Graphical Abstract
Graphical Abstract.
Cardioneuroablation (CNA) is an effective treatment for cardio-inhibitory vaso-vagal syncope (CI-VVS) that is under evaluation as a potential alternative to cardiac pacing in selected patients.1 The cornerstone of CNA is targeting groups of autonomic ganglia known as ganglionated plexi (GPs) embedded in epicardial fat pads (EFP) that are interconnected via intrinsic nerves with the sino-atrial and atrio-ventricular nodes. Ganglionated plexi are commonly identified by high frequency stimulation, fractionated electrogram (fEGM), or based on anatomical landmarks.1–5 Although varying in efficacy, these approaches may be hampered by limited sensitivity and specificity, unintended AF induction and interpatient variability. The CT-assisted CNA6 and CT-based identification of EFP to target GPs during AF ablation7 have been reported. We sought to assess feasibility of CT-based EFP-guided CNA in patients with CI-VVS.
We enrolled 12 patients (six males, 53 ± 13 years) with multiple episodes of CI-VVS. Ten had head-up tilt-test (HUT) with cardio-inhibitory response (type 2A in three patients, type 2B in five and mixed response in two). One patient had a negative HUT, and one did not undergo the test. These two patients experienced spontaneous transient symptomatic high-degree AV block. Patients underwent electrophysiology study, including atropine test that ruled out organic sinus and AV node dysfunction. After discussion with patients on management options, CNA was proposed prior to committing to pacing. The study was approved from the Ethic Committee, and informed consent was obtained.
Contrast-enhanced CTs displaying right, left atrial (LA) anatomy, aorta and EFP near the area of anticipated GPs8 with attenuation −190 to −30 HU were segmented and exported using ADAS3D software (Galgo Inc). The following GPs were identified as EFP: left superior GP between left superior pulmonary vein (PV) and LA appendage, Marshall tract GP in the carina between left PVs, left inferior GP posteriorly to the left inferior PV, inferior paraseptal ganglionated plexus (IPSGP) between the posterior wall of LA and coronary sinus, superior paraseptal ganglionated plexus (SPSGP) between the right superior PV and superior vena cava, right inferior GP (RIGP) between the two right PVs, and aorta-superior vena cava GP (Ao-SVC GP).
On the day of CNA, anatomical map of the LA was acquired using CARTO 3 (Biosense Webster, Diamond Bar, CA) and fused with CT. The EFP areas were searched for fEGMs (≥4 deflections) but ablated independently from fragmentation. The ablation end-point for each GP was defined as abolition of radiofrequency (RF)-induced vagal response for left PV GPs, an increase in basal HR ≥25% for SPSGP, RIGP and Ao-SVC GPs, and shortening of AH interval for IPSGP. In case RF over EFP did not elicit autonomic response, we targeted the closest GP area based on anatomical landmarks. Left PV GPs were ablated first, followed by right PV GPs, IPSGP and Ao-SVC GP.
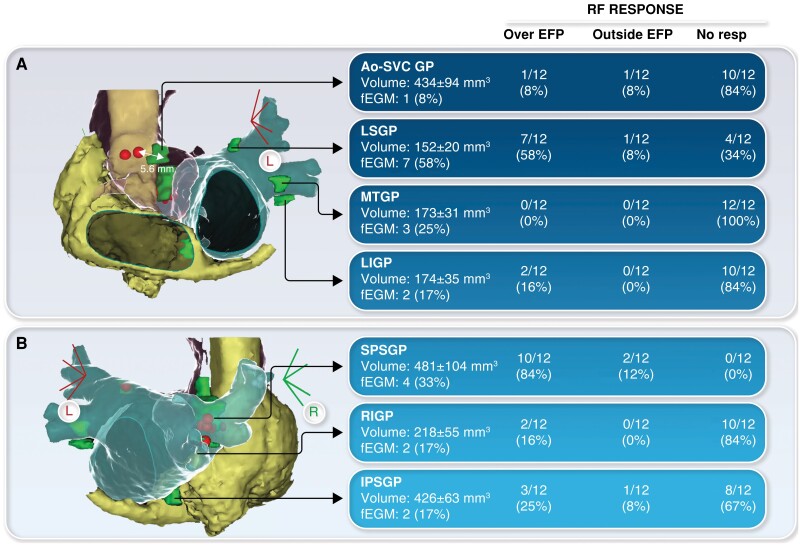
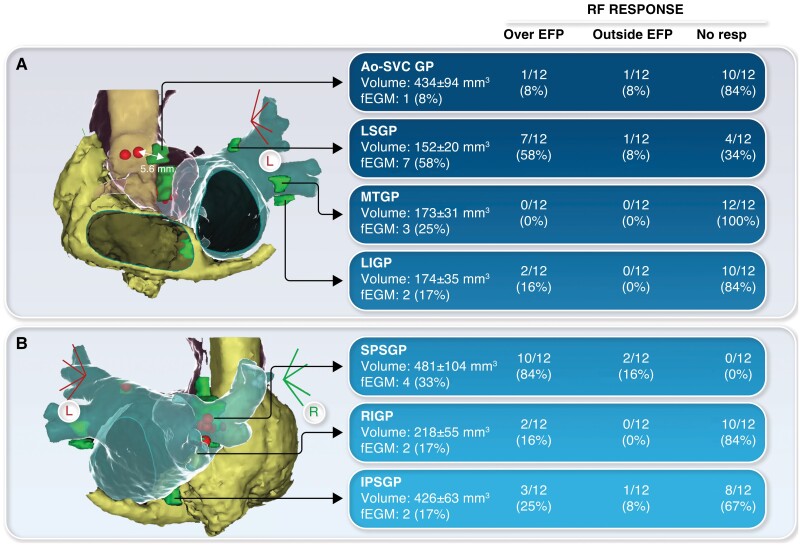
A total of 84 EFP were ablated in 12 patients (median RF applications: 2, IQR: 1–3; time: 57 s, IQR: 33–77). Response to RF for each EFP is shown in Figure 1. Overall, 25 (30%) EFP responded to RF. Superior paraseptal ganglionated plexus displayed the highest rate of RF response (100%) and the most consistent correlation between EFP position and post-RF HR increase (Figure 1). Five additional GPs (6%) responded close but outside the EFP, at the level of the predicted area (EFP-RF distance: 5.5 ± 1 mm). Therefore, out of 30 GPs that responded to RF, 25 (83%) were effectively ablated over the EFP. The RF delivery did not induce any response in 54 (64%) GPs either at the level of the EFP or at the predicted anatomical location. After CNA, atropine was administered showing flattened HR response (from 75 ± 24 to 78 ± 24 bpm) without substantial changes of sinus or AV node function in all but one patient, who still displayed significant HR increase (from 72 to 126 bpm). There were no procedure-related complications. Nine patients had follow-up >4 months (median: 5, IQR: 4–9), without syncope recurrences.
Figure 1.
Anatomic localization of epicardial fat pads (EFP) and corresponding ganglionated plexi (GPs). For each GP, radiofrequency (RF) response is reported according to the application site (over EFP, outside EFP, no response). (A) Disagreement between EFP and effective RF site at the level of the Ao-SVC GP. (B) In the same patient, effective RF over the EFP at the level of SPSGP. Ao-SVC GP, aorta-superior vena cava GP; IPSGP, inferior paraseptal GP; LIGP, left inferior GP; LSGP, left superior GP; MTGP, Marshall tract GP; RIGP, right inferior GP; SPSGP, superior paraseptal GP.
This study shows that CT-based EFP-guided CNA for CI-VVS is feasible, can assist RF delivery with high precision and has the potential to overcome the interpatient variability that affects CNA when performed solely by anatomic landmarks. Some EFP may not show autonomic response to RF due to previous successful ablation of a GP concealing subsequent GPs’ response. Alternatively, some CT-identified EFP might not contain GPs, as we did not use direct methods (i.e. extracardiac vagal stimulation) to prove EFP innervation. Of note, we did not target areas with vagal innervation (i.e. left interatrial septum)9 not associated with EFP. Further larger studies with longer follow-up are required to improve CT-based identification of GPs and our understanding of GP pathophysiology.
Contributor Information
Pietro Francia, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain; Cardiology Unit, Department of Clinical and Molecular Medicine, Sant’Andrea Hospital, University Sapienza, Rome, Italy.
Daniel Viveros, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain.
Giulio Falasconi, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain.
David Soto-Iglesias, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain.
Juan Fernández-Armenta, Cardiology Department, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Diego Penela, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain.
Antonio Berruezo, Arrhythmia Department, Teknon Heart Institute, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain.
Data availability
Data are available upon reasonable request.
References
- 1. Pachon JC, Pachon EI, Lobo TJ, Pachon MZ, Vargas RN, Jatene AD. ‘Cardioneuroablation’—new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 2005;7:1–13. [DOI] [PubMed] [Google Scholar]
- 2. Vandenberk B, Lei LY, Ballantyne B, Vickers D, Liang Z, Sheldon RSet al. Cardioneuroablation for vasovagal syncope: a systematic review and meta-analysis. Heart Rhythm doi: 10.1016/j.hrthm.2022.06.017 [DOI] [PubMed] [Google Scholar]
- 3. Pachon JC, Pachon EI, Cunha Pachon MZ, Lobo TJ, Santillana TG. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace 2011;13:1231–42. [DOI] [PubMed] [Google Scholar]
- 4. Pachon M JC, Pachon M EI, Pachon M JC, Lobo TJ, Pachon MZ, Vargas RNet al. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace 2004;6:590–601. [DOI] [PubMed] [Google Scholar]
- 5. Aksu T, De Potter T, John L, Osorio J, Singh D, Alyesh Det al. Procedural and short-term results of electroanatomic-mapping-guided ganglionated plexus ablation by first-time operators: a multicenter study. J Cardiovasc Electrophysiol 2022;33:117–22. [DOI] [PubMed] [Google Scholar]
- 6. Debruyne P, Rossenbacker T, Collienne C, Roosen J, Ector B, Janssens Let al. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction under computed tomographic guidance. Circ Arrhythm Electrophysiol 2018;11:e006604. [DOI] [PubMed] [Google Scholar]
- 7. Markman TM, Khoshknab M, Santangeli P, Marchlinski FE, Nazarian S. Feasibility of computed tomography-guided cardioneuroablation for atrial fibrillation. JACC Clin Electrophysiol 2022;8:1449–50. [DOI] [PubMed] [Google Scholar]
- 8. Aksu T, Gupta D, D’Avila A, Morillo CA. Cardioneuroablation for vasovagal syncope and atrioventricular block: a step-by-step guide. J Cardiovasc Electrophysiol 2022;33:2205–12. [DOI] [PubMed] [Google Scholar]
- 9. Pachon-M EI, Pachon-Mateos JC, Higuti C, Santillana-P TG, Lobo T, Pachon Cet al. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol 2020;13:e007900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.