Abstract
Osimertinib, a third-generation EGFR TKI, is the standard therapy for previously untreated EGFR-mutated non-small cell lung cancer patients following the landmark FLAURA study. However, resistance inevitably hinders patient prognosis, increasing the need for new therapeutic strategies beyond osimertinib. Frontline osimertinib-based combination strategies (platinum-based chemotherapy and angiogenesis inhibitors) are currently being tested primarily to prevent initial resistance. In the later-line setting after osimertinib, many next-line therapeutic candidates have been actively examined in clinical trials. Notably, several drugs with novel mechanisms of action, such as antibody–drug conjugates and EGFR -MET bispecific antibodies, have shown promising efficacy despite the resistance mechanisms and are close to clinical application. In addition, genotype-based target strategies have been investigated for a better understanding of osimertinib resistance mechanisms based on molecular profiling tests at relapse. The C797S mutation and MET gene alterations are commonly identified following osimertinib resistance, for which targeting strategies are actively tested. This review describes current pharmacotherapeutic strategies for EGFR-mutated non-small cell lung cancer based on the results of clinical trials and the latest published data, broadly grouped into two sections: 1) EGFR TKIs-based combination therapy in the front-line setting and 2) novel therapeutic strategies after osimertinib resistance.
Keywords: lung cancer, EGF receptor, tyrosine kinase inhibitors, precision medicine
The pharmacotherapeutic management of epidermal growth factor receptor-mutated advanced non-small cell lung cancer has been rapidly updated to a new phase with the advent of genomic medicine.
Introduction
Epidermal growth factor receptor (EGFR) mutation was the first driver oncogene identified in lung cancer (1). The development of EGFR tyrosine kinase inhibitors (TKIs) targeting activating EGFR mutations has led to a paradigm shift in pharmacotherapy for patients with advanced non-small cell lung cancer (NSCLC). Several other driver oncogenes have been discovered in NSCLC, and the corresponding molecular targeting agents are preferentially used in clinical practice (2).
EGFR TKIs are the major first-line (1L) therapy for patients with advanced NSCLC with activating EGFR mutations. Substantial evidence for EGFR TKI has historically been established in randomized controlled trials, demonstrating superior efficacy to conventional platinum-based chemotherapy. Gefitinib (3,4), erlotinib (5–7) and afatinib (8,9) have shown survival benefits over standard-of-care platinum-based chemotherapy in the 1L setting, with better response and significantly improved progression-free survival (PFS). Subsequently, studies were conducted for a head-to-head comparison of EGFR TKIs. Second-generation (2G) TKIs, afatinib and dacomitinib, were compared with the first-generation (1G) TKI, gefitinib, in randomized controlled trials, demonstrating prolonged PFS (10,11); however, both were more toxic than gefitinib (10–13).
Osimertinib is an irreversible third-generation (3G) EGFR TKI that selectively inhibits sensitizing EGFR and T790M resistance mutations (14). In the phase III AURA3 trial, osimertinib showed better efficacy over platinum-based chemotherapy for T790M-positive NSCLC patients previously treated with prior-generation EGFR TKIs approved as salvage therapy in this setting (15). Later, the phase III FLAURA trial demonstrated significantly prolonged PFS in the osimertinib arm compared with the gefitinib or erlotinib arm [18.9 vs. 10.2 months; hazard ratio (HR), 0.46; 95% confidence interval (CI), 0.37–0.57] in the 1L setting (16); therefore, osimertinib was also approved as a 1L regimen for EGFR-mutated advanced NSCLC. Furthermore, constant superiority in overall survival (OS) was observed in the osimertinib arm until the final OS analysis (38.6 vs. 31.8 months; HR, 0.80; 95% CI, 0.64–1.00) despite crossover to osimertinib being permitted in the comparator TKI arm upon T790M emergence at disease progression (17). In addition, osimertinib had a higher response rate to central nervous system (CNS) lesions and showed a reduced risk of CNS progression (18,19). Regarding toxicity, osimertinib was relatively well tolerated, with a comparable rate of grade ≥3 adverse events to the comparator EGFR TKIs, and no serious safety concerns were observed (16,17). This evidence supports the validity of choosing 1L treatment for EGFR-mutated advanced NSCLC patients, boosting osimertinib to a forefront agent in this setting.
Despite the robust evidence established by the AURA3 and FLAURA trials, most patients eventually develop osimertinib resistance. However, the next-line therapy for osimertinib resistance is yet to be confirmed. Therefore, it is necessary to establish effective and novel therapeutic strategies for this population. To date, various treatment strategies have been investigated in the clinic to address osimertinib resistance and improve patient prognosis. This review focuses on current therapeutic strategies for EGFR-mutated NSCLC, EGFR TKI-based combination strategies to outperform osimertinib monotherapy and the next-line novel drug candidates following 1L osimertinib, based on data from ongoing clinical trials and published literature.
EGFR TKI-based combination strategy
Osimertinib monotherapy prolonged PFS and OS in a 1L setting (16,17); however, there is room for improvement in survival outcomes. A strategy to either prevent or postpone resistance acquisition to 1L EGFR TKI is desired, offering a combination strategy of EGFR TKI with other anticancer agents. Furthermore, the organ-specific efficacy properties might also allow for a combination strategy after 1L EGFR TKI resistance. This section describes the current status of frontline combination strategies for EGFR-mutated advance NSCLC patients.
Combining EGFR TKI with platinum-based chemotherapy
Intratumoral and intrapatient molecular heterogeneity within EGFR-mutated NSCLC may be associated with intrinsic resistance to EGFR TKI. It has been assumed that EGFR TKI monotherapy cannot treat innate TKI-resistant cancer cells (20). In theory, a combination of cytotoxic chemotherapeutic agents with different mechanisms of action could regulate intratumor heterogeneity and delay resistance to EGFR TKIs (21,22). Therefore, combination strategies of EGFR TKI and platinum-based chemotherapy have been investigated.
This combination strategy for EGFR-mutated advanced NSCLC patients was examined before the launch of osimertinib. Therapeutic schemes, including the timing of platinum-based chemotherapy, vary across trials. The phase III IMPRESS trial assessed continuing gefitinib combined with cisplatin plus pemetrexed compared with chemotherapy alone when the disease progressed with 1L gefitinib. It failed to demonstrate the PFS benefit of continuing gefitinib [median PFS, 5.4 vs. 5.4 months; HR (95% CI), 0.86 (0.65–1.13)] (23), and OS was inferior in the continuing group [median OS, 13.4 vs. 19.5 months; HR (95% CI), 1.44 (1.07–1.94)] (24), showing the disadvantage of continuing gefitinib with cisplatin plus pemetrexed at disease progression on 1L gefitinib.
In contrast, a concurrent combination of gefitinib with platinum-based chemotherapy as a 1L regimen is an effective approach. In the phase III NEJ009 trial, gefitinib in combination with carboplatin plus pemetrexed (CGP) was compared with gefitinib alone in a 1L setting (25). PFS was significantly prolonged in the CGP group (median, 20.9 vs. 11.9 months; HR, 0.49; 95% CI, 0.39–0.62) (25), and OS was also extended in the CGP group (median, 49.0 vs. 38.5 months, HR, 0.82; 95% CI, 0.64–1.06) (26). Another phase III trial conducted in India to compare CGP and gefitinib alone in a 1L setting showed significantly improved PFS (median, 16 vs. 8 months; HR, 0.51; 95% CI, 0.39–0.66) and OS (median, not reached vs. 20.5 months; HR, 0.45; 95% CI, 0.31–0.65) (27). These results supported that the CGP regimen would be an effective 1L approach for EGFR-mutated advanced NSCLC patients.
Following the approval of 1L osimertinib, these combination strategies were investigated by replacing gefitinib with osimertinib. A combination strategy following osimertinib resistance in a scheme analogous to that in the IMPRESS trial is being investigated based on the higher CNS activity of osimertinib. In the phase III COMPEL trial (NCT04765059), patients with non-CNS progression after initial response to 1L osimertinib were randomized to platinum-pemetrexed plus osimertinib or placebo groups. The primary endpoint will be PFS, stratified by the presence or absence of stable CNS metastases (28). The randomized phase II TORG1938/EPONA trial (jRCTs071200029) being conducted in Japan is evaluating the efficacy of platinum-pemetrexed with or without continuation of osimertinib. In this trial, the feasibility of platinum-pemetrexed plus osimertinib for non-CNS progression after initial response to osimertinib in patients with brain metastatic EGFR-mutated NSCLC is being investigated with PFS as the primary endpoint (29). These trials will elucidate the plausibility of the beyond progressive disease (PD) combination strategy during the progression of extracranial lesions after 1L osimertinib.
The concurrent strategy of osimertinib plus platinum-based chemotherapy is also being tested. The phase II OPAL trial evaluated the safety and efficacy of osimertinib plus platinum-pemetrexed in the 1L setting, reporting an objective response rate (ORR) of 90.9% (95% CI, 84.0–97.8), median PFS of 31.0 months (95% CI, 26.8 months-not reached) and an acceptable safety profile (30). Moreover, the phase III FLAURA2 trial (NCT04035486) is investigating the efficacy of 1L osimertinib with or without platinum-pemetrexed chemotherapy for EGFR-mutated locally advanced or metastatic NSCLC with 587 patients (31). In the 30 patients who participated in the safety run-in component of the trial, no specific pattern of toxicities was reported in the combination therapy group (32). The initial data cutoff for the primary endpoint of PFS was April 3, 2023. Another treatment scheme involving the insertion of platinum pemetrexed during EGFR TKI therapy is also under investigation. The JCOG1404/WJOG8214L (UMIN000020242) is a randomized phase III trial that compares 1L osimertinib (or gefitinib) and inserted cisplatin plus pemetrexed with osimertinib (or gefitinib) alone for patients with advanced EGFR-mutated NSCLC (33). In the experimental group, gefitinib or osimertinib was administered on days 1–56. After a drug-free period of 2 weeks, three cycles of cisplatin and pemetrexed were administered on days 71, 92 and 113. Gefitinib or osimertinib was reinitiated on day 134 and continued until the disease progressed. The investigators hypothesized that initiating platinum doublet chemotherapy after the initial response to EGFR TKIs might prevent the emergence of acquired resistance to EGFR TKIs and increase patient survival. Unlike the FLAURA2 trial, OS is the primary endpoint of the JCOG1404/WJOG8214L trial. Patient enrollment was completed, and the results are expected to be published shortly. These trials provide instructive information regarding the optimal timing for combining 1L of osimertinib with platinum-based chemotherapy.
Overall, the combination strategy of EGFR TKI and platinum-based chemotherapy is feasible because the CGP regimen has shown survival benefits as a 1L regimen in randomized phase III trials. However, increased toxicities associated with the addition of chemotherapy should be considered (23–27).
To date, the superiority of combination regimens over osimertinib monotherapy is questionable; therefore, progress in ongoing randomized trials comparing osimertinib plus chemotherapy with osimertinib will draw further clinical interest.
Combining EGFR TKI with VEGF inhibitor
Combination therapies using EGFR TKI and angiogenesis inhibitors have also been extensively investigated. EGFR activation and VEGF expression are reportedly closely interrelated (34). These have common downstream pathways, and signaling from both can stimulate their shared pathway and act in a protumorigenic manner (35–38). Therefore, EGFR-VEGF dual inhibition is considered a rational strategy in EGFR-mutated NSCLC.
Several clinical studies have reported that adding the anti-VEGF antibody, bevacizumab, to the 1G EGFR TKI, erlotinib, prolonged PFS but not OS. A randomized phase II JO25567 trial first reported improved PFS of erlotinib plus bevacizumab over erlotinib alone in previously untreated EGFR-mutated NSCLC patients (median PFS, 16.0 vs. 9.7 months; HR, 0.54; 95% CI, 0.36–0.79) (39). The efficacy of this combination therapy was validated in subsequent phase III trials. The NEJ026 and ARTemis/CTONG1509 trials successfully demonstrated the prolonged PFS with erlotinib plus bevacizumab over erlotinib (NEJ026: median PFS, 16.9 vs. 13.3 months; HR, 0.605; 95% CI, 0.417–0.877 (40)) (ARTemis/CTONG1509: median PFS, 17.9 vs. 11.2 months, HR: 0.55; 95% CI, 0.41–0.73 (41)). In addition, subgroup analysis in both trials for types of EGFR mutations showed a PFS benefit in patients with exon19 deletion and patients with the L858R mutation, a population known to have a reduced response to EGFR TKIs.
The RELAY trial is a global, placebo-controlled, double-blinded phase III trial conducted to assess the efficacy of erlotinib plus the anti-VEGFR 2 antibody, ramucirumab, compared with erlotinib plus placebo in previously untreated advanced NSCLC patients with activating EGFR mutations (42). A total of 449 patients, of whom 336 East Asian, were enrolled. PFS, as the primary endpoint, was significantly improved with erlotinib plus ramucirumab compared with erlotinib plus placebo (median, 19.4 vs. 12.4 months; HR, 0.59; 95% CI, 0.46–0.76). A PFS benefit was consistently observed, irrespective of the EGFR mutation (exon19 deletion or L858R). Moreover, the frequency of acquiring the T790M mutation upon disease progression was similar in the two groups, suggesting that adding ramucirumab did not affect T790M emergence.
In summary, the EGFR-VEGF dual-inhibition strategy confers a PFS benefit for patients with advanced EGFR-mutated NSCLC in a 1L setting. It was also expected to be effective in patients with the L858R mutation based on the results of the subgroup analysis. However, these combinations have yet to improve OS (43,44). Patients receiving a combination regimen have a higher incidence of grade ≥3 treatment-related adverse events (40–42,45), and adverse events specific to angiogenesis inhibitors should be considered.
Currently, combination therapy with osimertinib, bevacizumab or ramucirumab in a 1L setting is being investigated. A phase I/II trial by Yu et al. evaluated osimertinib plus bevacizumab in the 1L setting with 49 patients. The study met the primary endpoint with a 12-month PFS rate of 76% (95% CI, 65–90%), an overall response rate of 80% (95% CI, 67–91%) and a CNS response rate of 100% (6 of 6; 95% CI, 50–100% (46)). However, the randomized phase II WJOG9717L trial, which investigated osimertinib with or without bevacizumab in previously untreated EGFR-mutated NSCLC, failed to show improved PFS with the combination regimen (combination vs. osimertinib alone: median PFS, 22.1 vs. 20.1 months; HR, 0.862; 95% CI, 0.531–1.397 (47)).
Regarding the feasibility of this combination, it will be necessary to await the results of ongoing randomized trials. Clinical trials of osimertinib-based combination regimens are summarized in Table 1.
Table 1.
Summary of clinical trials on osimertinib-based combination strategy
| Agent | Trial number (Trial name) | Phase | Number of patients | Arm | Year | Status |
|---|---|---|---|---|---|---|
| EGFR TKI + platinum-based chemotherapy | ||||||
| NCT04410796 | II | 571 | Osimertinib Osimertinib + CBDCA + PEM |
2020 | Recruiting | |
| NCT04695925 (TOP) | III | 291 | Osimertinib Osimertinib + CBDCA + PEM *concurrent TP53 mutation |
2021 | Not yet recruiting | |
| NCT04769388 (FLAME) | II | 150 | Osimertinib Osimertinib + CBDCA + PEM *3 weeks after 1 L osimertinib without PD |
2021 | Recruiting | |
| NCT04765059 (COMPEL) | III | 204 | Osimertinib + CBDCA/CDDP + PEM Placebo + CBDCA/CDDP + PEM *PD after 1 L osimertinib |
2021 | Recruiting | |
| jRCTs071200029 (TORG1938/EPONA) | II | 92 | Osimertinib + CBDCA/CDDP + PEM CBDCA/CDDP + PEM *PD after osimertinib |
2020 | Recruiting | |
| NCT05281406 (PACE-LUNG) | II | 50 | Osimertinib + CBDCA/CDDP + PEM *ctDNA EGFRm after 3w 1 L osimertinib |
2022 | Recruiting | |
| NCT05507606 | II | 50 | Osimertinib + bevacizumab + CBDCA + PEM *concurrent TP53 mutation |
2022 | Recruiting | |
| NCT04035486 (FLAURA2) | III | 587 | Osimertinib Osimertinib + CBDCA/CDDP + PEM |
2019 | Active, not recruiting | |
| NCT04335292 (OCELOT) | II | 200 | Osimertinib → CBDCA/CDDP+PEM → osimertinib | 2020 | Recruiting | |
| NCT05493501 | III | 420 | Aumolertinib Aumolertinib + CBDCA/CDDP + PEM Osimertinib |
2022 | Recruiting | |
| UMIN000020242 (JCOG1404/WJOG8214L, AGAIN) | III | 500 | Osimertinib (gefitinib) Osimertinib (gefitinib) → CDDP + PEM → osimertinib (gefitinib) |
2015 | Active, not recruiting | |
| EGFR TKI + VEGF inhibitor | ||||||
| Bevacizumab | NCT02971501 | II | 112 | Osimertinib Osimertinib + bevacizumab |
2016 | Active, not recruiting |
| NCT04181060 | III | 300 | Osimertinib Osimertinib + bevacizumab |
2019 | Recruiting | |
| NCT04988607 (FLAIR) | II | 90 | Osimertinib Osimertinib + bevacizumab *patients with EGFR L858R only |
2021 | Not yet recruiting | |
| Ramucirumab | NCT03909334 | II | 150 | Osimertinib Osimertinib + ramucirumab |
2019 | Recruiting |
| JapicCTI-184 146 (TORG1833) | II | 120 | Osimertinib Osimertinib + ramucirumab |
2018 | Progressing Recruitment completed |
|
| EGFR-MET bispecific antibody | ||||||
| Amivantamab | NCT04487080 (MARIPOSA) | III | 1074 | Amivantamab + lazertinib Osimertinib + placebo Lazertinib + placebo |
2020 | Active, not recruiting |
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CBDCA, carboplatin: CDDP, cisplatin; PEM, pemetrexed; PD, progressive disease.
Novel therapeutic strategy beyond osimertinib
Several mechanisms underlying acquired resistance to osimertinib have been identified. They can be broadly classified into EGFR-dependent (on-target) and EGFR-independent (off-target) resistance mechanisms. In on-target resistance, cell proliferation and tumor growth are driven by sustained activation of EGFR signaling, including secondary EGFR mutations, amplification, mutation loss and ligand overexpression. Conversely, in off-target resistance, tumor growth depends on pathways other than EGFR signaling, such as alternative pathway kinase activation, downstream signaling pathway activation, histological transformation and cell cycle gene alterations (Fig. 1) (48–52).
Figure 1.
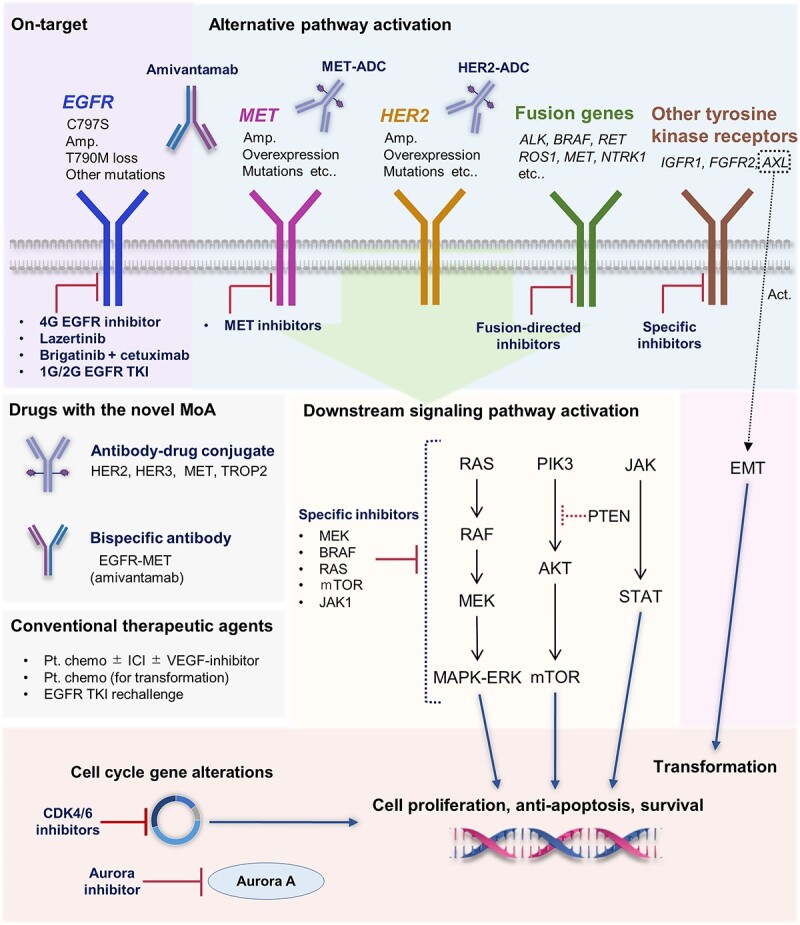
Overview of osimertinib resistance mechanisms and corresponding treatment options. Diverse targeting strategies might be available depending on the resistance mechanism. Abbreviations: 4G, fourth generation; 1G, first generation; 2G, second generation; TKI, tyrosine kinase inhibitor; MoA, mechanism of action; ADC, antibody–drug conjugate; amp., amplification; Pt. chemo, platinum-based chemotherapy; ICI, immune checkpoint inhibitor.
Available data regarding the resistance mechanisms to osimertinib, based on plasma and/or tissue biomarker analyses, have been compiled from several clinical and observational studies, including the AURA3 and FLAURA trials. Based on these studies, the frequency and description of osimertinib resistance mechanisms are summarized in Fig. 2 (53–61). EGFR C797S and MET amplifications are relatively common after the 1L and later-line osimertinib therapies and are considered important therapeutic targets. Other resistance mechanisms have been identified, including alterations in the RAS/MAPK and PIK3/AKT pathways, cell cycle gene alterations, oncogenic fusions and histological transformations (53–61).
Figure 2.
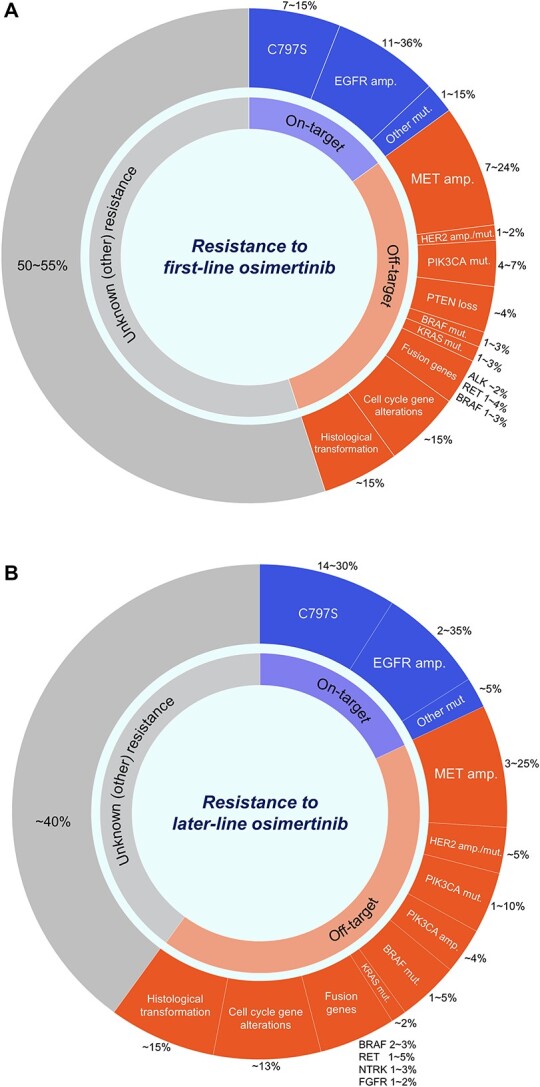
Breakdown of frequency and types of major resistance mechanisms to first-line (A) and later-line (B) osimertinib treatment based on previous reports (53–61). Note: different molecular aberrations might have been identified in the same patient. Abbreviations: amp., amplification; mut., mutation.
Along with the growing understanding of osimertinib resistance mechanisms, next-line novel therapeutic agents are being developed and intensively examined in clinical trials. Progress in ongoing clinical trials has shown promising efficacy of several novel agents such as a HER3-directed antibody–drug conjugate (ADC) and an EGFR-MET bispecific antibody in osimertinib-resistant patients (62). Importantly, these agents reportedly exhibit antitumor activity regardless of resistance mechanisms and are nearing clinical application. Other genotype-based strategies, focusing on EGFR C797S and MET gene alterations, are also being examined, highlighting the importance of molecular testing at relapse. This section provides an overview of the current status of novel therapeutic strategies following osimertinib resistance.
Antibody–drug conjugate
ADC is a novel drug class belonging to the immunotherapeutic agent category, which is rapidly emerging in oncology and especially lung cancer (63). ADCs are designed to bind a cytotoxic payload through a linker to a specific monoclonal antibody directed against a tumor antigen. Internalization occurs once the monoclonal antibody binds to the targeted antigen, resulting in linker degeneration and release of the cytotoxic payload, thereby exerting an antitumor effect (64,65). ADCs targeting HER2, HER3, TROP-2 and MET have potential applicability in NSCLC patients (66). These agents are being investigated in clinical trials, which include patients after they develop osimertinib resistance (MET-directed ADCs will be described in a later section).
A HER3-directed ADC, patritumab deruxtecan (U3–1402) (HER3-DXd), has the closest clinical application in osimertinib-resistant patients. HER3 is not implicated in oncogenicity, although it is involved in EGFR/HER2-mediated resistance through conjugation with MET and has recently been regarded as an important therapeutic target (67). A phase I dose escalation/expansion HER3-DXd study for EGFR-mutated NSCLC patients previously treated with EGFR TKIs, including osimertinib, was conducted (U31402-A-U102, NCT03260491). The participants were heavily pre-treated [median number of prior treatment lines: 4 (1–9)], with 89% of them receiving osimertinib. In the pooled population of patients treated with 5.6 mg/kg HER3-DXd using the recommended dose for expansion, the confirmed ORR was 39%. Median PFS was 8.2 months for osimertinib pretreated patients (68). Importantly, responses to HER3-DXd were observed notwithstanding different EGFR TKI resistance mechanisms not directly associated with HER3, such as EGFR C797S, MET, HER2 amplification and BRAF fusion (68). The phase II HERTHENA-Lung01 trial (NCT04619004) for HER3-DXd in patients with EGFR-mutated NSCLC previously treated with at least one EGFR TKI and a platinum-based chemotherapy regimen is ongoing. Similarly, the phase III HERTHENA-Lung02 trial (NCT05338970) is comparing HER3-DXd and platinum-based chemotherapy in EGFR-mutated NSCLC after progression using a 3G EGFR TKI. Furthermore, the efficacy of combining HER3-DXd and osimertinib for EGFR-mutated NSCLC with osimertinib progression is currently being evaluated in a phase I trial (NCT04676477).
HER2-directed ADCs, trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) are being investigated in NSCLC patients with HER2 gene alternations. The phase II TRAEMOS trial (NCT03784599) evaluated the efficacy of T-DM1 in combination with osimertinib for EGFR-mutated NSCLC patients previously treated with EGFR TKIs, including osimertinib who acquired HER2 overexpression and/or amplification with re-biopsy at relapse. However, the reported ORR and median PFS were 13% and 2.8 months, respectively, indicating the limited efficacy of this approach. Therefore, the trial was terminated early (69). On the other hand, T-DXd has demonstrated attractive antitumor efficacy for previously treated HER2 positive (overexpression or mutations) NSCLC (not including EGFR-mutated patients) with an ORR of 55% and a median PFS and OS of 8.2 and 17.8 months, respectively (DESTINY-Lung01, NCT03505710 (70)). An ongoing phase Ib trial (NCT04042701) is investigating T-DXd in combination with pembrolizumab in HER2-expressing or HER2-mutated patients, including those with EGFR-mutated NSCLC who were previously treated with EGFR TKIs.
TROP-2 is an intracellular calcium signaling transducer and an attractive target for ADC. Datopotamab deruxtecan (Dato-DXd), a TROP-2 targeting ADC, has demonstrated satisfactory therapeutic efficacy in patients with NSCLC in a phase I trial (TROPION-PanTumor01, NCT03401385 (71)) and is further being investigated for EGFR-mutated patients who were previously treated with osimertinib in the TROPION-Lung05 (NCT04484142) and TROPION-Lung01 (NCT04656652) trials.
Preliminary data on the efficacy of ADCs in the clinical trials described above underscore the viability of this approach in osimertinib-resistant populations. Despite their impressive efficacy, the reported negligible occurrence of interstitial lung disease (ILD) should be considered. In the DESTINY-Lung01 trial, 24 of 91 patients (26%) developed T-Dxd-related ILD, including four (6.6%) grade 3 and two (2.2%) grade 5 patients (70). A systematic review of advanced HER2-positive solid tumor patients reported an incidence of T-Dxd-related ILD of 11.40%, which was exceptionally high at 24.77% in NSCLC patients (72). Insufficient data are available; however, the frequency of ILD related to HER3-Dxd (68) and Dato-Dxd (71) was reported to be 5–10.6% and 8% in phase I trials, respectively. The definite mechanism is unknown, as it is assumed that these pulmonary toxicities may be associated with the cytotoxic payload carried by ADCs. Safety data for more patients are required in ongoing clinical trials.
In summary, positive preliminary data on ADCs in EGFR-mutated NSCLC after osimertinib resistance were provided. HER3-DXd has been evaluated extensively in clinical trials. If the promising efficacy of ADCs is demonstrated in the above clinical trials, it could change the treatment paradigm for EGFR-mutated NSCLC in TKI-resistant populations.
MET-targeting strategy
The MET oncogene encodes the receptor tyrosine kinase, c-Met, whose dysregulation is an oncogenic driver. MET signaling mediates osimertinib resistance by bypassing EGFR downstream signaling pathways such as PI3K/AKT, JAK/STAT3 and RAS/MAPK/ERK (73). Among the various genomic statuses of the MET oncogene, MET amplification is a common resistance mechanism to osimertinib, detectable in 6–24% of cases (53–61). MET gene alterations are considered an important therapeutic target after osimertinib resistance, and several potential therapeutic candidates have been developed and examined in clinical trials.
MET tyrosine kinase inhibitor
Clinical trials of MET TKIs for osimertinib-resistant patients are ongoing; the efficacy of savolitinib, tepotinib and capmatinib as single agents or combined with osimertinib in patients who acquired resistance to osimertinib has been evaluated.
The phase Ib TATTON trial (NCT02143466) investigated the efficacy of savolitinib and osimertinib for EGFR-mutated NSCLC patients previously treated with EGFR TKIs. The recently published data of the interim analysis revealed the acceptable safety profile and encouraging antitumor activity of this approach; an ORR of 30% and a median PFS of 5.4 months were obtained in patients previously treated with 3G EGFR TKI, reaching 64–67% and 9–11 months, respectively, in 3G EGFR TKI-naïve patients based on the study’s different cohorts (74). In the phase II SAVANNAH trial (NCT03778229), savolitinib in combination with osimertinib in EGFR-mutated NSCLC patients with MET-driven (amplification and/or overexpression) resistance mechanisms acquired after osimertinib is being evaluated. Preliminary data showed an ORR of 32% and a median PFS of 5.3 months in the overall population (75). Notably, the population with higher MET levels with amplification and/or overexpression had an ORR of 49% and a median PFS of 7.1 months, compared with an ORR of 9% and a median PFS of 2.8 months in the population without higher MET levels (75). In the phase II ORCHARD trial, MET-amplified patients are assigned to the osimertinib plus savolitinib arm (76), and the recent interim analysis results showed an ORR of 41% (77). The phase III SACHI trial (NCT05015608) compared osimertinib plus savolitinib with platinum-based chemotherapy in patients with MET amplification acquired after 1L EGFR TKI therapy. In addition, the phase III SAFFRON trial (NCT05261399) compared osimertinib plus savolitinib with platinum-based chemotherapy in MET-amplified and/or MET-overexpressed patients previously treated with osimertinib.
Tepotinib is also being investigated in several clinical trials. The phase Ib/II INSIGHT trial evaluated the efficacy and safety of tepotinib combined with gefitinib. In the phase II part with randomization against platinum-based chemotherapy, tepotinib plus gefitinib was assessed in EGFR-mutated NSCLC patients with MET amplification and/or overexpression previously treated with 1G- or 2G-EGFR TKIs. The survival outcomes were comparable in both arms; however, the median PFS and OS were longer in the tepotinib plus gefitinib arm than in the chemotherapy arm in a prespecified subgroup analysis of the high MET-expressing group (78). The phase II INSIGHT2 trial (NCT03940703) compared the combination of tepotinib plus osimertinib with tepotinib alone in patients previously treated with 1L of osimertinib. The initial results were recently reported; the ORR was high at 55% for tepotinib plus osimertinib compared with 8.3% for tepotinib alone (79). The combination of capmatinib and osimertinib is also being investigated in clinical trials [phase II A Lung-MAP trial (NCT05642572), phase III GEOMETRY-E trial (NCT04816214)].
EGFR-MET bispecific antibody
Amivantamab is a fully humanized bispecific antibody targeting EGFR mutations and MET mutations/amplifications. Amivantamab inhibits ligand binding of EGFR and MET, further inhibits downstream signaling pathways and has been reported to have antibody-dependent cellular cytotoxicity; therefore, it is expected to have a wider range of effects (80). Specifically, it has antitumor activity against various activating and resistant EGFR mutations (del19, L858R, T790M, C797S and exon20 insertion) (81,82) and might have potential clinical use for osimertinib-resistant patients.
The multiarm phase I CHRYSALIS trial (NCT02609776) investigated the combination of amivantamab and lazertinib (a potent brain-penetrant 3G EGFR TKI) immediately after osimertinib resistance in 45 patients with NSCLC with activating EGFR mutations. The ORR was 36%, and overall patients’ median PFS was 4.9 months (83). Notably, this combination approach had an ORR of 47% in patients with EGFR/MET-driven resistances and an ORR of 29% in patients without these resistances (83). In the recently reported CHRYSALIS-2 trial (NCT04077463) results, combining amivantamab with lazertinib showed antitumor activity for EGFR-mutated NSCLC patients who had disease progression with 1 or 2L osimertinib followed by platinum-based chemotherapy (including heavily pretreated patients) with an ORR of 33% and median PFS of 5.1 months (84). A combination of amivantamab and lazertinib is being investigated in phase III trials for EGFR-mutated patients. The MARIPOSA study (NCT04487080), a randomized phase III trial, compares the combination of amivantamab and lazertinib with osimertinib monotherapy in the 1L setting. In addition, the MARIPOSA-2 study (NCT04988295), a randomized, open-label, phase III trial, compared lazertinib, amivantamab, carboplatin and pemetrexed with carboplatin, amivantamab and pemetrexed in EGFR-mutated NSCLC patients following disease progression during or after osimertinib treatment. Regarding the toxicity of amivantamab, skin and mucous membrane disorders and edema associated with EGFR and MET inhibition have been frequently reported (85). Besides, it should be noted that infusion site reactions frequently occur at initial administration (85,86).
MET-directed ADC
Telisotuzumab vedotin (Teliso-V) is a MET-directed ADC composed of ABT-700, an anti-c-Met monoclonal antibody, and monomethyl auristatin E, a potent microtubule inhibitor as a cytotoxic payload (87). Teliso-V has shown antitumor activity in solid tumors with MET gene alterations (88,89) and has been investigated in clinical trials in patients after they acquire osimertinib resistance.
In a phase II trial (NCT03539536), the ORR of Teliso-V monotherapy was reported to be 13.3% in EGFR- and c-Met-positive nonsquamous NSCLC patients (90). The combination of Teliso-V and EGFR TKIs is also being tested in an ongoing phase I/Ib trial (NCT02099058), which involves patients who acquired c-Met overexpression following 1L osimertinib. The safety and efficacy of Teliso-V monotherapy or in combination with osimertinib, erlotinib, or nivolumab are currently being evaluated. In the reported interim analysis results, the combination of Teliso-V and osimertinib showed an ORR of 58% in patients previously treated with 1L osimertinib (91). Moreover, an ongoing randomized phase III trial (NCT04928846) compares Teliso-V and docetaxel in previously treated c-Met-overexpressing NSCLC patients, including EGFR-mutated patients.
In summary, in patients after osimertinib resistance, the MET-targeting strategy would be feasible. MET TKIs have shown a certain efficacy in early-phase clinical trials and are further being evaluated in phase III trials. Drugs with novel mechanisms of action are also promising for acquired MET gene alterations. Specifically, amivantamab in combination with lazertinib has broader antitumor activity and has demonstrated impressive efficacy in patients without EGFR/MET-driven resistances and in heavily pretreated patients. It should be noted, however, that the evaluation criteria for MET gene alterations is yet to be standardized and differs among trials. Amplification might be underestimated in liquid biopsy analysis with plasma specimens.
C797S-targeting strategy
Among on-target resistance mechanisms to osimertinib, the C797S mutation is common and emerging in 7–15 and 14–30% of cases resistant to 1L- and later-line osimertinib therapy, respectively (53–61). The C797S mutation occurs in exon 20 and is responsible for drug resistance by breaking the covalent bond between osimertinib and the mutant EGFR site (92). C797S imparts cross-resistance to other irreversible 3G EGFR TKIs by inhibiting binding to the active EGFR site (92–95).
C797S may have different drug susceptibilities depending on the acquired allelic status. When C797S occurs on a chromosome other than T790M (trans), it retains sensitivity to 1G- and 3G-TKI combination therapy (95). Preclinical data and some case reports have shown the efficacy of osimertinib in combination with gefitinib or erlotinib for EGFR-mutated NSCLC harboring T790M in trans with C797S mutation (96–98). In contrast, if C797S and T790M occur on the same chromosome (cis), they lose activity against any conventional EGFR TKIs (95–97). As a potential approach to C797S in cis, the efficacy of combining brigatinib, an EGFR-ALK dual inhibitor, with anti-EGFR antibodies has been reported. Preclinical data have demonstrated that combining brigatinib with cetuximab or panitumumab has antitumor activity against triple mutant cells with C797S in cis and T790M and activates EGFR mutation (98–100). A phase I/II trial conducted to ascertain the safety and efficacy of brigatinib combined with panitumumab in patients who acquired C797S after osimertinib treatment is ongoing (jRCT2031200231).
As another promising therapeutic agent against C797S mutation, a next-generation EGFR allosteric inhibitor is being developed (101). EGFR allosteric inhibitors that belong to fourth-generation (4G) EGFR inhibitors bind to EGFR far from the ATP-binding site, thereby selectively altering EGFR conformation and bypassing the C797S-mediated resistance mechanism (92,102). These agents have shown antitumor activity alone or combined with osimertinib in vivo/in vitro studies (ongoing clinical trials on 4G EGFR inhibitors are summarized in Table 2). EAI-045 is the first EGFR allosteric inhibitor targeting T790M and C797S mutations (103). EAI-045 exhibited limited activity in vivo as a single agent; however, the marked tumor regression for the triple mutant (L858R/T790M/C797S) Ba/F3 cell line was observed when combined with cetuximab (102). Several novel 4G EGFR inhibitors have shown antitumor activity in in vivo/in vitro research. JBJ-04-125-02 is a more potent EGFR allosteric inhibitor that selectively targets L858R and inhibits triple mutant (L858R/T790M/C797S) EGFR signaling. JBJ-04-125-02, combined with osimertinib, offered increased cell death, more effective inhibition of cell proliferation and increased efficacy compared with either single agent alone (104). Comparing the EAI-045 and JBJ-04-125-02, CH7233163, a potent and selective 4G EGFR inhibitor, showed activity against triple EGFR mutants with del19/T790M/C797S (105). Furthermore, some agents are being evaluated as candidates in clinical trials. BLU-701 is a new, highly selective and potent oral EGFR inhibitor that has demonstrated in vivo antitumor activity against sensitizing EGFR mutations (del19 or L858R) and double mutations with C797S (del19/C797S or L858R/C797S) (106). The phase I/II HARMONY trial (NCT05153408) evaluates the safety and efficacy of BLU-701 in EGFR-mutated NSCLC patients previously treated with EGFR TKIs, including osimertinib. The effectiveness of BLU-701 in patients with C797X mutations will be evaluated in a phase II expansion cohort study. BLU-945 is a selective and potent 4G EGFR inhibitor with activity against triple-resistant mutations (del19 or L858R/T790M/C797S) (107,108). A combination of BLU-945 with either gefitinib or osimertinib exhibited increased antitumor activity compared with BLU-945 alone for del19/T790M/C797S in a patient-derived xenograft model (109). The phase I/II SYMPHONY trial (NCT04862780) also evaluates the safety and efficacy of BLU-945, either a single agent or combined with osimertinib in NSCLC patients with activating EGFR mutations previously treated with EGFR TKIs including osimertinib. The efficacy of BLU-945 in patients with the C797S mutation will be evaluated in a phase II expansion cohort. Other 4G EGFR inhibitors, such as JIN-A02 (NCT05394831) and BBT-176 (NCT04820023), will also be assessed in phase I/II trials.
Table 2.
Summary of clinical trial after osimertinib resistance
| Agent | Trial number (Trial name) | Phase | Number of patients | Arm | Year | Status |
|---|---|---|---|---|---|---|
| Fourth-generation EGFR allosteric inhibitor | ||||||
| BLU-701 | NCT05153408 (HARMONY) | I/II | 160 | BLU-701 BLU-701 + osimertinib BLU-701 + CBDCA + PEM |
2021 | Recruiting |
| BLU-945 | NCT04862780 (SYMPHONY) | I/II | 190 | BLU-945 BLU-945 + osimertinib |
2021 | Recruiting |
| BBT-176 | NCT04820023 | I/II | 168 | BBT-176 | 2021 | Recruiting |
| JIN-A02 | NCT05394831 | I/II | 95 | JIN-A02 | 2022 | Not yet recruiting |
| MET inhibitor | ||||||
| Savolitinib | NCT02143466 (TATTON) | Ib | 344 | Savolitinib + osimertinib | 2014 | Active, not recruiting |
| NCT03778229 (SAVANNAH) | II | 360 | Savolitinib + osimertinib Sabolitinib + placebo |
2018 | Recruiting | |
| NCT05015608 (SACHI) | III | 250 | Savolitinib + osimertinib CDDP/CBDCA + PEM |
2021 | Recruiting | |
| NCT05261399 (SAFFRON) | III | 324 | Savolitinib + osimertinib CDDP/CBDCA + PEM |
2022 | Recruiting | |
| Tepotinib | NCT03940703 (INSIGHT2) | II | 120 | Tepotinib + osimertinib Tepotinib |
2019 | Active, not recruiting |
| Capmatinib | NCT05642572 (A Lung-MAP) | II | 66 | Capmatinib + osimertinib + ramucirumab Capmatinib + osimertinib |
2022 | Not yet recruiting |
| NCT04816214 (GEOMETRY-E) | III | 245 | Capmatinib + osimertinib CDDP/CBDCA + PEM |
2021 | Active, not recruiting | |
| Other specific inhibitor | ||||||
| Selumetinib (MEK1/2 inhibitor) | NCT02143466 (TATTON) | Ib | 344 | Selumetinib + osimertinib | 2014 | Active, not recruiting |
| DS-1205c (AXL inhibitor) | NCT03255083 | I | 13 | DS-1205c + osimertinib | 2017 | Terminated |
| ERAS-007 (ERK1/2 inhibitor) | NCT04959981 (HERKULES-2) | I/II | 200 | ERAS-601 + osimertinib | 2021 | Active, not recruiting |
| Sapanisertib (mTOR inhibitor) | NCT02503722 | I | 36 | Sapanisertib + osimertinib | 2015 | Active, not recruiting |
| TQ-B3525 (PIKα/δ inhibitor) | NCT05284994 | I/II | 160 | TQ-B3525 + osimetinib | 2022 | Recruiting |
| Itacitinib (JAK1 inhibitor) | NCT02917993 | I/II | 59 | Itacitinib + osimertinib | 2016 | Active, not recruiting |
| Abemaciclib (CDK4/6 inhibitor) | NCT04545710 | II | 18 | Abemaciclib + osimetinib | 2020 | Recruiting |
| Alisertib (AURKA inhibitor) | NCT04479306 | I | 48 | Alisertib + osimetinib | 2020 | Active, not recruiting |
| Antibody–drug conjugate | ||||||
| Trastuzumab deruxtecan (T-Dxd) (HER2-ADC) | NCT04042701 | Ib | 115 | T-DXd + pembrolizumab (part 2 dose expansion cohort) |
2019 | Recruiting |
| Patritumab deruxtecan (HER3-ADC) | NCT03260491 | I | 264 | HER3-DXd | 2017 | Recruiting |
| NCT04676477 | I | 252 | HER3-DXd + osimertinib | 2020 | Recruiting | |
| NCT04619004 (HERTHENA-Lung01) | II | 420 | HER3-DXd (fixed dose or up-titration) |
2020 | Recruiting | |
| NCT05338970 (HERTHENA-Lung02) | III | 560 | HER3-DXd CDDP/CBDCA + PEM |
2022 | Recruiting | |
(Continued)
Table 2.
Continued
| Agent | Trial number (Trial name) | Phase | Number of patients | Arm | Year | Status |
|---|---|---|---|---|---|---|
| Datopotamab deruxtecan (Dato-Dxd) (TROP2-ADC) | NCT04484142 (TROPION-Lung05) | II | 137 | Dato-DXd | 2020 | Active, not recruiting |
| NCT04656652 (TROPION-Lung01) | III | 590 | Dato-DXd Docetaxel |
2020 | Recruiting | |
| Telisotuzumab vedotin (Teliso-V) (MET-ADC) | NCT02099058 | I | 260 | Teliso-V Teliso-V + erlotinib Teliso-V + nivolumab Teliso-V + osimertinib |
2014 | Recruiting |
| NCT04928846 | III | 698 | Teliso-V Docetaxel |
2021 | Recruiting | |
| EGFR-MET bispecific antibody | ||||||
| Amivantamab | NCT02609776 (CHRYSALIS) | I | 780 | Amivantamab + lazertinib | 2015 | Recruiting |
| NCT04077463 (CHRYSALIS-2) | I/Ib | 460 | Lazertinib Amivantamab + lazertinib Amivantamab + Lazertinib + CBDCA + PEM |
2019 | Recruiting | |
| NCT04988295 (MARIPOSA-2) | III | 600 | Amivantamab + Lazertinib + CBDCA + PEM CBDCA + PEM Amivantamab + CBDCA + PEM |
2021 | Recruiting | |
| Immune checkpoint inhibitor | ||||||
| ICI + chemotherapy | NCT02864251 (Checkmate 722) | III | 367 | Nivolumab + CDDP/CBDCA + PEM CDDP/CBDCA + PEM |
2016 | Completed |
| NCT03515837 (KEYNOTE-789) | III | 492 | Pembrolizumab + CDDP/CBDCA + PEM CDDP/CBDCA + PEM |
2018 | Active, not recruiting | |
| NCT03802240 (ORIENT-31) | III | 600 | Sintilimab + IBI305 + CDDP + PEM Sintilimab + placebo + CDDP + PEM Placebo + placebo + CDDP + PEM |
2019 | Recruiting | |
| Multiple study treatment (platform study) | ||||||
| NCT03944772 (ORCHARD) | II | 250 | Osimertinib + savolitinib Osimertinib + gefitinib Osimertinib + necitumumab CBDCA + PEM + durvalumab Osimertinib + alectinib Osimertinib + selpercatinib CDDP/CBDCA + etoposide + durvalumab Osimertinib + CDDP/CBDCA + PEM Osimertinib + selumetinib Osimertinib + Dato-Dxd |
2019 | Recruiting | |
Abbreviations: CBDCA, carboplatin; PEM, pemetrexed; CDDP, cisplatin: ADC, antibody–drug conjugate; ICI, immune checkpoint inhibitor.
In summary, C797S mutation is a crucial on-target resistance mechanism that can be potentially targeted after osimertinib resistance. Treatment with brigatinib and EGFR antibodies is being evaluated based on the cis/trans strategy for the C797S mutation. Moreover, there are sufficient preclinical data for the efficacy of 4G EGFR inhibitors for C797S, and 4G EGFR inhibitors are currently being investigated in early-phase clinical trials.
Other specific inhibitors
Molecular aberrations other than C797S and MET gene alterations have also been identified following osimertinib resistance. Changes in downstream signaling pathways (i.e. RAS/MAPK/ERK, PI3K/AKT and JAK/STAT3), oncogenic fusion genes, alterations in other tyrosine kinase receptors and cell cycle genes are regarded as resistance mechanisms to osimertinib (Fig. 1) that are potentially targetable. However, each of these is a rare fraction detected in <10% of cases (53–61). Specific inhibitors targeting these mechanisms are currently being investigated in early-phase clinical trials. The ongoing clinical trials of these agents are summarized in Table 2. It is still being undetermined whether these specific inhibitors will be implemented in practice, as limited evidence has suggested their efficacy; therefore, the progress of clinical trials of individual agents should be monitored closely.
Chemoimmunotherapy combination
Immune oncology combinatorial treatment (chemo-IO) with a VEGF inhibitor may be a possible treatment option in the clinic following 1L osimertinib resistance. The potential applicability of chemo-IO for EGFR-mutated patients was first reported in a subgroup analysis of EGFR- and ALK-positive patients in the IMpower150 trial, showing survival benefits in terms of both PFS and OS in the atezolizumab, bevacizumab, paclitaxel and carboplatin groups compared with the bevacizumab, paclitaxel and carboplatin groups (110,111).
The chemo-IO ± VEGF inhibitor strategy for patients with EGFR mutations is being investigated in ongoing prospective trials. The CheckMate 722 (NCT02864251) and KEYNOTE-789 (NCT03515837) trials investigated chemo-IO in patients previously treated with EGFR TKIs. Both trials were recently reported to have failed to show the PFS benefit of chemo-IO over platinum chemotherapy (112,113). On the other hand, the ORIENT-31 trial (NCT03802240) that is investigating combination therapy of sintilimab (anti-PD-1 antibody), IBI305 (a bevacizumab biosimilar), and platinum-based chemotherapy in EGFR TKI-treated patients demonstrated significantly improved PFS in the sintilimab + IBI305 + chemotherapy arm compared with chemotherapy alone [median PFS, 6.9 vs. 4.3 months; HR (95% CI), 0.464 (0.337–0.639); P < 0.0001] (114). These results suggest a potential role for VEGF inhibitors combined with chemo-IO for EGFR-mutated patients.
Discussion and conclusion
This review discussed potential treatment strategies for EGFR-mutated advanced NSCLC based on clinical trials. Osimertinib monotherapy is currently the preferred therapeutic option in this population based on favorable efficacy and safety data from the AURA3 and FLAURA trials. However, since resistance to osimertinib would hinder persistent effectiveness for patients, strategies beyond osimertinib in 1L and later-line settings need to be provided.
In the 1L setting, strategies combining EGFR TKI and platinum-based chemotherapy or VEGF inhibitors are being investigated to surpass osimertinib monotherapy. In addition, the beyond PD osimertinib-based combination strategy is also being investigated, expecting to control CNS involvements. An encouraging data on combination strategies from clinical trials before the FLAURA era supports the feasibility of combining osimertinib with these anticancer agents. The results of the ongoing randomized trials of osimertinib-based combination therapy could set a new landmark in 1L therapy for EGFR-mutated NSCLC soon.
In later-line settings after osimertinib resistance, various novel therapeutic strategies have been challenged to address acquired resistance, linked to information on resistance mechanisms, with molecular profile testing gaining a better understanding in recent years. Drugs with a novel class of action, such as ADCs and EGFR-MET bispecific antibodies, have also shown specific clinical activity in osimertinib-resistant patients regardless of the resistance mechanism. The C797S mutation and MET amplification are important therapeutic targets in this setting. Several agents targeting a relatively rare fraction of resistance mechanisms are currently being investigated in early-phase clinical trials. Chemo-IO plus VEGF inhibitors may be an option for 1L osimertinib-resistant patients. However, most of these therapies still need to be investigated in clinical trials and are unavailable in practice. Therefore, performing a re-biopsy at relapse is necessary to expand the possibilities of available trial platforms for individual patients, which would facilitate enrollment in ongoing clinical trials.
In conclusion, the pharmacotherapeutic management of EGFR-mutated advanced NSCLC has been rapidly updated to a new phase with the advent of genomic medicine. Upfront combination strategies aimed at preventing or postponing the emergence of resistance are currently being evaluated. Strategies using antibody agents with novel mechanisms of action during a relapse should be implemented clinically. In addition, there is a growing need for repeated proactive re-biopsies for molecular profile testing. A greater understanding of osimertinib resistance mechanisms will contribute to further progress in the ongoing clinical trials discussed in this review, establishing new therapeutic evidence in this setting. Ultimately, this will maximize survival outcomes in patients with relapsed disease.
Acknowledgements
We would like to express our great appreciation to the Japan Clinical Oncology Group for providing us with the opportunity to author this article. We would like to thank Editage (www.editage.com) for English language editing.
Contributor Information
Taisuke Araki, First Department of Internal Medicine, Shinshu University School of Medicine, Nagano, Japan.
Shintaro Kanda, Department of Hematology and Oncology, Shinshu University School of Medicine, Nagano, Japan.
Hidehito Horinouchi, Department of Thoracic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Yuichiro Ohe, Department of Thoracic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Funding
This research was supported by the research fund of National Cancer Center Japan (Grant number: 2023-J-03).
Conflicts of interest statement
No author has any conflicts of interest related to this article. Hidehito Horinouchi reports research funding from MSD, Abbvie, AstraZeneca, BMS, Ono, Merck Biopharma, Daiichi-Sankyo, Janssen, Genomic Helath, Chugai, Roche, Novartis; personal fees as speakers' bureau from AstraZeneca, MSD, Eli Lilly, Ono, BMS, Chugai, Roche, Amgen; personal fees as advisory role from AstraZeneca, Eli Lilly, Chugai, Roche, ONO, BMS, outside the submitted work. Yuichiro Ohe reports research funding from AstraZeneca, Chugai, Lilly, ONO, BMS, Kyorin, Dainippon-Sumitomo, Pfizer, Taiho, Novartis, Takeda, Kissei, Daiichi-Sankyo, Janssen, LOXO; personal fees as expert testimony from AstraZeneca, Chugai, ONO, BMS, Kyorin, Celltrion, Amgen, Nippon Kayaku, Boehringer Ingelheim, AnHeart Therapeutics Inc; personal fees as speakers' bureau from AstraZeneca, Chugai, Eli Lilly, Ono, BMS, Boehringer Ingelheim, Bayer, Pfizer, MSD, Taiho, Nippon Kayaku, Kyowa Hakko Kirin, outside the submitted work.
References
- 1. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–74 [published correction appears in N Engl J Med 2009 Apr 9;360:1579]. [DOI] [PubMed] [Google Scholar]
- 2. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol 2022;40:611–25. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 4. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9. [DOI] [PubMed] [Google Scholar]
- 8. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- 10. Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. [DOI] [PubMed] [Google Scholar]
- 11. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- 12. Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-lung 7 trial. Ann Oncol 2017;28:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018;36:2244–50 [published correction appears in J Clin Oncol 2020 Nov 1;38:3725]. [DOI] [PubMed] [Google Scholar]
- 14. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 17. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 18. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018;36:2702–9. [DOI] [PubMed] [Google Scholar]
- 19. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to Osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with UntreatedEGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol 36:3290–7 [published online ahead of print, 2018 Aug 28]. [DOI] [PubMed] [Google Scholar]
- 20. Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci 2008;99:929–35 [published correction appears in Cancer Sci 2008 Sep;99:1869]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Z, Tam KY. Combination strategies using EGFR-TKi in NSCLC therapy: learning from the gap between pre-clinical results and clinical outcomes. Int J Biol Sci 2018;14:204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galvani E, Peters GJ, Giovannetti E. Thymidylate synthase inhibitors for non-small cell lung cancer. Expert Opin Investig Drugs 2011;20:1343–56. [DOI] [PubMed] [Google Scholar]
- 23. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990–8. [DOI] [PubMed] [Google Scholar]
- 24. Mok TSK, Kim SW, Wu YL, et al. Gefitinib plus chemotherapy versus chemotherapy in epidermal growth factor receptor mutation-positive non-small-cell lung cancer resistant to first-line gefitinib (IMPRESS): overall survival and biomarker analyses. J Clin Oncol 2017;35:4027–34. [DOI] [PubMed] [Google Scholar]
- 25. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol 2020;38:115–23. [DOI] [PubMed] [Google Scholar]
- 26. Miyauchi E, Morita S, Nakamura A, et al. Updated analysis of NEJ009: gefitinib-alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated EGFR. J Clin Oncol 2022;40:3587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 2020;38:124–36. [DOI] [PubMed] [Google Scholar]
- 28. Sequist LV, Peled N, Tufman A, et al. P47.11 COMPEL: chemotherapy with/without osimertinib in patients with EGFRm advanced NSCLC and progression on first-line osimertinib. J Thorac Oncol 2021;16:S1101. [Google Scholar]
- 29. Okuma Y, Nomura S, Ninomiya K, et al. 1186TiP EPONA, efficacy of osimertinib with platinum and pemetrexed in EGFR mutant non-small cell lung cancer patients bearing CNS metastasis, and have systemic progression but stable intracranial disease on osimertinib resistance (TORG 1938). Ann Oncol 2022;33:S1090–1. [Google Scholar]
- 30. Saito R, Sugawara S, Ko R, et al. Phase 2 study of osimertinib in combination with platinum and pemetrexed in patients with previously untreated EGFR-mutated advanced non-squamous non-small cell lung cancer: the OPAL study. Eur J Cancer 2023;185:83–93. [DOI] [PubMed] [Google Scholar]
- 31. Jänne P, Planchard D, Howarth P, Todd A, Kobayashi K. OA07.01 osimertinib plus platinum/pemetrexed in newly-diagnosed advanced EGFRm-positive NSCLC; the phase 3 FLAURA2 study. J Thorac Oncol 2019;14:S222–3. [Google Scholar]
- 32. Planchard D, Feng PH, Karaseva N, et al. Osimertinib plus platinum-pemetrexed in newly diagnosed epidermal growth factor receptor mutation-positive advanced/metastatic non-small-cell lung cancer: safety run-in results from the FLAURA2 study. ESMO Open 2021;6:100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanda S. A phase III study comparing gefitinib and inserted platinum-doublet chemotherapy with gefitinib as a first-line treatment for patients with advanced non-squamous non-small cell lung cancer harboring EGFR activating mutations. JJLC 2015;55:879–84 (in Japanese)]. [Google Scholar]
- 34. Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol 2021;16:205–15. [DOI] [PubMed] [Google Scholar]
- 35. Byers LA, Heymach JV. Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer 2007;8:S79–85. [DOI] [PubMed] [Google Scholar]
- 36. Xu L, Nilsson MB, Saintigny P, et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1 alpha in non-small cell lung cancer cells. Oncogene 2010;29:2616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ciardiello F, Caputo R, Bianco R, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res 2001;7:1459–65. [PubMed] [Google Scholar]
- 38. Jackson AL, Zhou B, Kim WY. HIF, hypoxia and the role of angiogenesis in non-small cell lung cancer. Expert Opin Ther Targets 2010;14:1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–44 [published correction appears in Lancet Oncol 2014 Oct;15:e475]. [DOI] [PubMed] [Google Scholar]
- 40. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625–35. [DOI] [PubMed] [Google Scholar]
- 41. Zhou Q, Xu CR, Cheng Y, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell 2021;39:1279–91.e3. [DOI] [PubMed] [Google Scholar]
- 42. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655–69. [DOI] [PubMed] [Google Scholar]
- 43. Stinchcombe TE, Jänne PA, Wang X, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol 2019;5:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawashima Y, Fukuhara T, Saito H, et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med 2022;10:72–82. [DOI] [PubMed] [Google Scholar]
- 45. Nadal E, Horinouchi H, Shih JY, et al. RELAY, ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated, epidermal growth factor receptor mutation-positive, metastatic non-small-cell lung cancer: safety profile and manageability. Drug Saf 2022;45:45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: a phase 1/2 single-group open-label trial. JAMA Oncol 2020;6:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kenmotsu H, Wakuda K, Mori K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol 2022;17:1098–108. [DOI] [PubMed] [Google Scholar]
- 48. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor tyrosine kinase-targeted cancer therapy. Int J Mol Sci 2018;19:3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santoni-Rugiu E, Melchior LC, Urbanska EM, et al. Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: differences and similarities with acquired resistance. Cancer 2019;11:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res 2016;5:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cell 2018;7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chmielecki J, Gray JE, Cheng Y, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun 2023;14:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 2020;26:2654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Akli A, Girard N, Fallet V, et al. Histomolecular resistance mechanisms to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer: a multicentric retrospective French study. Target Oncol 2022;17:675–82. [DOI] [PubMed] [Google Scholar]
- 56. Hartmaier RJ, Markovets A, Cho BC, et al. Abstract LB078: tumor genomics in patients (pts) with advanced epidermal growth factor receptor mutant (EGFRm) non-small cell lung cancer (NSCLC) whose disease has progressed on first-line (1L) osimertinib therapy in the phase II ORCHARD study. Cancer Res 2022;82:LB078. [Google Scholar]
- 57. Piotrowska Z, Ahn MJ, Pang YK, et al. LBA53 ELIOS: a multicentre, molecular profiling study of patients (pts) with epidermal growth factor receptor-mutated (EGFRm) advanced NSCLC treated with first-line (1L) osimertinib. Ann Oncol 2022;33:S1420–1. [Google Scholar]
- 58. Papadimitrakopoulou VA, Wu Y-L, Han J-Y, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol 2018;29:vii741. [Google Scholar]
- 59. Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov 2018;8:1529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018;4:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Le X, Puri S, Negrao MV, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res 2018;24:6195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Girard N. New strategies and novel combinations in EGFR TKI-resistant non-small cell lung cancer. Curr Treat Options Oncol 2022;23:1626–44. [DOI] [PubMed] [Google Scholar]
- 63. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther 2022;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol 2021;18:327–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jin Y, Schladetsch MA, Huang X, Balunas MJ, AJ. Stepping forward in antibody-drug conjugate development. Pharmacol Ther 2022;229:107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Desai A, Abdayem P, Adjei AA, Planchard D. Antibody-drug conjugates: a promising novel therapeutic approach in lung cancer. Lung Cancer 2022;163:96–106. [DOI] [PubMed] [Google Scholar]
- 67. Lyu H, Han A, Polsdofer E, Liu S, Liu B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm Sin B 2018;8:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jänne PA, Baik C, Su WC, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov 2022;12:74–89 [published correction appears in Cancer Discov 2022 Jun 2;12:1598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hotta K, Aoe K, Kozuki T, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol 2018;13:273–9. [DOI] [PubMed] [Google Scholar]
- 70. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 2022;386:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spira A, Lisberg A, Sands J, et al. OA03.03 Datopotamab deruxtecan (dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: updated results of TROPION-PanTumor01 phase 1 study. J Thorac Oncol 2021;16:S106–7. [Google Scholar]
- 72. Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol 2021;7:1873–81. [DOI] [PubMed] [Google Scholar]
- 73. Rosário M, Birchmeier W. How to make tubes: signaling by the met receptor tyrosine kinase. Trends Cell Biol 2003;13:328–35. [DOI] [PubMed] [Google Scholar]
- 74. Hartmaier RJ, Markovets AA, Ahn MJ, et al. Osimertinib + savolitinib to overcome acquired MET-mediated resistance in epidermal growth factor receptor-mutated, MET-amplified non-small cell lung cancer: TATTON. Cancer Discov 2023;13:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahn MJ, De Marinis F, Bonanno L, et al. EP08.02-140 MET biomarker-based preliminary efficacy analysis in SAVANNAH: savolitinib+osimertinib in EGFRm NSCLC post-Osimertinib. J Thorac Oncol 2022;17:S4690–70. [Google Scholar]
- 76. Yu HA, Goldberg SB, Le X, et al. Biomarker-directed phase II platform study in patients with EGFR sensitizing mutation-positive advanced/metastatic non-small cell lung cancer whose disease has progressed on first-line osimertinib therapy (ORCHARD). Clin Lung Cancer 2021;22:601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu HA, Ambrose H, Baik C, et al. 1239P ORCHARD osimertinib + savolitinib interim analysis: a biomarker-directed phase II platform study in patients (pts) with advanced non-small cell lung cancer (NSCLC) whose disease has progressed on first-line (1L) osimertinib. Ann Oncol 2021;32:S978–9. [Google Scholar]
- 78. Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (Insight study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020;8:1132–43 [published correction appears in Lancet Respir Med 2020 Jul;8:e59]. [DOI] [PubMed] [Google Scholar]
- 79. Mazieres J, Kim TM, Lim BK, et al. LBA52 Tepotinib + osimertinib for EGFRm NSCLC with MET amplification (METamp) after progression on first-line (1L) osimertinib: initial results from the INSIGHT 2 study. Ann Oncol 2022;33:S1419–20. [Google Scholar]
- 80. Cho BC, Simi A, Sabari J, Vijayaraghavan S, Moores S, Spira A. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer 2023;24:89–97. [DOI] [PubMed] [Google Scholar]
- 81. Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov 2020;10:1194–209. [DOI] [PubMed] [Google Scholar]
- 82. Haura EB, Cho BC, Lee JS, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:9009–9. [Google Scholar]
- 83. Bauml J, Cho BC, Park K, et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. J Clin Oncol 2021;39:9006–6. [Google Scholar]
- 84. Shu CA, Goto K, Ohe Y, et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: updated results from CHRYSALIS-2. J Clin Oncol 2022;40:9006–6. [Google Scholar]
- 85. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol 2021;39:3391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Krebs MG, Johnson ML, Cho BC, et al. Subcutaneous delivery of amivantamab in patients with advanced solid malignancies: initial safety and pharmacokinetic results from the PALOMA study. Cancer Res 2022;82:CT198. [Google Scholar]
- 87. Wang J, Anderson MG, Oleksijew A, et al. ABBV-399, a c-Met antibody-drug conjugate that targets both MET-amplified and c-Met-overexpressing tumors, irrespective of MET pathway dependence. Clin Cancer Res 2017;23:992–1000. [DOI] [PubMed] [Google Scholar]
- 88. Strickler JH, LoRusso P, Yen C-J, et al. Phase 1, open-label, dose-escalation, and expansion study of ABT-700, an anti-C-met antibody, in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts 2014;32:2507. [DOI] [PubMed] [Google Scholar]
- 89. Kang Y-K, LoRusso P, Salgia R, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). ASCO Meet Abstr 2015;33:167. [Google Scholar]
- 90. Camidge DR, Bair J, Horinouchi H, et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2022;40:9016–6. [Google Scholar]
- 91. Goldman JW, Horinouchi H, Cho BC, et al. Phase 1/1b study of telisotuzumab vedotin (Teliso-V) + osimertinib (Osi), after failure on prior Osi, in patients with advanced, c-met overexpressing, EGFR-mutated non-small cell lung cancer (NSCLC). J Clin Oncol 2022;40:9013–3. [Google Scholar]
- 92. Tsai CJ, Nussinov R. Emerging allosteric mechanism of EGFR activation in physiological and pathological contexts. Biophys J 2019;117:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang Z, Yang N, Ou Q, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res 2018;24:3097–107. [DOI] [PubMed] [Google Scholar]
- 95. Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 2015;21:3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arulananda S, Do H, Musafer A, Mitchell P, Dobrovic A, John T. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung cancer. J Thorac Oncol 2017;12:1728–32. [DOI] [PubMed] [Google Scholar]
- 97. Wang Z, Yang JJ, Huang J, et al. Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J Thorac Oncol 2017;12:1723–7. [DOI] [PubMed] [Google Scholar]
- 98. Zhou Z, Zhao Y, Shen S, et al. Durable clinical response of lung adenocarcinoma harboring EGFR 19Del/T790M/in trans-C797S to combination therapy of first- and third-generation EGFR tyrosine kinase inhibitors. J Thorac Oncol 2019;14:e157–9. [DOI] [PubMed] [Google Scholar]
- 99. Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 2017;8:14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang X, Zhou L, Yin JC, Wu X, Shao YW, Gao B. Lung adenocarcinoma harboring EGFR 19del/C797S/T790M triple mutations responds to brigatinib and anti-EGFR antibody combination therapy. J Thorac Oncol 2019;14:e85–8. [DOI] [PubMed] [Google Scholar]
- 101. Tripathi SK, Biswal BK. Allosteric mutant-selective fourth-generation EGFR inhibitors as an efficient combination therapeutic in the treatment of non-small cell lung carcinoma. Drug Discov Today 2021;26:1466–72. [DOI] [PubMed] [Google Scholar]
- 102. Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang S, Song Y, Liu D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett 2017;385:51–4. [DOI] [PubMed] [Google Scholar]
- 104. To C, Jang J, Chen T, et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. Cancer Discov 2019;9:926–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kashima K, Kawauchi H, Tanimura H, et al. CH7233163 overcomes Osimertinib-resistant EGFR-Del19/T790M/C797S mutation. Mol Cancer Ther 2020;19:2288–97. [DOI] [PubMed] [Google Scholar]
- 106. Conti C, Campbell J, Woessner R, et al. Abstract 1262: BLU-701 is a highly potent, brain-penetrant and WT-sparing next-generation EGFR TKI for the treatment of sensitizing (ex19del, L858R) and C797S resistance mutations in metastatic NSCLC. Cancer Res 2021;81:1262. [Google Scholar]
- 107. Eno MS, Brubaker JD, Campbell JE, et al. Discovery of BLU-945, a reversible, potent, and wild-type-sparing next-generation EGFR mutant inhibitor for treatment-resistant non-small-cell lung cancer. J Med Chem 2022;65:9662–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lim SM, Park CW, Zhang Z, et al. Abstract 1467: BLU-945, a fourth-generation, potent and highly selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with intracranial activity, demonstrates robust in vivo antitumor activity in models of osimertinib-resistant non-small cell lung cancer (NSCLC). Cancer Res 2021;81:1467. [Google Scholar]
- 109. Schalm SS, Dineen T, Lim SM, et al. 384P BLU-945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol 2020;31:S1391. [Google Scholar]
- 110. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387–401. [DOI] [PubMed] [Google Scholar]
- 111. Nogami N, Barlesi F, Socinski MA, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol 2022;17:309–23. [DOI] [PubMed] [Google Scholar]
- 112. Mok TSK, Nakagawa K, Park K, et al. Nivolumab (NIVO) + chemotherapy vs chemotherapy in patients (pts) with EGFR-mutated metastatic non-small cell lung cancer (mNSCLC) with disease progression after EGFR tyrosine kinase inhibitors (TKIs). In: Check Mate 722. Ann Oncol 2022;33:S1561–1562. [Google Scholar]
- 113. Merck provides update on phase 3 trials KEYNOTE-641 and KEYNOTE-789 https://www.merck.com/news/merck-provides-update-on-phase-3-trials-keynote-641-and-keynote-789/
- 114. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2022;23:1167–79 [published correction appears in Lancet Oncol 2022 Sep;23:e404]. [DOI] [PubMed] [Google Scholar]


