This cohort study evaluates long-term educational outcomes and quality of life over 13 years after cochlear implantation in adolescents.
Key Points
Question
Is cochlear implantation during early childhood associated with better educational and quality-of-life outcomes in adolescence?
Findings
In this cohort study of 188 children with bilateral severe to profound hearing loss with cochlear implants and 340 children with severe to profound hearing loss without implants, children with cochlear implants had better educational outcomes in reading, writing, and quality of life compared with children without cochlear implants. Children who received cochlear implants prior to 18 months of age had the highest levels of oral language and academic achievement.
Meaning
Findings from this study indicate that children with severe to profound hearing loss who use cochlear implants have better educational outcomes and quality of life, regardless of age at implantation, than children without cochlear implants.
Abstract
Importance
Cochlear implants (CIs) have been shown to be effective in improving auditory skills and speech and language development. However, less is known about the long-term outcomes of CIs on educational functioning or quality of life.
Objective
To evaluate long-term educational outcomes and quality of life in adolescents over 13 years postimplantation.
Design, Setting, and Participants
This longitudinal cohort study included 188 children with bilateral severe to profound hearing loss with CIs from the Childhood Development After Cochlear Implantation (CDaCI) study from hospital-based CI programs; a cohort of 340 children with severe to profound hearing loss without CIs from a nationally representative survey (National Longitudinal Transition Study-2; NLTS-2), and results from the literature of comparable children without CIs.
Exposure(s)
Cochlear implantation (early and late).
Main Outcomes and Measures
Adolescent performance on measures of academic achievement (Woodcock Johnson), language (Comprehensive Assessment of Spoken Language), and quality of life (Pediatric Quality of Life Inventory, Youth Quality of Life Instrument–Deaf and Hard of Hearing).
Results
The CDaCI cohort included 188 children, 136 of whom completed the wave 3 postimplantation follow-up visits (77 [55%] female) with CIs; mean [SD] age was 11.47 [1.27] years. The NLTS-2 cohort included 340 children (50% female) with severe to profound hearing loss without CIs. Children with CIs had better academic performance compared with children without CIs with similar levels of hearing loss. The largest benefits were seen for children who received implants early (prior to age 18 months), who performed at or above age and gender norms for language and academic achievement. Similarly, adolescents with CIs reported better quality of life on the Pediatric Quality of Life Inventory compared with children without CIs. On a condition-specific measure (Youth Quality of Life Instrument–Deaf and Hard of Hearing), children who received implants early scored higher across all 3 domains than comparisons without CIs.
Conclusions and Relevance
To our knowledge, this is the first study to evaluate long-term educational outcomes and quality of life in adolescents using CIs. This longitudinal cohort study showed better outcomes of CIs in terms of language, academic performance, and quality of life. While the greatest benefits were observed for children who received implants before age 18 months, benefits were also noted for children who received implants later, providing evidence that children with severe to profound hearing loss with CIs can achieve at or above expected levels compared with hearing peers.
Introduction
Hearing loss is a common condition affecting 1 to 3 per 1000 children.1 As with most other pediatric conditions, early identification and intervention is critical to ensuring that children are able to develop a wide variety of skills (ie, speech, language, academic, cognitive) commensurate with their hearing peers. Without proper hearing loss management and intervention, children with severe to profound hearing loss (SPHL) are at risk for language delays, poor academic performance, and increased behavioral and social difficulties.2,3,4,5 Due to newborn hearing screening, children with hearing loss are identified earlier, which leads to quicker referrals for hearing technology and rehabilitation services.6
For children with SPHL, cochlear implantation (CI) is the primary treatment for families pursuing a listening and spoken language approach. Age at implantation is crucial, with studies reporting that those receiving implants within the critical period for language development (0-3 years) achieve better outcomes in speech recognition, speech comprehension, and spoken language development than those receiving implants later; growing evidence supports that earlier implantation (ie, prior to 12 months of age) yields better outcomes.7,8 To date, the majority of literature has focused on the benefits of CIs on speech perception, vocabulary, and language abilities, with few studies investigating the longitudinal outcomes of implantation on academic performance and quality of life (QOL) in adolescence.
The Childhood Development After Cochlear Implantation (CDaCI) cohort is a longitudinal, multisite, nationally representative sample of pediatric CI recipients.9 The CDaCI study followed children from preimplantation (ages 5 months to 5 years) to 13 years postimplantation. The current study evaluated educational outcomes, including academic achievement and language, and QOL from the 9 to 13 years postimplantation follow-up visits when children were ages 9 to 19 years.
Methods
Participants
Data used for this study came from the CDaCI (NIDCD R01DC004797) cohort. The cohort consists of 188 children with bilateral SPHL. Children were recruited from 6 CI centers across the US. Eligibility for the cohort included age younger than 5 years and a screening score of 70 or higher on the Bayley Scales of Infant Development, Second Edition Mental Scale or Motor Scale or 66 or higher on the Leiter International Performance Scale–Revised. On average, children had a mean IQ of 101.3.10 The CDaCI cohort included a diverse sample of children, including 15% who had an additional diagnosis (eg, developmental disorder, attention deficit disorder). See Cruz et al11 for further details regarding the subset of children with additional disabilities. The University of Miami Institutional Review Board approved this study and determined it meets the criteria for exemption of informed consent as described in Federal Regulation 45 CFR 46.104.
At enrollment, prior to implantation, children were on average 2 years of age (mean [SD], 2.2 [1.2] years; Table 1). See Fink et al9 for a full description of the sample demographics. Children were assessed every 6 months for the first 3 years and annually for the subsequent 10 years. For the current study, measures of spoken language, academic achievement, and QOL were used from the third wave of data collection (wave 3) that covered the 5 follow-up visits from 9 to 13 years postimplantation.
Table 1. Childhood Development After Cochlear Implantation Study Demographics for Wave 3.
| Characteristic | No. (%) | Standardized mean difference | ||
|---|---|---|---|---|
| Full cohort (n = 136) | Earlier implantation (n = 45) | Later implantation (n = 91) | ||
| Age at beginning of wave 3, mean (SD), y | 11.47 (1.27) | 10.27 (0.30) | 12.03 (1.15) | 2.11 |
| Sex | ||||
| Female | 75 (55) | 22 (49) | 53 (58) | 0.19 |
| Male | 61 (45) | 23 (51) | 38 (42) | |
| Race and ethnicity | ||||
| Asian | 6 (4) | 0 | 6 (7) | 0.57 |
| Black | 11 (8) | 2 (4) | 9 (10) | |
| Hispanic White | 13 (10) | 3 (7) | 10 (11) | |
| Hispanic (race unspecified) | 7 (5) | 1 (2) | 6 (7) | |
| Non-Hispanic White | 93 (68) | 36 (80) | 57 (63) | |
| Othera/mixed | 6 (4) | 3 (7) | 3 (3) | |
| Income <$50 000 | 50 (37) | 11 (24) | 39 (43) | 0.40 |
| Less than high school education | 27 (20) | 8 (18) | 19 (21) | 0.08 |
| Onset age, mean (SD), y | 0.17 (0.47) | 0.03 (0.12) | 0.24 (0.56) | 0.52 |
| Amplification age, mean (SD), y | 1.00 (0.81) | 0.39 (0.26) | 1.29 (0.82) | 1.48 |
| Activation age, mean (SD), y | 2.37 (1.22) | 1.16 (0.19) | 2.96 (1.07) | 2.35 |
| Onset type | ||||
| Congenital | 79 (58) | 40 (89) | 39 (43) | 1.18 |
| Progressive | 43 (32) | 3 (7) | 40 (44) | |
| Sudden | 7 (5) | 2 (4) | 5 (6) | |
| Unknown | 7 (5) | 0 | 7 (8) | |
Participants who did not identify with the provided racial or ethnic categories were given the option to select “other” as their response.
A comparison sample was used from the National Longitudinal Transition Study-2 (NLTS-2) obtained from the Institute of Education Sciences. The NLTS-2 database collected academic achievement data for children enrolled in special education programs, including children with SPHL. Children were on average 13 to 16 years of age in December 2000 and were followed up over a span of 10 years. There were 370 children who completed academic achievement assessments and had a primary indication of SPHL, defined by the student’s parent/guardian stating that the youth’s hearing loss is profound (as opposed to moderate or mild). We also know if those students reported having a CI at the moment of the assessment or not. We compared the outcomes of individuals with and without CIs from this subsample, and we compared the 340 children reported as not having a CI with the CDaCI cohort (Table 2).
Table 2. NLTS-2 Subpopulation Proportion and Mean Estimates for Individuals With SPHL Without CIs.
| Subpopulation | Proportion (or average), % | Standard error |
|---|---|---|
| Female | 50 | 0.06 |
| Income <$50 000 | 59 | 0.08 |
| Race and ethnicity | ||
| Asian/Pacific Islander | 3 | 0.02 |
| Black | 14 | 0.05 |
| Hispanic | 19 | 0.06 |
| White | 64 | 0.06 |
| High school degree or below | 66 | 0.11 |
| Onset age | 1 y 8 mo | 5.5 mo |
Abbreviations: CIs, cochlear implants; NLTS-2, National Longitudinal Transition Study-2; SPHL, severe to profound hearing loss.
Comparative Samples for QOL
To provide more context for the results calculated from the CDaCI and NLTS-2, we examined the existing literature for studies of adolescents without CIs with bilateral SPHL that used the same measures as the CDaCI study. We identified 3 independent studies that included samples similar to the CDaCI cohort in terms of age and hearing loss severity. Study samples included were predominantly without implants. Some of the studies included some children with CIs; however, these studies did not provide separate estimates for those with and without implants. Given the assumption that CIs are associated with QOL, the resulting estimates will be closer to those from the CDaCI cohort, thus providing a more conservative comparison of adolescents’ QOL. All children in the comparative samples were included in our analyses.
Participants (n = 157) in the Meyer et al12 sample were aged 11 to 18 years with a mean (SD) age of 14.1 (2.3) years. All children were diagnosed with SPHL; 31.2% (n = 49) of the sample did not use hearing technology, 28.7% (n = 45) used hearing aids, and 40.1% (n = 63) used CIs.12
Participants (n = 226) in the Rachakonda et al13 sample were between the ages of 13 to 18 years with a mean (SD) age of 15.4 (1.7) years. Fifty-nine of the participants had bilateral SPHL, 25.5% (n = 15) used CIs (unilateral = 11, bilateral = 4), 69.5% (n = 41) used hearing aids, 5.1% (n = 3) used a bone conduction device, and 10.2% (n = 6) did not use hearing technology.13 Only results for bilateral SPHL were used as a comparison.
Participants (n = 115) in the Umansky et al14 sample were between the ages of 7 and 12 years; 35 had normal hearing, 35 had unilateral hearing loss, and 45 had bilateral hearing loss. Only results for those with bilateral hearing loss were used as a comparison. Of the participants with bilateral hearing loss, 25 participants were diagnosed with bilateral SPHL. Use of hearing devices included FM system (n = 9), hearing aids (n = 11), Baha system (n = 1), CIs (n = 3), no hearing device (n = 1), and unknown (n = 8). Mean (SD) age of participants with bilateral hearing loss was 9.8 (1.4) years.14
Measures
Academic Achievement
Academic achievement was assessed using the Woodcock-Johnson IV (WJ- IV) for the CDaCI cohort and the Woodcock-Johnson III (WJ-III) for the NLTS-2 cohort.15,16 The WJ-III and WJ-IV provide age- and gender-adjusted standard scores (SS) with a mean (SD) of 100 (15). The CDaCI cohort was administered the reading fluency, passage comprehension, writing fluency, math fluency, and calculation subscales at each yearly assessment during wave 3. The NLTS-2 administered an assessment that measures achievement in reading, math, science, and social studies in either 2002 or 2004 to youth who were aged 16 to 18 years at the time of the assessment.
Spoken Language
The Comprehensive Assessment of Spoken Language (CASL) was administered to the CDaCI cohort at each yearly assessment. The CASL is a well-established language measure for children aged 3 to 21 years and assesses lexical/semantic, syntactic, supralinguistic, and pragmatic skills.17 A core composite score is calculated based on subtests completed by the participant, including age- and gender-adjusted SS. We report the SS for the Core Composite as well as Synonyms and Antonyms. Antonyms were given to participants aged 5 to 12 years and Synonyms to those 13 21 years of age. We included the Antonyms and Synonyms to provide a comparison to the Synonym and Antonym scales from the WJ-III completed by the NLTS-2 cohort.
Quality of Life
Quality of life was measured using both a generic and condition-specific measure. The Pediatric Quality of Life Inventory (PedsQL) has 23 items rated on a 5-point Likert scale ranging from “never” to “almost always” and yields 3 summary scores: Total Scale Score, Physical Health Summary Score, and Psychosocial Health Summary Score. The PedsQL scores for the CDaCI cohort were compared with normative data for healthy children, as well as children without implants with bilateral SPHL.13,14,18 The Youth Quality of Life Instrument–Deaf and Hard of Hearing (YQOL-DHH) is a condition-specific self-report measure of QOL for youth aged 11 through 18 years and includes 32 items that assess 3 core domains: self-acceptance/advocacy, perceived stigma, and participation.19 As a point of comparison, we used published results from a sample of similar age and hearing loss that was predominantly without CIs.12
Statistical Analyses
There were 136 CDaCI participants who completed at least 1 performance measure or QOL measure during wave 3. Standardized mean difference (SMD) was used to estimate the size of the differences between those who received implants before vs after age 18 months. Standardized mean differences enable group comparisons using a common metric for continuous and categorical variables and can be interpreted in the metric of Cohen d.20 We used the same approach to compare those who participated vs those who did not participate in wave 3.
For the performance measures, there were 486 assessments within 134 participants (average number of assessments = 3.63). We used linear mixed-effects models to account for nesting of assessments within participants and to account for missing assessments. A separate model was fit to each scale and included intercept; age at implantation, which was coded as before vs after age 18 months; and household income coded as above vs below $50 000, as fixed effects and intercept as a random effect. We used general linear models for the NLTS-2 data that accounted for the survey weights; we used the successive difference replication method using the mean squared error formula for variance estimation.21 We chose to adjust for family income because it is a strong predictor of academic outcomes22,23 and was likely related to families’ ability to mobilize resources for earlier implantation during the early 2000s when the CDaCI cohort was recruited and when earlier implantation was less common due to insurance barriers.24 Both the CDaCI and NLTS-2 data sources documented family income using different sets of categories, yet both included a category starting with $50 000, providing a common scale for both data sources. We report the point estimates and 95% confidence intervals for each scale or subscale for those who were above the $50 000 cutoff. Results for the full cohorts and those below the $50 000 cutoff are provided as supplemental materials (eTables 1-6 in Supplement 1).
For the QOL assessments, there were 438 assessments nested within 126 participants (average number = 3.48). Again, we used linear mixed-effects modeling but did not adjust for income because the comparison data that were abstracted from previous articles12,13,14 did not adjust for income. For the comparison data, means and SDs were abstracted from Table 3 of Meyer et al,12 Table 5 of Rachakonda et al,13 and Table 3 of Umansky et al.14 Standard errors and 95% confidence intervals were calculated using reported sample sizes and SDs. Data from the CDaCI cohort were analyzed using the lme4 package in R. Data from the NLTS-2 cohort was analyzed using Stata 16 (StataCorp LLC). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Results
The CDaCI cohort included 188 children (77 [55%] female) with CIs; mean [SD] age was 11.47 [1.27] years. The NLTS-2 cohort included 340 children (50% female) with SPHL without CIs. The 136 participants from the CDaCI cohort who completed at least 1 assessment during wave 3 differed from the 52 who were lost to follow-up in terms of household income, age at onset, and amplification age, with noncompleters reporting lower income (noncompleters = 55%; completers = 37% less than $50 000; SMD = 0.39), older age at identification (noncompleters = 0.39 [0.87]; completers = 0.17 [0.47]; SMD = 0.31), and older at age at amplification (noncompleters = 1.40 [1.02]; completers = 1.00 [0.81]; SMD = 0.44). These differences suggested that our reported outcomes may be somewhat optimistic given that later detection and age at amplification could adversely be associated with access to early language, as well as the known association between higher family income and academic performance.
Educational Outcomes
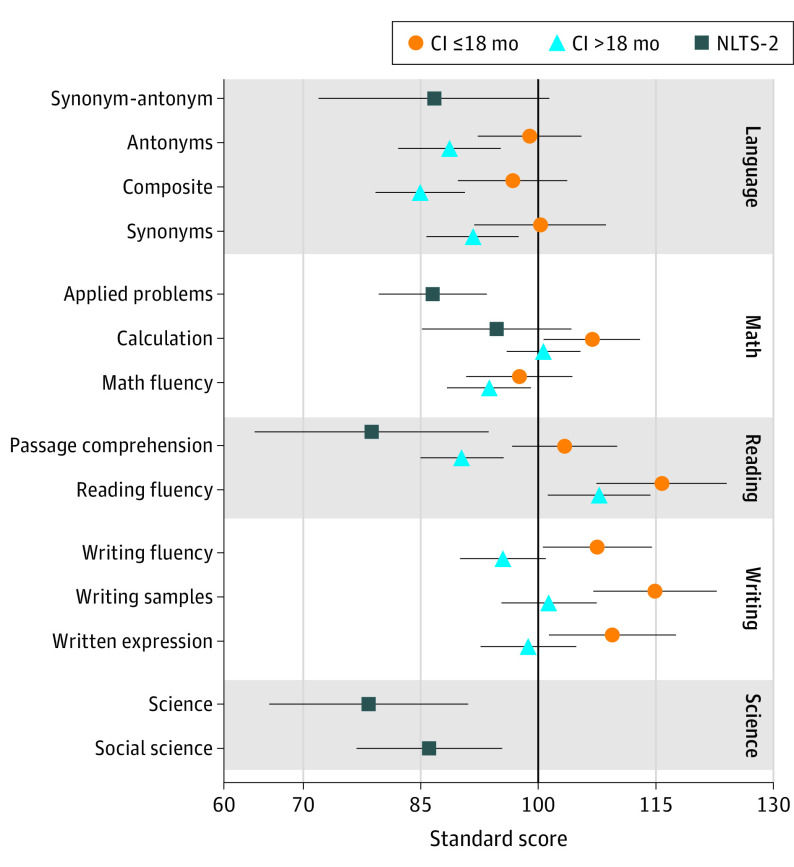
Results from the CDaCI cohort suggest that participants who received implants before 18 months of age performed at or above age and gender norms for spoken language and academic achievement (Figure 1). Their scores were in the average range (SS of 90-110) for all subscales of the CASL and WJ-IV except for reading fluency and writing samples, which were in the high average range (SS = 111-120). Participants who received implants after 18 months of age demonstrated lower scores on all subscales except math fluency. Their scores were in the average range for all subscales except for the CASL composite and Antonyms subscales, which were in the low average range (SS = 80-89). Notably, the results displayed are estimates for those with household incomes greater than $50 000. The association of household income was significant for all subscales ranging from 7 SS lower for mathematical fluency to 14 SS lower for reading fluency.
Figure 1. Comparison of Academic Achievement in Samples With and Without Cochlear Implants (CIs).
Marginal means and 95% confidence intervals are presented for each cohort for those with household incomes greater than $50 000. The scores can be interpreted as follows (very low, ≤69; low, 70-79; low average, 80-89; average, 90-110; high average, 111-120; superior, 121-130). The CI cohort was obtained from the Childhood Development after Cochlear Implantation study, and the sample without CIs was obtained from the National Longitudinal Transition Study-2 (NLTS-2).
Results from the NLTS-2 database suggested that children with SPHL had scores within the low average ranges: the upper bounds of the 95% confidence intervals were all below 100. This was also true when controlling for family income, with 2 exceptions: upper bounds for the Calculation subtest were slightly above 100 for 2 of the 3 income categories, and the upper bound for the Synonym-Antonym subtest was slightly above 100 for the higher income range. Within-sample comparisons between severe to profound and mild/moderate hearing loss and between participants with and without CIs were consistent with expectations and data presented above using the CDaCI cohort. In particular, students using CIs had better scores than children without CIs across 5 of the 6 subtests (no difference for the remaining scale). Even though the point estimates were different, these differences were not statistically significant due to limited power in the CI subsample. We also analyzed the scores of children without CIs with and without prescribed hearing aids (eTable 7 in Supplement 1). The results were similar between those subsamples (except for the Passage Comprehension subtest, though the difference was not significant). Additionally, all but 1 of the upper bounds (Calculation subtest for the children without prescribed hearing aids) were below 100.
Quality of Life
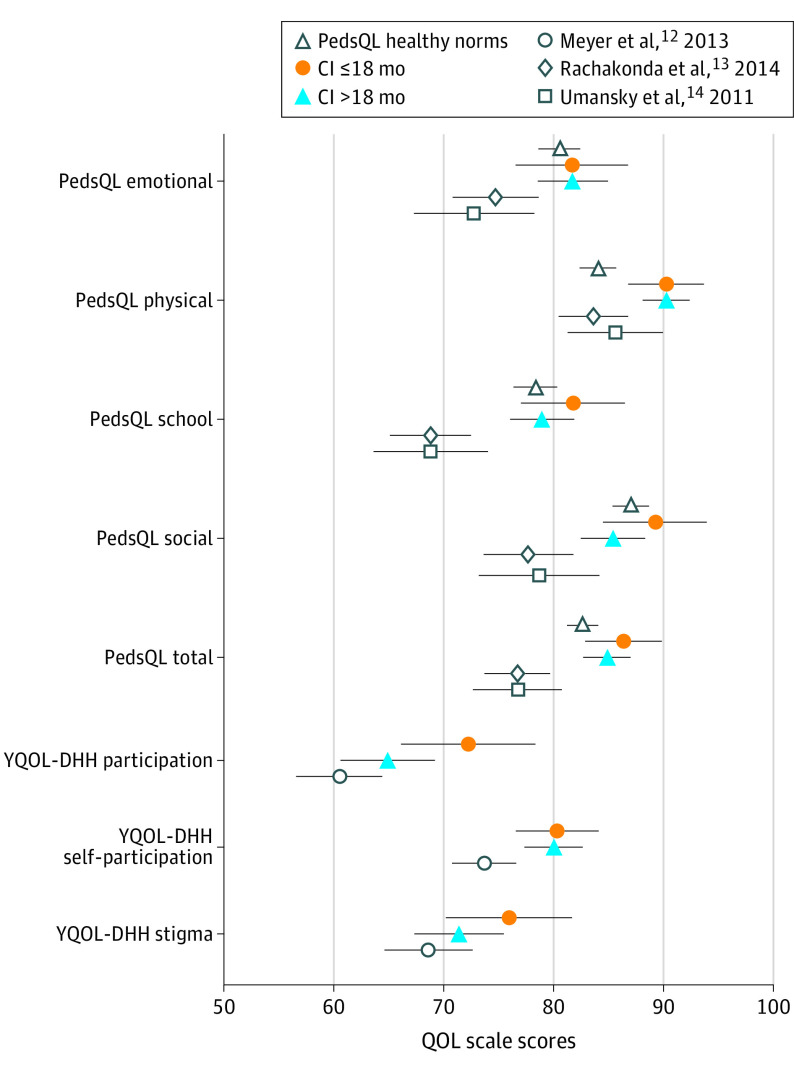
As seen in Figure 2, adolescents with CIs reported better QOL on the generic PedsQL measure across all subscales compared with the comparison samples without CIs abstracted from the Rachakonda et al13 and Umansky et al14 studies. The differences among the CDaCI cohort with CIs and samples without CIs were larger than the established minimally clinical important difference score of 4.4 for the PedsQL.18 Additionally, adolescents in the CDaCI cohort reported QOL scores within the same range as normative data for healthy controls on the PedsQL.18 Interestingly, there were minimal differences observed for the early vs late implantation groups, suggesting that there are benefits in QOL regardless of age at implantation.
Figure 2. Quality-of-Life Outcomes in Adolescents With Severe to Profound Hearing Loss With and Without Cochlear Implants (CIs).
Marginal means and 95% confidence intervals are depicted for all cohorts. Comparison data were abstracted from published articles of studies that primarily included adolescents without implants with bilateral moderate to profound hearing loss. Normative data for children with normal hearing are also presented. The CI cohort was obtained from the Childhood Development After Cochlear Implantation study. PedsQL indicates Pediatric Quality of Life Inventory; YQOL-DHH, Youth Quality of Life Instrument–Deaf and Hard of Hearing.
On the condition-specific YQOL measure, children who received implants early consistently scored higher across all 3 domains than the comparison sample from the Meyer et al12 study. In contrast, only differences were reported on the YQOL self-acceptance scale for the cohort who received implants later compared with the sample without implants, thus suggesting that using a condition-specific measure may be more sensitive in identifying differences in QOL in early vs later implanted groups.
Discussion
The current study supports the benefits of pediatric cochlear implantation in children with bilateral SPHL. Cochlear implants are the standard of care for children with this level of hearing loss when parents are focusing on the development of listening and spoken language. Existing literature has shown long-term benefits for CIs in terms of auditory skill development, speech perception, and language development.25,26,27 However, evidence of long-term educational outcomes and QOL during adolescence is limited. In the current study, children with CIs performed similarly to their same-age hearing peers on measures of academic achievement and general QOL. As expected, children using CIs reported better QOL across several scales (emotional, social, school functioning) compared with children without implants. Adolescents’ self-reported QOL was within the same range as normative data on the PedsQL for hearing peers. This is consistent with prior literature documenting similar QOL scores between children with CIs and hearing peers.28,29,30,31 They also consistently showed better outcomes compared with samples of adolescents with similar hearing loss who did not pursue CIs on academic achievement and QOL.
Children who received implants prior to 18 months of age consistently showed better educational outcomes than those receiving implants later. On average, those who received implants early achieved average to above-average performance on reading comprehension and writing, while those who received implants later achieved low-average to average performance. This is consistent with the extensive literature documenting better auditory performance and improved speech outcomes in comparisons of children receiving implants earlier rather than later.7,32,33,34,35,36,37 Moreover, these data support the recent lowering of age for the US Food and Drug Administration (FDA) criteria for CI. Up until 2019, the FDA criteria for cochlear implantation included age of 12 months or older; children are now able to undergo cochlear implantation as early as 9 months of age.
Importantly, CIs should not be discounted for those who are identified late or pursue CIs at a later age or who have an additional disability.38 The CDaCI included children who received implants up to 5 years old, including 15% with an additional disability11 as noted previously; these children consistently performed better on measures of academic achievement than the children followed up through the NLTS-2 database who did not use CIs. In addition, QOL was also higher compared with children without CIs obtained from the comparative studies. These findings are consistent with studies reporting the overall benefits of CIs at later ages, particularly in terms of QOL.39 Thus, while speech intelligibility may be poorer for children receiving implants later as reported in the literature, these children may still benefit in terms of QOL.40 Cochlear implant centers should routinely incorporate assessment of QOL into their practice, using newly developed adult and pediatric condition-specific measures (ie, CI-QoL, QoL-CI).41,42,43,44 This will allow centers to monitor their patients’ daily functioning and identify any needs for intervention that may be overlooked during routine audiological evaluations.
Limitations
To our knowledge, the CDaCI cohort is the largest longitudinal study evaluating the efficacy of pediatric CIs to date; however, a number of important limitations should be noted. The cohort was recruited between 2000 and 2004, when early implantation was not as common and those seeking early implantation likely being more educated and having access to more resources than the deaf and hard of hearing population at the time. Self-selection likely biases the results toward better outcomes. Although we accounted for household income in analyses, this bias likely remains, limiting comparisons between early vs late implantation. Similarly, the cohort did not include a comparison group without implants, requiring us to identify alternative comparisons from the NLTS-2 and other previously published samples. These samples may differ from one another on characteristics associated with the outcome (eg, age at amplification, household income, parental education), limiting direct comparisons. While the NLTS-2 is the only currently available database that uses similar academic achievement measures as the CDaCI cohort, their hearing loss data were obtained via parent report rather than audiological data (ie, thresholds). In addition, a systematic review on QOL was not conducted, as our goal was to compare the CDaCI cohort with samples that used the same QOL measures as the CDaCI study. Future studies should consider a scoping review of QOL in children with SPHL with and without implants. Understanding these limitations, our results represent a large prospective evaluation of adolescent outcomes following early childhood implantation, and the results incorporate some of the only information available on adolescents without implants using the same or similar measures to the CDaCI cohort.
Conclusions
Results from this cohort study have strong clinical implications for management of severe to profound sensorineural hearing loss. Cochlear implants were consistently associated with at or above expected levels of academic achievement in adolescence, with those children receiving CIs at earlier ages demonstrating the highest performance in reading and writing. These findings highlight the importance of early identification and intervention. Moreover, it also shows that the language and academic gains achieved postimplantation carry over to improved QOL through adolescence. Given this evidence, pediatricians are urged to refer children with SPHL for CI evaluation. Pediatric otology and audiology programs can then focus on guiding families on receiving timely intervention that will support comparable educational and QOL outcomes similar to hearing peers.
eTable 1. Woodcock Johnson Achievement Scores for individuals with SPHL and without CIs
eTable 2. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs, income levels comparison (standard errors in parenthesis)
eTable 3. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs and annual family income between $25,000 and $50,000
eTable 4. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs and annual family income above $50,000
eTable 5. Standard Scores from the CDaCI study for those implanted before 18 months
eTable 6. Standard Scores from the CDaCI study for those implanted after 18 months
eTable 7. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs according to whether they were prescribed hearing aids or not
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention . Research and tracking of hearing loss in children. Accessed July 11, 2022. https://www.cdc.gov/ncbddd/hearingloss/research.html#:~:text=CDC%20data%20have%20shown%20that,to%205%20per%201%2C000%20children
- 2.Kelly C, Morgan G, Freeth M, Siegal M, Matthews D. The understanding of communicative intentions in children with severe-to-profound hearing loss. J Deaf Stud Deaf Educ. 2019;24(3):245-254. doi: 10.1093/deafed/enz001 [DOI] [PubMed] [Google Scholar]
- 3.Sugaya A, Fukushima K, Takao S, et al. Impact of reading and writing skills on academic achievement among school-aged hearing-impaired children. Int J Pediatr Otorhinolaryngol. 2019;126:109619. doi: 10.1016/j.ijporl.2019.109619 [DOI] [PubMed] [Google Scholar]
- 4.Barker DH, Quittner AL, Fink NE, Eisenberg LS, Tobey EA, Niparko JK; CDaCI Investigative Team . Predicting behavior problems in deaf and hearing children: the influences of language, attention, and parent-child communication. Dev Psychopathol. 2009;21(2):373-392. doi: 10.1017/S0954579409000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman MF, Quittner AL, Cejas I. Comparisons of social competence in young children with and without hearing loss: a dynamic systems framework. J Deaf Stud Deaf Educ. 2015;20(2):115-124. doi: 10.1093/deafed/enu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehl AL, Thomson V. The Colorado newborn hearing screening project, 1992-1999: on the threshold of effective population-based universal newborn hearing screening. Pediatrics. 2002;109(1):E7. doi: 10.1542/peds.109.1.e7 [DOI] [PubMed] [Google Scholar]
- 7.Sharma SD, Cushing SL, Papsin BC, Gordon KA. Hearing and speech benefits of cochlear implantation in children: a review of the literature. Int J Pediatr Otorhinolaryngol. 2020;133:109984. doi: 10.1016/j.ijporl.2020.109984 [DOI] [PubMed] [Google Scholar]
- 8.Glaubitz C, Liebscher T, Hoppe U. Age-related language performance and device use in children with very early bilateral cochlear implantation. Int J Pediatr Otorhinolaryngol. 2021;147:110780. doi: 10.1016/j.ijporl.2021.110780 [DOI] [PubMed] [Google Scholar]
- 9.Fink NE, Wang NY, Visaya J, et al. ; CDACI Investigative Team . Childhood Development after Cochlear Implantation (CDaCI) study: design and baseline characteristics. Cochlear Implants Int. 2007;8(2):92-116. doi: 10.1179/cim.2007.8.2.92 [DOI] [PubMed] [Google Scholar]
- 10.Cejas I, Mitchell CM, Hoffman M, Quittner AL; CDaCI Investigative Team . Comparisons of IQ in children with and without cochlear implants: longitudinal findings and associations with language. Ear Hear. 2018;39(6):1187-1198. doi: 10.1097/AUD.0000000000000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz I, Vicaria I, Wang NY, Niparko J, Quittner AL; CDaCI Investigative Team . Language and behavioral outcomes in children with developmental disabilities using cochlear implants. Otol Neurotol. 2012;33(5):751-760. doi: 10.1097/MAO.0b013e3182595309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer A, Sie K, Skalicky A, et al. Quality of life in youth with severe to profound sensorineural hearing loss. JAMA Otolaryngol Head Neck Surg. 2013;139(3):294-300. doi: 10.1001/jamaoto.2013.35 [DOI] [PubMed] [Google Scholar]
- 13.Rachakonda T, Jeffe DB, Shin JJ, et al. Validity, discriminative ability, and reliability of the hearing-related quality of life questionnaire for adolescents. Laryngoscope. 2014;124(2):570-578. doi: 10.1002/lary.24336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umansky AM, Jeffe DB, Lieu JE. The HEAR-QL: quality of life questionnaire for children with hearing loss. J Am Acad Audiol. 2011;22(10):644-653. doi: 10.3766/jaaa.22.10.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrank F. Woodcock-Johnson III tests of cognitive abilities. In: Flanagan DP, Harrison PL, eds. Contemporary Intellectual Assessment: Theories, Tests, and Issues. Guilford Press; 2005:371-401. [Google Scholar]
- 16.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Riverside Publishing; 2001. [Google Scholar]
- 17.Carrow-Woolfolk E. CASL: Comprehensive Assessment of Spoken Language. American Guidance Services Circle Pines; 1999. [Google Scholar]
- 18.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Patrick DL, Edwards TC, Skalicky AM, et al. Validation of a quality-of-life measure for deaf or hard of hearing youth. Otolaryngol Head Neck Surg. 2011;145(1):137-145. doi: 10.1177/0194599810397604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay RE, Train GF. Aspects of Survey and Model-Based Postcensal Estimation of Income and Poverty Characteristics for States and Counties. Taylor & Francis; 1995:154-159. [Google Scholar]
- 22.Haveman R, Wolfe B. The determinants of children’s attainments: a review of methods and findings. J Econ Lit. 1995;33(4):1829-1878. [Google Scholar]
- 23.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822-829. doi: 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester EB, Dawson JD, Gantz BJ, Hansen MR. Barriers to the early cochlear implantation of deaf children. Otol Neurotol. 2011;32(3):406-412. doi: 10.1097/MAO.0b013e3182040c22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peixoto MC, Spratley J, Oliveira G, Martins J, Bastos J, Ribeiro C. Effectiveness of cochlear implants in children: long term results. Int J Pediatr Otorhinolaryngol. 2013;77(4):462-468. doi: 10.1016/j.ijporl.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 26.Wang NY, Eisenberg LS, Johnson KC, et al. ; CDaCI Investigative Team . Tracking development of speech recognition: longitudinal data from hierarchical assessments in the Childhood Development after Cochlear Implantation Study. Otol Neurotol. 2008;29(2):240-245. doi: 10.1097/MAO.0b013e3181627a37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Zhao F, Tao Y, Zhang L, Yao X, Zheng Y. Trajectory of auditory and language development in the early stages of pre-lingual children post cochlear implantation: a longitudinal follow up study. Int J Pediatr Otorhinolaryngol. 2020;128:109720. doi: 10.1016/j.ijporl.2019.109720 [DOI] [PubMed] [Google Scholar]
- 28.Warner-Czyz AD, Loy B, Tobey EA, Nakonezny P, Roland PS. Health-related quality of life in children and adolescents who use cochlear implants. Int J Pediatr Otorhinolaryngol. 2011;75(1):95-105. doi: 10.1016/j.ijporl.2010.10.018 [DOI] [PubMed] [Google Scholar]
- 29.Hofmann M, Meloche M, Zwolan TA. Health related quality of life in adolescent cochlear implant users. Cochlear Implants Int. 2020;21(4):198-205. doi: 10.1080/14670100.2020.1724676 [DOI] [PubMed] [Google Scholar]
- 30.Roland L, Fischer C, Tran K, Rachakonda T, Kallogjeri D, Lieu JE. Quality of life in children with hearing impairment: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2016;155(2):208-219. doi: 10.1177/0194599816640485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira SA, Sousa H, Barros E. Health-related quality of life after pediatric cochlear implantation. Int J Pediatr Otorhinolaryngol. 2022;155:111087. doi: 10.1016/j.ijporl.2022.111087 [DOI] [PubMed] [Google Scholar]
- 32.Yoshinaga-Itano C, Sedey AL, Wiggin M, Mason CA. Language outcomes improved through early hearing detection and earlier cochlear implantation. Otol Neurotol. 2018;39(10):1256-1263. doi: 10.1097/MAO.0000000000001976 [DOI] [PubMed] [Google Scholar]
- 33.Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27(3):286-298. doi: 10.1097/01.aud.0000215973.76912.c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Incerti PV, Ching TYC, Hou S, Van Buynder P, Flynn C, Cowan R. Programming characteristics of cochlear implants in children: effects of aetiology and age at implantation. Int J Audiol. 2018;57(suppl 2):S27-S40. doi: 10.1080/14992027.2017.1370139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niparko JK, Tobey EA, Thal DJ, et al. ; CDaCI Investigative Team . Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498-1506. doi: 10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes H, Geers AE, Treiman R, Moog JS. Receptive vocabulary development in deaf children with cochlear implants: achievement in an intensive auditory-oral educational setting. Ear Hear. 2009;30(1):128-135. doi: 10.1097/AUD.0b013e3181926524 [DOI] [PubMed] [Google Scholar]
- 37.Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Prospective long-term auditory results of cochlear implantation in prelinguistically deafened children: the importance of early implantation. Acta Otolaryngol Suppl. 2004;(552):55-63. doi: 10.1080/03655230410017148 [DOI] [PubMed] [Google Scholar]
- 38.Glaubitz C, Liebscher T, Hoppe U. Children with cochlear implant and additional disabilities benefit from consistent device use. Int J Pediatr Otorhinolaryngol. 2022;162:111301. doi: 10.1016/j.ijporl.2022.111301 [DOI] [PubMed] [Google Scholar]
- 39.Debruyne JA, Janssen AM, Brokx JPL. Systematic review on late cochlear implantation in early-deafened adults and adolescents: clinical effectiveness. Ear Hear. 2020;41(6):1417-1430. doi: 10.1097/AUD.0000000000000884 [DOI] [PubMed] [Google Scholar]
- 40.Ovari A, Hühnlein L, Nguyen-Dalinger D, et al. Functional outcomes and quality of life after cochlear implantation in patients with long-term deafness. J Clin Med. 2022;11(17):5156. doi: 10.3390/jcm11175156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman MF, Cejas I, Quittner AL. Health-related quality of life instruments for children with cochlear implants: development of child and parent-proxy measures. Ear Hear. 2019;40(3):592-604. doi: 10.1097/AUD.0000000000000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cejas I, Coto J, Sarangoulis C, Sanchez CM, Quittner AL. Quality of Life-CI: development of an early childhood parent-proxy and adolescent version. Ear Hear. 2021;42(4):1072-1083. doi: 10.1097/AUD.0000000000001004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McRackan TR, Hand BN, Velozo CA, Dubno JR; Cochlear Implant Quality of Life Development Consortium . Cochlear Implant Quality of Life (CIQOL): development of a profile instrument (CIQOL-35 Profile) and a global measure (CIQOL-10 Global). J Speech Lang Hear Res. 2019;62(9):3554-3563. doi: 10.1044/2019_JSLHR-H-19-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McRackan TR, Hand BN, Velozo CA, Dubno JR; Cochlear Implant Quality of Life Consortium . Validity and reliability of the Cochlear Implant Quality of Life (CIQOL)-35 Profile and CIQOL-10 Global instruments in comparison to legacy instruments. Ear Hear. 2021;42(4):896-908. doi: 10.1097/AUD.0000000000001022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Woodcock Johnson Achievement Scores for individuals with SPHL and without CIs
eTable 2. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs, income levels comparison (standard errors in parenthesis)
eTable 3. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs and annual family income between $25,000 and $50,000
eTable 4. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs and annual family income above $50,000
eTable 5. Standard Scores from the CDaCI study for those implanted before 18 months
eTable 6. Standard Scores from the CDaCI study for those implanted after 18 months
eTable 7. Woodcock Johnson Achievement Scores for individuals with SPHL without CIs according to whether they were prescribed hearing aids or not
Data Sharing Statement