Abstract
This systematic review and meta-analysis pool evidence available from clinical trials to verify the effect of antioxidants on the outcome of acute aluminum phosphide (AlP) poisoning. A systematic review complied with “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) Protocols. Meta-analysis was conducted on 10 studies that fulfill eligibility criteria. Four antioxidants were implemented: N-Acetyl cysteine (NAC), L-Carnitine, Vitamin E, and Co-enzyme Q10 (Co Q10). Risk of bias, publication bias, and heterogeneity were assessed to ensure the results’ reliability. Antioxidants significantly decrease mortality of acute AlP poisoning around three folds (OR = 2.684, 95% CI: 1.764–4.083; P < .001) and decrease the need for intubation and mechanical ventilation by two folds (OR = 2.391, 95% CI 1.480–3.863; P < .001) compared with control. Subgroup analysis revealed that NAC significantly decreases mortality by nearly three folds (OR = 2.752, 95% CI: 1.580–4.792; P < .001), and vitamin E significantly decreases mortality by nearly six folds (OR = 5.667, 95% CI: 1.178–27.254; P = .03) compared with control. L-Carnitine showed a borderline significance (P = .050). Co Q10 decreased the mortality compared with the control; however, the difference was not statistically significant (P = .263). This meta-analysis provides solid evidence regarding the efficacy of antioxidants in improving the outcome of acute AlP poisoning with reference to NAC. Wide confidence interval and small relative weight affect reliability regarding vitamin E efficacy. Future clinical trials and meta-analyses are recommended. To our knowledge, no previous meta-analysis was conducted to investigate the efficacy of treatment modalities for acute AlP poisoning.
Keywords: aluminum phosphide poisoning, antioxidants, L-carnitine, N-acetyl cysteine, vitamin E, co-enzyme Q10
Introduction
Aluminum phosphide (AlP) is a highly toxic phosphine-generating pesticide that is widely used in many countries. In the last few years, AlP gained popularity as a suicidal agent, with an escalating rise in cases of acute poisoning.1
Upon oral intake of AlP, it rapidly liberates the highly toxic phosphine (PH3) gas that interferes with cellular respiration. PH3 disrupts mitochondrial functions by inhibiting cytochrome oxidase and adenosine triphosphate (ATP) formation. Furthermore, phosphine induces oxidative stress by enhancing the generation of reactive oxygen species (ROS) and inhibition of enzymatic antioxidants such as catalase (CAT), glutathione reductase (GR), and superoxide dismutase (SOD). Disturbed redox status results in lipid peroxidation, destroying the various cellular structures and enhancing cellular death.2,3
Clinically, acute AlP poisoning manifested with severe metabolic acidosis and refractory cardiogenic shock. The management of phosphide poisoning is confined to supportive measures in the absence of an antidote. Unfortunately, acute AlP poisoning is associated with high mortality.4,5 Thus, adopting therapeutic interventions based on the mechanism of acute AlP poisoning is necessary.6–8
Oxidative stress is a well-established mechanism of acute phosphide poisoning.9,10 Therefore; several clinical trials proposed that antioxidants, including N-acetyl cysteine,11–16 L-carnitine,17,18 Vitamin E,19 and Co-enzyme Q10 (Co Q10),20 could counteract the fatal phosphine effects. However, clinical studies often have limitations, such as inconsistent results, heterogeneity, and small sample size. Therefore, sporadic trials assess the efficacy of therapeutic intervention with limited validity.21
Systematic reviews are done using a standardized approach better than traditional narrative reviews, randomly searching literature with a high possibility of escaping valuable studies. Thus, systematic reviews have been commonly applied in different medical specialties for a long time. However, in toxicology, the first systematic review was in 2014, and a limited number of systematic reviews have been published since then.22,23
Meta-analysis is a statistical method that combines the results of multiple individual studies to generate a new hypothesis. A meta-analysis is a powerful tool with the strongest statistical evidence in clinical research. It overcomes the problem of small numbers of patients in sporadic studies and assesses the effects of specific treatments with high precision. Therefore, meta-analysis provides the “platinum standard of evidence”.24,25
Objectives
The current study adopted a systematic review and meta-analysis to pool the evidence available from clinical trials that studied the effect of various antioxidants in improving the outcome of acute AlP poisoning.
Methods
In the current study, a systematic review was performed in compliance with the requirements of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols.26 The study was approved by the Research Ethics Committee of the Faculty of Medicine, Alexandria University (IRB Number: 00012098, FWA Number: 00018699, Approval Serial Number: 0305138).
(i) Study design types
The study included all published clinical studies that used antioxidants in the management of acute AlP poisoning and fulfilled illegibility criteria.
Participants: patients with acute AlP poisoning.
Intervention: antioxidant therapy along with conventional supportive measures.
Control: conventional supportive measures.
Outcome:
(a) Primary outcome: mortality percentage.
(b) Secondary outcome: the need for endotracheal intubation and mechanical ventilation.
(ii) Exclusion criteria
(a) Non-human studies that include in vitro and animal studies.
(b) Registered ongoing clinical trials that are unpublished at the time of conduction of the study.
(c) Co-exposure to other toxicants with AlP.
(d) One group without comparison arm, case series, and case reports.
(e) When additional interventions are used other than conventional supportive measures and antioxidants.
(iii) Information sources
The electronic retrieval method was implemented, and the retrieval time started from the established database on 2022 February 25. The retrieval database included advanced searches in Scopus, PubMed (National Library of Medicine), Web of Science (Clarivate), and Egyptian journals that were accessed through the Egyptian Knowledge Bank (EKB). The retrieved studies were in the English language.
(iv) Search strategy
The advanced search strategies of the Scopus, Web of Science, and Pubmed databases were performed, as illustrated in Table 1. Egyptian articles were retrieved from EKB using the exact keywords.
Table 1.
List of advanced search strategies in the Scopus, Web of Science, and Pubmed databases.
| Search items of query | |
|---|---|
| Scopus | (acute) AND (aluminum OR aluminium) AND (phosphide) AND (poisoning OR toxicity) AND (antioxid* OR nac OR n-acetyl AND cysteine OR l-carnitine OR vitamin OR co-enzyme) |
| Web of Science | ((((TS = ((acute))) AND TS = ((aluminum) OR (aluminium))) AND TS = ((phosphide))) AND TS = ((toxicity) OR (poisoning))) AND TS = ((antioxid*) OR (NAC) OR (N-acetyl cysteine) OR (L-carnitine) OR (vitamin) OR (co-enzyme)) |
| Pubmed | ((((((acute))) AND ((aluminum OR aluminium))) AND ((phoshide))) AND ((poisoning OR toxicity))) AND ((antioxid* OR NAC OR N-acetyl cysteine OR L-carnitine OR vitamin OR co-enzyme)) |
(v) Study selection
The search results were exported into reference management software (EndNote™ X8), and duplicated studies were omitted. Two reviewers screened titles and abstracts separately to exclude studies according to the eligibility criteria. Then, the reviewers obtained the full text for the articles and assessed them again based on the inclusion criteria.27
(vi) Data extraction
The two reviewers comprehensively read the full text of articles that fulfill the inclusion criteria. Then, they extracted relevant data in a designed Excel sheet that consisted of the following sections:
(a) General research data: title, authors, and year of publication.
(b) Study design: intervention (type of antioxidant) and the number of patients in intervention and control groups.
(c) Base-line or on-admission characteristics of the patients in intervention and control groups.
(d) Outcome: mortality percentage and need for endotracheal intubation and mechanical ventilation.
(vii) Data analysis
Data analysis was done by comprehensive meta-analysis software version 2. Effect size odds ratio for mortality proportion, the proportion of intubation, and Odds ratio (OR) were presented with an estimate of precision using a 95% confidence interval (CI).
Risk of bias assessment is a quality assessment that establishes transparency of generated hypothesis. The modified Cochrane Collaboration tool was used to assess the risk of bias for each included study.28
Heterogeneity denotes variations in observed effect(s) among researches, which, if excessive, suggest uncontrolled factors influencing the observed effect(s). In the current study, the heterogeneity was assessed using Cochrane Q statistic, which has Chi-Square statistic, and I2 statistic and P-value, statistical heterogeneity was considered with I2 of >50% or P < .1.29
The summary of the meta-analysis results was presented graphically using the forest plot.30 Subgroup analysis was considered based on the type of antioxidants for each subgroup.
Publication bias means that studies with small sample sizes could have less chance of publication than larger studies. The funnel plot is a graphical method to assess the presence of publication bias by plotting effect sizes against their precisions. The symmetry of the funnel plot denotes the absence of publication bias.31
Results
The search in Scopus, Web of Science, PubMed, and Egyptian Journals yielded 204, 13, 20, and 16 articles, respectively. One article was obtained by direct author contact. After removing duplicate articles and excluding articles that did not meet the inclusion criteria, 10 articles were included in the meta-analysis, as illustrated in the PRISMA flow chart (Fig. 1).
Fig. 1.
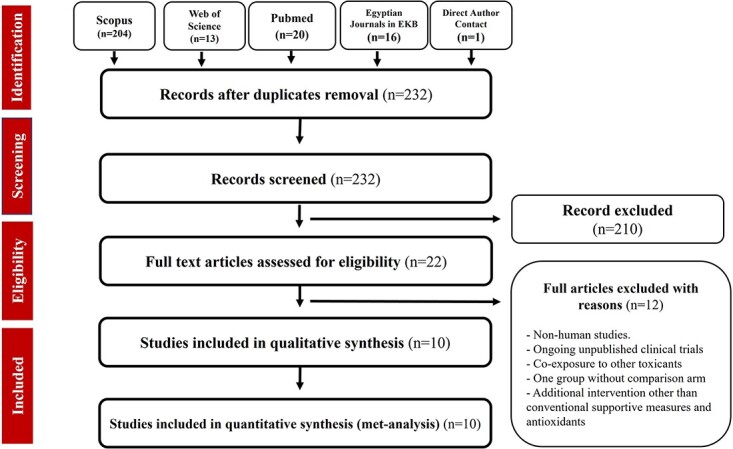
PRISMA flow diagram describing the phases of the current systematic review. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Assessment of risk of bias
The risk of bias was assessed using the modified Cochrane Collaboration tool. The bias was judged as high, low, or unclear for individual elements from five domains (selection, reporting, performance, detection, attrition).28–32 All studies included in the meta-analysis had a low or unclear risk of bias, except the study of Taghaddosinejad et al. 2016 had a high risk of selection bias, as demonstrated in Fig. 2.
Fig. 2.
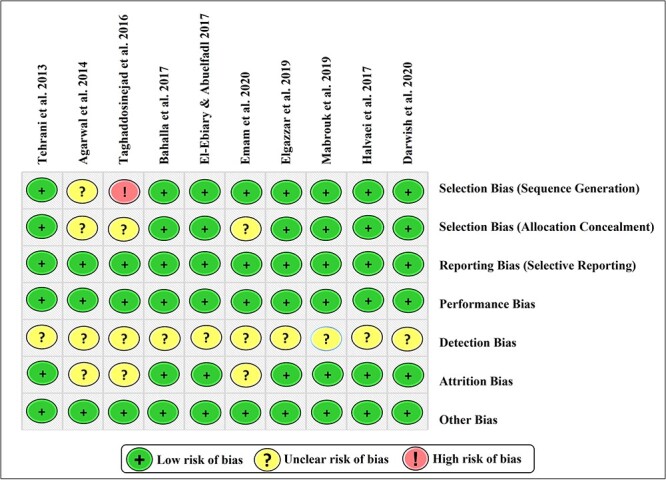
Risk of bias assessment of the studies included in a meta-analysis using the modified Cochrane Collaboration tool.
Data of included studies
Table 2 revealed general publication data of the studies included in the meta-analysis. The studies included 465 patients; 227 received the conventional supportive measures (control groups), and 238 received conventional supportive measures along with antioxidant therapy (intervention groups).
Table 2.
Description of the clinical studies included in the meta-analysis.
| Studies included in meta-analysis | Country | Groups | Antioxidant | Sample size |
|---|---|---|---|---|
| Tehrani et al. 201311 | Iran | Control | 15 | |
| Intervention | NAC | 22 | ||
| Agarwal et al. 201412 | India | Control | 22 | |
| Intervention | NAC | 24 | ||
| Taghaddosinejad et al. 201613 | Iran | Control | 23 | |
| Intervention | NAC | 23 | ||
| Bahalla et al. 201714 | India | Control | 26 | |
| Intervention | NAC | 24 | ||
| El-Ebiary & Abuelfadl 201715 | Egypt | Control | 15 | |
| Intervention | NAC | 15 | ||
| Emam et al. 202016 | Egypt | Control | 30 | |
| Intervention | NAC | 30 | ||
| Elgazzar et al. 201917 | Egypt | Control | 25 | |
| Intervention | L-Carnitine | 25 | ||
| Mabrouk et al. 201918 | Egypt | Control | 25 | |
| Intervention | L-Carnitine | 25 | ||
| Halvaei et al. 201719 | Iran | Control | 16 | |
| Intervention | Vit E | 20 | ||
| Darwish et al. 202020 | Egypt | Control | 30 | |
| Intervention | Co Q10 | 30 |
Four types of antioxidants were used in these studies as follows:
(i) N-Acetyl cysteine (NAC): Tehrani et al. (2013),11 Agarwal et al. (2014),12 Taghaddosinejad et al. (2016),13 Bahalla et al. (2017),14 El-Ebiary and Abuelfadl (2017),15 and Emam et al. (2020).16
(ii) L-Carnitine: Elgazzar et al. (2019)17 and Mabrouk et al. (2019).18
(iii) Vitamin E: Halvaei et al. (2017).19
(iv) Co-enzyme Q 10 (Co Q10): Darwish et al. (2020).20
On-admission data
Table 3 illustrates the admission data most relevant to acute AlP poisoning, including the mean patients’ age, exposure data, vital signs, and Arterial Blood Gases (ABG).
Table 3.
Baseline data (on admission) of acute AlP-poisoned patients in the studies included in the meta-analysis.
| Studies included in meta-analysis | Groups | Age (year) | Time till admission (minute) | Amount of AlP (gram) | SBP (90–120 mmHg). | DBP (60–80 mmHg) | Pulse (60–100 beat/ minute) | pH (7.35–7.45) | PaCO2 (35–45 mmHg). | HCO3 (22–26 mEq/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| Tehrani et al. 201311 | Control | 24.7 ± 6.4 | 78.9 ± 59.6 | 5.4 ± 3.3 | 87.3 ± 13.6 | ---- | 94.6 ± 21.1 | 7.35 ± .08 | ---- | 16.8 ± 4.1 |
| Intervention | 23.5 ± 7.8 | 102.1 ± 63 | 4.8 ± .9 | 93.7 ± 17.8 | ---- | 88.7 ± 17.1 | 7.36 ± .13 | ---- | 19.2 ± 5.8 | |
| Agarwal et al. 201412 | Control | 27.74 ± 8.86 | — | ---- | NS | NS | NS | 7.2 ± .2 | 33.7 ± 12 | 14.1 ± 7.8 |
| Intervention | — | — | 7.2 ± .2 | 31.3 ± 15.1 | 13.7 ± 6.1 | |||||
| Taghaddosinejad et al. 201613 | Control | 28.39 ± 1.11 | — | — | 106.63 ± 2.4 | — | 80.36 ± 3.9 | — | — | — |
| Intervention | 26.65 ± 1.06 | — | — | 100.15 ± 3.9 | — | 82.76 ± 3.1 | — | — | — | |
| Bahalla et al. 201714 | Control | S* | NS | — | NS | — | — | — | — | — |
| Intervention | — | — | — | — | — | — | ||||
| El-Ebiary & Abuelfadl 201715 | Control | NS | 168 | 1.680 | NS | NS | NS | 7.3 ± .1 | 23.8 ± 5.6 | — |
| Intervention | 180 | 1.680 | 7.4 ± .1 | 27.2 ± 8.1 | — | |||||
| Emam et al. 202016 | Control | 24.43 ± 9.66 | 70.8 ± 35 | NS | 94.83 ± 21.19 | 57.33 ± 15.44 | 104.27 ± 20.51 | 7.28 ± .13 | 30.43 ± 6.76 | 18.15 ± 4.40 |
| Intervention | 24.40 ± 10.55 | 75 ± 37 | NS | 92.16 ± 21.95 | 56.33 ± 16.00 | 104.57 ± 20.51 | 7.27 ± .14 | 30.46 ± 6.94 | 18.05 ± 4.24 | |
| Elgazzar et al. 201917 | Control | 23.9 ± 7.6 | 120 | 1.680 | NS | NS | NS | 7.21 ± .02 | 30.7 ± 9.4 | 13.9 ± 5.5 |
| Intervention | 22.9 ± 7.8 | 90 | 1.680 | 7.23 ± .10 | 35.0 ± 6.1 | 16.2 ± 5.1 | ||||
| Mabrouk et al. 201918 | Control | 26.6 | 120 | — | 89.5 | 57.5 | 97.2 | 7.2 ± .10 | 31.4 ± 9.1 | 14.9 ± 6.3 |
| Intervention | 25.3 | 120 | — | 96 | 56.7 | 96 | 7.3 ± .10 | 35.2 ± 5.6 | 17.1 ± 5.7 | |
| Halvaei et al. 201719 | Control | 26.31 ± 8.90 | 74.06 ± 43.29 | 2.64 | 89.81 ± 13.29 | 60.67 ± 1.91 | 95.07 ± 15.04 | 7.38 ± .09 | 27.81 ± 7.30 | 16.88 ± 4.24 |
| Intervention | 24.95 ± 8.11 | 98.25 ± 76.66 | 2.15 | 89.16 ± 17.95 | 55.76 ± 11.03 | 90.50 ± 16.68 | 7.40 ± .07 | 31.81 ± 7.06 | 20.06 ± 5.47 | |
| Darwish et al. 202020 | Control | 22.8 ± 7.03 | 193.2 ± 78 | 1.39 ± .6 | 91.43 ± 24.14 | 56.19 ± 17.46 | 108.33 ± 24.26 | 7.37 ± .10 | 27.87 ± 7.84 | 16.03 ± 4.47 |
| Intervention | 21.5 ± 4.98 | 147 ± 63 | 1.93 ± 1.2 | 90.00 ± 12.33 | 59.23 ± 12.30 | 96.47 ± 31.27 | 7.37 ± .14 | 26.97 ± 7.42 | 16.50 ± 4.27 |
NS, non-significant difference among categorized data of intervention and control group (mean values were not mentioned).
S*, significant difference among categorized data of intervention and control group (mean values were not mentioned).
Almost no statistical differences were recorded between each study’s control and intervention groups regarding baseline data. The classical clinical features of acute AlP poisoning were present in almost all studies, including decreased systolic and diastolic blood pressures and decreased pH and bicarbonate levels.
In addition, the studies investigated other laboratory parameters less relevant to acute AlP poisoning, which include liver enzymes [alanine transaminase (ALT), aspartate transaminase AST), and renal function test (creatinine, urea, blood urea nitrogen (BUN)]. There were no statistical differences between each study’s control and intervention groups regarding these parameters (Supplementary Table 1).
Outcome (Table 4)
Table 4.
Outcome of acute AlP-poisoned patients in the studies included in the meta-analysis.
| Studies included in meta-analysis | Groups | Mortality % | P | Intubation and mechanical ventilation % | P |
|---|---|---|---|---|---|
| Tehrani et al. 201311 | Control | 60 | < .05* | 73.30 | 0.040* |
| Intervention | 36 | 45.40 | |||
| Agarwal et al. 201412 | Control | 82 | 0.046* | — | — |
| Intervention | 54 | — | |||
| Taghaddosinejad et al. 201613 | Control | 43.47 | 0.36 | — | - |
| Intervention | 30.43 | — | |||
| Bahalla et al. 201714 | Control | 88.5 | 0.91 | — | - |
| Intervention | 87.5 | — | |||
| El-Ebiary & Abuelfadl 201715 | Control | 80 | 0.01* | 80 | 0.058 |
| Intervention | 33 | 47 | |||
| Emam et al. 202016 | Control | 43.30 | < .05* | 20.00 | 0.754 |
| Intervention | 20 | 23.30 | |||
| Elgazzar et al. 201917 | Control | 80 | 0.123 | 56 | 0.009* |
| Intervention | 60 | 20 | |||
| Mabrouk et al. 201918 | Control | 80 | 0.208 | 40 | 0.225 |
| Intervention | 64 | 24 | |||
| Halvaei et al. 201719 | Control | 50 | 0.02* | 62 | < .05* |
| Intervention | 15 | 30 | |||
| Darwish et al. 202020 | Control | 36.67 | 0.26 | 56.7 | 0.21 |
| Intervention | 23.33 | 40 |
*Significant difference between intervention and control group.
Out of 10 studies, 5 clinical studies showed significantly lower mortality percentages in intervention groups compared with control groups. These studies include Tehrani et al. (2013),11 Agarwal et al. (2014),12 El-Ebiary and Abuelfadl (2017),15 and Emam et al. (2020),16 which used NAC, and Halvaei et al. (2017),19 which use Vitamin E.
Out of seven studies, three clinical studies showed a significantly lower need for intubation and mechanical ventilation in intervention groups compared with control groups. These studies include Tehrani et al. (2013),11 which used NAC, Elgazzar et al. (2019),17 which used L-Carnitine, and Halvaei et al. (2017),19 which used Vitamin E.
Effects of antioxidants on outcomes of acute AlP poisoning
Primary outcome (mortality percentage)
Overall, 10 studies with 465 participants were included in the meta-analysis. The effect size of antioxidants was assessed at first pooling in all possible studies.
The statistical heterogeneity was assessed using Cochran’s Q test. Cochran’s Q value of 4.8 with P = .85 indicates no statistically significant heterogeneity of the intervention effects across the studies, I2 statistic that reflects the percentage of variation attributed to between-study heterogeneity showed 0% variation (I2 = .000, Tau2 = .000), So, the fixed-effect model was applied.
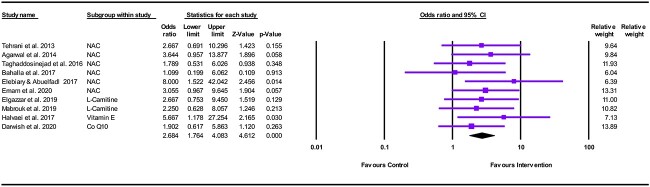
The meta-analysis showed a significant difference between antioxidants and control in favor of antioxidants in decreasing mortality among patients with acute AlP poisoning (OR = 2.684, 95% CI: 1.764–4.083; P < .001), as shown in Fig. 3.
Fig. 3.
Forest plot illustrating the effect of antioxidants on reducing mortality in acute AlP poisoning.
Subgroup analysis
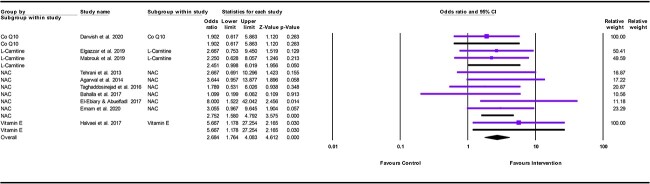
The meta-analysis showed a significant difference between antioxidants and control in favor of antioxidants in decreasing mortality among patients with acute AlP poisoning in both vitamin E, and NAC subgroups. Vitamin E significantly decreased the mortality by nearly six folds compared with the control (OR = 5.667, 95% CI: 1.178–27.254; P = .030). In addition, NAC significantly decreases the mortality by nearly three folds compared with the control (OR = 2.752, 95% CI: 1.580–4.792; P < .001). Nevertheless, the L-Carnitine subgroup showed a borderline statistical significance in decreasing mortality compared with the control (OR = 2.451, 95% CI: .998–6.019; P = .05). On the other hand, Co Q10 showed no statistically significant difference in reducing mortality (OR = 1.902, 95% CI: .617–5.863; P < .263) (Fig. 4).
Fig. 4.
Forest plot illustrating the effect of each type of antioxidant on reducing mortality in acute AlP poisoning (subgroup analysis).
Assessment of publication bias
The presence of publication bias was assessed visually by funnel plot. There was no visual asymmetry on visual inspection (Fig. 5). Statistically, Trim and fill analysis showed that when using the random effects model, no studies were missing to the left of the mean, suggesting no bias against the effect of antioxidants (adjusted effect size exactly as observed .98; 95% CI: .568: 1.47, P < .001).
Fig. 5.
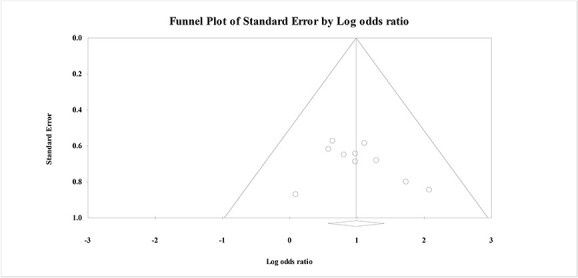
Funnel plot for the meta-analysis of the effects of antioxidants on reducing mortality in acute AlP poisoning.
Secondary outcome (need for intubation and mechanical ventilation)
The statistical heterogeneity was assessed using Cochran’s Q test. Cochran’s Q value of 6.6 with P = .356 indicates no statistically significant heterogeneity of the intervention effects across the studies, I2 statistic that reflects the percentage of variation attributed to between-study heterogeneity showed a 9.605% variation (I2 = 9.605, Tau2 = .043), So, the fixed-effect model was applied.
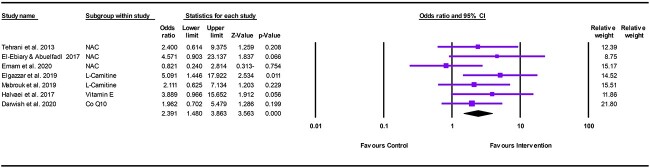
The meta-analysis showed a significant difference between antioxidants and control in favor of antioxidants in decreasing the number of patients with acute AlP poisoning who need intubation and mechanical ventilation (OR = 2.391, 95% CI: 1.480–3.863; P < .001) (Fig. 6).
Fig. 6.
Forest plot illustrating the effect of antioxidants on reducing the need for endotracheal intubation and mechanical ventilation in acute AlP poisoning.
Assessment of publication bias
The presence of publication bias was assessed visually by funnel plot. There was no visual asymmetry on visual inspection (Fig. 7). Statistically, Trim and fill analysis showed that when using the random effects model, no studies were missing to the left of the mean, suggesting no bias against the effect of antioxidants (adjusted effect size exactly as observed .88; 95% CI: .58: 1.65, P < .001).
Fig. 7.
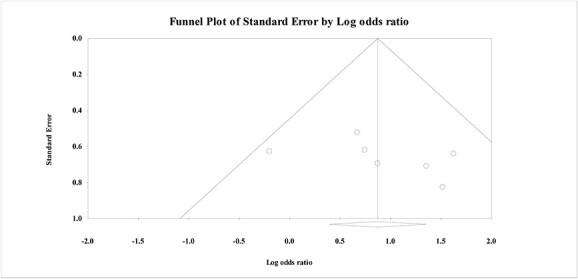
Funnel plot for the meta-analysis of the effects of antioxidants on reducing the need for endotracheal intubation and mechanical ventilation in acute AlP poisoning.
Discussion
Acute AlP poisoning remains one of the most gravid toxicities associated with poor prognosis. Currently, supportive measures are implemented empirically in attempts to rescue AlP-poisoned patients. Phosphine, the toxic constituent of AlP, is proven to exert its toxic effects through interference with cellular respiration and induction of oxidative stress.33,34 Thus, individual clinical studies have evaluated the efficacy of different antioxidants in improving the outcome of acute AlP poisoning. Each study applied a single antioxidant in a relatively limited number of AlP-poisoned patients.11–20 The results of these different studies did not yield an uninformed conclusion regarding the effect of antioxidants in improving the prognosis of acute AlP poisoning. Also, no previous studies compared the effects of different antioxidants in managing these cases.
A meta-analysis is a powerful statistical tool adopted to combine the results of multiple studies to provide a conclusion.35 The current study started with a comprehensive performance of a systematic review in compliance with the requirements of the reporting rules in PRISMA protocols. Then, all published clinical studies that used antioxidants in managing acute AlP poisoning were included in a meta-analysis according to the eligibility criteria.36
The meta-analysis encompasses 10 published articles that implemented four antioxidants. NAC was used in six studies; Tehrani et al. (2013),11 Agarwal et al. (2014),12 Taghaddosinejad et al. (2016),13 Bahalla et al. (2017),14 El-Ebiary and Abuelfadl (2017),15 and Emam et al. (2020).16 Two studies used L-Carnitine: Elgazzar et al. (2019)17 and Mabrouk et al. (2019).18 Although Vitamin E was used in a study by Halvaei et al. (2017),19 Co Q 10 was used in a study conducted by Darwish et al. (2020).20
Each antioxidant has a different mechanism by which it exerts its antioxidant effect. NAC is a potent antioxidant that acts as a disulfide bonds reductant, ROS scavenger, and glutathione precursor.37 L-Carnitine (4-N-trimethylammonium-3-hydroxybutyric acid) is a vital structure in mitochondrial membranes essential for cellular respiration. Also, L-Carnitine scavenger ROS and decreases lipid peroxidation.8,38,39 Vitamin E, or tocopherol, is a powerful antioxidant that decreases the generation of ROS during fat oxidation and free radical reactions.40 Considering Co Q10 or Ubiquinone, it is a constituent of mitochondrial membranes essential in oxidative phosphorylation and energy production at the cellular level. Also, Co Q10 selectively enhances systolic cardiac function.41,42
It was noticed that 6 studies out of 10 used NAC. The popularity of NAC could stand behind the increasing number of studies that used NAC in relation to other antioxidants. It is worth mentioning that NAC is a mucolytic commonly used to manage various bronchopulmonary diseases. In clinical toxicology, NAC is an effective antidote in treating acute acetaminophen poisoning. Also, NAC prevents the development of hepatotoxicity and cardiotoxicity following acute zinc phosphide poisoning.37 Thus, NAC is convenient in healthcare institutes, and prescribed for managing different disorders. In the field of research, NAC is one of the widely used antioxidants in in vitro and in vivo studies, as well as clinical studies.43 Most researchers considered NAC an ideal antioxidant that efficiently ameliorates oxidative stress.44,45
The clinical studies included in the meta-analysis were conducted in Egypt,46 Iran,4 and India47 that pointing to a high prevalence of acute pesticide poisoning, including AlP, in these developing agricultural countries. The mean ages of the patients in all studies were less than 30 years, which could be explained by the popularity of AlP as a suicidal agent among youth. Hypotensive shock and metabolic acidosis were evident in nearly all included studies on admission which represent classical manifestations of acute AlP poisoning. The statistical comparability between control and intervention groups was proved considering almost all baseline data of the included studies that ensure the reliability of their results.
The outcome of individual clinical studies highlighted the controversy regarding the ability of antioxidants to improve the outcome of acute AlP poisoning; therefore, this meta-analysis was conducted to formulate a universal conclusion in this context.
To ensure statistical inference, the current meta-analysis started with the assessment of the risk of bias for each included study using the modified Cochrane Collaboration tool.28 In addition, the heterogeneity was assessed and was found to be accepted. Lastly, publication bias was excluded as the funnel plot was symmetrical.31
The current meta-analysis proved that antioxidants significantly decreased the mortality of AlP-poisoned patients by nearly three folds. In addition, antioxidants significantly decrease the number of patients who need intubation and mechanical ventilation in relation to control by two folds.
Further subgroup analysis was done to evaluate the effect of each antioxidant on decreasing AlP-related mortality. The results revealed that Vit E and NAC significantly reduced mortality by almost six and three folds, respectively, compared with the control. However, a wide CI (1.178–27.254) and small relative weight (7.13%) could affect the reliability regarding the efficacy of vitamin E in decreasing the mortality of acute AlP poisoning, which needs future clinical trials and meta-analyses.
On the other hand, the L-Carnitine subgroup showed a borderline statistical significance in decreasing mortality compared with the control (P = .05). Also, Co Q10 decreased the mortality compared with the control; however, the differences were not statistically significant.
The current meta-analysis was restricted to the 10 clinical studies that complied with eligibility criteria and were published at the time of study conduction. This meta-analysis investigated the effects of four types of antioxidants, two of which, vitamin E and Co Q10, were represented by one study each. Many antioxidants’ efficacy in managing acute AlP poisoning, such as melatonin, vitamin C, and curcumin, could not be assessed. Thus, it is necessary to conduct clinical studies that implement various antioxidants in managing acute AlP poisoning. These future clinical studies should extend to investigating the efficacy of antioxidants not previously mentioned in published clinical trials, along with promising antioxidants that are not extensively investigated, such as L-Carnitine, vitamin E, and Co Q10. The availability of a large number of clinical studies will enable the conduct of more comprehensive meta-analyses in the future.
Despite the current study being limited to 10 available studies, it provides solid evidence regarding the efficacy of antioxidants in improving the prognosis of AlP-poisoned patients. This meta-analysis recommends the addition of NAC to national and international guidelines for the management of acute AlP poisoning. This research is the first meta-analysis to investigate the effect of one of the treatment modalities of acute AlP poisoning. Therefore, further meta-analyses are needed to verify the efficacy of all other treatment modalities in improving the patients’ outcome, then the adoption of evidence-based guidelines for managing acute AlP poisoning will be possible.
Conclusions
The current meta-analysis pooled the evidence available from published clinical studies for the effects of antioxidants in improving the outcome of AlP-poisoned patients. Meta-analysis proved that antioxidants decrease mortality of acute AlP poisoning three folds in relation to control. Subgroup analysis proposed NAC as the best antioxidant that reduced mortality by three folds. Also, antioxidants significantly decrease the need for intubation and mechanical ventilation by two folds. This meta-analysis provides solid evidence regarding the therapeutic benefit of antioxidants on the outcome of acute AlP poisoning. Subsequently, based on this meta-analysis, NAC should be included as a standard treatment in treating acute AlP-poisoned patients.
Supplementary Material
Acknowledgment
Our most profound appreciation goes to the authors of the researches included in the current meta-analysis. This study couldn’t be conducted without their valuable literature.
Contributor Information
Zahraa K Sobh, Forensic Medicine and Clinical Toxicology Department, Faculty of Medicine, Alexandria University Alexandria, 21517, Egypt.
Asmaa Abd-Elhameed, Biomedical Informatics and Medical Statistics Department, Medical Research Institute, Alexandria University, Alexandria 21524, Egypt.
Author contribution statement
Dr Zahraa Khalifa Sobh contributed to the research concept, work design, advanced search of databases, data extraction, results interpretation, and manuscript drafting. Dr Asmaa Abd Elhameed Ahmed contributed to the advanced search of databases, data extraction, statistical analysis, results interpretation, and manuscript drafting. All authors revised and approved the final manuscript.
Funding
None.
Conflict of Interest statement. The authors declare that there is no conflict of interest.
Data availability
The dataset used in the study is available from the corresponding author upon reasonable request.
References
- 1. Abdelhamid WG, Wahdan MM, Zaafar D. Acute toxic exposures in Egypt population: analysis of a five-year registry from 2015 to 2019. Toxicol Environ Health Sci. 2022:14(3):235–244. [Google Scholar]
- 2. Haghi Aminjan H, Abtahi SR, Hazrati E, Chamanara M, Jalili M, Paknejad B. Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci. 2019:232:116607. 10.1016/j.lfs.2019.116607. [DOI] [PubMed] [Google Scholar]
- 3. Naddafi M, Eghbal MA, Ghazi Khansari M, Sattari MR, Azarmi Y, Samadi M, Mehrizi AA. Sensing of oxidative stress biomarkers: the cardioprotective effect of taurine & grape seed extract against the poisoning induced by an agricultural pesticide aluminum phosphide. Chemosphere. 2022:287(3):132245. [DOI] [PubMed] [Google Scholar]
- 4. Dorooshi G, Mirzae M, Fard NT, Zoofaghari S, Mood NE. Investigating the outcomes of aluminum phosphide poisoning in Khorshid referral hospital, Isfahan, Iran: a retrospective study. J Res Pharm Pract. 2021:10(4):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naddafi M, Mehrizi AA, Eghbal MA, Khansari MG, Azarmi Y, Sattari MR, Karaman C, Karimi F, Alizadeh M, Yazdani MN, et al. . Reducing the risk of death induced by aluminum phosphide poisoning: the new therapies. Chemosphere. 2022:294:133800. [DOI] [PubMed] [Google Scholar]
- 6. Ghonem MM, El Sharkawy SI, Lashin HI. Predictive variables of acute aluminum phosphide poisoning outcome: a new proposed model. Egypt J Forensic Sci. 2020:20(2):45–60. [Google Scholar]
- 7. El Shehaby DM, Sayed SA, Abd El-Kareem DM, Elsherif R, Almaz D. Trimetazedine with hyperinsulinimea–euoglycemia, N-acetyl cysteine, and vitamin C: a new approach concept for management of aluminum phosphide poisoning. J Biochem Mol Toxicol. 2022:36(1):e22931. [DOI] [PubMed] [Google Scholar]
- 8. Abdelhamid WG, Sakr ML, Mostafa OE, Zaafar D, Abdelwahab HM. Comparing the effectiveness of L-carnitine and paraffin oil in acute aluminum phosphide poisoning using predictive biomarkers and scores: a randomized controlled clinical trial. Hum Exp Toxicol. 2023:42:9603271221149650. [DOI] [PubMed] [Google Scholar]
- 9. Mashali AA, Salama NH, Elsobky HA, Sobh ZK. Prediction of zinc phosphide-induced hepatotoxicity and cardiotoxicity from clinical, laboratory, and radiological indicators. Environ Sci Pollut Res Int. 2020:27(31):39547–39559. [DOI] [PubMed] [Google Scholar]
- 10. Helal NE, Lashin HI, Nagy AA, Shama MA, Mostafa TAH, Wahdan AA. Potential role of paraffin oil gastric lavage in acute aluminum phosphide poisoning: a randomized controlled trial. Environ Sci Pollut Res. 2022:29(22):33844–33855. [DOI] [PubMed] [Google Scholar]
- 11. Tehrani H, Halvaie Z, Shadnia S, Soltaninejad K, Abdollahi M. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clin Toxicol. 2013:51(1):23–28. [DOI] [PubMed] [Google Scholar]
- 12. Agarwal A, Robo R, Jain N, Gutch M, Consil S, Kumar S. Oxidative stress determined through the levels of antioxidant enzymes and the effect of N-acetylcysteine in aluminum phosphide poisoning. Indian J Crit Care Med. 2014:18(10):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taghaddosinejad F, Farzaneh E, Ghazanfari-Nasrabad M, Eizadi-Mood N, Hajihosseini M, Mehrpour O. The effect of N-acetyl cysteine (NAC) on aluminum phosphide poisoning inducing cardiovascular toxicity: a case-control study. Springerplus. 2016:5(1):1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhalla A, Jyothinath P, Singh S. Antioxidant therapy in patients with severe aluminum phosphide poisoning: a pilot study. Indian J Crit Care Med. 2017:21(12):836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Ebiary A, Abuelfad A. N-acetylcysteine as an adjuvant in the treatment of acute aluminum phosphide poisoning: a randomized clinical trial. Ain-Shams J Forensic Med Clin Toxicol. 2017:28(1):38–46. [Google Scholar]
- 16. Emam N, Shaaban S, Ahmed D, Could N. Acetyl cysteine prolong survival time in acute aluminum phosphide poisoning among Egyptian patients? Egypt Soc Clin Toxicol J. 2020:8(2):1–12. [Google Scholar]
- 17. Elgazzar F, Keshk W, Khalifa H. Early L-carnitine therapy in severe acute aluminum phosphide poisoning: a randomized controlled clinical trial. Egypt J Forensic Sci Appli Toxicol. 2019:19(2):147–164. [Google Scholar]
- 18. Mabrouk H, Abuelfadl A, Elkelany R, Eldakroory S, El-Bassuony A. L-carnitine in acute phosphide pesticide poisoning: a randomized, clinical trial. Nat Sci. 2019:17(8):86–91. [Google Scholar]
- 19. Halvaei Z, Tehrani H, Soltaninejad K, Abdollahi M, Shadnia S. Vitamin E as a novel therapy in the treatment of acute aluminum phosphide poisoning. Turk J Med Sci. 2017:47(3):795–800. [DOI] [PubMed] [Google Scholar]
- 20. Darwish RT, Sobh ZK, Hamouda EH, Saleh EM. The efficacy of coenzyme Q10 and liquid paraffin oil in the management of acute aluminum phosphide poisoning. Toxicol Res. 2020:9(4):444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bagherian F, Kalani N, Rahmanian F, Abiri S, Hatami N, Foroughian M, Mehramiz NJ, Shahi B. Aluminum phosphide poisoning mortality rate in Iran; a systematic review and meta-analysis. Archmeta-analysis Acad Emerg Med. 2021:9(1):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stephens ML, Betts K, Beck NB, Cogliano V, Dickersin K, Fitzpatrick S, Freeman J, Gray G, Hartung T, McPartland J, et al. . The emergence of systematic review in toxicology. Toxicol Sci. 2016:152(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wikoff DS, Miller GW. Systematic reviews in toxicology. Toxicol Sci. 2018:163(2):335–337. [DOI] [PubMed] [Google Scholar]
- 24. Stegenga J. Is meta-analysis the platinum standard of evidence? Stud Hist Philos Sci C. 2011:42(4):497–507. [DOI] [PubMed] [Google Scholar]
- 25. Lin YC, Liang YJ, Yang HC. Evaluating statistical significance in a meta-analysis by using numerical integration. Comput Struct Biotechnol J. 2022:20:3615–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021:10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sohrabi C, Franchi T, Mathew G, Kerwan A, Nicola M, Griffin M, Agha M, Agha R. PRISMA 2020 statement: what's new and the importance of reporting guidelines. Int J Surg. 2021:88:105918. [DOI] [PubMed] [Google Scholar]
- 28. Bo A, Hai AH, Chen DG, Hammock K. Risk of bias assessments in systematic reviews and meta-analyses of behavioral interventions for substance use outcomes. J Clin Epidemiol. 2021:139:20–27. [DOI] [PubMed] [Google Scholar]
- 29. Lin L. Comparison of four heterogeneity measures for meta-analysis. J Eval Clin Pract. 2020:26(1):376–384. [DOI] [PubMed] [Google Scholar]
- 30. Dettori JR, Norvell DC, Chapman JR. Seeing the forest by looking at the trees: how to interpret a meta-analysis forest plot. Global Spine J. 2021:11(4):614–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018:74(3):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flemyng E, Dwan K, Moore THM, Page MJ, Higgins JPT. Risk of bias 2 in Cochrane reviews: a phased approach for the introduction of new methodology. Cochrane Database Syst Rev. 2020:(11):ED000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheta AA, El-Banna AS, Elmeguid RA, Mohamed HE, Gad NH. A study of the predictive factors of mortality in acute poisoning with aluminum phosphide with special reference to echocardiography and SOFA score. Environ Sci Pollut Res. 2019:26(32):33135–33145. [DOI] [PubMed] [Google Scholar]
- 34. Hosseini SF, Forouzesh M, Maleknia M, Valiyari S, Maniati M, Samimi A. The molecular mechanism of aluminum phosphide poisoning in cardiovascular disease: pathophysiology and diagnostic approach. Cardiovasc Toxicol. 2020:20(5):454–461. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez AV, Marti KM, Roman YM. Meta-analysis. Chest. 2020:158(1s):97–102. [DOI] [PubMed] [Google Scholar]
- 36. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group . PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. J Med Libr Assoc. 2021:109(2):174–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwalfenberg GK. N-Acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J Nutr Metab. 2021:2021:9949453. 10.1155/2021/9949453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghonem MM, Lashin HI, Hodeib AA, Soliman NA. L-carnitine as an adjuvant treatment in acute organophosphorus pesticides poisoning: a randomized clinical trial. MJFMCT. 2018:26(2):37–52. [Google Scholar]
- 39. Sherif NA, El-Banna AS, ElBourini MM, Khalil NO. Efficacy of L-carnitine and propranolol in the management of acute theophylline toxicity. Toxicology research. 2020:9(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niki E, Noguchi N. Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic Res. 2021:55(4):352–363. [DOI] [PubMed] [Google Scholar]
- 41. Mwaeni VK, Nyariki JN, Jillani N, Omwenga G, Ngugi M, Isaac AO. Coenzyme Q10 protected against arsenite and enhanced the capacity of 2,3-dimercaptosuccinic acid to ameliorate arsenite-induced toxicity in mice. BMC Pharmacol Toxicol. 2021:22(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sifuentes-Franco S, Sánchez-Macías DC, Carrillo-Ibarra S, Rivera-Valdés JJ, Zuñiga LY, Sánchez-López VA. Antioxidant and anti-inflammatory effects of coenzyme Q10 supplementation on infectious diseases. Healthcare. 2022:10(3):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El-Banna AS, Badr El Dine FMM, Abd El Halim R. Assessment of protective role of green tea extract (GTE) and n-acetylcysteine (NAC) against the potential genotoxicity of nicotine in adult male albino rats. Egypt. J Forensic Sci. 2019:19(1):1–16. [Google Scholar]
- 44. El-Ebiary AA, Elsharkawy RE, Soliman NA, Soliman MA, Hashem AA. N-acetylcysteine in acute organophosphorus pesticide poisoning: a randomized, clinical trial. Basic Clin Pharmacol Toxicol. 2016:119(2):222–227. [DOI] [PubMed] [Google Scholar]
- 45. Pedre B, Barayeu U, Ezeriņa D, Dick TP. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol Ther. 2021:228:107916. 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- 46. Deraz RH, Elrafey DS, Mesallam DIA. Acute aluminium phosphide poisoning in East Delta, Egypt: a growing public health problem over the last five years. ESCTJ. 2022:10(1):49–61. [Google Scholar]
- 47. Bonvoisin T, Utyasheva L, Knipe D, Gunnell D, Eddleston M. Suicide by pesticide poisoning in India: a review of pesticide regulations and their impact on suicide trends. BMC Public Health. 2020:20(1):251. 10.1186/s12889-020-8339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in the study is available from the corresponding author upon reasonable request.