Abstract
Background
The scale-up of antiretroviral therapy programmes has resulted in increased life expectancy of people with HIV in Africa. Little is known of the menopausal experiences of African women, including those living with HIV. We aimed to determine the prevalence and severity of self-reported menopause symptoms in women at different stages of menopause transition, by HIV status, and evaluate how symptoms are related to health-related quality of life (HRQoL). We further sought to understand factors associated with menopause symptoms.
Methods
A cross-sectional study recruited women resident in Harare, Zimbabwe, sampled by age group (40–44/45–49/50–54/55–60 years) and HIV status. Women recruited from public-sector HIV clinics identified two similarly aged female friends (irrespective of HIV status) with phone access. Socio-demographic and medical details were recorded and women staged as pre-, peri- or post-menopause. The Menopausal Rating Scale II (MRS), which classified symptom severity, was compared between those with and without HIV. Linear and logistic regression determined factors associated with menopause symptoms, and associations between symptoms and HRQoL.
Results
The 378 women recruited (193[51.1%] with HIV), had a mean (SD) age of 49.3 (5.7) years; 173 (45.8%), 51 (13.5%) and 154 (40.7%) were pre-, peri and post-menopausal respectively. Women with HIV reported more moderate (24.9% vs. 18.1%) and severe (9.7% vs. 2.6%) menopause symptoms than women without HIV. Peri-menopausal women with HIV reported higher MRS scores than those pre- and post-menopausal, whereas in HIV negative women menopausal stage was not associated with MRS score (interaction p-value = 0.014). With increasing severity of menopause symptoms, lower mean HRQoL scores were observed. HIV (OR 2.02[95% CI 1.28, 3.21]), mood disorders (8.80[2.77, 28.0]), ≥ 2 falls/year (4.29[1.18, 15.6]), early menarche (2.33[1.22, 4.48]), alcohol consumption (2.16[1.01, 4.62]), food insecurity (1.93[1.14, 3.26]) and unemployment (1.56[0.99, 2.46]), were all associated with moderate/severe menopause symptoms. No woman reported use of menopausal hormone therapy.
Conclusions
Menopausal symptoms are common and negatively impact HRQoL. HIV infection is associated with more severe menopause symptoms, as are several modifiable factors, including unemployment, alcohol consumption, and food insecurity. Findings highlight an unmet health need in ageing women in Zimbabwean, especially among those living with HIV.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-023-02466-1.
Keywords: HIV, Menopause, Menopausal symptoms, Quality of life, Africa, Ageing
Background
People in Africa are now living longer than ever before; by 2025 the older population in Sub-Saharan Africa (SSA) is expected to have doubled from 2010 [1]. This ageing is attributed to improved living conditions, accessibility and availability of medicines such as antiretroviral therapy (ART), and means more women are now living through menopause [2].
Menopause is a physiological phase, defined retrospectively by 12 months of amenorrhea and loss of ovarian follicular function, typically occurring between the ages of 40 and 58 years [3]. The cessation of menstrual periods is often associated with a variety of adverse symptoms, including anxiety, mood disorders and vaginal dryness [4]. These symptoms begin as estrogen levels decline and may continue for years after menses end [4]. Although women experience similar hormonal changes, menopausal experiences vary greatly [5, 6]. Symptom severity is often evaluated using the validated menopause rating scale (MRS) II which characterizes psychological, somatic, and urogenital symptoms [7]. Severe menopausal symptoms can profoundly affect personal and social function [8], and health-related quality of life (HRQoL) [9–11], and are exacerbated by the effects of biological ageing, changing socio-demographics and comorbidities [12].
In Zimbabwe, 15.3% of adult women live with HIV and survival rates have greatly improved as a result of widespread ART roll-out [13]; hence increasing numbers of women with HIV are now reaching menopause. By contrast there is a paucity of data concerning the menopausal experiences of women living with HIV in SSA [14], with most data stemming from North American and European settings. However, one report from Nigeria suggests women with HIV may experience more menopausal symptoms than those without HIV [15]. Understanding menopause in a Zimbabwean population is important, as differences in culture, health-seeking behaviours, and availability of menopause care, invalidate extrapolation from high-income country settings [16, 17].
This study aimed to determine the prevalence and severity of self-reported menopause symptoms in Zimbabwean women at different stages of menopausal transition and determine how symptoms differ in the context of HIV infection. We further sought to explore other potential factors associated with menopausal symptoms, and to understand the extent to which menopausal symptoms relates to HRQoL in those with and without HIV.
Methods
Design and setting
A cross-sectional study of women in Harare, Zimbabwe was conducted between April and December 2020.
Recruitment
Women, resident in Harare, were sampled by four age groups (40–44, 45–49, 50–54 and 55–60 years) and by HIV status. Women living with HIV were recruited from the Sally Mugabe (formally Harare Central) and Parirenyatwa Hospitals HIV clinics – the two public hospitals that serve the city of Harare. Women routinely attending the HIV clinic to collect their ART were invited to participate. Women living without HIV had been planned to be recruited from local churches in Harare. However, on the second day of recruitment, Zimbabwe implemented a lockdown in response to the COVID-19 pandemic and as a result the protocol was revised to adhere to infection prevention and control measures. Thus, women without HIV were recruited by asking each participant recruited from the HIV clinics to identify two female friends (irrespective of HIV status) around the same age with phone access who might be willing to take part.
Inclusion criteria
The study included women aged 40–60 years, resident in Harare and who were willing to have an HIV test.
Exclusion criteria
Women who were pregnant, acutely unwell or failed to provide informed consent were excluded from the study.
Sample size calculation
Cochrane’s formula was used to calculate the power of the study (n = 378, p1 = 70.2%, p2 = 51.1%, z = 1.96, d = 0.05) [18]. The proportions of overall menopausal symptoms among women living with (p1) and without (p2) HIV were obtained from previous studies [19, 20]. The study had 84% power to detect a 15% difference in overall menopause symptoms between the two groups.
Data collection
A female nurse-administered questionnaire recorded socio-demographic and lifestyle information, medical history, and menopause symptoms (see below). Socio-demographic factors included education, employment, household income and food insecurity (using selected questions from the US adult food security survey module [21]). Identified comorbidities included tuberculosis, diabetes, hypertension, cardiac disease, arthritis and balance impairment. Mental health was assessed using the validated 14-item Shona symptom questionnaire with women scoring ≥ 10/14 points classified as having mood disorders [22]. HRQoL was quantified using the WHOQOL eight-item questionnaire (score range: 1–40) which assessed perceived physical, mental and social health status over the previous two-weeks [23, 24]. The Global Physical Activity Questionnaire (GPAQ) quantified self-reported physical activity, from which minutes/week of moderate to vigorous intensity physical activity (MVPA) were calculated by summing moderate and vigorous work, travel-related and recreational activities (low physical activity defined as < 150 min/week of moderate to vigorous physical activity) [25].
Two nurses measured height (in cm) and weight (in kg) using a Seca 213 stadiometer and Seca 875 digital scales (Seca Precision for health, Seca Mechanical Floor Scales Class III, Hamburg, Germany) respectively, with the mean of both measurements calculated. Body mass index (BMI) was calculated (kg/m2) and categorized as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25-29.9 kg/m2) and obese (≥ 30 kg/m2) [26].
Menopause status and symptoms
Participants were classified into one of three menopause categories based on self-reported final menstrual period (FMP) [27, 28]. Women currently having regular periods were classified as pre–menopausal. Women having irregular periods or having missed 3 months of consecutive menstrual periods within the past year, were classified as peri–menopausal. Women who had had no bleeding for more than 12 months were classified as post–menopausal. Women who had had a hysterectomy (+/- oophrectomy) could not be classified and were excluded from analyses. The menopausal rating scale II (MRS) was used to assess the severity of menopausal symptoms [no/little (0–4), mild [5–8], moderate [9–14, 29, 30] and severe (17+)], characterized by 11 questions measured on a five-point Likert scale. Three symptom sub-domains were rated: somatic (joint/muscle discomfort, sleep disturbance, heart discomfort, body temperature disturbance), psychological (irritability, anxiety, physical and mental exhaustion, mood disorders) and urogenital (vaginal dryness, urinary problems, sexual problems) [7]. The severity of MRS sub-domains were categorized into no/little, mild, moderate and severe groups according to sub-domain specific cut-offs, as previously described [7].
HIV testing
All women recruited by participants with HIV had a point-of-care HIV antibody test performed using Alere Determine™ HIV-1/2 (Alere San Diego, Inc. San Diego, CA). If negative, there were enrolled into the study. If positive after a confirmatory test (Chembio SURE CHECK® HIV 1/2 Assay) they were enrolled into the HIV-positive group and referred to local HIV services [31].
Statistical analysis
Data were cleaned, checked and analysed using Stata16 (StataCorp, College Station, TX). Unless otherwise stated, quantitative variables were summarised using the mean ± standard deviation (SD) if normally distributed, or otherwise as median with an interquartile range (IQR). Categorical variables were summarised as frequencies with percentages. Comparisons of quantitative variables by HIV status were performed using the Student’s t-test and the Mann Whitney U test if normally distributed or skewed respectively. Chi-squared and Fishers’ exact tests were used to compare categorical variables (including severity of overall MRS and the sub-domains) between women living with and without HIV. We evaluated whether the relationship between menopause stage and menopausal symptoms (and symptom sub-domains) was modified by HIV infection using a likelihood ratio test.
Univariate linear regression was used to determine the association between menopausal symptoms and HRQoL, reporting beta coefficients and their respective 95% confidence intervals. We tested whether the association between menopausal symptoms and HRQoL was modified by the presence of HIV infection using likelihood ratio testing. A test for trend was performed for HRQoL ranks across ordered total menopausal symptoms and menopausal symptoms sub-domains. Finally, univariate logistic regression was used to explore associations between socio-economic, obstetric, and maternal factors, as well as reported comorbidities, with menopausal symptoms (none/mild [MRS < 9], and moderate/severe [MRS ≥ 9]), reporting odds ratios (OR) with 95% confidence intervals.
Results
Participant characteristics
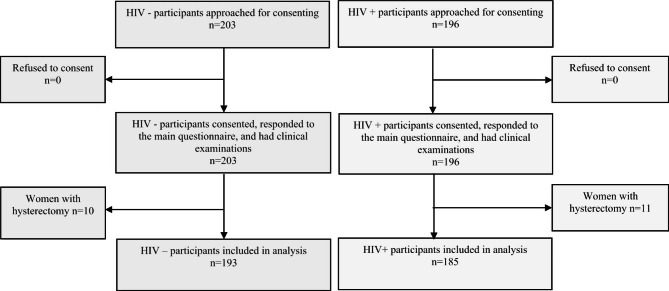
All 399 women who were approached consented to be recruited. After exclusion of 21 (5.3%) women who reported a hysterectomy (Fig. 1), analyses included 193 (51%) women with and 185 without HIV (Fig. 1); their overall mean (SD) age was 49.3 (5.7) years. Participant characteristics stratified by HIV status are reported in Table 1. Women living with HIV were frequently widowed (40.2%) or divorced/separated (25%), had lower educational attainment and were more often unemployed. Women living with HIV were less likely to report contraceptive use, reported bearing fewer children and were more often worried about food availability. Whilst the overall prevalence of obesity was high (37.8%), obesity was almost twice as common in women without HIV (26.5% vs. 48.7%). Correspondingly, whilst hypertension was common, identified in 31.6% of all women, it was less common in those living with HIV, than in those without HIV.
Fig. 1.
Participant recruitment flow diagram. Figure showing participant enrolment by HIV status
Table 1.
Characteristics of women recruited in Harare, by HIV status
| Total (n = 378) |
HIV – (n = 193) |
HIV + (n = 185) |
p value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age groups (years), n (%) | ||||
| 40–44 | 95(25.1) | 51(26.4) | 44(23.8) | 0.823 |
| 45–49 | 104(27.5) | 51(26.4) | 53(28.7) | |
| 50–54 | 95(25.1) | 46(23.8) | 49(26.5) | |
| 55–60 | 84(22.2) | 45(23.3) | 39(21.1) | |
| Socio-economic characteristics | ||||
| Highest level of education, n (%) | ||||
| None/Primary | 57(15.1) | 31(16.1) | 26(14.1) | < 0.001 |
| Secondary | 258(68.3) | 115(59.6) | 143(77.3) | |
| Tertiary education | 63(16.7) | 47(24.4) | 16(8.7) | |
| Employed, n (%) | 194(51.3) | 130(67.4) | 64(34.6) | < 0.001 |
| Household income ($US) per month, n (%) | ||||
| ≤100 | 329(87.0) | 168(87.1) | 161(87.0) | 0.352 |
| 101–200 | 34(9.0) | 15(7.8) | 19(10.3) | |
| >200 | 15(4.0) | 10(5.2) | 5(2.7) | |
| Worry of food insecurity in the household, n (%) | 258(68.3) | 120(62.2) | 138(74.6) | 0.010 |
| Marital status, n (%) ** | ||||
| Never married | 6(1.6) | 4(2.1) | 2(1.1) | < 0.001 |
| Currently married | 181(48.0) | 119(61.7) | 62(33.7) | |
| Separated/Divorced | 81(21.5) | 35(18.1) | 46(25.0) | |
| Widowed | 109(28.9) | 35(18.1) | 74(40.2) | |
| Smoking history, n (%) | ||||
| Never | 373(98.7) | 192(99.5) | 181(97.8) | 0.340 |
| Ex-smoker | 4(1.0) | 1(0.5) | 3(1.6) | |
| Current | 1(0.3) | 0 | 1(0.5) | |
| Current alcohol uptake, n (%) | 30(7.9) | 14(7.3) | 16(8.7) | 0.616 |
| Obstetric and maternal factors | ||||
| Age at menarche (years), n (%) ** | ||||
| ≤ 12 | 48(13.1) | 18(9.5) | 30(17.0) | 0.164 |
| 13–15 | 204(55.7) | 113(59.8) | 91(51.4) | |
| ≥16 | 114(31.2) | 58(30.7) | 56(31.6) | |
| Parity groups, n (%) | ||||
| Nulliparity | 10(2.7) | 2(1.0) | 8(4.3) | 0.046 |
| Low parity (1–3) | 229(60.6) | 112(58.0) | 117(63.2) | |
| Multi-parous (> 3) | 139(36.8) | 79(40.9) | 60(32.4) | |
| Types of contraceptives, n (%) | ||||
| None | 264(69.8) | 125(64.8) | 139(74.1) | 0.033 |
| Oral pill | 72(19.0) | 39(20.2) | 33(17.8) | |
| Intrauterine device | 15(4.0) | 12(6.2) | 3(1.6) | |
| Injectables (depo) | 27(7.1) | 17(8.8) | 10(5.4) | |
| Comorbidities | ||||
| Number of comorbidities, n (%) | ||||
| None | 201(53.2) | 113(58.6) | 88(47.6) | 0.078 |
| 1–2 | 168(44.4) | 77(39.9) | 91(49.2) | |
| >2 | 9(2.4) | 3(1.6) | 6(3.2) | |
| Depression (Shona symptom questionnaire), n (%) | 16(4.2) | 5(2.6) | 11(6.0) | 0.105 |
| Falls in the past year, n (%) | ||||
| None | 336(88.9) | 174(90.2) | 162(87.6) | 0.395 |
| One | 32(8.5) | 16(8.3) | 16(8.7) | |
| Two or more | 10(2.7) | 3(1.6) | 7(3.8) | |
| Low moderate to vigorous physical activity (< 150 minutes/week) | 19(5.0) | 9(4.7) | 10(5.4) | 0.741 |
| HIV history | ||||
| Years since HIV diagnosis, median (IQR) | 10.0(5.8–14.0) | - | 10.0(5.8–14.0) | - |
| ART duration (in years), median (IQR) ** | 9(5–13) | 9(5–13) | - | |
| Anthropometry | ||||
| BMI, n (%) | ||||
| Underweight | 6(1.6) | 0 | 6(3.2) | < 0.001 |
| Normal | 95(25.1) | 35(18.1) | 60(32.4) | |
| Overweight | 134(35.5) | 64(33.2) | 70(37.8) | |
| Obese | 143(37.8) | 94(48.7) | 49(26.5) | |
Summary of participant’s socio-demographic characteristics, maternal, clinical history, and anthropometry measurements. The Chi-square test p-values were generated to compare categorical variables by HIV status. No comparisons were performed for HIV specific variables (ART duration and years after HIV diagnosis) ** represents complete case analysis for marital status (missing one participant), Age at menarche (missing 12 participants) and ART duration (missing eight participants)
Comorbidities were frequently observed; overall 52 (13.8%) women reported a history of tuberculosis, 119 (31.5%) hypertension, 18 (4.8%) diabetes, 16 (4.2%) cardiac disease, 5 (1.3%) poor balance, and 33 (8.7%) arthritis. Almost half of the women living with HIV had at least one comorbidity (Table 1). As expected, a history of tuberculosis was more common in women with HIV (50 [27.0%] vs. 2 [1.0%]; p < 0.001), whereas more women without HIV reported having a diagnosis of arthritis (26 [13.5%] vs. 7 [3.8%]; p = 0.001). No women reported to be breastfeeding.
Menopausal symptoms
Most women were either pre- (n = 173, 45.8%) or post-menopausal (n = 154, 40.7%) (Table 2);. Similar proportions of women in each of the three menopausal stages were seen in those with and without HIV (Table 2). Overall, 140 (80.9%) pre-menopausal women had at least one menopausal symptom, as did 48 (94.1%) peri-menopausal and 119 (77.3%) post-menopausal women. Women with HIV were more likely to report moderate (24.9% vs. 18.1%) and severe (9.7% vs. 2.6%) menopausal symptoms, compared to women without HIV (Table 2). This pattern was also evident for the psychological and urogenital symptom sub-domains, with 25.4% of women with HIV reporting moderate or severe psychological symptoms, and 46.5% moderate or severe urogenital symptoms. However, there was no statistical evidence to suggest women living with HIV experienced more moderate or severe somatic symptoms (30.3% vs. 21.2%, p = 0.250, in those living with and without HIV respectively). In addition, HRQoL was no different between women living with and without HIV (Table 2).
Table 2.
Menopausal stage, menopausal symptoms and health-related quality of life in women resident in Harare, by HIV status
| Total (n = 378) |
HIV – (n = 193) |
HIV + (n = 185) |
p value * | |
|---|---|---|---|---|
| Menopausal stage, n (%) | ||||
| Pre-menopause | 173(45.8) | 94(48.7) | 79(42.7) | 0.278 |
| Peri-menopause | 51(13.5) | 28(14.5) | 23(12.4) | |
| Post menopause | 154(40.7) | 71(36.8) | 83(44.9) | |
| Total MRS symptoms, n (%) | ||||
| No, little (≤ 4) | 177(46.8) | 101(52.3) | 76(41.1) | 0.005 |
| Mild (5–8) | 97(25.7) | 52(26.9) | 45(24.3) | |
| Moderate (9–16) | 81(21.4) | 35(18.1) | 46(24.9) | |
| Severe (≥ 17) | 23(6.1) | 5(2.6) | 18(9.7) | |
| Somatic symptomsub-domain, n (%) | ||||
| No, little (0–2) | 190(50.3) | 102(52.9) | 88(47.6) | 0.250 |
| Mild (3–4) | 91(24.1) | 50(25.9) | 41(22.2) | |
| Moderate (5–8) | 82(21.7) | 35(18.1) | 47(25.4) | |
| Severe (≥ 9) | 15(4.0) | 6(3.1) | 9(4.9) | |
| Psychological symptomsub-domain, n (%) | ||||
| No, little (0–1) | 228(60.3) | 135(70.0) | 93(50.3) | < 0.001 |
| Mild (2–3) | 75(19.8) | 30(15.5) | 45(24.3) | |
| Moderate (4–6) | 53(14.0) | 26(13.5) | 27(14.6) | |
| Severe (≥ 7) | 22(5.8) | 2(1.0) | 20(10.8) | |
| Urogenital symptomsub-domain, n (%) | ||||
| No, little (0) | 172(45.5) | 100(51.8) | 72(38.9) | 0.009 |
| Mild (1) | 63(16.7) | 36(18.7) | 27(14.6) | |
| Moderate (2–3) | 88(23.3) | 35(18.1) | 53(28.7) | |
| Severe (≥ 4) | 55(14.5) | 22(11.4) | 33(17.8) | |
| HRQoL, mean (SD) | 26.4(3.5) | 26.5(3.3) | 26.3(3.7) | 0.642 |
Participants’ menopausal symptoms by HIV status. *p values generated using Chi-square tests of association for categorical variables and t-test for normally distributed continuous variables, MRS: Menopause Rating Scale, HRQoL: Health Related Quality of Life
Among women with HIV, those who were peri-menopausal reported higher MRS scores than those who were pre- and post-menopause, whereas in HIV negative women no clear association was seen between menopausal stage and MRS score (p-value for interaction = 0.014) (Table 3). Across the three menopausal symptom sub-domains, peri-menopausal women with HIV experienced the highest frequency of symptoms, with 78% experiencing somatic and/or urogenital symptoms, and 69% psychological symptoms. Pre-menopausal women with HIV also reported a high level of psychological symptoms (affecting 57%). Amongst women living with HIV, menopausal stage was strongly associated with the presence of somatic and psychological symptoms. A weaker relationship was seen between menopausal stage and somatic symptoms in women without HIV, with no relationship between menopausal stage and psychological or urogenital symptoms (Table 3). There was strong evidence for HIV infection modifying the association with menopausal stage and both somatic and psychological symptoms (interaction p-values 0.033 and 0.013 respectively), suggesting that women living with HIV experience more severe somatic and psychological symptoms through menopause.
Table 3.
Menopausal symptom prevalence and severity across menopausal stages in women living with and without HIV in Harare
| Menopausal symptoms | HIV negative (n = 193) | HIV positive (n = 185) | Interaction p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre- (n = 94) |
Peri- (n = 28) |
Post- (n = 71) |
p value | Pre- (n = 79) |
Peri- (n = 23) |
Post- (n = 83) |
p value | ||
| Total | |||||||||
| MRS II total, mean (SD) | 4.4(4.6) | 6.1(5.0) | 5.6(4.9) | 0.095 | 7.4(6.3) | 11.4(7.8) | 6.1(5.7) | 0.004 | 0.014 |
| Any symptoms, n (%) | 70(74.7) | 25(89.3) | 53(74.6) | 0.233 | 70(88.6) | 23(95.8) | 66(79.5) | 0.029 | |
| Any moderate or severe symptoms, n (%) | 14(14.9) | 7(25.0) | 19(26.8) | 0.147 | 27(34.2) | 11(45.8) | 26(31.3) | 0.337 | |
| Somatic sub-domain | |||||||||
| Sub-domain total, mean (SD) | 2.1(2.2) | 3.3(2.4) | 3.2(2.9) | 0.012 | 2.9(2.8) | 4.8(2.8) | 2.8(2.5) | 0.010 | 0.033 |
| Any symptoms, n (%) | 36(38.3) | 16(57.1) | 39(54.9) | 0.055 | 37(46.8) | 18(78.3) | 42(50.6) | 0.027 | |
| Any moderate or severe symptoms, n (%) | 11(11.7) | 8(28.6) | 22(31.0) | 0.005 | 22(27.9) | 13(56.5) | 21(25.3) | 0.013 | |
| Psychological sub-domain | |||||||||
| Sub-domain total, mean (SD) | 1.3(2.0) | 1.5(1.8) | 1.1(1.6) | 0.824 | 2.8(2.9) | 3.8(3.6) | 1.5(2.0) | 0.001 | 0.013 |
| Any symptoms, n (%) | 28(29.8) | 11(39.3) | 19(26.7) | 0.471 | 45(57.0) | 16(69.6) | 31(37.4) | 0.006 | |
| Any moderate or severe symptoms, n (%) | 12(12.8) | 6(21.4) | 10(14.1) | 0.516 | 25(31.7) | 9(39.1) | 13(15.7) | 0.018 | |
| Urogenital sub-domain | |||||||||
| Sub-domain total, mean (SD) | 1.0(1.5) | 1.4(1.9) | 1.3(1.8) | 0.494 | 1.6(1.9) | 2.8(2.8) | 1.8(2.4) | 0.081 | 0.280 |
| Any symptoms, n (%) | 41(43.6) | 16(57.1) | 36(50.7) | 0.393 | 48(60.8) | 18(78.3) | 47(56.6) | 0.169 | |
| Any moderate or severe symptoms, n (%) | 25(26.6) | 9(32.1) | 23(32.4) | 0.684 | 35(44.3) | 16(69.6) | 35(42.2) | 0.058 | |
Comparison of menopausal symptoms by menopausal stage stratified by HIV status. MRS II: Menopause Rating Scale II; All continuous variables presented as mean (SD), Menopausal status (Pre-menopause, Peri-menopause, and Post-menopausal) All comparisons for categorical data presented were performed using the Chi-square test, Whilst means (SD) are presented for MRS II and sub-domain scores, Kruskal Wallis tests were used to compare medians across the menopausal stages given skewness of the data. Interaction test for the effect of menopause stage* HIV on menopausal symptoms
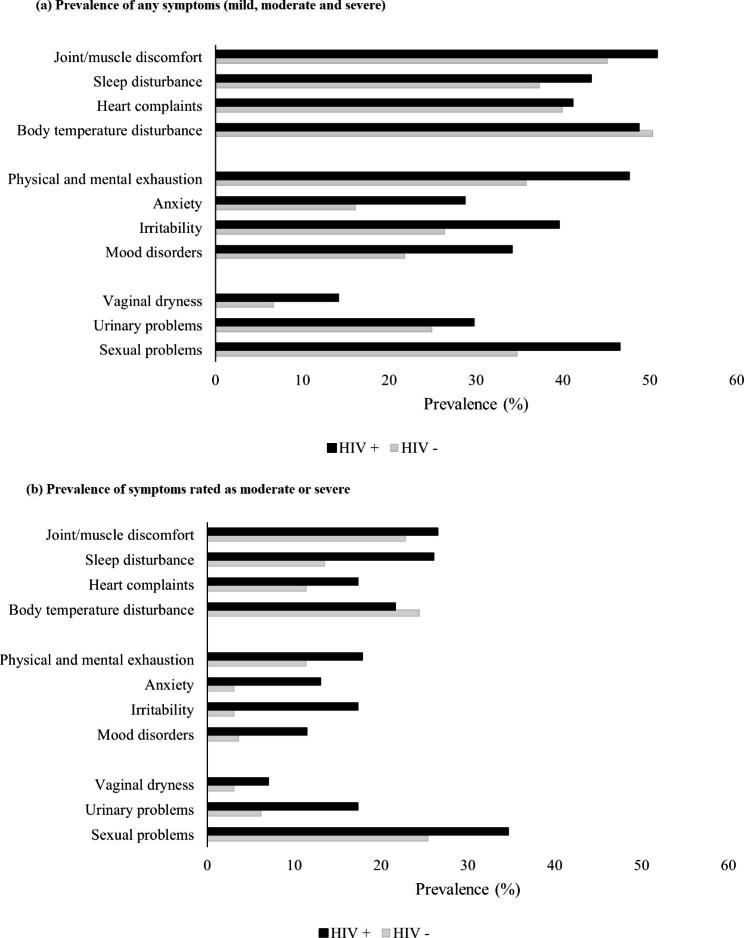
Other than hot flushes, all menopausal symptoms, whether mild or moderate/severe, were more commonly seen in women living with HIV (Fig. 2). Generally, somatic symptoms were most commonly reported as affecting at least 40% of all women, as was physical and mental exhaustion (affecting ≥ 45%); however, moderate and/or severe sexual problems were a particular issue for women with HIV reported by 35%, as compared to 25% of women without HIV (Fig. 2b).
Fig. 2.
Prevalence of Menopause Rating Scale (MRS) II symptoms by HIV status in Zimbabwean women
Menopausal symptoms and HRQoL
Generally, with increasing severity of menopause symptoms (total MRS, somatic and psychological sub-domains), trends were seen towards lower mean HRQoL scores in both women with and without HIV (Table 4). Women with HIV who experienced moderate or severe symptoms reported substantially reduced HRQoL compared against those with few or no symptoms overall (based on total MRS score) and by all symptom sub-domains. The greatest impact on HRQoL was reported by those women with HIV experiencing severe somatic symptoms. In women living with HIV, clear associations were seen between moderate and/or severe psychological and urogenital symptoms and lower HRQoL, which were less evident in women without HIV. HIV infection did not appear to modify the association between menopausal symptoms (either total MRS or symptom sub-domains) and HRQoL (p > 0.1 for all) (Table 4).
Table 4.
The association between menopausal symptoms and Health Related Quality of Life in Zimbabwean women living with and without HIV
| Menopausal symptoms | HIV negative (n = 193) | p-value for trend | HIV positive (n = 185) | p-value for trend | ||
|---|---|---|---|---|---|---|
| Mean (SD) HRQoL Score |
Beta [95% CI] |
Mean (SD) HRQoL Score |
Beta [95% CI] |
|||
| Total MRS score | ||||||
| No, little (0–4) | 27.0(3.1) | Ref | 27.1(3.5) | Ref | ||
| Mild (5–8) | 26.4(3.4) | -0.64 [-1.72, 0.45] | 0.001 | 26.8(3.4) | -0.38 [-1.71, 0.96] | 0.001 |
| Moderate (9–16) | 25.2(3.4) | -1.81 [-3.06, -0.56] | 25.7(2.9) | -1.41 [-2.74, -0.09] | ||
| Severe (≥ 17) | 25.4(2.6) | -1.65 [-4.50, 1.21] | 23.4(5.7) | -3.69 [-5.54, -1.83] | ||
| Somatic sub-domain | ||||||
| No, little (0–2) | 27.3(2.8) | Ref | 26.9(3.2) | Ref | ||
| Mild (3–4) | 26.2(3.6) | -1.09[-2.16, -0.02] | < 0.001 | 27.2(3.6) | 0.36[-0.98, 1.69] | 0.002 |
| Moderate (5–8) | 24.9(3.4) | -2.46[-3.67, -1.24] | 25.2(3.9) | -1.67[-2.95, -0.40] | ||
| Severe (≥ 9) | 24.5(3.4) | -2.81[-5.42, -0.21] | 22.6(4.7) | -4.33[-6.80, -1.86] | ||
| Psychological sub-domain | ||||||
| No, little (0–1) | 26.8(3.1) | Ref | 26.9(3.4) | Ref | ||
| Mild (2–3) | 25.4(3.5) | -1.41[-2.70, -0.12] | 0.027 | 26.8(3.6) | -0.16[-1.46, 1.15] | 0.004 |
| Moderate (4–6) | 26.1(3.5) | -0.77[-2.14, 0.61] | 24.9(4.6) | -2.00[-3.56, -0.42] | ||
| Severe (≥ 7) | 24.5(3.5) | -2.34[-6.91, 2.22] | 24.6(3.5) | -2.36[-4.14, -0.59] | ||
| Urogenital sub-domain | ||||||
| No, little (0) | 26.7(3.6) | Ref | 26.9(3.5) | Ref | ||
| Mild (1) | 26.4(3.1) | -0.24[-1.50, 1.02] | 0.120 | 27.3(3.6) | 0.34[-1.30, 1.98] | 0.064 |
| Moderate (2–3) | 26.5(2.9) | -0.12[-1.39, 1.16] | 25.7(3.1) | -1.18[-2.49, 0.13] | ||
| Severe (≥ 4) | 25.8(2.3) | -0.84[-2.37, 0.69] | 25.2(4.8) | -1.67[-3.20, -0.15] | ||
Beta [95% CI]: Univariate analysis of the association between menopausal symptoms on HRQoL. The univariate beta coefficients explain the difference in the effect of menopausal symptoms on HRQoL between different severity groups (mild, moderate and severe) when compared to the reference, with associated p-values. No evidence was detected for an interaction between menopausal symptoms and HIV infection on Health-Related Quality of Life for Total MRS or the symptom sub-domains
Associations with moderate/severe menopausal symptoms
Overall, 104 (18.8%) women reported moderate or severe menopausal symptoms. In addition to HIV described above, a number of other factors were associated with increased odds of moderate and/or severe symptoms (Table 5). By far the strongest association identified was for mood disorders (OR 8.80 [2.77, 27.3]), with other factors including history of two or more falls in the previous year (4.29 [1.18, 15.6]), early menarche (≤ vs. >12 years; 2.33 [1.22, 4.48]), alcohol consumption (2.16 [1.01, 4.62]), food insecurity (1.93 [1.14, 3.26]) and unemployment (1.56 [0.99, 2.46]). No association was seen between either BMI or comorbidities and moderate or severe menopausal symptoms (Table 5). In multivariate analysis, we identified consistent results though with attenuated associations between covariates and moderate/severe menopausal symptoms (Additional File Table 2).
Table 5.
Factors associated with moderate and/or severe menopausal symptoms in Zimbabwean women living in Harare
| Association between potential risk factors and menopausal symptoms | p-value | |||
|---|---|---|---|---|
| None / Mild (n = 274) |
Moderate / Severe (n = 104) |
Odds ratio [95% CI] | ||
| Socio-economic and household characteristics | ||||
| Highest level of education | ||||
| None/Primary | 40(14.6) | 17(16.4) | Ref | |
| Secondary | 184(67.2) | 74(71.2) | 0.94[0.50, 1.77] | 0.863 |
| Tertiary education | 50(18.3) | 13(12.5) | 0.61[0.27, 1.41] | 0.248 |
| Employment status | ||||
| Employed | 149(54.4) | 45(43.3) | Ref | |
| Unemployed | 125(45.6) | 59(56.7) | 1.56[0.99, 2.46] | 0.054 |
| Household income ($US) per month | ||||
| ≤100 | 237(86.5) | 92(88.5) | Ref | |
| 1:101–200 | 24(8.8) | 10(9.6) | 1.07[0.49, 2.33] | 0.858 |
| 2 > 200 | 13(4.7) | 2(1.9) | 0.40[0.09, 1.79] | 0.229 |
| Worry of food insecurity in the household | 177(64.6) | 81(77.9) | 1.93[1.14, 3.26] | 0.014 |
| Marital status | ||||
| Currently married | 130(47.6) | 51(49.0) | Ref | |
| Separated/Divorced | 62(22.7) | 19(18.3) | 0.76[0.41, 1.38] | 0.361 |
| Widowed | 75(27.5) | 34(32.7) | 1.10[0.66, 1.83] | 0.704 |
| Never married | 6(2.2) | 0 | - | - |
| Current alcohol uptake | 17(6.2) | 13(12.5) | 2.16[1.01, 4.62] | 0.047 |
| Obstetric and maternal factors | ||||
| Age at menarche (years) | ||||
| ≤ 12 | 27(10.1) | 21(21.1) | Ref | |
| 13–15 | 153(57.3) | 51(51.5) | 0.43[0.22, 0.82] | 0.011 |
| ≥16 | 87(32.6) | 27(27.3) | 0.40[0.20, 0.82] | 0.012 |
| Parity | ||||
| Low parity (1–3) | 169(61.7) | 60(57.7) | Ref | |
| Nulliparity | 7(2.6) | 3(2.9) | 1.21[0.30, 4.81] | 0.790 |
| Multi-parous (> 3) | 98(35.8) | 41(39.4) | 1.18[0.74, 1.88] | 0.492 |
| Types of contraceptives, n (%) | ||||
| None | 185(67.5) | 79(76.0) | Ref | |
| Oral pill | 56(20.4) | 16(15.4) | 0.67[0.36, 1.24] | 0.200 |
| Intrauterine device | 15(5.5) | 0 | - | - |
| Injectables (depo) | 18(6.6) | 9(8.7) | 1.17[0.50, 2.72] | 0.714 |
| HIV history and self-reported comorbidities | ||||
| HIV infection | 121(44.2) | 64(61.5) | 2.02[1.28, 3.21] | 0.003 |
| Total number of comorbidities, n (%) | ||||
| None | 153(55.8) | 48(46.2) | Ref | |
| 1–2 | 116(42.3) | 52(50.0) | 1.43[0.90, 2.26] | 0.129 |
| ≥3 | 5(1.8) | 4(3.9) | 2.55[0.66, 9.88] | 0.175 |
| Depression (Shona symptom questionnaire) | 4(1.5) | 12(11.5) | 8.80[2.77, 28.0] | < 0.001 |
| Falls in the past year | ||||
| None | 249(90.9) | 87(83.7) | Ref | |
| Once | 21(7.7) | 11(10.6) | 1.50[0.69, 3.24] | 0.302 |
| Twice or more | 4(1.5) | 6(5.8) | 4.29[1.18, 15.6] | 0.027 |
| Anthropometry | ||||
| BMI | ||||
| Normal | 68(24.8) | 27(26.0) | Ref | |
| Underweight | 3(1.1) | 3(2.9) | 2.63[0.50, 13.84] | 0.254 |
| Overweight | 99(36.1) | 35(33.7) | 0.89[0.50, 1.61] | 0.707 |
| Obese | 104(38.0) | 39(37.5) | 0.96[0.54, 1.69] | 0.889 |
| Low MVPA | 16(5.8) | 3(2.9) | 0.48[0.14, 1.68] | 0.250 |
Determinants of moderate and severe menopausal symptoms in ageing women. The logistic regression model is presenting unadjusted odds ratios
Nine women classified as post-menopausal reported using contraception (Additional File Table 1); 25 women reporting use of the oral contraceptive or the injectable depo reported moderate/severe menopausal symptoms, but no association between contraceptive use and menopausal symptoms was detected (Table 5). No woman reported use of menopausal hormone therapy (either vaginal or systemic oestrogens).
Discussion
This study showed that menopausal symptoms are common in urban-dwelling Zimbabwean women as they transition through menopause, and that women with HIV experience more moderate and severe menopausal symptoms, (particularly somatic and psychological symptoms) than women without HIV. The most common menopausal symptoms in women living with HIV are joint/muscle discomfort, body temperature disturbance, physical and mental exhaustion and sexual problems. Somatic (joint/muscle discomfort, sleep disturbance, heart discomfort, body temperature disturbance), and psychological (irritability, anxiety, physical and mental exhaustion, mood disorders) symptoms were particularly associated with lower HRQoL.
Our study showed that menopausal symptoms are prevalent among Zimbabwean women before comparing to other studies. There are few studies of menopausal symptoms in African women; it has even been suggested that the warm climate may mask menopausal symptoms [5]. Religious and cultural norms among African women have historically been thought to provide a platform for positive adaptation to menopausal symptoms when they do occur [32]. However, a cross-sectional study of Nigerian women aged 40–60 years showed, as we have, that menopausal symptoms are common, particularly joint/muscle pain and hot flashes (somatic), physical and mental exhaustion (psychological) and sexual problems (urogenital) [33]. In Nigeria peri- and post-menopausal women experienced the more severe symptoms, although association with HIV status was not examined. In our study, 50% of Zimbabwean women reported body temperature disturbances. A small study of 118 rural-dwelling Xhosa women age 45–61 years in South Africa, reported hot flushes in 80% of women and night sweats in 92%; however, women were biased in selection having been sampled based on attendance at the medical practice of the author [34]. Our findings highlight the importance of making effective treatments available to women experiencing menopause. While oral and transdermal menopausal hormone therapy offers effective symptom management [35], availability is limited in SSA where economically challenged healthcare services tend to prioritise essential medicines [36].
Few studies have examined menopausal symptoms among women with HIV in Africa. We found that women living with HIV were particularly prone to somatic and psychological symptoms. A cross-sectional study of 714 Nigerian women, age 40–80 years, reported women living with HIV were three times more likely to experience severe menopause symptoms; however, the numbers with HIV were few [15]. Consistent with our study, cross-sectional studies from Brazil (251 women age 40 years and older) and Spain (251 women age 45–60 years) have reported an increase in somatic symptoms and vaginal dryness among women living with HIV, though the prevalence and severity varied according to socio-cultural and clinical characteristics [37, 38]. It has been hypothesized that somatic symptoms such as body temperature disturbances, may be worsened by ART or by HIV-associated immunological dysfunction affecting estradiol metabolism/ function and further research is needed [39].
We found that almost half of women living with HIV and a third of those without HIV reported sexual problems, 35% of women with HIV described these as moderate or severe, making this a common and debilitating symptom. Of the few other small studies of menopausal symptoms in African women none specifically addresses sexual problems [40, 41]. In our experience, sexual health of older women is rarely addressed as part of HIV care and therefore problems remain unidentified. We recommend that sexual health be routinely assessed in clinical practice and appropriate advice, counselling and treatment offered to women.
The study identified lower HRQoL in women with more frequent menopausal symptoms. The association we identified between overall, somatic and psychological menopausal symptoms and poorer HRQoL, is consistent with other studies from North and South America and Europe [42–44]. For example, the US National Health and Wellness Survey of women age 40–64 years, identified associations between mood disorders, anxiety and mood changes with reduced HRQoL to a similar extent as we have reported in Zimbabwean women [44].
Our study identified three potentially modifiable factors associated with menopausal symptom severity - alcohol consumption, falls and food insecurity. However, a causal relationship cannot be inferred from these cross-sectional data. Alcohol consumption and food insecurity were associated with twice the frequency of moderate to severe menopausal symptoms, while those who reported at least two falls in the past year were found to have a four-fold increase in symptoms. A dose-response association between alcohol consumption and menopausal symptom severity has previously been reported in American women [45]. Though alcohol consumption is frequently under-reported [46], plus a cross-sectional association may be explained by reverse causality since women with menopausal symptoms may consume alcohol in an effort to manage symptoms, e.g. of anxiety. Food insecurity is associated with sleep disturbance and mood disorders in older women [47, 48], both symptoms are included in the MRS score. Whilst these factors may not be causal, they are potential clinical indictors of women in whom more severe menopausal symptoms are reported.
Our study found older age at menarche to be associated with fewer moderate and severe menopausal symptoms. Though not previously reported in an African setting, a pooled analysis of studies from the UK, US and Australia identified an association between age at menarche and somatic menopausal symptoms, particularly body temperature disturbances, which may be explained by associations with BMI [49–51]. Whereas Iranian and Korean studies have reported conflicting results with an older age at menarche associated with menopausal symptoms [52, 53]. These inconsistencies may be explained by different racial, cultural and socio-economic backgrounds of women, variation in the outcome measure, and/or inconsistent use of menarche age thresholds.
Strengths and limitations
Strengths of our study include a 100% participation rate despite a global pandemic, reflecting a desire to access health care for this otherwise neglected issue. In response to these findings, our research team conducted qualitative research to determine attitudes and understanding towards menopause, and went on to co-develop and co-design information resources on menopausal health with women in both South Africa and Zimbabwe, available in local languages [54]. Limitations include the cross-sectional study design which precludes conclusions on causal inference. We were unable to measure sex hormone levels to verify menopausal status. Although the MRS tool has been validated in Europe, America, and has been widely used in African settings, it has not been specifically validated in Zimbabwe, nor in people living with HIV. The study excluded women under 40 years of age, hence those with potential premature ovarian insufficiency were less likely to be studied.
Conclusions
This study has shown that menopausal symptoms are common in older urban-dwelling Zimbabwean women as they transition through menopause, and women living with HIV tend to experience a greater prevalence of somatic and psychological symptoms. Menopausal symptoms understandably negatively affect quality of life. Our findings highlight an unmet health need in ageing women living with HIV in Zimbabwe. As populations age, HIV services are expected to need to manage growing numbers of women approaching menopause. Our findings suggest these services should routinely enquire of, and give advice on the management of, menopausal symptoms and the potential benefits of MRT use.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional File Table 1: Different contraceptive methods by menopause status in women living with and without HIV
Additional File Table 2: Multivariate model for factors associated with moderate and/or severe menopausal symptoms in Zimbabwean women living in Harare
Acknowledgements
The authors are grateful to all research staff and study participants.
List of abbreviations
- SSA
Sub-Saharan Africa
- HIV
Human Immunodeficiency Virus
- ART
Antiretroviral Therapy
- MRS
Menopause Rating Scale
- HRQoL
Health-Related Quality of Life
- WHOQOL
World Health Organisation Quality of Life Scale
- GPAQ
Global Physical Activity Questionnaire
- MVPA
Moderate to Vigorous intensity Physical Activity
- BMI
Body Mass Index
- SD
Standard deviation
- IQR
Interquartile range
Authors’ contributions
The study was conceived by RAF, CLG. Design: RR, RAF, CLG. Data acquisition: TM, TB, RR. Analysis: TM, SH, CLG. Interpretation: TM, SH, CLG. Manuscript drafting: TM, SH, ED, TB, RR, RAF, CLG. Manuscript revision: TM, RR, SH, RAF, CLG. All authors take responsibility for their contributions outlined above and have read and approved the final manuscript.
Funding
This study is funded by University of Bristol QR Global Challenges Research Funding. RR is funded by the Wellcome Trust (206764/Z/17/Z). RAF is funded by the Wellcome Trust (206316/Z/17/Z).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
Ethical approval was granted by the Institutional Review Board of the Biomedical Research and Training Institute (Ref: AP152/2019), the Joint Research Ethics Committee for University of Zimbabwe College of Health Sciences and the Parirenyatwa Group of Hospitals, Harare Central Hospital Ethics Committee (Ref: HCHEC 181119/66), and the Medical Research Council of Zimbabwe (Ref: MRCZ/A/2551). Written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Ageing. Geneva. World Health Organization; 2017.
- 2.Victoria A, Velkoff . In: Kowal. Aging in Sub-Saharan Africa: the changing demography of the region. National Research Council (US) Committee on Population. Cohen B, Menken J, editors. Washington (DC): National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 3.Sherman S. Defining the menopausal transition. Am J Med. 2005 Dec;118(12):3–7. [DOI] [PubMed]
- 4.Bruce D, Rymer J. Symptoms of the menopause. Best Practice & Research Clinical Obstetrics & Gynaecology. 2009 Feb; 23(1):25–32. [DOI] [PubMed]
- 5.Agwu UM, Umeora OUJ, Ejikeme BN. Patterns of menopausal symptoms and adaptive ability in a rural population in south-east Nigeria. J Obstet Gynecol 2008 Jan; 28(2):217–21. [DOI] [PubMed]
- 6.Makuwa GN, Rikhotso SR, Mulaudzi FM. The perceptions of african women regarding natural menopause in Mamelodi, Tshwane district. Curationis 2015 Dec 17; 38(2):1531. [DOI] [PMC free article] [PubMed]
- 7.Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA, et al. The menopause rating cale (MRS) scale: a methodological review. Health Qual Life Outcomes. 2004 Sep;2(1):45. [DOI] [PMC free article] [PubMed]
- 8.Chedraui P, San Miguel G, Avila C. Quality of life impairment during the female menopausal transition is related to personal and partner factors. Gynecol Endocrinol. 2009 Feb;25(2):130–5. [DOI] [PubMed]
- 9.Karaçam Z, Seker SE. Factors associated with menopausal symptoms and their relationship with the quality of life among turkish women. Maturitas 2007 Sep 20; 58(1):75–82. [DOI] [PubMed]
- 10.Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, Ory M, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: study of women’s Health across the Nation. Menopause. 2009 Oct;16(5):860–9. [DOI] [PMC free article] [PubMed]
- 11.Schwarz S, Völzke H, Alte D, Schwahn C, Grabe HJ, Hoffmann W et al. Menopause and determinants of quality of life in women at midlife and beyond: the study of health in pomerania (SHIP). Menopause. 2007 Feb; 14(1):123–34. [DOI] [PubMed]
- 12.El Khoudary SR, McClure CK, VoPham T, Karvonen-Gutierrez CA, Sternfeld B, Cauley JA, et al. Longitudinal assessment of the menopausal transition, endogenous sex hormones, and perception of physical functioning: the study of women’s Health across the Nation. J Gerontol A Biol Sci Med Sci. 2014 Aug;69(8):1011–7. [DOI] [PMC free article] [PubMed]
- 13.Rwafa T, Shamu S, Christofides N. Relationship power and HIV sero-status: an analysis of their relationship among low-income urban zimbabwean postpartum women. BMC Public Health 2019 Jun 21; 19(1):792. [DOI] [PMC free article] [PubMed]
- 14.Chirwa M, Ma R, Guallar C, Tariq S. Managing menopause in women living with HIV: A survey of primary care practitioners. Post Reprod Health. 2017 Sep 1;23(3):111–5. [DOI] [PMC free article] [PubMed]
- 15.Agaba PA, Meloni ST, Sule HM, Ocheke AN, Agaba EI, Idoko JA, et al. Prevalence and predictors of severe menopause symptoms among HIV-positive and -negative nigerian women. Int J STD AIDS. 2017 Nov;28(13):1325–34. [DOI] [PubMed]
- 16.Ilankoon IMPS, Samarasinghe K, Elgán C. Menopause is a natural stage of aging: a qualitative study. BMC Womens Health. 2021 Feb;21(1):47. [DOI] [PMC free article] [PubMed]
- 17.Namazi M, Sadeghi R, Behboodi Moghadam Z. Social determinants of health in menopause: an integrative review. Int J Womens Health 2019 Dec 9; 11:637–47. [DOI] [PMC free article] [PubMed]
- 18.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013 Apr;35(2):121–6. [DOI] [PMC free article] [PubMed]
- 19.Okhai H, Sabin C, Haag K, Sherr L, Dhairyawan R, Shephard J, Richard G, Burns F, Post F, Jones R, Gilleece Y, Tariq S. The prevalence and patterns of menopausal symptoms in women living with HIV. AIDS Behav. 2022 Nov;26(11):3679–368. [DOI] [PMC free article] [PubMed]
- 20.Obalowu IA, Odeigah LO, Alabi KM, Ayinmode BA, Alabi NA, Muhammed A, et al. Pattern and severity of menopausal symptoms experienced by middle-aged nigerian women attending the family medicine clinic of the university of Ilorin Teaching Hospital, north-central Nigeria. Nigerian J Family Pract. 2021;12(3):32–9. [Google Scholar]
- 21.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999 Aug;89(8):1231–4. [DOI] [PMC free article] [PubMed]
- 22.Patel V, Simunyu E, Gwanzura F, Lewis G, Mann A. The shona symptom questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psychiatr Scand. 1997 Jun;95(6):469–75. [DOI] [PubMed]
- 23.Schmidt S, Mühlan H, Power M. The EUROHIS-QOL 8-item index: psychometric results of a cross-cultural field study. Eur J Public Health 2006 Aug 1; 16(4):420–8. [DOI] [PubMed]
- 24.World Health Organization. Division of Mental Health and Prevention of Substance abuse. WHOQOL: measuring quality of life. World Health Organization 1997.
- 25.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Activity Health. 2009 Nov;6(6):790–804. [DOI] [PubMed]
- 26.Weir CB, Jan A. BMI classification percentile and cut off points. PMID: In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2020. p. 31082114. [PubMed] [Google Scholar]
- 27.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–75. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 28.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012 Apr;19(4):387–95. [DOI] [PMC free article] [PubMed]
- 29.Mishra GD, Brown WJ, Dobson AJ. Physical and mental health: changes during menopause transition. Qual Life Res. 2003 Jun;12(4):405–12. [DOI] [PubMed]
- 30.Kumari M, Stafford M, Marmot M. The menopausal transition was associated in a prospective study with decreased health functioning in women who report menopausal symptoms. J Clin Epidemiol. 2005 Jul;58(7):719–27. [DOI] [PubMed]
- 31.Semá Baltazar C, Raposo C, Jani IV, Shodell D, Correia D, Gonçalves da Silva C, et al. Evaluation of performance and acceptability of two rapid oral fluid tests for HIV detection in Mozambique. J Clin Microbiol. 2014 Oct;52(10):3544–8. [DOI] [PMC free article] [PubMed]
- 32.Mashiloane CD, Bagratee J, Moodley J. Awareness of and attitudes toward menopause and hormone replacement therapy in an african community. Int J Gynecol Obstet. 2002 Jan;76(1):91–3. [DOI] [PubMed]
- 33.OlaOlorun FM, Lawoyin TO. Experience of menopausal symptoms by women in an urban community in Ibadan. Nigeria Menopause. 2009 Jul;16(4):822–30. [DOI] [PubMed]
- 34.Friderichs T, Hall D. Postmenopausal symptoms in a group of rural Xhosa women. South Afr Family Pract. 2005 Jun;47(5):56–8.
- 35.Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U et al. Menopausal hormone therapy and women’s health: An umbrella review. PLOS Medicine. 2021 Aug 2;18(8):e1003731. [DOI] [PMC free article] [PubMed]
- 36.World Health Organization model list of essential medicines – 22nd list., 2021. Geneva: World Health Organization; 2021 (WHO/MHP/HPS/EML/2021.02). Licence: CC BY-NC-SA 3.0 IGO.
- 37.Ferreira CE, Pinto-Neto AM, Conde DM, Costa-Paiva L, Morais SS, Magalhães J. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007 Jan;23(4):198–205. [DOI] [PubMed]
- 38.CoRIS Cohort, Suarez-García I, Alejos B, Pérez-Elías MJ, Iribarren JA, Hernando A, et al. How do women living with HIV experience menopause? Menopausal symptoms, anxiety and depression according to reproductive age in a multicenter cohort. BMC Women’s Health. 2021 Dec;21(1):223. [DOI] [PMC free article] [PubMed]
- 39.Looby SE, Shifren J, Corless I, Rope A, Pedersen MC, Joffe H, et al. Increased hot flash severity and related interference in perimenopausal human immunodeficiency virus–infected women. Menopause. 2014 Apr;21(4):403–9. [DOI] [PMC free article] [PubMed]
- 40.Dienye PO, Judah F, Ndukwu G. Frequency of symptoms and health seeking behaviours of menopausal women in an out-patient clinic in Port Harcourt, Nigeria. GJHS. 2013 Mar 18; 5(4):p39. [DOI] [PMC free article] [PubMed]
- 41.Ande A, Ande O, Omu O, Olagbuji N. Features and perceptions of menopausal women in Benin City, Nigeria. Ann Afr Med. 2011;10(4):300. doi: 10.4103/1596-3519.87048. [DOI] [PubMed] [Google Scholar]
- 42.Conde DM, Pinto-Neto AM, Santos-Sá D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006 Jan;22(8):441–6. [DOI] [PubMed]
- 43.Ayers B, Hunter MS. Health-related quality of life of women with menopausal hot flushes and night sweats. Climacteric. 2013 Apr;16(2):235–9. [DOI] [PubMed]
- 44.Whiteley J, DiBonaventura M, daCosta, Wagner JS, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Women’s Health. 2013 Nov;22(11):983–90. [DOI] [PMC free article] [PubMed]
- 45.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for african american and caucasian women. Volume 10. Journal of Women’s Health & Gender-Based Medicine; 2001 Jan. pp. 67–76. 1. [DOI] [PubMed]
- 46.Taneri PE, Kiefte-de Jong JC, Bramer WM, Daan NMP, Franco OH, Muka T. Association of alcohol consumption with the onset of natural menopause: a systematic review and meta-analysis. Hum Reprod Update 2016 Jun 1; 22(4):516–28. [DOI] [PubMed]
- 47.Ding M, Keiley MK, Garza KB, Duffy PA, Zizza CA. Food insecurity is associated with poor sleep outcomes among US adults. J Nutr. 2015 Mar;1(3):615–21. [DOI] [PubMed]
- 48.Lee SJ, Lee KW, Cho MS. Association of food insecurity with nutrient intake and depression among korean and US adults: data from the 2014 Korea and the 2013–2014 US National Health and Nutrition examination surveys. IJERPH. 2021 Jan;9(2):506. [DOI] [PMC free article] [PubMed]
- 49.Mishra GD, Chung HF, Pandeya N, Dobson AJ, Jones L, Avis NE, et al. The Interlace study: design, data harmonization and characteristics across 20 studies on women’s health. Maturitas. 2016 Oct;92:176–85. [DOI] [PMC free article] [PubMed]
- 50.Mishra GD, Anderson D, Schoenaker DAJM, Adami HO, Avis NE, Brown D, et al. InterLACE: a new international collaboration for a life course approach to women’s reproductive health and chronic disease events. Maturitas. 2013 Mar;74(3):235–40. [DOI] [PubMed]
- 51.Chung H, Zhu D, Dobson A, Kuh D, Gold E, Crawford S, et al. Age at menarche and risk of vasomotor menopausal symptoms: a pooled analysis of six studies. BJOG: Int J Obstet Gy. 2021 Feb;128(3):603–13. [DOI] [PMC free article] [PubMed]
- 52.Delavar MA, Hajiahmadi M. Factors affecting the age in normal menopause and frequency of menopausal symptoms in Northern Iran. Iran Red Crescent Med J. 2011 Mar;13(3):192–8. [PMC free article] [PubMed]
- 53.Jung SJ, Shin A, Kang D. Menarche age, menopause age and other reproductive factors in association with post-menopausal onset depression: results from Health Examinees Study (HEXA). J Affect Disord. 2015 Nov;187:127–35. [DOI] [PubMed]
- 54.Drew S, Khutsoane K, Buwu N, Gregson CL, Micklesfield LK, Ferrand RA, et al. Improving experiences of the menopause for women in Zimbabwe and South Africa: co-producing an information resource. Social Sci. 2022 Apr;11(4):143.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File Table 1: Different contraceptive methods by menopause status in women living with and without HIV
Additional File Table 2: Multivariate model for factors associated with moderate and/or severe menopausal symptoms in Zimbabwean women living in Harare
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.