Abstract
Fatty acid and polyketide biosynthetic enzymes exploit the reactivity of acyl- and malonyl-thioesters for catalysis. A prime example is FabH, which initiates fatty acid biosynthesis in many bacteria and plants. FabH performs an acyltransferase reaction with acetyl-CoA to generate an acetyl-S-FabH acyl-enzyme intermediate and subsequent decarboxylative Claisen-condensation with a malonyl-thioester carried by an acyl carrier protein (ACP). We envision crystal structures of FabH with substrate analogs can provide insight into the conformational changes and enzyme:substrate interactions underpinning the distinct reactions. Here we synthesize acetyl/malonyl-CoA analogs with esters or amides in place of the thioester and characterize their stability and behavior as E. coli FabH substrates or inhibitors to inform structural studies. We also characterize the analogs with mutant FabH C112Q that mimics the acyl-enzyme intermediate allowing dissection of the decarboxylation reaction. The acetyl- and malonyl-oxa(dethia)CoA analogs undergo extremely slow hydrolysis in the presence of FabH or C112Q mutant. Decarboxylation of malonyl-oxa(dethia)CoA by FabH or C112Q mutant was not detected. The amide analogs were completely stable to enzyme activity. In enzyme assays with acetyl-CoA and malonyl-CoA (rather than malonyl-ACP) as substrates, acetyl-oxa(dethia)CoA is surprisingly slightly activating, while acetyl-aza(dethia)CoA is a moderate inhibitor. The malonyl-oxa/aza(dethia)CoAs are inhibitors with Ki’s near the Km of malonyl-CoA. For comparison, we determine the FabH catalyzed decomposition rates for acetyl/malonyl-CoA, revealing some fundamental catalytic traits of FabH including hysteresis for malonyl-CoA decarboxylation. The stability and inhibitory properties of the substrate analogs makes them promising for structure-function studies to reveal fatty acid and polyketide enzyme:substrate interactions.
Acyl-CoA analogs have been used in a relatively small number of enzyme structure-function studies, where the analogs can clarify roles of catalytic residues.1 These acyl-CoA analogs have the relatively labile thioester substituted with esters/amides/ketones to examine acyl-transfer catalysis, or the acyl-thioester is substituted by acyl-enolate isosteres to examine carbon-carbon bond forming enzymes. The thioester bond of malonyl-CoA is considered to be especially labile compared to acetyl-CoA.2 Thus, stable malonyl-thioester analogs are desired for use in structure-function studies.3-5 Other uses for stable malonyl-CoA analogs include off-loading polyketide synthase metabolic intermediates, or as probes of enzymes like carboxymethylproline synthase, where the analogs can participate as slow substrates.6-8 To determine suitability of stable acetyl/malonyl-thioester analogs in fatty acid and polyketide synthase structure-function studies or as inhibitor warheads, we report alternative syntheses of acetyl-oxa(dethia)CoA/acetyl-aza(dethia)CoA (1/2), malonyl-oxa(dethia)CoA/malonyl-aza(dethia)CoA (3/4) and their reactivity with Escherichia coli FabH, a ketosynthase (ECS) initiating fatty acid biosynthesis,. We also determine the inhibition constants of 1-4, oxa(dethia)CoA (5) and aza(dethia)CoA (6) against FabH using a continuous spectrophotometric assay applicable to other KSs. Since FabH carries out the same fundamental catalytic reactions as KS in fatty acid and polyketide biosynthesis in other organisms, our findings here concerning the stability of the analogs reveal their potential use in structural studies is broadly applicable.
Carbon-carbon bonds in fatty acids and polyketides are generated by condensation of acyl-thioesters and malonyl-thioesters, carried by either CoA or an acyl carrier protein (ACP).9 In E. coli and related bacteria the first fatty acid carbon-carbon bond formed is between acetyl-CoA and malonyl-S-ACP by FabH, which is likely rate limiting in vivo.10 Inhibition of FabH with thiolactomycin or other natural products and a variety of synthetic compounds in vivo leads to overall growth inhibition, revealing FabH and homologs are valid drug targets.11-13 Thus, a better understanding of FabH catalysis, especially uncovering the presence or absence of substrate induced conformational changes, will inform drug discovery efforts.14
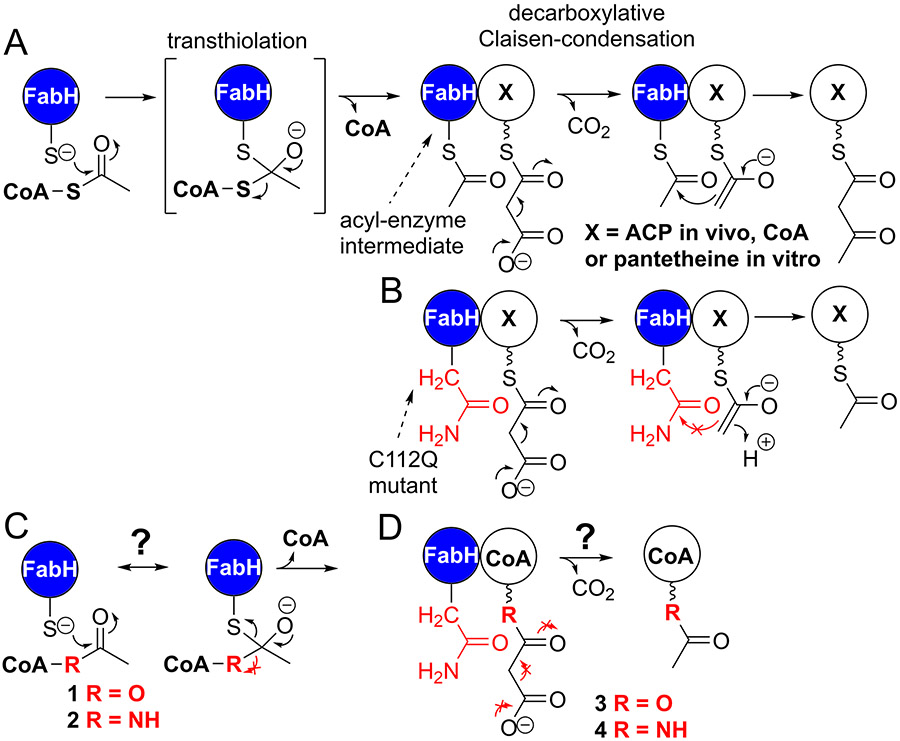
It is known that FabH transfers the acetyl group of acetyl-CoA onto its active site Cys112 followed by decarboxylation of malonyl-S-ACP forming an enolate intermediate that reacts with acetyl-S-FabH generating acetoacetyl-S-ACP, Figure 1A.12, 15-18 Mutation of the FabH active site cysteine to a glutamine is expected to generate a malonyl-S-ACP decarboxylase, similar to other β-ketoacyl-ACP synthases, Figure 1B and can enable studies of the decarboxylation reaction.19 We expect analogs 1 and 2 can reveal catalytic interactions for the transthiolation reaction if they are stable in the FabH active site, Figure 1C, while analogs 3 and 4 would reveal interactions leading to the decarboxylative Claisen condensation, Figure 1D. Structures of apo-FabH reveal many active site loops can be disordered and become ordered upon acetyl-CoA substrate presentation.20 Subsequent studies probing FabH structure and catalysis with CoA mixed disulfides revealed negative cooperativity between the active sites of the dimer.21 The FabH dimer appears to undergo acetyl-CoA transthiolation with the first active site at least ten times faster than with the second site, while the CoA mixed disulfide inhibitors react at the second site 90-times slower.21 Conformational differences in the active sites, which are apparent in PDB 3IL9,22 could explain the negative cooperativity. The conformational differences may impede drug design as one active site might have reasonable affinity for an inhibitor, while the other active site remains capable of catalysis. We expect that substrate analogs combined with structural biology can reveal the missing aspects of transthiolation negative cooperativity, conformational changes and decarboxylation mechanism, but a first step is synthesis and characterization of substrate analog:enzyme affinity and stability.
Figure 1.
Initiation of fatty acid biosynthesis and potential substrate analogs. A) FabH activity indicating the two fundamental reactions with an acyl-S-FabH enzyme intermediate. B) FabH C112Q mutant mimics the decarboxylation activity of wild-type, the reactions prevented are shown as red no go arrows. C) FabH transthiolation reaction prevented by stable acetyl-CoA analogs. D) Decarboxylation reaction of FabH C112Q prevented or slowed by stable malonyl-thioester analogs.
RESULTS AND DISCUSSION
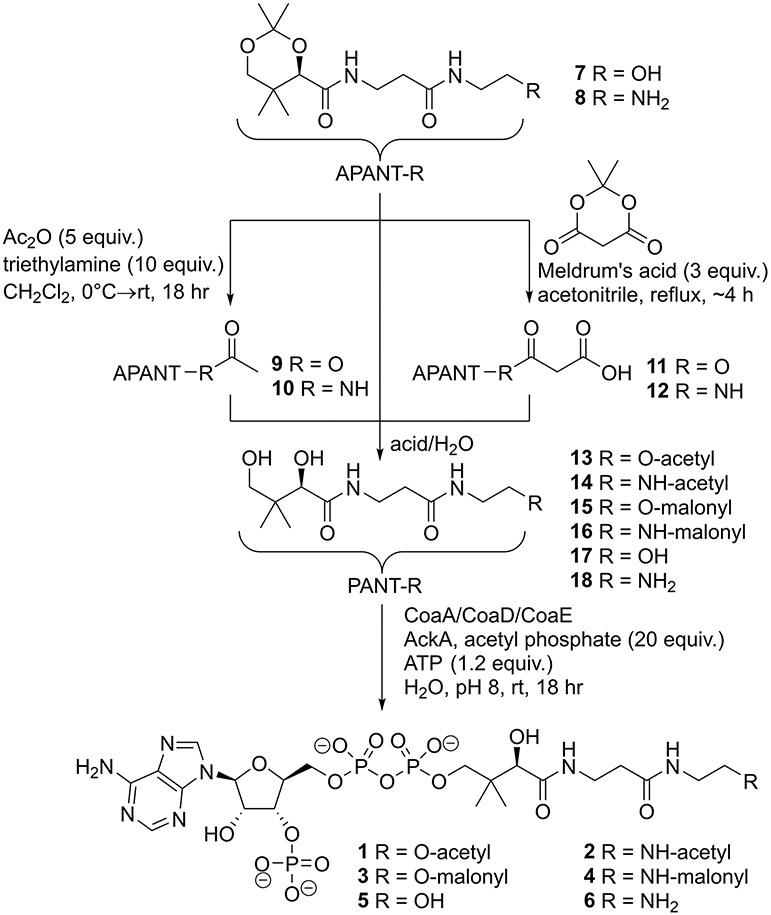
We synthesized the extremely polar acetyl-CoA and malonyl-CoA analogs using the strategy in scheme 1, similar to our previous synthesis of methylmalonyl-CoA analogs, with details in the supplemental information.23 Acetonide protected oxa(dethia)pantetheine (7) and aza(dethia)pantetheine (8) were generated as previously published.23 The acetonide pantetheine analogs 7/8 were treated with acetic anhydride and triethylamine to give acetyl-oxa(dethia)pantetheine acetonide (9) and acetyl-aza(dethia)pantetheine acetonide (10), respectively. Malonyl-oxa(dethia)pantetheine acetonide (11) and malonyl-aza(dethia)pantetheine acetonide (12) were generated through heating 7/8 with Meldrum’s acid for 4 hours, respectively. Decarboxylation of 11/12 to 9/10 was detected if the reactions were left refluxing for extended times. The acetonides 7-12 were deprotected with formic acid in water and chemoenzymatically converted to the CoA analogs 1-6 using an acetate kinase ATP regeneration system.24 The deprotection of esters 9 and 11 required slow dropwise addition of 50% formic acid to a pH of 2.2 to avoid ester hydrolysis.
Scheme 1.
The product standard aza(dethia)CoA (6) was a challenge to generate using our workflow due to side reactions or limited turnover during chemoenzymatic preparation. If the ATP regeneration system was used with aza(dethia)pantetheine (18), a large amount of 2 was generated due to acetylation by acetyl phosphate as an expected side product. If an excess of ATP is used rather than the ATP regeneration system 6 can be generated in small quantities but the major product is 3’-dephosphoaza(dethia)CoA, which appears to be a very poor substrate for E. coli CoaE. Therefore, we turned to alternative routes for 6, Scheme 2 with details in the supplemental information.
Scheme 2.
Starting with N-Boc-ethylenediamine and pantothenic acid acetonide, we produced intermediate 19 using mixed anhydride coupling. We generated the azide intermediate 20 using previously published methods.25 Selective acetonide deprotection of 19 to N-Boc-aza(dethia)pantetheine (21) and 20 to azide(dethia)pantetheine (22) was achieved with formic acid at pH 2.2. The N-protected pantetheines were converted to CoAs chemoenzymatically yielding 23 and 24. The final deprotection of 23 to 6 using TFA at a pH of 2 was possible with repeated HPLC monitoring until complete. The conversion of 24 to 6 was examined with a few reducing reagents with TCEP being superior to the reagents triphenylphosphine, tributylphosphine, tris(hydroxymethyl)phosphine, or borohydride. We confirmed the identity of 6 through comparison of products by HPLC, HRMS and NMR, Supplemental Figure S1.
Our synthesis of the malonyl-CoA analogs 3/4 via Meldrum’s acid used fewer steps than previous methods and gave high yields before purification as judged by analytical LCMS.6-8 Furthermore, our strategy of using Meldrum’s acid is applicable to 2-substituted analogs for use with various polyketide synthase and intermediary metabolic enzymes. Analogs 1-6 are completely stable overnight in buffer at pH 6, 7 or 8, which are conditions used for the subsequent enzymology experiments.
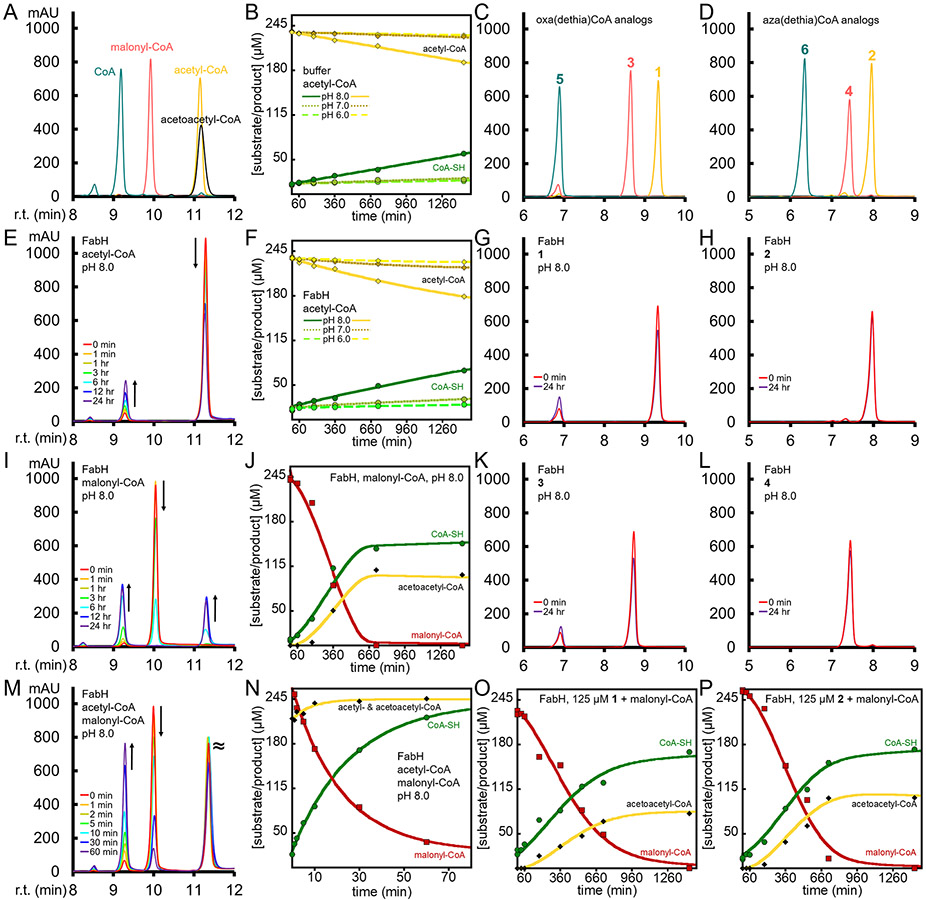
To establish our FabH enzyme is active we performed HPLC assays with acetyl-CoA and malonyl-CoA, Figure 2. We chose malonyl-CoA rather than malonyl-S-ACP for a few reasons. First to avoid the need for coupled enzyme assays with FabD to generate malonyl-S-ACP, since FabD is also expected to be inhibited by the malonyl-CoA analogs. In addition we needed to avoid generating ACPs with substrate analogs attached for use in enzyme inhibition assays. Another reason is that malonyl-CoA has been used as a FabH substrate in drug screening platforms or in other studies with FabH homologs, but is incompletely characterized as a substrate.26-29 One complication of our HPLC assay is that the substrate acetyl-CoA peak overlaps with the product, acetoacetyl-CoA, although the acetoacetyl-CoA peak is distinctly broader, Figure 2A. Nevertheless, the disappearance of malonyl-CoA and appearance of CoA is easily monitored to provide kinetics, Figure 2M and 2N. In these HPLC assays with 10 μM FabH and equimolar acetyl-CoA and malonyl-CoA at ~125 or ~250 μM at pH 8, the initial rates are 0.58±0.01 and 0.81±0.01 min−1 respectively, suggesting FabH is still not saturated even at these high concentrations. We also found FabH activity increases modestly with pH, Supplemental Figure S2 and Table 1
Figure 2.
Analyses of 10 μM FabH activity with substrates and analogs. HPLC traces for standards, with retention time (r.t.) and 254 nm milliabsorbance (mAU) for A) acetyl-CoA, malonyl-CoA and CoA, C) 1, 3, 5, and D) 2, 4, 6. HPLC traces for FabH activity with E) acetyl-CoA, G) 1, H) 2, I) malonyl-CoA, K) 3, L) 4. HPLC traces for FabH activity with M) both acetyl- and malonyl-CoA. Kinetic analysis of the reactions monitored by HPLC, B) non-enzymatic acetyl-CoA hydrolysis at various pH, F) acetyl-CoA hydrolysis with FabH at various pH, J) FabH with malonyl-CoA, N) FabH with acetyl- and malonyl-CoA, O) FabH with malonyl-CoA and 1, and P) FabH with malonyl-CoA and 2. The progress curves of B), F), and N) were fit with exponential decay or increase, while J), O), and P) were fit with a hysteretic/inhibition model as described in the supplemental information. The HPLC data points in the kinetic analysis are shown as green circles for CoA, red squares for malonyl-CoA and yellow or black diamonds for acetyl- or acetoacetyl-CoA. Notice the significant lag phase in J), O) and P) compared to N).
Table 1.
Initial rates of FabH or C112Q mutant reactivity with substrates and analogs*
| FabH wt (rates in min−1) | FabH C112Q (rates in min−1) | |||||
|---|---|---|---|---|---|---|
| reactants → products | pH 6 | pH 7 | pH 8 | pH 6 | pH 7 | pH 8 |
| acetyl- & malonyl-CoA → acetoacetyl-CoA | 0.47 ± 0.04 | 0.74 ± 0.01 | 0.81 ± 0.01 | |||
| malonyl-CoA → acetyl-CoA/acetoacetyl-CoA | n.d. | biphasic | 0.043 ± 0.001‡ | 0.0060 ± 0.0006 | 0.18 ± 0.01 | 0.20 ± 0.02 |
| acetyl-CoA → CoA | 0.0004 ± 0.0001 | 0.0010 ± 0.0001 | 0.0038 ± 0.0002 | 0.0005 ± 0.0001 | 0.0006 ± 0.0001 | 0.0030 ± 0.0001 |
| malonyl-CoA → CoA | 0.0005 ± 0.0001 | 0.0007 ± 0.0001 | 0.0032 ± 0.0005 | |||
| acetyl-oxa(dethia)CoA → oxa(dethia)CoA | ~ 0.003 | ~ 0.001 | ||||
| malonyl-oxa(dethia)CoA → oxa(dethia)CoA | ~ 0.001 | ~ 0.001 | ||||
Reactions determined by HPLC analysis have ~250 μM substrate or analog and 10 μM FabH wt or FabH C112Q. Not detected (n.d.)
Reaction contained 25 μM acetyl-CoA to alleviate lag phase, value is for acetoacetyl-CoA appearance, malonyl-CoA disappearance rate is approximately double.
In order to determine the reactivity or inhibition behavior of FabH with acetyl- and malonyl-CoA analogs, we first explored their stability in the presence of FabH, see Figure 2 panels G, H, K and L. Our synthesis of the standards oxa and aza(dethia)CoA, allowed us to confirm the presence or absence of the hydrolysis products and acetyl-oxa/aza(dethia)CoA allowed us to confirm decarboxylation products. As a baseline, we determined the stability of acetyl- and malonyl-CoA at pH 6, 7 and 8, Figure 2B and Supplemental Figure S2A. Non-enzymatic hydrolysis of acetyl- and malonyl-CoA is very slow at pH 6 and 7 with rates of ~0.0004 min−1, while at pH 8 the rate is ~10 times higher. The analogs are completely stable in buffer at pH 6, 7 and 8 over 24 hours. The hydrolysis of acetyl-CoA by FabH in the absence of malonyl-CoA is insignificant compared to the non-enzymatic rates of hydrolysis, while the oxa(dethia) analogs hydrolyze very slowly and the aza(dethia) analogs are completely stable over 24 hours, Figure 2E-H/K/L and Table 1.
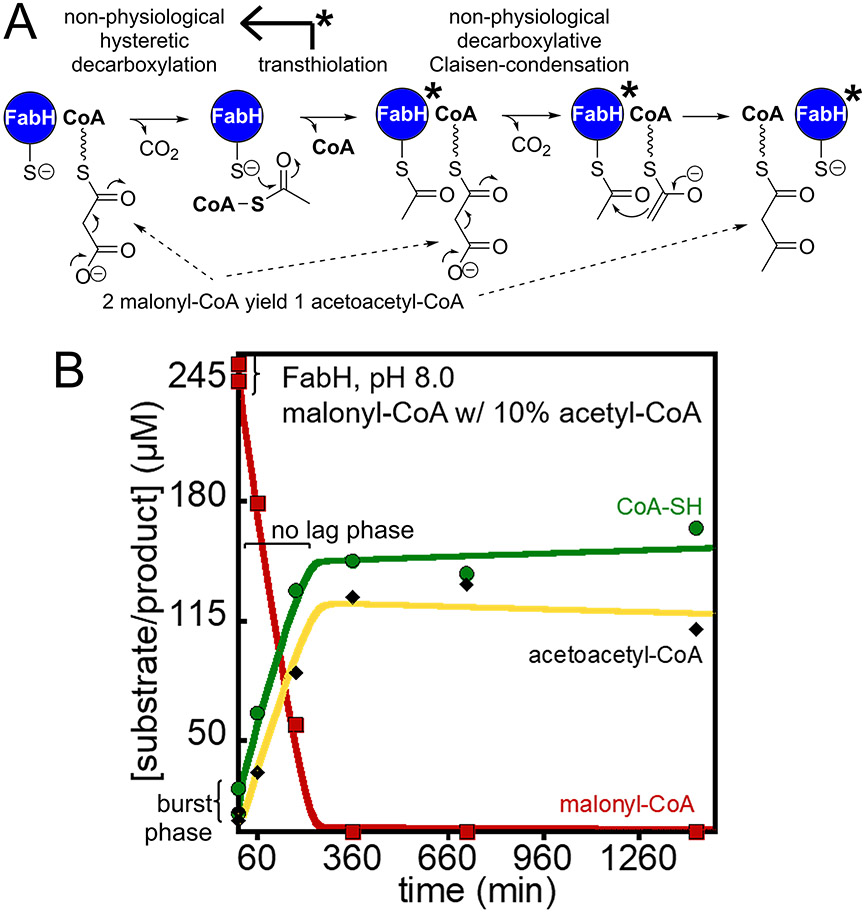
We found that FabH generates acetoacetyl-CoA via decarboxylation of malonyl-CoA to acetyl-CoA and reaction with a second malonyl-CoA (Figure 3A) at pH 7 and 8, but not pH 6, Figure 2I/J and Supplemental Figure S2. The progress curves display a significant lag phase that we modeled as hysteresis alleviated by acetyl-CoA as outlined in the supplemental information and Supplemental Figure S3.30 We noticed that the lag phase was dependent on the stock of malonyl-CoA used to initiate the reaction and concentration. All commercial stocks of malonyl-CoA we tested had ~5-10% acetyl-CoA and ~5-10% CoA contaminants. To determine the behavior of FabH in the absence of acetyl-CoA with malonyl-CoA alone, we synthesized and purified an appropriate stock of malonyl-CoA as described in the supplemental information. FabH behavior with the purified malonyl-CoA stock (1% acetyl-CoA) has a significant lag phase where little happens in the first hour, Figure 2I/J and Supplemental Figure 2D/E. The addition of acetyl-CoA at 10% of malonyl-CoA alleviates the lag phase completely with the introduction of a small burst phase within the first data points that we interpret as the rapid use of acetyl-CoA, Figure 3B. The second phase allowed us to determine the rate of malonyl-CoA conversion to acetoacetyl-CoA by activated FabH which is about 20 times slower than the complete reaction, Table 1. We interpret the lag phase to be FabH in an inactive state that only slowly decarboxylates malonyl-CoA, but acetyl-CoA binding generates a catalytically efficient enzyme that only slowly relaxes back to the inactive state, a similar state transition has been discussed previously.20-21
Figure 3.
Activity of FabH decarboxylation of malonyl-CoA to generate acetoacetyl-CoA. A) A scheme depicting the activity of FabH with malonyl-CoA in the absence of acetyl-CoA, the non-physiological malonyl-CoA decarboxylation reaction is slow in the absence of acetyl-CoA, but once formed acetyl-CoA modifies FabH to generate a more active decarboxylase, FabH*. B) With the addition of priming acetyl-CoA, there is a lack of a lag phase as in Figure 2J and instead within the first 5 minutes the reaction is ~10% complete (reaction is complete by about 250 min compared to 620 min)
The role of hysteresis, or in other terms, the observation that the catalytic behavior of FabH for the decarboxylation of malonyl-CoA changes in the presence and absence of starting acetyl-CoA, is likely tied to the role of FabH as a regulator of fatty acid biosynthesis.31-32 A crystal structure of FabH revealed that the active site loops were disordered in the absence of substrates (PDB 1HNK).20 It could be that the disordered loops become ordered in the presence of acetyl-CoA to generate an active enzyme through rapid acyl-enzyme intermediate formation. Conversely, in the presence of long chain acyl-S-ACP which is an inhibitor of FabH,31-32 the disordered loops may adopt conformations that bind the feedback inhibitor. A beneficial outcome of the hysteresis is that the disordered FabH does not readily decarboxylate malonyl-CoA.
The finding that acetyl-CoA activates FabH for decarboxylation, even after acetyl-CoA depletion from the reaction, inspired us to determine the activating potential of 1 and 2. Incubation of FabH with 125 μM of 1 or 2 then addition of 250 μM malonyl-CoA still yields progress curves with lag phases for the production of acetoacetyl-CoA, revealing 1 or 2 are not strong activators, Figure 2O/P. The data was fit similarly to the progress curves of malonyl-CoA alone, but with inhibition as shown in the supplemental information. Fitting suggested both 1 and 2 act as inhibitors since the time to completion is longer (compare Figure 2J with 2O or 2P), however more data is needed to generate reliable inhibition values. Rather than HPLC analysis, we describe a UV-Vis assay to assess inhibition below.
FabH with 1 or 2 and malonyl-CoA is expected to generate acetoacetyl-CoA, if the substrate analogs can react with the active site cysteine to generate an acyl-enzyme intermediate. However, the same amount of acetoacetyl-CoA was generated with malonyl-CoA alone and when the acetyl-CoA analogs were added at 50% of the malonyl-CoA concentration (Figure 2J/O/P). The identical levels of acetoacetyl-CoA product (Figure 2J/O/P) indicate the formation of acyl-enzyme intermediate from the analogs is insignificant compared to the background rate of malonyl-CoA decarboxylation to acetyl-CoA, explaining why no additional acetoacetyl-CoA product was generated in the presence of the acetyl-CoA analogs revealing they are not efficient substrates.
We examined the ability of the primed acetyl-FabH acyl-enzyme intermediate to decarboxylate 3 or 4 and produce the respective acetoacetyl-CoA analogs. In 24 hour reactions, neither 3 or 4 lead to the production of acetoacetyl-oxa(dethia)CoA or acetoacetyl-aza(dethia)CoA as judged by LCMS. The HPLC traces for these reactions were complicated by overlapping peaks, so we explored an alternative that would allow us to directly monitor the decarboxylation of 3 or 4 by an activated FabH.
The FabH Cys112→Gln mutation is expected to generate a FabH locked into an acyl-enzyme intermediate state primed for decarboxylation, Figure 1B. The stability of malonyl-CoA and analogs was tested with the FabH C112Q mutant. Again, while malonyl-CoA was relatively rapidly converted to acetyl-CoA at pH 7 or 8, malonyl-oxa(dethia)CoA was relatively unreactive and malonyl-aza(dethia)CoA was completely stable over 24 hours, Figure 4. The rate of malonyl-CoA decarboxylation by FabH C112Q was ¼ the rate of malonyl-CoA decarboxylative Claisen condensation by the wild-type enzyme at pH 8. The lack of an electrophile or appropriate proton donor in the active site is expected for FabH C112Q, thus water is likely to fulfill that role, accounting for the lower rate of decarboxylation. FabH C112Q decarboxylation of malonyl-CoA is 5 times faster than activated wild-type in the absence of acetyl-CoA and there is no lag phase, suggesting FabH C112Q represents an activated acyl-enzyme state. The stability of the analogs against decarboxylation by FabH C112Q again reveals they will be useful for structure-function studies.
Figure 4.
Analyses of 10 μM FabH C112Q activity with malonyl-CoA. HPLC traces for FabH activity with A) malonyl-CoA, C) 3, D) 4. Kinetic analysis of the reaction monitored by HPLC, B) FabH decarboxylation of malonyl-CoA. Fitting of the B) progress curves are described in the supplemental information. The HPLC data points in the kinetic analysis are shown as green circles for CoA, red squares for malonyl-CoA and yellow or black diamonds for acetyl- or acetoacetyl-CoA.
The pH rate profile of FabH C112Q and even wild-type with respect to non-physiological malonyl-CoA decarboxylation is puzzling. At pH 6 FabH C112Q displays a lag phase for the decarboxylation of malonyl-CoA and a dramatically reduced rate, Supplemental Figure S4. One might expect that at low pH, protonation of the enolate intermediate would proceed faster due to a higher concentration of proton, but that isn’t the case. In one proposed mechanism for decarboxylation, the active site histidine and asparagine form hydrogen bonds with the substrate malonyl-thioester ketone, Figure 5A.9 In the proposed mechanism where the histidine plays a role in decarboxylaton/enolate stabilization, the active site histidine is in a neutral state. One explanation for abolished decarboxylation activity in the absence of acetyl-CoA at pH 6 is that the histidine becomes protonated and changes conformation, Figure 5B. Yet the wild-type enzyme remains active at pH 6 with both substrates. Somehow, wild-type acyl-enzyme intermediate formation prevents protonation of the active site histidine. Comparison of structures of the FabH C112Q mutant at low and high pH should provide insight into the change in catalytic activity.
Figure 5.
Reactions in the active site of FabH and C112Q mutant. A) Putative interactions for malonyl-thioester decarboxylation and Claisen condensation reactions for wild-type. B) The C112Q mutant likely adopts a conformation similar to wild-type above pH 6.5, but below pH 6.5 protonation of the active site histidine is likely and prevents substrate binding through an unknown mechanism, possibly a conformational change.
In order to obtain inhibition constants, we used a continuous UV-Vis assay for the production of acetoacetyl-CoA. Previously a discontinuous UV-Vis assay based on 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB, Ellman’s reagent) as a probe of CoA production was used to examine the inhibition of FabH by small molecules.29 However, we found that incubation of FabH with DTNB resulted in some precipitation of the protein and we were concerned that the CoA-TNB adduct could inhibit FabH.33 Rather, we chose to follow the production of acetoacetyl-CoA, which has a reasonable extinction coefficient at 302 nm of 17.7 mM−1 cm−1 in the presence of 50 mM Mg2+ at pH 8.0 (see Supplemental Figure S5 for extinction coefficient determination under assay conditions) and has been previously described for FabH at pH 7 where the extinction coefficient is lower.28, 34 Comparison of FabH enzymatic reactions in the presence and absence of Mg2+ followed by HPLC revealed no inhibition by 50 mM Mg2+. Using the UV-Vis assay, we determined an apparent Km of 0.3±0.1 μM for acetyl-CoA with 125 μM malonyl-CoA, Figure 6. With acetyl-CoA at 125 μM and varying malonyl-CoA, we noticed substrate inhibition at high malonyl-CoA concentrations. Fitting the data to a substrate inhibition model gave a malonyl-CoA Km of 2.5±0.8 mM, a Ki of 1.9±0.6 mM, a kcat of 5.9±1.4 min−1 and kcat/Km of 0.0024 μM−1·min−1, Figure 6.
Figure 6.
Determination of catalytic parameters for FabH activity with acetyl- and malonyl-CoA. A) Varying acetyl-CoA while holding malonyl-CoA at 125 μM. B) Varying malonyl-CoA while holding acetyl-CoA at 125 μM. Error bars may be smaller than the dots used for the data points. Error reported is propagated from fitting based on 3 technical replicates.
In previous reports at pH 7.0 with malonyl-S-ACP as an acceptor, the observed kcats are on the order of 2-3 s−1, with an apparent KM of 5 μM for malonyl-ACP and 40 μM for acetyl-CoA, approximate kcat/KM of 24 μM−1·min−1 and 3 μM−1·min−1 respectively.32, 35 Previous experiments using malonyl-CoA as a substrate at 5 mM but pH 7 determined a Vi of ~3 min−1, which is close to our result of ~6 min−1 at pH 8.28 Comparison of kcat/KM demonstrates malonyl-S-ACP is a better substrate than malonyl-CoA by four orders of magnitude. To determine the importance of the malonyl-CoA nucleotide, we explored FabH catalysis with malonyl-S-pantetheine, see supplemental information for synthesis. Based on the poor FabH activity with malonyl-CoA, one could conclude that FabH has specifically evolved against using that substrate. Changing the adenosine triphosphate phosphate moiety of CoA to proton could yield a better substrate. Yet malonyl-S-pantetheine is an even poorer substrate than malonyl-CoA with a kcat/KM that is 20 times lower, suggesting FabH has evolved to use ACP interactions to increase activity. As such, we did not attempt to determine inhibition constants for the acetyl- and malonyl-pantetheine substrate analogs. We expect follow up studies comparing structures of FabH in the presence of malonyl-CoA and malonyl-S-ACP analogs will reveal how the significant substrate specificity is determined, but these studies need to be guided by knowledge of analog behavior with the enzyme.
In order to generate inhibition constants, we used the assumption that acetyl-CoA going to acyl-enzyme intermediate is very fast compared to decarboxylative Claisen condensation with malonyl-CoA (acetyl-CoA transthiolation rate 0.05 s−1 vs overall reaction 0.016 s−1 with malonyl-S-ACP as reported previously)21. As such, our inhibitors compete with malonyl-CoA binding. In the UV-Vis assays we did not find inhibition by acetyl-oxa(dethia)CoA and unexpectedly, we noticed a couple percent activation. This suggests acetyl-oxa(dethia)CoA somewhat mimics acetyl-CoA as far as promoting FabH activation. The activation suggests a dual role for acetyl-oxa(dethia)CoA, since there was some inhibition seen for FabH decarboxylation of malonyl-CoA to acetoacetyl-CoA in the HPLC assay. Inhibition by acetyl-aza(dethia)CoA is moderately stronger than substrate binding, Ki of 800 μM, see Table 2 for inhibition constants.
Table 2.
Inhibition constants of acetyl/malonyl-oxa/aza(dethia)CoA analogs.
| Substrate or Product analog | Ki (mM) |
|---|---|
| 1 acetyl-oxa(dethia)CoA | weak activation |
| 2 acetyl-aza(dethia)CoA | 0.8±0.1 |
| 3 malonyl-oxa(dethia)CoA | 0.8±0.4 |
| 4 malonyl-aza(dethia)CoA | 0.7±0.3 |
| 5 oxa(dethia)CoA | 0.4±0.2 |
| 6 aza(dethia)CoA | 0.9±0.4 |
| CoA | 0.15±0.07 |
With product analogs in hand, we explored their ability to inhibit the reaction, again based on an assumption they competed for malonyl-CoA binding. We found that CoA is a reasonable inhibitor with a Ki of 0.15 mM, while, oxa(dethia)CoA inhibits with a Ki of 0.4 mM and aza(dethia)CoA inhibits with a Ki of 0.9 mM.
When considering the malonyl-CoA substrate has a Km of ~2.5 mM the inhibition constants for the malonyl-CoA substrate analogs are respectable, Table 2. Recognizing that the kcat/KM for malonyl-ACP is 10,000 fold larger than that of malonyl-CoA, suggests appending the malonyl-thioester isosteres to the ACP will also engender them with tighter binding on the same order of magnitude.
There are two previous reports of malonyl- and methylmalonyl-aza(dethia)CoA synthesis and use in enzyme assays.7-8 In both cases the material was tested for the ability to undergo decarboxylative Claisen condensation or decarboxylative Michael addition. Minor enzymatic activity with the malonyl-oxa(dethia)CoA analog has been reported, while we here demonstrate again that the malonyl-aza(dethia)CoA is stable in the presence of an enzyme. The β-keto acid decarboxylation would normally be enabled by the formation of an enolate intermediate, however amide resonance competes with enolate formation. The malonyl-oxa(dethia)CoA analog also likely behaves similar to the amide analog with regard to ester resonance competing with enolate formation. For example, if we consider the methyl-group pKa of acetyl-CoA and analogs (pKa ~21 for the thioester, ~26 for the ester and ~28 for the amide),36 it is easiest to deprotonate the methyl-group of acetyl-thioester, then the acetyl-ester and hardest to deprotonate the acetyl-amide. Decarboxylation to generate the same enolate as deprototonation of the methyl-group is expected to follow a similar pattern. Stabilization of the enolate during malonyl-CoA analog decarboxylation by FabH is appropriately 5 orders of magnitude more difficult for the ester and 7 for the amide compared to the thioester. This phenomena reveals why the analogs are stable and likely promising isosteres for structure-function studies in experiments that take hours to days including NMR and crystallography.
In a previous study of fluoroacetyl-CoA hydrolase, the acetyl-CoA oxa/aza analogs were generated and compared with the corresponding fluorinated analogs as substrates.37 In those studies, the aza analogs were completely stable, whereas the oxa analogs were substrates at very low rates. In our study, we found that the oxa analogs undergo minor hydrolysis. With fluoroacetyl-CoA hydrolase, the fluoroacetyl-CoA Km is 8 μM,38 while the Kd for fluoroacetyl-oxa(dethia)CoA is 90 μM and fluoroacetyl-aza(dethia)CoA is 1700 μM, indicating large decreases in affinity for the oxa/aza substrate analogs. In our case, an assay that directly monitors the competition for generation of acyl-enzyme intermediate is needed to determine inhibition constants for the acetyl-oxa/aza(dethia)CoA analogs with FabH for the transthiolation reaction. However, such assays are inherently based on single-turnover kinetics and outside the scope of this report. Nevertheless, our data differ from fluoroacetyl-CoA hydrolase, where acetyl-aza(dethia)CoA is an inhibitor on the same order of magnitude as the malonyl-CoA Km. Furthermore, other examples where acetyl-aza(dethia)CoA has binding properties similar to acetyl-CoA are citrate synthase, where the acetyl-aza(dethia)CoA Ki is reported to be 1.6 μM, which is lower than the 8.5 μM acetyl-CoA Km, and phosphotransacetylase with a Ki of 130 μM and acetyl-CoA Km of 210 μM.39 Together these studies reveal the diverse behavior of acetyl-oxa/aza(dethia)CoA analogs with enzymes and their potential usefulness for enzyme structure-function studies.
In conclusion, we have demonstrated an alternate synthetic route to malonyl-CoA analogs where the thioester has been replaced with an ester or amide. These analogs were found to be generally stable over a 24-hour time course with FabH, a ketosynthase which performs acyl transfer and decarboxylative Claisen-condensation. Furthermore, while assessing substrate stability in the presence of FabH, we have revealed some interesting aspects of FabH catalysis, especially hysteresis. Previous reports of FabH reactivity with CoA disulfides revealed that there was likely a disordered conformation of FabH in the absence of acetyl-CoA along with negative cooperativity between the FabH active sites of the dimer for transthiolation activity.21 However, the structural basis for cooperativity was not apparent in crystal structures. We expect careful examination of FabH structures in the presence of acetyl-CoA analogs will reveal deeper insights into previously suggested FabH negative cooperativity. Furthermore, variation of wild-type and C112Q FabH activities as a function of pH poses further investigation, why is it that the mutant is inactive around pH 6, while wild-type remains active? The variation in pH sensitivity reveals that the active site cysteine to glutamine mutants should only be used at a pH above 7 for structural studies with malonyl-S-ACP analogs. We anticipate that neutron diffraction experiments combined with substrate analogs will provide evidence for activation of FabH in the presence of substrate and knowledge of the protonation state of titratable residues critical to catalysis.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge support from the Purdue University Center for Cancer Research’s American Cancer Society Institutional Grant program for new investigators and NIH R01 GM140290-03.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Synthesis and NMR data for 1-6, protein production, and enzymology procedures. Figures for kinetics of FabH and FabH C112Q at various pH.
REFERENCES
- 1.Mishra PK; Drueckhammer DG, Coenzyme A Analogues and Derivatives: Synthesis and Applications as Mechanistic Probes of Coenzyme A Ester-Utilizing Enzymes. Chem. Rev 2000, 100 (9), 3283–3310. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni RA; Worth AJ; Zengeya TT; Shrimp JH; Garlick JM; Roberts AM; Montgomery DC; Sourbier C; Gibbs BK; Mesaros C; Tsai YC; Das S; Chan KC; Zhou M; Andresson T; Weissman AM; Linehan WM; Blair IA; Snyder NW; Meier JF, Discovering Targets of Non-enzymatic Acylation by Thioester Reactivity Profiling. Cell Chem. Biol 2017, 24 (2), 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benning MM; Haller T; Gerlt JA; Holden HM, New reactions in the crotonase superfamily: structure of methylmalonyl CoA decarboxylase from Escherichia coli. Biochemistry 2000, 39 (16), 4630–9. [DOI] [PubMed] [Google Scholar]
- 4.Ellis BD; Milligan JC; White AR; Duong V; Altman PX; Mohammed LY; Crump MP; Crosby J; Fuo R; Vanderwal CD; Tsai SC, An Oxetane-Based Polyketide Surrogate To Probe Substrate Binding in a Polyketide Synthase. J. Am. Chem. Soc 2018, 140 (15), 4961–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancia F; Smith GA; Evans PR, Crystal structure of substrate complexes of methylmalonyl-CoA mutase. Biochemistry 1999, 38 (25), 7999–8005. [DOI] [PubMed] [Google Scholar]
- 6.Tosin M; Spiteller D; Spencer JB, Malonyl carba(dethia)- and malonyl oxa(dethia)-coenzyme A as tools for trapping polyketide intermediates. ChemBioChem 2009, 10 (10), 1714–23. [DOI] [PubMed] [Google Scholar]
- 7.Tosin M; Betancor L; Stephens E; Li WM; Spencer JB; Leadlay PF, Synthetic chain terminators off-load intermediates from a type I polyketide synthase. ChemBioChem 2010, 11 (4), 539–46. [DOI] [PubMed] [Google Scholar]
- 8.Hamed RB; Henry L; Gomez-Castellanos JR; Asghar A; Brem J; Claridge TD; Schofield CJ, Stereoselective preparation of lipidated carboxymethyl-proline/pipecolic acid derivatives via coupling of engineered crotonases with an alkylmalonyl-CoA synthetase. Org. Biomol. Chem 2013, 11 (47), 8191–6. [DOI] [PubMed] [Google Scholar]
- 9.White SW; Zheng J; Zhang YM; Rock, The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem 2005, 74, 791–831. [DOI] [PubMed] [Google Scholar]
- 10.Jackowski S; Rock CO, Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J. Biol. Chem 1987, 262 (16), 7927–7931. [PubMed] [Google Scholar]
- 11.Price AC; Choi KH; Heath RJ; Li Z; White SW; Rock CO, Inhibition of beta-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. Structure and mechanism. J. Biol. Chem 2001, 276 (9), 6551–9. [DOI] [PubMed] [Google Scholar]
- 12.Jackowski S; Murphy CM; Cronan JE; Rock CO, Acetoacetyl-acyl carrier protein synthase. J. Biol. Chem 1989, 264 (13), 7624–7629. [PubMed] [Google Scholar]
- 13.Radka CD; Rock CO, Mining Fatty Acid Biosynthesis for New Antimicrobials. Annu. Rev. Microbiol 2022, 76, 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Castillo Y; Froeyen M; Nowé A; Cabrera-Pérez MÁ, Bacterial FabH: Towards the Discovery of New Broad-Spectrum Antibiotics. In Recent Advances in Medicinal Chemistry, Vol. 1; Atta-ur-Rahman, Choudhary M, Perry G, Eds.; Elsiver, 2014; pp 131–158. [Google Scholar]
- 15.Williamson IP; Wakil SJ, Studies on the Mechanism of Fatty Acid Synthesis. XVII. Preparation and General Properties of Acetyl Coenzyme A and Malonyl Coenzyme A-Acyl Carrier Protein Transacylases. J. Biol. Chem 1966, 241 (10), 2326–2332. [PubMed] [Google Scholar]
- 16.Alberts AW; Majerus PW; Talamo B; Vagelos PR, Acyl-Carrier Protein. II. Intermediary Reactions of Fatty Acid Synthesis. Biochemistry 1964, 3, 1563–71. [DOI] [PubMed] [Google Scholar]
- 17.Tsay JT; Oh W; Larson TJ; Jackowski S; Rock CO, Isolation and characterization of the beta-ketoacyl-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12. J. Biol. Chem 1992, 267 (10), 6807–14. [PubMed] [Google Scholar]
- 18.Davies C; Heath RJ; White SW; Rock CO, The 1.8 Å crystal structure and active-site architecture of β-ketoacyl-acyl carrier protein synthase III (FabH) from Escherichia coli. Structure 2000, 8 (2), 185–195. [DOI] [PubMed] [Google Scholar]
- 19.Witkowski A; Joshi AK; Lindqvist Y; Smith S, Conversion of a β-Ketoacyl Synthase to a Malonyl Decarboxylase by Replacement of the Active-Site Cysteine with Glutamine. Biochemistry 1999, 38 (36), 11643–11650. [DOI] [PubMed] [Google Scholar]
- 20.Qiu X; Janson CA; Smith WW; Head M; Lonsdale J; Konstantinidis AK, Refined structures of β-ketoacyl-acyl carrier protein synthase III. J. Mol. Biol 2001, 307 (1), 341–56. [DOI] [PubMed] [Google Scholar]
- 21.Alhamadsheh MM; Musayev F; Komissarov AA; Sachdeva S; Wright HT; Scarsdale N; Florova G; Reynolds KA, Alkyl-CoA disulfides as inhibitors and mechanistic probes for FabH enzymes. Chem. Biol 2007, 14 (5), 513–24. [DOI] [PubMed] [Google Scholar]
- 22.Gajiwala KS; Margosiak S; Lu J; Cortez J; Su Y; Nie Z; Appelt K, Crystal structures of bacterial FabH suggest a molecular basis for the substrate specificity of the enzyme. FEBS Lett. 2009, 583 (17), 2939–46. [DOI] [PubMed] [Google Scholar]
- 23.Stunkard LM; Dixon AD; Huth TJ; Lohman JR, Sulfonate/Nitro Bearing Methylmalonyl-Thioester Isosteres Applied to Methylmalonyl-CoA Decarboxylase Structure-Function Studies. J. Am. Chem. Soc 2019, 141 (13), 5121–5124. [DOI] [PubMed] [Google Scholar]
- 24.Crans DC; Kazlauskas RJ; Hirschbein BF; Wong C-H; Abril O; Whitesides GM, Enzymatic regeneration of adenosine 5′-triphosphate: Acetyl phosphate, phosphoenolpyruvate, methoxycarbonyl phosphate, dihydroxyacetone phosphate, 5-phospho-α-d-ribosyl pyrophosphate, uridine-′-diphosphoglucose. Methods Enzymol. 1987, 136, 263–280. [DOI] [PubMed] [Google Scholar]
- 25.Meier JL; Burkart MD, Synthetic Probes for Polyketide and Nonribosomal Peptide Biosynthetic Enzymes. Methods Enzymol. 2009, 458, 219–254. [DOI] [PubMed] [Google Scholar]
- 26.Hou J; Zheng H; Tzou WS; Cooper DR; Chruszcz M; Chordia MD; Kwon K; Grabowski M; Minor W, Differences in substrate specificity of V. cholerae FabH enzymes suggest new approaches for the development of novel antibiotics and biofuels. FEBS J. 2018, 285 (15), 2900–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdeva S; Musayev FN; Alhamadsheh MM; Scarsdale JN; Wright HT; Reynolds KA, Separate entrance and exit portals for ligand traffic in Mycobacterium tuberculosis FabH. Chem. Biol 2008, 15 (4), 402–12. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YM; Rao MS; Heath RJ; Price AC; Olson AJ; Rock CO; White SW, Identification and analysis of the acyl carrier protein (ACP) docking site on β-ketoacyl-ACP synthase III. J. Biol. Chem 2001, 276 (11), 8231–8. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrom AG; Wang JT; Bella J; Campopiano DJ, Non-invasive (19)F NMR analysis of a protein-templated N-acylhydrazone dynamic combinatorial library. Org. Biomol. Chem 2018, 16 (43), 8144–8149. [DOI] [PubMed] [Google Scholar]
- 30.Frieden C., Slow transitions and hysteretic behavior in enzymes. Annu. Rev. Biochem 1979, 48, 471–89. [DOI] [PubMed] [Google Scholar]
- 31.Heath RJ; Rock CO, Regulation of Fatty Acid Elongation and Initiation by Acyl-Acyl Carrier Protein in Escherichia coli. Journal of Biological Chemistry 1996, 271 (4), 1833–1836. [DOI] [PubMed] [Google Scholar]
- 32.Heath RJ; Rock CO, Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J. Biol. Chem 1996, 271 (18), 10996–1000. [DOI] [PubMed] [Google Scholar]
- 33.Acheampong KK; Kokona B; Braun GA; Jacobsen DR; Johnson KA; Charkoudian LK, Colorimetric Assay Reports on Acyl Carrier Protein Interactions. Sci. Rep 2019, 9 (1), 15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern JR, Optical Properties Of Acetoacetyl-S-Coenzyme A and Its Metal Chelates. J. Biol. Chem 1956, 221 (1), 33–44. [PubMed] [Google Scholar]
- 35.Khandekar SS; Konstantinidis AK; Silverman C; Janson CA; McNulty DE; Nwagwu S; Van Aller GS; Doyle ML; Kane JF; Qiu X; Lonsdale J, Expression, purification, and crystallization of the Escherichia coli selenomethionyl β-ketoacyl-acyl carrier protein synthase III. Biochem. Bioph. Res. Co 2000, 270 (1), 100–7. [DOI] [PubMed] [Google Scholar]
- 36.Amyes TL; Richard JP, Substituent Effects on Carbon Acidity in Aqueous Solution and at Enzyme Active Sites. Synlett 2017, 28 (12), 2407–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weeks AM; Wang N; Pelton JG; Chang MCY, Entropy drives selective fluorine recognition in the fluoroacetyl-CoA thioesterase from Streptomyces cattleya. P. Natl. Acad. Sci. USA 2018, 115 (10), E2193–E2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weeks AM; Coyle SM; Jinek M; Doudna JA; Chang MC, Structural and biochemical studies of a fluoroacetyl-CoA-specific thioesterase reveal a molecular basis for fluorine selectivity. Biochemistry 2010, 49 (43), 9269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart CJ; Dixon RG; McClendon EM; Swearingen LW Synthesis of Acetylaminodesthio-Coenzyme A, a Competitive Inhibitor of Acetyl-Coenzyme A. In Federation Proceedings 1942-1987, Federation of American Societies for Experimental Biology; 1975, 34 (3), 690. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.