Abstract
The sarcoplasmatic reticulum (SR) cardiac ryanodine receptor/calcium release channel RyR2 is an essential regulator of cardiac excitation–contraction coupling and intracellular calcium homeostasis. Mutations of the RYR2 are the cause of rare, potentially lethal inherited arrhythmia disorders. Catecholaminergic polymorphic ventricular tachycardia (CPVT) was first described more than 20 years ago and is the most common and most extensively studied cardiac ryanodinopathy. Over time, other distinct inherited arrhythmia syndromes have been related to abnormal RyR2 function. In addition to CPVT, there are at least two other distinct RYR2-ryanodinopathies that differ mechanistically and phenotypically from CPVT: RYR2 exon-3 deletion syndrome and the recently identified calcium release deficiency syndrome (CRDS). The pathophysiology of the different cardiac ryanodinopathies is characterized by complex mechanisms resulting in excessive spontaneous SR calcium release or SR calcium release deficiency. While the vast majority of CPVT cases are related to gain-of-function variants of the RyR2 protein, the recently identified CRDS is linked to RyR2 loss-of-function variants. The increasing number of these cardiac ‘ryanodinopathies’ reflects the complexity of RYR2-related cardiogenetic disorders and represents an ongoing challenge for clinicians.
This state-of-the-art review summarizes our contemporary understanding of RYR2-related inherited arrhythmia disorders and provides a systematic and comprehensive description of the distinct cardiac ryanodinopathies discussing clinical aspects and molecular insights. Accurate identification of the underlying type of cardiac ryanodinopathy is essential for the clinical management of affected patients and their families.
Keywords: RYR2, Catecholaminergic polymorphic ventricular tachycardia, RYR2 exon-3 deletion syndrome, Cardiac ryanodinopathy, Calcium-release deficiency syndrome
Graphical Abstract
Graphical abstract.
What’s new?
This state-of-the-art review summarizes our contemporary understanding of RYR2-related inherited arrhythmia disorders and provides a systematic and comprehensive description of the distinct cardiac ryanodinopathies that have been identified over the past decades.
Accurate identification of the underlying type of cardiac ryanodinopathy is essential for the clinical management of affected patients and their families.
Introduction
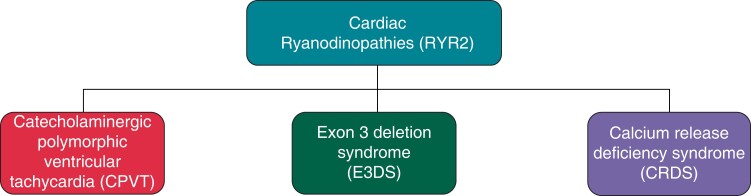
The sarcoplasmatic reticulum (SR) cardiac ryanodine receptor/calcium release channel RyR2 is an essential regulator of cardiac excitation–contraction coupling and intracellular calcium homeostasis.1 The RYR2 gene was first linked to cardiac disorders when studies by Swan et al. and Priori et al. identified RYR2 mutations as a genetic cause of catecholaminergic polymorphic ventricular tachycardia (CPVT) more than 20 years ago.2,3 Since that time, other distinct inherited arrhythmia syndromes have been related to abnormal RYR2 function (Figure 1). The increasing number of these cardiac ‘ryanodinopathies’ reflects the complexity of RYR2-related cardiogenetic disorders and represents an ongoing challenge for clinicians. In addition to CPVT, there are at least two other distinct RYR2-ryanodinopathies: exon 3 deletion syndrome (E3DS) and calcium release deficiency syndrome (CRDS; Figure 1). The pathophysiology of the different cardiac ryanodinopathies is characterized by complex mechanisms resulting in spontaneous diastolic SR calcium release or SR calcium release deficiency.
Figure 1.
Cardiac ryanodinopathies. CPVT, catecholaminergic polymorphic ventricular tachycardia; CRDS, calcium release deficiency syndrome; E3DS, RYR2 exon-3 deletion syndrome.
The aim of this review is to provide an overview about the clinical aspects and molecular insights of the known cardiac ryanodinopathies.
Molecular and genetic aspects of RYR2
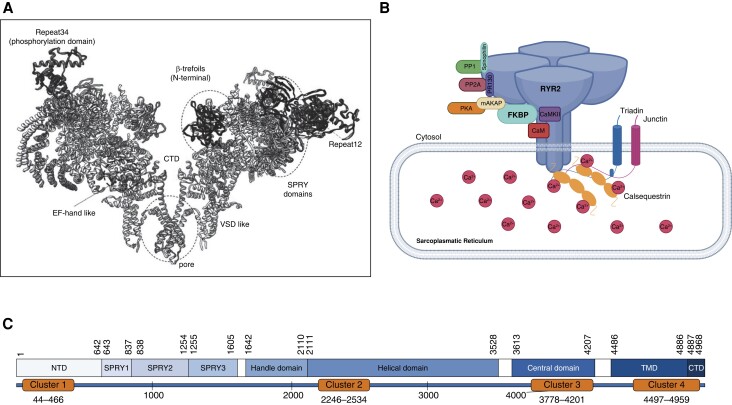
The cardiac ryanodine receptor (RyR2) is a calcium-release channel encoded by the RYR2 gene which is located on chromosome 1 (1q42.1-q43) and comprises 105 exons.1,4RYR2 translates into a large protein of 565 kDa that forms a large homotetrameric channel (∼2.2 MDa) that is located within the membrane of the SR (Figure 2).1,4 The N-terminal part of the protein accounts for ∼90% of the entire protein mass, while the actual transmembrane pore region accounts for only ∼10%.1,5 The complex regulation of the RyR2 channel activity is mediated by Ca2+ itself and numerous other modulators including complex protein interactions at both the cytosolic and intraluminal side (Figure 2B).5 An in-depth discussion of the complex function and regulation of the RyR2 channel would be beyond the scope of this article, and these aspects have been reviewed elsewhere.6–8
Figure 2.
RyR2 molecule. (A) Three-dimensional structure of RyR2 (Protein Data Bank identification code 6JI0). (B) Regulation of the RyR2 complex. CaM, calmodulin; CaMKII, Ca2+/calmodulin-dependent protein kinase II; FKBP, FK506-binding protein; mAKAP, muscle-specific A kinase–anchoring protein; PKA, protein kinase A; PM, plasma membrane; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; PR130, regulatory subunit of PP2A. (C) Schematic organization of the linear sequence of RyR2 with major structural domains of RyR2 (blue boxes). Orange boxes indicate four disease-associated variant clusters (variant hotspots). Adapted with permission from Zhong et al.85
The vast majority of pathogenic RYR2 mutations are missense variants (86–92%) (Human Gene Mutation Database version 2022.1, ClinVar April 2021).9–11 Bioinformatic data and in silico tools suggest that RyR2 poorly tolerates genetic variants that induce loss-of-function (LOF) properties.12 However, limitations of these predictive data as well as the significant rate of genetic background noise in the healthy population are challenges that may lead to incorrect interpretation of rare RYR2 variants.13,14 To compensate for this problem, approaching CPVT diagnosis with a probabilistic mindset can help to overcome the estimated 3% incidence of benign heterozygous rare RYR2 variants in the general population.14–16 In the case of CPVT, it has been shown that using a Bayesian approach that considers the pretest probability of disease may be useful in reclassifying variants of unknown significance.17 Keeping in mind the inherent limitations of statistical genetic methods (including Bayesian), it is important to highlight that diagnostic certainty can eventually only been achieved using a comprehensive multifactorial approach integrating statistical genetics into phenotype information (including familial segregation) and ideally functional data from cellular or animal models.
Catecholaminergic polymorphic ventricular tachycardia
Clinical aspects
Cardiac ryanodinopathies are rare disorders, with CPVT being the most common and most extensively studied phenotype. The estimated prevalence of classical CPVT is probably less than 1:10 000.18 Catecholaminergic polymorphic ventricular tachycardia is a pure channelopathy that is not associated with structural heart disease. Ventricular arrhythmia is characterized by multifocal and polymorphic ventricular ectopy and ventricular tachycardia/ventricular fibrillation that is frequently triggered by adrenergic stimulation. Typical clinical manifestations of CPVT include syncope or sudden cardiac arrest during exercise or with emotional triggers.18–20
In symptomatic patients, CPVT usually manifests during childhood with a median age of disease onset at 10–11 years; however, late onset during the 3rd or 4th decade has also been reported.21–24 Data from the international paediatric CPVT registry suggest that three quarters of symptomatic cases present with syncope and a quarter with sudden cardiac arrest.21,23,25 A family history of unexplained sudden cardiac death in individuals younger than 40 years is present in up to 30% of probands.20,26 However, the high symptom burden reported in many studies is often explained by proband-enriched cohorts and that the clinical spectrum of CPVT shows variable (typically mutation-dependent) phenotype expression and penetrance including a substantial proportion of more benign forms.27 For example, large CPVT kindreds, most notably related to the R420W variant in the Netherlands and the G357S variant in the Canary Islands, demonstrate that fairly benign familial forms of CPVT exist when extensive screening is undertaken.28,29
Unlike other hereditary channelopathies, the resting ECG in CPVT is normal. Prominent U waves and relative, asymptomatic sinus bradycardia on resting ECGs have been reported by some authors.19,24,30,31 Subclinical chronotropic insufficiency unmasked by exercise treadmill testing has been described in small series of paediatric patients with CPVT.32 At least in paediatric cohorts, subclinical sinus node dysfunction off beta blocker therapy has been associated with increased ventricular arrhythmia score on treadmill testing and seems to be a risk predictor for arrhythmic events during follow-up.32
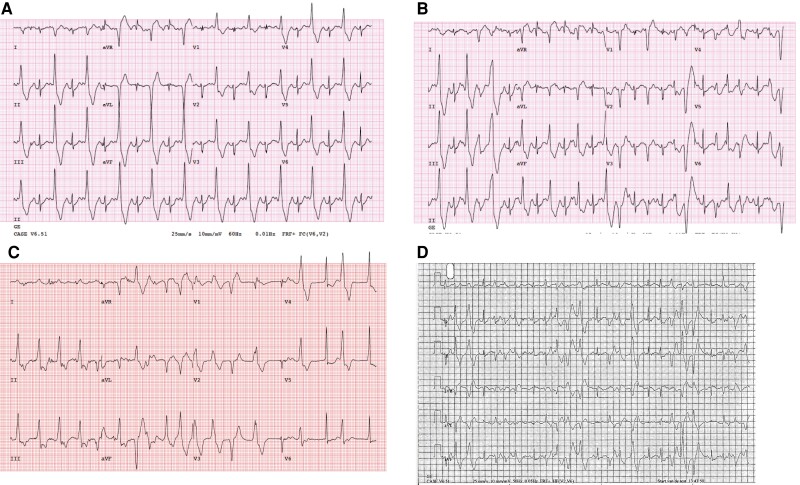
Exercise treadmill testing is the gold standard to unmask CPVT-related ventricular ectopy. Typical findings include a progressive burden of ventricular ectopy, with often a clear window of electrical vulnerability occurring early in exercise and before maximal exercise.33 The typical exercise threshold to unmask ventricular ectopy or tachycardia is a heart rate window from 110–150 b.p.m.24,33,34 With ongoing exercise, the ectopy burden may eventually decrease in a subset of CPVT patients.35 Ventricular ectopy typically starts with isolated, late-coupled premature ventricular contractions (PVCs) with increasing complexity (multifocal and/or bidirectional PVCs, and ventricular couplets/triplets) that may result in non-sustained or sustained polymorphic or bidirectional ventricular tachycardia (Figure 3). Although bidirectional ventricular tachycardia has been recognized as a signature arrhythmia of CPVT (Figure 3), its true prevalence remains unknown; it appears much less common than previously reported.20,23 QT dynamics at rest and during exercise are strictly normal in CPVT.23
Figure 3.
Catecholaminergic polymorphic ventricular tachycardia phenotype. (A) Exercise treatment test of a 32-year-old female patient with RYR2-related CPVT. Inducible ventricular ectopy with monomorphic bigeminal PVCs at Stage 2 (4:22 min) and a heart rate of 134 b.p.m. (B) Same patient as (A): at Stage 3 (6:11 min), intermittent manifestation of bidirectional PVCs. (C) 67-year-old male with RYR2-related CPVT. Run of non-sustained polymorphic ventricular tachycardia at peak Stage 4 of an exercise treadmill test (11:00 min). (D) Shown are intermittent runs of non-sustained bidirectional ventricular tachycardia.
In addition to ventricular arrhythmias, individuals with CPVT may also develop various types of supraventricular arrhythmias including atrial tachycardia, atrial flutter, or atrial fibrillation all reflecting the underlying abnormal calcium handling.7,19,36 In the context of CPVT, this may even result in atypical clinical scenarios such as the manifestation of atrial fibrillation during early infancy.37
Diagnosis of CPVT relies on appropriate phenotype assessment in conjunction with comprehensive genetic testing. Additional tools like a recently published CPVT scoring algorithm (see Supplementary material online, Figure S1) may facilitate the accuracy of CPVT diagnosis, in particular in suspected CPVT patients with RYR2 variants of unknown significance.17 Referral to a specialized cardiogenetic clinic is recommenced for all individuals with suspected or confirmed CPVT to ensure adequate diagnosis, including interpretation of genetic test results, optimizing medical treatment, stratification for the risk of sudden cardiac death, and appropriate family screening.38
The cornerstone of CPVT management is appropriate beta blocker treatment. Nadolol, an unselective beta blocker, is the most effective agent to prevent breakthrough arrhythmia in CPVT and should be titrated to target daily doses of at least 1 mg/kg, but a goal of ≥1.5 mg/kg is ideal if tolerated and/or signs of CPVT are still present on exercise testing.33,39 Nadolol access can be challenging in some countries, and propranolol is a recommended alternative.
In the case of beta blocker intolerance or failure despite adequate dosing, flecainide should be added (target dose of 2.0–3.0 mg/kg per day).28,30,40,41 Some patients will experience cardiac breakthrough events despite optimized antiarrhythmic medication. Invasive treatment options include left cardiac sympathetic denervation (LCSD) that should be considered in individuals with drug refractory CPVT, intolerance or non-adherence to drug therapy, and some very high risk individuals in addition to standard medication.42–44 When performed by experienced centres, LCSD is highly effective, reducing the risk of major cardiac events by 70–90% and the risk of appropriate implantable cardioverter defibrillator (ICD) shocks by 93%.44–47
Exercise stress testing is integral to optimizing these therapies. Early data showed that suppression of ventricular ectopy during exercise testing was associated with a lower risk of events and that over time, serial exercise testing results are generally reproducible.39,48 Emerging data also suggest that an exercise stress test protocol involving an initial sprint may be more sensitive and could be useful to confirm adequate arrhythmia suppression after therapies appear to be optimized.49
The role and benefits of ICD insertion in CPVT remain controversial. Current guidelines recommend ICD insertion for secondary prevention after resuscitated sudden cardiac arrest or for primary prevention in individuals with arrhythmic syncope while on appropriate antiarrhythmic medication.43,44 Special considerations may apply to paediatric CPVT patients given their particular vulnerability to device-related complications in the short and long term. The 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients suggest that ‘In selected patients with aborted SCA as the initial presentation of CPVT, pharmacologic therapy and/or cardiac sympathetic denervation without ICD may be considered as a possible alternative.’50
In the absence of prospective, large-scale studies, the question of a survival benefit in recipients of a secondary prevention ICD remains unanswered. Several studies reported no survival benefit and a high rate of device-related complications including inappropriate shocks in 20–25% and hardware-related complications in 29–32% over a median follow-up of 4–5 years.51–53 This is in contrast to the findings of another group reporting lower rates of device-related complications and a potential survival benefit.54 Appropriate ICD therapies typically occur in patients without appropriate antiarrhythmic medication.51 On the other hand, 1–4% of CPVT patients experience sudden cardiac death despite an ICD.51,52 This is in part due to shocks that are a powerful adrenergic trigger for recurrent polymorphic VT and subsequent degeneration to ventricular fibrillation, potentially leading to exhaustion of available ICD shock delivery (typically after six shocks). Overall, contemporary data suggest the importance of a careful and comprehensive patient evaluation in an expert setting with shared decision-making to avoid unnecessary, potentially harmful ICD implantation.55 If ICD implantation is inevitable, it is important to ensure adequate device programming including long detection delays and high cut-off rates for shock delivery to minimize the risk of inappropriate shocks and enabling spontaneous termination. In the absence of prospective ICD data in CPVT patients, the suggestions of device programming are extrapolated and adapted from the general concepts of adequate contemporary ICD programming.56,57
Genetic and electrophysiological aspects
Pathogenic RYR2 variants account for at least half but probably over 80% of all CPVT cases, and transmission is typically autosomal-dominant.21,58 In addition, there are rare cases of RYR2-related CPVT with autosomal-recessive transmission.59 Other genetic substrates of CPVT have been extensively reviewed elsewhere and will not be discussed in this article.7,58 The majority of CPVT-associated RYR2 mutations are missense variants (96%) resulting in a gain-of-function (GOF) of the RyR2 channel.7,58 The distribution of CPVT RYR2 variants shows a clustering within four distinct mutational hotspot regions including exons 3–15 (amino acids 44–466), 44–50 amino acids (2246–2534), 83–90 (amino acids 3778–4201), and exons 93–105 (amino acids 4497–4959) (Figure 2C).15,25,60 Three of these cluster regions are located within the N-terminal portion of the RYR2 protein. Only 10% of CPVT-related variants are located outside these mutational hotspot regions.15 A substantial proportion of monogenetic RYR2-related CPVT cases is related to de novo variants.27,61,62 Interestingly, RYR2 de novo variants are more likely to be located within the C-terminus domain compared to familial RYR2 variants (more likely within the N-terminal domain).62 Limited data suggest that disease manifestation in probands harbouring de novo variants is earlier and phenotype traits seem to be more severe compared to probands with familial forms of CPVT and RYR2 variants at other locations.62 Interestingly, an association between C-terminal RYR2 variants and increased risk for ventricular arrhythmia has also been observed in familial forms of CPVT.25,27,54 Disease penetrance is incomplete and may be mutation-dependent. Previous studies suggested a penetrance of 50–65%.27,63
The arrhythmogenesis of CPVT is complex and implies different, mutation-dependent mechanisms affecting the Ca2+ activation, protein folding, or binding sites of regulatory proteins of RyR2.7,18,64 At the cellular level, RYR2 GOF variants result in abnormal calcium handling with spontaneous diastolic calcium release which in turn triggers delayed afterdepolarizations that initiate ventricular arrhythmia.7,65,66 Corresponding cellular electrophysiological findings of CPVT are depicted in Tables 1 and 2 and in the Central Illustration. One important mechanism is mediated by a reduced threshold for the occurrence of store overload-induced Ca2+ release (SOICR) which is favoured by an increased sensitivity of the RyR2 channel to the activation by luminal and/or cytosolic Ca2+ (Central Illustration).67,68 Typical conditions leading to increased sarcoplasmic reticulum calcium load include stimulation with catecholamines, for example, isoproterenol.65,66 Another typical cellular electrophysiological finding of abnormal calcium handling is the enhanced response to caffeine-induced calcium release from the sarcoplasmic reticulum.66,68,69 Another prerequisite for the arrhythmogenesis in CPVT is the existence of an arrhythmic heart rate window as described above that determines the balance of SR calcium loading and spontaneous diastolic calcium release.35 Experiments in mice and humans showed that increasing the sinus rate through vagolytic pretreatment with atropine or atrial overdrive pacing significantly reduces the occurrence of exercise-induced ventricular arrhythmia.35,70 A likely explanation for this observation is that shortening the diastole prevents spontaneous SR calcium release.7,70
Table 1.
Arrhythmogenesis and cellular electrophysiological findings of cardiac ryanodinopathies
| Caffeine-induced Ca2+ release |
SOICR | Isoproterenol stimulation | DAD | EAD | |
|---|---|---|---|---|---|
| CPVT | ↑↑ | ↑↑ | ↑↑ DAD + ventricular arrhythmia | + | − |
| Exon 3 deletion syndrome | ND | Impaired termination | ↑↑ DAD + ventricular arrhythmia? | + | − |
| CRDS | ↓↓ | ↓↓ | − | − | + |
CPVT, catecholaminergic polymorphic ventricular tachycardia; CRDS, calcium release deficiency syndrome; DAD, delayed afterdepolarization; EAD, early afterdepolarization; ND, not determined; SOICR, store overload-induced Ca2+ release.
Table 2.
Clinical features and ECG characteristics of cardiac ryanodinopathies
| QTca | Exercise-induced ventricular ectopy | Syncope/presyncope prior to sudden cardiac arrest | Bidirectional VT | |
|---|---|---|---|---|
| CPVT | Normal | Common | Common | Yes |
| Exon 3 deletion syndrome | Normal | Common | Possible | Yes |
| CRDS | Normal | Infrequent | Possible | No |
CPVT, catecholaminergic polymorphic ventricular tachycardia; CRDS, calcium release deficiency syndrome; QTc, corrected QT interval.
At rest and during/post exercise.
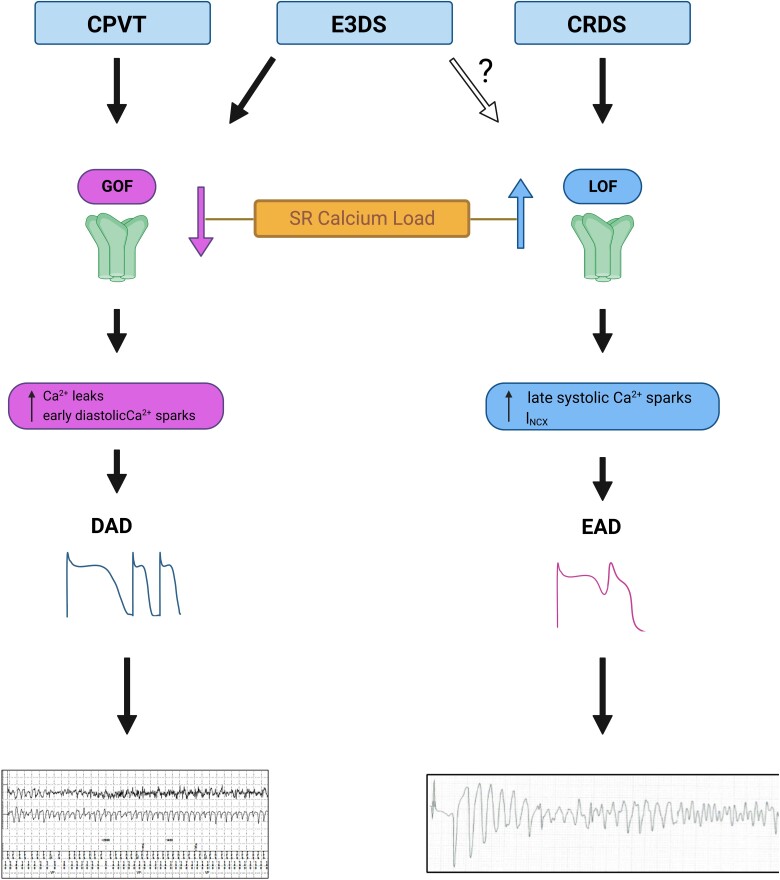
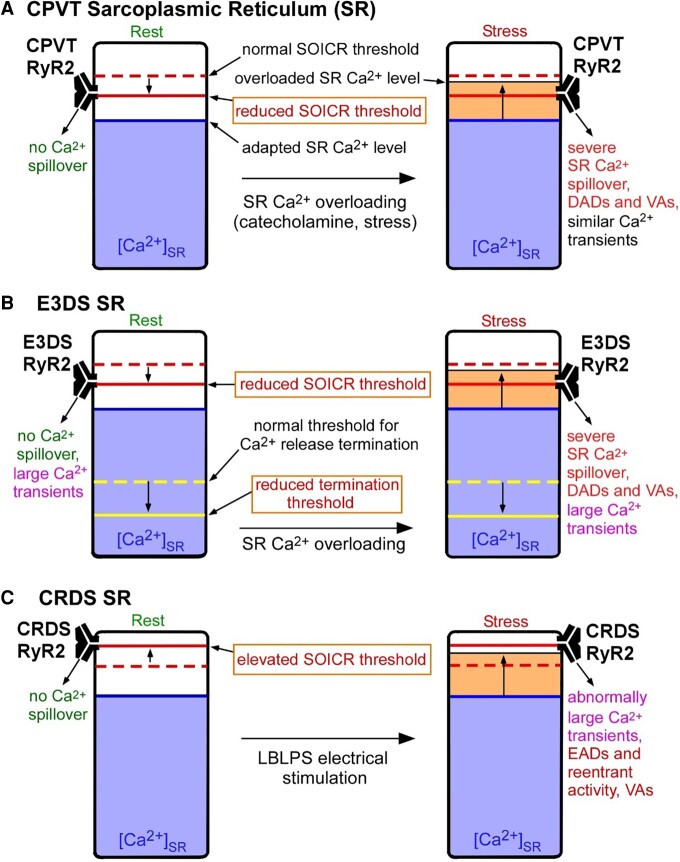
Central Illustration.
Proposed mechanisms for RyR2-associated catecholaminergic polymorphic ventricular tachycardia (CPVT), exon 3 deletion syndrome (E3DS), and calcium release deficiency syndrome (CRDS). The different thresholds for store overload-induced Ca2+ release (SOICR) and Ca2+ release termination and the free sarcoplasmic reticulum (SR) luminal Ca2+ levels in CPVT, E3DS, or CRDS associated with RyR2 mutations are illustrated in the resting state (Rest, left panels) and in the stress states (Stress, right panels). The normal thresholds for SOICR and Ca2+ release termination are depicted as red and yellow dashed bars, respectively. The reduced or elevated SOICR thresholds as a consequence of CPVT, E3DS, or CRDS RyR2 mutations are depicted as solid red bars. The reduced threshold for Ca2+ release termination as a consequence of E3DS RyR2 mutations are depicted as solid yellow bars. The SR free luminal Ca2+ level is represented as a blue area. The yellow areas above the blue areas in the right panels represent an elevation, even if only transient, in the free SR luminal Ca2+ levels, which, we propose, will occur when sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) activity is enhanced by catecholamines or during the long-burst, long-pause, short-coupled (LBLPS) programed electrical stimulation. When the SR-free luminal Ca2+ level surpasses, even transiently, the reduced SOICR threshold in the case of CPVT and E3DS, SOICR occurs, leading to a spillover of SR Ca2+ that can trigger spontaneous Ca2+ release, delayed afterdepolarizations (DADs) and ventricular arrhythmias (VAs). The reduced termination threshold in the case of E3DS will increase the fractional Ca2+ release, resulting in large Ca2+ transients at rest (left panels) and stress (right panels) that may promote cardiomyopathies in addition to cardiac arrhythmia. In the case of CRDS, the elevated SOICR threshold prevents spontaneous SR Ca2+ leak, leading to markedly elevated SR Ca2+ load upon LBLPS electrical stimulation and subsequently large Ca2+ transients that promote early afterdepolarizations (EADs), reentrant activity, and ventricular arrhythmias.
RyR2 exon 3 deletion syndrome
Clinical aspects
First described in 2007,71 the RyR2 E3DS has now been recognized as a distinct entity among the different cardiac ryanodinopathies.72–77 With less than 50 cases published to date, E3DS represents a very rare RYR2-ryanodinopathy. Assuming that deletion of exon 3 accounts for ∼1% of pathogenic RYR2 variants, the prevalence of E3DS might be estimated at 1:100 000.
In contrast to the exclusive tachyarrhythmias of other cardiac ryanodinopathies, E3DS is characterized by a complex pleiotropic tachycardia–bradycardia phenotype (Table 3). The mixed phenotype shows marked interindividual variability within affected families. A typical example of the marked phenotype variability is displayed in Figures 4 and 5, showing examples from a large French–Canadian family affected by E3DS.
Table 3.
Phenotype findings in E3DS
| Clinical findings in RYR2 exon 3 deletion syndromea | |
|---|---|
| Sinus node dysfunction | 58% |
| Atrial standstill | 7% |
| AV node conduction disordersb | 22% |
| Atrial fibrillation/atrial flutter | 18% |
| Atrial tachycardia or other SVT | 36% |
| CPVT-like ventricular arrhythmias | 56% |
| Sudden cardiac death | 11% |
| Dilated cardiomyopathy ± left ventricular non-compaction | 18% |
| Mitral valve prolapse | 4% |
AV, atrioventricular; CPVT, catecholaminergic polymorphic ventricular tachycardia; SVT, supraventricular tachycardia.
All estimates are based on a pooled analysis of published reports and the first author’s own cohort of E3DS patients. The definition of the different rhythm disorders and cardiomyopathies is based on current guidelines. Only rhythm disorders with appropriate clinical testing and description in the corresponding articles were retained for data collection and analysis. Given the variable extent of clinical testing and limited follow-up data in the reviewed literature, the pooled findings may only provide crude estimates of the frequency of E3DS-related rhythm disorders.
Including second-degree, third-degree, or high degree AV block.
Figure 4.
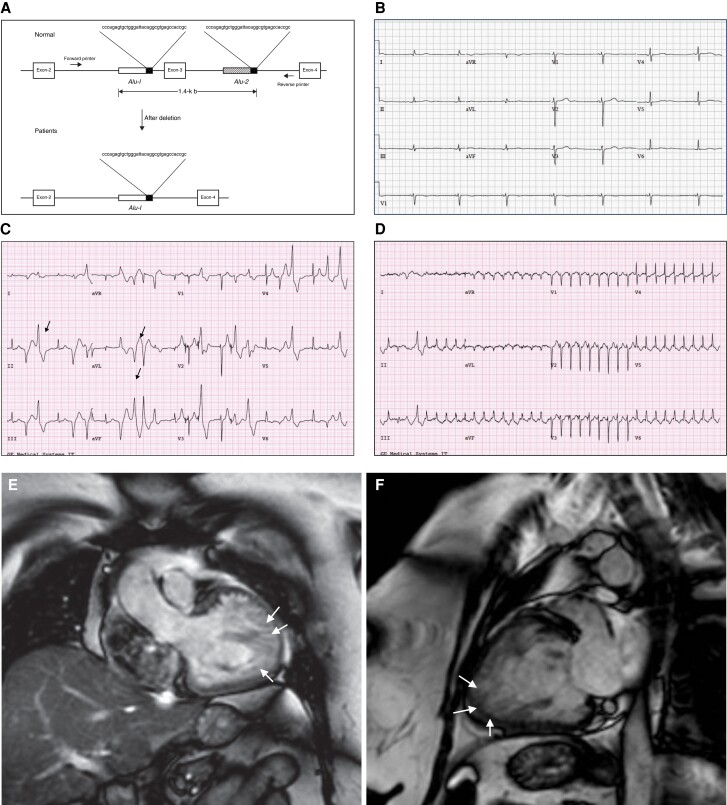
Exon 3 deletion syndrome clinical phenotype. (A) Alu repeat–mediated RYR2 exon 3 deletion. The diagram represents the Alu-Alu recombination. Alu sequences are located in intron 2, 190 bp upstream from exon 3 and also 536 bp downstream in intron 3. Adapted with permission from Bhuiyan et al.71 (B) 34-year-old female patient with E3DS. Resting ECG showing marked junctional bradycardia at 42 b.p.m. The patient had symptomatic sinus node disease with chronotropic insufficiency. (C) 48-year-old female patient with E3DS. Exercise treadmill testing showed inducible polymorphic ventricular ectopy with intermittent bidirectional ventricular PVCs (arrows). (D) Same patient as (C). Induction of supraventricular tachycardia at peak exercise. The echocardiogram showed a normal sized left ventricular with preserved ejection fraction. However, there was evidence of marked apical and apico-lateral trabeculation meeting criteria for LVNC. (E) and (F) Cardiac magnetic resonance imaging of a 52-year-old female patient with E3DS. The patient developed dilated cardiomyopathy with LVNC and marked systolic dysfunction requiring the insertion of a cardiac resynchronization therapy defibrillator. Note the marked left ventricular apical and apico-lateral hypertrabeculations (arrows).
Figure 5.
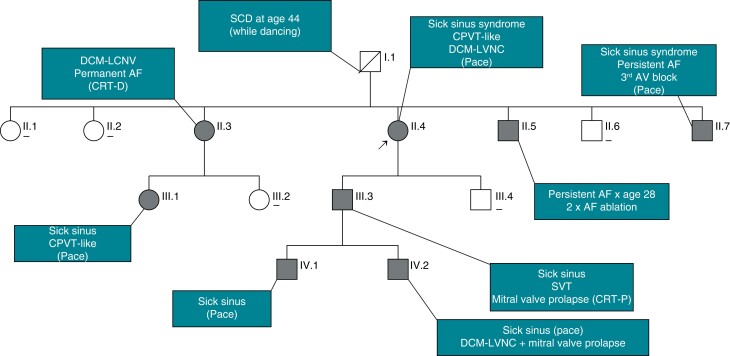
Exon 3 deletion syndrome phenotype diversity. Shown is the pedigree of a large family with RYR2 exon-3 deletion syndrome; [-] indicates gene-negative individuals; filled circles or squares indicates heterozygous carriers with E3DS phenotype; AF, atrial fibrillation; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; CRT-P, biventricular pacemaker; DCM–LCNV, dilated cardiomyopathy with left ventricular non-compaction; SCD, sudden cardiac death.
Given its rarity and the paucity of published data, the frequency of the various electrophysiological and structural features of E3DS can only be estimated. Table 3 depicts the spectrum of relevant clinical features associated with E3DS. Sinus node dysfunction manifesting with symptomatic sinus bradycardia, chronotropic insufficiency, sinus arrest, or atrial stillstand represents the leading bradyarrhythmia in E3DS (58%) (Table 3). Atrioventricular (AV) node conduction disorders have been described in up to 22% of gene carriers. Atrial fibrillation/flutter and other supraventricular tachycardias are common and will develop in more than half of affected patients over time. Ventricular arrhythmia is characterized by a CPVT including exercise-triggered ventricular ectopy, bidirectional ventricular tachycardia, as well as polymorphic VT/ventricular fibrillation.71–75,77,78 A family history of sudden cardiac death is present in at least 10% of affected individuals.
Isolated left ventricular non-compaction (LVNC) without ventricular enlargement or left ventricular systolic dysfunction has been reported in up to 31% of patients with E3DS.75,78 True dilated cardiomyopathy with or without LVNC seems to be less common and is observed in up to 18% of cases (Table 3). A novel but potentially underreported finding is the co-association of mitral valve prolapse that was observed our French–Canadian cohort (Figure 5).
The mixed tachycardia–bradycardia phenotype of E3DS represents a major challenge for pharmacologic management. Patients with E3DS are extremely sensitive and often intolerant to various antiarrhythmic or heart rate slowing medication (personal, unpublished observations), which is typically required to treat the CPVT phenotype and the common supraventricular arrhythmias. Even very small doses of cardioselective beta blockers or calcium channel blockers may result in excessive symptomatic bradycardia (personal unpublished observation).76,77 Consequences of the marked pharmacological intolerance and the frequent severe sinus node dysfunction include early insertion of cardiac implantable electronic devices. Appropriate device selection may be challenging and requires a thorough and comprehensive evaluation as most E3DS patients will become recipients at young age.
Genetic and electrophysiological aspects
The deletion of exon 3 (c.161 to c.272) and the subsequent genetic rearrangement result in an in-frame deletion of 35 amino acids of the RyR2 N-terminal domain A (p.Asn 57_Gly91del; NM_001035) (Figure 4A).71,79 The estimated size of genomic deletion is 241 bp affecting the entire exon 3 and the flanking introns 2 and 3.71,78 The molecular mechanisms leading to exon 3 deletion remain incompletely understood. Bhuiyan and coworkers proposed polymerase slippage as a result of Alu-I transposon repeats.71
Structural modelling suggests that the altered RyR2 protein facilitates pore opening and in turn diastolic spontaneous calcium release.79 At the cellular level, E3DS increases the propensity for SOICR by reducing the threshold for SOICR activation. Exon 3 deletion syndrome also increases the amplitude of SOICR by impairing calcium release termination or disinhibition of the RyR2 channel (Central Illustration).80
Exon 3 deletion syndrome shows full penetrance but variable disease expression. So far, no homozygous gene carriers and no asymptomatic carriers of E3DS have been reported. The marked phenotype variability even within a single family (Figure 5) may be related to yet unknown genetic modifiers and/or epigenetic factors. Transmission of E3DS is typically autosomal dominant, but de novo events have also been described.76 The age of onset is highly variable and can be as young as 7 years. Virtually all gene carriers will present with some phenotype traits by 45 years of age (personal unpublished observations). The totality of the data strongly supports classifying E3DS as a unique overlap of inherited arrhythmia and cardiomyopathy that is distinct from CPVT.
RyR2 calcium release deficiency syndrome
Clinical aspects
The RyR2 CRDS represents a novel, emerging cardiac ryanodinopathy and has only recently been characterized.81–84 Given its infancy as an established syndrome and the challenges surrounding diagnostic testing that will be discussed, the prevalence of CRDS is unknown. Average age of presentation appears to be in early adulthood, but index events during childhood and preadolescence are not uncommon.82
Despite some overlapping clinical features, there is growing evidence that CRDS is a phenotypically and mechanistically distinct RYR2-related channelopathy that differs from classic CPVT. Individuals with CRDS are susceptible to malignant ventricular arrhythmia (polymorphic ventricular tachycardia and ventricular fibrillation) resulting in syncope or sudden cardiac death.81–85
Unlike classic CPVT, only 50% of arrhythmic events in CRDS seem to occur during adrenergic stimulation.82 Similar to CPVT, the resting ECG is normal in CRDS, although one group has suggested that some patients may also exhibit mild corrected QT interval (QTc) prolongation.86 In contrast to CPVT, exercise treadmill testing has limited diagnostic value in CRDS and does not usually show complex polymorphic ventricular ectopy or bidirectional ventricular tachycardia.81–84 In the largest cohort of CRDS patients described so far (19 patients including probands and relatives), exercise treadmill testing was negative in 64% and showed only isolated ventricular PVCs in 34%.82 This lack of sensitivity is most likely related to the particular electrophysiological mechanisms that have been identified in CRDS (see below). The same mechanisms may also account for the incomplete penetrance and an overall lower rate of cardiac breakthrough events compared to classic CPVT.81,82 Sudden cardiac arrest or sustained ventricular arrhythmia occur in at least 23% of individuals with CRDS, though there is clear ascertainment bias in this estimate.82
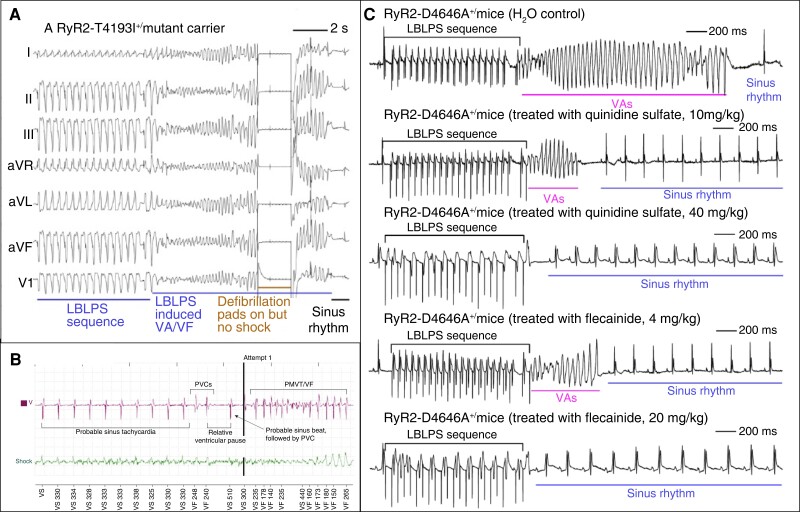
The most accurate clinical test to unmask CRDS is a specific protocol of programmed ventricular stimulation characterized by a long-burst, long-pause, and short-coupled ventricular extra stimulus (LBLPS) sequence (Figure 6A, B).81–83 Current data on the sensitivity and specificity of a dedicated LBLPS protocol are still limited. Initial experience in a limited number of individuals showed inducible ventricular arrhythmia in up to 78% of CRDS patients.81 The LBLPS sequence also reflects the arrhythmia onset of spontaneous events (Figure 6C).82 There is also emerging evidence that the same protocol may be useful to determine the efficacy of antiarrhythmic medication.81
Figure 6.
Ventricular arrhythmia induction in CRDS. (A) Ventricular fibrillation induction in CRDS using programmed ventricular stimulation. Shown are the ECG tracings of a CRDS proband carrying the RYR2-T4196I LOF variant. Ventricular arrhythmia is reproducibly precipitated using the LBLPS stimulation protocol (adapted with permission from Sun et al.83). (B) Spontaneous VF initiation in CRDS. Implantable cardioverter defibrillator tracings from a CRDS proband harbouring a RYR2 LOF variant. Shown is an episode of ventricular fibrillation preceded by sinus tachycardia. Ventricular tachycardia is then initiated by a sequence of two premature ventricular complexes, a long pause, a sinus beat, and a shorter coupled PVC (adapted with permission from Roston et al.82). (C) Effects of quinidine and flecainide on VA in D4646A +/− mutant mice. The tracing in the top row displays typical VF induction using a LBLPS stimulation (mice treated with H2O control). Tracings from the second and third row demonstrate a dose-dependent (10 mg/kg vs. 40 mg/kg per day) suppression of inducible ventricular arrhythmia after pretreatment with quinidine sulphate for 6 days. Similar effects on VA inducibility were also observed in mice after pretreatment with different doses (4 mg/kg vs. 20 mg/kg per day) of flecainide for 6 days. LBLPS, long-burst, long-pause, and short-coupled ventricular extra stimulus; PVC, premature ventricular contraction; VA, ventricular arrhythmia; VF, ventricular fibrillation (adapted with permission from Sun et al.83).
Data on the optimal medical management in CRDS remain sparse. Unlike CPVT, the benefit of beta blockers is less certain with breakthrough events in ∼20%,81,82 and experience with this class of medication almost exclusively stems from the initial misdiagnosis of CPVT that occurs in most patients. Potential antiarrhythmics include flecainide and quinidine, which effectively suppress ventricular fibrillation in a CRDS mouse model.83 Flecainide monotherapy also suppressed LBLPS-inducible ventricular arrhythmia in 89% of human adults with CRDS.81 In contrast, the combination of flecainide with metoprolol seems to abolish the protective effect of flecainide in a significant proportion of patients; however, convincing follow-up data supporting these findings are yet missing.81 Clearly, further studies with larger cohorts and longer follow-up periods will be required to identify the best antiarrhythmic treatment strategies in CRDS. In addition to the knowledge gaps with regard to medical treatment, the role of primary prevention ICDs in CRDS remains undefined at this point.’
Genetic and electrophysiological aspects
The genetic substrate of CRDS is RYR2 mutations resulting in LOF. These LOF variants are predominantly located in the C-terminal Cluster 4 region, which is also a well-known mutational hotspot for CPVT.15,60,81–84 The vast majority of CRDS-related mutations are RYR2 missense variants with autosomal dominant transmission. An exception is a particularly aggressive form of autosomal recessive CRDS that has been linked to a copy number variant mutation of RYR2.84 The tandem duplication including the 5′ untranslated region of the RYR2 promoter region and exons 1 through 4 has only been identified in Amish communities, so far.84 The resulting haploinsufficiency translates into a highly penetrant and lethal phenotype (78% of sudden cardiac arrest or death).84,87
With the exception of this very aggressive phenotype, current data on other CRDS-associated variants do not yet allow the determination of mutation-dependent disease severity. Unlike CPVT, CRDS has only been linked to RYR2, and no other genetic substrates have been described, so far.
Functional data from cellular and animal models suggest an arrhythmogenesis distinctly different from CPVT. Loss-of-function is characterized by a significant decrease of the propensity for stress-induced ventricular arrhythmia upon stimulation with caffeine or isoproterenol compared to wild type RyR2 channels.81–83,85 In a similar way, SOICR is markedly inhibited resulting in a decreased propensity for delayed afterdepolarizations (Central Illustration).81 Additional findings of the electrophysiological remodelling include increases of ICa-L, Ito, and INCX currents and a hyperpolarization shift of the voltage-dependent activation of the cardiac sodium channel Nav1.5.83,88 The result is a prolonged duration of the cardiac action potential with increased propensity to early afterdepolarizations that may trigger reentrant ventricular arrhythmia.83,87,88
Other cardiac ryanodinopathies and overlap features
In addition to the distinct phenotypes of the classical RyR2 CPVT, CRDS, or E3DS, there is some evidence suggesting the existence of other forms of yet unclassified cardiac ryanodinopathies with atypical phenotypes and unusual electrophysiological properties85,86,89–92 (see Supplementary material online, Table S2). Although data on these atypical cardiac ryanodinopathies are restricted to case reports with limited segregation and clinical follow-up, the published reports highlight the incredible complexity of RYR2-related inherited arrhythmia syndromes. Many questions still remain unanswered. Some of the cardiac ryanodinopathies that present with atypical abnormalities on exercise testing may represent subtypes of CRDS, given that not all LOF carriers exhibit identical phenotypes. Thus, the clinical spectrum of cardiac ryanodinopathies may be even broader than appreciated, with disease entities yet to be discovered. These concepts suggest that both the amount and timing of calcium release and inhibition from RyR2 play an integral role in the maintenance of cardiac rhythm and structure and thus is process prone to marked pleiotropy.
Future directions
Large-scale international registries exist for CPVT and have provided the basis for much of our knowledge surrounding this condition. Similar collaborative approaches are also required for other cardiac ryanodinopathies to improve our understanding of the different phenotypes and clinical evolution of disease. Larger cohorts are also fundamental to design future studies to shift our current approach towards genotype-based disease management.93 Refined disease modelling incorporating novel technologies such as three-dimensional human-engineered heart tissue may provide us with complementary functional data for tailored therapies.94,95 Human inducible pluripotent stem cell (hiPSC) platforms are already increasingly being used for CPVT research with similar utility anticipated for other cardiac ryanodinopathies.96–99 Promising pathways for potential future curative gene therapy may include allele-specific gene silencing and targeted in vivo gene editing using viral vectors.100,101
Complementary functional genomic studies in conjunction with data from cellular models and clinical registries may help us to identify additional genetic and non-genetic cofactors that affect disease severity and prognosis.
In addition to gene-therapeutic strategies, a number of novel RyR2-specific pharmacological therapies are currently under investigation, although very few of them have entered the stage of clinical testing so far. One of them is the oral RyR2 modulator S48168 (ARM210) that will be assessed in CPVT patients in an upcoming Phase II trial starting in 2023 (NCT05122975). Other molecules with pharmacological properties for potential RyR2-directed precision medicine like EL20, K201, or ent-(+)-verticilide are still at the preclinical stage.102–105
Supplementary Material
Acknowledgements
We are deeply grateful to our patients for their participation in the studies that are reviewed in this article.
Contributor Information
Christian Steinberg, Institut universitaire de cardiologie et pneumologie de Québec, Laval University, 2725, Chemin Ste-Foy, Quebec G1V 4G5, Canada.
Thomas M Roston, Centre for Cardiovascular Innovation, Division of Cardiology, St. Paul’s Hospital, University of British Columbia, 211-1033 Davie Street, Vancouver, BC, V6E 1M7, Canada.
Christian van der Werf, Amsterdam UMC, Department of Clinical and Experimental Cardiology, University of Amsterdam, Heart Centre, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Shubhayan Sanatani, Division of Cardiology, Department of Pediatrics, BC Children’s Hospital, University of British Columbia, Vancouver, Canada.
S R Wayne Chen, Department of Physiology and Pharmacology, Libin Cardiovascular Institute, University of Calgary, Calgary, Canada.
Arthur A M Wilde, Amsterdam UMC, Department of Clinical and Experimental Cardiology, University of Amsterdam, Heart Centre, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Andrew D Krahn, Centre for Cardiovascular Innovation, Division of Cardiology, St. Paul’s Hospital, University of British Columbia, 211-1033 Davie Street, Vancouver, BC, V6E 1M7, Canada.
Supplementary material
Supplementary material is available at Europace online.
Funding
A.D.K. receives support from the Sauder Family and Heart and Stroke Foundation Chair in Cardiology (Vancouver, BC) and the Paul Brunes Chair in Heart Rhythm Disorders (Vancouver, BC). Christian Steinberg is a clinical research scholar of the Fonds de Recherche du Québec—Santé.
References
- 1. Kushnir A, Wajsberg B, Marks AR. Ryanodine receptor dysfunction in human disorders. Biochim Biophys Acta Mol Cell Res 2018;1865:1687–97. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise Ret al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001;103:196–200. [DOI] [PubMed] [Google Scholar]
- 3. Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen Ket al. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol 1999;34:2035–42. [DOI] [PubMed] [Google Scholar]
- 4. Woll KA, Van Petegem F. Calcium-release channels: structure and function of IP3 receptors and ryanodine receptors. Physiol Rev 2022;102:209–68. [DOI] [PubMed] [Google Scholar]
- 5. Fowler ED, Zissimopoulos S. Molecular, subcellular, and arrhythmogenic mechanisms in genetic RyR2 disease. Biomolecules 2022;12:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santulli G, Lewis D, des Georges A, Marks AR, Frank J. Ryanodine receptor structure and function in health and disease. Subcell Biochem 2018;87:329–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wleklinski MJ, Kannankeril PJ, Knollmann BC. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J Physiol 2020;598:2817–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woll KA, Van Petegem F. Calcium-release channels: structure and function of IP(3) receptors and ryanodine receptors. Physiol Rev 2022;102:209–68. [DOI] [PubMed] [Google Scholar]
- 9. Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla Set al. Clinvar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res 2018;46:D1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DMet al. Clinvar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 2014;42:D980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenson PD, Mort M, Ball EV, Chapman M, Evans K, Azevedo Let al. The Human Gene Mutation Database (HGMD((R))): optimizing its use in a clinical diagnostic or research setting. Hum Genet 2020;139:1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell Tet al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziegler A, Colin E, Goudenege D, Bonneau D. A snapshot of some pLI score. Hum Mutat 2019;40:839–41. [DOI] [PubMed] [Google Scholar]
- 14. Kapplinger JD, Pundi KN, Larson NB, Callis TE, Tester DJ, Bikker Het al. Yield of the RYR2 genetic test in suspected catecholaminergic polymorphic ventricular tachycardia and implications for test interpretation. Circ Genom Precis Med 2018;11:e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JPet al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol 2009;54:2065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jabbari J, Jabbari R, Nielsen MW, Holst AG, Nielsen JB, Haunso Set al. New exome data question the pathogenicity of genetic variants previously associated with catecholaminergic polymorphic ventricular tachycardia. Circ Cardiovasc Genet 2013;6:481–9. [DOI] [PubMed] [Google Scholar]
- 17. Giudicessi JR, Lieve KVV, Rohatgi RK, Koca F, Tester DJ, van der Werf Cet al. Assessment and validation of a phenotype-enhanced variant classification framework to promote or demote RYR2 missense variants of uncertain significance. Circ Genom Precis Med 2019;12:e002510. [DOI] [PubMed] [Google Scholar]
- 18. Priori SG, Mazzanti A, Santiago DJ, Kukavica D, Trancuccio A, Kovacic JC. Precision medicine in catecholaminergic polymorphic ventricular tachycardia: JACC Focus Seminar 5/5. J Am Coll Cardiol 2021;77:2592–612. [DOI] [PubMed] [Google Scholar]
- 19. Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 1995;91:1512–9. [DOI] [PubMed] [Google Scholar]
- 20. Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini Met al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69–74. [DOI] [PubMed] [Google Scholar]
- 21. Kallas D, Roston TM, Franciosi S, Brett L, Lieve KVV, Kwok SYet al. Evaluation of age at symptom onset, proband status, and sex as predictors of disease severity in pediatric catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2021;18:1825–32. [DOI] [PubMed] [Google Scholar]
- 22. Roston TM, Vinocur JM, Maginot KR, Mohammed S, Salerno JC, Etheridge SPet al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sy RW, Gollob MH, Klein GJ, Yee R, Skanes AC, Gula LJet al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm Society 2011;8:864–71. [DOI] [PubMed] [Google Scholar]
- 24. Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann Get al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 2005;42:863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roston TM, Yuchi Z, Kannankeril PJ, Hathaway J, Vinocur JM, Etheridge SPet al. The clinical and genetic spectrum of catecholaminergic polymorphic ventricular tachycardia: findings from an international multicentre registry. Europace 2018;20:541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe H, van der Werf C, Roses-Noguer F, Adler A, Sumitomo N, Veltmann Cet al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2013;10:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Werf C, Nederend I, Hofman N, van Geloven N, Ebink C, Frohn-Mulder IMet al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol 2012;5:748–56. [DOI] [PubMed] [Google Scholar]
- 28. van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace 2012;14:175–83. [DOI] [PubMed] [Google Scholar]
- 29. Wanguemert F, Bosch Calero C, Perez C, Campuzano O, Beltran-Alvarez P, Scornik FSet al. Clinical and molecular characterization of a cardiac ryanodine receptor founder mutation causing catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2015;12:1636–43. [DOI] [PubMed] [Google Scholar]
- 30. Aizawa Y, Komura S, Okada S, Chinushi M, Aizawa Y, Morita Het al. Distinct U wave changes in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT). Int Heart J 2006;47:381–9. [DOI] [PubMed] [Google Scholar]
- 31. Sumitomo N, Harada K, Nagashima M, Yasuda T, Nakamura Y, Aragaki Yet al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart 2003;89:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franciosi S, Roston TM, Perry FKG, Knollmann BC, Kannankeril PJ, Sanatani S. Chronotropic incompetence as a risk predictor in children and young adults with catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol 2019;30:1923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leren IS, Saberniak J, Majid E, Haland TF, Edvardsen T, Haugaa KH. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with beta1-selective beta-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:433–40. [DOI] [PubMed] [Google Scholar]
- 34. Lieve KVV, Dusi V, van der Werf C, Bos JM, Lane CM, Stokke MKet al. Heart rate recovery after exercise is associated with arrhythmic events in patients with catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2020;13:e007471. [DOI] [PubMed] [Google Scholar]
- 35. Faggioni M, Hwang HS, van der Werf C, Nederend I, Kannankeril PJ, Wilde AAet al. Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circ Res 2013;112:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita Met al. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J 2007;71:1606–9. [DOI] [PubMed] [Google Scholar]
- 37. Di Pino A, Caruso E, Costanzo L, Guccione P. A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm 2014;11:1480–3. [DOI] [PubMed] [Google Scholar]
- 38. Wilde AAM, Semsarian C, Marquez MF, Shamloo AS, Ackerman MJ, Ashley EAet al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Europace 2022;24:1307–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peltenburg PJ, Pultoo SNJ, Tobert KE, Bos JM, Lieve KVV, Tanck Met al. Repeatability of ventricular arrhythmia characteristics on the exercise-stress test in RYR2-mediated catecholaminergic polymorphic ventricular tachycardia. Europace 2023;25:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lieve KV, van der Werf C, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia. Circ J 2016;80:1285–91. [DOI] [PubMed] [Google Scholar]
- 41. Kannankeril PJ, Moore JP, Cerrone M, Priori SG, Kertesz NJ, Ro PSet al. Efficacy of flecainide in the treatment of catecholaminergic polymorphic ventricular tachycardia: a randomized clinical trial. JAMA Cardiol 2017;2:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 43. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190–252. [DOI] [PubMed] [Google Scholar]
- 44. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 45. De Ferrari GM, Dusi V, Spazzolini C, Bos JM, Abrams DJ, Berul CIet al. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation 2015;131:2185–93. [DOI] [PubMed] [Google Scholar]
- 46. Sgro A, Drake TM, Lopez-Ayala P, Phan K. Left cardiac sympathetic denervation in the management of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: a meta-regression. Con Heart Dis 2019;14:1102–12. [DOI] [PubMed] [Google Scholar]
- 47. Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul Tet al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Eng J Med 2008;358:2024–9. [DOI] [PubMed] [Google Scholar]
- 48. Hayashi M, Denjoy I, Hayashi M, Extramiana F, Maltret A, Roux-Buisson Net al. The role of stress test for predicting genetic mutations and future cardiac events in asymptomatic relatives of catecholaminergic polymorphic ventricular tachycardia probands. Europace 2012;14:1344–51. [DOI] [PubMed] [Google Scholar]
- 49. Roston TM, Kallas D, Davies B, Franciosi S, De Souza AM, Laksman ZWet al. Burst exercise testing can unmask arrhythmias in patients with incompletely penetrant catecholaminergic polymorphic ventricular tachycardia. JACC Clin Electrophysiol 2021;7:437–41. [DOI] [PubMed] [Google Scholar]
- 50. Writing Committee Members; Shah MJ, Silka MJ, Silva JNA, Balaji S, Beach CMet al. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Heart Rhythm 2021; 18: 1888–924. [DOI] [PubMed] [Google Scholar]
- 51. Roston TM, Jones K, Hawkins NM, Bos JM, Schwartz PJ, Perry Fet al. Implantable cardioverter-defibrillator use in catecholaminergic polymorphic ventricular tachycardia: a systematic review. Heart Rhythm 2018;15:1791–9. [DOI] [PubMed] [Google Scholar]
- 52. van der Werf C, Lieve KV, Bos JM, Lane CM, Denjoy I, Roses-Noguer Fet al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J 2019;40:2953–61. [DOI] [PubMed] [Google Scholar]
- 53. Nordkamp LR O, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AAet al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–54. [DOI] [PubMed] [Google Scholar]
- 54. Mazzanti A, Kukavica D, Trancuccio A, Memmi M, Bloise R, Gambelli Pet al. Outcomes of patients with catecholaminergic polymorphic ventricular tachycardia treated with beta-blockers. JAMA Cardiol 2022;7:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corrado D, Link MS, Schwartz PJ. Implantable defibrillators in primary prevention of genetic arrhythmias. A shocking choice? Eur Heart J 2022;43:3029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral Jet al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2016;18:159–83. [DOI] [PubMed] [Google Scholar]
- 57. Stiles MK, Fauchier L, Morillo CA, Wilkoff BL; Group ESCSD . 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2019; 21: 1442–3. [DOI] [PubMed] [Google Scholar]
- 58. Paludan-Muller C, Ahlberg G, Ghouse J, Herfelt C, Svendsen JH, Haunso Set al. Integration of 60,000 exomes and ACMG guidelines question the role of catecholaminergic polymorphic ventricular tachycardia-associated variants. Clin Gen 2017;91:63–72. [DOI] [PubMed] [Google Scholar]
- 59. Shauer A, Shor O, Wei J, Elitzur Y, Kucherenko N, Wang Ret al. Novel RyR2 mutation (G3118R) is associated with autosomal recessive ventricular fibrillation and sudden death: clinical, functional, and computational analysis. J Am Heart Assoc 2021;10:e017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 2011;108:871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ohno S, Hasegawa K, Horie M. Gender differences in the inheritance mode of RYR2 mutations in catecholaminergic polymorphic ventricular tachycardia patients. PLoS One 2015;10:e0131517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shimamoto K, Ohno S, Kato K, Takayama K, Sonoda K, Fukuyama Met al. Impact of cascade screening for catecholaminergic polymorphic ventricular tachycardia type 1. Heart 2022;108:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bauce B, Rampazzo A, Basso C, Bagattin A, Daliento L, Tiso Net al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: early diagnosis of asymptomatic carriers. J Am Coll Cardiol 2002;40:341–9. [DOI] [PubMed] [Google Scholar]
- 64. Roston TM, Van Petegem F, Sanatani S. Catecholaminergic polymorphic ventricular tachycardia: a model for genotype-specific therapy. Curr Opin Cardiol 2017;32:78–85. [DOI] [PubMed] [Google Scholar]
- 65. Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O’Connell R, Berenfeld Oet al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res 2007;101:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri Set al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res 2006;99:292–8. [DOI] [PubMed] [Google Scholar]
- 67. Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang Let al. Ryr2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc Natl Acad Sci U S A 2004;101:13062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi Pet al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res 2005;97:1173–81. [DOI] [PubMed] [Google Scholar]
- 69. George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res 2003;93:531–40. [DOI] [PubMed] [Google Scholar]
- 70. Kannankeril PJ, Shoemaker MB, Gayle KA, Fountain D, Roden DM, Knollmann BC. Atropine-induced sinus tachycardia protects against exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. Europace 2020;22:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders Met al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 2007;116:1569–76. [DOI] [PubMed] [Google Scholar]
- 72. Campbell MJ, Czosek RJ, Hinton RB, Miller EM. Exon 3 deletion of ryanodine receptor causes left ventricular noncompaction, worsening catecholaminergic polymorphic ventricular tachycardia, and sudden cardiac arrest. Am J Med Genet A 2015;167A:2197–200. [DOI] [PubMed] [Google Scholar]
- 73. Dharmawan T, Nakajima T, Ohno S, Iizuka T, Tamura S, Kaneko Yet al. Identification of a novel exon3 deletion of RYR2 in a family with catecholaminergic polymorphic ventricular tachycardia. Ann Noninv Electrocardiol 2019;24:e12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marjamaa A, Laitinen-Forsblom P, Lahtinen AM, Viitasalo M, Toivonen L, Kontula Ket al. Search for cardiac calcium cycling gene mutations in familial ventricular arrhythmias resembling catecholaminergic polymorphic ventricular tachycardia. BMC Med Genet 2009;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ohno S, Omura M, Kawamura M, Kimura H, Itoh H, Makiyama Tet al. Exon 3 deletion of RYR2 encoding cardiac ryanodine receptor is associated with left ventricular non-compaction. Europace 2014;16:1646–54. [DOI] [PubMed] [Google Scholar]
- 76. Kohli U, Aziz Z, Beaser AD, Nayak HM. A large deletion in RYR2 exon 3 is associated with nadolol and flecainide refractory catecholaminergic polymorphic ventricular tachycardia. PACE 2019;42:1146–54. [DOI] [PubMed] [Google Scholar]
- 77. Szentpali Z, Szili-Torok T, Caliskan K. Primary electrical disorder or primary cardiomyopathy? A case with a unique association of noncompaction cardiomyopathy and cathecolaminergic polymorphic ventricular tachycardia caused by ryanodine receptor mutation. Circulation 2013;127:1165–6. [DOI] [PubMed] [Google Scholar]
- 78. Leong IU, Sucich J, Prosser DO, Skinner JR, Crawford JR, Higgins Cet al. Array comparative genomic hybridization identifies a heterozygous deletion of exon 3 of the RYR2 gene. Ups J Med Sci 2015;120:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lobo PA, Kimlicka L, Tung CC, Van Petegem F. The deletion of exon 3 in the cardiac ryanodine receptor is rescued by beta strand switching. Structure 2011;19:790–8. [DOI] [PubMed] [Google Scholar]
- 80. Tang Y, Tian X, Wang R, Fill M, Chen SR. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ Res 2012;110:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ormerod JOM, Ormondroyd E, Li Y, Taylor J, Wei J, Guo Wet al. Provocation testing and therapeutic response in a newly described channelopathy: RyR2 calcium release deficiency syndrome. Circ Genom Precis Med 2022;15:e003589. [DOI] [PubMed] [Google Scholar]
- 82. Roston TM, Wei J, Guo W, Li Y, Zhong X, Wang Ret al. Clinical and functional characterization of ryanodine receptor 2 variants implicated in calcium-release deficiency syndrome. JAMA Cardiol 2022;7:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sun B, Yao J, Ni M, Wei J, Zhong X, Guo Wet al. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med 2021;13:eaba7287. [DOI] [PubMed] [Google Scholar]
- 84. Tester DJ, Bombei HM, Fitzgerald KK, Giudicessi JR, Pitel BA, Thorland ECet al. Identification of a novel homozygous multi-exon duplication in RYR2 among children with exertion-related unexplained sudden deaths in the Amish community. JAMA Cardiol 2020;5:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhong X, Guo W, Wei J, Tang Y, Liu Y, Zhang JZet al. Identification of loss-of-function RyR2 mutations associated with idiopathic ventricular fibrillation and sudden death. Biosci Rep 2021;41:BSR20210209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hirose S, Murayama T, Tetsuo N, Hoshiai M, Kise H, Yoshinaga Met al. Loss-of-function mutations in cardiac ryanodine receptor channel cause various types of arrhythmias including long QT syndrome. Europace 2022;24:497–510. [DOI] [PubMed] [Google Scholar]
- 87. Tester DJ, Kim CSJ, Hamrick SK, Ye D, O’Hare BJ, Bombei HMet al. Molecular characterization of the calcium release channel deficiency syndrome. JCI Insight 2020;5:e135952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RLet al. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci U S A 2015;112:E1669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shigemizu D, Aiba T, Nakagawa H, Ozaki K, Miya F, Satake Wet al. Exome analyses of long QT syndrome reveal candidate pathogenic mutations in calmodulin-interacting genes. PLoS One 2015;10:e0130329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Roston TM, Guo W, Krahn AD, Wang R, Van Petegem F, Sanatani Set al. A novel RYR2 loss-of-function mutation (I4855M) is associated with left ventricular non-compaction and atypical catecholaminergic polymorphic ventricular tachycardia. J Electrocardiol 2017;50:227–33. [DOI] [PubMed] [Google Scholar]
- 91. Taniguchi Y, Miyazaki A, Sakaguchi H, Hayama Y, Ebishima N, Negishi Jet al. Prominent QTc prolongation in a patient with a rare variant in the cardiac ryanodine receptor gene. Heart Vess 2017;32:229–33. [DOI] [PubMed] [Google Scholar]
- 92. Fujii Y, Itoh H, Ohno S, Murayama T, Kurebayashi N, Aoki Het al. A type 2 ryanodine receptor variant associated with reduced Ca2+ release and short-coupled torsades de pointes ventricular arrhythmia. Heart Rhythm 2017;14:98–107. [DOI] [PubMed] [Google Scholar]
- 93. Fatkin D, Huttner IG, Kovacic JC, Seidman JG, Seidman CE. Precision medicine in the management of dilated cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:2921–38. [DOI] [PubMed] [Google Scholar]
- 94. Mills RJ, Hudson JE. Bioengineering adult human heart tissue: how close are we? APL Bioeng 2019;3:010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nugraha B, Buono MF, von Boehmer L, Hoerstrup SP, Emmert MY. Human cardiac organoids for disease modeling. Clin Pharmacol Ther 2019;105:79–85. [DOI] [PubMed] [Google Scholar]
- 96. Arslanova A, Shafaattalab S, Ye K, Asghari P, Lin L, Kim Bet al. Using hiPSC-CMs to examine mechanisms of catecholaminergic polymorphic ventricular tachycardia. Curr Protoc 2021;1:e320. [DOI] [PubMed] [Google Scholar]
- 97. Bezzerides VJ, Caballero A, Wang S, Ai Y, Hylind RJ, Lu Fet al. Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca(2+)/calmodulin-dependent kinase II. Circulation 2019;140:405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Park SJ, Zhang D, Qi Y, Li Y, Lee KY, Bezzerides VJet al. Insights into the pathogenesis of catecholaminergic polymorphic ventricular tachycardia from engineered human heart tissue. Circulation 2019;140:390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sleiman Y, Souidi M, Kumar R, Yang E, Jaffre F, Zhou Tet al. Modeling polymorphic ventricular tachycardia at rest using patient-specific induced pluripotent stem cell-derived cardiomyocytes. EBioMedicine 2020;60:103024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bongianino R, Denegri M, Mazzanti A, Lodola F, Vollero A, Boncompagni Set al. Allele-specific silencing of mutant mRNA rescues ultrastructural and arrhythmic phenotype in mice carriers of the R4496C mutation in the ryanodine receptor gene (RYR2). Circ Res 2017;121:525–36. [DOI] [PubMed] [Google Scholar]
- 101. Pan X, Philippen L, Lahiri SK, Lee C, Park SH, Word TAet al. In vivo Ryr2 editing corrects catecholaminergic polymorphic ventricular tachycardia. Circ Res 2018;123:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Klipp RC, Li N, Wang Q, Word TA, Sibrian-Vazquez M, Strongin RMet al. EL20, A potent antiarrhythmic compound, selectively inhibits calmodulin-deficient ryanodine receptor type 2. Heart Rhythm 2018;15:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda Set al. FKBP12.6-mediated Stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 2003;107:477–84. [DOI] [PubMed] [Google Scholar]
- 104. Batiste SM, Blackwell DJ, Kim K, Kryshtal DO, Gomez-Hurtado N, Rebbeck RTet al. Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic. Proc Natl Acad Sci U S A 2019;116:4810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Szentandrassy N, Magyar ZE, Hevesi J, Banyasz T, Nanasi PP, Almassy J. Therapeutic approaches of ryanodine receptor-associated heart diseases. Int J Mol Sci 2022;23:4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.