Abstract
Diagnostic and therapeutic advances over the last decades have substantially improved health outcomes for patients with acute coronary syndrome (ACS). Both age-related physiologic changes and accumulated cardiovascular risk factors increase the susceptibility to ACS over a lifetime. Compared with younger patients, outcomes for ACS in the large and growing demographic of older adults are relatively worse. Increased atherosclerotic plaque burden and complexity of anatomic disease, compounded by age-related cardiovascular and non-cardiovascular comorbid conditions, contribute to the worse prognosis observed in older individuals. Geriatric syndromes including frailty, multimorbidity, impaired cognitive and physical function, polypharmacy and other complexities of care can undermine therapeutic efficacy of guidelines-based treatments as well as the resiliency of older adults to survive and recover.
In this American Heart Association (AHA) Scientific Statement, we: (1) review age-related physiologic changes that predispose to ACS and management complexity; (2) describe the influence of commonly encountered geriatric syndromes on cardiovascular disease (CVD) outcomes; and (3) recommend age-appropriate and guideline-concordant revascularization and ACS management strategies, including transitions of care, the use of cardiac rehabilitation, palliative care services, and holistic approaches. The primacy of individualized risk assessment and patient-centered care decision-making is highlighted throughout.
Keywords: Myocardial Infarction, Acute; Acute Coronary Syndrome; Older Adults; Aging; Geriatric Syndromes; Patient-cntered care
INTRODUCTION
Approximately 720,000 Americans suffer an acute myocardial infarction (MI)- or coronary artery disease (CAD)-related death per year, and more than 335,000 Americans suffer recurrent events annually.1 The older adult population is disproportionately affected. Adults ≥75 years of age constitute approximately 30-40% of all hospitalized ACS patients and the majority of ACS-related mortality is observed in this segment of the population.2-4 These patients often present with heavy atherosclerotic plaque burden, anatomic complexities, calcifications, vessel tortuosity, ostial lesions, multivessel disease, and left main stenosis. In addition to such disease-specific therapeutic challenges, older adults are also more likely to present with concomitant geriatric syndromes that compound aggregate prognostic risk.5 Frailty, multimorbidity, cognitive impairment, functional decline, nutritional deficiencies, and polypharmacy are among a long list of geriatric domains that are endemic in the large and growing older population.6 Consideration of these geriatric complexities is crucial for optimal management of ACS amidst contemporary healthcare trends.5
Clinical trials that are considered standards regarding the efficacy and safety of ACS therapeutics predominantly enrolled patients <75 years of age, and most did not include candidates with geriatric complexities. However, in contemporary clinical practice, cardiovascular (CV) as well as geriatric risks are entwined, and both are relevant in regard to determining therapeutics that minimize adverse outcomes and achieve health states that are most likely to be valued by older patients.7 Timely differentiation between the different types of MI, especially MI with nonobstructive coronary arteries, is essential with related goals to avoid superfluous diagnostic tests, heterogeneity in clinical decisions, and underuse of treatments that are vital. These gaps highlight the need for updated recommendations for ACS management in older adults, with a synthesis of the information that has proliferated since the publication of the first American Heart Association (AHA) Scientific Statements on ACS in older adults.8, 9 The 2007 AHA statement established the need for clinical studies enrolling older adult populations.10 This document is primarily concerned with adults ≥75 years of age because most of the gaps in knowledge exist in this age group.11
In this AHA Scientific Statement, we review age-related physiologic changes in the heart and vascular systems that predispose to cardiovascular disease (CVD) and management complexities. We review commonly encountered “geriatric syndromes” and their influence on CVD outcomes. Guidelines-based revascularization and adjunctive strategies are emphasized, with relevant considerations regarding transitions of care, cardiac rehabilitation (CR), palliative care services, futility, and a holistic approach to care. Broader considerations regarding risk assessment and decision-making are highlighted.
CARDIOVASCULAR AGING
Cardiovascular Physiology
Normal aging is associated with multiple changes in CV structure and function that predispose older adults to CAD, myocardial ischemia, and ACS (Table 1).12 A hallmark of aging is increased stiffness of the aorta and large central arteries primarily due to increased collagen deposition and cross-linking in conjunction with degradation of elastin fibers. These changes lead to increased impedance to the left ventricular (LV) ejection and a widening of central aortic pulse pressure reflected in an age-related increase in systolic blood pressure and a decline in diastolic blood pressure, especially after age 75.13 To compensate for increased aortic impedance and central systolic blood pressure, aging myocytes tend to hypertrophy, provoking both increased apoptosis as well as left ventricular hypertrophy. In turn, the increased impedance to ejection and higher systolic blood pressure give rise to increased myocardial work and myocardial oxygen demand, whereas the lower diastolic pressure is associated with decreased coronary perfusion pressure. Thus, aging increases susceptibility to an imbalance between myocardial oxygen demand and supply.
Table 1.
Normal cardiovascular aging and risk for acute coronary syndromes.
| Age-related Change | Implications for ACS |
|---|---|
| Increased central aortic stiffness due to increased collagen crosslinking and elastin fiber degeneration | Increased impedance to LV ejection, increased SBP and pulse pressure, increased myocardial work and O2 demand, decreased coronary perfusion pressure |
| Altered LV diastolic relaxation and increased myocardial stiffness | Increased resistance to coronary perfusion; predisposition to atrial fibrillation and HFpEF |
| Decreased responsiveness to β-adrenergic stimulation | Decreased maximum HR and contractility, decreased peak cardiac output, decreased peripheral vasodilation |
| Impaired endothelium-mediated vasodilation | Decreased peak coronary blood flow and coronary flow reserve; increased atherogenesis; increase in vascular impedance as a result of impaired endothelium mediated vasodilatation. |
| Altered balance between intrinsic thrombosis and fibrinolysis | Increased risk for venous and arterial thromboembolism |
| Chronic low-grade inflammation (inflammaging†) | Increased atherogenesis and geriatric syndromes, including frailty |
Not a normal age-related change but prevalent at advanced age.
Abbreviations: ACS: acute coronary syndrome; HFpEF: heart failure with preserved ejection fraction; HR: heart rate; LV: left ventricular; SBP: systolic blood pressure.
Aging is also associated with increased collagen deposition in the myocardial interstitium as well as deposition of lipofuscin and other moieties. These factors and others lead to an increase in myocardial stiffness and impedance to LV filling with alteration in the diastolic filling pattern and increased force of left atrial contraction to maintain LV end diastolic volume.12 Slowing of LV relaxation also contributes to age-related diastolic dysfunction. The net effect is an increase in LV diastolic pressure that further lowers coronary perfusion pressure, which is defined as the difference between aortic diastolic pressure and LV diastolic pressure.
Aging is also associated with impaired coronary endothelial function mediated primarily by a decline in nitric oxide synthase activity.14 As a result, aged coronary arteries have less capacity to upregulate coronary blood flow in response to increased myocardial oxygen demands, which predisposes older adults to type II MI and non-ST-elevation MI (NSTEMI). In addition, endothelial dysfunction, along with inflammation, is a fundamental driver of atherosclerosis, thereby contributing to the increasing prevalence of both subclinical and overt CAD with advancing age. Further, while chronic inflammation is not inherent to normal aging, it is a common accompaniment to many age-associated diseases and conditions, including diabetes mellitus, arthritis, and frailty.15 The combination of endothelial dysfunction and chronic inflammation provides a fertile milieu for the development and progression of CAD in older adults.
Additional age-related CV changes include diminished responsiveness to beta-adrenergic stimulation and a nearly linear decline in maximum attainable heart rate (i.e., maximum heart rate ~ 220-age), both of which contribute to an age-associated decline in peak cardiac output in response to physiologic or pathologic stress.16 Although this decline in CV reserve with increasing age does not directly lead to ischemia under normal circumstances, it increases the risk for the development of acute and chronic heart failure in older patients with CAD.17 Apart from the effects on the CV system, aging is associated with a shift in the intrinsic balance between thrombosis and fibrinolysis in favor of thrombosis.18 As a result, older adults are more likely to develop venous thromboembolic disease, as well as arterial clots, including coronary thrombosis, the hallmark of type I MI, as well as left atrial appendage thrombus in patients with atrial fibrillation.17
Renal Aging
While the physiology of aging has broad impact on the CV system, kidney function is particularly pertinent to ACS as it exacerbates morbidity and mortality, and also diminishes accuracy of diagnosis and difficulty of therapeutic decision-making. Aging kidneys undergo macro- and microanatomical changes such as kidney cysts, focal inflammation, decreased cortical volume, nephrosclerosis (age-related histologic changes), and renal artery atherosclerosis. Other pathophysiologic changes in the kidney include loss of glomeruli and tubules and increased interstitial fibrosis, which need to be differentiated from other disease syndromes, such as those associated with diabetes mellitus and hypertension. The aging kidney is also characterized by tubular dysfunction, decreased sodium reabsorption, potassium excretion and urine concentrating ability, leading to volume depletion and risk of drug toxicity, all of which may predispose to acute kidney injury (AKI). Chronic kidney disease (CKD) is also more likely to develop in older patients due to the longitudinal loss of kidney function associated with aging.19 CKD is associated with increased coronary calcium, which has significant implications on revascularization strategies and outcomes.
Kidney disease evaluation includes assessment of kidney function by estimated glomerular filtration rate (eGFR). The National Kidney Foundation and the American Society of Nephrology have recently recommended race-neutral equations or use of the CKD-EPI equation with cystatin C.20 Additional indices for kidney function include urine test for albuminuria, serum electrolytes to evaluate for disorders of sodium, potassium, calcium and phosphate, as well as for volume assessment. Serum creatinine in older adults is less reliable than in younger adults as it may be spuriously low in the context of low muscle mass.
Implication of Kidney Disease on ACS
Cardiorenal syndrome (CRS) is defined as a bidirectional pathophysiologic disorder of both the heart and kidneys, which was initially classified into 5 different categories by Ronco.21 These 5 subtypes of CRS are categorized based on acuity and sequential organ involvement, which is discussed in detail in a prior AHA Scientific Statement and summarized in Supplemental Table 1.22 CRS type 1 is defined as an acute cardiac disease leading to acute kidney injury (AKI), typically caused by ACS, acute heart failure or cardiac surgery. Based on a systemic review and meta-analysis, Vandenberghe23 found that the prevalence of AKI in patients with ACS is 12.7%. A greater severity of AKI was associated with worse outcomes, including mortality (risk ratio 3.53, 95% adjusted hazard ratio (aHR) 2.04-6.10), length of stay in the intensive care unit (ICU), and rehospitalizations. AKI in older patients typically presents with multiple comorbidities, including diabetes mellitus, hypertension, heart failure, and peripheral arterial disease.
Key Points for Clinical Practice
The CV aging process is characterized by increased stiffness of the aorta and large central arteries, concentric LV hypertrophy, elevated LV end diastolic pressure, collagen deposition in the myocardial interstitium and impaired myocardial relaxation, impairment in the endothelial function of coronary arteries, diminished responsiveness to beta-adrenergic stimulation, and an imbalance between thrombosis and fibrinolysis.
The aging kidney is characterized by tubular dysfunction, decreased sodium reabsorption, potassium excretion and urine concentrating ability, predisposing to volume depletion and risk of drug toxicity, all of which increased risk for contrast induced AKI in the context of ACS.
Understanding of these pathophysiological changes is critical when evaluating guideline-directed medical therapy for ACS and for managing age-related risks in order to prevent physical, cognitive, or functional decline in older individuals.
GERIATRIC SYNDROMES
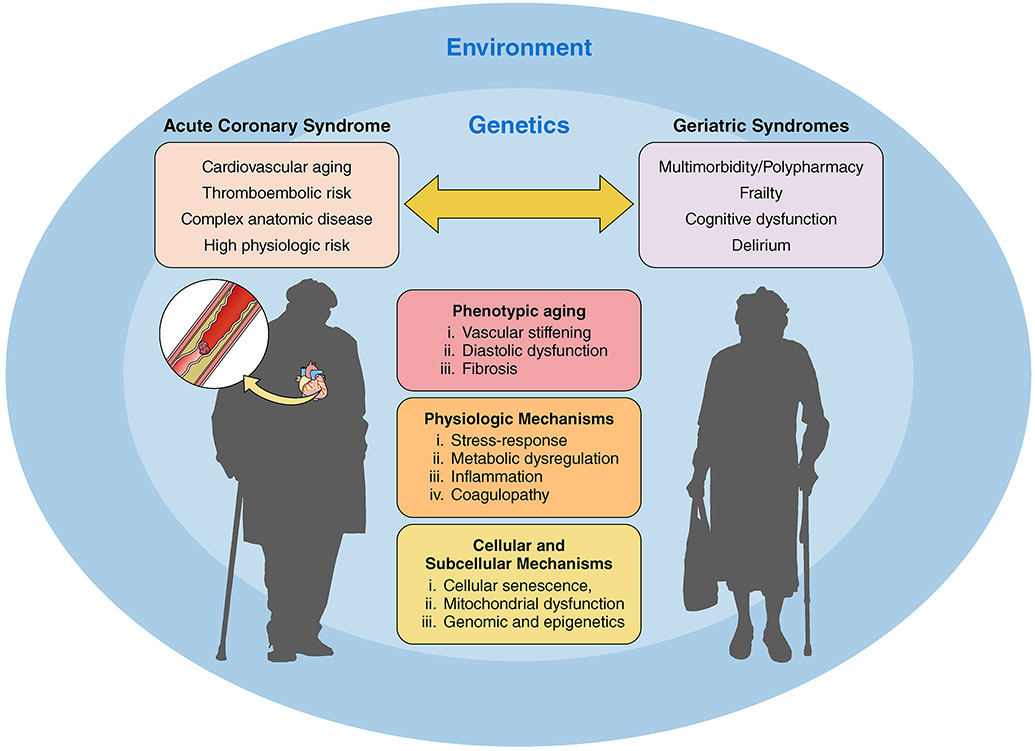
Geriatric syndromes are multifactorial conditions that are increasingly prevalent at older age. Whereas they are clinical syndromes, most stem from the same age-related physiologic vulnerabilities that underlie CVD.15 Examples include multimorbidity, frailty, functional decline (cognitive and physical), delirium, sensory decline (hearing, vision, and pain), falls, and polypharmacy.17 Notably, the incidence and prevalence of geriatric syndromes largely parallels the CV risk profile, such that older adults with low CV risk profiles are also at lower risk for the development of geriatric syndromes (Table 2).24 Conversely, older adults presenting with ACS are more likely to have a spectrum of diminished functional (physical and cognitive) reserves ranging from mild impairment to more significant decline. In addition, the presence of one or more geriatric syndromes may substantially impact clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment for ACS. It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and to be able to integrate them into the care plan when appropriate (Figure 1).
Table 2:
Geriatric Syndromes and Clinical Implications
| Geriatric Syndrome | Diagnosis/Prevalence | Prognosis | Disease Management |
|---|---|---|---|
| Multimorbidity | 2 or more chronic conditions (cardiac and non-cardiac) that are active simultaneously Prevalence: 63% of adults 65-74 years, 77% of adults 75-84 years, and 83% of adults ≥85 years. |
↑Short and long-term prognostic risks due to CVD as well as uncontrolled CVD and non-CVD risk factors. | Confounds customary CVD symptoms and signs Multiple diseases and providers often result in desynchronized or even contradictory aspects of care ↑ likelihood that patients will experience high therapeutic burden |
| Frailty | State of vulnerability relating to diminished physiological reserves across multiple physiologic systems.
|
↑Risk from CVD as well as medical, device, percutaneous catheter, and surgical therapies used to treat CVD. ↑Risks, disability, falls, rehospitalization, poor quality of life, mortality |
Guidelines-based therapy and procedures commonly overlook the impact of frailty on recommendations. Intensive care, bedrest and functional decrements associated with many conventional therapies can exacerbate frailty and functional decline. Nutrition and exercise may help mitigate frailty and risks of frailty |
| Cognitive decline | Mild cognitive impairment (MCI)→↓ cognitive function without loss of function;
|
↓Independence ↓Adherence ↓Shared decision making ↓QOL ↑Hospitalization ↑Mortality |
Often confounds assessments of symptoms Often confounds accounts of present illness and PMHx Often confounds adherence Does not negate the potential value of therapeutic intervention, but it impacts the decision and implementation process. |
| Delirium | Disturbance in cognition, attention, and consciousness or perception with fluctuating course
|
↑LOS ↑Rehospitalization ↑Functional Decline ↑Falls ↑Long-term Care ↑Mortality |
Prevention of delirium should take priority by optimizing the environment to increase orientation, avoid sedation, reduce meds, reduce pain. Predisposing risks include cognitive deficit, sensory limitations, and disorienting medications. Treat by optimizing environment to increase orientation, avoid sedation, reduce meds, reduce pain |
| Polypharmacy |
|
↑Adverse Events (errors and drug interactions) ↑Rehospitalizations ↑Mortality |
↑Medication errors ↑Drug-drug and drug-body interactions ↓Adherence is common Under- and Over-treatment both commonly occur Consider deprescribing |
| Disability | The inability to care for oneself or to manage one’s own home | ↑Risk progressive functional and cognitive declines ↓Self-reliance and self-efficacy ↑Long-term care ↑Mortality |
Conventional care for CVD often contributes to a cycle of progressive disability, which highlights rationale for shared decision making for each aspect of therapy. Conventional care for ACS (including pharmacotherapy, procedural care, and bedrest) can result in temporary immobility, delirium, disturbance in sleep pattern, and increase the risk of loss of independence. Suboptimal transitions are common contributors to disability, (e.g., hospital to home, and even hospital to post-acute care or hospital to home if support is inadequate or patient education has been deficient) |
| Sensory Loss | Vision and hearing deficits are common | ↑Risk progressive functional and cognitive declines ↓Self-reliance and self-efficacy ↑Long-term care ↑Mortality |
Same as disability |
Modified with permission from O’Neil DE and Forman DE. BMJ 2021; 374 :n1593 doi:10.1136/bmj.n1593.
Abbreviations: ACS = Acute Coronary Syndrome; CVD = Cardiovascular Disease.
Figure 1.
The bidirectional association between acute coronary syndrome and geriatric syndromes. Several factors influence this bidirectional association including phenotypic aging, physiologic mechanisms, cellular and subcellular mechanisms, genetics, and the environment.
Multimorbidity and polypharmacy.
Multimorbidity, defined as two or more chronic conditions, is ubiquitous in older adults with CV disease.17 In Medicare fee-for-service beneficiaries with ischemic heart disease, 48% have 5 or more comorbid conditions, 32% have 3-4 co-existing conditions, 17.5% have 1-2 other conditions, and only about 2.5% have isolated ischemic heart disease.25 Thus, ACS in older patients almost always occurs in the context of multiple comorbidities, many of which may intersect with the approach to treatment. In addition, high comorbidity burden is often associated with polypharmacy (usually defined as chronic use of 5 or more medications) and hyperpolypharmacy (usally defined as chronic use of 10 or more medications), which greatly increases the risk of adverse drug interactions and hospitalization.26, 27 It has been estimated, for example, that patients taking 10 or more medications have >90% likelihood of having at least one clinically important drug-drug interaction. To mitigate the impact of multimorbidity and polypharmacy, it becomes important to consider treatment for ACS in the context of these competing risks and engage in a shared decision-making process with the patient and family to ensure that the approach to management is patient-centered and aligned with the patient’s goals of care. A pharmacist with expertise in geriatric drug prescribing can provide guidance for discontinuing (i.e., deprescribing) non-essential prescription and over-the counter medications, as well as nutraceuticals, with goals to minimize the potential for drug-drug and drug-disease interactions and related susceptibilities to falls, confusion, and other age-related risks.28
Frailty.
Frailty, characterized by physiological decline across multiple organ systems resulting in increased vulnerability to stressors, is associated with an increased risk for adverse health outcomes, including functional decline, institutionalization, and death.29, 30 As noted above, older adults often have chronic low grade inflammation (“inflammaging”), which represents a shared risk factor for frailty and CV disease (Figure 1).15, 31, 32
In community-dwelling older adults, the prevalence of frailty increases from 16% at ages 65-69 years to about 22.2% at ages 80-84.29 The prevalence of frailty is higher in women than in men.33 Commonly used tools to assess frailty include the Fried Frailty Index34, Rockwood Clinical Frailty Score35, and the Frail Scale.36 In addition, slow gait speed (<0.8 m/sec), as assessed by a 4-6 meter walk, has been shown to be a reliable surrogate for frailty in ambulatory older adults. While there is still no consensus on the optimal method to assess frailty in ACS, there is consensus that this dimension of health status merits assessment as a criterion for clinical decisions in a manner that is at least standardized within each institution.6 Notably, it is often not feasible to assess frailty by gait speed or other performance metrics in the context of ACS management; alternative approaches are provided in the percutaneous coronary intervention (PCI) and surgical sections below.
Because frailty heightens risk for adverse outcomes in patients undergoing procedures, a less aggressive approach may yield superior outcomes. Given that frail patients are at high risk for functional decline during and after hospitalization37, physical therapy and CR with progressive mobilization are also particularly important to consider early in the course of treatment (See Cardiac Rehabilitation). Likewise, outpatient CR and nutrition are key components of long-term ACS care. Future clinical trials and observational studies evaluating patients admitted with ACS must include older patients living with geriatric syndromes including frailty.
Cognitive impairment.
In the United States, the prevalence of mild cognitive impairment (MCI), a condition associated with increased risk for subsequent dementia, is about 10% in people 70-79 years of age and about 25% in those 80 years of age or older.38 The prevalence of Alzheimer’s Disease, which accounts for 60-80% of dementia, increases from 5.3% at ages 65-74, to 13.8% at ages 75-84, and 34.6% after age 85.39 Vascular cognitive impairment and dementia (VCID) is also present in 5-10% of older adults and often coexists with other dementia types. Women are about 50% more likely to develop dementia than men, and women account for nearly two-thirds of dementia cases in the U.S. In addition, the prevalence of dementia is 2-fold higher in older African Americans and 1.5-fold higher in older Hispanic-Americans compared to whites. While formal testing is required to establish a diagnosis of dementia, the Mini-Mental State Exam40, Mini-Cog41, and Montreal Cognitive Assessment42 are commonly used screening tools to identify individuals who may benefit from further evaluation.
In the setting of ACS, patients with MCI or dementia may experience deterioration in cognitive function relative to their baseline due to the stress of the acute event, the unfamiliar environment, or medication side effects. The medical history may be unreliable and decision-making capacity may be impaired. When feasible, relevant history and goals of care, including resuscitation status, should be reviewed with a surrogate historian. It is also important to clarify if the patient has an advance directive and durable power of attorney for healthcare.
Delirium.
Delirium is an acute state of confusion characterized by a fluctuating course with alterations in attention, orientation, awareness of environment, cognition and behavior. The incidence of delirium in hospitalized patients over age 65 is about 20% and increases to over 70% among those requiring intensive care. Delirium is associated with increased risk for complications, longer length of stay, and higher likelihood of discharge to a post-acute care facility.43 Risk factors for delirium include older age, pre-existing dementia, and certain psychoactive medications (especially benzodiazepines), which should therefore be avoided.44 Several screening tools are available but the Confusion Assessment Method45 is the most widely used. Patients at increased risk for delirium should be identified (including all older patients admitted to intensive care), as up to 40% of incident delirium cases can be prevented by implementing a series of specific interventions. Management of delirium requires attention to all potential causes, non-pharmacologic interventions, and judicious use of medications if necessary.44
Holistic approach to care
Given the complexity of disease management in older adults with ACS, optimal approaches to management usually entail care that is more individualized and patient-centered than among younger adults, as each patient’s unique set of circumstances has bearing on their sense of optimal outcomes. Comorbid medical and geriatric conditions, medications, pre-existing functional status, patient preferences and goals of care are among the issues that shape treatment decisions and extend beyond the parameters utilized in traditional disease-based ACS risk scores. In critically ill patients for whom an urgent therapeutic decision is required (e.g., emergency PCI or surgery vs. medical management), there may not be sufficient time to explore all relevant aspects of the patient’s situation, in which case clinicians must use assessments incorporating retrospective perspectives and best clinical judgement based on the information at hand, including discussions with the patient, health care proxy and family.46, 47 In other cases requiring less urgent decision making, a more considered approach, including a determination of what matters most to the patient as a means to inform goals of care and therapeutic direction, coupled with shared decision-making, will likely yield outcomes that are most satisfying to the patient, health care proxy, family and clinical care team. It is optimal to have an advance directive or a durable power of attorney for older patients prior to hospitalization for acute ACS event.
Key Points for Clinical Practice
Geriatric syndromes are age-related physiologic vulnerabilities that include multimorbidity, polypharmacy, frailty, functional decline (cognitive and physical), delirium, sensory decline (hearing, vision, and pain), and falls.
Geriatric syndromes influence health outcomes for older patients with ACS, but ACS can also worsen the burden of preexisting geriatric syndromes.
A holistic approach to ACS care is commensurate with the relatively more complex issues pertaining to ACS in older adults, and includes an individualized and patient-centric approach to care, taking into consideration coexisting and overlapping healthcare domains.
DIAGOSTIC AND DISEASE-BASED CLASSIFICATION OF ACS IN OLDER ADULTS
Clinical History and Exam
The first step in the evaluation of patients presenting with possible ACS, either in the emergency or office setting, is classification of symptoms.48 A comprehensive history captures the characteristics of chest pain or associated types of symptoms, including the nature, onset and duration, location, radiation, and precipitating or relieving factors. Nonetheless, in older adults, accounts of symptoms are more likely to be affected by multimorbidity, altered sensory capacities, and cognitive impairment, such that their diagnostic specificity and sensitivity are diminished. In a nationwide study of emergency department (ED) patients ≥80 years of age with chest pain, more than half were ultimately found to have a non-cardiac cause of chest pain, 5.7% had decompensated heart failure, 7.8% had CAD without MI, and only 3.7% had an ACS.49 Thus, the vast majority of chest pain presentations do not represent ACS. ACS in older adults is also more likely to manifest with symptomology that is considered “non-ischemic” in younger populations, including shortness of breath, syncope, or sudden confusion.50 In the prospective SILVER-AMI study of more than 3,000 patients ≥75 years of age hospitalized with AMI, 44% of patients did not report chest pain as their primary symptom, including 40% of patients presenting with STEMI. Women presenting with NSTEMI were less likely than men to report chest pain as a primary symptom (50.0% versus 58.6%, P<0.001).51 In another analysis from the SILVER-AMI cohort, cognitive impairment was present in 17% of participants.52 Women had a higher prevalence of cognitive impairment compared with men (NSTEMI: 20.6% versus 14.3%, P<0.001) – posing challenges for timely presentation to the ED and the description and recall of symptoms.51 Heart failure, symptoms other than chest pain, and being African- or Hispanic-American were also associated with delayed arrival to the hospital.53
The electrocardiogram (ECG) is a key step in initial evaluation for ACS. The majority of older adults (~70%) in clinic and hospital settings have some form of baseline ECG abnormality. These include left ventricular hypertrophy (~20%), conduction system disease (10% right bundle branch block and ~5% left bundle branch block), paced rhythm (~5%) or atrial fibrillation (~12%).54 Such high prevalence of pre-existing ECG abnormalities often complicates interpretation of the presenting ECG in older patients with suspected ACS, and comparison with prior ECGs, when available, is essential. For older patients with non-diagnostic ECG presentations (e.g., LBBB or paced QRS), a high level of suspicion for ACS diagnosis is needed, especially if older patients present with cardiac symptoms, unstable hemodynamics, or the typical rise and fall pattern of cardiac biomarkers. The physical examination for older adults with ACS may be informative of conditions that alter myocardial oxygen supply and demand, including hypoxia, tachycardia (e.g., atrial fibrillation), hypotension, uncontrolled hypertension, or acute stressors such as an injurious fall – all contributors to type 2 MI. Aortic stenosis can be readily detected by physical examination and is a common confounder among patients with angina.
Due to the inherent complexities in older patients presenting with suspected ACS, the initial history, physical examination, and ECG alone often do not confirm or exclude the diagnosis. However, the initial evaluation can stratify those with possible ACS presentations into low, intermediate, or high risk to better inform the initial approach to care and interpretation of biomarker testing (Figure 2).55 While the 4th Universal Definition of MI is widely used, it is often not ideal for guiding risk stratification in older patients with chest pain or ACS. There is need for refinements to estimate overall prognosis that incorporate concomitant (and biologically related) aging phenomena that exacerbate risk (reduced longevity as well as greater disability) when present (frailty, sarcopenia, multimorbidity, etc.). Older patients living with geriatric syndromes and presenting with NSTEMI are at increased risk for hospital associated complications. Characteristics that predict higher risks of complications requiring ICU admission in older patients include heart failure, initial heart rate, systolic blood pressure, initial troponin, initial serum creatinine, prior revascularization, chronic lung disease, ST-segment depression, and age.56 The ACTION ICU risk score can be a helpful guide for older patient disposition into the CICU vs. intermediate care units.56 The ACTION ICU risk score included patients ≥ 65 years old in the ACTION registry with a median [IQR] age of 77 [71-84] years.
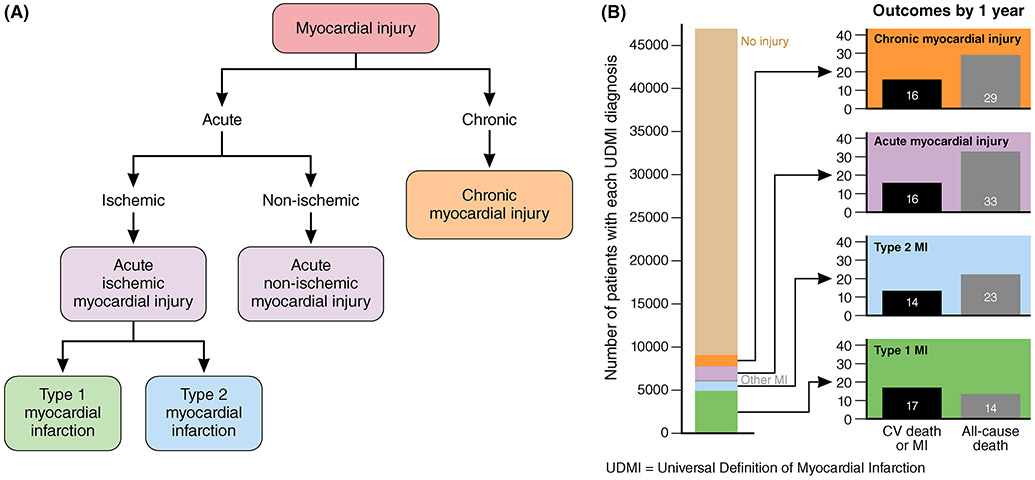
Figure 2.
A. Classification system for acute and chronic myocardial injury. B. The number of patients with each universal definition of myocardial infarction (UDMI). Modified with permission from Morrow et al.55
Sex-related Differences in ACS
While men constitute the majority of ACS encounters, the proportion of women in the ACS population increases with age from 20-25% among patients <50 years of age to approximately 50% among older adults >80 years of age.57, 58 These patients tend to be described in practice as “older and sicker” highlighting the sex-related differences among men and women.59 The burden of CV risk factors among older women is higher than men including hypertension, diabetes mellitus, and older women may be more likely to have a prior heart failure or heart failure with preserved ejection fraction, but not heart failure with reduced ejection fraction.59 In the Italian Elderly ACS collaboration, older women had a higher prevalence of cardiovascular risk factors, including diabetes, and non-cardiovascular comorbidities including kidney dysfunction.60 While older men had longer duration of coronary disease, history of MI, revascularization, and more complex anatomic disease.60 Symptoms like nausea or abdominal pain are more common among women, and pain in the jaw, neck, or back are also frequently observed. Weakness, dyspnea, or total lack of pain (“silent” ischemia) may affect interpretation of symptoms and result in delays in presentation. These issues can result in poor outcomes due to delays in implementation of reperfusion therapies. In the LADIES ACS study, the Gensini score, a surrogate for coronary artery disease severity, was higher in men than women at all ages.61 In the CURE trial, high-risk women with ACS underwent less coronary angiography, CABG surgery and had higher incidence of MACE outcomes including recurrent MI, stroke, and refractory angina.62 These sex-related differences should trigger improvement in quality care for older women presenting with ACS.
Biomarkers (hs-cTn and NT pro BNP)
The addition of high sensitivity troponin (hs-Tn) provides important diagnostic sensitivity for the presence of ACS in older adults, but that comes at the expense of reduced specificity, particularly among older adults with underlying kidney disease.63,64 hs-cTn assays are the standard of care for identifying myocardial injury, although questions remain about whether minimal elevations, which carry prognostic value, are actionable in a manner that improves outcomes.65 If the hs-cTn continue to rise, the biomarker is helpful in identifying the underlying cause of the injury and indicating myocardial ischemia. Chronic elevations of hs-Tn levels are associated with replacement fibrosis and progressive changes in left ventricular structure that are common in the older population.66 Fluctuations in the levels of hs-Tn are also associated with age-related decline in kidney function (especially in the context of ACS), hormonal changes (menopause in women), and age-related changes in body composition.65, 67 For this reason, evaluating patterns of rise and fall of hs-Tn is essential.67 Independent determinants of higher levels of troponin in asymptomatic populations include older age, male sex, diabetes mellitus, lower eGFR, left ventricular mass, black race, hypertension, sarcopenia, and history of heart failure. Therefore, many asymptomatic older adults live with biomarkers that fluctuate or are persistently above the diagnostic thresholds for MI. Population studies such as Dallas Heart Study, Atherosclerosis Risk in Communities, and Cardiovascular Health Study demonstrate that the proportion of hs-Tn above the 99% URL is upwards of 19-28 ng/L (depending on vender) in asymptomatic individuals over the age of 75.
Classification of Myocardial Injury
Myocardial infarction is defined as myocardial cell death attributable to a prolonged period of inadequate oxygen supply to the myocardium. All four types of myocardial injury are more common in older adults. The taxonomies for myocardial injury include acute non-ischemic myocardial injury identified by a rise and fall of hs-Tn without a primary ischemic cause and chronic myocardial injury identified by persistent elevations of hs-Tn above the 99th URL over time. Type 1 MI is caused by atherothrombotic CAD, usually the result of plaque rupture. Type 2 MI is caused by a mismatch between oxygen supply and demand and indicates myocardial stress related to another condition (i.e. not due to plaque rupture). Older adults are predisposed to type 2 MI in part because of an age-related decline in endothelium-mediated coronary vasodilation and resultant impaired ability to increase coronary blood flow in response to increased myocardial oxygen demand.17 Type 2 MI is particularly common in older adults admitted with comorbid conditions such as hypotension (e.g. due to sepsis), uncontrolled hypertension, chronic obstructive pulmonary disease, pneumonia, acute anemia, heart failure exacerbations, or chronic kidney disease.68 Among patients with type 2 MI, the incidence of recurrent type 2 MI at five-year follow-up was 9.7%, compared to 1.7% five-year incidence of type I MI.68 The survival prognosis among patients with type 2 MI is also determined by the underlying cause for the supply-demand mismatch, with arrhythmia having a more favorable prognosis than hypotension, anemia, or hypoxia. Adjusted long-term mortality after type 2 MI is markedly higher than after type 1 MI (52% vs. 31% at 5 years), driven by early non-cardiovascular death.68 Patients with myocardial injury are also at increased risk for future cardiovascular events irrespective of pathogenesis.
Coronary CT Angiogram (CCTA) for Risk Stratification
Whereas coronary angiography has traditionally been regarded as the most definitive means to assess for CAD, it is associated with relatively greater risks in older adults. After MI has been ruled out by troponin, CCTA is a less invasive way than coronary angiography to exclude obstructive CAD in patients with intermediate or undetermined risk. CCTA can visualize the extent and severity of non-obstructive and obstructive CAD, as well as atherosclerotic plaque composition and high-risk features (e.g., positive remodeling, low attenuation plaque). Calculation of fractional flow reserve with CT (FFR-CT) may provide an estimation of lesion-specific ischemia. Clinicians may be able to use CCTA for triaging patients with suspected ACS by looking for features of high-risk plaque, but the reliability of the test is somewhat diminished particularly in very old patients.69 High-risk plaque characteristics on CCTA may assist in identifying culprit lesions of ACS.70 When evaluating older adults for CCTA, the use of contrast (typically 50-100 ml) must be considered, especially given the age-related vulnerabilities of acute and chronic kidney disease. The heart rhythm must be regular (i.e. not atrial fibrillation or atrial arrhythmia) with rate preferably less than 70 beat per minute. Heavily calcified coronary arteries in older patients may render interpretation of lesion severity difficult, and motion artifact (e.g. if patient is delirious) can obscure interpretation. Downstream testing is also a relevant consideration as CCTA may lead to additional testing that diverges from a patient’s goals and preferences.
Key Points for Clinical Practice
ACS represents a minority of all chest pain presentations in younger and older adults. ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more likely to occur in older adults.
hs-cTn assays are standard of care for identifying acute and chronic myocardial injury. Many older adults have persistent elevations due to myocardial fibrosis and CKD that lessen the positive predictive value. For this reason, evaluating patterns of rise and fall is essential.
Myocardial injury can be classified in 4 subtypes: Acute non-ischemic injury, chronic myocardial injury, myocardial infarction (type 1) and myocardial infarction (type 2). All types are more common in older than younger adults.
GUIDELINE-DIRECTED MEDICAL THERAPY FOR ACS IN OLDER ADULTS
Pharmacodynamics and Pharmacokinetics
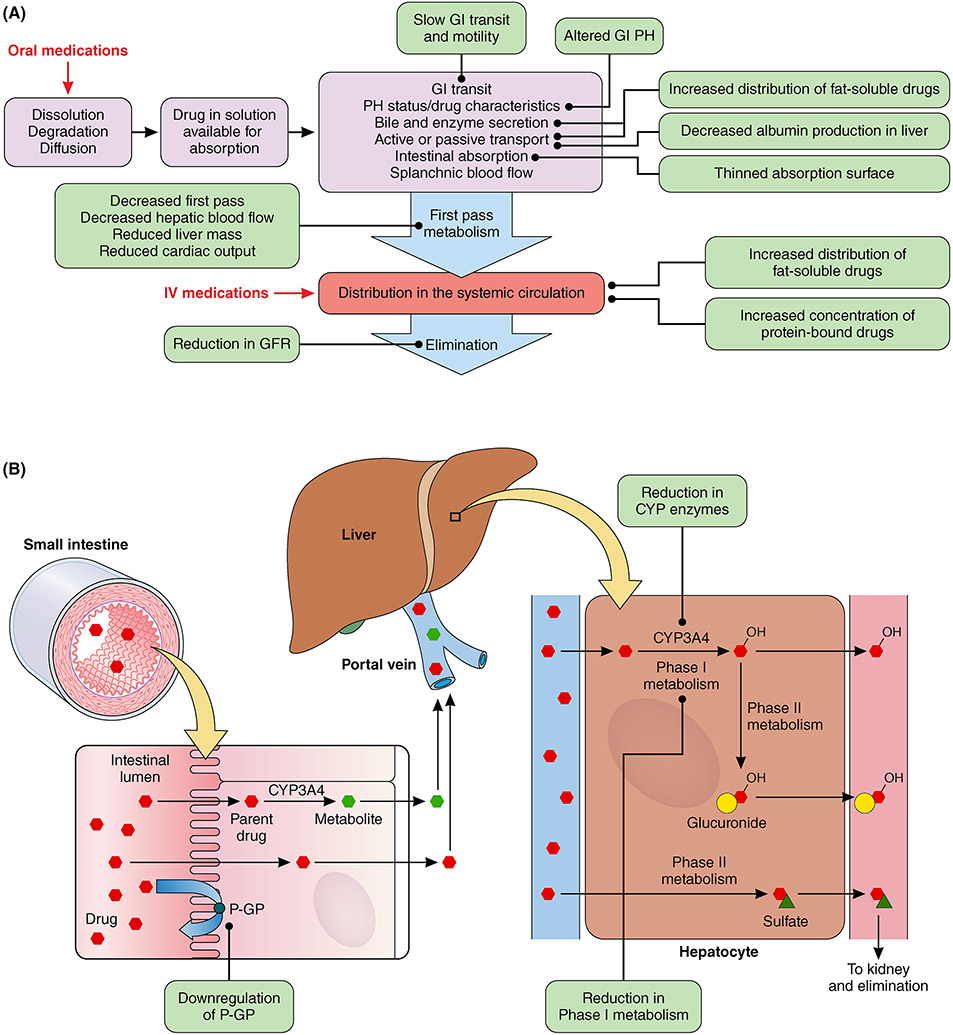
With aging, multiple physiological changes affect both the pharmacokinetics and pharmacodynamics of many medications used for ACS. Older adults can exhibit reductions in kidney function, hepatic blood flow, and muscle mass with an increase in body fat that in turn impact the absorption, distribution, metabolism, and elimination of certain guideline-directed medical therapies (Figure 3).71 These physiologic changes greatly affect the pharmacokinetics of orally administered medications that must undergo dissolution in order to be absorbed by the gut, then undergo first pass hepatic metabolism before entering the systemic circulation, and then potentially be renally eliminated. For intravenously administered medications, first pass metabolism is bypassed, but protein binding and elimination can be altered.6 With a reduction in p-glycoprotein and cytochrome P450 isoenzymes, the potential exists for elevated serum concentrations anticoagulants (warfarin, apixaban, dabigatran, and rivaroxaban), β-adrenergic blockers (carvedilol), and statins (atorvastatin, simvastatin). Anticoagulants (bivalirudin, eptifibatide, tirofiban, enoxaparin, fondaparinux), as well as all the oral direct anticoagulants are eliminated renally and doses must be adjusted according to the patient’s glomerular filtration rate.72, 73 As older adults may exhibit altered plasma protein concentrations, primarily due to underlying comorbid conditions, medications that are highly protein bound (e.g., > 90%) such as warfarin, heparin, amiodarone, lidocaine, furosemide, bumetanide, nicardipine, and all stains except pravastatin can exhibit higher free concentrations with the potential for greater distribution, which may accentuate the risk for toxicity.6, 74
Figure 3.
A. Absorption, metabolism, distribution, and elimination of guideline directed medical therapy in older adults with acute coronary syndromes. B. Changes in small intestine and liver that influence drug metabolism in older adults with acute coronary syndrome.
Low body weight and lean mass are common in many older adults with ACS, which can impact medication dosing in order to avoid adverse events. Based on US package labeling, in patients <60 Kg, a lower maintenance dose of prasugrel (5mg/daily) should be considered in order to reduce the risk of bleeding.75 In the case of apixaban, dosing adjustment is warranted if a patient meets two of the following: age ≥ 80 years of age, body weight ≤ 60 kg, or a serum creatinine ≥ 1.5 mg/dl.73 For dabigatran, observational studies suggest increased dabigatran-induced bleeding at low body mass indexes (<23.9 kg/m2), and while no conclusive consensus exists regarding rivaroxaban dosing in patients weighing <60 kg, increased systemic exposure in cachectic patients has been suggested.73 Finally, body weight is critical when dosing many intravenous antiplatelet medications such as the glycoprotein IIb/IIIa inhibitors (eptifbatide and tirofiban), prasugrel, and cangrelor, as well as unfractionated heparin, low molecular weight heparins (enoxaparin and dalteparin), and the factor Xa inhibitor fondaparinux.72
From a pharmacodynamic perspective, adaptive and structural vascular and myocardial changes due to aging can lead to exaggerated response to certain medications.12 Diminished carotid sinus baroreceptor sensitivity as well as a slowing of both sinus node activity and atrioventricular conduction can increase the risk of bradycardia with β-adrenergic blockers, non-dihydropyridine calcium channel blockers, and amiodarone. With an increase in arterial and ventricular stiffness, older adults may become preload sensitive, leading to a greater risk of hemodynamic lability from vasodilator therapies and diuretics.6, 76
Pharmacotherapy for ACS in Older Adults
Medical therapy for ACS in the older adult is generally similar to that in younger patients but needs to take into consideration the increased atherothrombotic risk, higher bleeding risk compared with younger patients in the context of the physiologic changes described above, and prevalent geriatric syndroms. As such, risk versus benefit assessments, tailored to each individual, guide the use and dosing of ACS therapies. Therapies initiated in the hospital should be evaluated on a recurring basis during outpatient follow-up with escalation of treatment as needed to reduce overall CV risk, but also de-escalation or deprescribing as needed to relieve or prevent side effects in the context of patients’ goals and preferences.28
While both clopidogrel and ticagrelor are recommended for older patients with ACS in the recent revascularization guidelines77, clopidogrel may be the preferred P2Y12 inhibitor in older patients because it carries a lower bleeding risk profile when compared to ticagrelor. In the Elderly ACS 2 trial, prasugrel did not show superiority over clopidogrel in the primary endpoints of mortality, MI, disability stroke, rehospitalization, or bleeding among older patients.78 However, the bleeding rate was numerically higher in the prasugrel group.78 In the POPular AGE Trial that enrolled older patients with NSTE-ACS, clopidogrel had a similar efficacy to ticagrelor in reducing MACE events and had fewer bleeding events.79 In the recent SWEDEHEART (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) study, patients ≥80 years with MI who were discharged alive with aspirin combined with either clopidogrel or ticagrelor were evaluated. Older patients treated with ticagrelor did not have lower risk for the combined outcomes of death, MI, or stroke, but these patients had a 32% higher risk of bleeding.80 A network metanalysis (n=14,485) comparing clopidogrel, prasugrel and ticagrelor in older adults with ACS has been recently published and showed that clopidogrel has the most favorable profile for reducing bleeding events.81 While the benefit of ticagrelor over clopidogrel was consistent across age groups, there was a significantly higher risk of major bleeding in ticagrelor-treated older patients.82 Further, there is a black box warning on the use of full dose prasugrel of 10mg/day in patients more than 75 years of age due to increased risk of serious bleeding. Limited evidence exists for P2Y12 inhibitor switching. In older patients at high risk of both thrombosis and long-term bleeding, higher potency P2Y12 inhibitors, such as ticagrelor or reduced dose (5mg) prasugrel, may be superior in the first month after PCI for ACS, then transitioning to clopidogrel for long-term bleeding risk reduction.83 If more potent DAPT are used in older patients, DAPT de-escalation by switching from more potent drugs (e.g., ticagrelor or lower dose prasugrel) to clopidogrel can be considered 30 days after initial ACS event or guided by platelet function testing because a switch within the first 30 days after index admission may increase risk of ischemic events.84-86
Beta-blockers may be of particular benefit due to their anti-ischemic effects and influence on the burden of arrhythmia in the setting of acute myocardial ischemic injury. However, cautious dosing is warranted along with vigilance for bradycardia and incident heart failure in the acute setting, as well as chronotropic incompetence and fatigue during chronic management. In patients with severe asymptomatic bradycardia (heart rate <40 beats per minute), reduction or cessation of beta-blockade and other nodal agents is preferred to see if pacemaker implantation can be avoided. In a large observational study of patients over age 65 who were more than 3 years post-MI, no association between beta-blocker use and long-term cardiovascular outcomes was observed, suggesting that de-prescribing may be considered at that time.87
A recent meta-analysis confirmed that low density lipoprotein (LDL) cholesterol lowering had similar efficacy in reducing the risk of cardiovascular death, ACS, stroke, or coronary revascularization in patients over and under 75 years of age; the results were also consistent across trials of statins, ezetimibe, and PCSK9 inhibitors. While post-market reports have raised concern for cognitive impairment, particularly in the aging population, most recent data have shown that there is no impact of statins or PCSK9 inhibitors on patient reported cognition even with very low LDL levels.88 Furthermore, randomized trials of statins89, ezetimibe90, and PCSK9 inhibitors91 have not observed between-group differences in cognitive function. Studies with longer term follow-up of PCSK9 inhibitor treatment are needed. Moderate- or high-intensity statin therapy is recommended for patients older than 75 years with clinical atherosclerotic cardiovascular disease.92 Myalgias can be dose dependent in older adults and influence mobility and quality of life, particularly among patients from Asian descent. Careful attention to statin dosing, dietary intake, and drug-drug interactions will allow older adults to benefit from secondary prevention lipid lowering effectively and safely.
Drugs targeting the renin-angiotensin-aldosterone (RAAS) system, namely angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), and aldosterone antagonists, have been associated with mortality benefit in ACS patients with large infarcts and left ventricular dysfunction.93 Of note, in patients with heart failure with reduced ejection fraction, low dose compared to higher doses of lisinopril, losartan, and sacubitril valsartan showed no difference in mortality, thus suggesting lower doses of these agents may be beneficial, particularly in patients with renal and hemodynamic vulnerabilities.94-96 These are often under-utilized in older ACS patients due to clinician concerns regarding expected benefit in the face of shorter life expectancy, and potential complications (e.g., syncope, hyperkalemia, or worsening kidney function) of treatment. When possible, shared decision making is important, and kidney function, blood pressure, and potassium levels are best monitored within the first 12 weeks of initiation, with each dose increase, and on a yearly basis thereafter. Dose reduction or drug discontinuation may be needed for severe or symptomatic hypotension, hyperkalemia, or persistent >30% creatinine increases.97 Recent data suggest that ACEi/ARBs that cross the blood brain barrier (ACEi: captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril; ARB: telmisartan and candesartan) were associated with better memory recall at 3 years than those that did not.98 The use of angiotensin receptor-neprilysin inhibitor (ARNI) vs ACEi to reduce MACE events following MI was investigated in the PARADISE-MI trial.99 In that study, more than one-third of the patients were older than 70 years but only 24% were women. The primary outcome of death, first HF hospitalization or outpatient HF was not different between the ARNI vs. ACEi (11.9 vs. 13.2, p=0.17).100
Pharmacologic Management of Atrial Fibrillation in Context of ACS
Older adults are at higher risk for recurrent thrombotic events, and they may derive the most benefit from appropriately dosed anticoagulation therapy. The prevalence of atrial fibrillation increases with age and concomitant anticoagulation therapy increases bleeding risk. Among older adults in sinus rhythm treated with PCI for ACS, 1-4 weeks of triple anticoagulation therapy (aspirin, clopidogrel, and a direct oral anticoagulant [DOAC]) is recommended, followed by up to 1 year of clopidogrel and DOAC, after which DOAC monotherapy is continued.101 For patients with chronic atrial fibrillation undergoing PCI for ACS, double antithrombotic therapy is superior to a strategy of triple therapy. This is achieved using a DOAC and a P2Y12 inhibitor preferably clopidogrel without aspirin.102 In older patients at increased thrombotic risk with acceptable bleeding risk, it is reasonable to continue triple therapy including aspirin for one month post PCI.103 Double therapy is continued for 6 to 12 months, after which discontinuation of antiplatelet therapy is achieced and treatment with oral anticoagulation therapy alone is maintained thereafter.103
Enhanced communication between the hospital cardiology team and outpatient cardiology team (e.g., cardiologist, nurse practitioner, or physician assistant) caring for the older patient is critical to coordinate the medication regimen and to provide the rationale for longterm care. With this alignment between clinicians, older adults, particularly those with mobility or cognitive difficulties, may benefit from more simplified medication and dosing regimens than one that checks all the guideline indication boxes. Once daily medications are often more conducive to adherence but require careful assessment of potential interactions when multiple drugs are taken together. While within class medication switches or dosing changes can occur after discharge from an ACS hospitalization, 90-day prescriptions are often feasible and have been shown to improve post-ACS medication adherence.104 Facilitating medication supplies in other ways, such as using mail order pharmacies105, can make a big difference in long-term evidence-based medication persistence of older adults after ACS.
Key Points for Clinical Practice
In the older ACS patient, clopidogrel is the preferred P2Y12 inhibitor because of significantly lower bleeding profile than ticagrelor or prasugrel, but for patients with STEMI or complex anatomy, the use of ticagrelor is reasonable.
In patients with chronic atrial fibrillation undergoing PCI for ACS, the duration of triple therapy should be minimized, with discontinuation of ASA and transition to dual antithrombotic therapy with clopidogrel and a NOAC, ideally within 4 weeks of PCI.
Therapies initiated in the hospital are best evaluated on a recurring basis during outpatient follow-up with escalation of treatment as needed to reduce CV risk, but also de-escalation or deprescribing to relieve or prevent side effects.
Older adults, particularly those with mobility or cognitive difficulties, may benefit from relatively simpler medication and dosing regimens than those commonly indicated by existing guidelines. Comorbidities, geriatric syndromes, and personal healthcare goals and preferences are also relevant factors to integrate within tailored regimens of care.
PERCUTANEOUS REVASCULARIZATION IN OLDER ADULTS
The management for STEMI in older adults follows the same general principles as for younger patients. The indication for primary PCI in older adults presenting with STEMI should consider STEMI-related variables including delay in presentation, extent of ECG changes, and left ventricular dysfunction, and patient-related variables, including severe comorbidity, severe cognitive impairment and limited life expectancy. Barring these end stage disease processes, primary PCI is safe and effective even among patients with very advanced age, as long as this is aligned with their preferences of care.106, 107 For older patients with NSTEMI, cardiovascular and non-cardiovascular risk evaluation prior to revascularization are critical elements to achieve optimal outcomes, both in respect to cardiovascular (e.g. symptom control and low bleeding risk)108 and non-cardiovascular (e.g., function, independence, quality of life, and overall well-being).
Cardiovascular Risk Evaluation for PCI
TIMI/Grace Risk Score:
The ACC/AHA guideline recommends risk stratification of patients with suspected NSTEMI to determine the choice of management, e.g. through use of risk assessment scores such as Thrombolysis In Myocardial Infarction (TIMI) risk score and GRACE risk score.77 The GRACE score is heavily age-weighted109, 110 leading to older adults with NSTEMI being classified as high-risk warranting consideration of early invasive therapy. Type 2 MI is frequently observed in older adults111 and the GRACE risk score provides only moderate discrimination of mortality risk for those with type 2 MI.112 The baseline risk of bleeding during NSTEMI care among older adults is increased when compared to younger patients, and the CRUSADE bleeding score has the best discriminative function for in-hospital major bleeding across all postadmission treatments.113 Importantly, these risk scores do not take into account characteristics that are especially prevalent in older adults, such as frailty, multimorbidity, polypharmacy, and cognitive dysfunction. The relationship between frailty and risk of adverse outcome following STEMI and NSTEMI has been demonstrated in many studies using different frailty assessment tools.114-116
Among NSTEMI patients, the composite outcome of MI, need for urgent repeat revascularization, stroke, significant bleeding, and all-cause mortality at one year occurred in more frail patients than in robust patients (39% vs. 18%, HR: 2.79; 95% CI: 1.28-6.08).117 In a systematic review and meta-analysis, frailty was associated with a several-fold increase in the adjusted risk of mortality for patients with STEMI (HR: 6.51; 95% CI: 2.01–21.10) and NSTEMI (HR: 2.63; 95%CI: 1.51–4.60).118 Multimorbidity is increasingly prevalent in the older population, particularly in those presenting with ACS. Comorbidity burden, as measured by the Charlson Comorbidity Index (CCI), predicts in-hospital and 1-year mortality in patients with ACS and is independently associated with adverse short-, medium- and long-term outcomes after PCI.119, 120 In another study, older adults with NSTE-ACS referred for coronary angiography, the presence of multi-morbidity was associated with an increased risk of long-term adverse cardiovascular events, driven by a higher risk of all-cause mortality. On average, each additional CCI co-morbidity was associated with a 31% increased adjusted risk of all-cause mortality at five-years.121
Undiagnosed cognitive impairment is common in older patients with NSTEMI receiving coronary angiography, and these patients are more likely to experience adverse events at 1 year.122 While the optimal risk indices for older adults with ACS remain undefined, considerations of frailty, cognition and comorbity are critical perspectives to foster in respect to guiding optimal care, particularly for prognostic stratification, and identifying those who are most as well as least likely to benefit from early invasive therapy. There is a need for refinements of standard risk scores to estimate ischemic and bleeding risks for older patients with ACS.
Geriatric and Patient-Centric Outcomes for the Older ACS Populations
While the older adult population is rapidly expanding, these patients do not represent a homogeneous group. They differ based on the presence or absence of frailty, comorbidities, physical or functional limitations, and social status when presenting with ACS.123 Older patients living with a greater burden of geriatric syndromes are more likely to develop disability, loss of independence, and impairment in quality of like and ability to selfcare after an ACS event. When managing older patients presenting with ACS, the goals of care should extend beyond traditional cardiovascular outcomes including MI, need for urgent and repeat revascularization, stroke, significant bleeding, and all-cause mortality. Assessment of patient-centeric goals and outcomes remains necessarily with a focus on quality of life outcomes.124 Other key outcomes for older patients are the ability to live independently and to return to their previous life environment/style. . For older patients with high burden of geriatric syndromes or with terminal cardiovascular illness, goals like “days at home” and other personal preferences that maximize quality of life should be prioritized as important outcomes for guiding ACS management.124 With these considerations in mind, the most recent clinical trial designs in older frail patients with ACS included secondary outcomes like functional capacity, instrumental activities, cognitive capacity, and quality of life during follow-up when compared to baseline.125 It should be noted that issues of senescence, inflammation, and other hallmarks of aging are variable from person to person, thus adding to the heterogeneity of older adults.
Key Points:
When managing older patients presenting with ACS, the goals of care should extend beyond traditional cardiovascular outcomes to include patient-aligned goals and preferences that maximize quality of life outcomes.
For older ACS patients at end of life, goals like “days spent at home in the last 6 months of life” and relief of pain/discomfort are important metrics for quality care.
Correlation between Frailty and Coronary Anatomical Complexity
Frail adults presenting with NSTEMI have more procedurally challenging angiographic findings independent of age (i.e., severe culprit lesion calcification, high SYNTAX scores, high risk lesions). In one study, frail patients had more than a five-fold increase in odds of severe culprit lesion calcification when compared to robust patients (unadjusted OR 5.40, 95% CI 1.75–16.8, p=0.03).126 Frail patients also had a greater prevalence of high-risk lesions on intravascular ultrasound imaging, with a 2.81 increased adjusted odds (95% CI 1.06–7.48, P=0.039) of presence of thin-cap fibroatheroma.127 During follow-up, frail older adults who underwent angiography for NSTEMI is associated were at increased risk of all-cause mortality, unplanned revascularization, myocardial infarction, stroke, and bleeding.128
Timing of Invasive Strategy
The current revascularization guidelines describe high-risk groups that may benefit from early invasive approach, which results in lower rate of death, myocardial infarction, or refractory angina during follow-up. Older patients with ACS are disproportionately affected with high-risk features favouring and early invasive approach to management. The GRACE score has been used to estimate the risk of death attributed to ischemic burden in patients with ACS. A GRACE score >140 indicates a high-risk patient group that benefits from revascularization within the 24 hours, resulting in lower incidence of recurrent ischemia, need for revascularization, and shorter hospital length of stay.129 For patients ≥ 75 years of age, ST-segment deviation on ECG coupled with elevated hs-Tn levels yields a GRACE score >140, categorizing them as high-risk. When more precarious conditions coexist, the risk of mortality and major adverse cardiac events increases significantly. These conditions include the presence of cardiogenic shock, refractory angina, and hemodynamic or electrical instability, which require an immediate invasive assessment (preferably within 2 hours) to provide information on the extent and severity of coronary disease, hemodynamics, left ventricular function, and suitability for revascularization.129 However, other factors, including patient’s preferences and burden of geriatric syndromes that may affect life expectancy, must be taken into account when considering immediate revascularization strategies in older patients.
Key Points:
Older patients with ACS are often high risk (Grace Score >140) and should be considered for an early invasive approach to reduce the incidence of recurrent ischemia, need for revascularization, and shorten hospital length of stay
When cardiogenic shock, refractory angina, and hemodynamic or electrical instability are present immediate invasive approach may be associated with improved outcomes.
Patient preferences and geriatric syndromes affecting life expectancy must be considered when deciding on an invasive vs. conservative approach to ACS management.
Timing of DAPT Therapy
The use of DAPT is critical in the medical management of ACS because of its role in reducing ischemic and thrombotic complications. For older patients being considered for immediate or early invasive therapy, a loading dose of non-enteric coated aspirin 325mg, followed by a daily dose of 81mg is recommended prior to invasive assessment to reduce ischemic risks. Available P2Y12 inhibitors include clopidogrel, ticagrelor, and prasugrel. Of these, clopidogrel and ticagrelor are most commonly used in older patients with ACS because prasugrel is associated with adverse outcomes including major bleeding in the older adult populations. The timing of the loading dose of P2Y12 inhibitors remains an area of debate, but a strategy of introducing the loading dose after the anatomy is known was recently endorsed by the ACC/AHA/SCAI revascularization guidelines. This should be followed by daily dose to reduce ischemic events.129 This strategy seems most reasonable because of the need to discuss approach to revascularization with the Heart Team and whether surgical evaluation is needed.
Key Points:
A loading dose of aspirin 325mg followed by a daily dose of 81mg should be given prior to invasive approach to management to reduce ischemic events.
A loading dose of a P2Y12 inhibitor should be given after the anatomy is known in patients proceeding to PCI. A P2Y12 inhibitor should be withheld in patients for whom cardiac surgery is being contemplated.
Efficacy of Percutaneous Revascularization in Older Adults
NSTEMI:
To date, of the five randomized clinical trials (RCTs) specifically investigating an invasive strategy in older patients with NSTEMI, four have found no benefit of invasive treatment on the primary endpoint compared to conservative management,130-133 whilst the other showed that an invasive strategy reduced recurrent MI and urgent repeat revascularization (Table 3).134, 135 The Italian Elderly ACS trial randomized patients ≥75 years of age with NSTEACS to early invasive vs. initially conservative strategy. The trial did not report geriatric syndromes including frailty and multimorbidity.136 Patients randomized to the initially conservative strategy had higher rates of ischemic events during index hospitalization, which prompted urgent angiography. While there was 20% reduction in the rate of death, reinfarction, disability stroke, or rehospitalization, this reduction was not statistically significant because the trial was powered to detect a 40% difference in event rates. Patients with elevated troponins who were randomized to the early invasive group showed a significant 57% reduction in the primary endpoint on subgroup analysis.136 The After Eighty trial (2016) randomized patients aged 80 years or older with NSTEMI or unstable angina to an invasive vs. conservative strategy.134 Similar to the Italian Elderly ACS study, frailty or other geriatric syndromes were not explicitly reported. During a median follow-up of 18 months, the invasive strategy was found to be superior to the conservative strategy in reducing MI, urgent revascularization, and stroke (41% vs. 61%; OR 0.48, 95% CI 0.37-0.63), p<0.001).134 The MOSCA trial randomized patients ≥70 years of age with higher comorbidity burden to invasive vs. conservative strategy for NSTEMI. While there were numerically fewer primary endpoint events (death, reinfarction, or cardiac readmission) in the invasive arm, the difference did not reach statistical significance.131 This study was followed by the MOSCA-Frail trial, which is currently ongoing (Table 3). Other smaller trials also did not show benefit of an early invasive strategy but were underpowered to detect clinically meaningful differences.137, 138 The larger British Heart Foundation SENIOR-RITA Trial is currently randomizing patients ≥75 years of age with type 1 NSTEMI to either invasive or conservative treatment strategy. With an anticipated enrollment of 1,668 older participants, this trial is poised to inform practice in older NSTEMI patients living with geriatric syndromes (ClinicalTrials.gov/Identifier: NCT03052036). Using both observational and randomized data, systematic reviews and meta-analyses have shown a likely reduction in MI and recurrent revascularization associated with an invasive strategy, but no survival benefit and a higher risk of bleeding relative to a conservative strategy.139, 140 More recently non-randomized data from the SENIOR-NSTEMI study showed a survival advantage associated with invasive strategy among patients older than 80 years of age.141 Thus, the value of an invasive strategy in the management of patients ≥75 years of age remains uncertain, and additional studies are needed.
Table 3.
Randomized control trials comparing early or routine invasive strategies with conservative approaches in older patients with non-ST-elevation acute coronary syndromes
| Study | Population | Primary Outcome | Frailty | Comorbidity |
|---|---|---|---|---|
| Savonitto et al. Italian Elderly ACS (2012) | n = 313 NSTEACS ≥ 75 years old |
No difference in death, reinfarction, disabling stroke, repeat hospital stay for cardiovascular causes and severe bleeding at 1 year
|
No frailty assessment | No assessment of comorbidity burden or severity |
| Tegn et al. After Eighty (2016) | n = 457 NSTEACS ≥ 80 years old |
Reduction in death, reinfarction, need for urgent revascularization and stroke at 18 months, driven by lower rates of reinfarction and urgent repeat revascularization
|
No frailty assessment | No assessment of comorbidity burden or severity |
| Sanchis et al. MOSCA (2016) | n = 106 NSTEMI ≥ 70 years old |
No difference in death, reinfarction or readmission for cardiac causes at 2.5 years
|
No frailty assessment | Participants had at least two comorbidities Comorbidity burden or severity assessed with CCI Findings not stratified by CCI score or comorbidities |
| MOSCA-Frail (2019) | n = 313 NSTEMI ≥ 70 years old |
|
Clinical Frailty Scale ≥ 4 | Comorbidity burden and total number of drugs used (polypharmacy) |
| Hirlekar et al. (2020) | n = 186 NSTEACS ≥ 80 years old |
No difference in all-cause mortality, reinfarction, stroke, urgent revascularization or rehospitalization for cardiac causes at 1 year
|
Frailty assessed with the CSHA Clinical Frailty Scale Low prevalence of frailty Findings not stratified by frailty status |
No assessment of comorbidity burden or severity |
| De Belder et al. RINCAL (2021) | n = 251 NSTEMI ≥ 80 years old |
No difference in all-cause mortality or reinfarction at 1 year
|
No frailty assessment | No assessment of comorbidity burden or severity |
Abbreviations: ACS = acute coronary syndromes; PCI = percutaneous coronary intervention; NSTEACS = non-ST elevation acute coronary syndrome; HR = hazards ratio; CI = confidence interval; NSTEMI = non-ST elevation myocardial infarction; CCI = Charlson comorbidity index; CSHA = Canadian Study of Health and Aging.
STEMI:
In the context of STEMI, a pooled analysis of the TRIANA, SENIOR-PAMI and Zwolle trials showed a significant reduction in the risk of a composite outcome of death, reinfarction or disabling stroke (OR 0.64; 95% CI 0.45-0.91, p=0.013) with primary PCI compared to fibrinolysis.142 In a meta-analysis, patients aged 70-80 years who were randomized to primary PCI had reduced all-cause mortality (OR 0.55; 95% CI 0.39-0.76), reinfarction (OR 0.37; 95% CI 0.23-0.62), stroke (OR 0.36; 95% CI 0.20-0.68) and a composite of all three endpoints (OR 0.45; 95% CI 0.34-0.59).143 The superiority of primary PCI over fibrinolysis appears to extend to older patients, although trials have been small, affected by slow recruitment, and findings are limited in the ‘very old’ cohort, with no evaluation of frailty or comorbidity included. However, the results also suggest that fibrinolysis may be safe and effective, providing rationale for fibrinolytic therapy in situations where prompt PCI is not readily available or the risk of invasive intervention is considered to be prohibitive. In patients over age 75, fibrin selective agents, such as recombinant tPA, appear to be more effective in reperfusing the occluded artery but with the tradeoff of increased risk for intracranial hemorrhage.144 In the context of cardiogenic shock and cardiac arrest, age is an independent predictor of mortality following PCI.145, 146 In addition, the value of mechanical circulatory support in these cases, e.g. with intraaortic balloon counterpulsation or an Impella left ventricular assist device, is uncertain. Therefore careful selection of patients most likely to benefit from PCI is required. In the CULPRIT-SHOCK trial, in which the mean age of the study population was 70 years and 31% were >75 years-old, no age x treatment interaction was observed with regard to the benefit in favor of culprit vessel PCI only, as compared to multivessel PCI during the index procedure.147
Adverse Outcomes of PCI in Older Adults: Management to Minimize Risks
While an invasive approach appears to reduce reinfarction and the need for further revascularization, a meta-analysis shows increased bleeding among patients undergoing invasive management (OR 2.19; 95% CI 1.12-4.28; P=0.02; I2 = 0%) compared to those treated conservatively.139 In contemporary practice, bleeding risk is reduced by the use of radial access, even among patients presenting with cardiogenic shock.148, 149 Bleeding risk is further reduced with the use of latest generation drug eluting stents and shorter duration of dual antiplatelet therapy (DAPT).150, 151 The SENIOR trial showed that in older adults with high bleeding risk, patients treated with drug-eluting stents and short duration DAPT had lower incidence of the composite endpoint of all cause-mortality, MI, stroke, or urgent revascularization compared to patients treated with bare-metal stents and short duration DAPT with no difference in risk of bleeding.151 Additional approaches to reducing bleeding include age, weight and kidney function adjusted dosing of antithrombotic agents, shortening the duration of DAPT, and using double rather than triple antithrombotic therapy in patients who require anticoagulation.
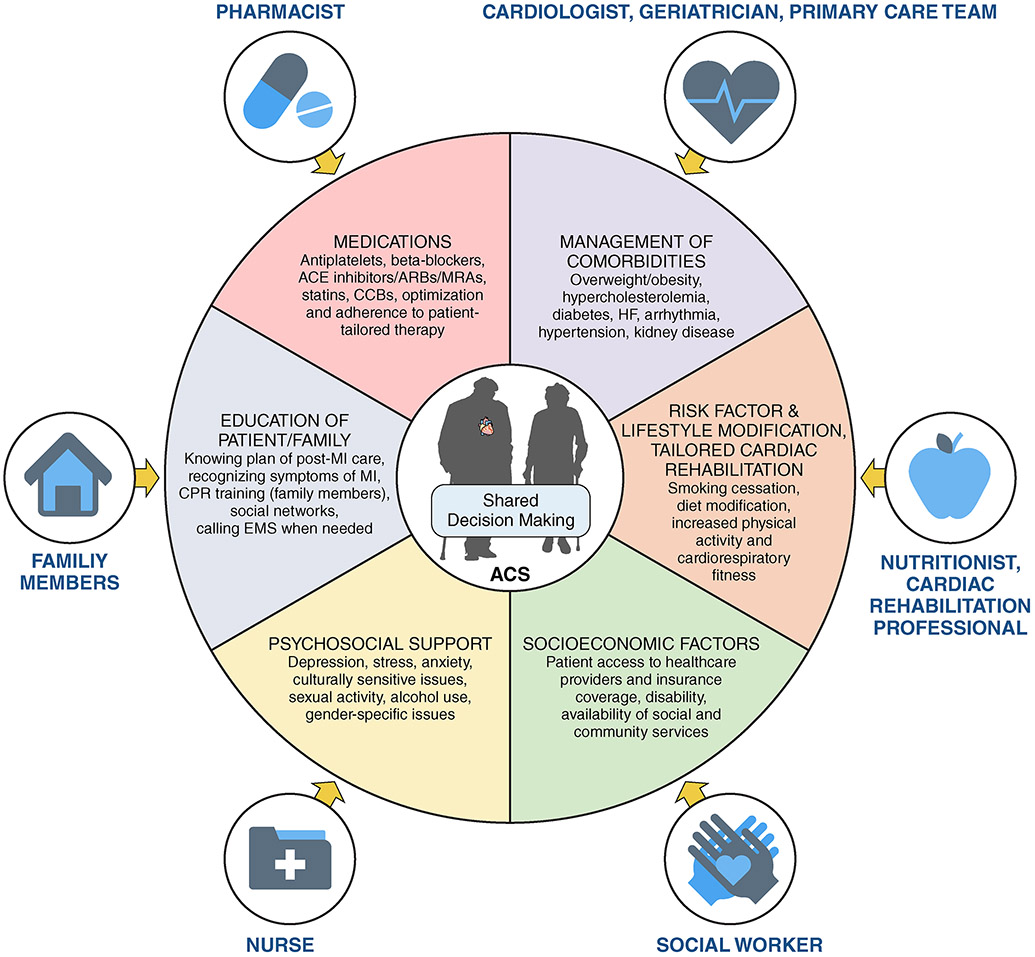
As the population ages, the significant advancements in interventional strategies and technologies is often applied as rationale to prioritize this option of care for the increasing number of older adults with ACS. Nonetheless, robust RCT evidence in older patients with ACS is lacking, and geriatric domains (e.g., frailty, multimorbidity, polypharmacy, cognitive function, and healthcare goals), which often impact outcomes, have not been incorporated into most studies. The multidisciplinary heart team, integrating geriatrics experts with cardiologists and surgeons, providess an important opportunity to enhance decision-making and clinical process.
Key Points for Clinical Practice
Until a more specific risk score for older adults with ACS becomes available, the ACC/AHA chest pain and revasccularization guidelines recommend risk stratification for patients with suspected NSTEMI using the TIMI and GRACE risk scores, with added emphasis placed on high sensitivity troponins. Geriatric syndromes are acknowledged as relevant, but they are not formally integrated into risk assessment.
Immediate myocardial reperfusion by primary PCI is beneficial in older patients with STEMI, and it may also reduce recurrent MI and repeat revascularization in patients with NSTEMI, but for patients with cardiogenic shock and cardiac arrest, careful selection of older patients undergoing PCI is warranted given their high inherent risk for adverse outcomes and futility.
Strategies to mitigate the risk of major bleeding include the use of radial access; age, weight, and kidney function adjusted dosing of anticoagulant and antithrombotic therapy, and shorter duration of DAPT with clopidogrel, particularly among older adults at high bleeding risk.
Ideally, the multidisciplinary team that cares for older patients with ACS includes cardiologists, surgeons, geriatricians, primary care clinicians, nutritionist, cardiac rehabilitation professionals, social workers, nurses, family members, and pharmacists, but centers should tailor their team according to available resources and patient needs.
Among older patients advanced DNR directives, careful discussion with the patients, family, or their power of attorney must occur before invasive management and utilization of primary PCI. If the patient undergoes invasive treatment or primary PCI, the advanced DNR directive should be suspended for the duration of the invasive procedure.
SURGICAL REVASCULARIZATION IN OLDER ADULTS
Surgical Risk Evaluation and Patient Selection
Currently, the predicted risk of operative mortality is less than 0.5% for an 82 year old man with no major comorbidities undergoing coronary bypass surgery and defined as death within 30-days of surgery or during the index admission.152, 153 Over the last two decades risk adjusted operative mortality associated with surgical revascularization in older patients has steadily decreased, which has been attributed to incremental improvements in case selection and perioperative management.154-156 During this time the number of older patients undergoing surgical revascularization annually in the US hardly changed, while the prevalence of risk factors and comorbidities including diabetes, pre-operative dialysis, chronic obstructive airways disease, heart failure, cerebrovascular disease and prior stroke, prior percutaneous intervention, left main stem disease and urgent presentation, have all significantly increased.154, 155
Like other risk stratification systems, most surgical risk scores do not include a formal assessment of frailty or other geriatric domains that are important determinants of outcomes after cardiac surgery in older patients.156 Frailty stands out as a particularly relevant risk. Both clinical and administrative data-based approaches to defining frailty may increase the accuracy of standard risk-prediction systems in older patients. For example, in an analysis of over two million patients undergoing coronary artery bypass graft surgery (CABG) between 2005 and 2016 identified using a national administrative claims database, 4.0% were considered frail according to the Johns Hopkins Adjusted Clinical Groups frailty indicator, a binary frailty variable derived from a diagnostic code cluster.157 In this analysis, frailty (i.e. the presence of any one of those diagnoses) was an independent predictor of major complications and costs and associated with a more than two-fold increase in the odds of operative mortality (aOR 2.49, 95% CI 2.3-2.7). The same frailty indicator has also been reported to be associated with significantly reduced long-term survival after coronary artery bypass surgery: in an analysis of 40,083 patients operated on between 2008 and 2015 in Ontario, Canada in which 22% were defined as frail, adjusted mortality at 4 years was significantly higher in frail patients (adjusted hazard ratio 1.2, 95% CI 1.12-1.28).158 Gait speed has also been reported to predict long-term survival after cardiac surgery.159
Efficacy of surgical revascularization in older patients with ACS
Medical literature has indicated that surgical revascularization is associated with an event-free survival benefit compared to PCI in select cohorts of older patients with left main or complex disease. Three overlapping meta-analyses of pivotal randomized trials of percutaneous versus surgical revascularization of multi-vessel and left main disease have evaluated mid-term outcomes in older patients, reporting that coronary bypass was associated with superior freedom from major adverse cardiac events compared to drug eluting stents.160-162 In the EXCEL trial, patients aged over 75 years had an all-cause mortality of 16.6% in the PCI arm versus 8.4% in the CABG arm (OR: 1.96, 95% CI 1.00-3.83), and meta-regression analysis showed increasing mortality with advanced age with PCI.161 It should be noted that approximately 60% of the EXCEL trial cohort had either stable angina or silent ischemia, while those who presented with recent ACS events (within 7 days of randomization) are approximately 40% of the cohort. Thus, the generalizability of the results to all older patients with ACS is limited. However, caution should be exercised when interpreting data from the EXCEL trial because the study population mostly consisted of stable angina patients rather than those with ACS and complex geriatric syndromes (stable angina ~ 53%; recent MI within 7 days of randomization ~ 15%; unstable angina with negative biomarkers ~ 24%). The event-free survival benefit observed in patients who received coronary bypass was primarily driven by lower risk of repeat revascularization and MI.160-162 Lesion complexity appears to be highly relevant: in a meta-analysis of 4,686 patients with unprotected left main stem disease randomized in six trials, PCI was associated with lower mortality than coronary bypass in patients with SYNTAX score ≤22, but higher mortality in patients with SYNTAX scores ≥33.160 In these meta-analyses the risk of early stroke was higher in surgical patients; however a recent analysis of 1,680 patients who underwent either PCI or CABG while participating in a prospective study of 23,860 adults undergoing two per year memory tests as part of a national longitudinal study on aging showed no significant differences between PCI and CABG in memory score or dementia up to 10 years after revascularization.163
Adverse Events of Surgical Revascularization in Older Adults: Management to Minimize Risk
Less invasive surgical approaches such as off-pump and hybrid revascularization are aimed at reducing post-operative morbidity particularly in older patients. However, the German Off-Pump Coronary Artery Bypass Grafting in Elderly Patients (GOBCABE) trial, in which 2,539 patients aged over 75 years were randomized to on-pump or off-pump surgery, suggests that off-pump techniques have no impact on stroke or new dialysis, and the most important surgical determinant of event-free survival after coronary bypass surgery in older patients is completeness of revascularization.164, 165 In this landmark trial, the rate of death at five years was similar in patients who underwent off-pump (361, 31%) versus on-pump (352, 30%) surgery, as was the composite outcome of death, MI and repeat revascularization (33% versus 34%, respectively).164 Incomplete revascularization, which was more common in off-pump compared to on-pump coronary bypass surgery, was associated with significantly worse survival at five years (HR 1.2, 95% CI 1.01-1.39). In this trial, there was no significant difference at 30-days in the incidence of stroke between on- and off-pump surgery, or between the incidence of stroke in off-pump patients stratified according to aortic clamping versus no-touch techniques.165 Stroke rates may be minimized by individualized operative techniques and peri-operative management including aggressive prevention and treatment of atrial fibrillation and tailored pre-and intra-operative imaging of aortic and cerebrovascular disease.166 Post operative delirium remains an important adverse outcome during index MI hospitalization, and proactive efforts to identify and prevent delirium postoperatively are warranted.71 Rate of memory decline has been reported to be worse after off-pump compared to on-pump coronary bypass surgery, which may predominantly reflect patient selection but serves as a caution against pursuing less-invasive surgical revascularization strategies such as hybrid procedures in older patients with multi-vessel disease who may derive greatest long-term benefit from complete and durable revascularization.163
Prevention of AKI in Older Patients undergoing Revascularization
More than half a million percutaneous or surgical revascularization procedures are performed annually in the US.167 Older patients are more likely to develop adverse outcomes such as contrast-induced acute kidney injury (CI-AKI) than younger patients due to underlying comorbid conditions such as CKD, DM, hypertension, and volume depletion. CI-AKI is defined as ≥ 0.5 mg/dL or 25% increase of serum creatinine from the baseline value at 48 h after contrast media administration.168 The risk of AKI among patients undergoing revascularization varies with the volume of contrast and the patient’s baseline risk.169
Several measures can be implemented to prevent CI-AKI following coronary interventions. Radial artery PCI is a potential method for decreasing AKI by prevention of bleeding or by reduction and avoidance of direct embolization into the renal circulation. The AKI-MATRIX trial show that radial artery PCI compared with femoral artery PCI resulted in a 13% reduction in AKI (aHR=0.87, 95% CI 0.77-0.98), defined as either an absolute (0.5mg/dL) or relative (>25%) increase in serum creatinine from baseline. In subgroup analyses, radial compared to femoral access was associated with 20% less AKI in patients age ≥ 75yrs. Overall, age >75 years (OR = 1.99, 1.75-2.27), DM (OR=1.33, 1.16-1.52), anemia (OR=1.20, 1.04-1.39), and contrast media volume per 100ml (OR=1.34, 1.26-1.72) were independent risk factors for AKI.170 Evaluation of data from the National Cardiovascular Data Registry showed that a patient-centered approach to contrast use can attenuate the risk of AKI.171 Using the formula 3 x estimated glomerular filtration rate (eGFR) to define contrast volume threshold, the authors found an AKI rate of 14.5% when the contrast volume threshold was exceeded vs. 9.8% if the volume was below the threshold. Meaningful reductions in CI-AKI risk can be attained by decreasing contrast volume among patients undergoing PCI. Best kidney practices for older adults with ACS are summarized in Table 4.172-174
Table 4.
Best Kidney Practices for Older Adults with acute coronary syndrome.
| Best Kidney Practices |
|---|
| Pre-procedure identification of older patients at risk for CI-AKI with the aim to stabilize renal function prior to coronary intervention in the context of ACS. Risk factors include preexisting kidney disease, DM, hemodynamic instability, and those on nephrotoxic medications. |
| If patients are not at risk for acute pulmonary edema associated with reduced LV function, assess volume status and consider gentle pre-procedure volume resuscitation and/or holding of diuretics to protect against CI-AKI. |
| Avoid hyperosmolar CM and minimize dose of isoosmolar or low-osmolar CM to avoid CA-AKI. A recommended dose of CM to avoid AKI: 3 X eGFR. |
| The use of transradial PCI to lower risk of AKI, bleeding, and death is important and avoidance of renal toxins, NSAIDS, diuretics, and aminoglycosides), adjustment of medication doses for reduced eGFR, and reduction of polypharmacy in the older patients are critical. |
| Avoid hypotensive episodes to protect renal function particularly among patients with left ventricular impairment. |
| Rule out other causes of AKI prior to procedures (obstruction, volume depletion, sepsis). |
| In case of multi-vessel PCIs, procedures can be staged to allow renal recovery. |
| Among patients already on dialysis, coordination of PCI procedures and dialysis with the renal team. |
| Short duration (1-3 months) of DAPT with DES in CKD patients reduces bleeding events post PCI. |
Abbreviations: ACS = acute coronary syndrome; CI-AKI = contrast induced kidney injury; CM = contrast media; DAPT = dual antiplatelet therapy; DES = drug-eluting stent; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; LV = left ventricular; NSAIDS = nonsteroidal anti-inflammatory drugs; PCI = percutaneous coronary intervention.
Key Points for Clinical Practice
Among selected older adults with left main or multi-vessel coronary disease, CABG has been associated with improved survival and lower risk of repeat revascularization and myocardial infarction compared to PCI, but for older patients a Heart Team approach to revascularization strategy is suggested, including geriatrics expertise to assess frailty, multimorbidity, cognition, and other pertinent age-related elements of care.
STS PROM score quantifies the risk of early mortality after CABG at all ages; however, important additional considerations that may reduce the feasibility of safe and effective surgical revascularization or affect the goals of care are commonly encountered in older patients, including severe cognitive impairment, frailty, cerebrovascular disease and aortic calcification.
Among older adults with left main or multi-vessel coronary disease, CABG surgery with goal of complete revascularization has shown survival benefits, but shared decision making with patient and family is critical particularly among very old adults. The utilization of percutaneous revascularization with medical therapy or medical therapy alone are also reasonable options if symptom control is a primary patient-centered goal.
Stroke risk can be minimized by tailoring operative techniques and peri-operative management to include pre-and intra-operative imaging of aortic and cerebrovascular disease, and aggressive prevention and treatment of atrial fibrillation.
FUTILITY
Futility has been defined as (1) “as a lack of medical efficacy, particularly when the therapy is unlikely to produce its intended clinical result, as judged by the physician; or lack of a meaningful survival, as judged by the personal values of the patient”175; (2) “advanced life-prolonging treatments that would almost certainly result in a quality of life that the patient has previously stated that he/she would not want”; or (3) “when there is no reasonable expectation that the patient will improve sufficiently to survive outside the acute care setting”.176 It is frequently challenging to predict or recognize futility or gain consensus from the patient and caregivers, particularly after percutaneous or surgical revascularization when concerns around publicly reported outcomes may influence the decision to pursue aggressive care in the end-of-life setting. Establishing goals of care in older patients from the outset may help avoid unwanted or futile intervention.6 Mechanical circulatory support for surgical revascularization is generally futile in older patients with advanced geriatric syndromes (such as delirium and cognitive impairment, frailty, multimorbidity, and polypharmacy), very poor baseline functional status, or prohibitive surgical risk (defined as STS PROM >20%).
The confirmation of futility often remains ambiguous and even misinterpreted within contemporary management. Those who are not candidates for procedures due to perceived futility often benefit significantly from palliative care, such that the concept of futility does not imply withdrawal of care.
Key Points:
Futility is a lack of medical efficacy when the therapy is unlikely to produce its intended clinical result or meaningful survival as judged the Heart Team.
Determining a priori goals of care in older patients may help avoid unwanted or futile intervention.
For older patients at a high-risk for mortality and adverse outcomes, a major challenge is to identify futility before rather than after revascularization but establishing goals of care in older patients from the outset may help avoid unwanted or futile intervention.
TRANSITIONS, CARDIAC REHABILITATION, AND FOLLOW-UP
Transitions of Care in Older Adults
ACS is associated with significant morbidity and mortality and older adults are at highest risk.177 The 30-day readmission rate following ACS ranged from 11-14% in a meta-analysis of 14 studies.178 Mortality rates vary among populations and range from 7-18% in the first year following STEMI.179 These adverse outcomes suggest that transitions in care from hospital to home and to primary care are important and are a target for improvement. Continuity in care is particularly critical for aging adults who are more likely to experience frailty, multimorbidity, depression, cognitive decline, diminished functional status, and a higher symptom burden.180 For example, depression is three times more common in patients following MI and complicates adherence to guideline-directed therapies, self-care, and clinic visits.180 In addition, coronary disease predominantly affects older adults so multimorbidity is expected and contributes to MACE following ACS, making coordinated care during transitions critical.
Based on a Get with the Guidelines approach, Goldman et al.177 recommended that the post-ACS discharge plan address 6 elements including: (1) medications, (2) lifestyle modification/cardiac rehabilitation, (3) management of comorbidities, (4) psychosocial support, (5) socioeconomic factors, and (6) patient/family education to include teaching and preparation of patients for their own self-care. (Figure 4). Polypharmacy is a frequent issue for older adults and transitions in care should include consideration of deprescribing potentially inappropriate medications and medications that are no longer needed.181 To achieve optimal care coordination, the multidisciplinary team benefits from inclusion of the cardiologist and surgeon (when relevant), as well as the primary care clinician, geriatrician, nurses, social worker, patient, and the patient’s family or significant other(s), with ready access to pharmacists, dieticians, psychologists, occupational therapists, and case managers as needed. The older adult’s general health status is often a key patient-centered outcome that must be considered in addition to improving survival.180 The American Heart Association recommends prioritizing three components of the patient’s health status, including symptom burden, functional status, and quality of life.182-184 Monitoring these domains during post-discharge follow-up can provide insight into how the patient is progressing relative to their goals of care and where there is potential for improvement.
Figure 4.
Post discharge plan with shared decision making for older adults with acute coronary syndrome focusing on (1) medications, (2) lifestyle modification/cardiac rehabilitation, (3) management of comorbidities, (4) psychosocial support, (5) socioeconomic factors, and (6) patient/family education. Family and patient education is a collaborative effort performed by the primary medical team, and corroborated by cardiologist, geriatrician and social worker to improve overall comprehensive and health literacy.
The complexity of care required for many older adults following hospital discharge can lead to gaps in care, and multidisciplinary coordination is essential to ensure that all aspects of care are integrated into a cohesive plan. High-quality transitional care and case management can reduce the risk of recurring adverse events and improve patient-centered outcomes such as functional status and symptom control.
Key Points for Clinical Practice
Transitions of care are high risk times for older adults following ACS coordination of surgeons (when relevant), cardiologists, geriatricians, nurses, pharmacists, and primary care clinician is integral to successful discharge/transitional care plan for older patients with ACS. Goals to better harmonize diverse clinicians involved with the care of older patients with ACS remains a challenging goal of care.
A detailed medication review and reconciliation is essential, ideally in collaboration with a pharmacist, to ensure that once at home, the patient has access to and is taking the medications prescribed at discharge and that they are integrated with any additional pharmaceuticals acquired over-the-counter and from other clinicians.
Palliative and End of Life Care in Older Adults
ACS is a sentinel event that can lead to deterioration in cognitive function, increasing frailty, loss of overall function, independence, and self-confidence among older patients. This presents a challenge to patients, their caregivers, and clinicians providing primary and specialty care, particularly when evaluating decision making. Palliative care is an important step in providing optimal treatment and support to patients and caregivers. The World Health Organization estimates that those with CVD have the greatest need for palliative care. Warraich et al. note that aggressive symptom management should be the cornerstone of management of CVD at the end of life rather than persistent life-prolonging therapies.185
Palliative care is defined as an interdisciplinary approach to improving quality of life and reducing suffering among patients with serious illness. Core domains include: (1) treating physical symptoms, (2) psychosocial care, 3) identifying the patient’s priorities for care, and (4) patient and caregiver support for decision-making. The National Coalition for Hospice and Palliative Care published the Clinical Practice Guidelines for Quality Palliative Care and included eight domains: (1) structure and processes of care, (2) physical aspects of care, (3) psychological and psychiatric aspects, (4) social aspects of care, (5) spiritual, religious, and existential aspects of care, (6) cultural aspects of care, (7) care of the patient nearing the end of life, and 8) ethical and legal aspects of care. It is especially important to assess patient-reported symptoms and quality of life since symptoms and well-being do not necessarily correlate with objective measures of disease severity. Palliative care differs from hospice care in that hospice requires an estimated life expectancy of 6 months or less. Further, palliative care does not preclude hospitalization or life-prolonging interventions, whereas hospice care generally promotes comfort measures only.
While there are few studies that focused on ACS alone, for patients with ACS complicated by severe heart failure, home and team-based palliative interventions improved patient-centered outcomes, health related quality of life, documentation of preferences, and healthcare utilization. The burden of CVD is increasing and evidence in support of various palliative care delivery methods is growing. This provides an opportunity to adopt and integrate palliative care measures into the standards of cardiovascular care. Aggressive management of patients’ symptoms is critical to improving their quality of life. Rigorous studies are needed to better define optimal timing, utilization and implementation of palliative care into practice for older patients with ACS.
Key Points for Clinical Practice
End of life management should be made available to all patients with progressive CVD to treat symptoms and improve quality of life.
Research is needed to better determine the utility and timing of palliative care interventions in older patients with ACS and how to best integrate palliative care precepts into standard post ACS-care.
Patient-reported outcome measures (PROMS) should encompass measure of function and health-related quality of life, symptoms and symptom burden, and health behavior.
Cardiac Rehabilitation in Older Adults
Cardiac Rehabilitation (CR) is a comprehensive secondary prevention CVD management program that utilizes exercise training, behavioral modification, education, and psychosocial counseling to improve outcomes in patients with ACS and other conditions. CR has been demonstrated to reduce recurrent CVD events, mortality, and rehospitalizations, as well as improve cardiorespiratory fitness, physical function, self-efficacy, and quality of life. It is an important component of ACS care for older adults with benefits applying across the clinical spectrum from those who are vigorous, robust, and confident to those who are weak, sedentary, and fearful.186 CR has particular value for older adults as it provides opportunity to develop personalized approaches to CV health in the context each patient’s aggregate healthcare challenges.186
Key components of CR include an initial medical assessment to determine a patient’s CVD status as well as the comorbidities that may affect symptoms, signs, and cumulative medication effects.187 Carefully prescribed exercise training regimens are also fundamental, with the goal to assess baseline capacity and to design tailored approaches that improve physical function over time.183, 184 Medical assessments provide opportunity for medication review and reconciliation, and referral to clinicians who can address relevant medical issues (CVD and non-CVD).183, 184 Risk reduction and psychosocial support can also be implemented, as well as steps to align overall care with each patient’s personal goals of care. Most CR programs incorporate significant care partners (spouses, partners, adult children) to reinforce learning and support structures for the patient, and also to advance the premise of healthfulness that benefits the whole family.183, 184
Despite age-related benefits of CR, participation declines with increasing patient age, and only 25% of older patients eligible for CR actually participate.188 Women also tend to participate less often than men across all ages, but since women outlive men, these gaps tend to worsen among older adults.189 Common barriers include a lack of understanding among patients and clinicians of CR’s value, prohibitive logistics particularly as many older adults cannot drive, unaffordable co-payments for CR for patients on fixed incomes, and inflexibility in format to achieve personalized therapeutic approaches in CR that are tailored to the wide range of needs and circumstances among older adults. While many contemporary initiatives now aim to improve CR participation using remote-based formats, often incorporating smartphones and wearable devices to facilitate and inform this care, the usability and safety of these novel methods for older adults who are frail, cognitively limited, and medically complex is still largely uncertain.
Benefits of CR in Older ACS patients
CR addresses heterogeneity of older adults:
Principles of ACS disease management are potentially transformed by each patient’s health circumstances. An older adult who is relatively robust is likely to have benefitted from revascularization and well-tolerated adjunctive therapies. Such a patient derives valuable benefit from CR through exercise and activity regimens that aim to increase cardiorespiratory fitness in combination with guidance to mitigate risks of over-exertion and injury. Likewise, CR provides a robust patient, i.e., those without physical frailty or pre-frailty, the opportunities to better understand ACS pathophysiology and treatment precepts, insights that correlate to improved adherence and diminished anxiety. Robust patients also benefit from improved understanding of symptoms, sleep hygiene, diet, and other aspects of care.
CR also benefits patients who are frail and sedentary. In this case, CR fosters safe and effective exercise strategies that promote physical function and confidence. Initial emphasis on strength and balance training in CR is often essential to provide a foundation before aerobic training is feasible. The effects of comorbidity, medications, nutrition, sleep, cognition, and fear are also factored into therapeutic goals and strategies. Patients who are the most functionally impaired often benefit the most from CR in terms of their relative improvements in function and quality of life.190
CR targets improved functional capacity:
Whereas CVD research is typically predicated primarily on endpoints of Major Adverse Cardiovascular Events (MACE), CR benefits include enhanced physical function.191 For many older adults, this is their primary goal of care, as it is associated with self-efficacy, independence, and improved quality of life. CR fosters opportunity for patients to maintain or regain vigor despite disease by targeting progressive strength, endurance, and cardiorespiratory fitness, with the associated aims of increased daily activity and long-term health.192
While a definitive therapy for frailty is still not known, exercise training and nutrition show promise.193, 194 CR provides critical supervision for patients who are easily fatigued and highly susceptible to falls and injury. Multiple studies demonstrate the utility of CR to restore physical function and improve outcomes.190, 195 While a cardiac hospitalization has the potential to trigger a cascade of progressive weakening and disablements for older adults who are frail, CR can potentially restore sufficient function for independence to be preserved. The concept of prehabilitation extends from CR as a complementary strategy to mitigate frailty risks even before surgical revascularization is undertaken.196
Whereas older patients with severe frailty are often excluded from cardiac surgery, patients who are treated with PCI or medical management may be relatively more functionally impaired. Ironically, the rate of referral of surgical patients to CR exceeds that for PCI and medical management, with the implication that many older PCI and medically managed ACS patients do not get the much-needed benefits of CR.197 A secondary goal of a CR program is to identify older patient at a low socioeconomic status with of lack of social support, and at a high risk for chronic stress, anxiety, and depression. The CR program can thus provide a tailored interventions to enhance psychosocial support and care for older patients after ACS admission.197
Key Points for Clinical Practice
CR targets functional enhancement as a key outcome benefit. For many older adults this is a vital clinical priority that is not fostered by other aspects of treatment, thereby reinforcing the distinctive value of CR.
CR is best utilized with a tailored approach that addresses each patient’s distinctive circumstances and goals of care.
Frailty is biologically linked to CAD and plays a key role in ACS management. CR provides opportunity for strength training, nutritional emphasis and other strategies to mitigate frailty effects and improve patient-centered outcomes. Whereas many clinicians avoid CR for patients who are frail, in fact patients who are frail often benefit the most.
CONCLUSION
The management of ACS in the older adult population is more complex than in younger patients owing to their anatomic complexity, physiologic vulnerability, age-related risks (including prevalent geriatric syndromes), and heterogeneity in life expectancy and goals of care. In this document, we propose a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning. Post MI care should include CR tailored to address each patient’s aggregate circumstances and personal goals of care.
Supplementary Material
ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- AKI
Acute Kidney Injury
- CAD
Coronary Artery Disease
- CKD
Chronic Kidney Disease
- CR
Cardiac Rehabilitation
- CRS
Cardiorenal Syndrome
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- eGFR
estimated Glomerular Filtration Rate
- ED
Emergency Department
- HRQoL
Health-related Quality of Life
- LV
Left Ventricular
- MI
Myocardial Infarction
- MINOCA
Myocardial Infarction with No Obstructive Coronary Artery
- NSTEMI
Non-ST elevation myocardial infarction
- PCI
Percutaneous Coronary Intervention
- STEMI
ST-Elevation Myocardial Infarction
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.De Luca L, Marini M, Gonzini L, Boccanelli A, Casella G, Chiarella F, De Servi S, Di Chiara A, Di Pasquale G, Olivari Z, et al. Contemporary Trends and Age-Specific Sex Differences in Management and Outcome for Patients With ST-Segment Elevation Myocardial Infarction. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Luca L, Olivari Z, Bolognese L, Lucci D, Gonzini L, Di Chiara A, Casella G, Chiarella F, Boccanelli A, Di Pasquale G, et al. A decade of changes in clinical characteristics and management of elderly patients with non-ST elevation myocardial infarction admitted in Italian cardiac care units. Open Heart. 2014;1:e000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaman MJ, Stirling S, Shepstone L, Ryding A, Flather M, Bachmann M and Myint PK. The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the Myocardial Ischaemia National Audit Project (MINAP). Eur Heart J. 2014;35:1551–8. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill DE and Forman DE. Cardiovascular care of older adults. BMJ. 2021;374:n1593. [DOI] [PubMed] [Google Scholar]
- 6.Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL 2nd, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, et al. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e6–e32. [DOI] [PubMed] [Google Scholar]
- 7.Bendz B and Aaberge L. Acute coronary syndromes in older patients: does older age matter? The Lancet. 2020;396:585–587. [DOI] [PubMed] [Google Scholar]
- 8.Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–69. [DOI] [PubMed] [Google Scholar]
- 9.Alexander KP, Newby LK, Armstrong PW, Cannon CP, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–89. [DOI] [PubMed] [Google Scholar]
- 10.Morici N, De Servi S, De Luca L, Crimi G, Montalto C, De Rosa R, De Luca G, Rubboli A, Valgimigli M and Savonitto S. Management of acute coronary syndromes in older adults. Eur Heart J. 2022;43:1542–1553. [DOI] [PubMed] [Google Scholar]
- 11.Rich Michael W, Chyun Deborah A, Skolnick Adam H, Alexander Karen P, Forman Daniel E, Kitzman Dalane W, Maurer Mathew S, McClurken James B, Resnick Barbara M, Shen Win K, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population. Journal of the American College of Cardiology. 2016;67:2419–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singam NSV, Fine C and Fleg JL. Cardiac changes associated with vascular aging. Clinical cardiology. 2020;43:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirinos JA, Segers P, Hughes T and Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seals DR, Jablonski KL and Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond). 2011;120:357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrucci L and Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singam NSV, Fine C and Fleg JL. Cardiac changes associated with vascular aging. Clin Cardiol. 2020;43:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damluji AA, Ramireddy A and Forman DE. Management and Care of Older Cardiac Patients. In: Vasan RS and Sawyer DB, eds. Encyclopedia of Cardiovascular Research and Medicine Oxford: Elsevier; 2018: 245–265. [Google Scholar]
- 18.Tzoran I, Hoffman R and Monreal M. Hemostasis and Thrombosis in the Oldest Old. Semin Thromb Hemost. 2018;44:624–631. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan ED, Hughes J and Ferenbach DA. Renal Aging: Causes and Consequences. J Am Soc Nephrol. 2017;28:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79:268–288.e1. [DOI] [PubMed] [Google Scholar]
- 21.Ronco C. Cardiorenal and renocardiac syndromes: clinical disorders in search of a systematic definition. Int J Artif Organs. 2008;31:1–2. [DOI] [PubMed] [Google Scholar]
- 22.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e840–e878. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, Herck I, Decruyenaere J and Hoste EA. Acute Kidney Injury in Cardiorenal Syndrome Type 1 Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2016;6:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkins JL, Delgado J, Pilling LC, Bowman K, Masoli JAH, Kuchel GA, Ferrucci L and Melzer D. Impact of Low Cardiovascular Risk Profiles on Geriatric Outcomes: Evidence From 421,000 Participants in Two Cohorts. J Gerontol A Biol Sci Med Sci. 2019;74:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts. Last Access: November 2021.
- 26.Schwartz JB, Schmader KE, Hanlon JT, Abernethy DR, Gray S, Dunbar-Jacob J, Holmes HM, Murray MD, Roberts R, Joyner M, et al. Pharmacotherapy in Older Adults with Cardiovascular Disease: Report from an American College of Cardiology, American Geriatrics Society, and National Institute on Aging Workshop. J Am Geriatr Soc. 2019;67:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medication Safety in Polypharmacy. Geneva: World Health Organization; 2019. (WHO/UHC/SDS/2019.11). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 28.Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK, Goodlin SJ, Maurer MS, Alexander KP, Rich MW, et al. Deprescribing in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2019;73:2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damluji AA, Chung S-E, Xue Q-L, Hasan RK, Moscucci M, Forman DE, Bandeen-Roche K, Batchelor W, Walston JD, Resar JR, et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. European Heart Journal. 2021;42:3856–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G and Fried LP. Frailty: implications for clinical practice and public health. The Lancet. 2019;394:1365–1375. [DOI] [PubMed] [Google Scholar]
- 31.Soysal P, Arik F, Smith L, Jackson SE and Isik AT. Inflammation, Frailty and Cardiovascular Disease. Adv Exp Med Biol. 2020;1216:55–64. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi C, Garagnani P, Parini P, Giuliani C and Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology. 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- 33.Damluji AA, Chung SE, Xue QL, Hasan RK, Walston JD, Forman DE, Bandeen-Roche K, Moscucci M, Batchelor W, Resar JR, et al. Physical Frailty Phenotype and the Development of Geriatric Syndromes in Older Adults with Coronary Heart Disease. Am J Med. 2021;134:662–671 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 35.https://www.haringey.gov.uk/sites/haringeygovuk/files/clinical_frailty_scale_poster.pdf. Last Accessed: November 2021.
- 36.https://www.mass.gov/doc/frail-scale-screening-tool/download. Last Accessed: November 2021.
- 37.Ekerstad N, Pettersson S, Alexander K, Andersson D, Eriksson S, Janzon M, Lindenberger M, Swahn E and Alfredsson J. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: A follow-up after more than 5 years. Eur J Prev Cardiol. 2018;25:1813–1821. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, Gronseth GS, Marson D, Pringsheim T, Day GS, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. [DOI] [PubMed] [Google Scholar]
- 40.https://www.oxfordmedicaleducation.com/wp-content/uploads/2015/08/MMSE-printable-mini-mental-state-examination.pdf. Last Accessed: November 2021.
- 41.http://mini-cog.com/wp-content/uploads/2018/03/Standardized-English-Mini-Cog-1-19-16-EN_v1-low-1.pdf. Last Accessed: November 2021.
- 42.https://www.parkinsons.va.gov/resources/MOCA-Test-English.pdf. Last Accessed: November 2021.
- 43.Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB and Stevens RD. Outcome of delirium in critically ill patients: systematic review and meta-analysis. Bmj. 2015;350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh ES, Fong TG, Hshieh TT and Inouye SK. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA. 2017;318:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.https://www.mnhospitals.org/Portals/0/Documents/ptsafety/LEAPT%20Delirium/Confusion%20Assessment%20Method%20-%20CAM.pdf. Last Accessed: November 2021.
- 46.Ekerstad N, Javadzadeh D, Alexander KP, Bergström O, Eurenius L, Fredrikson M, Gudnadottir G, Held C, Ängerud KH, Jahjah R, et al. Clinical Frailty Scale classes are independently associated with 6-month mortality for patients after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2022;11:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I and Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 49.Hsia RY, Hale Z and Tabas JA. A National Study of the Prevalence of Life-Threatening Diagnoses in Patients With Chest Pain. JAMA Intern Med. 2016;176:1029–32. [DOI] [PubMed] [Google Scholar]
- 50.Grosmaitre P, Le Vavasseur O, Yachouh E, Courtial Y, Jacob X, Meyran S and Lantelme P. Significance of atypical symptoms for the diagnosis and management of myocardial infarction in elderly patients admitted to emergency departments. Arch Cardiovasc Dis. 2013;106:586–92. [DOI] [PubMed] [Google Scholar]
- 51.Nanna MG, Hajduk AM, Krumholz HM, Murphy TE, Dreyer RP, Alexander KP, Geda M, Tsang S, Welty FK, Safdar B, et al. Sex-Based Differences in Presentation, Treatment, and Complications Among Older Adults Hospitalized for Acute Myocardial Infarction: The SILVER-AMI Study. Circ Cardiovasc Qual Outcomes. 2019;12:e005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajduk AM, Saczynski JS, Tsang S, Geda ME, Dodson JA, Ouellet GM, Goldberg RJ and Chaudhry SI. Presentation, Treatment, and Outcomes of Older Adults Hospitalized for Acute Myocardial Infarction According to Cognitive Status: The SILVER-AMI Study. Am J Med. 2021;134:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouellet GM, Geda M, Murphy TE, Tsang S, Tinetti ME and Chaudhry SI. Prehospital Delay in Older Adults with Acute Myocardial Infarction: The ComprehenSIVe Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction Study. J Am Geriatr Soc. 2017;65:2391–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman A, Chudow J, Merritt Z, Shulman E, Fisher JD, Ferrick KJ and Krumerman A. Electrocardiogram abnormalities in older individuals by race and ethnicity. J Electrocardiol. 2020;63:91–93. [DOI] [PubMed] [Google Scholar]
- 55.Morrow DA. The Fourth Universal Definition of Myocardial Infarction and the Emerging Importance of Myocardial Injury. Circulation. 2020;141:172–175. [DOI] [PubMed] [Google Scholar]
- 56.Fanaroff AC, Chen AY, Thomas LE, Pieper KS, Garratt KN, Peterson ED, Newby LK, de Lemos JA, Kosiborod MN, Amsterdam EA, et al. Risk Score to Predict Need for Intensive Care in Initially Hemodynamically Stable Adults With Non–ST-Segment–Elevation Myocardial Infarction. Journal of the American Heart Association. 2018;7:e008894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao Y, Liu J, Liu J, Yang N, Smith SC, Huo Y, Fonarow GC, Ge J, Taubert KA, Morgan L, et al. Sex Differences in In-Hospital Management and Outcomes of Patients With Acute Coronary Syndrome. Circulation. 2019;139:1776–1785. [DOI] [PubMed] [Google Scholar]
- 58.Gurwitz JH, Col NF and Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. Jama. 1992;268:1417–22. [PubMed] [Google Scholar]
- 59.Pagidipati NJ and Peterson ED. Acute coronary syndromes in women and men. Nature Reviews Cardiology. 2016;13:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Rosa R, Morici N, De Luca G, De Luca L, Ferri LA, Piatti L, Tortorella G, Grosseto D, Franco N, Misuraca L, et al. Association of Sex with Outcome in Elderly Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. The American Journal of Medicine. 2021;134:1135–1141.e1. [DOI] [PubMed] [Google Scholar]
- 61.Savonitto S, Colombo D, Franco N, Misuraca L, Lenatti L, Romano IJ, Morici N, Lo Jacono E, Leuzzi C, Corrada E, et al. Age at Menopause and Extent of Coronary Artery Disease Among Postmenopausal Women with Acute Coronary Syndromes. Am J Med. 2016;129:1205–1212. [DOI] [PubMed] [Google Scholar]
- 62.Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S, Fox KA and Yusuf S. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol. 2005;46:1845–51. [DOI] [PubMed] [Google Scholar]
- 63.Fanaroff AC, Rymer JA, Goldstein SA, Simel DL and Newby LK. Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review. Jama. 2015;314:1955–65. [DOI] [PubMed] [Google Scholar]
- 64.de la Fuente A. The economic consequences of Covid in Spain and how to deal with them. Applied Economic Analysis. 2021;29:90–104. [Google Scholar]
- 65.deFilippi CR and Damluji AA. At the Crossroad Between Skeletal and Cardiac Muscle Cells. Circulation. 2022;145:1780–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A and deFilippi CR. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, Rihal CS, Gersh BJ, Lewis B, Lennon RJ, et al. Incidence, Trends, and Outcomes of Type 2 Myocardial Infarction in a Community Cohort. Circulation. 2020;141:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laggoune J, Nerlekar N, Munnur K, Ko BS, Cameron JD, Seneviratne S and Wong DT. The utility of coronary computed tomography angiography in elderly patients. Journal of geriatric cardiology : JGC. 2019;16:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferencik M, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Liu T, Ghemigian K, Kitslaar P, Broersen A, Bamberg F, et al. Computed tomography-based high-risk coronary plaque score to predict acute coronary syndrome among patients with acute chest pain--Results from the ROMICAT II trial. J Cardiovasc Comput Tomogr. 2015;9:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, et al. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e6–e32. [DOI] [PubMed] [Google Scholar]
- 72.Washam JB, Herzog CA, Beitelshees AL, Cohen MG, Henry TD, Kapur NK, Mega JL, Menon V, Page RL 2nd and Newby LK. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. 2015;131:1123–49. [DOI] [PubMed] [Google Scholar]
- 73.Chen A, Stecker E and B AW. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J Am Heart Assoc. 2020;9:e017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roberts JA, Pea F and Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52:1–8. [DOI] [PubMed] [Google Scholar]
- 75.Effient Package insert. Prasugrel hydrochloride. Eli Lilly, Indianapolis, IN. Available at: https://uspl.lilly.com/effient/effient.html#pi. Accessed July 7, 2022. [Google Scholar]
- 76.Menichelli M, Neumann FJ, Ndrepepa G, Mayer K, Wöhrle J, Bernlochner I, Richardt G, Witzenbichler B, Sibbing D, Gewalt S, et al. Age- and Weight-Adapted Dose of Prasugrel Versus Standard Dose of Ticagrelor in Patients With Acute Coronary Syndromes : Results From a Randomized Trial. Ann Intern Med. 2020;173:436–444. [DOI] [PubMed] [Google Scholar]
- 77.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4–e17. [DOI] [PubMed] [Google Scholar]
- 78.Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, et al. Comparison of Reduced-Dose Prasugrel and Standard-Dose Clopidogrel in Elderly Patients With Acute Coronary Syndromes Undergoing Early Percutaneous Revascularization. Circulation. 2018;137:2435–2445. [DOI] [PubMed] [Google Scholar]
- 79.Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, Heestermans T, Tjon Joe Gin M, Waalewijn R, Hofma S, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–1381. [DOI] [PubMed] [Google Scholar]
- 80.Szummer K, Montez-Rath ME, Alfredsson J, Erlinge D, Lindahl B, Hofmann R, Ravn-Fischer A, Svensson P and Jernberg T. Comparison Between Ticagrelor and Clopidogrel in Elderly Patients With an Acute Coronary Syndrome. Circulation. 2020;142:1700–1708. [DOI] [PubMed] [Google Scholar]
- 81.Montalto C, Morici N, Munafò AR, Mangieri A, Mandurino-Mirizzi A, D’Ascenzo F, Oreglia J, Latib A, Porto I, Colombo A, et al. Optimal P2Y12 inhibition in older adults with acute coronary syndromes: a network meta-analysis of randomized controlled trials. European Heart Journal - Cardiovascular Pharmacotherapy. 2022;8:20–27. [DOI] [PubMed] [Google Scholar]
- 82.Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, Cannon CP, Heras M, Lopes RD, Morais J, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5:680–8. [DOI] [PubMed] [Google Scholar]
- 83.Crimi G, Morici N, Ferrario M, Ferri LA, Piatti L, Grosseto D, Cacucci M, Mandurino Mirizzi A, Toso A, Piscione F, et al. Time Course of Ischemic and Bleeding Burden in Elderly Patients With Acute Coronary Syndromes Randomized to Low-Dose Prasugrel or Clopidogrel. J Am Heart Assoc. 2019;8:e010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal. 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 85.Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 86.Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van 't Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, et al. A Genotype-Guided Strategy for Oral P2Y(12) Inhibitors in Primary PCI. N Engl J Med. 2019;381:1621–1631. [DOI] [PubMed] [Google Scholar]
- 87.Shavadia JS, Holmes DN, Thomas L, Peterson ED, Granger CB, Roe MT and Wang TY. Comparative Effectiveness of β-Blocker Use Beyond 3 Years After Myocardial Infarction and Long-Term Outcomes Among Elderly Patients. Circulation: Cardiovascular Quality and Outcomes. 2019;12:e005103. [DOI] [PubMed] [Google Scholar]
- 88.Gencer B, Mach F, Guo J, Im K, Ruzza A, Wang H, Kurtz CE, Pedersen TR, Keech AC, Ott BR, et al. Cognition After Lowering LDL-Cholesterol With Evolocumab. Journal of the American College of Cardiology. 2020;75:2283–2293. [DOI] [PubMed] [Google Scholar]
- 89.Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M and Trikalinos TA. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, Califf RM, Braunwald E and Blazing MA. Effect of Simvastatin-Ezetimibe Compared With Simvastatin Monotherapy After Acute Coronary Syndrome Among Patients 75 Years or Older: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiology. 2019;4:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, et al. Cognitive Function in a Randomized Trial of Evolocumab. New England Journal of Medicine. 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 92.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. [DOI] [PubMed] [Google Scholar]
- 94.Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K and Uretsky BF. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–8. [DOI] [PubMed] [Google Scholar]
- 95.Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–8. [DOI] [PubMed] [Google Scholar]
- 96.Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winkelmayer WC, Fischer MA, Schneeweiss S, Wang PS, Levin R and Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46:1080–7. [DOI] [PubMed] [Google Scholar]
- 98.Ho JK, Moriarty F, Manly JJ, Larson EB, Evans DA, Rajan KB, Hudak EM, Hassan L, Liu E, Sato N, et al. Blood-Brain Barrier Crossing Renin-Angiotensin Drugs and Cognition in the Elderly: A Meta-Analysis. Hypertension. 2021;78:629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, et al. Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021;23:1040–1048. [DOI] [PubMed] [Google Scholar]
- 100.Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, Mann DL, McMurray JJV, Rouleau JL, Solomon SD, et al. Angiotensin Receptor-Neprilysin Inhibition in Acute Myocardial Infarction. N Engl J Med. 2021;385:1845–1855. [DOI] [PubMed] [Google Scholar]
- 101.Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. New England Journal of Medicine. 2019;381:1103–1113. [DOI] [PubMed] [Google Scholar]
- 102.Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay J-F, Granger CB, Mauri L, et al. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention. Circulation. 2018;138:527–536. [DOI] [PubMed] [Google Scholar]
- 103.Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, Granger CB, Holmes DR, Lopes RD, Mehran R, et al. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention. Circulation. 2021;143:583–596. [DOI] [PubMed] [Google Scholar]
- 104.Rymer JA, Fonseca E, Bhandary DD, Kumar D, Khan ND and Wang TY. Difference in Medication Adherence Between Patients Prescribed a 30-Day Versus 90-Day Supply After Acute Myocardial Infarction. J Am Heart Assoc. 2021;10:e016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernandez EV, McDaniel JA and Carroll NV. Examination of the Link Between Medication Adherence and Use of Mail-Order Pharmacies in Chronic Disease States. J Manag Care Spec Pharm. 2016;22:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Gara Patrick T, Kushner Frederick G, Ascheim Deborah D, Casey Donald E, Chung Mina K, de Lemos James A, Ettinger Steven M, Fang James C, Fesmire Francis M, Franklin Barry A, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Journal of the American College of Cardiology. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 107.Damluji AA, Resar JR, Gerstenblith G, Gross AL, Forman DE and Moscucci M. Temporal Trends of Percutaneous Coronary Interventions in Older Adults With Acute Myocardial Infarction. Circulation: Cardiovascular Interventions. 2019;12:e007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 109.D'Ascenzo F, Biondi-Zoccai G, Moretti C, Bollati M, Omedè P, Sciuto F, Presutti DG, Modena MG, Gasparini M, Reed MJ, et al. TIMI, GRACE and alternative risk scores in Acute Coronary Syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials. 2012;33:507–14. [DOI] [PubMed] [Google Scholar]
- 110.Fox KAA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ (Clinical research ed). 2006;333:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Putot A, Jeanmichel M, Chague F, Manckoundia P, Cottin Y and Zeller M. Type 2 Myocardial Infarction: A Geriatric Population-based Model of Pathogenesis. Aging Dis. 2020;11:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hung J, Roos A, Kadesjö E, McAllister DA, Kimenai DM, Shah ASV, Anand A, Strachan FE, Fox KAA, Mills NL, et al. Performance of the GRACE 2.0 score in patients with type 1 and type 2 myocardial infarction. Eur Heart J. 2021;42:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, et al. Baseline Risk of Major Bleeding in Non–ST-Segment–Elevation Myocardial Infarction. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung K, Wilkinson C, Veerasamy M and Kunadian V. Frailty Scores and Their Utility in Older Patients with Cardiovascular Disease. Interv Cardiol. 2021;16:e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Damluji AA, Chung SE, Xue QL, Hasan RK, Walston JD, Forman DE, Bandeen-Roche K, Moscucci M, Batchelor W, Resar JR, et al. Physical Frailty Phenotype and the Development of Geriatric Syndromes in Older Adults with Coronary Heart Disease. Am J Med. 2021;134:662–671.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Damluji AA, Huang J, Bandeen-Roche K, Forman DE, Gerstenblith G, Moscucci M, Resar JR, Varadhan R, Walston JD and Segal JB. Frailty Among Older Adults With Acute Myocardial Infarction and Outcomes From Percutaneous Coronary Interventions. Journal of the American Heart Association. 2019;8:e013686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Batty J, Qiu W, Gu S, Sinclair H, Veerasamy M, Beska B, Neely D, Ford G and Kunadian V. One-year clinical outcomes in older patients with non-ST elevation acute coronary syndrome undergoing coronary angiography: An analysis of the ICON1 study. Int J Cardiol. 2019;274:45–51. [DOI] [PubMed] [Google Scholar]
- 118.Dou Q, Wang W, Wang H, Ma Y, Hai S, Lin X, Liu Y, Zhang X, Wu J and Dong B. Prognostic value of frailty in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. BMC Geriatr. 2019;19:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Radovanovic D, Seifert B, Urban P, Eberli FR, Rickli H, Bertel O, Puhan MA and Erne P. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart. 2014;100:288–94. [DOI] [PubMed] [Google Scholar]
- 120.Mamas MA, Fath-Ordoubadi F, Danzi GB, Spaepen E, Kwok CS, Buchan I, Peek N, de Belder MA, Ludman PF, Paunovic D, et al. Prevalence and Impact of Co-morbidity Burden as Defined by the Charlson Co-morbidity Index on 30-Day and 1- and 5-Year Outcomes After Coronary Stent Implantation (from the Nobori-2 Study). Am J Cardiol. 2015;116:364–371. [DOI] [PubMed] [Google Scholar]
- 121.Beska B, Mills GB, Ratcovich H, Wilkinson C, Damluji AA and Kunadian V. Impact of multimorbidity on long-term outcomes in older adults with non-ST elevation acute coronary syndrome in the North East of England: a multi-centre cohort study of patients undergoing invasive care. BMJ Open. 2022;12:e061830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu SZ, Beska B, Chan D, Neely D, Batty J, Adams-Hall J, Mossop H, Qiu W and V. K. Cognitive Decline in Older Patients With Non-ST Elevation Acute Coronary Syndrome. J Am Heart Assoc. 2019;8:e011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Montilla Padilla I, Martín-Asenjo R and Bueno H. Management of Acute Coronary Syndromes in Geriatric Patients. Heart Lung Circ. 2017;26:107–113. [DOI] [PubMed] [Google Scholar]
- 124.Groff AC, Colla CH and Lee TH. Days Spent at Home - A Patient-Centered Goal and Outcome. N Engl J Med. 2016;375:1610–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanchis J, Ariza-Solé A, Abu-Assi E, Alegre O, Alfonso F, Barrabés JA, Baz JA, Carol A, Díez Villanueva P, García Del Blanco B, et al. Invasive Versus Conservative Strategy in Frail Patients With NSTEMI: The MOSCA-FRAIL Clinical Trial Study Design. Rev Esp Cardiol (Engl Ed). 2019;72:154–159. [DOI] [PubMed] [Google Scholar]
- 126.Beska B, Ratcovich R, Bagnall A, Burrell A, Edwards E, Egred M, Jordan R, Khan A, Mills G, Morrison E, et al. Angiographic and Procedural Characteristics in Frail Older Patients with Non-ST Elevation Acute Coronary Syndrome. Interventional Cardiology Review. 2022;(In-Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gu SZ, Qiu W, Batty JA, Sinclair H, Veerasamy M, Brugaletta S, Neely D, Ford G, Calvert PA, Mintz GS, et al. Coronary artery lesion phenotype in frail older patients with non-ST-elevation acute coronary syndrome undergoing invasive care. EuroIntervention. 2019;15:e261–e268. [DOI] [PubMed] [Google Scholar]
- 128.Ratcovich H, Beska B, Mills G, Holmvang L, Adams-Hall J, Stevenson H, Veerasamy M, Wilkinson C and Kunadian V. Five-year clinical outcomes in patients with frailty aged ≥75 years with non-ST elevation acute coronary syndrome undergoing invasive management. European Heart Journal Open. 2022:oeac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lawton Jennifer S, Tamis-Holland Jacqueline E, Bangalore S, Bates Eric R, Beckie Theresa M, Bischoff James M, Bittl John A, Cohen Mauricio G, DiMaio JM, Don Creighton W, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. Journal of the American College of Cardiology. 2022;79:e21–e129. [DOI] [PubMed] [Google Scholar]
- 130.Savonitto S, Cavallini C, Petronio AS, Murena E, Antonicelli R, Sacco A, Steffenino G, Bonechi F, Mossuti E, Manari A, et al. Early Aggressive Versus Initially Conservative Treatment in Elderly Patients With Non–ST-Segment Elevation Acute Coronary Syndrome: A Randomized Controlled Trial. JACC: Cardiovasc Interv. 2012;5:906–916. [DOI] [PubMed] [Google Scholar]
- 131.Sanchis J, Núñez E, Barrabés JA, Marín F, Consuegra-Sánchez L, Ventura S, Valero E, Roqué M, Bayés-Genís A, del Blanco BG, et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur J Intern Med. 2016;35:89–94. [DOI] [PubMed] [Google Scholar]
- 132.de Belder A, Myat A, Blaxill J, Haworth P, O'Kane P, Hatrick R, Aggarwal RK, Davie A, Smith W, Gerber R, et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: the RINCAL randomised trial. EuroIntervention. 2021;17:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirlekar G, Libungan B, Karlsson T, Bäck M, Herlitz J and Albertsson P. Percutaneous coronary intervention in the very elderly with NSTE-ACS: the randomized 80+ study. Scandinavian Cardiovascular Journal. 2020;54:315–321. [DOI] [PubMed] [Google Scholar]
- 134.Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl-Hofseth O, Ranhoff AH, Gullestad L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387:1057–1065. [DOI] [PubMed] [Google Scholar]
- 135.Mills GB, Ratcovich H, Adams-Hall J, Beska B, Kirkup E, Raharjo DE, Veerasamy M, Wilkinson C and Kunadian V. Is the contemporary care of the older persons with acute coronary syndrome evidence-based? European Heart Journal Open. 2022;2:oeab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Savonitto S, Cavallini C, Petronio AS, Murena E, Antonicelli R, Sacco A, Steffenino G, Bonechi F, Mossuti E, Manari A, et al. Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: a randomized controlled trial. JACC Cardiovasc Interv. 2012;5:906–16. [DOI] [PubMed] [Google Scholar]
- 137.Hirlekar G, Libungan B, Karlsson T, Bäck M, Herlitz J and Albertsson P. Percutaneous coronary intervention in the very elderly with NSTE-ACS: the randomized 80+ study. Scand Cardiovasc J. 2020;54:315–321. [DOI] [PubMed] [Google Scholar]
- 138.de Belder A, Myat A, Blaxill J, Haworth P, O'Kane PD, Hatrick R, Aggarwal RK, Davie A, Smith W, Gerber R, et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: the RINCAL randomised trial. EuroIntervention. 2021;17:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gnanenthiran SR, Kritharides L, D'Souza M, Lowe HC and Brieger DB. Revascularisation compared with initial medical therapy for non-ST-elevation acute coronary syndromes in the elderly: a meta-analysis. Heart. 2017;103:1962–1969. [DOI] [PubMed] [Google Scholar]
- 140.Ma W, Liang Y and Zhu J. Early Invasive Versus Initially Conservative Strategy in Elderly Patients Older Than 75 Years with Non-ST-Elevation Acute Coronary Syndrome: A Meta-Analysis. Heart Lung Circ. 2018;27:611–620. [DOI] [PubMed] [Google Scholar]
- 141.Kaura A, Sterne JAC, Trickey A, Abbott S, Mulla A, Glampson B, Panoulas V, Davies J, Woods K, Omigie J, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): a cohort study based on routine clinical data. Lancet. 2020;396:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bueno H, Betriu A, Heras M, Alonso JJ, Cequier A, García EJ, López-Sendón JL, Macaya C, Hernández-Antolín R, on behalf of the TI, et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011;32:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Boer SP, Westerhout CM, Simes RJ, Granger CB, Zijlstra F and Boersma E. Mortality and morbidity reduction by primary percutaneous coronary intervention is independent of the patient's age. JACC Cardiovasc Interv. 2010;3:324–31. [DOI] [PubMed] [Google Scholar]
- 144.Mehta RH, Granger CB, Alexander KP, Bossone E, White HD and Sketch MH. Reperfusion strategies for acute myocardial infarction in the elderly: Benefits and risks. Journal of the American College of Cardiology. 2005;45:471–478. [DOI] [PubMed] [Google Scholar]
- 145.Kunadian V, Qiu W, Ludman P, Redwood S, Curzen N, Stables R, Gunn J and Gershlick A. Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc Interv. 2014;7:1374–85. [DOI] [PubMed] [Google Scholar]
- 146.Kunadian V, Bawamia B, Maznyczka A, Zaman A and Qiu W. Outcomes following primary percutaneous coronary intervention in the setting of cardiac arrest: a registry database study. Eur Heart J Acute Cardiovasc Care. 2015;4:6–15. [DOI] [PubMed] [Google Scholar]
- 147.Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. New England Journal of Medicine. 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 148.Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. [DOI] [PubMed] [Google Scholar]
- 149.Tehrani BN, Damluji AA, Sherwood MW, Rosner C, Truesdell AG, Epps KC, Howard E, Barnett SD, Raja A, deFilippi CR, et al. Transradial access in acute myocardial infarction complicated by cardiogenic shock: Stratified analysis by shock severity. Catheter Cardiovasc Interv. 2021;97:1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. New England Journal of Medicine. 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 151.Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. The Lancet. 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 152.O'Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC Jr., Lobdell KW, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann Thorac Surg. 2018;105:1419–1428. [DOI] [PubMed] [Google Scholar]
- 153.https://www.sts.org/resources/risk-calculator Last Accessed: November 2021.
- 154.Alkhouli M, Alqahtani F, Kalra A, Gafoor S, Alhajji M, Alreshidan M, Holmes DR and Lerman A. Trends in Characteristics and Outcomes of Patients Undergoing Coronary Revascularization in the United States, 2003-2016. JAMA Netw Open. 2020;3:e1921326. [DOI] [PubMed] [Google Scholar]
- 155.ElBardissi AW, Aranki SF, Sheng S, O'Brien SM, Greenberg CC and Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81. [DOI] [PubMed] [Google Scholar]
- 156.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Silvay G and Adams DH. Results and predictors of early and late outcomes of coronary artery bypass graft surgery in octogenarians. J Cardiothorac Vasc Anesth. 2007;21:784–92. [DOI] [PubMed] [Google Scholar]
- 157.Dobaria V, Hadaya J, Sanaiha Y, Aguayo E, Sareh S and Benharash P. The Pragmatic Impact of Frailty on Outcomes of Coronary Artery Bypass Grafting. Ann Thorac Surg. 2021;112:108–115. [DOI] [PubMed] [Google Scholar]
- 158.Tran DTT, Tu JV, Dupuis JY, Bader Eddeen A and Sun LY. Association of Frailty and Long-Term Survival in Patients Undergoing Coronary Artery Bypass Grafting. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Afilalo J, Sharma A, Zhang S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr., Smith PK, Shahian DM, et al. Gait Speed and 1-Year Mortality Following Cardiac Surgery: A Landmark Analysis From the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J Am Heart Assoc. 2018;7:e010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang M, Lee CW, Ahn JM, Cavalcante R, Sotomi Y, Onuma Y, Park DW, Kang SJ, Lee SW, Kim YH, et al. Outcomes of Coronary Artery Bypass Graft Surgery Versus Drug-Eluting Stents in Older Adults. J Am Geriatr Soc. 2017;65:625–630. [DOI] [PubMed] [Google Scholar]
- 161.Khan MR, Kayani WT, Ahmad W, Manan M, Hira RS, Hamzeh I, Jneid H, Virani SS, Kleiman N, Lakkis N, et al. Effect of increasing age on percutaneous coronary intervention vs coronary artery bypass grafting in older adults with unprotected left main coronary artery disease: A meta-analysis and meta-regression. Clin Cardiol. 2019;42:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palmerini T, Serruys P, Kappetein AP, Genereux P, Riva DD, Reggiani LB, Christiansen EH, Holm NR, Thuesen L, Makikallio T, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: A meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017;190:54–63. [DOI] [PubMed] [Google Scholar]
- 163.Whitlock EL, Diaz-Ramirez LG, Smith AK, Boscardin WJ, Covinsky KE, Avidan MS and Glymour MM. Association of Coronary Artery Bypass Grafting vs Percutaneous Coronary Intervention With Memory Decline in Older Adults Undergoing Coronary Revascularization. Jama. 2021;325:1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Diegeler A, Börgermann J, Kappert U, Breuer M, Böning A, Ursulescu A, Rastan A, Holzhey D, Treede H, Rieß FC, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med. 2013;368:1189–98. [DOI] [PubMed] [Google Scholar]
- 165.Diegeler A, Börgermann J, Kappert U, Hilker M, Doenst T, Böning A, Albert M, Färber G, Holzhey D, Conradi L, et al. Five-Year Outcome After Off-Pump or On-Pump Coronary Artery Bypass Grafting in Elderly Patients. Circulation. 2019;139:1865–1871. [DOI] [PubMed] [Google Scholar]
- 166.Böning A, Diegeler A, Hilker M, Zacher M, Reents W, Faerber G and Doenst T. Preoperative atrial fibrillation and outcome in patients undergoing on-pump or off-pump coronary bypass surgery: lessons learned from the GOPCABE trial. Interact Cardiovasc Thorac Surg. 2015;20:74–8. [DOI] [PubMed] [Google Scholar]
- 167.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 168.Ozkok S and Ozkok A. Contrast-induced acute kidney injury: A review of practical points. World J Nephrol. 2017;6:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang C, Li SX, Mahajan S, Testani JM, Wilson FP, Mena CI, Masoudi FA, Rumsfeld JS, Spertus JA, Mortazavi BJ, et al. Development and Validation of a Model for Predicting the Risk of Acute Kidney Injury Associated With Contrast Volume Levels During Percutaneous Coronary Intervention. JAMA Netw Open. 2019;2:e1916021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andò G, Cortese B, Russo F, Rothenbühler M, Frigoli E, Gargiulo G, Briguori C, Vranckx P, Leonardi S, Guiducci V, et al. Acute Kidney Injury After Radial or Femoral Access for Invasive Acute Coronary Syndrome Management: AKI-MATRIX. Journal of the American College of Cardiology. 2017;69:2592–2603. [DOI] [PubMed] [Google Scholar]
- 171.Malik AO, Amin A, Kennedy K, Qintar M, Shafiq A, Mehran R and Spertus JA. Patient-centered contrast thresholds to reduce acute kidney injury in high-risk patients undergoing percutaneous coronary intervention. Am Heart J. 2021;234:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yuan N, Latif K, Botting PG, Elad Y, Bradley SM, Nuckols TK, Cheng S and Ebinger JE. Refining Safe Contrast Limits for Preventing Acute Kidney Injury After Percutaneous Coronary Intervention. J Am Heart Assoc. 2021;10:e018890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann FJ, Saito S, et al. 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:1870–1883. [DOI] [PubMed] [Google Scholar]
- 174.Stefanini GG, Briguori C, Cao D, Baber U, Sartori S, Zhang Z, Dangas G, Angiolillo DJ, Mehta S, Cohen DJ, et al. Ticagrelor monotherapy in patients with chronic kidney disease undergoing percutaneous coronary intervention: TWILIGHT-CKD. Eur Heart J. 2021;42:4683–4693. [DOI] [PubMed] [Google Scholar]
- 175.Lindman Brian R, Alexander Karen P, O'Gara Patrick T and Afilalo J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC: Cardiovascular Interventions. 2014;7:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kon AA, Shepard EK, Sederstrom NO, Swoboda SM, Marshall MF, Birriel B and Rincon F. Defining Futile and Potentially Inappropriate Interventions: A Policy Statement From the Society of Critical Care Medicine Ethics Committee. Crit Care Med. 2016;44:1769–74. [DOI] [PubMed] [Google Scholar]
- 177.Goldman JD and Harte FM. Transition of care to prevent recurrence after acute coronary syndrome: the critical role of the primary care provider and pharmacist. Postgraduate medicine. 2020;132:426–432. [DOI] [PubMed] [Google Scholar]
- 178.Wang H, Zhao T, Wei X, Lu H and Lin X. The prevalence of 30-day readmission after acute myocardial infarction: A systematic review and meta-analysis. Clinical cardiology. 2019;42:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, Ettinger SM, Fang JC, Fesmire FM and Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 180.Singh M, Spertus JA, Gharacholou SM, Arora RC, Widmer RJ, Kanwar A, Sanjanwala RM, Welle GA and Al-Hijji MA. Comprehensive geriatric assessment in the management of older patients with cardiovascular disease. Mayo Clinic Proceedings. 2020;95:1231–1252. [DOI] [PubMed] [Google Scholar]
- 181.Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK, Goodlin SJ, Maurer MS, Alexander KP and Rich MW. Deprescribing in older adults with cardiovascular disease. Journal of the American College of Cardiology. 2019;73:2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS and Smolderen KG. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. [DOI] [PubMed] [Google Scholar]
- 183.Fagundes A, Berg DD, Bohula EA, Baird-Zars VM, Barnett CF, Carnicelli AP, Chaudhry SP, Guo J, Keeley EC, Kenigsberg BB, et al. End-of-life care in the cardiac intensive care unit: a contemporary view from the Critical Care Cardiology Trials Network (CCCTN) Registry. Eur Heart J Acute Cardiovasc Care. 2022;11:190–197. [DOI] [PubMed] [Google Scholar]
- 184.Bueno H. Managing end of life in intensive cardiac care units. European Heart Journal Acute Cardiovascular Care. 2022;11:198–200. [DOI] [PubMed] [Google Scholar]
- 185.Warraich HJ, Mentz RJ and Hernandez AF. Paving a Better Path for Patients Dying of Heart Disease. Circulation. 2018;137:1216–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O'Neill D and Forman DE. Never Too Old for Cardiac Rehabilitation. Clin Geriatr Med. 2019;35:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.American Association of Cardiovascular & Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. Champaign, IL: Human Kinetics; 2020. [Google Scholar]
- 188.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking Cardiac Rehabilitation Participation and Completion Among Medicare Beneficiaries to Inform the Efforts of a National Initiative. Circulation: Cardiovascular Quality and Outcomes. 2020;13:e005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Supervía M, Medina-Inojosa JR, Yeung C, Lopez-Jimenez F, Squires RW, Pérez-Terzic CM, Brewer LC, Leth SE and Thomas RJ. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clin Proc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baldasseroni S, Pratesi A, Francini S, Pallante R, Barucci R, Orso F, Burgisser C, Marchionni N and Fattirolli F. Cardiac Rehabilitation in Very Old Adults: Effect of Baseline Functional Capacity on Treatment Effectiveness. J Am Geriatr Soc. 2016;64:1640–5. [DOI] [PubMed] [Google Scholar]
- 191.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, et al. Prioritizing Functional Capacity as a Principal End Point for Therapies Oriented to Older Adults With Cardiovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135:e894–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schopfer DW and Forman DE. Cardiac Rehabilitation in Older Adults. Can J Cardiol. 2016;32:1088–96. [DOI] [PubMed] [Google Scholar]
- 193.Walston J, Buta B and Xue QL. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin Geriatr Med. 2018;34:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA and Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. [DOI] [PubMed] [Google Scholar]
- 195.Lutz AH, Delligatti A, Allsup K, Afilalo J and Forman DE. Cardiac Rehabilitation Is Associated With Improved Physical Function in Frail Older Adults With Cardiovascular Disease. J Cardiopulm Rehabil Prev. 2020;40:310–318. [DOI] [PubMed] [Google Scholar]
- 196.Stammers AN, Kehler DS, Afilalo J, Avery LJ, Bagshaw SM, Grocott HP, Légaré JF, Logsetty S, Metge C, Nguyen T, et al. Protocol for the PREHAB study-Pre-operative Rehabilitation for reduction of Hospitalization After coronary Bypass and valvular surgery: a randomised controlled trial. BMJ Open. 2015;5:e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pogosova N, Saner H, Pedersen SS, Cupples ME, McGee H, Höfer S, Doyle F, Schmid J-P, von Känel R, on behalf of the Cardiac Rehabilitation Section of the European Association of Cardiovascular P, et al. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. European Journal of Preventive Cardiology. 2015;22:1290–1306.25059929 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.