Abstract
The Programmed death-1 (PD-1) and its programmed death-ligand 1 (PD-L1) comprise the PD-1/PD-L1 axis and maintain tumor immune evasion. Cancer immunotherapy based on anti-PD-1/PD-L1 antibodies is the most promising anti-tumor treatment available but is currently facing the thorny problem of unsatisfactory outcomes. Traditional Chinese Medicine (TCM), with its rich heritage of Chinese medicine monomers, herbal formulas, and physical therapies like acupuncture, moxibustion, and catgut implantation, is a multi-component and multi-target system of medicine known for enhancing immunity and preventing the spread of disease. TCM is often used as an adjuvant therapy for cancer in clinical practices, and recent studies have demonstrated the synergistic effects of combining TCM with cancer immunotherapy. In this review, we examined the PD-1/PD-L1 axis and its role in tumor immune escape while exploring how TCM therapies can modulate the PD-1/PD-L1 axis to improve the efficacy of cancer immunotherapy. Our findings suggest that TCM therapy can enhance cancer immunotherapy by reducing the expression of PD-1 and PD-L1, regulating T-cell function, improving the tumor immune microenvironment, and regulating intestinal flora. We hope this review may serve as a valuable resource for future studies on the sensitization of immune checkpoint inhibitors (ICIs) therapy.
Keywords: traditional Chinese medicine, cancer immunotherapy, sensitization, immune checkpoint inhibitor, PD-1/PD-L1 axis
Introduction
Cancer immunotherapy is an immunological principles-based treatment to enhance the host’s immune response against tumor cells, with the ultimate goal of tumor clearance (1, 2). As a revolutionary advancement in tumor treatment after surgery, radiation, and chemotherapy, it has the benefits of good efficacy, broad indications, long-lasting remission, and high targetability (3, 4). One of the most intriguing and efficient methods for enabling the body’s immune cells to eradicate tumors is immune checkpoint blockage. An emerging trend in oncology (5, 6) is next-generation combination therapies that simultaneously use several pathways to enhance tumor immunosuppression and provide an anti-tumor effect. However, drug toxicity, drug resistance, and immune-related adverse effects are only a few obstacles faced when using cancer immunotherapy, whether as a single agent or in combination (7–9). Therefore, we focused on the existing cancer immunotherapy sensitization (10).
Traditional Chinese medicine, including monomers, Chinese herbal formulas, acupuncture, moxibustion, and other types, is often used as adjuvant therapy for cancer patients. As a result of its ability to directly inhibit or kill tumor cells (11) or to decrease tumor immune escape through innate and adaptive immunity modulation, it shows considerable potential as a cancer immunotherapy (12, 13). Several recent in vivo and in vitro investigations have shown that TCM may increase the therapeutic sensitivity of cancer immunotherapy and that TCM, in conjunction with PD-1/PD-L1 monoclonal antibodies, can succeed even more. This review aimed to provide an understanding of the potential sensitization mechanisms of PD-1/PD-L1 axis-based TCM therapy and the synergistic effects of cancer immunotherapy.
PD-1/PD-L1 axis
First identified in 1992 by Tasuku Honjo (14), Programmed death-1 (PD-1) is a transmembrane glycoprotein with a molecular weight of 50-55 kDa (15) expressed on activated T cells, B cells, and macrophages. Under normal body conditions, it helps immune cells distinguish between healthy and damaged tissue, hence maintaining peripheral immune tolerance. In addition to its involvement in regulating autoimmunity, cancer immunity, and infection immunity, it also significantly regulates other types of immune responses (16).
The term “PD-1/PD-L1 axis”, often mentioned in cancer immunity, refers to the signaling pathway in which PD-1 and its PD-L1 ligand are the primary molecules (17). PD-L1, also known as CD274 or B7-H1, can be expressed on various immune cells such as T cells, B cells, NK cells, macrophages, dendritic cells, and epithelial cells, and can also exist in an extracellular form as exosomes or soluble proteins (18, 19). In pathological conditions, PD-L1 can be overexpressed on the surface of multiple tumor cells, leading to tumor invasion (20). Immune checkpoints PD-1 and PD-L1 have been the subject of numerous investigations, and PD-1/PD-L1 blockade-based immunotherapy has had unprecedented success. Additionally, PD-L2, a less well-studied PD-1 ligand that can be expressed independently of PD-L1 in tumor tissue (21), is mainly found on antigen-presenting cells and inhibits CD4+ T cell activation and proliferation upon binding to PD-1 (22).
Tumor immune escape and PD-1/PD-L1 axis
Cancer immunity is a cyclical process that begins with releasing cancer cell antigens and their presentation to T lymphocytes by antigen-presenting cells. Naive T cells then attach to MHC class I antigen fragments and differentiate into CD8+ T cells, which are cytotoxic effector T cells (23). The primed and activated effector T cells gradually migrate and infiltrate into the tumor tissues, identify the tumor by specific T cell receptor (TCR) and antigen binding, and then release perforin, granzyme B (GZMB), and interferon-gamma (IFN-γ) to induce killing of tumor cells (24). Tumor immune escape can result from ineffectiveness at any stage of the cancer immune cycle, including loss of antigenic epitopes, decreased antigen presentation, unsuccessful activation of tumor-specific T cells, and inadequate infiltration of effector T cells (25). Due to a chronic inflammatory environment and constant exposure to tumor antigen stimulation (26, 27), CD8+ T cells — the primary driver of anti-tumor immunity — might gradually become dysfunctional or exhausted. The PD-1/PD-L1 axis, on the other hand, may aggravate the induction of T cell apoptosis, anergy, and exhaustion, hence contributing to primary tumor immune escape and poor cancer prognosis (28–30). PD-1 destroys T cell functions by dephosphorylating molecules downstream of the TCR by recruiting Src homology 2-containing protein tyrosine phosphatase 2 (SHP2) (31).
Tumor immune escape occurs in the complex tumor immune microenvironment (TME), which is the site of antagonism between immune effects and immune suppression. The natural killer (NK) cells, macrophages, T helper 1 (Th1) cells, and Th17 cells, differentiated from CD4+ T cells, positively promote tumor immunity (32–34). In contrast, regulatory T (Treg) cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) mainly act as negative characters and together promote tumor immune escape and tumor metastasis (35, 36). These cells can then release cytokines that regulate the PD-1/PD-L1 axis directly, and most of them can activate the PD-1/PD-L1 axis, producing a superimposed suppressive effect on tumor immunity (37). Interferon-γ (IFN-γ), produced by activated T cells, can boost the immune system’s capacity to kill tumors in the preprophase. It may also up-regulate PD-L1 expression in tumor cells by activating the JAK/STAT1/IRF1 signaling pathway (38, 39). PD-L1 is up-regulated in tumor cells when TNF-α activates the NF-κB signaling pathway, increasing expression of the IFN-γ receptor (IFNGR1 and IFNGR2) (40). Through activation of NF-κB and ERK1/2 signaling pathways in tumor cells, interleukin-17(IL-17) released by Th17 cells may up-regulate PD-L1 expression (41). The transcription factor STAT3 is responsible for the effects of macrophage-derived IL-6 on CD8+ T cells (42). Infiltrating TAM may cause PD-L1 expression by secreting IL-6 and TNF-α (43). However, PD-1 expression in T cells may also be up-regulated by common gamma-chain cytokines, such as IL-2, IL-7, IL-15, and IL-21 (44). The PD-1/PD-L1 axis may also regulate cytokines in the TME. Transforming growth factor-β (TGF-β) has been shown to inhibit T-cell-induced immunological infiltration by stimulating collagen production to form a physical barrier (45). However, PD-L1 has increased tumor cell proliferation and invasion by up-regulating BAG-1 expression, lowering TGF-β levels, and subsequently down-regulating SMAD4 (46).
Cancer Immunotherapy and the sensitization strategy of TCM
Cancer immunotherapy can be divided into two categories based on the host immune system’s role in fighting tumors: active and passive immunotherapy (47). Active immunotherapy utilizes tumor antigens to increase the tumor’s immunogenicity and directly stimulate an immune response (48). This includes various therapeutic cancer vaccines. On the other hand, passive immunotherapies, such as monoclonal antibodies, adoptive cell therapy, and oncolytic virotherapy (49), rely on pre-induced antibodies or immune cells to mediate the host immune system’s response against the tumor cells (50). It has been suggested that passive immunotherapies may also possess moderate active immunological activity (51). Immune checkpoint inhibitors (ICIs) therapy targeting the PD-1/PD-L1 axis has been developed to address this (52, 53). This therapy utilizes monoclonal antibodies against PD-1 and PD-L1 to bind to tumor cells and T cells and overcome immunosuppression to enable effector functions. When tumor cells utilize the PD-1/PD-L1 axis to prevent an immunological attack, cancer immunotherapy using PD-1/PD-L1 inhibition can help the immune system re-identify and eliminate cancerous cells. Anti-PD-1 antibodies such as pembrolizumab and nivolumab, as well as anti-PD-L1 antibodies like atezolizumab and durvalumab, have been approved for the treatment of advanced melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and other cancers making them the foremost therapeutic agents in the oncology field (54). However, despite being the leading therapeutic agent in oncology, the current population that benefits from ICIs therapy falls short of expectations. This has prompted a search for more sensitization techniques to maximize anti-cancer effectiveness. Existing sensitization methods aim to directly impact low-antigen and effector T cells or indirectly influence tumor growth by inhibiting angiogenesis and mesenchymal transition (10, 55). Studies on the sensitization of this axis should receive adequate attention considering the importance of the PD-1/PD-L1 axis in tumor immunosuppression.
Based on the fundamental principles of Chinese medicine, TCM treatment seeks to restore a healthy Yin-Yang equilibrium inside the body. “Yin” denotes all adverse, inhibitive bodily processes, whereas “Yang” denotes all advantageous, stimulating processes. This action can be regarded as Yin when the PD-1/PD-L1 axis is activated and further inhibits the organism’s immune system. The following four diagnostic techniques (“Si Zhen” in Chinese) are used by TCM practitioners to obtain data: 1) Inspection (“Wang Zhen”) refers to the doctors’ ability to examine the patient’s bodily and mental conditions, especially the tongue. 2) Auscultation and olfaction (“Wen Zhen”) refer to the medical term for the process by which a doctor makes a diagnosis by hearing and smelling the patient’s speech (including breathing, coughing, belching, etc.) and the smell of feces to conclude. 3) Inquiry (Wen Zhen) refers to the TCM physician’s use of conversation to provoke information from the patient and others about the disease’s onset, progression, present-day symptoms, and any prior treatments. 4) Pulse-feeling and palpation (“Qie Zhen”) necessitate the medical professional to feel the patient’s pulse as well as their skin, chest, and abdomen to look for any abnormal symptoms. The patient’s state can be determined by these four criteria, which can assist in selecting an optimal personalized TCM treatment and increase the efficacy of cancer immunotherapy. The disease will be treated with Chinese medicines and physical procedures once the relevant therapeutic parameters have been determined through the empirical debate. Herbal monomers have been the research subject for their potential in herbal medicine. Natural products like polysaccharides, flavonoids, alkaloids, saponins, and organic acids may be obtained from either animal or plant extracts and are considered endogenous active ingredients. Various herbal remedies may share the same active ingredient (56).
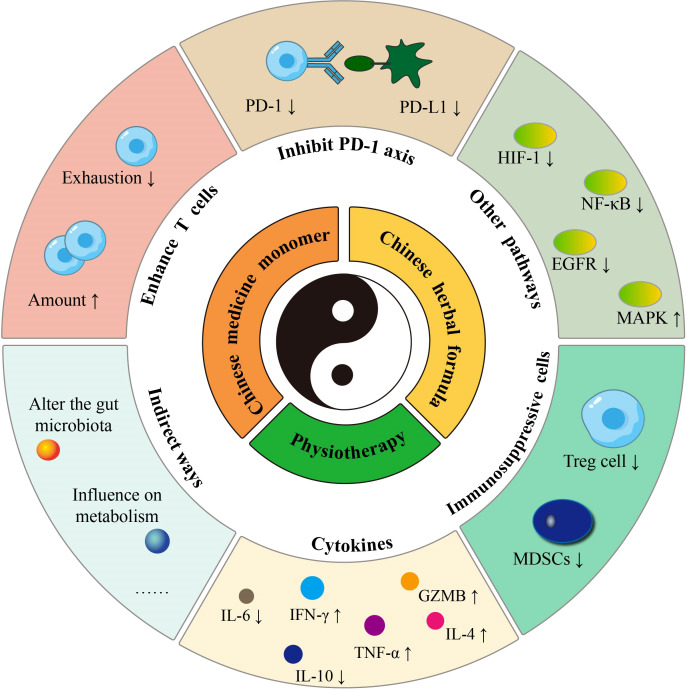
Consequently, a single herbal medicine often contains numerous active substances and exhibits multi-component, multi-target, and multi-pathway characteristics. Herbal monomers have been shown in pharmacological studies to have anti-inflammatory, antibacterial, antiviral, antioxidant, anti-tumor, and immune system-modulating activities (57). Several herbs with different therapeutic effects are combined into a single Chinese herbal formula according to precise combination criteria. (a) Sovereign (the principal medicinal herb for the major signs), (b) Minister (the herb that assists the sovereign), (c) Assistant (the herb that acts as an adjuvant or restraining agent), and (d) Guide (the herb that can harmonize all medicinal properties and induce them to specific meridians) are the four standard components of each procedure. As a result, herbs that kill tumors, modulate the immune system and mitigate immunotherapy’s adverse effects are frequently combined in a single formula to assist cancer immunotherapy. It may be broken down further into tonifying methods, pathogen elimination approach, and both based on the general guidelines. According to a case report study, Chinese herbal formula successfully treated cases of immune-related lichenoid dermatitis caused by sintilimab treatment in NSCLS patients (58). Acupuncture, moxibustion, cupping, and other methods from TCM are also often employed as adjuvant therapies for cancer patients. These physiotherapies primarily have anti-tumor and immune-boosting benefits by stimulating the acupuncture points of meridians and collaterals. The TCM-sensitized tumor immunotherapy includes killing tumor cells and enhancing T cell function, inhibiting the PD-1/PD-L1 axis, and improving the tumor immunosuppressive microenvironment to exert a sensitizing effect (Figure 1 by Adobe Illustrator). The latter can be manifested in promoting tumor-killing cells, suppressing immunosuppressive cells, and regulating cytokine secretion. Several animal investigations of TCM therapies in tumor immunotherapy sensitization have been carried out, and clinical trials with future translation studies are anticipated to be carried out subsequently.
Figure 1.
The sensitization strategy of TCM therapy.
Chinese medicine monomers sensitize cancer immunotherapy
Polysaccharides
Shortness of breath, exhaustion, palpitations, and thirst are all signs of qi deficit, and ginseng is an effective tonic for restoring vital energy. According to studies, ginseng polysaccharides (GPs), a key component of Panax ginseng, have been shown to reduce inflammation, boost adaptive immunity, and enhance gut flora metabolism (59–61). Research by Jumin Huang et al. (62) showed that GPs could positively impact cancer immunotherapy, both alone and in combination with the αPD-1 monoclonal antibody (αPD-1mAb). In Lewis lung cancer mice, GPs alone inhibited tumor growth and reduced tumor volume and weight. The combination of GPs and αPD-1mAb had an even more substantial effect, resulting in 65.1% tumor growth suppression than αPD-1mAb alone. This combined treatment also increased the CD8+/CD4+ T cells’ ratio. It enhanced the release of immune effector cytokines (IFN-γ, TNF-α, GZMB) in both peripheral and tumor tissue and suppressed Treg cells to improve tumor immunosuppression. Furthermore, GPs improved gut microbiota and further affected tryptophan metabolism through gut microbes. It also converted the immune response phenotype of the tumor, restoring response to αPD-1mAb in mice transplanted with fecal samples from PD-1 non-responders, thereby expanding the potential for PD-1 blockade therapy.
In addition to treating edema, perspiration, and diarrhea, Attractylodes macrocephala strengthens the spleen and improves Qi. The Polysaccharide of Atractylodis Macrocephalae Rhizoma (PAMR) could function as an immune adjuvant that can activate macrophages through NF-κB and JAK/STAT signaling pathways (63, 64). Yicun Han et al. (65) found that PAMR could inhibit the proliferation of PD-L1 high-expressing esophageal cancer cells in vitro and reduce the expression of PD-L1. MicroRNA-34a (miR-34a), on the other hand, could effectively inhibit PD-L1 expression (66). By performing silencing and overexpression assays on miR-34, this study discovered that PAMR inhibited PD-L1 by increasing miR-34a levels. Regarding the regulatory mechanism, microRNAs are a class of small noncoding RNAs that can control gene expression by degrading mRNA or inhibiting translation. Various microRNAs have been shown to exert anti-tumor effects in tumors; therefore, studying Chinese herbal medicine to sensitize cancer immunotherapy from this perspective is a good idea.
Astragalus may relieve chronic ulcers, indigestion, coughing, and sweating by mobilizing the spleen, stomach, qi, and blood. Astragalus polysaccharides (APS), a vital component of the Astragalus, have been found to possess anti-inflammatory, antiviral, immune-enhancing, and gut-microbiome-regulating properties (67, 68). According to a study by Guiqing Ding et al. (69), treatment with APS significantly reduced the size of melanoma tumors in mice after just 14 days compared to the model group. Analysis of the TME showed that APS increased the number of CD8+ T cells and dendritic cells while reducing the proportion of immunosuppressive MDSCs. Additionally, APS reduced the expression of cytokines such as IL-1β, IL-6, TGF-β, and arginase-1, making it easier for CD8+ T cells to target and attack the tumors. 16S rRNA next-generation sequencing results showed that APS could reshape the gut microbiome and regulate metabolism, enhancing the body’s anti-cancer immune response.
Furthermore, research by Xinrui Sha et al. (70) showed that honey-processed Astragalus polysaccharides (HP-APS) could directly inhibit the proliferation of A549, MC38, and B16 tumor cell lines and trigger apoptosis. It also supported the maturation and activation of dendritic cells, improving the presentation of tumor antigens. In melanoma mice, HP-APS treatment reduced the size of tumors, increased the proportion of CD8+ T cells, and resulted in a slower rate of tumor growth when combined with PD-1 monoclonal antibody (PD-1 mAb) treatment, indicating a possible synergistic effect.
Alkaloids
Tetradium ruticarpum dissipates cold, increases Yang, and can be used to treat cold-related pain, vomiting, and diarrhea. Evodiamine, a new cancer-fighting alkaloid extracted from Tetradium fruit, has shown promise in treating prostate cancer (71, 72), hepatocarcinoma (73), and gastric cancer (74). Zebo Jiang et al. (75) found that Evodiamine directly stopped the growth of lung cancer cell lines (H1650 and H1975), causing them to undergo apoptosis and stopping their cell cycle in the G2 phase. This is due to its effects on the MUC1-C gene, triggering a cancer cell’s transition from epithelial to mesenchymal (EMT) and promoting cancer cell invasion (76). Evodiamine was observed to decrease the expression of PD-L1 mRNA and protein 24 hours later; it also held for IFN-induced PD-L1 cell membrane expression. In co-culture with peripheral blood mononuclear cells, Evodiamine reduced T-cell apoptosis and increased the IFN-γ and GZMB to enhance immune effector capability. In the Lewis lung cancer mouse model, Evodiamine inhibited tumor growth, improved mouse survival, and improved the abundance, viability, and effectiveness of CD8+ T cells. Furthermore, when combined with PD-1 mAb, the treatment resulted in more tumor-infiltrating CD8+ T cells and greater secretion of tumor-killing factors like IFN-γ, TNF-α, and GZMB.
Sophora flavescens is a herbal remedy that helps treat diarrhea, bloody stools, jaundice, dermatitis, and clear heat and moisture. Oxymatrine, a quinazine alkaloid extracted from it, has been shown to have antiviral properties in treating chronic hepatitis B (77). Cellular tests have been performed by S. Hua et al. (78). They observed that oxymatrine inhibited cell viability in colorectal cancer cells, and the inhibition became more pronounced with increasing concentrations. When IFN-γ stimulated PD-L1 production in tumor cells, oxymatrine dramatically decreased this stimulation by controlling DNA demethylation.
Polyphenols
Clinical applications of the Chinese herb Rhus chinensis Mill include the treatment of coughing, bloody coughing, sweating, chronic diarrhea, and early ejaculation. Gallic acid (GA) is a polyphenolic compound derived by fermentation from Rhus chinensis Mill that can suppress tumor and tumor-induced oxidative damage (79). According to a study by Biaolong Deng et al. (80), GA negatively impacts the phosphorylation of STAT3 and the expression of the Usp21 gene. The expression of the Usp21 gene leads to the degradation of the Foxp3 protein, which is crucial for the immunosuppressive function of Treg cells and results in the formation of Th1-like Treg cells. Similarly, GA also deubiquitinates PD-L1 expression in Treg cells. In colon cancer, MC38 tumor-bearing mice, both GA alone and with anti-PD-1 antibodies, inhibited tumor growth and prolonged survival. The combination therapy inhibited Foxp3 and PD-L1 protein expression in Treg cells more effectively than the anti-PD-1 antibody alone. The combination therapy also improved the effect of CD8+ cytotoxic T cells by producing more IFN-γ, reducing T-cell exhaustion, core factor Thymocyte selection-associated high-mobility group box (TOX), and immune checkpoint LAG3, increasing TNF-α and delaying the progression of colorectal cancer.
A rhizome from the plant Curcuma longa L., turmeric contains blood-activating properties. It can treat bruises, dysmenorrhea, joint discomfort, and bruises, as well as hypochondriacal and chest pain. A polyphenol from turmeric called curcumin has immune-boosting, anti-cancer, anti-inflammatory, and antioxidant properties (81, 82). It has been suggested to reduce paclitaxel resistance in the treatment of cancer (83). Curcumin’s therapeutic advantages for head and neck squamous cell carcinoma (HNSCC) were studied by Lihua Liu et al. (84). Curcumin decreased PD-L1 and PD-L2 expression in HNSCC cell lines in an in vitro cellular experiment without negatively impacting healthy human fibroblast cells. When curcumin and PD-L1 antibody were combined, tumor development was considerably inhibited, and co-cultured CD8+ T cells released more IFN-γ and GZMB. Curcumin demonstrated a comparable inhibition on tumor growth in oral cancer model mice, and SCC15 cell-established HNSCC tumor-bearing animals, and PD-L1 and PD-L2 expression inhibition was also reported. Higher CD4+ and CD8+ T cells were observed in the mouse spleen and blood, indicating immune system activation and reduced Treg cell numbers.
Flavonoids
Psoralea corylifolia L. is a kidney tonic that treats impotence, frequent urination, and back pain caused by insufficient kidney supply. Neobavaisoflavone (NBIF) is a crucial component of Psoralea corylifolia L. that has been shown to have chemosensitizing benefits for treating glioma (85). It also can inhibit STAT3 activation and suppress NSCLC cell proliferation (86). Jufeng Guo et al. (87) found that NBIF inhibited tumor growth in breast and small-cell lung cancer mice. In breast cancer 4T1 mice, it increased the number of CD4+ and CD8+ T cells in the TME and spleen. Moreover, it also supported the production of IFN-γ, perforin, GZMB, and Ki-67, which enhanced the immune effector function. In addition, the study found that NBIF reduced spleen MDSC activity by decreasing STAT3 phosphorylation and lowered intracellular arginase-1 and reactive oxygen species (ROS) levels, which unrestricted T cells. In combination therapy, NBIF enhanced anti-PD-1 efficacy in 4T1 tumors insensitive to anti-PD-1 treatment and improved the peripheral immune environment. It not only increased spleen IFN-γ+CD4+ T cells, IFN-γ+CD8+ T cells, and memory CD4+ and CD8+ T cells in the blood.
Numerous plants contain quercetin, also present in Chinese herbal remedies derived from radix bupleuri, mulberry leaf, hawthorn, etc. It possesses antioxidant, antimicrobial, and anti-inflammatory properties (88, 89). In a competitive enzyme-linked immunosorbent test developed by Lei Jing et al. (90), it was discovered that quercetin dihydrate could prevent 90% of the interaction between glycosylated PD-1 or PD-L1 in HEK293 cells. Quercetin dihydrate encouraged Jurkat T cells to secrete IL-2 in a co-culture system with a cell line with high PD-L1 expression. On the other hand, it killed tumor cells in unstimulated peripheral blood mononuclear cells. In tumor-bearing mice, quercetin dihydrate decreased tumor growth without affecting body weight, increasing CD8+ T cells and secreting more GZMB and IFN-γ.
Lactones
The herb known as Andrographis paniculata is used to detoxify the body, clear heat, and treat various diseases such as colds, sore throats, lung abscesses, skin infections, etc. Andrographolide (AD) is a diterpene lactone compound found in the Andrographis paniculata (Burm.F.) Nees has been shown to have anti-cancer and antiviral properties. According to a study by Xuanrun Wang et al. (91), AD effectively inhibited the growth and promoted apoptosis of NSCLC cell lines (H1299, H1975). It works by binding to STAT3 and inhibiting its phosphorylation, significantly enhancing p62-dependent autophagy and reducing IFN-γ-induced PD-L1 expression. The study also found that AD increased levels of ROS in tumor cells, promoting oxidative stress and autophagy. However, this effect could be mitigated by the antioxidant N-acetyl-L-cysteine (NAC). In mice models with Lewis lung cancer and H1975 xenografts, AD prolonged the survival time and inhibited tumor growth without causing harm to vital organs. When combined with PD-1 mAb treatment, AD further reduced tumor volume and weight, suppressed PD-L1 expression, and positively regulated the TME. The treatment increased the number of CD8+ T cells infiltrating the tumor and increased levels of IFN-γ, GZMB, and TNF-α while decreasing the number of Treg cells in the tumor, blood, and spleen.
The root of Inula helenium L., known as Inulae Radix, is used to treat chest, stomach, and abdomen distension and pain, as well as gastrointestinal problems such as vomiting and diarrhea. Jaemoo Chun et al. (92) discovered that despite its moderate anti-tumor effect, a sesquiterpene lactone-rich fraction of I. helenium (SFIH) alone, substantially reduced tumor volume in MC38-bearing mice, when combined with PD-1 monoclonal antibody, and survival rate, increased significantly. Immunohistochemical analysis revealed that the combined treatment enhanced CD8+ T cell and macrophage infiltration increased GZMB secretion, and decreased TGF-β1 expression. While the combination treatment group demonstrated a decrease in MDSCs and an increase in M1-like macrophages, no significant effect on TAMs was observed for the duration of treatment. Furthermore, the study used RNA sequencing to discover that combined treatment enhanced apoptosis, interferon-gamma, and inflammatory responses.
Herbal extracts sensitize cancer immunotherapy
Platycodon grandiflorum is a medicinal plant used primarily to treat pulmonary diseases. It contains active ingredients, such as Platycodin D (93), which has numerous pharmacological benefits, including anti-angiogenic, anti-tumor, and antioxidant properties (94, 95). Ruijie Yang et al. (96) conducted network pharmacology and HPLC tests and discovered that Platycodon D and Platycodon D3 were abundant in Platycodon grandiflorum. Further bioinformatics analysis showed that this herb had strong anti-tumor and immunomodulatory potential. In vitro, studies showed that PG (Platycodon D: Platycodon D3 = 1.5:1) significantly reduced the survival of several tumor cell lines (LLC, H1975, A549, CT26, and B16-F10), with LLC cell lines being the most sensitive. Using a mouse model of LLC-bearing C57BL/6 mice, PG was found to have significant inhibitory effects on tumor growth and prolonged lifespan. PG inhibited the phosphorylation of STAT3, down-regulated the expression of VEGF-A, and reduced the PD-1 membrane expression, leading to a decrease in PD-1+ CD8+ T cells in the TME. Additionally, PG promoted the proliferation of CD8+ T cells and increased the secretion of IFN-γ and GZMB to improve the killing effect on tumors. The study found that although PG alone had strong immunomodulatory and tumor-suppressive effects, raising tumor growth inhibition (TGI) to 57% at day 18 (compared to 36% with anti-PD-L1 antibodies), combination treatment did not show any appreciable improvement in survival time or reduction in tumor size.
Traditional Chinese herb Taxus chinensis var. mairei, formerly employed as an anthelmintic in ancient texts, has been discovered to have anti-tumor properties. Its aqueous extract (AETC) induced apoptosis in NSCLC cells via the Hippo-YAP pathway, which may drastically reduce tumor growth in tumor-bearing nude mice (97). Shuying Dai et al. (98) confirmed the standardization of the AETC by using HPLC assays to measure the levels of ginkgetin and quercetin. In vitro, studies showed that AETC decreased the survival of LLC and HCC827 lung cancer cells by breaking down CD47 through ubiquitination. CD47 on tumor cells interacts with the phagocytes’ SIRPα, sending a “do not eat me” signal and hindering the innate immune response (99). AETC was also found to enhance the phagocytic function of macrophages, which was demonstrated by the increased average phagocytic index. In a study with LLC-bearing mice, combining AETC with anti-PD-1 therapy resulted in a greater tumor size and weight reduction than monotherapy. It was accompanied by a lower level of PD-1 in macrophages and a smaller percentage of Treg cells, indicating a reversal of the tumor’s immune-suppressing microenvironment.
Chinese herbal formulas sensitize cancer immunotherapy
Eliminating pathogen method
The Gegen Qinlian decoction (GQD) is a formula from the “Treatise On Cold Damage Disease” book by the ancient Chinese physician Zhongjing Zhang. It is commonly used to treat ulcerative colitis (100) and acute gastroenteritis, and its purpose is to relieve external evil and remove internal heat, thereby restoring the balance between the exterior and interior. In a study by Ji Lv et al. (101), the combination of GQD with anti-PD-1 mAb therapy inhibited tumor growth in colorectal cancer CT26 tumor-bearing mice, with a maximum TGI value of 70.526% at day 32 (vs. 48.216% in the anti-PD-1 group). Furthermore, this study discovered that GQD maintained modulation of the gut microbiome and that the combination treatment modulated glycerophospholipid metabolism and sphingolipid metabolic pathways, thereby encouraging an indirect immune-enhancing effect. While PD-1 inhibition was more pronounced in the combination treatment group, it was also observed that the CD8+ T-cell ratio and the immune infiltration density were higher in TME in the combination treatment group, suggesting a beneficial synergistic effect.
Additionally, the study by Yihua Xu et al. (102) explored the anti-tumor effects of Dahuang Fuzi Baijiang decoction (DFB), another classic formula from Zhongjing Zhang, in treating colorectal cancer. The results showed that DFB effectively limited tumor growth in mice with MC38 tumors. In TME, DFB enhanced the infiltration of CD8+ T cells while suppressing their exhaustion and PD-1 expression. The treatment also reduced the IL-6 level in tumor tissues, but no synergistic effect was found with the combination therapy. Therefore, the suppression of terminal T cells by DFB helps to preserve the killing function of tumor immunity, making it a valuable addition to immune checkpoint blockade therapy.
Qingfei Jiedu decoction (QFJDD) is a formula that acts on the lung system, clears heat, and removes toxins. Junjie Pan et al. (103) found that serum containing QFJDD could downregulate PD-L1 expression in lung cancer A549 cells. Moreover, multiple signaling pathways can regulate the PD-1/PD-L1 axis, such as HIF-1, EGFR, NF-κB, and MAPK. Among them, hypoxia-inducible transcription factors (HIFs) are the primary molecules of the hypoxic phenotype in the TME. They can up-regulate PD-L1 levels by binding the hypoxia-response element in the PD-L1 proximal promoter (104, 105). The expression of PD-L1 can be directly induced by the epidermal growth factor receptor (EGFR) and mitogen-activated protein kinase (MAPK) (106, 107). At the same time, NF-κB is a downstream molecule of PD-1 that activates further by recruiting SHP2 through phosphorylation (108). Pan’s study found that QFJDD decreased the expression of critical genes involved in the HIF-1, EGFR, and NF-κB signaling pathways and increased the activity of the MAPK/AKT signaling pathway. In mice with Lewis lung cancer, the treatment also reduced PD-L1 levels in the tumor tissue and increased the proportion of CD8+PD-1+ T cells in the spleen, promoting the immune response.
Tonifying method
The Buzhong Yiqi decoction (BYD) is an ancient Chinese herbal formula created by physician Dongyuan Li, used to treat digestive problems by promoting Yang energy and nourishing the spleen and stomach. It can be utilized in visceral prolapse, chronic gastroenteritis, muscle fatigue, and excessive menstruation. Ruihan Xu et al. (109) found that combining modified Buzhong Yiqi decoction (mBYD) with 5-fluorouracil prolonged survival time and improved thymus and spleen indexes in mice with gastric cancer. The combined treatment also increased CD4+/CD8+ T cells in peripheral blood, and a reduction in CD8+ PD-1+ T cells and PD-1+ Treg cells was observed. The study found that mBYD inhibits the PD-1/PD-L1 axis in tumors by downregulating the PI3K/AKT signaling pathway and directly promoting T cell proliferation and IFN-γ concentration to increase effector T cell toxicity. Clinical studies also confirmed the immunomodulatory abilities of mBYD, as chemotherapy combined with mBYD significantly reduced the proportion of CD8+ PD-1 + T cells.
Shu Yu Pill (SYP) is one of the few multi-herb prescriptions of Zhongjing Zhang that is typically prepared for long-term administration. Its effect is to support righteousness by nourishing qi and blood, and it can treat symptoms including fatigue, stomach ache, and irregular periods. SYP, in combination with cisplatin treatment, resulted in more dramatic tumor suppression and significantly reduced mortality in mice with hepatocellular carcinoma, as reported by Zhe Deng et al. (110). Moreover, HIF-1α, PD-1, and PD-L1 expression levels in tumor tissues decreased significantly. Although SYP alone could up-regulate CD4+ T cells, the combination treatment group showed increased CD4+ and CD8+ T cells, demonstrating the synergistic impact of mixing Chinese and Western medicine in T cell detection.
Yuqing Xie’s team (111) investigated the therapeutic role of Yangyin Fuzheng Jiedu’s prescription (YFJP) in hepatocellular carcinoma. They found that Fuzheng prescription (FZP), one of the three dismantled formulas of YFJP, was the most effective in inhibiting tumor growth, with a TGI of 63.1%. It significantly increased thymus and spleen indexes in H22 tumor-bearing mice and the levels of CD8+ and CD3+ T cells in the spleen and peripheral blood. In TME, FZP promoted tumor cell apoptosis, increased tumor-infiltrating CD8+ T cells, and significantly inhibited the inhibitory receptors PD-1, Tim-3, and TIGIT on the membrane. In addition, it reduced IL-1β, IL-4, IL-6, IL-10, and IL-13 levels, down-regulated PI3K, ERK1/2, and up-regulated EGFR levels, improving the anti-tumor effects of the tumor suppressor microenvironment.
Yu Zhang et al. studied the therapeutic facilitation of a herbal in-hospital formula known as CFF-1 for metastatic castration-resistant prostate cancer (112). It was discovered that adding CFF-1 to bicalutamide, goserelin acetate, and docetaxel, the Western primary therapy, can lower patients’ levels of prostate-specific antigen and lessen fatigue, acting as an anti-cancer and quality-of-life enhancement effect. Additionally, studies on animals demonstrated that CFF-1 might block the JAK1/STAT3 signaling pathway to reduce PD-L1 expression and hence have an anti-tumor impact.
Physiotherapy Sensitizes tumor immunotherapy
Moxibustion
Moxibustion is a traditional Chinese medicine treatment that involves placing a small amount of burning mugwort on the skin, either a specific area or an acupuncture point, to produce a thermal effect to treat the disease and improve the flow of qi in the meridians and blood circulation. A study found that moxibustion combined with cyclophosphamide chemotherapy effectively inhibited tumor growth in mice with hepatocellular cancer (113). Another study showed that moxibustion treatment, which caused tumor cell necrosis, combined with paclitaxel, significantly reduced tumor growth, improved weight loss, and prolonged survival in breast cancer mice (114). It can also repair the decrease in the thymus index caused by paclitaxel and improve the immune response by increasing IFN-γ and IL-2 and decreasing IL-10 and TGF-β1. Additionally, it inhibited tumor neovascularization through down-regulation of the HIF-1α-VEGF pathway and facilitated immune checkpoint inhibition by down-regulating PD-1 and PD-L1 expression in tumor tissues. Although moxibustion-assisted cancer immunotherapy has not been investigated as part of any clinical trials, it does enhance the quality of life for cancer patients. It has been demonstrated to reduce cancer-related fatigue (115), and Chunhui Wang et al. (116) discovered that moxibustion effectively reduces female breast cancer-related lymphedema.
Catgut implantation at Acupoint
The catgut implantation at acupoint (CIAA) method, which uses continuous stimulation of acupuncture points to enhance metabolism, improve qi and blood circulation, and relieve symptoms, has been widely used for conditions such as obesity (117, 118) and type 2 diabetes (119). A study by ShiHua Xu et al. (120) found that CIAA reduced the mortality of mice with hepatocellular carcinoma and reduced hepatic histopathological damage. It also decreased the expression of AFP and p-AKT and improved the immune environment by down-regulating PD-1, CTLA-4, and IL-10. Although the study did not include positive controls, it provides valuable insight into the potential benefits of CIAA in cancer immunotherapy. However, more research is needed to fully understand the sensitizing impact of CIAA on tumor immunity.
Summary and prospects
The down-regulation of PD-1 and PD-L1 prevents their binding and reduces the misidentification of T cells, thereby reducing the immune escape of tumor cells. This is the underlying principle behind ICIs therapy. However, some patients do not respond to these treatments due to tumor heterogeneity and individual drug resistance. Finding drugs that can sensitize cancer immunotherapy, reduce toxicity, and enhance immune function is a crucial challenge in medical oncology. To address this issue, the combination therapy approach should consider sensitization from a sophisticated, coordinated molecular perspective. With this in mind, a review of the PD-1/PD-L1 axis and its regulatory mechanisms was conducted, and it was found that several cytokines and cross-talk pathways can impact its regulation.
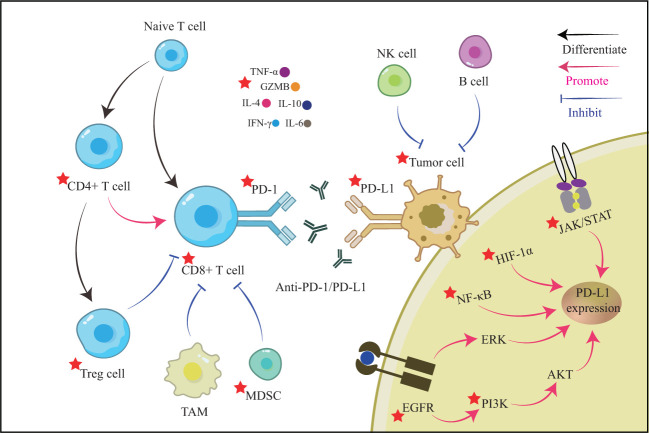
We hypothesize that TCM can overcome the limitations of ICIs therapy by serving as an adjuvant to sensitize and reduce toxicity. This hypothesis is based on our observation of existing cancer treatments in China. A thorough review of TCM treatment options, including Chinese medicine monomers, herbal extracts, Chinese herbal formulas, and physiotherapy, was conducted. The findings indicate that TCM can sensitize cancer immunotherapy in multiple ways, such as directly inhibiting the PD-1/PD-L1 axis, regulating T cell function, suppressing immunosuppressive cells, modulating cross-talk signaling pathways, improving the tumor microenvironment, regulating intestinal flora, and more (Table 1; Figure 2). When combined with PD-1 or PD-L1 mAb treatment, TCM has shown promising synergistic benefits in suppressing tumor volume, increasing longevity, and reducing mortality in tumor-bearing mice.
Table 1.
TCM therapies sensitize cancer immunotherapy.
| Treatment | Disease | PD-1/PD-L1 axis | Other sensitization methods | Cytokines & Molecules | Immune cells | Reference |
|---|---|---|---|---|---|---|
| Ginseng polysaccharides | NSCLC | \ | 1. Modulate the TME 2. Alter the gut microbiota 3. Influence tryptophan metabolism |
IFN-γ↑, TNF-α↑, GZMB↑, IDO↓, L- tryptophan↑, L- kynurenine↓ | CD8+ T cell ↑, Treg cell ↓, Th17 cells↓ | Jumin Huang (62) |
| Polysaccharide of Atractylodis Macrocephalae Rhizoma | Esophageal Cancer | PD-L1↓ | Regulate microRNAs | miR-34a↑ | \ | Yicun Han (65) |
| Honey-processed Astragalus polysaccharides | Melanoma | \ | 1. Induce tumor cell apoptosis 2. Modulate the TME |
\ | CD4+T cell ↓, CD8+T cell ↑ | Xinrui Sha (70) |
| Evodiamine | NSCLC | PD-L1↓ | 1. Induce tumor cell apoptosis 2. Enhance T cell function 3. Modulate the TME |
IFN-γ↑, TNF-α↑, GZMB↑, MUC1-C↓ | CD8+ T cells↑, Treg cell↓ | Zebo Jiang (75) |
| Oxymatrine | CRC | PD-L1↓ | Modulate DNA demethylation | \ | \ | S.Hua (78) |
| Gallic acid | CRC | PD-1↓, PD-L1↓ | 1. Promot Treg fragility 2. Modulate the TME 3. Improve T-cell exhaustion |
IFN-γ↑, TNF-α↑, STAT3↓ | Treg cells↓ | Biaolong Deng (80) |
| Curcumin | HNSCC | PD-1↓, PD-L1↓, PD-L2↓ | 1. Modulate the TME 2. Enhance T cell function 3. Suppress epithelial-mesenchymal transition |
IFN-γ↑, GZMB↑ | CD4+ T cells↑, CD8+ T cells ↑, Treg cells↓ | Lihua Liu (84) |
| Neobavaisoflavone | Breast cancer | \ | 1. Modulate the TME 2. Promote peripheral immunity |
IFN-γ↑, GZMB↑, STAT3↓, Ki-67↑, ROS↓, perforin↑, arginase-1↓ | MDSCs↓, CD4+ T cells↑, CD8+ T cells ↑, memory CD4+ T cells↑, memory CD8+ T cells↑, | Jufeng Guo (87) |
| Quercetin | Breast cancer | PD-1↓, PD-L1↓, | 1. Modulate the TME 2. Strengthen PBMC |
IFN-γ↑, GZMB↑, IL-2↑ | CD8+ T cells ↑ | Lei Jing (90) |
| Andrographolide | NSCLC | PD-L1↓ | 1. Induce tumor autophagy 2. Modulate the TME 3. Inhibit JAK/STAT pathway |
IFN-γ↑, TNF-α↑, GZMB↑, STAT3↓, Ki67↓, ROS↑ | CD8+ T cells ↑ | Xuanrun Wang (91) |
| Sesquiterpene lactone-rich fraction of Inula helenium L. | CRC | \ | 1. Modulate the TME 2. Promote T-cell infiltration |
GZMB↑, TGF-β1↓ | CD8+ T cells ↑, M1-like macrophages↑ | Jaemoo Chun (92) |
| Platycodon grandiflorum | NSCLC | PD-1↓ | 1. Modulate the TME 2. Inhibit the VEGF pathway |
IFN-γ↑, GZMB↑, STAT3↓, VEGF-A↓, IL-6↓, IL-10↓ | CD8+ T cell ↑ | Ruijie Yang (96) |
| Aqueous extract of Taxus chinensis var. mairei | NSCLC | PD-1↓ | 1. Restore macrophage capacity 2. Modulate the TME |
CD47↓ | Treg cell ↓ | Shuying Dai (98) |
| Gegen Qinlian decoction | CRC | PD-1↓ | 1. Modulate the TME 2. Alter the gut microbiota |
IFN-γ↑, IL-2↑ | CD8+T cell↑ | Ji Lv (101) |
| Dahuang Fuzi Baijiang decoction | CRC | PD-1↓ | 1. Alleviate T-cell exhaustion 2. Modulate the TME |
IL-6↓, CCL2↓ | CD8+T cell↑ | Yihua Xu (102) |
| Qingfei Jiedu decoction | NSCLC | PD-L1↓ | 1. Inhibit EGFR, HIF-1, NF-κB pathway 2. Promote MAPK/AKT pathway |
AKT1↑, MAPK1↑, EGFR↓, HIF1A↓, JUN↓, RELA↓, NFKBIA↓ | CD8+T cell↑ | Junjie Pan (103) |
| Modified Buzhong Yiqi decoction | Gastric Cancer | PD-L1↓, PD-1↓ | Modulate the TME | IFN-γ↑, PI3K↓, AKT↓ | CD4+/CD8+ T cells ↑, CD8+ T cell↓, Treg cells↓ | Ruihan Xu (109) |
| Shuyu pills | HCC | PD-1↓, PD-L1↓ | 1. Modulate the TME 2. Inhibit the HIF-1 pathway |
HIF-1α↓ | CD4+T cell↑, CD8+T cell↑ | Zhe Deng (110) |
| Fuzheng prescription | HCC | PD-1↓ | 1. Alleviate T-cell exhaustion 2. Modulate the TME 3. Inhibit the PI3K pathway |
PI3K↓, EGFR↑, ERK1/2↓, IL-1β↓, IL-4↓, IL-6↓, IL-10↓, IL-13↓ | CD8+T cell↑ | Yuqing Xie (111) |
| CFF-1 | prostate cancer | PD-L1↓ | 1. Modulate the TME 2. Inhibit the JAK1/STAT3 pathway |
JAK1↓, STAT3↓ | \ | Yu Zhang (112) |
| Moxibustion | Breast cancer | PD-1↓, PD-L1↓ | 1. Modulate the TME 2. Suppress tumor neovascularization |
IFN-γ↑, TGF-β↓, IL-2↑, IL-10↓, HIF-1α↓, VEGFA↓ | \ | Ning Xue (114) |
| Catgut Implantation at Acupoint | HCC | PD-1↓ | 1. Modulate the TME 2. Inhibit the AKT pathway |
AFP↓, p-AKT↓, CTLA-4↓, IL-10↓ | \ | Shihua Xu (120) |
NSCLC, Non-small cell lung cancer; CRC, Colorectal Cancer; HNSCC, Head and neck squamous cell carcinoma; HCC, Hepatocellular carcinoma; TME, Tumor microenvironment; PBMC, Peripheral blood mononuclear cell; IFN-γ, Interferon-γ; TNF-α, Tumor necrosis factor-α; GZMB, granzyme B; IDO, Indoleamine -2, 3-dioxygenase; ROS, Reactive oxygen species; PI3K, Phosphatidylinositol-3-kinase; STAT3, Signal Transducer And Activator Of Transcription 3; IL-2, Interleukin-2; IRF1, Interferon Regulatory Factor 1; MAPK1, Mitogen-activated protein kinase 1; EGFR, Epidermal growth factor receptor; HIF1A, Hypoxia inducible factor 1; CTLA-4, Cytotoxic T lymphocyte–associated antigen 4.
“↑” means the expression of this indicator is upregulated.
“↓” means the expression of this indicator is downregulated.
“\” means that this literature does not contain such indicators.
Figure 2.
The illustrates TCM sensitization sites in the tumor microenvironment.
Advancements in technology, such as fingerprinting, have led to a standardization of the quality of Chinese medicines (121). The potent tumor-killing and immune-boosting effects of various herbal compounds have been acknowledged. However, Chinese herbal formula use is still limited by factors like the concentration of the compounds and a lack of clarity in their pharmacodynamic ingredients. New technologies such as microfluidic microarrays, organoid microarrays, and artificial intelligence are being developed to identify the critical efficacy molecules to address this issue. Additionally, the growth and advancement of herb genomics research and the formation of a comprehensive Chinese medicine database are helping to create a complete mechanism for understanding and further clinical application and industrial development of Chinese medicine (122).
In conclusion, TCM can enhance cancer immunotherapy by sensitizing immune checkpoints through various mechanisms, making combination therapy a potentially more beneficial option for oncology patients. Further research is crucial in exploring the role of TCM in cancer immunotherapy, as it holds the promise of boosting immune response and overcoming the limitations of ICIs treatments.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding Statement
This work was supported by grants from the “Hundred Talents Project” of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Grant No. P2020042), a randomized controlled clinical study of Sanhuang Zengmian Decoction in treating mild to moderate SLE, no. 2021ZD03, and a clinical study on treating simple obesity with combined catgut embedding combined with traditional Chinese medicine, no. 2021MS035.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest (2015) 125(9):3335–7. doi: 10.1172/jci83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Sci (New York NY) (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2020) 124:109821. doi: 10.1016/j.biopha.2020.109821 [DOI] [PubMed] [Google Scholar]
- 4. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11(1):3801. doi: 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt C. The benefits of immunotherapy combinations. Nature (2017) 552(7685):S67–s9. doi: 10.1038/d41586-017-08702-7 [DOI] [PubMed] [Google Scholar]
- 6. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol (2021) 14(1):156. doi: 10.1186/s13045-021-01164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discovery (2021) 11(4):838–57. doi: 10.1158/2159-8290.Cd-20-1680 [DOI] [PubMed] [Google Scholar]
- 8. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbari C, Fontaine T, Parajuli P, Lamichhane N, Jakubski S, Lamichhane P, et al. Immunotherapies and combination strategies for immuno-oncology. Int J Mol Sci (2020) 21(14):5009. doi: 10.3390/ijms21145009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egen JG, Ouyang W, Wu LC. Human anti-tumor immunity: insights from immunotherapy clinical trials. Immunity (2020) 52(1):36–54. doi: 10.1016/j.immuni.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Liang Y, He C. Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chin Med (2017) 12:20. doi: 10.1186/s13020-017-0140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2020) 121:109570. doi: 10.1016/j.biopha.2019.109570 [DOI] [PubMed] [Google Scholar]
- 13. Huo JL, Fu WJ, Liu ZH, Lu N, Jia XQ, Liu ZS. Research advance of natural products in tumor immunotherapy. Front Immunol (2022) 13:972345. doi: 10.3389/fimmu.2022.972345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of pd-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J (1992) 11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the pd-1 antigen on the surface of stimulated mouse T and b lymphocytes. Int Immunol (1996) 8(5):765–72. doi: 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- 16. Chamoto K, Al-Habsi M, Honjo T. Role of pd-1 in immunity and diseases. Curr topics Microbiol Immunol (2017) 410:75–97. doi: 10.1007/82_2017_67 [DOI] [PubMed] [Google Scholar]
- 17. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20(11):651–68. doi: 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, Co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med (1999) 5(12):1365–9. doi: 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 19. Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal Pdl1 in tumour immune evasion. Nat Rev Immunol (2020) 20:209–15. doi: 10.1038/s41577-019-0264-y [DOI] [PubMed] [Google Scholar]
- 20. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in pd-L1/Pd-1-Mediated tumor immune escape. Mol Cancer (2019) 18(1):10. doi: 10.1186/s12943-018-0928-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, et al. Pd-L2 expression in human tumors: relevance to anti-Pd-1 therapy in cancer. Clin Cancer Res (2017) 23(12):3158–67. doi: 10.1158/1078-0432.Ccr-16-1761 [DOI] [PubMed] [Google Scholar]
- 22. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. Pd-L2 is a second ligand for pd-1 and inhibits T cell activation. Nat Immunol (2001) 2(3):261–8. doi: 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 23. Kumar S, Singh SK, Rana B, Rana A. Tumor-infiltrating Cd8(+) T cell antitumor efficacy and exhaustion: molecular insights. Drug Discovery Today (2021) 26(4):951–67. doi: 10.1016/j.drudis.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 25. Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int Immunopharmacol (2020) 86:106700. doi: 10.1016/j.intimp.2020.106700 [DOI] [PubMed] [Google Scholar]
- 26. van der Leun AM, Thommen DS, Schumacher TN. Cd8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer (2020) 20(4):218–32. doi: 10.1038/s41568-019-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang Y, Jia A, Wang Y, Liu G. Cd8(+) T cell exhaustion in anti-tumor immunity: the new insights for cancer immunotherapy. Immunology (2022) 168(1):30–48. doi: 10.1111/imm.13588 [DOI] [PubMed] [Google Scholar]
- 28. Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. Cd8(+) T cell exhaustion in cancer. Front Immunol (2021) 12:715234. doi: 10.3389/fimmu.2021.715234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu Y, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, et al. Disturbed mitochondrial dynamics in Cd8 tils reinforce T cell exhaustion. Nat Immunol (2020) 21(12):1540–51. doi: 10.1038/s41590-020-0793-3 [DOI] [PubMed] [Google Scholar]
- 30. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol (2008) 8(6):467–77. doi: 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]
- 31. Asmamaw M, Shi X, Zhang L, Liu H. A comprehensive review of Shp2 and its role in cancer. Cell Oncol (Dordrecht) (2022) 45(5):729–53. doi: 10.1007/s13402-022-00698-1 [DOI] [PubMed] [Google Scholar]
- 32. Kim HJ, Cantor H. Cd4 T-cell subsets and tumor immunity: the helpful and the not-So-Helpful. Cancer Immunol Res (2014) 2(2):91–8. doi: 10.1158/2326-6066.Cir-13-0216 [DOI] [PubMed] [Google Scholar]
- 33. Kagamu H, Yamasaki S, Kitano S, Yamaguchi O, Mouri A, Shiono A, et al. Single-cell analysis reveals a Cd4+ T cell cluster that correlates with pd-1 blockade efficacy. Cancer Res (2022) 82(24):4641–53. doi: 10.1158/0008-5472.Can-22-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. Cd4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol (2018) 18(10):635–47. doi: 10.1038/s41577-018-0044-0 [DOI] [PubMed] [Google Scholar]
- 35. Ahrends T, Borst J. The opposing roles of Cd4(+) T cells in anti-tumour immunity. Immunology (2018) 154(4):582–92. doi: 10.1111/imm.12941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim JH, Kim BS, Lee SK. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune network (2020) 20(1):e4. doi: 10.4110/in.2020.20.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JM, Chen DS. Immune escape to pd-L1/Pd-1 blockade: seven steps to success (or failure). Ann Oncol (2016) 27(8):1492–504. doi: 10.1093/annonc/mdw217 [DOI] [PubMed] [Google Scholar]
- 38. Zhang X, Wang S, Zhu Y, Zhang M, Zhao Y, Yan Z, et al. Double-edged effects of interferons on the regulation of cancer-immunity cycle. Oncoimmunology (2021) 10(1):1929005. doi: 10.1080/2162402x.2021.1929005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu C, Talukder A, Savage NM, Singh N, Liu K. Jak-Stat-Mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-Pd-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology (2017) 6(3):e1291106. doi: 10.1080/2162402x.2017.1291106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li N, Wang J, Zhang N, Zhuang M, Zong Z, Zou J, et al. Cross-talk between tnf-Α and ifn-Γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer immunology immunotherapy CII (2018) 67(2):271–83. doi: 10.1007/s00262-017-2086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, et al. Inflammatory cytokines il-17 and tnf-Α up-regulate pd-L1 expression in human prostate and colon cancer cells. Immunol Lett (2017) 184:7–14. doi: 10.1016/j.imlet.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuo IY, Yang YE, Yang PS, Tsai YJ, Tzeng HT, Cheng HC, et al. Converged Rab37/Il-6 trafficking and Stat3/Pd-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics (2021) 11(14):7029–44. doi: 10.7150/thno.60040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ju X, Zhang H, Zhou Z, Chen M, Wang Q. Tumor-associated macrophages induce pd-L1 expression in gastric cancer cells through il-6 and tnf-α signaling. Exp Cell Res (2020) 396(2):112315. doi: 10.1016/j.yexcr.2020.112315 [DOI] [PubMed] [Google Scholar]
- 44. Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, et al. The common gamma-chain cytokines il-2, il-7, il-15, and il-21 induce the expression of programmed death-1 and its ligands. J Immunol (Baltimore Md 1950) (2008) 181(10):6738–46. doi: 10.4049/jimmunol.181.10.6738 [DOI] [PubMed] [Google Scholar]
- 45. Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. Tgfβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature (2018) 554(7693):538–43. doi: 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- 46. Chen MJ, Wang YC, Wang L, Shen CJ, Chen CY, Lee H. Pd-L1 expressed from tumor cells promotes tumor growth and invasion in lung cancer Via modulating tgf-Β1/Smad4 expression. Thorac Cancer (2022) 13(9):1322–32. doi: 10.1111/1759-7714.14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy–revisited. Nat Rev Drug Discovery (2011) 10(8):591–600. doi: 10.1038/nrd3500 [DOI] [PubMed] [Google Scholar]
- 48. Chodon T, Koya RC, Odunsi K. Active immunotherapy of cancer. Immunol investigations (2015) 44(8):817–36. doi: 10.3109/08820139.2015.1096684 [DOI] [PubMed] [Google Scholar]
- 49. Zhang Z, Lu M, Qin Y, Gao W, Tao L, Su W, et al. Neoantigen: a new breakthrough in tumor immunotherapy. Front Immunol (2021) 12:672356. doi: 10.3389/fimmu.2021.672356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baxter D. Active and passive immunization for cancer. Hum Vaccines immunotherapeutics (2014) 10(7):2123–9. doi: 10.4161/hv.29604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buqué A, Senovilla L, Baracco EE, et al. Classification of current anticancer immunotherapies. Oncotarget (2014) 5(24):12472–508. doi: 10.18632/oncotarget.2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharma P, Allison JP. The future of immune checkpoint therapy. Sci (New York NY) (2015) 348(6230):56–61. doi: 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 53. Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. Application of pd-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J (2019) 17:661–74. doi: 10.1016/j.csbj.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of fda-approved immune checkpoint inhibitors. Int Immunopharmacol (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 55. Ren D, Hua Y, Yu B, Ye X, He Z, Li C, et al. Predictive biomarkers and mechanisms underlying resistance to Pd1/Pd-L1 blockade cancer immunotherapy. Mol Cancer (2020) 19(1):19. doi: 10.1186/s12943-020-1144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Natural products (2020) 83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285 [DOI] [PubMed] [Google Scholar]
- 57. Atanasov A, Zotchev S, Dirsch V, Supuran C. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery (2021) 20(3):200–16. doi: 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Tang J, Yu LY, Jiang Q. Successful treatment of immune-related lichenoid dermatitis by weiling decoction in a patient with non-small cell lung cancer: a case report and review of literature. Explore (New York NY) (2023). doi: 10.1016/j.explore.2023.02.008 [DOI] [PubMed] [Google Scholar]
- 59. Wang D, Shao S, Zhang Y, Zhao D, Wang M. Insight into polysaccharides from c. a. Meyer in improving intestinal inflammation: modulating intestinal microbiota and autophagy. Front Immunol (2021) 12:683911. doi: 10.3389/fimmu.2021.683911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li S, Qi Y, Chen L, Qu D, Li Z, Gao K, et al. Effects of panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol macromolecules (2019) 124:931–7. doi: 10.1016/j.ijbiomac.2018.11.271 [DOI] [PubMed] [Google Scholar]
- 61. Hwang S, Shin M, Yoon T, Shin K. Immunoadjuvant activity in mice of polysaccharides isolated from the leaves of panax ginseng C.A. Meyer. Int J Biol macromolecules (2018) 107:2695–700. doi: 10.1016/j.ijbiomac.2017.10.160 [DOI] [PubMed] [Google Scholar]
- 62. Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, et al. Ginseng polysaccharides alter the gut microbiota and Kynurenine/Tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/Programmed cell death ligand 1 (Anti-Pd-1/Pd-L1) immunotherapy. Gut (2022) 71(4):734–45. doi: 10.1136/gutjnl-2020-321031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cui YS, Li YX, Jiang SL, Song AN, Fu Z, Dong CX, et al. Isolation, purification, and structural characterization of polysaccharides from atractylodis macrocephalae rhizoma and their immunostimulatory activity in Raw264.7 cells. Int J Biol Macromol (2020) 163:270–8. doi: 10.1016/j.ijbiomac.2020.06.269 [DOI] [PubMed] [Google Scholar]
- 64. Xu W, Fang S, Wang Y, Zhang T, Hu S. Molecular mechanisms associated with macrophage activation by rhizoma atractylodis macrocephalae polysaccharides. Int J Biol Macromol (2020) 147:616–28. doi: 10.1016/j.ijbiomac.2020.01.081 [DOI] [PubMed] [Google Scholar]
- 65. Han Y, Chen Y, Fan X, Shang Y, Chen X, Wang G, et al. Polysaccharide of atractylodis macrocephalae rhizoma inhibits expression of immune checkpoint pd-L1 by targeting mir-34a in esophageal carcinoma cells. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin materia Med (2022) 47(6):1658–65. doi: 10.19540/j.cnki.cjcmm.20211203.701 [DOI] [PubMed] [Google Scholar]
- 66. Meng F, Chen Y, Yang M, Zhang H, Wang W. Concomitant inhibition of B7-H3 and pd-L1 expression by a novel and synthetic microrna delivers potent antitumor activities in colorectal tumor models. Investigational New Drugs (2021) 39(5):1267–74. doi: 10.1007/s10637-021-01123-4 [DOI] [PubMed] [Google Scholar]
- 67. Liu J, Kong L, Shao M, Sun C, Li C, Wang Y, et al. Astragalusseabuckthorn polysaccharide combined with polysaccharide ameliorate alcoholic fatty liver by regulating intestinal flora. Front Endocrinol (2022) 13:1018557. doi: 10.3389/fendo.2022.1018557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aleebrahim-Dehkordi E, Heidari-Soureshjani E, Aryan A, Ganjirad Z, Soveyzi F, Hoseinsalari A, et al. Antiviral compounds based on natural astragalus polysaccharides (Aps): research and foresight in the strategies for combating sars-Cov-2 (Covid-19). Mini Rev medicinal Chem (2022) 22(17):2299–307. doi: 10.2174/1389557522666220301143113 [DOI] [PubMed] [Google Scholar]
- 69. Ding G, Gong Q, Ma J, Liu X, Wang Y, Cheng X. Immunosuppressive activity is attenuated by astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci (2021) 112(10):4050–63. doi: 10.1111/cas.15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sha X, Xu X, Liao S, Chen H, Rui W. Evidence of immunogenic cancer cell death induced by honey-processed astragalus polysaccharides in vitro and in vivo. Exp Cell Res (2022) 410(1):112948. doi: 10.1016/j.yexcr.2021.112948 [DOI] [PubMed] [Google Scholar]
- 71. Cheng P, Zhang X, Wang X, Liu C, Zhao X, Fan J, et al. Identification of evodiamine as a suppressor of prostate cancer progression by reducing ar transcriptional activity Via targeting src. Endocrine (2022) 75(2):635–45. doi: 10.1007/s12020-021-02907-7 [DOI] [PubMed] [Google Scholar]
- 72. Lei Y, Chan M, Liu H, Lyu W, Chen L, Zhong Y, et al. Evodiamine as the active compound of evodiae fructus to inhibit proliferation and migration of prostate cancer through Pi3k/Akt/Nf-b signaling pathway. Dis Markers (2022) 2022:4399334. doi: 10.1155/2022/4399334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yun U, Bae S, Song Y, Kim Y. A critical yap in malignancy of hcc is regulated by evodiamine. Int J Mol Sci (2022) 23(3):1855. doi: 10.3390/ijms23031855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang J, Woo H, Lee P, Kim S. Induction of apoptosis and effect on the Fak/Akt/Mtor signal pathway by evodiamine in gastric cancer cells. Curr Issues Mol Biol (2022) 44(9):4339–49. doi: 10.3390/cimb44090298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang ZB, Huang JM, Xie YJ, Zhang YZ, Chang C, Lai HL, et al. Evodiamine suppresses non-small cell lung cancer by elevating Cd8(+) T cells and downregulating the Muc1-C/Pd-L1 axis. J Exp Clin Cancer Res CR (2020) 39(1):249. doi: 10.1186/s13046-020-01741-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rajabi H, Kufe D. Muc1-c oncoprotein integrates a program of emt, epigenetic reprogramming and immune evasion in human carcinomas. Biochim Biophys Acta Rev Cancer (2017) 1868(1):117–22. doi: 10.1016/j.bbcan.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gu XB, Yang XJ, Hua Z, Lu ZH, Zhang B, Zhu YF, et al. Effect of oxymatrine on specific cytotoxic T lymphocyte surface programmed death receptor-1 expression in patients with chronic hepatitis b. Chin Med J (2012) 125(8):1434–8. doi: 10.3760/cma.j.issn.0366-6999.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 78. Hua S, Gu M, Wang Y, Ban D, Ji H. Oxymatrine reduces expression of programmed death-ligand 1 by promoting DNA demethylation in colorectal cancer cells. Clin Trans Oncol (2021) 23(4):750–6. doi: 10.1007/s12094-020-02464-x [DOI] [PubMed] [Google Scholar]
- 79. Pedra NS, Bona NP, de Aguiar MSS, Spohr L, Alves FL, Santos F, et al. Impact of Gallic acid on tumor suppression: modulation of redox homeostasis and purinergic response in in vitro and a preclinical glioblastoma model. J Nutr Biochem (2022) 110:109156. doi: 10.1016/j.jnutbio.2022.109156 [DOI] [PubMed] [Google Scholar]
- 80. Deng B, Yang B, Chen J, Wang S, Zhang W, Guo Y, et al. Gallic Acid induces T-Helper-1-Like T(Reg) cells and strengthens immune checkpoint blockade efficacy. J immunotherapy Cancer (2022) 10(7):e004037. doi: 10.1136/jitc-2021-004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao X, Zhang R, Song Z, Yang K, He H, Jin L, et al. Curcumin suppressed the proliferation and apoptosis of hpv-positive cervical cancer cells by directly targeting the E6 protein. Phytotherapy Res PTR (2023). doi: 10.1002/ptr.7868 [DOI] [PubMed] [Google Scholar]
- 82. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods (Basel Switzerland) (2017) 6(10):92. doi: 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang SL, Chang TC, Sun NK. Curcumin reduces paclitaxel resistance in ovarian carcinoma cells by upregulating Snip1 and inhibiting nfκb activity. Biochem Pharmacol (2023) 212:115581. doi: 10.1016/j.bcp.2023.115581 [DOI] [PubMed] [Google Scholar]
- 84. Liu L, Lim MA, Jung SN, Oh C, Won HR, Jin YL, et al. The effect of curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine (2021) 92:153758. doi: 10.1016/j.phymed.2021.153758 [DOI] [PubMed] [Google Scholar]
- 85. Maszczyk M, Banach K, Karkoszka M, Rzepka Z, Rok J, Beberok A, et al. Chemosensitization of U-87 mg glioblastoma cells by neobavaisoflavone towards doxorubicin and etoposide. Int J Mol Sci (2022) 23(10):5621. doi: 10.3390/ijms23105621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cai X, Zhou F, Xie X, Zheng D, Yao Y, Zhao C, et al. Neobavaisoflavone demonstrates valid anti-tumor effects in non-small- cell lung cancer by inhibiting Stat3. Combinatorial Chem High throughput screening (2022) 25(1):29–37. doi: 10.2174/1386207323666201204135941 [DOI] [PubMed] [Google Scholar]
- 87. Guo J, Shen Y, Hu S, Rui T, Liu J, Yuan Y. Neobavaisoflavone inhibits antitumor immunosuppression Via myeloid-derived suppressor cells. Int Immunopharmacol (2022) 111:109103. doi: 10.1016/j.intimp.2022.109103 [DOI] [PubMed] [Google Scholar]
- 88. Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, El-Hack MEA, Taha AE, et al. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods (Basel Switzerland) (2020) 9(3):374. doi: 10.3390/foods9030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qi W, Qi W, Xiong D, Long M. Quercetin: its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules (2022) 27(19):6545. doi: 10.3390/molecules27196545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jing L, Lin J, Yang Y, Tao L, Li Y, Liu Z, et al. Quercetin inhibiting the pd-1/Pd-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytotherapy Res PTR (2021) 35(11):6441–51. doi: 10.1002/ptr.7297 [DOI] [PubMed] [Google Scholar]
- 91. Wang XR, Jiang ZB, Xu C, Meng WY, Liu P, Zhang YZ, et al. Andrographolide suppresses non-Small-Cell lung cancer progression through induction of autophagy and antitumor immune response. Pharmacol Res (2022) 179:106198. doi: 10.1016/j.phrs.2022.106198 [DOI] [PubMed] [Google Scholar]
- 92. Chun J, Park SM, Lee M, Ha IJ, Jeong MK. The sesquiterpene lactone-rich fraction of inula helenium l. enhances the antitumor effect of anti-Pd-1 antibody in colorectal cancer: integrative phytochemical, transcriptomic, and experimental analyses. Cancers (2023) 15(3):653. doi: 10.3390/cancers15030653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Q, Yang T, Zhao S, Zheng Q, Li Y, Zhang Z, et al. Distribution, biotransformation, pharmacological effects, metabolic mechanism and safety evaluation of platycodin d: a comprehensive review. Curr Drug Metab (2022) 23(1):21–9. doi: 10.2174/1389200223666220202090137 [DOI] [PubMed] [Google Scholar]
- 94. Son J, Lee S, Park J, Jung M, An S, Yang H, et al. Platycodin d inhibits vascular endothelial growth factor-induced angiogenesis by blocking the activation of mitogen-activated protein kinases and the production of interleukin-8. Am J Chin Med (2022) 50(6):1645–61. doi: 10.1142/s0192415x22500690 [DOI] [PubMed] [Google Scholar]
- 95. Choi Y. Activation of the Nrf2/Ho-1 signaling pathway contributes to the protective effects of platycodin d against oxidative stress-induced DNA damage and apoptosis in C2c12 myoblasts. Gen Physiol biophysics (2020) 39(6):519–30. doi: 10.4149/gpb_2020030 [DOI] [PubMed] [Google Scholar]
- 96. Yang R, Pei T, Huang R, Xiao Y, Yan J, Zhu J, et al. Platycodon grandiflorum triggers antitumor immunity by restricting pd-1 expression of Cd8(+) T cells in local tumor microenvironment. Front Pharmacol (2022) 13:774440. doi: 10.3389/fphar.2022.774440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang G, Dai S, Chen Y, Wang H, Chen T, Shu Q, et al. Aqueous extract of taxus chinensis var. mairei regulates the hippo-yap pathway and promotes apoptosis of non-small cell lung cancer Via Atf3 in vivo and in vitro. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2021) 138:111506. doi: 10.1016/j.biopha.2021.111506 [DOI] [PubMed] [Google Scholar]
- 98. Dai S, Liu Y, Zhao F, Wang H, Shao T, Xu Z, et al. Aqueous extract of taxus chinensis var. mairei targeting Cd47 enhanced antitumor effects in non-small cell lung cancer. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2022) 154:113628. doi: 10.1016/j.biopha.2022.113628 [DOI] [PubMed] [Google Scholar]
- 99. Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. Cd47/Sirpα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci (2021) 17(13):3281–7. doi: 10.7150/ijbs.60782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang W, Yang M, Rietz S, Li P. Effects of gegen qinlian decoction combined with Chinese herbal hot package on the expression of pct, crp and il-6 in patients with acute gastroenteritis. Cell Mol Biol (Noisy-le-Grand France) (2022) 68(2):189–96. doi: 10.14715/cmb/2022.68.2.27 [DOI] [PubMed] [Google Scholar]
- 101. Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, et al. Gegen qinlian decoction enhances the effect of pd-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis (2019) 10(6):415. doi: 10.1038/s41419-019-1638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xu Y, Wang H, Wang T, Chen C, Sun R, Yao W, et al. Dahuang fuzi baijiang decoction restricts progenitor to terminally exhausted T cell differentiation in colorectal cancer. Cancer Sci (2022) 113(5):1739–51. doi: 10.1111/cas.15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pan J, Yang H, Zhu L, Lou Y, Jin B. Qingfei jiedu decoction inhibits pd-L1 expression in lung adenocarcinoma based on network pharmacology analysis, molecular docking and experimental verification. Front Pharmacol (2022) 13:897966. doi: 10.3389/fphar.2022.897966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 is a novel direct target of hif-1α, and its blockade under hypoxia enhanced mdsc-mediated T cell activation. J Exp Med (2014) 211(5):781–90. doi: 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shurin M, Umansky V. Cross-talk between hif and pd-1/Pd-L1 pathways in carcinogenesis and therapy. J Clin Invest (2022) 132(9):e159473. doi: 10.1172/jci159473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the pd-1 pathway contributes to immune escape in egfr-driven lung tumors. Cancer Discovery (2013) 3(12):1355–63. doi: 10.1158/2159-8290.Cd-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stutvoet TS, Kol A, de Vries EG, de Bruyn M, Fehrmann RS, Terwisscha van Scheltinga AG, et al. Mapk pathway activity plays a key role in pd-L1 expression of lung adenocarcinoma cells. J Pathol (2019) 249(1):52–64. doi: 10.1002/path.5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mirzaei R, Gordon A, Zemp FJ, Kumar M, Sarkar S, Luchman HA, et al. Pd-1 independent of pd-L1 ligation promotes glioblastoma growth through the nfκb pathway. Sci Adv (2021) 7(45):eabh2148. doi: 10.1126/sciadv.abh2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xu R, Wu J, Zhang X, Zou X, Li C, Wang H, et al. Modified bu-Zhong-Yi-Qi decoction synergies with 5 fluorouracile to inhibits gastric cancer progress Via pd-1/Pd- L1-dependent T cell immunization. Pharmacol Res (2020) 152:104623. doi: 10.1016/j.phrs.2019.104623 [DOI] [PubMed] [Google Scholar]
- 110. Deng Z, Teng YJ, Zhou Q, Ouyang ZG, Hu YX, Long HP, et al. Shuyu pills inhibit immune escape and enhance chemosensitization in hepatocellular carcinoma. World J gastrointestinal Oncol (2021) 13(11):1725–40. doi: 10.4251/wjgo.v13.i11.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xie Y, Yan F, Wang X, Yu L, Yan H, Pu Q, et al. Mechanisms and network pharmacological analysis of yangyin fuzheng jiedu prescription in the treatment of hepatocellular carcinoma. Cancer Med (2022) 12(3):3237–59. doi: 10.1002/cam4.5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang Y, Wei Y, Jiang S, Dang Y, Yang Y, Zuo W, et al. Traditional Chinese medicine cff-1 exerts a potent anti-tumor immunity to hinder tumor growth and metastasis in prostate cancer through Egfr/Jak1/Stat3 pathway to inhibit pd-1/Pd-L1 checkpoint signaling. Phytomedicine (2022) 99:153939. doi: 10.1016/j.phymed.2022.153939 [DOI] [PubMed] [Google Scholar]
- 113. Zhu T, Ma Y, Wang J, Chen X, Li J, Meng L, et al. Grain-sized moxibustion heightens the antitumor effect of cyclophosphamide in Hepa1-6 bearing mice. Evidence-Based complementary Altern Med eCAM (2022) 2022:3684899. doi: 10.1155/2022/3684899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xue N, Fu X, Zhu Y, Da N, Zhang J. Moxibustion enhances chemotherapy of breast cancer by affecting tumor microenvironment. Cancer Manage Res (2020) 12:8015–22. doi: 10.2147/cmar.S249797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Han K, Kim M, Kim E, Park Y, Kwon O, Kim A, et al. Moxibustion for treating cancer-related fatigue: a multicenter, assessor-blinded, randomized controlled clinical trial. Cancer Med (2021) 10(14):4721–33. doi: 10.1002/cam4.4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang C, Yang M, Fan Y, Pei X. Moxibustion as a therapy for breast cancer-related lymphedema in female adults: a preliminary randomized controlled trial. Integr Cancer therapies (2019) 18:1534735419866919. doi: 10.1177/1534735419866919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Inprasit C, Huang Y, Lin Y. Evidence for acupoint catgut embedding treatment and Trpv1 gene deletion increasing weight control in murine model. Int J Mol Med (2020) 45(3):779–92. doi: 10.3892/ijmm.2020.4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sheng J, Jin X, Zhu J, Chen Y, Liu X. The effectiveness of acupoint catgut embedding therapy for abdominal obesity: a systematic review and meta-analysis. Evidence-Based complementary Altern Med eCAM (2019) 2019:9714313. doi: 10.1155/2019/9714313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Piao C, Zhang Q, Fu H, Wang L, Tang C. Effectiveness comparisons of catgut implantation at acupoint for obese type 2 diabetes: a protocol for systematic review and meta analysis. Medicine (2020) 99(30):e21316. doi: 10.1097/md.0000000000021316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Xu SH, Luo HX, Huang BJ, Yu L, Luo SJ, Hu H, et al. Therapeutic effect of catgut implantation at acupoint in a mouse model of hepatocellular carcinoma by suppressing immune escape. Evidence-Based complementary Altern Med eCAM (2022) 2022:5572869. doi: 10.1155/2022/5572869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ma J, Li K, Shi S, Li J, Tang S, Liu L. The application of uhplc-hrms for quality control of traditional Chinese medicine. Front Pharmacol (2022) 13:922488. doi: 10.3389/fphar.2022.922488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li Y, Lin Z, Wang Y, Ju S, Wu H, Jin H, et al. Unraveling the mystery of efficacy in Chinese medicine formula: new approaches and technologies for research on pharmacodynamic substances. Arabian J Chem (2022) 15(11):104302. doi: 10.1016/j.arabjc.2022.104302 [DOI] [PMC free article] [PubMed] [Google Scholar]