Abstract
OBJECTIVES
The Centers for Disease Control and Prevention identifies in-school COVID-19 testing as a key mitigation strategy to protect students and staff during the COVID-19 pandemic. Both nasal and saliva samples are acceptable, but existing school guidance does not state a preferred test method.
METHODS
From May 2021 through July 2021, we performed a randomized, crossover study in kindergarten through 12th grade (K-12) schools to evaluate student and staff preference for self-collected nasal or saliva testing. Participants performed both collection types and participated in a standardized questionnaire assessing the preferred method.
RESULTS
A total of 135 students and staff participated. Staff, middle school, and high school students preferred the nasal swab (80/96, 83%), whereas elementary students were mixed (20/39, 51% preferred saliva). Reasons reported for preferring the nasal swab included being faster and easier. Reasons reported for preferring saliva included being easier and more fun. Despite their preference, 126 (93%) and 109 (81%) participants would take the nasal swab or saliva test again, respectively.
CONCLUSIONS
The anterior nasal test was the preferred testing method by students and staff, although preference varied by age group. Willingness to perform both tests again in the future was high. Identifying the preferred testing modality is important to increase acceptance and participation in COVID-19 in-school testing programs.
The Centers for Disease Control and Prevention (CDC) includes testing for SARS-CoV-2 as an important mitigation strategy to keep students and staff safe during the COVID-19 pandemic. Consequently, the CDC recommended in-school COVID-19 testing during the 2021 to 2022 school year,1 but national guidance regarding the best specimen collection method in the school setting is not available. COVID-19 testing can be performed on samples obtained via various collection methods, including deep nasal specimens, superficial nasal specimens, or saliva, but not all these collection methods may be practical in the school setting. Schools need to consider resources, efficiency, and testing acceptance when determining the ideal testing strategy.
School-based testing programs, particularly screening programs, may require large numbers of students and staff to be tested in a short period to be effective and minimize learning disruptions. Therefore, self-collection methods may be more efficient than health care provider–administered testing. In higher educational settings, students and staff reported high confidence (98%) and acceptability (91%) of self-testing for COVID-19,2 but few data are available on the feasibility of COVID-19 testing in younger children. Participation in school-based testing programs is key to their efficacy as a mitigation strategy. If only small numbers of students and staff participate in testing, then the program is unlikely to identify positive cases. In a qualitative study assessing the attitudes of parents and students toward school-based COVID-19 testing, physical discomfort from deep nasal swabs was identified as a leading barrier to acceptance of frequent testing. Students reported a preference for less invasive testing and a willingness to participate in regular testing as long as the testing was not painful.3
Despite the multiple specimen types and factors associated with each strategy, a preferred testing strategy has not been identified.1,4,5 Anterior nasal and saliva specimens are sensitive, specific, can be self-collected, and are minimally invasive.4,6,7 We sought to determine the preferred COVID-19 sample collection method and reason for preference among kindergarten through 12th grade (K-12) students and staff.
Methods
Participants
Students, students’ parents/legal guardians, and staff from 3 public schools (1 elementary, 1 middle, and 1 high school) in Kansas City, Missouri, were approached for participation in the School Testing, Learning, and Consultation Study. Eligible students and staff members were required to attend or work at 1 of the 3 participating schools during the study period. The study was performed during summer school, May–July 2021, to inform COVID-19 testing decisions for the subsequent academic year, starting in August 2021. Participants and families were approached through standard school communications (eg, text, e-mail), school events (eg, virtual forums), and school encounters (eg, student dropoff and pickup). Consent was available electronically in English and Spanish via a web link or QR code. English and Spanish paper copies were also available. Before study procedures, consent was obtained from staff, students ≥18 years, or parents/legal guardians of students <18 years. Child verbal assent was obtained at the time of study procedure. All participants were deemed capable of performing self-testing. ICF International Inc.’s institutional review board approved this study.
Instrumentation
Testing Preference Survey
After collection of nasal and saliva specimens, study staff documented whether the participant was able to perform self-collection without assistance. In cases in which assistance was needed, the type of assistance provided was recorded (eg, assistance with opening packaging, collecting the specimen, opening/closing the sampled collection container). Participants were asked which type of COVID-19 test they preferred. The testing preference survey was administered in English or Spanish, based on participant’s request. Participants were asked to provide primary and secondary reasons for their preferred testing method, and study staff categorized both of these responses under predefined categories. Participants were similarly queried about the reasons why the other (unselected) testing method was less preferred, and study staff again categorized their responses. Last, participants were asked if they would take their preferred testing method again, as well as their less preferred testing, if it was offered.
Study Data
At the time of consent, participant demographics, including age, race, ethnicity, language spoken at home, and gender identity were collected using the pediatric Rapid Acceleration of Diagnostics Underserved Populations common data elements.8 During the testing preference survey, provided reasons for preferred and less preferred testing methods were categorized into predetermined categories. Study data were collected and managed using REDCap electronic data capture tools, which were hosted at Children’s Mercy Kansas City (Kansas City, Missouri).9,10
Procedure
COVID-19 Nasal and Saliva Testing Procedure
Using a crossover design, participants were randomized to perform a self-administered anterior nasal swab or saliva collection followed by the alternate sample collection method in the school setting while supervised by study staff. Nasal swabs were self-collected with a sterile dry polyester swab (Copan Diagnostics) and placed in viral transport media (BD Universal Viral Transport Medium, BD Diagnostics, California). Saliva collection and SARS-CoV-2 polymerase chain reaction testing have been previously described.11 Only nasal specimens were tested.
Data Analysis
A descriptive analysis was performed. Categorical variables were classified as percent and total number. Continuous variables were classified as using median and interquartile range. The distribution of responses was evaluated by participant type (student versus staff). Because of the sample size, students were grouped into middle/high school or elementary school for analysis based on developmental skills needed to perform testing (eg, opening a conical tube).
Results
From May 13, 2021, to July 22, 2021, a total of 152 participants were enrolled; 135 subjects participated, including 68 staff and 67 students, all of whom were asymptomatic (Tables 1 and 2). Incompletion of the study by participants was due to participants being unavailable at scheduled testing times. All K-12 grades, other than eighth grade, were represented (Table 3). Thirty-nine (58%) students were in elementary school (Table 2). Among participating students, 23 (34%) identified as white, 22 (33%) as Black, and 33 (49%) as Hispanic/Latino. Some students identified as more than 1 category. Primary spoken languages at home included English (n = 45, 67%) and Spanish (n = 17, 25%) with French, Somali, Marshallese, and Kinyarwanda also reported. Forty-three (64%) students identified as male. Among participating staff, 43 (63%) identified as white, 14 (20%) as Black, and 10 (15%) as Hispanic/Latino. Fifty (74%) staff identified as female.
TABLE 1.
Characteristics of Staff Participants
| All Staff Participants, n (%) | Prefer Nasal Swab, n (%) | Prefer Saliva, n (%) | |
|---|---|---|---|
| n = 68 | n = 62 | n = 6 | |
| Gender | |||
| Female | 50 (74) | 48 (77) | 2 (33) |
| Male | 16 (24) | 12 (19) | 4 (67) |
| Prefer not to answer/other | 2 (3) | 2 (3) | 0 (0) |
| Race/ethnicitya | |||
| White | 43 (63) | 41 (66) | 2 (33) |
| Black/African American | 14 (21) | 12 (19) | 2 (33) |
| Hispanic/Latino | 10 (23) | 8 (13) | 2 (33) |
| Other | 7 (10) | 5 (8) | 2 (33) |
Numbers total greater than 100% because participants could identify with more than 1 race/ethnicity.
TABLE 2.
Characteristics of Student Participants
| All Student Participants, n (%) | Prefer Nasal Swab, n (%) | Prefer Saliva, n (%) | |
|---|---|---|---|
| n = 67 | n = 37 | n = 30 | |
| Middle or high school | 28 (42) | 18 (64) | 10 (36) |
| Gender | |||
| Female | 8 (30) | 7 (39) | 1 (10) |
| Male | 19 (68) | 11 (61) | 8 (80) |
| Prefer not to answer | 1 (1) | 0 (0) | 1 (10) |
| Race/ethnicitya | |||
| White | 4 (6) | 1 (3) | 3 (10) |
| Black/African American | 19 (28) | 15 (41) | 4 (13) |
| Hispanic/Latino | 6 (9) | 2 (5) | 4 (13) |
| Other | 4 (14) | 2 (5) | 2 (5) |
| Elementary school | 39 (58) | 19 (49) | 20 (51) |
| Gender | |||
| Female | 15 (38) | 6 (32) | 9 (45) |
| Male | 24 (62) | 13 (68) | 11 (55) |
| Race/ethnicitya | |||
| White | 19 (49) | 12 (63) | 7 (35) |
| Black/African American | 3 (8) | 2 (11) | 1 (5) |
| Hispanic/Latino | 27 (69) | 10 (53) | 17 (85) |
| Other | 4 (10) | 2 (11) | 2 (10) |
Numbers total greater than 100% since participants could identify with more than one race/ethnicity.
TABLE 3.
Grade Range of Student Participants
| Grade Completed | Number Participating |
| Kindergarten | 6 |
| 1st | 7 |
| 2nd | 5 |
| 3rd | 6 |
| 4th | 7 |
| 5th | 6 |
| 6th | 1 |
| 7th | 3 |
| 8th | 0 |
| 9th | 5 |
| 10th | 4 |
| 11th | 4 |
| 12th | 12 |
| Not reported | 1 |
All staff members and 26 (93%) middle and high students performed both tests without assistance. Two high school students needed assistance with the saliva test and with snapping the swab and closing the lid of the nasal test. Among elementary students, 13 (33%) required assistance with the nasal swab, and a specimen was unable to be obtained in 1 child. Assistance varied from needing help with any part of specimen collection (n = 8) to only needing help with breaking the swab in the collection vial and closing the lid (n = 5). Seven (18%) required assistance with saliva testing, including 5 requiring help with specimen collection and 2 requiring help with closing the specimen lid. Three children were unable to provide a saliva specimen. Notably, all the children who were unable to provide a saliva specimen were able to successfully provide a nasal specimen (1 with help, 2 without assistance). Five elementary students, ranging from kindergarten to fourth grade, required assistance with both nasal and saliva tests.
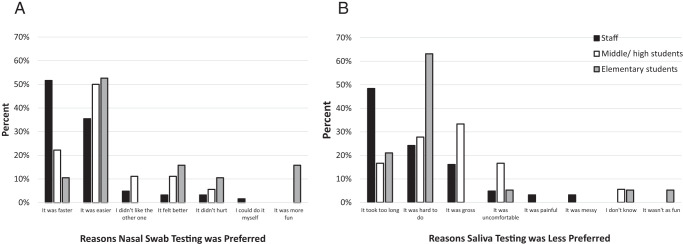
Overall, 99 (73%) participants preferred the nasal swab to saliva method, including 62 (91%) staff and 37 (55%) students (Table 1). The primary reason that staff indicated a preference for the nasal swab was that it was faster (n = 32, 52%), followed by it was easier (n = 22, 35%) (Fig 1A). Fewer than half (n = 19, 49%) of elementary students preferred the nasal swab compared with 18 (64%) middle and high school students. Students’ primary reason for preferring the nasal swab was that it was easier (middle and high school students = 9, 50%; elementary students = 10, 53%) (Fig 1A). Among staff who preferred the nasal swab, the primary reason given for not preferring the saliva test was that it took longer (n = 30, 48%) (Fig 1B). Middle and high school students reported the saliva test as being “gross” (n = 6, 33%), whereas elementary students reported it was harder (n = 12, 63%) (Fig 1B).
FIGURE 1.
Nasal swab preference results. (A) Reasons participants provided for preferring the nasal swab test and (B) reasons provided for the saliva test being less preferred.
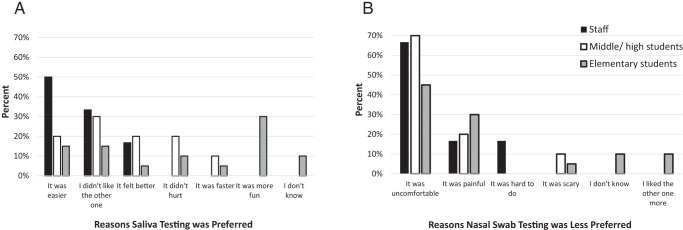
Thirty-six (27%) participants preferred the saliva test. Of the 6 staff that preferred the saliva test, 3 (50%) listed the primary reason as it being easier. Middle and high school (n = 10) and elementary (n = 20) students had various reasons for preferring the saliva test, including that it was more fun, felt better, was easier, and less scary (Fig 2A). Staff who preferred the saliva method reported preferring the nasal swab less because it was uncomfortable (n = 4, 67%). Middle and high school students reported that the nasal swab was uncomfortable (n = 3), hurt (n = 2), and felt weird (n = 2), whereas elementary students reported that it was uncomfortable (n = 8) and hurt (n = 6) (Fig 2B).
FIGURE 2.
Saliva test preference results. (A) Reasons participants provided for preferring the saliva test and (B) reasons provided for the nasal swab test being less preferred.
Despite the preference for 1 sample collection method, 126 (93%) and 109 (81%) of participants reported they would take the nasal swab or saliva test again, respectively. Of the 9 participants not wanting to take the nasal swab again, 1 was a staff member, 2 were middle or high school students, and 6 were elementary students. The 25 participants who reported not wanting to take the saliva test again included 8 staff, 9 middle or high school students, and 9 elementary students. Three people reported that they would not take either test again and 1 reported not knowing whether they would take the saliva test again. No subjects had a positive SARS-CoV-2 test.
Discussion
In this pilot study of 135 K-12 students and school staff, self-collected anterior nasal specimens were preferred to saliva specimens. Expert guidance has not identified a preferred sample collection method for school-based testing,4 and factors influencing sample type in the school setting may differ from the medical setting. School administrators may prioritize using a preferred sample type to increase participation in school testing programs, which strengthens their impact as a mitigation strategy. The ability to self-collect the specimen may decrease the number of personnel needed to implement the testing program. Last, efficient specimen collection can minimize learning disruptions.
Although secondary students and staff were mostly able to self-collect both specimens, one-third of elementary students needed assistance, regardless of the type. This underscores the need for support personnel to oversee test administration, particularly for younger students. Time needed for self-collection was not measured as part of this study; however, the most common reason that staff preferred the nasal test was that it was faster. If testing is performed during class time, then the speed of testing is important to minimize learning and teaching disruptions.
Preferences varied by participant age, with more staff preferring nasal swabs and elementary students preferring saliva testing. Implementing 2 test collection methods may not be practical; therefore, considerations based on the school population (eg, elementary versus high school) or target for testing (eg, students versus staff) may be important when determining the collection method for COVID-19 testing. Despite a preference for 1 sample type, >80% of staff and students reported they would take the alternate test in future situations. These findings highlight the acceptance of either nasal swab or saliva testing in the school setting and identify an overall preference for nasal swabs among school participants. Identifying the preferred COVID-19 testing method for students and staff is important to maximize participation in school-based testing programs, which are a key mitigation strategy in preventing the spread of COVID-19.
Our study had some limitations. First, our examination was only conducted in 3 schools and therefore may not be generalizable to all schools nationally. Second, schools included in our analysis represent an urban, diverse population, which may not be representative of all schools nationally. Third, testing was primarily performed during summer school, which may have different constraints than the academic school year. Fourth, we only tested 1 type of nasal swab and saliva collection method; other types of nasal and saliva collection methods may be more or less preferred. Finally, we did not collect the time needed to perform each test, but 1 of the more common reasons reported for nasal swab preference was that it was faster.
Conclusions
Overall, students and staff were able to perform both self-collected anterior nasal swabs and saliva specimens for COVID-19 testing in the school setting. Various age groups may prefer different collection methods. The preferred modality of COVID-19 testing may differ among age groups, but acceptance of either method was high. Developing systems for widespread COVID-19 testing in schools will be translatable for other infectious diseases or future pandemics that may disrupt in-person learning.
Acknowledgments
Erin Campbell, MS, provided editorial review and submission of this manuscript but Campbell did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Glossary
- CDC
Centers for Disease Control and Prevention
- K-12
kindergarten through 12th grade
Footnotes
Drs Schuster and Goldman conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Potts performed the analyses and reviewed and revised the manuscript; Dr Selvarangan conceptualized and designed the study and reviewed and revised the manuscript; Dr Keener Mast conceptualized and designed the study, designed the data collection instrument, coordinated and supervised the analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The School TLC Study Group members: Shannon M. Hill, RN, BSN, Abigail Kietzman, ACRP-CP, and Atenas Mena, MSN.
FUNDING: This research was funded by the Rapid Acceleration of Diagnostics (RADx) Underserved Populations (RADx-UP) Support for Safe Return to in-Person School: COVID-19 Testing, Learning, and Consultation (School TLC) OT2 HD107555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
CONFLICT OF INTEREST DISCLOSURES: Dr Schuster reports funding from Merck for unrelated studies. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Centers for Disease Control and Prevention (CDC) . Operational guidance for K-12 schools and early care and education programs to support safe in-person learning. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/k-12-childcare-guidance.html. Accessed September 21, 2022 [PubMed]
- 2. Hirst JA, Logan M, Fanshawe TR, et al. Feasibility and acceptability of community coronavirus disease 2019 testing strategies (FACTS) in a university setting. Open Forum Infect Dis. 2021;8(12):ofab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger JB, Soto D, Lee R, Deva S, Shanker K, Sood N. COVID-19 testing in schools: perspectives of school administrators, teachers, parents, and students in Southern California. Health Promot Pract. 2021;24(2):350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Rockefeller Foundation and Skoll Foundation . Covid-19 testing in K-12 settings: a playbook for educators and leaders. Available at: https://www.rockefellerfoundation.org/wp-content/uploads/2021/02/The-RockefellerFoundation-Covid-19-K-12-Testing-Playbook-for-Educators-and-Leaders.pdf. Accessed September 22, 2022
- 5. The Shah Family Foundation . The COVID-19 educational testing toolkit. Available at: https://covidedtesting.com/. Accessed September 22, 2022
- 6. Altamirano J, Govindarajan P, Blomkalns AL, et al. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for severe acute respiratory syndrome coronavirus 2 testing. JAMA Netw Open. 2020;3(6):e2012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(13):1283–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RADxUP . Previous CDE versions. Available at: https://radx-up.org/research/cdes/previous-cde-versions/. Accessed February 24, 2023
- 9. Harris PA, Taylor R, Minor BL, et al. REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee D, Sasidharan A, Abdulhamid A, et al. Diagnostic yield of saliva for SARS-CoV-2 molecular testing in children. J Pediatric Infect Dis Soc. 2021;10(10):967–969 [DOI] [PMC free article] [PubMed] [Google Scholar]