Abstract
OBJECTIVE
In April 2021, the US government made substantial investments in students’ safe return to school by providing resources for school-based coronavirus disease 2019 (COVID-19) mitigation strategies, including COVID-19 diagnostic testing. However, testing uptake and access among vulnerable children and children with medical complexities remained unclear.
METHODS
The Rapid Acceleration of Diagnostics Underserved Populations program was established by the National Institutes of Health to implement and evaluate COVID-19 testing programs in underserved populations. Researchers partnered with schools to implement COVID-19 testing programs. The authors of this study evaluated COVID-19 testing program implementation and enrollment and sought to determine key implementation strategies. A modified Nominal Group Technique was used to survey program leads to identify and rank testing strategies to provide a consensus of high-priority strategies for infectious disease testing in schools for vulnerable children and children with medical complexities.
RESULTS
Among the 11 programs responding to the survey, 4 (36%) included prekindergarten and early care education, 8 (73%) worked with socioeconomically disadvantaged populations, and 4 focused on children with developmental disabilities. A total of 81 916 COVID-19 tests were performed. “Adapting testing strategies to meet the needs, preferences, and changing guidelines,” “holding regular meetings with school leadership and staff,” and “assessing and responding to community needs” were identified as key implementation strategies by program leads.
CONCLUSIONS
School-academic partnerships helped provide COVID-19 testing in vulnerable children and children with medical complexities using approaches that met the needs of these populations. Additional work is needed to develop best practices for in-school infectious disease testing in all children.
The Centers for Disease Control and Prevention recommended the provision of coronavirus disease 2019 (COVID-19) testing in prekindergarten-12 (Pre-K-12) schools during the 2021 to 2022 school year to minimize COVID-19 outbreaks in schools and provide readily accessible testing for students and staff.1 In April 2021, the US government made billions of dollars available to support Pre-K-12 mitigation strategies in schools, including COVID-19 screening testing.2 State health agencies provided resources to support testing in Pre-K-12 schools.3,4 Coinciding with this influx of support, the National Institutes of Health launched the Safe Return to School Diagnostic Testing Initiative (RTS) as part of the Rapid Acceleration of Diagnostics Underserved Populations (RADx-UP) program to increase access to COVID-19 testing specifically for underserved and vulnerable populations.5
Under the RADx-UP RTS initiative, researchers from academic institutions collaborated with school districts on the design, implementation, and/or analysis of COVID-19 testing programs in schools and communities that may not have readily available access to testing.6,7 On-site infectious disease testing was a new endeavor for most school districts. Pre-K-12 schools that introduced testing early in the pandemic identified considerations for the planning, design, set-up, and evaluation of testing programs, and resources needed for testing implementation.8 However, real-world data were unavailable on the implementation of testing programs across various school settings and diverse student populations. Herein, we evaluated program implementation, enrollment rates, and tests performed by the RADx-UP RTS Pre-K-12 testing programs during the 2021 to 2022 school year. We also identified key implementation strategies for infectious disease testing in the Pre-K-12 school setting on the basis of consensus assessment by the program leads.
Methods: COVID-19 Testing Programs
RADx-UP Safe Return to School Diagnostic Testing Initiative Projects
In the RADx-UP RTS initiative, a total of 16 projects were awarded by July 2021. An additional RADx-UP project that was funded before the RTS initiative in July 2020 also focused on COVID-19 testing in underserved/vulnerable pediatric populations and was included in this study (Washington University School of Medicine in St. Louis/Special School District of St. Louis County). All projects involved academic researchers who partnered with local school communities serving underserved populations. Disadvantaged school settings were defined by the RADx-UP program as school or early education programs that have >50% of students eligible for free or reduced-price meals and schools, Head Start programs, and school districts or networks that serve a large proportion of individuals from racial and ethnic minority groups.9 Timing of initiation, target populations, and testing strategies varied by program.
Data Collection on COVID-19 Testing
All 17 funded RADx-UP projects were surveyed on the COVID-19 testing programs. Project leaders and principal investigators were contacted to participate in a survey in May 2022; contributing programs are listed in Table 1. Surveys were administered through REDCap electronic data capture tools hosted at Children’s Mercy Kansas City.10,11 The survey included descriptive information about participating school/school districts, student demographics, student populations eligible to participate in the testing programs, and actual student enrollment in testing. Participant-level race and ethnicity were self-reported, and district-level race and ethnicity data were obtained from publicly available sources. Survey respondents also reported on logistical features, including testing location, type of COVID-19 testing platform, estimated turnaround time from test to communication of results, the number of COVID-19 tests performed, and overall test positivity. All RADx-UP projects obtained individual institutional review board approval per their respective institutions for testing program implementation.
TABLE 1.
Baseline Testing Characteristics by RADx-UP Return to School Programs
| Site | School Type (Pre-K-12) | County Size | Participants | Participant Population | Type of Testing Program | Type of Test | Sample Collection Method | Location of Collection | Number of Tests Performed | Test Results Turnaround Time | Percent Positivity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arizona State University | Pre-K/ECE | Large metro (1 million or more people) | Student, staff, family | Hispanic/Latino populations, socioeconomically disadvantaged populations | Screening/asymptomatic, Test to Stay | Individual PCR/NAAT | Saliva, anterior nares | School | 449 | 25–48 h | 0.04% |
| Children’s Mercy/ICF | K-12 | Large metro (1 million or more people) | Student, staff | Black/African American and Hispanic/Latino populations, socioeconomically disadvantaged populations | Screening/asymptomatic, diagnostic/symptomatic, exposure | Individual PCR/NAAT | Anterior nares | School | 4014 | 25–48 h | 3.6% |
| Duke University | Pre-K-9 | Micropolitan (10 000–49 999) | Student, staff | American Indian/Alaska Native, Asian American, Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations; excluded populations unable to mask (eg, special needs) | Test to Stay | Antigen | Anterior nares | School | 10 568 | 0–1 h | 3.7% |
| University of Hawaii | K-12 | Small metro (<250 000) | Student, staff, family | Asian American and Native Hawaiian and other Pacific Islander populations, socioeconomically disadvantaged populations | Screening/asymptomatic | Antigen | Anterior nares | School, community-based site, pop-up site | 9569 | 0–1 h | 2.41% |
| San Diego State University | Middle | Large metro (1 million or more people) | Student, staff, family | Asian American and Hispanic/Latino populations, sexual and gender minorities, socioeconomically disadvantaged populations | Screening/asymptomatic, diagnostic/symptomatic, exposure | Antigen | Anterior nares | School, home | 13 781 | 0–1 h | 2.24% |
| University of Miami | K-12 | Large metro (1 million or more people) | Student | Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations, socioeconomically disadvantaged populations, underserved urban population | Screening/asymptomatic, diagnostic/symptomatic, exposure | Individual PCR/NAAT | Anterior nares | School | 697 | 0–1 h | 9% |
| University of Rochester | Pre-K-12 | Medium metro (250 000–999 999) | Student, staff | Children with developmental disabilities and American Indian/Alaska Native, Asian American, Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations | Screening/asymptomatic, diagnostic/symptomatic, Test to Stay, exposure | Individual PCR/NAAT | Anterior nares | School, home | 1378 | 25–48 h | 2% |
| University of Washington | K-5 | Micropolitan (10 000–49 999) | Student, staff | Hispanic/Latino populations, socioeconomically disadvantaged populations, underserved rural populations | Screening/asymptomatic, Test to Stay | Antigen | Anterior nares | School | 11 026 | 0–1 h | 14.93% |
| Washington University School of Medicine in St. Louis | Pre-K-12 | Medium metro (250 000–999 999) | Student, staff, household members | Children with developmental disabilities; American Indian/Alaska Native, Asian American, Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations; socioeconomically disadvantaged populations | Screening/asymptomatic, diagnostic/symptomatic, exposure | Individual PCR/NAAT | Saliva | School, home, community-based site, pop-up site | 11 913 | 2–12 h, 13–24 h, 25–48 h | 3.18% |
| Washington University School of Medicine in St. Louis/Special School District of St Louis County | K-12 | Medium metro (250 000–999 999) | Student, staff | Children with developmental disabilities and American Indian/Alaska Native, Asian American, Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations | Screening/asymptomatic, diagnostic/symptomatic, exposure | Individual PCR/NAAT | Saliva | School, home | 11 757 | 2–12 h, 13–24 h | 1.2% |
| Kennedy Krieger Institute Schools | K-12 | Medium metro (250 000–999 999) | Student, staff | Children with developmental disabilities; American Indian/Alaska Native, Asian American, Black/African American, Hispanic/Latino, and Native Hawaiian and other Pacific Islander populations; socioeconomically disadvantaged populations | Screening/asymptomatic | Individual PCR/NAAT | Saliva | School | 6764 | 25–48 h | 0.62% |
ECE, early care and education.
Statistical Analysis
This study provides descriptive statistics on school type, county size, type of participant (eg, student, staff, family member), participant population (eg, children with developmental disabilities, underserved populations, etc), testing program (eg, screening testing, Test to Stay, etc), tests administered (eg, pooled or individual nucleic acid amplification tests or antigen), collection method, collection locations, number of tests performed, test result turnaround time, and percent positivity of administered tests from July 1, 2021 to May 1, 2022. We examined student demographic characteristics between testing program participants and all students eligible to participate in testing programs using a t test of mean differences. A range plot of enrollment and eligibility was created to further describe the number of eligible students and the number of students enrolled by site. Data from programs that involved multiple school districts were aggregated to the program level. Analysis was performed in SAS 9.4, and figures were created by using the R statistical package.
Identifying Key Program Implementation Strategies
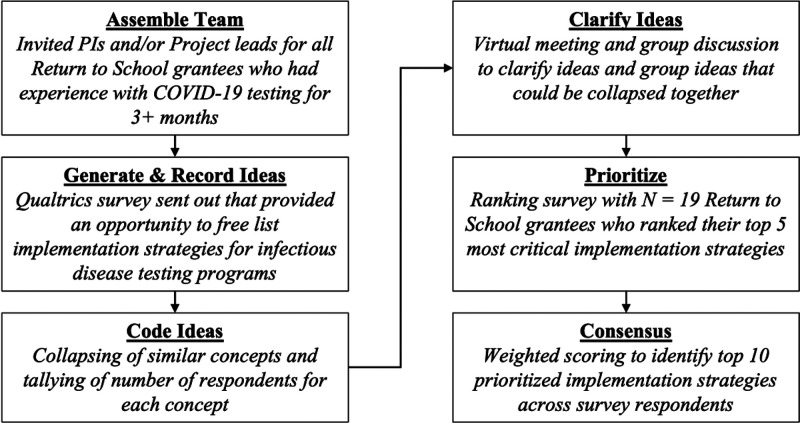
We used a modified Nominal Group Technique (NGT) with the purposive sample of 30 project leads, principal investigators, and other academic research team members across the RADx-UP RTS projects that implemented COVID-19 testing for ≥3 months. Fifteen projects had been testing for at least 3 months at the time of survey deployment. These individuals represent a broad range of child health experts, including pediatricians, epidemiologists, behavioral scientists, program implementers, and infectious diseases specialists. The modified NGT is a structured process for arriving at a consensus that encourages participation from all group members. Our NGT process involved 6 steps: (1) assemble the team, (2) generate and record ideas, (3) code the ideas generated, (4) clarify and refine the set of ideas, (5) prioritize ideas, and (6) construct a consensus set of ideas that can be recommended on the basis of expert opinion and experience (Fig 1).
FIGURE 1.
Modified NGT process.
A 2-step survey approach was used in July 2022 to identify key strategies for school-based COVID-19 testing. First, the authorship team fielded an initial Qualtrics electronic survey to 23 potential participants. These participants ranged from project principal investigators to persons appointed by the project principal investigators who were deemed knowledgeable about implementation strategies employed by schools. Participants could complete the survey on their own or based on group feedback for a specific project. The survey included a brief description of the goal (eg, to create a taxonomy of strategies schools can use to implement infectious disease testing programs) with definitions and examples of potential implementation strategies. Consistent with implementation science literature,12 we defined implementation strategies as “the actions taken to enhance adoption, implementation, and sustainability of infectious disease testing programs.” Participants were asked to list up to 10 strategies per domain of the Consolidated Framework for Implementation Research (CFIR).13 The CFIR was used to elicit strategies that target determinants of COVID-19 testing implementation barriers under 5 different empirically supported domains of implementation (Outer Setting, Inner Setting, Intervention Characteristics, Characteristics of Individuals, and Process).
A single coder (author EH) collapsed strategies identified from the first survey that were similar in meaning and applied a cover term for each strategy described. The set of coded strategies was then reviewed during a virtual conference call meeting with the survey respondents to further collapse and distinguish strategies and ensure agreement on a finalized list. The agreed-on list was compiled into a second Qualtrics electronic survey that was sent to the same 23 potential participants. The second survey asked participants to identify and rank their top 5 most important strategies for the successful implementation of infectious disease testing programs in schools.
From these results, the study authors compiled a consensus list of 10 strategies. Final rankings were based on whether ≥10% of participants ranked the strategy as their top choice, and weighted prioritization was based on the cumulative percentage of participants who placed the strategy among their top 5 choices. The final set of 10 strategies was then produced, representing a consensus from participants on the most important strategies needed to implement COVID-19 testing programs in schools.
Results
COVID-19 Testing Programs
In this study, 11 funded programs submitted programmatic details (Table 1). Participating educational programs were located across the United States and in county sizes ranging from micropolitan to large metropolitan counties. Overall, 4 (36%) programs included Pre-K and early care education, 8 (73%) programs engaged socioeconomically disadvantaged populations, and 4 (36%) programs focused on children with developmental disabilities.
Across programs, 81 916 COVID-19 tests were performed. Most programs provided COVID-19 screening testing (ie, testing those without symptoms and no known exposure), whereas Test to Stay/exposure testing (ie, testing those who have been exposed to someone with COVID-19 but remain asymptomatic), and symptomatic testing were offered at fewer sites. For the COVID-19 testing platform, 7 programs used individual polymerase chain reaction (PCR)/nucleic acid amplification test (NAAT), and 4 used antigen testing. Turnaround time from testing to result was typically 25 to 48 hours for PCR/NAAT and <1 hour for antigen testing. Anterior nares swabs were the primary sample collection method. All programs performed testing at school, and 5 programs offered testing at home and/or in a nonschool community setting.
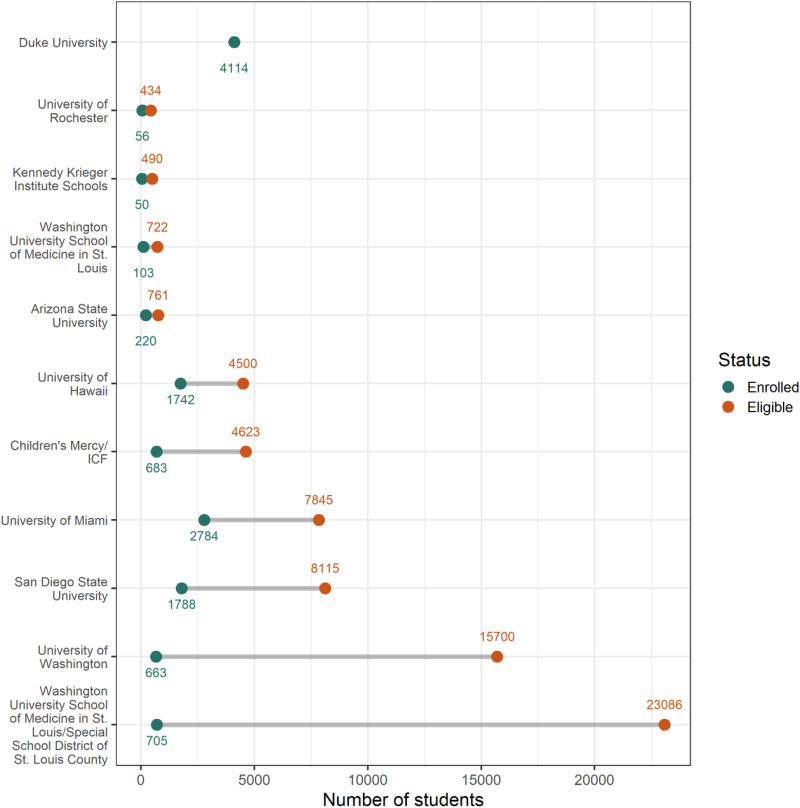
Student participation in the COVID-19 testing programs ranged from 3.1% to 38.7% of the eligible population. In general, programs with smaller eligible populations enrolled a higher percentage of students (Table 2 & Fig 2).
TABLE 2.
Participant Demographics for Those Eligible and Enrolled in Testing Programs
| Male | White | Black | Hispanic, Latino, or Spanish | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | Total students eligible | Total students enrolleda (%) | Enrolledb (%) | Eligiblec | Enrolledb (%) | Eligiblec | Enrolledb (%) | Eligiblec | Enrolledb (%) | Eligiblec |
| Arizona State University | 761 | 220 (28.9) | 99 (45) | —e | 46 (20.9) | — | 8 (3.6) | — | 61 (27.7) | — |
| Children’s Mercy/ICF | 4623 | 683 (14.8) | 303 (44.4) | 51.8% | 213 (31.2) | 11.9% | 247 (36.2) | 40.4% | 231 (33.8) | 35.4% |
| Duke University | — | 4114 (.)f | 2051 (49.9) | 51.3% | 3224 (78.4) | 71.9% | 552 (13.4) | 17.7% | 496 (12.1) | 19.6% |
| University of Hawaii | 4500 | 1742 (38.7) | 526 (30.2) | 50% | 37 (2.1) | 10% | 2 (0.1) | 3% | 64 (3.7) | 1% |
| San Diego State University | 8115 | 1788 (22) | 785 (43.9) | 41.4% | 94 (5.3) | 38.6% | 44 (2.5) | 3.3% | 1379 (77.1) | 77% |
| University of Miami | 7845 | 2784 (35.5) | 1308 (47) | 52% | 747 (26.8) | 26% | 1946 (69.9) | 71% | 776 (27.9) | 28% |
| University of Rochester | 434 | 56 (12.9) | 31 (55.4) | 69% | 31 (55.4) | 60% | 7 (12.5) | 33% | 3 (5.4) | 15% |
| University of Washington | 15700 | 663 (4.2) | 289 (43.6) | 52% | 418 (63) | 15% | — | 0.6% | 486 (73.3) | 80% |
| Washington University School of Medicine in St. Louis | 23086 | 705 (3.1) | 352 (49.9) | — | 178 (25.2) | — | 424 (60.1) | — | 42 (6) | — |
| Washington University School of Medicine in St. Louis/Special School District of St. Louis County | 722 | 103 (14.3) | 81 (78.6) | 76% | 41 (39.8) | 40% | 50 (48.5) | 52% | 3 (2.9) | 3% |
| Kennedy Krieger Institute Schools | 490 | 50 (10.2) | 41 (82) | 81% | 27 (54) | 45% | 16 (32) | 40% | 5 (10) | 8% |
| Overall mean difference (95% CI)d | — | — | −6.5 (−20.1 to 7.2) | 1.2 (−20.6 to 22.9) | −1.1 (−25.3 to 23.1) | −4.2 (−30.9 to 22.4) | ||||
CI, confidence interval.
Percentages are calculated on the basis of the total number of students eligible.
Percentages are calculated on the basis of the total number of participants enrolled.
Percentages are directly collected in the RedCap survey and are approximated by the school district.
t test of mean difference of eligible versus enrolled populations.
Data unavailable.
Unable to calculate due to unavailable data.
FIGURE 2.
Difference between the number of students participating in a COVID-19 testing program compared with the number of students eligible for the program.
Key Program Implementation Strategies
For the implementation strategies survey, 11 of the 15 programs (73%) that had been testing for ≥3 months participated in a component of the survey. A total of 11 participants (47.8% of full sample) completed the initial survey, which resulted in 255 strategies listed across the 5 CFIR domains. After initial coding, these 255 strategies were reduced to a total of 64 strategies. During a virtual meeting, 9 participants refined these strategies to a final list of 45 strategies. At the meeting, participants agreed that the list of strategies could not be specific to each CFIR domain as some strategies spanned multiple domains. As a result, further prioritization and consensus activities did not include strategies separated by specific CFIR domain.
For the final survey, a total of 19 participants responded (82.6%). Overall, 19 of the 45 strategies were not prioritized by any respondent as one of their top 5 most important strategies. All participants considered “Adapting testing strategies to meet the needs, preferences, and changing guidelines” as the most important implementation strategy. Participants shared the following examples of this strategy: (1) “instead of setting [testing] up in a room and bringing the person being tested to the room, go to the room where the person is located,” (2) “modify the testing strategy to align with the preferred testing strategy,” and (3) “design intentional opportunities to adapt and change course.” The second most important strategy was “Holding regular meetings with school leadership and staff.” Participants described this strategy as follows: (1) “regularly scheduled meetings with district testing champions, supervisors, and support staff as well as our local team and members of [the local health facility] to discuss problems and problem-solving,” (2) “regular meetings with school officials,” and (3) “continuously communicating with health center and school staff/administration.” The third most important strategy was “Assessing and responding to community needs.” All other prioritized strategies are included in Table 3.
TABLE 3.
Final List of Implementation Strategies Ranked in Order of Importance
| Strategy | Percentage Top Choice | Cumulative Percentage of Participants Who Ranked in Top 5 | Final Importance Ranking |
|---|---|---|---|
| Adapt testing strategies to needs and preferences; change guidelines | 32% | 84% | 1 |
| Meet regularly with school leadership and staff | 16% | 37% | 2 |
| Assess and respond to community needs | 11% | 32% | 3 |
| Establish/maintain Community Advisory Board | 11% | 21% | 4 |
| Integrate into existing infrastructure when possible | 0% | 53% | 5 |
| Identify, empower, and train champions | 5% | 47% | 6 |
| Develop materials tailored to community you serve (eg, communication, consent forms) | 0% | 37% | 7 |
| Report and disseminate data regularly | 5% | 32% | 8 |
| Meet with local stakeholders | 5% | 16% | 9 |
| Raise awareness through outreach and strategic communications | 0% | 21% | 10 |
Discussion
The US federal government invested substantial COVID-19 testing resources in Pre-K-12 schools to directly support a safe return to schools 1 year into the pandemic. Implementing infectious disease testing in schools during the 2021 to 2022 school year involved the rapid deployment of severe acute respiratory syndrome coronavirus 2 testing by school administrators and nurses, state and federal agencies, and testing companies. Despite this influx of support, testing program uptake and access in Pre-K-12 schools remain poorly understood. The RADx-UP RTS programs provide new information about testing uptake in pediatric populations and highlight key recommended strategies for testing program implementation in school populations.
With support from the National Institutes of Health, a variety of testing programs were developed to meet the needs of underserved and vulnerable children. We learned that a one-size-fits-all approach does not apply to all children, schools, and school systems when considering testing approaches and that testing enrollment can vary greatly across districts. A consensus was met by the RADx-UP RTS project leads and principal investigators, identifying key considerations for the implementation of diagnostic testing programs focused on underserved and/or vulnerable children.
RADx-UP RTS programs sought to implement testing strategies on the basis of community needs and implementation capabilities. Although individual states and the Centers for Disease Control and Prevention have provided broad programmatic guidance for COVID-19 school testing programs, little information is available on testing methods for vulnerable children or children with medical complexities. Challenges to testing included obtaining consent when most schools were not allowing caregivers inside school buildings because of the pandemic, communicating with non-English-, non-Spanish-speaking families, and developing safe and effective processes for testing young children and those with developmental disabilities outside of the medical setting.
Models to determine the efficacy of COVID-19 testing programs should be based on the realities of program enrollment and participation. Minimizing COVID-19 infections and transmission in schools is multifaceted and complex, with vaccination rates, community COVID-19 rates, mitigation strategies, and activities all contributing to transmission risk.14–16 Recent studies reveal that in-school testing programs can help reduce in-school transmission when the participation rates of students and staff are high (∼90% to 100%).8,17,18 However, inferences from existing studies of school testing programs across the United States are limited because they provide the number of tests performed but lack testing participation or enrollment data.19–21 We observed variable enrollment across RADx-UP RTS testing programs. Participant enrollment mirrored eligible participant demographics, suggesting that programs were able to effectively provide COVID-19 testing to diverse populations.
Little guidance is available for best practices related to in-school COVID-19 testing programs. Pilot testing programs in K-12 schools provided early insights, recommendations, and implications to guide testing programs.8 Adapting testing strategies and modifying guidelines were identified as the most important implementation strategy among RADx-UP project leaders. This likely reflects both the focus of the RADx-UP RTS program to bring testing to vulnerable pediatric populations and highlights the need to be flexible and adapt to ongoing updates and modifications of COVID-19 school protocols. Engaging communities and meeting with school leadership were also identified as key integration factors. These implementation strategies should be considered by vendors and program developers when constructing initiatives for school communities.
The limitations of this study include the self-reported implementation details, the variation in testing program design and context, and the potential lack of generalizability to schools across the country. However, the RADx-UP RTS programs represent a wide variety of pediatric populations in geographically distinct locations. We did not look specifically at the impact of COVID-19 testing on safe return to school as this was beyond the scope of this study and is being explored in additional work.22,23 Additionally, the key implementation strategies were identified by the project leads and principal investigators only, and input from schools and families was not evaluated in this study.
Conclusions
In conclusion, collaborations between academic centers and schools helped to provide COVID-19 testing for vulnerable children and children with medical complexities. Testing can be conducted by using different approaches that meet the unique needs of the population. Additional work is needed to develop best practices for the implementation of infectious disease testing for all children at school.
Acknowledgments
Brooke Walker, MS, Duke Clinical Research Institute, provided editorial review and submission. Ms Walker did not receive compensation for her contributions, apart from her employment at the institution in which this study was conducted.
Glossary
- CFIR
Consolidated Framework for Implementation Research
- COVID-19
coronavirus disease 2019
- NAAT
nucleic acid amplification test
- NGT
Nominal Group Technique
- PCR
polymerase chain reaction
- Pre-K-12
prekindergarten-12
- RADx-UP
Rapid Acceleration of Diagnostics Underserved Populations
- RTS
Safe Return to School Diagnostic Testing Initiative
Footnotes
Drs Goldman, Schuster, Kalu, and Haroz conceptualized and designed the study, designed the data collection instruments, conducted the initial analyses, and critically reviewed and revised the manuscript for important intellectual content; Drs Zimmerman, Benjamin, Newland, Foxe, Zand, Ko, Gurnett, Kalb, Inkelas, Manuel, Lee, Okihiro, Gwynn, McDaniels-Davidson, Coller, DeMuri, McCulloh, Keener Mast, and Wu conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed and revised the manuscript; Dr Brookhart and Mr Erickson conducted data analyses and critically reviewed and revised the manuscript; Drs Dewhurst, Oh, Wetter, Murugan, Kramer, Broadhurst, Pulgaron, Kiene, Oren, and Stump and Mr Sherby, Mr Walsh, Mr Watterson, Ms Godambe, Ms Shinde, Ms Archuleta, and Mr Fist coordinated and supervised data collection and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
FUNDING: Funded by the National Institutes of Health (NIH). Research reported in this publication was supported by the Office of the Director of the NIH under award number U24MD016258; NIH Agreement No.’s OT2HD107543, OT2HD107544, OT2HD107553, OT2HD107555, OT2HD107556, OT2HD107557, OT2HD107558, OT2HD107559, OT2HD108103, OT2HD108101, OT2HD108105, OT2HD108111, OT2HD108106, OT2HD108112, OT2HD108097, OT2HD108110, 3P0HD103525-03S1; the National Center for Advancing Translational Sciences of the NIH under award number U24TR001608; and the National Institute of Child Health and Human Development of the NIH under contract HHSN275201000003I.
CONFLICT OF INTEREST DISCLOSURES: Dr Kalu reports funding from the Centers for Disease Control and Prevention (CDC) Epicenter and the NIH, and receives consultancy fees from IPEC Experts and Wayfair. Dr Zimmerman reports funding from the NIH and the United States Food and Drug Administration (FDA). Dr Goldman reports funding from the NIH. Dr Schuster reports funding from the NIH, CDC, and Merck. Dr Newland reports funding from NIH, Pfizer, and Merck. The other authors have no conflicts of interest to disclose.
References
- 1. US Centers for Disease Control and Prevention . School testing for COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/school-testing.html. Accessed July 20, 2022
- 2. US Centers for Disease Control and Prevention . ELC reopening schools: support for COVID-19 screening testing to reopen and keep schools operating safely. Available at: https://www.cdc.gov/ncezid/dpei/elc/covid-response/index.html. Accessed July 20, 2022
- 3. North Carolina Department of Health and Human Services . NCDHHS selects state vendors to support K-12 schools implementing COVID-19 testing this fall. Available at: https://www.ncdhhs.gov/news/press-releases/2021/08/05/ncdhhs-selects-state-vendors-support-k-12-schools-implementing-covid-19-testing-fall. Accessed July 19, 2022
- 4. Missouri Department of Health and Senior Services . Missouri DHSS and DESE bring pooled COVID-19 testing to K-12 classrooms in partnership with Concentric by Ginkgo. Available at: https://health.mo.gov/news/newsitem/uuid/45e76509-1a4e-4166-8a5c-ba82ed8f9704/missouri-dhss-and-dese-bring-pooled-covid-19-testing-to-k-12-classrooms-in-partnership-with-concentric-by-ginkgo. Accessed July 20, 2022
- 5. Cernich AN, Lee S, Bianchi DW. Building the evidence for safe return to school during the COVID-19 pandemic. Pediatrics. 2022;149(12 Suppl 2):e2021054268B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health . NIH-funded COVID-19 testing initiative aims to safely return children to in-person school. Available at: https://www.nih.gov/news-events/news-releases/nih-funded-covid-19-testing-initiative-aims-safely-return-children-person-school. Accessed July 20, 2022
- 7. Haroz EE, Kalb LG, Newland JG, et al. Implementation of school-based COVID-19 testing programs in underserved populations. Pediatrics. 2022;149(12 Suppl 2):e2021054268G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Divya VRP, Goyal R, Hotchkiss J, O-Neil S. Mathematica and The Rockefeller Foundation . Early insights and recommendations for implementing a Covid-19 antigen testing program in K-12 schools: lessons learned from six pilot sites. Available at: https://www.rockefellerfoundation.org/wp-content/uploads/2021/02/Early-Insights-and-Recommendations-for-K-12-Schools-Covid-19-Testing-Lessons-Learned-from-Six-Pilot-Sites.pdf. Accessed February 20, 2023
- 9. National Institutes of Health RADx-UP . Return to school diagnostic testing approaches. Available at: https://www.nih.gov/sites/default/files/research-training/initiatives/radx/20210429-Phase-II-ROA.pdf. Accessed February 20, 2023
- 10. Harris PA, Taylor R, Minor BL, et al. REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brownson RC, Colditz GA, Proctor EK, eds.. Dissemination and Implementation Research in Health: Translating Science to Practice. Oxford, UK: Oxford University Press; 2017 [Google Scholar]
- 13. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2021;21(3):333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnaratne S, Littlecott H, Sell K, et al. Measures implemented in the school setting to contain the COVID-19 pandemic. Cochrane Database Syst Rev. 2022;1(1):CD015029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eyre DW, Taylor D, Purver M, et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022;386(8): 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bilinski A, Ciaranello A, Fitzpatrick MC, et al. Estimated transmission outcomes and costs of SARS-CoV-2 diagnostic testing, screening, and surveillance strategies among a simulated population of primary school students. JAMA Pediatr. 2022;176(7):679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilinski A, Salomon JA, Giardina J, et al. Passing the test: a model-based analysis of safe school-reopening strategies. Ann Intern Med. 2021;174(8):1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mendoza RP, Bi C, Cheng HT, et al. Implementation of a pooled surveillance testing program for asymptomatic SARS-CoV-2 infections in K-12 schools and universities. EClinicalMedicine. 2021;38:101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NYC Department of Education . School based testing report. Available at: https://testingresults.schools.nyc/. Accessed July 20, 2022
- 21. Massachussetts Department of Elementary and Seconday Education . Positive COVID-19 cases in schools. Available at: https://www.doe.mass.edu/covid19/positive-cases/default.html. Accessed July 20, 2022
- 22. Berke EM, Newman LM, Jemsby S, et al. Pooling in a pod: a strategy for COVID-19 testing to facilitate a safe return to school. Public Health Rep. 2021;136(6):663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samson ME, Still WL, Mark-Carew M, et al. Utility of a test-to-return strategy to identify individuals with coronavirus disease 2019 (COVID-19) in the prekindergarten through grade 12 school setting - District of Columbia, January 2022. Clin Infect Dis. 2022; 75(Suppl 2):S231–S235 [DOI] [PMC free article] [PubMed] [Google Scholar]