Abstract
In the United States, the frequency of using percutaneous mechanical circulatory support devices (MCS) for acute myocardial infarction (AMI) complicated by cardiogenic shock is increasing. These devices require large-bore vascular access to provide left-, right-, or bi-ventricular cardiac support, frequently under urgent/emergent circumstances. Significant technical and logistical variability exists in device insertion, care, and removal in the cardiac catheterization laboratory and in the cardiac intensive care unit. This variability in practice may contribute to adverse outcomes observed in centers that receive patients with cardiogenic shock, who are at higher risk for circulatory insufficiency, venous stasis, bleeding, and arterial hypoperfusion.
In this position statement, we aim to (1) describe the public health impact of bleeding and vascular complications in cardiogenic shock; (2) highlight knowledge gaps for vascular safety and provide a roadmap for a regulatory perspective necessary for advancing the field; (3) propose a minimum core set of process elements or “vascular safety bundle”; and (4) develop a possible study design for a pragmatic trial platform to evaluate which structured approach to vascular access drive most benefit and prevent vascular and bleeding complications in practice.
Keywords: Vascular Access Devices; Shock, Cardiogenic; Myocardial Infarction; Complications
INTRODUCTION
In the United States, cardiogenic shock affects 5 to 12% of patients with acute myocardial infarction (AMI), and significantly increases the risk of morbidity and mortality.(1) In centers specializing in cardiogenic shock, patients often receive mechanical circulatory support (MCS) devices to decrease the degree of hemodynamic compromise.(2,3) The devices are inserted through a large-bore vascular access, which is frequently obtained under emergent circumstances.(3) Vascular access and closure techniques remain highly variable among operators and this variability exists even among those working within institutions that have a standardized approach to management.(4) Cardiogenic shock care in the intensive care unit is complex and includes prolonged use of systemic anticoagulation and antiplatelet therapies to minimize thrombotic events.(5) To date, no well-accepted guidelines from professional organizations have been forthcoming.
Such variability in managing vascular access combined with the complexity of management during their intensive care unit stay are factors that may contribute to the high rate of complications observed in centers that accept shock patients from regional hospitals. Defining a minimum core set of process elements for a “vascular safety bundle” has the potential to improve outcomes and address safety concerns. In this Expert Opinion, we aim to (1) describe the public health impact of bleeding and vascular complications in cardiogenic shock; (2) highlight knowledge gaps for vascular safety and provide a roadmap for a regulatory perspective necessary for advancing the field; (3) propose a minimum core set of process elements for a “vascular safety bundle”; and (4) propose a possible study design for a pragmatic trial platform to evaluate which elements drive benefit and prevent complications in practice.
This document was developed as part of the Cardiac Safety Research Consortium (CSRC) conference meetings. The CSRC is a public-private academic partnership that consists of clinicians specializing in interventional cardiology and cardiogenic shock, academic cardiologists from different subspecialties including cardiac critical care, the U.S. Food and Drug Administration (FDA), the Centers for Medicare and Medicaid Services (CMS), industry leaders, and patients. The goal of the consortium is to fill a void in the evidence around the use of MCS devices in cardiogenic shock and establish reusable data collection mechanisms to enhance consistency for clinical trial design and development.(6,7) The CSRC Working Group identified many gaps in evidence that warrant rigorous prospective investigation. Prevention of vascular complications was the first clinical question that achieved the highest public health impact.
THE PROBLEM: VASCULAR COMPLICATION
Despite improvement in outcomes, vascular complications remain strongly associated with adverse events during cardiogenic shock admissions.(8–10) Vascular complications are attributed to several coexisting physiologic and therapeutic factors. These include end-organ hypoperfusion with associated coagulopathy, microcirculatory dysfunction, concomitant administration of antithrombotic and vasoactive agents, and targeted temperature management for cardiac arrest.(2) Many patients may also receive one or more percutaneous MCS devices to treat hemodynamic instability.(3,11) The insertion of these devices requires technical expertise in large-bore vascular access under emergent circumstances.(12) Despite efforts to standardize the process, variability in insertion techniques is frequently observed because of personal preferences, institutional restrictions, or regional differences.(3,4,13). A definition of the most commonly encountered vascular complications is summarized in Table 1.
Table 1.
Definitions of major bleeding and vascular complications during acute myocardial infarction.
| COMPLICATION | DEFINITION |
|---|---|
| Bleeding, BARC( 10 ) | |
| Type 0 | No bleeding |
| Type 1 | Bleeding that is not actionable and does not cause the patient to seek treatment |
| Type 2 | Any clinically overt or actionable sign of hemorrhage that leads to hospitalizations or intervention |
| Type 3 | a. Overt bleeding + Hgb ↓ 3-5 g/dL; transfusion + bleeding |
| b. Overt bleeding + Hgb ↓ <5 g/dL; tamponade; surgical intervention for control; use of vasoactive agents | |
| c. Intracranial hemorrhage; intraocular bleeding affecting vision | |
| Type 4 | CABG-related bleeding within 48 hours |
| Type 5 | a. Probable fatal bleeding |
| b. Definitive fatal bleeding; confirmed with autopsy or imaging | |
| Retroperitoneal Hematoma | Bleeding that extends to the retroperitoneal space due vascular access above the inguinal ligament and above the inferior epigastric artery. It can also be caused by inadvertent direct injury to the inferior epigastric artery |
| Arterial Access Site Complications | |
| Arterial Thrombosis | Local coagulopathy or clotting at the access site predisposed by larger arterial sheaths and prolonged dwell time. It can lead to painful and white extremity with loss of neurologic or motor function |
| Atheroembolism | Microembolization of necrotic tissue, atherosclerotic debris, or cholesterol particle secondary to manipulation of large sheath in the arterial space. It can present as a cutaneous manifestation (e.g., livedo reticularis) or the triad: (1) leg and foot pain; (2) livedo reticularis; (3) intact peripheral pulses. Atheroembolism can result in ischemic stroke if embolization affects the cerebrovascular space |
| Pseudoaneurysm | A locally contained hematoma that remains in continuity with arterial access site by a neck or a sinus track (e.g., false lumen) |
| Aneurysm | A 50% enlargement of the normal arterial wall diameter resulting from rupture, thrombosis, or distal embolization |
| Arteriovenous fistula | A communication between the arterial vascular access site with an adjacent venous puncture resulting in a bruit over the vascular access site |
| Femoral neuropathy | Femoral nerve dysfunction occurs because of direct trauma of the femoral nerve during vascular access or secondary to a compression from a hematoma or a pseudoaneurysm |
| ALI | Acute cessation in blood flow to peripheral limb resulting in ischemia and may result in amputation |
Abbreviations: Hgb = hemoglobin; CABG = coronary artery bypass graft surgery; ALI = Acute Limb Ischemia.
The rates of bleeding and vascular complication associated with percutaneous MCS are not insignificant (Table 2).(14–22) Prospective studies in patients who underwent percutaneous coronary intervention (PCI) with hemodynamic support for ischemic heart disease reported bleeding complications in <10% of cases, but for patients with AMI complicated by cardiogenic shock, the incidence is significantly higher and may approach 60%.(23–27) For patients undergoing PCI, the use of axial flow MCS was associated with increased risk of major bleeding (31.4% vs. 16.0% [95% CI, 12.5-18.2], p <0.001) and hospital mortality (45.0% vs. 4.1% [95% CI, 7.6-14.2], p <0.001), when compared with use of intra-aortic balloon pump. These findings were consistent whether the device was inserted prior to or after PCI.(28) In a pooled analysis of 1,866 patients with cardiogenic shock supported with venoarterial extracorporeal membrane oxygenation (va-ECMO), there was 41% increased rate of major bleeding.(29) Vascular access techniques, operator proficiency with large-bore access, intensive care unit management, and use of closure devices for hemostasis, and definition of data elements relevant to vascular access safety were all risk factors for this high rate of adverse events. Bleeding may further be enhanced because of heparin-induced thrombocytopenia, acquired von Willebrand syndrome, and coagulopathies, which are frequently encountered during cardiogenic shock (Figure 1).(19,30–33)
Table 2.
The incidence rates of major bleeding and vascular complications in select study population of acute myocardial infarction complicated by cardiogenic shock.
| IABP vs TANDEMHEART | IABP-SHOCK II TRIAL | IMPRESS TRIAL | va-ECMO | CATHPCI CHEST PAIN-MI | CULPRIT-SHOCK SUBANALYSIS | |
|---|---|---|---|---|---|---|
| Author (year) | Thiele (2005) | Thiele (2012) | Ouweneel (2017) | Cheng (2014) | Dhurva (2020) | Freund (2020) |
| Study Arms | TandemHeart vs IABP | IABP vs OPT | Impella CP vs IABP | va-ECMO | LVAD vs IABP | Culprit-only vs complete revascularization |
| Study Population | AMI-CS† | AMI-CS† | AMI-CS | AMI-CS | AMI-CS | AMI-CS‡ |
| Sample Size | 41 | 300 | 48 | 1,866 | 1,680 | 684 |
| Study Design | RCT | RCT | RCT | Meta-analysis | Retrospective cohort study | RCT‡ |
| Definitions | ||||||
| Bleeding | Transfusion* | GUSTO criteria | Hgb ↓ ≥ 5 g/dL Transfusion* Surgery to control bleeding |
Variable | Hgb ↓ ≥ 3 g/dL Transfusion Surgery to control bleeding Retroperitoneal GI or GU bleed |
BARC 3b-5 GUSTO criteria TIMI bleeding§ |
| Vascular Complications | Lower extremity ischemia requiring surgical or interventional action | Peripheral ischemic vascular complications requiring surgical or interventional therapy | Device extraction Thrombotic occlusion of CFA ALI Surgery |
Lower extremity ischemia Fasciotomy or compartment syndrome Amputation |
- | - |
| Major Findings | ||||||
| Bleeding | ↑↑↑ *TandemHeart 90% vs. 40%, p=0.002 |
No Difference IABP vs. Control: 3.3% vs. 4.4%, p =0.51 |
↑↑↑ *Impella Retroperitoneal bleeding (n=1) Puncture site bleeding (n=2) |
↑↑↑ * va-ECMO Major bleeding = 40.8% (95% CI 26.8%-56.6%) |
↑↑↑ *LVAD (LVAD vs IABP: 31.3% vs 16.0, P<0.001) |
↑↑↑ *va-ECMO *Impella Estimate = 21.5% |
| Vascular Complication | ↑↑ Limb ischemia *TandemHeart 33% vs. 0%, p=0.009 |
No Difference IABP vs. Control: 4.3% vs. 3.4%, p =0.53 |
↑↑ *Impella Vascular repair for retroperitoneal bleeding (n=1) |
↑↑ *va-ECMO ALI = 16.9% (95% CI 12.6%-22.6%); Amputation = 4.7% (95%CI 2.3%-9.3%) |
- | Among bleeding group=14.3% |
| Other outcomes | Improved hemodynamics *TandemHeart Cardiac Power Index: 0.37 vs. 0.28, p=0.004 |
- | ↑↑ hemolysis ↑retroperitoneal bleeding *Impella |
↑↑ fasciotomy compartment syndrome ↑Amputation *va-ECMO |
- | Dialysis among bleeding group Estimate = 25.2% |
Abbreviations: AMI = Acute Myocardial Infarction; OPT = optimal medical therapy; BARC = Bleeding Academic Research Consortium; CFA = Common Femoral Artery; CABG = coronary artery bypass graft surgery; CS = cardiogenic shock; GUSTO = Global Use of Strategies to Open Occluded Coronary Arteries; Hgb = hemoglobin; IABP=intra-aortic balloon pump; LVAD = left ventricular assist device; PCI = percutaneous coronary intervention; RCT=randomized clinical trial; TIMI = Thrombolysis in Myocardial Infarction.
Intended to undergo revascularization (PCI or CABG); va-ECMO = venoarterial-Extracorporeal Membrane Oxygenation; CI = Confidence Interval.
Pre-defined sub-analysis of the CULPRIT-SHOCK trial.
Major bleeding requiring transfusion.
In case of > 1 bleeding event, only the most severe was counted.
Figure 1.
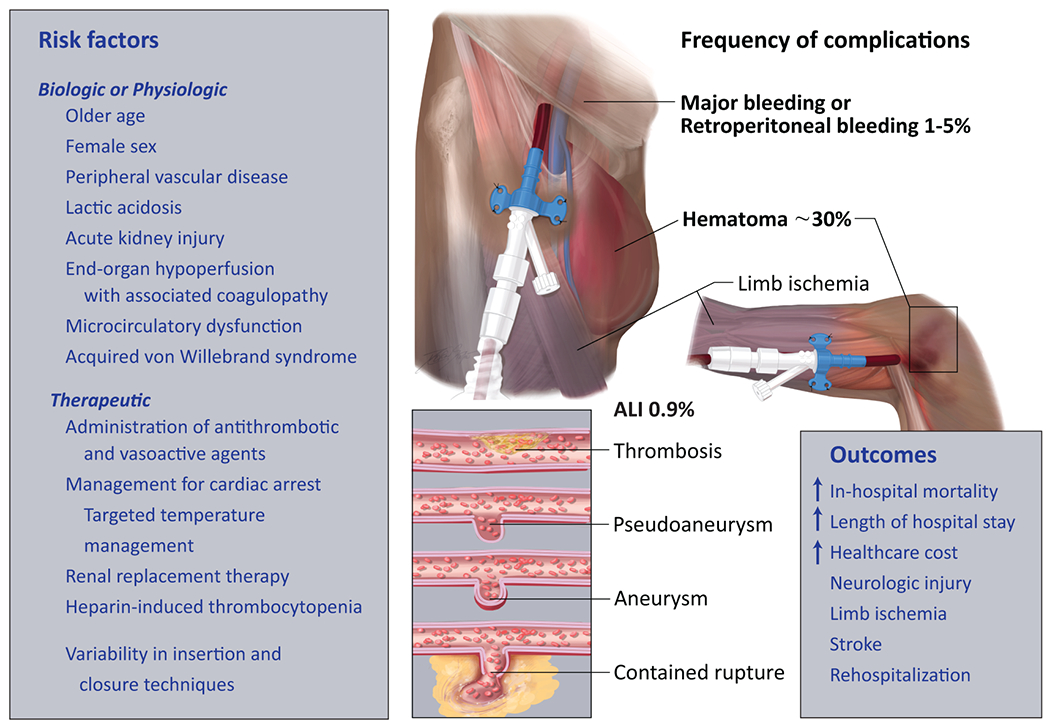
Vascular access-related complications for percutaneous mechanical support devices. The illustration shows different vascular access-related complications including access site hematoma, limb ischemia, thrombosis, pseudoaneurysm, aneurysm and contained rupture with callout data to risk factors, frequency, and associated outcomes. The above panel illustrates percutaneous mechanical support device in the right axillary artery and the lower panel illustrates the device in the right common femoral artery. All vascular and bleeding complications listed can occur using transfemoral, transaxillary, or transcaval (not shown in Figure) access. The Figure is meant to highlight the most common complications associated with large bore access in all types of MCS.
Acute limb ischemia (ALI) due to sheath-related vessel occlusion, thrombosis or atheroembolism, is common during placement of percutaneous MCS.(16,34) In an analysis of 172,491 admissions with ST-segment elevation myocardial infarction (STEMI) and cardiogenic shock, the incidence of ALI was 0.9%, and there was a 4 and 8.5-fold increased risk of ALI with a percutaneous ventricular assist device or va-ECMO, respectively.(33) The concomitant presence of symptomatic or asymptomatic peripheral artery disease can complicate vascular access, increase risk of limb related events, and may change the access closure strategy.(35) Compared with those without limb ischemia, patients with ALI had 20% higher rate of in-hospital mortality, increased length of stay, and higher healthcare cost.(33) Other vascular complications include bleeding, aortic dissection, arteriovenous fistula formation, and in the most severe cases, ALI requiring limb amputation.(30) Older age, female sex, peripheral vascular disease, lactic acidosis, and acute kidney injury requiring renal replacement therapy have been associated with bleeding and vascular complications in cardiogenic shock.(18,22,36) As a result, patients with these characteristics who are treated with MCS for hemodynamic stability are often at increased risk for complications. These considerations underscore not only the need for best practices when obtaining vascular access, but also investigation to discriminate which practices are associated with vascular injury, bleeding, and thrombotic complications. Conceptually, the domains of care can be trisected into (1) initial vascular access, (2) intensive care unit management, and (3) device removal and vascular closure.
Key Points Helpful for Clinical Practice:
Vascular complications are very common during cardiogenic shock admission and remain strongly associated with adverse cardiovascular events.
The incidence of vascular complications during cardiogenic shock is secondary to coexisting physiologic and therapeutic factors including end-organ hypoperfusion, coagulopathy, microcirculatory dysfunction, use of antithrombotic and vasoactive agents, and targeted temperature management for cardiac arrest.
Until clinical trial data become available to guide the choice of therapy, efforts aimed at encouraging best practices to prevent vascular injury, bleeding, and thrombotic complications are needed, and prompt recognition and management of these complications are critical to improve outcomes in the care of cardiogenic shock patients.
Initial Vascular Access
Patients who undergo large bore access for percutaneous MCS are underrepresented or excluded from randomized trials and mandatory public reporting. Thus, data on quality improvement are not readily available to improve outcomes.(27) In the context of cardiogenic shock, radial access is less likely utilized to minimize vascular complications due to weak/absent radial pulses and the convenience of working in one versus multiple sterile fields, especially when there is a high likelihood of using MCS device that can only be placed via femoral access.(4)
When considering femoral access, the ideal arteriotomy site is the common femoral artery because it is a relatively large vessel, less commonly involved with the development of atherosclerosis, and easily compressible. To avoid ischemic complications, the common femoral artery should ideally be large enough to accommodate a large-bore sheath based on non-invasive imaging (37,38). With advancement of technology, newer MCS sheaths expand in the iliac artery, and iliac sizing may be more meaningful than common femoral artery. Femoral angiography is needed in the absence of non-invasive data on vessel size to assess the puncture location, the presence of complications, and the size of the femoral artery. Both standard and digital subtraction angiography can be used to assess the access site, but based on the opinion of the writing group, digital subtraction is more sensitive for measurements and risk stratification particularly among patients with peripheral vascular disease. In addition, angiography and direct pressure/waveform recording also allow for an assessment of obstructive atherosclerotic disease and vessel tortuosity. The presence of both conditions is associated with an increased risk of complications and may preclude the placement of a percutaneous device in the vascular structure.(39,40) The use of fluoroscopy to identify bony landmarks at the beginning of the procedure is critical to highlight the anatomic relationship between the inguinal ligament to the pelvic radiographic landmarks.(41) The inguinal ligament is located between the anterior superior iliac spine and the pubic tubercle and the ideal site for puncture is 2 to 3 cm below the mid-inguinal point and 1 cm lateral to the most medial aspect of femoral head (lower third of femoral head).(41,42) Ultrasound guidance is increasingly used for vascular access during cardiac catheterization to reduce complications. Benefits of ultrasound during vascular access include direct visualization of the common femoral artery, its position relative to the femoral vein, and the location of the femoral artery bifurcation. In the Femoral Arterial Access with Ultrasound Trial (FAUST), routine real-time ultrasound guidance resulted in higher success rates in patients with high bifurcations, reduced the number of attempts and time to access, avoided accidental venous puncture in anatomic variants, and prevented cannulation in diseased segments of the common femoral artery.(43)
Using the smallest access needle feasible should logically be beneficial in minimizing vessel trauma at the initiation of access. A 21-gauge micropuncture needle reduces the size of the initial puncture and potentially the associated extravasation of blood during wire/sheath exchanges. Furthermore, a smaller gauge arteriotomy may reduce the risk of damage resulting from errant sticks or back-wall punctures. While the use of micropuncture system is reasonable because of their smaller profile (21 ga vs. 18 ga for standard needles), data derived from prospective trials to show superiority are lacking.(44) Two randomized clinical trials testing micropuncture vs. standard common femoral artery access were terminated early due to low vascular complication rates (NCT02026180).(44,45) Even after the use of a micropuncture needle, however, the insertion of a large-bore sheath into hostile vascular pathology may still result in damage at the arteriotomy site, bleeding, or complete vessel occlusion and ALI. Therefore, when the large-bore sheath is positioned and secured, the distal vessel should be evaluated for patency to ensure distal perfusion is maintained.
In the event that distal flow is compromised after large bore sheath placement, distal vessel perfusion can be achieved using ultrasound-guided antegrade access to either the superficial femoral or profunda femoris arteries using a 5- or 6-Fr braded sheath.(46) The donor vessel can be (1) the ipsilateral common femoral artery by creating an “external bypass” from the large bore sheath side port to the side port of the 5- or 6-F sheath in the superficial femoral artery; (2) contralateral femoral external bypass to the antegrade ipsilateral superficial femoral artery; or less commonly (3) contra-lateral femoral internal bypass from a contralateral retrograde 7-F common femoral artery sheath into the ipsilateral profunda femoris or superficial femoral artery through an “up-and-over” internal 4-F sheath inserted via the 7F contralateral sheath.(46) The use of radial artery as a donor vessel has been reported(46), but based on the opinion of the author group, prolonged sheath dwelling time in the radial artery may increase the risk of thrombosis and ischemic complications to the hand. The sheath in the donor vessel should ideally be larger Fr size than the recipient to promote flow from high pressure to low pressure gradient to the recipient vessel. The use of braided sheath can be helpful to reduce catheter kinks in the recipient vessel. In the setting of distal occlusion, there are several available options to restore perfusion, including peel-away sheaths, external-, and internal-bypass circuits. The placement of antegrade perfusion catheters for ECMO patients is usually performed at the outset, ideally before large bore access cannulation, but for Impella it is typically performed after placement of large bore devices when there are signs of compromise to distal perfusion. While the use of distal perfusion techniques to maintain blood flow distal to large bore sheaths is reasonable particularly in setting of limb ischemia, it should be noted that these practices are derived from clinical experience and prospective randomized data on best practices are needed.
Other techniques to reduce vascular and bleeding complications have been reported. The use of single access for High-risk PCI (SHiP) technique has gained popularity in clinical practice with the use of Impella CP (Abiomed, Danvers, MA) which can further simplify vascular access and limit it to one versus multiple sterile fields.(47) Radial access has the potential to reduce iatrogenic consequences of interventional therapies when a second arterial access is needed for coronary intervention by using non-femoral access and avoidance of a second entry into the femoral artery.(48,49)
While the common femoral artery remains the default vessel for implantation, several factors may preclude the ability to deploy large-bore (>8 Fr) sheaths. These factors include severe peripheral arterial disease, concentric vessel calcification, iliofemoral tortuosity, morbid obesity, and small vessel caliber, either due to inherent body habitus or reactive vasoconstriction stemming from heightened afterload commonly encountered in the low output shock state and high-dose vasopressor use.(50–52) Advances to prevent ALI, such as pre-emptive treatment of iliofemoral peripheral vascular disease using angioplasty and intravascular lithotripsy, as well as placement of distal perfusion catheters, may not always be feasible during emergency setting.(53–55) ALI is typically a result of thrombosis and embolism, but in the context of large bore access for cardiogenic shock, limb ischemia due to an occlusive sheath is most common etiology. In addition, certain patients with cardiogenic shock may require prolonged circulatory support to optimize end-organ perfusion, nutritional status, and physical conditioning while awaiting cardiac replacement therapy, thus rendering prolonged bedrest following femoral access counterproductive.(56,57) Given these considerations, proficiency with alternative vascular access can be helpful when they are performed at experienced centers, where high volume complex coronary and structural heart interventions are performed.(58) For patients in rural areas, shock patients are taken to the nearest hospital and the use of alternate access techniques may not be feasible. In the absence of clinical trial data, the complexity of shock will require formation of networks to facilitate early recognition and initiate management strategies for patients residing in rural areas including use of large bore access for MCS.(59)
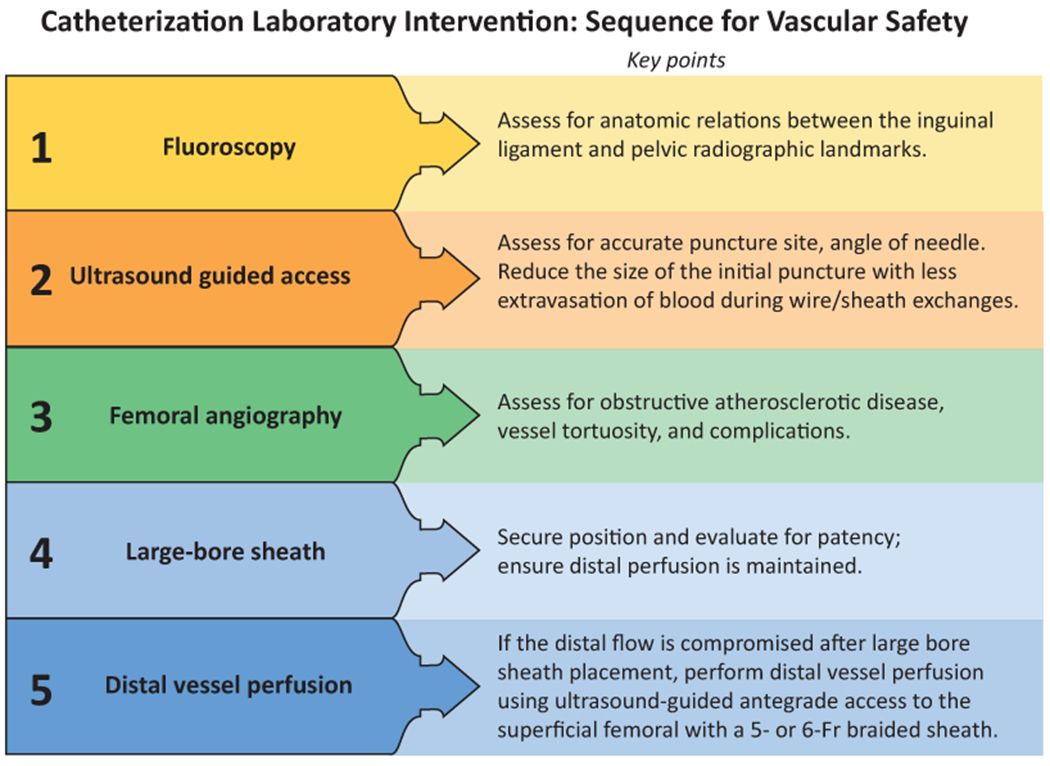
The transaxillary and caval-aortic techniques are two percutaneous approaches for alternative access. Potential concerns of using the axillary artery as a conduit for large-bore access exist with the thinner adventitia of the axillary artery, which may increase the risk of failure to achieve hemostasis when compared with the femoral artery. Further, the proximity of the axillary artery to the brachial plexus carries the risk of direct neurologic injury during vascular access. Despite these concerns, increasing experience with utilizing transaxillary access for percutaneous MCS and structural heart procedures has been encouraging.(60–63) Data from the prospective multicenter Axillary Access Registry to Monitor Safety study demonstrated a 98% success rate and <10% incidence of bleeding and vascular complications following implantation of axial flow percutaneous MCS using the axillary artery when performed at high volume centers with standardized algorithms.(64) Evidence on the learning curve or generalizability of such experience to smaller centers has not been reported. On average, the diameter of the axillary artery is 1.7 to 1.9 mm smaller than the common femoral artery and has less burden of calcification. Data derived from computed tomographic scans showed that the mean diameter of axillary artery in most patients is >6.3 mm, which is large enough to accommodate sheaths up to 18 Fr sheaths.(59) Endovascular repair with chimney graft technique can be utilized to achieve hemostasis after MCS device removal.(65) The sequence of “vascular safety bundle” in the cardiac catheterization laboratory is summarized in Central Illustration A.
Central Illustration.
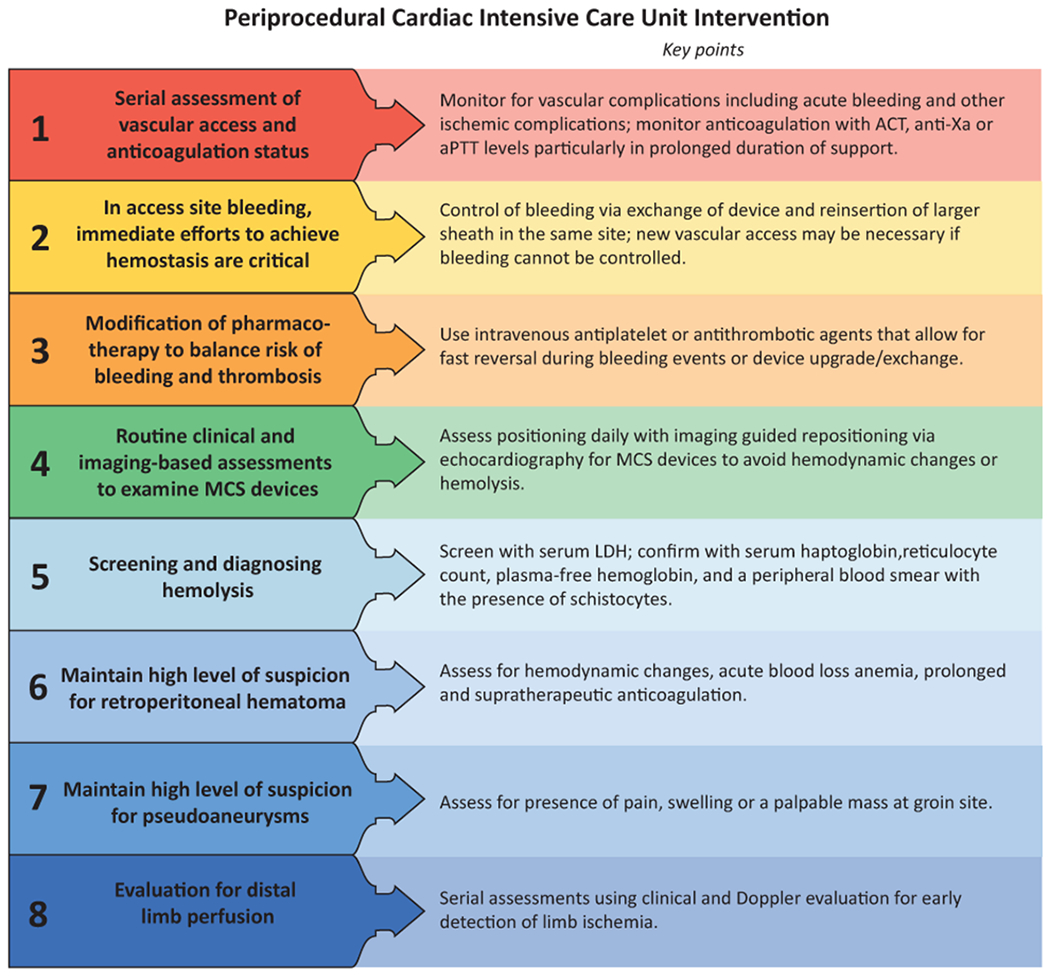
Illustrative algorithm to prevent vascular complications in the (A) cardiac catheterization laboratory; (B) Cardiac Intensive Care Unit.
Key Points Helpful for Clinical Practice:
The ideal transfemoral access site is the common femoral artery because it is relatively large and easily compressible.
Access is best achieved using a single anterior wall puncture with a 21-gauge micropuncture needle with combined ultrasound and fluoroscopy guidance.
Femoral angiography right after obtaining vascular access is often necessary to assess for the location of punction, size of femoral artery, and presence of complications.
Intensive Care Unit Management
Meticulous care aimed at the prevention of vascular complications extends into clinical management in the intensive care unit; the importance of which has been magnified by recent trends toward increasing duration of MCS.(28,66) Common practices reviewed by consensus of the Writing Group include: (a) minimizing the duration of vascular access whenever possible; (b) avoidance of excessive anticoagulation and tailoring antiplatelet therapy; (c) vigilance for bleeding after mobilizing the patient for turns and off-unit studies; (d) attention to the angulation of vascular access catheter insertion and securement to avoid tenting of the arteriotomy site; (e) prompt removal of the MCS device when bleeding cannot be controlled with the vascular access in place; and (f) device removal in the catheterization laboratory or operating room. Multidisciplinary collaboration among cardiologists, intensivists, interventional cardiologists, and vascular surgeons is rational.(2,67) While specific protocols for management may differ by institution, the first step is generally to ensure a safe hand-off from the referring proceduralist. In some centers, a multidisciplinary hand-off is performed at the patient’s bedside in the intensive care unit so that salient aspects of the patient’s care can be shared and all key stakeholders, including physicians and nurses, may ask questions.
The following practices may be considered based on expert consensus from the CSRC Working Group. Upon admission to the intensive care unit, providers ought to perform serial assessments to monitor for vascular complications, including access site bleeding or rapid hematoma expansion. When these complications occur, manual compression to achieve hemostasis is warranted until more definite approach of percutaneous or surgical repair is performed. Additional alternatives include exchanging the device with the insertion of a larger sheath and re-insertion of an MCS device in the same site. If control of bleeding cannot be achieved, a new vascular access with MCS removal and closure/compression of the previous site may be necessary. The reduction of anti-thrombotic therapies must be balanced against the risk of thrombosis. The use of intravenous antiplatelet or antithrombotic agents that allow for fast reversal should be utilized for any device upgrade/change or surgical left ventricular assist device consideration.
Chest radiography for IABP and point-of-care echocardiography for intracardiac devices are ideal to examine the device position or guide repositioning. Patients should be screened for hemolysis and thrombocytopenia via daily laboratory assessments, including complete blood count and serum lactate dehydrogenase (LDH). If the clinician is suspicious of hemolysis, serum haptoglobin, reticulocyte count, plasma-free hemoglobin, and a peripheral blood smear with the presence of schistocytes and elevated LDH may aid in confirming the diagnosis. Hemoglobinuria is a late manifestation of ongoing hemolysis and should be addressed expediently by either repositioning, removing, or replacing the MCS device. The advanced heart failure and critical care teams typically make at least daily assessments of the need for escalation or de-escalation of MCS. It is important to highlight the value of the “Shock Team” in the decision making to implant va-ECMO cannulas, Impella 5.0 via axillary access, or surgical removal of these devices if deemed necessary.(2,68)
Intensive Care Unit Complications
The intensive care unit physician should maintain awareness of several other vascular complications that may not be readily apparent from the clinical exam. These complications include retroperitoneal hematomas and arterial pseudoaneurysms. The risk of retroperitoneal hematoma significantly increases when the arteriotomy site is too high.(69) In patients who received a high arteriotomy access site, i.e., above the inferior epigastric artery, prolonged duration of support and/or supratherapeutic anticoagulation, increases the risk of retroperitoneal bleeding. Diagnosis is typically confirmed by computed tomographic imaging. Once identified, the primary objective is to prevent ongoing extravasation. Percutaneous management may be achieved by inflating a balloon at low pressure to cover the site of perforation. While bleeding is controlled, a covered stent may be rapidly deployed, or a vascular coil is delivered. An operator with advanced experience in peripheral vascular disease interventions should be concurrently available.
Clinical suspicion of pseudoaneurysm should be prompted by the presence of pain, swelling, or a palpable mass in the groin. The mass may be pulsatile with a palpable thrill or audible bruit. Diagnosis is typically confirmed with ultrasound or radiographic imaging. Risk factors include arteriotomies performed below the common femoral artery bifurcation and multiple arterial punctures. Clinical management includes (1) conservative therapy, as small pseudoaneurysms may thrombose spontaneously within four weeks; (2) percutaneous ultrasound-guided thrombin injection into the pseudoaneurysm; (3) embolization; or even (4) operative repair.(70) The latter is warranted for rapid expansion, rupture, infection, and mass effect resulting in distal or cutaneous ischemia or peripheral neuropathy. In most cases, pseudoaneurysms are managed with either ultrasound-guided manual compression or thrombin injection. Operative repair is warranted for rapid expansion, rupture, infection, and mass effect resulting in distal or cutaneous ischemia or peripheral neuropathy. The presence of arteriovenous fistula should be suspected in the presence of a pulsatile mass and the presence of a bruit over the arteriotomy site. Noninvasive imaging is often necessary for diagnosis. Endovascular therapies including coil embolization, stent grafts, cover stents are often successful in treating arteriovenous fistulae. Surgical interventions are reserved for large communication or failed endovascular approach.(71) Contemporary management of post-catheterization pseudoaneurysms entails the possible transfer of the patient for emergency surgery if conservative or percutaneous management of this complication fails.
In addition to awareness for bleeding, it is reasonable to implement systematic practices to foster early detection of ALI due to vessel occlusion, arterial dissection, or thromboembolism. intensive care unit clinicians should perform serial assessments with frequent clinical and Doppler evaluation of distal extremity pulses. Near-infrared spectroscopy monitoring is a noninvasive modality that can be used to monitor regional tissue oxygenation and early detection of limb ischemia.(72) The neurovascular checks should be performed every 2-4 hours to enhance early recognition of ALI. Serial measurement of lactic acid and/or lactate clearance may assist in evaluating limb perfusion and tissue necrosis. In this manner, intensive care unit clinicians can maintain awareness and address common vascular complications resulting from AMI with cardiogenic shock.(73)
Invasive arterial monitoring is often indicated with MCS and the radial access carries many of the same advantages seen in the cardiac catheterization laboratory. Introducer sheaths from the catheterization laboratory are not appropriate for this indication as they are generally occlusive in the radial artery and are constructed with thin walls that easily kink as they are not designed for chronic use. Ultrasound-guided access improves success, especially in hemodynamically compromised patients or those on hemodynamic support. In some situations of hemodynamic instability or physiologic changes such as hypothermia, radial pressure measurements may underestimate central pressures.(74) Improvements in support devices with central arterial monitors may obviate these issues in the future, but until then, some have demonstrated excellent results with small caliber brachial arterial lines.(75) The caveat is that these brachial lines were managed very carefully, and while brachial artery complications are rare, those that occur can be severe. In addition to the advantages for using transradial access for intervention, the forearm arteries are suggested over femoral arterial monitoring lines by the Center for Disease Control Healthcare Infection Control Practices Advisory Committee to reduce the risk of intravascular infection.(76)
The risk for both bleeding and ischemic complications is increased with the utilization of percutaneous MCS. Because the lack of consensus on what constitutes an optimal anticoagulation strategy, there is significant variability in use of pharmacotherapies in the intensive care unit.(77) Beavers and colleagues(78) assembled a panel of experts to review available evidence on anticoagulation practices and had 42 recommendations to improve anticoagulation management related to use of percutaneous MCS. Briefly, the use of Dextrose 5% in water as a standard purge solution is the mainstay of pharmacotherapy with a heparin concentration of 25 units/mL for Impella support outside of procedural settings.(78) Heparin-free purge solutions should be utilized in patients with heparin-induced thrombocytopenia.(78) For intravenous anticoagulation, two biomarkers can be used to check the effectiveness of anticoagulation therapy: anti-Xa target range of 0.2-0.4 IU/mL or serum aPTT level. Plasma-free hemoglobin was suggested to monitor for hemolysis within 72 hours of device insertion. In patients who received an Impella before ECMO cannulation, lower dose of heparin (maximum 50 units per kg) was recommended by the panel of experts. For patients with distal perfusion catheter, full dose systematic anticoagulation is recommended because of blood stasis between the access sheath and distal catheter which predisposes to thrombosis and limb ischemia. A full discussion on best anticoagulation practices is included in the referenced expert consensus statement (78). The periprocedural intensive care unit intervention is summarized in Central Illustration B.
Key Points Helpful for Clinical Practice:
Monitor for vascular complications including acute bleeding and other ischemic complications; Check anticoagulation status with ACT; anti-Xa and/or aPTT levels particularly in prolonged duration of support.
Control for bleeding via exchange of device and reinsertion of larger sheath in the same site; new vascular access may be necessary if bleeding cannot be controlled.
Use intravenous antiplatelet or antithrombotic agents that allow for fast reversal during bleeding events or device upgrade/exchange.
Assess positioning daily and imaging guided repositioning with echocardiography for interactive devices to avoid hemodynamic changes or hemolysis.
Screen for hemolysis with serum LDH; confirm the diagnosis with serum haptoglobin, reticulocyte count, plasma-free hemoglobin, and a peripheral blood smear with the presence of schistocytes.
Maintain high level of suspicion for pseudoaneurysm by assessing for presence of pain, swelling or a palpable mass at groin site.
Assess for early signs of distal limb ischemia using regional tissue oxygenation.
Device Removal and Vascular Closure
Device removal and successful hemostasis should be achieved in a controlled setting, preferably in the cardiac catheterization laboratory, or the hybrid operating room, to minimize risk of bleeding and vascular complications. Several methods for device removal are utilized in practice, but a comprehensive discussion by the shock team at each institution is advised to standardize the practice for device removal and periodically check outcomes related to the method used.
Several vascular closure techniques exist in clinical practice, but based on the experience of the writing group, the use of Perclose ProGlide devices (Abbott Vascular, Santa Clara, CA) prior to the insertion of the large-bore sheath has been associated with favorable outcomes. While many contemporary programs are now using one device in practice, two ProGlide devices can be used by deploying the needle into the arteriotomy sites at perpendicular angles prior to the placement of the large sheath. The use of one device combined with other active vascular closure devices, e.g., AngioSeal Closure device (St. Jude Medical, Inc, Minnetonka, Minnesota), has also been used to achieve hemostasis with variable success rates. Via the radial or contra-lateral femoral access, dry field closure can be utilized by advancing a peripheral balloon that sized one-to-one with the ipsilateral external iliac artery and inflated to achieve hemostasis during deployment of sutured based vascular closure device.(79) Inflation to 2-4 atm is often enough to achieve complete occlusive pressure to the femoral access site.(79) The proper sizing of the balloon and application of low pressure inflation are critical to avoid iatrogenic iliac artery injury.(79)
Other techniques with variable efficacy and safety profiles have been reported. For example, manual pressure to achieve hemostasis can be used, but based on the expertise of this writing group, it often results in the inability to attain hemostasis or results in ischemic complications. Percutaneous closure of femoral vascular access site can also be achieved using ProStar™ XL Percutaneous Vascular Surgical Systems (Abbott Vascular, Santa Clara, CA) for 8.5 to 10F sheaths, but outcomes are more favorable with Perclose device. The MANTA device (Teleflex, Wayne, PA) can provide effective closure of large bore access,(37) but apprehensions regarding safety events due failure of deployment and device-associated costs remain concerns for wide use adoption in practice.(80) The MANTA vs. Suture-based vascular closure after transcatheter aortic valve replacement (MASH) trial has shown that the plug-based MANTA closure device was not superior to suture based 2-ProGlides devices, but the bailout technique for MANTA involved the use of covered stents or surgical repair versus additional closure devices with 2-ProGlide devices.(81) Caution should be exercised when interpreting this data because it is derived from single-center study. Surgical repair of the arteriotomy site after device removal has been utilized, but with the increasing use of large-bore access for percutaneous MCS in smaller centers, the rapid availability of surgical expertise may not always be feasible. It should be noted that the study populations of the MASH and CHOICE-Closure trials are derived from TAVR cohorts, and generalizability to cardiogenic shock study populations may be limited. However, TAVR experience using large bore devices can be instructive to shock operators because the process of vascular access management was standardized. This has led to reduction in the rate of complications as operators gained more experience with the management of large bore access and closure.
After achieving hemostasis, a final angiographic image of the vascular access may improve early recognition of vascular access-related complications. The angiography is preferably achieved via ultrasound-guided radial access, but contralateral femoral access can also be utilized. After hemostasis is achieved, protamine can be used to reverse the effects of systemic anticoagulation and stop any skin oozing from the vascular access site. The systematic approach to vascular closure combined with continuous feedback based on clinical outcomes is necessary for improvement in quality and outcomes. When developing a shock program, operators with expertise in vascular closure of large-bore access should be available to supervise operators with less experience to minimize vascular access-related complications.
Building a Successful Shock Program
Health systems should actively engage expertise from interventional cardiology and vascular surgery to build successful shock programs to achieve optimal outcomes for patients with cardiogenic shock. The shock team is a multidisciplinary team that consists of an interventional cardiologist, cardiothoracic surgeon, cardiac intensivist, and advanced heart failure specialist.(82) Despite increased operator experience, improvement in delivery systems and devices, and volumes across centers, the rate of major vascular complications or major bleed after closure are still not insignificant.(83,84) Prompt recognition of vascular complications and immediate management are essential for preventing adverse outcomes as discussed elsewhere. The use of digital subtraction imaging can diagnose ileo-femoral dissection, rupture, arterial avulsion, stenosis thrombosis or occlusion. Operators with advanced skills in peripheral vascular disease interventions can manage these complications when recognized.
Key Points Helpful for Clinical Practice:
Several vascular closure techniques exist in clinical practice, but the use of Perclose ProGlide devices (Abbott Vascular, Santa Clara, CA) prior to the insertion of the large-bore sheath has been associated with favorable outcomes.
After achieving hemostasis, a final angiographic image of the vascular access may prevent delayed recognition of vascular access-related complications.
Health systems should actively engage expertise from interventional cardiology and vascular surgery to build successful shock programs to achieve optimal outcomes for patients with cardiogenic shock.
GAPS IN KNOWLEDGE
Although the epidemiology of vascular complications and access site bleeding points to a pressing need for effective interventions to mitigate these risks, there are substantial gaps in the evidence that might guide optimization of such interventions. As described in preceding sections, radial arterial access, combined use of palpation, angiography, and ultrasound to guide the selection of the vascular access location, use of micropuncture needles, angiographic confirmation of positioning and patency post-insertion, and rigorous vascular access closure procedures, all have plausible potential to reduce vascular access complications, including bleeding. However, except for radial access, none of these interventions have yet been studied in adequately sized, controlled clinical trials to establish their efficacy. Moreover, even the mechanisms of the established benefit of radial access are not completely understood. While some centers have adopted standardized approaches to vascular access, device removal, and closure that incorporate all these interventions, it is uncertain which elements may be necessary to achieving a benefit. Moreover, the tension between bleeding and thrombotic risks with manipulation of anticoagulation adds complexity to the management of vascular access complications. The wide variability in practices for vascular access underscores both the uncertainty regarding their benefit and highlights the potential for meaningful improvement in safety outcomes if specific interventions were confirmed to be effective.
The conduct of randomized controlled trials in patients with AMI complicated by cardiogenic shock is challenging.(6) The impediments to a successful execution of robustly sized clinical trials include (a) the severity of the clinical condition and its impact on the ability of the patient to provide informed consent for research; (b) the emergent nature of management decisions; (c) the inherent operational barriers to support round-the-clock clinical research in that context, and; (d) the ambiguity of clinical equipoise among some physicians when considering specific interventions. These potential barriers are present when considering trials of approaches to vascular access in patients with AMI complicated by cardiogenic shock. The inclusion of a “vascular safety bundle” as part of ongoing trials in AMI complicated by cardiogenic shock has the potential to inform the safety of MCS in this high-risk population.
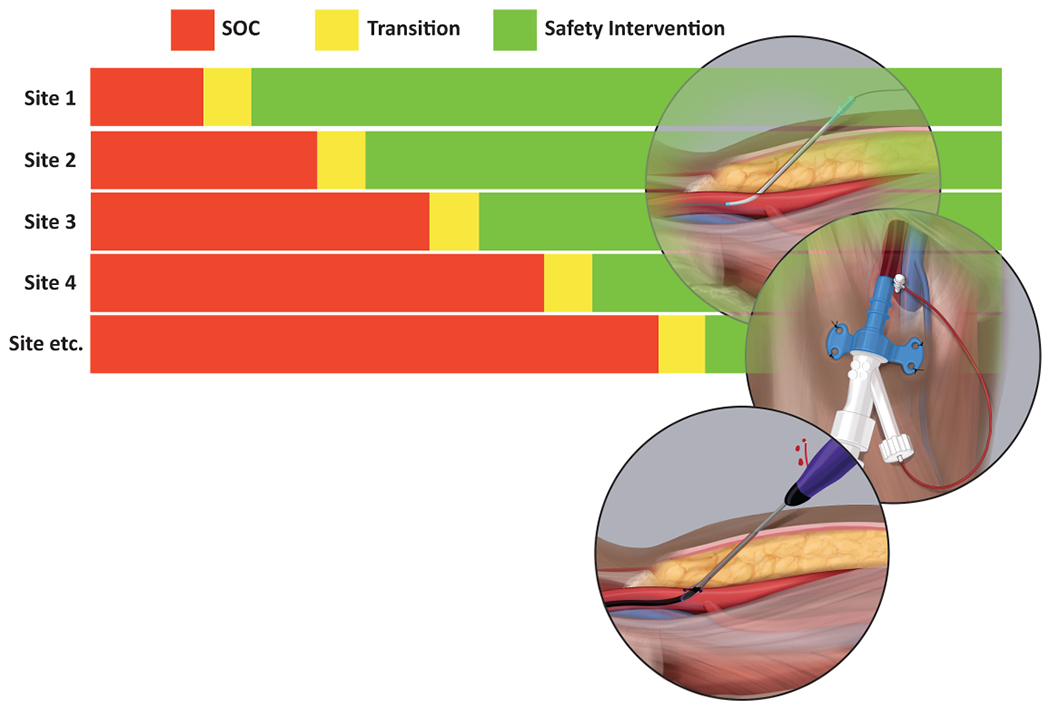
Incorporation of harmonized instruments for data collection using standardized definitions into registries and ongoing clinical trials offers an initial opportunity to accelerate the collection of observational data to more rigorously expand what we know regarding current practice patterns, the incidence of vascular access complications, and their associated outcomes in the contemporary care of patients with AMI complicated by cardiogenic shock.(85) Such data would help guide the design and implementation of clinical trials of specific interventions or processes of care. As one example, a stepped wedge, cluster randomized trial in which centers would be randomized at site-level to their timing of the transition from their prevailing usual care as the comparator to the investigational vascular access safety package would substantially advance the evidence available to guide mitigation of vascular access complications (Figure 2). Because of their very high incidence in this population, potential endpoints, including vascular access site bleeding and all-cause mortality, would be simple and clinically compelling. Such a pragmatic trial design has the possibility to be incorporated into registries to leverage parallel infrastructure and achieve lower costs because of existing data collection.
Figure 2.
Stepped wedge cluster randomized trial design. This clinical trial design aims to investigate the efficacy and safety of a “vascular access safety bundle” for percutaneous mechanical support devices in patients with acute myocardial infarction complicated by cardiogenic shock. Abbreviations: SOC = Standard of Care.
Key Points Helpful for Clinical Practice:
There is an urgent need to include a vascular safety “bundle” as part of ongoing trials in AMI complicated by cardiogenic shock to improve outcomes of large bore vascular access in this high-risk patient population.
A stepped wedge, cluster randomized trial in which centers would be randomized at site-level to their timing of the transition from their prevailing usual care as the comparator to the investigational vascular access safety package would substantially advance the evidence available to guide mitigation of vascular access complications.
REGULATORY PERSPECTIVE
The FDA has not required sponsors to show a benefit other than an increase in blood pressure to treat hypotension in cardiogenic shock, reasoning that treatment of hypotension provides time for definitive treatment. In the setting of cardiogenic shock, the benefit of a treatment strategy designed to avert significant morbidity and mortality is weighed against the risks associated with such treatment. Vascular access complications are aggravating features in device development to optimize the management of shock. Variability in clinical practice relating to vascular access complicates the evaluation of efficacy/safety and thus the benefit/risk analysis. Standardization of care (i.e., safety bundles) would homogenize the datasets and permit a more substantive review.
A stepped-wedge trial design strategy, whereby an intervention is rolled-out sequentially to trial participants (either as individuals or clusters), provides for all trial subjects to receive the intervention being tested, compared to a standard randomized clinical trial. The order in which the individuals/clusters receive the intervention is randomized. This pragmatic design may reconcile the need for a substantial evaluation under conditions of logistical constraints, as in the setting of cardiogenic shock. If substantial cluster-level effects are present (i.e., there is a large intra-cluster correlation), the stepped-wedge design is more powerful than a randomized clinical trial. If clusters are relatively homogenous (intra-cluster correlation is small), parallel studies tend to provide better statistical performance.(86) Given the variability in clinical practice, the optimal trial design would be based on the intra-cluster correlation (ICC) coefficient. A pilot trial can estimate the ICC as a prelude to the ultimate trial design with the aim of improving outcomes in patients with cardiogenic shock.
CONCLUSION
Major bleeding and vascular complications are common in patients with AMI complicated by cardiogenic shock and contribute significantly to morbidity and mortality. There is substantial variability in practice relating to vascular access, intensive care unit management, device removal, and vascular closure between operators within the same institution, and even more variability across different institutions worldwide. “Vascular safety bundles” that standardize vascular care during cardiogenic shock admissions have the potential to improve quality and outcomes. Controlled studies that examine the efficacy and safety of such vascular safety bundles are a compelling next priority of clinical investigations in field of cardiogenic shock.
HIGHLIGHTS.
Major bleeding and vascular complications are common in patients with acute myocardial infarction complicated by cardiogenic shock and contribute to morbidity and mortality.
There is substantial variability in practice relating to vascular access, cardiac critical care delivery, device removal, and vascular closure among institutions and individual operators.
Standardization of vascular access and closure techniques and approach to cardiac critical care management using “safety bundles” have the potential to favorably impact health outcomes during cardiogenic shock care.
ACKNOWLEDEMENT:
The authors would like to acknowledge Ms. Devon Stuart for her assistance with the medical illustration.
FUNDING AND FINANCIAL DISCLOSURE:
Dr. Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute (NHLBI) K23-HL153771-01. Dr. Tehrani is a consultant for Medtronic, and he is on the advisory board for Abbott Medical and Retriever Medical. Dr. Samsky receives salary support from NIH T32 Training Grant HL069749. Dr. Smilowitz is supported, in part, by the NHLBI under Award Number K23HL150315 and serves on an advisory board for Abbott Vascular. Dr. Truesdell is a consultant and a speaker for Abiomed Inc. Dr. Rao receives institutional research funding from Bayer, NHLBI, American College of Cardiology. Dr. Amin received research grant from Terumo. Dr. Dangas receives research grants from Mt. Sinai Hospital, Abbott Vascular, Boston Scientific, Medtronic, and Biotronik. Dr. West is an employee of Abbott Vascular. Dr. Moazami is on an independent provider quality panel for Medtronic related to safety evaluation of mechanical support devices. Dr. Krucoff receives consulting & research Grants from Edwards, Medtronic; Abbott Vascular; Abiomed; CSI; Boston Scientific; Bard; Cook Medical; Terumo. Drs. Sinha, Henry, DeVore, Senatore, Thiele, Cigarroa, Morrow, and Gilchrist have no disclosures.
ABBREVIATIONS:
- ALI
Acute Limb Ischemia
- AMI
Acute Myocardial Infarction
- BARC
Bleeding Academic Research Consortium
- FDA
Food and Drug Administration
- MCS
Mechanical Support Devices
- PCI
Percutaneous Coronary Intervention
- STEMI
ST-elevation Myocardial Infarction
- va-ECMO
venoarterial-Extracorporeal Membrane Oxygenation
Footnotes
PRESENTATION: None.
DISCLAIMER: The content and opinions expressed in this manuscript do not necessarily reflect guidance or policies of the U.S. Food and Drug Administration.
REFERENCES
- 1.Damluji AA, Bandeen-Roche K, Berkower C et al. Percutaneous Coronary Intervention in Older Patients With ST-Segment Elevation Myocardial Infarction and Cardiogenic Shock. J Am Coll Cardiol 2019;73:1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tehrani BN, Truesdell AG, Psotka MA et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail 2020;8:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin AP, Spertus JA, Curtis JP et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation 2020;141:273–284. [DOI] [PubMed] [Google Scholar]
- 4.Damluji AA, Nelson DW, Valgimigli M et al. Transfemoral Approach for Coronary Angiography and Intervention: A Collaboration of International Cardiovascular Societies. JACC Cardiovasc Interv 2017;10:2269–2279. [DOI] [PubMed] [Google Scholar]
- 5.Fordyce CB, Katz JN, Alviar CL et al. Prevention of Complications in the Cardiac Intensive Care Unit: A Scientific Statement From the American Heart Association. Circulation 2020;142:e379–e406. [DOI] [PubMed] [Google Scholar]
- 6.Samsky M, Krucoff M, Althouse AD et al. Clinical and regulatory landscape for cardiogenic shock: A report from the Cardiac Safety Research Consortium ThinkTank on cardiogenic shock. Am Heart J 2020;219:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samsky MD, Krucoff MW, Morrow DA et al. Cardiac safety research consortium “shock II” think tank report: Advancing practical approaches to generating evidence for the treatment of cardiogenic shock. Am Heart J 2020;230:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman PM, Gurm Hitinder S, McNamara R et al. Percutaneous Coronary Intervention Complications and Guide Catheter Size. JACC: Cardiovascular Interventions 2009;2:636–644. [DOI] [PubMed] [Google Scholar]
- 9.Leonardi S, Gragnano F, Carrara G et al. Prognostic Implications of Declining Hemoglobin Content in Patients Hospitalized With Acute Coronary Syndromes. J Am Coll Cardiol 2021;77:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton JS, Tamis-Holland JE, Bangalore S et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4–e17. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhajosyula S, O’Horo JC, Antharam P et al. Venoarterial Extracorporeal Membrane Oxygenation With Concomitant Impella Versus Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO Journal 2020;66:497–503. [DOI] [PubMed] [Google Scholar]
- 12.Ancona MB, Montorfano M, Masiero G et al. Device-related complications after Impella mechanical circulatory support implantation: an IMP-IT observational multicentre registry substudy. Eur Heart J Acute Cardiovasc Care 2021;10:999–1006. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval Y, Burke MN, Lobo AS et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC Cardiovasc Interv 2017;10:2233–2241. [DOI] [PubMed] [Google Scholar]
- 14.Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83. [DOI] [PubMed] [Google Scholar]
- 15.Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–8. [DOI] [PubMed] [Google Scholar]
- 16.Thiele H, Zeymer U, Neumann FJ et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 17.Ouweneel DM, Eriksen E, Sjauw KD et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 18.Freund A, Jobs A, Lurz P et al. Frequency and Impact of Bleeding on Outcome in Patients With Cardiogenic Shock. JACC: Cardiovascular Interventions 2020;13:1182–1193. [DOI] [PubMed] [Google Scholar]
- 19.Kaura A, Sterne JAC, Trickey A et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): a cohort study based on routine clinical data. The Lancet 2020;396:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson KI, Hillman C, Stillman CM et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc 2019;51:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrage B, Ibrahim K, Loehn T et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 22.Ratcovich H, Josiassen J, Helgestad OKL et al. Incidence, Predictors, and Outcome of In-Hospital Bleeding in Patients With Cardiogenic Shock Complicating Acute Myocardial Infarction. Am J Cardiol 2021;144:13–19. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill WW, Kleiman NS, Moses J et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717–27. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber T, Wah Htun W, Blank N et al. Real-world supported unprotected left main percutaneous coronary intervention with impella device; data from the USpella registry. Catheter Cardiovasc Interv 2017;90:576–581. [DOI] [PubMed] [Google Scholar]
- 25.Sjauw KD, Konorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol 2009;54:2430–4. [DOI] [PubMed] [Google Scholar]
- 26.Karami M, den Uil CA, Ouweneel DM et al. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. European Heart Journal: Acute Cardiovascular Care 2020;9:164–172. [DOI] [PubMed] [Google Scholar]
- 27.Damluji AA, Gilchrist IC. Lies, damned lies, and statistics, but bleeding and acute limb ischemia are facts! Catheter Cardiovasc Interv 2021;97:1139–1140. [DOI] [PubMed] [Google Scholar]
- 28.Dhruva SS, Ross JS, Mortazavi BJ et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng R, Hachamovitch R, Kittleson M et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610–6. [DOI] [PubMed] [Google Scholar]
- 30.Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiology and Therapy 2019;8:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kappetein AP, Head SJ, Généreux P et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg 2012;42:S45–60. [DOI] [PubMed] [Google Scholar]
- 32.Abaunza M, Kabbani LS, Nypaver T et al. Incidence and prognosis of vascular complications after percutaneous placement of left ventricular assist device. J Vasc Surg 2015;62:417–23. [DOI] [PubMed] [Google Scholar]
- 33.Pahuja M, Ranka S, Chehab O et al. Incidence and clinical outcomes of bleeding complications and acute limb ischemia in STEMI and cardiogenic shock. Catheter Cardiovasc Interv 2021;97:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmerini T, Serruys P, Kappetein AP et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: A meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J 2017;190:54–63. [DOI] [PubMed] [Google Scholar]
- 35.Mihatov N, Mosarla RC, Kirtane AJ et al. Outcomes Associated With Peripheral Artery Disease in Myocardial Infarction With Cardiogenic Shock. J Am Coll Cardiol 2022;79:1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed B, Piper WD, Malenka D et al. Significantly Improved Vascular Complications Among Women Undergoing Percutaneous Coronary Intervention. Circulation: Cardiovascular Interventions 2009;2:423–429. [DOI] [PubMed] [Google Scholar]
- 37.Dodson JA, Hajduk A, Curtis J et al. Acute Kidney Injury Among Older Patients Undergoing Coronary Angiography for Acute Myocardial Infarction: The SILVER-AMI Study. Am J Med 2019;132:e817–e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leon MB, Smith CR, Mack M et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New England Journal of Medicine 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 39.Redfors B, Watson BM, McAndrew T et al. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiol 2017;2:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damluji Abdulla A, Bandeen-Roche K, Berkower C et al. Percutaneous Coronary Intervention in Older Patients With ST-Segment Elevation Myocardial Infarction and Cardiogenic Shock. Journal of the American College of Cardiology 2019;73:1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rupp SB, Vogelzang RL, Nemcek AA, Jr., Yungbluth MM. Relationship of the inguinal ligament to pelvic radiographic landmarks: anatomic correlation and its role in femoral arteriography. J Vasc Interv Radiol 1993;4:409–13. [DOI] [PubMed] [Google Scholar]
- 42.Bangalore S, Bhatt DL. Femoral arterial access and closure. Circulation 2011;124:e147–56. [DOI] [PubMed] [Google Scholar]
- 43.Seto AH, Abu-Fadel MS, Sparling JM et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv 2010;3:751–8. [DOI] [PubMed] [Google Scholar]
- 44.Damluji Abdulla A, Nelson Daniel W, Valgimigli M et al. Transfemoral Approach for Coronary Angiography and Intervention. JACC: Cardiovascular Interventions 2017;10:2269–2279. [DOI] [PubMed] [Google Scholar]
- 45.Ambrose JA, Lardizabal J, Mouanoutoua M et al. Femoral Micropuncture or Routine Introducer Study (FEMORIS). Cardiology 2014;129:39–43. [DOI] [PubMed] [Google Scholar]
- 46.The Lichaa H. “lend a hand” external bypass technique: External radial to femoral bypass for antegrade perfusion of an ischemic limb with occlusive large bore sheath - A novel and favorable approach. Catheterization and Cardiovascular Interventions 2020;96:E614–E620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wollmuth J, Korngold E, Croce K, Pinto DS. The Single-access for Hi-risk PCI (SHiP) technique. Catheter Cardiovasc Interv 2020;96:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damluji AA, Rodriguez G, Noel T et al. Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am Heart J 2020;224:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijers TA, Aminian A, van Wely M et al. Randomized Comparison Between Radial and Femoral Large-Bore Access for Complex Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2021. [DOI] [PubMed] [Google Scholar]
- 50.Cheney AE, McCabe JM. Alternative Percutaneous Access for Large Bore Devices. Circulation: Cardiovascular Interventions 2019;12:e007707. [DOI] [PubMed] [Google Scholar]
- 51.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- 52.Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamb KM, DiMuzio PJ, Johnson A et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J Vasc Surg 2017;65:1074–1079. [DOI] [PubMed] [Google Scholar]
- 54.Das T, Mustapha J, Indes J et al. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc Interv 2014;83:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Mario C, Goodwin M, Ristalli F et al. A Prospective Registry of Intravascular Lithotripsy-Enabled Vascular Access for Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019;12:502–504. [DOI] [PubMed] [Google Scholar]
- 56.Esposito ML, Jablonski J, Kras A, Krasney S, Kapur NK. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int J Artif Organs 2018;41:236–239. [DOI] [PubMed] [Google Scholar]
- 57.Wood DA, Krajcer Z, Sathananthan J et al. Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Percutaneous Vascular Closure Device. Circulation: Cardiovascular Interventions 2019;12:e007258. [DOI] [PubMed] [Google Scholar]
- 58.Basir MB, Eng MH, Villablanca P et al. Alternative Access for Mechanical Circulatory Support. Structural Heart 2020;4:458–467. [Google Scholar]
- 59.Tehrani Behnam N, Sherwood Matthew W, Rosner C et al. A Standardized and Regionalized Network of Care for Cardiogenic Shock. JACC: Heart Failure;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnett DM, Lee JC, Harms MA et al. Caliber and fitness of the axillary artery as a conduit for large-bore cardiovascular procedures. Catheter Cardiovasc Interv 2018;91:150–156. [DOI] [PubMed] [Google Scholar]
- 61.Kajy M, Laktineh A, Blank N et al. Deploying Mechanical Circulatory Support Via the Axillary Artery in Cardiogenic Shock and High-Risk Percutaneous Coronary Intervention. Am J Cardiol 2020;128:127–133. [DOI] [PubMed] [Google Scholar]
- 62.Schäfer U, Ho Y, Frerker C et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach”. JACC Cardiovasc Interv 2012;5:477–486. [DOI] [PubMed] [Google Scholar]
- 63.Tayal R, Hirst CS, Garg A, Kapur NK. Deployment of acute mechanical circulatory support devices via the axillary artery. Expert Rev Cardiovasc Ther 2019;17:353–360. [DOI] [PubMed] [Google Scholar]
- 64.McCabe JM, Kaki AA, Pinto DS et al. Percutaneous Axillary Access for Placement of Microaxial Ventricular Support Devices. Circulation: Cardiovascular Interventions 2021;14:e009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moulakakis KG, Mylonas SN, Dalainas I et al. The chimney-graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg 2013;2:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afilalo J, Sharma A, Zhang S et al. Gait Speed and 1-Year Mortality Following Cardiac Surgery: A Landmark Analysis From the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J Am Heart Assoc 2018;7:e010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tehrani BN, Truesdell AG, Sherwood MW et al. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol 2019;73:1659–1669. [DOI] [PubMed] [Google Scholar]
- 68.Papolos Alexander I, Kenigsberg Benjamin B, Berg David D et al. Management and Outcomes of Cardiogenic Shock in Cardiac ICUs With Versus Without Shock Teams. Journal of the American College of Cardiology 2021;78:1309–1317. [DOI] [PubMed] [Google Scholar]
- 69.Kaki A, Blank N, Alraies MC et al. Access and closure management of large bore femoral arterial access. J Interv Cardiol 2018;31:969–977. [DOI] [PubMed] [Google Scholar]
- 70.Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007;115:2666–74. [DOI] [PubMed] [Google Scholar]
- 71.Numan F, Omeroglu A, Kara B, Cantaşdemir M, Adaletli I, Kantarci F. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx). J Vasc Interv Radiol 2004;15:939–46. [DOI] [PubMed] [Google Scholar]
- 72.Chang M, Lee CW, Ahn JM et al. Outcomes of Coronary Artery Bypass Graft Surgery Versus Drug-Eluting Stents in Older Adults. J Am Geriatr Soc 2017;65:625–630. [DOI] [PubMed] [Google Scholar]
- 73.Kapur NK, Whitehead EH, Thayer KL, Pahuja M. The science of safety: complications associated with the use of mechanical circulatory support in cardiogenic shock and best practices to maximize safety. F1000Res 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuda G, Denault A, Deschamps A et al. Risk Factors Involved in Central-to-Radial Arterial Pressure Gradient During Cardiac Surgery. Anesth Analg 2016;122:624–32. [DOI] [PubMed] [Google Scholar]
- 75.Singh A, Bahadorani B, Wakefield BJ et al. Brachial Arterial Pressure Monitoring during Cardiac Surgery Rarely Causes Complications. Anesthesiology 2017;126:1065–1076. [DOI] [PubMed] [Google Scholar]
- 76.O’Grady NP, Alexander M, Burns LA et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis 2011;52:1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reed BN, DiDomenico RJ, Allender JE et al. Survey of Anticoagulation Practices with the Impella Percutaneous Ventricular Assist Device at High-Volume Centers. Journal of Interventional Cardiology 2019;2019:3791307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beavers CJ, DiDomenico RJ, Dunn SP et al. Optimizing Anticoagulation for Patients Receiving Impella Support. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2021;n/a. [DOI] [PubMed] [Google Scholar]
- 79.Lichaa H, Wollmuth J, Tayal R. Dry Field Closure of Large-Bore Access With Iliac Artery Angioplasty Through the Ipsilateral Sheath: The Single-Access Dry-Closure Technique. J Invasive Cardiol 2021;33:E516–e521. [DOI] [PubMed] [Google Scholar]
- 80.Moccetti F, Brinkert M, Seelos R et al. Insights From a Multidisciplinary Introduction of the MANTA Vascular Closure Device. JACC: Cardiovascular Interventions 2019;12:1730–1736. [DOI] [PubMed] [Google Scholar]
- 81.van Wiechen MP, Tchétché D, Ooms JF et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure: A Pilot Randomized Controlled Trial. JACC: Cardiovascular Interventions 2021;14:149–157. [DOI] [PubMed] [Google Scholar]
- 82.Tehrani Behnam N, Truesdell Alexander G, Psotka Mitchell A et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC: Heart Failure 2020;8:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thyregod HG, Steinbrüchel DA, Ihlemann N et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184–94. [DOI] [PubMed] [Google Scholar]
- 84.Holmes DR Jr., Nishimura RA, Grover FL et al. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. J Am Coll Cardiol 2015;66:2813–2823. [DOI] [PubMed] [Google Scholar]
- 85.Damluji AA, van Diepen S, Katz JN et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021;144:e16–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ : British Medical Journal 2015;350:h391. [DOI] [PubMed] [Google Scholar]


