Abstract
Objective:
To determine risk factors and outcomes of NEC-associated sepsis in infants with necrotizing enterocolitis (NEC).
Methods:
A retrospective review comparing demographic and clinical information in infants with and without NEC-associated sepsis (defined as positive blood culture at the time of NEC onset.
Results:
209 infants with medical (n=98) and surgical NEC (n=111) had a median gestational age of 27 weeks (IQR 25; 30.5) and a median birth weight of 910g [IQR 655; 1138]. Fifty of 209 (23.9 %) infants had NEC-associated sepsis. Infants with NEC-associated sepsis had lower median GA (26.4 vs. 27.4 wks.; p=0.01), lower birth weight (745g vs. 930g; p=0.009), were more likely mechanically ventilated [p <0.001], received dopamine [p<0.001], had more evidence of acute kidney injury [60% vs. 38.4%, p=0.01], longer postoperative ileus (16d [13.0;22.0] vs. 12d [8;16]; p=0.006), higher levels of C-reactive protein, lower platelet counts, longer hospitalization compared to infants without NEC-associated sepsis. On multivariate regression, cholestasis was an independent risk factor for NEC-associated sepsis (OR 2.94; 95% CI 1.1–8.8, p=0.038).
Conclusion:
NEC-associated sepsis was associated with greater hemodynamic support, acute kidney injury, longer postoperative ileus, and hospitalization on bivariate analysis, and cholestasis was associated with higher odds of sepsis on multi regression analysis.
Introduction
Necrotizing enterocolitis (NEC) is the most common acute gastrointestinal illness during the neonatal period, affecting about 5–10% of preterm infants with a birth weight of less than 1500 grams 1,2. NEC, especially surgical type, remains a leading cause of morbidity and mortality among premature neonates and leads to increased cost of care and resource utilization 3–9.
The etiology of NEC is still unknown and is likely multifactorial. Many studies report the role of bacteria, viruses, and fungi in the pathogenesis of NEC and late-onset sepsis 10,11. A recent study reported higher mortality in infants with bloodstream infections within three days following NEC diagnosis 12. However, other important outcomes such as postoperative ileus, time to reach full feeds, brain injury on MRI, and surgical and clinical characteristics including length of bowel resected, type of stoma, and the levels of inflammatory biomarkers such as C - reactive protein (CRP) have not been extensively studied in association with bloodstream infections, and thus require further research 13,14.
This study sought to determine the demographics and clinical variables associated with bloodstream infections and the outcomes as mentioned above in neonates with NEC. Our primary hypothesis was to determine whether NEC-associated sepsis is associated with a longer length of hospital stay and higher mortality in infants with both medical and surgical NEC. Additionally, we sought to determine the clinical impact of NEC-associated sepsis on the postoperative outcomes in infants with surgical NEC. Another objective was to uncover the clinical and demographic risk factors linked with NEC-associated sepsis in preterm infants. We compared demographic and clinical information in NICU patients with and without NEC-associated sepsis to investigate these objectives.
Methods:
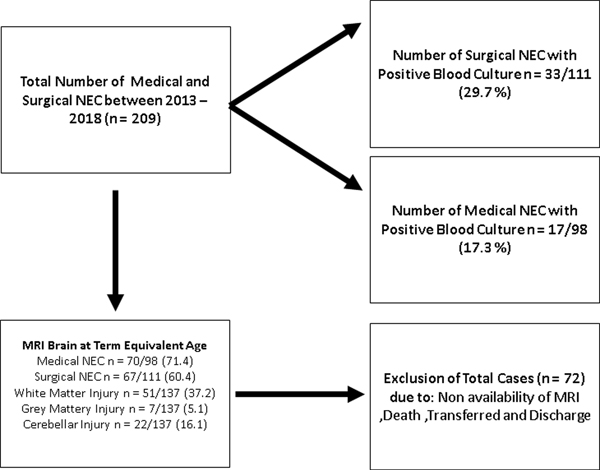
This single-center, retrospective observational cohort study was conducted at the University of Mississippi Medical Center (UMMC) at Jackson, Mississippi, after the Institutional Review Board (2017–0127) approval. UMMC’s level 4 neonatal intensive care unit (NICU) is the only referral center for neonates with surgical NEC in Mississippi. From a detailed review of the electronic medical records, we identified 209 patients with medical and surgical NEC (Bell stage II and above) who underwent management of NEC in the period between January 2013 and June 2018 (Figure 1). We excluded infants with incomplete clinical data or confounding medical conditions such as congenital heart disease, intestinal atresia, or spontaneous intestinal perforation (SIP).
Figure 1:
Flow diagram showing the patient included in the study.
Clinical information:
We recorded demographic characteristics, including birth weight, gestational age, sex, race/ethnicity (African-American, Caucasian, or Latino), mode of delivery (cesarean section/vaginal delivery), and inborn versus outborn status. We collected information regarding maternal factors, including pregnancy-induced hypertension, chorioamnionitis, and antenatal steroids.
NEC data:
The diagnosis of NEC was primarily made by abdominal X-ray by board-certified pediatric radiologists based upon established radiological NEC findings such as pneumatosis intestinalis, pneumoperitoneum, and portal venous gas. Infants who did not show obvious pneumatosis on radiology were classified as ≥ stage 2 NEC only if they displayed highly suggestive clinical (intestinal) signs with loss of bowel sounds, abdominal wall erythema, and abdominal distension; systemic instability with increased needs for respiratory and hemodynamic support; and radiological signs such as intestinal dilatation, fixed bowel loops and/or portal venous gas. The age of NEC onset in days, presence of pneumatosis, and clinical presentation (abdominal distension, feeding intolerance, and bloody stools) were also recorded. The practice of surgical management of NEC did not change substantially throughout the study period. . At our center, preterm infants with pneumoperitoneum who weigh less than 1 kg at the time of NEC diagnosis and are hemodynamically unstable are treated first with a Penrose drain at the bedside but may later receive laparotomy.
Bloodstream infection and outcome data:
Our institution’s routine management of neonates with NEC involved obtaining one peripheral blood culture and initiating broad-spectrum antimicrobial therapy with vancomycin, amikacin, +/− metronidazole. Infants with medical NEC were treated with 7–10 days of antibiotics. Infants with surgical NEC were treated with 10–21 days of antibiotics. We collected information about blood culture drawn at NEC onset (defined as NEC-associated sepsis). Our hospital policy is to collect a minimum of 1 ml of blood for blood culture. We collected data on C-reactive protein (CRP) values (mg/dl) and antibiotic therapy up to two weeks after NEC onset. We recorded complete blood cell count results at NEC onset, 24 hours, and 48 hours following NEC onset. All blood cultures were assessed using a fluorescent detection system for the presence of CO2 production (Bactec II or 9240; Becton Dickinson, Sandy, Utah), and species were identified using standard microbiology techniques. We collected information on clinical variables such as patent ductus arteriosus (PDA), PDA treatment (medical or surgical), use of inotropes at 24 hours, assisted ventilation, and cholestasis (direct bilirubin >2 mg/dL) at the time of NEC onset and every week up to two months after NEC onset. We also collected data on platelet transfusion before and after the NEC onset. In addition, we collected postoperative information on the duration of ileus (defined as a number of days the infant was without oral feeds), days on parenteral nutrition, the time to reach full enteral feedings (≥120 mL/kg/day), and development of short bowel syndrome (SBS; the need for parenteral nutrition ≥ 90 days following intestinal resection15).
We examined serum creatinine measurements and daily urine output the day before NEC diagnosis, at NEC onset, and 24, 48, 72, 96 hours after NEC diagnosis. The incidence of acute kidney injury (AKI) after NEC onset was determined using the modified neonatal staging criteria previously described in the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI 16–20. We also gathered information on the length of stay and mortality. The length of stay was defined as the total duration of hospitalization from the day of admission until discharge or death. Mortality was defined as death due to any cause before hospital discharge.
Neonatal MRI data:
At our institution, MRIs are obtained routinely at 36 weeks corrected gestational age or before discharge whenever clinically feasible in all infants with birth weight less than 1500 grams. MRI data can be missing in death cases or transferred to another hospital. All MRI scans without contrast were scored independently by two pediatric neuro-radiologists unaware of the infants’ clinical course. We used a standardized scoring system developed by Woodward et al. consisting of eight different scales for white and gray matter injury 21. White-matter abnormality was graded according to five scales, which assessed the nature and extent of white-matter signal abnormality, the loss in the volume of periventricular white matter, and the extent of any cystic abnormalities, ventricular dilatation, or the thinning of the corpus callosum. Gray-matter abnormalities were graded according to three scales, which assessed the extent of gray matter signal abnormality, gyral maturation quality, and subarachnoid space size. Composite white matter scores and composite gray matter scores were created and used to categorize infants according to the extent of their cerebral abnormalities 21. The categories of white matter abnormality were none (a score of 5 to 6), mild (a score of 7 to 9), moderate (a score of 10 to 12), or severe (a score of 13 to 15). Gray matter was categorized as normal (a score of 3 to 5) or abnormal (a score of 6 to 9). We also assessed cerebellar lesions on MRI.
Statistical Methods:
In this study, we first compared demographic and clinical factors in infants with medical and surgical NEC (n=209), then subdivided by NEC-associated sepsis status and created a sub-cohort of infants with surgical NEC (n=111). For normally distributed continuous variables, we summarized the data as mean and standard deviation (S.D.), and comparisons between groups were performed using Student’s t-test and ANOVA. For continuous data exhibiting skewed distribution, differences in the data were tested using the Mann-Whitney U test or Kruskal-Wallis test. Categorical data were summarized as counts with relative frequencies as percentages. Differences in the groups were analyzed using the Chi-squared test (χ2 test) or Fisher’s exact test (when cell count less than 5). Finally, bloodstream infection risk factors for surgical NEC were analyzed with multivariate logistic regression. A p-value < 0.05 was considered statistically significant for all the analyses. All the statistical analyses were performed in R statistical software (version 3.6.3; The R Foundation for Statistical Computing).
Results:
Bloodstream infections in infants with medical and surgical NEC
Cohort Characteristics:
The study included two hundred and nine infants diagnosed with medical (n=98) and surgical NEC (n=111). Of these, 50 infants (24%) had NEC-associated sepsis. The median gestational age was 27 weeks, with a median birth weight of 910g (Table 1). The median age of NEC onset was 11.0 days. The median length of hospital stay was 95 days, and forty-eight neonates (23 %) died before discharge. Detailed demographic, inflammatory, hematological, and clinical information is listed in Table 1 and Supplemental Table 1.
Table 1:
Demographic and Clinical Data in Infants with Medical and Surgical NEC with and without NEC associated Sepsis
| Total Cohort | NEC associated Sepsis | NEC without associated sepsis | p value | |
|---|---|---|---|---|
| N=209 | N=50 | N=159 | ||
| Prenatal Information | ||||
| Pregnancy-Induced Hypertension, n (%) | 60 (28.8%) | 13 (26.0%) | 47 (29.7%) | 0.74 |
| Chorioamnionitis, n (%) | 18 (8.65%) | 4 (8.00%) | 14 (8.86%) | 1 |
| Antenatal Steroids, n (%) | 126 (63.0%) | 31 (62.0%) | 95 (63.3%) | 1 |
| Infant Demographics | ||||
| Gestational Age (weeks), median (IQR) | 27.0 [25.0;30.5] | 26.4 [24.3;28.1] | 27.4 [25.0;31.5] | 0.011 |
| Birth Weight (grams), median (IQR) | 910 [680;1400] | 745 [652;1019] | 930 [690;1618] | 0.009 |
| Male Gender, n (%) | 127 (60.8%) | 32 (64.0%) | 95 (59.7%) | 0.71 |
| Ethnicity, n (%) | 0.047 | |||
| Caucasian | 44 (21.2%) | 9 (18.0%) | 35 (22.2%) | |
| Latino | 5 (2.40%) | 1 (2.00%) | 4 (2.53%) | |
| African American | 154 (74.0%) | 36 (72.0%) | 118 (74.7%) | |
| Other | 5 (2.40%) | 4 (8.00%) | 1 (0.63%) | |
| Mode of Delivery, n (%) | 1 | |||
| C-section | 149 (71.3%) | 36 (72.0%) | 113 (71.1%) | |
| Vaginal | 60 (28.7%) | 14 (28.0%) | 46 (28.9%) | |
| Outborn, n (%) | 122 (58.7%) | 31 (63.3%) | 91 (57.2%) | 0.55 |
| Infant Medical Information Prior to NEC | ||||
| Patent Ductus Arteriosus, n (%) | 104 (50.0%) | 31 (62.0%) | 73 (46.2%) | 0.07 |
| Patent Ductus Arteriosus, Indomethacin Treated, n (%) | 26 (12.8%) | 8 (16.7%) | 18 (11.6%) | 0.50 |
| Patent Ductus Arteriosus, Surgically Ligated, n (%) | 9 (4.35%) | 2 (4.00%) | 7 (4.46%) | 1 |
| NEC Disease Features | ||||
| Clinical Presentation, n (%) | 0.001 | |||
| Abdominal Distension | 156 (74.6%) | 47 (94.0%) | 109 (68.6%) | |
| Bloody Stools | 41 (19.6%) | 2 (4.00%) | 39 (24.5%) | |
| Feeding Intolerance | 12 (5.74%) | 1 (2.00%) | 11 (6.92%) | |
| Radiological Findings, n (%) | ||||
| Pneumatosis | 87 (42.0%) | 18 (36.0%) | 69 (43.9%) | 0.40 |
| Pneumoperitoneum | 62 (30.0%) | 17 (34.0%) | 45 (28.7%) | 0.58 |
| Portal Venous Gas | 9 (4.35%) | 3 (6.00%) | 6 (3.82%) | 0.45 |
| Age of NEC Onset (days), median (IQR) | 15.0 [8.00;29.0] | 13.5 [8.00;25.0] | 16.0 [7.00;31.0] | 0.69 |
| Length of Bowel Resected (cm), median (IQR) | 7.25 [0.00;23.0] | 10.0 [0.00;25.3] | 4.65 [0.00;20.9] | 0.28 |
| Region of Bowel Resected, n (%) | 1 | |||
| Small Bowel Resected | 63 (63.0%) | 18 (62.1%) | 45 (63.4%) | |
| Combined Large and Small Bowel Resected | 37 (37.0%) | 11 (37.9%) | 26 (36.6%) | |
| Short Bowel Syndrome, n (%) | 50 (45.5%) | 20 (58.8%) | 30 (39.5%) | 0.09 |
| Post-Operative Intestinal Features | ||||
| Post-Operative Ileus Days (days), median (IQR) | 13.0 [9.00;18.0] | 16.0 [13.0;22.0] | 12.0 [8.00;16.0] | 0.006 |
| Day of Attainment of Full Enteral Feedings (120 mL/kg), median (IQR) | 61.0 [30.0;87.0] | 71.0 [40.0;108] | 54.0 [28.5;76.8] | 0.05 |
| Duration of Parenteral Nutrition (days), median (IQR) | 68.5 [31.0;108] | 82.0 [31.0;130] | 65.0 [31.0;98.0] | 0.11 |
| Post-Operative Systemic Course | ||||
| Assisted Ventilation (intubated), n (%) | 133 (65.8%) | 42 (84.0%) | 91 (59.9%) | 0.001 |
| 24h Pressor Support, n (%) | 109 (53.4%) | 37 (77.1%) | 72 (46.2%) | <0.001 |
| AKI by Serum Creatinine Present, n (%) | 68 (34.9%) | 20 (43.5%) | 48 (32.2%) | 0.22 |
| AKI by Urine Output Present, n (%) | 49 (25.1%) | 16 (34.8%) | 33 (22.1%) | 0.12 |
| AKI by Urine Output and Serum Creatinine Present, n (%) | 91 (43.5%) | 30 (60.0%) | 61 (38.4%) | 0.01 |
| Platelet Transfusion before NEC onset, n (%) | 106 (52.7%) | 35 (71.4%) | 71 (46.7%) | 0.004 |
| Sepsis Variables | ||||
| Central Line Present (days), median (IQR) | 169 (83.3%) | 42 (84.0%) | 127 (83.0%) | 1 |
| Duration of Antibiotics, median (IQR) | 7.00 [5.00;10.0] | 9.00 [5.00;14.0] | 7.00 [5.00;10.0] | 0.08 |
| CRP on Day of NEC Onset, median (IQR) | 2.45 [0.72;6.27] | 3.10 [1.00;8.30] | 2.20 [0.70;6.00] | 0.05 |
| CRP at 24 Hours after NEC Onset, median (IQR) | 4.80 [1.45;14.8] | 6.70 [1.80;17.7] | 3.85 [1.20;13.6] | 0.09 |
| CRP at 48 Hours after NEC Onset, median (IQR) | 4.60 [1.35;17.9] | 11.8 [2.32;22.6] | 3.70 [1.20;15.7] | 0.029 |
| CRP at 2 Weeks after NEC Onset, median (IQR) | 2.40 [0.90;5.10] | 4.20 [1.90;6.30] | 1.85 [0.88;4.50] | 0.025 |
| Cholestasis at NEC Onset, n (%) | 89 (44.3%) | 29 (59.2%) | 60 (39.5%) | 0.024 |
| Hematological Factors | ||||
| Platelets after NEC, median (IQR) | 170 [97.5;254] | 139 [79.5;209] | 177 [108;271] | 0.012 |
| Platelets 1 Day after NEC, median (IQR) | 140 [89.0;231] | 106 [72.2;144] | 158 [96.2;246] | 0.001 |
| Discharge | ||||
| Length of Stay (days), median (IQR) | 95.0 [46.8;150] | 122 [78.8;169] | 89.5 [43.2;134] | 0.05 |
| Death, n (%) | 48 (23.0%) | 14 (28.0%) | 34 (21.4%) | 0.43 |
Abbreviations: NEC = necrotizing enterocolitis; AKI = acute kidney injury; CRP = c-reactive protein (mg/L); WBC = white blood cell; ANC = absolute neutrophil count
Continuous variables are presented as mean (standard deviation) if normality has been met and, if not normally distributed, as median with interquartile range. If normality criteria were satisfied, differences in continuous measures’ statistical associations were tested using a t-test. When the normality assumption was not satisfied, continuous measures’ statistical associations were tested with the Kruskal-Wallis Test.
Categorical variables are presented as count (column percentage). Differences in categorical measures’ associations were tested using the Chi- square test when cell counts were adequate, otherwise Fisher’s Exact was used with low expected cell counts. The presence of bold values signifies p < 0.05.
Clinical outcomes:
Preterm infants with NEC-associated sepsis had a lower median gestational age, lower birth weight than NEC infants without sepsis (Table 1). Infants with NEC-associated sepsis presented more frequently with abdominal distension than NEC infants without sepsis (Table 1). Assisted ventilation, dopamine use at 24 hours after NEC onset, acute kidney injury by urine output and serum creatinine criteria, and platelet transfusions before NEC diagnosis were more common among neonates with positive blood culture at NEC onset. Infants with NEC-associated sepsis had significantly higher CRP levels and lower platelet counts on the day of NEC onset and 24 hours after the onset compared to NEC infants without sepsis (Table 1 and Supplemental Table 1). Compared to NEC infants without sepsis, infants with NEC-associated sepsis received more blood transfusion before NEC onset (35/37 (94.5%) vs. 102/137(77.9%); p=0.038).
Bloodstream infection profile:
At NEC onset, the blood culture was positive in about one quarter (n=50/209, 23.9%) of infants with NEC (Table 2). Blood culture at NEC onset was positive for gram-positive bacteria in 32/209 (15.3%) infants and gram-negative bacteria in 22/209 (10.5%) cases. Fungal growth was seen in six (2.8%) patients. Compared to infants with medical NEC, infants with surgical NEC had higher rates of blood culture positivity (66% vs. 34%). In our cohort with surgical NEC, Klebsiella pneumoniae (n=6/33, 18%) and E. coli (n=5/33, 15 %) were the most common gram-negative organisms and Staphylococcus epidermidis (n=9/33, 27%) and Enterococcus faecalis (n=6/33, 18%) were the most common gram-positive bacteria. Candida albicans (n=3, 9 %) was the most common fungal infection.
Table 2:
Microbiology data in neonates with necrotizing enterocolitis
| Total Cohort | Medical NEC | Surgical NEC | Short Bowel Present | Short Bowel Absent | |
|---|---|---|---|---|---|
| n = 50 | n = 17 | n = 33 | n = 20 | n = 13 | |
| Monomicrobial, n (%) | 41 (82) | 17 (100.0) | 24 (72) | 13 (65) | 11 (84.6) |
| * Polymicrobial, n (%) | 9 (18) | 0 | 9 (28) | 7 (35) | 2 (15.3) |
| Gram Negative, n (%) | 22 (44) | 7 (41.2) | 15 (45) | 12 (60) | 4 (30.6) |
| Klebsiella pneumoniae, n (%) | 8 (16) | 2 (11.8) | 6 (18) | 4 (20) | 2 (15.3) |
| Escherichia coli, n (%) | 6 (12) | 1 (5.9) | 5 (15) | 3 (15) | 2 (15.3) |
| Serratia marcescens, n (%) | 4 (8) | 2 (11.8) | 2 (6) | 2 (10) | 0 |
| Enterobacter cloacae, n (%) | 3 (6) | 1 (5.9) | 2 (6) | 2 (10) | 0 |
| Acinetobacter baumannii, n (%) | 1 (2) | 0 | 1 (3) | 1 (5) | 0 |
| Klebsiella oxytoca, n (%) | 1 (2) | 1 (5.9) | 0 | 0 | 0 |
| Gram Positive, n (%) | 32 (66) | 9 (52.9) | 24 (72) | 14 (70) | 10 (77) |
| Staphylococcus epidermidis, n (%) | 11 (22) | 2 (11.8) | 9 (27) | 4 (20) | 5 (38.4) |
| Enterococcus faecalis, n (%) | 7 (14) | 1 (5.9) | 6 (18) | 5 (25) | 1 (7.6) |
| Staphylococcus capitis, n (%) | 5 (10) | 2 (11.8) | 3 (9) | 2 (10) | 1 (7.6) |
| Staphylococcus aureus, n (%) | 3 (6) | 1 (5.9) | 2 (6) | 0 | 2 (15.3) |
| Streptococci viridans, n (%) | 1 (2) | 0 | 1 (3) | 0 | 1 (7.6) |
| Enterococcus faecium, n (%) | 1 (2) | 0 | 1 (3) | 1 (5) | 0 |
| Staphylococcus hominis, n (%) | 1 (2) | 0 | 1 (3) | 1 (5) | 0 |
| Streptococcus mitis/oralis, n (%) | 1 (2) | 0 | 1 (3) | 1 (5) | 0 |
| Streptococcus agalactiae, n (%) | 2 (2) | 2 (11.8) | 0 | 0 | 0 |
| Fungal, n (%) | 6 (12) | 1 (5.9) | 5 (15) | 3 (15) | 2 (15.3) |
| Candida albicans, n (%) | 3 (12) | 0 | 3 (9) | 2 (10) | 1 (7.6) |
| Candida parapsilosis, n (%) | 3 (12) | 1 (5.9) | 2 (6) | 1 (5) | 1 (7.6) |
Abbreviations: NEC = necrotizing enterocolitis
The data presented as n (%).
In the surgical cohort, 9 neonates with NEC-associated positive blood cultures had polymicrobial infections. Organisms involved in the polymicrobial cultures were as follows: 1. Candida parapsilosis + Staphylococcus epidermidis; 2. Enterococcus faecalis + Staphylococcus capitis; 3. Escherichia coli + Candida albicans + Enterococcus faecalis; 4. Streptococci viridans + Staphylococcus capitis; 5. Enterococcus faecalis + Enterococcus faecium; 6. Enterococcus faecalis + Staphylococcus hominis; 7. Klebsiella pneumoniae + Enterococcus faecalis; 8. Klebsiella pneumoniae + Enterobacter cloacae; 9. Enterococcus faecalis + Staphylococcus epidermidis.
Gram-positive Sepsis:
Infants with NEC-associated sepsis with gram-positive bacteria had a longer duration of postoperative ileus (15.5 [13.0;19.2] vs 12.0 [7.25;15.8]; p=0.017), increased need for assisted ventilation (86.2% vs 58.7%; p=0.006), increased incidence of AKI based on urine output plus serum creatinine (65.5% vs 36.9%; p=0.008), an increased median CRP level 48 hours after NEC onset (11.1 [2.38;23.2] vs 3.70 [1.12;15.6]; p=0.038), and increased length of hospitalization (127 [82.0;171.0] vs 87.5 [41.5;133.0]; p=0.013) when compared to infants without NEC-associated sepsis (See Table 3).
Table 3:
Outcomes in Neonates with Gram positive and Gram negative sepsis compared with neonates with negative blood culture at NEC onset
| Total Cohort | Gram Positive sepsis | Negative Blood Culture | p value | Total Cohort | Gram Negative Sepsis | Negative blood Culture | p value | |
|---|---|---|---|---|---|---|---|---|
| N=186 | N=29 | N=157 | N=174 | N=17 | N=157 | |||
| Prenatal Information | ||||||||
| Pregnancy-Induced Hypertension, n (%) | 52 (28.1%) | 5 (17.2%) | 47 (30.1%) | 0.233 | 54 (31.2%) | 7 (41.2%) | 47 (30.1%) | 0.511 |
| Chorioamnionitis, n (%) | 16 (8.65%) | 2 (6.90%) | 14 (8.97%) | 1 | 16 (9.25%) | 2 (11.8%) | 14 (8.97%) | 0.66 |
| Antenatal Steroids, n (%) | 113 (63.8%) | 19 (67.9%) | 94 (63.1%) | 0.789 | 105 (63.3%) | 11 (64.7%) | 94 (63.1%) | 1 |
| Infant Demographics | ||||||||
| Gestational Age (weeks), median (IQR) | 27.1 [25.0;31.1] | 26.4 [24.3;27.5] | 27.6 [25.0;31.6] | 0.018 | 27.5 [25.0;31.3] | 27.5 [24.4;29.6] | 27.6 [25.0;31.6] | 0.249 |
| Birth Weight (grams), median (IQR) | 915 [680;1500] | 760 [630;1000] | 940 [690;1620] | 0.032 | 930 [686;1585] | 790 [670;1080] | 940 [690;1620] | 0.246 |
| Male Gender, n (%) | 112 (60.2%) | 18 (62.1%) | 94 (59.9%) | 0.988 | 106 (60.9%) | 12 (70.6%) | 94 (59.9%) | 0.55 |
| Ethnicity, n (%) | 0.742 | 0.025 | ||||||
| Caucasian | 40 (21.6%) | 5 (17.2%) | 35 (22.4%) | 36 (20.8%) | 1 (5.88%) | 35 (22.4%) | ||
| Latino | 5 (2.70%) | 1 (3.45%) | 4 (2.56%) | 4 (2.31%) | 0 (0.00%) | 4 (2.56%) | ||
| African American | 139 (75.1%) | 23 (79.3%) | 116 (74.4%) | 130 (75.1%) | 14 (82.4%) | 116 (74.4%) | ||
| Other | 1 (0.54%) | 0 (0.00%) | 1 (0.64%) | 3 (1.73%) | 2 (11.8%) | 1 (0.64%) | ||
| Mode of Delivery, n (%) | 0.735 | 0.159 | ||||||
| C-section | 130 (69.9%) | 19 (65.5%) | 111 (70.7%) | 126 (72.4%) | 15 (88.2%) | 111 (70.7%) | ||
| Vaginal | 56 (30.1%) | 10 (34.5%) | 46 (29.3%) | 48 (27.6%) | 2 (11.8%) | 46 (29.3%) | ||
| Outborn, n (%) | 109 (58.9%) | 20 (71.4%) | 89 (56.7%) | 0.211 | 98 (56.3%) | 9 (52.9%) | 89 (56.7%) | 0.969 |
| Infant Medical Information Prior to NEC | ||||||||
| PDA, n (%) | 90 (48.6%) | 18 (62.1%) | 72 (46.2%) | 0.17 | 83 (48.0%) | 11 (64.7%) | 72 (46.2%) | 0.231 |
| PDA, Indomethacin Treated, n (%) | 20 (11.0%) | 2 (7.14%) | 18 (11.8%) | 0.744 | 22 (13.0%) | 4 (25.0%) | 18 (11.8%) | 0.231 |
| NEC Disease Features | ||||||||
| Clinical Presentation, n (%) | 0.051 | 0.016 | ||||||
| Abdominal Distension | 133 (71.5%) | 26 (89.7%) | 107 (68.2%) | 124 (71.3%) | 17 (100%) | 107 (68.2%) | ||
| Bloody Stools | 41 (22.0%) | 2 (6.90%) | 39 (24.8%) | 39 (22.4%) | 0 (0.00%) | 39 (24.8%) | ||
| Feeding Intolerance | 12 (6.45%) | 1 (3.45%) | 11 (7.01%) | 11 (6.32%) | 0 (0.00%) | 11 (7.01%) | ||
| Radiological Findings, n (%) | ||||||||
| Pneumatosis | 78 (42.6%) | 11 (37.9%) | 67 (43.5%) | 0.725 | 75 (43.9%) | 8 (47.1%) | 67 (43.5%) | 0.982 |
| Pneumoperitoneum | 52 (28.4%) | 9 (31.0%) | 43 (27.9%) | 0.907 | 50 (29.2%) | 7 (41.2%) | 43 (27.9%) | 0.269 |
| Portal Venous Gas | 6 (3.28%) | 0 (0.00%) | 6 (3.90%) | 0.592 | 9 (5.26%) | 3 (17.6%) | 6 (3.90%) | 0.047 |
| Age of NEC Onset (days), median (IQR) | 16.0 [8.00;30.0] | 12.0 [8.00;25.0] | 17.0 [8.00;31.0] | 0.495 | 16.5 [8.00;31.0] | 15.0 [8.00;25.0] | 17.0 [8.00;31.0] | 0.744 |
| Length of Bowel Resected (cm), median (IQR) | 5.00 [0.00;23.0] | 15.0 [0.00;27.2] | 4.20 [0.00;16.9] | 0.201 | 5.00 [0.00;16.9] | 9.05 [1.72;20.3] | 4.20 [0.00;16.9] | 0.395 |
| Region of Bowel Resected, n (%) | 1 | 1 | ||||||
| Small Bowel Resected | 53 (61.6%) | 10 (58.8%) | 43 (62.3%) | 51 (63.0%) | 8 (66.7%) | 43 (62.3%) | ||
| Combined Large and Small Bowel Resected | 33 (38.4%) | 7 (41.2%) | 26 (37.7%) | 30 (37.0%) | 4 (33.3%) | 26 (37.7%) | ||
| Short Bowel Syndrome, n (%) | 38 (40.9%) | 10 (50.0%) | 28 (38.4%) | 0.495 | 37 (43.0%) | 9 (69.2%) | 28 (38.4%) | 0.077 |
| Post-Operative Intestinal Features | ||||||||
| Post-Operative Ileus Days (days), median (IQR) | 13.0 [9.00;16.2] | 15.5 [13.0;19.2] | 12.0 [7.25;15.8] | 0.017 | 12.0 [9.00;16.5] | 14.0 [11.0;18.0] | 12.0 [7.25;15.8] | 0.171 |
| Attainment of Full Enteral Feedings (120 mL/kg) (days), median (IQR) | 55.0 [28.5;79.0] | 63.0 [28.5;94.0] | 53.0 [29.0;76.5] | 0.617 | 57.0 [30.0;85.5] | 89.5 [62.2;120] | 53.0 [29.0;76.5] | 0.044 |
| Duration of Parenteral Nutrition (days), median (IQR) | 65.0 [31.0;99.5] | 79.0 [38.0;143] | 61.5 [30.2;95.8] | 0.093 | 65.0 [27.5;99.5] | 113 [25.0;130] | 61.5 [30.2;95.8] | 0.155 |
| Post-Operative Systemic Course | ||||||||
| Assisted Ventilation (intubated), n (%) | 113 (63.1%) | 25 (86.2%) | 88 (58.7%) | 0.006 | 102 (61.1%) | 14 (82.4%) | 88 (58.7%) | 0.2 |
| 24h Pressor Support, n (%) | 90 (50.0%) | 18 (66.7%) | 72 (47.1%) | 0.095 | 87 (51.2%) | 15 (88.2%) | 72 (47.1%) | 0.003 |
| AKI by Serum Creatinine Present, n (%) | 57 (32.9%) | 9 (33.3%) | 48 (32.9%) | 1 | 58 (35.8%) | 10 (62.5%) | 48 (32.9%) | 0.038 |
| AKI by Serum Creatinine, n (%) | 0.388 | 0.034 | ||||||
| Normal | 116 (67.1%) | 18 (66.7%) | 98 (67.1%) | 104 (64.2%) | 6 (37.5%) | 98 (67.1%) | ||
| Stage 1 | 27 (15.6%) | 2 (7.41%) | 25 (17.1%) | 30 (18.5%) | 5 (31.2%) | 25 (17.1%) | ||
| Stage 2 | 15 (8.67%) | 4 (14.8%) | 11 (7.53%) | 12 (7.41%) | 1 (6.25%) | 11 (7.53%) | ||
| Stage 3 | 15 (8.67%) | 3 (11.1%) | 12 (8.22%) | 16 (9.88%) | 4 (25.0%) | 12 (8.22%) | ||
| AKI by Urine Output Present, n (%) | 43 (24.9%) | 13 (48.1%) | 30 (20.5%) | 0.005 | 34 (21.0%) | 4 (25.0%) | 30 (20.5%) | 0.747 |
| AKI by Urine Output, n (%) | 0.008 | 0.422 | ||||||
| Normal | 130 (75.1%) | 14 (51.9%) | 116 (79.5%) | 128 (79.0%) | 12 (75.0%) | 116 (79.5%) | ||
| Stage 1 | 4 (2.31%) | 2 (7.41%) | 2 (1.37%) | 2 (1.23%) | 0 (0.00%) | 2 (1.37%) | ||
| Stage 2 | 26 (15.0%) | 8 (29.6%) | 18 (12.3%) | 22 (13.6%) | 4 (25.0%) | 18 (12.3%) | ||
| Stage 3 | 13 (7.51%) | 3 (11.1%) | 10 (6.85%) | 10 (6.17%) | 0 (0.00%) | 10 (6.85%) | ||
| AKI by Urine Output and Serum Creatinine Present, n (%) | 77 (41.4%) | 19 (65.5%) | 58 (36.9%) | 0.008 | 70 (40.2%) | 12 (70.6%) | 58 (36.9%) | 0.015 |
| Platelet Transfusion, n (%) | 86 (48.3%) | 16 (59.3%) | 70 (46.4%) | 0.305 | 86 (51.2%) | 16 (94.1%) | 70 (46.4%) | 0.001 |
| Sepsis Variables | ||||||||
| Central Line Present (days), median (IQR) | 148 (82.7%) | 23 (82.1%) | 125 (82.8%) | 1 | 139 (82.7%) | 14 (82.4%) | 125 (82.8%) | 1 |
| Duration of Antibiotics, median (IQR) | 7.00 [5.00;10.0] | 10.0 [6.00;13.5] | 7.00 [5.00;10.0] | 0.177 | 7.00 [5.00;10.0] | 7.00 [4.00;13.5] | 7.00 [5.00;10.0] | 0.992 |
| CRP on Day of NEC Onset, median (IQR) | 2.30 [0.70;6.05] | 3.10 [0.90;6.80] | 2.15 [0.70;5.90] | 0.296 | 2.35 [0.70;6.20] | 4.95 [2.22;15.3] | 2.15 [0.70;5.90] | 0.026 |
| CRP at 24 Hours after NEC Onset, median (IQR) | 3.90 [1.20;14.2] | 7.70 [1.80;17.7] | 3.80 [1.20;13.2] | 0.253 | 4.60 [1.37;14.7] | 15.3 [8.35;19.7] | 3.80 [1.20;13.2] | 0.004 |
| CRP at 48 Hours after NEC Onset, median (IQR) | 4.30 [1.30;15.9] | 11.1 [2.38;23.2] | 3.70 [1.12;15.6] | 0.038 | 4.00 [1.25;16.5] | 20.1 [8.90;22.4] | 3.70 [1.12;15.6] | 0.021 |
| Discharge | ||||||||
| Length of Stay (days), median (IQR) | 94.0 [46.0;142] | 127 [82.0;171] | 87.5 [41.5;133] | 0.013 | 89.0 [40.0;136] | 122 [26.0;161] | 87.5 [41.5;133] | 0.57 |
| Death, n (%) | 40 (21.5%) | 6 (20.7%) | 34 (21.7%) | 1 | 40 (23.0%) | 6 (35.3%) | 34 (21.7%) | 0.228 |
Abbreviations: NEC = necrotizing enterocolitis; AKI = acute kidney injury; CRP = c-reactive protein; WBC = white blood cell; ANC = absolute neutrophil count Continuous variables are presented as mean (standard deviation) and differences were tested with 2-sample t-test if normality has been met and, if not normally distributed, as median with interquartile range and Mann-Whitney U test were applied.
Categorical variables are presented as count (column percentage). Differences in categorical measures’ associations were tested using the Chi- squared test when cell counts were adequate, otherwise Fisher’s Exact were used with low expected cell counts. The presence of bold values signifies p < 0.05.
Gram-Negative Sepsis:
NEC-associated sepsis with gram-negative bacteria in neonates with surgical NEC was associated with higher evidence of portal venous gas on abdominal x-ray (17.6% vs 3.90%; p=0.047), increased time to reach full feeds postoperatively (89.5 [62.2;120] vs 53.0 [29.0;76.5]; p=0.044), increased requirement of dopamine at 24 hours after NEC onset (88.2% vs 47.1%; p=0.003), increased incidence of based on serum creatinine and urine output combined (70.6% vs 36.9%; p=0.015), and received more platelet transfusions before NEC onset (94.1% vs 46.4%; p=0.001). These infants also had significantly higher CRP levels on the day of NEC onset, at 24 hours, 48 hours, 96 hours, one week, and two weeks after NEC onset ( all p-value <0.05). Infants with gram-negative bacteremia had increased frequency of cholestasis at any time after NEC onset (75% vs. 38.9%; p=0.012), lower absolute lymphocyte count, and platelet count on the day and one day after NEC onset ( p <0.005) when compared to those without NEC-associated sepsis. (Table 3).
Comparison between Gram Positive vs. Gram Negative sepsis:
Compared to NDEC patients growing Gram-positive organisms in blood culture, NEC patients with Gram negative sepsis had a higher incidence of portal venous gas on abdominal X-ray (17.6% vs 0%; P=0.037), received more platelet transfusion prior to NEC onset, had higher CRP levels at 96 hours (22.2 [6.00; 25.0] vs. 4.55 [1.28; 10.6]; p=0.036), and lower median lymphocyte counts at 24 hour after NEC onset (2.11 [1.05;4.05] vs. 3.78 [2.48;5.95];p=0.042) (Supplemental Table 2).
Outcomes of bloodstream infection in neonates with surgical NEC:
The study included one hundred and eleven infants diagnosed with surgical NEC (n=111). Their demographic, inflammatory, hematological, and clinical information is summarized in Supplemental Table 3.
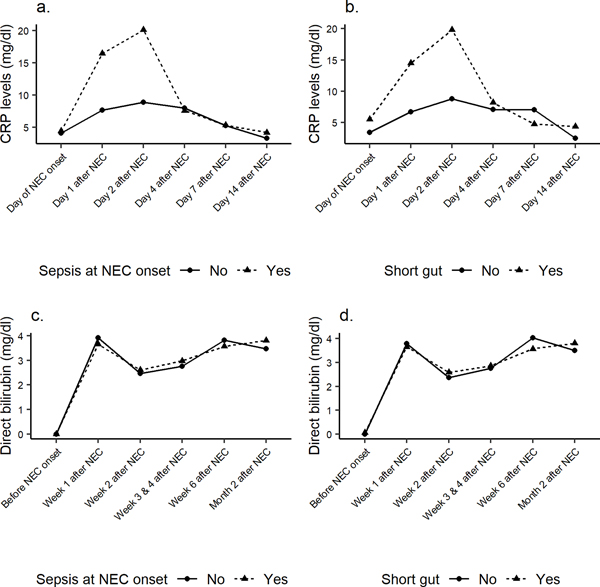
Of 111 Infants with surgical NEC, 33 infants had NEC-associated sepsis, and 78 infants were without NEC-associated sepsis. In this surgical cohort, 13 (11.7%) received a Penrose drain, and 98 (88.3%) underwent laparotomy. NEC-associated sepsis in neonates with surgical NEC was significantly associated with increased duration of postoperative ileus (16.0 [13.0;22.0] vs 12.0 [8.0;16.0]; p=0.006), increased requirement of dopamine at 24 hours after NEC onset (90.0% vs 71.4%; p=0.047), more platelet transfusions prior to NEC onset (93.8% vs 74.7%; p=0.044) and increased incidence of cholestasis at the time of NEC diagnosis (81.2% vs 58.7%; p=0.043). In our study, 52% (50/98) infants with surgical NEC developed the short gut syndrome. The microbiology of the microorganisms in neonates with surgical NEC and short gut is shown in Table 2. On laboratory evaluation, infants with NEC-associated sepsis had a lower absolute lymphocyte count on the day of NEC onset and a lower median platelet count at 24 hours after NEC onset (84.5 [67.5;125] vs. 127 [79.0; 165]; p=0.031) compared to infants with no positive blood culture at the time of NEC. The CRP and direct bilirubin trends following surgical NEC diagnosis in neonates with and without NEC-associated sepsis and for the short gut group are shown in Figure 2.
Figure 2:
Trend of CRP levels at different time points when comparing infants with sepsis at NEC onset (a) and infants with the short gut syndrome (b). The trend of direct bilirubin levels at different time points in Infants with sepsis at NEC onset (c) and in infants with the short gut syndrome (d).
On multivariate logistic regression, infants with cholestasis at surgical NEC onset had higher odds of NEC-associated sepsis (OR 2.94; 95% CI 1.1–8.8, p=0.038). The data has been summarized in Tables 4A and 4B.
Table 4A:
Multiple Logistic Regression Analysis for Combined Medical and Surgical NEC Cohort
| Positive Blood Culture at NEC Onset | Gram Positive | Gram Negative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p-value | aOR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Gestational Age | 0.92 | 0.82 – 1.02 | 0.116 | 1.12 | 1.00 – 1.28 | 0.069 | 1.06 | 0.93 – 1.24 | 0.423 |
| Clinical Presentation | |||||||||
| Abdominal Distension | Ref. | Ref. | |||||||
| Bloody Stools | 0.17 | 0.03 – 0.61 | 0.02 | 3.26 | 0.87 – 21.22 | 0.127 | |||
| Feeding Intolerance | 0.3 | 0.02 – 1.74 | 0.268 | 1.83 | 0.31 – 34.95 | 0.579 | |||
| Cholestasis | 1.6 | 0.80 – 3.25 | 0.185 | 1.06 | 0.48 – 2.36 | 0.882 | 0.37 | 0.12 – 1.02 | 0.064 |
| Portal Venous Gas | 1.17 | 0.15 – 6.35 | 0.86 | ||||||
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval The presence of bold values signifies p < 0.05.
Table 4B:
Multiple Logistic Regression Analysis for Infants with Surgical NEC
| Positive Blood Culture at NEC | Gram Positive | Gram Negative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p-value | aOR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Gestational Age | 0.96 | 0.83 – 1.09 | 0.533 | 1.09 | 0.94 – 1.30 | 0.306 | 0.97 | 0.82 – 1.16 | 0.702 |
| Cholestasis | 2.94 | 1.11 – 8.83 | 0.038 | 0.61 | 0.18 – 1.76 | 0.383 | 0.3 | 0.04 – 1.20 | 0.13 |
| Portal Venous Gas | 1.02 | 0.12 – 6.39 | 0.987 | ||||||
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval The presence of bold values signifies p < 0.05.
Brain Injury and NEC associated sepsis:
In this study, 137 infants received a brain MRI at term equivalent age for brain injury assessment. White matter injury was present in 51/137 (37.2%) preterm infants with medical and surgical NEC. White matter injury was more common in preterm infants with NEC-associated sepsis than in infants with NEC but without sepsis. In addition, preterm infants with gram-positive sepsis at NEC onset had greater white matter injury (57.9% vs. 35%) and cerebellar injury (42.9% vs. 31.9%) than NEC infants without NEC sepsis. The information is shown in Supplemental Table 4. In our cohort, neonates with surgical NEC had significantly higher white matter abnormality (52.2% vs. 22.9%; p=0.001) and grey matter injury (10.6% vs. 0%; p=0.019) on brain MRI obtained at term equivalent age compared to the medical NEC group. In addition, compared to neonates with medical NEC, those with surgical NEC had a higher number of positive blood cultures at NEC onset (33/111, 29.7% vs. 17/98, 17.3%) with higher rates of gram-positive (18% vs. 12.2 %), gram-negative (12.6% vs. 6.1%) and fungal infection (3.6% vs.1 %) (Supplemental Table 5).
Discussion:
Our study demonstrates that NEC-associated sepsis was present in one out of four cases in infants with medical and surgical NEC. Gram-positive bacteria, gram-negative bacteria, and Candida were cultured in blood in 15.3%, 10.5%, and 2.8% of NEC cases. In addition, infants with NEC-associated sepsis had lower birth weight, earlier gestational age, greater inflammatory response (CRP levels), frequently needed assisted ventilation, ionotropic support, and acute kidney injury after NEC onset compared to NEC cases without positive blood culture.
There are very few published reports on NEC-associated sepsis and clinical outcomes. A study by Bizzaro et al. reported NEC-associated sepsis (blood culture positive within 72 hours and >72 hours after NEC onset) in neonates (158/410, 38.5%) with NEC 12. The median gestational age of the infants with NEC-associated sepsis was (28 [25, 31] vs. 29 [26, 32]; p=<0.001), and birth weight was lower compared to neonates without NEC-associated sepsis. Gram-negative bacteria were more commonly cultures in the NEC-associated sepsis group than in cases of BSI without NEC. E coli was the most common gram-negative organism. Patients with NEC-associated sepsis were more frequently mechanically ventilated, received parenteral nutrition, and diagnosed bronchopulmonary dysplasia and retinopathy of prematurity. Infants with NEC-associated sepsis had a longer stay and higher mortality (32/69, 46.4% vs. 57/252, 22.6%, p=<0.001) 12. In comparison, our cohort with NEC-associated sepsis also had significantly lower median gestational age and lower birth weight; however, we found more episodes of gram-positive sepsis (63.5% vs. 44.2%) compared to infants without NEC-associated sepsis, implying a more immature immune response and intestinal barrier function. We had similar rates of poly-microbial and monomicrobial infection. Infants in our cohort with gram-positive sepsis also had a significantly longer length of hospital stay.
Another multicenter study by Coleman et al. reported outcomes of BSI in 932 infants with NEC (intestinal failure, IF, n=78; surgical NEC without IF, n=452; medical NEC, n=402). The overall blood culture positive rate at NEC diagnosis (obtained within seven days after NEC onset) was 20%. 151/452 (33%) and 80/402 (20%) in infants with surgical NEC and medical NEC, respectively. Our cohort of NEC-associated sepsis patients had a similar blood culture positivity rate (23.5%) for the whole cohort, for surgical NEC (33/111, 29.7%) and medical NEC (17/98, 17.3%). Klebsiella and Staphylococcus epidermidis were the most common gram-negative and gram-positive microorganisms in this cohort, similar to the study by Coleman et al. In our study, infants with NEC associated sepsis stayed 32 days longer (122 days vs. 89 days) and trended towards higher mortality (28 % vs. 21.4%). However, it did not reach statistical significance, most likely due to the smaller sample size.
In our cohort, patients with NEC-associated sepsis were more likely to suffer from AKI by urine output and serum creatinine criteria [60% vs. 38.4%, p=0.011] than neonates with NEC without sepsis. We have recently reported sepsis as an independent predictor (aOR = 3.5; 95% CI = 1.3, 10.0) of severe AKI (stage 2 and 3 by KDIGO classification) in neonates with NEC 22. Coggins et al. also reported Infants (n=203) with late-onset sepsis had increased odds (aOR, 3.0; 95% CI, 1.5–6.2; P = .002) of AKI and greater AKI severity within seven days of sepsis evaluation, compared to age-matched control infants without sepsis 23. The published reports have suggested most likely explanation of sepsis-associated AKI in neonates is due to altered hemodynamics, inflammation, and nephrotoxic medications 24,25.
On laboratory evaluation, infants with NEC-associated sepsis had a significantly higher CRP level at 48 hours in gram-positive sepsis and for up to 2 weeks in gram-negative sepsis, suggesting a more extended pro-inflammatory response in gram-negative NEC-associated infections. We also noted lower platelet and lymphocyte counts in neonates with culture-positive sepsis following NEC onset, suggesting the involvement of these cell lines in NEC pathogenesis and outcomes. The exact mechanism of thrombocytopenia in NEC is unclear, although consumptive disorders and the formation of microthrombi in the diseased intestine are deemed likely [25]. They may also be due to platelet activation from bacterial products leading to aggregation in the microvasculature26. Low percentages of lymphocytes may reflect sequestration of lymphocytes in the intestine and brain tissue, as shown in a recent study by Zhou et al. demonstrating the brains of mice and humans with NEC contained CD4+ T lymphocytes that were required for the development of brain injury27. In our cohort, cholestasis (direct bilirubin >2 mg/dl) was an independent risk factor for bloodstream infections in neonates with NEC, which points to sepsis-associated liver injury and the effects of pro-inflammatory cytokines induced by the induced effect by bacteria on the hepatocellular and ductal bile formation and ischemic liver injury 28. We noticed a trend toward higher rates of bloody stools in infants without NEC-associated sepsis, which points towards inflammation-induced vascular injury by stimuli other than infectious agents, suggesting the need for further investigation.
In infants with surgical NEC, we noted a higher gram-positive infection frequency than infants with medical NEC. We acknowledge that some isolates may be contaminants. Recent studies suggest that S. epidermidis is associated with neonatal morbidities, including NEC 29. In a recent meta-analysis, S. epidermidis sepsis was associated with higher odds for neurodevelopmental impairment (OR 1.31, 95% CI: 1.09–1.57) compared to control30. Neonatal host response to S. epidermidis sepsis has not been fully understood. It is most likely due to immature innate immunity with a distinctive regulation pattern of the inflammatory response 29. Another prospective study of 192 neonates (gestational age <30 weeks) reported 100 episodes of confirmed sepsis in 68 infants, and nine infants (5%) had confirmed NEC. The sepsis-associated NEC group had predominantly gram-positive infection, as noticed in our study cohort, and had significantly more white matter injury on the brain MRI than those with no sepsis-associated NEC 31. Bacteremia-induced brain injury may be explained by the release of lipopolysaccharide or peptidoglycan and modulating pro-inflammatory genes in the brain such as Toll-like receptors, nuclear factor-κB, antioxidants, oxidants, and cytokines 32.
This study’s strengths include a detailed description of NEC-associated sepsis and its impact on outcomes at term equivalent age. In addition, our data suggest that NEC-associated sepsis contributes to worse postoperative outcomes, which partially depend on the type of isolated organism.
Our study has some important limitations. First, this was a single-center experience, reducing the study’s generalizability. Second, the retrospective study design lacks uniformity and completeness in each neonate’s available data and documentation. Third, NEC is a relatively rare condition. While a relatively high volume neonatal intensive care unit center, sample size also limits our power to detect associations between clinical factors, NEC, and outcomes.
Further, the small sample size coupled with multiple factors and outcomes may result in type I errors from multiple comparisons. Finally, in our cohort, most of the neonates with NEC were African American. While this is partly due to race distribution in Mississippi, this may also be related to adverse social determinants of health and/or genetic risk for NEC. Therefore, future studies investigating the role of genetic variants and social determinants of health, race, and their impact on NEC and outcomes are needed.
This study raises some important clinical questions. First, we do not know if NEC or sepsis occurs first. Second, was surgical NEC leading to more intestinal barrier disruption resulting in higher percentages of blood culture positivity, or was the bloodstream infection leading up to surgical NEC? These questions can be best addressed in a prospective multicenter clinical trial or animal studies.
In conclusion, one-fourth of preterm infants with NEC have blood culture-positive sepsis associated with a lower gestational age and birth weight. In addition, these infants are more likely to receive hemodynamic support, suffer from AKI, have longer postoperative ileus, cholestasis, and a higher pro-inflammatory state (e.g., higher CRP levels and lower platelet counts), compared to NEC patients without sepsis. Future studies are needed to investigate the role of sepsis-induced inflammation in NEC by type of microorganism. In addition, there is a need to develop new immunomodulation strategies in NEC to restore a balanced neonatal inflammatory response and prevent inflammation-associated organ damage 33.
Supplementary Material
Impact.
NEC-associated sepsis was present in 24% of infants with NEC. Gram-positive bacteria, gram-negative bacteria, and Candida were found in 15.3%, 10.5%, and 2.8% of cases, respectively.
Infants with NEC-associated sepsis had a greater inflammatory response (CRP levels), received more blood transfusion before NEC onset, frequently needed assisted ventilation ionotropic support, and had acute kidney injury after NEC onset
NEC infants with gram-negative sepsis had higher portal venous gas, received more platelet transfusions before NEC onset, and had higher CRP levels and lower median lymphocyte counts at 24 hours after NEC onset than those with gram-positive sepsis.
Acknowledgment:
The Mississippi Clinical and translational research center for supporting the NEC research.
Funding:
Dr. Parvesh Mohan Garg is partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM115428. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Consent: Patient consent is not required as per IRB
Category of study: Clinical science
Data User Agreement: All data generated and analyzed during this study are included in this article and its supplementary information files.
Conflicts of interest: The authors disclose no conflicts.
References:
- 1.Neu J. & Walker WA Necrotizing enterocolitis. N Engl J Med 364, 255–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaran K, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr 39, 366–372 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Sjoberg Bexelius T, et al. Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ paediatrics open 2, e000316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allin BSR, Long AM, Gupta A, Lakhoo K. & Knight M. One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Archives of disease in childhood. Fetal and neonatal edition 103, F461–f466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knell J, Han SM, Jaksic T. & Modi BP Current Status of Necrotizing Enterocolitis. Curr Probl Surg 56, 11–38 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. Jama 314, 1039–1051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santulli TV, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics 55, 376–387 (1975). [PubMed] [Google Scholar]
- 8.Mowitz ME, Dukhovny D. & Zupancic JAF The cost of necrotizing enterocolitis in premature infants. Seminars in fetal & neonatal medicine 23, 416–419 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy V, Hay JW, Kim JH, Lee ML & Rechtman DJ Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC pediatrics 13, 127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu IH, et al. Incidence, clinical features, and implications on outcomes of neonatal late-onset sepsis with concurrent infectious focus. BMC Infect Dis 17, 465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggins SA, Wynn JL & Weitkamp JH Infectious causes of necrotizing enterocolitis. Clin Perinatol 42, 133–154, ix (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bizzarro MJ, Ehrenkranz RA & Gallagher PG Concurrent bloodstream infections in infants with necrotizing enterocolitis. J Pediatr 164, 61–66 (2014). [DOI] [PubMed] [Google Scholar]
- 13.An Y, Liu L, Li QY, Ran YL & Li LQ [Risk factors for concurrent sepsis in neonates with necrotizing enterocolitis]. Zhongguo Dang Dai Er Ke Za Zhi 18, 677–682 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole CR, et al. Bloodstream infections in very low birth weight infants with intestinal failure. J Pediatr 160, 54–59.e52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin SC, Pappas C, Iyengar H. & Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol 40, 53–68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selewski DT, et al. Neonatal Acute Kidney Injury. Pediatrics 136, e463–473 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Jetton JG, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1, 184–194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jetton JG, et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Frontiers in pediatrics 4, 68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jetton JG & Askenazi DJ Acute kidney injury in the neonate. Clin Perinatol 41, 487–502 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Zappitelli M, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatric research 82, 569–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodward LJ, Anderson PJ, Austin NC, Howard K. & Inder TE Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355, 685–694 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Garg PM, et al. Severe acute kidney injury in neonates with necrotizing enterocolitis: risk factors and outcomes. Pediatr Res (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coggins SA, et al. Acute Kidney Injury Associated with Late-Onset Neonatal Sepsis: A Matched Cohort Study. J Pediatr 231, 185–192.e184 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Murphy HJ, et al. Nephrotoxic medications and acute kidney injury risk factors in the neonatal intensive care unit: clinical challenges for neonatologists and nephrologists. Pediatr Nephrol 35, 2077–2088 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Seely KA, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 301, F209–217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsueh W, et al. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 6, 6–23 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, et al. Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci Transl Med 13(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geier A, Fickert P. & Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol 3, 574–585 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, Speer CP & Glaser K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 9, 621–633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alshaikh B, Yusuf K. & Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol 33, 558–564 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Shah DK, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 153, 170–175, 175.e171 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Volpe JJ Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr 153, 160–163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn JL, Neu J, Moldawer LL & Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol 29, 79–88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.