioned an explosive growth of “click chemistry” as a versatile technology in the surface modifications of EXOs. Taking use of the same strategy, Xu et al have clicked azide-integrated fluorescence on the pancreatic cancer cell-derived EXOs that were functionalized by DBCO moieties. Unsurprisingly, their findings have confirmed a claim of intrinsic tropism of EXOs for their mother cells, i.e., insight into the “homing” capabilities of EXOs.101 Besides, another study demonstrated that DBCO moieties were fabricated on the membrane proteins of MSC-derived EXOs by NHS-mediated amide coupling, so as to be further conjugated by azide-linked cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyk)], which could actively target the ischemic brain by recognizing integrin avb3 in reactive cerebral vascular endothelial cells. Next, cholesterol-modified miRNA-210 was loaded onto the c(RGDyk)-conjugated EXOs by incubation, preventing miRNA-210 degradation in the body. When c(RGDyk) targets integrin avb3 within the lesion region, EXOs carried miRNA-210 cross the blood–brain barrier and were specifically accumulated in ischemia to promote microvascular angiogenesis, by which it ultimately attenuated stroke symptoms.102 It is conceivable that the inherent advantages of “click chemistry” that have great potentials in EXO-based drug delivery for diagnosis/treatment of diseases are: (i) cargos conjugated by “click chemistry” will not greatly disturb intrinsic properties of themselves and EXOs; (ii) “click chemistry” has excellent selectivity and specificity and extraordinary stoichiometry; (iii) “click chemistry” greatly expands the scalability of cargos loaded on EXOs for diagnosis/treatment. Apart from azide–alkyne cycloaddition, we hope more subtypes that fall within “click chemistry” such as thiol–click reaction, Diels–Alder reaction, nitril oxide cycloaddition, tetrazole cycloaddition, and oxime formation will be utilized in the for bioconjugation of theranostic agents to the surface of EXOs.
Imaging probes and medicines loaded on exosomes by thiol-Michael addition
Except for chemical transfection and saponin-assisted drug loading on the EXOs as previously mentioned, other chemical engineering did not dramatically affect the size and integrity of EXOs. Michael addition, characterized as a reaction of an enolate-type nucleophile to an a, b-unsaturated carbonyl, has a long history of implementation in organic synthesis to afford highly selective products under environment-friendly conditions.103 This type of chemical reaction has evolved into highly efficient & versatile methods and seemingly arisen in approximate parallel to “click chemistry” in materials science. Exactly, what we are going to next look at is the applications of thiol-Micheal addition in the modifications of EXOs, as typically illustrated in Figure 5A, with particular emphasis on the recent developments in theranostics over the past years. The thiol-Micheal addition has been tailored to progress under mild and solventless conditions to yield a highly efficient reaction. Generally, sulfhydryl is widely distributed in most membrane proteins on the EXOs and can be employed as the binding site for drug loading via interlinkage between sulfhydryl and maleimide. To date, considerable attempts have been devoted to a practical application of thiol-Michael addition on the EXOs. For example, to characterize endocytosis of EXOs by live cells, Roberts-Dalton et al have firstly adopted thiol/maleimide reaction to conjugate Mal-Alexa488 and Mal-Alexa633 onto the prostate cancer cell-derived EXOs. This chemical technique arguably provided flexibility with regard to fluorescent labeling on the EXOs. By the fluorescent signals, they were able to directly observe their interactions with cells and endocytic traffic.104 In the same way, Luther et al have carried out Mal-Alexa488 to label MSC-derived EXOs, by which they could real-time monitor the uptake of cardioprotective miRNA-21a-5p by recipient cardiac cells to realize cardiac repair.105 Apart from fluorophores, quantum dots (QDs) anchored onto the EXOs by thiol-Michael addition also enables a moderate and biocompatible labeling strategy. In this study, a DNA hinge was first functionalized with maleimide or biotin on each terminal. Then, the DNA ligament could be conjugated onto the M1 macrophage-derived EXOs by the chemical reaction between build-in maleimides of DNA and thiols on the EXOs. Afterward, the streptavidin-labeled QDs facilely realized the terminal biotin of DNA hinge to integrate onto EXOs for tumor imaging.106 Apart from in vivo imaging, another aspect of thiol-maleimide chemistry will focus on the role it plays in the therapeutics of diseases.
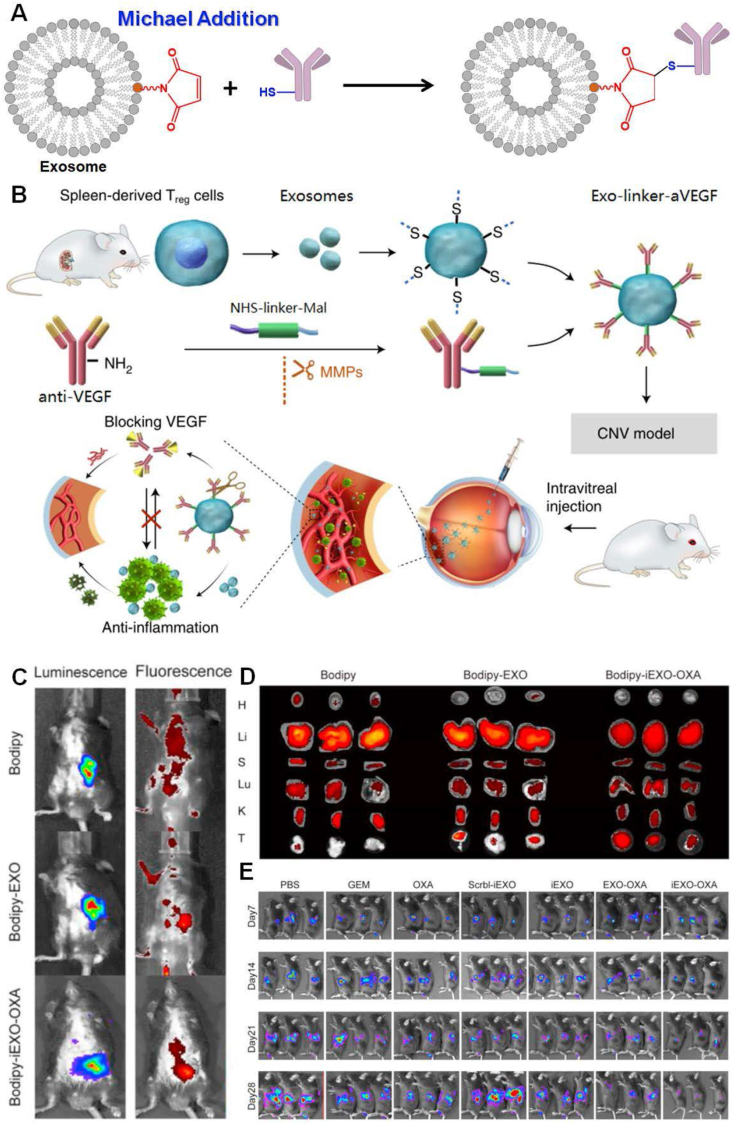
Figure 5.
Chemical engineering on the EXOs by Michael addition. (A) Schematic diagram of Michael addition on the EXOs. A maleimide-functionalized EXOs specifically reacted with thiol-labeled payloads by the thiol-maleimide reaction to carry probes and biomolecules for diagnosis/treatment of diseases. The thiol-maleimide reaction selectively produces thiosuccinimide linkage. (B) Conjugation of anti-VEGFs by Michael addition onto EXOs for suppression of choroidal or retinal neovascularization. The thiol groups were embedded on the surface of EXOs derived from regulatory T cells. Meanwhile, the anti-VEGFs were labeled with NHS-linker-maleimide via NHS-mediated coupling reaction. Finally, anti-VEGFs were successfully integrated onto regulatory T cell-derived EXOs via Michael reaction, which were used for EXOs. Referenced from Springer Nature (Copyright 2021). Oxaliplatin (OXA) and galectin-9-loaded EXOs from bone marrow mesenchymal stem cells (BM-MSC) for treatments of pancreatic cancer: maleimide-functionalized oxaliplatin molecules were successfully conjugated onto EXOs. (C)in vivo targeting evaluation of OXA-loaded EXOs by a fluorescent signal from Bodipy. (D) Accumulation assessments of OXA-loaded EXOs in major organs and tumor tissues by a fluorescent signal from Bodipy. (E) Real-time in vivo bioluminescence image at days 7, 14, 21, and 28 after injections with phosphate buffer saline (PBS), gemcitabine (GEM), oxaliplatin (OXA), scrambled siRNA-loaded EXOs (Scrbl-iEXO), gal-9 siRNA-loaded EXOs (iEXO), OXA-modified EXOs (EXO-OXA), and gal-9 siRNA & OXA-coloaded EXOs (iEXO-OXA), respectively. Prior to these performances, luciferase stable-transfected PANC-02 cells were implanted in the pancreas of C57BL/6 mice. Referenced with permission from Elsevier.
Considering the accessibility of thiol-maleimide chemistry, the conjugation of a new class of affinity ligands onto EXO in this way is receiving significant attention in the targeted therapy. Han et al have conjugated aptamers onto the HEK293T cell-derived EXOs by thiol-Michael addition to recognizing prostatic cancer cells. Under the guidance of aptamers, EXOs carried a high dose of siRNA into tumor cells to silence sirtuin 6 (SIRT6) expression, positively correlating with prostate cancer progression.107 As the report goes, the most effective treatment for ocular neovascular disease focuses on vascular endothelial growth factor (VEGF), which significantly provokes the aberrant growth of blood vessels.108 Intravitreal injection of anti-VEGF antibodies can block the activity of VEGF and suppress pathogenic angiogenesis in the eyes. Taking the use of thiol-Michael addition, anti-VEGF antibodies were successfully integrated onto the regulatory T cell-derived EXOs to suppress ocular neovascularization. First of all, anti-VEGF antibodies were functionalized with maleimide groups using a peptide linker, which would be subject to cleavage by matrix metalloproteinases in inflammatory lesions. Then, the anti-VEGF antibodies could be crosslinked onto the regulatory T cell-derived EXOs by thiol/maleimide reaction in mild conditions. After intravitreal injection, the engineered EXOs selectively accumulated within neovascularization lesions and the anti-VEGFs on them were released to suppress ocular and choroidal neovascularization, as explicitly shown in Figure 5B.109 Besides, immunotherapy has raised concern in treating pancreatic cancer since conventional therapies such as chemotherapy could not provide satisfactory survival outcome. A paradigm has described siRNA/oxaliplatin (OXA)-loaded EXOs for therapeutics of pancreatic cancer. In this study, galectin-9 siRNA and OXA were successively loaded on the bone marrow mesenchymal stem cell (BM-MSC)-derived EXOs by electroporation and thiol-Michael addition, respectively. OXA, as an important antitumor agent, was modified with N-(2-Aminoethyl) maleimide to synthesize OXA-maleimide. Then, OXA-maleimide was integrated onto the galectin-9 siRNA-loaded EXOs by thiol/maleimide reaction.110 Obviously, the use of BM-MSC-derived EXOs could significantly improve tumor targeting efficacy and effectively diffuse into the entire pancreatic tumor mass in contrast with the control group, as dramatically shown in Figure 5C and D. With time, the antitumor effects of siRNA & OXA-loaded EXOs preceded over than other groups such as PBS, gemcitabine, oxaliplatin, scrambled siRNA-loaded EXOs, galectin-9 siRNA-loaded EXOs, and OXA-modified EXOs, as clearly demonstrated in Figure 5E. Except for immune recovery of macrophages by galectin-9 siRNA, the OXA integrated onto EXOs by thiol-Michael addition was able to trigger immunogenic cell death within pancreatic tumors. More recently, anticytotoxic T lymphocyte-associated protein 4 antibody (aCTLA-4) was conjugated onto the dendritic cell-derived EXOs using thiol-maleimide chemistry to activate tumor-specific cytotoxic T lymphocytes to potentiate tumor therapeutic efficacy.111 Not only maleimide moiety is successfully conjugated onto the sulfhydryl on the EXOs, but citraconic anhydride can be integrated onto the amine of EXOs by thiol-Michael addition to modifying the surface charge of EXOs. Upon crosslinking of citraconic anhydride to the amine of EXOs, the reduced surface charge made for their superior intracellular uptake by macrophages.112 Of note, thiol-Michael addition is one of the popular chemical technologies to modify EXOs by specifically aiming at sulfhydryl of their surface proteins, which sufficiently enrich the cargos of interest for diagnosis/treatment. Nevertheless, traditional thiol-Michael addition tremendously depends on susceptible factors such as the basicity of the catalyst, thiol pKa, types of the thiol, and many others.103 Even so, we still hope that other types of Michael addition paradigms such as carbon-Michael reaction, oxa-Michael reaction, and aza-Michael reaction will be adopted in the EXO modifications for diagnosis/treatment in the future.
Imaging probes and medicines loaded on exosomes by other chemical engineering
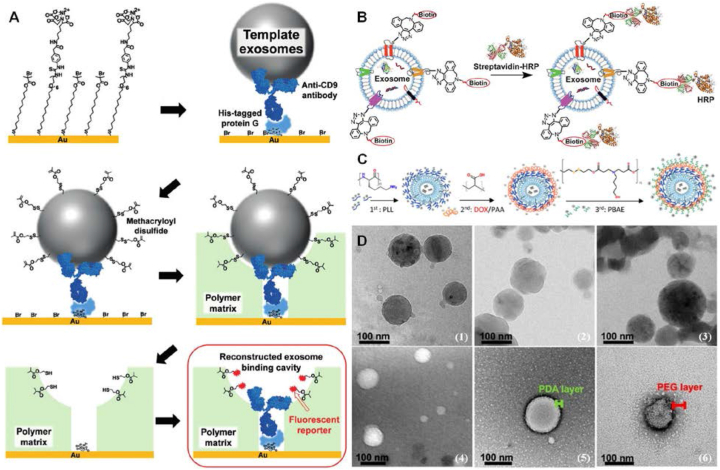
Many studies also have shown other chemical engineering emerges to reprogram EXOs as “bioscaffolds” for diagnosis/treatment. Serving as attractive surrogates for specific tumor cell-associated counterparts, intact EXOs' detection is of great significance for early-stage diagnosis of cancer.113 A pioneering work has recently developed molecular imprinting for creating EXO-binding cavities and introducing antibodies and fluorescent reporters inside the binding cavities to detect intact EXOs, as shown in Figure 6A, in which the surface of the template EXOs was modified with a disulfide bridge. In this study, His-tagged protein G was firstly anchored through the NiII complex with nitrilotriacetic acids (NTAs) on the gold substrate. Then, by interaction with His-tagged protein G, anti-CD9 antibodies were immobilized to capture CD9 highly expressed-EXOs. The EXOs were specifically captured through antigen/antibody binding and their surface was subsequently modified with a disulfide bridge using thiolated oleyl poly (ethylene glycol) and 2-(2-pyridyl) dithioethyl methacrylate. Afterward, the captured EXOs were imprinted by a polymer matrix. After removal of the template EXOs, the disulfide bridge would be transformed into a free thiol inside EXO-binding cavities, ensuring the fluorescent tags are site-specifically conjugated on the thiol groups.114 By monitoring the fluorescent signal inside EXO-binding cavities, this observation signified a successful detection of CD9-specific and intact EXOs. More importantly, such a rapid and highly sensitive “platform” was able to ultimately distinguish cancer-secreted EXOs from normal ones without tedious and time-consuming treatments. Ahead of the disulfide bridge, the surface modification of EXOs with cationized pullulan through electrostatic interaction has been proposed for the development of a new class of therapeutics for liver injury. By a simple mixing of EXOs and cationized pullulan, pullulan-loaded EXOs could be beneficial for targeting ability to hepatocyte asialoglycoprotein receptors. When intravenous administration, more internalization of pullulan-loaded EXOs by hepatocytes resulted in a greater therapeutic effect on liver injury.115 The regent-free approach was simple to operate while avoiding complex purification steps. Besides, EXOs can be also integrated with biotin on their surface, thereby establishing a specific conjugation of streptavidin-functionalized cargos onto EXOs for diagnosis/treatment of different diseases,99 as shown in Figure 6B.
Figure 6.
Chemical engineering on the EXOs by different methodologies. (A) Disulfide bridging-modified template EXOs for detecting intact EXOs. Firstly, His-tagged protein G was anchored through NiII complex with nitrilotriacetic acid (NTA) groups on the gold substrate modified with a mixed self-assembled monolayer, by which anti-CD9 antibodies were immobilized by interaction with His-tagged protein G. The template EXOs were then specifically captured on the substrate through antigen/antibody binding and subsequently, their surface was modified with methacryloyl disulfide. The captured EXOs were imprinted by a polymer matrix on the substrate. After removal of the template EXOs, the disulfide linker would be transformed into a free thiol inside EXO-binding cavities, allowing the fluorescent reporter to be conjugated on the thiol groups. By monitoring the fluorescent signal inside cavities, intact EXOs could be sufficiently detected with high sensitivity. Referenced with permission from Wiley-VCH. (B) Biotinylation of EXOs for intracellular delivery. EXOs were firstly biotinylated with DBCO-PEG4-biotin by “click chemistry”. Afterward, the streptavidin-functionalized cargos could be specifically crosslinked onto biotin conjugated on EXOs. (C) Trlayered shell assembly of polyelectrolytes formed on the siRNA-loaded EXOs by layer-by-layer deposition serves as the carrier for DOX loading. 1st layer: cationic poly(l-lysine) (PLL); 2nd layer: anionic poly(acrylic acids) (PAA); 3rd layer: poly(b-amino esters) (PBAE). Referenced from American Chemical Society (Copyright 2020). (D) Morphology characterization of polymer-modified EXOs. Cryo-TEM images of (1) bare EXOs, (2) EXO@PDA, and (3) EXO@PDA@PEG; TEM images of (4) bare EXOs, (5) EXO@PDA, and (6) EXO@PDA@PEG2000. Referenced with permission from Wiley-VCH.
Emerging as promising drug delivery vehicles, amphiphilic polymers are good candidates for surface functions of EXOs, facilitating the development of polymer-engineered EXOs for cargo loading. Another study showed, by incubation of siRNA-loaded EXOs with hydrophilic polyethylene glycol (PEG), the surface of EXOs became coated with a PEG corona. When oral drug delivery, the PEG coating could significantly reduce EXOs degradation in the acidic gastric environment and efficiently diffuse across mucosal barriers to enhance their overall permeability.116 On the other hand, PEGylation allows for decorating EXOs with targeting ligands. To improve EXO characteristics for drug delivery to tumor cells, Kooijmans et al have integrated phospholipid (DMPE)-PEG derivatives onto Neuro2A cell-derived EXOs, by which it facilitated conjugations of nanobodies specific for epidermal growth factor receptor (EGFR) by Michael addition. In this way, the modified EXOs had better specificity to EGFR highly expressed in tumor cells and longer circulation time within the mice body.117 For different purposes, polyethyleneimine (PEI) coated on the FA-functionalized EXOs could be complex with nucleic acids through ionic interaction. Under the guidance of FA molecules, PEI on the EXOs could effectively deliver a high dose of siRNA to silence gene expression in tumor therapy.74 Similarly to PEI, one more intricate polymer architecture was successfully constructed on the A549 cell-shed EXOs for selective codelivery of siRNA and chemotherapeutics to cancer cells, as shown in Figure 6C. The layer-by-layer architecture was designed by sequential deposition of oppositely charged polyelectrolytes. The trilayered shell assembly of polyelectrolytes on the surface of EXOs was composed of poly (l-lysine) (PLL), poly (acrylic acids) (PAA), and poly (b-amino ester) (PBAE) from inside to outside, which served as the carrier for DOX loading.118 The final layer PBAE was chosen owing to its high gene transfection efficiency and excellent intracellular delivery.119 Except for this polymer matrix, a recent study employed polydopamine coating to control the surface properties of EXOs, ensuring tailorable conjugations of other cargos.120 In this study, self-polymerization of dopamine on the EXOs was a simple and mild method, on which the catechol and amine groups conferred conjugations of other cargos by Michael addition or Schiff-base reaction.121 After PDA coating, the EXO@PDA could be also integrated with PEG-thiol to form EXO@PDA@PEG and fluorescently labeled EXO@PDA@fluorescein isothiocyanate (FITC)-PEG, as shown in Figure 6D. Collectively, modifications by polymers are able to improve the stability of EXOs and facilely incorporate functional molecules, which will expand the potential of EXOs in biomedical applications. Disappointingly, one of the major drawbacks associated with an encapsulation of polymers on the EXOs is a block of the communications of membrane proteins, resulting in a loss of their intrinsic characteristics, e.g., “crosstalk” and tissue-homing capabilities.
Conclusions and perspectives
EXOs secreted naturally by human cells are emerging as a new generation of delivery systems for future clinical development. As one of the natural “bioscaffolds” from the human body, they can carry not only medicines but also bioimaging agents for precise theranostics. It is worth mentioning that a phase II clinical trial of tumor-antigen loaded dendritic cell-derived EXOs as vaccination was successfully carried out in 2018.122 This review mainly discussed the chemical engineering of surface functionalization on the EXOs. Although each of them differs at the molecular level, their purpose is the same, i.e., to conjugate theranostic agents to EXOs for diagnosis/treatment. Compared with physical and genetic approaches, some of them own better scalability of cargos, more selectivity and specificity at binding sites on the EXOs, and higher stability and reproducibility. Regardless of the unique properties of chemically-engineered EXOs to develop smart drug delivery vehicles that circumvent phagocytosis, immunogenicity, and short circulation half-life, some key points are major hurdles to clinical translation of chemically-engineered EXOs, such as selection of proper donors, preservation of intrinsic characteristics, and standardization of quality control: (i) Firstly, prudent selection of mother cells is one of the critical upstream process steps for chemically-engineered EXOs. The properties of EXOs such as tissue-homing, immunogenicity, and oncogenicity should be considered, which are greatly associated with the mother cells. Of note, contamination and genetic drift occasionally occur during mother cell culture. On demand of theranostic goals, EXOs from mother cells should steadily express desired cargos.12 (ii) More disastrously, some of the chemical engineering greatly results in rearrangements of EXO membranes and destruction of their integrity, for example, by chemical transfection and saponin. Besides, the EDC/NHS chemistry and thiol-Michael addition are mainly operated on the functional groups (e.g., carboxyl, amine, and thiol) in membrane proteins or phospholipids of EXOs. If possible, their membrane proteins will be capped when modified by polymers. Collectively, chemical engineering partly alters the original distribution and density of membrane proteins on the EXOs and even denatures them, both of which will disturb their intrinsic functionality such as “crosstalk” and “homing” propensities to affect therapeutic efficacy. Thus, what kind of chemical engineering to choose to modify EXOs should be taken seriously. (iii) The stability and reproducibility of chemically-engineered EXll remain controversial when loading a variety of cargos on them. These important parameters of chemically-engineered EXOs require control of quality and clinical investigation. To further enhance rigor and reproducibility, the International Society for Extracellular Vesicles (ISEV) should initiate the standards of chemically-engineered EXOs and characterization, e.g., the amounts of functional groups and cargos, by which they can achieve equivalent or superior efficacies. Thus, these limitations may render their clinic translation cumbersome and risky. Despite these challenges, the chemical engineering of EXOs is still a rising star as drug delivery vehicles. This technique is still in its infancy and will offer a new route for precise diagnosis/treatment of human devastating diseases, including cancer and neurodegenerative disorders. In this regard, chemically engineered EXOs will be expected to carry other cargos, such as paramagnetic metals for MRI, contrast agents for CT imaging, or microbubbles for sonography. To this end, more ways of chemical cross-linking on the EXOs are expected to be exploited in the future. With concerted efforts from organic chemists, molecular biologists, and clinicians, the chemical engineered-EXOs will become a new theranostic frontier for human devastating diseases.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972023), the Natural Science Foundation of Chongqing City, China (No. cstc2021jcyj-msxm0172), the Science and Technology Research Program of Chongqing Education Commission of China (No. KJQN201900425), Creative Research Group of CQ University (China) (No. CXQT21017), and the Program for Youth Innovation in Future Medicine from Chongqing Medical University (China).
Conflict of interests
The authors declare no competing financial interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Ning Jiang, Email: jiangning@cqmu.edu.cn.
Qiling Peng, Email: pqlpzy@cqmu.edu.cn.
Lei Zheng, Email: nfyyzhenglei@smu.edu.cn.
References
- 1.Johnstone R.M., Adam M., Hammond J.R., et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 2.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Y., Duan L., Lu J., et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 5.Milane L., Singh A., Mattheolabakis G., et al. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L., Regnault A., Lozier A., et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B., Dorval T., Chaput N., et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse M.A., Garst J., Osada T., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M., Nickoloff E., Abramova T., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117(1):52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan C.Y., Lai R.C., Wong W., et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran P.H.L., Xiang D., Tran T.T.D., et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 2020;32(18) doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 13.Tkach M., Kowal J., Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018;373(1737) doi: 10.1098/rstb.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das C.K., Jena B.C., Banerjee I., et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16(1):24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 15.Kim M.S., Haney M.J., Zhao Y., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan Y., Wang L., Zhu C., et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78(3):798–808. doi: 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donoso-Quezada J., Ayala-Mar S., González-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit Rev Biotechnol. 2020;40(6):804–820. doi: 10.1080/07388551.2020.1785385. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Erviti L., Seow Y., Yin H., et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Zhang X., Chen X., et al. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L., Faruqu F.N., Lim Y.M., et al. Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. 2021;264 doi: 10.1016/j.biomaterials.2020.120369. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.H., Lechman E.R., Bianco N., et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174(10):6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y.T., Umezaki K., Sawada S., et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6 doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajipour H., Farzadi L., Roshangar L., et al. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 2021;275 doi: 10.1016/j.lfs.2021.119351. [DOI] [PubMed] [Google Scholar]
- 24.Haney M.J., Klyachko N.L., Zhao Y., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Contr Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalimuthu S., Gangadaran P., Rajendran R.L., et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra S., Dumoga S., Sirohi P., et al. Red blood cells-derived vesicles for delivery of lipophilic drug camptothecin. ACS Appl Mater Interfaces. 2019;11(25):22141–22151. doi: 10.1021/acsami.9b04827. [DOI] [PubMed] [Google Scholar]
- 27.Sun D., Zhuang X., Xiang X., et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang X., Xiang X., Grizzle W., et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh W.J., Lee C.K., Zou S., et al. Doxorubicin-loaded cell-derived nanovesicles: an alternative targeted approach for anti-tumor therapy. Int J Nanomed. 2017;12:2759–2767. doi: 10.2147/IJN.S131786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai C.P., Mardini O., Ericsson M., et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Q., Guo D., Liu G., et al. Exosomes from miR-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44(6):2105–2116. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 32.Anticoli S., Manfredi F., Chiozzini C., et al. An exosome-based vaccine platform imparts cytotoxic T lymphocyte immunity against viral antigens. Biotechnol J. 2018;13(4) doi: 10.1002/biot.201700443. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Zhang H., Bai M., et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26(3):774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra A., Singh P., Qayoom I., et al. Current strategies in tailoring methods for engineered exosomes and future avenues in biomedical applications. J Mater Chem B. 2021;9(32):6281–6309. doi: 10.1039/d1tb01088c. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A., Bisht B., Dutta S., et al. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol Sin. 2022;43(11):2759–2776. doi: 10.1038/s41401-022-00902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betzer O., Barnoy E., Sadan T., et al. Advances in imaging strategies for in vivo tracking of exosomes. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(2) doi: 10.1002/wnan.1594. [DOI] [PubMed] [Google Scholar]
- 37.Liu W., Huang Y., Li Z., et al. Multivalent engineering of exosomes with activatable aptamer probes for specific regulation and monitoring of cell targeting. Anal Chem. 2022;94(9):3840–3848. doi: 10.1021/acs.analchem.1c04741. [DOI] [PubMed] [Google Scholar]
- 38.You D.G., Lim G.T., Kwon S., et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7(23) doi: 10.1126/sciadv.abe0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan F., Zhong Z., Wang Y., et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnol. 2020;18:115. doi: 10.1186/s12951-020-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavasolian F., Hosseini A.Z., Soudi S., et al. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20(4):297–312. doi: 10.2174/1566523220666200916120708. [DOI] [PubMed] [Google Scholar]
- 41.Zhu L.P., Tian T., Wang J.Y., et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai R.C., Chen T.S., Lim S.K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 43.Bellin G., Gardin C., Ferroni L., et al. Exosome in cardiovascular diseases: a complex world full of hope. Cells. 2019;8(2):166. doi: 10.3390/cells8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarko D.K., McKinney C.E. Exosomes: origins and therapeutic potential for neurodegenerative disease. Front Neurosci. 2017;11:82. doi: 10.3389/fnins.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howitt J., Hill A.F. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem. 2016;291(52):26589–26597. doi: 10.1074/jbc.R116.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riazifar M., Mohammadi M.R., Pone E.J., et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D., Lee H., Wang X., et al. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther. 2018;26(9):2119–2130. doi: 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosseini Shamili F., Alibolandi M., Rafatpanah H., et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Contr Release. 2019;299:149–164. doi: 10.1016/j.jconrel.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Jiang K., Yang J., Guo S., et al. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27(10):1758–1771. doi: 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H., Jang H., Cho H., et al. Recent advances in exosome-based drug delivery for cancer therapy. Cancers. 2021;13(17):4435. doi: 10.3390/cancers13174435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou J., Shi M., Liu X., et al. Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem. 2019;91(3):2425–2430. doi: 10.1021/acs.analchem.8b05204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang F., Guo J., Zhang Z., et al. Application of engineered extracellular vesicles for targeted tumor therapy. J Biomed Sci. 2022;29:14. doi: 10.1186/s12929-022-00798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Y., Dong S., Li X., et al. Extracellular vesicles: an emerging nanoplatform for cancer therapy. Front Oncol. 2021;10 doi: 10.3389/fonc.2020.606906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan A., Rajadas J., Seifalian A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65(3):357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Chen S., Chen X., Luo Q., et al. Retinoblastoma cell-derived exosomes promote angiogenesis of human vesicle endothelial cells through microRNA-92a-3p. Cell Death Dis. 2021;12(7):695. doi: 10.1038/s41419-021-03986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng W., Wang K., Zhao Z., et al. Exosomes-mediated transfer of miR-125a/b in cell-to-cell communication: a novel mechanism of genetic exchange in the intestinal microenvironment. Theranostics. 2020;10(17):7561–7580. doi: 10.7150/thno.41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang D., Lee H., Zhu Z., et al. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L110–L121. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sayyed A.A., Gondaliya P., Mali M., et al. MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol Pharm. 2021;18(8):3010–3025. doi: 10.1021/acs.molpharmaceut.1c00213. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y., Ao X., Ding W., et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piffoux M., Silva A.K.A., Wilhelm C., et al. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12(7):6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 61.Lin Y., Wu J., Gu W., et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5(4) doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lv Q., Cheng L., Lu Y., et al. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7(18) doi: 10.1002/advs.202000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng L., Zhang X., Tang J., et al. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 64.Fuhrmann G., Serio A., Mazo M., et al. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Contr Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 65.Cammarata C.R., Hughes M.E., Ofner C.M., 3rd Carbodiimide induced cross-linking, ligand addition, and degradation in gelatin. Mol Pharm. 2015;12(3):783–793. doi: 10.1021/mp5006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermanson G.T. Bioconjugate Techniques. Academic Press; Boston: 2013. [Google Scholar]
- 67.Fischer M.J. Amine coupling through EDC/NHS: a practical approach. Methods Mol Biol. 2010;627:55–73. doi: 10.1007/978-1-60761-670-2_3. [DOI] [PubMed] [Google Scholar]
- 68.Dempsey D., Jiang H., Kalin J.H., et al. Site-specific protein labeling with N-hydroxysuccinimide-esters and the analysis of ubiquitin ligase mechanisms. J Am Chem Soc. 2018;140(30):9374–9378. doi: 10.1021/jacs.8b05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smyth T., Petrova K., Payton N.M., et al. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 2014;25(10):1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu H., Liao C., Liang S., et al. A novel peptide-equipped exosomes platform for delivery of antisense oligonucleotides. ACS Appl Mater Interfaces. 2021;13(9):10760–10767. doi: 10.1021/acsami.1c00016. [DOI] [PubMed] [Google Scholar]
- 71.Bagheri E., Abnous K., Farzad S.A., et al. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118369. [DOI] [PubMed] [Google Scholar]
- 72.Lu Y., Sega E., Leamon C.P., et al. Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Deliv Rev. 2004;56(8):1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Zwicke G.L., Ali Mansoori G., Jeffery C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012;3(1) doi: 10.3402/nano.v3i0.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munagala R., Aqil F., Jeyabalan J., et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021;505:58–72. doi: 10.1016/j.canlet.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao T.G.N., Kang J.H., You J.Y., et al. Safe and targeted sonodynamic cancer therapy using biocompatible exosome-based nanosonosensitizers. ACS Appl Mater Interfaces. 2021;13(22):25575–25588. doi: 10.1021/acsami.0c22883. [DOI] [PubMed] [Google Scholar]
- 76.Tian T., Wang Y., Wang H., et al. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 77.Koh E., Lee E.J., Nam G.H., et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Hong Y., Nam G.H., Koh E., et al. Exosome as a vehicle for delivery of membrane protein therapeutics, PH20, for enhanced tumor penetration and antitumor efficacy. Adv Funct Mater. 2018;28(17) [Google Scholar]
- 79.Kim H., Wang S.Y., Kwak G., et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6(20) doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jia G., Han Y., An Y., et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and. in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 81.Zhu H., Wang K., Wang Z., et al. An efficient and safe MUC1-dendritic cell-derived exosome conjugate vaccine elicits potent cellular and humoral immunity and tumor inhibition. in vivo. Acta Biomater. 2022;138:491–504. doi: 10.1016/j.actbio.2021.10.041. [DOI] [PubMed] [Google Scholar]
- 82.Tian T., Zhang H.X., He C.P., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 83.Xu J., Filion T.M., Prifti F., et al. Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry. Chem Asian J. 2011;6(10):2730–2737. doi: 10.1002/asia.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shelbourne M., Brown T., El-Sagheer A.H., et al. Fast and efficient DNA crosslinking and multiple orthogonal labelling by copper-free click chemistry. Chem Commun. 2012;48(91):11184–11186. doi: 10.1039/c2cc35084j. [DOI] [PubMed] [Google Scholar]
- 85.Moses J.E., Moorhouse A.D. The growing applications of click chemistry. Chem Soc Rev. 2007;36(8):1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 86.Agrahari A.K., Bose P., Jaiswal M.K., et al. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem Rev. 2021;121(13):7638–7956. doi: 10.1021/acs.chemrev.0c00920. [DOI] [PubMed] [Google Scholar]
- 87.Song S., Shim M.K., Lim S., et al. In situ one-step fluorescence labeling strategy of exosomes via bioorthogonal click chemistry for real-time exosome tracking in vitro and in vivo. Bioconjugate Chem. 2020;31(5):1562–1574. doi: 10.1021/acs.bioconjchem.0c00216. [DOI] [PubMed] [Google Scholar]
- 88.Salunkhe S., Basak M., Chitkara D., Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Z., Jing B., Lan X., et al. Goat milk-derived exosomes endowed with radioactive and targeting properties: potential to provide PET/CT monitoring for exosomes-based drug delivery system in gliomas therapy. J Nucl Med. 2020;61(supplement 1):1064. [Google Scholar]
- 90.Qian R., Jing B., Jiang D., et al. Multi-antitumor therapy and synchronous imaging monitoring based on exosome. Eur J Nucl Med Mol Imag. 2022;49(8):2668–2681. doi: 10.1007/s00259-022-05696-x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang L., Qin Z., Sun H., et al. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact Mater. 2022;18:1–14. doi: 10.1016/j.bioactmat.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.An Y., Jin T., Zhu Y., et al. An ultrasensitive electrochemical aptasensor for the determination of tumor exosomes based on click chemistry. Biosens Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111503. [DOI] [PubMed] [Google Scholar]
- 93.Fan C., Zhao N., Cui K., et al. Ultrasensitive exosome detection by modularized SERS labeling for postoperative recurrence surveillance. ACS Sens. 2021;6(9):3234–3241. doi: 10.1021/acssensors.1c00890. [DOI] [PubMed] [Google Scholar]
- 94.Lee T.S., Kim Y., Zhang W., et al. Facile metabolic glycan labeling strategy for exosome tracking. Biochim Biophys Acta Gen Subj. 2018;1862(5):1091–1100. doi: 10.1016/j.bbagen.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu L., Xu Y., Wei X., et al. Coupling aptamer-based protein tagging with metabolic glycan labeling for in situ visualization and biological function study of exosomal protein-specific glycosylation. Angew Chem Int Ed Engl. 2021;60(33):18111–18115. doi: 10.1002/anie.202103696. [DOI] [PubMed] [Google Scholar]
- 96.Nie W., Wu G., Zhang J., et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–2022. doi: 10.1002/anie.201912524. [DOI] [PubMed] [Google Scholar]
- 97.Zhang P., Dong B., Zeng E., et al. In vivo tracking of multiple tumor exosomes labeled by phospholipid-based bioorthogonal conjugation. Anal Chem. 2018;90(19):11273–11279. doi: 10.1021/acs.analchem.8b01506. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y., Wang L., Zhang P., et al. Chemoenzymatic labeling of extracellular vesicles for visualizing their cellular internalization in real time. Anal Chem. 2020;92(2):2103–2111. doi: 10.1021/acs.analchem.9b04608. [DOI] [PubMed] [Google Scholar]
- 99.Wang M., Altinoglu S., Takeda Y.S., et al. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang X., Xie B., Peng H., et al. Eradicating intracellular MRSA via targeted delivery of lysostaphin and vancomycin with mannose-modified exosome. J Contr Release. 2021;329:454–467. doi: 10.1016/j.jconrel.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 101.Xu L., Faruqu F.N., Liam-Or R., et al. Design of experiment (DoE)-driven in vitro and in vivo uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. J Extracell Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1779458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Wu J., Wu J., et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnol. 2019;17(1):29. doi: 10.1186/s12951-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nair D.P., Podgórski M., Chatani S., et al. The thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry. Chem Mater. 2014;26(1):724–744. [Google Scholar]
- 104.Roberts-Dalton H.D., Cocks A., Falcon-Perez J.M., et al. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale. 2017;9(36):13693–13706. doi: 10.1039/c7nr04128d. [DOI] [PubMed] [Google Scholar]
- 105.Luther K.M., Haar L., McGuinness M., et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125–137. doi: 10.1016/j.yjmcc.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Fan Z., Xiao K., Lin J., et al. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15(47) doi: 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- 107.Han Q., Xie Q.R., Li F., et al. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics. 2021;11(13):6526–6541. doi: 10.7150/thno.53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ozawa C.R., Banfi A., Glazer N.L., et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian Y., Zhang F., Qiu Y., et al. Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells. Nat Biomed Eng. 2021;5(9):968–982. doi: 10.1038/s41551-021-00764-3. [DOI] [PubMed] [Google Scholar]
- 110.Zhou W., Zhou Y., Chen X., et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 111.Jung M., Kang M., Kim B.S., et al. Nanovesicle-mediated targeted delivery of immune checkpoint blockades to potentiate therapeutic efficacy and prevent side effects. Adv Mater. 2022;34(9) doi: 10.1002/adma.202106516. [DOI] [PubMed] [Google Scholar]
- 112.Kim Y., Mok H. Citraconylated exosomes for improved internalization into macrophages. Appl Biol Chem. 2019;62:26. [Google Scholar]
- 113.Hong C.S., Funk S., Muller L., et al. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5 doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mori K., Hirase M., Morishige T., et al. A pretreatment-free, polymer-based platform prepared by molecular imprinting and post-imprinting modifications for sensing intact exosomes. Angew Chem Int Ed Engl. 2019;58(6):1612–1615. doi: 10.1002/anie.201811142. [DOI] [PubMed] [Google Scholar]
- 115.Tamura R., Uemoto S., Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–284. doi: 10.1016/j.actbio.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 116.Warren M.R., Zhang C., Vedadghavami A., et al. Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater Sci. 2021;9(12):4260–4277. doi: 10.1039/d0bm01497d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kooijmans S.A.A., Fliervoet L.A.L., van der Meel R., et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Jhan Y.Y., Palou Zuniga G., Singh K.A., et al. Polymer-coated extracellular vesicles for selective codelivery of chemotherapeutics and siRNA to cancer cells. ACS Appl Bio Mater. 2021;4(2):1294–1306. doi: 10.1021/acsabm.0c01153. [DOI] [PubMed] [Google Scholar]
- 119.Routkevitch D., Sudhakar D., Conge M., et al. Efficiency of cytosolic delivery with poly(beta-amino ester) nanoparticles is dependent on the effective pKa of the polymer. ACS Biomater Sci Eng. 2020;6(6):3411–3421. doi: 10.1021/acsbiomaterials.0c00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang C., Kimura K., Li J., et al. Polydopamine-mediated surface functionalization of exosomes. ChemNanoMat. 2021;7(6):592–595. [Google Scholar]
- 121.Lee H., Dellatore S.M., Miller W.M., et al. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rayamajhi S., Aryal S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B. 2020;8(21):4552–4569. doi: 10.1039/d0tb00744g. [DOI] [PubMed] [Google Scholar]