Abstract
Optimizing antibiotic prescribing is a crucial element of the fight against antibiotic resistance. Antibiotic prescribing patterns in jails have not been studied. We established a baseline of antibiotic prescribing between Massachusetts jails. We detected heterogeneity in quantity and duration of antibiotic prescriptions, revealing an opportunity for improved practice.
Antimicrobial resistance is a growing public health emergency that has sparked collaboration and innovation across disciplines. Efforts to improve antibiotic prescribing practices have focused on traditional medical settings, such as hospitals, with an increasing focus on emergency departments, ambulatory clinics, and skilled nursing facilities. 1 However, antibiotic prescribing in correctional healthcare settings, including jails and prisons, has gone largely unstudied. Although core elements of antimicrobial stewardship have been recommended by the CDC that include application to low-resource settings, there are no guidelines for antimicrobial stewardship in correctional facilities. Approximately 2 million people are incarcerated each year in the United States, so investment in improving antimicrobial delivery to people who are incarcerated will benefit a sizeable portion of the country. 2 We compared antibiotic use across Massachusetts jails as a first step to improve antibiotic prescribing practices.
Methods
Data set
We collected deidentified antibiotic prescription data (drug name, dose frequency, number of doses, order start and end date) for all 12 county jails in Massachusetts for the 2021 calendar year. One jail was excluded due to its relatively small size and census. The data set was analyzed across 4 categories of oral and intramuscular antibiotic prescriptions: (1) anti-MRSA agents (clindamycin, doxycycline hyclate, doxycycline monohydrate, linezolid, minocycline, tetracycline HCl, sulfamethoxazole-trimethoprim), (2) fluoroquinolones (ciprofloxacin and levofloxacin), (3) other antibiotics (amoxicillin, azithromycin, cefadroxil, cefuroxime axetil, cefpodoxime proxetil, ceftriaxone, cephalexin, clarithromycin, erythromycin, and penicillin), and (4) all.
Analysis
We chose 2 metrics for comparison of antibiotic use between jails: antibiotic days per patient and an estimated defined daily dose (DDD) per 1,000 inhabitants. Antibiotic days per patient was used to model the most prescribed duration of therapy. The DDD methodology is recommended by the WHO and the CDC for monitoring medication use. 3 Due to the limited availability of jail census data, we estimated each jail’s population by converting the average daily population (ADP) to an annual population (Supplementary Fig. 2). The ADP was converted to an average weekly population, then multiplied by the average weekly turnover rate for a jail of that size to account for jail flux, and finally multiplied by 52 weeks. 4,5
Results
We detected heterogeneity in antibiotic prescribing practices across the Massachusetts state jail system. Our analysis revealed differences in the quantity of prescriptions and duration of therapy.
Duration of therapy
The most frequently prescribed duration of therapy with antibiotics varied between jails. For fluoroquinolones, jail 2 had the shortest duration of therapy at 5 days, whereas jail 11 had the longest duration of therapy at 30 days (Supplementary Fig. 1). The duration of therapy with anti-MRSA drugs ranged from 7 days in some jail to 10 days in others. Duration of therapy for “other” and “all” antibiotics ranged from 7 to 14 days.
Quantity of prescriptions
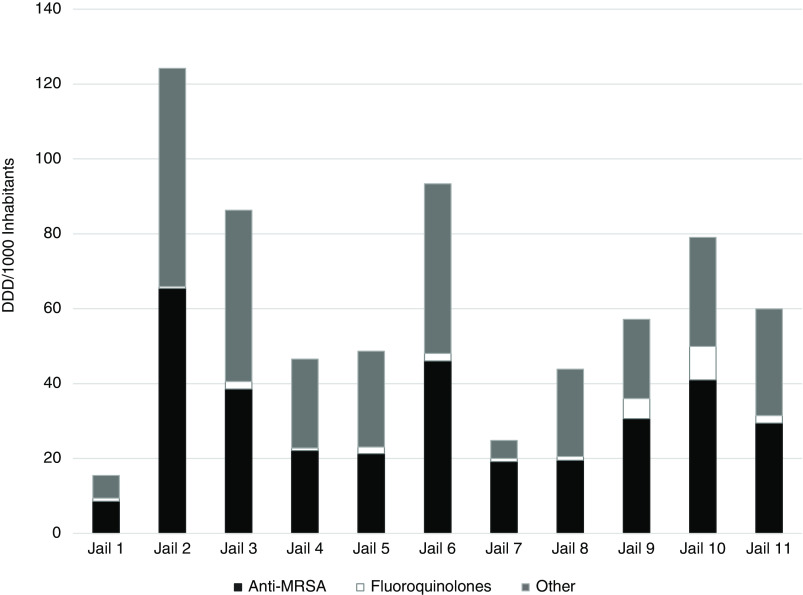
The estimated DDD per 1,000 inhabitants for “all” antibiotics ranged from 15.5 to 124.2 (Fig. 1). Jail 1 had the lowest estimated DDD per 1,000 inhabitants of 15.5. The equivalent of 1.5% (15.5 per 1,000) of the population in jail 1 received an antibiotic that year. Jail 2 had the highest estimated DDD per 1,000 inhabitants of 124.2; that is, the equivalent of 12.4% of the population of jail 2 received an antibiotic that year.
Figure 1.
The defined daily dose (DDD) of 19 antibiotics prescribed across 11 Massachusetts jails in 3 categories: anti-MRSA agents (clindamycin, doxycycline hyclate, doxycycline monohydrate, linezolid, minocycline, tetracycline HCl, sulfamethoxazole-trimethoprim), fluoroquinolones (ciprofloxacin, levofloxacin), and other (amoxicillin, azithromycin, cefadroxil, cefuroxime axetil, cefpodoxime proxetil, ceftriaxone, cephalexin, clarithromycin, erythromycin, and penicillin).
Discussion
Variability in prescribing practices reflects an opportunity to optimize antibiotic prescribing. 6 Although further investigation into the indication for therapy is needed, evaluating duration of therapy and selection of antibiotics can help to identify specific areas for detailed review. For example, anti-MRSA antibiotic use contributed to 50% or greater of the antimicrobial DDD per jail (Fig. 1), with 10 days of therapy as the common mode for many jails (Supplementary Fig. 1). For uncomplicated skin and soft-tissue infections, a common indication for oral anti-MRSA treatment, shorter courses of therapy (5–7 days) are recommended. 7 Therefore, there may be an opportunity to educate clinicians on the selection of agents within this setting.
Contrary to jails, the federal prison system has developed strategies aimed at improving antibiotic prescribing, including implementation of antimicrobial stewardship programs (ASPs). 8 Although ASPs may exist at jails or state prisons, we could find no publicly available data about the process or outcomes. Better understanding antibiotic prescribing practices in the carceral healthcare setting comes with an additional urgency owing to the significant health equity and public health concerns associated with the incarcerated population. The carceral system is the primary health provider to a large portion of the United States, with disproportionate incarceration of poor, minoritized, and marginalized communities. 9 Carceral facilities in the United States have high rates of multidrug-resistant infections, and prudent antibiotic use in these settings is highly relevant. 10
This study had several limitations. Jail healthcare delivery models vary by county in Massachusetts (ie, direct oversight vs medical vendor oversight), and this was not considered in our analysis. Because our data unit was deidentified prescriptions, we could not ascertain nor control for how pre-existing conditions affected antibiotic utilization nor evaluate the appropriateness of antibiotic prescriptions.
Despite these limitations, our research is novel as the first publication evaluating variability in prescribing practices among jails within the same state system. Having established this baseline of antibiotic use, our next steps will include analyzing disaggregated data to identify underlying trends and appropriateness of prescriptions. Our goal is to work with key stakeholders in jail and prison healthcare to promote integration of antimicrobial stewardship practices, decrease exposure to unnecessary antibiotics, and decrease the development of resistant bacteria.
Acknowledgments
We thank the sheriffs of Massachusetts counties, Wellpath, and Correctional Psychiatric Services for their collaboration.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.175.
click here to view supplementary material
Financial support
A.G.W. received grant support from the Tufts University Springboard Grant. R.T. received grant support from the Tufts University CIMAR Summer Student Grant.
Competing interests
All authors report no conflicts of interest relevant to this article.
References
- 1. Marcelin JR, Chung P, Van Schooneveld TC. Antimicrobial stewardship in the outpatient setting: a review and proposed framework. Infect Control Hosp Epidemiol 2020;41:833–840. [DOI] [PubMed] [Google Scholar]
- 2. Sawyer W. Mass incarceration: the whole pie, 2023. Prison Policy Initiative website. https://www.prisonpolicy.org/reports/pie2023.html#:∼:text=Together%2C%20these%20systems%20hold%20almost,centers%2C%20state%20psychiatric%20hospitals%2C%20and). Accessed May 30, 2023.
- 3. Introduction to DDD indicators. World Health Organization website. https://www.who.int/tools/atc-ddd-toolkit/indicators. Accessed May 30, 2023.
- 4. Wang L. Quarterly report on the status of prison capacity, third quarter, 2020. Report no. 20-328-DOC-01. Massachusetts Department of Correction; 2020.
- 5. Minton T. ZZ. Jail inmates in 2020—statistical tables. NCJ 303308. US Department of Justice website. https://bjs.ojp.gov/content/pub/pdf/ji20st.pdf. Published December 2020. Accessed May 30, 2023.
- 6. Gerber JS, Prasad PA, Russell Localio A, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatr Infect Dis Soc 2014;4:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–e52. [DOI] [PubMed] [Google Scholar]
- 8. Long MJ, LaPlant BN, McCormick JC. Antimicrobial stewardship in the Federal Bureau of Prisons: approaches from the national and local levels. J Am Pharm Assoc 2017;57:241–247. [DOI] [PubMed] [Google Scholar]
- 9. Kajeepeta S, Mauro PM, Keyes KM, El-Sayed AM, Rutherford CG, Prins SJ. Association between county jail incarceration and cause-specific county mortality in the USA, 1987–2017: a retrospective, longitudinal study. Lancet Public Health 2021;6:e240–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malcolm B. The rise of methicillin-resistant Staphylococcus aureus in US correctional populations. J Correct Health Care 2011;17:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.175.
click here to view supplementary material