Abstract
Background
Insulin resistance (IR) has been confirmed that getting involved in the pathophysiological process of cardiovascular diseases (CVD). Recently, increasing evidence suggests metabolic score for insulin resistance (METS-IR), triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, triglyceride and glucose (TyG) index, triglyceride glucose-body mass (TyG-BMI) index are simple and reliable surrogates for IR. However, their abilities in predicting cardiovascular outcomes in patients undergoing percutaneous coronary intervention (PCI) are not well explored. Therefore, this study aimed to investigate the association and evaluate the predictive performance of each index.
Methods
A total of 2533 consecutive participants undergoing PCI were included in this study, and the data from 1461 patients were used to determine the correlation of these non-insulin-based IR indices with major adverse cardiac and cerebrovascular events (MACCEs) via performing the multivariate logistic models and restricted cubic splines (RCS).
Results
During a median of 29.8 months follow-up, 195 cases of 1461 patients experienced incident MACCEs. In the overall population, both univariate and multivariate logistic regression analyses indicated no statistically significant connection between these IR indices and MACCEs. Subgroup analyses revealed significant interactions between age subgroups and TyG-BMI index, as well as METS-IR, and between sex subgroups and TyG index. In elderly patients, per 1.0-SD increment in TyG-BMI index and METS-IR had a significant association with MACCEs, with odds ratios (ORs) [95% confidence interval (CI)] of 1.24 (1.02–1.50) and 1.27 (1.04–1.56), respectively (both P < 0.05). Moreover, in female patients, all the IR indices showed significant associations with MACCEs. Multivariable-adjusted RCS curves demonstrated a linear relationship between METS-IR and MACCEs in elderly and female patients, respectively. However, all the IR indices failed to enhance the predictive performance of the basic risk model for MACCEs.
Conclusion
All the four IR indices showed a significant association with MACCEs in female individuals, whereas only TyG-BMI index and METS-IR showed associations in elderly patients. Although the inclusion of these IR indices did not improve the predictive power of basic risk model in either female or elderly patients, METS-IR appears to be the most promising index for secondary prevention of MACCEs and risk stratification in patients undergoing PCI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01898-1.
Keywords: Coronary artery disease, Insulin resistance, Cardiovascular outcomes, Percutaneous coronary intervention, Metabolic score for insulin resistance
Introduction
Coronary artery disease (CAD) has been the first major cause of death worldwide, posing a heavy burden on public health and healthcare costs [1, 2]. Patients undergoing PCI for CAD should receive guideline-directed medical treatment as part of secondary prevention strategies to improve the clinical outcomes [3–5]. Although the excellent performance of drug-eluting stents (DES) in reducing restenosis and advancement in medical care have noticeably improved the outcomes, some individuals, like with hypertension or diabetes mellitus (DM), still face a high risk of recurrences of cardiovascular events [6, 7]. Therefore, early and optimized risk stratification has far-reaching significance in improving the secondary prevention of patients who undergo PCI.
Insulin resistance (IR), which refers to the diminished or impaired insulin sensitivity of target organs or tissues shown as impairments in absorbing and oxidizing the glucose, has been confirmed as an important risk factor in pathogenesis of DM and CVD [8, 9]. The underlying mechanisms may account for the role of IR in endothelial impairment [8], ectopic angiotensinogen production [10], and inappropriate activation of renin-angiotensin-aldosterone system (RAAS) [11]. Although the hyperinsulinemic-euglycemic clamp is regarded as the gold-standard method for assessing IR [12], its clinical application is limited due to cost and complexity. Fortunately, several alternative measures of IR have been established that are more convenient and still valid, such as METS-IR [13], TG/HDL-C [14, 15], TyG index [16], and TyG-BMI index [17]. Previous evidence has shown that these four IR indices are significantly associated with various CVD risk factors, including DM, metabolic syndrome, and arterial stiffness progression [10, 18–21]. Moreover, accumulating evidence suggests that these IR indices can predict the presence and severity of CAD, as well as adverse cardiovascular outcomes like stroke, acute myocardial infarction (AMI), and mortality [22–25].
However, there is limited research specifically investigating the correlation between these IR indices and cardiovascular outcomes in patients undergoing PCI with DES, and no studies have compared their predictive abilities for MACCEs in this patient population. Therefore, there is an urgent need to explore the association between these IR indices and cardiovascular events in individuals undergoing PCI with DES, and to assess whether incorporating these IR indices into the basic predictive model improves its performance.
Methods
Study population
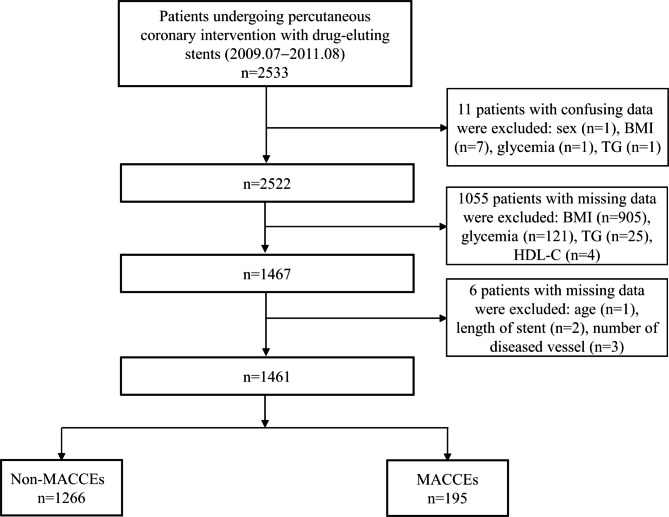
The data used in the study were obtained from a public dataset (10.5061/dryad.13d31) uploaded by Yao HM et al. [26, 27]. This study with a waiver of informed consent has obtained the approval from the ethics committee of the First Affiliated Hospital of Zhengzhou University. Considering the nature of public dataset, no further research ethic was needed in the present study. The detailed study design has been described by Yao HM et al. [26]. In brief, a total of 2533 consecutive participants with CAD who underwent PCI with DES were recruited between July 2009 and August 2011. The participants were followed up for a median of 29.8 months (25.6−34.0 months). PCI procedures were performed by the experienced surgeons following standard protocols. Loading doses of aspirin (300 mg) and clopidogrel (300 mg) were administered before the PCI, unless the patients were already on standard antiplatelet medication. Medication use after discharge was in accordance with the guidelines at the time. As shown in Fig. 1, a total of 1461 patients were enrolled in the present study after excluded the confusing or missing data.
Fig. 1.
The flowchart of study participants
Data collection and definitions
The data on demographics, medical history and clinical presentation were recorded at admission. Fasting peripheral venous-blood samples were collected before PCI to obtain the laboratory data, including glycemia, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), creatinine, uric acid, triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C). Angiographic and procedural information, such as the location of the culprit vessel, characteristics of lesions, length and diameters of stents, were extracted from the medical records. Information on medication was also collected. The standardized spreadsheets were designed to collect these retrospective data.
Hypertension was diagnosed based on a self-reported physician diagnosis, recent use of an antihypertensive agent, or a BP ≥ 140/90 mmHg [28]. DM was defined as glycemia > 6.1 mmol/L, HbA1c level ≥ 6.5%, or recent use of a hypoglycemic agent (insulin and/or any hypoglycemic drug) [26]. The elderly patients referred to individuals with age ≥ 60 years [29]. BMI was calculated as: BMI (kg/m2) = weight/height2. Smokers were considered as those who had smoked within the past 10 years. Repeat revascularization in the target vessel was adjudicated as target vessel revascularization (TVR). The formulars of the four indices [13, 14, 16, 17] are summarized as Supplementary file 1, Table S1.
Endpoints and follow up
The endpoint of the study was defined as MACCEs, including all-cause death, AMI, stroke and TVR. The follow-up data were obtained through outpatient clinic visits, telephone interviews, or readmission, and the endpoints were adjudicated by an independent committee.
Statistical analysis
Data analyses were performed using STATA MP version 17.0, RStudio 4.2.1 and SPSS 24.0. The normal distribution and equality of variance of continuous datasets were evaluated using the Kolmogorov-Smirnov test and Levene test, respectively. The continuous datasets were described using mean ± standard deviation (SD) or median (25th and 75th percentiles), and compared by one-way analysis of variance or Mann-Whitney U test, as appropriate. Nominal variables were described as counts and percentages, and the chi-square test or Fisher’s exact test was used to identify differences between the groups, as appropriate.
The four IR indices were standardized (Z-score) and added to the unadjusted or adjusted models to evaluate the impact of per 1.0-SD increment in the indices on MACCEs. The univariate logistic regression was conducted prior to the multivariate model to select the covariates. Based on a significance level of P < 0.05, the following covariates were adjusted for: age, hypertension, diabetes mellitus, heart failure, previous AMI, creatinine, uric acid (UA), angiotensin converting enzyme inhibitor (ACEI), number of diseased vessels, left anterior descending (LAD), right coronary artery (RCA), length and diameters of stents to identify the association between the four IR indexes and MACCEs in overall population. All the adjusted variables were evaluated for collinearity, and no clear evidence of multicollinearity was found in overall population (all-variance inflation factor of the included variables was < 5) (Supplementary file 2, Figure S1A).
Next, we also conducted subgroup analyses stratified by age, sex, DM and hypertension, and the P value for interaction was calculated. Then the association between the four IR indices and MACCEs was further explored in elderly and female patients, respectively, and no multicollinearity was detected using the above criteria (Supplementary file 2, Figure S1B and C). Multivariate adjusted restricted cubic splines (RCS) were used to assess whether there was a linear or nonlinear correlation between METS-IR and MACCEs in the gender and age subgroups (with a threshold of P < 0.10). The receiver operating characteristic (ROC) curves were used for diagnostic value analysis, and the area under the curve, as measured by the C-statistic, was computed to quantify the predictive power of logistic models for MACCEs [30]. Additionally, the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) index were calculated to further assess the additional predictive value of the four indices beyond established risk factors for MACCEs. A two-side P < 0.05 was considered as statistically significant.
Results
The study excluded 11 patients with confusing data and an additional 1061 patients with missing data, as shown in Fig. 1. The clinical features of the included and excluded patients were presented at Supplementary file 1, Table S2. The included patients had a higher prevalence of DM, a higher proportion of smoking, and use of ACEI and stain, a higher prevalence of acute coronary syndromes (ACS), and higher levels of lipid parameters. They also had a lower incidence of MACCEs, a lower prevalence of stable angina (SA), previous AMI and stroke. No significant differences in age, BMI, proportion of females, and angiographic characteristics were observed between the excluded and included patients.
Baseline characteristics of the included participants by MACCEs
The study included a total of 1461 patients receiving at least one DES at baseline. During the 34-month follow-up period, 195 cases (13.35%) experienced incident MACCEs. The baseline characteristics of the participants were compared between those who experienced MACCEs and those who did not, and the results are presented in Table 1. As expected, several variables showed obvious differences between the patients with MACCEs and those without MACCEs. In brief, compared patients without MACCEs, those with MACCEs tended to be older and had a higher prevalence of previous AMI, heart failure, hypertension, and DM. They also had higher levels of creatinine and UA, and had a higher proportion of ACEI use. Additionally, there was a higher prevalence of chronic total occlusions (CTO) and ≥ 3-vessel disease, as well as a higher occurrence of target lesions in LAD and RCA. Furthermore, the length of stents used in patients with MACCEs was longer compared to those without MACCEs (all P < 0.05). However, no discernible differences were found in the proportion of females, BMI, lipid parameters, glycemia, and any of the four IR indices between the MACCEs and non-MACCEs groups (all P > 0.05). In addition, Supplementary file 1, Table S3 and S4 showed the baseline data for the participants, classified according to age and sex.
Table 1.
Baseline characteristics of participants by MACCEs.
| Characteristics | Overall (n = 1461) |
Non-MACCEs (n = 1266) |
MACCEs (n = 195) |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60.10 ± 11.11 | 59.40 ± 10.94 | 64.67 ± 11.14 | < 0.001 |
| Female, (%) | 459 (31.4) | 395 (31.2) | 64 (32.8) | 0.650 |
| BMI, kg/m2 | 23.85 ± 3.80 | 23.79 ± 3.79 | 24.27 ± 3.89 | 0.102 |
| Medical history | ||||
| Heart failure, n (%) | 161 (11.0) | 129 (10.2) | 32 (16.4) | 0.010 |
| Atrial fibrillation, n (%) | 25 (1.7) | 21 (1.7) | 4 (2.1) | 0.764 |
| Previous AMI, n (%) | 130 (8.9) | 103 (8.1) | 27 (13.8) | 0.009 |
| Previous stroke, n (%) | 64 (4.4) | 55 (4.3) | 9 (4.6) | 0.863 |
| Previous PCI, n (%) | 82 (5.6) | 68 (5.4) | 14 (7.2) | 0.308 |
| Hypertension, n (%) | 726 (49.7) | 616 (48.7) | 110 (56.4) | 0.045 |
| Diabetes mellitus, n (%) | 329 (22.5) | 268 (21.2) | 61 (31.4) | 0.001 |
| Smoking, n (%) | 502 (34.4) | 430 (34.0) | 72 (36.9) | 0.418 |
| Clinical presentation | 0.842 | |||
| STEMI, n (%) | 377 (25.8) | 329 (26.0) | 48 (24.6) | |
| NSTE-ACS, n (%) | 909 (62.2) | 784 (61.9) | 125 (64.1) | |
| SA, n (%) | 175 (12.0) | 153 (12.1) | 22 (11.3) | |
| Laboratory data | ||||
| Glycemia, mmol/L | 6.10 ± 2.76 | 6.10 ± 2.80 | 6.11 ± 2.47 | 0.963 |
| Creatinine, µmol/L | 72.36 ± 30.25 | 71.43 ± 28.42 | 78.51 ± 39.85 | 0.003 |
| Uric acid, µmol/L | 305.60 ± 97.38 | 303.33 ± 91.92 | 320.46 ± 126.79 | 0.023 |
| TG, mmol/L | 1.61 (1.16, 2.33) | 1.62 (1.17, 2.35) | 1.56 (1.09, 2.25) | 0.363 |
| TC, mmol/L | 4.30 ± 1.08 | 4.29 ± 1.06 | 4.39 ± 1.20 | 0.232 |
| HDL-C, mmol/L | 1.08 ± 0.34 | 1.08 ± 0.32 | 1.09 ± 0.42 | 0.610 |
| LDL-C, mmol/L | 2.74 ± 0.95 | 2.72 ± 0.94 | 2.84 ± 1.03 | 0.091 |
| Treatment | ||||
| Aspirin, n (%) | 1438 (98.6) | 1243 (98.3) | 195 (100.0) | 0.070 |
| Clopidogrel, n (%) | 1412 (96.6) | 1224 (96.7) | 188 (96.4) | 0.774 |
| Beta blocker, n (%) | 1017 (69.6) | 872 (68.9) | 145 (74.4) | 0.121 |
| ACEI, n (%) | 854 (58.5) | 714 (56.4) | 140 (71.8) | < 0.001 |
| CCB, n (%) | 360 (24.6) | 310 (24.5) | 50 (25.6) | 0.728 |
| Statin, n (%) | 1365 (93.4) | 1177 (93.0) | 188 (96.4) | 0.071 |
| Number of diseased vessels | < 0.001 | |||
| 1-vessel disease, n (%) | 545 (37.3) | 496 (39.2) | 49 (25.1) | |
| 2-vessel disease, n (%) | 554 (37.9) | 484 (38.2) | 70 (35.9) | |
| 3-vessel disease, n (%) | 362 (24.8) | 286 (22.6) | 76 (39.0) | |
| Location of target lesions | ||||
| LM, n (%) | 46 (3.1) | 36 (2.8) | 10 (5.1) | 0.089 |
| LAD, n (%) | 1221 (83.6) | 1046 (82.6) | 175 (89.7) | 0.012 |
| LCX, n (%) | 717 (49.1) | 609 (48.1) | 108 (55.4) | 0.058 |
| RCA, n (%) | 736 (50.4) | 614 (48.5) | 122 (62.6) | < 0.001 |
| Characteristics of lesions | ||||
| Occlusion, n (%) | 197 (13.5) | 173 (13.7) | 24 (12.3) | 0.605 |
| CTO, n (%) | 121 (8.3) | 88 (7.0) | 33 (16.9) | < 0.001 |
| Ostial lesion, n (%) | 170 (11.6) | 146 (11.5) | 24 (12.3) | 0.753 |
| Bifurcation lesion, n (%) | 251 (17.2) | 217 (17.1) | 34 (17.4) | 0.919 |
| Number of treated vessels | < 0.001 | |||
| 1-vessel disease, n (%) | 853 (58.4) | 747 (59.0) | 106 (54.4) | |
| 2-vessel disease, n (%) | 481 (32.9) | 424 (33.5) | 57 (29.2) | |
| ≥ 3-vessel disease, n (%) | 127 (8.7) | 95 (7.5) | 32 (16.4) | |
| Length of stents, (mm) | 48.55 ± 31.24 | 47.11 ± 29.95 | 57.90 ± 37.33 | < 0.001 |
| Diameter of stents, (mm) | 3.12 ± 1.09 | 3.14 ± 1.16 | 3.00 ± 0.40 | 0.098 |
| TyG index | 8.94 ± 0.67 | 8.95 ± 0.66 | 8.94 ± 0.69 | 0.912 |
| TyG-BMI index | 213.54 ± 38.93 | 212.95 ± 38.51 | 217.40 ± 41.50 | 0.137 |
| TG/HDL-C ratio | 3.65 (2.37, 5.67) | 3.70 (2.37, 5.67) | 3.18 (2.38, 5.67) | 0.280 |
| METS-IR | 38.53 ± 7.73 | 38.42 ± 7.69 | 39.25 ± 8.01 | 0.162 |
ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infraction; BMI, body mass index; CCB, calcium channel blocker; CTO, chronic total occlusions; HDL-C, high-density lipoprotein cholesterol; LAD, left anterior descending; LCX, left circumflex artery; LDL-C, low-density lipoprotein cholesterol; LM, left main coronary artery; MACCEs, major adverse cardiac and cerebrovascular events; METS-IR, metabolic score for insulin resistance; NSTEMI, ono-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SA, stable angina; STEMI, ST elevation myocardial infarction; TC, total cholesterol; TG, triglyceride; TG/HDL-C, triglyceride to high-density lipoprotein cholesterol ratio; TyG, triglyceride and glucose; TyG-BMI, triglyceride glucose-body mass index
Association of IR indices with MACCES in overall population
The logistic analyses were conducted to assess the impact of per 1.0-SD increment in the four IR indices on MACCEs, as shown in Table 2. To screen for the adjusted covariates, the univariate logistic regression analysis was performed (Supplementary file 1, Table S5), and the identified risk factors included age, heart failure, previous AMI, hypertension, DM, creatinine, UA, use of ACEI, target lesions in LAD and RCA, CTO, number of diseased vessels, and the diameters and length of stents. Regrettably, no great association were found between the four IR indices and MACCEs in either univariate or multivariate logistic models.
Table 2.
Association between insulin resistance indexes and MACCEs in overall population
| OR (95% CI)a | P value | OR (95% CI)b | P value | OR (95% CI)c | P value | |
|---|---|---|---|---|---|---|
| TyG index | 0.99 (0.85–1.15) | 0.912 | 0.98 (0.83–1.16) | 0.844 | 0.94 (0.79–1.12) | 0.508 |
| TyG-BMI index | 1.12 (0.97–1.30) | 0.137 | 1.05 (0.89–1.23) | 0.575 | 1.03 (0.87–1.22) | 0.738 |
| TG/HDL-C ratio | 1.04 (0.90–1.19) | 0.610 | 1.09 (0.95–1.25) | 0.225 | 1.05 (0.90–1.21) | 0.550 |
| METS-IR | 1.11 (0.96–1.29) | 0.162 | 1.08 (0.92–1.27) | 0.349 | 1.05 (0.89–1.24) | 0.578 |
CI, confidence interval; OR, odds ratio
a Model 1: unadjusted
b Model 2: adjusted for age, hypertension, and diabetes mellitus
c Model 3: model 2 + further adjusted for heart failure, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, length of stents, and diameters of stents
Subgroup analyses
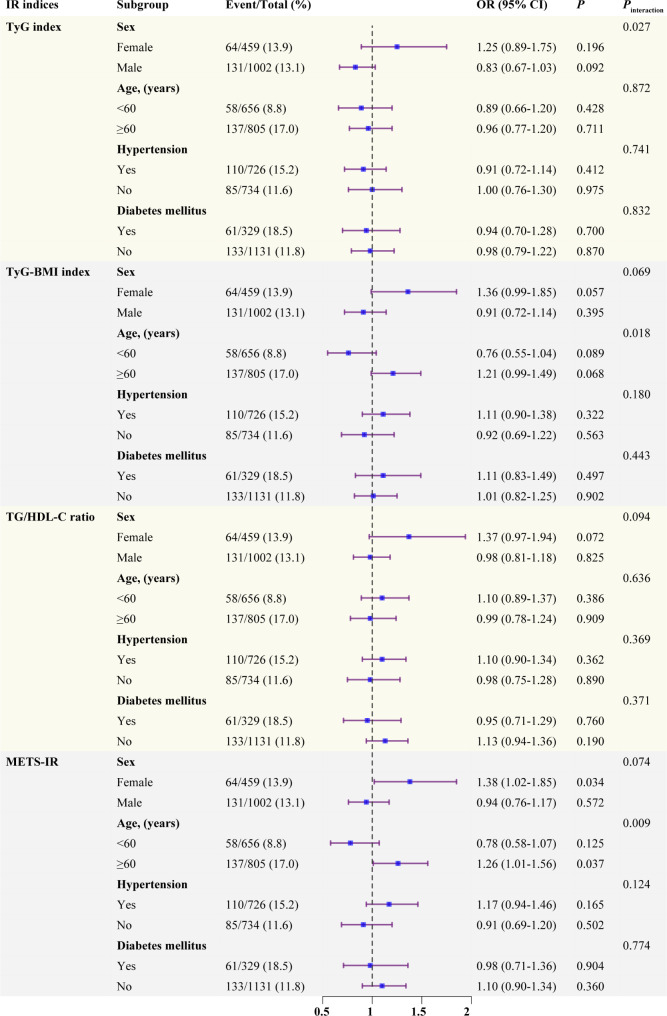
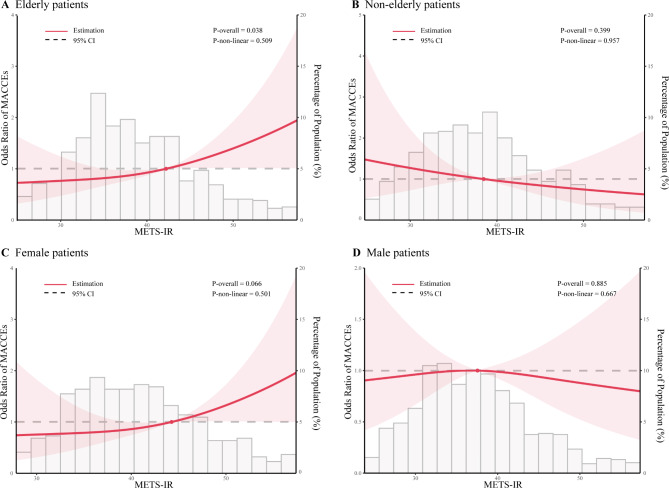
Next, we conducted the exploratory subgroups analyses stratified by age, sex, DM and hypertension. As shown in Fig. 2, the results indicated a significant interaction between age subgroups and the impact of TyG index on the incidence of MACCEs (P for interaction = 0.027). Similarly, significant interactions were detected between sex subgroups and either TyG-BMI index or METS-IR (P for interaction = 0.018 and 0.009, respectively). Furthermore, a positive correlation was found between per 1.0-SD increment in METS-IR and the incidence of MACCEs in elderly (OR: 1.26, 95% CI: 1.01−1.56, P = 0.037) and female patients (OR: 1.38, 95% CI: 1.02−1.85, P = 0.034), respectively. Encouraged by the results, we assessed whether a linear or nonlinear association existed between METS-IR and the subgroups stratified by age and sex using multivariate adjusted RCS. The results, as shown in Fig. 3, indicated a significantly linear relationship between METS-IR and MACCEs in elderly patients (P for overall = 0.038, P for nonlinear = 0.509) and female patients (P for overall = 0.066, P for nonlinear = 0.501). However, no apparent association was observed in non-elderly and male patients (all P for overall > 0.10).
Fig. 2.
Association between IR indices and MACCEs among people undergoing PCI in different subgroups. Each subgroup was adjusted for age, hypertension, diabetes mellitus, heart failure, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, length of stents, and diameters of stents. Odds ratios are presented as per 1.0-SD increase in the IR indices for MACCEs. ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infraction; CI, confidence interval; IR, insulin resistance; LAD, left anterior descending; MACCEs, major adverse cardiac and cerebrovascular events; METS-IR, metabolic score for insulin resistance; RCA, right coronary artery; TG/HDL-C, triglyceride to high-density lipoprotein cholesterol ratio; TyG, triglyceride and glucose; TyG-BMI, triglyceride glucose-body mass index
Fig. 3.
Restricted cubic spline curves for MACCEs by METS-IR after covariate adjustment. (A) Relationship in the elderly patients; (B) Relationship in the non-elderly patients; (C) Relationship in the female patients; (D) Relationship in the male patients. The threshold of statistical significance was set as P < 0.10. In A and B, age, heart failure, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, CTO, and length of stents were adjusted; in C and D, age, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, RCA, CTO, and length of stents were adjusted. Odds ratios are indicated by solid lines and 95% CIs by shaded areas. ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infraction; CI, confidence interval; CTO, chronic total occlusions; IR, insulin resistance; LAD, left anterior descending; MACCEs, major adverse cardiac and cerebrovascular events; METS-IR, metabolic score for insulin resistance; RCA, right coronary artery
Association of IR indices with MACCEs in elderly patients
Then the correlations between the four IR indices and MACCEs in elderly patients were explored. Covariates were selected based on the results from univariate logistic model (Supplementary file 1, Table S6). Three models were established, including an unadjusted, partly adjusted and fully adjusted model (Table 3). The results indicated that per 1.0-SD increment in TyG-BMI index and METS-IR showed a significant association with MACCEs in elderly patients. The OR for the TyG-BMI index was 1.24 (95% CI: 1.02−1.50, P = 0.033), while for METS-IR, the OR was 1.27 (95% CI: 1.04−1.56, P = 0.020).
Table 3.
Association between insulin resistance indexes and MACCEs in elderly patients
| OR (95% CI)a | P value | OR (95% CI)b | P value | OR (95% CI)c | P value | |
|---|---|---|---|---|---|---|
| TyG index | 1.01 (0.84–1.22) | 0.904 | 1.09 (0.90–1.32) | 0.390 | 1.04 (0.85–1.27) | 0.719 |
| TyG-BMI index | 1.21 (1.01–1.44) | 0.036 | 1.26 (1.06–1.51) | 0.011 | 1.24 (1.02–1.50) | 0.033 |
| TG/HDL-C ratio | 1.00 (0.83–1.22) | 0.967 | 1.06 (0.87–1.28) | 0.570 | 1.01 (0.81–1.26) | 0.918 |
| METS-IR | 1.25 (1.04–1.50) | 0.017 | 1.32 (1.09–1.59) | 0.004 | 1.27 (1.04–1.56) | 0.020 |
CI, confidence interval; OR, odds ratio
a Model 4: unadjusted
b Model 5: adjusted for age, heart failure, and previous AMI
c Model 6: model 5 + further adjusted for creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, CTO, and length of stents
Association of IR indices with MACCEs in female patients
We further explored the relationship of these IR indices with MACCEs in female patients. Similar with the above analysis, the univariate logistic model was performed prior to the three models constructed (Supplementary file 1, Table S7). All the four IR indices had a positive correlation with MACCEs in the female patients in whichever of the three models (Table 4).
Table 4.
Association between insulin resistance indexes and MACCEs in female patients
| OR (95% CI)a | P value | OR (95% CI)b | P value | OR (95% CI)c | P value | |
|---|---|---|---|---|---|---|
| TyG index | 1.36 (1.05–1.76) | 0.021 | 1.39 (1.06–1.83) | 0.018 | 1.39 (1.04–1.86) | 0.024 |
| TyG-BMI index | 1.37 (1.07–1.76) | 0.013 | 1.35 (1.04–1.75) | 0.023 | 1.37 (1.04–1.80) | 0.027 |
| TG/HDL-C ratio | 1.40 (1.05–1.85) | 0.021 | 1.52 (1.12–2.07) | 0.008 | 1.49 (1.08–2.06) | 0.015 |
| METS-IR | 1.32 (1.04–1.69) | 0.024 | 1.35 (1.05–1.74) | 0.020 | 1.38 (1.05–1.81) | 0.021 |
CI, confidence interval; OR, odds ratio
a Model 7: unadjusted
b Model 8: adjusted for age, previous AMI
c Model 9: model 8 + further adjusted for creatinine, uric acid, ACEI, number of diseased vessels, RCA, CTO, and length of stents
Incremental predictive performance of IR indexes in the risk assessment of MACCEs
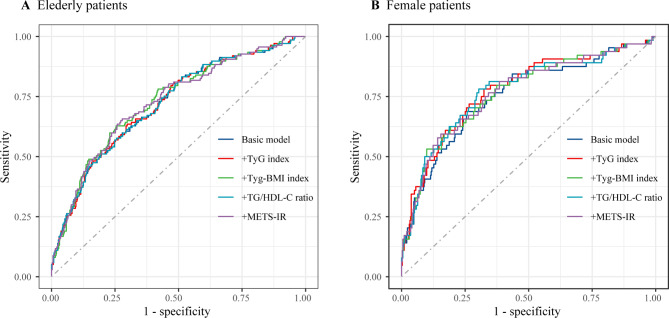
In the analysis of elderly patients, the ROC curves were constructed to assess the predictive power of the basic model (including age, heart failure, previous AMI, creatinine, UA, use of ACEI, number of diseased vessels, LAD, RCA, CTO, and length of stents) and the basic model plus each of the four IR indices for MACCEs, respectively (Fig. 4A). The C-statistic, NRI and IDI were presented in Table 5. Unfortunately, the results showed no significant incremental predictive ability of the four IR indices to the basic risk model in elderly patients. The similarly results also were observed in female patients (Fig. 4B; Table 6).
Fig. 4.
The receiver operating characteristic curves of the IR indices as a marker to predict MACCEs. (A) Basic risk model vs. + the IR indices in the elderly patients. Basic risk model includes age, heart failure, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, CTO, and length of stents; (B) Basic risk model vs. + the IR indices in the female patients. Basic risk model includes age, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, RCA, CTO, and length of stents. ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infraction; CI, confidence interval; CTO, chronic total occlusions; IR, insulin resistance; LAD, left anterior descending; MACCEs, major adverse cardiac and cerebrovascular events; METS-IR, metabolic score for insulin resistance; RCA, right coronary artery
Table 5.
Improvement in discrimination and risk reclassification for MACCEs after adding IR indices in elderly patients
| Model | C-statistic (95% CI) |
P value | NRI (95% CI) |
P value | IDI (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Basic model | 0.719 (0.672–0.767) | Ref. | Ref. | Ref. | ||
| +TyG index | 0.720 (0.673–0.768) | 0.562 | 0.065 (-0.092–0.244) | 0.452 | 0.000 (-0.001–0.001) | 0.905 |
| +TyG-BMI index | 0.730 (0.683–0.777) | 0.150 | 0.243 (0.009–0.419) | 0.020 | 0.006 (-0.001–0.014) | 0.109 |
| +TG/HDL-C ratio | 0.720 (0.672–0.767) | 0.854 | -0.062 (-0.147–0.288) | 0.578 | 0.000 (-0.001–0.001) | 0.855 |
| +METS-IR | 0.729 (0.681–0.779) | 0.228 | 0.158 (-0.008–0.373) | 0.109 | 0.009 (0.001–0.016) | 0.034 |
CI, confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification improvement
The basic model included age, heart failure, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, LAD, RCA, CTO, and length of stents
Table 6.
Improvement in discrimination and risk reclassification for MACCEs after adding IR indices in female patients
| Model |
C-statistic (95% CI) |
P value | NRI (95% CI) |
P value | IDI (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Basic model | 0.753 (0.684–0.823) | Ref. | Ref. | Ref. | ||
| +TyG index | 0.776 (0.709–0.842) | 0.040 | 0.178 (-0.046–0.569) | 0.257 | 0.013 (-0.002–0.027) | 0.087 |
| +TyG-BMI index | 0.767 (0.698–0.835) | 0.169 | 0.241 (-0.027–0.538) | 0.092 | 0.012 (-0.002–0.027) | 0.103 |
| +TG/HDL-C ratio | 0.772 (0.704–0.841) | 0.175 | 0.101 (-0.182–0.507) | 0.564 | 0.014 (-0.004–0.033) | 0.122 |
| +METS-IR | 0.766 (0.698–0.835) | 0.194 | 0.174 (-0.055–0.523) | 0.235 | 0.014 (-0.001–0.028) | 0.066 |
CI, confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification improvement
The basic model included age, previous AMI, creatinine, uric acid, ACEI, number of diseased vessels, RCA, CTO, and length of stents
Discussion
Considering the limited availability of data on evaluating the predictive performance of IR indices in patients undergoing PCI, we designed the present study that assessed the association of the four IR indices with MACCEs in patients undergoing PCI with at least one DES. This study not only provides new evidence for risk stratification of these patients but also represents the first investigation of the association between METS-IR and MACCEs in PCI patients. By addressing this research gap, our findings may exert far-reaching significance for the secondary prevention of CVD. The main findings could be summarized as follows: (1) the TyG-BMI index and METS-IR were markedly associated with MACCEs in elderly patients, while all the four IR indexes were obviously associated with MACCEs in female patients; (2) more importantly, the METS-IR demonstrated a strong linear association with MACCEs in either elderly or female individuals, suggesting that METS-IR might be a promising biomarker in predicting the adverse cardiovascular outcomes in those patients; (3) the four IR indices did not significantly optimize the predictive performance of basic risk model for MACCEs in either elderly or female patients.
The value and need of IR assessment on cardiovascular outcomes
Increasing evidence suggested that IR, as the major characteristic of type 2 DM, has a major role in the pathogenesis of CVD [31]. Currently, growing evidence also suggests that elevated IR can initiate and contribute to the development of CVD, as well as predict cardiovascular events in patients with pre-existing CVD [32–34]. More importantly, although remarkable advancements have been made in clinical practice, such as timely PCI and medication treatment, the incidence of CVD in general population continues to rise, and the long-term cardiovascular events, like restenosis in stents, heart failure, stroke and cardiac death, remain high. Therefore, there is an urgent need to identify the IR promptly and accurately, with an expectation of improving primary and secondary prevention through establishing effective cardiovascular risk stratification. While the hyperinsulinemic-euglycemic clamp is regarded as the gold-standard method of assessing IR [12], its complexity limits its application in clinical practice and epidemiological investigation. Consequently, several reliable and valid alternatives for non-insulin-based IR assessment have been developed [13–17]. However, to date, the relationship between IR and cardiovascular outcomes of patients undergoing PCI have not been well elucidated. Thus, this study was designed to explore the association of METS-IR with MACCEs and compare the predictive abilities of the four IR indices for MACCEs in elderly or female patients undergoing PCI.
The previous evidence on association of IR with MACCEs
Data from a study of 1092 ACS patients undergoing PCI suggested that patients with the upper quartile of TyG index had an increased risk of MACCEs within 1 year after PCI (hazard ratio (HR): 1.53, 95% CI: 1.00−2.06, P = 0.003) [9]. Another study by Xiong et al., which included 633 consecutive patients with type 2 DM undergoing PCI and followed up for 18.83-month, found that TyG index independently predicted the major adverse cardiac events (HR: 1.80, 95% CI: 1.26−2.57, P = 0.001). Furthermore, the inclusion of TyG index in basic risk model significantly improved the predictive performance [35]. Evidence from the Korean population indicated that TyG-BMI index was superior to TyG index for IR prediction [36]. The TyG-BMI index has also demonstrated a robust and independent association with ischemic stroke [37]. However, the inconsistent results were found in a rural Chinese cohort study involving 14,595 participants. The study suggested that the relative risk (95% CI) for stroke with per 1.0-SD increment in TyG index was 1.23 (1.15−1.32) and in TyG-BMI index was 1.17 (1.09−1.26) (both P < 0.05), and the C-statistics (95% CIs) for predicting stroke were 0.614 (0.606−0.621) for TyG index and 0.598 (0.590−0.606) for the TyG-BMI index, respectively [25]. Furthermore, a study by Yunke et al. involving 317 participants revealed that TG/HDL-C ratio could independently predict cardiovascular events [38]. Two other studies also demonstrated that the TG/HDL-C ratio was an effective biomarker for evaluating the severity of CAD [22, 39]. However, there are inconsistent findings regarding the priority between TyG index and TG/HDL-C ratio.
Stronger associations of METS-IR with MACCEs than other IR indices
More recently, the METS-IR has been proposed as a more promising and reliable indicator for evaluating IR and predicting cardiovascular outcomes [13]. Mounting evidence suggests that METS-IR is positively associated with the severity of CAD and has a better predictive ability than the other three indices [22, 39]. These findings are consistent with our own findings, as only METS-IR showed a positive correlation with MACCEs in both female and elderly patients in the subgroup analyses (Fig. 2). The positive association of METS-IR with MACCEs remains robust in elderly and female patients (Tables 4 and 5), but not in the overall population. There are several possible reasons for the findings. Firstly, age is a well-known risk factor for CAD, and elderly individuals with CAD are more likely to experience cardiovascular events [40]. Secondly, the elderly patients in our study had a higher proportion of multi-vessel (2- and 3-vessel) disease, higher BMI, higher prevalence of DM, and longer length of stents. Regarding the sex-specific difference, female patients in our study were much older than male patients (63.24 ± 10.04 vs. 58.67 ± 11.28). Thirdly, the prospective effect of estrogen on circulating and endocrine systems may diminish or disappear for females in menopausal transition or postmenopausal period [41, 42]. Aging women, especially those in the menopausal transition or postmenopausal period, are more likely to have increased abdominal fat accumulation and a higher prevalence of metabolic dysfunction, such as dyslipidemia, IR, and chronic low-grade inflammation [41, 43]. Several previous studies have also reported a more pronounced association of IR with cardiovascular events in females compared to males, which is consistent with our results [34, 44]. Moreover, differences in endothelial function between elderly and younger patients may contribute to the observed results. Age-related arterial stiffness and endothelial dysfunction can impact treatment response and long-term outcomes in elderly patients [45]. Additionally, chronic low-grade inflammation is common in elderly or perimenopausal women, which may increase the risk of MACCEs [43]. Finally, elderly and female CAD patients often have a higher burden of comorbidities and coexisting conditions, such as hypertension, DM, abdominal obesity, and chronic kidney disease [46], which can interact with CAD and influence treatment outcomes.
Furthermore, a prospective cohort study of 6489 adults suggested that the highest quintile and per 1.0-SD increasement in METS-IR were highly correlated with a higher risk of CVD incidence (HR: 1.80, 95% CI: 1.24−2.61, P < 0.01; HR: 1.17, 95% CI: 1.05−1.31, P < 0.01, respectively) [47]. These inconsistent results may be attributed to the heterogeneity of the included population and study design. Therefore, there is an urgent need to conduct our study to provide additional evidence on whether non-insulin IR indices can predict cardiovascular outcomes in patients undergoing PCI.
General mechanisms of IR on MACCEs
The association between these indices and MACCEs in patients undergoing PCI with at least one DES may be explained, at least in part, by the following mechanisms. First, involvement of IR in the pathogenesis of CAD: high circulating insulin concentrations can reduce the production of nitric oxide via the activation of serum and glucocorticoid kinase 1, leading to decreased nitric oxide concentration. This, in turn, can result in matrix protein deposition and fibrosis [48]. Second, the metabolic impairment of lipid and glucose induced by IR: IR can lead to the overproduction of reactive oxide species through the activation of signaling pathways, such as the protein kinase C pathway and the nuclear factor (NF)κB pathway. These pathways can trigger cardiovascular events [49]. Third, the ectopic synthesis of angiotensinogen and the inappropriate activation of the RAAS caused by IR contribute to fluid retention and high blood pressure [10, 11]. Finally, impact of insulin on thrombosis and platelet aggregation: insulin can impair fibrinolysis by increasing the circulating concentration of plasminogen activator inhibitor 1. This can lead to a pro-thrombotic state and promote platelet aggregation in the cardiovascular system [50].
Strengths and limitations
In this study, we explored the correlation of METS-IR with MACCEs and respectively assessed the predictive ability of the four IR indices for MACCEs in CAD patients undergoing PCI. While we found no positive association between these IR indices and MACCEs in overall population, and no additional predictive performance was observed, our results suggest that both METS-IR and TyG-BMI index could serve as effective and reliable predictors for MACCEs in elderly patients (age ≥ 60 years) and female patients with CAD undergoing PCI. These findings have far-reaching significance for secondary prevention and risk stratification in these patient populations. However, there are several limitations that should be acknowledged. Firstly, the lack of follow-up time in the dataset prevented us from conducting the time-to-event analysis, which may underestimate the association between IR and MACCEs. Secondly, due to the observational nature of the study, obtain dynamic data on the four IR indices during the follow-up period were unavailable. Having longitudinal data could have added value to the risk stratification of MACCEs. Therefore, future prospective, large-scale, multicenter randomized controlled trials are needed to address this limitation. Thirdly, the dataset did not provide information on hypoglycemic treatment, which could potentially introduce bias in the analysis. Additionally, the lack of data on patients’ dietary habits is another notable limitation, as diet could be a crucial confounder. Including detailed dietary assessments in future studies would be helpful to a more comprehensive understanding of the association between IR and MACCEs and enhance the validity of the results. Finally, despite adjusting for many potential confounders, there may still be residual or unmeasured confounders that could influence the observed associations, such as sleep duration and physical activities. Future studies should consider incorporating these factors to further improve the accuracy and validity of the results.
Conclusions
As widely recognized surrogates of IR, all the four indices demonstrated a clear association with MACCEs in female individuals, while only the TyG-BMI index and METS-IR exhibited such an association in elderly patients. Although incorporating these indices into the basic risk model did not lead to an improvement in predictive performance for MACCEs in either female or elderly patients, METS-IR appears to hold the most promise as an index for secondary prevention and risk stratification of MACCEs in these patient populations. Further investigations are urgently required to validate and build upon these findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully thank Haimu Yao (Department of Cardiology, The First Affiliated Hospital of Zhengzhou University) for providing the datasets.
List of abbreviations
- ACEI
Angiotensin converting enzyme inhibitor
- ACS
Acute coronary syndrome
- AMI
Acute myocardial infraction
- BMI
Body mass index
- CAD
Coronary artery disease
- CTO
Chronic total occlusions
- CVD
Cardiovascular disease
- CI
Confidence interval
- DBP
Diastolic blood pressure
- DES
Drug-eluting stent
- DM
Diabetes mellitus
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard ratio
- IDI
Integrated discrimination improvement
- IR
Insulin resistance
- LAD
Left anterior descending
- LDL-C
Low-density lipoprotein cholesterol
- MACCEs
Major adverse cardiac and cerebrovascular events
- METS-IR
Metabolic score for insulin resistance
- NRI
Net reclassification improvement
- OR
Odds ratio
- PCI
Percutaneous coronary intervention
- RAAS
Renin-angiotensin-aldosterone system
- RCS
Restricted cubic spline
- RCA
Right coronary artery
- ROC
Receiver operating characteristic
- SA
Stable angina
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglyceride
- TG/HDL-C
Triglyceride to high-density lipoprotein cholesterol ratio
- TyG
Triglyceride and glucose
- TyG-BMI
Triglyceride glucose-body mass index
- TVR
Target vessel revascularization
- UA
Uric acid
Authors’ contributions
Zenglei Zhang, Xu Meng and Xianliang Zhang designed this study. Zenglei Zhang performed the statistical analysis. Zenglei Zhang wrote the first draft of the manuscript. Lin Zhao and Yiting Lu wrote some sections of the manuscript. Xu Meng and Xianliang Zhou revised the manuscript.
Funding
This study was supported by grants from the National Key Research and Development Program of China (2016YFC1300100), Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320057), CAMS Innovation Fund for Medical Science (CIFMS, 2022-I2M-C&T-A-010).
Data Availability
The datasets generated and/or analyzed during the current study are available in the Dryad repository 10.5061/dryad.13d31.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study with a waiver of informed consent were approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University and conducted in line with the Declaration of Helsinki.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xu Meng, Email: mengxu1219@hotmail.com.
Xianliang Zhou, Email: zhouxianliang0326@hotmail.com.
References
- 1.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: a compass for Future Health. J Am Coll Cardiol. 2022;80(25):2361–71. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Global. burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020, 396(10258):1223-49. [DOI] [PMC free article] [PubMed]
- 3.Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019, 40(2). [DOI] [PubMed]
- 4.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 5.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022, 79(2). [DOI] [PubMed]
- 6.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. Predictive effect of triglyceride–glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43. doi: 10.1186/s12933-021-01236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park D-W, Kang D-Y, Ahn J-M, Yun S-C, Yoon Y-H, Hur S-H, et al. Routine functional testing or Standard Care in High-Risk patients after PCI. N Engl J Med. 2022;387(10):905–15. doi: 10.1056/NEJMoa2208335. [DOI] [PubMed] [Google Scholar]
- 8.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–85. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. doi: 10.1186/s12933-019-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Q, Lin Y, Xu R, Luo F, Chen R, Li P, et al. Positive association of triglyceride-glucose index with new-onset hypertension among adults: a national cohort study in China. Cardiovasc Diabetol. 2023;22(1):58. doi: 10.1186/s12933-023-01795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44. doi: 10.1530/EJE-17-0883. [DOI] [PubMed] [Google Scholar]
- 14.Uruska A, Zozulinska-Ziolkiewicz D, Niedzwiecki P, Pietrzak M, Wierusz-Wysocka B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J Clin Lipidol. 2018;12(3):734–40. doi: 10.1016/j.jacl.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Iwani NAKZ, Jalaludin MY, Zin RMWM, Fuziah MZ, Hong JYH, Abqariyah Y, et al. Triglyceride to HDL-C ratio is Associated with insulin resistance in overweight and obese children. Sci Rep. 2017;7:40055. doi: 10.1038/srep40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 17.Er L-K, Wu S, Chou H-H, Hsu L-A, Teng M-S, Sun Y-C, et al. Triglyceride glucose-body Mass Index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park B, Lee HS, Lee Y-J. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the korean genome and epidemiology study cohort. Transl Res. 2021;228:42–51. doi: 10.1016/j.trsl.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604. doi: 10.1016/j.numecd.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Gao Y, Han J, Li L, Wang M, Peng H, et al. Comparison of longitudinal changes in four surrogate insulin resistance indexes for incident T2DM in middle-aged and elderly chinese. Front Public Health. 2022;10:1046223. doi: 10.3389/fpubh.2022.1046223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134. doi: 10.1186/s12933-021-01330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang R, Fu X, Song H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol Metab Syndr. 2022;14(1):191. doi: 10.1186/s13098-022-00967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y-C, Huang J-C, Lin C-I, Chien H-H, Lin Y-Y, Wang C-L et al. Comparison of innovative and traditional cardiometabolic indices in estimating atherosclerotic Cardiovascular Disease risk in adults. Diagnostics (Basel). 2021, 11(4). [DOI] [PMC free article] [PubMed]
- 25.Zhao Y, Zhang J, Chen C, Qin P, Zhang M, Shi X, et al. Comparison of six surrogate insulin resistance indexes for predicting the risk of incident stroke: the rural chinese cohort study. Diabetes Metab Res Rev. 2022;38(7):e3567. doi: 10.1002/dmrr.3567. [DOI] [PubMed] [Google Scholar]
- 26.Yao H-M, Wan Y-D, Zhang X-J, Shen D-L, Zhang J-Y, Li L, et al. Long-term follow-up results in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents: results from a single high-volume PCI centre. BMJ Open. 2014;4(8):e004892. doi: 10.1136/bmjopen-2014-004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao H-M, Dataset et al. 10.5061/dryad.13d31.
- 28.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 29.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet (London England) 2016;387(10033):2145–54. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 31.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Reviews Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 32.Uetani T, Amano T, Harada K, Kitagawa K, Kunimura A, Shimbo Y, et al. Impact of insulin resistance on post-procedural myocardial injury and clinical outcomes in patients who underwent elective coronary interventions with drug-eluting stents. JACC Cardiovasc Interv. 2012;5(11):1159–67. doi: 10.1016/j.jcin.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. doi: 10.1186/s12933-020-01006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Liu L, Wang W, Cui H, Zhang Y, Xu J, et al. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(1):142. doi: 10.1186/s12933-022-01576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong S, Chen Q, Zhang Z, Chen Y, Hou J, Cui C, et al. A synergistic effect of the triglyceride-glucose index and the residual SYNTAX score on the prediction of intermediate-term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21(1):115. doi: 10.1186/s12933-022-01553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in korean adults: an analysis of the 2007–2010 korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14(3):e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Z, Xing L, Lin M, Sun Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 2020;20(1):483. doi: 10.1186/s12872-020-01768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yunke Z, Guoping L, Zhenyue C. Triglyceride-to-HDL cholesterol ratio. Predictive value for CHD severity and new-onset heart failure. Herz. 2014;39(1):105–10. doi: 10.1007/s00059-013-3788-0. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Cui H, Li W, Zhang Y, Liu L, Liu Z, et al. Comparison of three non-insulin-based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front Cardiovasc Med. 2022;9:918359. doi: 10.3389/fcvm.2022.918359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capranzano P, Angiolillo DJ. Antithrombotic Management of Elderly patients with coronary artery disease. JACC Cardiovasc Interv. 2021;14(7):723–38. doi: 10.1016/j.jcin.2021.01.040. [DOI] [PubMed] [Google Scholar]
- 41.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32(6):949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Lancet Diabetes E Menopause: a turning point for women’s health. The Lancet Diabetes & Endocrinology. 2022;10(6):373. doi: 10.1016/S2213-8587(22)00142-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Tang J, Cui X, Qin B, Zhang J, Zhang L, et al. New Insights and Novel Therapeutic Potentials for Macrophages in myocardial infarction. Inflammation. 2021;44(5):1696–712. doi: 10.1007/s10753-021-01467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19. doi: 10.1186/s12933-020-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159–76. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Kuan V, Denaxas S, Patalay P, Nitsch D, Mathur R, Gonzalez-Izquierdo A, et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit Health. 2023;5(1):e16–e27. doi: 10.1016/S2589-7500(22)00187-X. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Cui H, Zhang Y, Liu L, Zhang W, Xiong W, et al. The impact of the metabolic score for insulin resistance on cardiovascular disease: a 10-year follow-up cohort study. J Endocrinol Invest. 2023;46(3):523–33. doi: 10.1007/s40618-022-01925-0. [DOI] [PubMed] [Google Scholar]
- 48.Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res. 2022;118(1):130–40. doi: 10.1093/cvr/cvaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Wang X, Chen J, You C, Ma L, Zhang W, et al. Household air pollution, adherence to a healthy lifestyle, and risk of cardiometabolic multimorbidity: results from the China health and retirement longitudinal study. Sci Total Environ. 2023;855:158896. doi: 10.1016/j.scitotenv.2022.158896. [DOI] [PubMed] [Google Scholar]
- 50.Brazionis L, Rowley K, Jenkins A, Itsiopoulos C, O’Dea K. Plasminogen activator inhibitor-1 activity in type 2 diabetes: a different relationship with coronary heart disease and diabetic retinopathy. Atertio Thromb Vasc Biol. 2008;28(4):786–91. doi: 10.1161/ATVBAHA.107.160168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Dryad repository 10.5061/dryad.13d31.