Abstract
Background
Systemic lupus erythematosus (SLE) is a clinically and biologically heterogeneous autoimmune disease. We explored whether the deconvolution of whole blood transcriptomic data could identify differences in predicted immune cell frequency between active SLE patients, and whether these differences are associated with clinical features and/or medication use.
Methods
Patients with active SLE (BILAG-2004 Index) enrolled in the BILAG-Biologics Registry (BILAG-BR), prior to change in therapy, were studied as part of the MASTERPLANS Stratified Medicine consortium. Whole blood RNA-sequencing (RNA-seq) was conducted at enrolment into the registry. Data were deconvoluted using CIBERSORTx. Predicted immune cell frequencies were compared between active and inactive disease in the nine BILAG-2004 domains and according to immunosuppressant use (current and past).
Results
Predicted cell frequency varied between 109 patients. Patients currently, or previously, exposed to mycophenolate mofetil (MMF) had fewer inactivated macrophages (0.435% vs 1.391%, p = 0.001), naïve CD4 T cells (0.961% vs 2.251%, p = 0.002), and regulatory T cells (1.858% vs 3.574%, p = 0.007), as well as a higher proportion of memory activated CD4 T cells (1.826% vs 1.113%, p = 0.015), compared to patients never exposed to MMF. These differences remained statistically significant after adjusting for age, gender, ethnicity, disease duration, renal disease, and corticosteroid use. There were 2607 differentially expressed genes (DEGs) in patients exposed to MMF with over-representation of pathways relating to eosinophil function and erythrocyte development and function. Within CD4 + T cells, there were fewer predicted DEGs related to MMF exposure. No significant differences were observed for the other conventional immunosuppressants nor between patients according disease activity in any of the nine organ domains.
Conclusion
MMF has a significant and persisting effect on the whole blood transcriptomic signature in patients with SLE. This highlights the need to adequately adjust for background medication use in future studies using whole blood transcriptomics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13075-023-03089-5.
Keywords: Systemic lupus erythematosus, Transcriptomics, Deconvolution, Mycophenolate mofetil
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune, immune complex-mediated disease associated with systemic inflammation and the production of autoantibodies. The marked heterogeneous nature of SLE, both clinically and biologically, has continued to impose challenges in terms of understanding the pathogenesis of SLE, in drug development and in clinical care [1, 2].
Transcriptomic profiling has the potential to accelerate our understanding of the biology of SLE. It may contribute to improving the classification of SLE, allow exploration of disease mechanisms, and help to identify novel treatment targets or personalised approaches to treatment [2]. Previous transcriptomic studies in SLE have demonstrated the contribution of IFNβ and IFNγ (in addition to IFNα) to SLE pathogenesis and the presence of distinct gene signatures for susceptibility, disease activity, and severity [2, 3]. However, the marked clinical and molecular heterogeneity of SLE can make transcriptomic analysis at either the transcript or gene level challenging and may lead to discrepancies between studies. Consideration of the cell proportions within bulk RNA sequencing (RNA-seq) data may yield important insights and improve the statistical power of transcriptomic analyses.
CIBERSORTx is a deconvolution-based computational method which uses support vector regression in combination with the knowledge of expression profiles in a signature matrix to accurately estimate the relative immune proportions of cells from bulk tissue transcriptomes [4, 5]. CIBERSORTx has been used to estimate immune cell proportions in peripheral blood in several conditions including ischaemic stroke [6], schizophrenia [7], and liver cirrhosis [8]. The data obtained from the CIBERSORTx pipeline has been validated against clinical laboratory measurements and/or flow cytometry data [7, 8]. Recently, it has been reported that immune cell scoring systems, based on CIBERSORTx data, can predict prognosis in patients with myelodysplastic syndromes [9].
Deconvolution of microarray data has been used in studies comparing patients with SLE to healthy controls. Patients with SLE had increased monocytes and fewer NK cells in the peripheral blood compared to healthy controls [10]. Similarly, deconvolution of microarray data from renal tissue identified differences in cell proportions between glomeruli from patients with lupus nephritis compared to healthy donors with increased monocytes, macrophages, and activated natural killer (NK) cells and fewer memory B cells and T follicular helper cells in LN tissue [11].
In this study, we aimed to use CIBERSORTx to determine whether deconvolution of whole blood RNA-seq data could identify differences in predicted immune cell proportions between active SLE patients and whether these differences are associated with the clinical phenotype of patients or with concomitant medication use.
Methods
Study cohort
Patients with active SLE who fulfilled the 1997 Updated American College of Rheumatology (ACR) classification criteria for SLE [12] or the SLICC 2012 criteria [13] were registered with the BILAG-Biologics Registry (BILAG-BR). Ethical approval was granted by North West Greater Manchester West Research Ethics Committee (09/H1014/64) and the local Research and Development departments at participant sites. This cohort formed a key component of the UK Medical Research Council (MRC) Precision Medicine Consortium ‘Maximising SLE Therapeutic Potential by the Application of Novel and Stratified Approaches’ (MASTERPLANS). Disease activity was quantified using the BILAG-2004 index [14]. Blood samples were collected at enrolment into the registry for whole blood RNA-seq and autoantibody profiles were measured in a central laboratory. Routine biochemical, haematological, and serological parameters were measured locally.
Whole blood bulk RNA sequencing (RNA-seq)
RNA was extracted from PAXgene tubes and RNA integrity was analysed using the Agilent 2100 Bioanalyzer. Complementary DNA synthesis was performed using the Illumina® TruSeq RNA Sample Preparation Kit (Illumina), and pooled cDNA libraries were sequenced using the HiSeq 2000 Illumina® platform (Illumina). Quality assessment was performed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), quality filtering with Trimmomatic [15], read mapping to hg38, and counting into genes with STAR [16] using annotation from GENCODE v24 (http://www.gencodegenes.org/). Fragments per kilobase million (FPKM) were determined for downstream analysis using RNAAgeCalc [17]. Differential gene expression was determined using DESeq2 [18]. Gene set enrichment analysis (GSEA) was performed using ClusterProfiler using the genome wide annotation for human (org.Hs.eg.db). Gene ontology (GO) analysis was conducted using GOnet [19]. Hierarchical clustering with bootstrapping was performed using factoextra.
Deconvolution of gene expression profiles
Bulk RNA-seq deconvolution and cell type estimation was performed using CIBERSORTx and the LM22 leukocyte gene signature matrix [4, 5]. LM22 comprises 547 genes, derived from human microarray data, to distinguish 22 human immune cell types [5]. The following specifications were used: B-mode batch correction, quantile normalisation disabled, 100 per mutations and relative-mode. For some analyses, absolute mode was used. For further details, see Supplementary methods. For high resolution mode, only protein-coding transcripts were used.
The relative cell proportions obtained from CIBERSORTx were compared between set groups using non-parametric tests (Mann–Whitney U test with Benjamini–Hochberg correction or Kruskal–Wallis test with Dunn’s correction where appropriate) with the FDR set at 0.05. In the adjusted models, cell frequency was converted into quartiles and ordered logistic regression models to determine the odds ratio for moving between quartiles. Data were analysed using IBM® SPSS® Statistics 26, STATA v.16.0 and R v4.0.3.
Results
Patient characteristics
Whole blood transcriptomic data were available for 110 patients; one of whom had incomplete clinical data and was therefore excluded from analyses. The cohort included 104/109 (95.4%) females, with a median (IQR) age and disease duration of 38 (29–49) and 10 (6.5–16.5) years respectively. Patients had high disease activity with a median SLEDAI score of 8 (4–14) (Table 1).
Table 1.
Description of the study population
| Patient characteristics, total (n = 109) | No (%)/median (IQR) |
|---|---|
| Age, years (n = 98) | 38 (29–49) |
| Sex | |
| Female | 104 (95.4%) |
| Male | 5 (4.6%) |
| Ethnicity | |
| White | 61 (56%) |
| Black | 14 (12.8%) |
| South Asian | 20 (18.3%) |
| Other | 11 (10.1%) |
| Not Specified | 3 (2.8%) |
| SLE duration, years | 10 (6.5–16.5) |
| 1997 ACR criteria | |
| Malar rash | 60 (55%) |
| Discoid rash | 15 (13.8%) |
| Photosensitivity | 59 (54.1%) |
| Oral ulcers | 68 (62.4%) |
| Arthritis | 97 (89%) |
| Serositis | 33 (30.3%) |
| Renal disorder | 44 (40.4%) |
| Neurologic disorder | 8 (7.3%) |
| Haematologic disorder | 63 (57.8%) |
| Immunologic disorder | 81 (74.3%) |
| Positive ANA | 96 (88.1%) |
| Disease activity | |
| SLEDAI score (n = 99) | 8 (4–14) |
| BILAG 2004, A or B in organ domain (n = 98) | |
| Constitutional | 8 (8.2%) |
| Mucocutaneous | 51 (52%) |
| Neuropsychiatric | 10 (10.2%) |
| Musculoskeletal | 51 (52%) |
| Cardiorespiratory | 13 (13.3%) |
| Gastrointestinal | 5 (5.1%) |
| Ophthalmic | 5 (5.1%) |
| Renal | 39 (39.8%) |
| Haematological | 3 (3.1%) |
| Low C3/C4 (n = 99) | 53 (53.5%) |
| Raised anti-dsDNA (n = 104) | 43 (41.4%) |
| Autoantibodies (n = 104) | |
| ANA antibodies | 99 (95.2%) |
| dsDNA antibodies | 58 (55.8%) |
| U1RNP antibodies | 34 (32.7%) |
| U3RNP antibodies | 1 (1%) |
| SSa/Ro52 antibodies | 20 (19.2%) |
| SSa/Ro60 antibodies | 37 (35.6%) |
| SSb/La antibodies | 6 (5.8%) |
| Medication | |
| Current oral corticosteroid use (n = 108) | 94 (87%) |
| Usual oral corticosteroid dose (mg) (n = 75) | 10 (7.5–20) |
| Current or previous antimalarial therapy | |
| Hydroxychloroquine | 54 (49.5%) |
| Mepacrine | 2 (1.8%) |
| Current or previous immunosuppressant exposure | |
| Azathioprine | 9 (8.3%) |
| Methotrexate | 5 (4.6%) |
| Mycophenolate mofetil | 53 (48.6%) |
| Tacrolimus | 1 (0.9%) |
| Cyclophosphamide | 8 (7.3%) |
Values are n (%) or median (IQR) as appropriate
Deconvolution of whole blood RNA-seq data
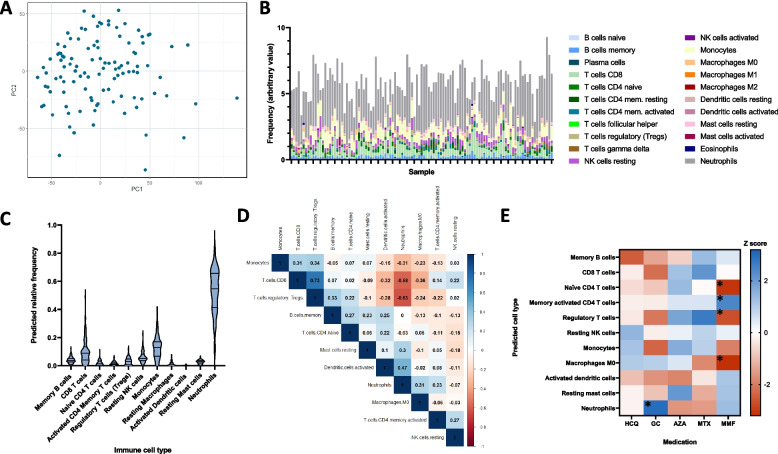
We analysed protein-coding genes in 110 patients. Principal component analysis did not identify any clear patient subgroups (Fig. 1A). Of 19,986 protein-coding genes, after removal of those with a raw count of zero in all samples, and those not matched during FPKM processing, there remained 17,231 for analysis. For further details of the workflow, see the Supplementary data file. For 11 of the cell types, frequencies could not be estimated in > 50% of samples, and therefore, we focussed on the remaining 11 cells: memory B cells, CD8 T cells, naïve CD4 T cells, memory activated CD4 T cells, regulatory T cells (Treg), resting NK cells, monocytes, M0 macrophages, activated dendritic cells, and resting mast cells (see Supplementary data). Of these, only monocytes and resting mast cells had a normal distribution. Neutrophils were the most abundant cell type (median [IQR] 54.7% [41.3–65.7] followed by monocytes (12.9% [6.80–17.0]). The distribution of the 11 cell types is shown in Fig. 1B and C.
Fig. 1.
Predicted frequency of immune cell types in whole blood. A Principal component (PC) plot of PC1 and PC2 for all patient samples. B Relative frequency of each of the 22 cell types predicted using CIBERSORTx in each sample. C The predicted frequency of the 11 selected cell types in the whole cohort. D Correlation matrix of the 11 cell types in the whole cohort. Spearman r coefficients are shown and the intensity of the colour indicates the strength of the correlation. E Pseudoheatmap of the 11 cell types according to exposure to GC, HCQ and immunosuppressants. The colour shows the magnitude of the Z score, *p < 0.05. HCQ, hydroxychloroquine; GC, glucocorticoids; AZA, azathioprine; MTX, methotrexate; MMF, mycophenolate mofetil; CYC, cyclophosphamide
The estimated absolute number of neutrophils was negatively correlated with Treg (r = − 0.634, p < 0.0001), CD8 T cells (r = − 0.560, p < 0.0001), and monocytes (r = − 0.321, p = 0.0006) and positively correlated with activated dendritic cells (r = 0.413, p < 0.0001). Similarly, there was a strong correlation between Treg and CD8 T cells (r = 0.706, p < 0.0001) (Fig. 1D).
Association between immune cell populations and disease phenotype
To assess whether organ involvement was associated with differing immune cell frequencies, patients with active disease (defined as a BILAG A or B score) in an organ domain were compared to those with inactive disease (C, D, or E score). There were no significant differences in immune cell frequency between active and inactive disease in any of the nine organ domains after correction for multiple testing (data not shown). In a sensitivity analysis in which active disease was defined as a BILAG A, B, or C score, again, no differences were observed. Similarly, there was no correlation between these immune cell frequencies and SLEDAI score or between patients with lower disease activity (SLEDAI < 10) and high disease activity (≥ 10) (data not shown).
Association between immune cell populations and medication exposure
Most patients (94, 87%) were taking oral glucocorticoids (GCs) which was associated with increased predicted frequency of neutrophils compared to those not taking oral GCs (56.6% [44.1–66.8] vs 44.3% [33.4–50.3], p = 0.003) (Fig. 1E and Supplementary data S2). No statistically significant differences were observed in the other cell types between patients taking GC and those not taking GC. There were no differences in immune cell frequency in patients exposed (currently or previously) to azathioprine (AZA), methotrexate (MTX), hydroxychloroquine (HCQ), or cyclophosphamide (CYC).
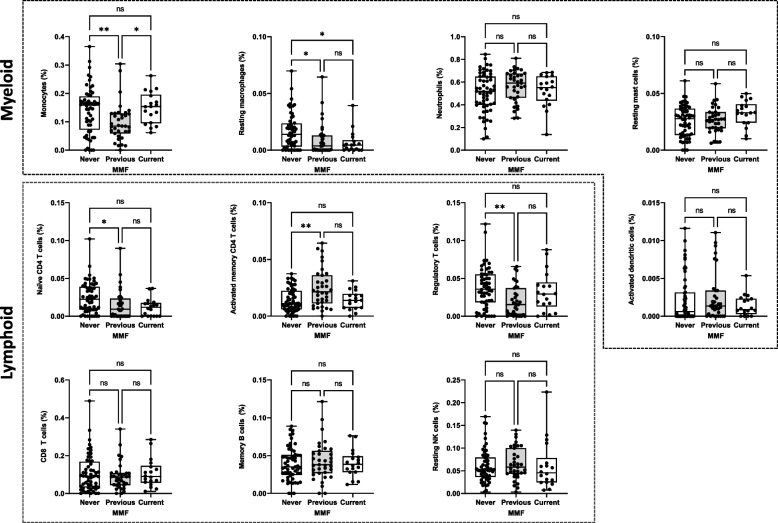
Compared to patients never exposed to mycophenolate mofetil (MMF), patients exposed to MMF had a significantly lower proportion of resting macrophages (0.44% [0–1.25] vs 1.39% [0.32–2.36], unadjusted p = 0.001), naïve CD4 T cells (0.96% [0–1.96] vs 2.25% [0.86–3.88], p = 0.002), and regulatory T cells (1.86% [0.29–3.93] vs 3.57% [1.84–5.52], p = 0.006), as well as a higher proportion of memory activated CD4 memory T cells (1.83% [0.87–2.78] vs 1.11% [0.60–2.23], p = 0.015). After Benjamini–Hochberg correction, these remained statistically significant (Supplementary file S3) and when MMF exposure was considered separately as current, previous, or never exposed, statistically significant differences remained for these four immune cell types (Fig. 2).
Fig. 2.
Differences in estimated cell proportions in patients receiving mycophenolate mofetil (MMF). Box plots show differences in estimated cell populations between patients never exposed, previously exposed, or currently receiving MMF. Horizontal line shows the median value and the box shows the IQR. Error bars show minimum and maximum values. Comparisons made using Kruskal–Wallis tests with Dunn’s correction for multiple comparisons. *p < 0.05, **p < 0.01
Patients exposed to MMF were more likely to have current or previous renal disease and were less likely to have a history of photosensitivity (Table 2). Similarly, patients with active mucocutaneous or musculoskeletal disease were less likely to have been prescribed MMF (57.9% never, 28.6% previous, 4.44% current, p = 0.023 and 61.4% never, 28.6% previous, 33.3% current, p = 0.004, respectively). Conversely, patients with active renal disease were more likely to be currently taking MMF (61.6% current, 25.7% ever, 33.3% never, p = 0.034). To exclude the effect of confounding by indication, multivariable ordered logistic regression analyses, using quartiles of cell frequency as the dependent variable, adjusted for age, gender, ethnicity, disease duration, renal disease, and corticosteroid use, were constructed which remained statistically significant for the 4 cell types above (Table 3).
Table 2.
Clinical characteristics of patients related to exposure to MMF
| Never (n = 57) |
Previous (n = 35) |
Current (n = 18) |
p | |
|---|---|---|---|---|
| Age | ||||
| Sex (female) | 54 (96.4%) | 33 (94.3%) | 17 (94.4%) | 0.873 |
| Ethnic background | 0.408 | |||
| White | 34 (60.7%) | 18 (54.6%) | 10 (55.6%) | |
| Black | 4 (7.14%) | 6 (18.2%) | 4 (22.2%) | |
| Asian | 13 (21.2%) | 4 (12.1%) | 3 (16.7%) | |
| Other | 5 (8.93%) | 5 (15.2%) | 1 (5.56%) | |
| Disease duration | 18 (8, 17) | 11 (7, 17) | 8 (5, 13) | 0.273 |
| ACR criteria | ||||
| Malar rash | 33 (58.9%) | 17 (48.6%) | 10 (55.6%) | 0.626 |
| Discoid rash | 7 (12.5%) | 5 (14.3%) | 3 (16.7%) | 0.900 |
| Photosensitivity | 37 (66.1%) | 15 (42.9%) | 7 (38.9%) | 0.035 |
| Oral ulcers | 38 (67.9%) | 18 (51.4%) | 12 (66.7%) | 0.266 |
| Arthritis | 49 (87.5%) | 34 (97.1%) | 14 (77.8%) | 0.090 |
| Serositis | 18 (32.1%) | 9 (25.7%) | 6 (33.3%) | 0.772 |
| Neurological | 4 (7.14%) | 3 (8.57%) | 1 (5.56%) | 0.921 |
| Renal | 21 (37.5%) | 11 (31.4%) | 12 (67.7%) | 0.038 |
| Haematological | 30 (53.6%) | 22 (62.9%) | 11 (61.1%) | 0.651 |
| Immunological | 39 (69.6%) | 27 (77.1%) | 15 (83.3%) | 0.460 |
| ANA | 49 (87.5%) | 32 (91.4%) | 15 (83.3%) | 0.678 |
| SLICC-DI | 0 (0, 1) | 0 (0, 2) | 0 (0, 1) | 0.862 |
| Current prednisolone | 46 (83.6%) | 31 (88.6%) | 17 (94.4%) | 0.470 |
| Daily prednisolone dose (mg/day) | 10 (5–20) | 10 (7.5–20) | 10 (10–20) | 0.280 |
| SLEDAI score | 10 (5.5–13.5) | 6 (4–10) | 10 (6–14) | 0.051 |
| Active disease (BILAG A/B) | ||||
| Constitutional (A or B) | 6 (10.5%) | 1 (2.86%) | 1 (5.56%) | 0.371 |
| Mucocutaneous (A or B) | 33 (57.9%) | 10 (28.6%) | 8 (44.4%) | 0.023 |
| Neuropsychiatric (A or B) | 4 (7.02%) | 4 (11.4%) | 2 (11.1%) | 0.735 |
| Musculoskeletal (A or B) | 35 (61.4%) | 10 (28.6%) | 6 (33.3%) | 0.004 |
| Cardiorespiratory (A or B) | 8 (14.0%) | 2 (5.71%) | 3 (16.7%) | 0.382 |
| Gastrointestinal (A or B) | 3 (5.26%) | 0 | 2 (11.1%) | 0.172 |
| Ophthalmic (A or B) | 2 (3.51%) | 2 (5.71%) | 1 (5.56%) | 0.863 |
| Renal (A or B) | 19 (33.3%) | 9 (25.7%) | 11 (61.1%) | 0.034 |
| Haematological (A or B) | 0 | 2 (5.71%) | 1 (5.56%) | 0.190 |
| Low C3/C4 | 29 (51.2%) | 11 (44.0%) | 13 (72.2%) | 0.173 |
| Raised dsDNA | 24 (42.9%) | 12 (48.0%) | 13 (72.2%) | 0.094 |
Table 3.
Ordered logistic regression models of the association between MMF exposure and quartiles of peripheral blood immune cell frequency
| Cell type (quartiles) | Unadjusted | Model 1a | Model 2b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Resting macrophages | 0.354 | 0.176, 0.771 | 0.004 | 0.386 | 0.178, 0.840 | 0.016 | 0.329 | 0.177, 0.872 | 0.022 |
| Naïve CD4 + T cells | 0.390 | 0.195, 0.776 | 0.007 | 0.398 | 0.184, 0.859 | 0.019 | 0.369 | 0.167, 0.816 | 0.014 |
| Regulatory T cells | 0.363 | 0.181, 0.728 | 0.004 | 0.227 | 0.099, 0.521 | < 0.001 | 0.216 | 0.092, 0.508 | < 0.001 |
| Activated CD4 + memory T cells | 1.952 | 0.984, 3.869 | 0.055 | 2.174 | 1.014, 1.659 | 0.046 | 2.447 | 1.120, 5.346 | < 0.001 |
| Monocytes | 0.528 | 0.266, 1.045 | 0.067 | 0.527 | 0.244, 1.139 | 0.103 | 0.579 | 0.262, 1.279 | 0.176 |
aModel 1: adjusted for age, gender, ethnicity, and disease duration
bModel 2: as for model 1 plus presence of renal disease (BILAG A/B) and corticosteroid use (Y/N)
Transcriptomic signature associated with MMF exposure
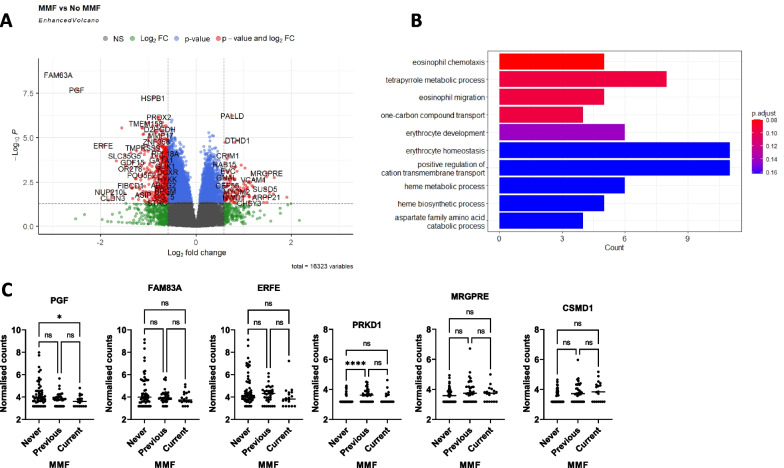
There were 2607 differentially expressed genes (DEGs) between patients who were exposed (current or ever) to MMF compared to those who were not (log2 fold change of 0.5 and adjusted p value of < 0.05). Of these, 767 were upregulated, and 1840 were downregulated (Fig. 3A). Gene set enrichment analysis (GSEA) of DEGs identified over-representation of genes sets related to erythrocyte development and eosinophil migration (Fig. 3B). The genes upregulated or downregulated with smallest adjusted p-value were further examined (3 of each). Of the 3 downregulated DEGs, expression of PGF was statistically significantly lower in patients currently taking or ever exposed to MMF. Similarly, of the 3 upregulated DEGS, CSMD1 was increased in patients currently taking or ever exposed to MMF (Fig. 3C).
Fig. 3.
Whole blood gene signature in patients exposed to MMF. A Volcano plot to show differentially expressed genes between patients exposed to MMF or not. The y-axis shows -log10 adjusted p value and x-axis shows log2 fold change. Genes in red are differentially upregulated or downregulated with log2 fold change > 0.5 and adjusted p value < 0.05. B Gene set enrichment analysis of the 2607 differentially expressed genes. The x-axis shows the number of genes contributing to the term and the colour of the bar represents the p value. C Box plots of the 3 genes most significantly upregulated or downregulated according to MMF exposure. Horizontal bar shows median. Comparisons with Kruskal–Wallis test with Dunn’s correction. *p < 0.05, ****p < 0.0001
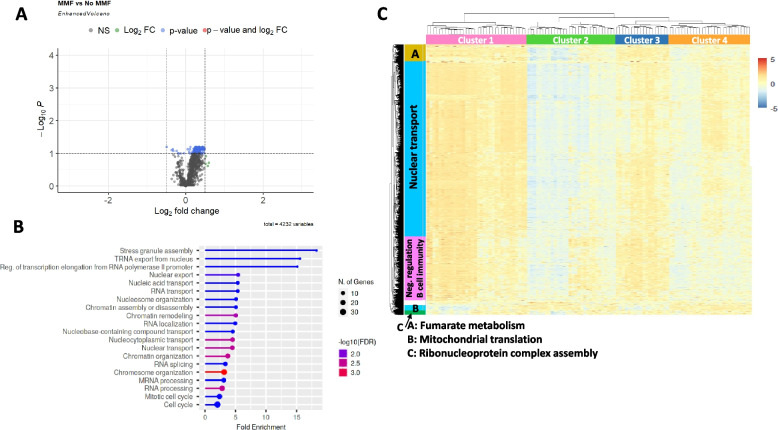
As MMF exposure was associated with changes to T cell frequency, gene expression within the CD4 cell subset was predicted using CIBERSORTx high resolution mode. Of 4232 genes, only 157 were differentially expressed (adjusted p < 0.05) according to exposure to MMF (Fig. 4A). There were insufficient genes to perform GSEA, but gene ontology (GO) analysis of biological function identified over-representation of genes involved in nucleocytoplasmic transport (Fig. 4B). Hierarchical clustering of CD4 T cell transcripts identified 4 patients clusters (cluster 1; 42 patients, cluster 2; 30 patients, cluster 30; 14 patients, cluster4; 21 patients) (Fig. 4C) and 5 gene clusters with identifiable pathway enrichment. GO analysis of the gene clusters identified pathways related to nuclear transport (fold enrichment 2.67, enrichment false discovery rate [FDR] 9.97 × 10−10), negative regulation of B cell immunity (fold enrichment 11.9, enrichment FDR 0.013), and fumarate metabolism (fold enrichment 73.5, enrichment FDR 0.030). The pathway genes for each of the top 3 GO terms in each of the 5 gene clusters are shown in the Supplementary Table S4. The characteristics of patients in each of the 4 clusters is shown in Table 4. Patients in clusters 2 and 4 were older and were more likely to be receiving prednisolone and less likely to be exposed to methotrexate. Overall, the expression of most transcripts was higher in clusters 2 and 4 compared to clusters 1 and 3, except for a small group of transcripts which were enriched with genes related to mitochondrial translation (GO: 0070125). There was no difference in lupus disease activity by BILAG organ system, exposure to MTX or AZA, between the clusters. Patients in clusters 2 and 3 were more likely to have a history of mucosal ulceration.
Fig. 4.
Predicted gene expression in CD4 + T cells in patients exposed to MMF. A Volcano plot of DEGs predicted in the CD4 T cell subset according to exposure to MMF. Horizontal line shows -log10 adjusted p value and vertical line shows log2 fold change. B GO analysis of over-represented biological pathways in the 157 DEGs with adjusted p < 0.05 between patients exposed or not to MMF. C Heatmap of genes in CD4 T cells with hierarchical clustering of samples (horizontal) and genes (vertical). The vertical bars show the top GO biological process for each cluster of genes
Table 4.
Characteristics of patients in each CD4 T cells transcriptional cluster
| Cluster 1 N = 42 |
Cluster 2 N = 30 |
Cluster 3 N = 14 |
Cluster 4 N = 24 |
p | |
|---|---|---|---|---|---|
| Age (years) | 35 (27–46) | 41 (34–53) | 34 (19–38) | 42 (34–52) | 0.027 |
| Gender (female) | 30 (95.1%) | 29 (96.7%) | 14 (100%) | 22 (91.7%) | 0.790 |
| Ethnicity | |||||
| White | 27 (65.9%) | 17 (56.7%) | 6 (46.2%) | 11 (50.0%) | 0.396 |
| Black | 5 (11.9%) | 5 (16.7%) | 3 (23.1%) | 1 (4.55%) | |
| Asian | 6 (14.3%) | 4 (13.3%) | 4 (30.8%) | 6 (27.3%) | |
| Other | 3 (7.14%) | 4 (13.3%) | 0 | 4 (18.2%) | |
| Disease duration (years) | 8 (6–15) | 11 (9–17) | 8.5 (6–11) | 11.5 (8–20.5) | 0.228 |
| SLICC DI | 0 (0–1) | 0 (0–1) | 0 (0–0.5) | 0 (0–1) | 0.561 |
| ACR criteria | |||||
| Malar rash | 21 (51.2%) | 14 (46.7%) | 9 (64.3%) | 16 (66.7%) | 0.409 |
| Discoid rash | 9 (22.0%) | 1 (3.33%) | 1 (7.14%) | 4 (16.7%) | 0.124 |
| Photosensitivity | 21 (51.2%) | 15 (50.0%) | 9 (64.3%) | 14 (58.3%) | 0.777 |
| Oral ulcers | 24 (58.5%) | 23 (76.7%) | 11 (78.6%) | 10 (41.7%) | 0.032 |
| Arthritis | 38 (92.7%) | 26 (86.7%) | 12 (85.7%) | 21 (87.5%) | 0.815 |
| Serositis | 14 (34.2%) | 7 (23.3%) | 4(28.6%) | 8 (33.3%) | 0.777 |
| Neurological | 3 (7.32%) | 2 (6.67%) | 1 (7.14%) | 2 (8.33%) | 0.997 |
| Renal | 15 (36.6%) | 14 (46.7%) | 7 (50.0%) | 8 (33.3%) | 0.621 |
| Haematological | 22 (52.7%) | 14 (46.7%) | 9 (64.3%) | 18 (75.0%) | 0.174 |
| Immunological | 29 (70.7%) | 24 (80.0%) | 12 (85.7%) | 16 (66.7%) | 0.480 |
| ANA | 37 (90.2%) | 25 (85.3%) | 12 (85.7%) | 22 (91.7%) | 0.754 |
| Disease activity | |||||
| SLEDAI score | 8 (4–2) | 8 (4–12) | 12 (8–18) | 6 (4–12) | 0.145 |
| Active disease (BILAG A/B) | |||||
| Constitutional | 4 (9.76%) | 3 (10.0%) | 1 (7.14%) | 0 | 0.463 |
| Mucocutaneous | 20 (48.8%) | 15 (50.0%) | 6 (42.9%) | 10 (41.7%) | 0.912 |
| Neuropsychiatric | 4 (9.76%) | 3 (10.0%) | 1 (7.14%) | 2 (8.33%) | 0.988 |
| Musculoskeletal | 21 (5.12%) | 17 (56.7%) | 7 (50.0%) | 6 (25.0%) | 0.105 |
| Cardiorespiratory | 4 (9.76%) | 4 (13.3%) | 0 | 5 (20.83%) | 0.267 |
| Gastrointestinal | 2 (4.88%) | 1 (33.3%) | 0 | 2 (8.33%) | 0.669 |
| Ophthalmic | 1 (2.44%) | 2 (6.67%) | 0 | 2 (8.33%) | 0.538 |
| Renal | 13 (31.7%) | 12 (40.0%) | 6 (42.9%) | 8 (33.3%) | 0.826 |
| Haematological | 1 (2.44%) | 2 (6.67%) | 0 | 0 | 0.422 |
| Medication | |||||
| Oral prednisolone | 32 (78.1%) | 29 (96.7%) | 10 (76.9%) | 23 (95.8%) | 0.042 |
| Oral prednisolone dose (mg/day) | 10 (5–20) | 10 (7.5–20) | 10 (5–15) | 12.5 (10–20) | 0.495 |
| MMF exposed | 23 (56.1%) | 13 (43.3%) | 4 (28.6%) | 13 (54.2%) | 0.284 |
| MMF current | 8 (19.5%) | 5 (16.7%) | 3 (21.4%) | 2 (8.33%) | 0.642 |
| AZA exposed | 5 (12.2%) | 2 (6.67%) | 1 (7.14%) | 1 (4.17%) | 0.684 |
| AZA current | 3 (7.32%) | 2 (6.67%) | 1 (7.14%) | 0 | 0.613 |
| MTX exposed | 1 (2.44%) | 0 | 4 (28.7%) | 0 | < 0.001 |
| MTX current | 1 (2.44%) | 0 | 3 (21.4%) | 0 | 0.002 |
As MMF exposure was associated with fewer predicted resting macrophages, predicted gene expression in monocytes was also investigated in high resolution mode. There were no differentially expressed genes in patients exposed or not exposed to MMF with adjusted p < 0.05. Cluster analysis suggested 7 patient groups and 9 gene clusters (see Supplementary Figure S2).
Discussion
We used CIBERSORTx to predict the immune cell composition in the blood of patients with active SLE. In the interpretation of deconvoluted transcriptomic data, it is important to recognise that, whilst this may not be directly comparable to studies using flow cytometric methods, CIBERSORT has been validated against flow cytometry data in human blood [20]. Of the 22 cell types, 11 were reliably identified in over 50% of patients and used for further analysis. In our data, there were notable correlations between the predicted numbers of neutrophils, Treg, monocytes, and CD8 + T cells, which have not been observed in other clinical contexts [6, 21] suggesting that these finding may be disease- or medication-specific.
In this study, we did not find any associations between predicted immune cell frequency and active disease in any of the nine BILAG-2004 domains. This contrasts with a small flow cytometry study which identified increased frequency of T cells and reduced B cell and NK cell frequency in patients with active glomerulonephritis (lupus nephritis class III or IV) [22]. Absolute counts of NK cells were also decreased in patients with renal involvement. This discrepancy between the studies could be attributed to using BILAG A/B scores, rather than a histological definition of nephritis, which is likely to encompass a wider group of patients, or to important differences between flow cytometry and transcriptomic analyses. Our findings in this study suggest that the principal immune cell composition in the peripheral blood of patients with SLE does not relate to specific organ activity.
Oral GCs were noted to be associated with a significant increase in the proportion of neutrophils in the peripheral blood, consistent with established literature validating our approach [23]. Of the remaining immunosuppressants assessed in this study, MMF use associated with statistically significant differences in the predicted frequency of immune cells (macrophages, memory activated CD4 T cells, naïve CD4 T cells and regulatory T cells). Although patients currently or previously treated with MMF were more likely to have renal disease and less likely to have musculoskeletal or mucocutaneous disease, in multivariable models, the relative frequency of these 4 immune cell populations was independently associated with MMF exposure.
Recently a large study by Northcott et. al. (2022) investigated gene module expression in 210 patients with SLE using a commercially available assay [24]. In this study, current MMF use was associated with a significantly reduced plasmablast signature. Similarly, AZA use was associated with a lower B cell signature and higher IFN signature. The number of patients exposed to AZA in our study was small, and so it was likely underpowered to identify differences in gene expression related to AZA use. Patients treated with prednisolone had lower pDC, B cell, T cell, and plasmablast signatures, and supporting our findings, higher doses of prednisolone (> 7.5 mg/day) was associated with higher expression of neutrophil-related genes, which may reflect an absolute increase in neutrophil number in the circulation.
A previous small study examined the effects of MMF and cyclophosphamide treatment on peripheral blood lymphocytes and NK cells after 4 weeks using flow cytometry [25]. It identified a significant increase in CD3+CD4+ Th cells over the baseline (pre-treatment) level when taking either MMF or CYC. Whilst CIBERSORTx identified higher memory activated CD4 T cells with MMF use in our study, which would be consistent with more CD4 Th cells, there were also fewer naïve CD4 T cells. This could be because our study analysed multiple subgroups of CD4 T cells, rather than CD4 Th cells as a single group. A similar study reported that the frequencies and absolute numbers of CD27-IgD + CD38 + + transitional and CD27-IgD + CD38 + naïve B cells, absolute numbers of CD27 + IgD + pre-switched memory B cells, and B cell counts overall were lower in patients taking AZA compared to both MMF-treated patients or patients not taking immunosuppressive therapy [26]. Conversely, patients taking MMF had significantly lower frequencies and counts of antibody-producing cells than patients taking AZA or no immunosuppressants. In our study, plasma cells were not predicted to be present in the peripheral blood for many patients. This is in contrast to the study by Northcott et. al. [24] in which a plasmablast/plasma cell signature was detected. This is likely due to differences in the gene panel between the commercially available assay and the LM22 dataset. Furthermore, the plasmablast signature was lower in patients receiving higher doses of prednisolone (defined by Northcott as > 7.5 mg/day). In their study, 15% of patients were receiving this > 7.5 mg/day, compared to 50% in our cohort which may have suppressed the plasma cell signature further.
We did not identify any significant differences in cell frequency in patients receiving either CYC or AZA although the number of patients in each of these groups was low in our study. A study focusing on juvenile-onset SLE in 2020 identified four patient groups based on eight immune cell subsets and defined predominantly by differences in the frequency of CD4 and CD8 T cells. Interestingly, there were significant differences in MMF use, but not other immunosuppressants, between the groups [27].
In our study, changes in predicted immune cell frequency were observed according to current/previous exposure to MMF rather than whether patients were currently receiving MMF. We were unable to determine whether, in those patients previously exposed, MMF withdrawal was recent enough to still affect cell proportions. In patients with SLE, others have demonstrated prolonged changes in the B cell compartment following withdrawal of MMF [28].
Although there were a significant number of DEGs related to MMF exposure in whole blood, only a small number of DEGs were identified within the CD4 T cell subset. This suggests that the transcriptomic difference observed in whole blood may be driven by changes in immune cell frequency, rather than significant differences in the transcriptome within an individual cell type. Further studies should confirm this observation using sorted cells. Within the CD4 T cell population, the patient clusters differed by age, previous mucosal ulceration, prednisolone use, and MTX exposure. Genes related to nuclear transport including NUP88 and NUP105 appeared to be expressed at lower levels in clusters 2 and 4, which comprised older patients, consistent with reports of reduced nuclear transport protein expression in ageing [29].
An important limitation of our study is that the number of patients currently immunosuppressants other than MMF was relatively small, which may be because patients were recruited just prior to an escalation in therapy (mostly commonly starting rituximab). Furthermore, in our study, low estimated cell numbers were obtained for 11/22 cells in the LM22 matrix. This included plasma cells which have been reported to be increased in the peripheral blood in active SLE patients [30] along with other potentially relevant cell types including naïve B cells and activated macrophages. As our data does not include a healthy control population, we were unable to directly compare SLE with heathy controls, but for the 11 cell types we analysed, their estimated frequencies were similar to those reported by others [3]. Our study was cross-sectional, and so we were unable to study whether these cell populations were stable over time or how they might change in response to drug treatment. Finally, whilst estimations of cell populations based on transcriptomic profile have been shown to correlate with flow cytometry findings in other contexts, this methodology has not been validated in patients with SLE.
Conclusion
Drug therapy is associated with important differences in the whole blood transcriptomic signature in patients with SLE which may persist even after the medication is withdrawn. A better understanding of the short- and medium-term consequences of these changes and how they relate to prognosis in SLE is needed. Persistent immune system changes could influence refractoriness to future treatments. Importantly, our results demonstrate the need to adequately adjust for background medication use, especially in studies using whole blood transcriptomics.
Supplementary Information
Acknowledgements
The authors would also like to acknowledge the work of Patrick Doherty (MASTERPLANS data architect), Gillian Armitt (MASTERPLANS project manager), and the MASTERPLANS Patient Partners.
British Isles Lupus Assessment Group Biologics Register (BILAG BR) Consortium
The register is supported by restricted income from UK pharmaceutical companies, presently Roche and GSK, through a contract with the University of Manchester. The principal investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions regarding analyses, interpretation, and publication are made autonomously of any industry contribution. The registry has also received funding support from Lupus UK, a registered charity. BILAG-BR collaborators—to be indexed by The National Library of Medicine (NLM): Patrick Gordon, Department of Rheumatology, King’s College Hospital, London, UK; Steven Young-Min, Portsmouth Hospitals National Health Service (NHS) Trust, Portsmouth, UK; Robert Stevens, Department of Rheumatology, Doncaster and Bassetlaw Hospitals NHS Foundation Trust, Doncaster, UK; Athiveer Prabu, Worcestershire Acute Hospitals NHS Trust and Sandwell and West Birmingham Hospitals NHS Trust, Tunbridge Wells, UK; Mike Batley, Maidstone and Tunbridge Wells NHS Trust, UK; Nagui Gendi, Basildon and Thurrock University Hospitals NHS Trust, Basildon, UK; Bhaskar Dasgupta, Southend University Hospital, Westcliff-on-Sea, Essex, UK; Munther Khamashta, St Thomas’ Hospital, London, UK; Peter Hewins, Queen Elizabeth Hospital, Birmingham, UK; Richard J. Stratton, Royal Free Hospital, London, UK; Antoni Chan, Royal Berkshire Hospital, Reading, UK; Denise De Lord, Queen Elizabeth Queen Mary Hospital, East Kent, UK; Jon King, Derriford Hospital, Plymouth, UK; Shirish Dubey, University Hospital of Coventry and Warwickshire, UK; Edmond O’Riordan, Salford Royal Foundation Trust, Manchester, UK; Shireen Shaffu, Leicester Royal Infirmary, Leicester, UK; Cathy Laversuch, Musgrove Park Hospital, Taunton, Somerset, UK; Thomas P. Sheeran, Cannock Chase Hospital, Cannock, Staffordshire, UK; Erin Vermaak, Haywood Hospital, Stoke-on-Trent, Staffordshire, UK; Nicola Erb, Dudley Group of Hospitals NHS Foundation Trust, West Midlands, UK; Debasish Pyne, Barts Lupus Centre, Royal London Hospital, London, UK; Rachel Jeffrey, Northampton General Hospital, Northampton, UK; Hazem Youssef, Department of Rheumatology, Aberdeen Royal Infirmary, Aberdeen, UK; Wahab Al-Allaf, New Cross Hospital, Wolverhampton, UK and University of Birmingham, Birmingham, UK; Marian Regan, Royal Derby Hospital, Derby, UK; Arvind Kaul, St George’s, University of London, Cranmer Terrace, London, UK.
MASTERPLANS Consortium
MASTERPLANS Consortium Members: The University of Manchester: Ian N Bruce, Katherine Payne, Mark Lunt, Niels Peek, Nophar Geifman, Sean Gavan, Gillian Armitt, Patrick Doherty, Jennifer Prattley, Narges Azadbakht, Angela Papazian, Helen Le Sueur, Carmen Farrelly, Clare Richardson, Zunnaira Shabbir, Lauren Hewitt. University of Bath: Neil McHugh. University of Birmingham: Caroline Gordon, John A Reynolds, Stephen Young. University of Cambridge: David Jayne, Vern Farewell, Li Su. Imperial College London: Matthew Pickering, Elizabeth Lightstone, Alyssa Gilmore, Marina Botto. King's College London: Timothy Vyse, David Lester Morris, David D’Cruz. University of Leeds: Edward M Vital, Miriam Wittmann, Paul Emery. University of Liverpool: Michael Beresford, Christian Hedrich, Angela Midgley, Jenna Gritzfeld. University College London: Michael Ehrenstein, David Isenberg, Mariea Parvaz. MASTERPLANS Patient and Public Involvement Group: Jane Dunnage, Jane Batchelor, Elaine Holland, Pauline Upsall.
Abbreviations
- AZA
Azathioprine
- BILAG
British Isles Lupus Assessment Group
- CYC
Cyclophosphamide
- DEG
Differentially expressed gene
- FDR
False discovery rate
- FPKM
Fragments per kilobase million
- GC
Glucocorticoids
- GSEA
Gene set enrichment analysis
- HCQ
Hydroxychloroquine
- IFN
Interferon
- MMF
Mycophenolate mofetil
- MTX
Methotrexate
- NK
Natural killer
- RNA-seq
Ribonucleic acid sequencing
- SLE
Systemic lupus erythematosus
- SLEDAI
Systemic lupus erythematosus disease activity index
- SLICC
Systemic lupus international collaborating clinics
Authors’ contributions
MA performed the data analysis and wrote the initial draft of the manuscript. NN produced the laboratory data and contributed to the manuscript. LMC and EMV contributed to data analysis and data interpretation and review of the manuscript. ES participated in data collection and data integrity. NM derived and provided autoantibody data used in this study. INB and JAR conceived and developed the study, contributed to data analysis including generation of figures, and interpretation and review of the manuscript. All authors contributed to the manuscript and approved the submitted version.
Funding
The MASTERPLANS Consortium is funded by a grant from the Medical Research Council (MR/M01665X/1). The BILAG-BR acknowledges funding support from Roche, Lupus UK, and GSK. Professor Bruce is a National Institute for Health Research (NIHR) senior investigator and is funded by the NIHR Manchester Biomedical Research Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Availability of data and materials
The data underlying this article are available on reasonable request to the corresponding author dependent upon the nature of the request, the availability of the data, and its intended use.
Declarations
Ethics approval and consent to participate
Ethical approval was granted by North West Greater Manchester West Research Ethics Committee (09/H1014/64) and the local Research and Development departments at participant sites.
Consent for publication
Not applicable.
Competing interests
INB has received grant/research support from Genzyme/Sanofi, GlaxoSmithKline, Roche, and UCB; received consulting fees from Eli Lilly, GlaxoSmithKline, ILTOO, Merck Serono, and UCB; and was a speaker for AstraZeneca, GlaxoSmithKline, and UCB. EMV has received consultancy fees from Roche, GSK, AstraZeneca, Aurinia Pharmaceuticals, Lilly and Novartis. All other authors have declared no competing interests.
Footnotes
The original online version of this article was revised: an error to the last name of Mumina Akthar was identified.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/4/2023
A Correction to this paper has been published: 10.1186/s13075-023-03160-1
Contributor Information
John A. Reynolds, Email: j.a.reynolds.1@bham.ac.uk
British Isles Lupus Assessment Group Biologics Register (BILAG BR) Consortium:
Patrick Gordon, Steven Young-Min, Robert Stevens, Athiveer Prabu, Mike Batley, Nagui Gendi, Bhaskar Dasgupta, Munther Khamashta, Peter Hewins, Richard J. Stratton, Antoni Chan, Denise De Lord, Jon King, Shirish Dubey, Edmond O’Riordan, Shireen Shaffu, Cathy Laversuch, Thomas P. Sheeran, Erin Vermaak, Nicola Erb, Debasish Pyne, Rachel Jeffrey, Hazem Youssef, Wahab Al-Allaf, Marian Regan, and Arvind Kaul
MASTERPLANS Consortium:
Katherine Payne, Mark Lunt, Niels Peek, Nophar Geifman, Sean Gavan, Gillian Armitt, Patrick Doherty, Jennifer Prattley, Narges Azadbakht, Angela Papazian, Helen Le Sueur, Carmen Farrelly, Clare Richardson, Zunnaira Shabbir, Lauren Hewitt, Caroline Gordon, Stephen Young, David Jayne, Vern Farewell, Li Su, Matthew Pickering, Elizabeth Lightstone, Alyssa Gilmore, Marina Botto, Timothy Vyse, David Lester Morris, David D’Cruz, Miriam Wittmann, Paul Emery, Michael Beresford, Christian Hedrich, Angela Midgley, Jenna Gritzfeld, Michael Ehrenstein, David Isenberg, Mariea Parvaz, Jane Dunnage, Jane Batchelor, Elaine Holland, and Pauline Upsall
References
- 1.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano M, Iwasaki Y, Fujio K. Transcriptomic studies of systemic lupus erythematosus. Inflamm Regen. 2021;41:11. doi: 10.1186/s41232-021-00161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panousis NI, Bertsias GK, Ongen H, Gergianaki I, Tektonidou MG, Trachana M, et al. Combined genetic and transcriptome analysis of patients with SLE: distinct, targetable signatures for susceptibility and severity. Ann Rheum Dis. 2019;78(8):1079–1089. doi: 10.1136/annrheumdis-2018-214379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37(7):773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Cui Y, Feng J, Guo Y. Identifying the pattern of immune related cells and genes in the peripheral blood of ischemic stroke. J Transl Med. 2020;18(1):296. doi: 10.1186/s12967-020-02463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpiński P, Frydecka D, Sąsiadek MM, Misiak B. Reduced number of peripheral natural killer cells in schizophrenia but not in bipolar disorder. Brain Behav Immun. 2016;54:194–200. doi: 10.1016/j.bbi.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Weiss E, de la Grange P, Defaye M, Lozano JJ, Aguilar F, Hegde, et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF. Front Immunol 2021;11:619039. [DOI] [PMC free article] [PubMed]
- 9.Wang YH, Hou HA, Lin CC, Kuo YY, Yao CY, Hsu CL, et al. A CIBERSORTx-based immune cell scoring system could independently predict the prognosis of patients with myelodysplastic syndromes. Blood Adv. 2021;5(22):4535–4548. doi: 10.1182/bloodadvances.2021005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Zhang L, Wang J, Zhang M, Song Z, Ni B, You Y. Identification of key biomarkers and immune infiltration in systemic lupus erythematosus by integrated bioinformatics analysis. J Transl Med. 2021;19(1):35. doi: 10.1186/s12967-020-02698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Tang W, Tang W. Immune cell infiltration characteristics and related core genes in lupus nephritis: results from bioinformatic analysis. BMC Immunol. 2019;20(1):37. doi: 10.1186/s12865-019-0316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004.Σ Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44(7):902–6. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 15.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X, Kuan PF. RNAAgeCalc: a multi-tissue transcriptional age calculator. PLoS ONE. 2020;15(8):e.0237006. doi: 10.1371/journal.pone.0237006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomaznoy M, Ha B, Peters B. GOnet: a tool for interactive gene ontology analysis. BMC Bioinformatics. 2018;19(1):470. doi: 10.1186/s12859-018-2533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Cai B, Ren C, Xu H, Du W, Wu Y, et al. Identification of immune related cells and crucial genes in the peripheral blood of ankylosing spondylitis by integrated bioinformatics analysis. PeerJ. 2012;9:e12125. doi: 10.7717/peerj.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus. 1997;6(9):708–712. doi: 10.1177/096120339700600905. [DOI] [PubMed] [Google Scholar]
- 23.Ronchetti S, Ricci E, Migliorati G, Gentil M, Riccardi C. How glucocorticoids affect the neutrophil life. Int J Mol Sci. 2018;19(12):4090. doi: 10.3390/ijms19124090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Northcott M, Gearing LJ, Bonin J, Koelmeyer R, Hoi A, Hertzog PJ, Morand EF. Immunosuppressant exposure confounds gene expression analysis in systemic lupus erythematosus. Front Immunol. 2022;13:964263. doi: 10.3389/fimmu.2022.964263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Jiang Z, Jiang Y, Ma N, Wang K, Zhang Y. Changes in immune cell frequencies after cyclophosphamide or mycophenolate mofetil treatments in patients with systemic lupus erythematosus. Clinical Rheumatol. 2021;31(6):951–959. doi: 10.1007/s10067-012-1958-8. [DOI] [PubMed] [Google Scholar]
- 26.Eickenberg S, Mickholz E, Jung E, Nofer JR, Pavenstädt HJ, Jacobi AM. Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res Ther. 2012;14(3):R110. doi: 10.1186/ar3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson GA, Peng J, Dönnes P, Coelewij L, Naja M, Radziszewska A, et al. 2020. Disease-associated and patient-specific immune cell signatures in juvenile-onset systemic lupus erythematosus: patient stratification using a machine-learning approach. Lancet Rheumatol. 2020;2(8):e485–496. doi: 10.1016/S2665-9913(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandyil RM, Kamdar AA, Warren R, Hanson IC. Serial immunphenotyping and prolonged B cell dysfunction in systemic lupus erythematosus (SLE) treated with mycophenolate mofetil (MMF) J Allergy Clin Immunol. 2009;123(2):S162. doi: 10.1016/j.jaci.2008.12.611. [DOI] [Google Scholar]
- 29.Kim SY, Ryu SJ, Ahn HJ, Choi HR, Kang HT, Park SC. Senescence-related functional nuclear barrier by down-regulation of nucleo-cytoplasmic trafficking gene expression. Biochem Biophys Res Commun. 2010;391(1):28–32. doi: 10.1016/j.bbrc.2009.10.154. [DOI] [PubMed] [Google Scholar]
- 30.Lugar PL, Love C, Grammer AC, Dave SS, Lipsky PE. Molecular characterization of circulating plasma cells in patients with active systemic lupus erythematosus. PLoS ONE. 2012;7(9):e44362. doi: 10.1371/journal.pone.0044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available on reasonable request to the corresponding author dependent upon the nature of the request, the availability of the data, and its intended use.