Abstract
Introduction
As a CGM-derived indicator, ‘time in range’ (TIR) is emerging as a key indicator for accurate assessment of glycaemic control. However, there is few report focusing on the correlation of TIR with albumuria and renal fuction. The aim of this work was to investigate whether TIR, as well as nocturnal TIR and hypoglycaemic events is related to the presence and severity of albuminuria and decrease of eGFR in type 2 diabetes.
Research design and methods
A total of 823 patients were enrolled in this study. All patients received continuous glucose monitoring, TIR indicating the percentage of time that blood glucose was in the range of 3.9–10.0 mmol/L. The Spearman analysis was applied to analyze the relationship between TIR (or nocturnal TIR) and ACR. Logistic regression was used to explore whether TIR (or nocturnal TIR) is an independent risk factor for albuminuria.
Results
The prevalence of albuminuria decreased with increasing TIR quartiles. Binary logistic regression revealed that TIR as well as nocturnal TIR was obviously related to the presence of albuminuria. Multiple regression analysis found that only nocturnal TIR was obviously related to the severity of albuminuria. In our study, eGFR was significantly associated with the number of hypoglycemic events.
Conclusions
In T2DM patients, TIR and nocturnal TIR is associated with the presence of albuminuria independent of HbA1c and GV metrics. Nocturnal TIR shows better correlation than TIR. The role of TIR especially nocturnal TIR in the evaluation of diabetes kidney disease should be emphasized.
Keywords: Albuminuria, Hypoglycemia, Glomerular filtration rate, Time in range, Nocturnal time in range, Continuous glucose monitoring, Hemoglobin A1C, Type 2 diabetes mellitus
Introduction
According to the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS) [1, 2], hemoglobin A1C is the gold standard for assessing glycaemic control for decades and is strongly associated with the risk of long-term diabetic complications. However, as an indirect measure of average blood glucose levels over three months, HbA1c is considered to have some limitations [3]. HbA1c does not provide details about hypoglycaemia or hyperglycaemia and does not reflect glycemic variability [4]. HbA1c also has limitations in interpreting the risk of chronic complications of diabetes. For instance, HbA1c accounted for only 11% of the variation in risk of diabetic retinopathy observed in the Diabetes Control and Complications Trial (DCCT) [5].
Continuous glucose monitoring (CGM) provides an accurate reflection of an individual’s blood glucose status throughout the day. Compared to glycosylated haemoglobin, CGM technology can better reflect blood glucose variability. As a CGM-derived indicator, ‘time in range’ (TIR) is simple, intuitive and responsive to treatment and lifestyle changes, and has become a key indicator for assessing glycaemic control. It is negatively correlated with glycated haemoglobin and the American Diabetes Association 2021 guidelines [6] stated that it can be used to assess glycaemic control and might be an acceptable endpoint for future clinical trials. Several different studies [7] have reported that time range (TIR) is associated with the risk of microvascular complications and can predict the risk of future diabetic complications. Furthermore, according to the study [8], average blood glucose levels at night, but not daytime blood glucose values or glucose variability, were independently associated with the degree of vascular remodelling. This suggests that nighttime blood glucose may be a better indicator of the risk of diabetic complications than full-day blood glucose status. Circadian rhythms have received increasing attention in recent years with the award of the 2017 Nobel Prize to Young MW et al. [9] for their discoveries of molecular mechanisms controlling the circadian rhythm. Circadian rhythms also exist for various hypoglycemic hormones and baseline blood glucose levels, and studies have shown that baseline pre-meal glucose levels in animals and healthy humans show circadian rhythms under a regular light/dark cycle, with a trough during sleep and a peak during wakefulness [10–12]. Nevertheless, the clinical significance of circadian rhythms in blood glucose is not clear.
Diabetic kidney disease (DKD) is a common chronic microvascular complication of diabetes that is now a major cause of CKD and end-stage renal disease, manifested mainly by a urinary albumin/creatinine ratio (UACR) ≥ 30 mg/g and/or an estimated glomerular filtration rate (eGFR) < 60 ml-min -¹-(1.73 m²)-¹ that persists for more than 3 months. In early screening, a random urine measurement of UACR is recommended to reflect urinary albumin excretion. TIR was found to be strongly associated with albuminuria in type 2 diabetes [13]. Furthermore, respective study found that each 10% treatment-induced increase in TIR was associated with 18% reduction in albuminuria in patients with T1D [14]. However, there is little report on the impact of nocturnal TIR and albuminuria in T2D.
The aim of this work was to investigate whether TIR measured by CGM, especially nocturnal TIR and hypoglycaemic events is related to the presence and severity of albuminuria and decrease of eGFR in type 2 patients with diabetes.
Research design and methods
Participants
A total of 823 patients (aged ≥ 18 years) with T2DM admitted to the Department of Endocrinology, Jinling Hospital, Nanjing University from April 2018 to July 2020 were recruited, all of whom met the 1999 WHO diagnostic criteria for type 2 diabetes mellitus. Exclusion criteria included (1) patients with acute complications of diabetes, acute stress such as trauma, surgery and severe infections, severe respiratory disease, malignant disease, pregnancy; (2) patients with definite hepatic disease; (3) patients with narcotic and psychotropic drugs, and a recent history of alcoholism; (4) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula to calculate, patients with an estimated glomerular filtration rate (eGFR) of less than 30 ml/min/1.73m2. The study was supported by the local ethics committee.
Clinical and biochemical measurements
General clinical information and physical examination such as age, sex, duration of diabetes, hypertension, diabetic retinopathy and diabetic kidney disease were recorded. Height, weight, systolic blood pressure (SBP) were measured. Body mass index (BMI) was computed. Biochemical measurements such as blood and urine samples were tested after a 12-hour overnight fast. Hemoglobin A1C (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), blood urea nitrogen (BUN), and serum creatinine (SCr) were detected. Albumin creatinine ratio (ACR) was calculated from albumin and creatinine measured in urine samples, and based on ACR, patients was classified as normoalbuminuria (ACR < 30 mg/g), microalbuminuria (ACR 30–299 mg/g), and macroalbuminuria (ACR ≥ 300 mg/g). Estimate glomerular filtration rate (eGFR) was calculated by the CKD-EPI creatinine equation [15] using the clinical data of patients.
CGM parameters
Patients were asked to wear a continuous glucose monitoring system of Meiqi Company to monitor blood glucose levels every 5 min for 72 h continuously during the study. Capillary blood glucose at least four times was measured on each day of use of CGM to update the monitor. All 72 h of glucose data collected were calculated, TIR indicating the percentage of time that blood glucose was in the range of 3.9–10.0 mmol/L. Nocturnal TIR was defined as the percentage of time that blood glucose was in the range of 3.9–10.0 mmol/L between 00.00 am and 06:00 am (6 h). Patients were considered to be hypoglycaemic if they had at least one documented hypoglycaemic event during CGM monitoring. A blood glucose level < 3.9 mmol/L was recorded as one hypoglycaemic event. Nocturnal hypoglycaemic events are those that occur between 00.00 am and 06:00 am (6 h). Based on the original blood glucose data recorded by this system, a number of metrics concerning mean blood glucose (MBG) and glycemic variability (GV), including standard deviation (SD), average daily risk range (ADDR), mean amplitude of glucose excursions (MAGE), largest amplitude of plasma glucose excursions (LAGE), coefficient of variation (CV), and M-value were calculated using the EasyGV Version 9.0R2 provided by Oxford University.
Statistical analysis
The SPSS 22.0 software package was used for statistical analysis. Continuous data for normal distributions were expressed as mean ± SD, while data for abnormal distributions were expressed as median (upper and lower quartiles). Categorical data were expressed as numbers (percentages). Two normally distributed samples were compared using the Student’s t-test. One-way ANOVA was used for multi-sample comparisons and the KruskalWallis test was used for abnormal distributions. Categorical variables were tested using the χ2 test. The Spearman analysis was applied to analyze the relationship between TIR (or nocturnal TIR) and ACR. The binary logistic regression was applied to examine the independent connection between TIR (or nocturnal TIR) and ACR by adjusting age, sex, diabetes duration, BMI, lipid situation, SBP, SCr, HbA1c (%), and GV metrics. In addition, the multinomial logistic regression was applied to examine the independent connection between TIR (or nocturnal TIR) and different stages of ACR by adjusting age, P < 0.05 was considered statistically significant.
Results
Clinical characteristics among stages of ACR Groups
The median (upper and lower quartiles) age of all 823 patients was 56.0[38.0,65.0] years, the median (upper and lower quartiles) duration of diabetes was 7[2, 12] years. There were 570 diabetic individuals without albuminuria (normoalbuminuria), 188 individuals with microalbuminuria and 65 diabetic individuals with macroalbuminuria. The prevalences of microalbuminuria and macroalbuminuria were 22.8% and 7.9%, respectively. With the aggravation of albuminuria, patients showed increased levels of SCr, SBP, MBG, ADDR, and M-value (P < 0.05), and lower levels of nocturnal TIR (P < 0.001). Median (upper and lower quartiles) nocturnal TIR was 97.50%(71.18%, 100.00%), 88.61%(56.88%, 100.00%), and 79.86%(47.85%, 99.23%) in normoalbuminuria (ACR < 30 mg/g), microalbuminuria (ACR 30–299 mg/g), and macroalbuminuria, respectively. TIR was significant different with the aggravation of albuminuria (P < 0.001). However, no significant difference between microalbuminuria and macroalbuminuria was evident(P = 0.98). In addition, there was no significant difference in HbA1c (%) among different groups.
The comparison of clinical characteristics by quartiles (Q1-Q4) of TIR
Further analysis after dividing patients into groups was done according to quartiles of TIR((Q1):≤ 42.92%; (Q2): 42.92–69.13%; (Q3): 69.13–85.45%; (Q4): >85.45%). The characteristics were shown in Table 1. Patients with the highest quartiles of TIR had lower TC, TG, HbA1c (%), ACR, SD, MAGE, MBG, ADDR, CV and M-value (P < 0.001). Notably, there was no significant difference in eGFR between the different TIR groups (P = 0.702).
Table 1.
The comparison of clinical characteristics by quartiles (Q1-Q4) of TIR.
| Quartiles (Q1-Q4) of TIR | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | χ2/t/z | P | |
| N | 206 | 206 | 206 | 205 | ||
| Age (y) | 56.0(47.5,65.0) | 57.0(48.0,66.0) | 54.0(44.3,63.0) | 56.0(48.0,64.0) | 4.247 | 0.236 |
| Diabetes duration (y) | 8(3,15) | 10(4,15) | 6(2,12) | 5(1,10) | 31.323 | < 0.001 |
| Male (n, %) | 66 | 63 | 70 | 72 | 3.834 | 0.28 |
| SCr (µmol/L) | 54.00(46.00,67.50) | 56.00(46.00,67.00) | 58.00(48.00,68.75) | 59.00(48.00,70.00) | 4.622 | 0.202 |
| TC (mmol/L) | 4.49(3.77,5.39) | 4.46(3.72,5.35) | 4.29(3.73,5.06) | 4.27(3.53,4.88) | 12.611 | 0.006 |
| TG (mmol/L) | 1.74(1.15,2.84) | 1.59(1.59,2.56) | 1.47(1.1,2.17) | 1.37(1.02,2.12) | 12.11 | 0.007 |
| HDL (mmol/L) | 0.97(0.86,1.19) | 1.06(0.92,1.24) | 1.06(0.9,1.24) | 1.05(0.9,1.19) | 8.794 | 0.032 |
| LDL (mmol/L) | 2.54(1.96,3.32) | 2.66(2.07,3.37) | 2.6(2.07,3.18) | 2.51(1.89,3.12) | 5.211 | 0.157 |
| BMI | 24.54(22.60,27.46) | 25.34(22.0,27.24) | 25.39(23.14,27.68) | 25.39(22.60,27.78) | 5.891 | 0.117 |
| SBP (mmHg) | 128(120,142) | 128(120,140) | 130(120,140) | 129(120,138) | 0.886 | 0.829 |
| HbA1C (%) | 9.9(8.9,11.7) | 9(7.8,10.4) | 7.9(7,9.2) | 7(6.3,8.1) | 256.012 | < 0.001 |
| eGFR, mL/min per 1.73 m2 | 107.74(98.50,121.56) | 107.34(98.28,118.58) | 111.59(100.4,123.42) | 110.50(99.86,119.56) | 1.417 | 0.702 |
| ACR, mg/g | 16.7(8.8,67.7) | 13.7(7.5,59.0) | 11.2(5.6,31.8) | 10.4(5.7,31.3) | 20.556 | < 0.001 |
| SD (mmol/L) | 2.94(2.3,3.84) | 2.79(2.29,3.38) | 2.23(1.86,2.63) | 1.49(1.27,1.8) | 454.174 | < 0.001 |
| MAGE (mmol/L) | 5.07(3.79,6.25) | 5.12(4.06,6.21) | 4.31(3.54,5.29) | 3.38(2.89,4.04) | 192.068 | < 0.001 |
| MBG (mmol/L) | 11.96(11.25,13.04) | 9.8(9.42,10.26) | 8.46(8.17,8.86) | 7.39(6.84,7.81) | 793.17 | < 0.001 |
| CV | 0.25(0.19,0.3) | 0.28(0.24,0.35) | 0.26(0.22,0.31) | 0.2(0.17,0.25) | 154.467 | < 0.001 |
| ADDR (mmol/L) | 36.31(29.84,45.34) | 36.31(29.84,45.34) | 19.26(15.87,23.22) | 11.71(8.75,14.46) | 636.16 | < 0.001 |
| M-value (mmol/L) | 24.72(16.64,34.93) | 10.3(8.26,14.1) | 4.71(3.77,5.95) | 1.81(1.11,2.52) | 784.969 | < 0.001 |
TIR: time in range; Nocturnal TIR: nocturnal time in range; HbA1C: hemoglobin A1C; SD: standard deviation; MAGE: mean amplitude of glucose excursions; CV: coefficient of variation; ADDR: average daily risk range; eGFR: estimated glomerular filtration rate
Prevalence of all stages of albuminuria in different quartiles (Q1-Q4) of TIR
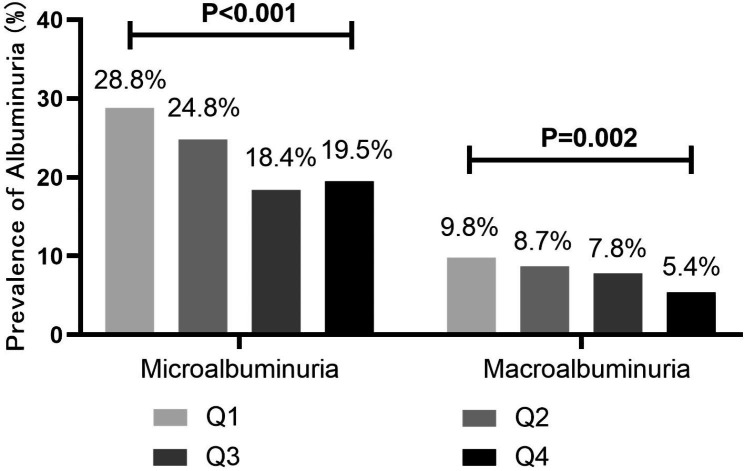
As shown in Fig. 1, individuals were classified into groups according to quartiles of the TIR, the proportion of “Normoalbuminuria” increased with the increase of TIR (P < 0.001). What else, the proportion of microalbuminuria and macroalbuminuria decreased with the increase of TIR (P < 0.05) (Fig. 2).
Fig. 1.
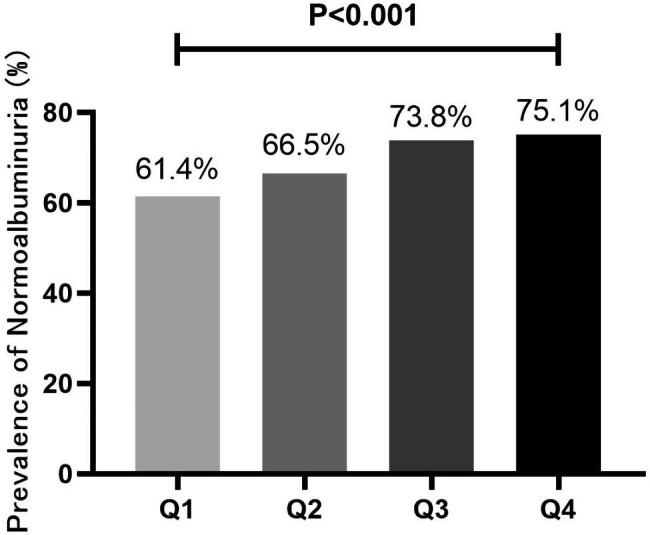
Prevalence of “Normalbuminuria” in different quartiles (Q1-Q4) of TIR. aTIR (Q1):≤ 42.92%; (Q2): 42.92–69.13%; (Q3): 69.13–85.45%; (Q4): >85.45%. bAs shown in this figure, patients were divided into groups according to quartiles of the time in range (TIR), the proportion of “Normoalbuminuria” increased with the increase of TIR (P < 0.001). P value for the significant
Difference among the groups was determined by χ2-test
Fig. 2.
Prevalence of Albuminuria in different quartiles (Q1-Q4) of TIR. aTIR (Q1):≤ 42.92%; (Q2): 42.92–69.13%; (Q3): 69.13–85.45%; (Q4): >85.45%. bAs shown in this figure, patients were divided into groups according to quartiles of the time in range (TIR), the proportion of microalbuminuria and macroalbuminuria decreased with the increase of TIR (P < 0.05). P value for the significant difference among the groups was determined by χ2-test
The correlation of TIR and nocturnal TIR with HbA1c, eGFR and ACR
Spearman analysis was used to analyse the correlation between TIR and nocturnal TIR with HbA1c, eGFR and ACR and the results are shown in Table 2. Both TIR and nocturnal TIR were negatively correlated with HbA1c (P < 0.001, P < 0.001), ACR (P < 0.001, P < 0.001) and insignificantly correlated with eGFR (P = 0.367,P = 0.495). Notably, the correlation coefficient between nocturnal TIR and ACR was higher than that of TIR (R=-0.159 vs. R=-0.147).
Table 2.
The Correlation of TIR and nocturnal TIR with HbA1c, eGFR and ACR.
| HbA1c | eGFR | ACR | ||
|---|---|---|---|---|
| TIR (3.9–10 mmol/L) | R | -0.544 | 0.029 | -0.147 |
| P | < 0.001 | 0.367 | < 0.001 | |
| Nocturnal TIR (3.9–10 mmol/L) | R | -0.425 | 0.022 | -0.159 |
| P | < 0.001 | 0.495 | < 0.001 |
TIR: time in range; Nocturnal TIR: nocturnal time in range; HbA1C: hemoglobin A1C; ACR: albumin/creatinine ratio; eGFR: estimated glomerular filtration rate
Associations between TIR, nocturnal TIR and ACR
Association of TIR and nocturnal TIR with the presence of albuminuria were investigated using binary logistic regression (Tables 3 and 4). After adjusting for age, diabetes duration, sex, BMI, lipid profile, blood pressure, and HbA1c (%) (model 1), the data revealed that TIR (odds ratio(OR): 0.992, 95% confidence interval (CI): 0.985–0.999, P = 0.026) as well as nocturnal TIR (OR: 0.990, 95% CI: 0.984–0.996, P = 0.002) was obviously related to the presence of albuminuria. After adjusting for SD, MAGE, CV and ADDR (model 2,3,4,5), the association persisted. However, after adjustment of M-value (model 6), the link between albuminuria and TIR was weakened (p = 0.197), the association between the presence of albuminuria and nocturnal TIR was still strong (p = 0.015).
Table 3.
Associations between between TIR and albuminuria
| Microalbuminuria | Macroalbuminuria | Any Albuminuria | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Model 1 | ||||||
| TIR | 0.993(0.985-1.000) | 0.062 | 0.988(0.976-1.000) | 0.053 | 0.992(0.985–0.999) | 0.026 |
| Model 2 | ||||||
| TIR | 0.994(0.986–1.002) | 0.126 | 0.99(0.977–1.002) | 0.112 | 0.993(0.986–1.001) | 0.082 |
| SD | 1.069(0.926–1.233) | 0.363 | 1.113(0.941–1.316) | 0.213 | 1.091(0.955–1.247) | 0.2 |
| Model 3 | ||||||
| TIR | 0.989(0.981–0.997) | 0.008 | 0.987(0.974-1) | 0.052 | 0.989(0.981–0.996) | 0.004 |
| MAGE | 0.91(0.821–1.008) | 0.072 | 0.948(0.816–1.101) | 0.484 | 0.92(0.838–1.01) | 0.079 |
| Model 4 | ||||||
| TIR | 0.993(0.985-1) | 0.065 | 0.987(0.975-1) | 0.05 | 0.992(0.985–0.999) | 0.026 |
| CV | 0.626(0.086–4.558) | 0.644 | 13.67(1.45-128.892) | 0.022 | 2.168(0.437–10.764) | 0.344 |
| Model 5 | ||||||
| TIR | 0.983(0.973–0.994) | 0.003 | 0.988(0.973–1.003) | 0.118 | 0.986(0.977–0.996) | 0.006 |
| ADDR | 0.972(0.95–0.994) | 0.014 | 1(0.975–1.025) | 0.989 | 0.983(0.965–1.002) | 0.078 |
| Model 6 | ||||||
| TIR | 0.994(0.985–1.003) | 0.216 | 0.992(0.979–1.005) | 0.224 | 0.995(0.986–1.003) | 0.197 |
| M-value | 1.005(0.992–1.017) | 0.482 | 1.01(0.998–1.022) | 0.105 | 1.007(0.997–1.018) | 0.164 |
a TIR: time in range; HbA1C: hemoglobin A1C; SD: standard deviation; MAGE: mean amplitude of glucose excursions; CV: coefficient of variation; ADDR: average daily risk range; CI: confidence interval
b Model 1 was adjusted for age, diabetes duration, sex, BMI, lipid profile, blood pressure, and HbA1c (%); model 2 was adjusted for variables as in model 1 and for SD; model 3 was adjusted for variables as in model 1 and for MAGE; model 4 was adjusted for variables as in model 1 and for CV; model 5 was adjusted for variables as in model 1 and for ADDR; model 6 was adjusted for variables as in model 1 and for M-value
Table 4.
Associations between between nocturnal TIR and albuminuria
| Microalbuminuria | Macroalbuminuria | Any Albuminuria | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Model 1 | ||||||
| Nocturnal TIR | 0.992(0.985–0.998) | 0.015 | 0.984(0.974–0.994) | 0.002 | 0.990(0.984–0.996) | 0.002 |
| Model 2 | ||||||
| Nocturnal TIR | 0.992(0.985–0.999) | 0.027 | 0.992(0.985–0.999) | 0.027 | 0.991(0.984–0.997) | 0.002 |
| SD | 1.068(0.931–1.225) | 0.348 | 1.068(0.931–1.225) | 0.348 | 1.087(0.956–1.236) | 0.002 |
| Model 3 | ||||||
| Nocturnal TIR | 0.99(0.983–0.997) | 0.005 | 0.983(0.972–0.993) | 0.002 | 0.988(0.982–0.995) | 0.001 |
| MAGE | 0.928(0.842–1.024) | 0.137 | 0.954(0.827–1.101) | 0.522 | 0.935(0.856–1.022) | 0.139 |
| Model 4 | ||||||
| Nocturnal TIR | 0.992(0.985–0.999) | 0.018 | 0.984(0.973–0.994) | 0.002 | 0.99(0.983–0.996) | 0.001 |
| CV | 0.72(0.1-5.183) | 0.744 | 13.837(1.455-131.599) | 0.022 | 2.392(0.487–11.761) | 0.283 |
| Model 5 | ||||||
| Nocturnal TIR | 0.988(0.98–0.996) | 0.003 | 0.984(0.972–0.996) | 0.007 | 0.987(0.98–0.995) | 0.001 |
| ADDR | 0.981(0.963–0.999) | 0.04 | 0.998(0.975–1.021) | 0.867 | 0.987(0.972–1.003) | 0.109 |
| Model 6 | ||||||
| Nocturnal TIR | 0.992(0.985-1) | 0.051 | 0.986(0.975–0.997) | 0.012 | 0.991(0.985–0.998) | 0.015 |
| M-value | 1.003(0.991–1.014) | 0.648 | 1.008(0.998–1.018) | 0.128 | 1.005(0.997–1.014) | 0.234 |
a TIR: time in range; Nocturnal TIR: nocturnal time in range; HbA1C: hemoglobin A1C; SD:standard deviation; MAGE: mean amplitude of glucose excursions; CV: coefficient of variation; ADDR: average daily risk range; CI: confidence interval; eGFR: estimated glomerular filtration rate
b Model 1 was adjusted for age, diabetes duration, sex, BMI, lipid profile, blood pressure, and HbA1c (%); model 2 was adjusted for variables as in model 1 and for SD; model 3 was adjusted for variables as in model 1 and for MAGE; model 4 was adjusted for variables as in model 1 and for CV; model 5 was adjusted for variables as in model 1 and for ADDR; model 6 was adjusted for variables as in model 1 and for M-value
The multinomial Logistic regression found the weak relationship between TIR and severe stage of albuminuria (Microalbuminuria: P = 0.062. Macroalbuminuria: P = 0.053.) (Tables 4 and 6) (model 1). However, nocturnal TIR was obviously related to the severity of albuminuria (Microalbuminuria: OR: 0.992, 95% CI: 0.985–0.998, P = 0.015. Macroalbuminuria: OR: 0.984, 95% CI: 0.974–0.994, P = 0.002.) (model 1). Furthermore, the association persisted after adjusting for SD, MAGE, CV and ADDR (model 2,3,4,5), after adjustment of M-value (model 6), the link between the severe stage of albuminuria and nocturnal TIR was weakened (Microalbuminuria: p = 0.051; Macroalbuminuria: P = 0.012).
Table 6.
The correlation between eGFR and the number of hypoglycaemic events
| B | 95%CI | p | |
|---|---|---|---|
| number of hypoglycaemic events | -0.090 | (-2.769, -0.761) | 0.001 |
| age | -0.497 | (-1.103, -0.876) | < 0.001 |
| duration of diabetes | -0.149 | (-0.774, -0.347) | < 0.001 |
| blood pressure | -0.073 | (-0.188, -0.033) | 0.005 |
The correlation of hypoglycaemic events and nocturnal hypoglycaemic events with HbA1c, eGFR and ACR
The number of nocturnal hypoglycaemic events was used as a statistic for hypoglycaemia. Based on Spearman analysis, Table 5 presents the correlations between hypoglycaemicevents and nocturnal hypoglycaemic events with HbA1c, eGFR, and ACR. Hypoglycaemia and nocturnal hypoglycaemia were both negatively correlated with HbA1c (P < 0.001, P = 0.005), eGFR (P < 0.001, P = 0.003), and not significantly correlated with ACR (P = 0.476, P = 0.351). A multiple linear regression model was used to assess the correlation between eGFR and the number of hypoglycaemic events. The model showed that the number of hypoglycaemic, age, duration of diabetes and blood pressure were independent risk factors for eGFR. (B = -0.090, -0.497, -0.149, -0.073, all P < 0.05, Table 6).
Table 5.
The Correlation of hypoglycaemic events and nocturnal hypoglycaemic events with HbA1c, eGFR and ACR.
| HbA1c | eGFR | ACR | ||
|---|---|---|---|---|
| Hypoglycaemic events | R | -0.138 | -0.131 | -0.023 |
| P | < 0.001 | < 0.001 | 0.476 | |
| Nocturnal hypoglycaemic events | R | -0.091 | -0.096 | -0.030 |
| P | 0.005 | 0.003 | 0.351 |
HbA1C: hemoglobin A1C; ACR: albumin/creatinine ratio; eGFR: estimated glomerular filtration rate
Discussion
In our cohort of 823 T2D patients, the prevalence of albuminuria decreased with increasing TIR quartiles. Binary logistic regression revealed that TIR as well as nocturnal TIR was obviously related to the presence of albuminuria after adjusting for age, diabetes duration, sex, BMI, lipid profile, blood pressure, HbA1c (%), SD, MAGE, CV and ADDR. However, after adjustment of M-value, the link between albuminuria and TIR was weakened, the association between the presence of albuminuria nocturnal TIR was still significant. Multiple regression found the relationship between TIR and severity of albuminuria. However, nocturnal TIR was obviously related to the severe stage of albuminuria regardless of SD, MAGE, CV and ADDR. In our study, eGFR was not significantly associated with TIR, but was significantly associated with the number of hypoglycemic events and the number of nocturnal hypoglycemic events.
As the gold standard for assessing glycaemic management, hemoglobin A1c (HbA1c) is considered to have a strong correlation with the microvascular complications of diabetes, including diabetic nephropathy [16, 17]. However, HbA1c does not reflect information on hyperglycaemia, hypoglycaemia and fluctuations in blood glucose, nor does it reflect the magnitude and frequency of intra- and inter-day glucose changes [18, 19]. In addition, the measurement of glycated haemoglobin can be affected by specific conditions including kidney insufficiency, anaemia, pregnancy, haemoglobinopathies and iron deficiency [20–23]. Due to the shortcomings of HbA1c, CGM-derived indicators, especially TIR has become an alternative marker of glycemic control over the last few years [24, 25].
There is insufficient evidence in previous studies to demonstrate the association of glycaemic variability with diabetic nephropathy in T2DM. The study by S.-M. Jin et al. did not find an independent association between glycaemic variability and the degree of proteinuria in patients with type 2 diabetes [26]. Subramanian S et al. suggest that glycaemic variability may be a factor in the development of DKD, but clear evidence is lacking [27]. Wakasugi S et al. demonstrated that FLP-CGMderived metrics related to intraday and interday glucose variability, including TIR, SD, MAGE and MODD, were significantly associated with albuminuria severity, and these associations remained significant after adjustment for HbA1c [28]. Our study also found that TIR was obviously related to the presence of albuminuria after adjusting for glycaemic variability Indicators including SD, MAGE, CV and ADDR. However, in contrast to their results, multiple regression analysis found a weaker relationship between TIR and the stage of albuminuria severity.
A series of studies have demonstrated a correlation between CGM indicators, represented by TIR, and the development or progression of albuminuria in T2DM. Yoo JH et al. demonstrated that CGM-derived TIR significantly associated with the risk of albuminuria, even after adjusting for various confounding factors including CV. With a 10% increase in TIR, the risk of albuminuria was reduced by 6% [9]. However, after further adjustment for HbA1c, the study did not show a significant association between TIR and albuminuria. In contrast to their results, we found that TIR as well as nocturnal TIR was obviously related to the presence of albuminuria even after adjustment for HbA1c. In the study by Varghese JS, participants were divided into three groups based on glycemic profile (‘TIR profile’, ‘hyper’ and ‘hypo’). Compared with ‘TIR profile’, both ‘hyper’ and ‘hypo’ profiles had higher odds of macroalbuminuria and higher odds of diabetic kidney disease [29]. TIR was demonstrated to be associated with UACR, DPN and T2DM duration in the multicentre, prospective cohort study by Kuroda N et al. [30]. Consistent with those fndings, we found that the prevalence of albuminuria decreased as the TIR quartiles increased. The independently negative association between TIR and the presence of albuminuria exists even after adjusting for several risk factors including HbA1c.
There is little previous research on the relationship between nocturnal blood glucose and diabetic complications. A study of T1DM showed that reduced nocturnal TIR was more closely associated with function of sudomotor nerves function of sudomotor nerves [31]. In our study, nocturnal TIR showed a better correlation with albuminuria compared to TIR. Binary logistic regression analysis showed that TIR as well as nocturnal TIR were significantly correlated with the presence of albuminuria, after adjustment for a range of indicators. However, after further correction for M-value, only nocturnal TIR remained significant. Furthermore, multiple regression analysis showed that TIR did not correlate significantly with the severity of albuminuria, while nocturnal TIR correlated significantly, even after adjustment for a number of indicators including HbA1c. One possible explanation is attributed to the effect of growth hormone secreted at night on the kidneys. Growth hormone (GH) is widely used to treat short stature in children, including children with chronic kidney disease (CKD). GH-excess can affect kidney health by causing glomerular hyperfiltration, hypertrophy, and glomerulosclerosis [32]. GH-excess is also an important promoter of diabetes nephropathy in T1DM patients. Studies in patients with T1DM showed that urinary GH and IGF-1 levels were related to microalbuminuria in patients [33]. In addition, GH also has a renal protective effect on humans. Studies have shown that GH therapy can protect cisplatin-induced nephropathy in rats [34]. To sum up, GH secreted at night has an impact on blood glucose and kidneys, and the nocturnal TIR can better include its impact on blood glucose, which may explain the better correlation between nocturnal TIR and albuminuria. In addition, cortisol as a circadian hormone is not easy to ignore. a study by Roy et al. [35] found a tendency for elevated cortisol secretion in patients with diabetic retinopathy or diabetic cardiovascular complications. Chiodini I et al. [36] showed that the degree of midnight cortisol secretion was directly related to the presence and number of complications of type 2 diabetes, including DKD. In contrast, other parameters of cortisol secretion were not significantly correlated with the presence or number of diabetic complications. We can therefore speculate that nocturnal TIR could incorporate the effect of nocturnal cortisol levels on blood glucose and therefore correlate better with diabetic nephropathy. In addition, diabetes significantly affects melatonin secretion levels at night. Hikichi et al. [37] compared melatonin secretion at night and during the day in non-diabetic and diabetic subjects and found that melatonin levels were lower at night in patients with diabetes, but not during the day as affected by diabetes. The study by Baris Afsar et al. [38] suggests that melatonin activates cardiovascular system and renal receptors to protect DN in preclinical models. Considering that elevated blood glucose at night may inhibit melatonin secretion, thus depriving the kidneys of the protective effect of melatonin, the association between nocturnal TIR and proteinuria is more significant compared to full-day TIR.
Unlike albuminuria and TIR, our study found no significant correlation between eGFR and TIR. In recent years, the role of the renal tubules has received increasing attention in studies on the pathogenesis of DKD. As a traditional indicator of kidney disease, the increase of microalbuminuria or creatinine based glomerular filtration rate (eGFR) may occur long after the decline of renal function [39]. Some biomarkers of proximal renal tubular injury, such as kidney injury molecule 1 (KIM-1), N-acetyl-b-D-glucosaminidase (NAG), and liver fat acid binding protein (L-FABP), appear abnormal before proteinuria, and may become new biomarkers for DKD prediction [40]. At present, it is believed that the mechanism of albuminuria is the excessive inflammatory reaction of proximate tubular epithelial cells (PTECs) under the condition of diabetes [41, 42]. Considering the central role of renal tubules rather than glomeruli in the occurrence and development of early stage DKD, it can be explained why TIR, as a blood glucose control indicator, has a good correlation with albuminuria but not with eGFR. A notable result was that hypoglycaemic and nocturnal hypoglycaemic events were negatively correlated with eGFR. According to the study by Khanimov I [43], eGFR was strongly associated with an increased incidence of hypoglycaemia during hospitalization in non-critically ill patients. However, this study also showed that renal function was a strong predictor of hypoglycaemia regardless of the presence of diabetes, implying that hypoglycaemic events may not be specific for the prediction of diabetic nephropathy.
Our study had several limitations that should be noted. Firstly, this is a retrospective, single-centre study and it can only describe the correlation, not the causal relationship. Secondly, all subjects received CGM for 72 h, which may not be representative of overall glucose status. Some glucose-raising hormones that may play a role in the development of diabetic complications such as cortisol, growth hormone and melatonin were not measured.
In conclusion, our study revealed that TIR and nocturnal TIR is associated with the presence and severity of albuminuria independent of HbA1c and GV metrics. Nocturnal TIR shows better correlation than TIR. Hypoglycemia and nocturnal hypoglycemia events were negatively correlated with eGFR, while TIR was not correlated with eGFR. The role of TIR and nocturnal TIR in the evaluation of diabetes kidney disease should be emphasized.
Abbreviations
- TIR
time in range
- T2DM
type 2 diabetes mellitus
- eGFR
estimated glomerular filtration rate
- GV
glycemic variability
- CV
cardiovascular
- HbA1C
hemoglobin A1C
- CGM
continuous glucose monitoring
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- BMI
body mass index
- TC
total cholesterol
- TG
triglyceride
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- Scr
serum creatinine
- ACR
urinary albumin/creatinine ratio
- DKD
diabetic kidney disease
- DR
diabetic retinopathy
- MBG
mean blood glucose.
- SD
standard deviation.
- MAGE
mean glucose fluctuation amplitude
- CV
coefficient of variation
- MODD
mean absolute difference of daytime glucose
- ADDR
mean daily risk range
- TBR
time below range
- TAR
time above range
- OR
odds ratio
- CI
confidence interval
- T1DM
type 1 diabetes mellitus
Authors’ contributions
Ping Gu conceived and designed the research. Xuguang Jin, Xinyi Yang and Yixin Xu collected the data. Xuguang Jin analyzed and interpreted the data. Xuguang Jin performed the statistical analysis. Xuguang Jin wrote the manuscript. Ping Gu, Jiaqing Shao critically revised the manuscript and contributed to the discussion. Xuguang Jin is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Jinling Hospital affiliated with Nanjing University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors’ information
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuguang Jin, Xinyi Yang, Yixin Xu contributed equally to this work.
Contributor Information
Jiaqing Shao, Email: shaojiaq@hotmail.com.
Yong Zhong, Email: zhongyongnj@163.com.
Ping Gu, Email: guping@nju.edu.cn.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352(9131):837–53. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 2.The DCCTR, Group, “The Diabetes Control and Complications Trial (DCCT) Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes. 1986;35(5):530–45. doi: 10.2337/diab.35.5.530. [DOI] [PubMed] [Google Scholar]
- 3.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess Glycemic Control can be misleading. Diabetes Care Aug. 2017;40(8):994–9. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulintreated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther Nov. 2012;14(11):1008–12. doi: 10.1089/dia.2012.0099. [DOI] [PubMed] [Google Scholar]
- 5.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial–revisited. Diabetes. 2008;57(4):995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 7.Bellido V, Pinés-Corrales PJ, Villar-Taibo R, Ampudia-Blasco FJ. Time-in-range for monitoring glucose control: is it time for a change? Diabetes Res Clin Pract. 2021;177:108917. doi: 10.1016/j.diabres.2021.108917. [DOI] [PubMed] [Google Scholar]
- 8.Yano Y, Hayakawa M, Kuroki K, et al. Nighttime blood pressure, nighttime glucose values, and target-organ damages in treated type 2 diabetes patients. Atherosclerosis. 2013;227(1):135–9. doi: 10.1016/j.atherosclerosis.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Young MW, Time Travels A 40-Year journey from Drosophila’s clock mutants to Human Circadian Disorders (Nobel lecture) Angew Chem Int Ed Engl. 2018;57(36):11532–9. doi: 10.1002/anie.201803337. [DOI] [PubMed] [Google Scholar]
- 10.La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11(8):643–52. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 11.Challet E, Malan A, Turek FW, Van Reeth O. Daily variations of blood glucose, acid-base state and PCO2 in rats: effect of light exposure. Neurosci Lett. 2004;355(1–2):131–5. doi: 10.1016/j.neulet.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Cailotto C, La Fleur SE, Van Heijningen C, et al. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005;22(10):2531–40. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in Range, other Core Metrics, and Albuminuria in Type 2 diabetes. Diabetes Technol Ther. 2020;22(10):768–76. doi: 10.1089/dia.2019.0499. [DOI] [PubMed] [Google Scholar]
- 14.Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved Time in Range over 1 year is Associated with reduced Albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care. 2020;43(11):2882–5. doi: 10.2337/dc20-0909. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408] Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995;47(6):1703–20. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 17.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DJ, Kovatchev BP, Julian DM, et al. Frequency of severe hypoglycemia in insulinde-pendent diabetes mellitus can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79:1659–62. doi: 10.1210/jcem.79.6.7989471. [DOI] [PubMed] [Google Scholar]
- 19.Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted fromglycemic variability data. Diabetes Technol Ther. 2012;14:1008–12. doi: 10.1089/dia.2012.0099. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center. Sickle cell trait & other hemoglobinopathies & diabetes (for providers) [Internet]. Available from https://www.niddk.nih.gov/health-information/diagnostic-tests/sickle-cell-trait-hemoglobinopathiesdiabetes. Accessed 12 January 2018.
- 21.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153–63. doi: 10.1093/clinchem/47.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Cowie CC, Li C, Handelsman Y, Bloomgarden ZT. Iron-deficiency anemia, noniron-deficiency anemia and HbA1c among adults in the US. J Diabetes. 2011;3:67–73. doi: 10.1111/j.1753-0407.2010.00100.x. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200–1. doi: 10.2337/diacare.27.5.1200. [DOI] [PubMed] [Google Scholar]
- 24.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose Monitoring Data Interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellido V, Aguilera E, Cardona-Hernandez R, et al. Expert Recommendations for using Time-in-range and other continuous glucose monitoring Metrics to achieve patient-centered Glycemic Control in people with diabetes [published online ahead of print, 2022 Apr 26]. J Diabetes Sci Technol. 2022;19322968221088601. 10.1177/19322968221088601. [DOI] [PMC free article] [PubMed]
- 26.Jin SM, Kim TH, Oh S, et al. Association between the extent of urinary albumin excretion and glycaemic variability indices measured by continuous glucose monitoring. Diabet Med. 2015;32(2):274–9. doi: 10.1111/dme.12607. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian S, Hirsch IB. Diabetic kidney disease: is there a role for glycemic variability? Curr Diab Rep. 2018;18(3):13. doi: 10.1007/s11892-018-0979-3. [DOI] [PubMed] [Google Scholar]
- 28.Wakasugi S, Mita T, Katakami N, et al. Associations between continuous glucose monitoring-derived metrics and diabetic retinopathy and albuminuria in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e001923. doi: 10.1136/bmjdrc-2020-001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varghese JS, Ho JC, Anjana RM, et al. Profiles of intraday glucose in type 2 diabetes and their association with complications: an analysis of continuous glucose Monitoring Data. Diabetes Technol Ther. 2021;23(8):555–64. doi: 10.1089/dia.2020.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda N, Kusunoki Y, Osugi K, et al. Relationships between time in range, glycemic variability including hypoglycemia and types of diabetes therapy in japanese patients with type 2 diabetes mellitus: Hyogo Diabetes Hypoglycemia Cognition Complications study. J Diabetes Investig. 2021;12(2):244–53. doi: 10.1111/jdi.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng ZQ, Guo QY, Wang W, et al. Time in range, especially overnight time in range, is associated with sudomotor dysfunction in patients with type 1 diabetes. DiabetolMetabSyndr. 2021;13(1):119. doi: 10.1186/s13098-021-00739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffner D, Grund A, Leifheit-Nestler M. Renal effects of growth hormone in health and in kidney disease. Pediatr Nephrol. 2021;36(8):2511–30. doi: 10.1007/s00467-021-05097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verrotti A, Cieri F, Petitti MT, Morgese G, Chiarelli F. Growth hormone and IGF-I in diabetic children with and without microalbuminuria. Diabetes NutrMetab. 1999;12(4):271–6. [PubMed] [Google Scholar]
- 34.Mahran YF. New insights into the protection of growth hormone in cisplatin-induced nephrotoxicity: the impact of IGF-1 on the Keap1-Nrf2/HO-1 signaling. Life Sci. 2020;253:117581. doi: 10.1016/j.lfs.2020.117581. [DOI] [PubMed] [Google Scholar]
- 35.Roy MS, Roy A, Brown S. Increased urinary-free cortisol outputs in patients with diabetes. J Diabetes Complications. 1998;12(1):24–7. doi: 10.1016/s1056-8727(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 36.Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30(1):83–8. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- 37.Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:655–60. doi: 10.2147/OPTH.S19559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afsar B, ElsurerAfsar R, Sag AA, et al. Sweet dreams: therapeutic insights, targeting imaging and physiologic evidence linking sleep, melatonin and diabetic nephropathy. Clin Kidney J. 2020;13(4):522–30. doi: 10.1093/ckj/sfz198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2015;38(6):954–62. doi: 10.2337/dc15-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert RE. Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic kidney disease. Diabetes. 2017;66(4):791–800. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Jia Y, Xue M, et al. Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci. 2020;261:118347. doi: 10.1016/j.lfs.2020.118347. [DOI] [PubMed] [Google Scholar]
- 43.Khanimov I, Zingerman B, Korzetz A, et al. Association between estimated GFR and incident hypoglycaemia during hospitalization. Nephrol (Carlton) 2022;27(2):162–70. doi: 10.1111/nep.13984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.